Chemical modification method of endostatin and its use
An endostatin, chemical modification technology, applied in chemical instruments and methods, peptide preparation methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] 1. Preparation of PEG-ES:
[0030] (1) Coupling of PEG and ES
[0031] 100ml ES stock solution (pH4.5, 20mM, HAC / NaAc Buffer, protein content is 1.07mg / ml) adjust the pH to 5.52 with 1M NaAc, add the reducing agent sodium cyanoborohydride NaCNBH 3 To the final concentration of 20mM, add 267.5mg of monomethoxy polyethylene glycol propionaldehyde to make the molar ratio of ES to 1:1. Stir gently at 4℃ for 10 hours, and finally adjust the pH to 4.0 with 100mM HCl. The reaction is to form a PEG-ES modification, and the product is identified by SDS-PAGE. (Attached figure 1 )
[0032] (2) Purification of PEG-ES
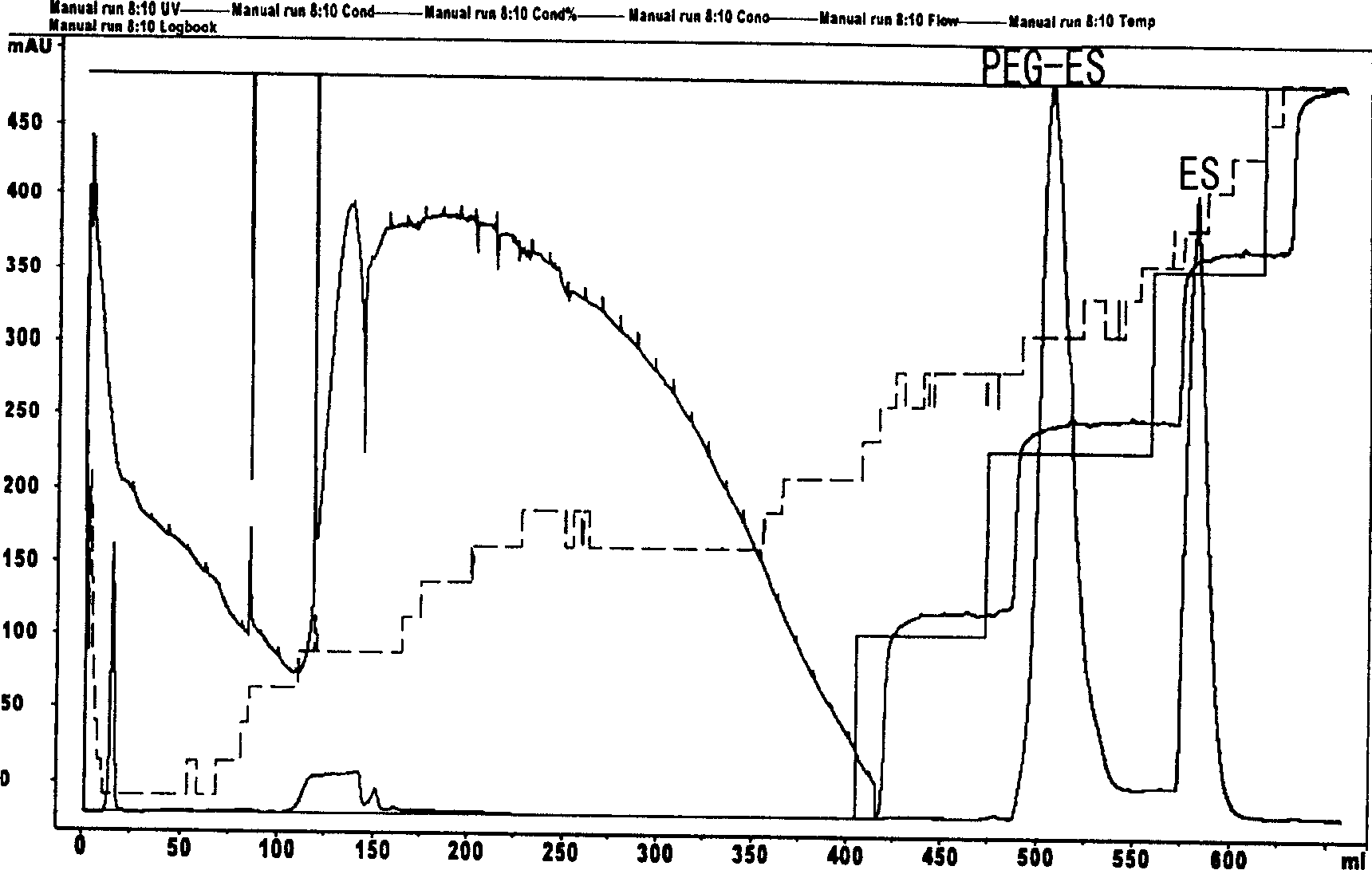
[0033] The above reaction product was separated and purified with a CM-Sepharose column (bed volume 15ml) with a KTA chromatograph. After loading the sample, 20mM containing 0.1M, 0.2M, 0.3M, NaCl, pH4.5, HAC / NaAc Buffer for stepwise elution (attached figure 2 ) Collect the eluate containing 0.2M NaCl, which is PEG-ES, and freeze-dry after dialysis to obtain 60 mg.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com