Compound sustained-release preparation and preparation method thereof
The technology of a sustained-release preparation and a compound formula, which is applied to the compound sustained-release preparation composed of aplite and terazosin hydrochloride and the field of preparation thereof, can solve the problem that there are no compound eplite sustained-release preparations, patients and doctors have not yet seen. Inconvenience, failure to achieve treatment and other problems, to achieve obvious clinical application advantages, improve drug safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
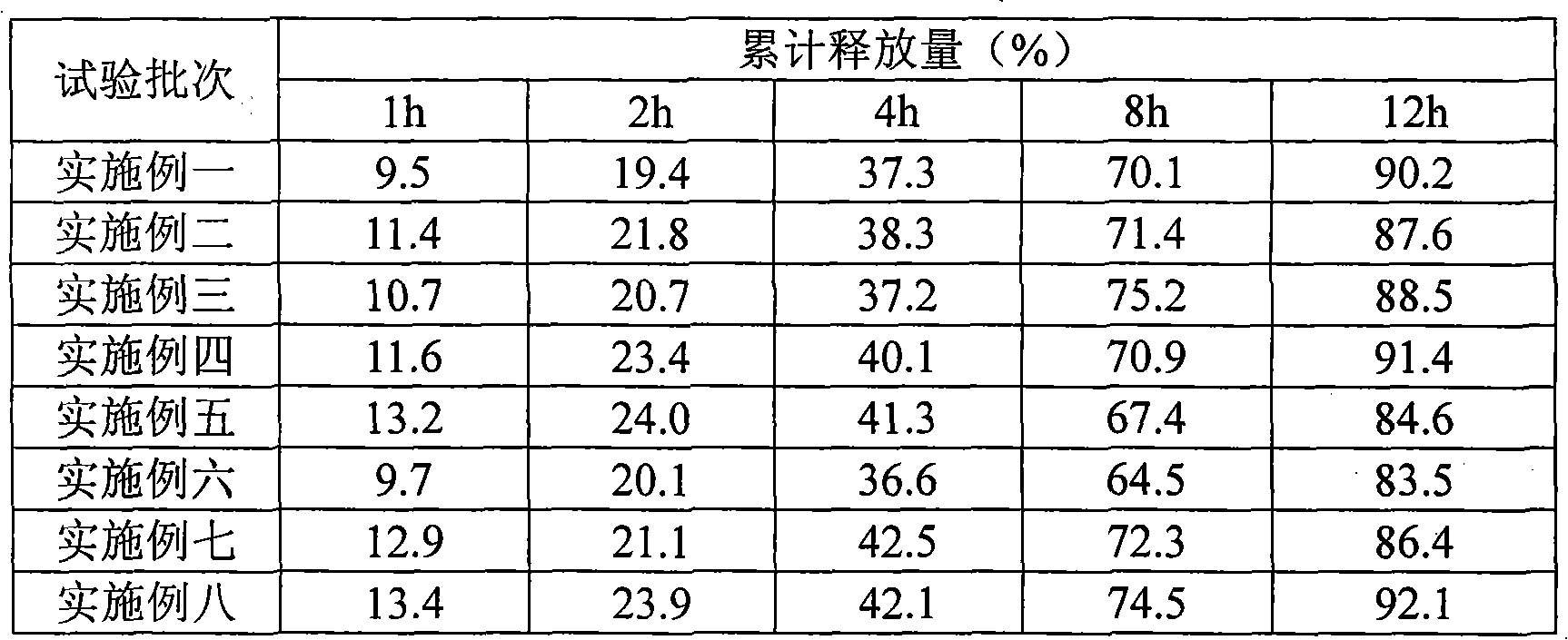
Embodiment 1
[0035] Compound Apretide Double Layer Sustained Release Tablets
[0036] Sustained release part:
[0037] Aprilite 10g
[0038] Lactose 100g
[0039] Microcrystalline Cellulose 60g
[0040] Hypromellose (K15M) 30g
[0041] Ethanol 150ml
[0042] Magnesium Stearate Appropriate amount
[0043] Regular part:
[0044] Terazosin hydrochloride 2g (calculated as terazosin)
[0045] Sodium carboxymethyl starch 2.5g
[0046] Microcrystalline Cellulose 50g
[0047] Starch 40g
[0048] Lactose 30g
[0049] Hypromellose (4% aqueous solution) 50g
[0050] Magnesium Stearate Appropriate amount
[0051] Preparation process operation is as follows:
[0052]The raw and auxiliary materials are crushed through a 100-mesh sieve, and the sustained-release part of Eprelate, lactose, microcrystalline cellulose, and hypromellose (K15M) is weighed according to the prescription. granules, dried at 50-60°C, granulated with 24 mesh sieve, added magnesium stearate and mixed evenly, and set a...
Embodiment 2
[0054] Compound Aprelate Film Coated Sustained Release Tablets
[0055] Applet chip
[0056] Aprilite 10g
[0057] Lactose 110g
[0058] Microcrystalline Cellulose 80g
[0059] Ethanol 150ml
[0060] Magnesium Stearate Appropriate amount
[0061] Coating solution containing terazosin hydrochloride
[0062] Terazosin hydrochloride 2g (calculated as terazosin)
[0063] Ethylcellulose 25g
[0064] Acrylic II 10g
[0065] Macrogol 6000 5g
[0066] Absolute ethanol 500ml
[0067] Preparation process operation is as follows:
[0068] The raw and auxiliary materials are crushed and passed through a 100-mesh sieve, and weighed according to the prescription, eprelate, lactose, and microcrystalline cellulose. After mixing, use ethanol as a soft material, granulate with a 24-mesh sieve, dry at 50-60°C, and sieve through a 24-mesh sieve. Add magnesium stearate and mix evenly, press into tablets, and set aside; weigh ethyl cellulose, acrylic resin II, and polyethylene glycol 600...
Embodiment 3
[0070] Compound Epulet Sustained-release Granules
[0071] Epulet Sustained Release Pellets
[0072] Aprilite 10g
[0073] Terazosin hydrochloride 2g (calculated as terazosin)
[0074] Cetyl Alcohol 80g
[0075] Lactose 80g
[0076] Microcrystalline Cellulose 100g
[0077] Povidone K30 20g
[0078] 50% ethanol solution appropriate amount
[0079] 2% HPMC aqueous solution appropriate amount
[0080] Magnesium Stearate Appropriate amount
[0081] Preparation process operation is as follows:
[0082] The raw and auxiliary materials are crushed through a 100-mesh sieve, weighed Apret according to the prescription, added to the molten cetyl alcohol and stirred evenly, and then added povidone K30 as a porogen and mixed evenly, cooled and solidified, crushed, and added 50g Mix microcrystalline cellulose and 50g lactose evenly, make soft material with 50% ethanol solution, granulate with 12-18 mesh sieve, dry at 50-60°C, and set aside; weigh terazosin hydrochloride according ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com