Method for quantitative determination of in vivo activity of recombination human endothelial chalone
A technology for quantitative determination of endostatin, applied in anti-angiogenesis therapy, in the field of quantitative determination of in vivo activity, can solve the problems of difficult quantification, not obvious dose-effect relationship, and only qualitative, etc., to achieve stable results, Good repeatability and stable experimental results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Establishment of an animal model for the in vivo activity determination of recombinant endostatin
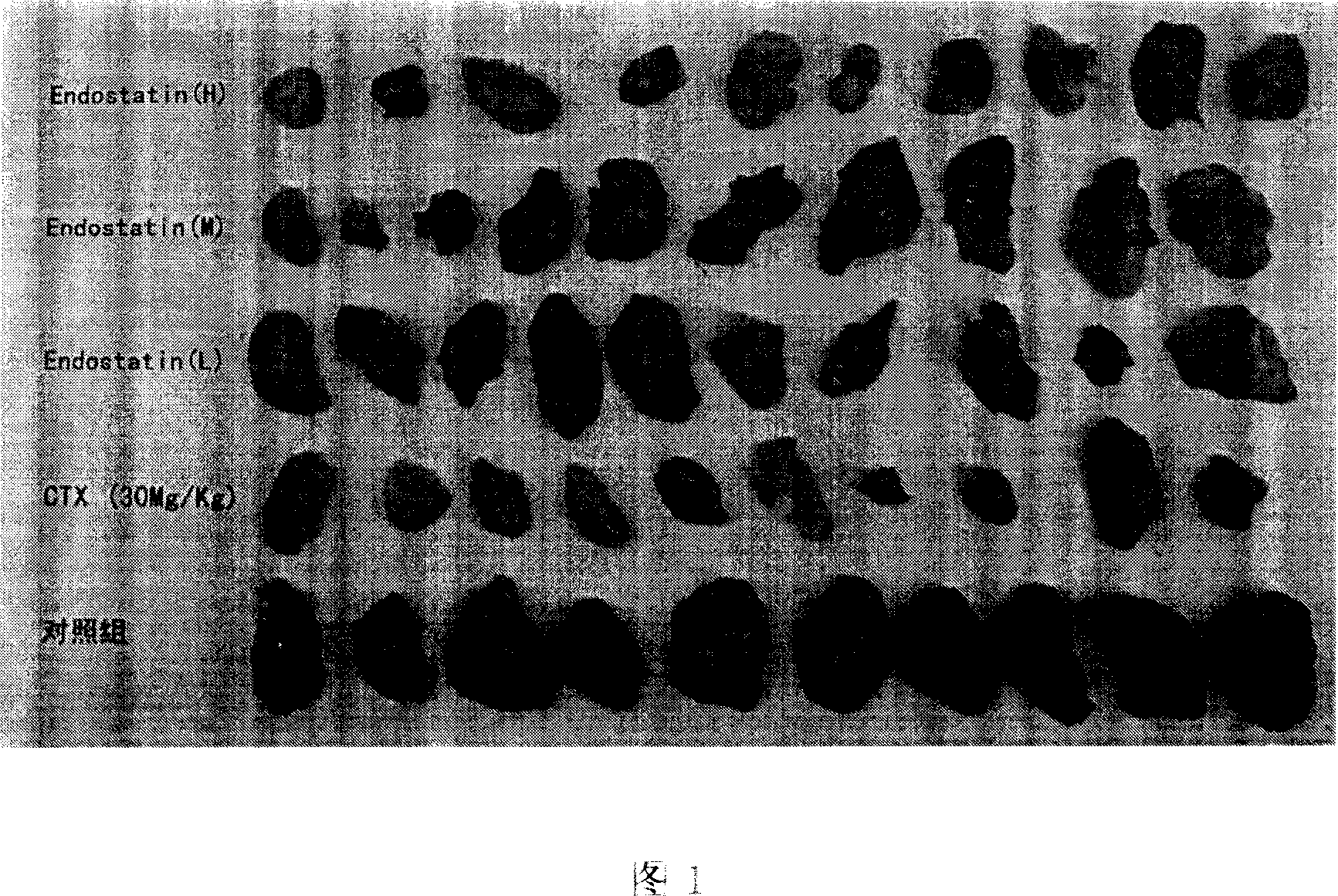
[0022] The main function of recombinant endostatin is to inhibit the growth of tumor vascular endothelial cells and other abnormally proliferating vascular endothelial cells. When measuring its activity in vivo, it is considered that the abnormal proliferation of vascular endothelial cells in tumor tissues is the fastest and the tissue with the largest blood vessel density is the first choice. Liver cancer The density of blood vessels is large, the proliferation of vascular endothelial cells is fast, and there is obvious migration phenomenon, so it is more appropriate to choose mouse liver cancer as an animal model for in vivo testing. However, it does not exclude other solid tumors as animal models for quantitative determination of activity in vivo as the scope of protection of this patent.
