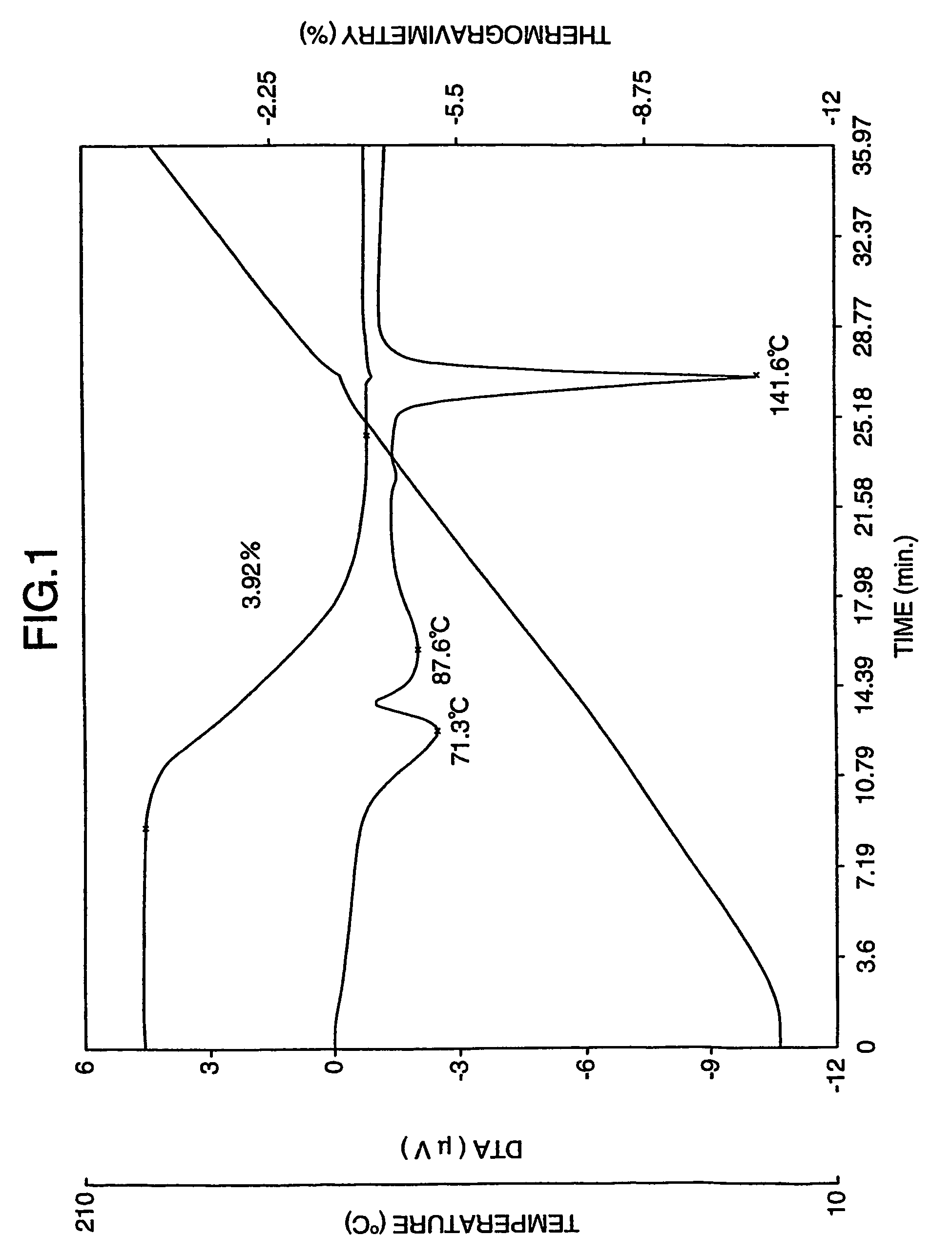
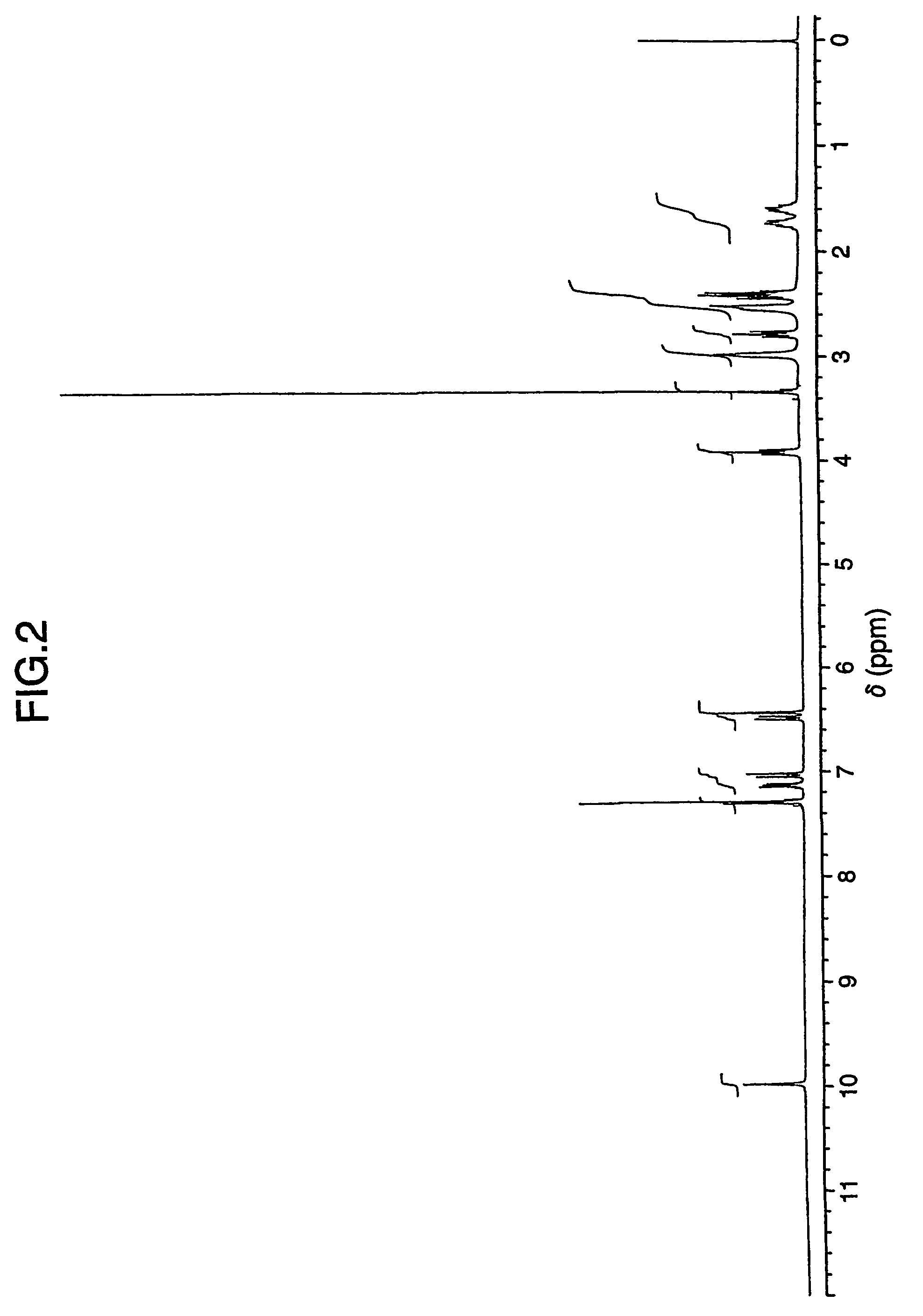
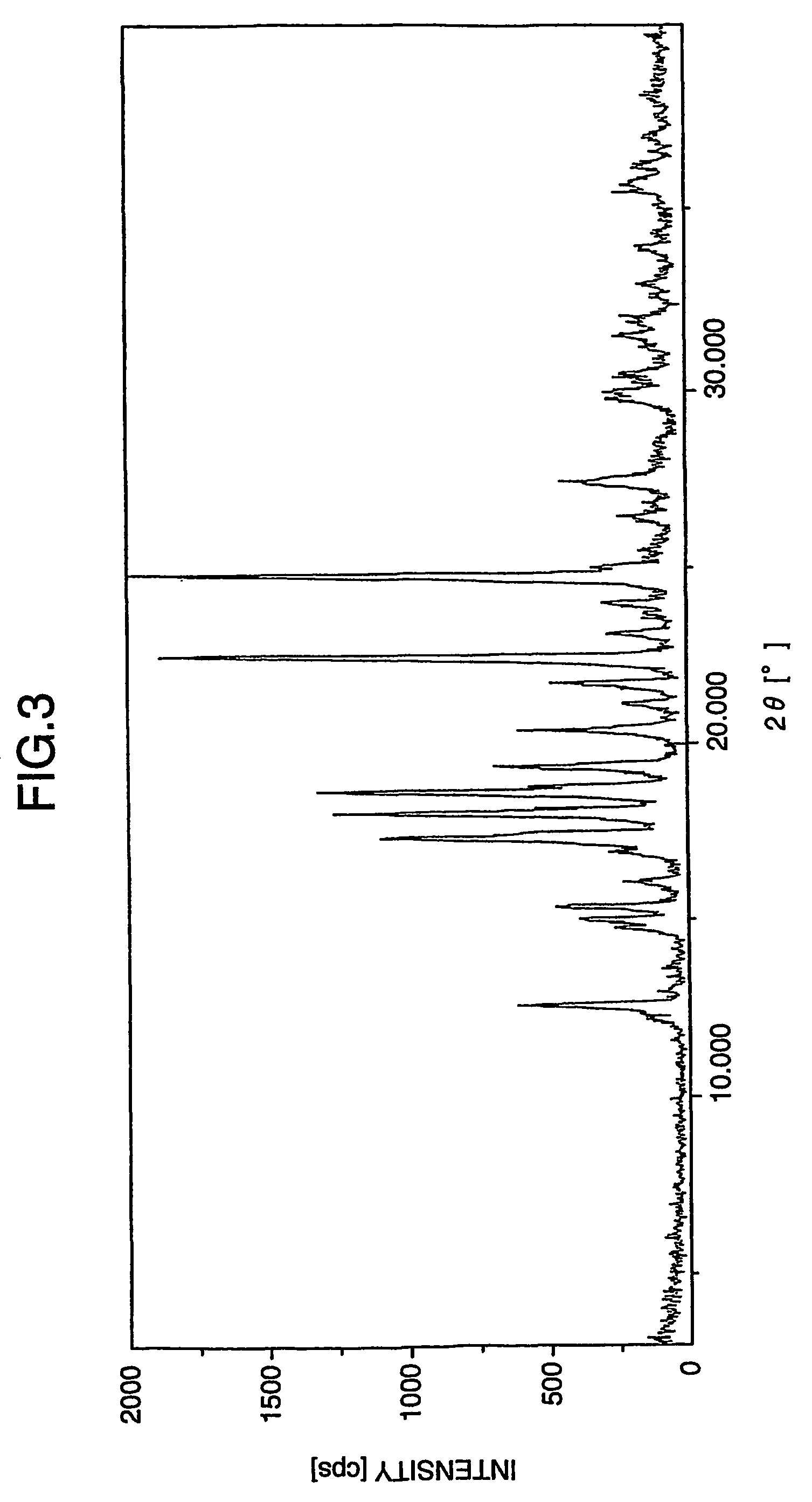
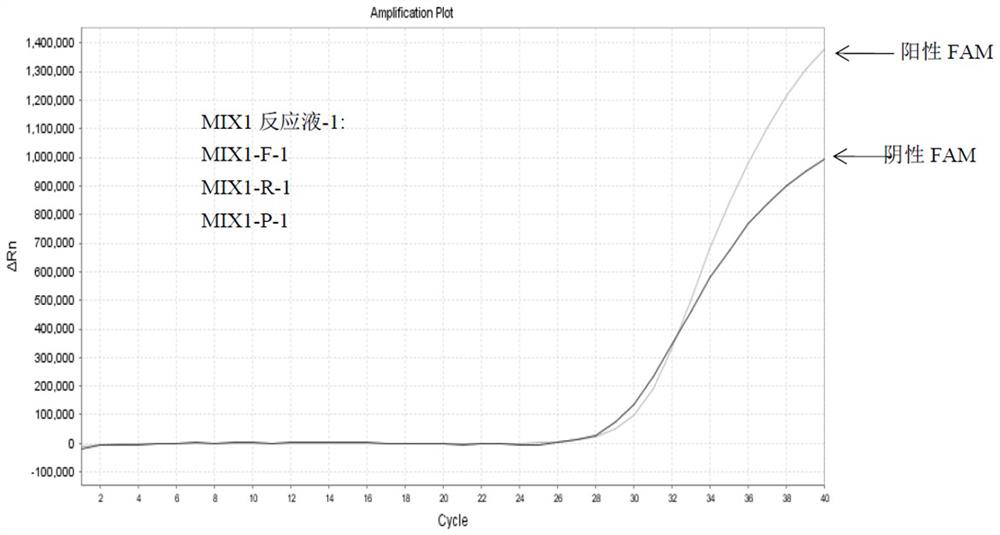
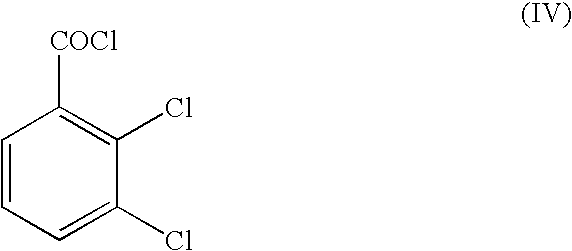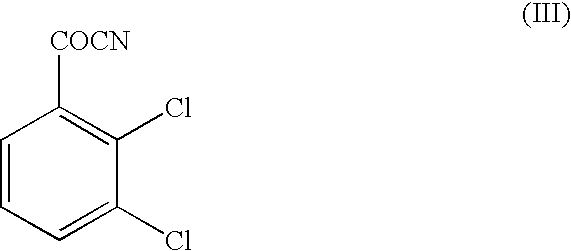Patents
Literature
85 results about "Lamotrigine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lamotrigine is used alone or with other medications to prevent and control seizures. It may also be used to help prevent the extreme mood swings of bipolar disorder in adults.
