Skeleton type sulfamethoxazole sulfadiazine and trimethoprim sustained-release pellet and preparation method thereof
A technology of trimethoprim and sustained-release pellets is applied in the field of medicine, which can solve the problems of reducing the number of administrations, difficult to control the administration dosage, and complicated administration, so as to alleviate the toxic and side effects and optimize the clinical treatment effect. , the effect of maintaining effective blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 The ratio of raw and auxiliary materials of trimethoprim sustained-release pellets of the present invention.
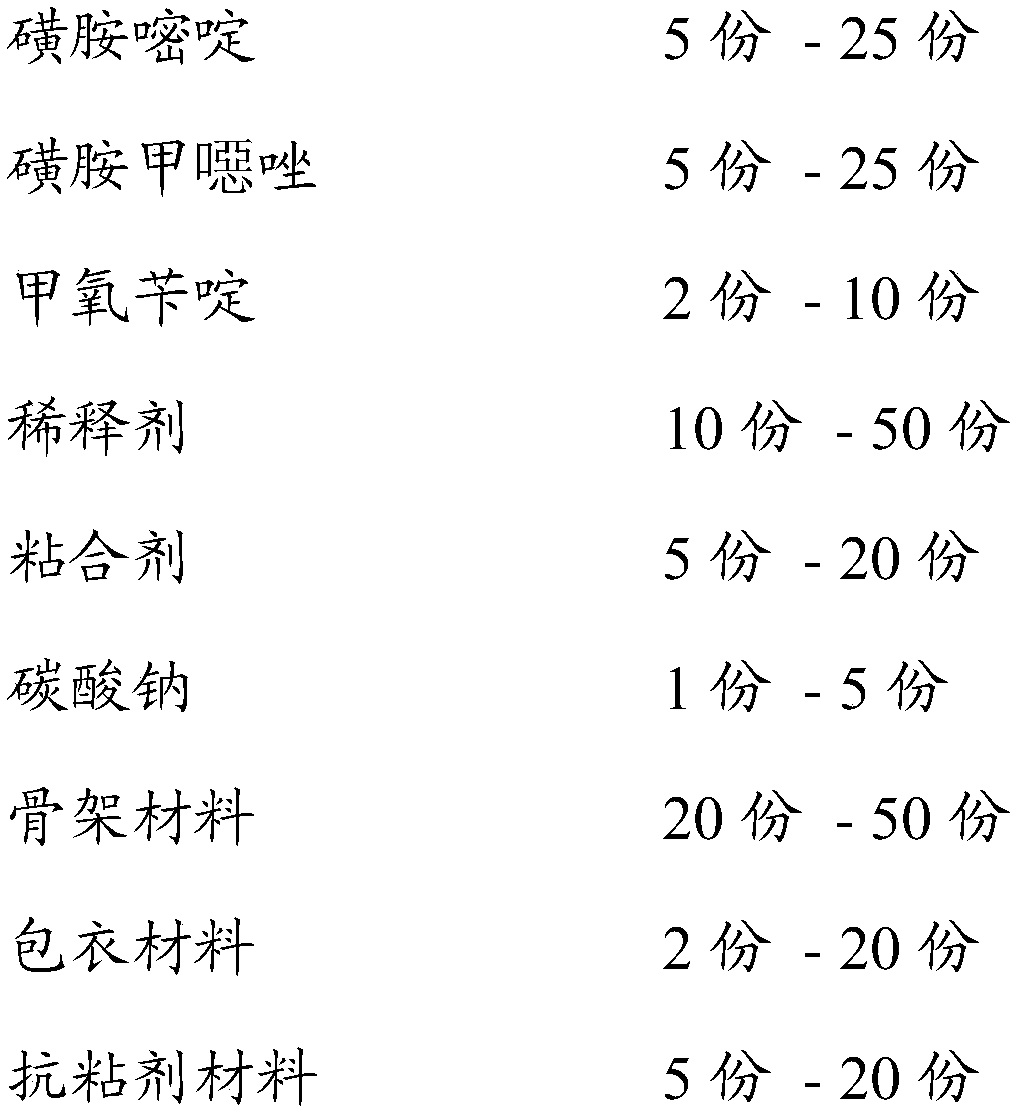
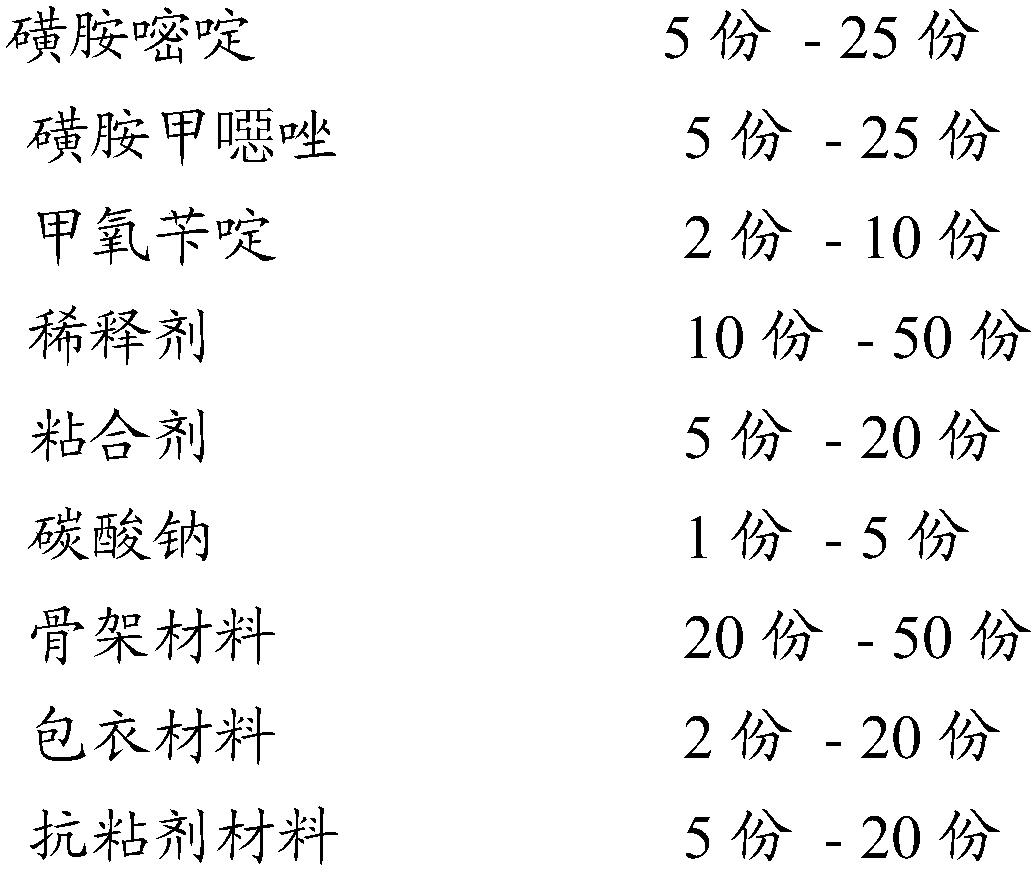
[0029] The screening formula is according to the following ratio of raw materials in parts by weight, formula 1-formula 3.
[0030]
[0031]
[0032] Take the raw materials of the formula weight parts in the above table respectively, take sulfadiazine, sulfamethoxazole, trimethoprim, sucrose, polyethylene glycol 400, put them in a pulverizer and pulverize them and pass through an 80 mesh sieve, put them in a fluidized bed Boil at medium temperature for 20 minutes to mix the raw materials evenly, take another binder and dilute it with water, and then turn on the nozzle.
[0033] Take formula 4 and sulfamethoprim premix (100g: sulfadiazine 20g + sulfamethoxazole 20g + trimethoprim 8g) commonly used in clinical practice for pharmacodynamics test, through the change of drug concentration in serum within a certain period of time Analyze whether t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com