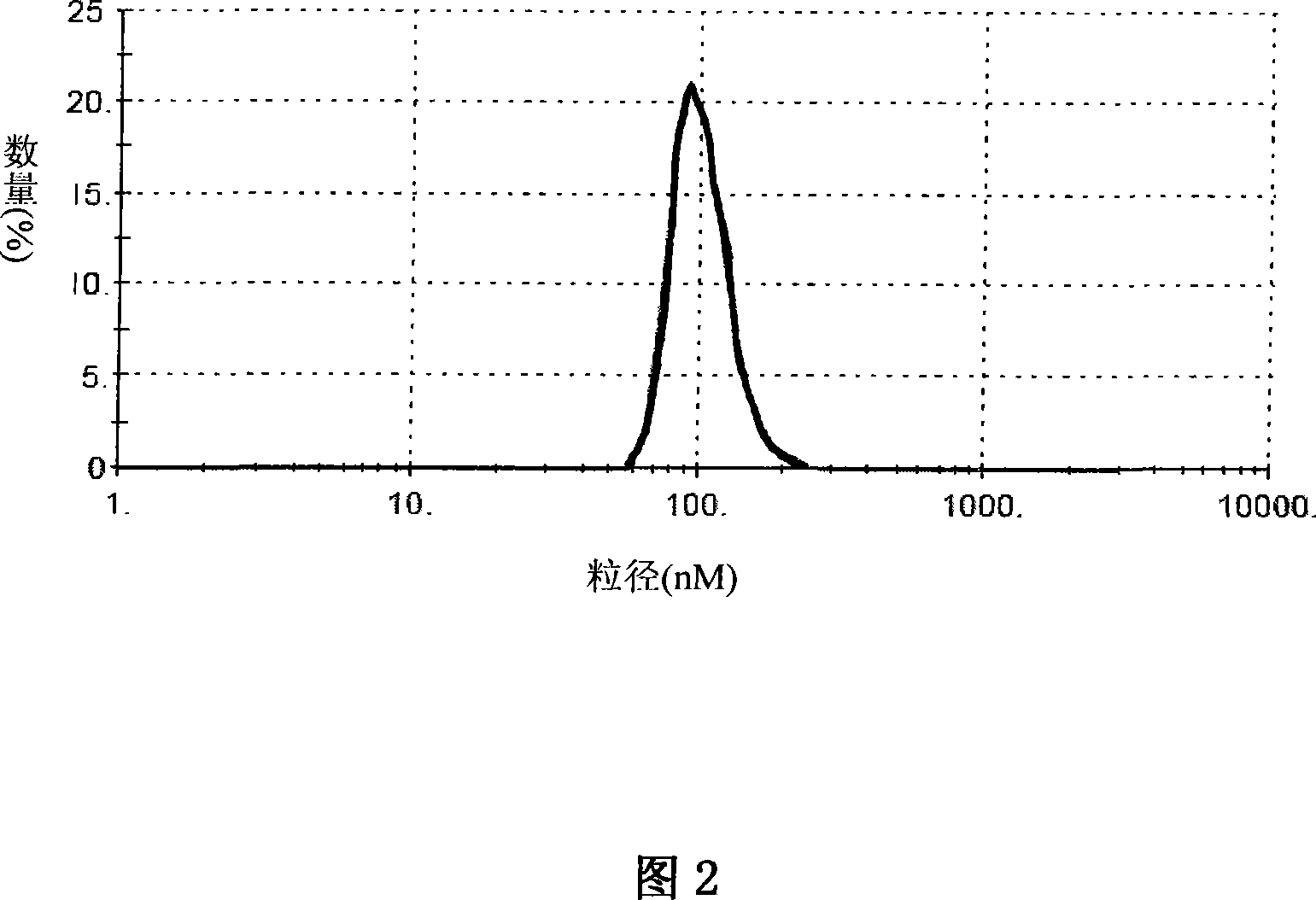
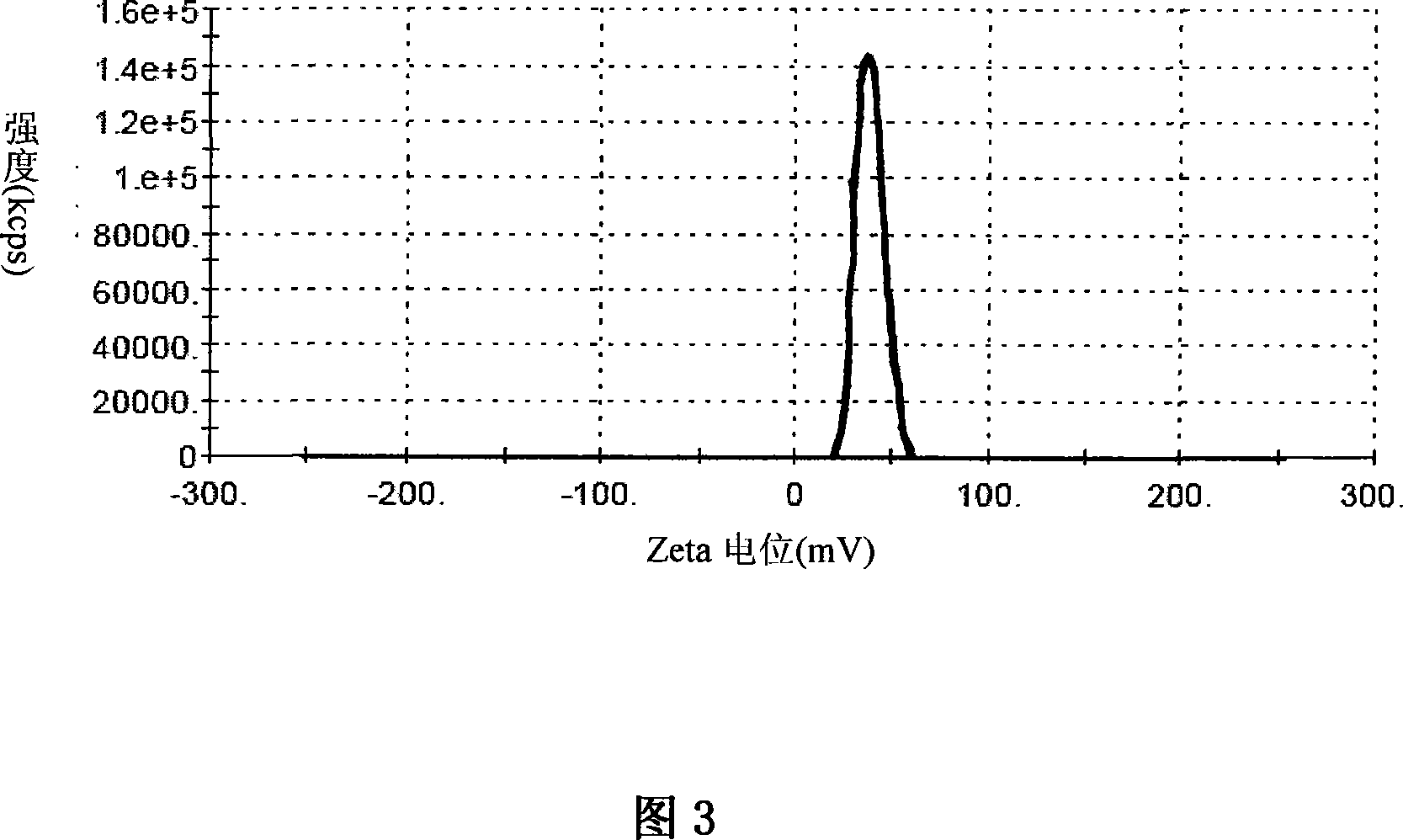
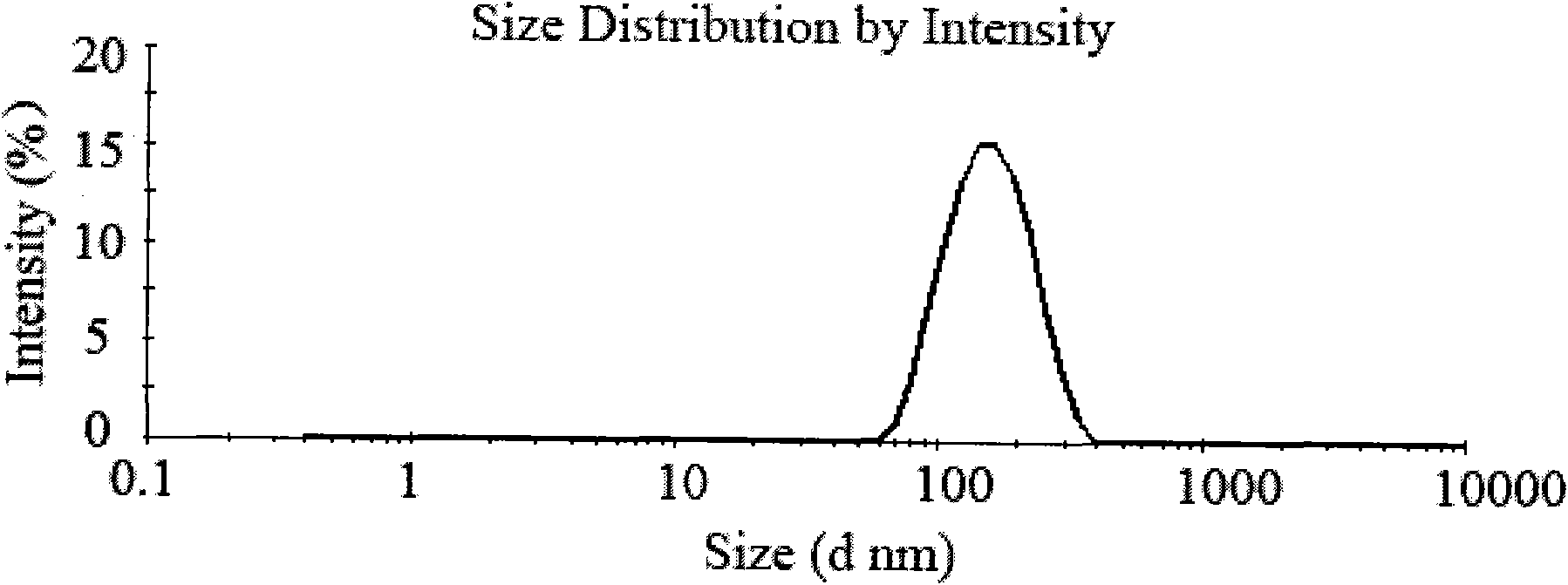
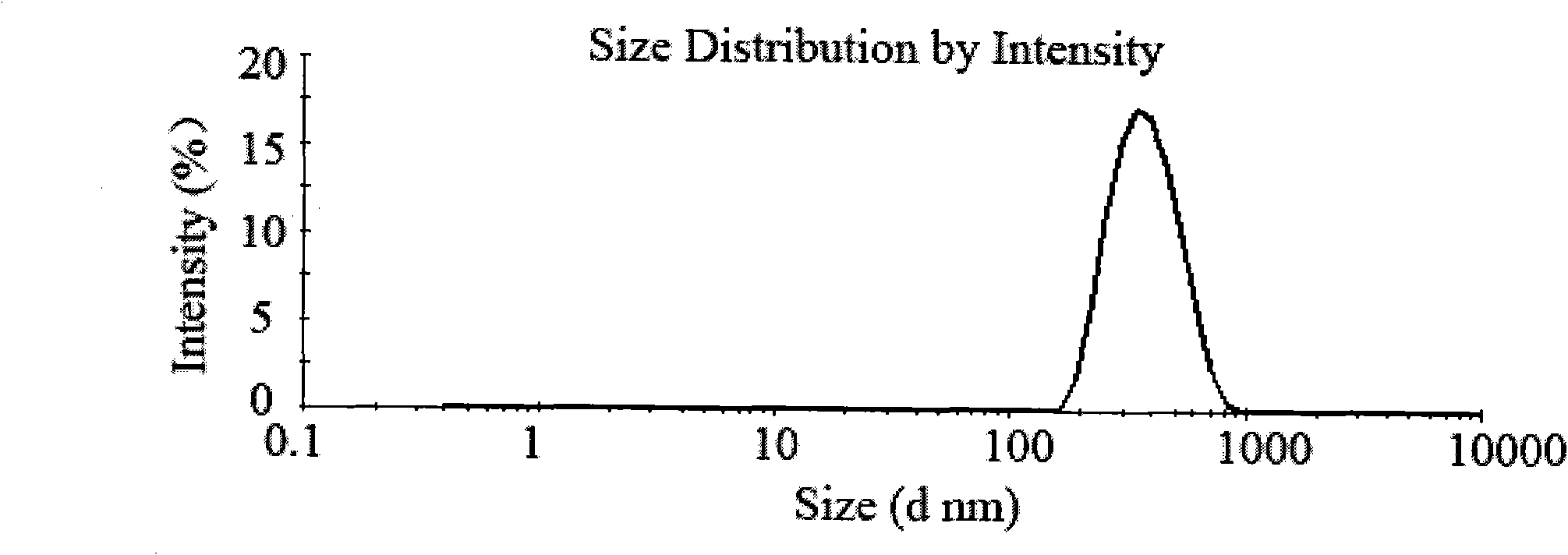
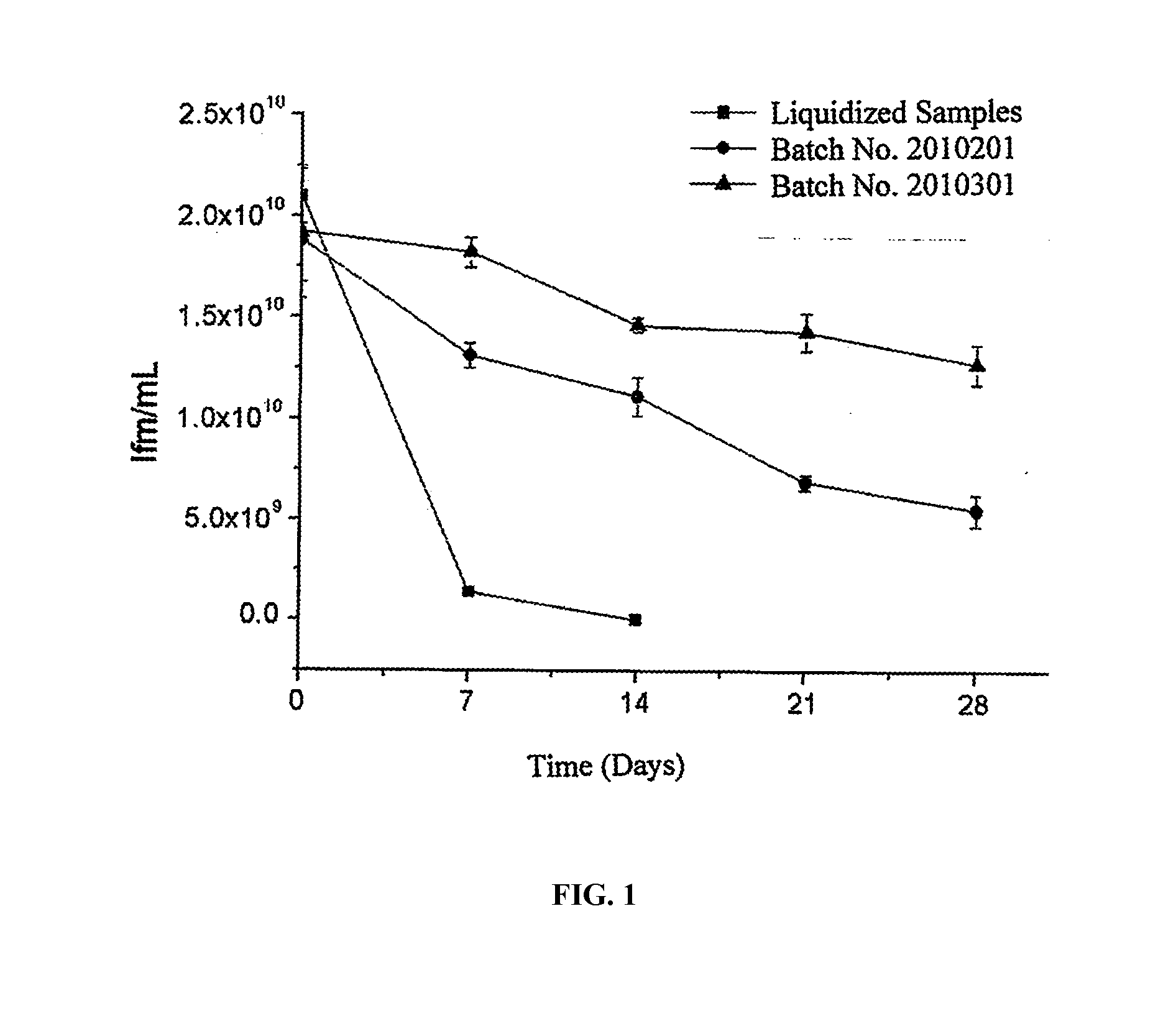
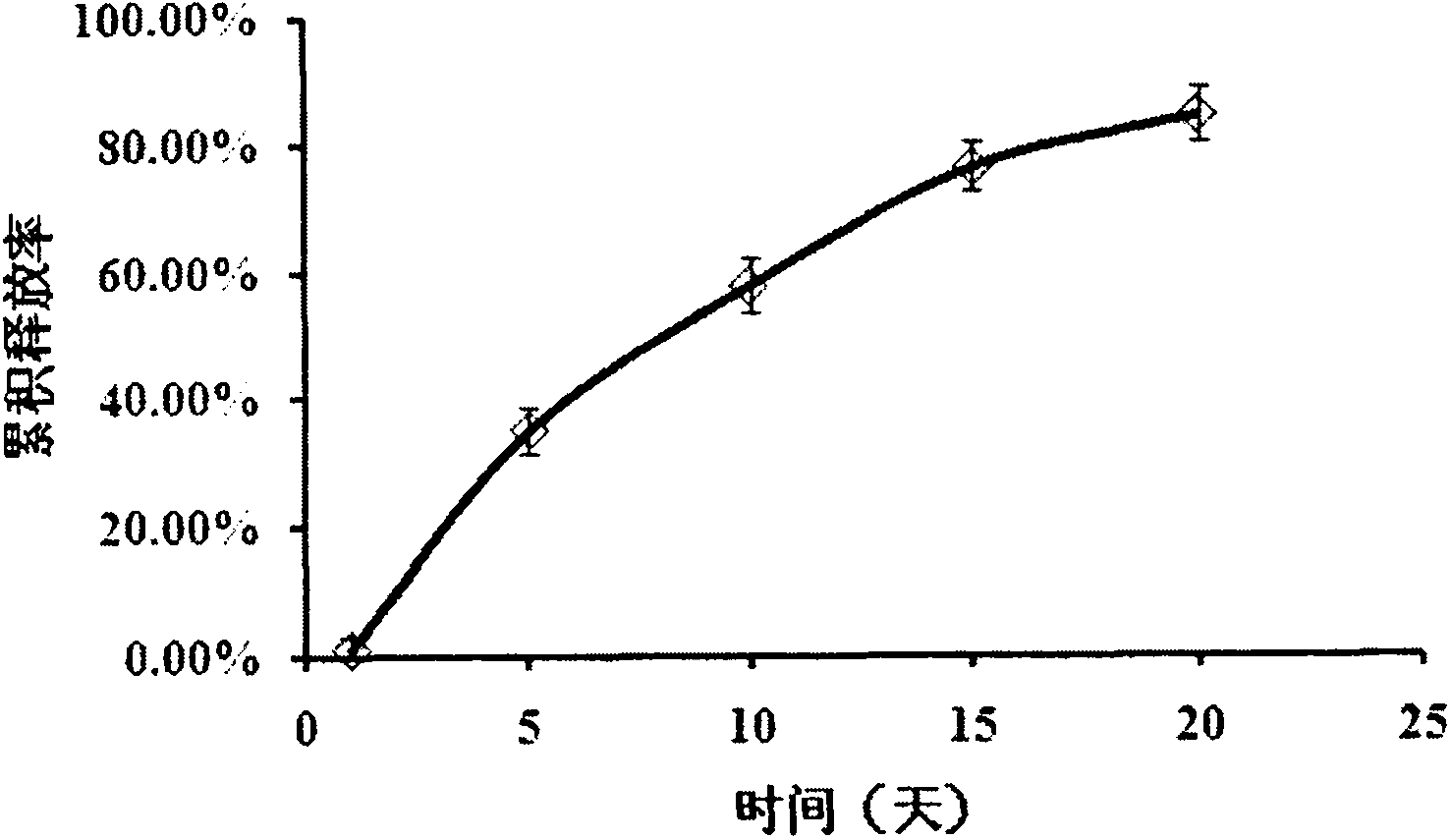
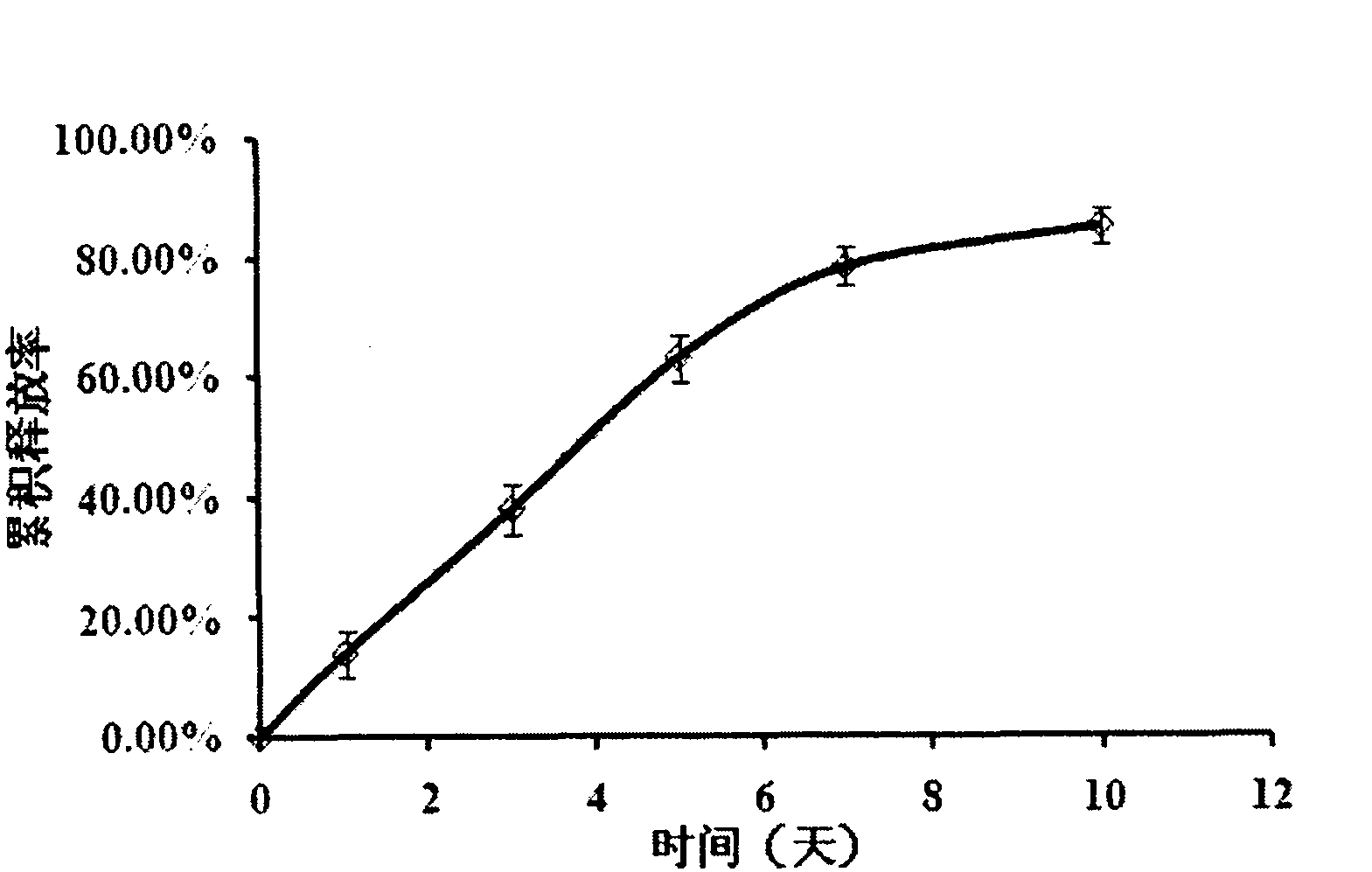
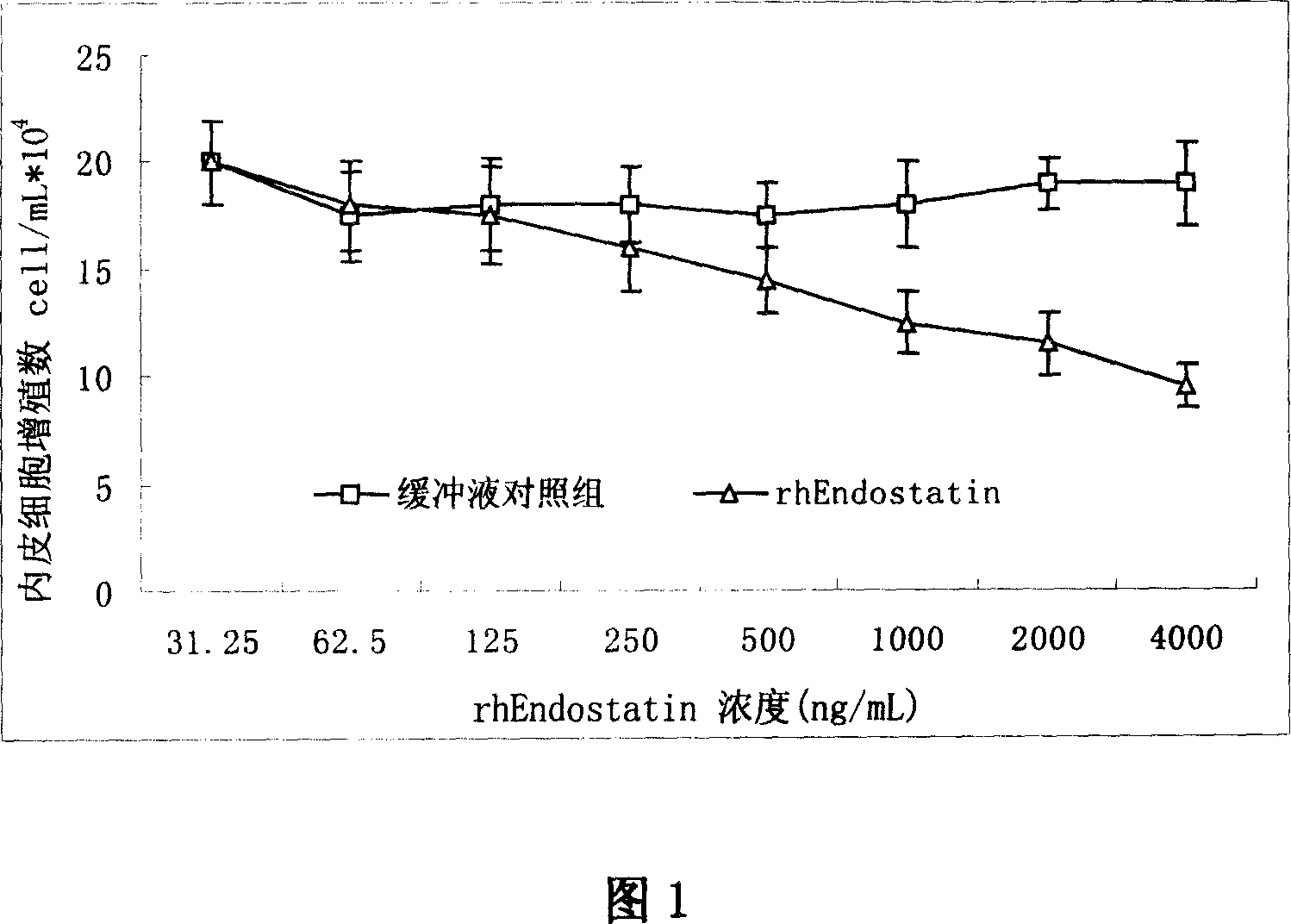
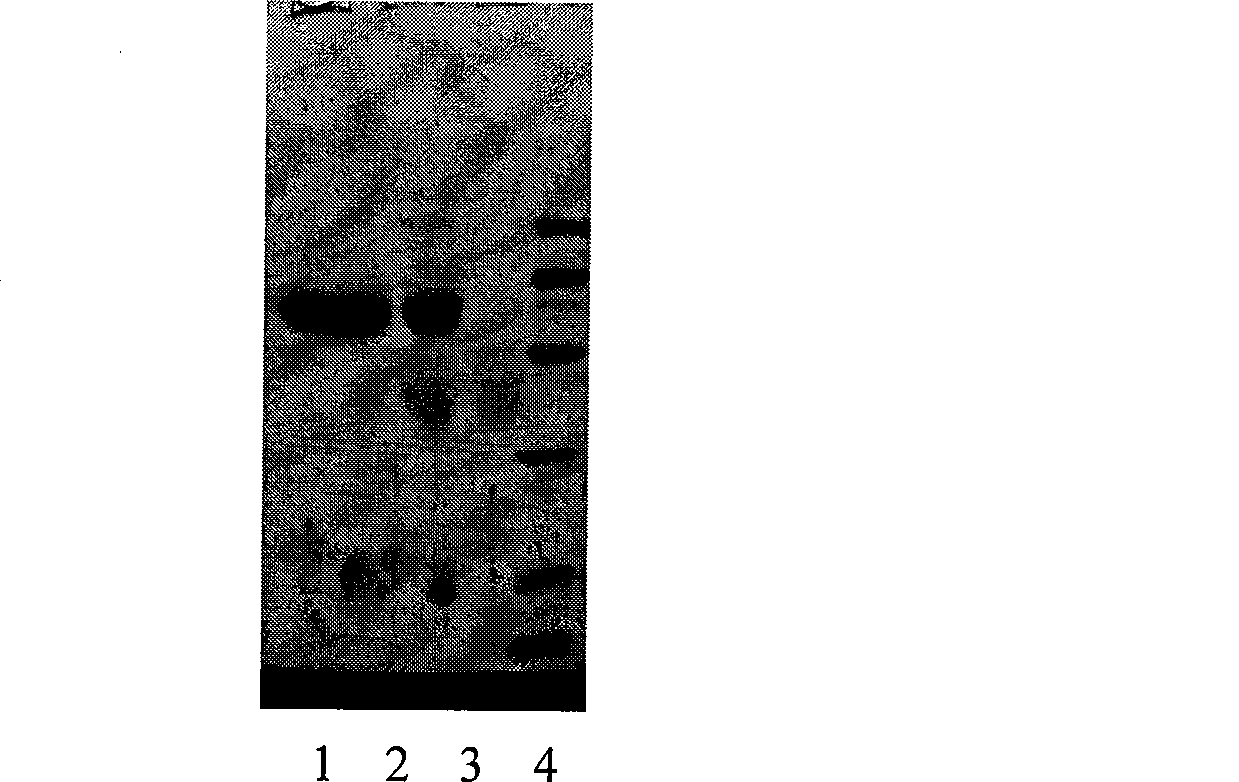
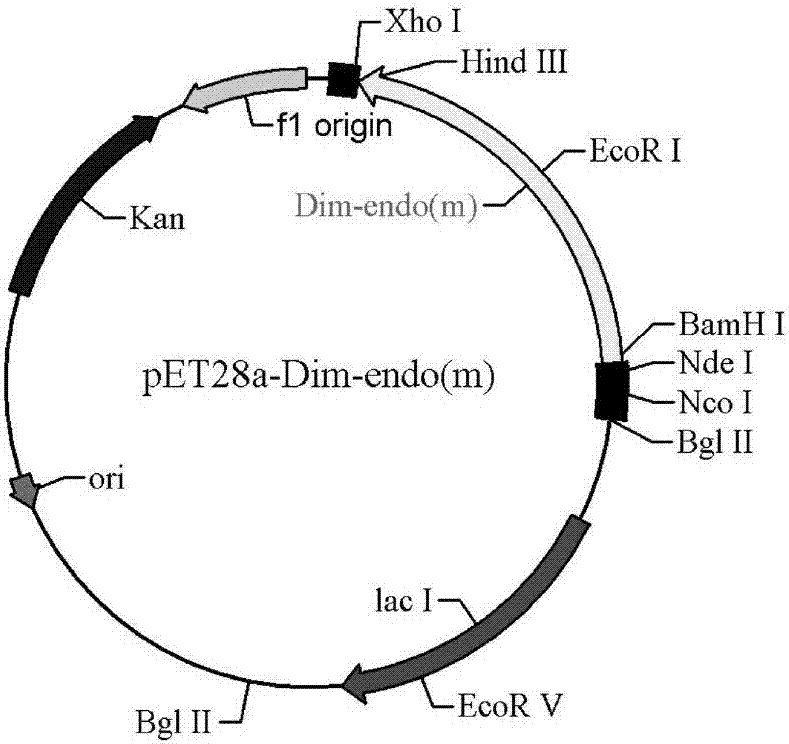
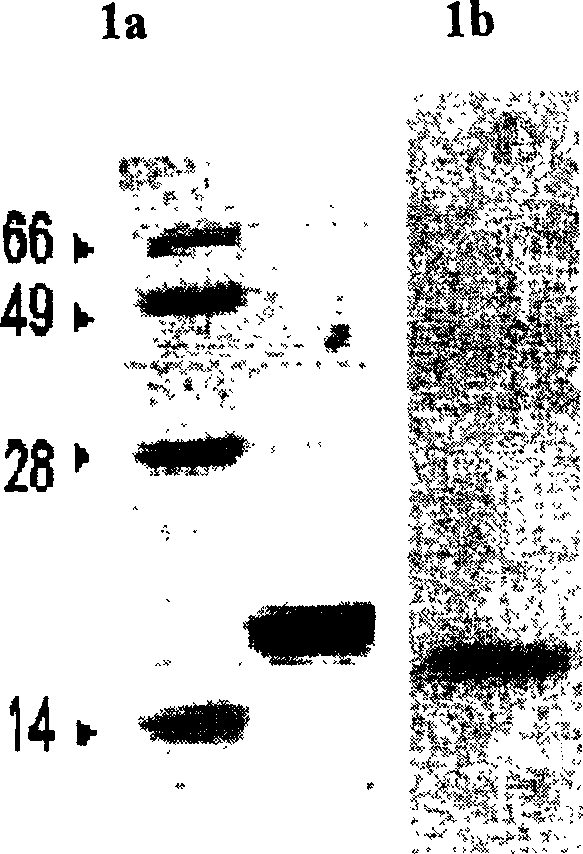
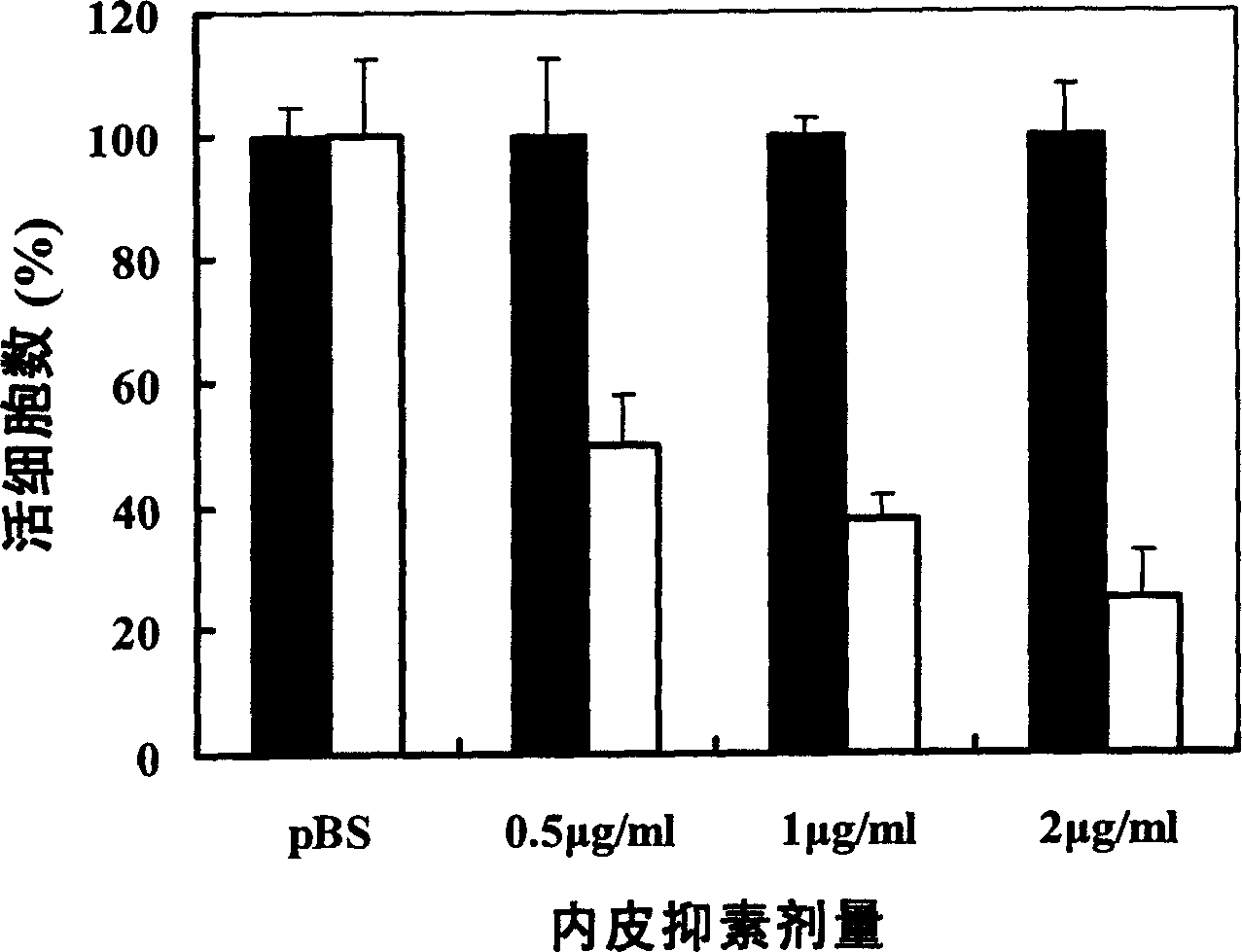
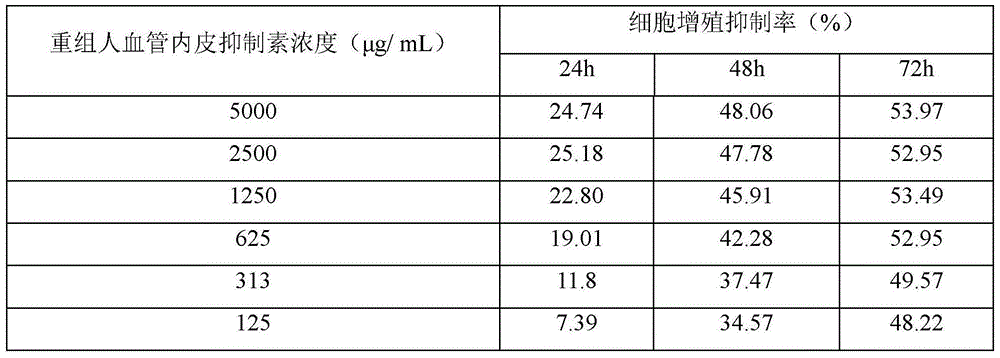
