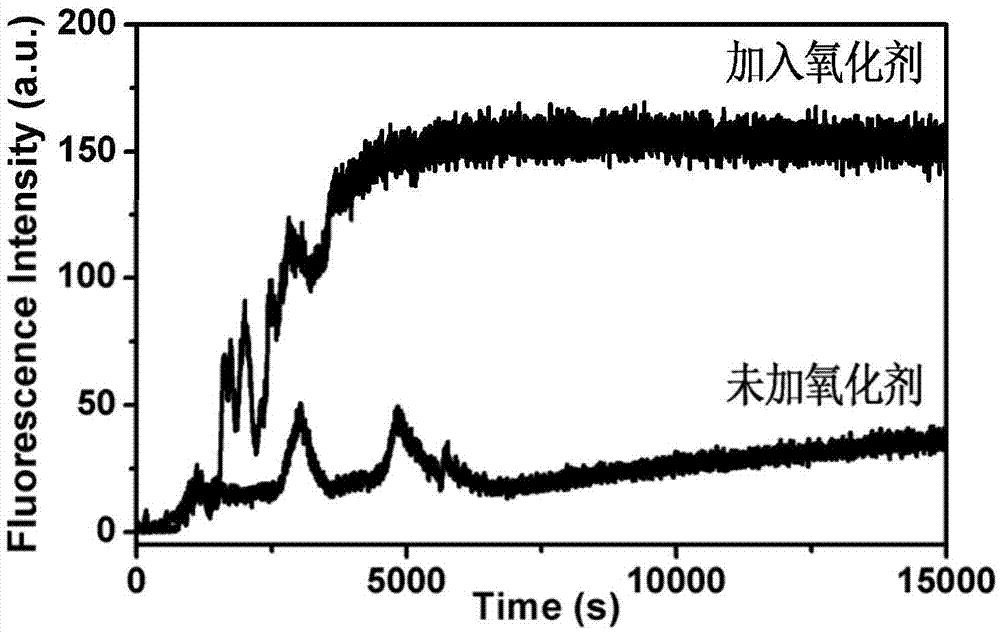
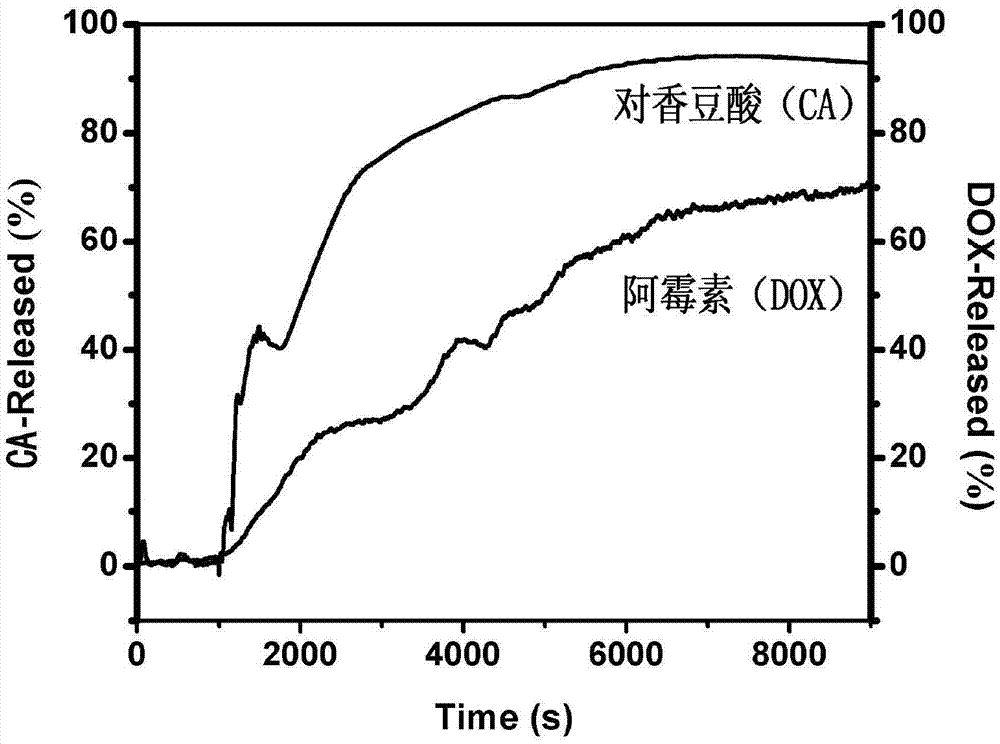
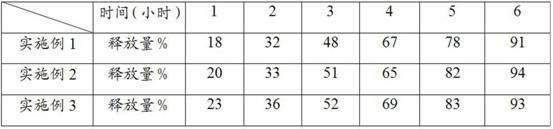
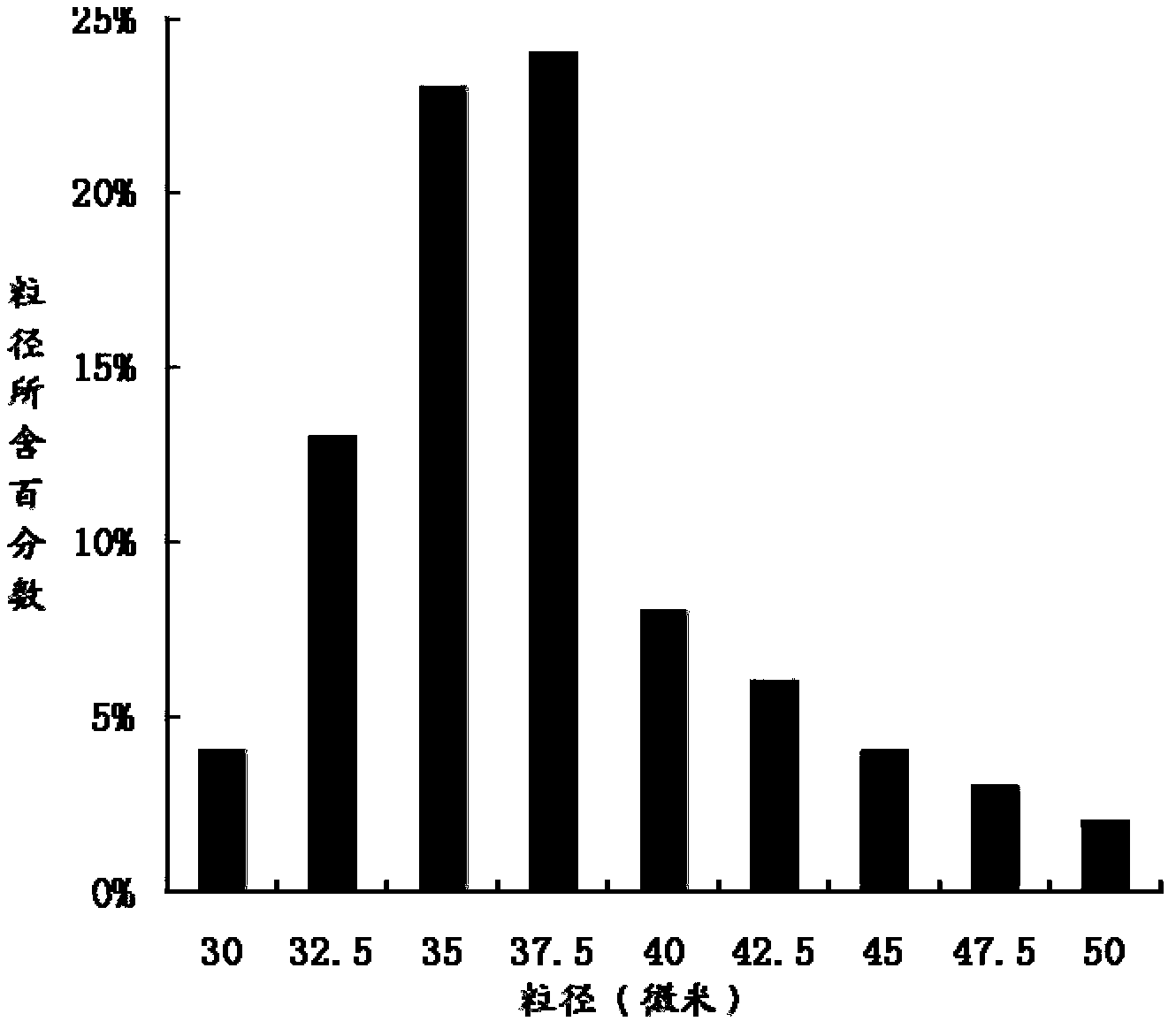Patents
Literature
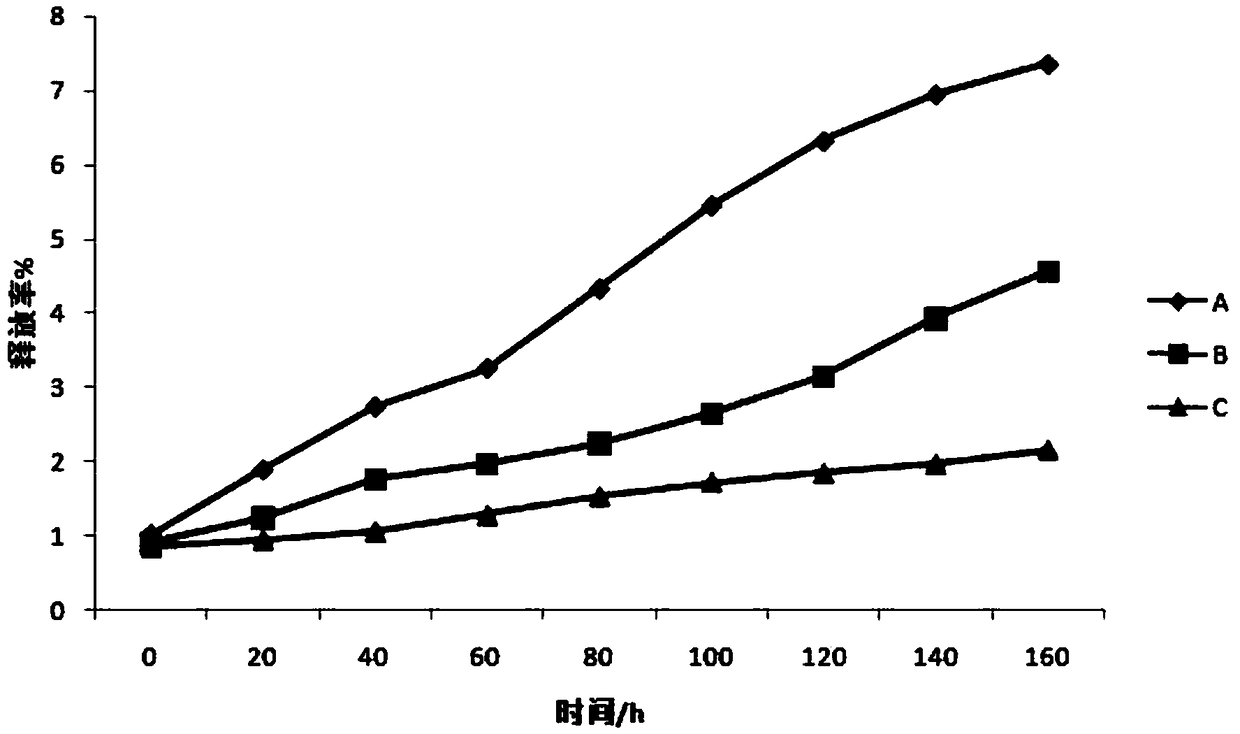
309 results about "Administration time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Analyte Measurement and Management Device and Associated Methods
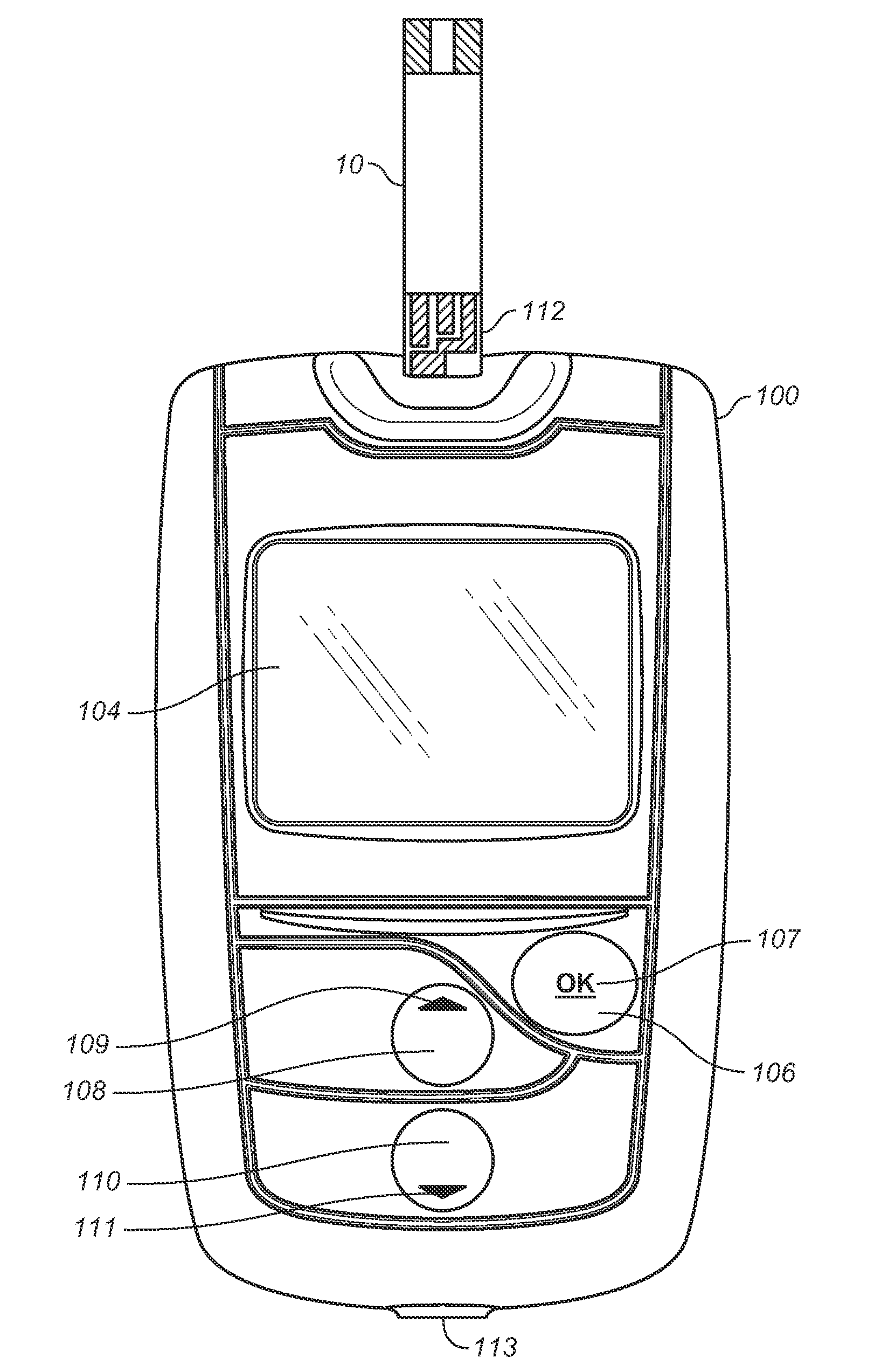
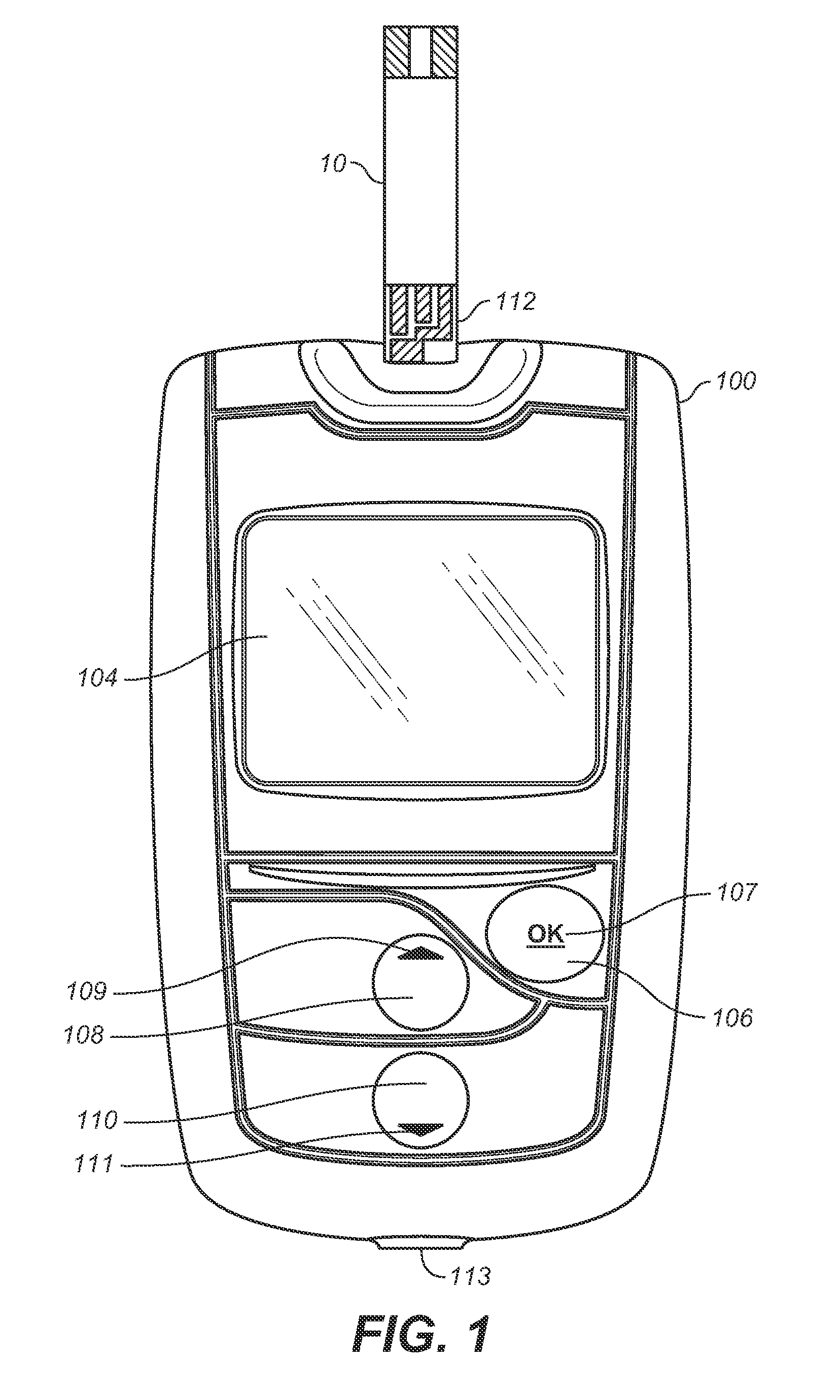
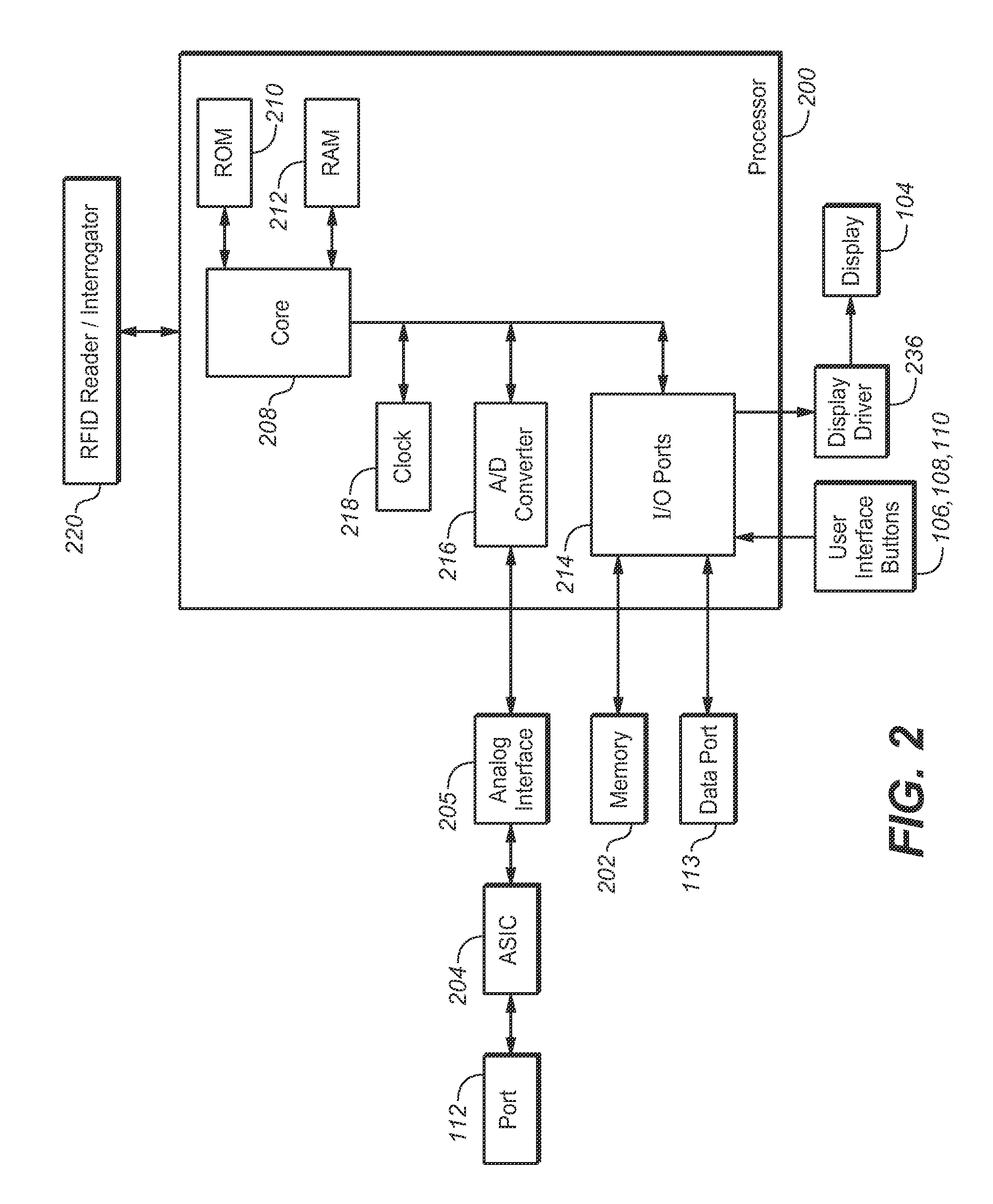
A method for measuring and managing an analyte (e.g., blood glucose) in a bodily fluid includes storing a therapeutic administration protocol in a memory module of an analyte measurement and management device and measuring the analyte in the bodily fluid sample using an analyte measurement module of the device. The method also includes calculating, with a processor module of the device, a recommended therapeutic agent dosage (for example, an insulin dosage) and a recommended administration time for user-activated delivery of the dosage by employing the therapeutic administration protocol. The method further includes displaying the recommended therapeutic agent dosage and administration time to a user on a visual display of the device, delivering a therapeutic agent dosage to the user via a user-activated therapeutic agent delivery device, and detecting the user-activated administration of the therapeutic agent using a delivery device communication module of the device. In addition, the method includes communicating the