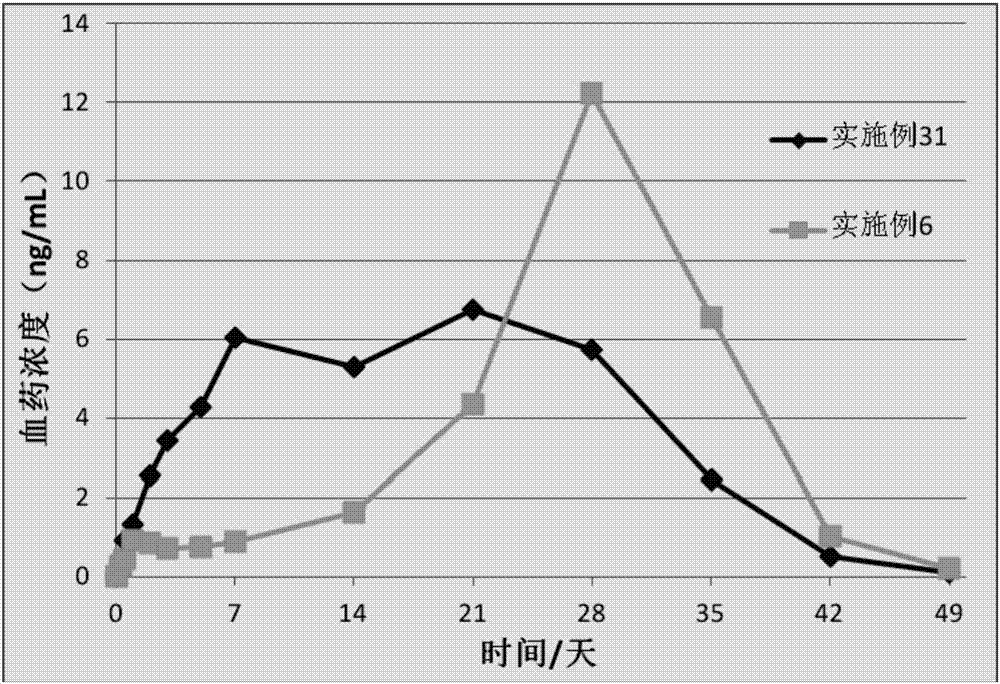
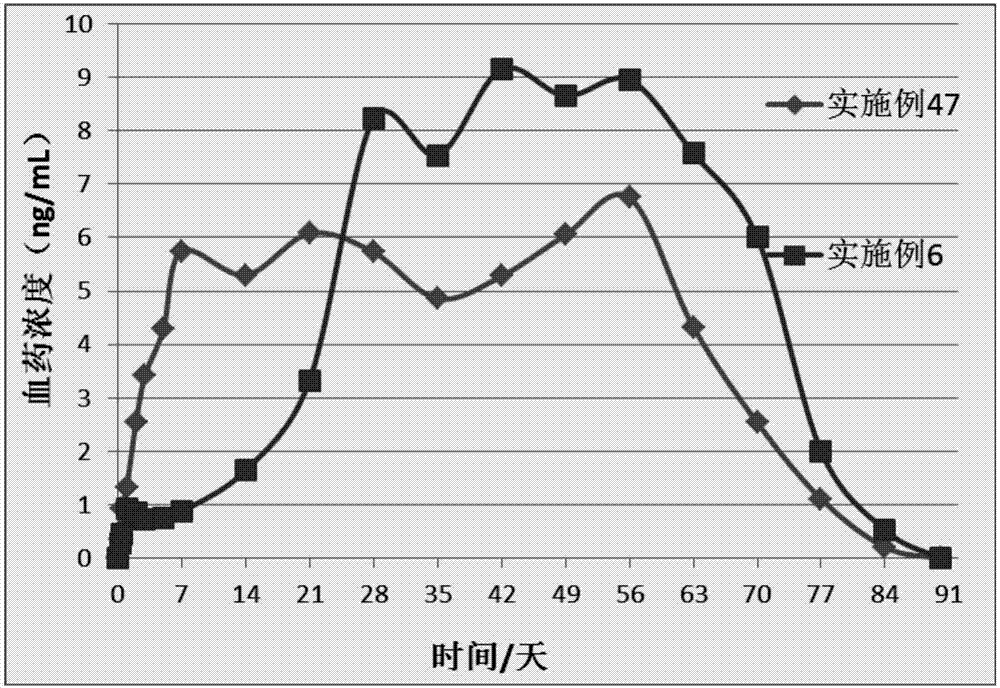
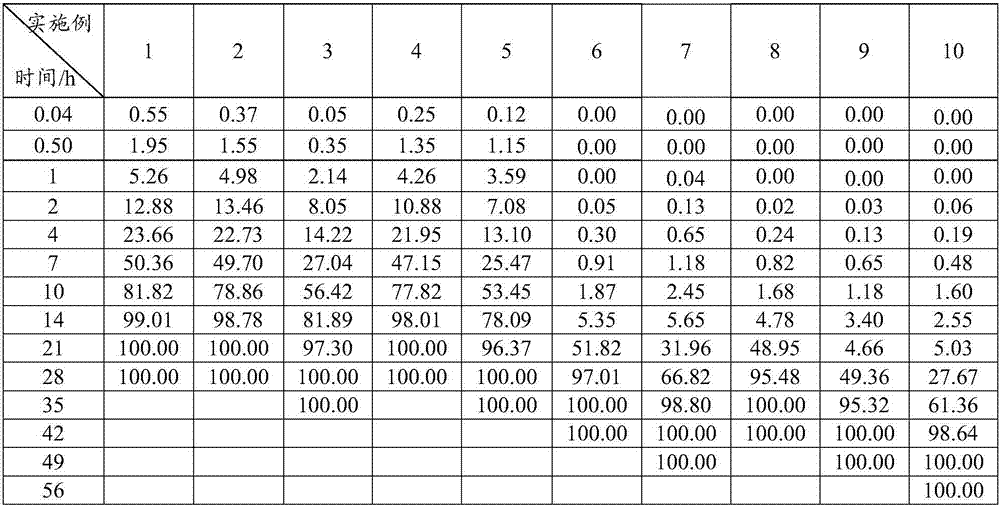
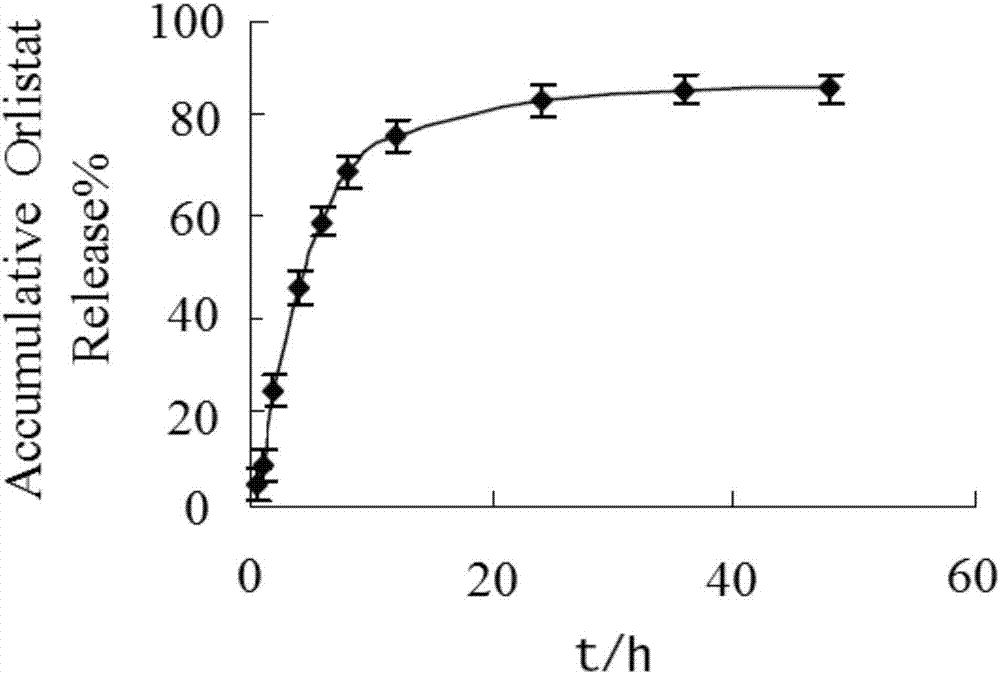
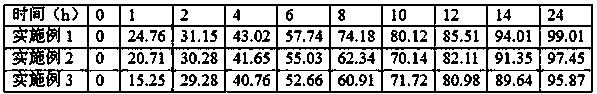
Patents
Literature
34results about How to "Maintain effective plasma concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
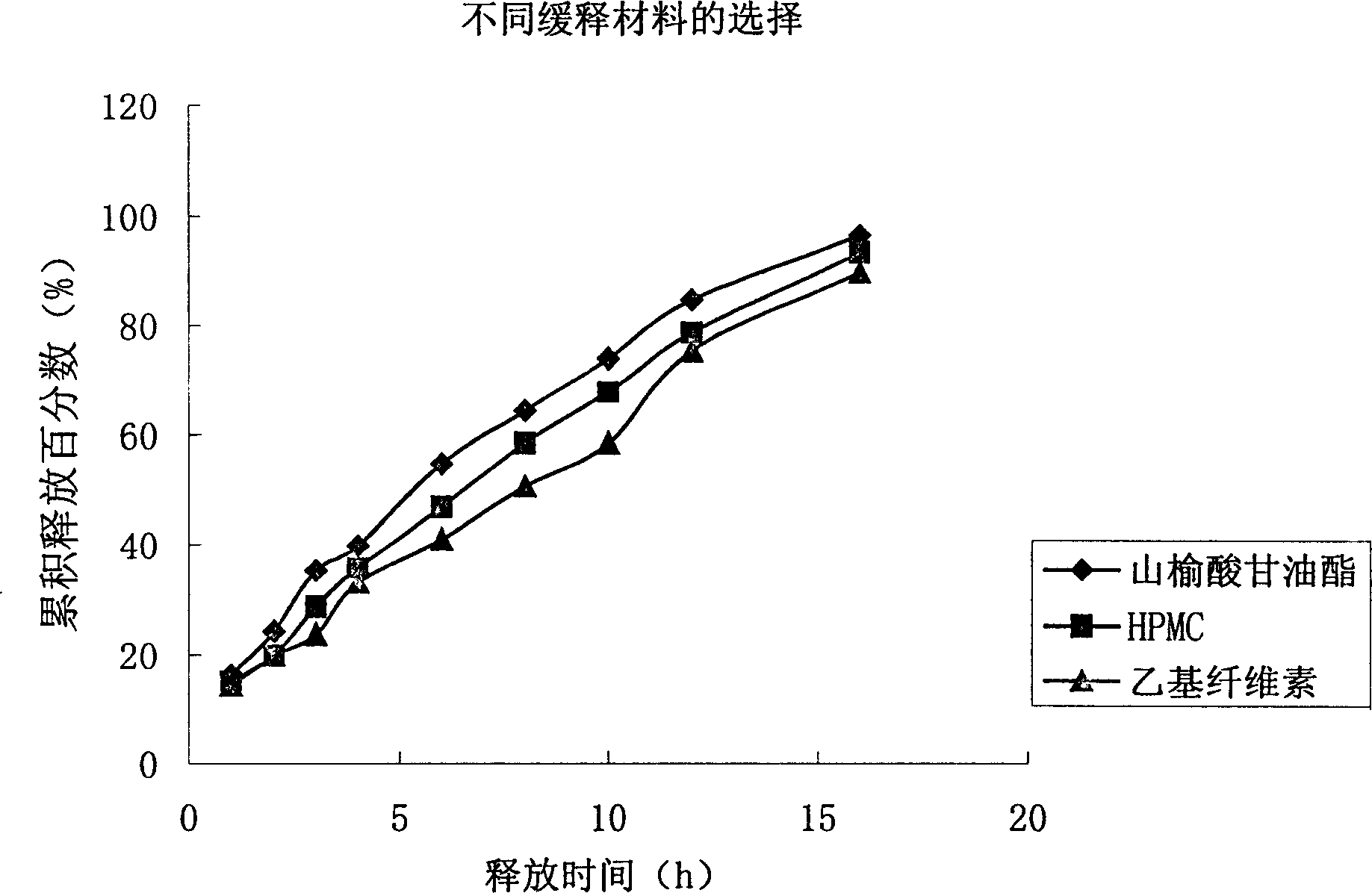
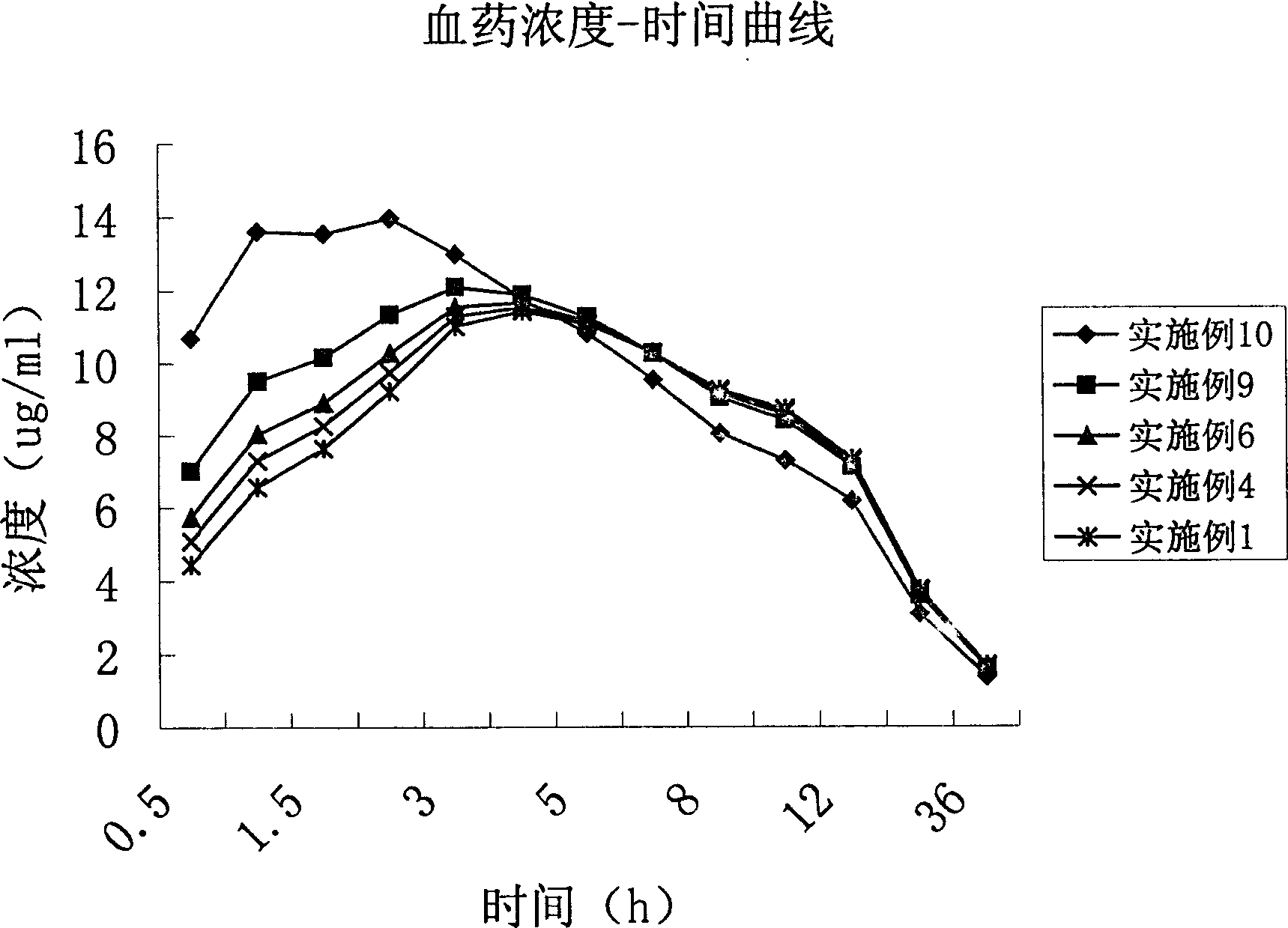
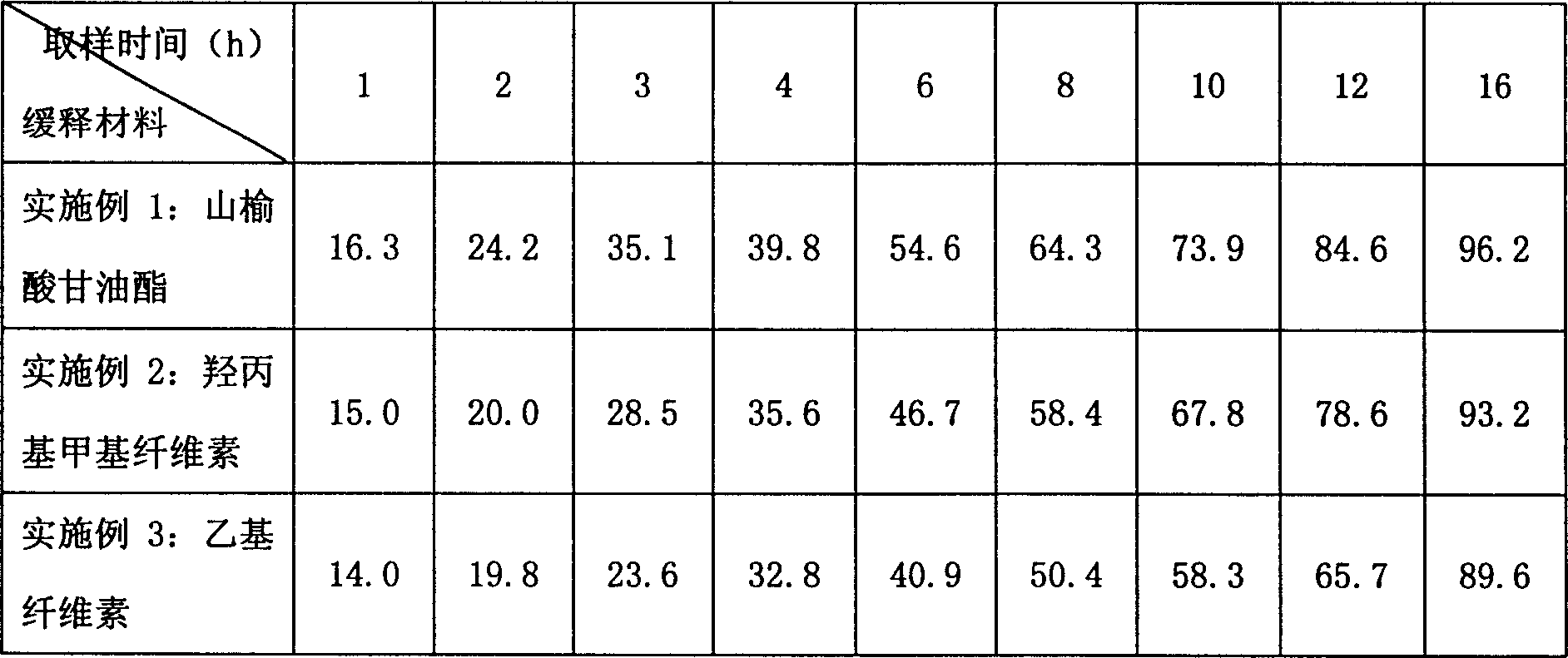
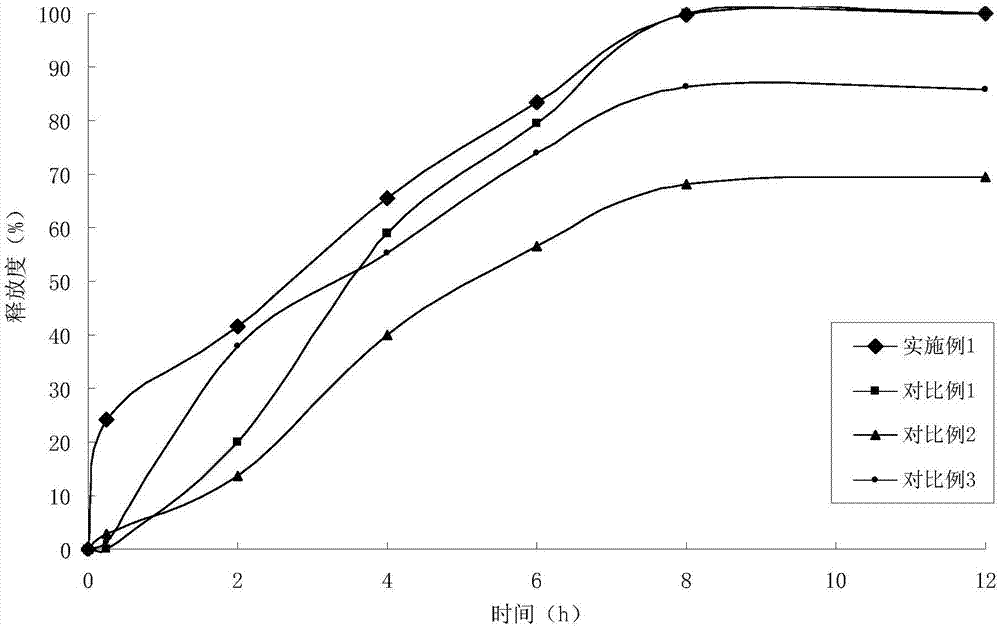
Water indissolvable/micro-dissolvable drug sustained release composition
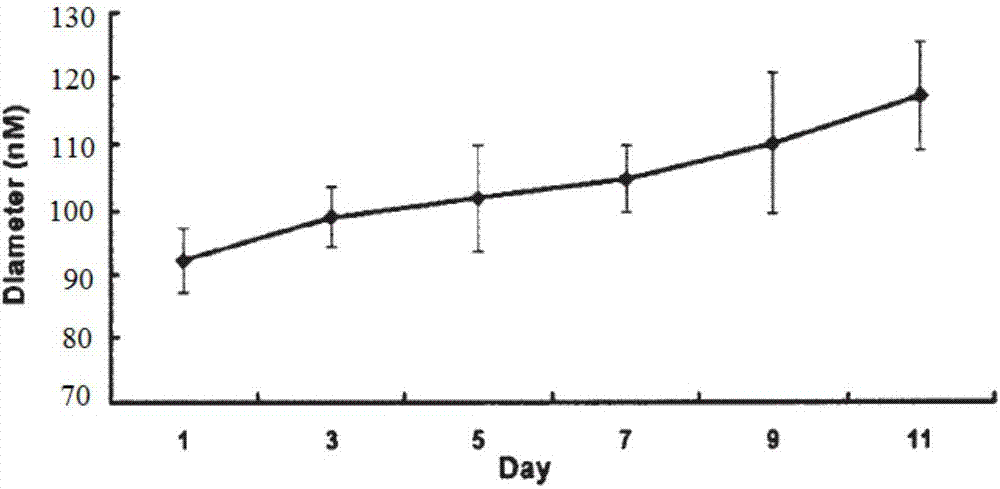
PendingCN106963746AGood sustained release performanceMaintain effective plasma concentrationPharmaceutical non-active ingredientsNanocapsulesDrugDelaying periods
The invention relates to a sustained release composition, and particularly relates to a water indissolvable / micro-dissolvable drug sustained release composition. The indissolvable / micro-dissolvable drug sustained release composition comprises more than two indissolvable / micro-dissolvable drug sustained release microspheres with different release behaviors. Through combining more than two drug sustained release microspheres with different release behaviors, the slow release composition of the indissolvable / micro-dissolvable drug is free from obvious release delay period or short delay release period, and also free from sudden release; the composition has good sustained release performance, and can maintain effective blood concentration within several weeks or above, thus the drug applying frequency is effectively reduced, the composition can facilitate patient's life and work; moreover, the composition is good for improving the drug applying compliance and convenience of the patient.
Owner:AC PHARMA
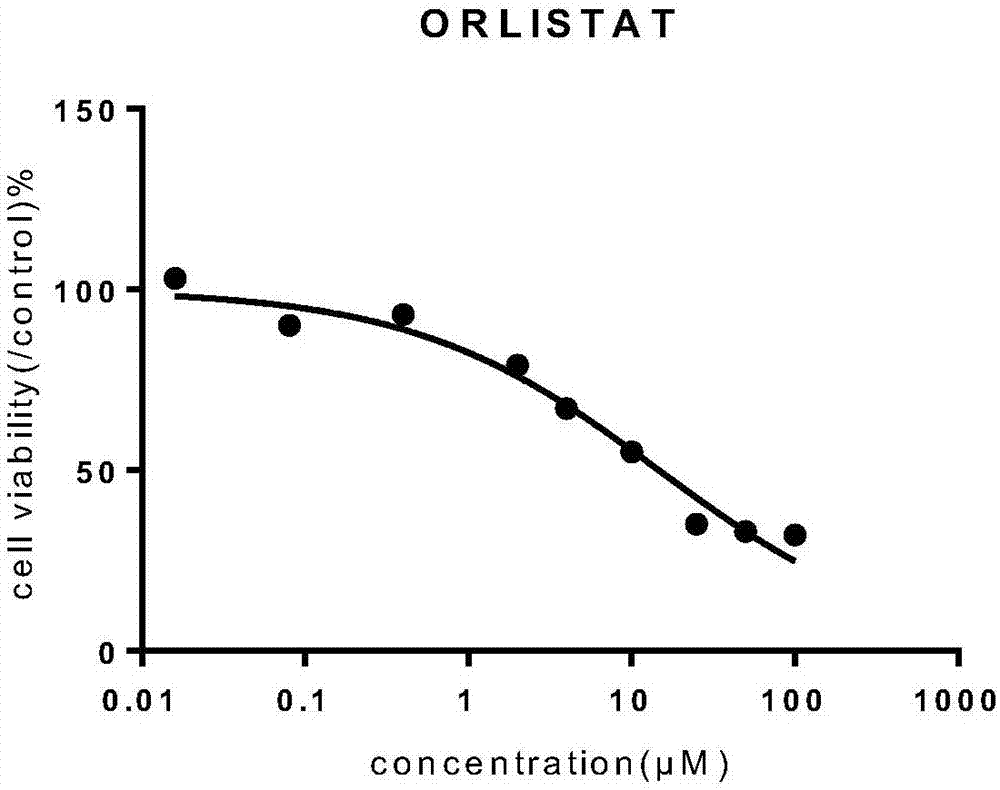
Orlistat nano-microsphere as well as preparation method and application thereof in anti-tumor drugs
ActiveCN107412196AChange hydrophobic propertiesHigh clinical valueOrganic active ingredientsInorganic non-active ingredientsOrlistatDisease
The invention belongs to the technical field of bio-medicine, and in particular relates to an orlistat nano-microsphere as well as a preparation method and an application thereof in anti-tumor drugs. The orlistat nano-microsphere provided by the invention is prepared by mixing orlistat and a drug-carrying material, wherein the drug-carrying material is polyethylene glycol-polycaprolactone (mPEG-PCL); the mass ratio of the orlistat to the mPEG-PCL is at (1-2) to (3-10); and the molecular weight ratio of mPEG to PCL is at 2000 to 10000 or 2000 to 20000. The polymer nano-microsphere provided by the invention is prepared by loading the orlistat on the nano-material, so that a hydrophobic property of the nano-microsphere is changed; and through the sustained-release property of the microsphere, the microsphere can be used for preparing drugs for resisting tumor diseases; therefore, the nano-microsphere is huge in potential clinical value.