[0023] (1) Mouse liver cancer H 22 (Ascites type) tumor type passag...
Embodiment 2
[0040] Embodiment 2: the ED of recombinant human endostatin activity quantitative determination working reference substance 50 and determination of potency
[0041] (1) Determination of tumor inhibition rate
[0042] According to the above-mentioned method for measuring the activity of recombinant human endostatin, the reference substance was used to carry out 6 independent activity measurements, and the results of 6 tumor inhibition rate measurements were obtained (see Table 1). Figure 6.
[0043] (2) ED of 6 experiments of the reference substance 50 and potency calculation
[0044] The regression curve was made with the tumor inhibition rate against the dose, and x was ED 50 When the actual dose, in mg / kg, when calculating the ED 50 When the value of y is 50, the formula is y=a+bx, where a is the intercept and b is the slope. Through the regression curve, the correlation coefficient r can be obtained. Taking the results of the control experiment 1 as an example, use a ...
Embodiment 3
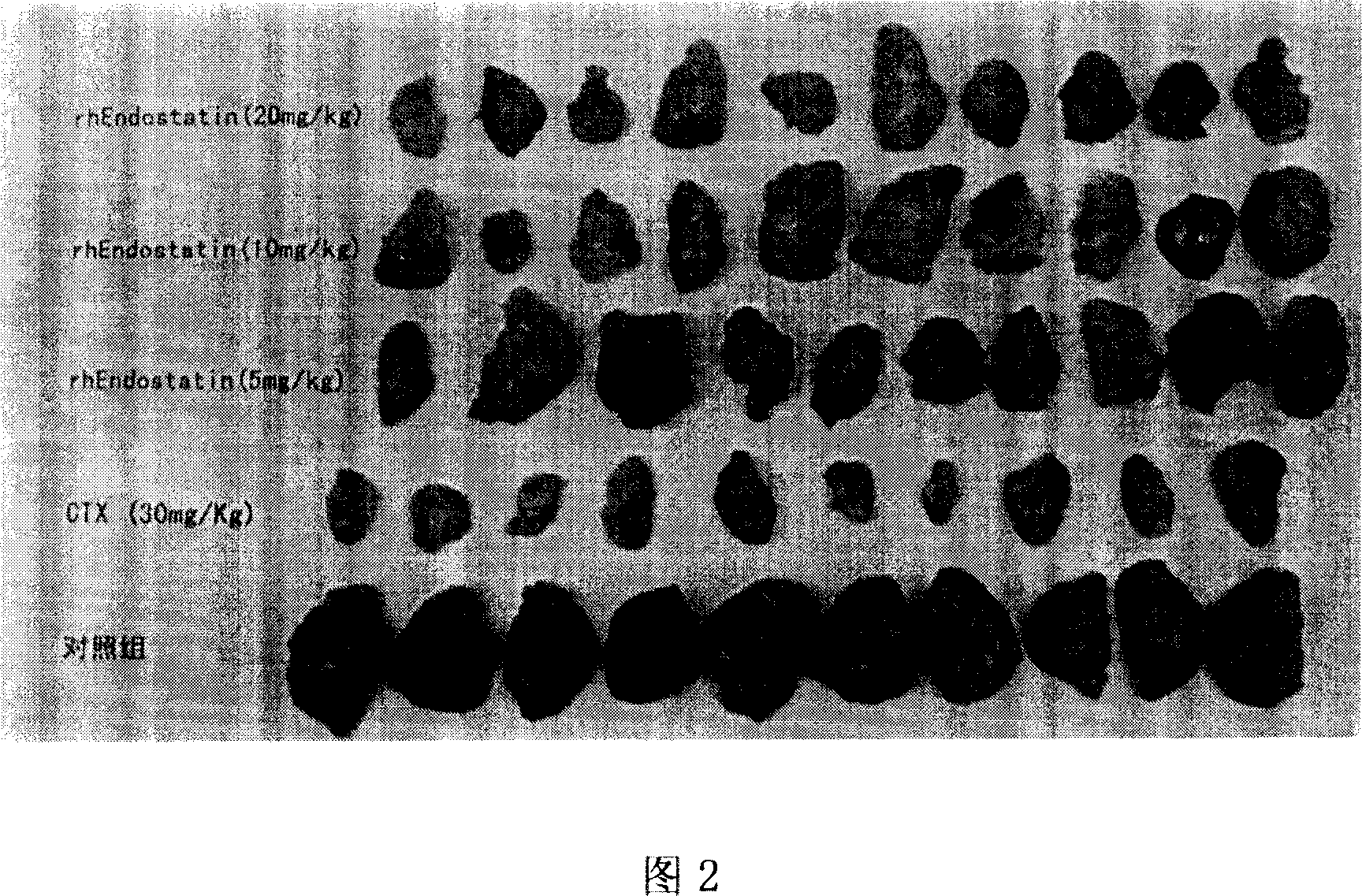
