Lamotrigine orally disintegrating sustained release tablets
A technology for lamotrigine and sustained-release tablets, which is applied in the field of pharmaceutical preparations and can solve problems such as inconvenient administration and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
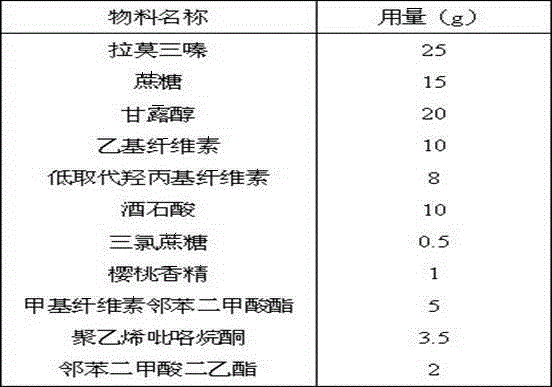
[0026] The prescription quantity is 1000 tablets, and the specification is 25mg.
[0027]
[0028] Process:
[0029] Fast-dissolving microparticles: Mix appropriate amount of crospovidone, mannitol and microcrystalline cellulose evenly, granulate, and coat with hydroxypropyl cellulose;
[0030] Organic acid core: Mix appropriate amount of fumaric acid, microcrystalline cellulose and lactose, granulate, use ethyl cellulose and cellulose acetate succinate mixture as coating barrier, coating weight gain is about 6%, and prepare Coated granules with long-lasting release of organic acids;
[0031] Lamotrigine IR beads: Layer a hydroxypropyl cellulose solution containing an appropriate amount of lamotrigine on a partially barrier-coated organic acid core, and coat an ethyl cellulose coating containing hydroxypropyl cellulose Coating liquid, to carry out sealing coating to particle;
[0032] Lamotrigine SR Beads: Ethylcellulose coating at approximately 5% weight gain ba...
Embodiment 2
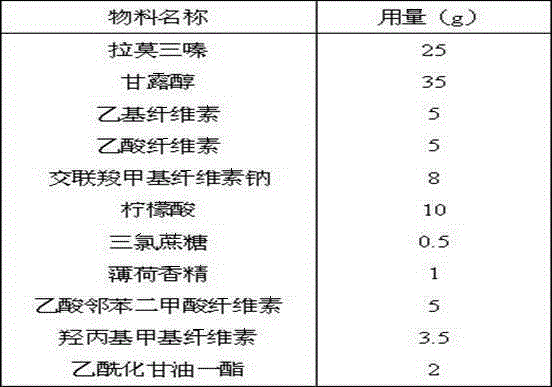
[0036] The prescription quantity is 1000 tablets, and the specification is 25mg.
[0037]
[0038] Process:
[0039] Fast-dissolving microparticles: Mix an appropriate amount of cross-linked povidone and mannitol evenly, granulate, and coat with methylcellulose;
[0040] Organic acid core: mix an appropriate amount of citric acid and mannitol, granulate, use a mixture of ethyl cellulose and cellulose acetate succinate as a coating barrier, and the weight gain of the coating is about 8%, and the preparation can release organic acids for a long time coated granules;
[0041] Lamotrigine IR beads: layer the methylcellulose solution containing an appropriate amount of lamotrigine on the organic acid core of the barrier coating, and coat the ethylcellulose coating solution containing methylcellulose , seal-coat the granules;
[0042] Lamotrigine SR Beads: Ethylcellulose coating at approximately 5% weight gain based on dry weight of IR uncoated seal-coated beads;
[004...
Embodiment 3
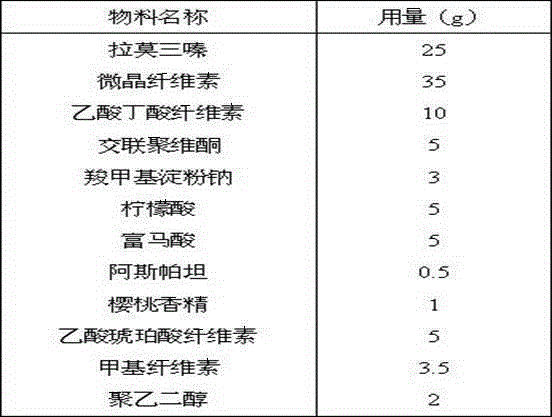
[0046] The prescription quantity is 1000 tablets, and the specification is 25mg.
[0047]
[0048] Process:
[0049] Fast-dissolving microparticles: Mix an appropriate amount of crospovidone and microcrystalline cellulose evenly, granulate, and coat with methylcellulose;
[0050] Organic acid core: Mix appropriate amount of citric acid, fumaric acid and microcrystalline cellulose, granulate, use the mixture of cellulose acetate butyrate and cellulose acetate succinate as the coating barrier, and the weight gain of the coating is about 8% , to prepare coated granules that can release organic acids for a long time;
[0051] Lamotrigine IR beads: A layer of methylcellulose solution containing an appropriate amount of lamotrigine is layered on a partially barrier-coated organic acid core and coated with a cellulose acetate butyrate coating containing methylcellulose liquid to seal coat the granules;
[0052] Lamotrigine SR beads: cellulose acetate butyrate coating at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com