Owner:ZEIN BIOTECHNOLOGY CO LTD
Felodipine sustained-release tablet and preparation technology thereof
ActiveCN104758266ARapid drug releaseStable drug releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention discloses a felodipine sustained-release tablet and a preparation technology thereof. According to the preparation technology of the felodipine sustained-release tablet, fast-release solid dispersions, sustained-release micro-pills and pharmaceutically acceptable accessories are mixed and the mixture is compressed to obtain the preparation. The fast-release solid dispersions comprise felodipine, copovidone and polacrilin potassium; the sustained-release micro pills are obtained by coating the fast-release solid dispersions with the ethyl cellulose film with a pore-forming agent. The sustained-release tablet disclosed by the invention has the advantages of fast early-stage releasing speed and slow and stable later-stage releasing speed, so that the drug can be completely released and an effective blood drug concentration can be maintained for a long time; the felodipine sustained-release tablet is high in bioavailability, simple in preparation technology and applicable to industrial mass production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
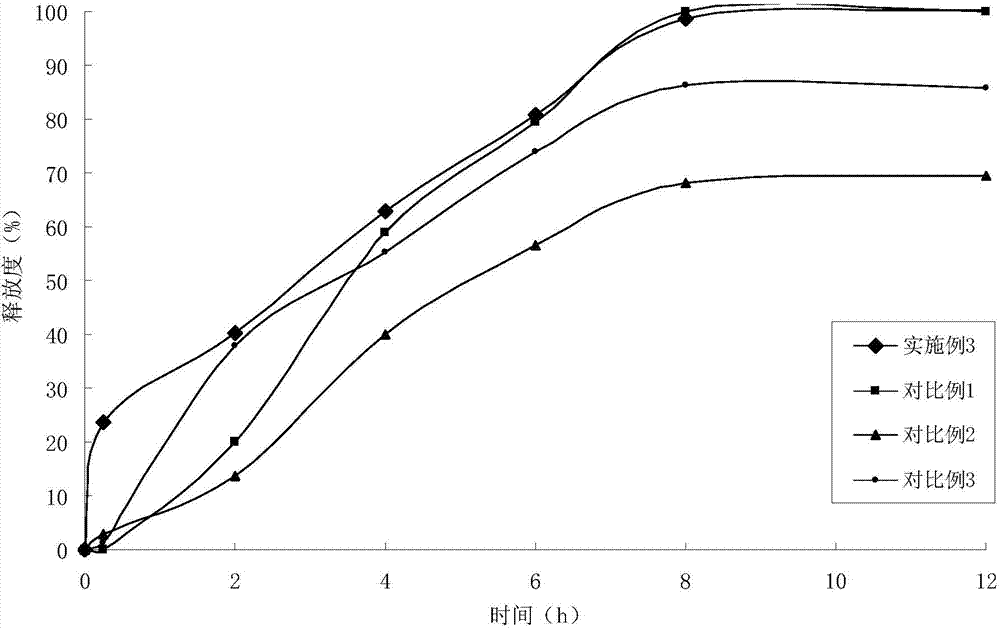
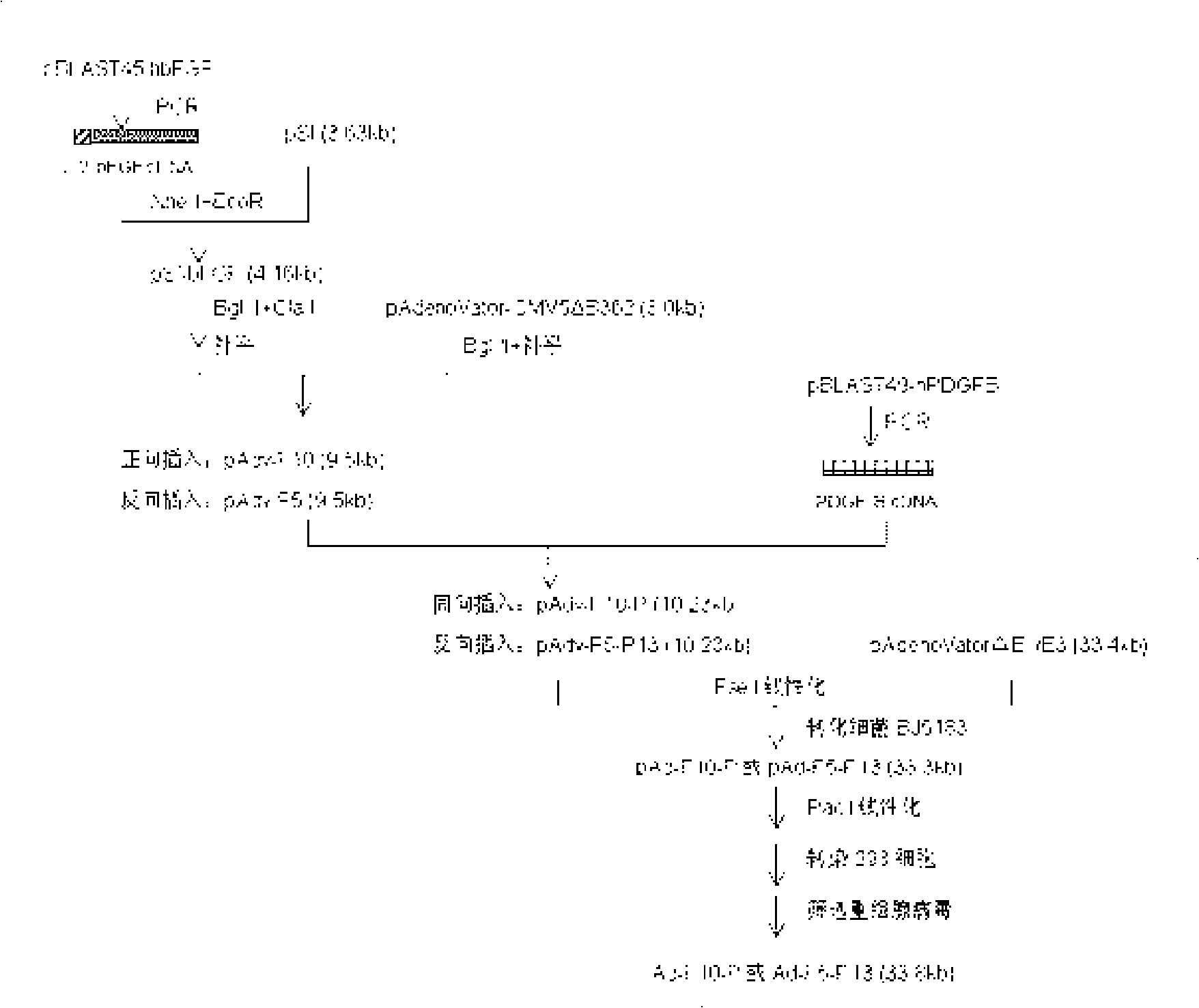
Recombined human bFGF and PDGF-B duplicate adenovirus carrier and uses thereof
ActiveCN101319229AGood treatment effectReduce manufacturing costGenetic material ingredientsFermentationDiseaseCardiac muscle
The invention belongs to the gene therapy field. The technical problem to be solved is to provide a new gene therapy product capable of effectively treating diseases of a cardiovascular system. The invention particularly relates to an expression vector containing recombinant human bFGF and PDGF-B double genes, and a preparation method and an application thereof. The vector which contains the gene capable of encoding bFGF protein and the gene capable of encoding PDGF-B protein can simultaneously express the bFGF protein and the PDGF-BB protein in a eukaryotic cell. An optimal proposal is to make the gene capable of encoding the bFGF protein and the gene capable of encoding the PDGF-B protein be expressed respectively under the control of different promoters. Experiments show that: the recombinant vector has good application prospect for treating myocardial ischemia of coronary heart disease and provides a new choice for treating the diseases of the cardiovascular system.