Patents
Literature
56 results about "Recombinant Human Endostatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chemical modification method of endostatin and its use
InactiveCN1891717ANot easy to hydrolyzeStable pHPeptide/protein ingredientsPeptide preparation methodsMonomethoxypolyethylene glycolChymotrypsin
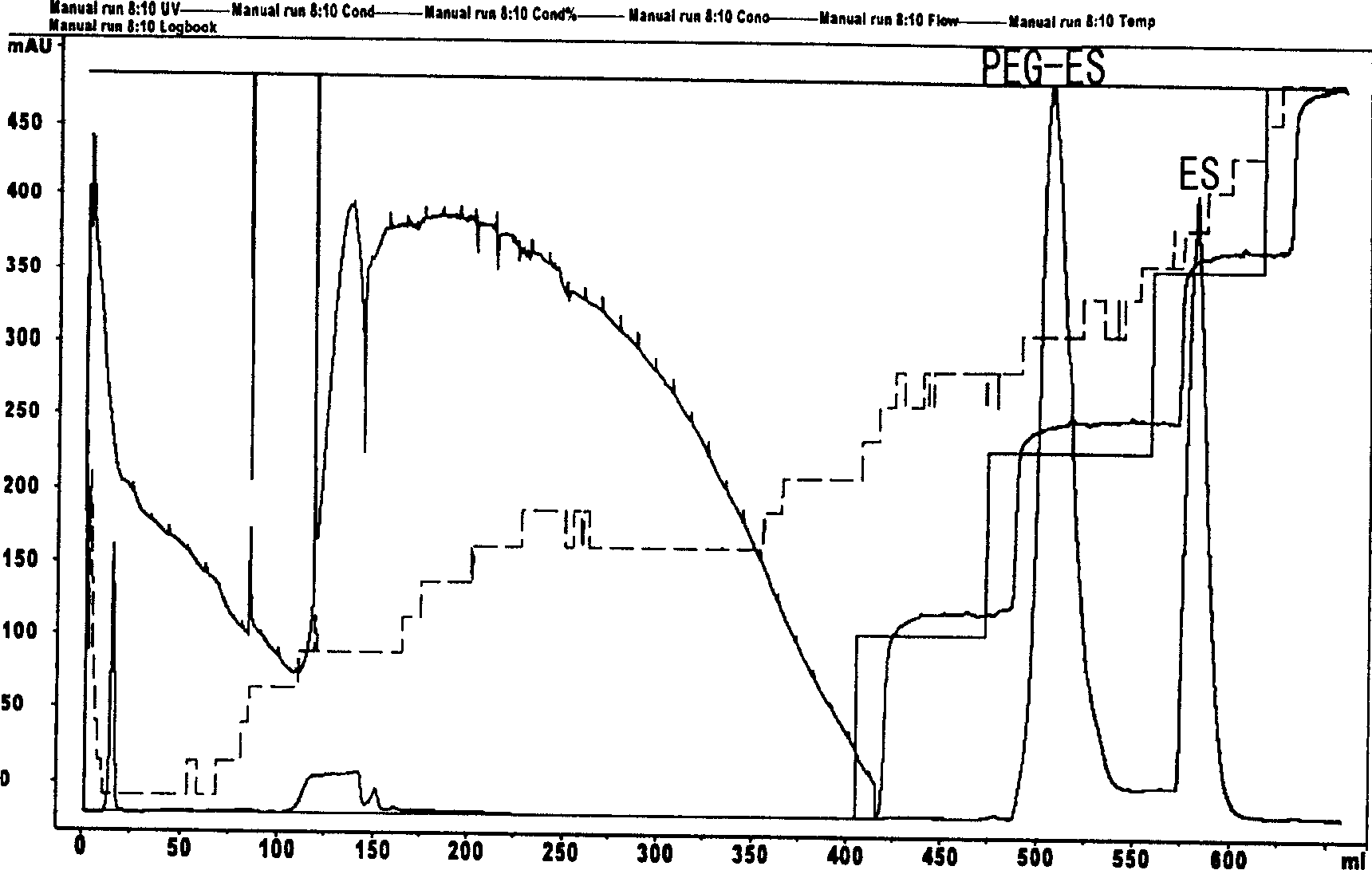
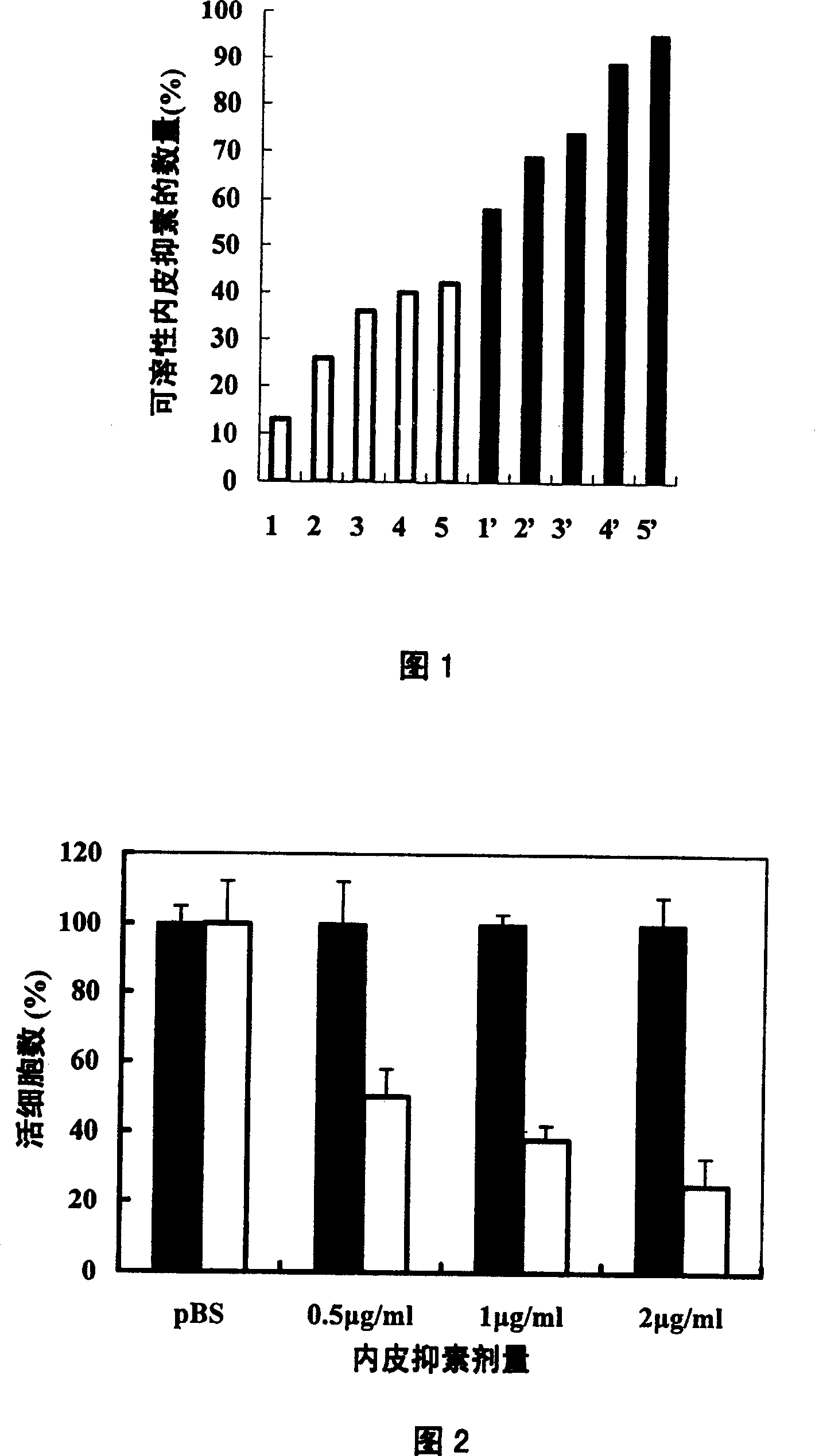
The invention belongs to the field of bio-pharmaceutical products, which provides a chemical modification method for recombinate human rh-Endostatin ('rh-ES' is called for short) through the way of using mPEG-propionaldehyde to be combined on the fixed point of free amino of N-terminal amino acid of rh-ES. Mono-PEG-rhES is the output material, its purity meet the requirements of biological products. It plays a key role in the hydrolysis resistance of trypsin and chymotrypsin, it can be used as long-acting formulations for its stability for pH and temperature and there is a great perspective in the clinical treatment of cancer.