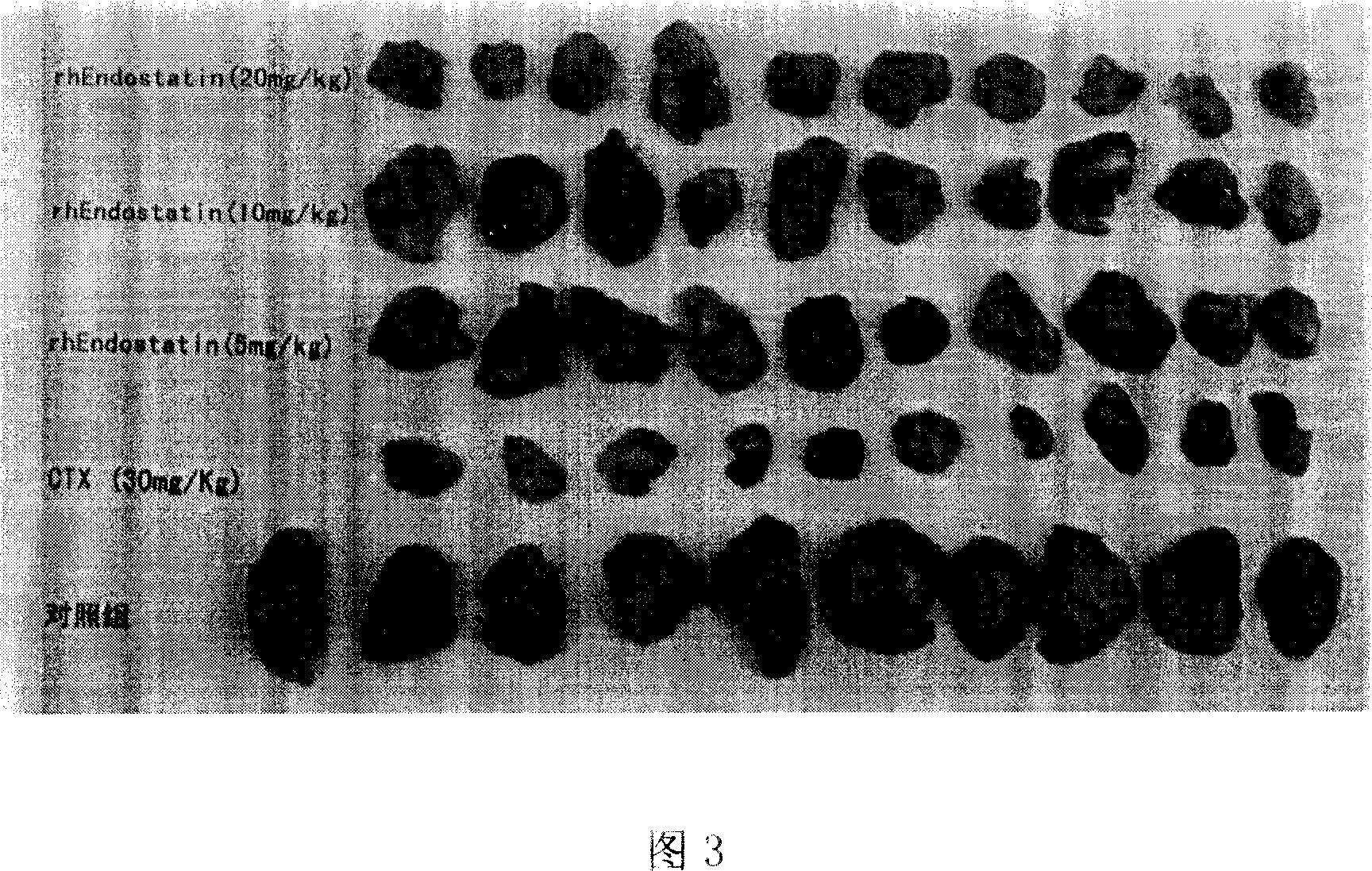
[0052] Example 3: In vivo activity determination and potency calculation of recombinant human endostatin to be tested
[0053] Measure the randomized block design method by volume response parallel line to carry out recombinant human endostatin titer determination (tumor inhibition test method), obtain the tumor inhibition test result in recombinant human endostatin titer determination (tumor weight, see Table 3), Activity assay titers were calculated by the formula. Among them, S is the recombinant human endostatin active reference substance, the titer is 86.2U, and the diluent ds 1 : 0.2mg / ml, ds 2 : 0.4mg / ml, ds 3 : 0.8mg / ml; T is the finished product of recombinant human endostatin, the marked potency is 80U, the diluent dt 1 : 0.2mg / ml, dt 2 : 0.4mg / ml, dt 3 : 0.8mg / ml, r=0.8:0.4=2, I=lgr=lg2=0.3010. The calculated results are shown in the table below (tumor inhibition weight is the average tumor weight of the negative control group minus each tumor weight):
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com