Owner:GUANGXI WUZHOU PHARMA GRP
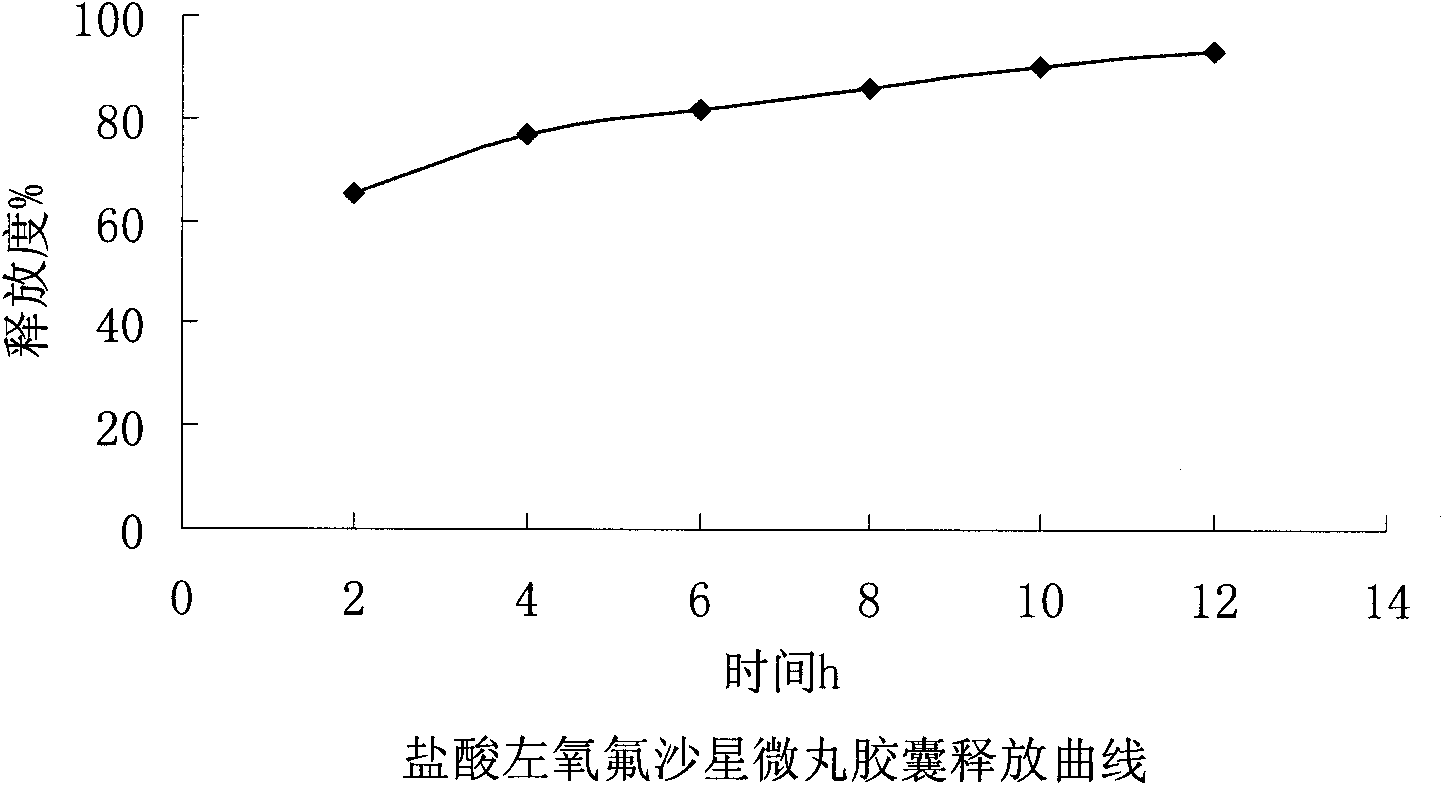
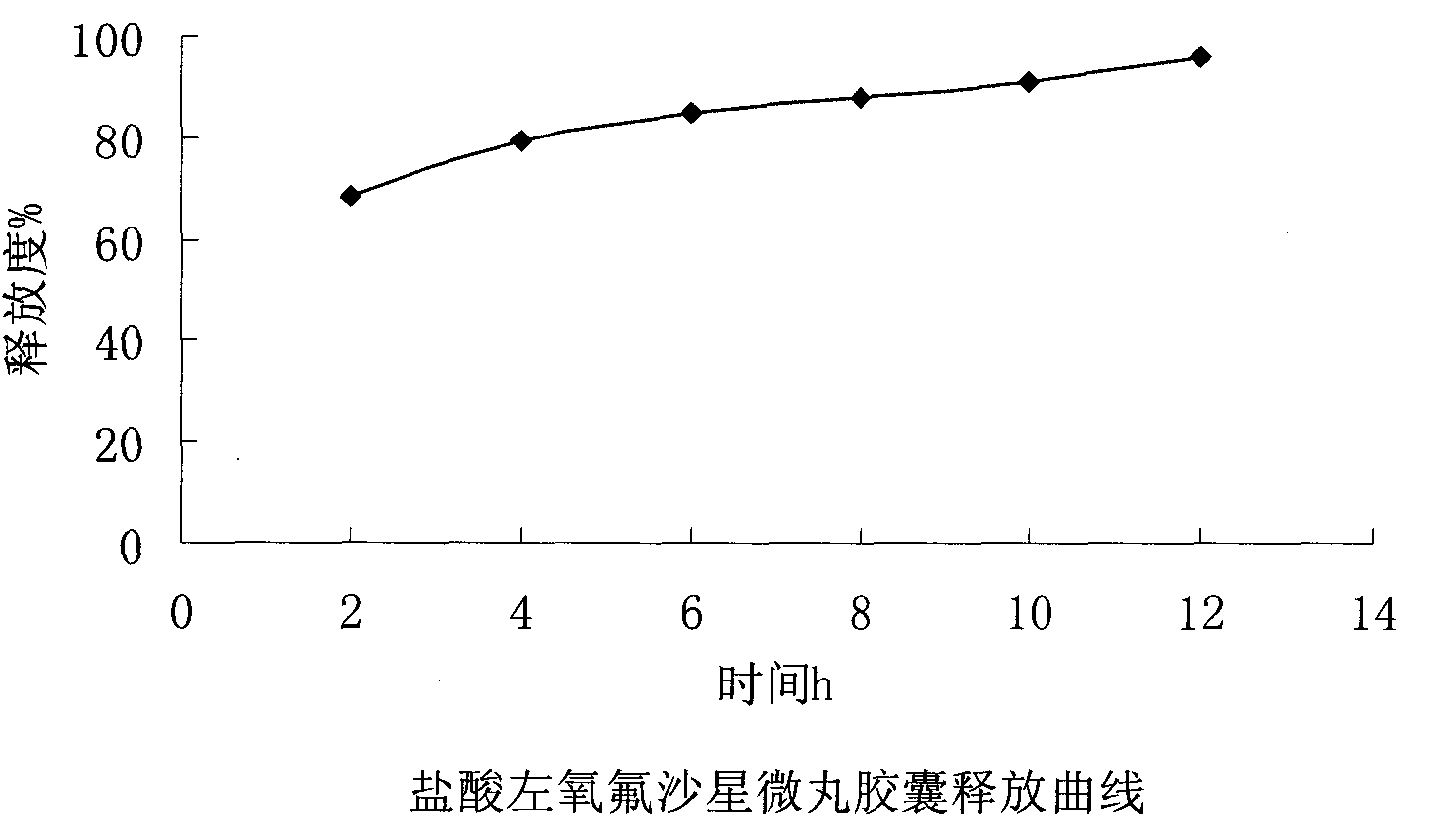
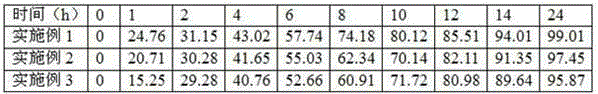
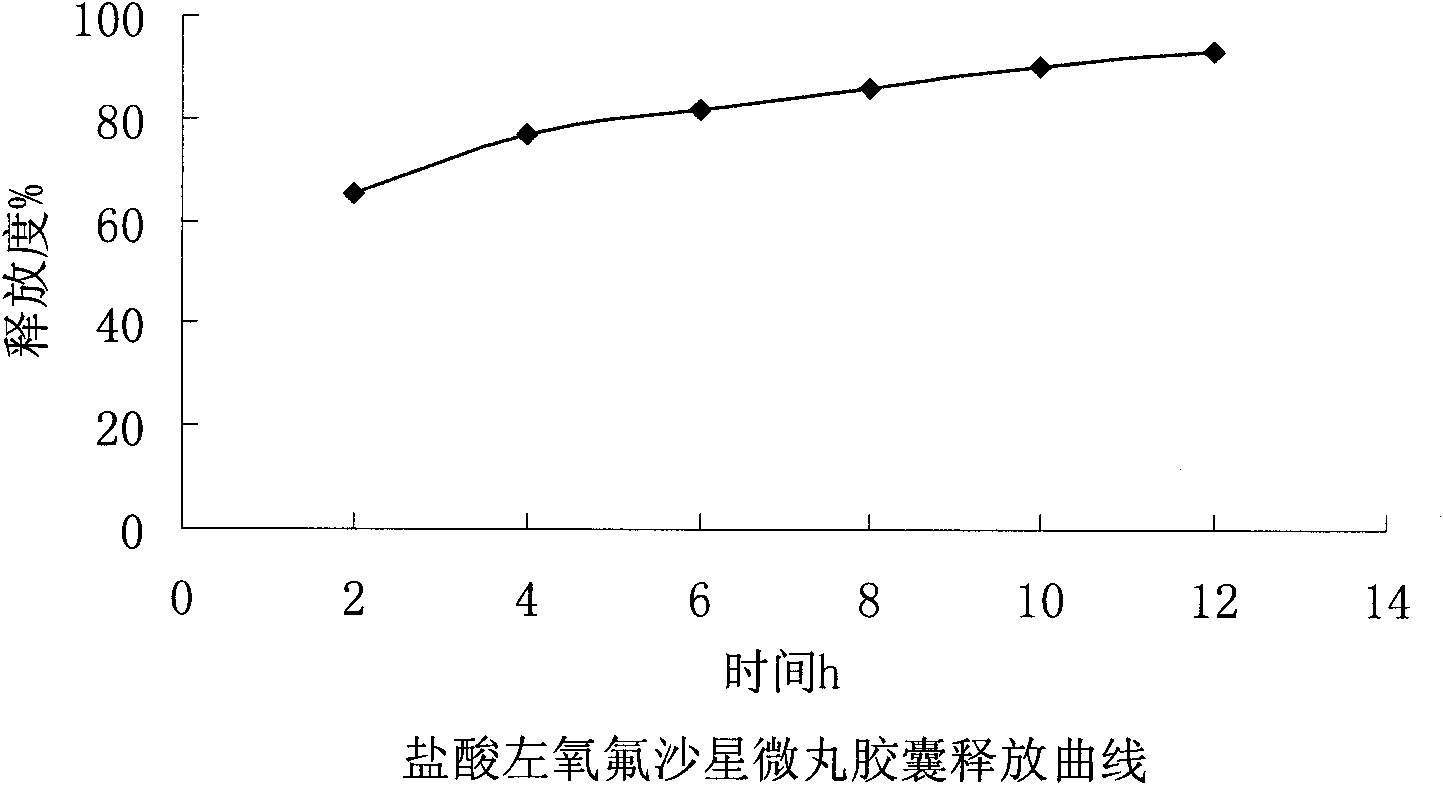
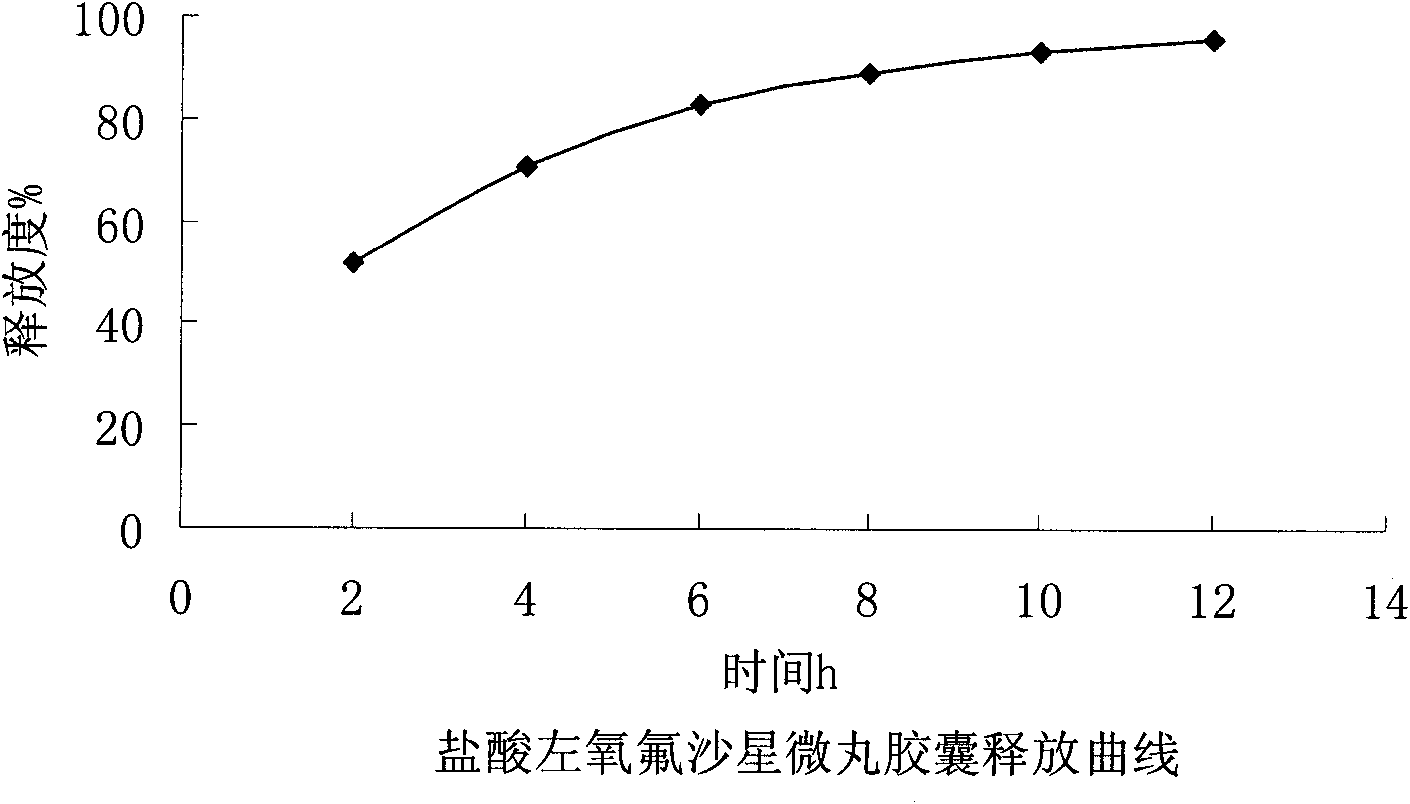
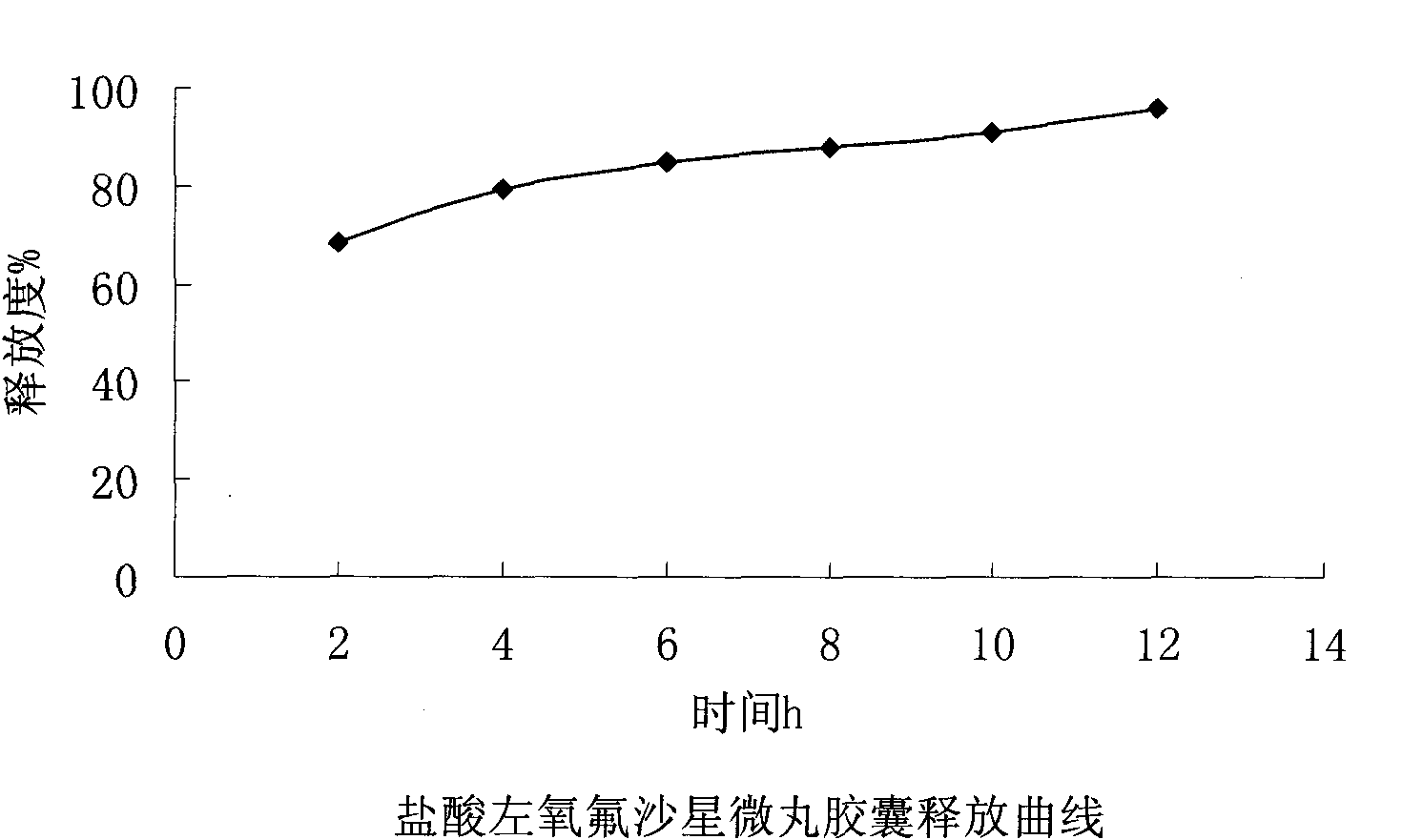
Levofloxacin hydrochloride micropill capsule and preparation method thereof
ActiveCN102106842AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsPlasticizerFluidized bed
The invention relates to a levofloxacin hydrochloride micropill capsule and a preparation method thereof. The levofloxacin hydrochloride micropill capsule comprises a pill core, a medicament-containing layer, a sustained-release coating layer and a quick-release layer, wherein levofloxacin hydrochloride is contained in the medicament-containing layer and the quick-release layer; and the sustained-release coating layer comprises the following materials in percentage by weight: 20 to 60 percent of levofloxacin hydrochloride, 30 to 55 percent of pill core, 10 to 25 percent of binding agent, 3 to 5 percent of sustained-release coating material, 0.3 to 3 percent of pore-forming agent and 0.1 to 1 percent of plasticizer. The levofloxacin hydrochloride micropill capsule has the advantages of high stability, small local stimulation of medicaments, high bioavailability and the like. Due to the adoption of a fluidized bed, the problems of large dust and low yield in a method of powder agglomerating in the background technology are solved.
