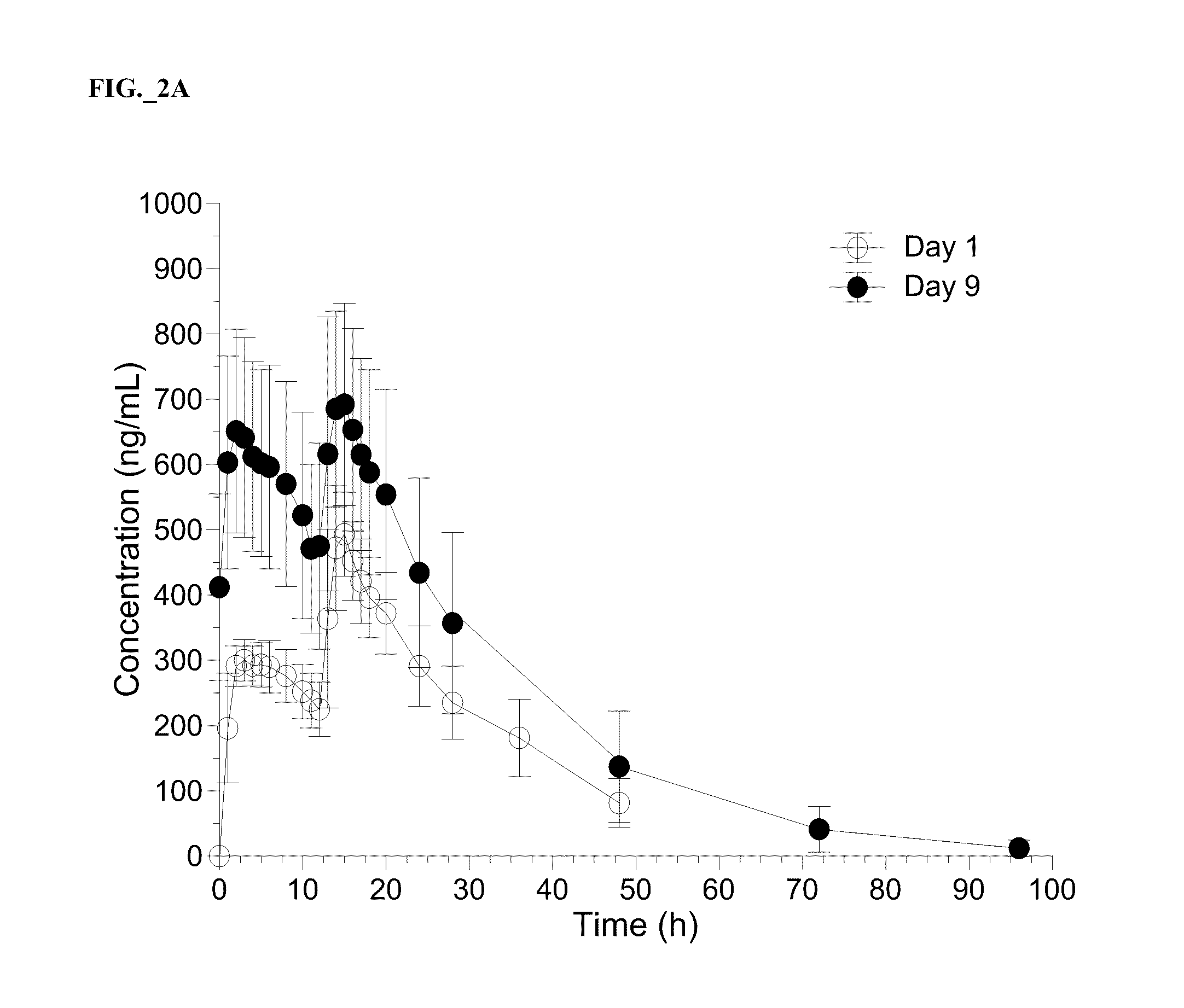
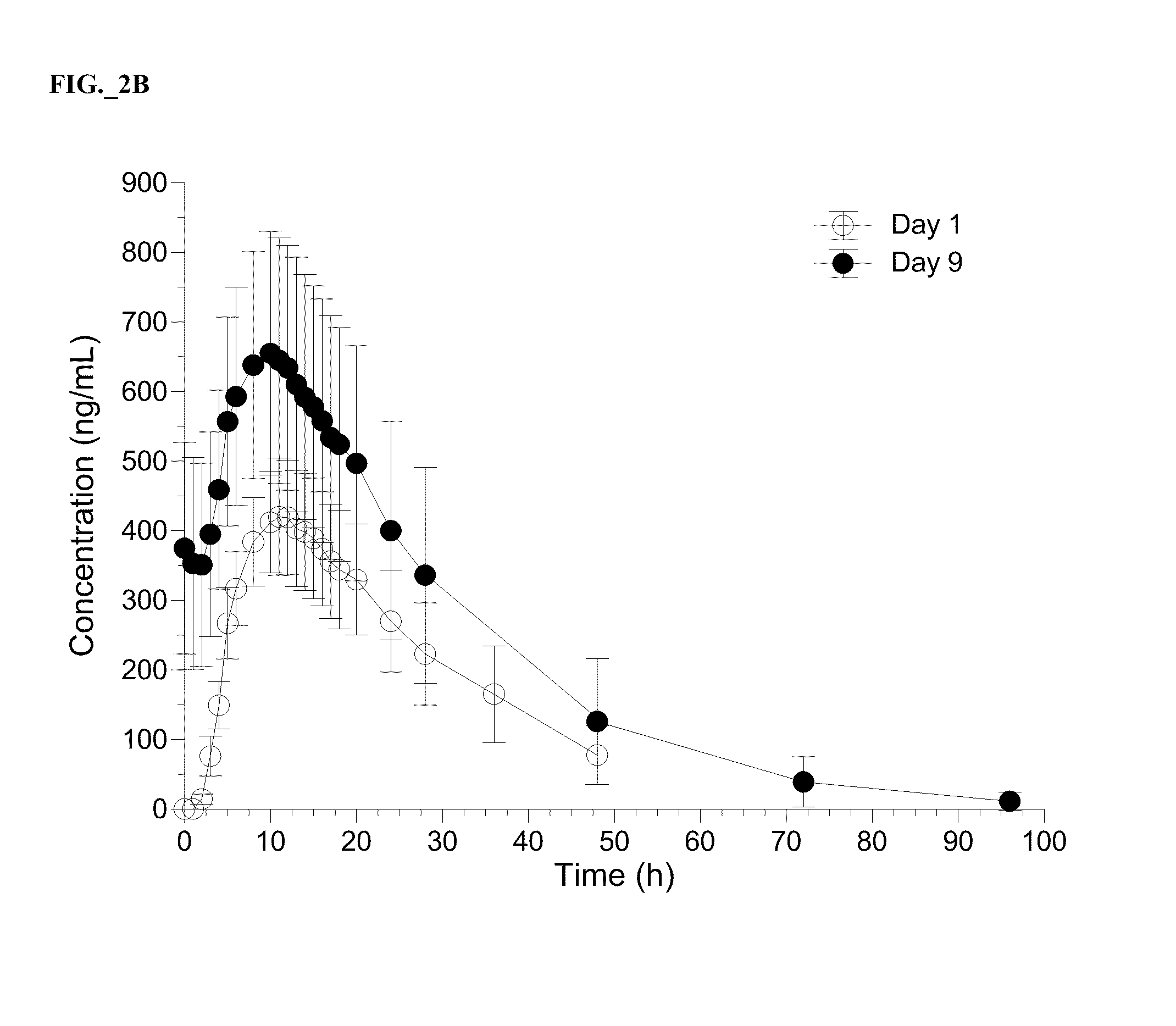
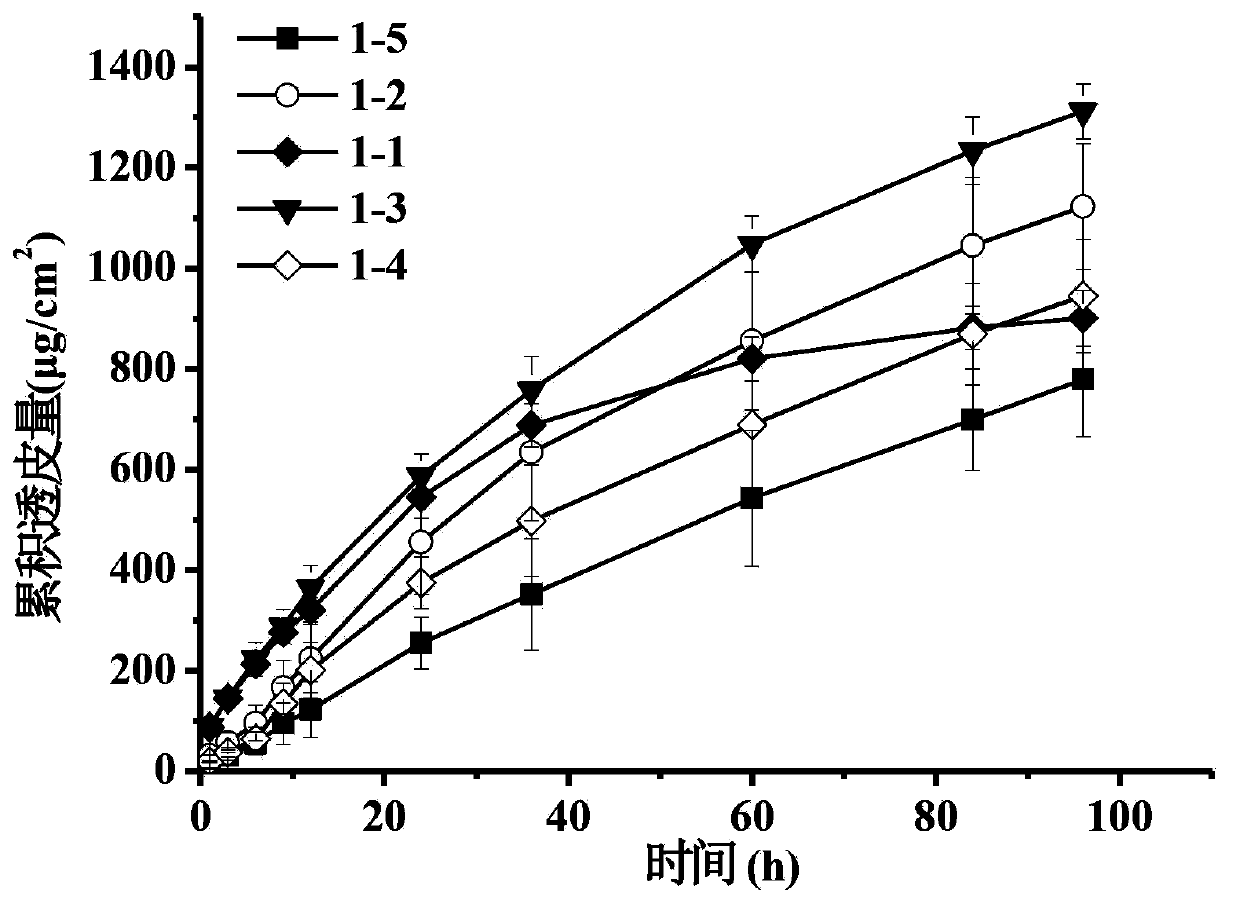
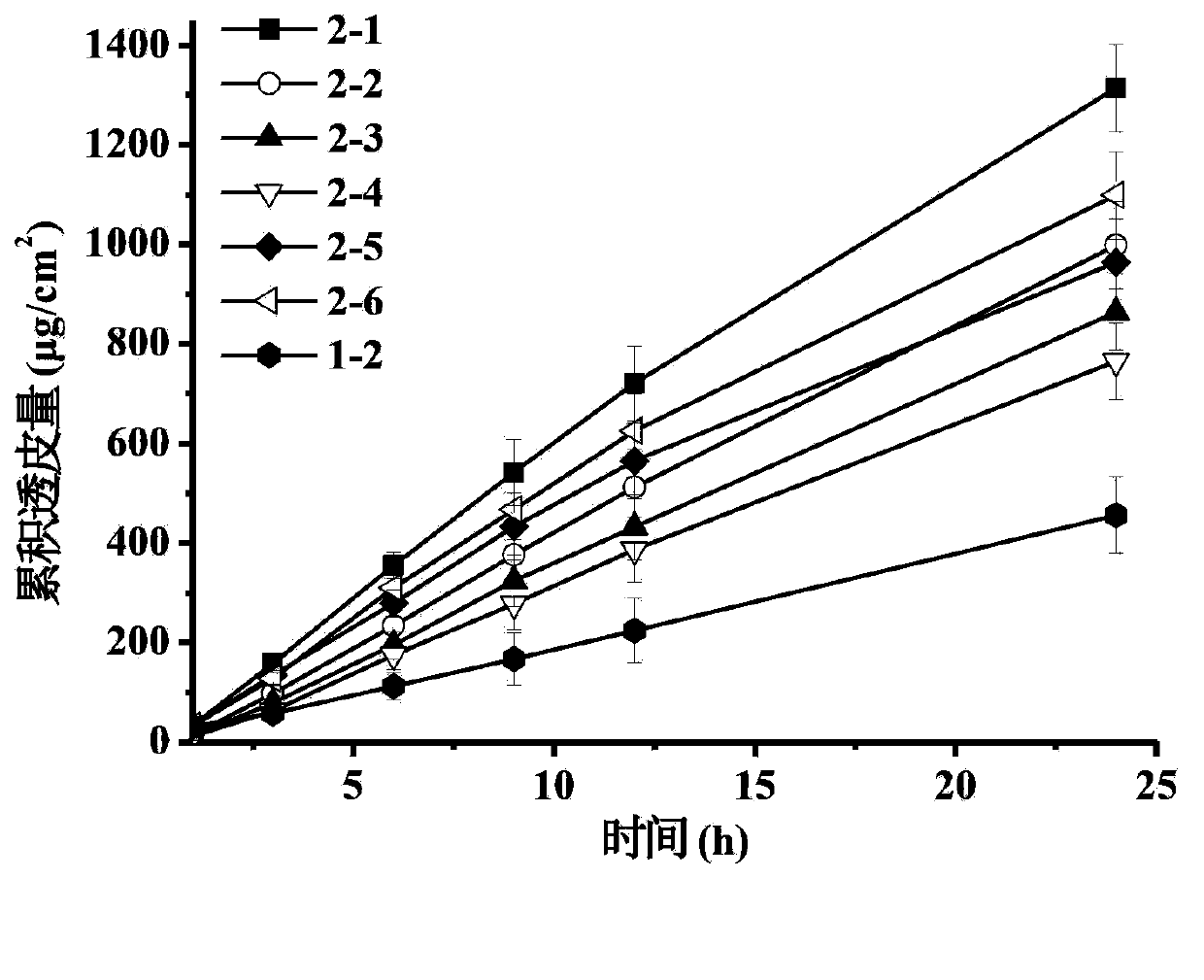
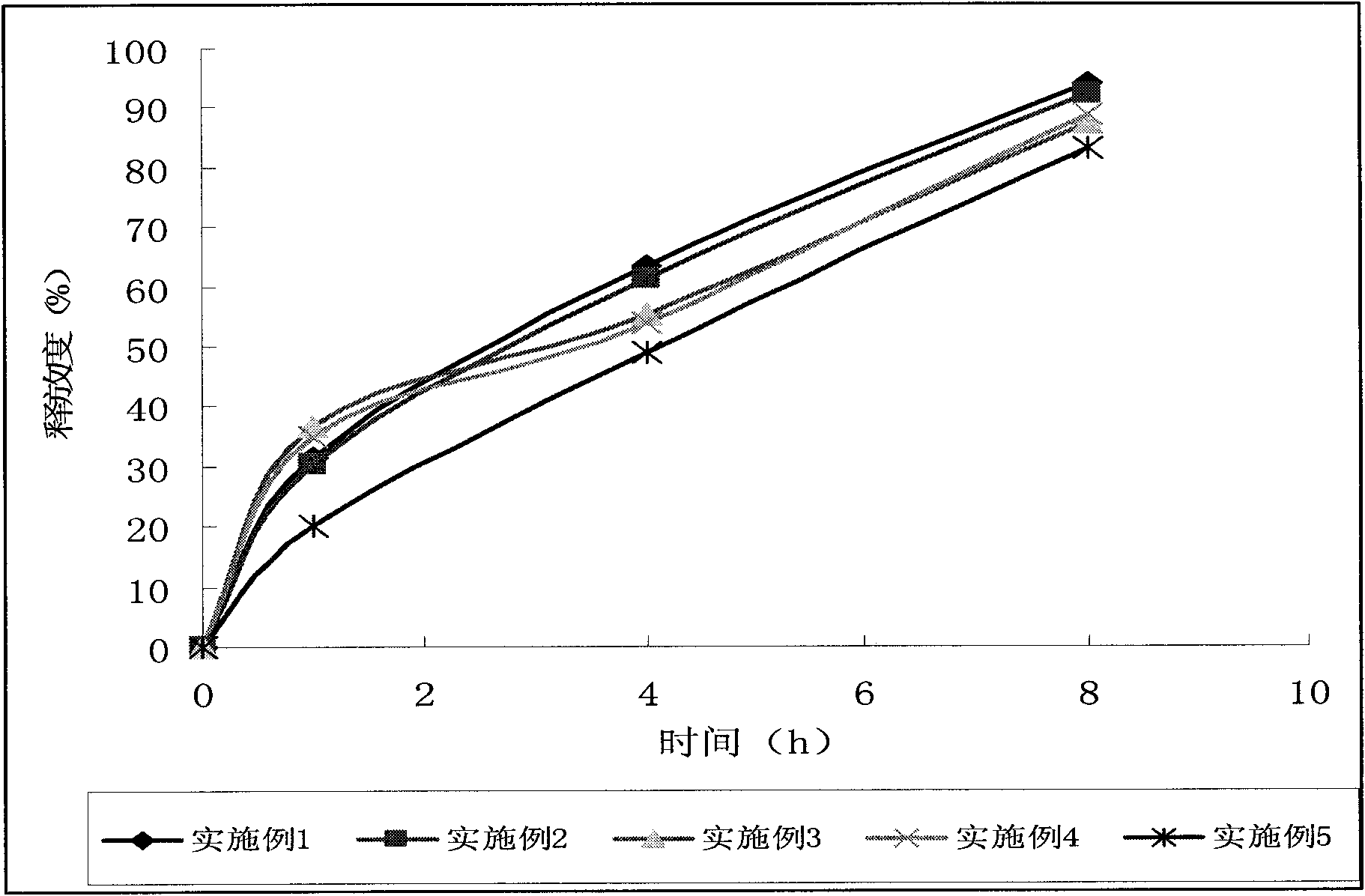
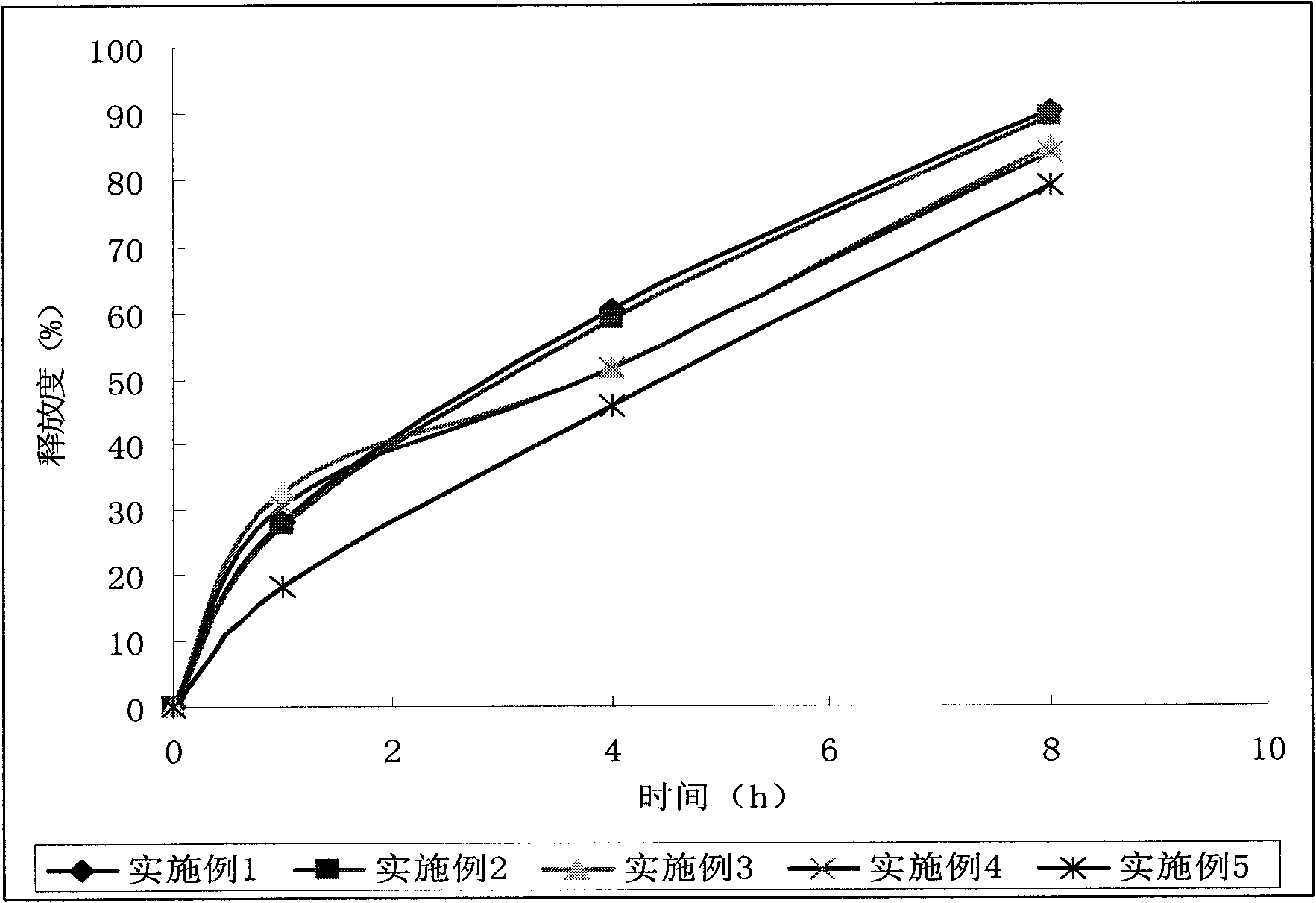
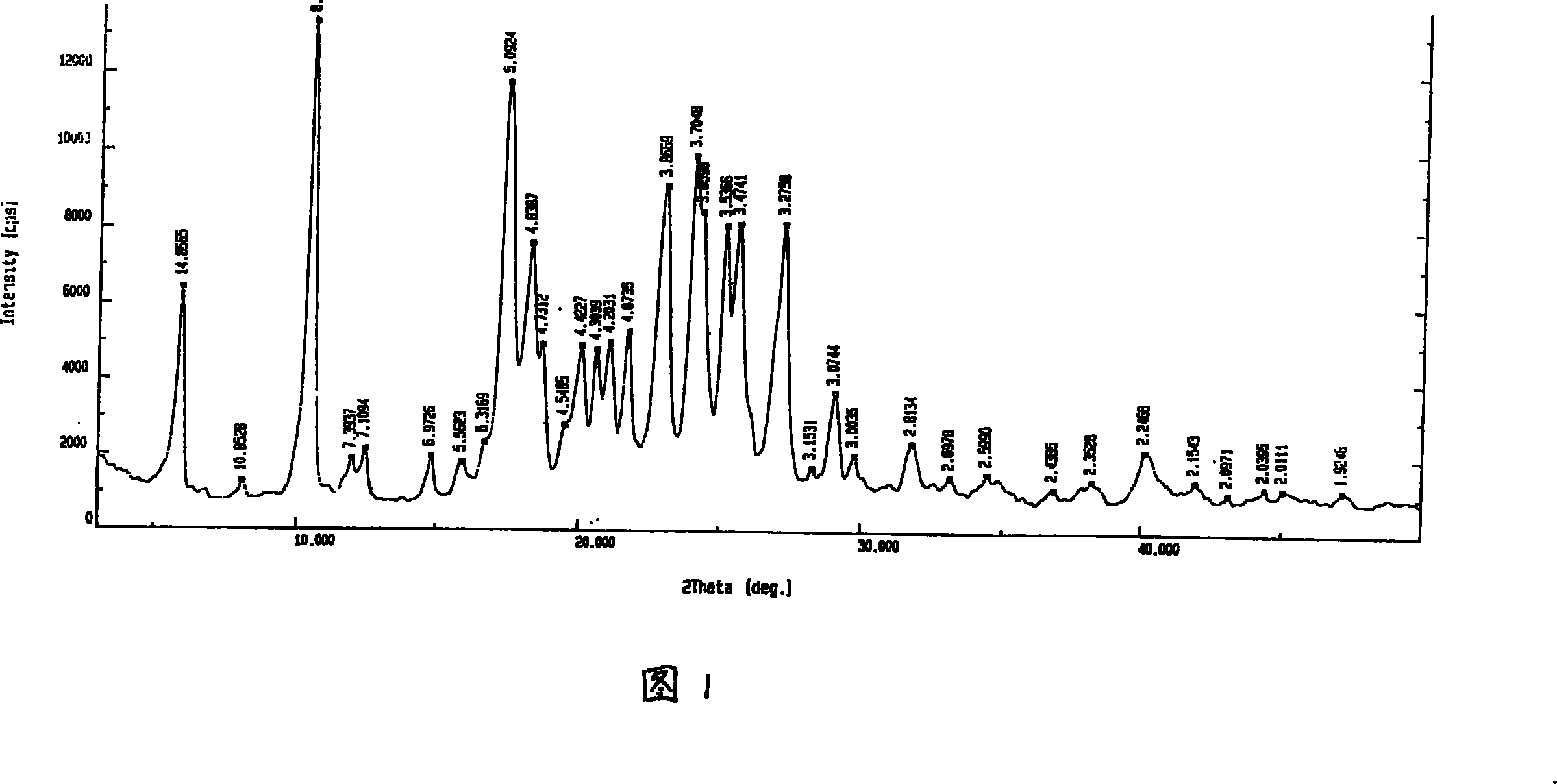
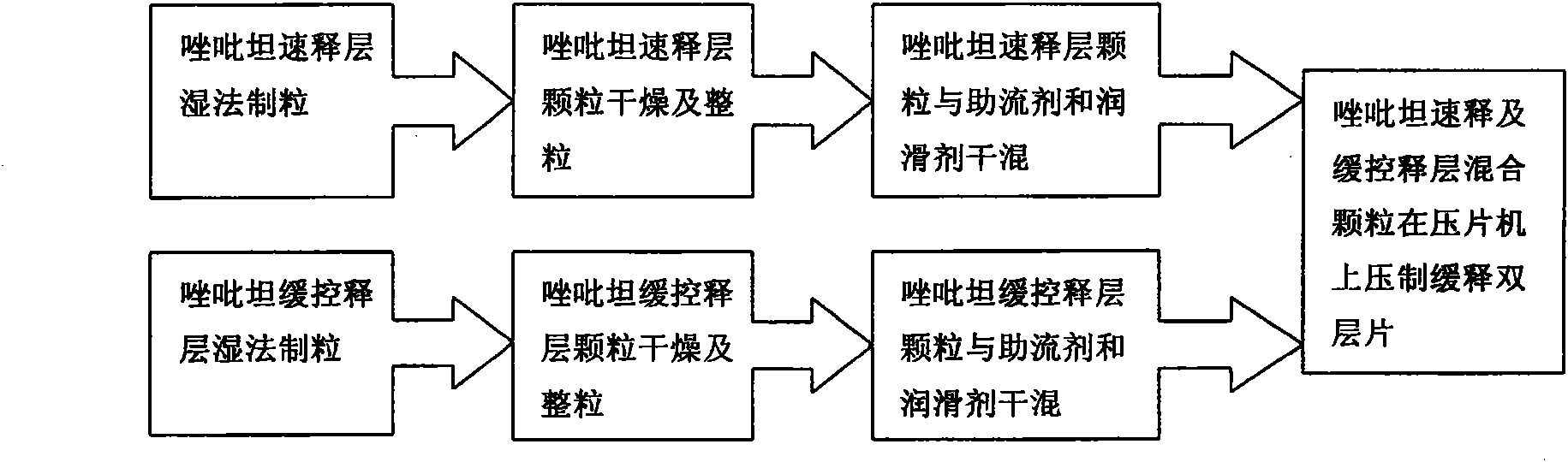
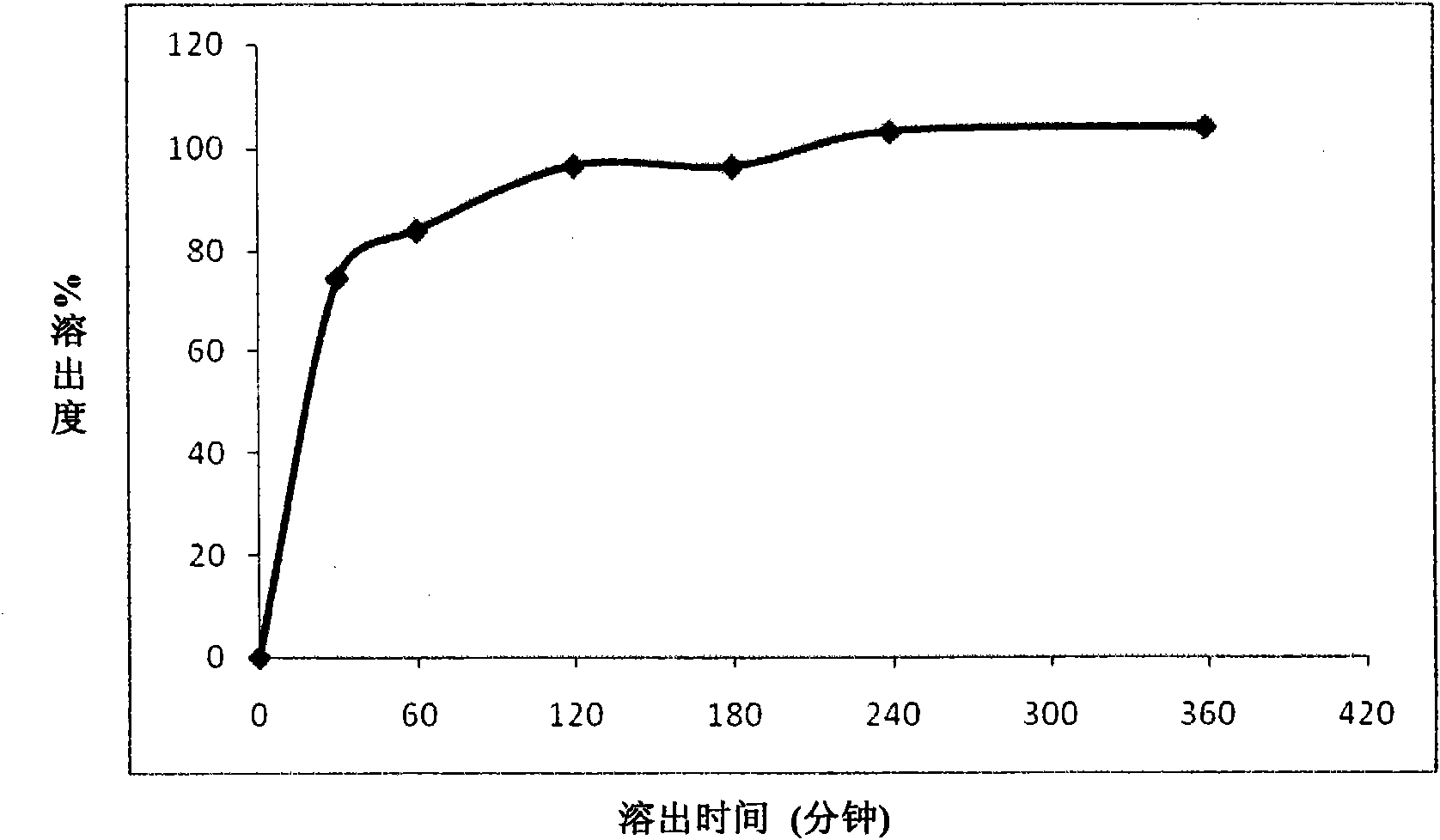
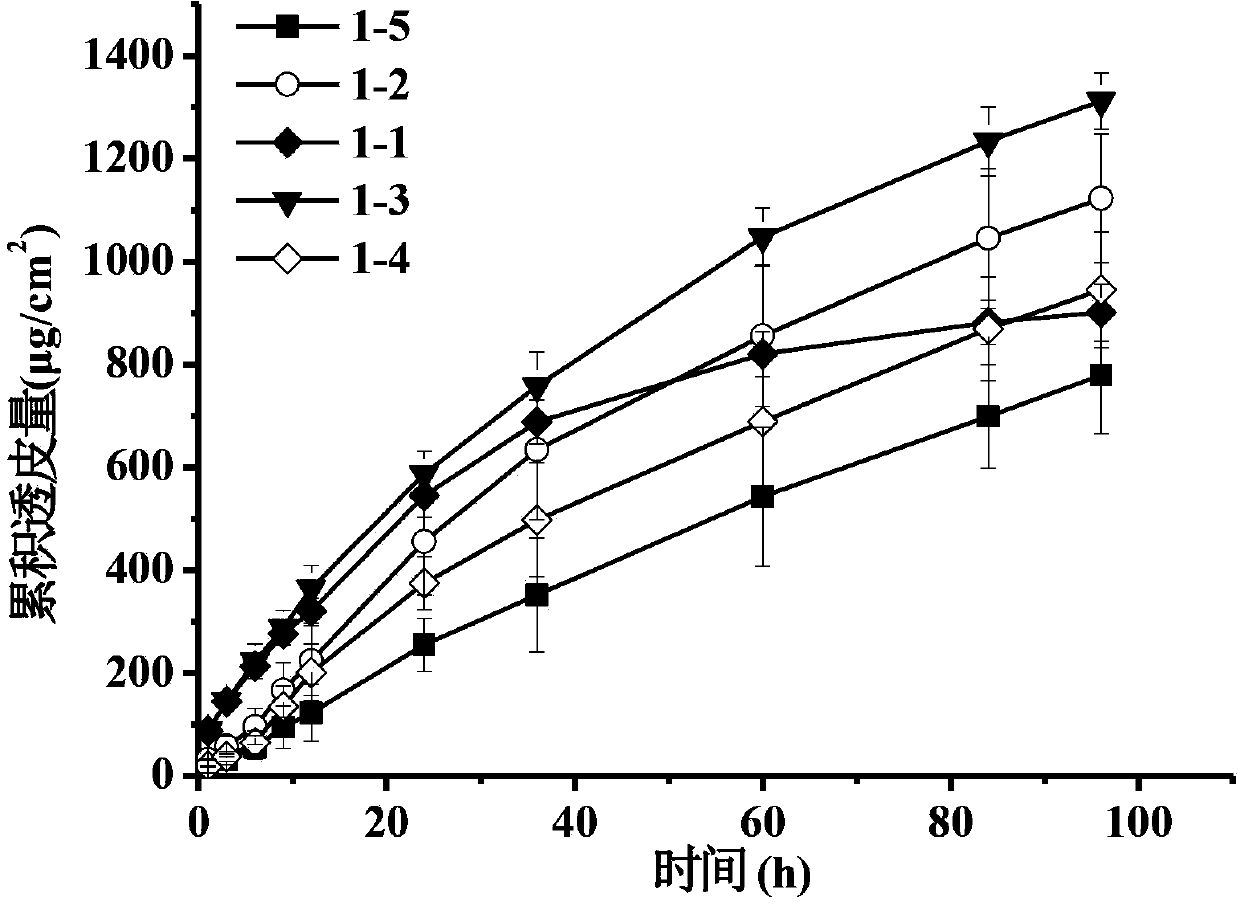