aforementioned detection to the processor module and / or memory module using the delivery device communication module. The method employs analyte measurement, memory, processor, and delivery device modules, as well as a visual display, and user interface that are integrated as a single hand-held unit.
Owner:LIFESCAN INC
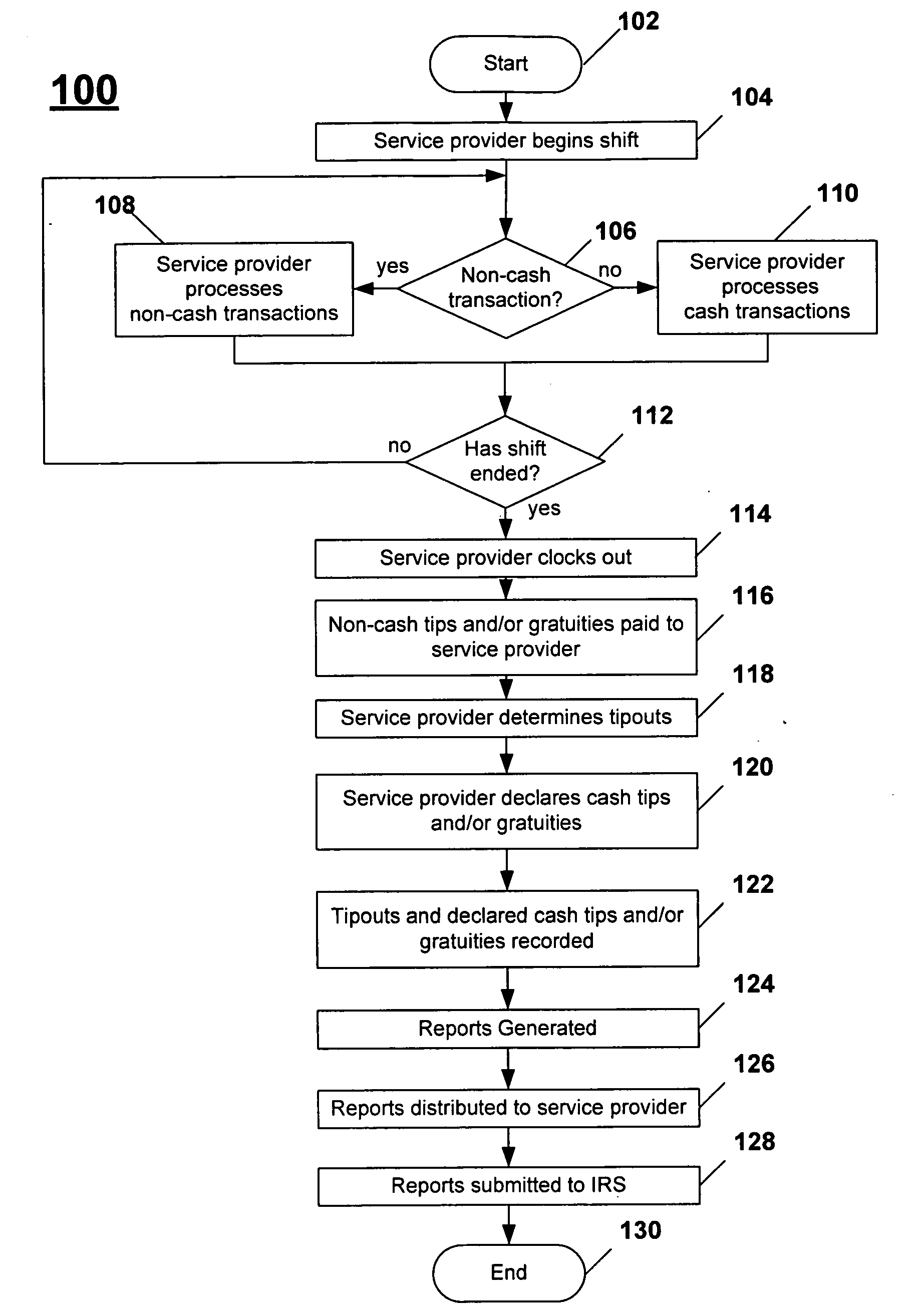
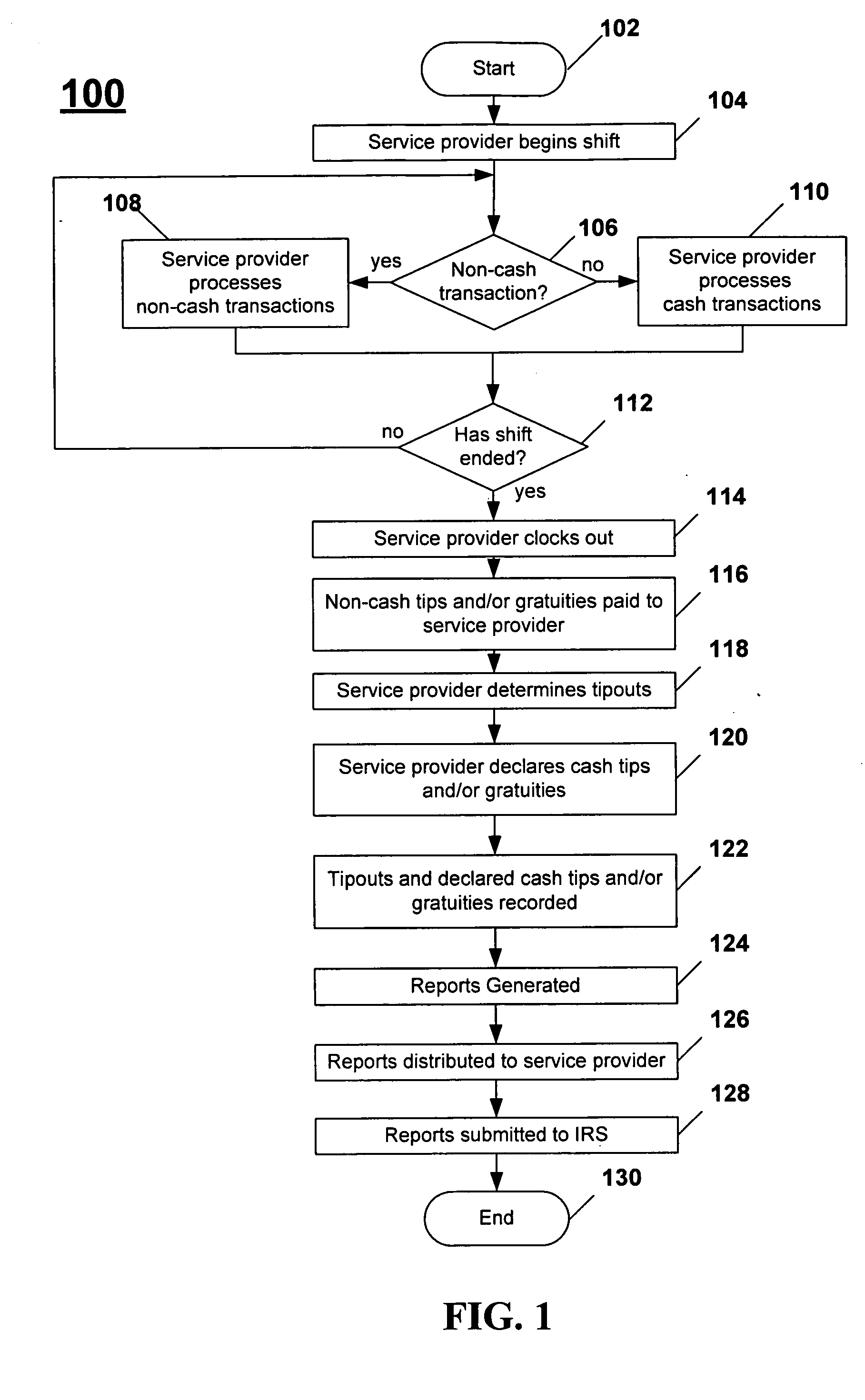
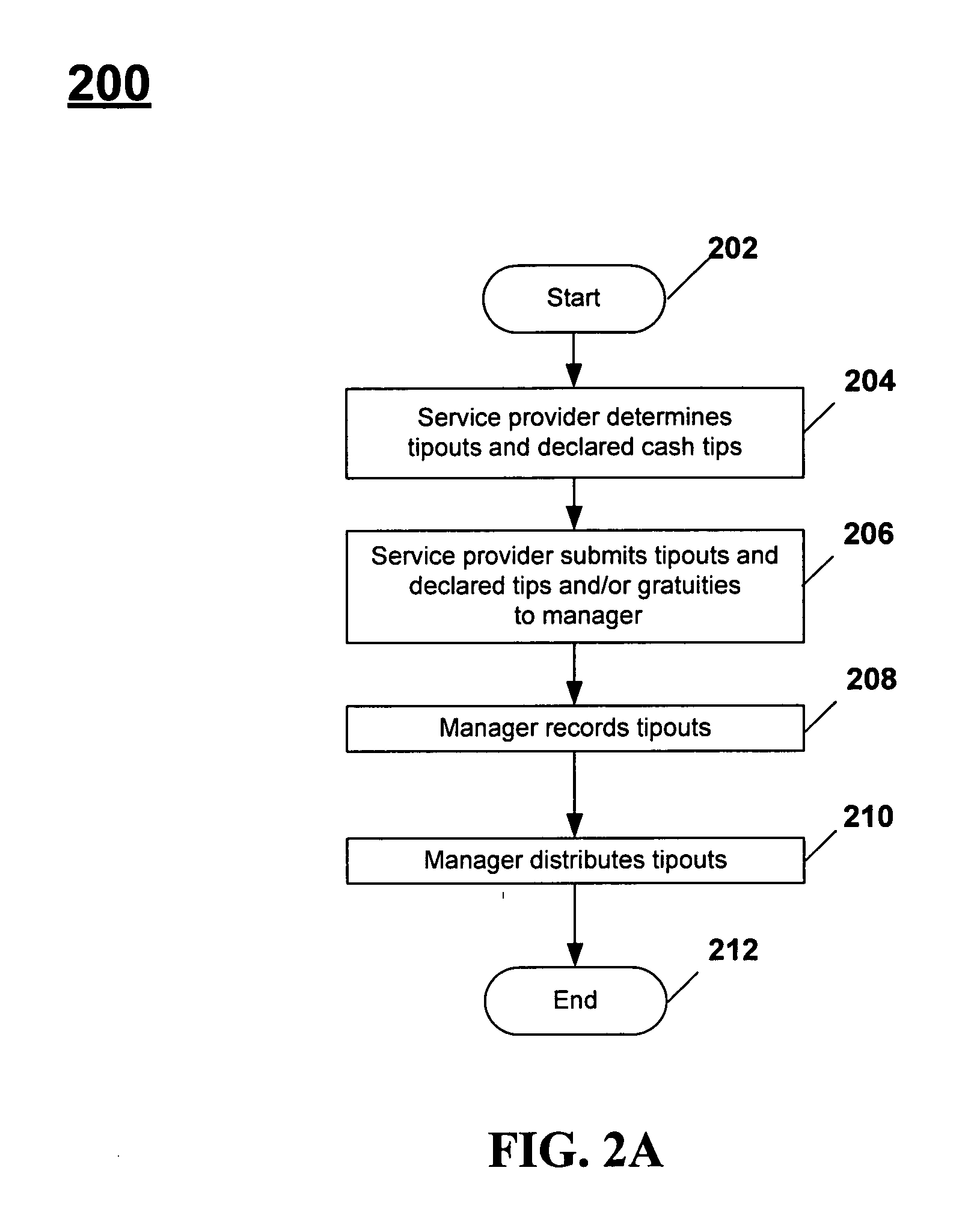
Systems and methods for managing tips and gratuities
Disclosed are systems and methods for recording, maintaining, and reporting tips and gratuities, optionally including details such as employee, task performed, and shift. In one aspect, inter-employee tip and gratuity transactions are tracked and verified. In another aspect, taxing authority forms and / or returns are automatically generated with substantially decreased administration time and cost, thereby facilitating a user's participation in voluntary taxing authority programs. Additionally, the training required by such programs is automatically monitored. In yet another aspect, tips and / or gratuities may be paid as wages. In another aspect of the present invention, employees are provided an opportunity to increase declared tips if the originally declared tips place employee in jeopardy of an audit. In another aspect, interfaces to third-party systems such as POS systems, payroll systems, and the like are provided to further minimize system administration time and data accuracy. In another aspect, Web-based systems are provided.