Owner:HAINAN PULIN PHARMA +1
Slow-release tablet comprising succinate frovatriptan
ActiveCN104800184AMaintain effective plasma concentrationLow frequency of useOrganic active ingredientsNervous disorderBlood concentrationPolyethylene glycol
The invention relates to a slow-release tablet comprising succinate frovatriptan. The slow-release tablet comprises a tablet core and a coating, wherein the tablet core adopts a matrix tablet, and comprises succinate frovatriptan, hydroxypropyl methylcellulose, pregelatinized starch, magnesium stearate, talcum powder, and a 95% ethanol solution; the coating comprises a slow-release coating material, polyethylene glycol and succinate frovatriptan. The slow-release tablet has the advantages that the administration number of times is reduced, the compliance of a patient is improved, and the effective blood concentration is kept for a relatively long time.
Owner:CP PHARMA QINGDAO CO LTD
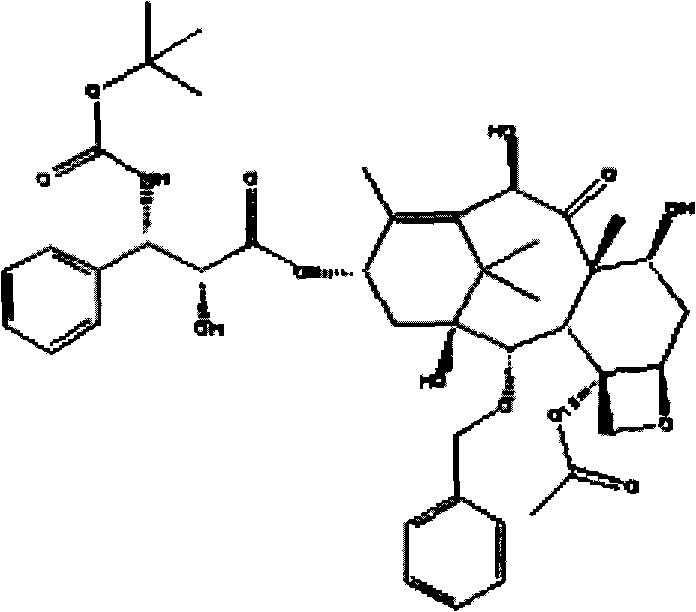
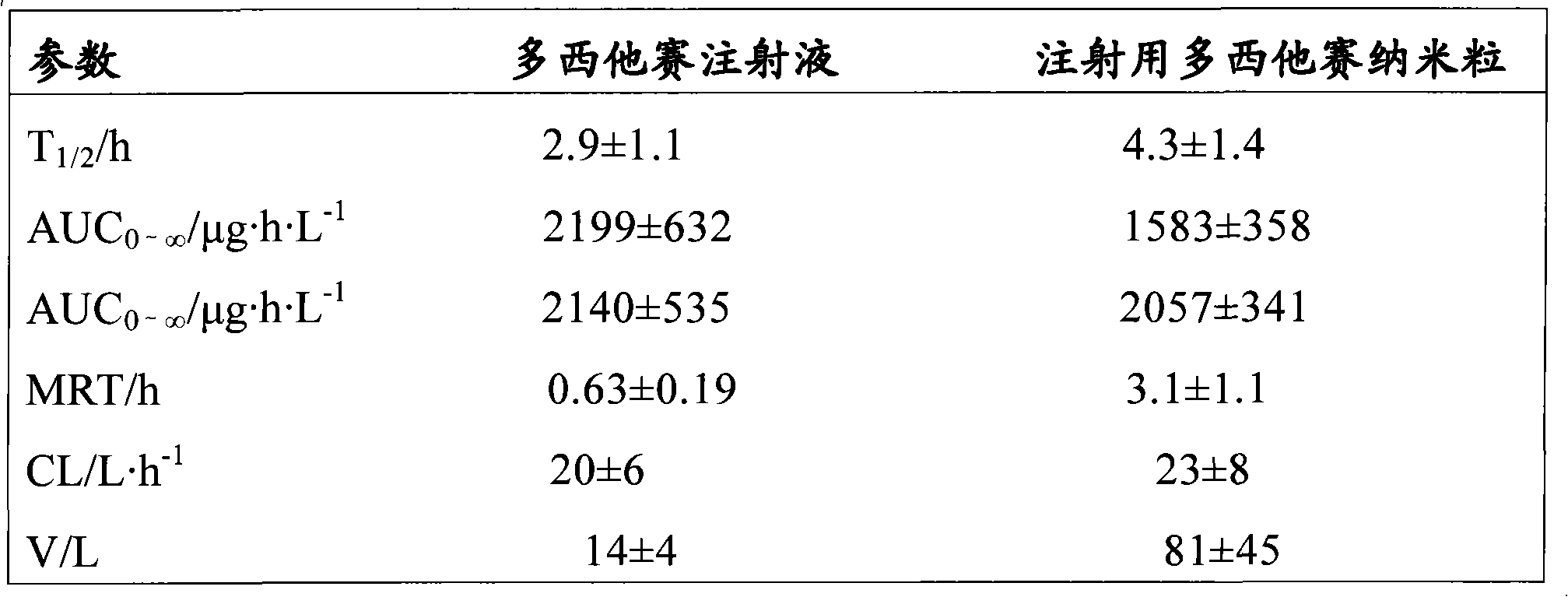
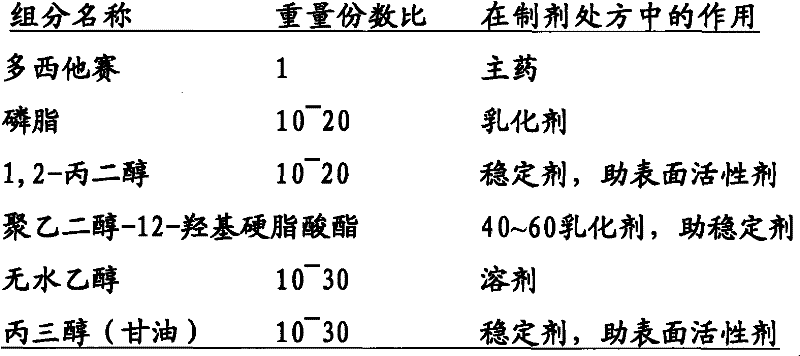
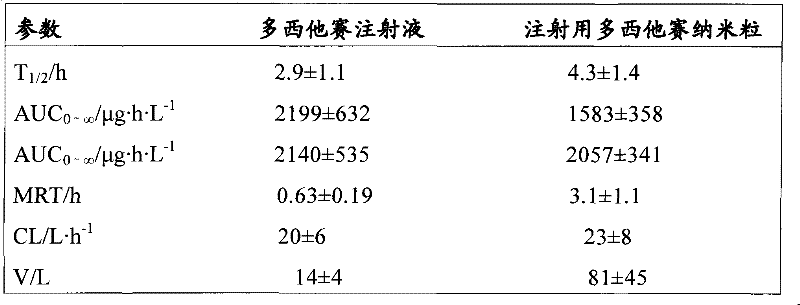
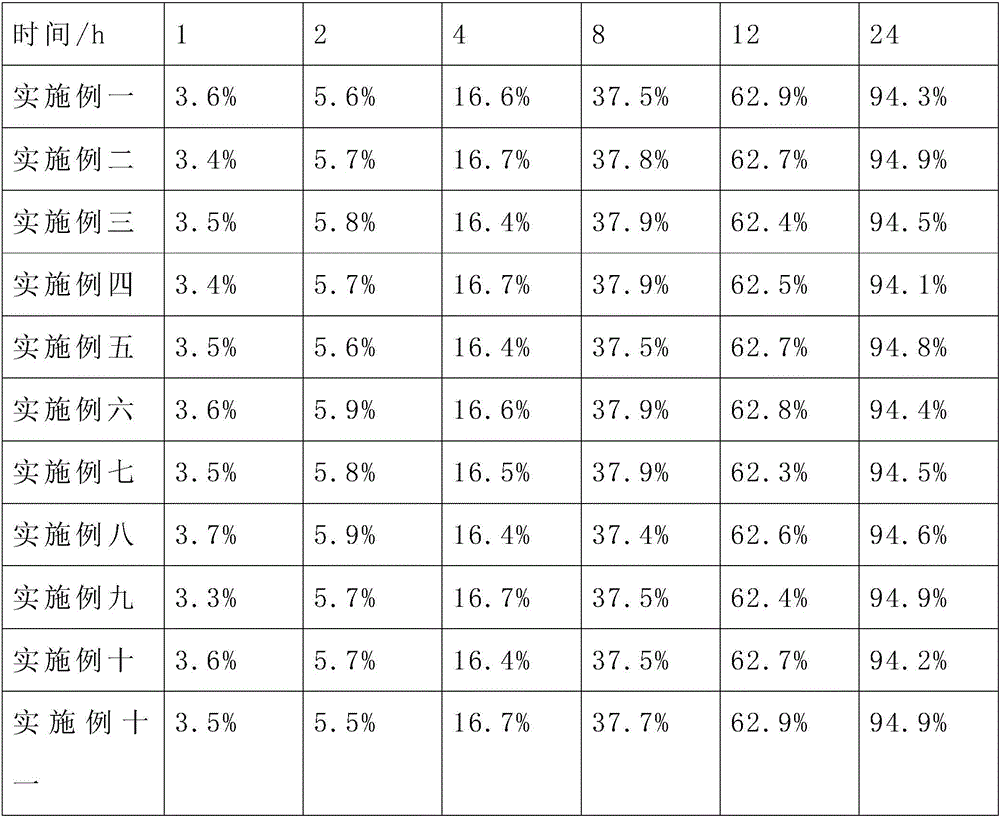
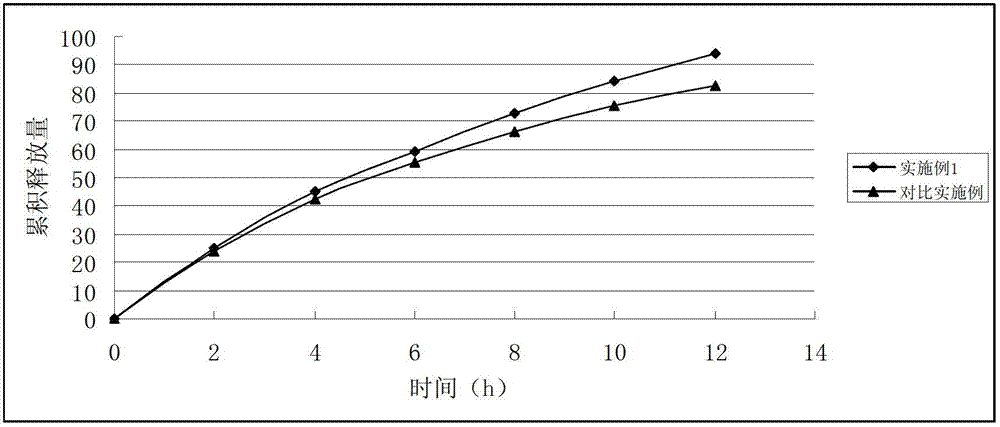
Docetaxel nanometer lipid injection, preparation method and purpose thereof
ActiveCN101548947AImprove bioavailabilityPromote modernizationOrganic active ingredientsSolution deliverySide effectDocetaxel
The present invention relates to a docetaxel nanometer lipid injection. The invention also relates to a method for preparing the docetaxel nanometer lipid injection. The invention further relates to the application of the docetaxel nanometer lipid injection for preparing medicament for treating a tumor. The nanometer preparation of the invention is capable of prolonging the medicament action time, conveying the medicament with a target, reducing the administration dose under the precondition of ensuring the medicament action, alleviating or avoiding medicament toxin and side effects, improving the medicament action stability, and being benefited for the medicament storage, thus it is capable of implementing the development of the medicament which has advantages in the aspects of a high yield, automatization, large scale, low cost, convenient carry and storage, small dose and low side-effect.
