Recombined human bFGF and PDGF-B duplicate adenovirus carrier and uses thereof
A PDGF-B and PDGF-BB technology, applied in the field of adenovirus bivalent vectors, can solve the problems of impossible to control the infection efficiency of two adenoviruses, difficult to control the expression amount of two genes, difficult to control the expression amount, etc. The effect of reducing the frequency of medication, improving the treatment effect, and reducing the cost of production and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
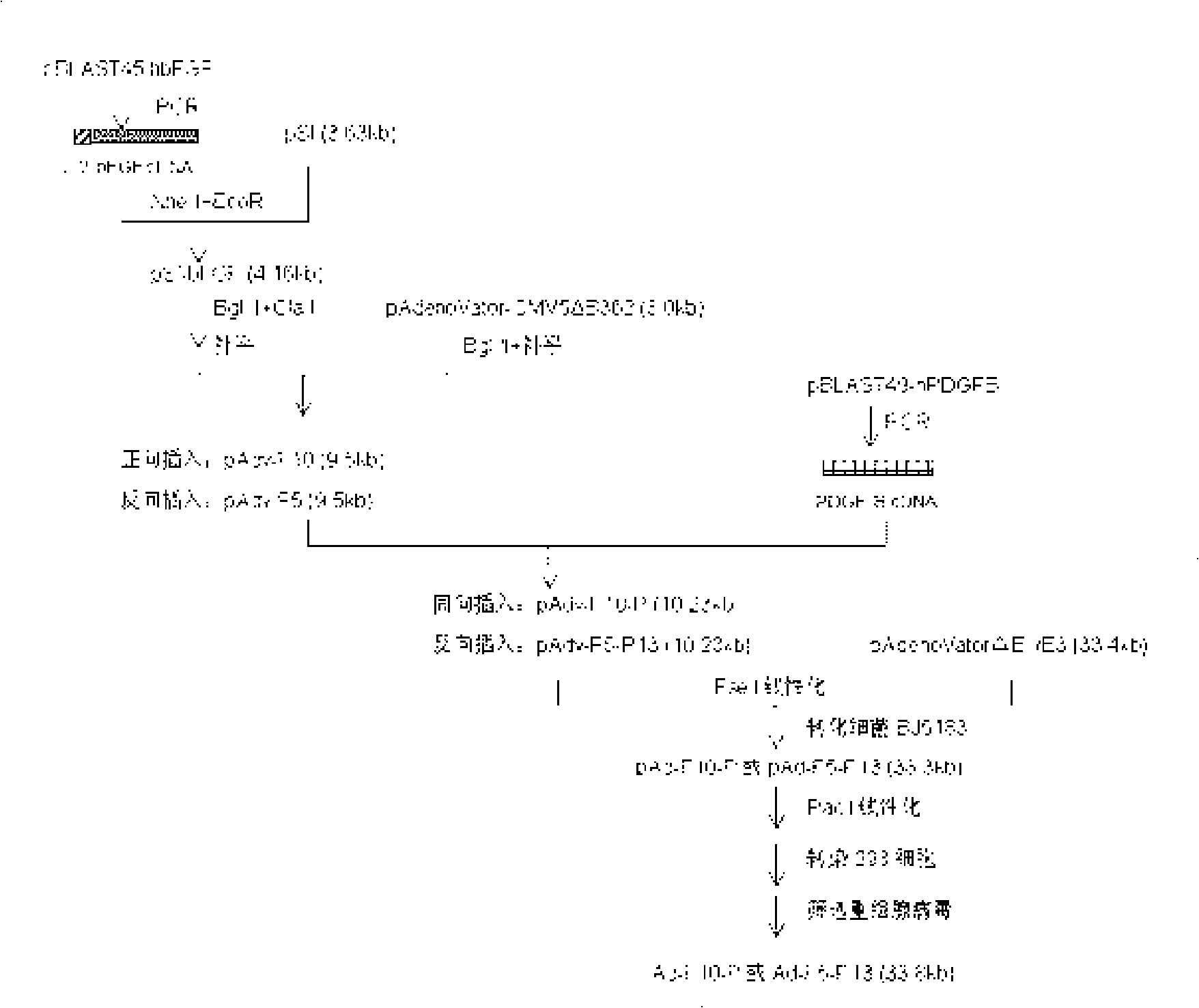
[0041] Example 1 Construction and screening of recombinant bFGF and PDGF-B double gene adenovirus of the present invention
[0042] See the detailed construction process of recombinant bFGF and PDGF-B double gene adenovirus figure 1 In order to obtain an expression vector containing two independent expression frames (including different promoters, multiple cloning sites, and tailing signals) in the adenovirus, the bFGF gene was first inserted into the eukaryotic expression vector pSI (purchased from Promega), and then used The restriction site on the vector cuts out the sequence containing the complete bFGF expression framework (SV40 promoter-secreted bFGF-SV40 tailing signal), and inserts it into the modified shuttle vector pAdenoVator-CMV5△B362 (pAdenoVator-CMV5 purchased from Qbiogen Company), the bFGF shuttle plasmids inserted in the same direction or reverse direction—pAdv-F10 and pAdv-F5, respectively; insert the PDGF-B cDNA fragment into the recombinant bFGF shuttle vector...