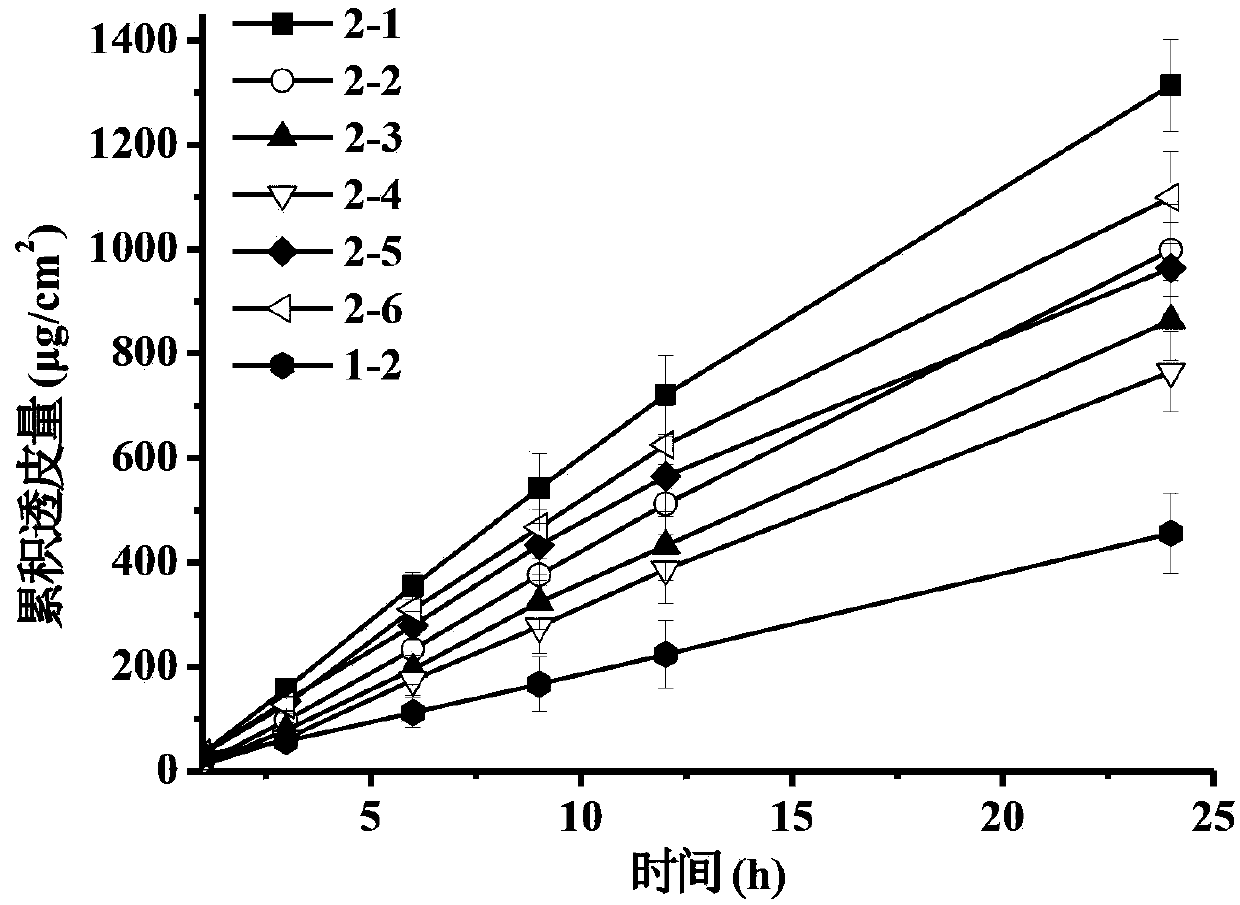
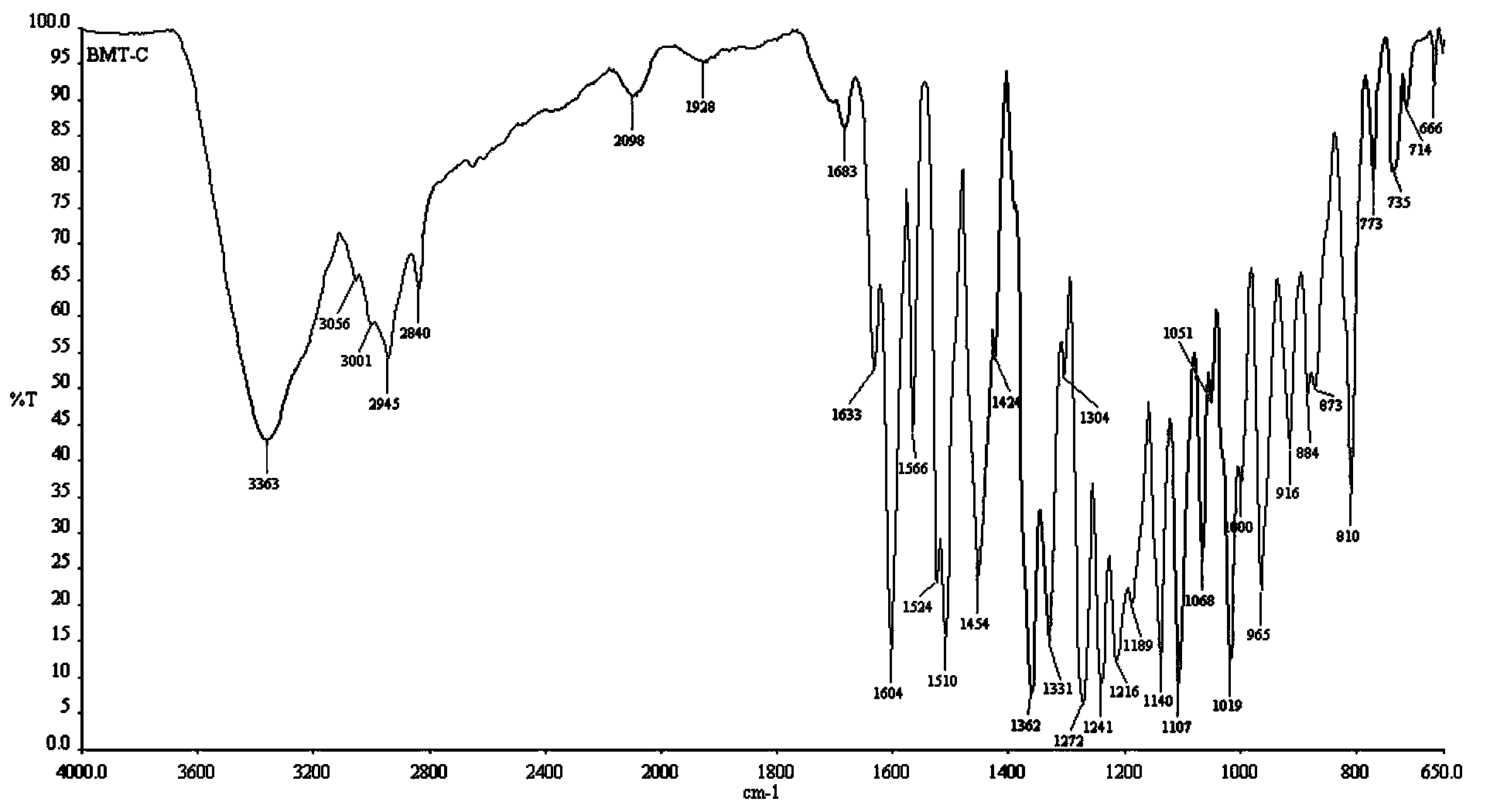
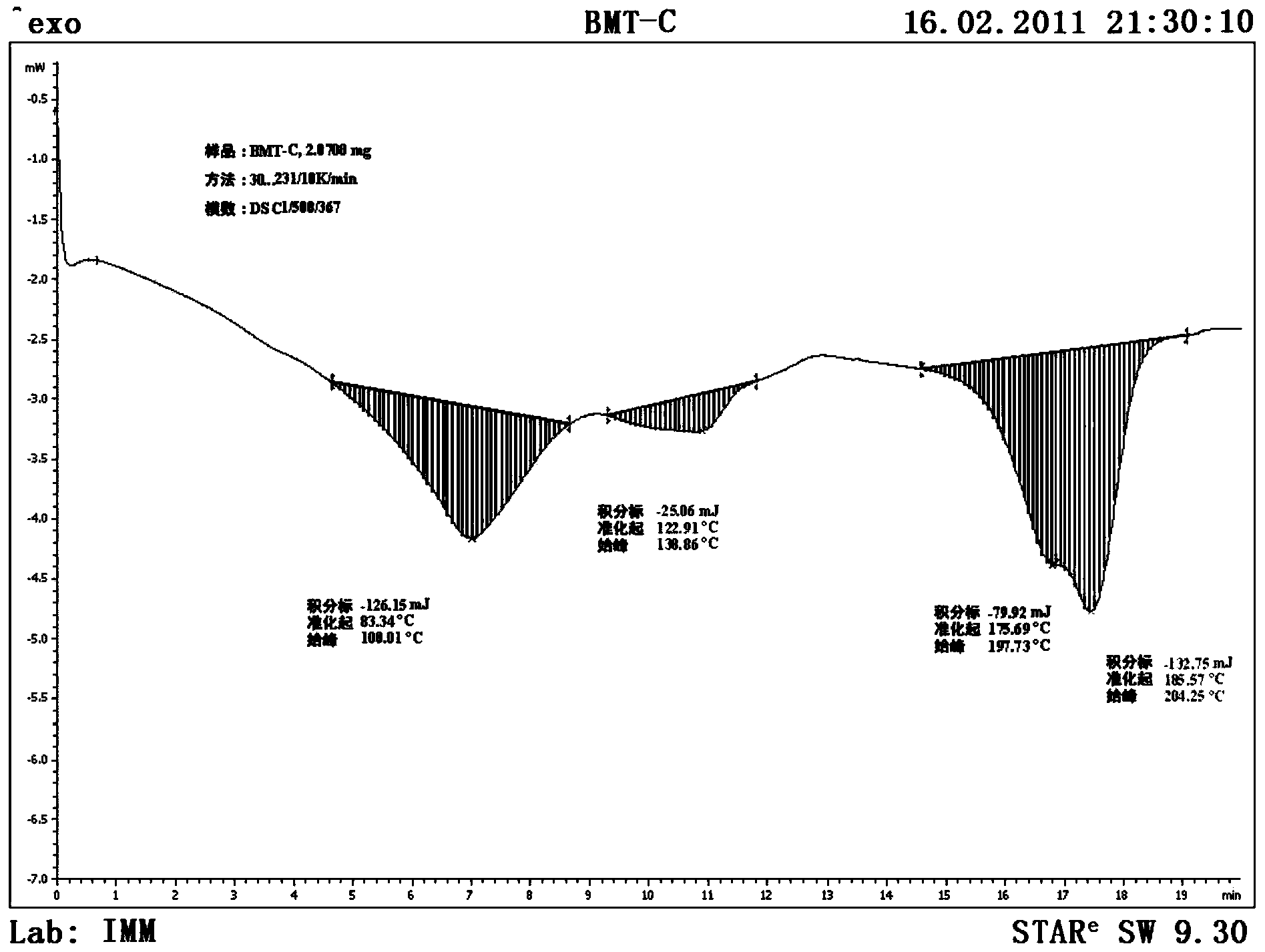
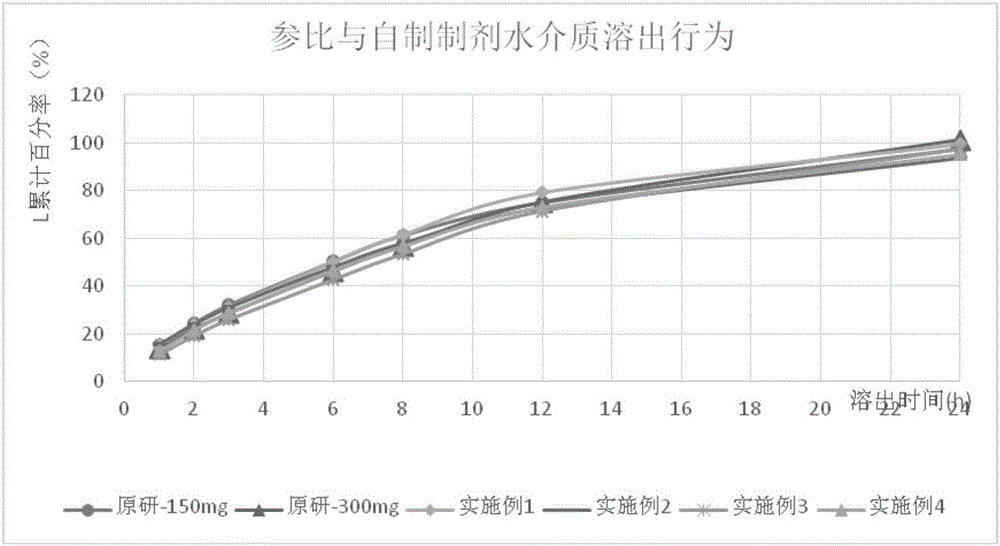
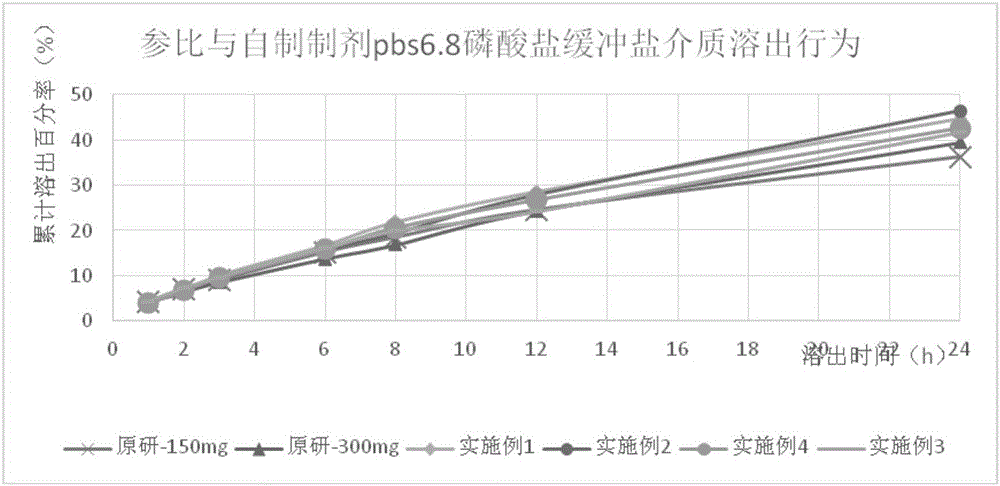
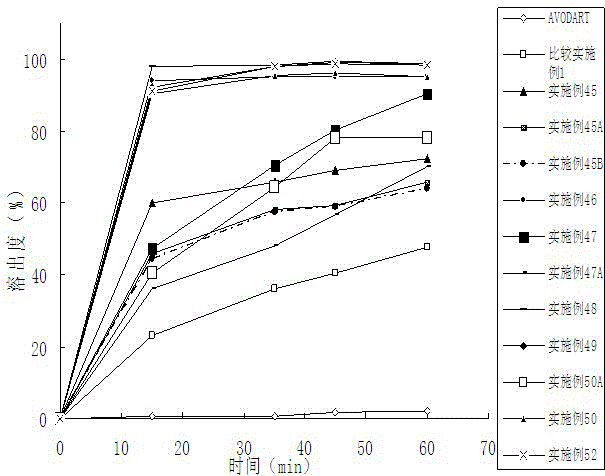
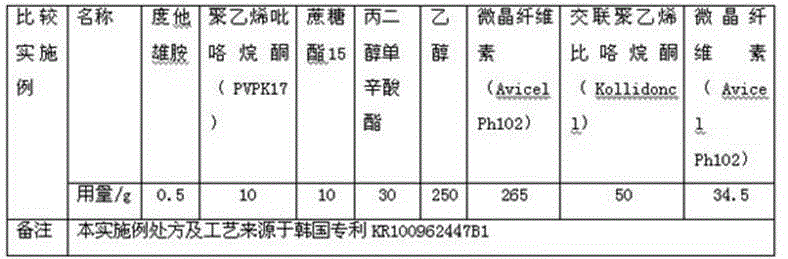
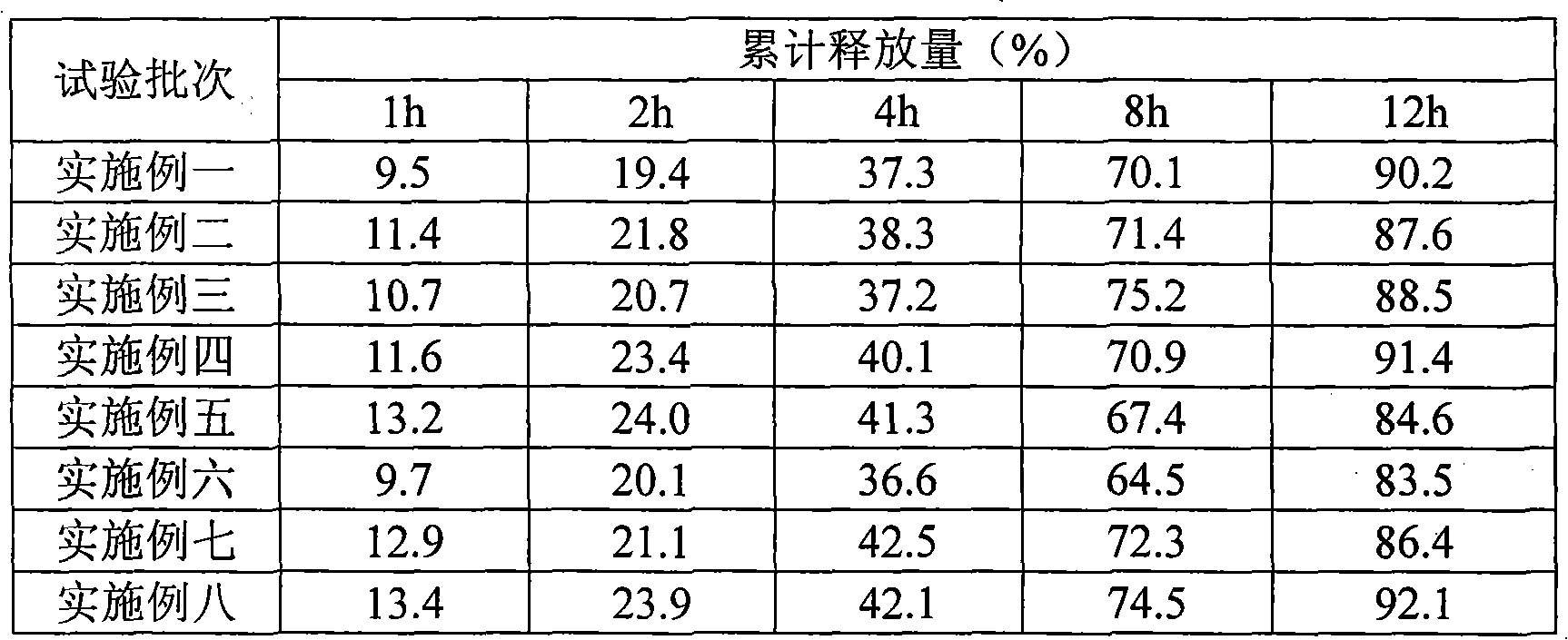
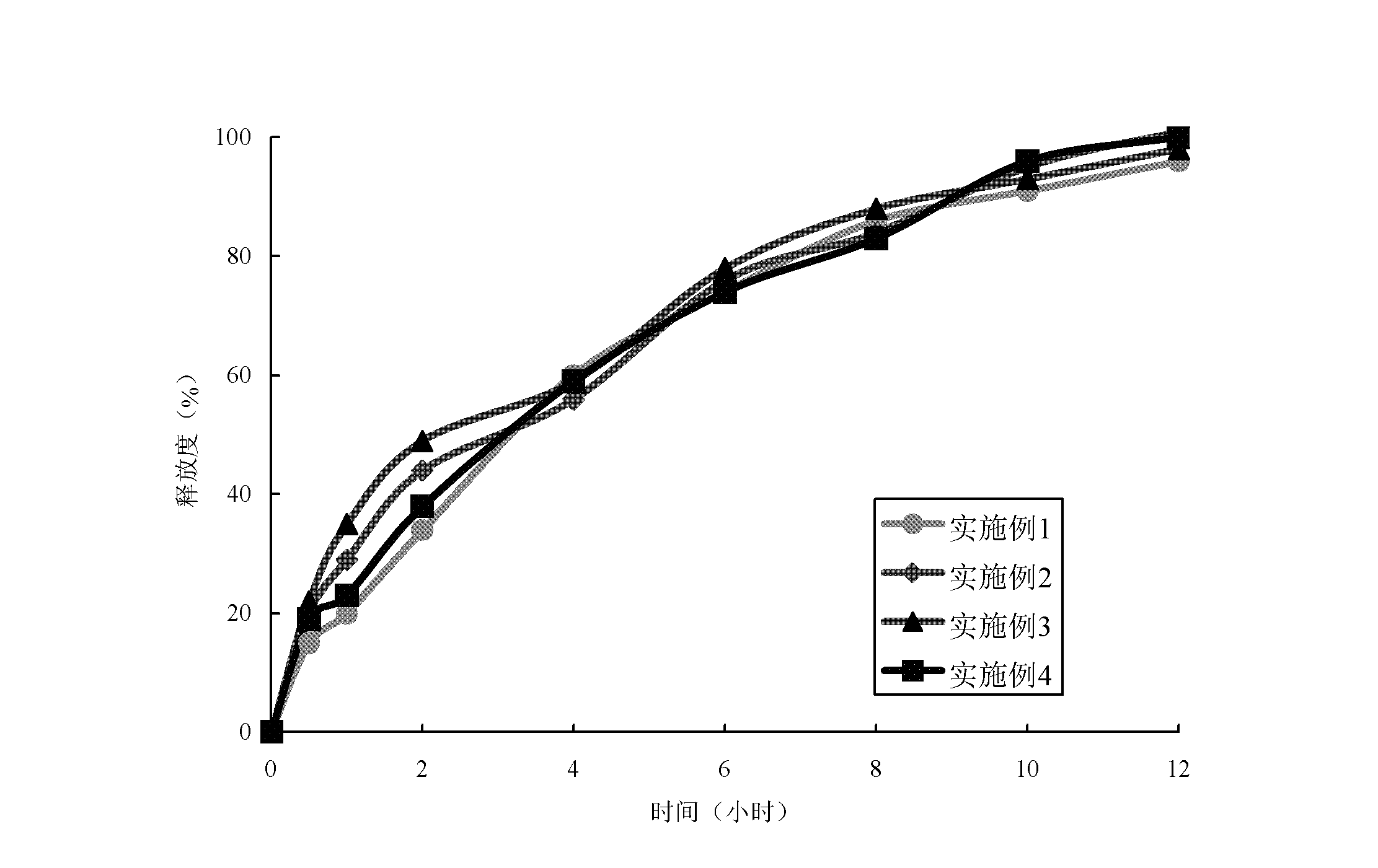Patents
Literature
85results about How to "Effective plasma concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dosage forms for movement disorder treatment
InactiveUS20070003621A1Reduce effectEffective plasma concentrationBiocideOrganic active ingredientsDrugDisease
The invention relates to the improvement in the treatment of certain neural disorders / diseases, such as Parkinson's disease and other motor disorders. One aspect of the invention relates to drug compositions and dosage forms comprising said drug composition. Another aspect of the invention relates to methods of manufacturing the drug compositions and dosage forms. Another aspect of the invention relates to methods of treatment, comprising administering the drug composition and dosage form to an individual.