Owner:HAIKOU PHARMA FACTORY +1
Amoxicillin sustained release solid medicinal composition and preparation method thereof
ActiveCN102397262ANo significant difference in releaseMaintain effective plasma concentrationAntibacterial agentsCapsule deliveryHydrophilic coatingMedicine
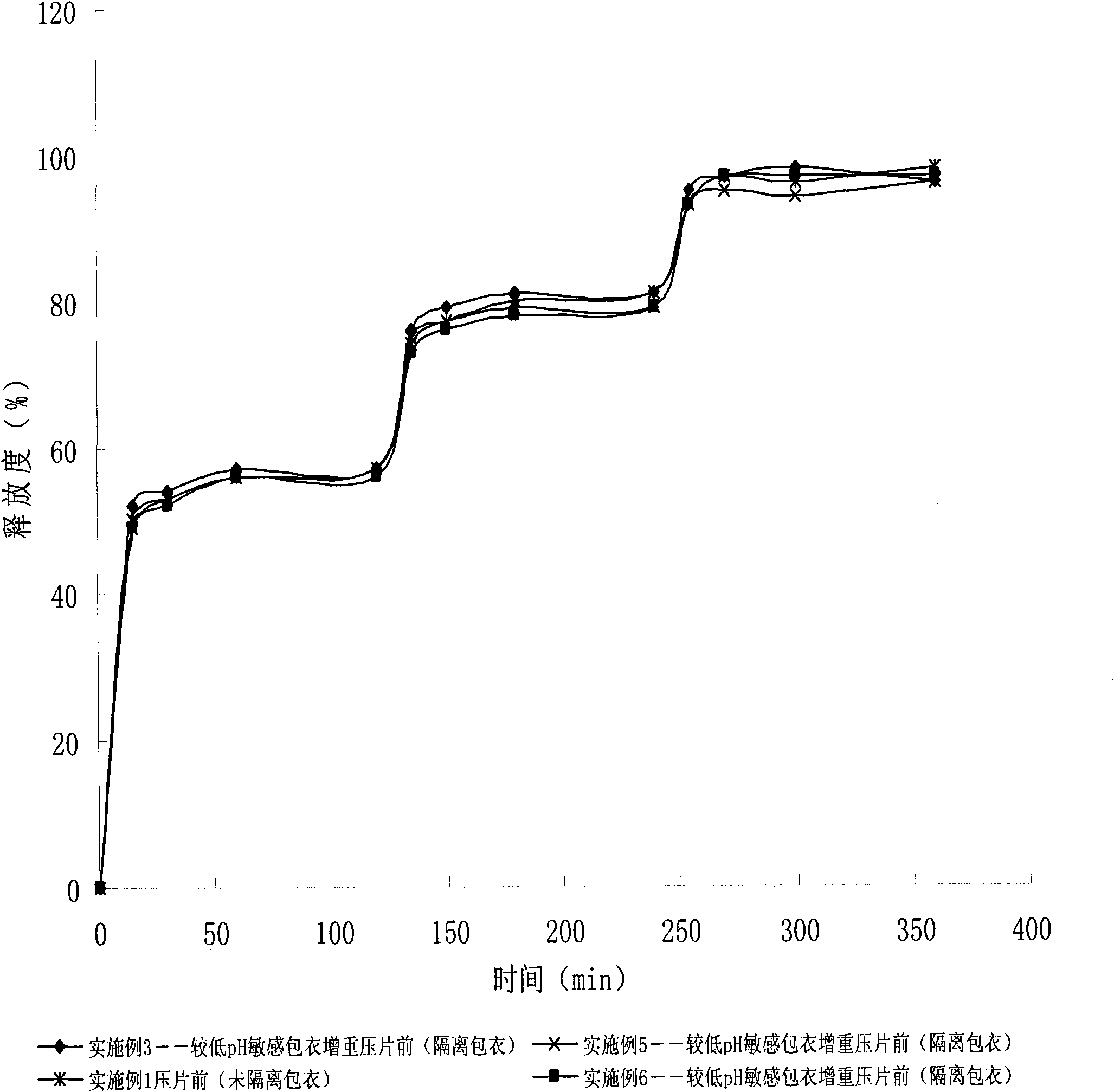
The invention relates to an amoxicillin sustained release solid medicinal composition and a preparation method thereof. The composition which comprises a fast release unit containing amoxicillin, a first delayed release unit containing amoxicillin, and a second delayed release unit containing amoxicillin is characterized in that: the two delayed release units are a micropill or a particle which comprises a pill core containing amoxicillin, an inner pH-sensitive coating material which is coated on the inner layer of the pill core, and an outer hydrophilic coating material which is coated on the outer layer of the pill core, wherein pH-sensitive coating materials of the first delayed release unit and the second delayed release unit are different; the pH of the pH-sensitive release point of the coating materials of the first delayed release unit is equal to or more than 5.0; and the pH of the pH-sensitive release point of the coating materials of the second delayed release unit is equal to or more than 6.0. The composition allows the release degree of the tableted amoxicillin pH-sensitive coating micropill in the initial release stage to be improved.
Owner:CHONGQING PHARMA RES INST
Double layer laminating sustained release tablet of metronidazole and its preparation method
InactiveCN1593404AExtended release timeEffective inhibitory concentrationOrganic active ingredientsAntimycoticsMass ratioLubricant
Disclosed is a metronidazole double layer laminated slow release tablet comprising two layers, the first layer being a slow release layer consisting of metronidazole, slow release frame, binding agent and lubricating agent, the second layer being a quick release layer consisting of metronidazole, bulking agent, crumbling agent, binding agent and lubricating agent, wherein the mass ratio of metronidazole content in the slow release layer and in the quick release layer is 1 : 0.36-2.75. The invention also discloses the process for preparing the tablet.
Owner:南京亿华药业有限公司
Compound sustained-release preparation and preparation method thereof
InactiveCN101780054AMaintain blood levelsMaintain effective plasma concentrationOrganic active ingredientsUrinary disorderBlood concentrationActive component
The invention relates to a compound sustained-release preparation and a preparation method thereof, and the compound sustained-release preparation comprises Epristeride, terazosin hydrochloride and pharmaceutically acceptable auxiliary materials, wherein the Epristeride and the terazosin hydrochloride are used as the active components, the Epristeride is prepared into a sustained-release part, and the terazosin hydrochloride is prepared into an ordinary-release part, so that the two medicines are kept in an effective blood concentration range in vivo, the adverse effect of the medicines and the administration frequency are reduced, and the safety and effectiveness of pharmacy and the compliance of patients are improved.
Owner:JIANGSU LIANHUAN PHARMA
Pentoxifylline tablet controlled relasease tablet
InactiveCN1444943AMaintain blood levelsMaintain effective plasma concentrationOrganic active ingredientsNervous disorderDiseaseCellulose acetate
A slow-releasing torental table for treating peripheral obliteration vasculitis, sequelae of arteriosclerosis, and local ischemia is prepared from torental (20-70 wt.%), slow releasing agent (cellulose acetate and / or ethyl cellulose) (30-80 wt.%), and other assistants. Its advantages are high curative effect, durable action and low by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Fentanyl double-layer buccal tablet and preparation method thereof
InactiveCN103340833BConsider pain severitySustainability is fully consideredOrganic active ingredientsNervous disorderCyclodextrinDrug release
The invention discloses a fentanyl double-layer buccal tablet and a preparation method thereof. The buccal tablet is composed by a quick release layer (an outer layer) and a sustained release layer (an inner layer) which form a biphasic drug release system. The quick release layer is prepared by effervescence and cyclodextrin inclusion technologies, a transient change of a pH value happens around the tablet by utilizing an effervescence reaction, and the quick release layer rapidly disintegrates and releases fentanyl in a few minutes; the sustained release layer slowly releases drugs to maintain an effective plasma concentration for up to a few hours. The buccal tablet releases the drugs from fast to slow, maintains the effective plasma concentration for a long time, is easy to use, is safe and reliable, and has the characteristics of rapid and continuous analgesia.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Sustained-release tablet containing isradipine
InactiveCN104208035AMaintain blood levelsGuaranteed efficacyOrganic active ingredientsPharmaceutical delivery mechanismDrugAdministration time
The invention discloses a sustained-release tablet containing isradipine. The sustained-release tablet containing the isradipine is mainly prepared from the following raw materials in percentage by mass: 1-10% of isradipine, 20-60% of a framework material, 30-70% of a filler, 2-5% of an adhesive and 0.5-3% of a lubricant. The sustained-release tablet containing the isradipine can be used for treating hypertension. The sustained-release tablet containing the isradipine has the effects of sustained release of medicines, lasting medicine effect and stable blood concentration, has the advantages of reducing medicine administration times, improving medication compliance of patients, reducing adverse effects and improving the safety of medicine administration, and is more suitable for the patients; meanwhile, a preparation method of the sustained-release tablet containing the isradipine is simple in process and favorable for industrial mass production.