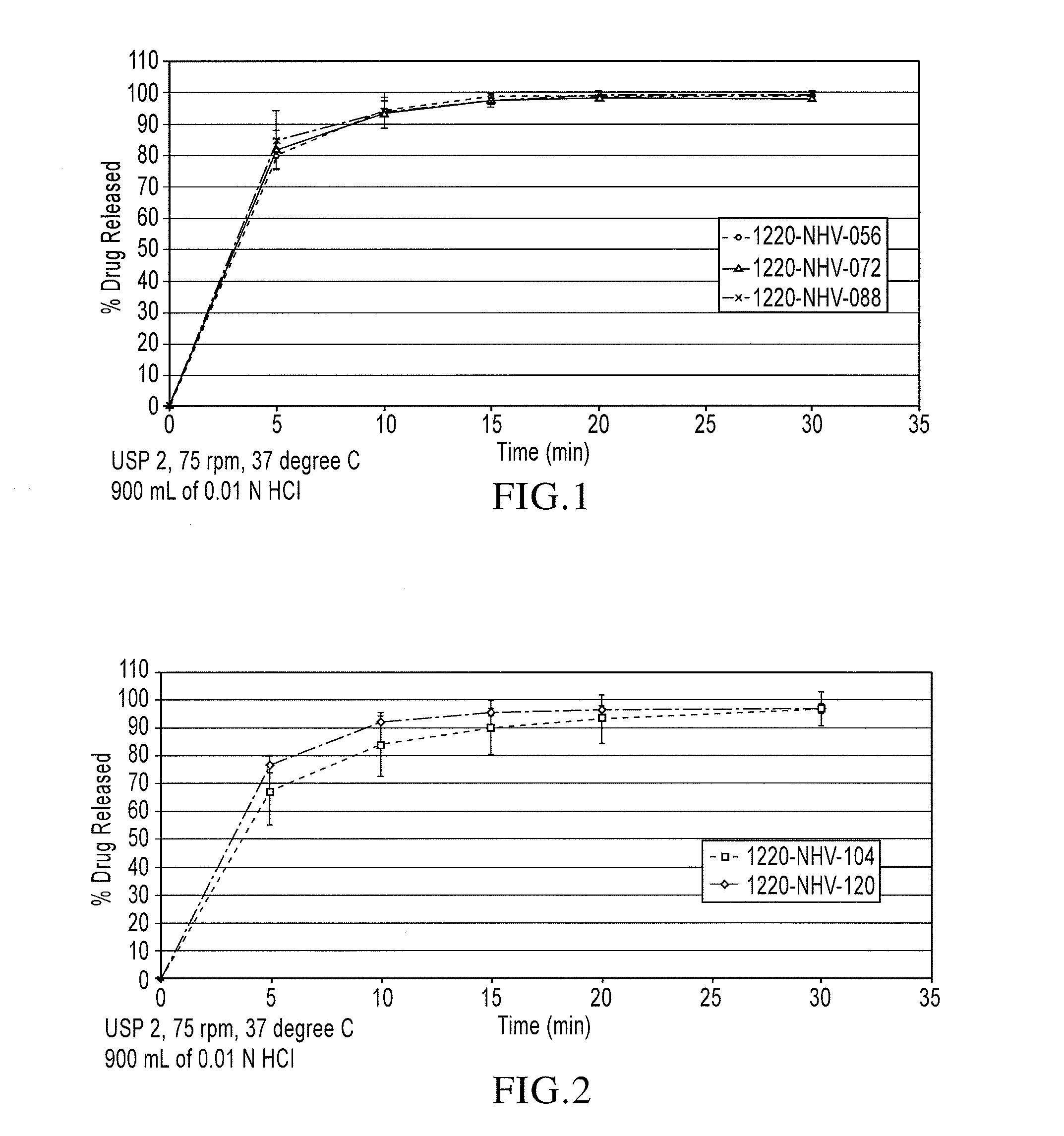
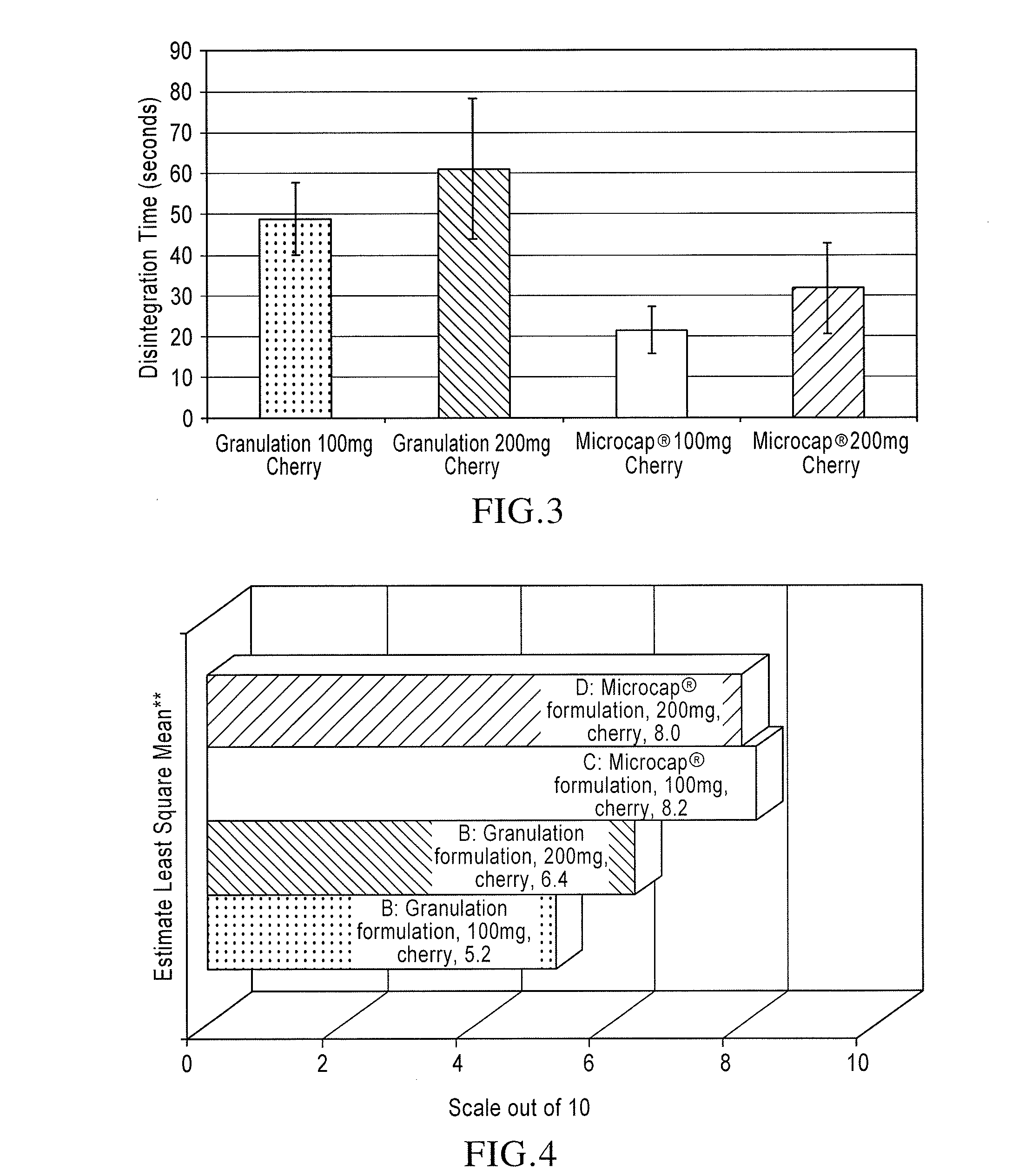
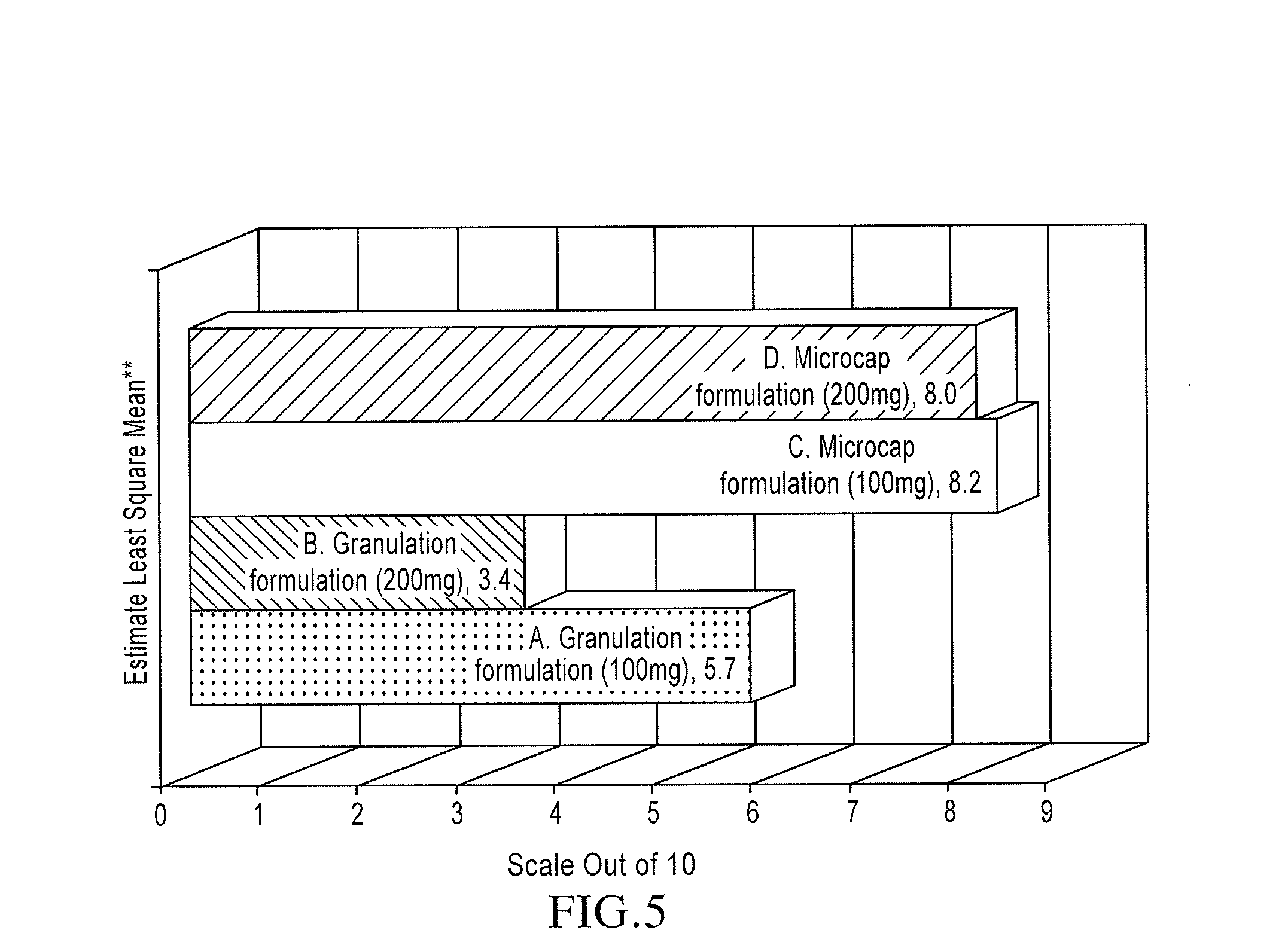
Orally disintegrating tablet compositions of lamotrigine
The compositions of the present invention composition comprise a therapeutically effective amount of particles comprising lamotrigine, in combination with granules comprising a disintegrant, and a sugar alcohol and / or a saccharide. These compositions are useful in treating epilepsy and bipolar disorder, particularly for patients with dysphagia, and to improve compliance with bipolar patients.