Owner:COMBINATORX
Amantadine compositions and methods of use
ActiveUS20110189273A1Without adversely affecting sleepEliminate side effectsPowder deliveryBiocideAnesthesiaMedicinal chemistry
Methods of nighttime administration of amantadine to reduce sleep disturbances in patient undergoing treatment with amantadine are described, as well as compositions of extended release amantadine that are suitable for nighttime administration.
Owner:ADAMAS PHARMA LLC
Slow-releasing preparation containing metformin hydrochloride and glipizide and its preparation method
InactiveCN101057849AEvenly distributedReduce local irritationOrganic active ingredientsMetabolism disorderMedicineMetformin Hydrochloride
The invention discloses a diabecron and glipizide -containing slow-release agent and the method for preparing the same. The glipizide micro-pill takes blank micro-pill as carrier, and combines glipizide and other medical findings with it. The diabecron-containing slow-release micro-pill comprises diabetosan pill, slow-release coating membrane material or other medical findings. The method for preparing diabecron-containing slow-release micro-pill takes extrusion rolling method or blank micro-pill loading method. The product is characterized by safety, high efficient, low toxicity and convenient usage. It can be used to treat non-insulin-dependent diabetes mellitus.
Owner:QIQIHAR MEDICAL UNIVERSITY
Melatonin two-layer release-controlled tablet and preparing process thereof
InactiveCN1488346AEffective plasma concentrationEffective hypnosisOrganic active ingredientsNervous disorderVitamin b6Polyethylene glycol
The invention is a melatonin double-layer controlled release tablet and making technique, using melatonin as main raw material, and composed of quick-release layer and sustained-release layer. The quick-release layerí»s components: melatonin 1-5%, Vitamin B6 3-15%, lactose 10-70%, pre-gel amylum 10-70%, hypromellose 0.03-0.3%, magnesium stearate 0.3-2% and SiO2 1.0-3.0%; the sustained layerí»s components: melatonin 2-6%, hypromellose 15-60%, stearic acid 15-65%, polyethylene glycol 10000 10-30%, and magnesium stearate 1.0-5.0%. It has efficacies of quickly hypnotizing and prolonging effective time of sleeping.
Owner:CHONGQING TAIJI MEDICAL RES INST CO LTD
Transdermal patch containing pramipexole
ActiveCN103432104AGood solubilityImprove uniformityOrganic active ingredientsNervous disorderSolventMicrogram
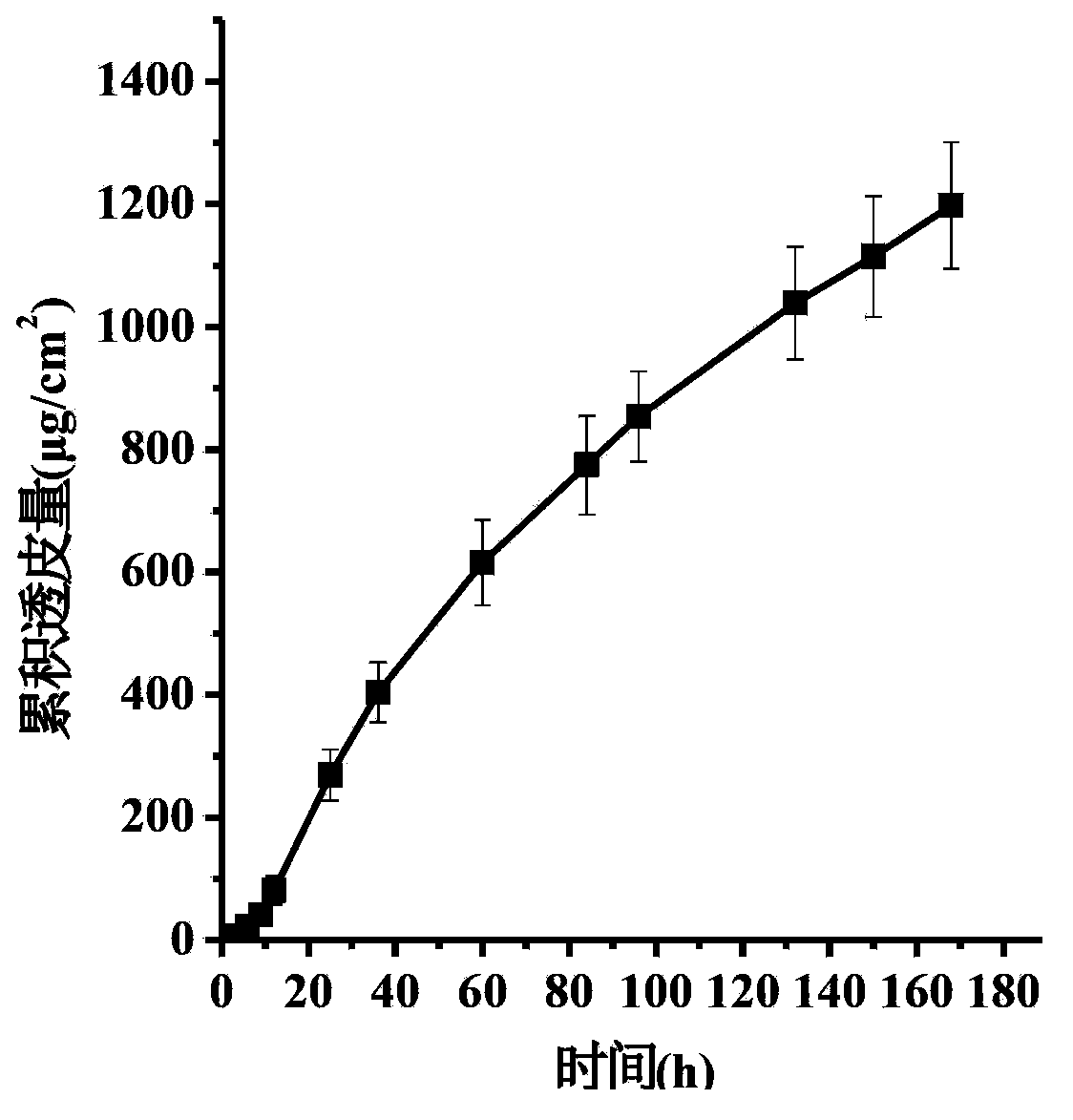
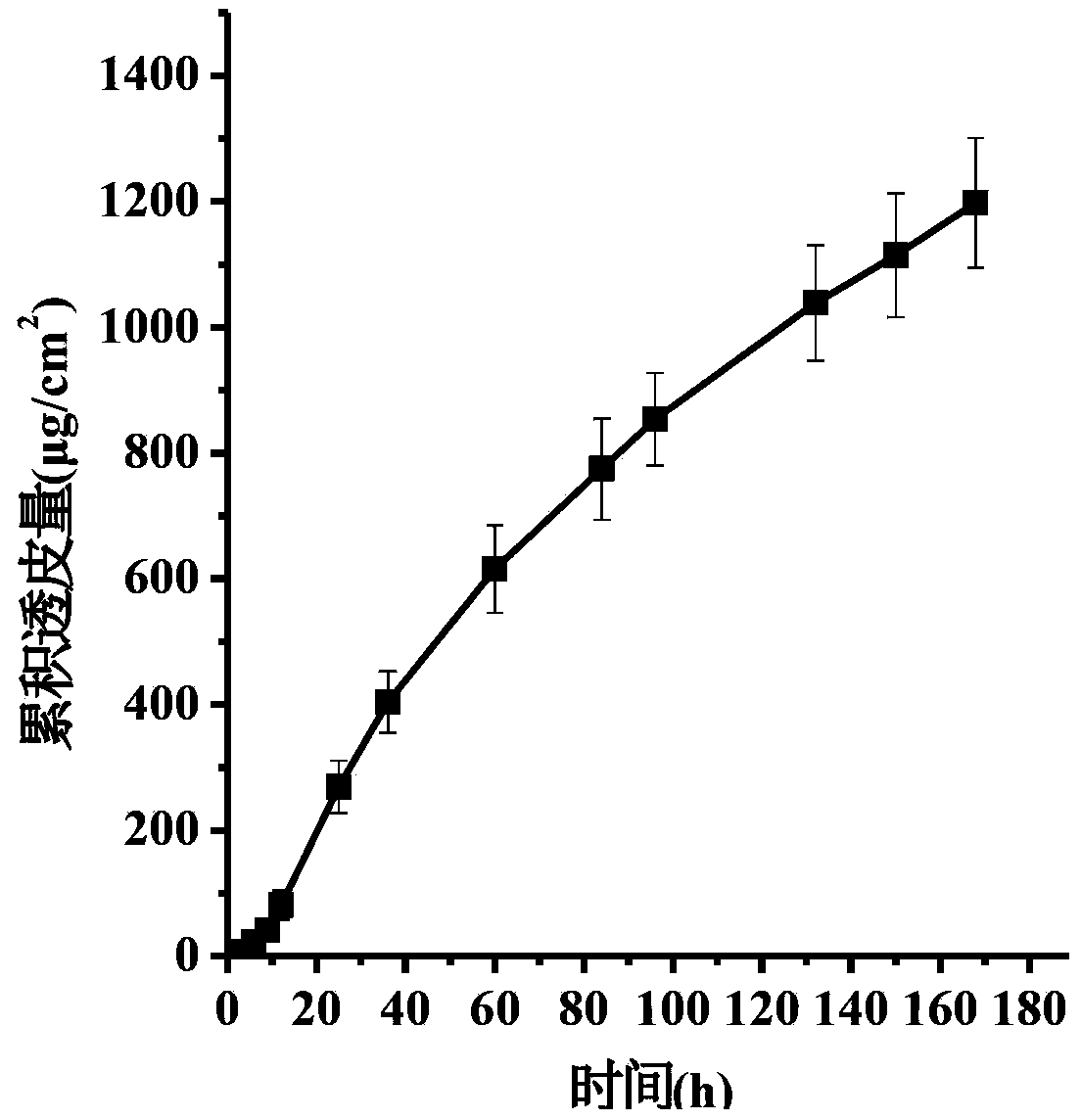
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Nimodipine lipid microsphere injection and preparation method thereof
ActiveCN101485632AImprove solubilityImprove stabilityOrganic active ingredientsNervous disorderSolubilityLipid formation
The invention provides a nimodipine lipid microsphere injection, which is prepared from the following components in percentage by weight: 0.08 percent of nimodipine, 0.5 to 2.3 percent of lecithin for injection, 2 to 8 percent of soybean oil for injection, 2 to 8 percent of medium chain fatty acid for injection, 1 to 3 percent of glycerin, 0.1 to 0.2 percent of tween-80, 0.03 to 0.05 percent of sodium oleic acid, and the balance being water for injection. The preparation method comprises steps of preparation of an oil phase, preparation of water phase, preparation of colostrum, homogenization and canning. In the nimodipine lipid microsphere injection, the soybean oil for injection and the medium chain fatty acid for injection are used to prepare the oil phase, the nimodipine is a fat soluble drug and can be better dissolved in the oil phase, the lipid microsphere in which the soybean oil for injection is the main component has solvent characteristics, is non-toxic, and can guide the fat soluble drugs to be dissolved in emulsion particles and perform the metabolism along with lipid oil drops and slowly release, thereby maintaining the effective blood concentration, lowering toxic and side effects of the drugs, increasing the solubility and stability of the nimodipine drug, improving the drug-loading rate and reducing the hydrolysis of the drugs.
Owner:沈阳信康药物研究有限公司
Allopurinol dual-release preparation and preparation method thereof
ActiveCN102091051AInsufficient improvementEffective plasma concentrationOrganic active ingredientsSkeletal disorderSpecific gravityAdministration time
The invention relates to an allopurinol dual-release preparation and a preparation method thereof, which is characterized in that the dual-release preparation consists of a quick-release part and a slow-release part, wherein the ratio of the base allopurinol contained in the quick-release part to the base allopurinol contained in the slow-release part is 1:1 to 1:24. The invention belongs to the technical field of medicine preparation and aims at providing the allopurinol dual-release preparation with good patient compliance and small side effect and the preparation method thereof, wherein the allopurinol dual-release preparation has the advantages of taking effect quickly and maintaining effective blood concentration stably. The dual-release preparation can not only be used for reducing the side effect on the gastrointestinal tract, but also decreasing the administration times of a patient and adverse effect and promoting the patient compliance.
Owner:COSCI MED TECH CO LTD
Diclofenac sodium hydrogel microballoon with pH sensitivity, preparation method and application thereof
InactiveCN102018674ANo adverse reactionAvoid morning stiffnessOrganic active ingredientsAntipyreticStomach mucous membraneRheumatism
The invention relates to a diclofenac sodium hydrogel microballoon with pH sensitivity, a preparation method and application thereof. In the invention, chitosan and sodium alginate are used as drug carriers to prepare a diclofenac sodium hydrogel microballoon slow release preparation; the mass ratio of a used diclofenac sodium drug to the chitosan to the sodium alginate is (1-3):(5-8):(5-8); and the chitosan is N-succinyl-chitosan. The microballoon preparation can durably develop the drug effect to ensure that diclofenac sodium can be regularly and quantitatively released for a long time; after the microballoon preparation is taken, the blood concentration is stable, the fluctuation is small, and the duration time is long; the microballoon preparation has pH sensitivity, thereby irritations and toxic hazards to the stomach mucous membrane, which are caused by directly and orally taking diclofenac sodium tablets, are reduced; and the microballoon preparation has good effects of cooling down and easing pains and is suitable for various rheumatisms, rheumatoid arthritis, lupus erythematosus, ankylosing spondylitis, various pains caused after operations, fevers caused by various reasons, and the like.