Owner:SUZHOU UNIV
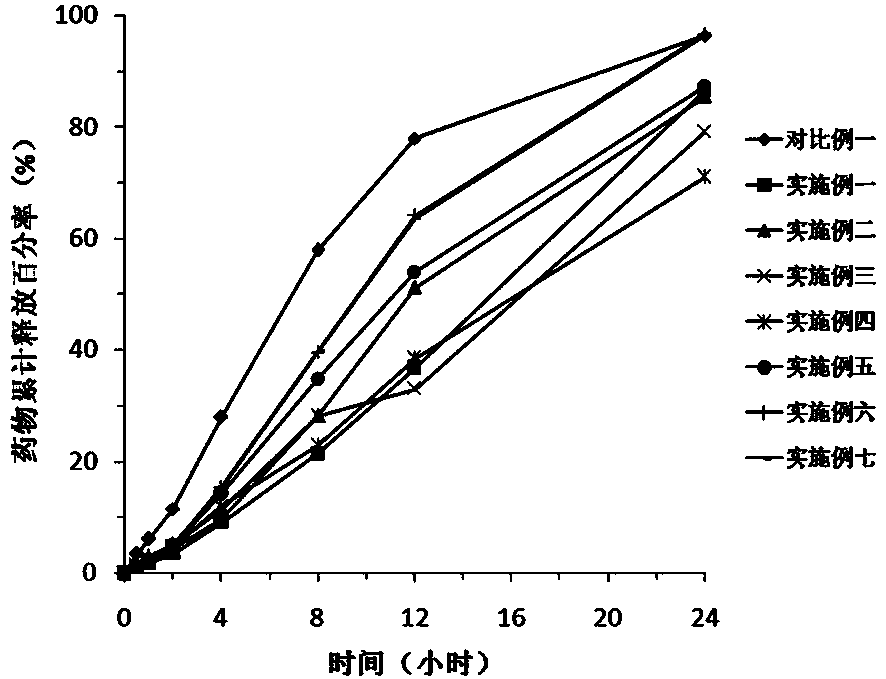
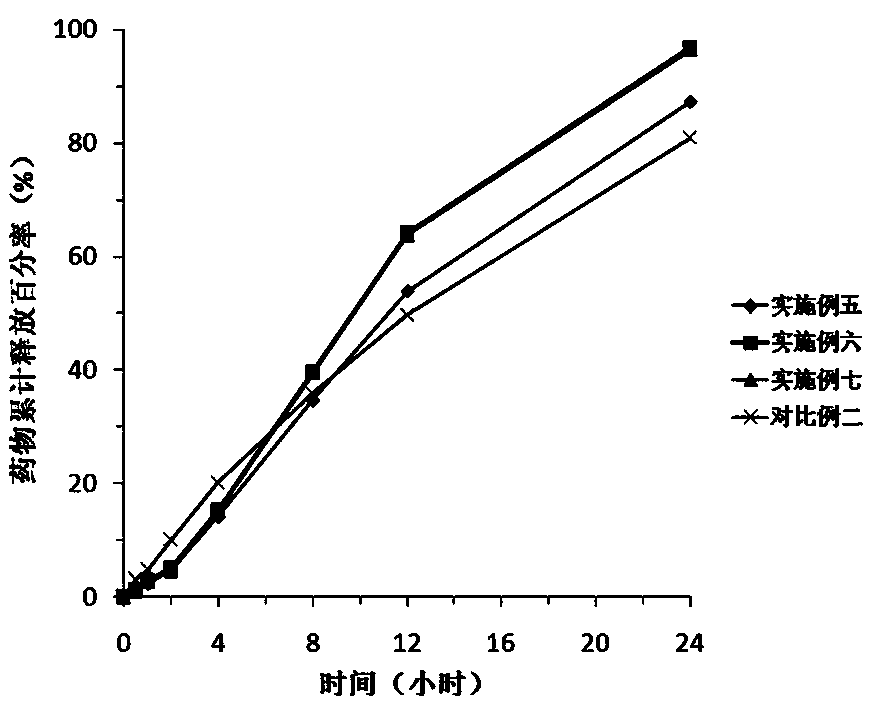
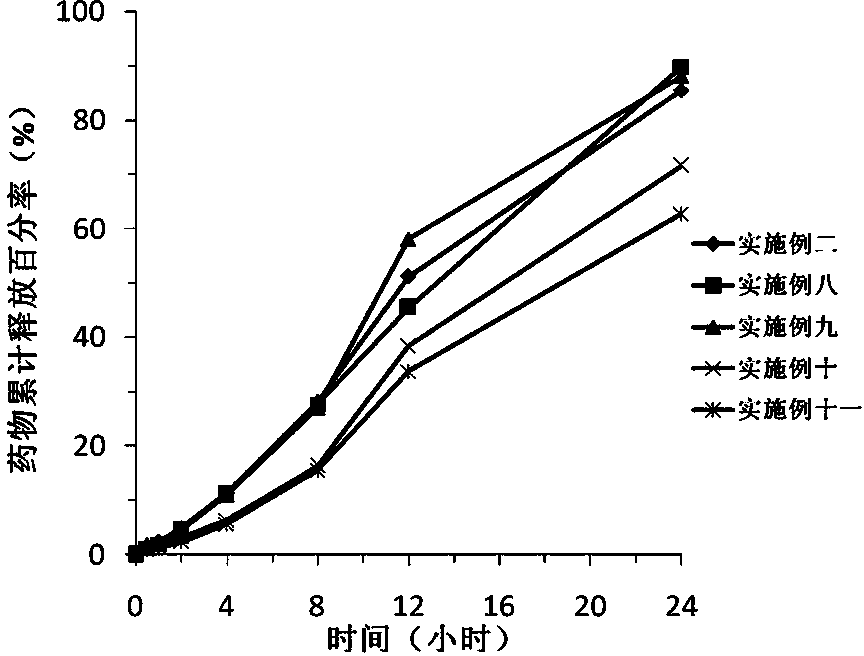
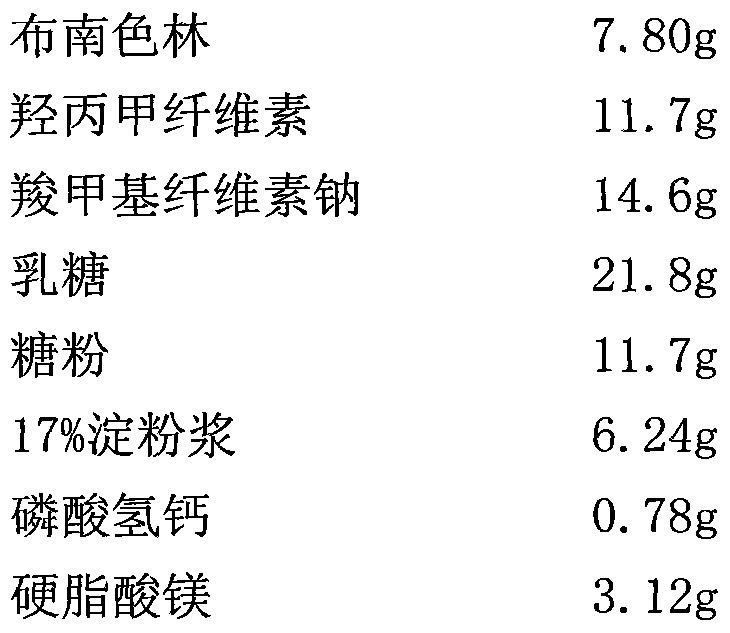
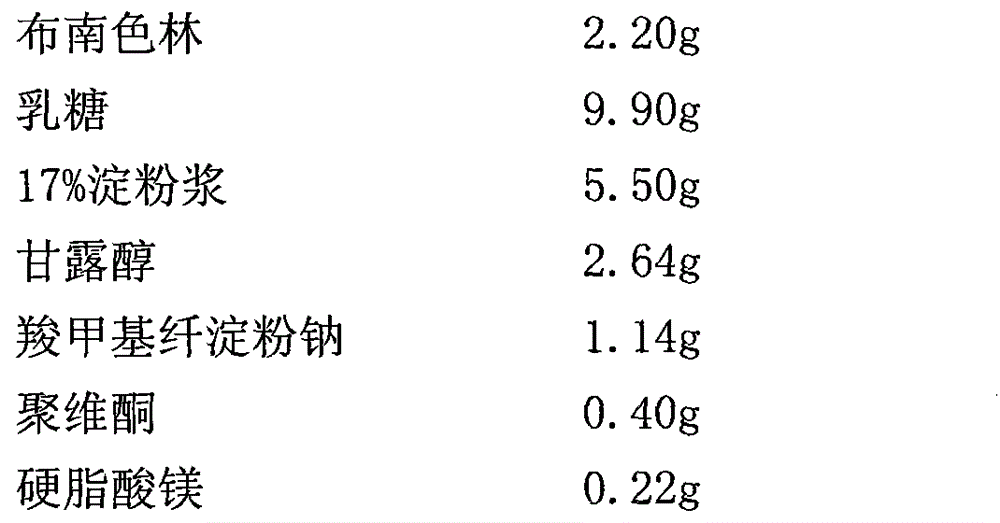
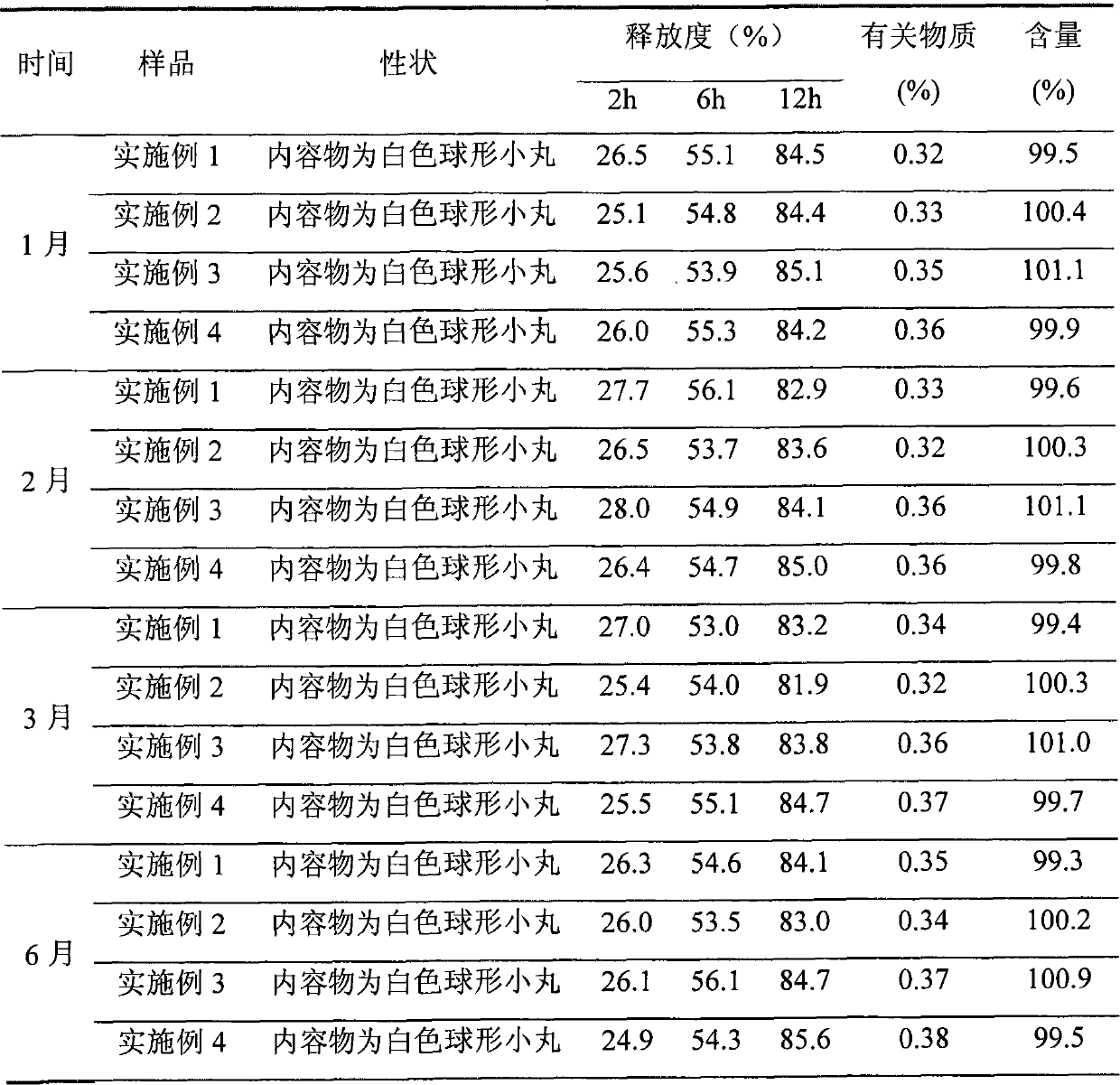
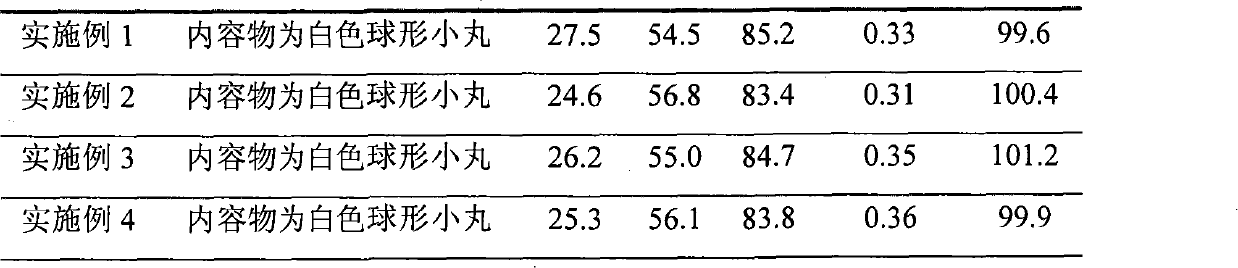
Sustained release preparation of blonanserin and preparation method thereof
ActiveCN104306346AReduce the frequency of takingEasy to takeOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationBlonanserin
The invention relates to a sustained release preparation of blonanserin and a preparation method of the sustained release preparation of the blonanserin. A blonanserin tablet is prepared by mixing slow release particles prepared by slow release components and common components according to the prescription proportion; the mass ratio of the slow release components to the common components ranges from 75:25 to 80:20; the slow release components of the blonanserin tablet mainly comprise, by mass, 8.00%-12.0% of the blonanserin, 15.0%-20.0% of hydroxypropyl methylcellulose, 15.0%-25.0% of sodium carboxymethylcellulose and the like; the common components mainly comprise, by mass, 8.00%-12.0% of the blonanserin, 30.0%-50.0% of lactose and the like. By means of the tablet prepared from the sustained release preparation, the number of medicine taking can be decreased, the stable blood concentration can be maintained, and side effects can be reduced.
Owner:LIVZON PHARM GRP INC
Moxifloxacin hydrochloride medicine composition as well as preparation method and application thereof
ActiveCN109045034AReduce direct stimulationLittle side effectsAntibacterial agentsOrganic active ingredientsDrugIrritation
The invention relates to the technical field of medicines and provides a moxifloxacin hydrochloride medicine composition as well as a preparation method and application thereof. The moxifloxacin hydrochloride medicine composition comprises the following components in parts by weight: 40-50 parts of moxifloxacin hydrochloride, 4-5 parts of pregelatinized starch, 10-15 parts of microcrystalline cellulose, 3-4 parts of croscarmellose sodium and 0.3-1 part of magnesium stearate. The preparation method comprises the following steps: uniformly mixing the moxifloxacin hydrochloride, the pregelatinized starch, the microcrystalline cellulose and the croscarmellose sodium, adding water for pelletizing, further adding the magnesium stearate, and uniformly mixing, thereby obtaining capsules or tablets. According to the embodiment of the invention, contents of components are limited, medicines are slowly released in bodies; direct irritation of the moxifloxacin hydrochloride to gastrointestinal tracts can be alleviated; side effects, particularly serious gastrointestinal tract reactions, can be reduced; the compliance and the security of medicine taking can be improved for patients; patients can tolerate the composition; long-term treatment can be facilitated.
Owner:HARBIN ZHENBAO PHARMA +1
Osmotic pump type controlled release preparation of tolterodine
InactiveCN1827092AMaintain blood levelsMaintain effective plasma concentrationOrganic active ingredientsPill deliveryDiseaseSide effect
This invention supplies a Totelode osmosis pump type controlled release formulation, whose constitutes weight percentages are: Totelode 0.5-10.0 percent, findings for controlling release 80.0-99.0 percent and other findings. The type of this invention is osmosis pump troche. Findings for controlling release are separated to controlled release film material and controlled release osmotic pressure active compounds; the other findings contain bond, lubricant and thin film materials that usually used in troche preparation. This Totelode osmosis pump troche can keep effective medicine concentration for a day, advance curative effect and decrease side effect, and its release assumes zeroth-order characteristic in certain period. It is convenient to eat and taking, and it can cut down medicine-taking times. It can used broadly to cure urination frequent, urination urgent, precipitant urine incontinence, and so on caused by bladder pubovesical muscle excess excitation.
Owner:ZHEJIANG UNIV
Piclofenac potassium sustained release capsule and preparing technique thereof
InactiveCN101322695BSmall toxicityGood formulation stabilityOrganic active ingredientsAntipyreticSustained release pelletsDiclofenac Acid
The invention provides a diclofenac potassium sustained-release capsule which is anti-inflammation and analgesic drug, and a production method thereof. The diclofenac potassium sustained-release capsule is formed by preparing diclofenac potassium into sustained-release pellets and then filling the pellets into the capsule; the diclofenac potassium sustained-release pellet consists of a blank pellet core taken as mother nucleus, a main drug layer which is coated outside of the pellet core and contains diclofenac potassium and a sustained-release coating layer covered on the main drug layer. The diclofenac potassium sustained-release capsule of the invention has fine preparation stability and prominent release effect.
Owner:海南华旗药业销售有限公司
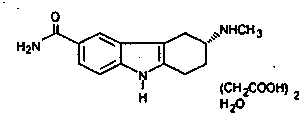
Percutaneous absorption type cerebral protective agent
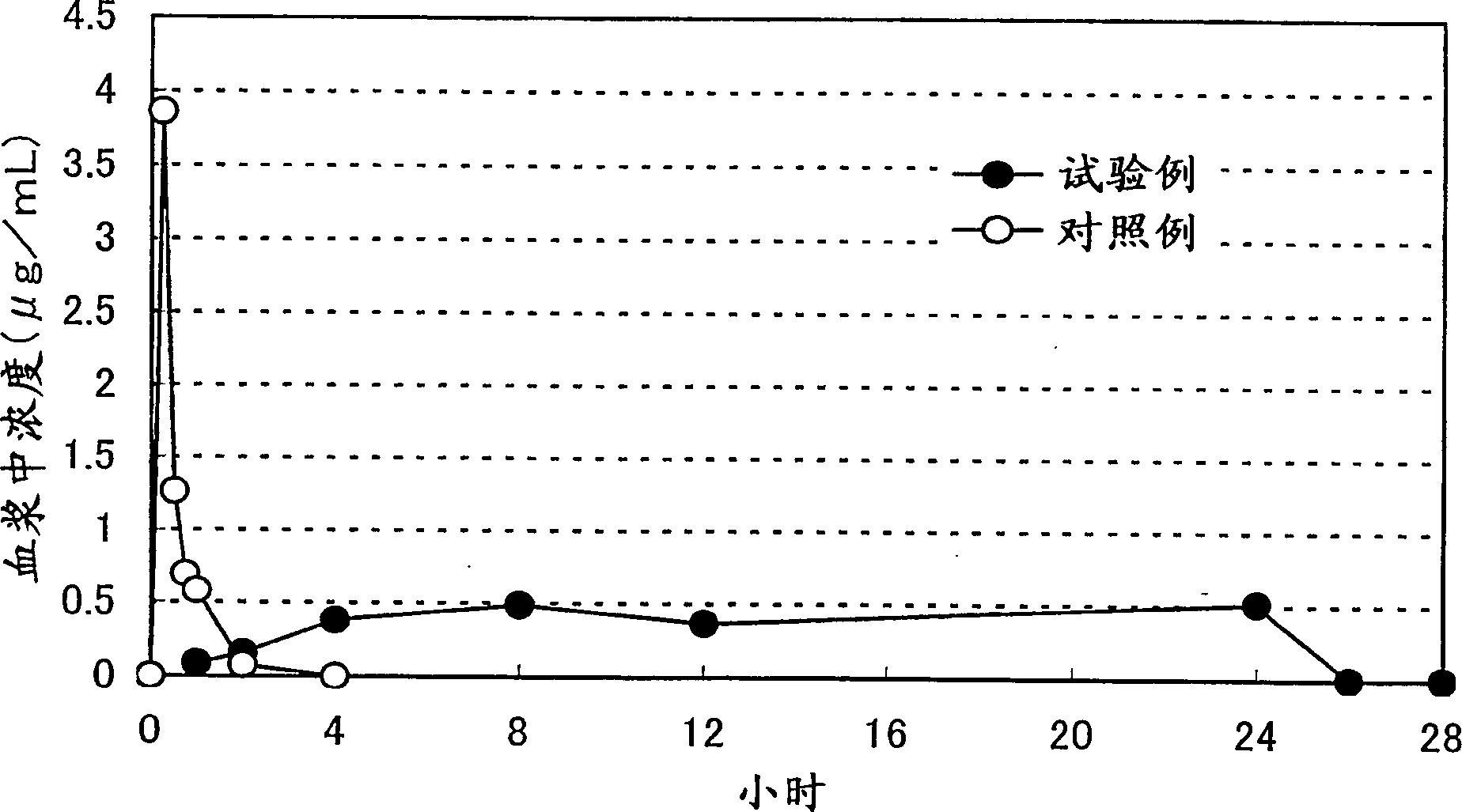
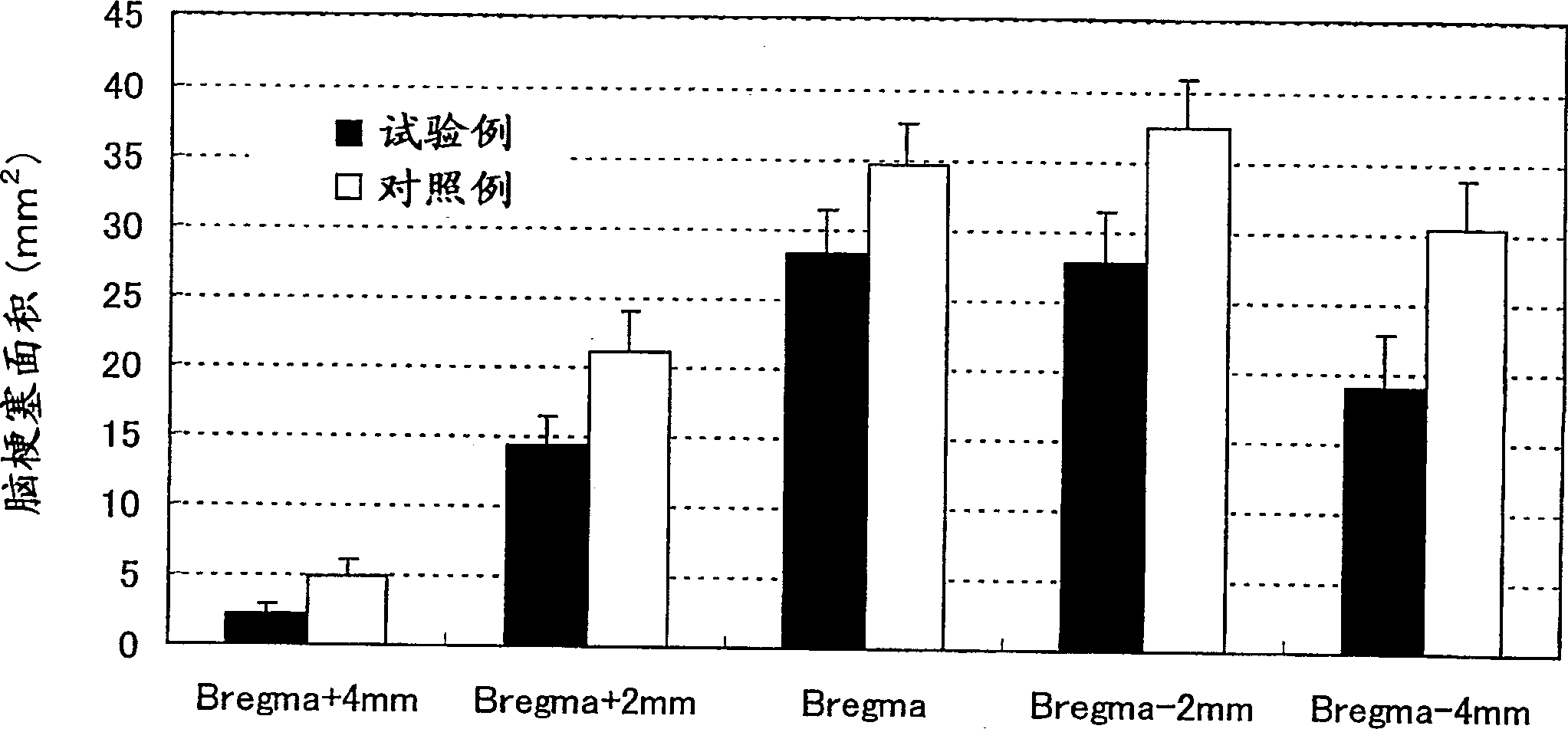
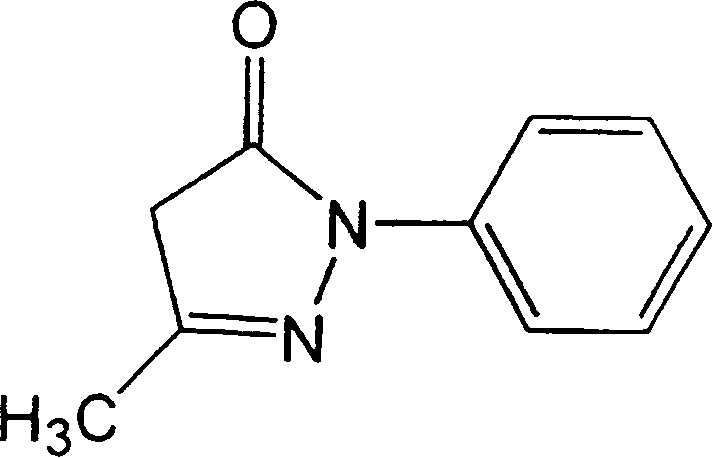
ActiveCN1878549ASuppress deathMaintain effective plasma concentrationOrganic active ingredientsNervous disorder3-methyl-1-phenyl-2-pyrazolin-5-onePercutaneous absorption
A percutaneous absorption type cerebral protective agent containing as an active ingredient, 0.1 to 30 percent by mass of 3-methyl-1-phenyl-2-pyrazolin-5-one represented by the following formula: or a medically acceptable salt thereof in a base; a use of the compound as an active ingredient for the manufacture of a percutaneous absorption type pharmaceutical composition for protecting brain; and a method of protecting brain comprising administering to a patient the pharmaceutical composition as an active ingredient.