Owner:ADARE PHARM INC
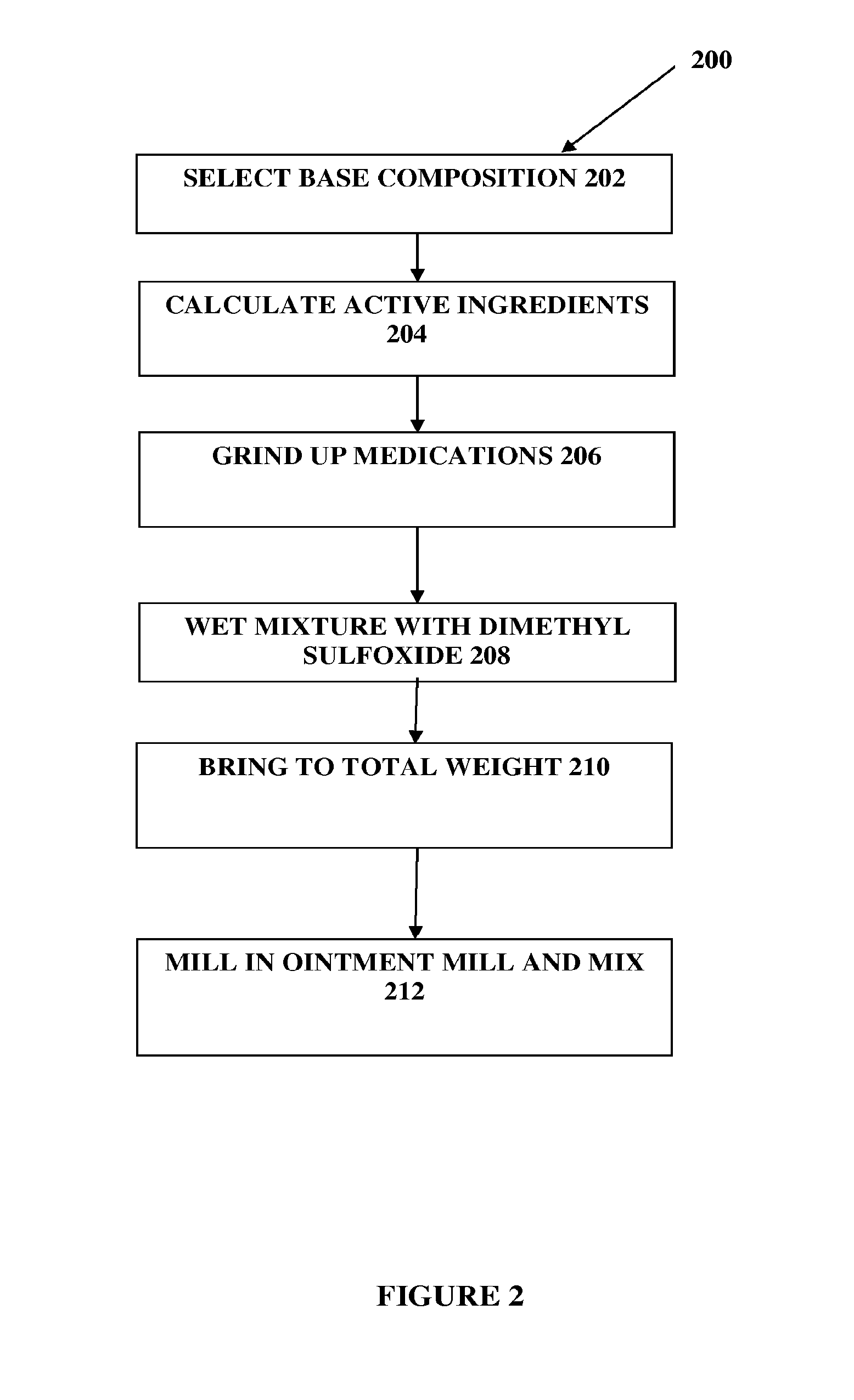
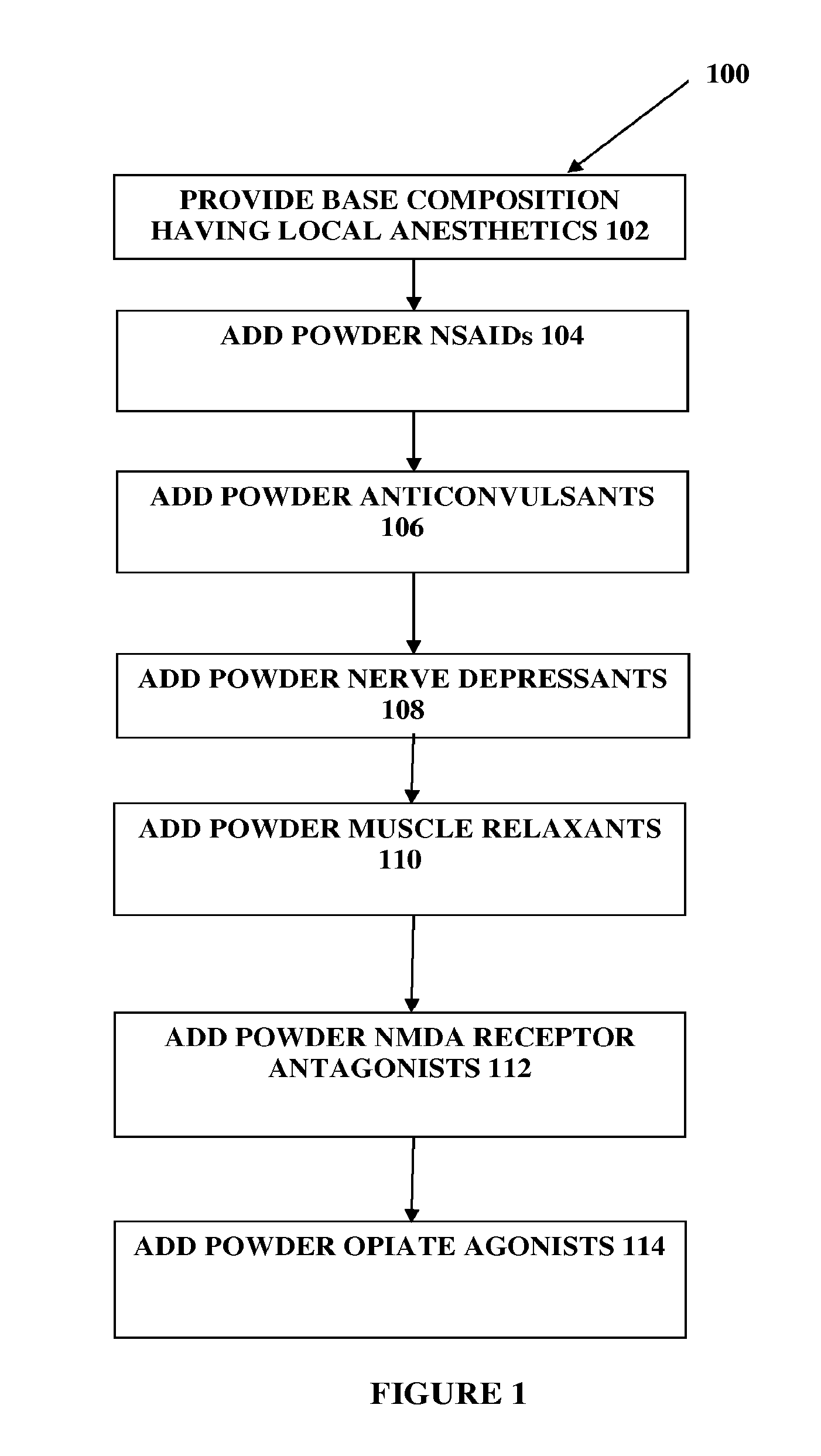
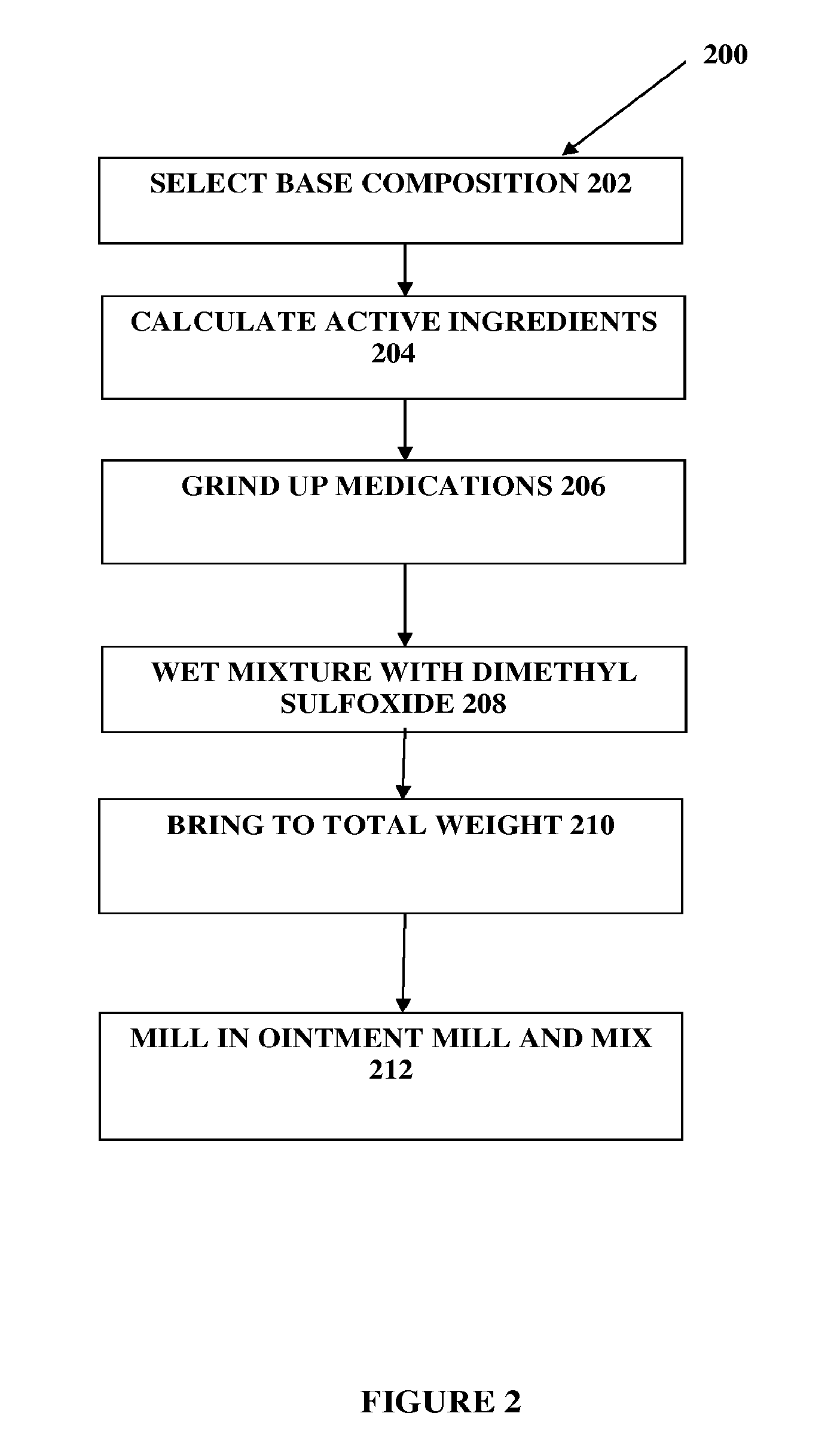
Composition and method for compounded therapy
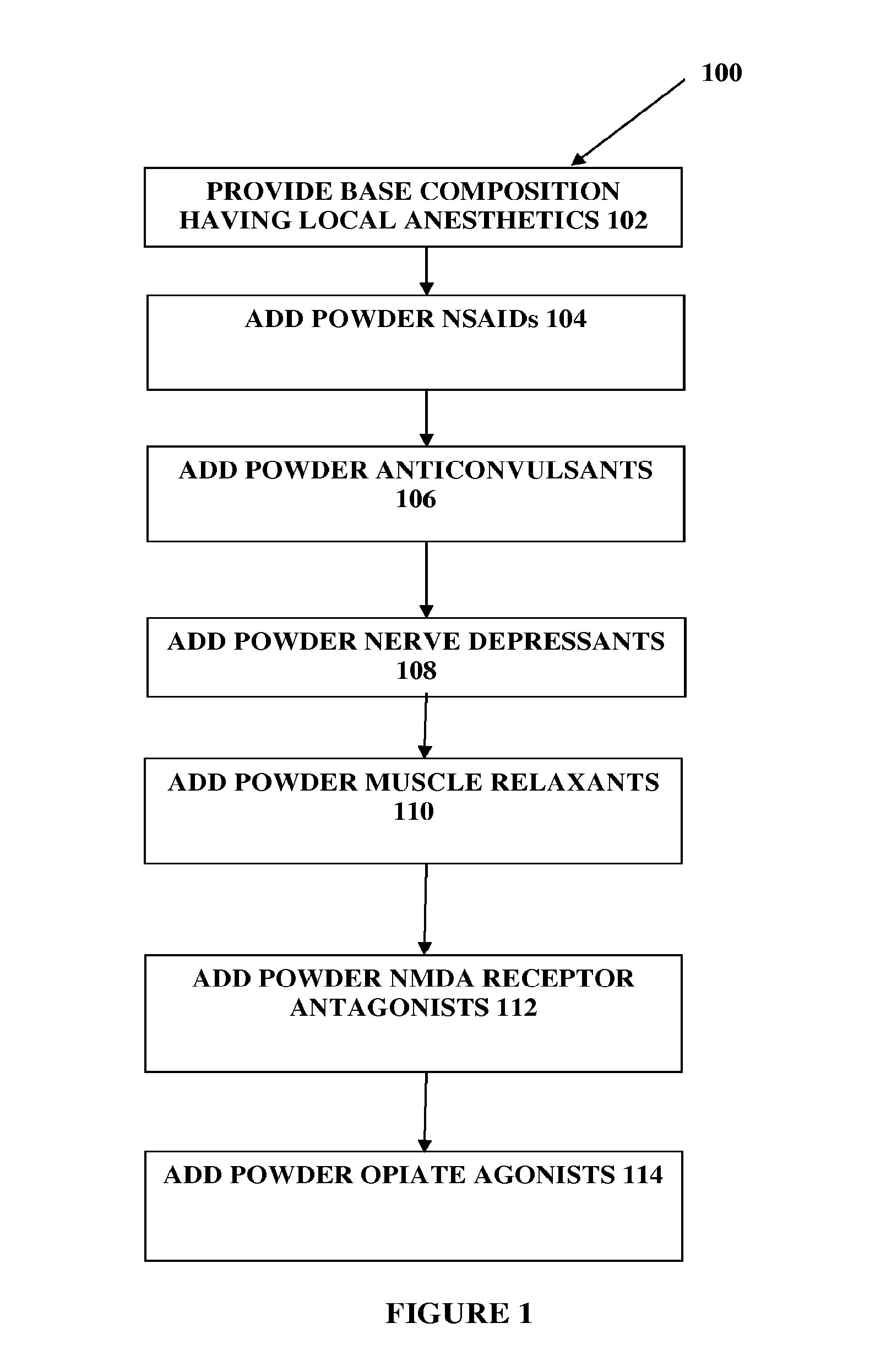
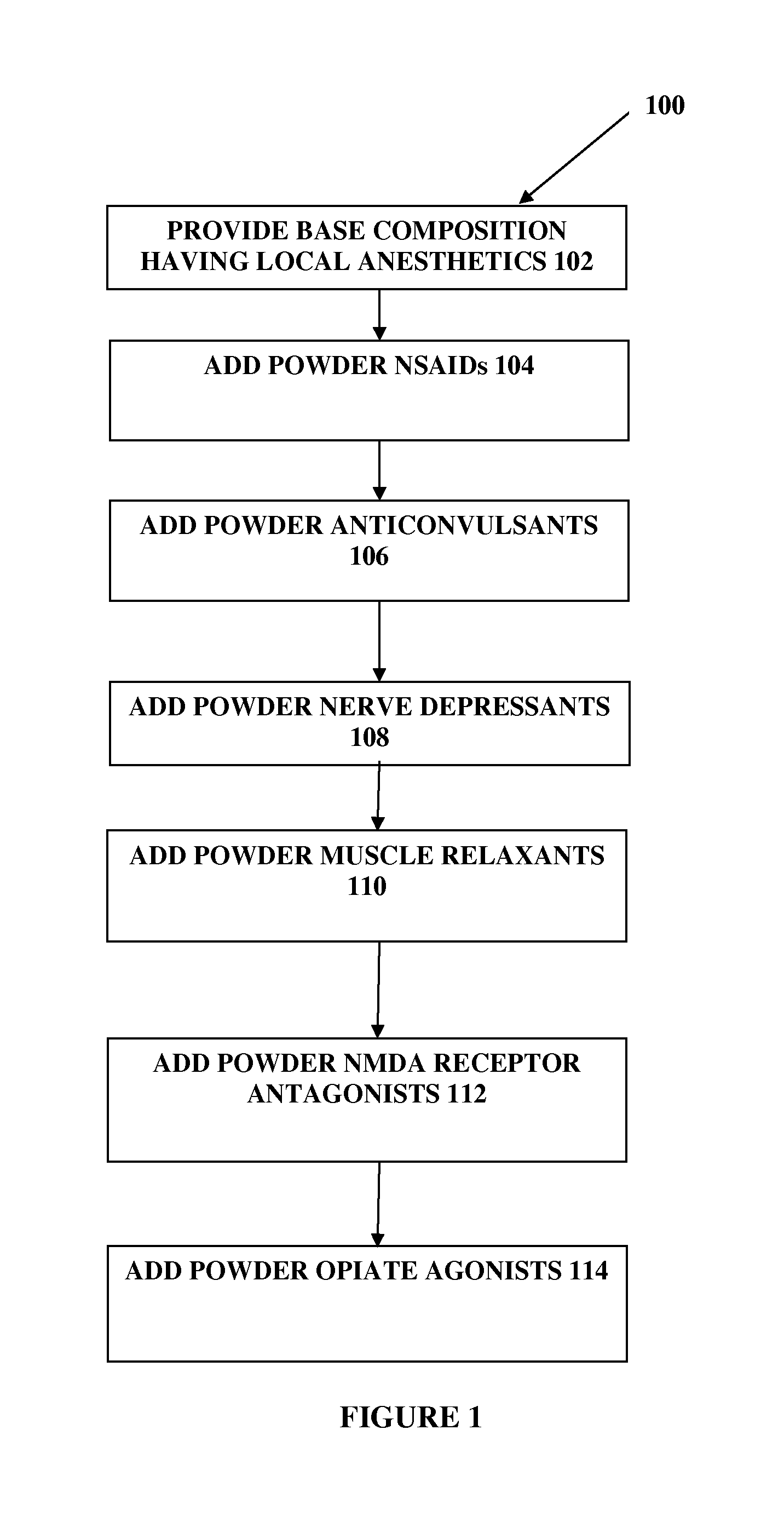
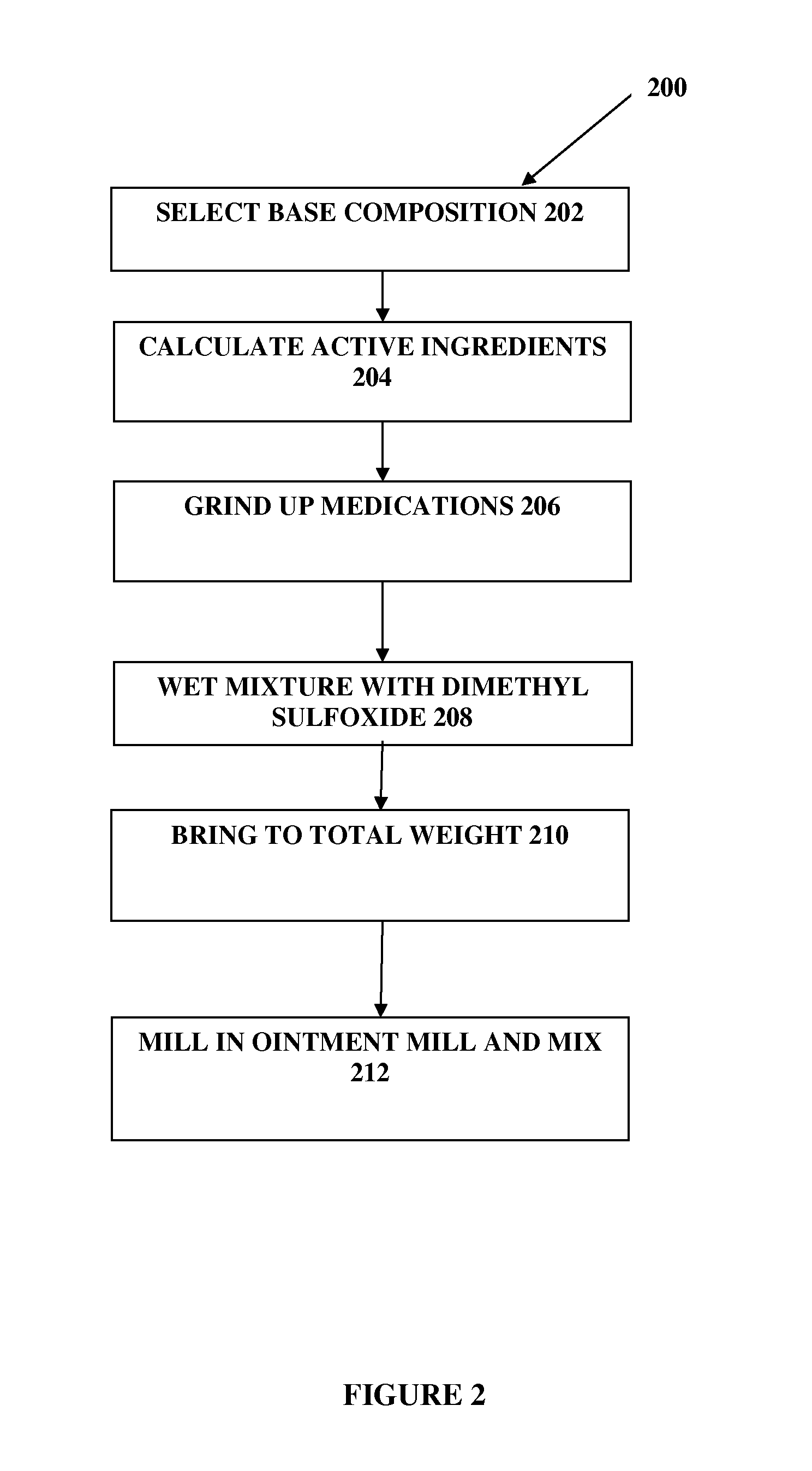
The present embodiments relate to topically delivered compounded medications. A transdermal cream may provide the effective topical administration of multiple medications simultaneously; may include low concentrations of local anesthetics, a NSAID, an anticonvulsant, and / or other active ingredients; and may include lidocaine, prilocaine, meloxicam, and lamotrigine and / or topiramate. Alternatively, the transdermal cream may include a lidocaine / prilocaine base cream to which is added a fine powder of one or more ground up medications to form a compounded medication. The compounded medication in powder form may be generated from grinding up tablets of NSAIDs, anticonvulsants, nerve depressants, antidepressants, muscle relaxants, NMDA receptor antagonists, opiate or opioid agonists, and / or other agents. The compounded medication in powder form may include meloxicam, lamotrigine, topiramate, other active ingredients, and DMSO or Sterile Water for Irrigation. In another aspect, the present embodiments relate to methods of compounding medications and transdermal creams or gels.