Owner:MARSHALL JOHN STEVEN
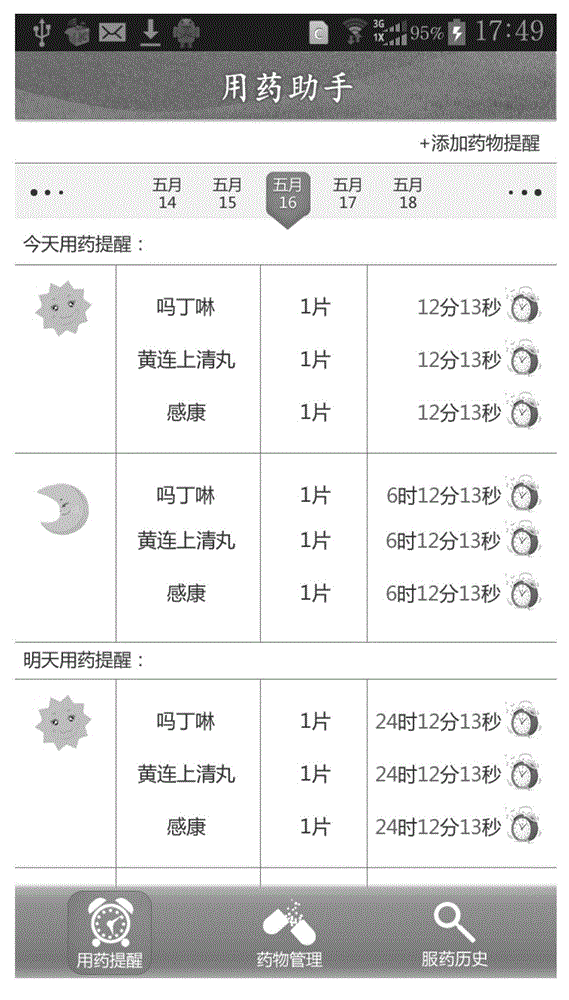
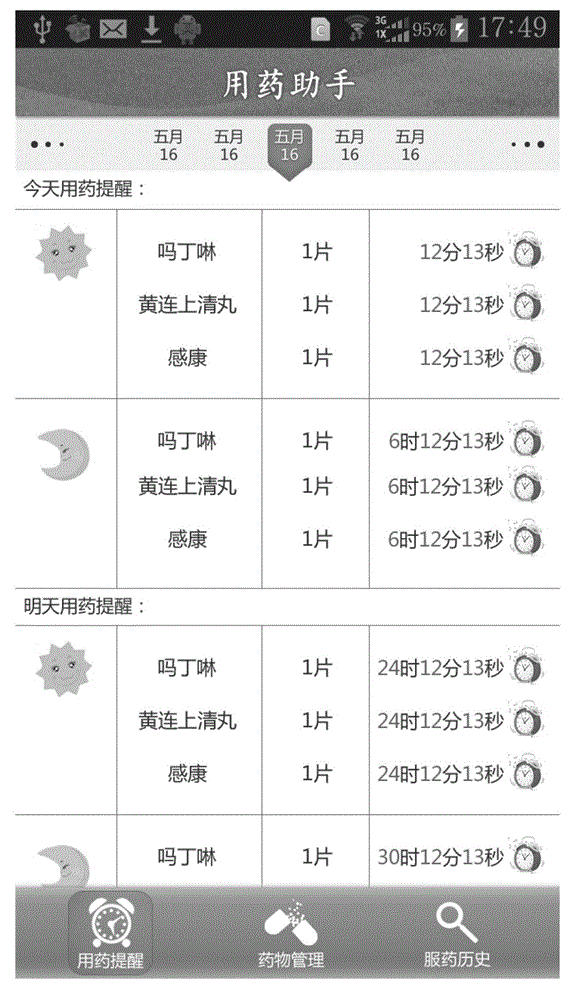
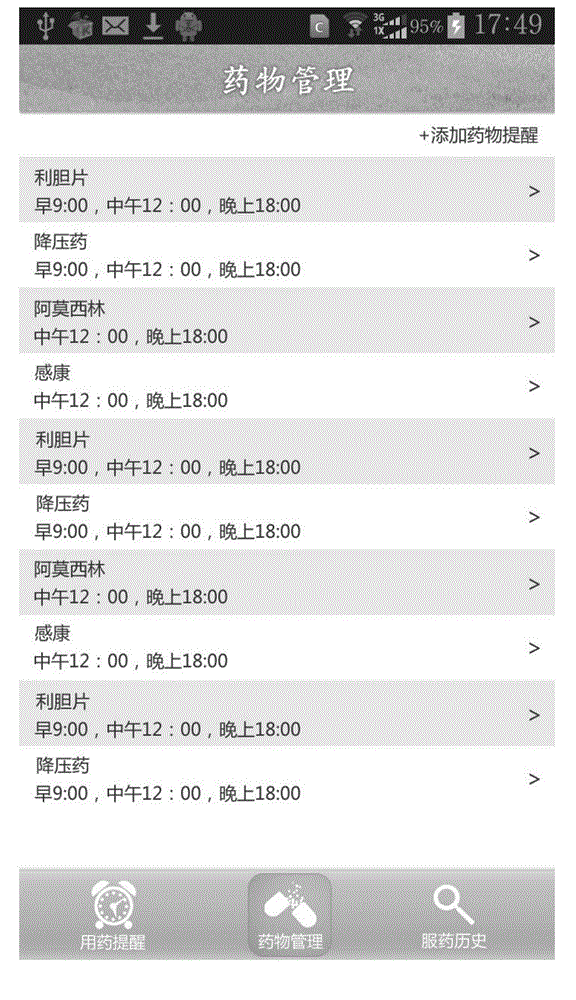
Medicine administration reminding and statistical system and medicine administration intelligent electronic system
The invention provides a medicine administration reminding and statistical system and a medicine administration intelligent system of intelligent electronic equipment and an intelligent electronic medicine box. The medicine administration reminding and statistical system comprises a login module, a reminding setting module, a current day reminding module, a medicine administration history reminding module and a medicine administration statistic uploading module; the intelligent electronic medicine box comprises a plurality of medicine storage cups, a pushing device, an transparent upper cover, an LED (Light Emitting Diode) display screen, an electronic chip, a transmitting device, a receiving device and a movable chassis. According to the medicine administration reminding and statistical system and the intelligent system, a person who takes medicines can be reminded of the medicine administration time and helped to manage the own medicine administration behavior; in the meantime, the medicine administration situation of the person who takes the medicines can be recorded so as to provide evidences for the person who takes the medicines as well as the family members (guardians) of the person who takes the medicines and a doctor to monitor the treatment condition of the person who takes the medicines.
Owner:王宇轩 +2
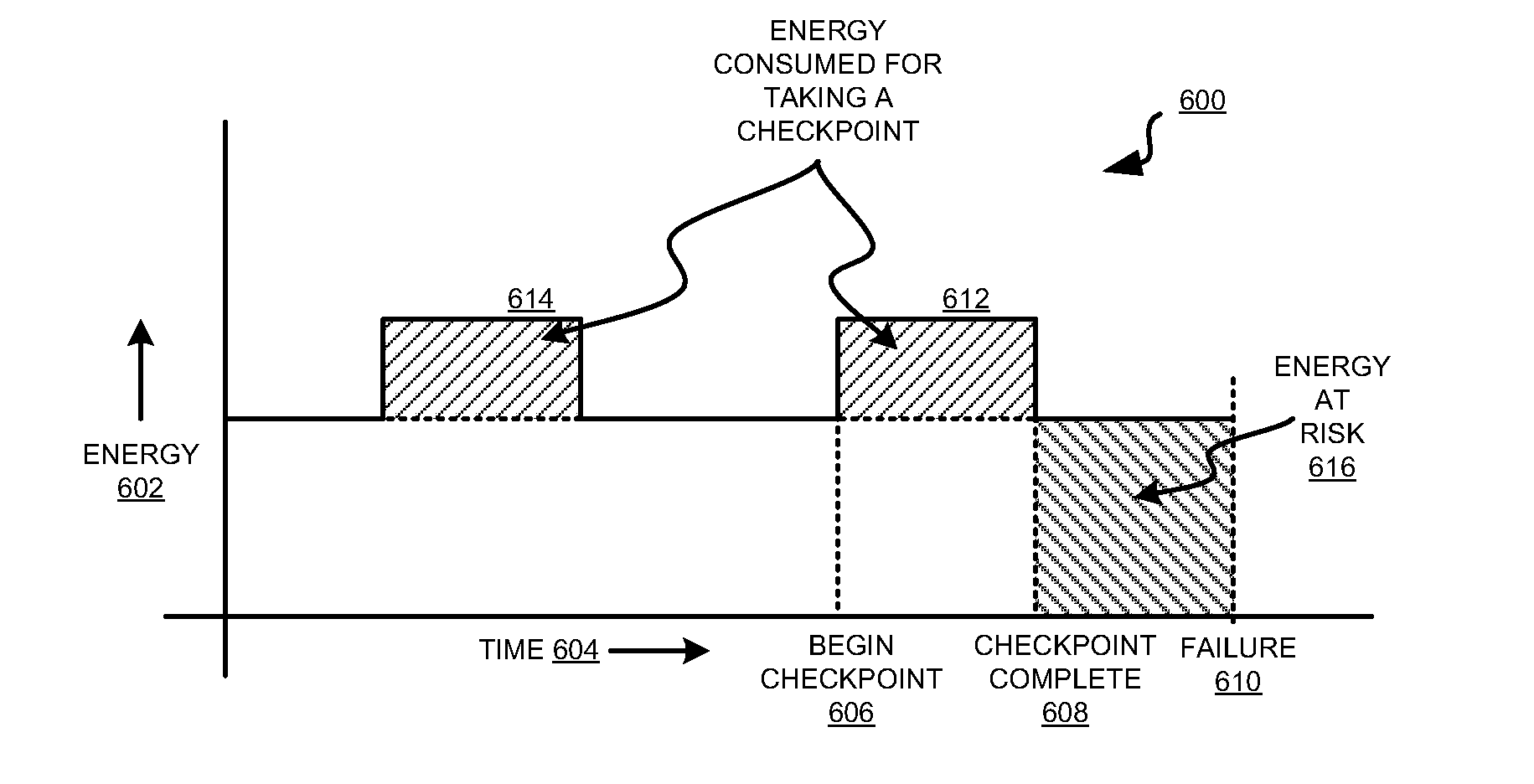
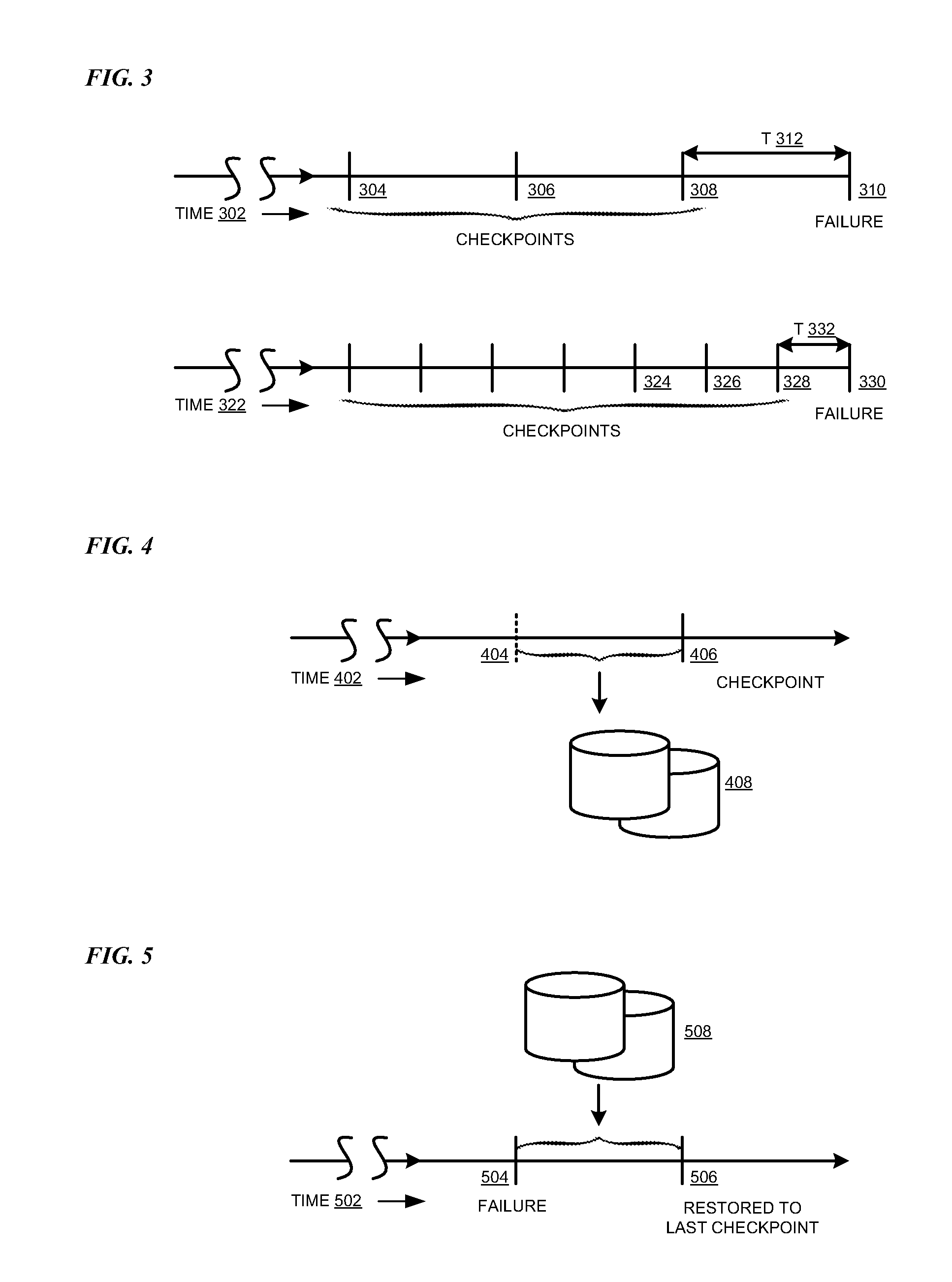
Total cost based checkpoint selection
InactiveUS20100088494A1Data processing applicationsElectric devicesAdministration timeReliability engineering
A method, system, and computer usable program product for total cost based checkpoint selection are provided in the illustrative embodiments. A cost associated with taking a checkpoint is determined. The cost includes an energy cost. An interval between checkpoints is computed so as to minimize the cost. An instruction is sent to schedule the checkpoints at the computed interval. The energy cost may further include a cost of energy consumed in collecting and saving data at a checkpoint, a cost of energy consumed in re-computing a computation lost due to a failure after taking the checkpoint, or a combination thereof. The cost may further include, converted to a cost equivalent, administration time consumed in recovering from a checkpoint, computing resources expended in taking a checkpoint, computing resources expended after a failure in restoring information from a checkpoint, performance degradation of an application while taking a checkpoint, or a combination thereof.