Owner:LEAD CHEM
A kind of felodipine sustained-release tablet and its preparation process
ActiveCN104758266BRapid drug releaseStable drug releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletFast release
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
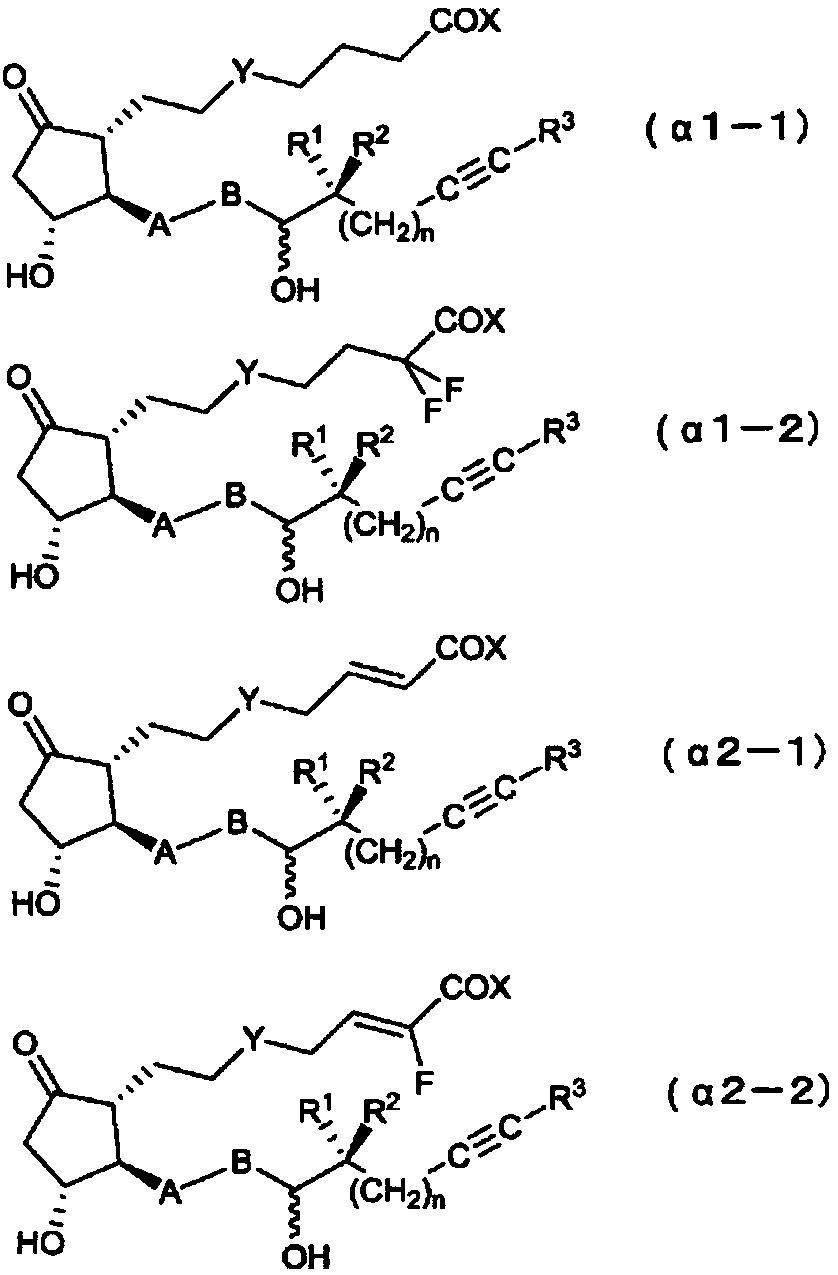
Novel prostaglandin derivative
ActiveCN109071427AEnhanced inhibitory effectExcellent blood flow increasing effectOrganic active ingredientsOligosaccharidesArterial Occlusive DiseasesBlood flow
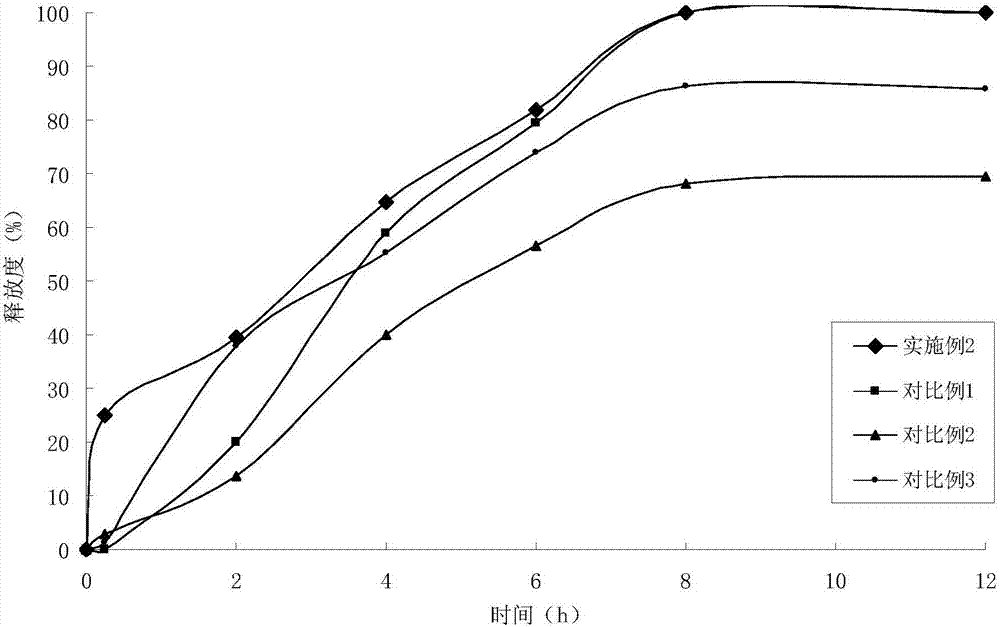
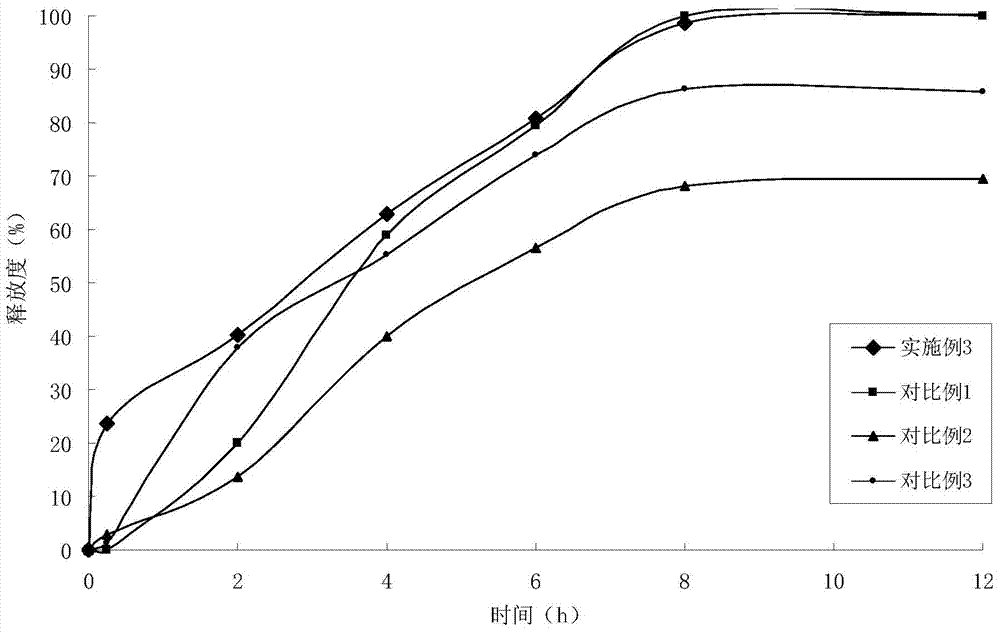
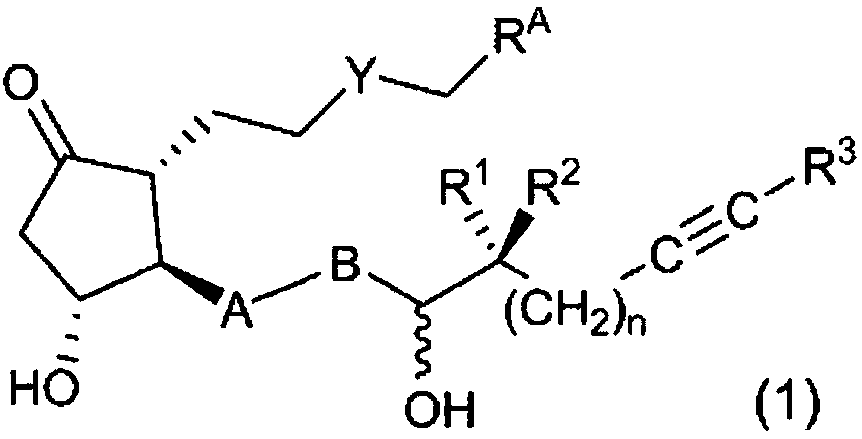
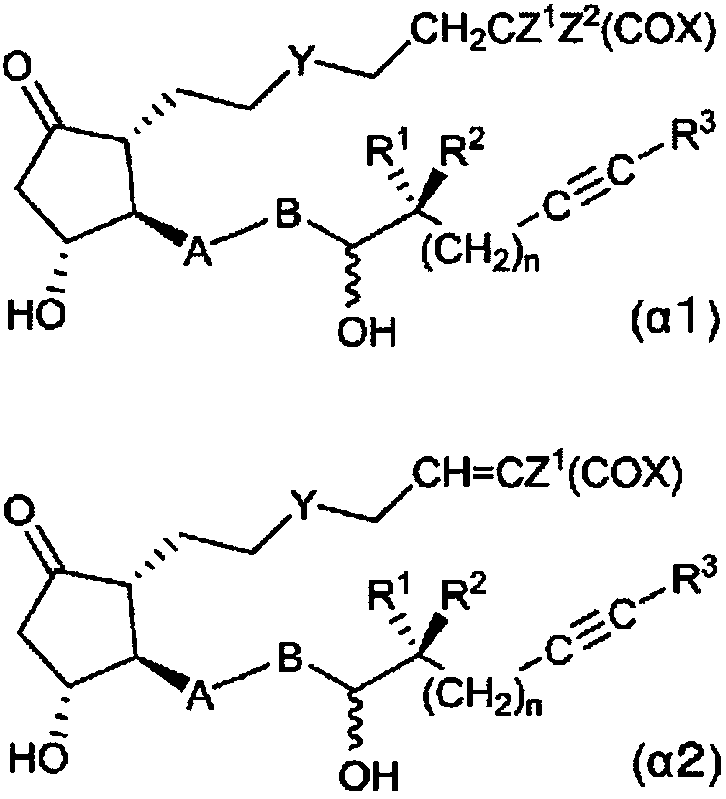
The present invention relates to: a novel prostaglandin derivative having an alkynyl group in a [omega]-chain, particularly a novel prostaglandin derivative having a double bond at position-2 and alsohaving an alkynyl group in a [omega]-chain; and a medicine containing the compound as an active ingredient. According to the present invention, it becomes possible to provide: a compound representedby formula (1) [wherein each symbol is as defined in the description], a pharmaceutically acceptable salt of the compound, or a cyclodextrin inclusion complex of the compound or the pharmaceutically acceptable salt; and a medicine which contains the compound as an active ingredient, particularly which can be used for the prevention or treatment of a blood flow disturbance associated with spinal canal stenosis or chronic arterial occlusive disease.
Owner:AGC INC +1
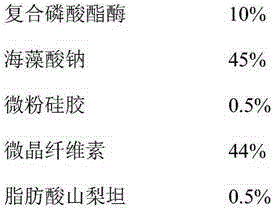
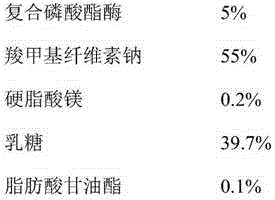
Technology for extracting phosphoesterase by ion exchange resin
InactiveCN107174656AMaintain effective plasma concentrationReduce dosePeptide/protein ingredientsDigestive systemPatient complianceIon exchange
The invention discloses a technology for extracting phosphoesterase by ion exchange resin in the field of biological pharmacy. The phosphoesterase is prepared from the following ingredients in parts by weight: 3-35% of compound phosphoesterase, 20-55% of releasing agent and the balance of other medical ingredients. The preparation method for a sustained release tablet comprises the following steps that: preparing materials, pelletizing, carrying out size stabilization and tabletting. The sustained release tablet can realize a purpose that medicines can be slowly released in vivo for a long time, blood drug concentration is stable, and fluctuation is small so as to lower drug administration frequency and improve patient compliance. Through the control of the concentration of effective drug ingredients in blood, a drug effect can be conveniently maintained, a drug releasing peak receiving frequency is reduced, and certain protection is given to the visceral organs of a patient.
Owner:RUGAO YONGXING CASING
Furotriptan Succinate Extended Release Tablets
ActiveCN104800184BMaintain effective plasma concentrationLow frequency of useOrganic active ingredientsNervous disorderBlood concentrationPolyethylene glycol
The invention relates to a slow-release tablet comprising succinate frovatriptan. The slow-release tablet comprises a tablet core and a coating, wherein the tablet core adopts a matrix tablet, and comprises succinate frovatriptan, hydroxypropyl methylcellulose, pregelatinized starch, magnesium stearate, talcum powder, and a 95% ethanol solution; the coating comprises a slow-release coating material, polyethylene glycol and succinate frovatriptan. The slow-release tablet has the advantages that the administration number of times is reduced, the compliance of a patient is improved, and the effective blood concentration is kept for a relatively long time.
Owner:CP PHARMA QINGDAO CO LTD
Patch used for angina pectoris and preparation method and use method thereof
InactiveCN106727931AFast absorptionFully absorbedUnknown materialsSheet deliveryMedicineCoronary heart disease
The invention discloses a patch used for angina pectoris and a preparation method and a use method thereof, belonging to the technical field of externally-used Chinese traditional medicine used for treating angina pectoris. The invention aims at solving the technical problems of the existing angina plaster that the acupoints need to be pasted are more, the time for pasting is short, patients are difficult to accept and the overall curing rate needs to be improved. The patch used for angina pectoris in the invention includes a non-woven fabrics layer and a medicine layer, wherein the medicine layer is mainly prepared by complex salviae miltiorrhizae drills. The preparation method of the invention comprises the following steps: grinding the complex salvia miltiorrhizae drills into fine powder, mixing with honey and binding in the annular groove. The use method of the patch in the invention is to paste the patch on the shenque acupoint. The total curing rate of the patch reaches 96.66%.
