D-type non-natural amino acid containing antimicrobial peptide analog, synthesis therefor and application of D-type non-natural amino acid containing antimicrobial peptide analog
A technology of unnatural amino acids and antimicrobial peptides, applied in the field of biochemistry, can solve problems such as limiting the development of natural antimicrobial peptides, destroying mammalian cells, and poor enzymatic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
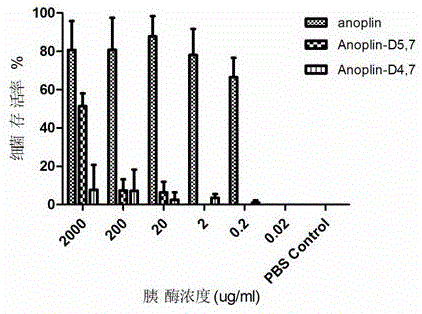
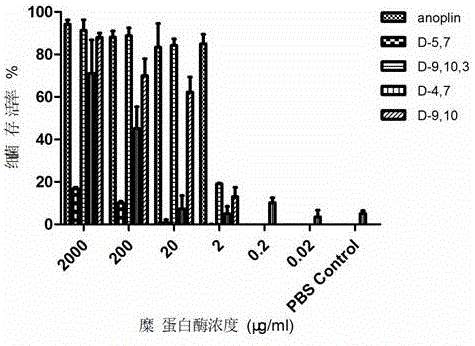
[0045] Embodiment 1, the synthesis of analogue Anoplin-D9,10
[0046] Weigh Rink-MBHA resin, the substitution value is 0.43mmol / g, the amount of peptide synthesis is 0.15mmol, add redistilled dichloromethane solution, fully stir and swell the resin for 30min, then add 10% hexahydropyridine to remove the resin Fmoc protection, wash 2 minutes each time, wash 4 times in total, then wash the resin with redistilled DMF solution, wash the resin 2 minutes each time, wash 4 times in total, and finally use indene detection indicator (phenol: potassium cyanide pyridine: ninhydrin = 1:2:1) Check whether the Fmoc protecting group has been removed from the resin, boil the resin for 1 min, observe immediately, and the resin turns blue, which proves that the Fmoc group has been removed. According to the sequence of the peptide, start from the C-terminus and weigh The first amino acid is d-Leu, 1.5-fold excess, add HOBT (1-fold excess), HBTU (1-fold excess), after the amino acid is dissolved,...
Embodiment 2
[0047] Example 2 Synthesis of Analog Anoplin-D9,10,3
[0048] Weigh Rink-MBHA resin, the substitution value is 0.43mmol / g, the amount of peptide synthesis is 0.25mmol, add redistilled dichloromethane solution, fully stir and swell the resin for 30min, then add 15% hexahydropyridine to remove the resin Fmoc protection, wash 2 minutes each time, wash 4 times in total, then wash the resin with redistilled DMF solution, the same 2 minutes each time, wash 4 times in total, and then detect the indicator (phenol: potassium cyanide pyridine: ninhydrin = 1 :2:1) test, the resin turns blue, which proves that the Fmoc group on the resin has been removed. According to the sequence of the peptide, starting from the C-terminus, weigh the first amino acid d-Leu amino acid, and add HOBT to a 2-fold excess (3 times excess), HBTU (3 times excess), after the amino acid is dissolved, add starter DIEA (6 times excess). The above amino acid and the resin were stirred and reacted together for 1.5 h...
Embodiment 3
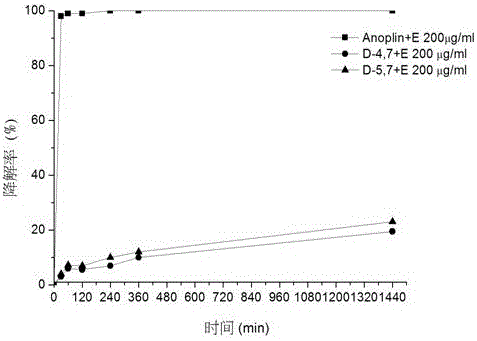
[0049] Embodiment 3, the synthesis of analogue Anoplin-D4,7
[0050] Weigh the Rink-MBHA resin, the substitution value is 0.43mmol / g, the amount of peptide synthesis is 0.2mmol, add redistilled dichloromethane solution, fully stir and swell the resin for 30min, then add 15% hexahydropyridine to remove the resin Fmoc protection, wash 2 minutes each time, wash 4 times in total, then wash the resin with redistilled DMF solution, the same 2 minutes each time, wash 4 times in total, and then detect the indicator (phenol: potassium cyanide pyridine: ninhydrin = 1 :2:1) test, the resin turns blue, which proves that the Fmoc group has been released. According to the sequence of the peptide, starting from the C-terminus, weigh the first amino acid Leu, 3-fold excess, add HOBT (5-fold excess), HBTU (5-fold excess), after the amino acid is dissolved, add starter DIEA (6-fold excess). Stir and react the above amino acid with the resin for 1 hour. After the reaction, check with ninhydrin....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com