Owner:CMPD LICENSING
Composition and method for compounded therapy
Owner:CMPD LICENSING
Lamotrigine dry suspension and preparation method thereof
ActiveCN106491539AEasy to takeFast physical stabilityPowder deliveryNervous disorderMedicineLamotrigine
The invention discloses a lamotrigine dry suspension. The lamotrigine dry suspension comprises lamotrigine and other pharmaceutical-grade auxiliary materials, wherein the auxiliary materials include a filling agent, a suspending aid, a buffer agent, a sweetening agent, a flavor agent and a flow aid. The lamotrigine dry suspension has the advantages of liquid suspensions that the taste of medicines is improved, the medicines are easy to be taken, and the stability of medication dosage is improved; furthermore, the chemical and physical stabilities of a preparation can be improved, and the quality of the lamotrigine dry suspension is improved.
Owner:SHANGHAI AUCTA PHARMA CO LTD
Preparation method for lamotrigine oral solution
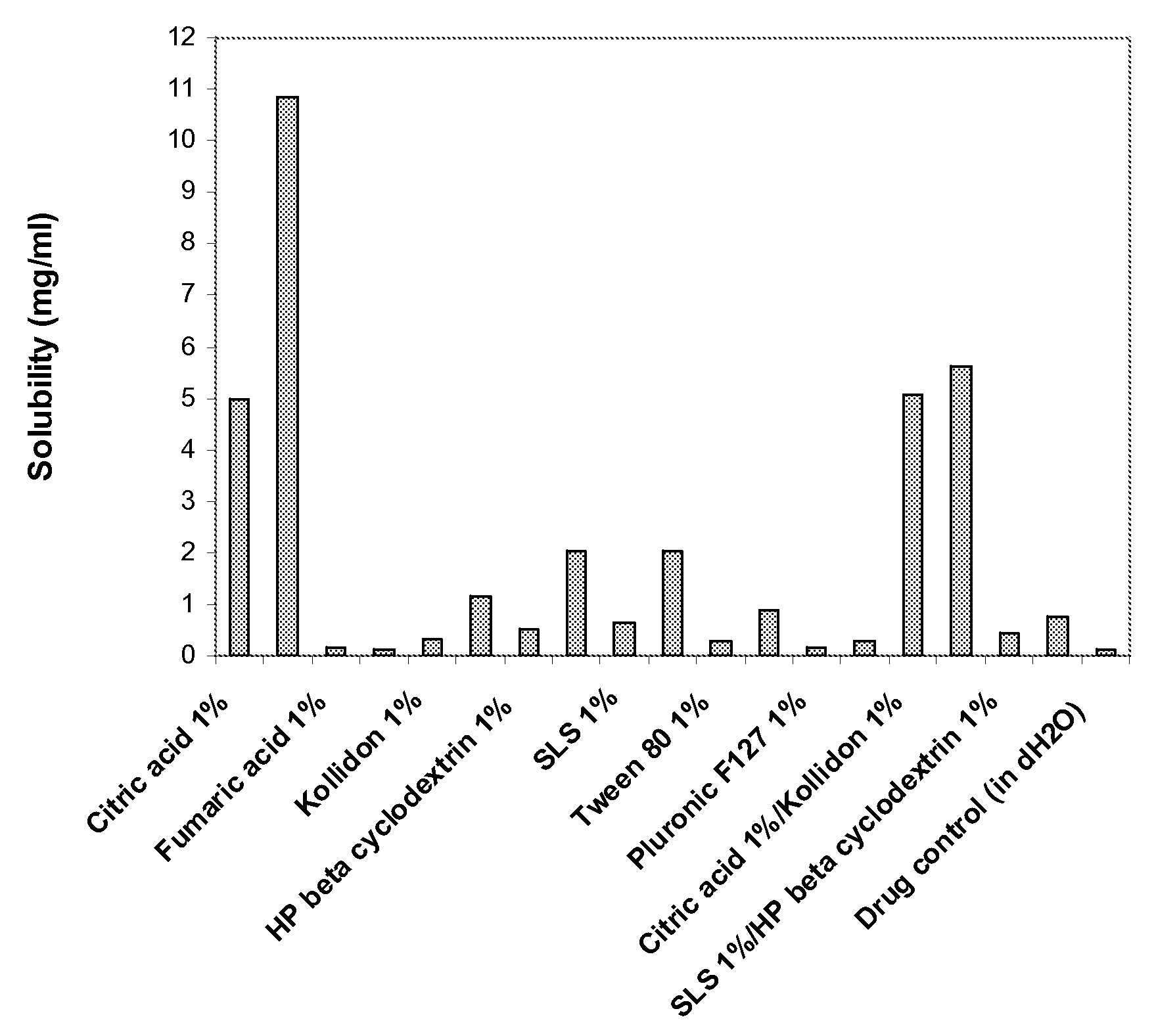
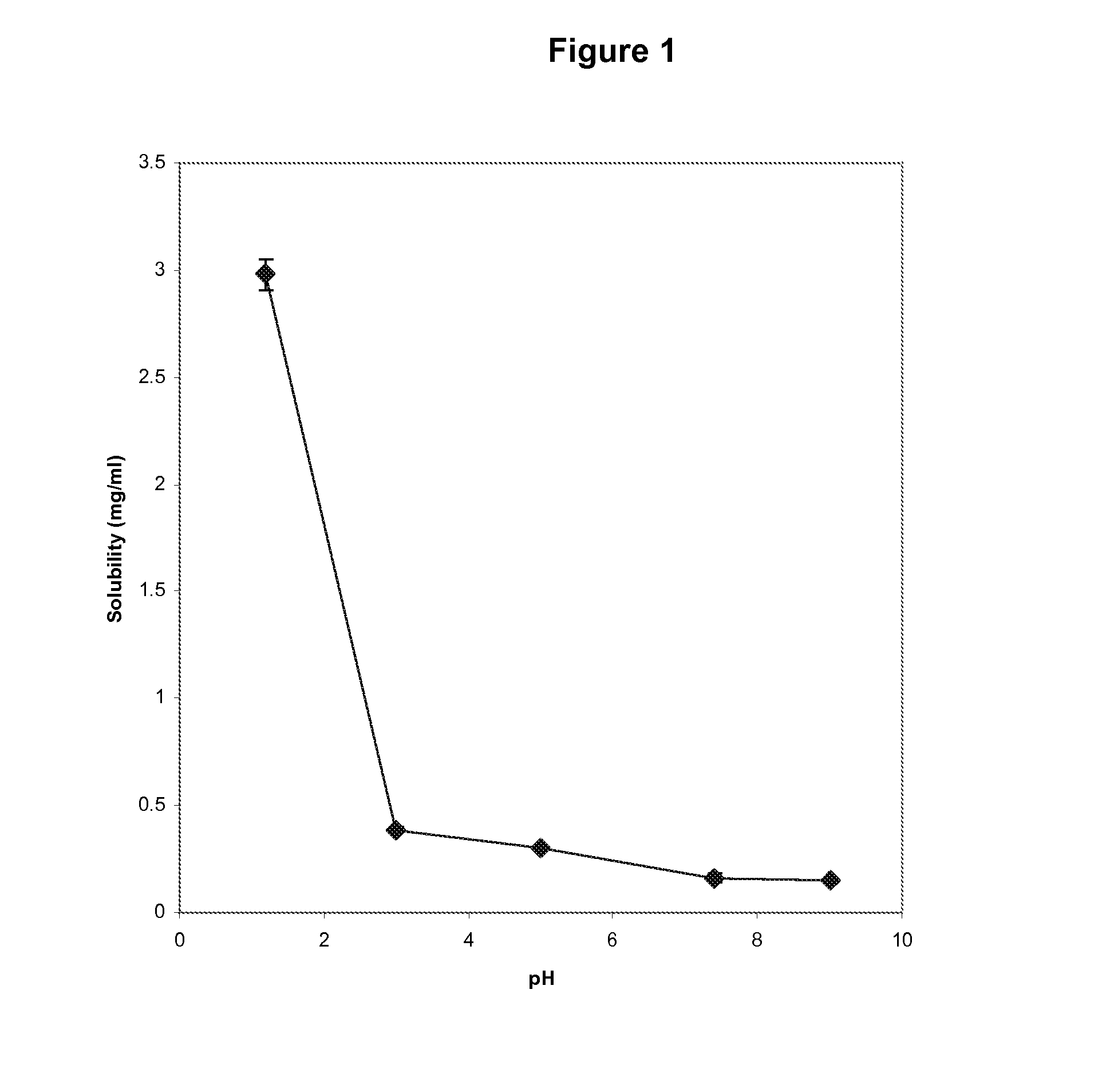
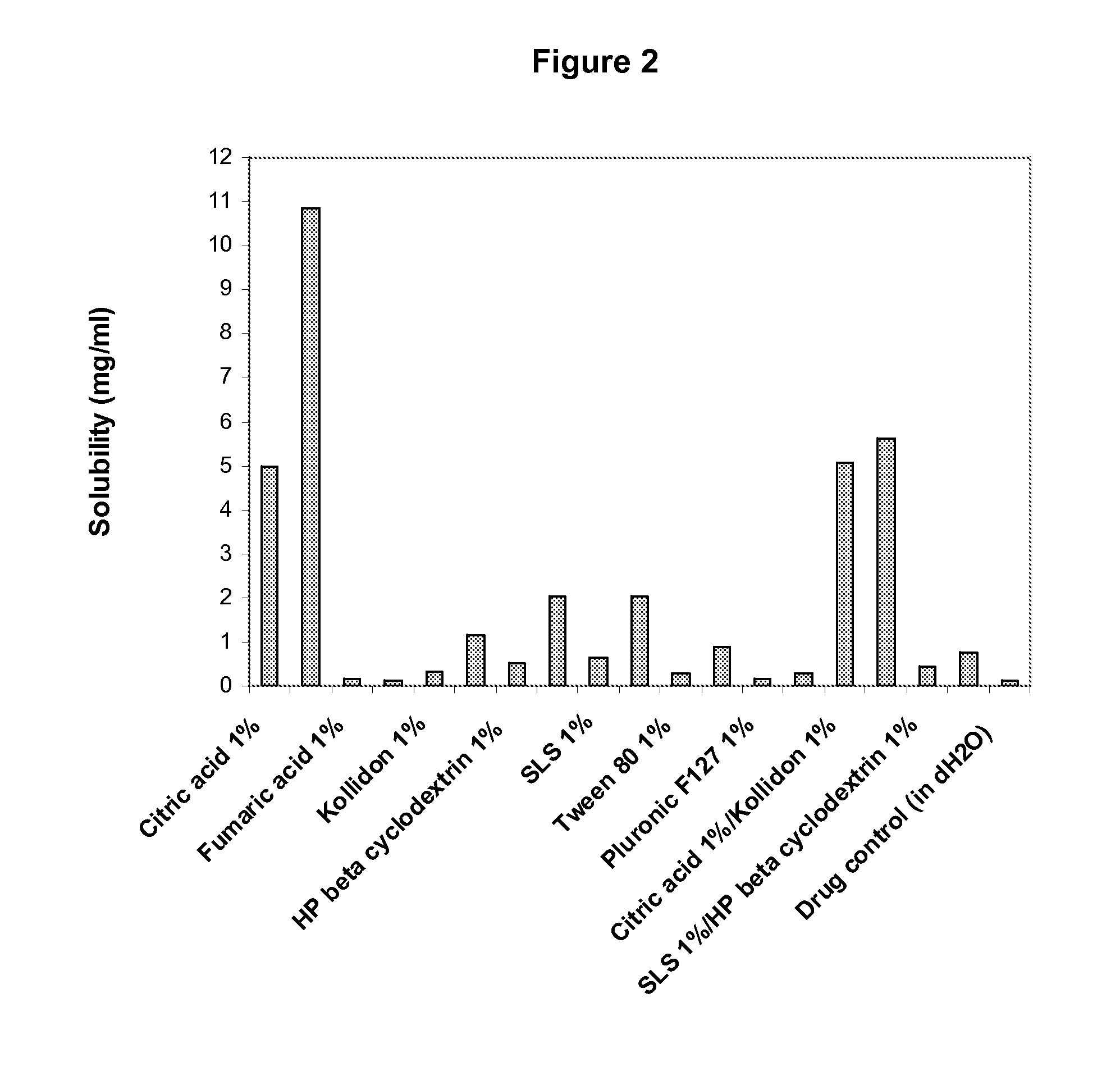
The invention provides a medicine solution suitable for oral administration. The preparation method for a lamotrigine oral solution comprises lamotrigine or a lamotrigine eutectic matter which can improve the solubility, a suitable medicinal solvent system, one or multiple flavour masking agents, one or multiple preservatives, one or multiple matters selecting from lactic acid, acetic acid, tartaric acid and citric acid and the like. The lamotrigine oral solution has the PH value ranging from two to four. The lamotrigine oral solution can be used for treating epilepsy and bipolar disorder, especially for a patient who suffers from dysphagia and for improving the compliance of a patient sufferring from the bipolar disorder.