Owner:IBM CORP
Installation and method for individually tailored filling of blister packs with medication according to predetermined prescription data
InactiveUS20150353219A1Improve efficiencyEasy maintenanceDrug and medicationsArticle unpackingBlister packAdministration time
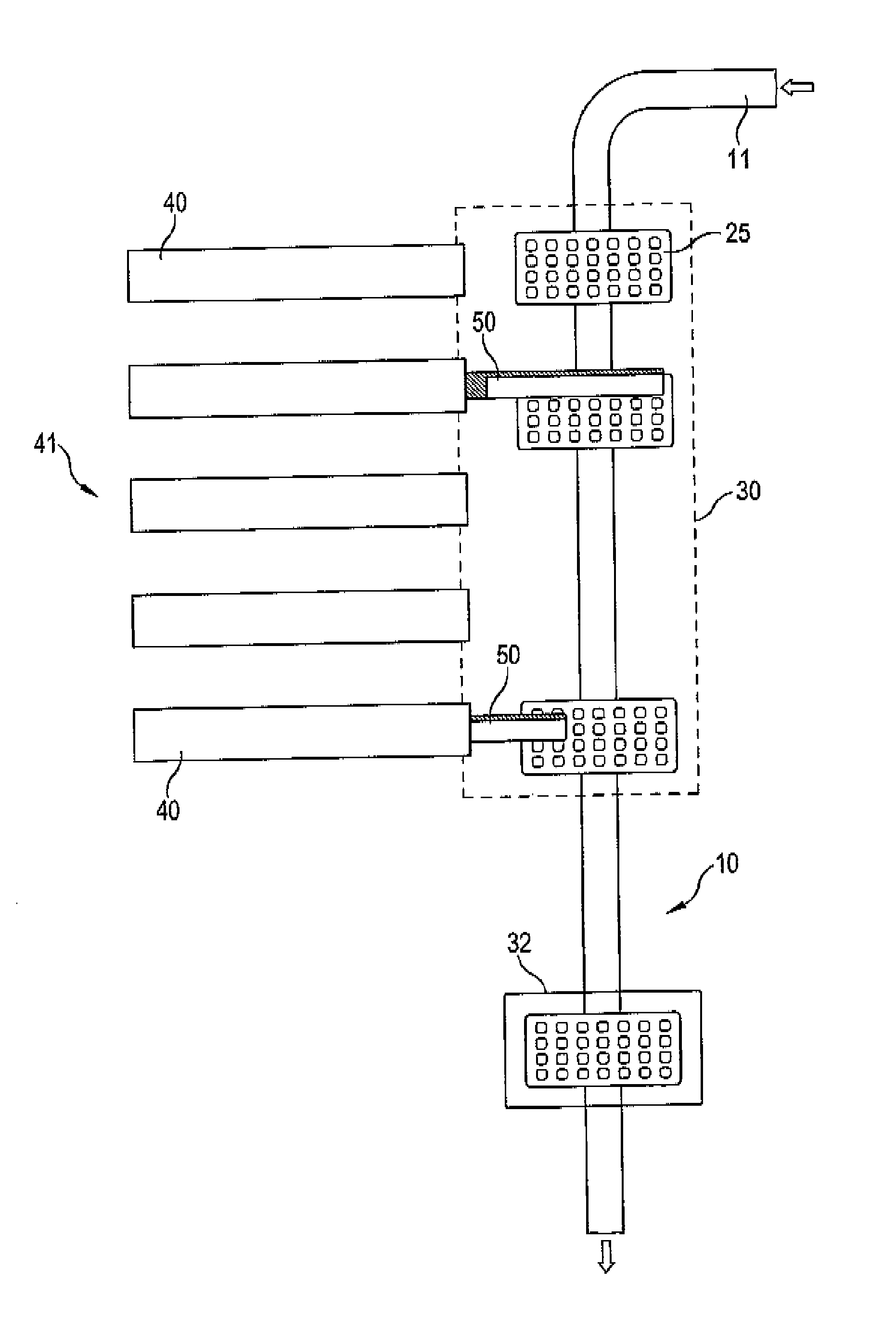
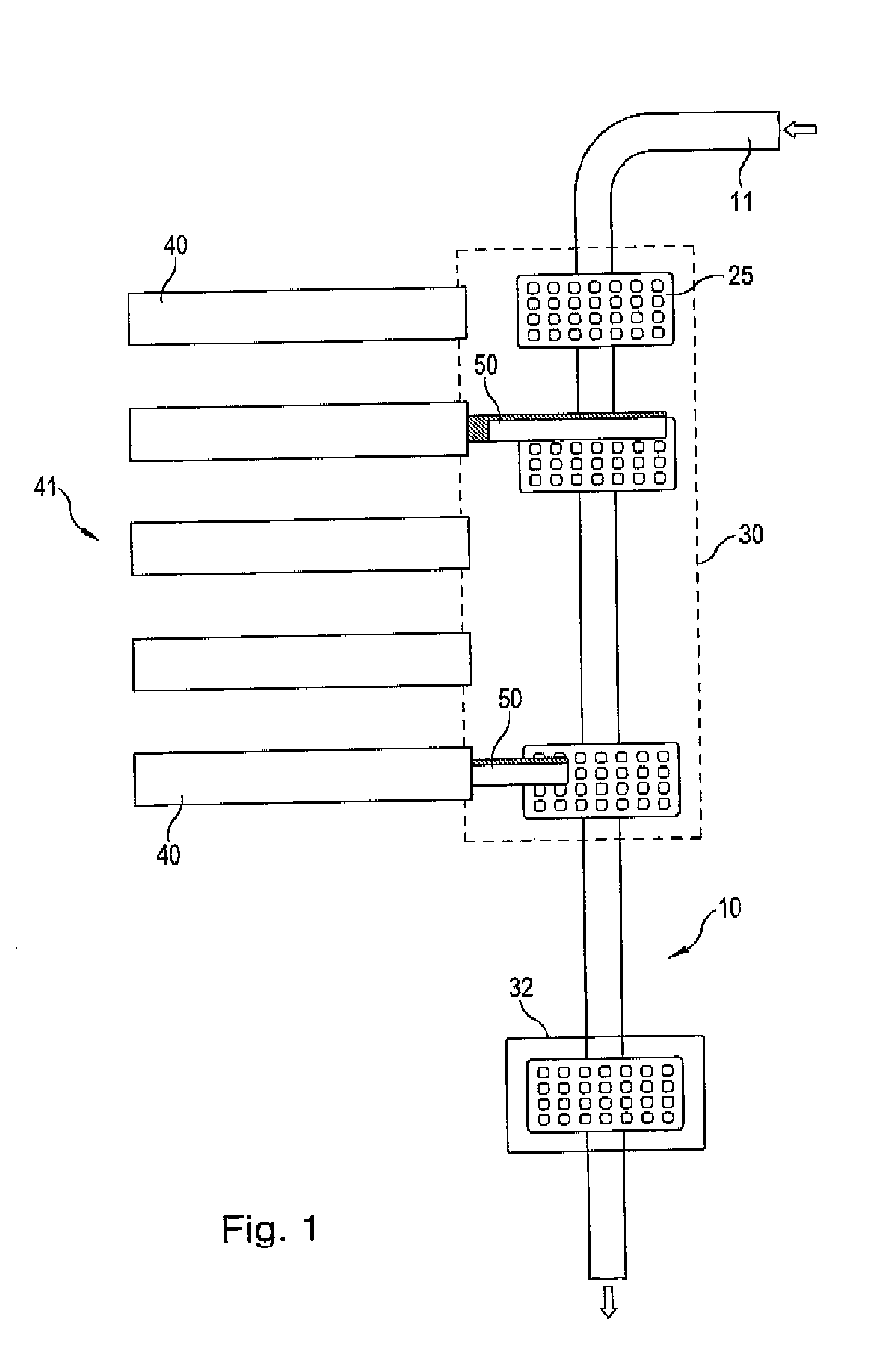
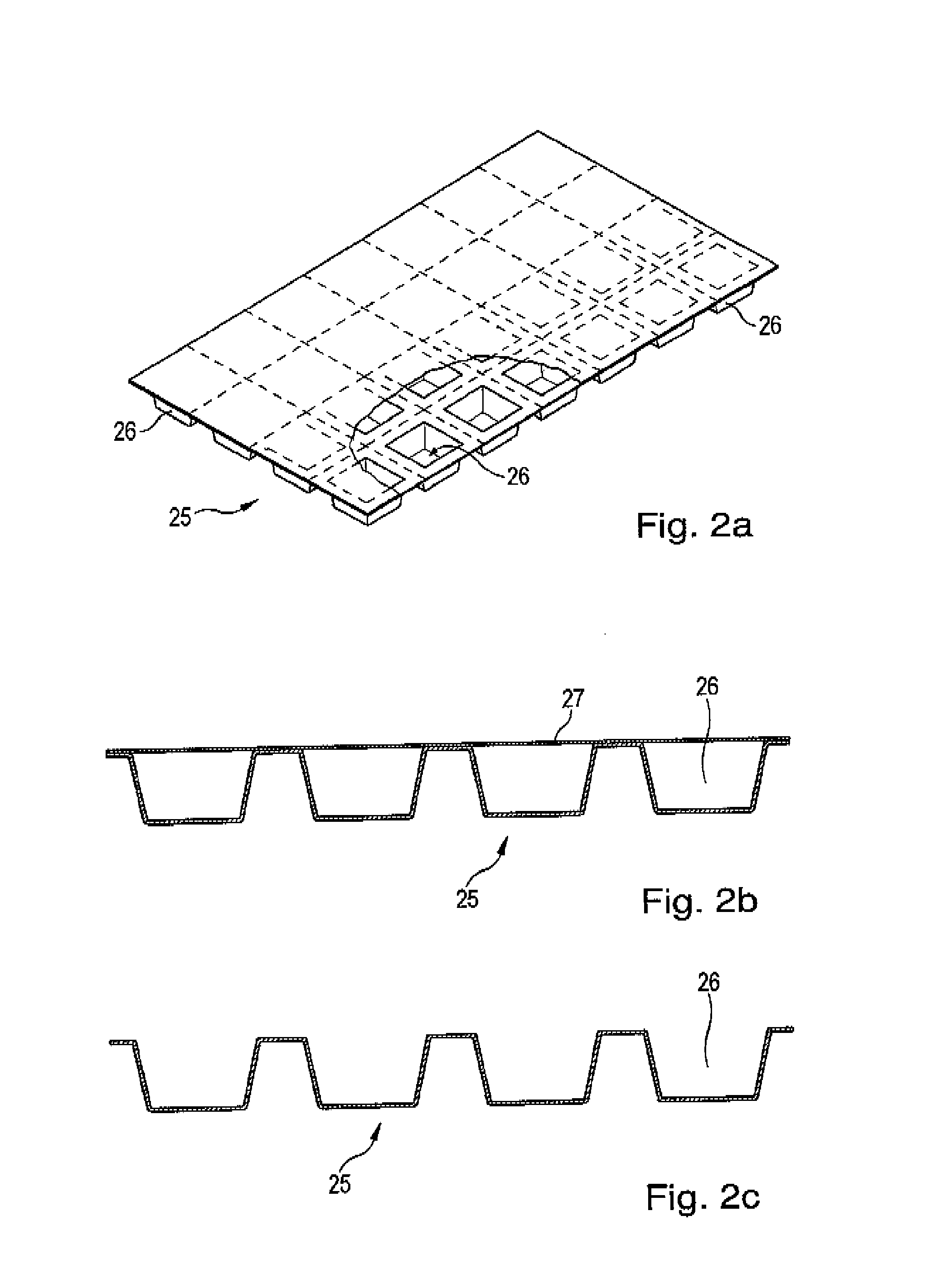
The invention relates to an installation and a method for the individually tailored filling of blister packs (25) with medication according to predetermined prescription data, the blister packs (25) having receiving compartments for respective medication administration units, said compartments being arranged in a matrix configuration in rows corresponding to a number of administration times during a day and columns corresponding to a number of days, for example weekdays. The installation comprises a plurality of medication filling stations, arranged one behind the other, for filling each blister pack (25) with the respective specified medication and a transport device (10) designed to transport the blister packs (25) individually one behind the other in a direction of travel alongside the medication filling stations (40). Each blister back (25) is individually guided in such a way that said pack only approaches those medication filling stations (40) that are required by the prescription data for a filling operation, bypassing the other medication filling stations, and is directly conveyed onwards to the next required medication filling station or to a position behind a preceding blister pack (25) that is currently at a medication filling station (40). The individual guiding of the individual blister packs without a fixed cyclic operation to only those medication filling stations required by the prescription data permits a more rapid passage of the individual blister packs through the installation and thus increased efficiency. In addition, the operation of the installation is not brought to a standstill as a result of maintenance work on an individual medication filling station.
