Method for absolutely quantifying mass spectrum of histidine tag-containing recombinant human endostatin
A technology of histidine tag and endostatin, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that there is no quantitative method of mass spectrometry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Determination of pharmacokinetics of recombinant human endostatin containing histidine tag in rats
[0029] The main steps are as follows:
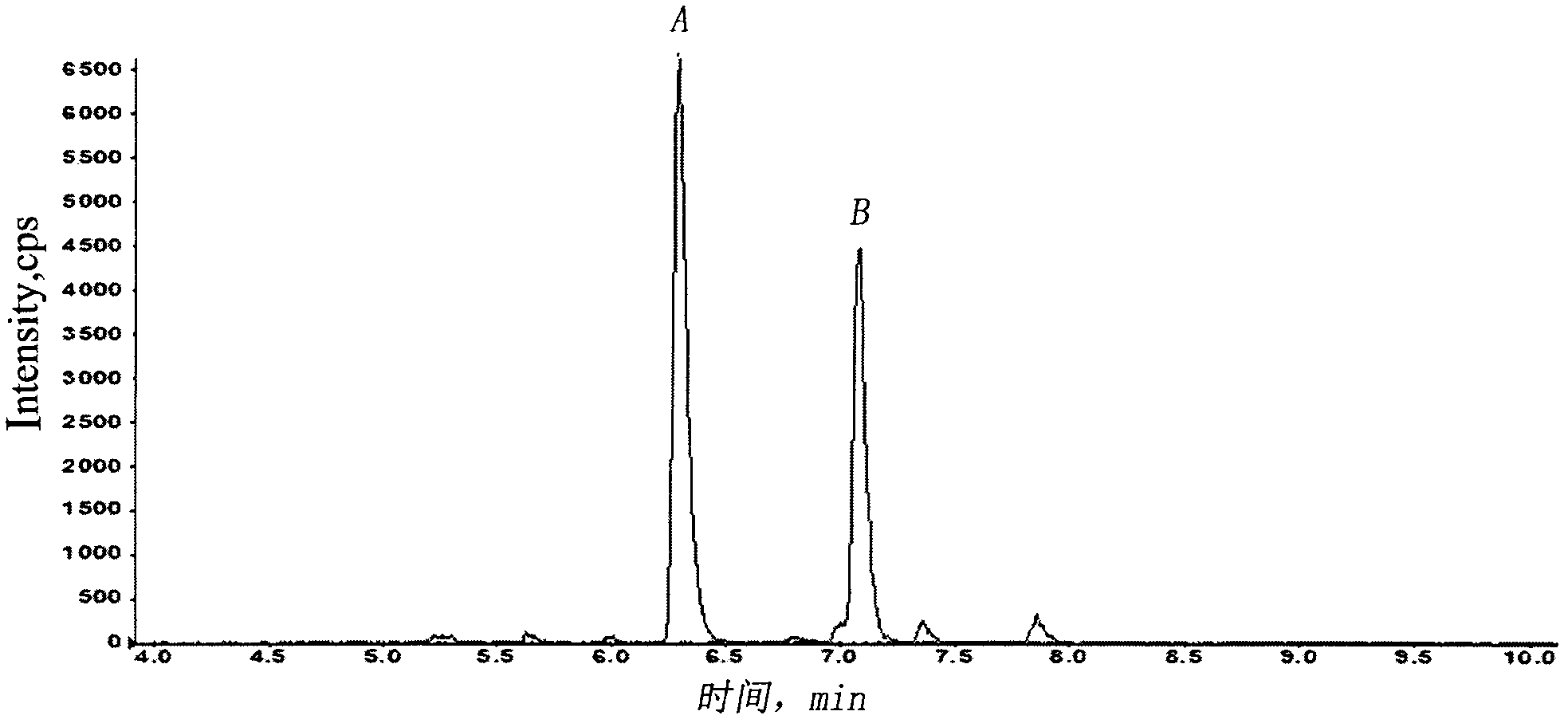
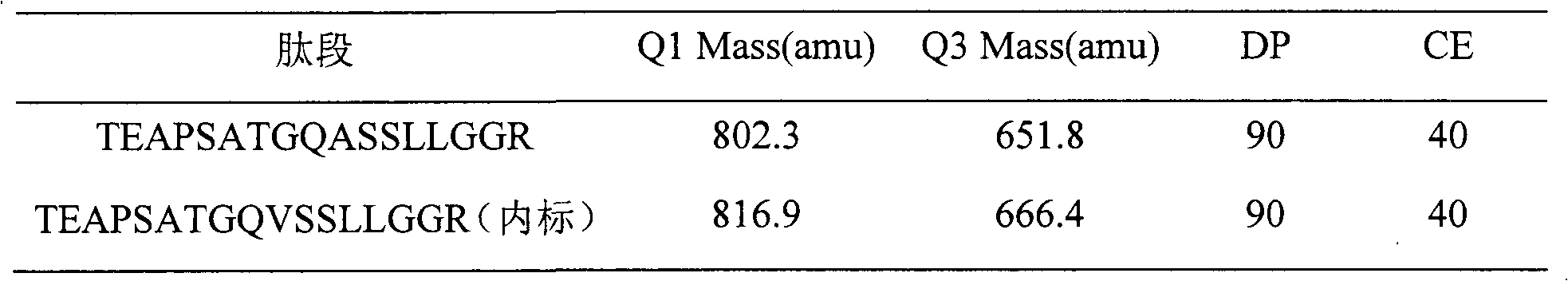
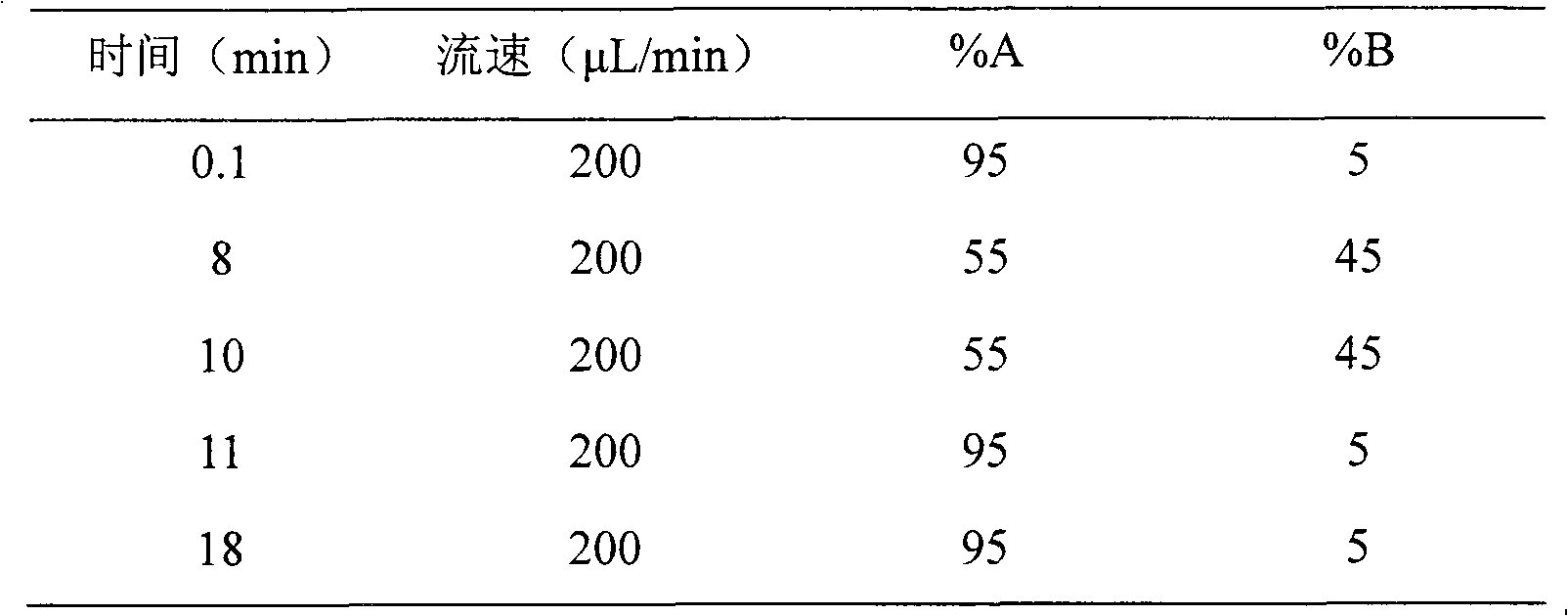
[0030] Eighteen SD rats were randomly divided into three groups (half male and half male, body weight 200±20 g), 6 rats in each group. Tail vein injection of recombinant human endostatin injection 1.5, 4.5, or 13.5mg / kg, after administration, 15 and 30 minutes, 1, 2, 3, 4, 6, 8, 12, 24 hours blood collection about 300μL , placed in an anticoagulant test tube, centrifuged at 3000 rpm at 4°C, and then transferred to another sterile dry tube, and stored in a freezer at -80°C. After the plasma sample was extracted and prepared according to the above biological sample preparation method, the blood drug concentration was determined by LC-MS / MS. Use the pharmacokinetic calculation software WinNonlin to calculate the kinetic behavior of the drug in the blood, and the half-life of the distribution phase t 1 / 2α , Elimination ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com