Owner:NANJING UNIV +1
Medicinal composition and application thereof
ActiveCN101890155AHigh anti-hepatoma activityOrganic active ingredientsPeptide/protein ingredientsVascular endotheliumTransplanted liver
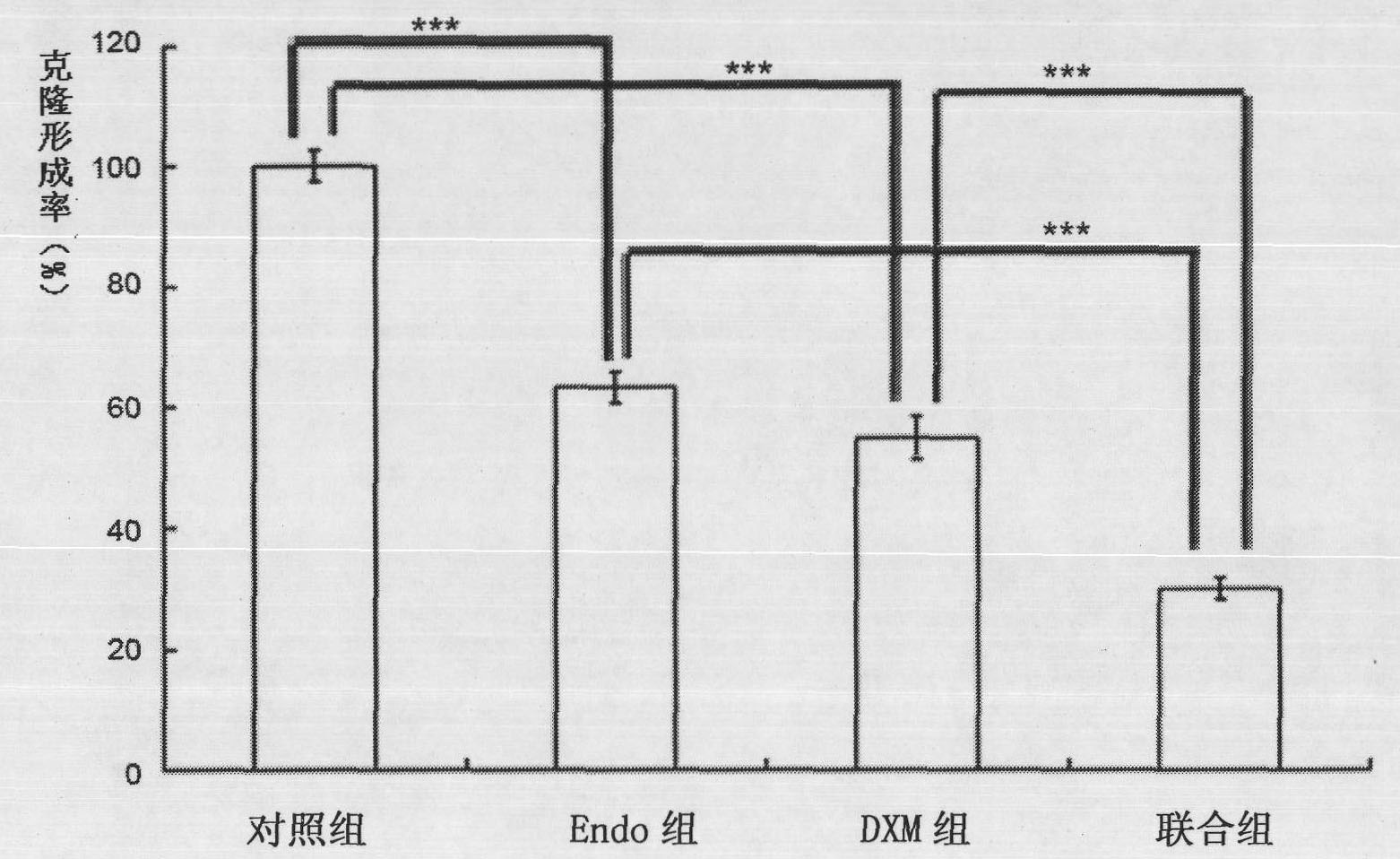
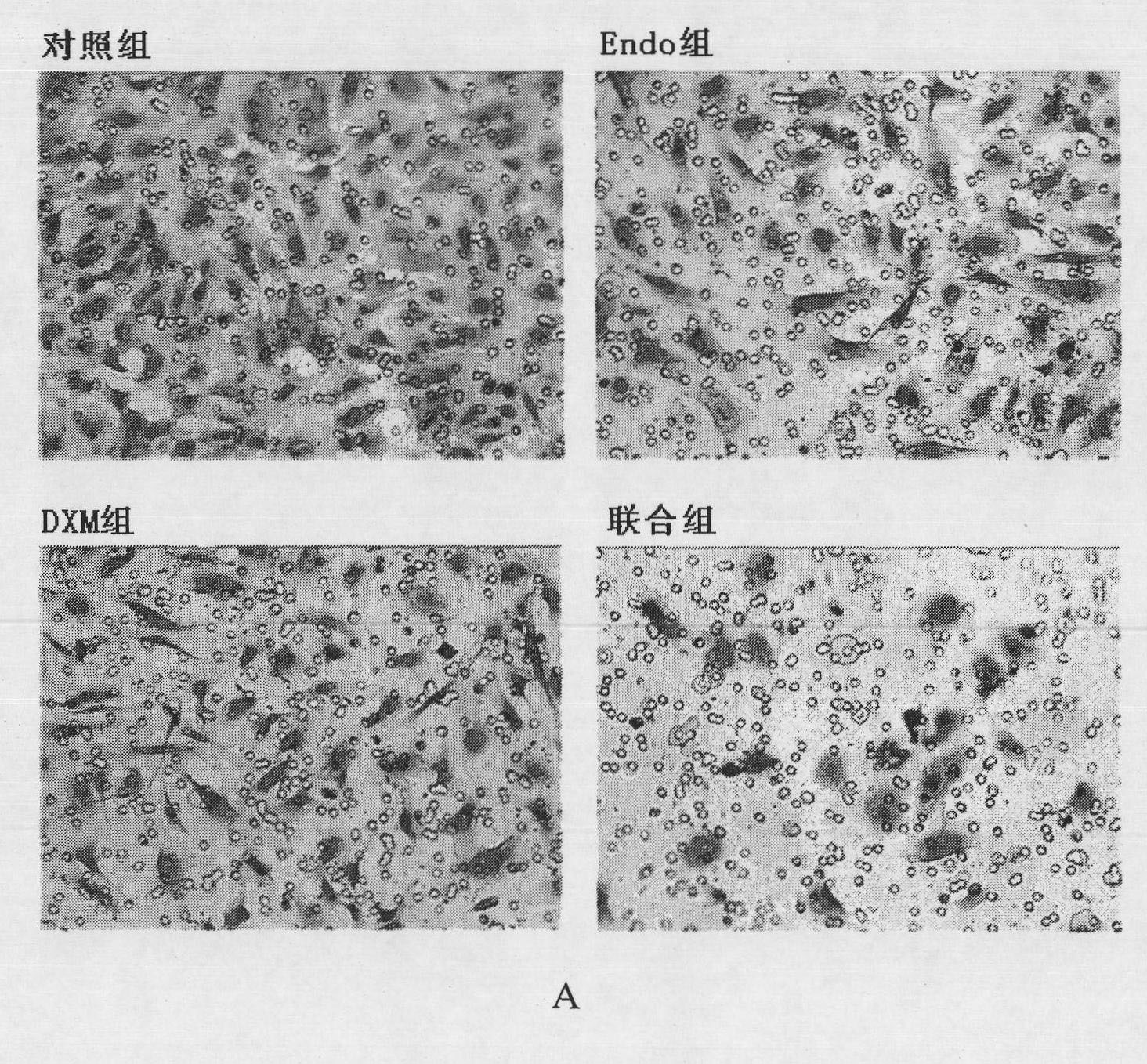
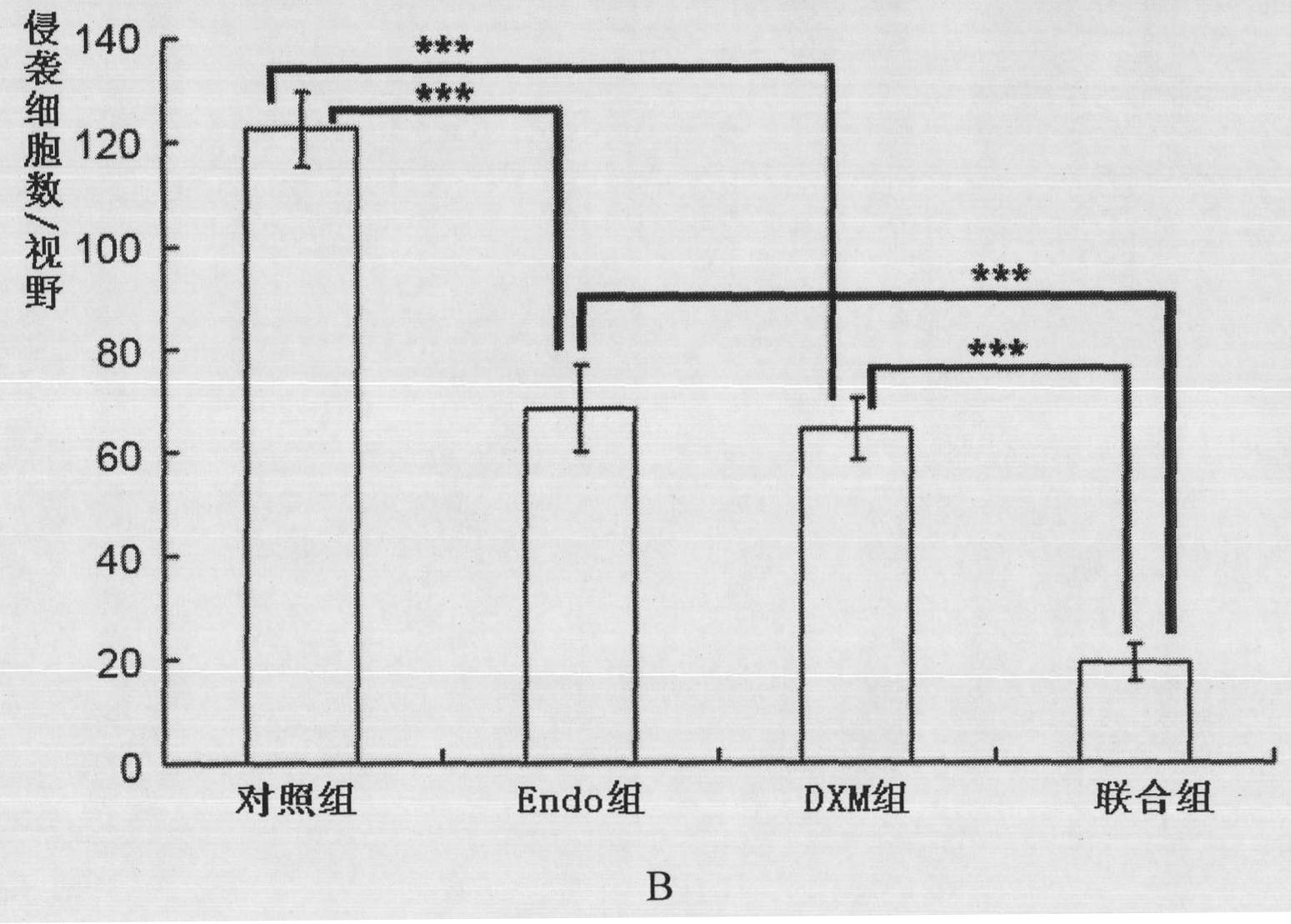
The invention belongs to the technical field of biological products and discloses a medicinal composition and application thereof. The composition takes recombinant human endostatin and hexadecadrol as effective constituents, wherein the mole ratio of the recombinant human endostatin to the hexadecadrol is 10-100:1. The recombinant human endostatin in the combination can co-act with the hexadecadrol to restrain the multiplication, invasion, migration and angiogenesis of endothelial cells. An in-vivo test result proves that the recombinant human endostatin and the hexadecadrol co-act with each other to restrain the growth of the mice H22 transplanted liver tumor and human Bel7402 transplanted liver tumor. Therefore, the composition can be widely applied to medicaments for treating liver cancer.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Cationic elaioplast and its adenovirus composition, its preparing method and use
ActiveCN101045037AImprove stabilityUnique formulaGenetic material ingredientsPharmaceutical non-active ingredientsCholesterolElaioplast
A cationic liposome is proportionally prepared from DOPE, DOTMA and cholesterol and can be used to encapsulate the object (adenovirus carrier) to obtain a recombinant human endostatin adenovirus-cationic liposome composition carrying the active antineoplastic genes for suppressing the growth of tumor. Its preparing process is also disclosed.