Owner:TONGJI UNIV
Iguratimod oral double-layer sustained-release preparation
InactiveCN101095671AAccelerate time to peak blood concentrationImprove in vitro dissolutionOrganic active ingredientsAntipyreticSide effectEffective action
The invention relates to oral double iguratimod controlled release formulation, which comprises fast release layer and slow release layer that are composed of 8-30% micronizing iguratimod crystal powder and medical findings, and the granule size of iguratimod crystal powder is 1-10 um. The effective component in fast release layer is released in short time and reaches to effective blood chemical concentration for effective action; the iguratimod in sloe release layer is released gradually and maintains effective blood medical concentration for continuous effective action. The invention overcomes shortcomings of short effective action time and a little high toxic effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Doxazosin-mesylate controlled-releasing tablet and preparation method thereof
ActiveCN101167728AImprove complianceStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectGeneration rate
The invention discloses methanesulfonic acid doxazosin controlling releasing tablets and a process for preparation, which contains a single-layer core, insoluble semi-permeable membrane, single drug-releasing eyelet on each side of the tablet, and depends on the osmotic pressure difference between inside and outside of the semi-permeable membrane medium. The invention realizes the control of linear releasing of methanesulfonic acid doxazosin by single-layer core structure, and can remain effective and steady blood and drug concentration. The curative effect is improved, the generation rate of side effect is reduced, and the productive technology is greatly simplified.
Owner:HEFEI LIFEON PHARMA
Zolpidem tartrate controlled-release double-layer tablet and preparation process of zolpidem tartrate controlled-release double-layer tablet
InactiveCN102600097AControl releaseImprove bioavailabilityOrganic active ingredientsNervous disorderControlled releaseZolpidem Tartrate
The invention discloses a zolpidem tartrate controlled-release double-layer tablet. The tablet comprises a zolpidem tartrate quick-release medicine releasing layer and a zolpidem tartrate controlled-release medicine releasing layer and shows the quick dissolving in 1h and the subsequent slow dissolving in 6h. In addition, the invention also discloses a preparation process of the zolpidem tartrate controlled-release double-layer tablet. The preparation process comprises the steps of 1) preparing zolpidem tartrate quick-release particles and zolpidem tartrate controlled-release particles through wet granulation process, and drying and straightening the particles; 2) respectively mixing the quick-release particles and the controlled-release particles with a flow aid and a lubricant in a mixer through a dry method; and 3) pressing the mixed zolpidem tartrate quick-release mixed particles and the controlled-release mixed particles into the zolpidem tartrate controlled-release double-layer tablet.
Owner:李宁
A transdermal patch containing pramipexole
ActiveCN103432104BImprove uniformityImprove solubilityOrganic active ingredientsNervous disorderHigh concentrationTransdermal patch
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Nefopam hydrochloride bilayer slow-release tablet and preparation method thereof
InactiveCN102085196AInsufficient improvementEffective plasma concentrationOrganic active ingredientsNervous disorderSustained Release TabletNefopam Hydrochloride
The invention relates to a nefopam hydrochloride bilayer slow-release tablet and a preparation method thereof. The nefopam hydrochloride bilayer sustained-release tablet is characterized by being prepared by the step of jointly tabletting a quick release layer and a slow release layer into a bilayer tablet, wherein the proportion of nefopam hydrochloride as the main drug contained in the quick release layer and the slow release layer is (1:1)-(1:5). The invention belongs to the technical field of medical preparations and aims at providing the nefopam hydrochloride bilayer slow-release tablet capable of relieving pain rapidly and relieving and easing pain uniformly and persistently and a preparation method thereof. The bilayer slow-release tablet has the advantages of not only decreasing side effects of a common nefopam hydrochloride preparation to the gastrointestinal tract, but also lowering the medicine-taking frequency of patients, reducing adverse effects and improving patient compliance.
Owner:COSCI MED TECH CO LTD
Compound olanzapine subcutaneous implantable sustained-release preparation
InactiveCN103877005AGood curative effectSlight side effectsOrganic active ingredientsNervous disorderBlood concentrationControl release
The invention discloses a compound olanzapine subcutaneous implantable sustained-release preparation which comprises olanzapine and a medicinal carrier. The medicinal carrier is a controlled release agent. The invention further discloses a medicinal preparation of the compound olanzapine subcutaneous implantable sustained-release preparation, a preparation method and an application method. The compound olanzapine subcutaneous implantable sustained-release preparation disclosed by the invention has the advantages of high blood concentration stability after medicament, long time of effective effect of medicines, economical and practical benefits, convenience in delivery and the like.
Owner:陈德香 +2
Florfenicol premixing agent and preparation method thereof
InactiveCN108096191AImprove biological activityEliminate side effectsAntibacterial agentsPowder deliverySide effectMedicine
The invention discloses a florfenicol premixing agent. The florfenicol premixing agent is prepared from the following raw materials in parts by weight: 2 to 3 parts of florfenicol bulk drugs, 2 to 15parts of active auxiliary materials, 2 to 10 parts of carriers, 10 to 25 parts of dissolution agent, 2 to 10 parts of stabilizer, and the balance of filling agent. The obtained florfenicol premixing agent has the advantages of high biological activity, high bioavailability, high stability, low cost, small toxicity and side effect, multiple effects and capability of promoting the growth of animals.By adopting the preparation method of the invention, the defects of a conventional complicated solid inclusion process can be avoided, the use of expensive reagent can be avoided, the process flow issimple, the cost is simple, and the preparation method has positive popularization significance.
Owner:HEBEI LIHUA PHARMA CO LTD
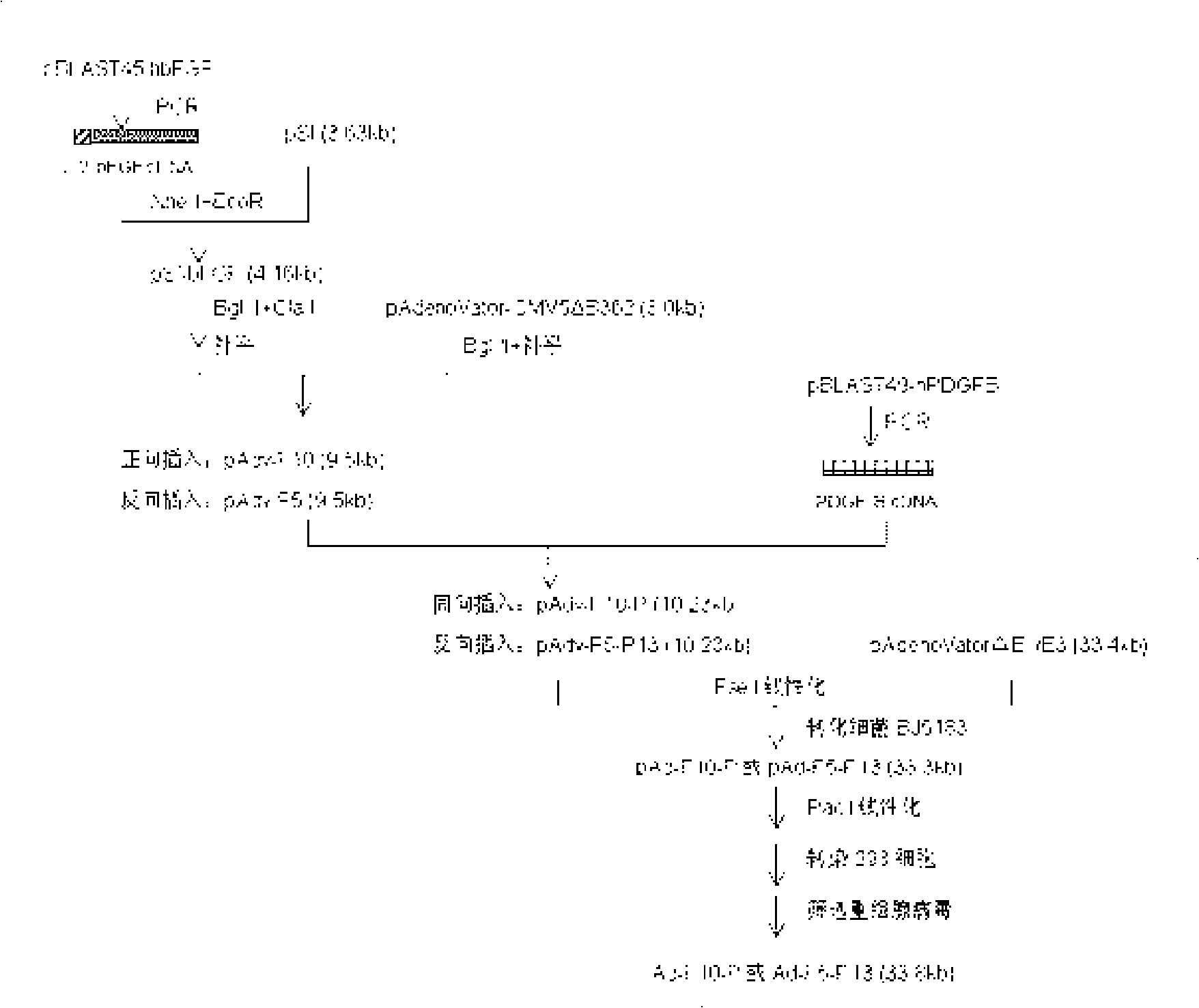
Recombined human bFGF and PDGF-B duplicate adenovirus carrier and uses thereof
ActiveCN101319229AGood treatment effectReduce manufacturing costGenetic material ingredientsFermentationDiseaseCardiac muscle
The invention belongs to the gene therapy field. The technical problem to be solved is to provide a new gene therapy product capable of effectively treating diseases of a cardiovascular system. The invention particularly relates to an expression vector containing recombinant human bFGF and PDGF-B double genes, and a preparation method and an application thereof. The vector which contains the gene capable of encoding bFGF protein and the gene capable of encoding PDGF-B protein can simultaneously express the bFGF protein and the PDGF-BB protein in a eukaryotic cell. An optimal proposal is to make the gene capable of encoding the bFGF protein and the gene capable of encoding the PDGF-B protein be expressed respectively under the control of different promoters. Experiments show that: the recombinant vector has good application prospect for treating myocardial ischemia of coronary heart disease and provides a new choice for treating the diseases of the cardiovascular system.
Owner:GUANGXI WUZHOU PHARMA GRP
Medication composition of containing
InactiveCN1795870AImprove solubilityEffective plasma concentrationOrganic active ingredientsPowder deliveryOrganic acidPEG 400
A composite medicine containing etoposide in the form of freeze-dried powder injection contains etoposide, organic acid, polyethanediol 400, propanediol and excipient. It has high stability.