Owner:AVENTIS PHARMA HAINAN
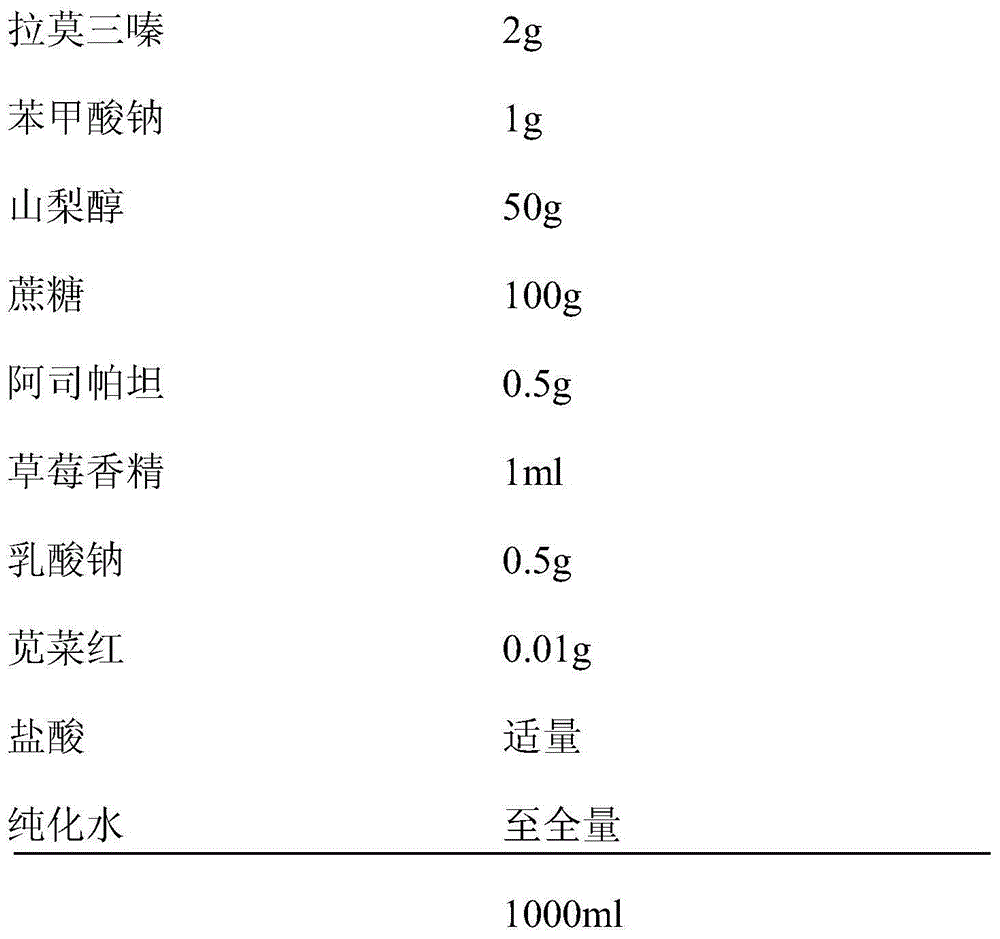
Lamotrigine oral liquid preparation and preparation method thereof
InactiveCN104940128APromote absorptionImprove accuracyNervous disorderPharmaceutical delivery mechanismMedicineLamotrigine
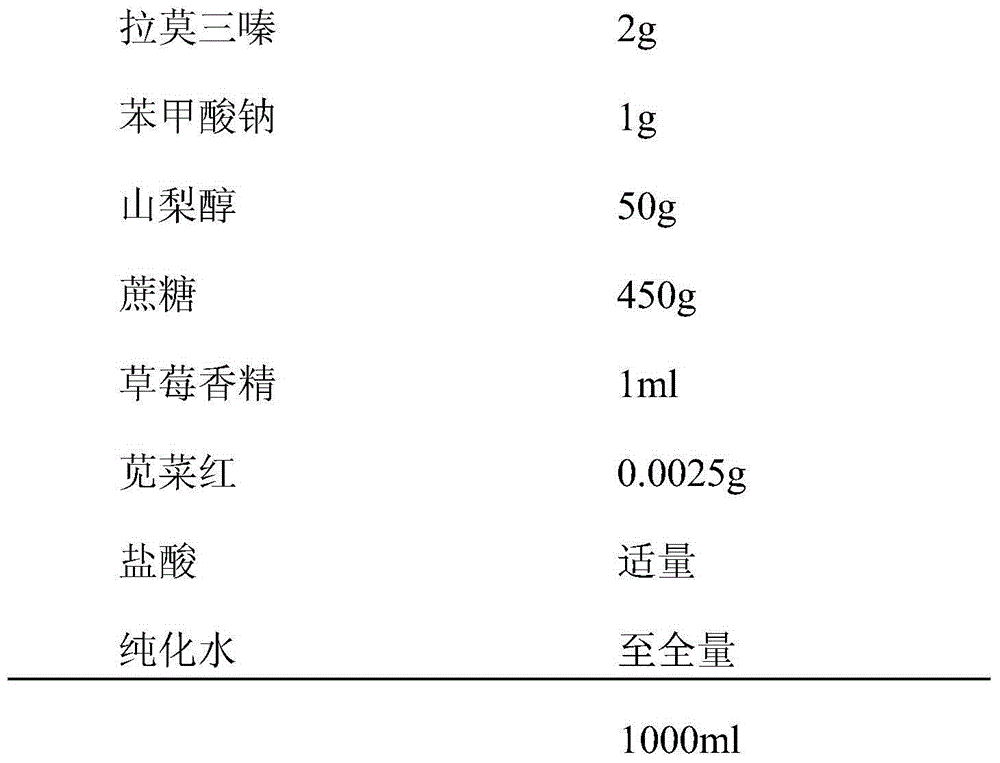
The invention discloses a lamotrigine oral liquid preparation. Each 1000ml of the lamotrigine oral liquid preparation contains 0.4-2g of lamotrigine, 10-500g of a sweetener, 0.5-1.5ml of a perfume, 0.5-1.5g of an antiseptic, 0.001-0.1g of a coloring agent, a proper amount of a pH adjusting agent, and the balance of purified water, and the pH value of the lamotrigine oral liquid preparation is 2.5-4.0. The invention also discloses a preparation method of the lamotrigine oral liquid preparation. The lamotrigine oral liquid preparation can effectively cover the bad taste of medicines, can be conveniently swallowed, can be accepted by patients (especially children), and has good administration compliance.
Owner:黑龙江童医生儿童生物制药有限公司
Enhanced formulations of lamotrigine
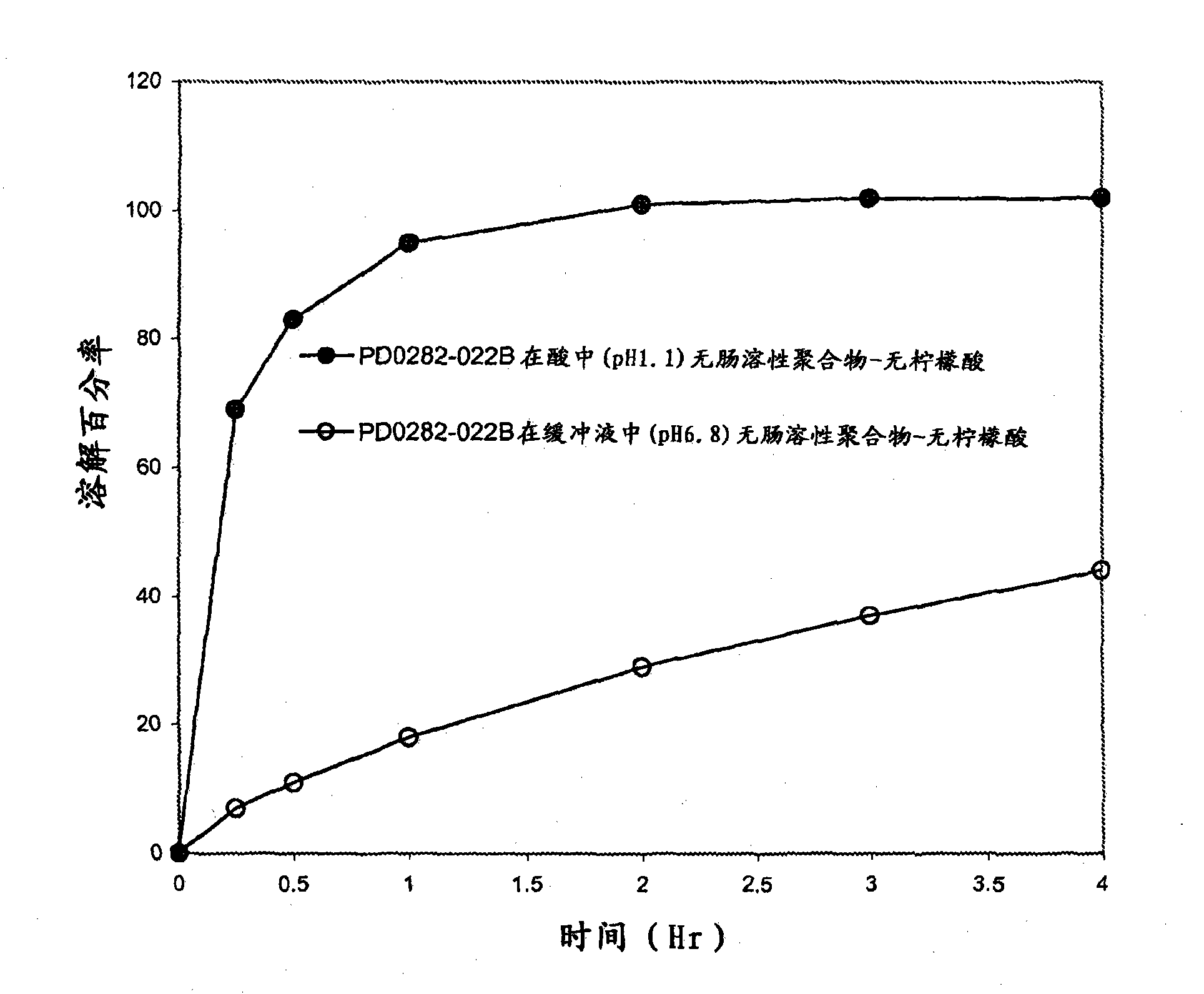
A once-a-day, extended-release formulation of lamotrigine, exhibiting a significantly similar release rate throughout the GI tract irrespective of the pH of the environment, is provided. The formulation comprises lamotrigine, an organic acid, a release enhancing polymer and a release controlling polymer. The use of the formulation for the treatment of the neurological disorders is also disclosed.