Owner:SICHUAN UNIV
Recombinant human endostatin protein with different amino acid structures, method for preparing recombinant human endostatin protein and application thereof
ActiveCN105646701AAntiproliferative activityGrowth inhibitionConnective tissue peptidesPeptide/protein ingredientsDiseaseVascular endothelium
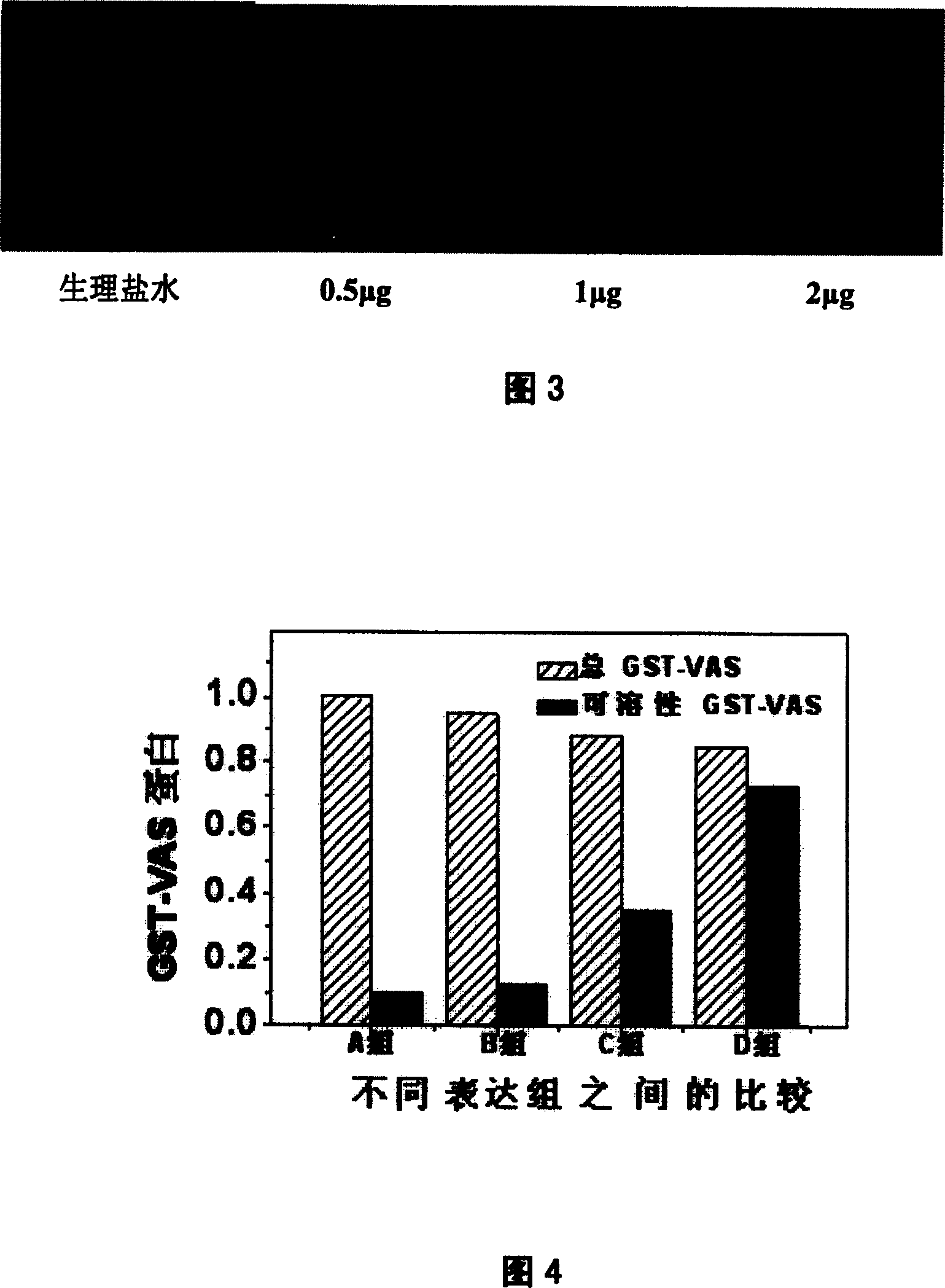
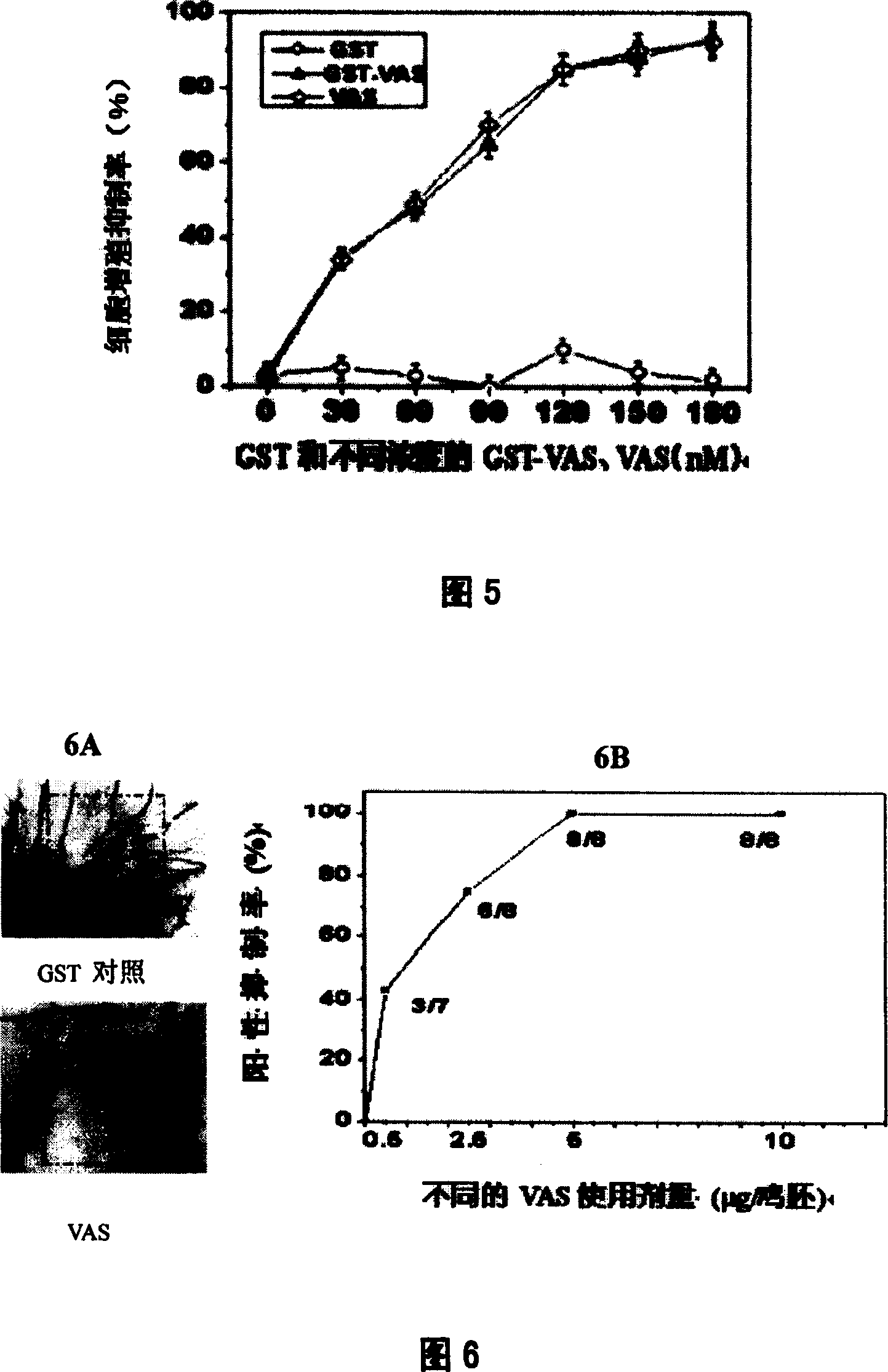
The invention discloses a recombinant human endostatin protein with different amino acid structures, a method for preparing the recombinant human endostatin protein and application thereof. Amino acid sequences of a recombinant human endostatin protein 1 are shown as SEQ ID NO.1, and amino acid sequences of a recombinant human endostatin protein 2 are shown as SEQ ID NO.2. The recombinant human endostatin protein, the method and the application have the advantages that the two types of recombinant proteins are active in the aspects of inhibiting the proliferation activity of vascular endothelial cells under the induction actions of basic fibroblast growth factors (bFGF) and inhibiting the angiogenesis activity of chick chorioallantoic membranes (CAM) and are easy to enter the vascular endothelial cells and vascular endothelial cells of the chick chorioallantoic membranes, and transmembrane effects of the recombinant human endostatin protein and the structural stability of N ends can be obviously improved; effects of directly inhibiting tumor cell growth can be realized, effects of inhibiting generation of neonatal vascular endothelial cells can be effectively realized, and the recombinant human endostatin protein can be used for treating various diseases, including solid tumor, retinal diseases due to diabetes mellitus and rheumatoid arthritis, due to angiogenesis.
Owner:点斗基因科技(南京)有限公司
Method for preparing recombinant human endostatin chitosan nanoparticles for injection
ActiveCN101953796AUniform particle size controlHigh drug loadingPowder deliveryPeptide/protein ingredientsChitosan nanoparticlesBlood vessel
The invention relates to a method for preparing recombinant human endostatin chitosan nanoparticles for injection, in particular to the preparation of the recombinant human endostatin chitosan nanoparticles by taking biodegradable chitosan as a carrier and sodium tripolyphosphate as an ion crosslinking agent and performing an ion crosslinking reaction on chitosan solution of recombinant human endostatin and the solution of sodium tripolyphosphate under a continuously stirring condition.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD +1
Compositions of recombinant human endostatin adenovirus injections and methods of production
The invention generally relates to compositions of and methods for production of recombinant adenoviruses that carry therapeutic genes. More particularly, the invention relates to lyophilized recombinant adenoviruses injection and its related production procedures, including production procedures for the recombinant adenovirus vectors (or other viral vectors) that carry the genes of human endostatins.
Owner:HUANG WENLIN
Recombinant human endostatin temperature-sensitive gel composition for injection
ActiveCN101933897AReduce releaseAvoid destructionPeptide/protein ingredientsAerosol deliveryVascular endotheliumAdministration time
The invention relates to a recombinant human endostatin temperature-sensitive gel composition for injection can release medicaments slowly and stably through one time of medicament administration, so the medicament administration times are reduced, physiological pain in patients is relieved and the clinical compliance of the patients is improved.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD +1
Quantitative measurement method of extracorporeal activity of recombinant human endostatin
InactiveCN101045941ASimple and fast operationGood for quantitative determinationMicrobiological testing/measurementRecombinant DNA-technologyVascular endotheliumEndothelial NOS
The present invention is quantitative measurement method of extracorporeal activity of high activity soluble recombinant human endostatin produced through a genetic engineering process, and features that in the test, the vascular endothelial cell with introduced bFGF and / or VEGF gene and without need of adding additional bFGF or VEGF for excitation is added directly into the measured sample to calculate the activity quantitatively. The method is simple and accurate, and may be applied in industrial production and clinical angiogenesis antagonizing treatment.
