'Huilaxitan' injection, and its prepn. method
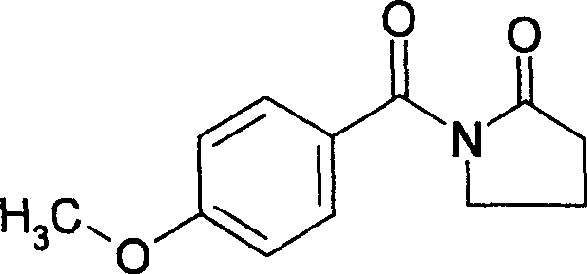
The technology of a racetam injection and aniracetam, which is applied in the field of brain function improving drugs, can solve problems such as poor water solubility, and achieve the effects of improving compliance, reducing side effects and ensuring therapeutic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Prescription: 4g of aniracetam, 100ml of propylene glycol, 40ml of ethanol, and a preparation volume of 200ml. Aniracetam API, propylene glycol, ethanol, etc. All raw materials, solvents, and additives should meet the standards for injection.
[0028]Dissolve 4g of aniracetam raw material in 100ml of propylene glycol, heat to 70-90 degrees Celsius to promote dissolution, add 100ml of 40% ethanol prepared in advance with water for injection during or after dissolution, mix well, and let stand One hour later, the preparation of the injection was completed. Add 0.001% to 0.05% activated carbon for needles according to the weight and volume ratio of the prepared liquid, mix for half an hour, coarse filter, fine filter, fill into 10ml per tube (10ml: 200mg) or fill into each tube 5ml specification (5ml: 100mg) or filled into each 1ml specification (1ml: 20mg), sealed, sterilized, leak tested, light inspected, printed, packaged, checked and put into storage, and finally anir...
Embodiment 2
[0030] Prescription: 4g of aniracetam, 100ml of propylene glycol, 40ml of ethanol, 0.2g of sodium bisulfite, 10% sodium hydroxide solution to adjust the pH to meet the requirements of the injection, and the preparation volume is 200ml. Aniracetam API, propylene glycol, ethanol, etc. All raw materials, solvents, and additives should meet the standards for injection.
[0031] Dissolve 4g of aniracetam crude drug in 100ml of propylene glycol, heat to 70-90 degrees Celsius to promote dissolution, add 100ml of 40% ethanol prepared in advance with water for injection during or after dissolution, and mix well. Add 0.2 g of sodium bisulfite, adjust the pH to meet the standard with 10% sodium hydroxide solution, mix evenly, and complete the preparation of the injection after standing for one hour. Add the prepared liquid to 0.001% to 0.05% of the weight-to-volume ratio, add activated carbon for needles and mix for half an hour, then coarsely filter, finely filter, and fill into 10ml pe...
Embodiment 3
[0033] Prescription: 4g of aniracetam, 100ml of propylene glycol, 40ml of ethanol, and a preparation volume of 200ml. Aniracetam API, propylene glycol, ethanol, etc. All raw materials, solvents, and additives should meet the standards for injection.
[0034] Dissolve 4g of aniracetam raw material in 100ml of propylene glycol, heat to 70-90 degrees Celsius to promote dissolution, add 40ml of ethanol during or after dissolution, and mix well. Add water for injection to make the volume to 200ml, mix evenly, and complete the preparation of the injection after standing for one hour. Add the prepared liquid to 0.001% to 0.05% of the weight-to-volume ratio, add activated carbon for needles and mix for half an hour, then coarsely filter, finely filter, and fill into 10ml per bottle (10ml: 200mg) or 5ml per bottle specifications (5ml: 100mg) or filled into 1ml specifications (1ml: 20mg) per vial, sealed, sterilized, leak tested, light inspected, printed, packaged, checked and put into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com