Owner:KOHL EDWIN
Ibuprofen preparation and preparation method thereof
InactiveCN104248767ASolve the problem of slow onsetImprove complianceOrganic active ingredientsAntipyreticAdministration timeRelease time
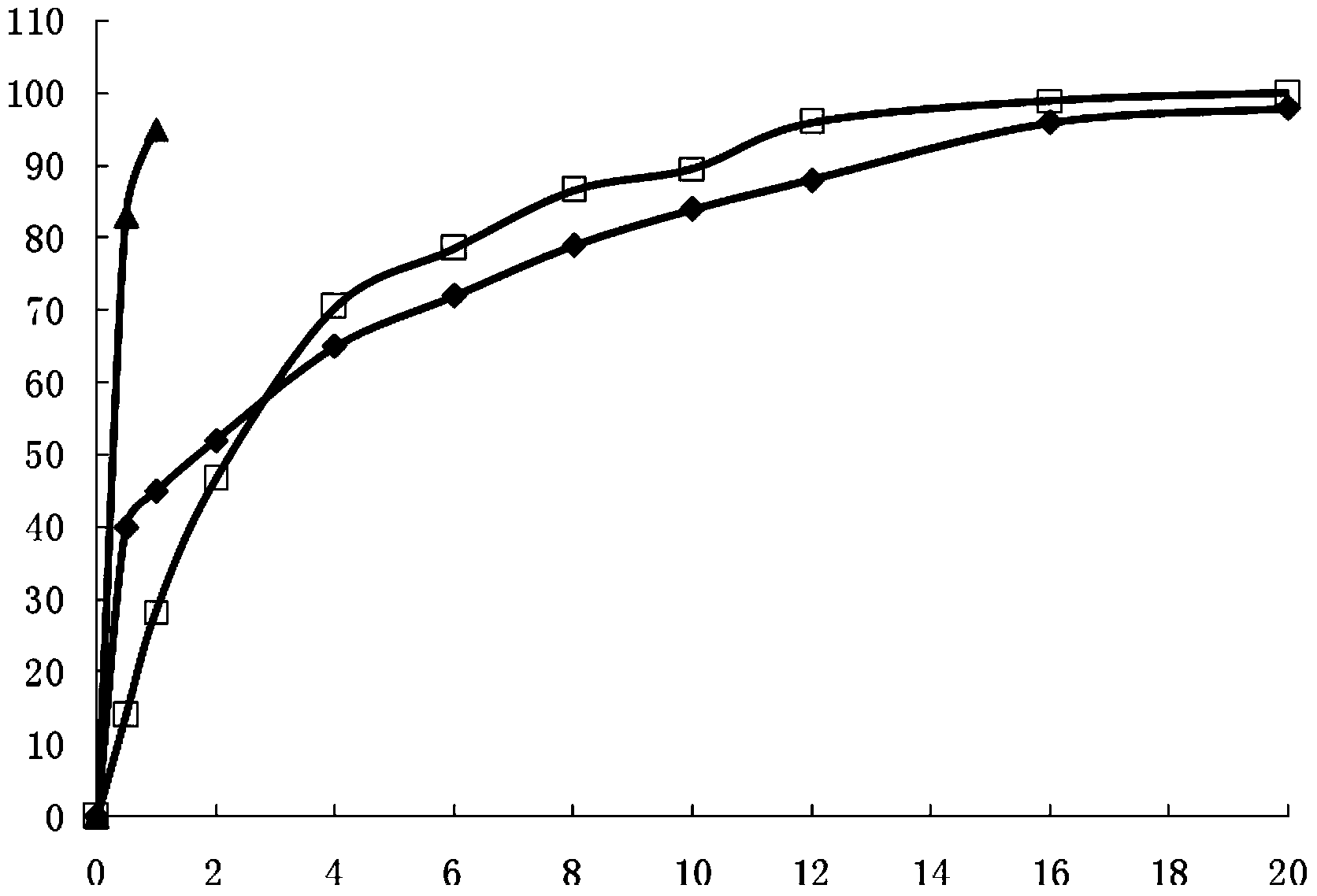
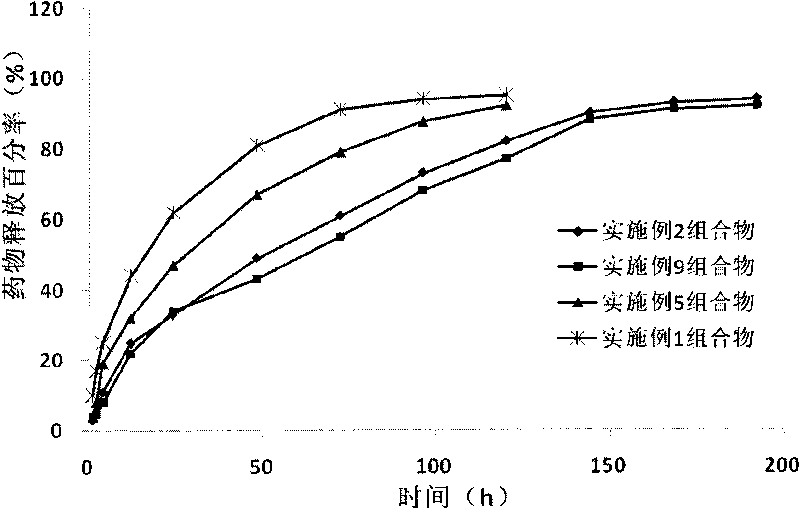
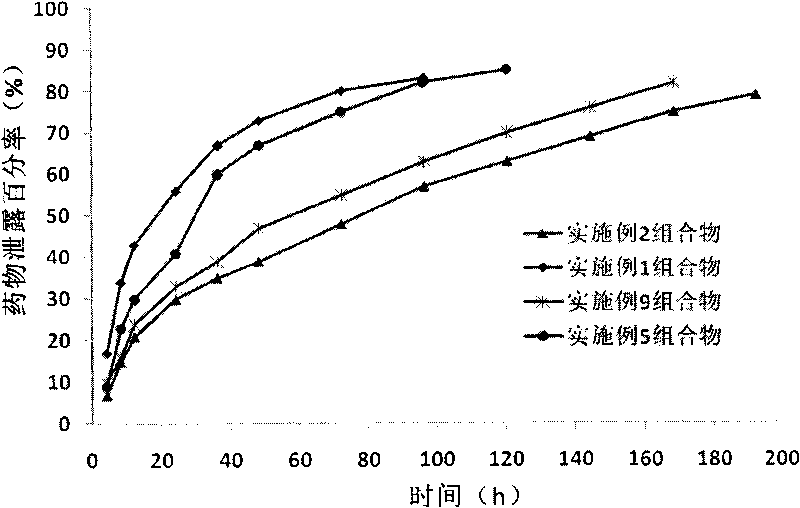
The invention provides a novel ibuprofen preparation comprising a fast release part and a sustained release part. The ibuprofen as an active component in the fast release part accounts for 20% to 60% by weight of the active component ibuprofen in the novel ibuprofen preparation, and preferably accounts for 30% to 50%, and the ibuprofen as an active component in the sustained release part accounts for 80% to 40%by weight of the active component ibuprofen in the novel ibuprofen preparation, and preferably accounts for 70% to 50%. Single dose of the novel ibuprofen preparation contains 200-1000mg of ibuprofen, and preferably contains 300mg or 600mg of ibuprofen. After the product is taken, according to Chinese pharmacopoeia releasing rate detection method, the fast release part reach the maximum release value in 30 minutes, the slow release time of the sustained release part can maintain 12 to 20 hours. Drugs can relatively much release during the early stage of medication, effective drug concentration in the blood can be rapidly reached, the problem of two slow drug effect can be solved, because of the existence of the sustained-release part, the drugs can slowly and sustainedly release in the body, maintain proper blood drug concentration, play the role of continue treatment and reduce administration times.
Owner:SHANGHAI SUNTECH PHARMA
Preparation and applications of mesoporous silica/insulin nanoparticles modified by phenylboronic acid
InactiveCN106236734AStabilize blood sugar levelsAutomatic sensing of glucose concentration changesPeptide/protein ingredientsMetabolism disorderPhenylboronic acidNanoparticle
The invention relates to preparation and applications of mesoporous silica / insulin nanoparticles modified by phenylboronic acid, for effectively solving the problem that the traditional drug carriers release medicines unidirectionally. The technical scheme is as follows: the preparation comprises the following steps: firstly, synthesizing mesoporous silica nanoparticles, modifying amino groups on the surfaces of the mesoporous silica nanoparticles, loading medicine insulin in the mesoporous structure through physical absorption, and further modifying with phenylboronic acid and polysaccharide, thus obtaining the mesoporous silica / insulin nanoparticles modified by phenylboronic acid. The preparation and applications have the advantages that the synthesis process is simple, the prepared nanoparticles have the good biocompatibility, and the releasing valve of medicines can be repeatedly opened and closed, so that the sustained-release effect is achieved on the release of medicines; the mesoporous silica / insulin nanoparticles have a long-time circulation in the body, so that the administration times can be reduced, and thus the mesoporous silica / insulin nanoparticles belong to an innovation in diabetes treatment medicines.
Owner:ZHENGZHOU UNIV
Chitosan-based hepatic-targeted nano-particle drug delivery system and preparation method thereof
InactiveCN101642573AGood biocompatibilityFunctionalOrganic active ingredientsDigestive systemDiseaseSide effect
The invention relates to a chitosan-based hepatic-targeted nano-particle drug delivery system which takes a derivative of glycyrrhetinic acid-modified chitosan as a carrier material and is prepared byembedding an anti-cancer drug, the particle size of nano-particles is 50nm-300nm, and the drug-loading rate is 5-40%; and the carrier material is glycyrrhetinic acid-sulfate chitosan or glycyrrhetinic acid-carboxymethyl chitosan, and the embedded anti-cancer drug is doxorubicin, paclitaxel or hydroxycamptothecin. The chitosan-based hepatic-targeted nano-particle drug delivery system has the beneficial effects that the chitosan and the derivative thereof are non-toxic and have good biocompatibility and anti-tumor effect, the hepatic targeting tendency of glycyrrhetinic acid and excellent biological performance of the chitosan derivative are combined and a novel hepatic-targeted drug delivery system,is developed and prepared; and the hepatic-targeted nano-particle drug delivery system has drug sustained-release function and hepatic targeting property and can reduce the using amount of the drug and the administration times, reduce the toxicity or the side effects of the drug and improvethe efficacy by being used in the treatment of liver diseases, thereby having good application prospects.
Owner:NANKAI UNIV
Medicinal composition for treating bacterial vaginitis
ActiveCN101744833ASpecial bioadhesionReduce leakageOrganic active ingredientsSexual disorderContact timeRelease time
The invention relates to a medicinal composition for treating bacterial vaginitis, which comprises main medicaments, substrates, a bioadhesive material, a release rate regulator and other additives. The medicinal composition for treating bacterial vaginitis has specific biological adhesion, keeps longer time, reduces leakage of the medicaments, prolongs the contact time of the medicaments and the vagina mucosa, and guarantees the curative effect. The composition has specific vagina slow release performance, and can continuously release the medicaments for 2 to 7 days. Compared with the common preparation, the medicinal composition prolongs the releasing time of the medicaments, can reduce the administration times, and is convenient for the patient to take. As simultaneously the medicinal composition has the characteristics of biological adhesion and slowly-released long effect, the medicinal composition has no requirement for the administration time, and the administration can be carried out at any time.