Owner:HEILONGJIANG UNIV OF CHINESE MEDICINE
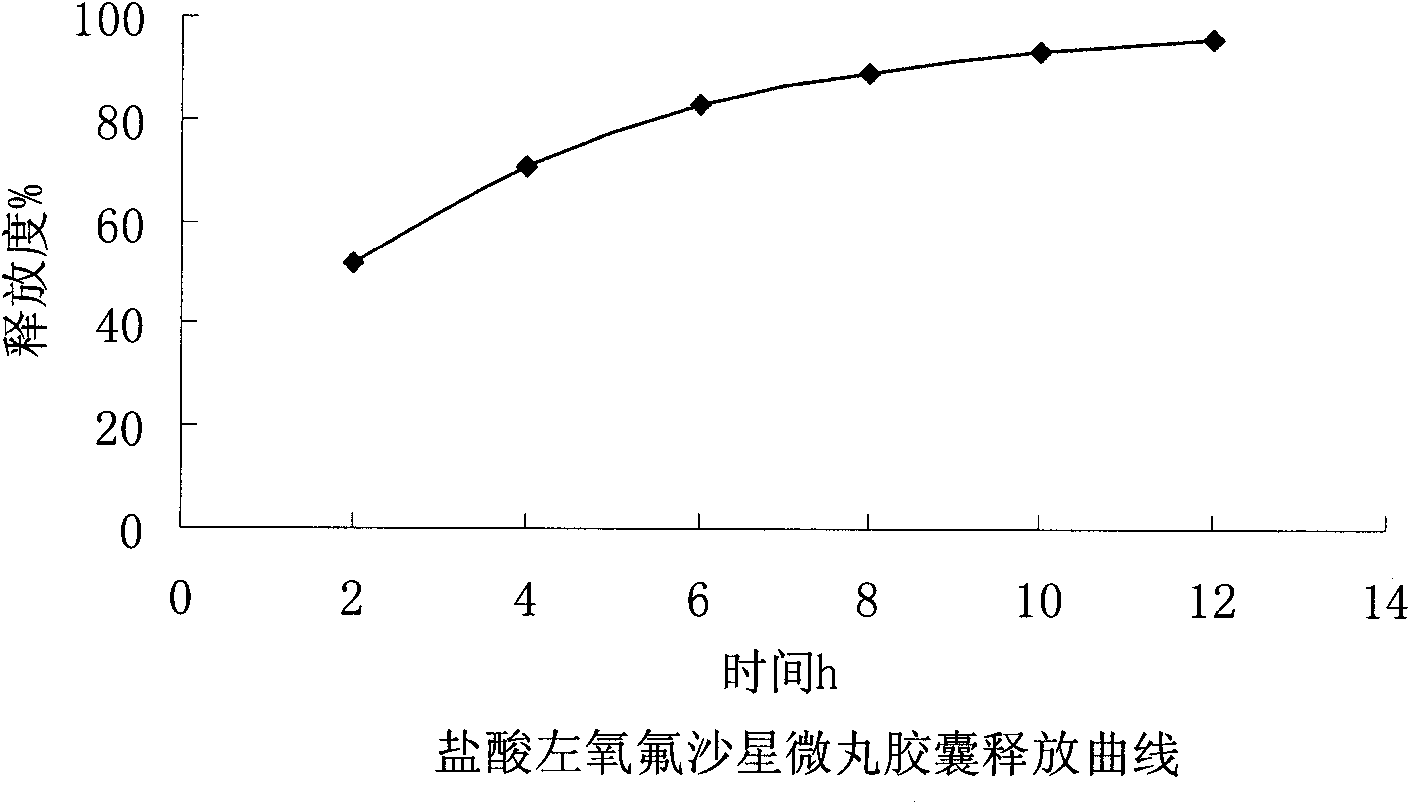
Levofloxacin hydrochloride micropill capsule and preparation method thereof
ActiveCN102106842BUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsPlasticizerFluidized bed
The invention relates to a levofloxacin hydrochloride micropill capsule and a preparation method thereof. The levofloxacin hydrochloride micropill capsule comprises a pill core, a medicament-containing layer, a sustained-release coating layer and a quick-release layer, wherein levofloxacin hydrochloride is contained in the medicament-containing layer and the quick-release layer; and the sustained-release coating layer comprises the following materials in percentage by weight: 20 to 60 percent of levofloxacin hydrochloride, 30 to 55 percent of pill core, 10 to 25 percent of binding agent, 3 to 5 percent of sustained-release coating material, 0.3 to 3 percent of pore-forming agent and 0.1 to 1 percent of plasticizer. The levofloxacin hydrochloride micropill capsule has the advantages of high stability, small local stimulation of medicaments, high bioavailability and the like. Due to the adoption of a fluidized bed, the problems of large dust and low yield in a method of powder agglomerating in the background technology are solved.
Owner:HAINAN PULIN PHARMA +1
A kind of metformin hydrochloride pharmaceutical composition and its preparation method and application
ActiveCN109044988BImprove digestive tract side effectsReduce direct stimulationOrganic active ingredientsHydrolysed protein ingredientsMetformin hclMagnesium stearate
The invention relates to the technical field of medicines and provides a metformin hydrochloride medicine composition as well as a preparation method and application thereof. The metformin hydrochloride medicine composition comprises the following components in parts by weight: 100-150 parts of metformin hydrochloride, 5-10 parts of pregelatinized starch, 5-10 parts of mannitol and 1-5 parts of magnesium stearate. The preparation method comprises the following steps: uniformly mixing the metformin hydrochloride, the pregelatinized starch and the mannitol, adding water to pelletize, further adding the magnesium stearate, and uniformly mixing, thereby obtaining capsules or tablets. According to the embodiment of the invention, contents of the metformin hydrochloride, the pregelatinized starch and the mannitol are limited, medicines are slowly released in bodies, direct irritation of the metformin hydrochloride to gastrointestinal tracts can be alleviated, side effects, particularly serious gastrointestinal tract reactions, can be reduced, an effective blood concentration can be maintained, the compliance and the security of medicine taking can be improved for patients, the patients can tolerate the composition, and long-term treatment can be facilitated.
Owner:HARBIN ZHENBAO PHARMA +1
A kind of moxifloxacin hydrochloride pharmaceutical composition and its preparation method and application
ActiveCN109045034BImprove digestive tract side effectsReduce direct stimulationAntibacterial agentsOrganic active ingredientsIrritationMoxifloxacin
Owner:HARBIN ZHENBAO PHARMA +1
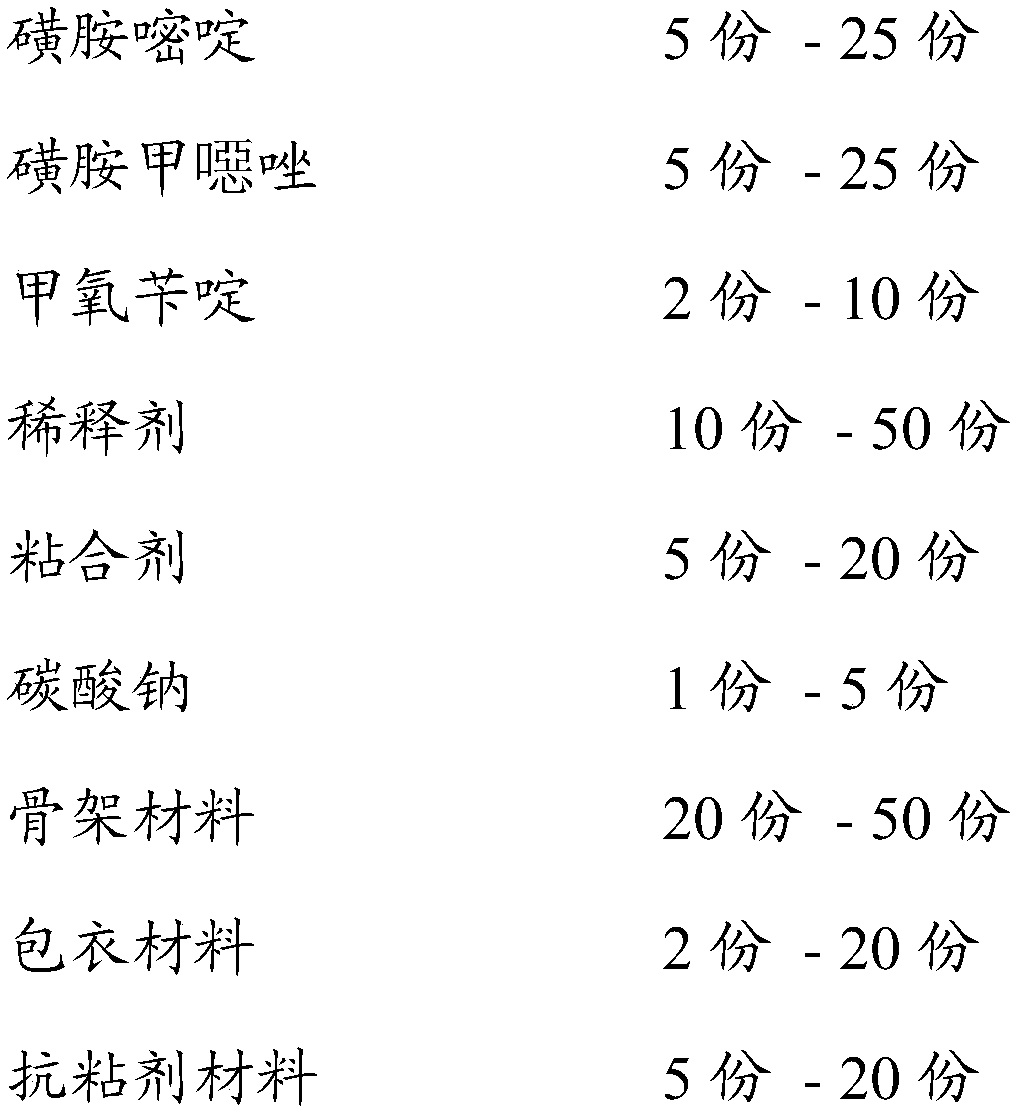
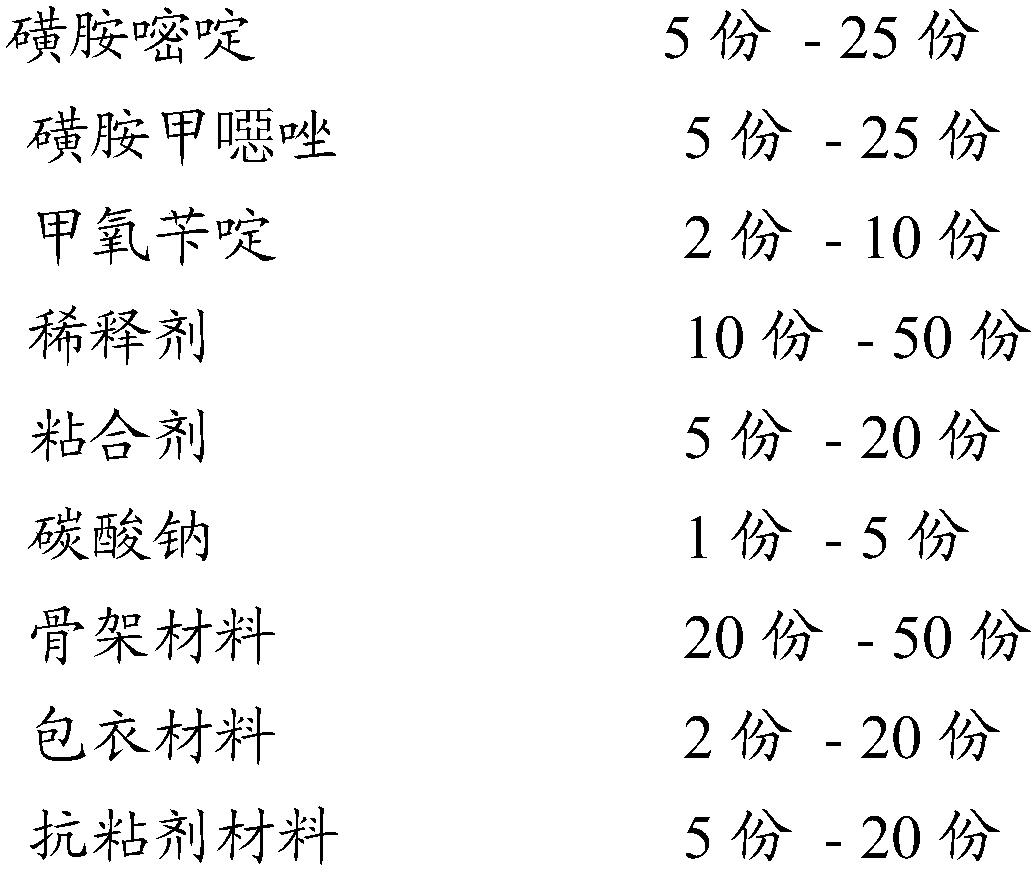
Skeleton type sulfamethoxazole sulfadiazine and trimethoprim sustained-release pellet and preparation method thereof
InactiveCN107669684ARealize one machine with multiple functionsRelieve Toxic Side EffectsAntibacterial agentsOrganic active ingredientsOral medicineSustained release pellets
The invention relates to an oral medicine sustained-release preparation and a preparation method thereof, in particular relates to a skeleton type sulfamethoxazole sulfadiazine and trimethoprim sustained-release pellet and a preparation method thereof, and belongs to the technical field of medicine. The skeleton type sulfamethoxazole sulfadiazine and trimethoprim sustained-release pellet comprisesa skeleton type sulfamethoxazole sulfadiazine and trimethoprim sustained-release pill core and a coating, wherein the sulfamethoxazole sulfadiazine and trimethoprim sustained-release pellet is prepared from sulfadiazine, sulfamethoxazole, trimethoprim, a diluent, adhesive, sodium carbonate, a skeleton material, a coating material and an anti-adhesion material according to a fluidized bed method.The sulfamethoxazole sulfadiazine and trimethoprim sustained-release pellet can fulfill the aims of reducing administration times, reducing the total dose of administration and increasing the recoveryrate through sustained release, and can optimize the clinical effect of sulfamethoxazole sulfadiazine and trimethoprim.
Owner:LUOYANG RUIHUA ANIMAL HEALTH PROD
Docetaxel nanometer lipid injection, preparation method and purpose thereof
ActiveCN101548947BImprove bioavailabilityPromote modernizationOrganic active ingredientsSolution deliverySide effectDocetaxel
The present invention relates to a docetaxel nanometer lipid injection. The invention also relates to a method for preparing the docetaxel nanometer lipid injection. The invention further relates to the application of the docetaxel nanometer lipid injection for preparing medicament for treating a tumor. The nanometer preparation of the invention is capable of prolonging the medicament action time,conveying the medicament with a target, reducing the administration dose under the precondition of ensuring the medicament action, alleviating or avoiding medicament toxin and side effects, improvingthe medicament action stability, and being benefited for the medicament storage, thus it is capable of implementing the development of the medicament which has advantages in the aspects of a high yield, automatization, large scale, low cost, convenient carry and storage, small dose and low side-effect.
Owner:HAIKOU PHARMA FACTORY +1
Preparation method of medicine for treating hypertension
InactiveCN105769798AGood storage stabilityGood sustained releaseOrganic active ingredientsPill deliveryCelluloseMicrosphere
The invention discloses a preparation method of a medicine for treating hypertension. The method comprises the following steps: firstly mixing shell powder, organic microspheres and polyvinyl alcohol to prepare a green body; sintering the green body into solid and carrying out ball-milling to form powder to obtain a support material; carrying out sealed reaction on equimolar 1,4-butanolide and 6-caprolactone in presence of an alcohol initiator and an organic zinc catalyst in a nitrogen environment; terminating the reaction with glacial acetic acid, carrying out precipitating in ice diethyl ether, filtering and vacuum-drying to obtain a carbonic ester polymer; weighing an active medicine and the carbonic ester polymer, mixing evenly, adding the support material and a hydroxy propyl cellulose alcohol solution, pelletizing through a 30-mesh sieve and drying the product at 60 DEG C for 4 hours; and granulating through a 24-mesh sieve, mixing evenly, and tabletting to obtain the medicine for treating hypertension. According to the medicine, a few of components are fused into a whole through a splicing system to form a stable system, so that release of the medicine is relatively well controlled.
Owner:张阳
Diclofenac sodium sustained-release capsule and preparation method thereof
ActiveCN102860987BSmall toxicityGood formulation stabilityOrganic active ingredientsComponent separationSustained release pelletsSustained Release Capsule
The invention provides an anti-inflammation analgesia medicine, particularly provides a diclofenac sodium sustained-release capsule and a production method thereof. The preparation method of the diclofenac sodium sustained-release capsule includes that diclofenac sodium is prepared to be a sustained-release pellet, and the sustained-release pellet is filled in the capsule. The diclofenac sodium sustained-release pellets is composed of a blank pellet core serving as a parent nucleus, a main medicine layer and a sustained-release coating layer, the main medicine layer contains the diclofenac sodium and is coated outside the pellet core, and the sustained-release coating layer is coated outside the main medicine layer. The diclofenac sodium sustained-release capsule has the advantages that the preparation stability is good, the releasing effect is remarkably excellent, and compared with common capsules, the diclofenac sodium sustained-release capsule is safe and convenient for a patient to take in.
Owner:NANJING CHANGAO PHARM CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com