Owner:云南创立生物医药集团股份有限公司
Preparation method of oral slow/controlled-release preparation containing febuxostat
ActiveCN101773498BImprove securityEffective plasma concentrationOrganic active ingredientsSkeletal disorderAdhesiveImmediate release
Owner:QINGDAO HUANGHAI PHARM CO LTD
Levofloxacin hydrochloride micropill capsule and preparation method thereof
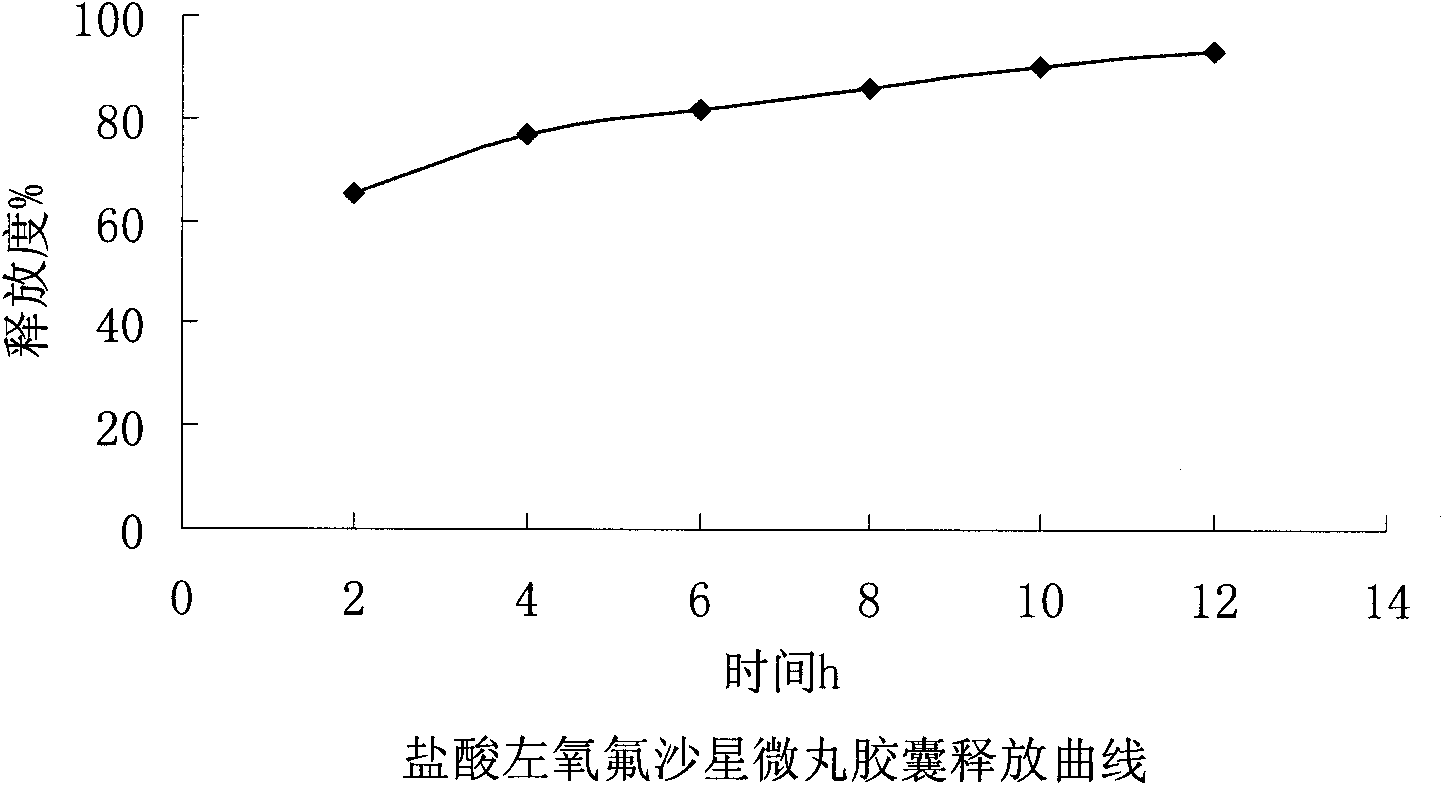
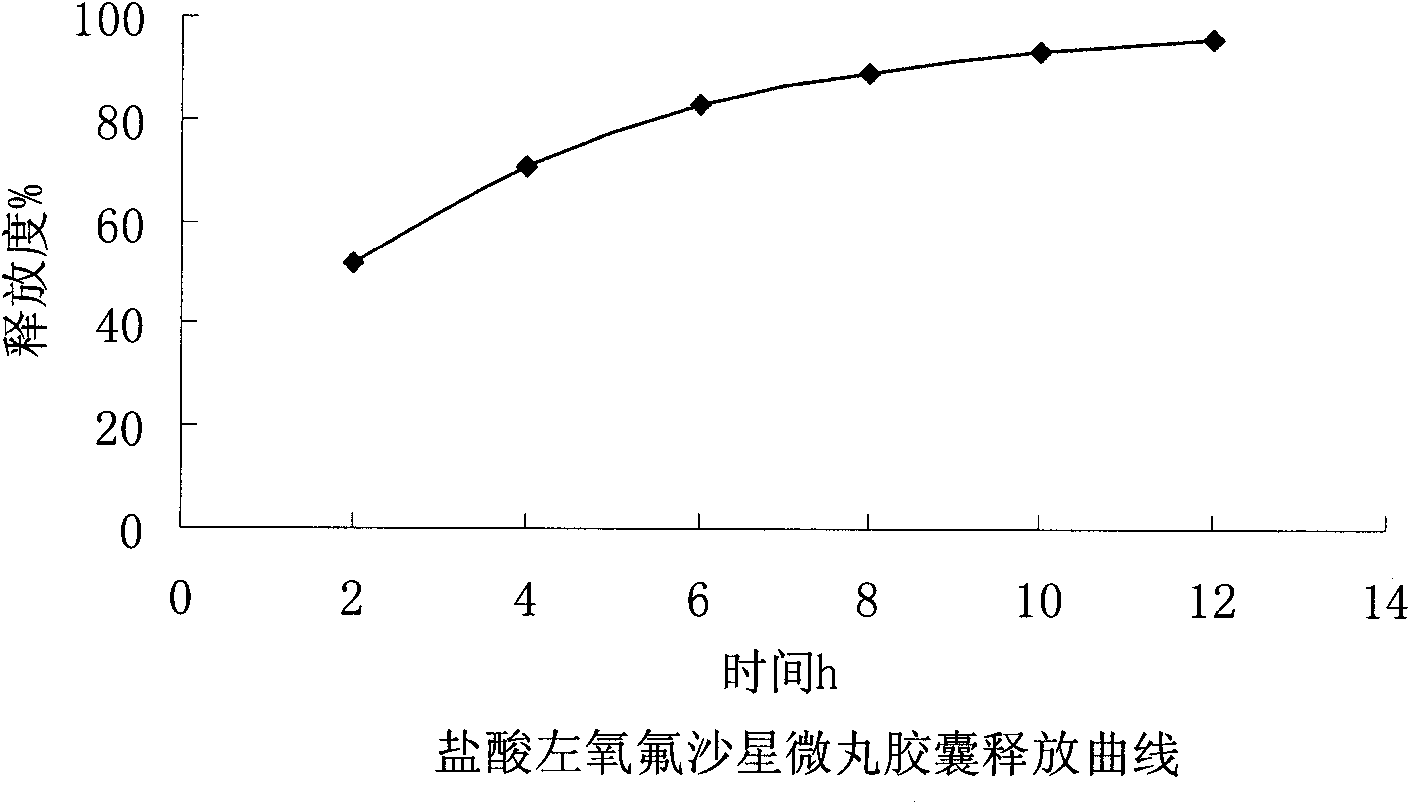
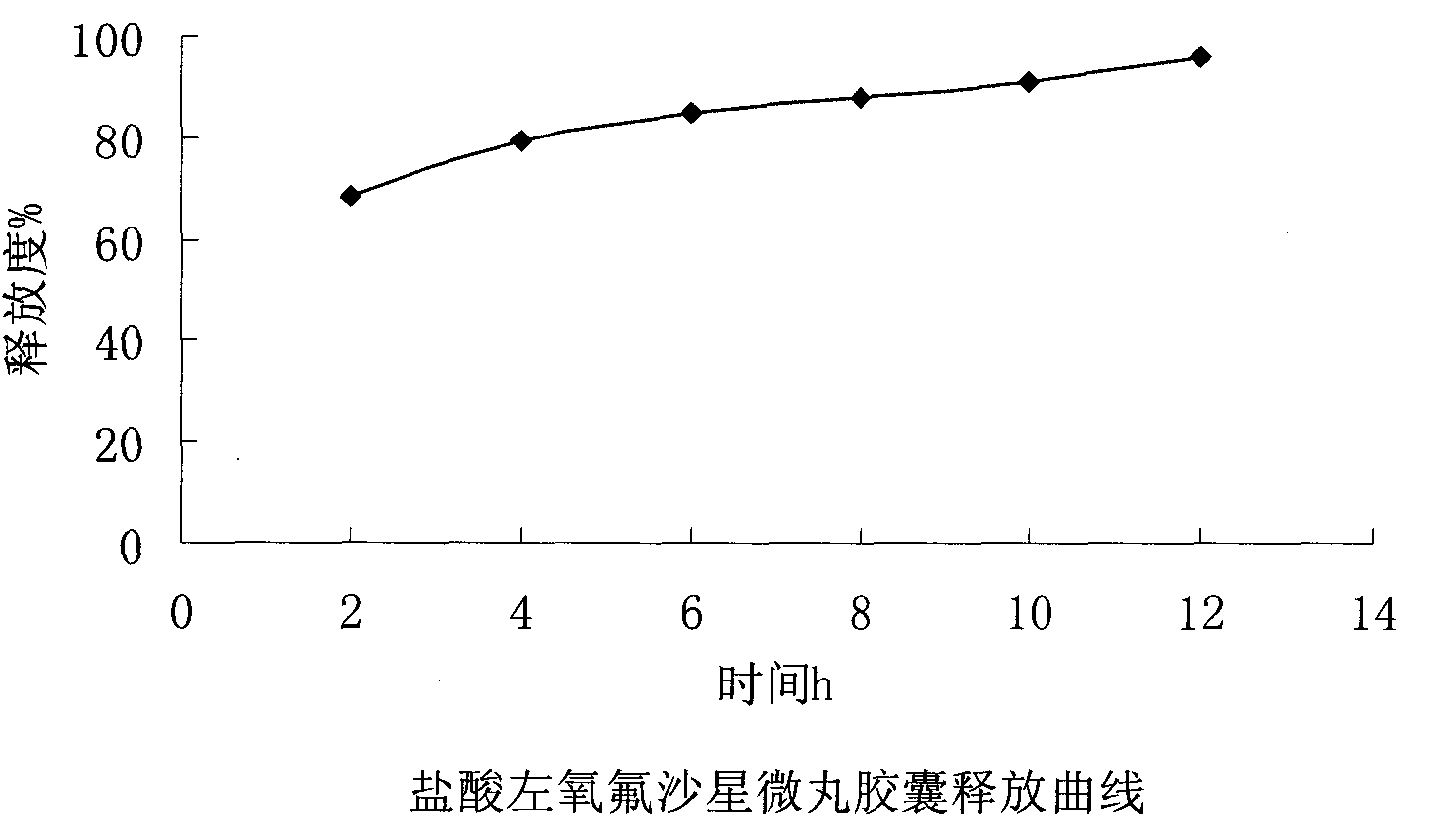
ActiveCN102106842AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsPlasticizerFluidized bed
The invention relates to a levofloxacin hydrochloride micropill capsule and a preparation method thereof. The levofloxacin hydrochloride micropill capsule comprises a pill core, a medicament-containing layer, a sustained-release coating layer and a quick-release layer, wherein levofloxacin hydrochloride is contained in the medicament-containing layer and the quick-release layer; and the sustained-release coating layer comprises the following materials in percentage by weight: 20 to 60 percent of levofloxacin hydrochloride, 30 to 55 percent of pill core, 10 to 25 percent of binding agent, 3 to 5 percent of sustained-release coating material, 0.3 to 3 percent of pore-forming agent and 0.1 to 1 percent of plasticizer. The levofloxacin hydrochloride micropill capsule has the advantages of high stability, small local stimulation of medicaments, high bioavailability and the like. Due to the adoption of a fluidized bed, the problems of large dust and low yield in a method of powder agglomerating in the background technology are solved.
Owner:HAINAN PULIN PHARMA +1
Palmatine hydrochloride crystal form C as well as preparation method thereof and application thereof in medicament composition or health-care product
ActiveCN104016978AGuaranteed absorption characteristicsEffective plasma concentrationAntibacterial agentsOrganic active ingredientsAnti hypertensionCardiac arrhythmia
The invention relates to a palmatine hydrochloride crystal form C as well as a preparation method thereof and an application thereof in a medicament composition or a health-care product. The invention also relates to the medicament composition or the health-care product containing a crystal, and an application of the crystal in preparing the medicament composition or the health-care product with anti-inflammation, anti-bacterium, pain alleviating and tranquilizing, anti-hypertension, cardiac arrhythmia prevention and anti-tumor effects.
Owner:北京健坤和医药科技有限公司
Sustained-release tablet containing trazodone hydrochloride and preparation method of sustained-release tablet
ActiveCN105748421ARelease stabilitySustained releaseOrganic active ingredientsNervous disorderSustained Release TabletTrazodone Hydrochloride
The invention discloses a sustained-release tablet containing trazodone hydrochloride and a preparation method of the sustained-release tablet. The sustained-release tablet is prepared from, in percentage by weight of the whole tablet, 15%-65% of trazodone hydrochloride, 30%-85% of a sustained-release framework and 0.1%-10% of other medical auxiliaries, wherein the sustained-release framework is prepared from high-viscosity hydroxypropyl methylcellulose and water-soluble filler in the weight ratio being 1: (0.3-1.2), and other medical auxiliaries comprise a flow aid and a lubricating agent. Through matched use of high-viscosity hydroxypropyl methylcellulose and the water-soluble filler, the microporous sustained-release framework is formed; the prepared sustained-release tablet containing trazodone hydrochloride can effectively control the release speed of trazodone hydrochloride and can completely release trazodone hydrochloride contained in a sustained release tablet core within certain time, so that water-soluble trazodone hydrochloride is easily stabilized and effectively released, plasma concentration is prevented from fluctuating substantially, the prescription is simple, and the process is simple and convenient.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
Esterase-responsive polycurcumin thiodipropionic acid copolymer prodrug nano-micelle, preparation method and applications thereof
ActiveCN108175860AHigh drug loading rateReduce usageKetone active ingredientsPharmaceutical non-active ingredientsCancer cellMonomer
The invention belongs to the field of novel nanometer drugs, and discloses an esterase-responsive polycurcumin thiodipropionic acid copolymer prodrug nano-micelle, a preparation method and applications thereof, wherein the esterase-responsive polycurcumin thiodipropionic acid copolymer prodrug nano-micelle comprises, by mass, 28-93.6% of a carrier auxiliary material and 6.4-72% of a curcumin drug,the nano-micelle monomer is an amphiphilic block and comprises a hydrophilic segment and a hydrophobic segment, the hydrophilic block is methoxy polyethylene glycol with a molecular weight of 1500-6000, the hydrophobic chain segment is a curcumin poly prodrug short chain co-polymerized from thiodipropionic acid and an anticancer drug curcumin and having a molecular weight of 800-12000, and a weight ratio of the hydrophilic segment methoxypolyethylene glycol to the curcumin poly prodrug short chain is 0.1-20:1. According to the present invention, the novel nano-micelle monomer can be degradedand reduced under the action of the esterase in the cancer cells to obtain the anticancer drug curcumin bulk drug so as to achieve the precise controlled-release effect.
Owner:SOUTH CHINA UNIV OF TECH
Chlorretadin oral disintegrating tablet and its preparation method
InactiveCN1657044AImprove complianceQuick effectOrganic active ingredientsPill deliveryDiseaseChronic urticaria
An oral disintegrating tablet of loratadine for treating allergic rhinitis, urticaria and other allergic skin diseases is prepared from loratadine and medicinal auxiliary.
Owner:浙江现代中药与天然药物研究院有限公司
Slow release levodropropizine pharmaceutical composition
InactiveCN1520820AEffective plasma concentrationLower peak plasma concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectLevodropropizine
The present invention provides one kind of delayed releasing levohydroxypropyl piperazine medicine composition and the medicine composition consists of levohydroxypropyl piperazine 10-80 weight portions, supplementary material for delayed releasing 0.5-60 weight portions and other supplementary material 0.5-90 weight portions. The delayed releasing levohydroxypropyl piperazine medicine composition is cough relieving medicine and the supplementary material for delayed releasing results in the effective blood medicine concentration maintained for relatively long period, relatively low blood medicine concentration, raised curative effect, reduced toxic side effect and less medicine taking times.
Owner:王志刚
Sustained release compound capsules and its preparation method
ActiveCN1593413AImprove complianceEffective plasma concentrationOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsPseudoephedrine
The invention discloses a sustained release compound capsules and its preparation method, wherein each capsule comprises a desloratadine tablet and a plurality of pseudo ephedrine slow release particles, the active components include desloratadine 2.5mg-5mg, pseudo ephedrine 120mg-240mg. The compound slow release capsule can increase the biological availability and compliableness for the patients.