Owner:SHENZHEN SOUTH CHINA PHARMA
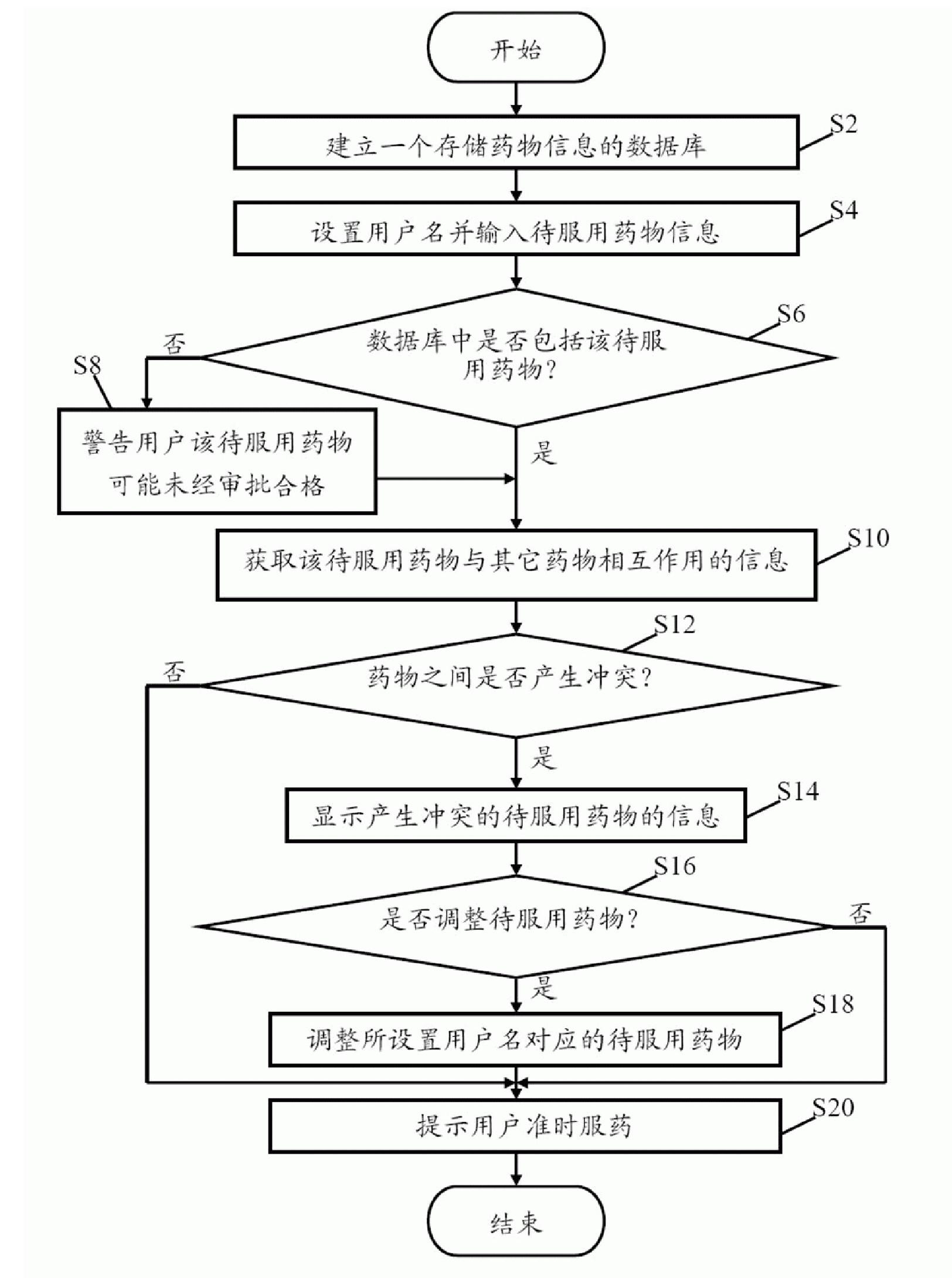
Medicine-taking prompt system and method
InactiveCN101773451AAvoid takingOral administration deviceMedication informationRelevant information
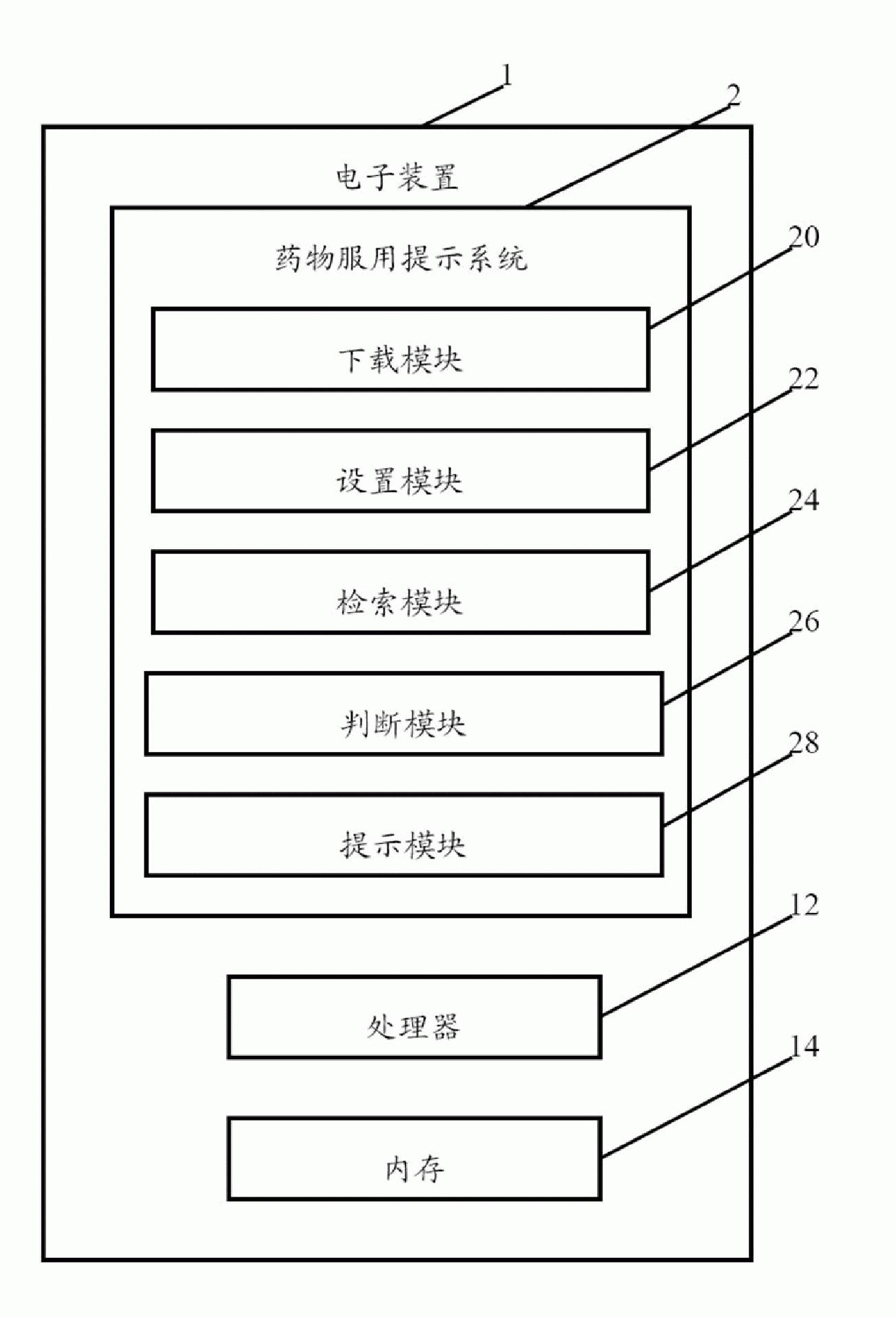
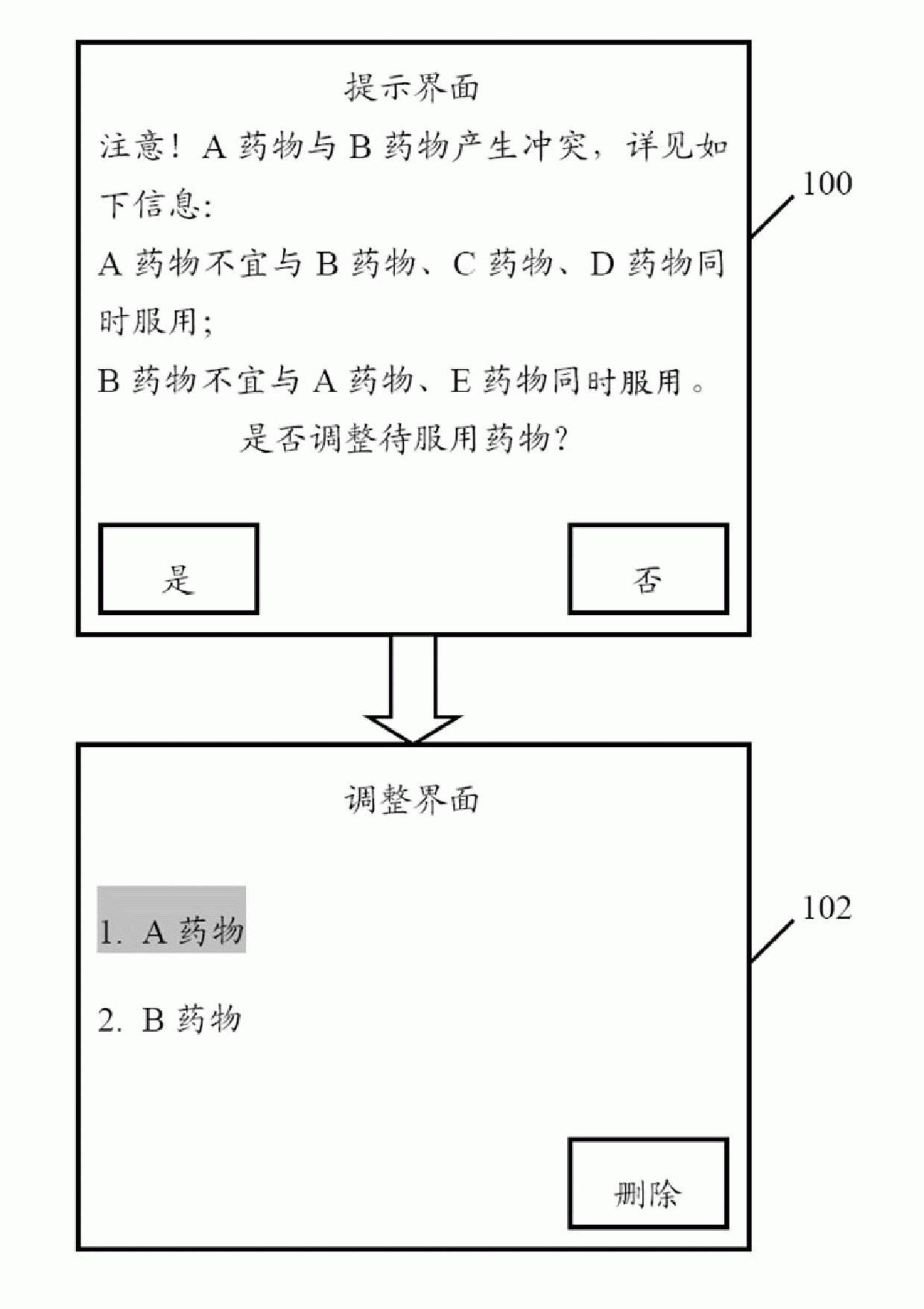
The invention provides a medicine-taking prompt system arranged in an electronic device. The system comprises a download module, a setting module, a retrieval module, a judging module and a prompt module, wherein the download module is used to establish a database for storing medicine information; the setting module is used to set a user name and the information of medicines to be taken corresponding to the user name; the retrieval module is used to judge whether related information of the medicines to be taken are contained in the database, if so, the information about the interaction of the medicines to be taken and other medicines is obtained by the retrieval module, if not, the retrieval module prompts the user to obtain the interaction information of the medicines to be taken and other medicines; the judging module is used to judge whether interactions are between the medicines to be taken, if so, the judging module displays the interacted medicines to be taken and prompts the user change the medicines to be taken; and the prompt module is used to prompt the user to take medicines on time according to the set administration time and the amount of medicines. The invention also provides a medicine-taking prompt method. By using the prompt system and method of the invention, the user can be prompted to take medicines.
Owner:SHENZHEN FUTAIHONG PRECISION IND CO LTD +1
Allopurinol dual-release preparation and preparation method thereof
ActiveCN102091051AInsufficient improvementEffective plasma concentrationOrganic active ingredientsSkeletal disorderSpecific gravityAdministration time
The invention relates to an allopurinol dual-release preparation and a preparation method thereof, which is characterized in that the dual-release preparation consists of a quick-release part and a slow-release part, wherein the ratio of the base allopurinol contained in the quick-release part to the base allopurinol contained in the slow-release part is 1:1 to 1:24. The invention belongs to the technical field of medicine preparation and aims at providing the allopurinol dual-release preparation with good patient compliance and small side effect and the preparation method thereof, wherein the allopurinol dual-release preparation has the advantages of taking effect quickly and maintaining effective blood concentration stably. The dual-release preparation can not only be used for reducing the side effect on the gastrointestinal tract, but also decreasing the administration times of a patient and adverse effect and promoting the patient compliance.
Owner:COSCI MED TECH CO LTD
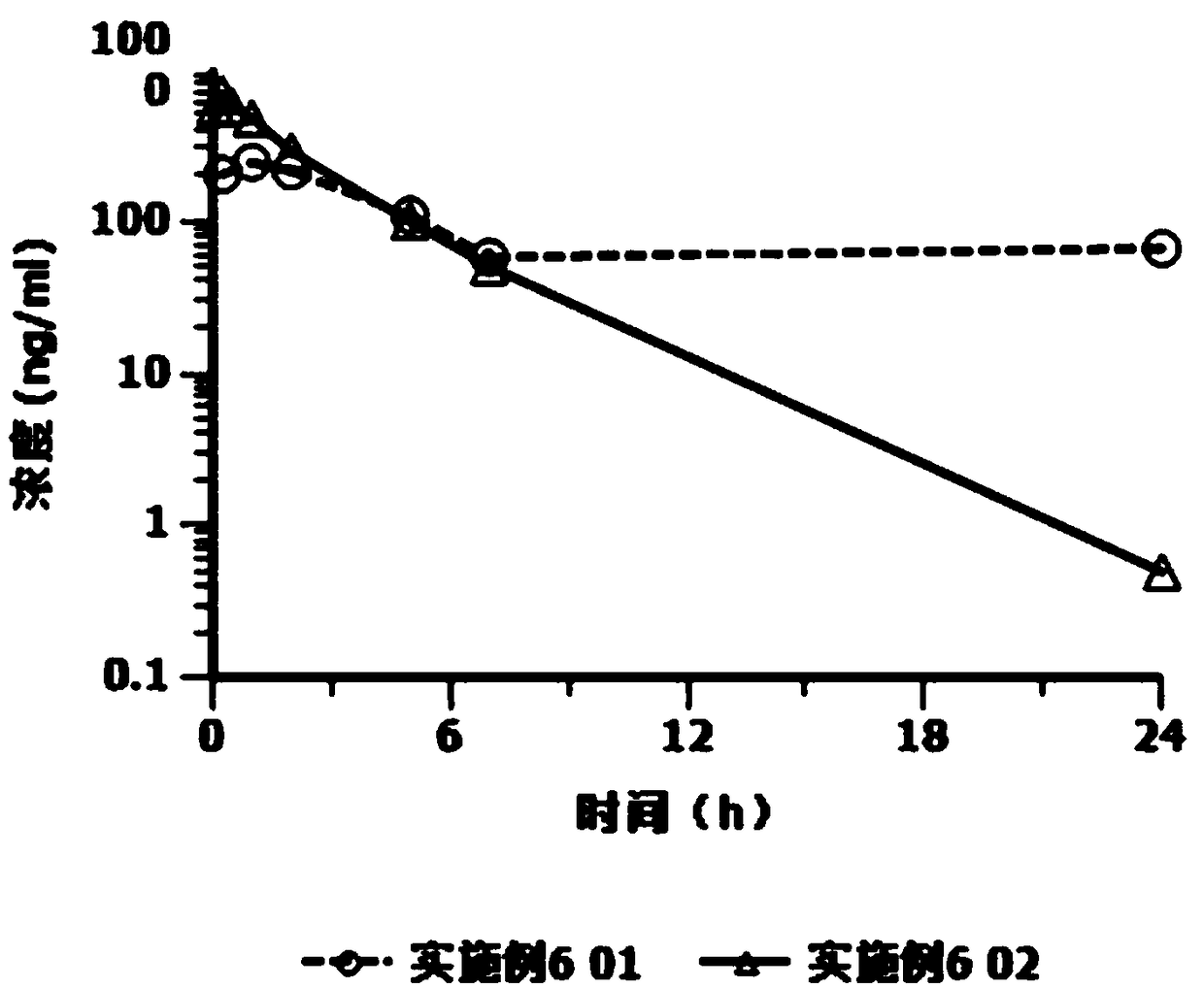
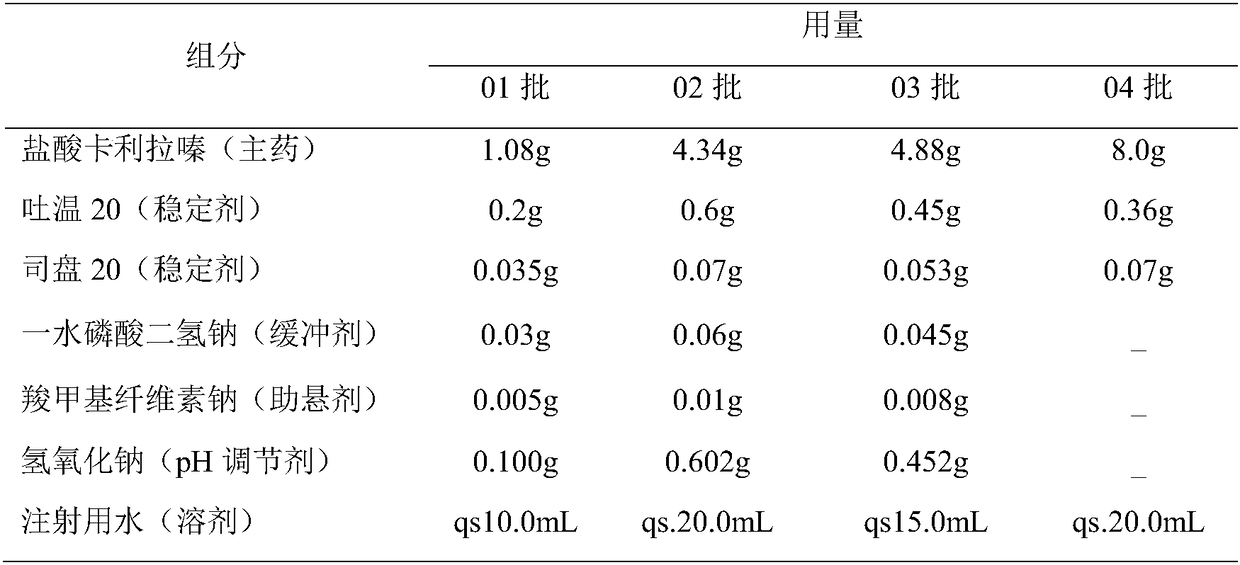
Cariprazine hydrochloride injection preparation, and preparation method and use thereof
ActiveCN108261394AImprove drug stabilitySmall batch-to-batch varianceOrganic active ingredientsPowder deliveryHigh concentrationInjection volume
The invention provides a cariprazine hydrochloride injection preparation, and a preparation method and a use thereof. The injection preparation is an aqueous suspension when used, cariprazine hydrochloride has a high concentration and a good stability, a high dosage can be obtained within a limited injection volume, the dosage can be flexibly adjusted according to the long-acting administration time, the particle size distribution and the injection dosage are controlled to achieve the long-acting effect, the cariprazine hydrochloride is continuously released in at least one week after the preparation is injected, and the preparation is administrated every one week or more to increase the compliance of patients. The invention also provides the preparation method of the cariprazine hydrochloride injection preparation. The injection preparation prepared by the method has the advantages of good stability and high safety, and the method is simple, is easy to implement, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Slow-release preparation for postoperation analgesia and preparation method of slow-release preparation
ActiveCN108379269AProlong the action timeImprove bioavailabilityAntipyreticAerosol deliveryProcaineSide effect
The invention discloses a slow-release preparation for postoperation analgesia and a preparation method of the slow-release preparation. The slow-release preparation is mainly prepared from the following raw materials: 10-15 parts of phospholipid, 4-7 parts of glyceride, 0.3-5 parts of poloxamer, 0.8-5 parts of a cosolvent and 0.5-2 parts of a local anesthetic mixture of cocaine, procaine and lidocaine, wherein the mass ratio of the cocaine to the procaine to the lidocaine in the local anesthetic mixture is 1:1:(4-7.5). The phospholipid is phosphatidylcholine, and preferably soybean phosphatidylcholine; the glyceride is dinolin; the cosolvent is ethanol; the slow-release preparation is easy to inject, and medicines can be slowly released in situ; the action time of the medicines is prolonged, the administration times are reduced, and the bioavailability of the medicines is improved; meanwhile, toxic and side effects caused by too high medicine concentrations can be avoided.