Owner:江苏省基因药物工程技术研究中心
Modified recombinant human endostatin and use thereof
InactiveCN101381413AHigh effective blood concentrationIncrease proliferative activityConnective tissue peptidesPeptide/protein ingredientsPharmaceutical drugBiochemistry
The invention discloses modified recombinant human endostatin and an application thereof. The modified recombinant human endostatin has the following structure: CH3O-(CH2CH2O)m-CH2CH2CH2-N<alpha>H-Endosta, wherein the weight average molecular weight of CH3O-(CH2CH2O)m-CH2CH2CH2- is between 20 and 40Kd. The modified recombinant human endostatin strengthens in vivo stability, improves plasma concentration, prolongs half decay period, and remarkably increases the proliferative activity of anti-endothelial cells, thereby reducing administration dosage, reducing administration frequency, and relieving the body pain and the economical burden of patients. The modified recombinant human endostatin can be used to prepare antineoplastic medicine.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD +2
Recombinant human endostatin fusion protein as well as preparation method and application thereof
ActiveCN107236046AHigh binding affinityReduce use costConnective tissue peptidesHybrid immunoglobulinsTumor cellsLesion
The invention discloses fusion protein composed of Her2 nano antibody dipolymer and recombinant human endostatin as well as a preparation method and application thereof. The recombinant human endostatin fusion protein comprises the Her2 nanobody dipolymer and recombinant human endostatin protein, and the fusion protein is easy to purify, can specifically target to breast cancer cells with high expression of Her2, has good transmembrane effect and can effectively inhibit the growth of tumor cells. The invention discloses the application of the recombinant human endostatin fusion protein in preparing a medicine for detecting or treating breast cancer in a targeting mode clinically, the concentration of the medicine in the location of tumor lesion can be increased, and correspondingly the dose-effect ratio is increased. The invention also discloses the recombinant expression vectors, transgenic cell lines or transgenic recombinant bacteria of the encoding gene of the recombinant human endostatin fusion protein as well as a gene engineering preparation method to be applied in clinical production and preparation of the fusion protein.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP +1
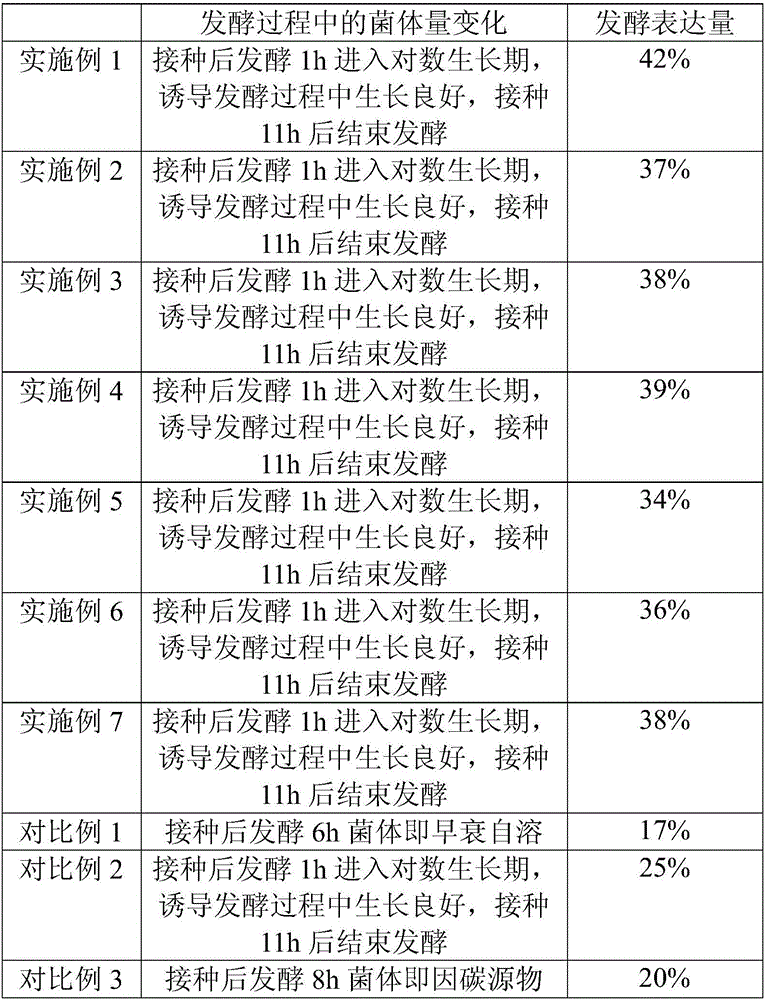
Induced expression method of recombinant human endostatin
ActiveCN103993057AMeet mass production requirementsPrevent premature agingMicroorganism based processesFermentationThiogalactosidesNitrogen source
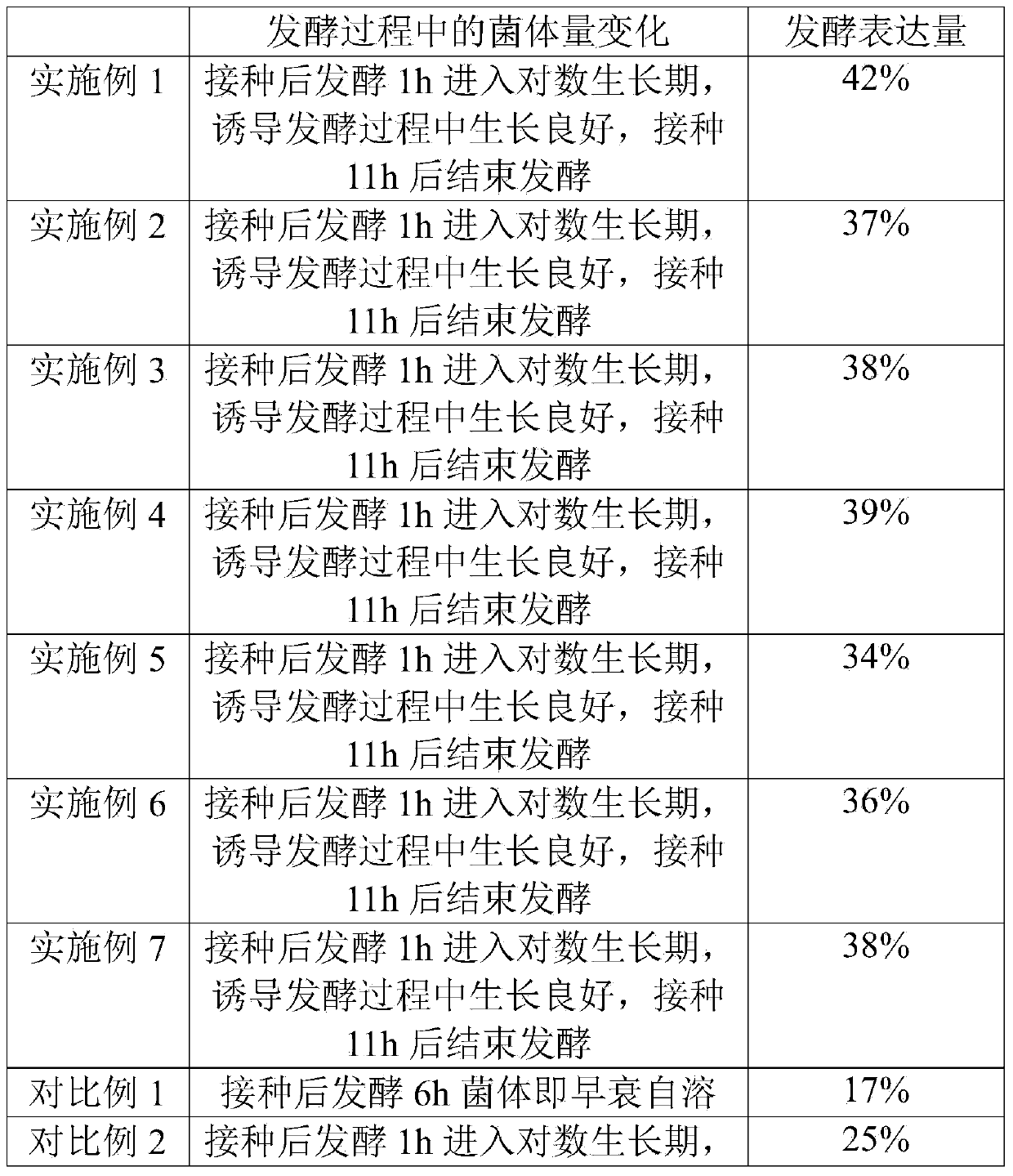
The invention discloses an induced expression method of recombinant human endostatin. The method comprises the following steps: fermenting engineering bacteria until the OD600 value of fermentation liquor is 10-15, adding an inducer isopropyl-beta-d-thiogalactoside (IPTG), a carbon source substance and a nitrogen source substance, so that the concentration of the IPTG in the fermentation liquor is 0.2-0.4mmol / L; cultivating at 30-37 DE C for 2.5-3.5 hours; adding the inducer IPTG, so that the concentration of the IPTG in the fermentation liquor is 0.1-0.3mmol / L, and continuing to cultivate for 3-5 hours. By adopting the induced expression method of the recombinant human endostatin disclosed y the invention, the requirements of large-scale production of the recombinant human endostatin can be met, and efficient induced expression is achieved at high cell concentration.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
Recombinant human endostatin liposome microbubble and application thereof in preparing medicament for inhibiting tumor angiogenesis under ultrasound mediation
InactiveCN102727893AEnhanced inhibitory effectPowerful generationPeptide/protein ingredientsEnergy modified materialsPolyethylene glycolP phosphate
The invention discloses a recombinant human endostatin liposome microbubble, which is prepared by a method in the following steps: dispersing DSPE-PEG (distearoyl phosphoethanolamine-polyethylene glycol) 2000-Biotin and DSPC (disaturated phosphatidylcholine) in a molar ratio of 1:(0.5-4) in a phosphate buffer; introducing inert gas, mechanically oscillating, adding streptavidin, and incubating at room temperature, wherein the molar ratio of the streptavidin to the DSPE-PEG2000-Biotin is 1:(1-2); and adding biotinylated recombinant human endostatin, and incubating at room temperature to prepare the recombinant human endostatin liposome microbubble, wherein the molar ratio of the streptavidin to the biotinylated recombinant human endostatin is 1:(1-2). The invention also discloses application of the recombinant human endostatin liposome microbubble in preparing a medicament for inhibiting tumor angiogenesis under ultrasound mediation. The recombinant human endostatin liposome microbubble has a tumor inhibition rate of 41.3 percent, which is remarkably superior to the inhibition effect of a single recombinant human endostatin liposome microbubble.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Method and use of producing soluble recombinant protein in colibacillus
ActiveCN100334216CEasy to produceBiologically activeBacteriaMicroorganism based processesAngiostatinEscherichia coli
The invention was involved in that target protein expression strain and molecular partner were expressed in E.coli.at the same time at low temperature to prevent the formation of target protein inclusion body effectively and increase yields of soluble production largely. Soluble recombinant human endostatin and human angiostatin were increased largely by the technique. It provided a new simple and cheap technique to produce soluble recombinant protein with biological activity. It was applied in bioengineering pharmaceutical factory, gene engineering, biochemistry and molecular biology with high efficiency, cheap price, wide application and strong promotion.
Owner:NANJING JIRUIKANG BIOTECHNOLOGY RES INST CO LTD
Recombinant human endostatin nanoparticle composition for injection and preparation method thereof
InactiveCN101954065AFacilitated releaseSimple process routePowder deliveryPeptide/protein ingredientsCarrying capacityFreeze-drying
The invention relates to a recombinant human endostatin nanoparticle composition for injection and a preparation method thereof. The recombinant human endostatin nanoparticle composition can be prepared by methods such as a covalent bond linking method and an ionic induction method and by using biologically degradable polymer as a carrier. The recombinant human endostatin nanoparticle composition can be prepared into injection and freeze-dried powder injection, has high medicament carrying capacity and uniform particle size distribution, is suitable for intravenous injection and can delay the release of recombinant human endostatin.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD
Method for preparing chitosan nanoparticles
ActiveCN101905017AGood biocompatibilityPromote degradationPeptide/protein ingredientsPharmaceutical non-active ingredientsChitosan nanoparticlesCentrifugation
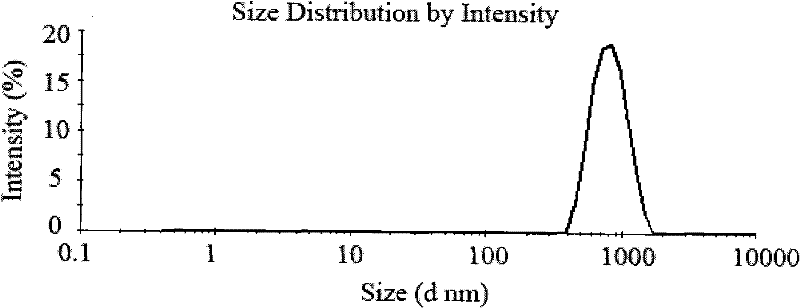
The invention relates to a method for preparing recombinant human endostatin (rh-endostatin) nanoparticles for injection. The nanoparticles comprise chitosan and the rh-endostatin. A coacervation process is adopted for preparing the nanoparticles, namely a coagulant is added into chitosan solution containing the rh-endostatin, the mixture is subjected to stirring, ultrasonic sound treatment and centrifugation, and finally the deposit is re-mixed and suspended in water to form the nanoparticles. The preparation method is simple, and the particle size of the obtained nanoparticles is 400 to 900 nanometers.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Method for producing recombined endothelium chalone in bacillus colis and application
InactiveCN1661019AEasy to produceBiologically activePeptide/protein ingredientsFermentationBio engineeringOrganism
A process for producing soluble bioactive recombinant human endothelium inhibin in colibacillus and separating and purifying it is disclosed. The resultant recombinant human endothelium inhibin can specifically and dosage-dependently suppress the reproduction of endothelium cells and the generation of new blood vessel.
Owner:NANJING UNIV
Application of recombinant human endostatin in preparing drugs for treating ocular neovascular diseases
InactiveCN104548067AInhibition formationNo drug resistanceSenses disorderPeptide/protein ingredientsDiseaseVascular endothelium
The invention discloses the application of recombinant human endostatin in preparing drugs for treating ocular neovascular diseases. Researches show that the recombinant human endostatin can remarkably inhibit the formation of ocular neovasculars, is safe, non-toxic and free of drug resistance, and has wide application prospects in preparing drugs for treating ocular neovascular diseases.
Owner:ZHEJIANG PROVINCIAL PEOPLES HOSPITAL +1
Method for quantitative determination of in vivo activity of recombination human endothelial chalone
InactiveCN101045167ARelatively small errorReduce mistakesRecombinant DNA-technologyIn-vivo testing preparationsObservational errorQuantitative determination
A method for quantitatively testing the in vivo activity of recombinant human endostatin includes such steps as using mouse' s liver cancer as animal model, quantitatively converting the tumor suppressing rate to activity units, measuring the valence of reference, testing the reliability , calculating the confidence, tumor suppressing experiment of the specimen to be tested and reference specimen, and calculating the confidence of the valence of the specimen to be tested to quantitatively measure said in vivo activity and error.