Owner:HAINAN PULIN PHARMA +2
Pharmaceutical composition for treating or preventing bacterial and mycoplasma diseases of livestock and use thereof
ActiveCN103211818ANo stress responseReduce releaseAntibacterial agentsOrganic active ingredientsDiseaseMedicine
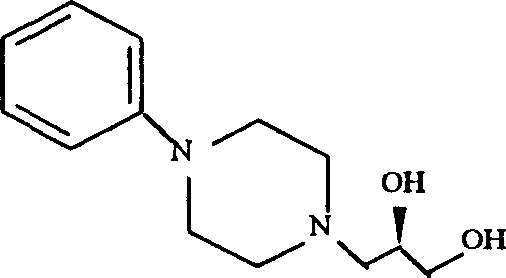
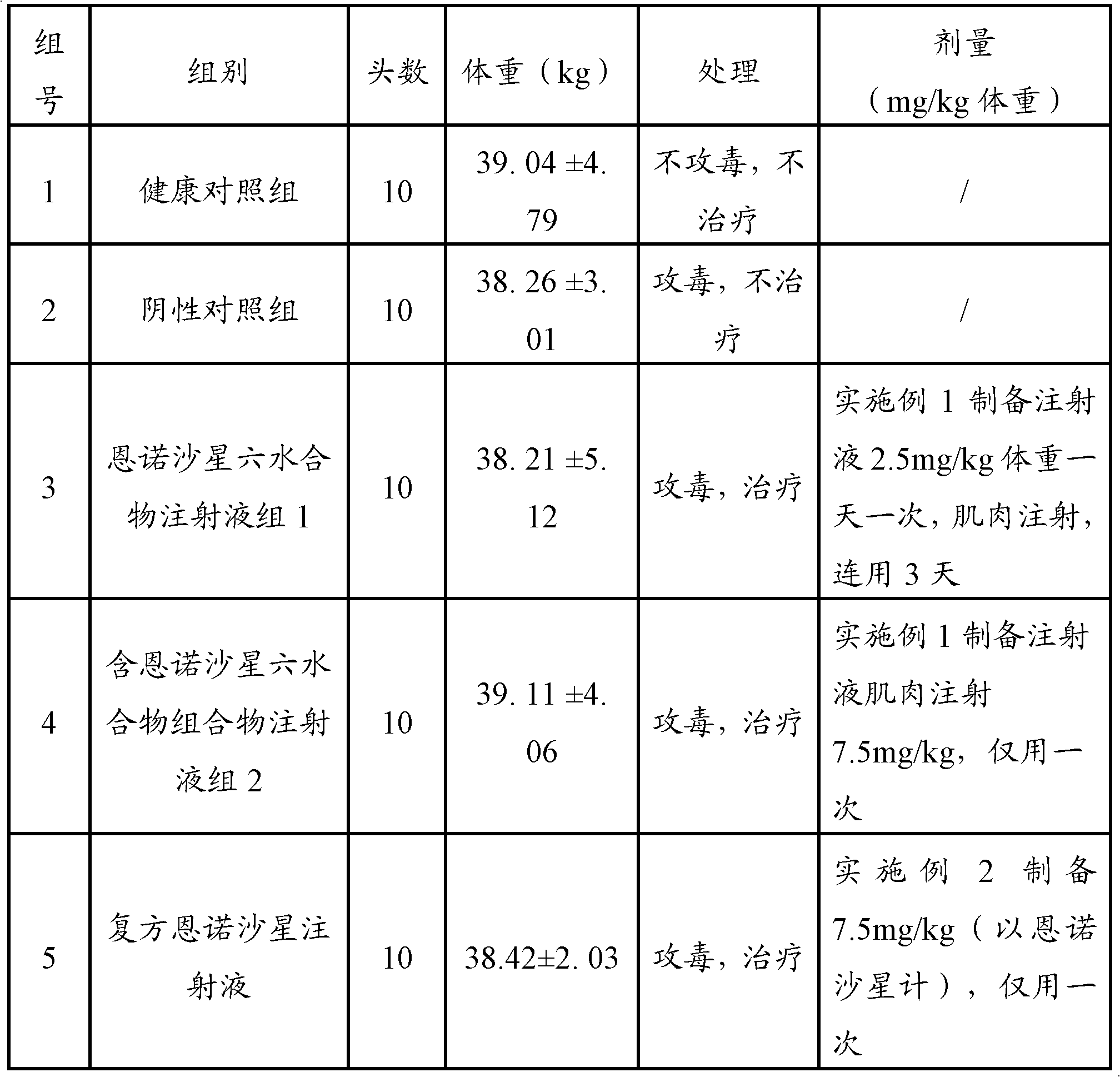
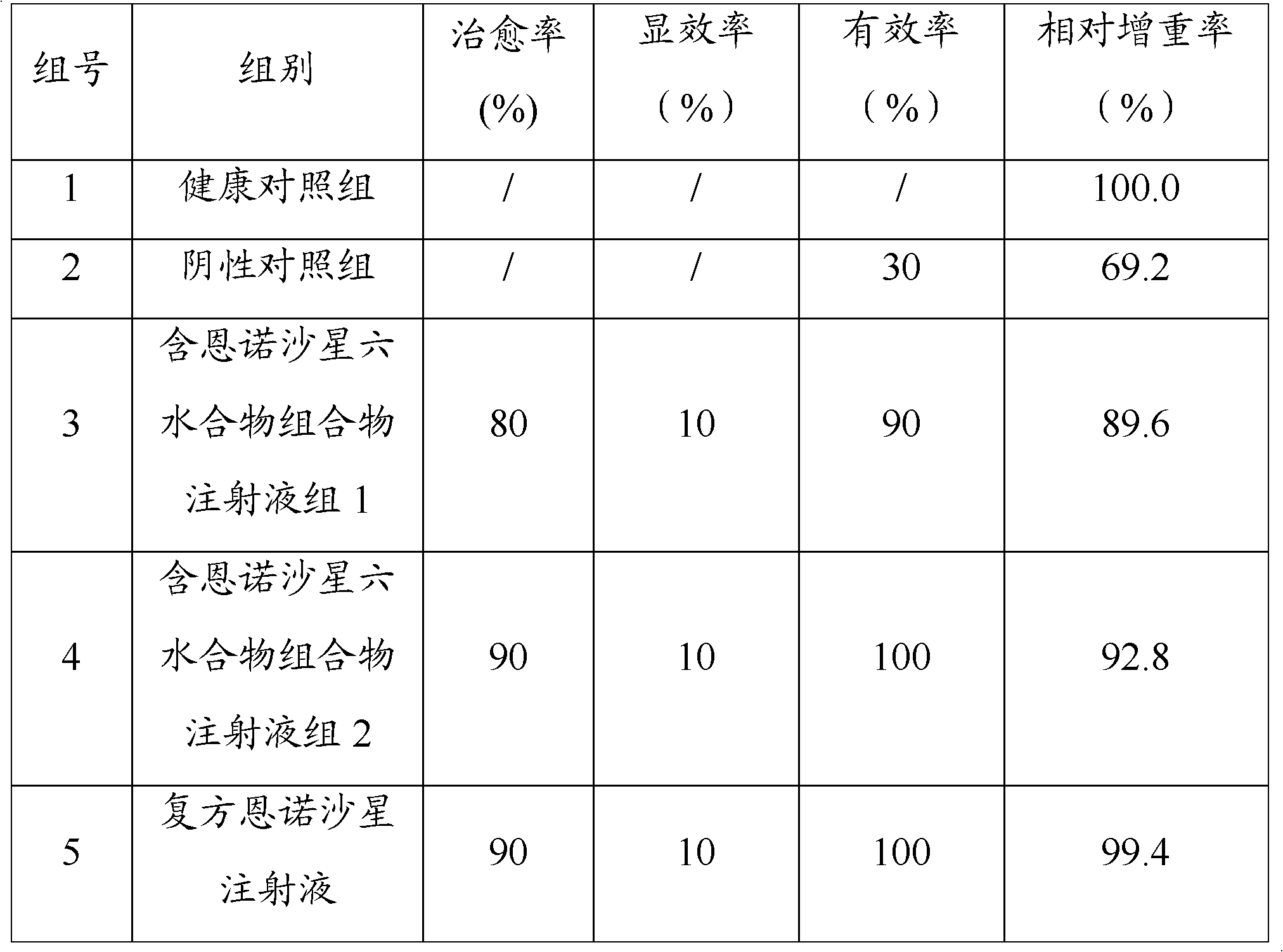
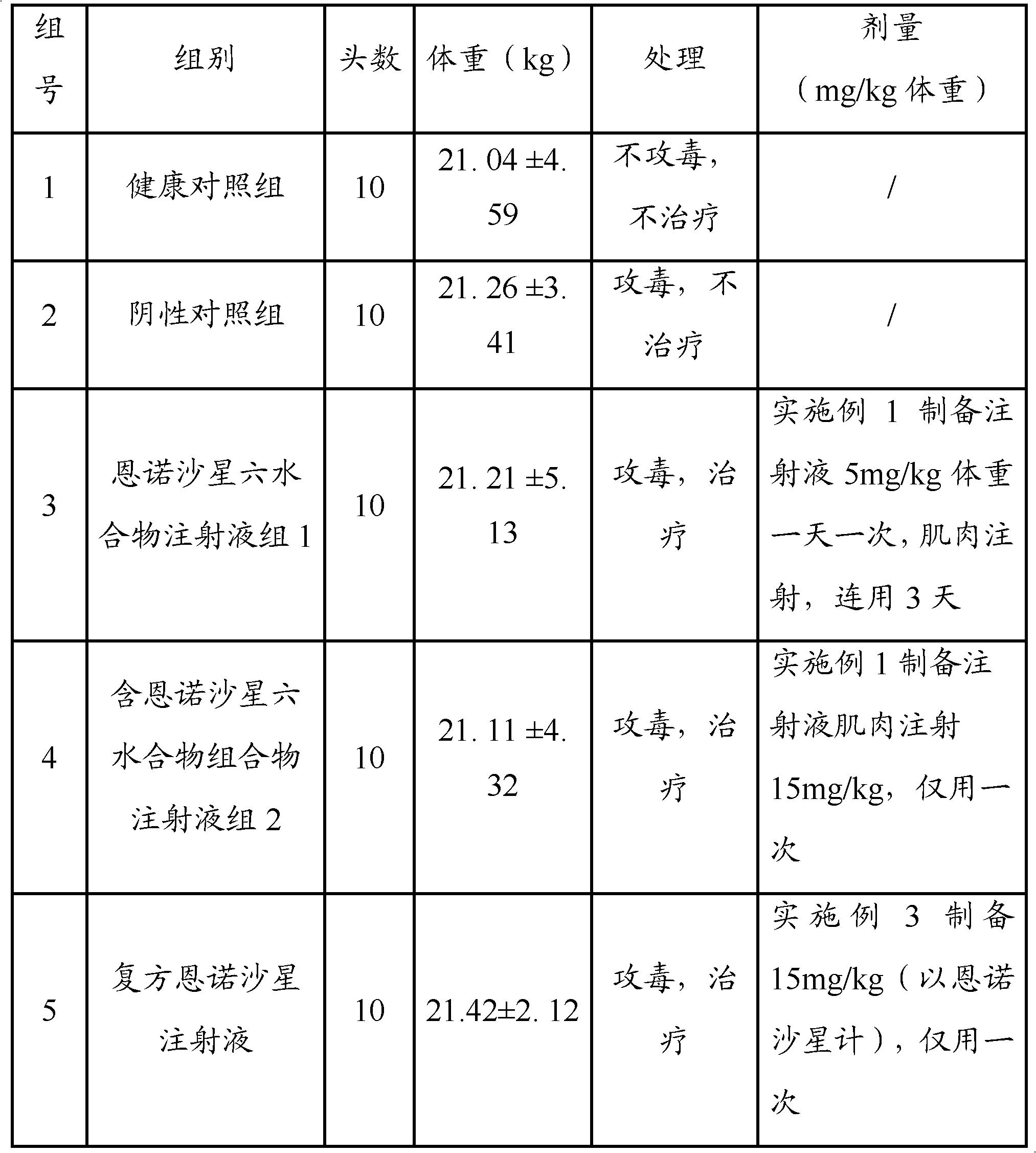
The invention relates to a pharmaceutical composition for treating or preventing bacterial and mycoplasma diseases of livestock and a use thereof. The pharmaceutical composition comprises 5 to 15% w / v of enrofloxacin, an enrofloxacin hydrate or an enrofloxacin derivative (such as enrofloxacin base) and 1 to 10% w / v of butafosfan. The pharmaceutical composition containing enrofloxacin can reduce injection frequency, can treat or prevent bacterial and mycoplasma diseases of livestock, and can improve piglet weight gain after being injected into the piglet.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Double layer laminating sustained release tablet of metronidazole and its preparation method
InactiveCN1593404AExtended release timeEffective inhibitory concentrationOrganic active ingredientsAntimycoticsMass ratioLubricant
Disclosed is a metronidazole double layer laminated slow release tablet comprising two layers, the first layer being a slow release layer consisting of metronidazole, slow release frame, binding agent and lubricating agent, the second layer being a quick release layer consisting of metronidazole, bulking agent, crumbling agent, binding agent and lubricating agent, wherein the mass ratio of metronidazole content in the slow release layer and in the quick release layer is 1 : 0.36-2.75. The invention also discloses the process for preparing the tablet.
Owner:南京亿华药业有限公司
Dihydroartemisinin controlled-release preparation used for treating lupus erythematosus
InactiveCN103181901AControl release speedImprove performanceOrganic active ingredientsSkeletal disorderSide effectOral medication
The invention discloses a dihydroartemisinin controlled-release preparation used for treating lupus erythematosus. The controlled-release preparation shows at least one property of the following: (1) according to an in-vitro release curve, when the preparation is placed for 0.5h according to a standard dissolution test, an accumulative release rate of dihydroartemisinin is 10-25%; and (2) according to a healthy adult single-dose oral administration dihydroartemisinin in-vivo absorption curve, an average time for 40% absorption is longer than approximately 2h, and / or an average time for 70% absorption is longer than approximately 6h. With the preparation, dihydroartemisinin in-vivo release speed can be controlled. The preparation can be taken only once every day, and long-time effective blood concentration can be maintained, such that medication is more convenient. The preparation has stable performance, and low toxic and side effect. Because the preparation only needs to be taken once a day, treatment cost is reduced.
Owner:GUILIN PHARMA
Dutasteride self-microemulsion freeze-dried composition and preparation method thereof
ActiveCN104146966ASolve the problem of low oral dissolutionLittle side effectsPowder deliveryOrganic active ingredientsOil phaseMicroemulsion
The invention discloses a dutasteride self-microemulsion freeze-dried composition and a preparation method thereof. The dutasteride self-microemulsion freeze-dried composition disclosed by the invention comprises dutasteride self-microemulsion and a freeze-dried excipient in the weight ratio of (0.5-2):1, wherein the dutasteride self-microemulsion is mainly composed of the following components in percentage by weight: 0.05-0.3% of dutasteride, 5-75% of oil phase, 20-60% of emulsifier, and 4-50% of co-emulsifier. The dutasteride self-microemulsion freeze-dried composition disclosed by the invention is more suitable for preparing dutasteride solid preparations and is capable of increasing the bioavailability of dutasteride.
Owner:CHONGQING PHARMA RES INST
Compound sustained-release preparation and preparation method thereof
InactiveCN101780054AMaintain blood levelsMaintain effective plasma concentrationOrganic active ingredientsUrinary disorderBlood concentrationActive component
The invention relates to a compound sustained-release preparation and a preparation method thereof, and the compound sustained-release preparation comprises Epristeride, terazosin hydrochloride and pharmaceutically acceptable auxiliary materials, wherein the Epristeride and the terazosin hydrochloride are used as the active components, the Epristeride is prepared into a sustained-release part, and the terazosin hydrochloride is prepared into an ordinary-release part, so that the two medicines are kept in an effective blood concentration range in vivo, the adverse effect of the medicines and the administration frequency are reduced, and the safety and effectiveness of pharmacy and the compliance of patients are improved.
Owner:JIANGSU LIANHUAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com