Owner:SUPERNUS PHARM INC
Method for measuring density of anti-epileptic in blood
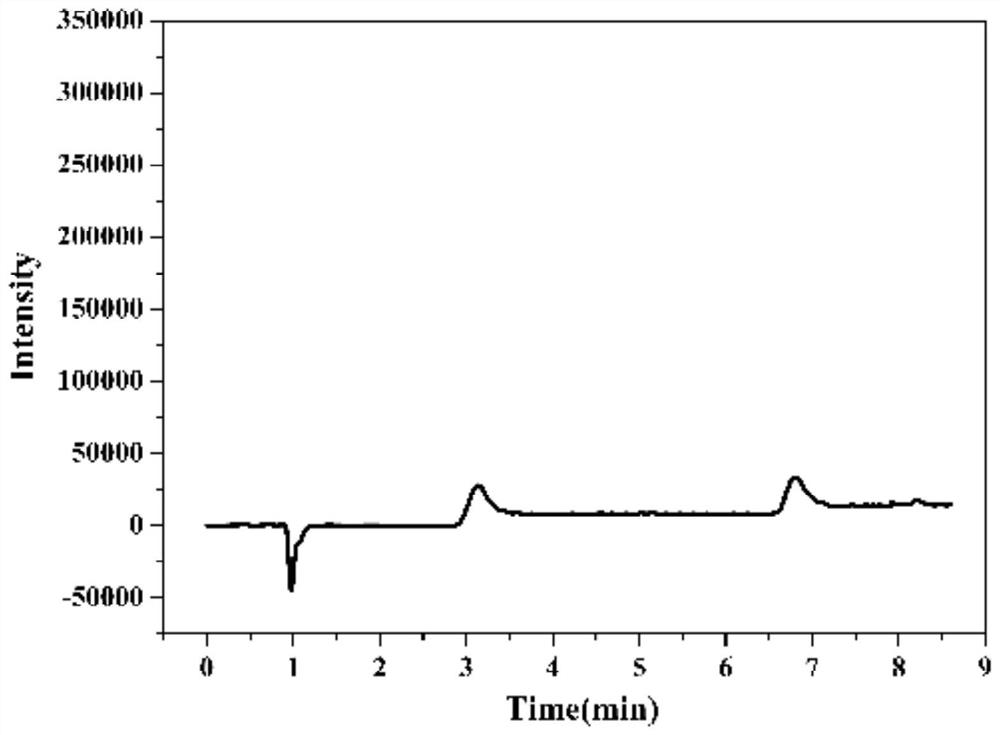
InactiveCN101093214ASimple and fast operationEasy to operateComponent separationColor/spectral properties measurementsUltraviolet detectorsAntiepileptic drug
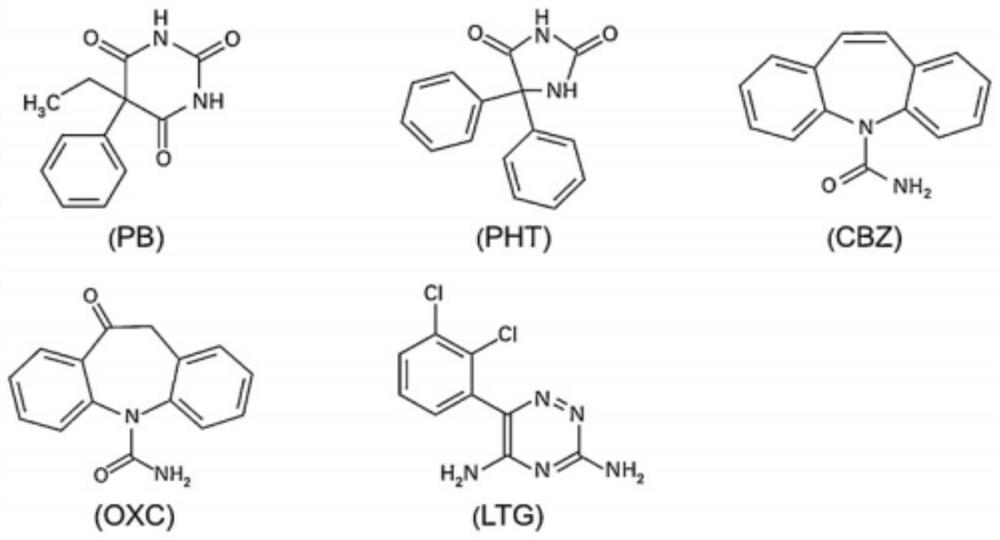
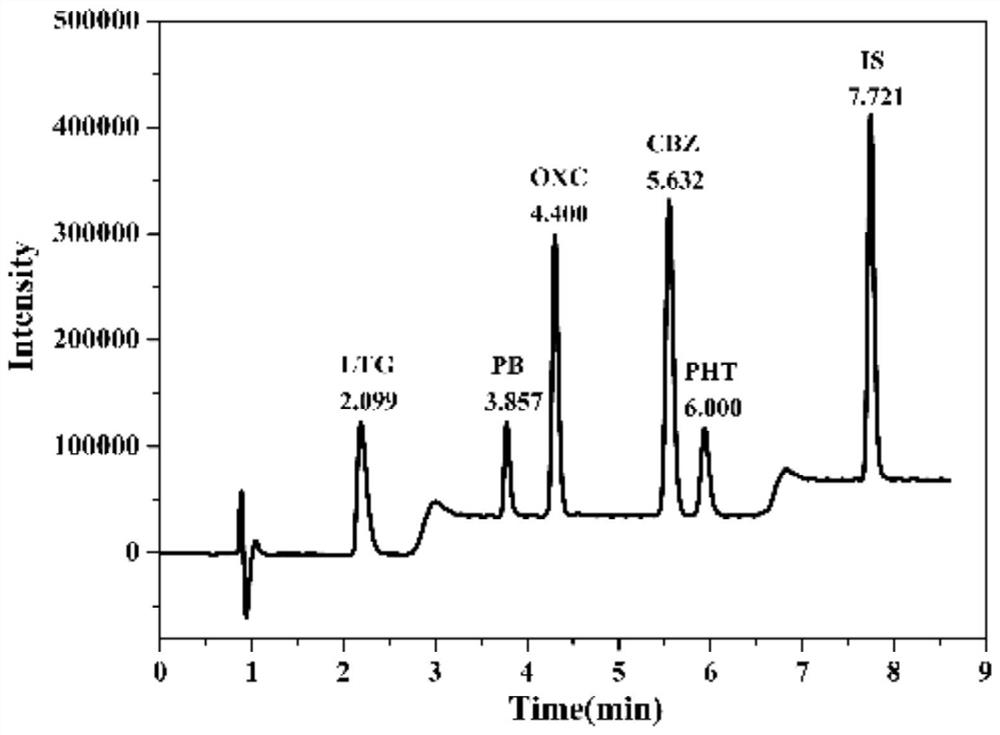
A method for determining blood-medicine concentration of antiepileptic medicine includes pre-treating sample to be determined and separating pretreated sample by acidic mobile phase in chromatographic column then utilizing ultraviolet detector to simultaneously detect quantitative concentration of phenobarbital, lamotrigine, oxcarbazepin and adipomono- oxcarbazepin as antiepileptic medicine.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Synthetic method of drug lamotrigine for curing bipolar disorder and epilepsy
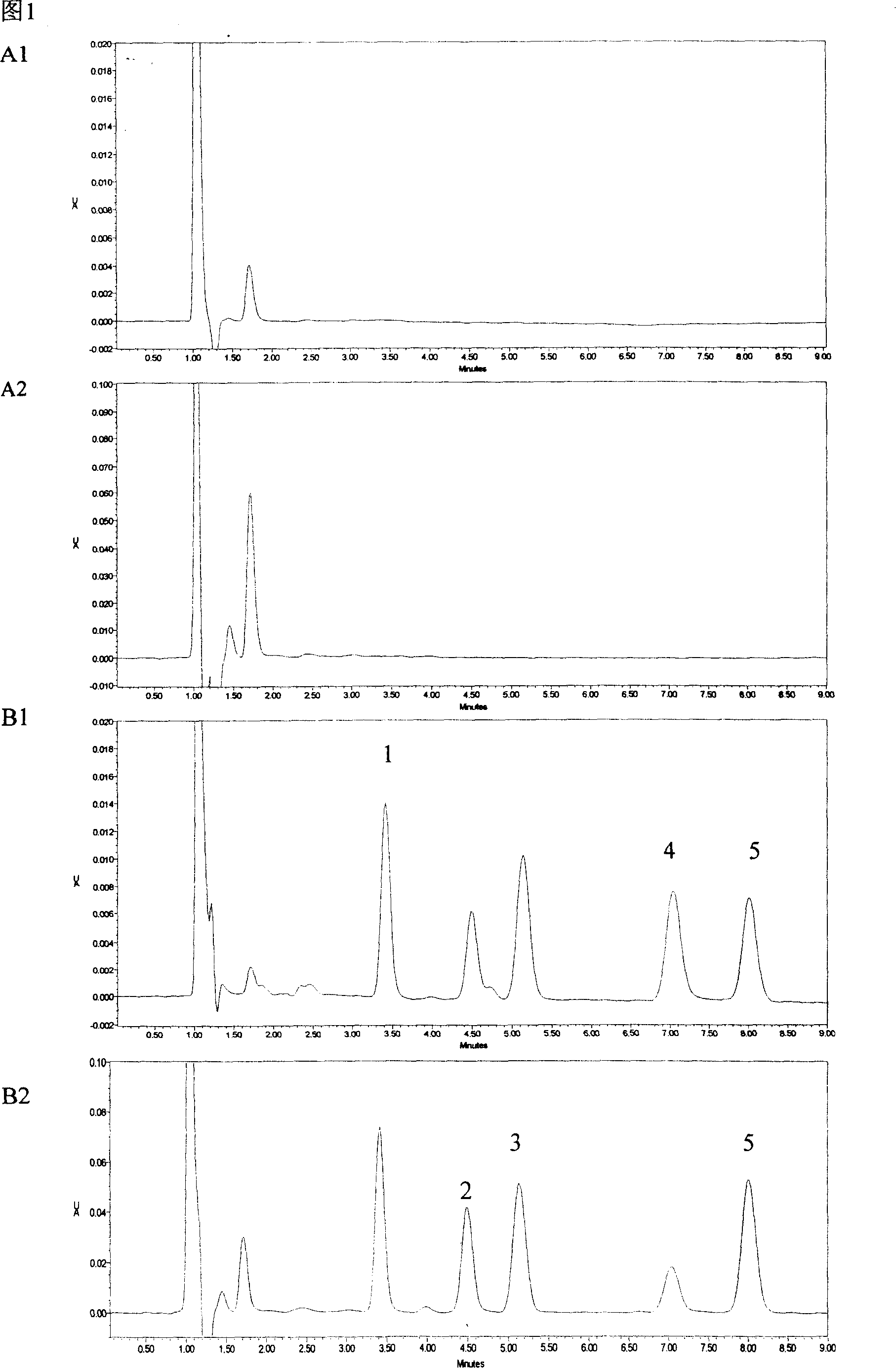
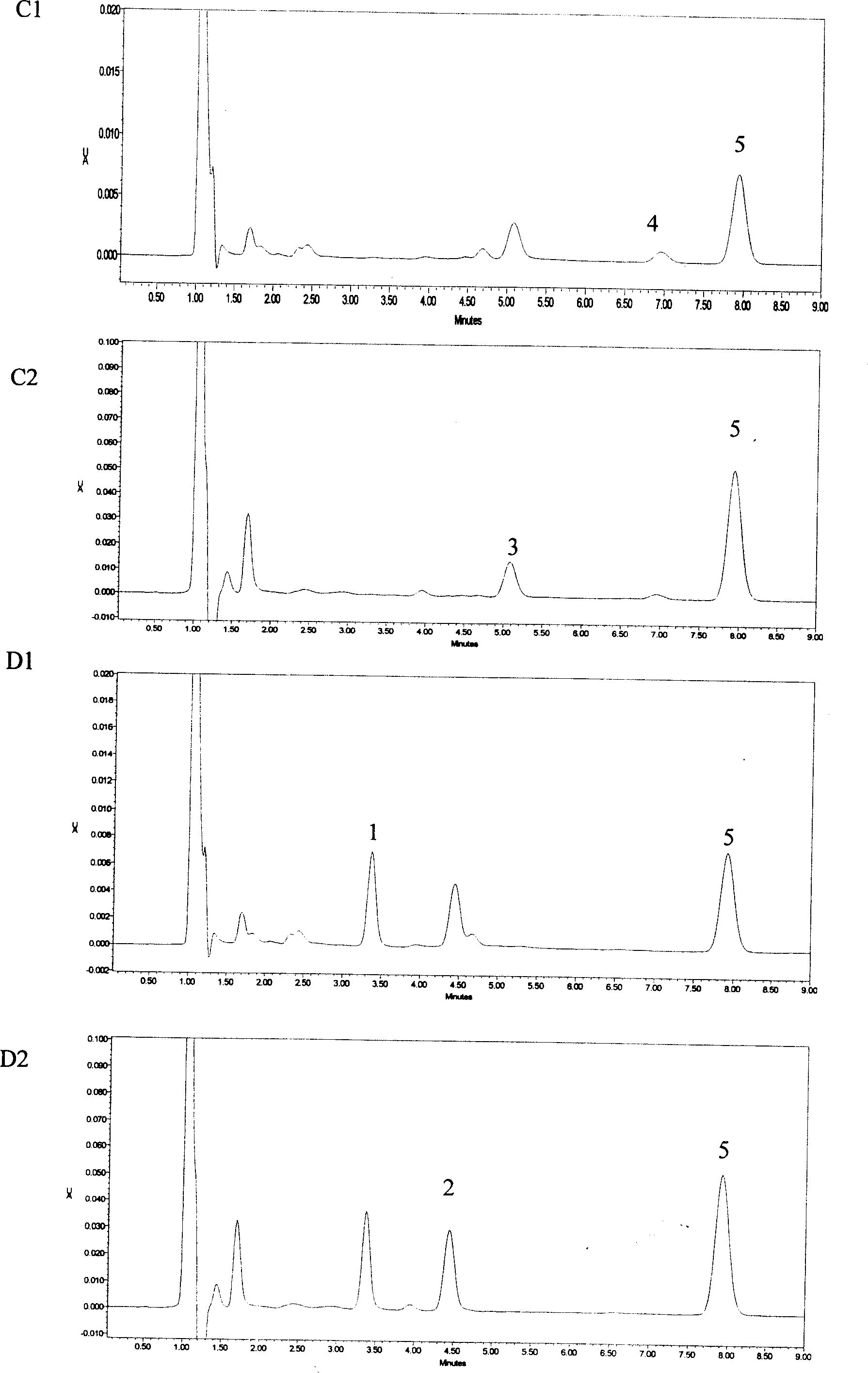
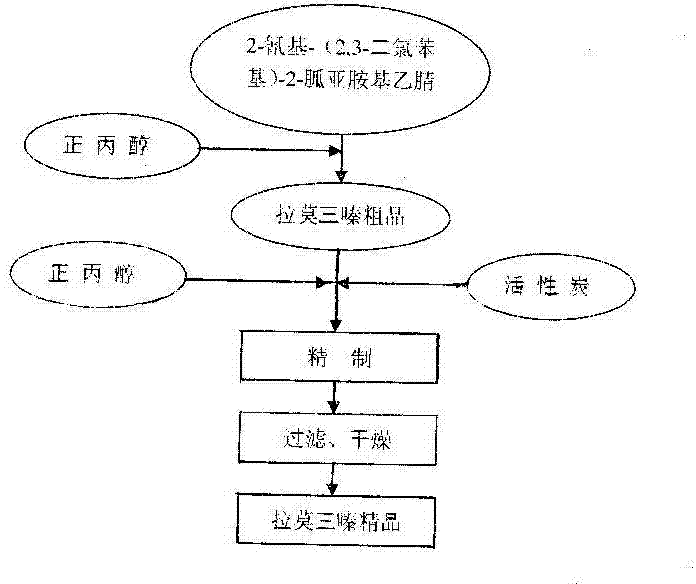
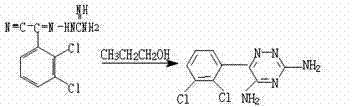
The invention provides a synthetic method of drug lamotrigine for curing bipolar disorder and epilepsy. 2-cyano-(2, 3-dichlorophenyl)-2-guanidine amino acetonitrile and n-propyl alcohol (CH3CH2CH2OH) solvent are used as a raw material, and the lamotrigine is generated through one-step cyclization reaction in a reaction tank. The synthetic method of the drug lamotrigine includes material preparation, crude product manufacturing, purification, filtration and drying. The raw material of the synthetic method comprises only the 2-cyano-(2, 3-dichlorophenyl)-2-guanidine amino acetonitrile as a starting material and the n-propyl alcohol (CH3CH2CH2OH) solvent and has no strong acid, strong base and other toxic substances, the lamotrigine is manufactured through the one-step cyclization reaction, and therefore the synthetic method is small in reaction steps, high in yield, simple and convenient to operate and high in raw material utilization rate, avoids using toxic and harmful substances in abundance, reduces production cost remarkably, and has high social benefits and social benefits.
Owner:SANJIN GROUP HUNAN SANJIN PHARMA
Pharmaceutical composition containing lamotrigine particles of defined morphology