Owner:江苏省基因药物工程技术研究中心
Recombinant human endostatin sustained-release injection oil preparation and preparation process thereof
ActiveCN102166187ASimple technical processLow costPeptide/protein ingredientsPharmaceutical delivery mechanismIntramuscular injectionBlood Vessel Endothelium
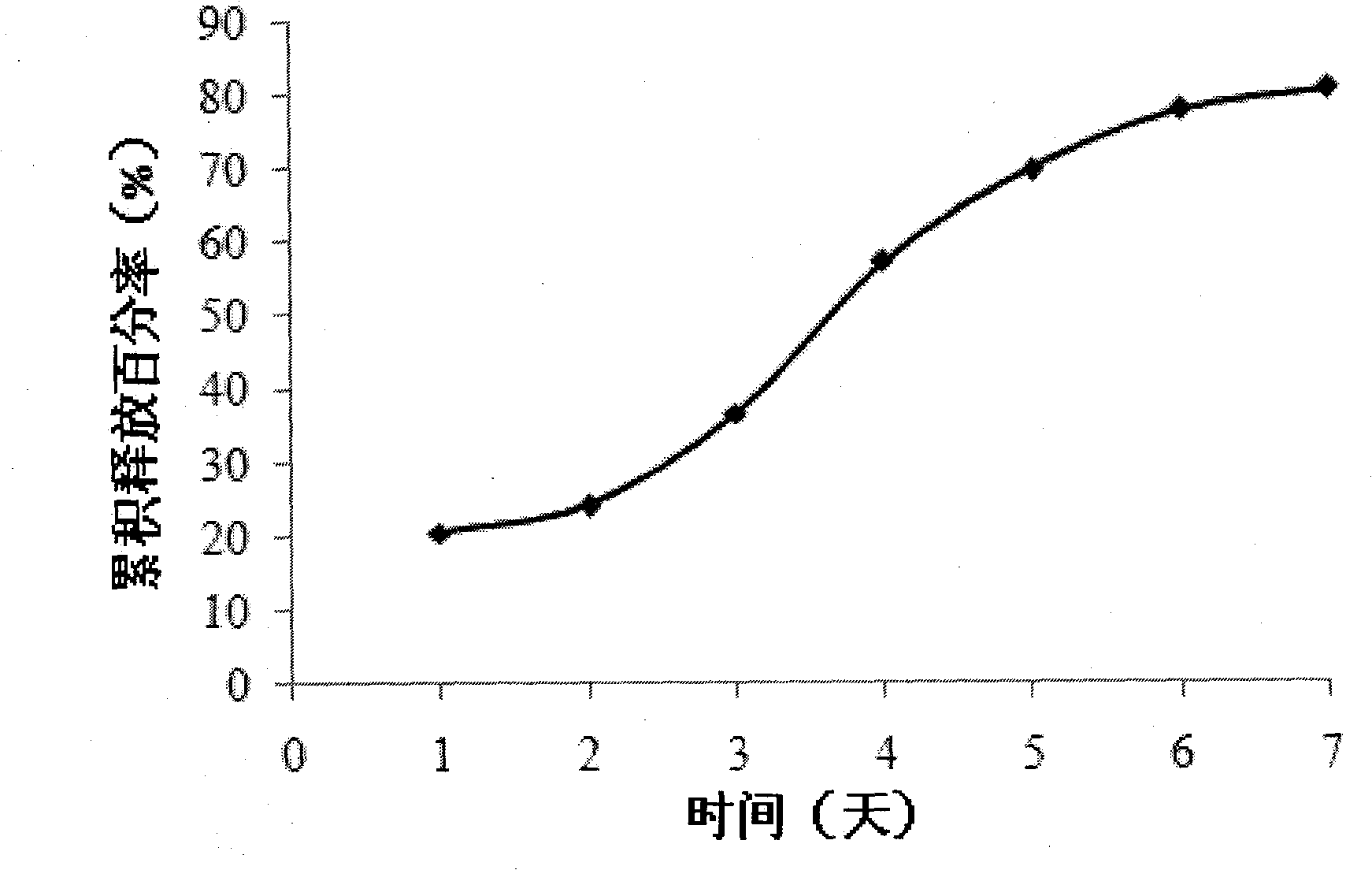
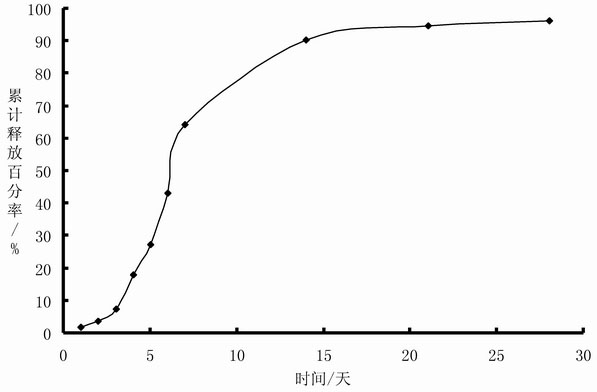
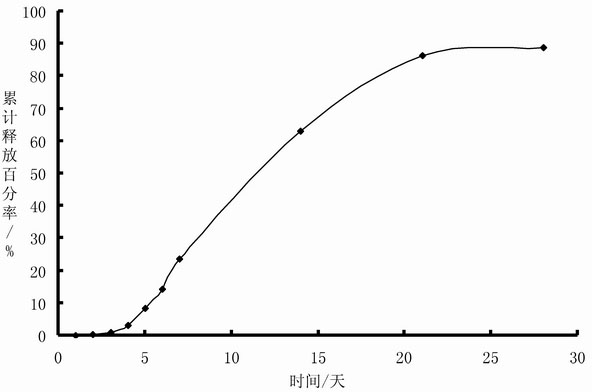
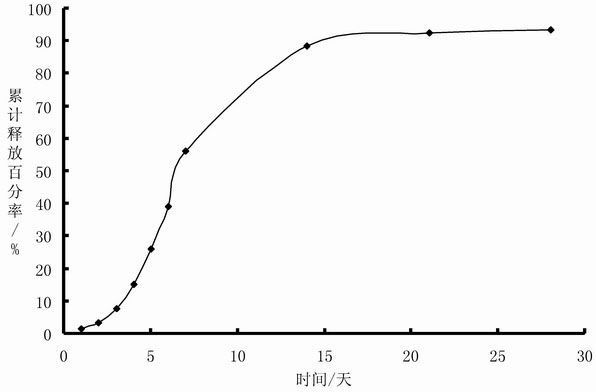
The invention belongs to the technical field of medicinal preparation, and provides a sustained-release injection oil preparation containing recombinant human endostatin and a preparation process thereof. The sustained-release injection oil preparation is mainly characterized in that by taking injection oil as main solvent, the recombinant human endostatin is evenly dispersed in the oily solvent by different methods such as a freeze-drying composite dissolving method to form oil solution, and an oily medicine storage tank can be automatically formed after subcutaneous or intramuscular injection, so as to delay the release of the recombinant human endostatin, and be used for continuous treatment of tumor. Results of release rate in vitro show that the sustained-release injection oil preparation containing recombinant human endostatin can enable the medicine in the preparation to continuously release for 3-28 days. The preparation overcomes the shortcomings of existing recombinant human endostatin injection for clinical use that the dosage of the injection is once a day, and patients need to be subjected to injection every day, thus greatly reducing the physical pain of the patients, and relieving the economic burden of the patients.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
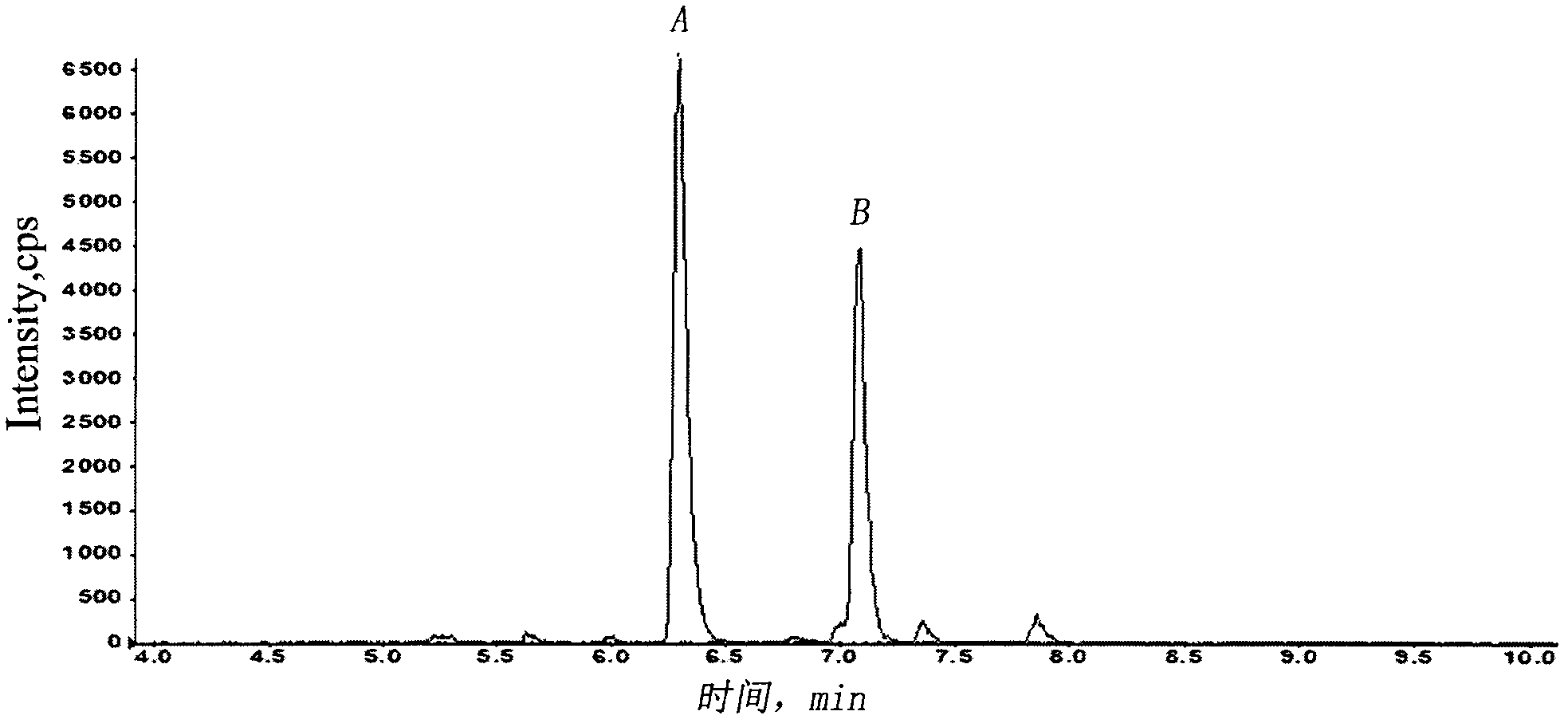
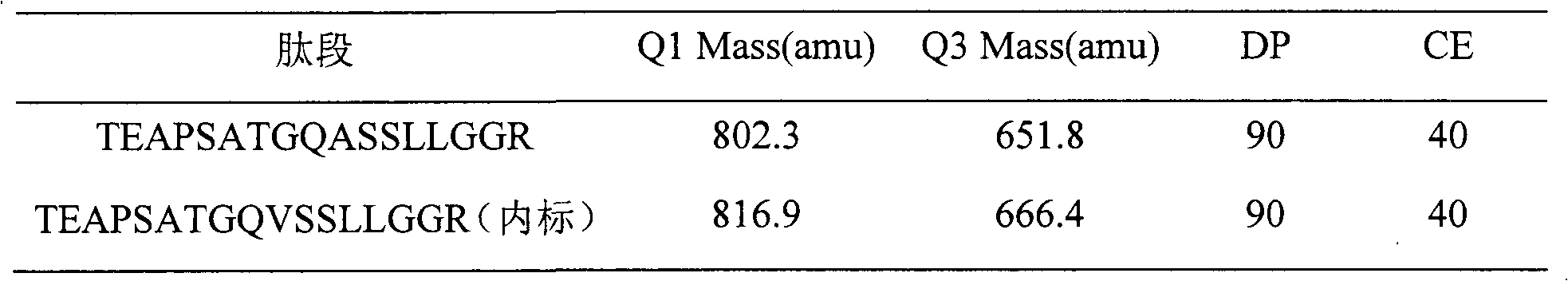
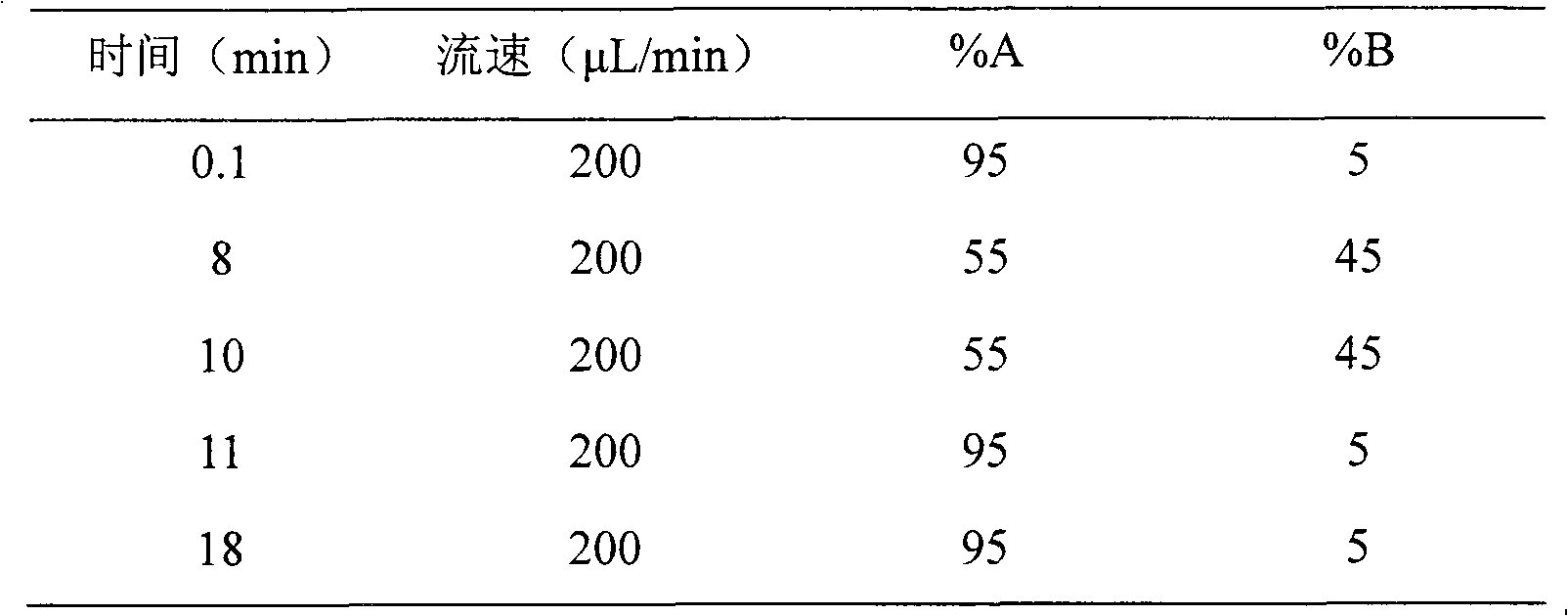
Method for absolutely quantifying mass spectrum of histidine tag-containing recombinant human endostatin
The invention belongs to the technical field of analysis, and relates to a method for absolutely quantifying mass spectrum of histidine tag-containing recombinant human endostatin. The method is based on LC-MS / MS, and extracts histidine tag-containing recombinant human endostatin from a plasma sample through utilizing Ni<2+> sepharose gel resin, and realizes absolute quantification of histidine tag-containing recombinant human endostatin in the plasma sample through utilizing a quantifying strategy for a specific peptide generated during hydrolysis of recombinant human endostatin with trypsin and taking a synthetic peptide having a sequence similar to that of the specific peptide as an interior label. The invention mainly includes a biological sample preparation method, an LC-MS / MS measuring method, standard curve preparation and precision degree measurement. The method has good linear relation, high accuracy degree and excellent reproducibility, and can be used for measuring the blood concentration of histidine tag-containing recombinant human endostatin and studying the pharmacokinetics.
Owner:CHINA PHARM UNIV
Recombinant human endostatin liposome microbubble and application thereof in preparing medicament for inhibiting tumor angiogenesis under ultrasound mediation
InactiveCN102727893BEnhanced inhibitory effectPowerful generationPeptide/protein ingredientsEnergy modified materialsPolyethylene glycolP phosphate
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Recombination human endothelium chalene expression strain and solubility expression method
InactiveCN1724671AEasy to purifyOvercoming technical deficienciesBacteriaVector-based foreign material introductionSolubilityInclusion bodies
The invention relates to a method to rebuilding human vivo skin chalone strain and the solubility expression that belongs to gene engineering field. The strain contains expression carrier pET-Endo that could be gained by inserting the human vivo skin chalone into carrier pET43.1a. For cultivating the strain, it is induced by using IPTG at the concentration of 0.1-1.0mmol / L and the temperature in 25-30 degree centigrade, and for 6-10 hours time period; the external albumen solubility expression quantity could reach over 80% of the total expression quantity. And the total expression quantity is about 50% of the thallus total albumen quantity. Thus, the invention conquers the disadvantage of expressing the human vivo by inclusion body mode. It could be used to rebuild the human vivo skin chalone and the other solubility expression of rebuilding albumen.