Owner:武汉百纳礼康生物制药有限公司
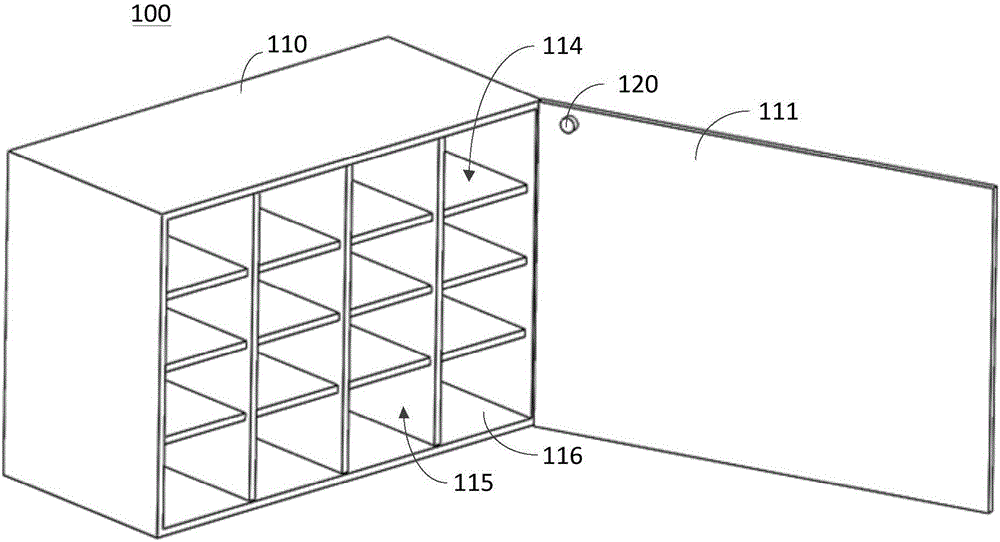
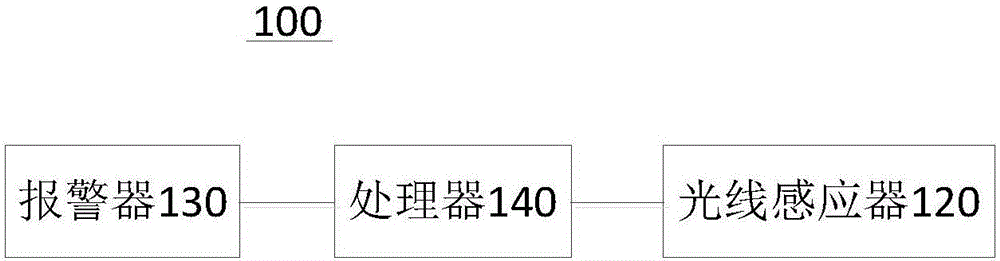
Intelligent medicine kit and intelligent medicine kit system
The invention provides an intelligent medicine kit and an intelligent medicine kit system and relates to the technical field of medical equipment. The intelligent medicine kit comprises a kit body which is opened on the side face and a cover plate capable of covering the opening, and the cover plate is movably connected to the kit body. The intelligent medicine kit comprises a light sensor, an alarm and a processor. The light sensor and the alarm are electrically connected to the processor. The processor is used for controlling the alarm to alarm when a preset administration time arrives. The light sensor is arranged on a face, facing the kit body, of the cover plate, for detecting the radiance of the kit body and converting the detected radiance information into a current which is transferred to the processor. The processor is used for controlling the alarm to stop alarming when the current reaches a preset value. By way of the design, a patient can be reliably reminded of taking medicines.
Owner:JIANGSU PANASIA MEDICAL TECH GRP CO LTD
Formulations of bendamustine
InactiveUS20140094496A1Avoid side effectsSmall volumeBiocideOrganic active ingredientsComing outBULK ACTIVE INGREDIENT
Methods of treatment using bendamustine formulations designed for small volume intravenous administration are disclosed. The methods conveniently allow shorter administration time without the active ingredient coming out of solution as compared to presently available formulations.
Owner:EAGLE PHARMACEUTICALS INC
Huperzine a lyotropic liquid crystal preparation and preparation method thereof
ActiveCN106924172AImprove solubilityOvercome the disadvantages of insoluble in waterOrganic active ingredientsNervous disorderTO-18Organic solvent
The invention provides a huperzine a lyotropic liquid crystal preparation and a preparation method thereof. The huperzine a lyotropic liquid crystal preparation is prepared from the following components in parts by weight: 0.12 to 0.7 part of huperzine a, 10 to 18 parts of organic solvent, 12 to 70 parts of phospholipid, and 12 to 59.5 parts of grease. According to the huperzine a lyotropic liquid crystal preparation provided by the invention, the phospholipid, the grease and the organic solvent are used as carriers of the huperzine a, so that the solubleness of the huperzine a is effectively improved, the defect that the huperzine a is insoluble in water is overcome, the pharmacological function of the huperzine a is played favorably; meanwhile, under the condition of the limited component proportion, the huperzine a lyotropic liquid crystal preparation encounters water to form a lyotropic liquid crystal after being injected into a human body, so that the adhesion of a medicine and the preparation is improved, the medicine diffusion speed is retarded, the administration period is increased, the administration times are reduced, and thus the compliance of a patient is greatly improved.
Owner:武汉百纳礼康生物制药有限公司
Dual-response multiple-medicine delivery system based on cyclodextrin
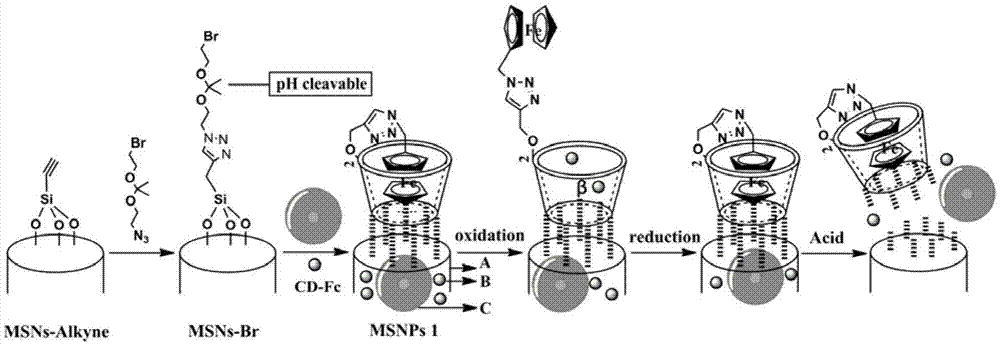
InactiveCN106902356ADual stimulus responseImmediate response to stimuliPowder deliveryOrganic active ingredientsDual responseMicrosphere
The invention discloses a dual-response multiple-medicine delivery system based on cyclodextrin. Firstly, through the action of a donor and a receptor, a macrocyclic molecule cyclodextrin serving as a host and a molecule ferrocene group serving as a guest form a self-inclusion complex; the dissociation and the inclusion of the host to the guest can be realized by utilizing redox regulation and control; the effect that a micromolecular medicine is subjected to adsorption-release through a cavity of the cyclodextrin is realized; secondly, a carbon chain containing an acetal group is introduced to connect a derivative of the cyclodextrin and a silicon dioxide microsphere; in an acid environment, an acetal bond is ruptured; the derivative of the cyclodextrin is quitted; a macromolecule is further released. The medicine delivery system provided by the invention has the characteristics of redox and dual response in the acid environment, moreover, can be used for seriatim or synchronously releasing medicine molecules in different sizes, can be used for controlling administration time, a position and a dosage, and has a favorable application prospect.
Owner:NANJING UNIV OF SCI & TECH
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
ActiveCN102319225ASmooth releaseImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention belongs to the field of sustained release medicament preparations, and particularly relates to a trimetazidine hydrochloride sustained release tablet and a preparation method thereof. The trimetazidine hydrochloride sustained release tablet is prepared from 40 to 45 parts of trimetazidine hydrochloride, 100 to 200 parts of polyoxyethylene, 100 to 200 parts of dextrin, 60 to 100 parts of 3-10 percent ethyl cellulose solution and 3 to 5 parts of magnesium stearate through material mixing, soft material preparing, drying, tabletting and other steps. In the trimetazidine hydrochloride sustained release tablet, the polyoxyethylene serves as an auxiliary material, and the sustained release tablet is prepared from the medicaments by a method of direct tabletting or tabletting aftergranulating. The drug dissolution of the trimetazidine hydrochloride sustained release tablet reaches about 90 percent 6 hours later, so the sustained release tablet is only required to be taken twice a day; therefore, the sustained release tablet has the advantages of releasing drug slowly and uniformly to reduce release rate and postpone peak time, reducing the number of administration times per day, improving the compliance of patients to the medicament and the like. Furthermore, the preparation method of the invention is simple and easy to operate.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Tacrolimus sustained release tablets and preparation method thereof
InactiveCN101361722AImprove medication complianceReduce the number of dosesOrganic active ingredientsPharmaceutical delivery mechanismLiver and kidneySide effect
The invention relates to a sustained release tablet dosage, in particular to a Tacrolimus sustained release tablet used for liver and kidney transplantation patients and a preparation method thereof, belonging to the field of medicine technology. The invention is characterized in that Tacrolimus and adhesive are taken and dissolved in ethanol to prepare Tacrolimus solution; sustained release materials, thinner and flow agent are taken, mixed and dispersed uniformly and then added into the Tacrolimus solution to prepare wet particles, which are dried, ground and added and mixed with lubricant; finally, the Tacrolimus sustained release tablet is prepared after tabletting and drying. The sustained release tablet has the unique advantages of: reducing administration times, improving the administration adaptability of patients; after administration, having stable blood concentration, small toxic and side effects and improving the drug effect and safety; reducing the total dosage of drugs so as to achieve the greatest curative effect by the lowest dosage; being capable of being realized on most of tablet production lines and easily accepted by manufacturing units and manufacturers due to relatively simple preparation process; and having relatively low manufacturing cost and broad clinical application prospect.
Owner:贾祥波 +1
Teriparatide sustained-release microsphere and preparation method thereof
ActiveCN103157096AEasy to useImprove compliancePeptide/protein ingredientsSolution deliveryPolyesterMicrosphere
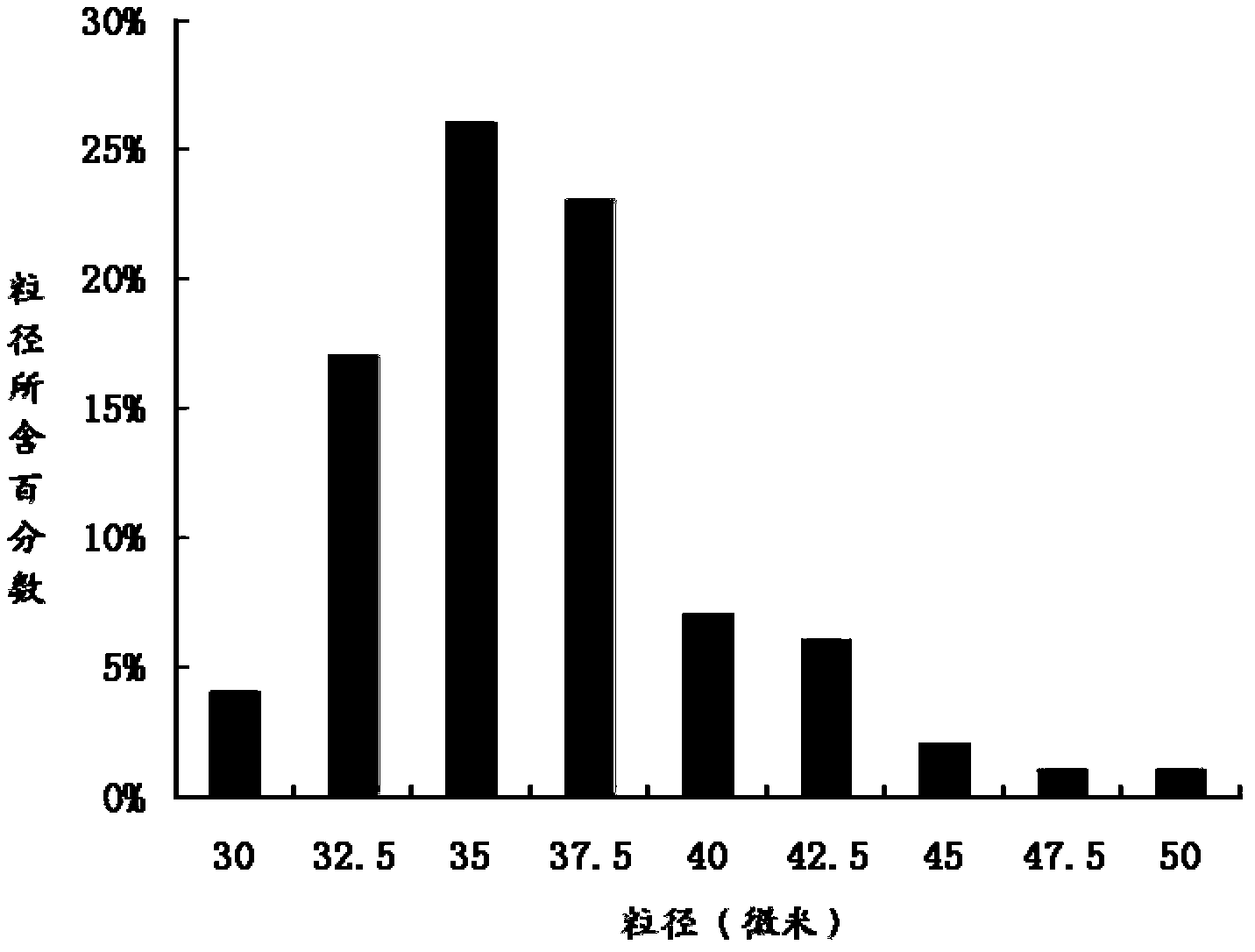
The invention relates to the technical field of medicines, and in particular relates to a teriparatide sustained-release microsphere and a preparation method thereof. The teriparatide sustained-release microsphere comprises a teriparatide and a carrier, wherein the particle size of the teriparatide is 30 micrometers-50 micrometers; and the carrier is a mixture of one or two of polyester, starch, gelatin, Arabic gum, albumin, alginate and chitosan. The teriparatide microsphere is prepared by adjusting the constitution and molecular weight of the carrier and the medicine-loading rate and particle size of the microsphere according to the properties of the teriparatide. The prepared microsphere is stable in releasing and small in sudden releasing, the dose administration times can be reduced, the probability of infection is decreased, patients can conveniently take the microsphere, and the compliance of the patients is improved. Experiments show that the teriparatide has uniform particle size distribution, high encapsulation efficiency and large medicine-loading capacity. The sustained-release microsphere can be externally released for 10 days.