Embodiment 2
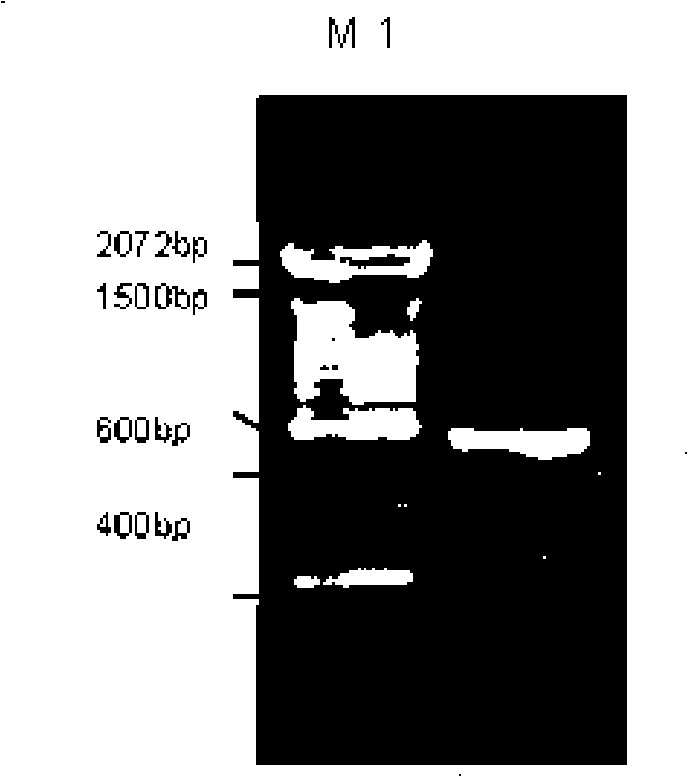
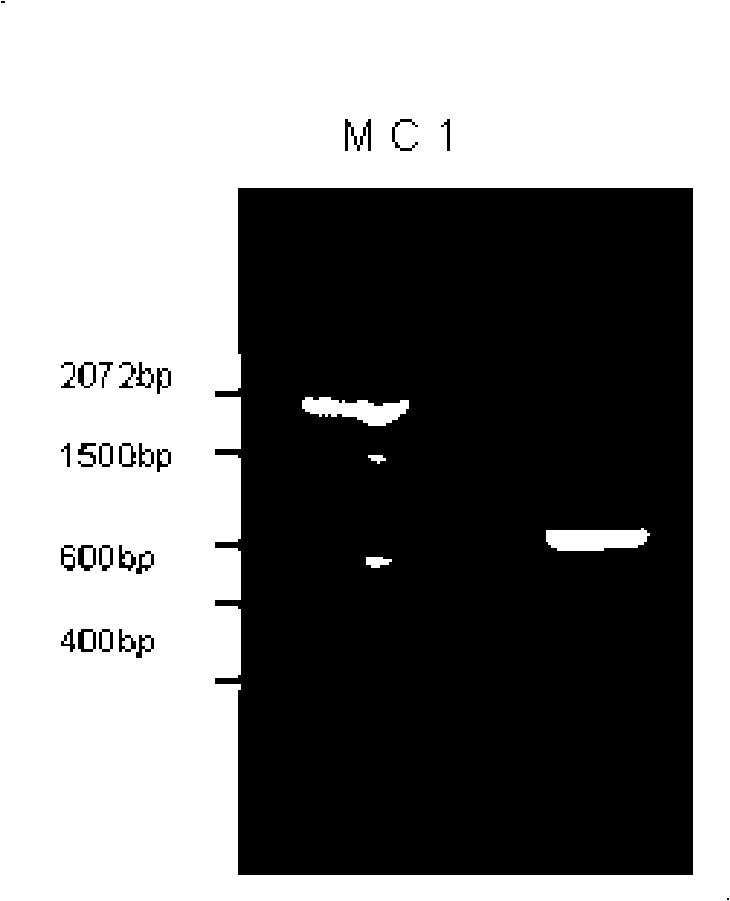
[0068] Example 2 Construction and screening of recombinant bFGF adenovirus and recombinant PDGF-B adenovirus
[0069] The detailed construction process of the recombinant bFGF adenovirus is as follows: It is carried out on the basis of the recombinant bFGF shuttle vector pAdv-F5 and pAdv-F10 in Example 1, respectively, with the circular plasmid containing the entire adenovirus genome (E1, E3 deletion) pAdenoVator△E1E3 was electroporated into BJ5183 to obtain pAd-F10 and pAd-F5; the pAd-F5 and pAd-F10 linearized by Pac I were co-transfected with lipofectamine 2000 using conventional co-transfection methods to transfect 293 cells, and the results were obtained by screening Recombinant adenovirus Ad-F5 and Ad-F10. After extracting the viral DNA from the primary virus seed solution, PCR amplifies the inserted bFGF DNA fragment (PF1+PF2) to identify whether it is a recombinant adenovirus, and at the same time amplifies the E2B region fragment (PB5+PB6) that identifies the characteristi...
Embodiment 3
[0072] Example 3 Mass amplification and purification of recombinant adenovirus of the present invention
[0073] After the different recombinant adenoviruses constructed in the above examples were amplified by 293 cells, the recombinant adenovirus-Ad-F, Ad-P and Ad-FP were purified by two-step CsCl density gradient ultracentrifugation. The first step was discontinuous The CsCl gradient removes most of the cell debris and defective virus particles (that is, virus particles that do not have infectious activity). In the second step, a continuous CsCl density gradient is used to completely separate infectious virus particles from defective virus particles, and finally Desalting by dialysis, remove CsCl, and exchange to the required preservation solution.
[0074] See the results of the virus discontinuous CsCl density gradient centrifugation Picture 11 -A, the two white bands formed by the light-visible virus particles, the upper band is weaker and more diffuse, which are virus parti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com