Owner:SHAN DONG DONG E E JIAO +1
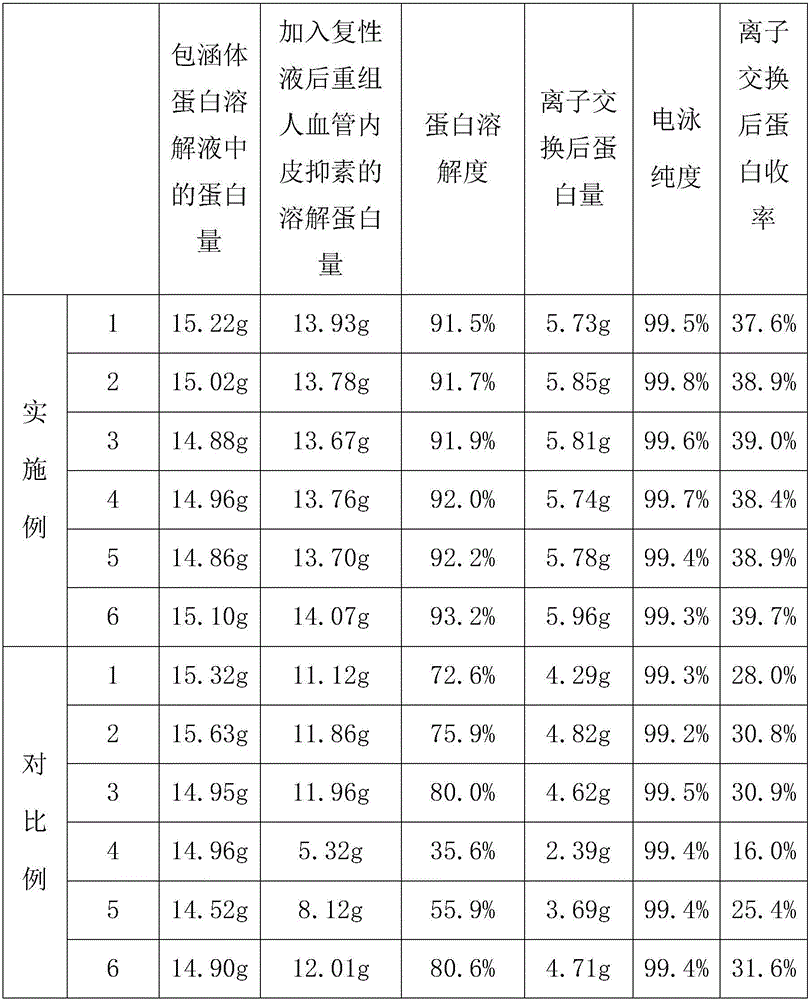
Refolding solution of recombinant human endostatin and its preparation and use method
ActiveCN103992400BGuaranteed mass productionImprove solubilityConnective tissue peptidesPeptide preparation methodsOxidized GlutathioneGuanidine
The invention relates to a refolding solution of recombinant human endostatin. The refolding solution comprises the following components: guanidine hydrochloride, reduced glutathione, oxidized glutathione and a complexing agent and the pH value of the refolding solution is 4.0-5.0. The invention also provides a preparation and application methods of the refolding solution of recombinant human endostatin. By virtue of the refolding solution of recombinant human endostatin disclosed by the invention, the high refolding yield of recombinant human endostatin can be realized, thereby ensuring the mass production of the recombinant human endostatin.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
Induced Expression Method of Recombinant Human Vascular Endostatin
ActiveCN103993057BMeet mass production requirementsPrevent premature agingMicroorganism based processesFermentationVascular endotheliumThiogalactosides
The invention discloses an induced expression method of recombinant human endostatin. The method comprises the following steps: fermenting engineering bacteria until the OD600 value of fermentation liquor is 10-15, adding an inducer isopropyl-beta-d-thiogalactoside (IPTG), a carbon source substance and a nitrogen source substance, so that the concentration of the IPTG in the fermentation liquor is 0.2-0.4mmol / L; cultivating at 30-37 DE C for 2.5-3.5 hours; adding the inducer IPTG, so that the concentration of the IPTG in the fermentation liquor is 0.1-0.3mmol / L, and continuing to cultivate for 3-5 hours. By adopting the induced expression method of the recombinant human endostatin disclosed y the invention, the requirements of large-scale production of the recombinant human endostatin can be met, and efficient induced expression is achieved at high cell concentration.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
Fusion protein of liver-targeting peptide and recombinant human endostatin and its preparation method and application
ActiveCN103319606BStrong specificityImprove the dose-effect ratioBacteriaPeptide/protein ingredientsSide effectWhole body
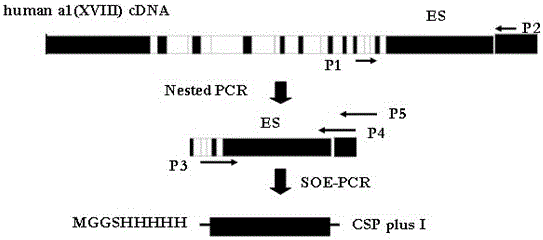
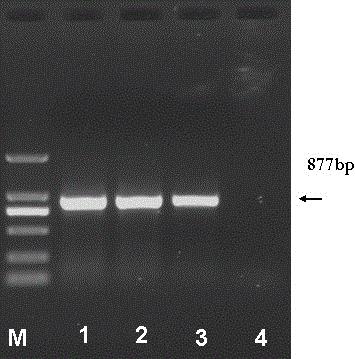
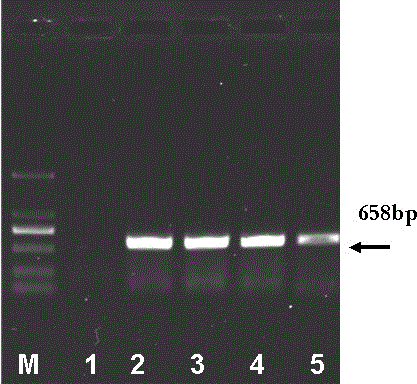
The invention discloses a fusion protein of liver-targeting peptide and recombinant human endostatin, its preparation method and application. The present invention selects 19 amino acids that can be specifically combined with the receptor--heparan sulfate proteoglycan HSPG on the surface of liver cells in the conserved I region of the N-terminal of the Plasmodium circumsporozoite protein CSP, and uses genetic engineering to fuse it in The carboxy-terminus of the novel human endostatin nhES with 9 amino acid sequences (MGGSHHHHH) added to the amino-terminus, and the liver-targeting peptide and recombinant human endostatin fusion protein (ES‑CSP) were prepared. The fusion protein is easy to purify, can specifically target the liver to inhibit the formation of new blood vessels, increase the local concentration of the drug at the lesion site, reduce the systemic dosage, and reduce its toxic and side effects. It provides ideas and scientific basis for the clinical development of drugs that target angiogenesis to treat liver cancer, and has great scientific significance and practical value for further improving the treatment level of liver cancer in my country.
Owner:GUANGDONG PHARMA UNIV
Method for preparing chitosan nanoparticles
ActiveCN101905017BGood biocompatibilityPromote degradationPeptide/protein ingredientsPharmaceutical non-active ingredientsChitosan nanoparticlesCentrifugation
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Recombination human endothelium chalene expression strain and solubility expression method
InactiveCN1724671BEasy to purifyOvercoming technical deficienciesBacteriaMicroorganism based processesSolubilityInclusion bodies
The invention relates to a method to rebuilding human vivo skin chalone strain and the solubility expression that belongs to gene engineering field. The strain contains expression carrier pET-Endo that could be gained by inserting the human vivo skin chalone into carrier pET43.1a. For cultivating the strain, it is induced by using IPTG at the concentration of 0.1-1.0mmol / L and the temperature in 25-30 degree centigrade, and for 6-10 hours time period; the external albumen solubility expression quantity could reach over 80% of the total expression quantity. And the total expression quantity isabout 50% of the thallus total albumen quantity. Thus, the invention conquers the disadvantage of expressing the human vivo by inclusion body mode. It could be used to rebuild the human vivo skin chalone and the other solubility expression of rebuilding albumen.
Owner:SHAN DONG DONG E E JIAO +1
Preparation method of recombined human blood-vessel endothelia inhibin sustained-released microsphere
ActiveCN101396347BPlay the role of suspension stabilityRound shapePowder deliveryPeptide/protein ingredientsVascular endotheliumMicrosphere
The invention discloses a method for preparing a recombinant human endostatin sustained release microsphere. In the method, recombinant human endostatin is solved in buffer solution containing protein stabilizer to obtain an inner water phase; lacto-glycolic acid copolymer is solved in mixed organic solvent to obtain an inner oil phase; the mixed solution of the inner water phase and the inner oil phase is dispersed fast to obtain primary emulsion; the obtained primary emulsion is arranged in a stirring vessel which is filled with plant oil or mineral oil containing emulsifier to be stirred into water-in-oil type compound emulsion; the water-in-oil type compound emulsion is stirred continuously to volatilize the mixed organic solvent in the inner oil phase and then is filtered and separated by a microporous filtering film after the microsphere is solidified; the residual plant oil or mineral oil on the surface of the microsphere is washed by the solvent; and the microsphere finished product is obtained after vacuum drying. The microsphere prepared by the method has high drug loading quantity, high entrapment rate, proper burst release quantity and long sustained release period.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Refolding solution of recombinant human endostatin as well as preparation and application method thereof
ActiveCN103992400AGuaranteed mass productionImprove solubilityConnective tissue peptidesPeptide preparation methodsOxidized GlutathioneGuanidine
The invention relates to a refolding solution of recombinant human endostatin. The refolding solution comprises the following components: guanidine hydrochloride, reduced glutathione, oxidized glutathione and a complexing agent and the pH value of the refolding solution is 4.0-5.0. The invention also provides a preparation and application methods of the refolding solution of recombinant human endostatin. By virtue of the refolding solution of recombinant human endostatin disclosed by the invention, the high refolding yield of recombinant human endostatin can be realized, thereby ensuring the mass production of the recombinant human endostatin.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
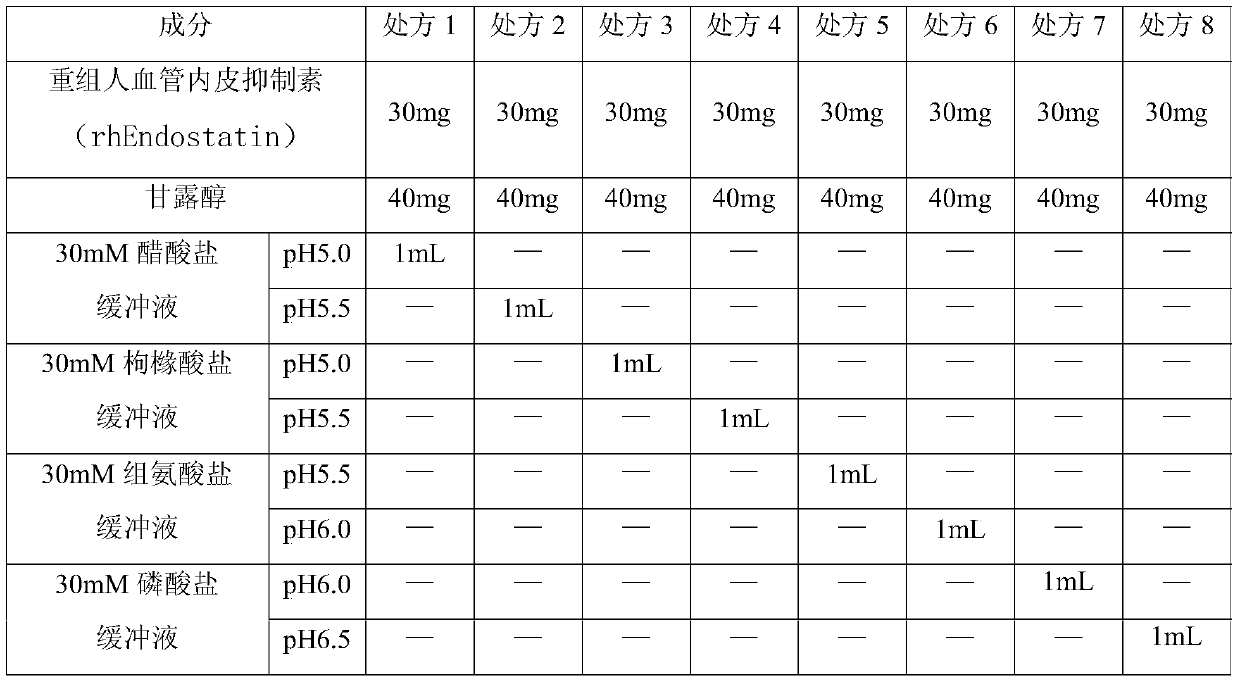
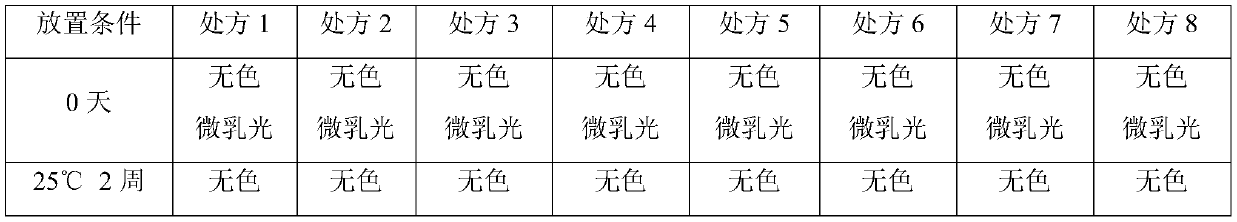
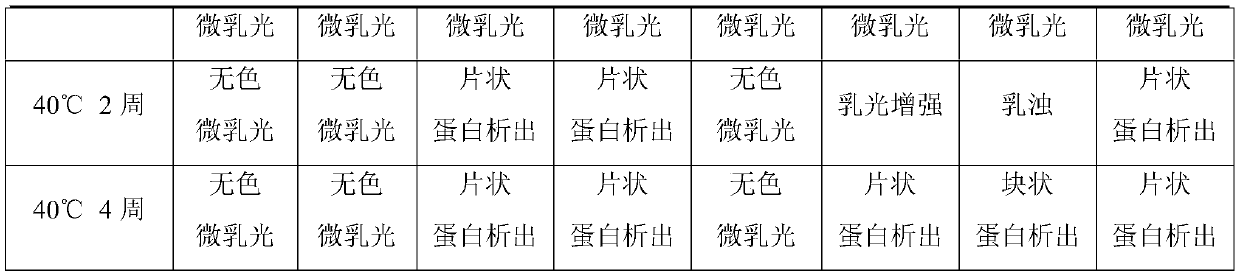
A stable recombinant human endostatin composition for subcutaneous injection
ActiveCN107865824BInhibition formationImprove stabilizerPeptide/protein ingredientsPharmaceutical delivery mechanismBlood Vessel EndotheliumSubcutaneous injection
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com