Owner:HYBIO PHARMA
Vonoprazan oral quick-dissolving film agent and method for preparing same
ActiveCN105663096AAvoid difficultiesImprove complianceOrganic active ingredientsDigestive systemVonoprazanPharmaceutical formulation
The invention discloses a Vonoprazan oral quick-dissolving film agent and a method for preparing the same, and belongs to the field of medicine preparations.The Vonoprazan oral quick-dissolving film agent particularly comprises, by weight, 10-15% of Vonoprazan active monomers, 50-55% of hydroxypropyl methylcellulose, 25-30% of maltodextrin, 0.8-1.0% of hyaluronic acid and 5-10% of plasticizers.The Vonoprazan oral quick-dissolving film agent and the method have the advantages that the water-soluble hydroxypropyl methylcellulose, the water-soluble maltodextrin and the water-soluble hyaluronic acid are preferably used as film-forming materials, the appropriate plasticizers with the appropriate weight ratio are screened, and accordingly the film agent with excellent disintegration time and excellent mechanical performance can be prepared; the disintegration time limit of the Vonoprazan oral quick-dissolving film agent can be obviously shortened, accordingly, the shortcoming that water is required when existing most oral solid preparations are about to be administered can be overcome, the medicine administration time cannot be delayed even under the condition of deficiency of water resources, and the medication compliance of patients can be improved.
Owner:NANJING GRITPHARMA CO LTD
Cetirizine hydrochloride gel
InactiveCN1457765AImprove complianceAvoid gastrointestinal irritationOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideOral medication
The present invention relates to the field of medicine technology and is one externally applied form of Cetirizine hydrochloride gel as one histamine resisting medicine and its preparation. The present invention prepares the gel with Cetirizine hydrochloride as main medicine component and different supplementary material, and the gel is administrated via skin or mucous membrane. The present invention can avoid liver reaction and stimulation to gstrointestinal tract of the medicine, reduce medicine concentration in blood, reduce central nerve suppressing effect, raise the medicine concentration in local affected part, decrease the administration times and raise patient's compatibility.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
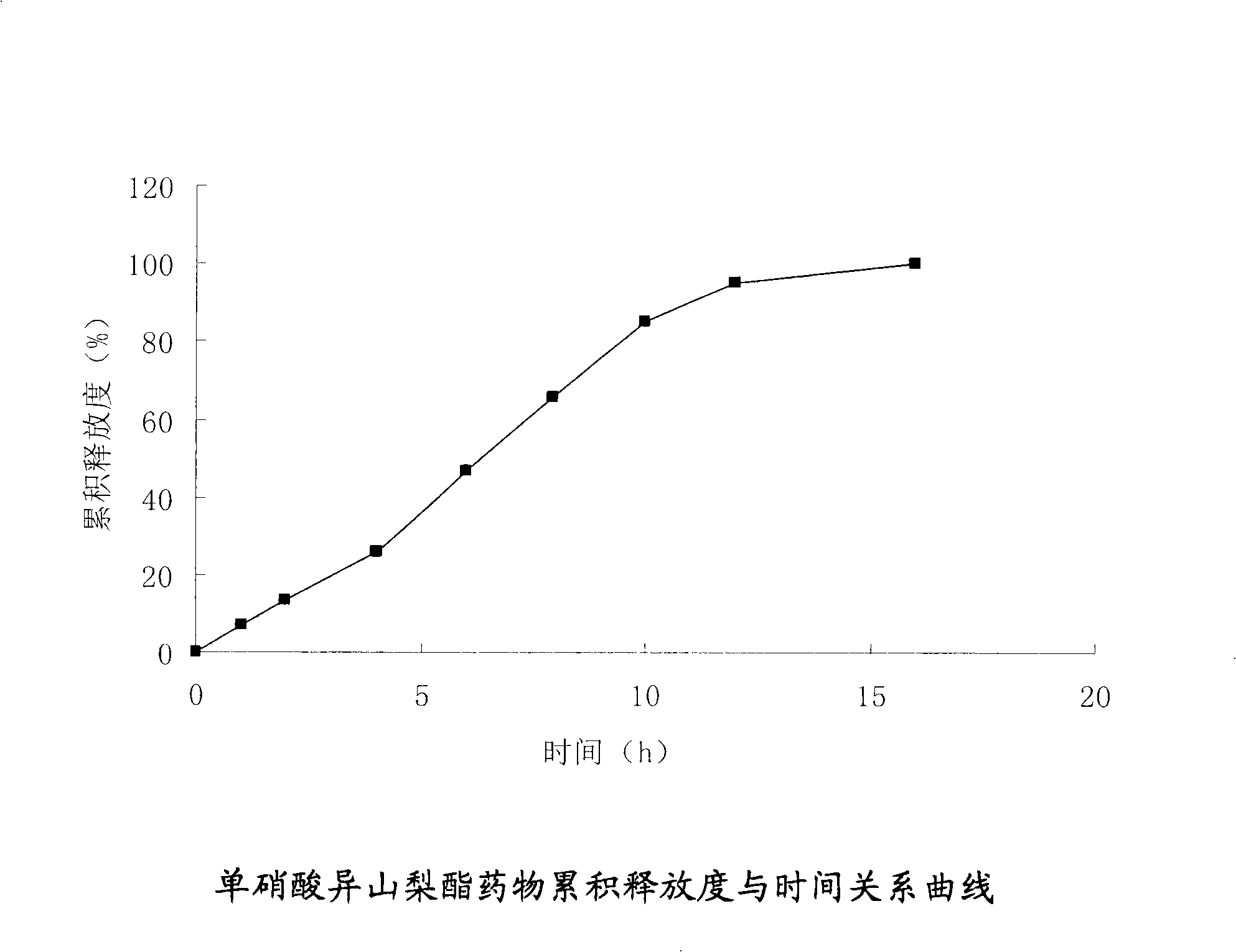
Isosorbide mononitrate osmotic pump type controlled release formulation and preparation method thereof
InactiveCN101342151AImprove complianceThe effect of small individual differencesPharmaceutical delivery mechanismCardiovascular disorderSide effectExcipient
The invention relates to an isosorbide mononitrate osmotic pump type controlled release preparation and a preparation method thereof. The osmotic pump type controlled release preparation is composed of a tablet core and semipermeable film coating with medicine releasing holes; wherein, the tablet core is composed of isosorbide mononitrate, penetration enhancer, bond and other excipient, and the semipermeable film includes coating material and plasticizer; the punching mode includes mechanical drilling and laser drilling. The preparation of the invention can effectively control the constant speed release rate of medicine and has the advantages of few administration times, less side effect, long efficacy lasting time, avoidance of medicine tolerance, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Compound bactericide of osthole and Ningnanmycin
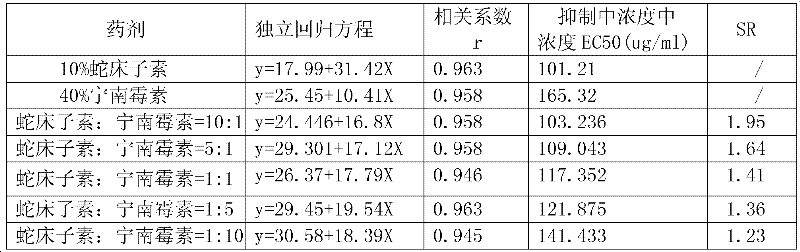
InactiveCN102293205AExpanded bactericidal spectrumReduce dosageBiocideFungicidesFungicideCnidium monnieri
The invention belongs to the technical field of pesticides, and relates to a compound fungicide of osthole and Ningnanmycin for preventing and treating powdery mildew of strawberries, grapes and tomatoes and sheath blight of rice. The active ingredients of the fungicide are osthole and Ningnanmycin, and the weight ratio of the osthole and Ningnanmycin in the composition is 1:10-10:1. The advantages of the present invention are: 1. The combined use of the two fungicides expands the spectrum of fungicides. 2. After compounding, compared with a single agent, the dosage of osthole is reduced, and the effective dependence on plant resources is reduced. 3. The invention is suitable for preventing and controlling crop powdery mildew and sheath blight, and can reduce the selection pressure of chemical agents for preventing and controlling crop powdery mildew and sheath blight. 4. Reduce the amount of spraying, reduce the frequency of application, and reduce the cost of use. It has the advantages of high efficiency, quick effect, and long-lasting effect.
Owner:JIANGSU LVDUN PLANT PROTECTION AGROCHEM EXPERIMENTAL
Preparation of ticagrelor or pharmaceutical salt thereof
ActiveCN106074357AOrganic active ingredientsInorganic non-active ingredientsTicagrelorAcute thrombosis
The invention relates to a preparation of ticagrelor or a pharmaceutical salt thereof. Specifically, the invention relates to the improved preparation of ticagrelor or the pharmaceutical salt thereof for administration for once daily. The ticagrelor plasma concentration of a tested person can reach about more than 0.2 [mu]g / mL within 2 hours; the ticagrelor plasma concentration of the tested person can still reach about more than 0.2 [mu]g / mL 12 hours later after the drug is taken; and the maximum plasma concentration (Cmax) of the ticagrelor or the pharmaceutical salt thereof between about 0.2 [mu]g / mL-about 0.8 [mu]g / mL is generated in the tested person. The preparation of ticagrelor or the pharmaceutical salt thereof can make the administration times reduced, thereby improving the administration compliance of patients, reducing the risks of myocardial infarction or stroke caused by acute thrombosis caused because the patients miss taking ticagrelor.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Preparation method for carbostyril injection
ActiveCN101690713AReduced fitnessReduce clinical workloadAntibacterial agentsOrganic active ingredientsOrganic filmFiltration
The invention relates to a preparation method for a carbostyril injection, in particular to a veterinary medicament. The injection comprises the following components: 1 to 30 percent of a carbostyril medicament or salt and hydrate thereof,, 0.5 to 20 percent of fatty acid, 0 to 30 percent of other medicaments combined to augment antimicrobial spectrum, 0 to 20 percent of one or more than two of blocker, local anesthesia, a stabilizing agent and an antioxidant, and the balance of an organic solvent. The preparation method comprises the following steps of: taking the carbostyril medicament or the salt thereof, the fatty acid and the organic solvent; heating until all the components are dissolved in the process of stirring; cooling to the room temperature; adding the combined antimicrobial medicament; stirring until all the components are dissolved; adding activated carbon; evenly stirring; and carrying out organic film filtration to obtain the carbostyril long-acting injection. A novel composition is formed through the reaction of the carbostyril medicament or the salt and the hydrate thereof with the fatty acid. The medicament release time of the prepared carbostyril long-acting injection is prolonged, and the time of maintaining effective blood concentration of the medicament in vivo is prolonged because the release time is prolonged so as to reduce administration times and stress of the medicament.
Owner:PU LIKE BIO ENG
Preparation method of pharmaceutical composition for treating type II diabetes
ActiveCN101984974AStable drug releaseSmall fluctuations in blood concentrationMetabolism disorderSulfonylurea active ingredientsSide effectPatient compliance
The invention relates to a preparation method of a pharmaceutical composition for treating type II diabetes, belonging to the medical preparations containing organic ingredients, particularly relating to a preparation method of a pharmaceutical composition of metformin hydrochloride and glimepiride. In the preparation method, the metformin hydrochloride and the glimepiride are taken as active ingredients of the pharmaceutical composition, the metformin hydrochloride is firstly prepared into pill cores by adopting an osmotic pump technology and then the metformin hydrochloride pill cores are coated with the glimepiride by using a coating technology. The preparation method comprises the following steps: 1), preparing the metformin hydrochloride and pharmaceutically acceptable auxiliary materials into pills which are coated with semipermeable membrane layers, punching by laser and producing the pill cores; and 2), preparing the glimepiride and the pharmaceutically acceptable auxiliary materials into a coating liquid dissolved in stomach and carrying out coating on the pill cores in step 1. The invention provides the preparation method of the pharmaceutical composition for treating the type II diabetes, wherein the pharmaceutical composition steady and slowly releases drugs, has reduced administration times, good patients compliance, small side effect and small tablet volume, is less influenced by pH values of different segments of a gastrointestinal tract, and is convenient for administration for the patients.
Owner:SHANDONG XINHUA PHARMA CO LTD
Gabapentin stomach retention sustained-release composition
InactiveCN101120929AReduce releaseReduce doseNervous disorderPeptide/protein ingredientsGabapentinTreatment effect
A Gabapentin slow-release compound for gastric retention is a slow-release drug delivery system for gastric retention, which comprises the Gabapentin served as the active component and the slow-release skeleton material with slow-release property and the other excipient with pharmaceutical approval. The composition proportion (weight ratio) is as following. The ratio of slow-release skeleton material to the other excellent with pharmaceutical approval is from one to 0.2 till 2 to 0.0 till 2. The compound can stay in the stomach for more than 2 hours. The compound is also capable of controlling the drug release, reducing the administration times, reducing toxic and side effects and improving the treatment effect.
Owner:珠海天翼医药技术开发有限公司
Chinese medicinal composition for treating chronic pancreatitis
InactiveCN101480478AEffective treatmentConvenient treatmentDigestive systemSulfur/selenium/tellurium inorganic active ingredientsAdministration timeAdemetionine
The invention discloses a traditional Chinese medicine composition for treating chronic pancreatitis, which is characterized by comprising the following medicaments by weight: 10 to 12 portions of rhubarb, 9 to 10 portions of glauber salt, 10 to 15 portions of citrus aurantium, 15 to 18 portions of bupleurum, 5 to 6 portions of liquorice, 12 to 15 portions of white peony roots, 20 to 30 portions of coix seeds, 12 to 15 portions of cyperus rotundus, 10 to 15 portions of burreed tuber and 10 to 15 portions of white atractylodes rhizome. The preparation method of the composition comprises the steps of adding 1000 portions of water into the mixed medicaments, boiling for two times, filtering and recovering, extracting juice, and sterilizing at high temperature after being bottled for standby application. The prescription takes the rhubarb, the glauber salt and the citrus aurantium as the principle medicine, the bupleurum root and the white peony root as the assistant medicine, the cyperus rotundus, the burreed tuber and the white atractylodes rhizome as the adjuvant medicine, and the coix seed and the liquorice as the dispatcher medicine. Aiming at sthenia and asthenia, the chronic pancreatitis is treated by adopting a purgative and antiphlogistic method. The traditional Chinese medicine composition for treating the chronic pancreatitis can effectively treat the chronic pancreatitis, has the advantages of shorter administration time, obvious effect, convenient and safe taking medicaments, no side effect and low treatment cost, and not only has the preventive effect on patients suffering from the chronic pancreatitis at one time and frequently taking the traditional Chinese medicine composition, but also has the efficacy of treating both the secondary and the primary symptoms.
Owner:重庆市合川区生产力促进中心
Chinese medicinal composition for treating chronic appendagitis, and preparation method thereof
InactiveCN102600419APromote absorptionConvenient treatmentSexual disorderPlant ingredientsAdministration timePolyporus
The invention provides a Chinese medicinal composition for treating chronic appendagitis, which comprises Radix Stemonae, Polyporus, Rhizoma Ligustici Chuanxiong, Radix Semiaquilegiae, Semen Abutili, Lignum Dalbergiae Odoriferae, Cortex Ilicis Rotundae, Herba Clinopodii Polycephali, Folium Pyrrosiae, Rhizoma Alpiniae Officinarum, Fructus Malvae, Fructus Zanthoxyli, Flos Rosae Rugosae, Caulis Aristolochiae, Radix Rubiae, Semen Impatientis, Exocarpium Citri Rubrum, Ginseng Radix, Rhizoma Dioscoreae, Radix Platycodi and Caulis Bambusae In Taenia. The composition directly permeates into inflammatory mass to facilitate local drug absorption; and has the advantages of short treatment course, rapid on-set, and short administration time.
Owner:高丽丽
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com