The present invention provides a pharmaceutical composition comprising a plurality of lamotrigine particles having a specific surface area of from about two to about three and a half meters per gram. Pharmaceutical compositions falling within the surface area criteria for the lamotrigine particles include those having a particle diameter equal to or less than about 100 μm, preferably about 50 μm, and most preferably 10 μm. The pharmaceutical composition can be formulated into a wide variety of dosage forms.
Owner:TEVA PHARM USA INC
Lamotrigine sustained release tablet and preparation method of lamotrigine sustained release tablet
InactiveCN103948553AStable release rateOvercome the "peak and valley" phenomenonNervous disorderAntipyreticSustained Release TabletSide effect
The invention aims to provide a lamotrigine sustained release tablet, which is stable in drug release and higher in medication safety and is characterized by comprising lamotrigine with the effective therapeutic dose and physiologically acceptable pharmaceutical accessories. The lamotrigine sustained release tablet disclosed by the invention has the characteristics of being convenient for drug delivery, lasting in effect, stable in curative effect, low in toxic and side effect and the like.
Owner:QINGDAO CENT HOSPITAL
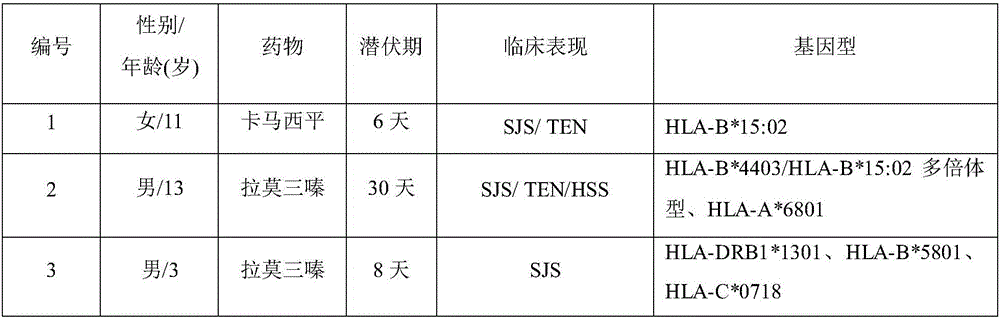
Biomarker for forecasting severe drug-induced cutaneous adverse reaction of child patient and application
The invention discloses a biomarker for forecasting severe drug-induced cutaneous adverse reaction of a child patient and an application. The biomarker is capable of forecasting the risk of severe cutaneous adverse reaction of the child patient using beta-lactam antibiotics such as penicillin, cephalosporin, carbamazepine, lamotrigine, oxcarbazepine, phenytoin, allopurinol, nevirapine, abacavir, methazolamide and dapsone.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
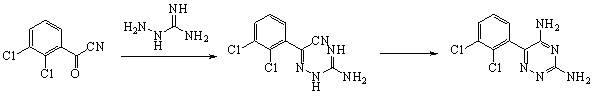
Method for preparing lamortrigine and its intermediate 2,3-dichlorobenzoyl chloride
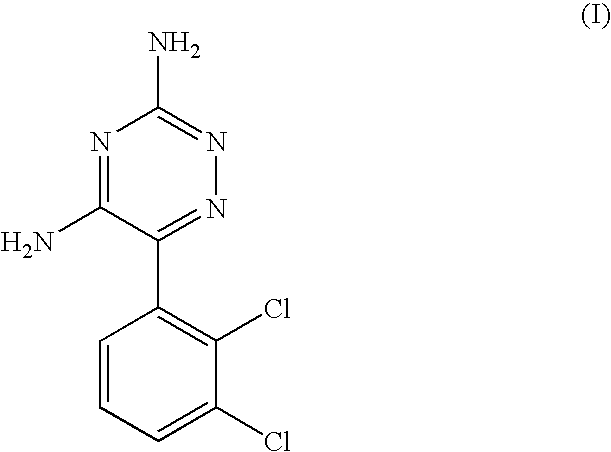
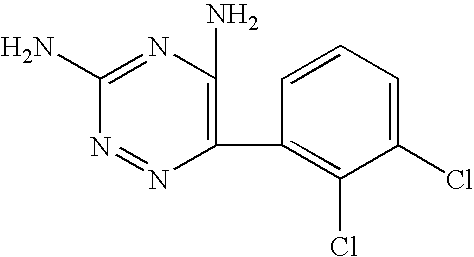
A method for preparing 3,5-diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine, commonly known as lamotrigine, is disclosed. A method of preparing the intermediate, 2,3-dichlorobenzoyl chloride, by photochlorination of 2,3-dichlorobenzotrichloride followed by hydrolysis is also disclosed. The intermediate may then be used in the preparation of lamotrigine.
Owner:CALAIRE CHEM
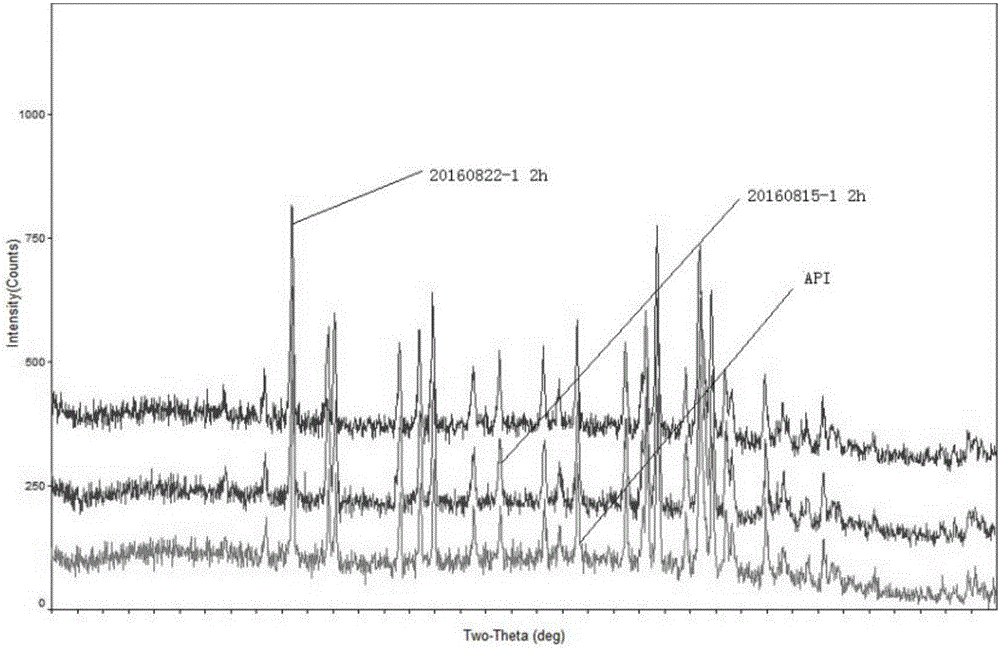
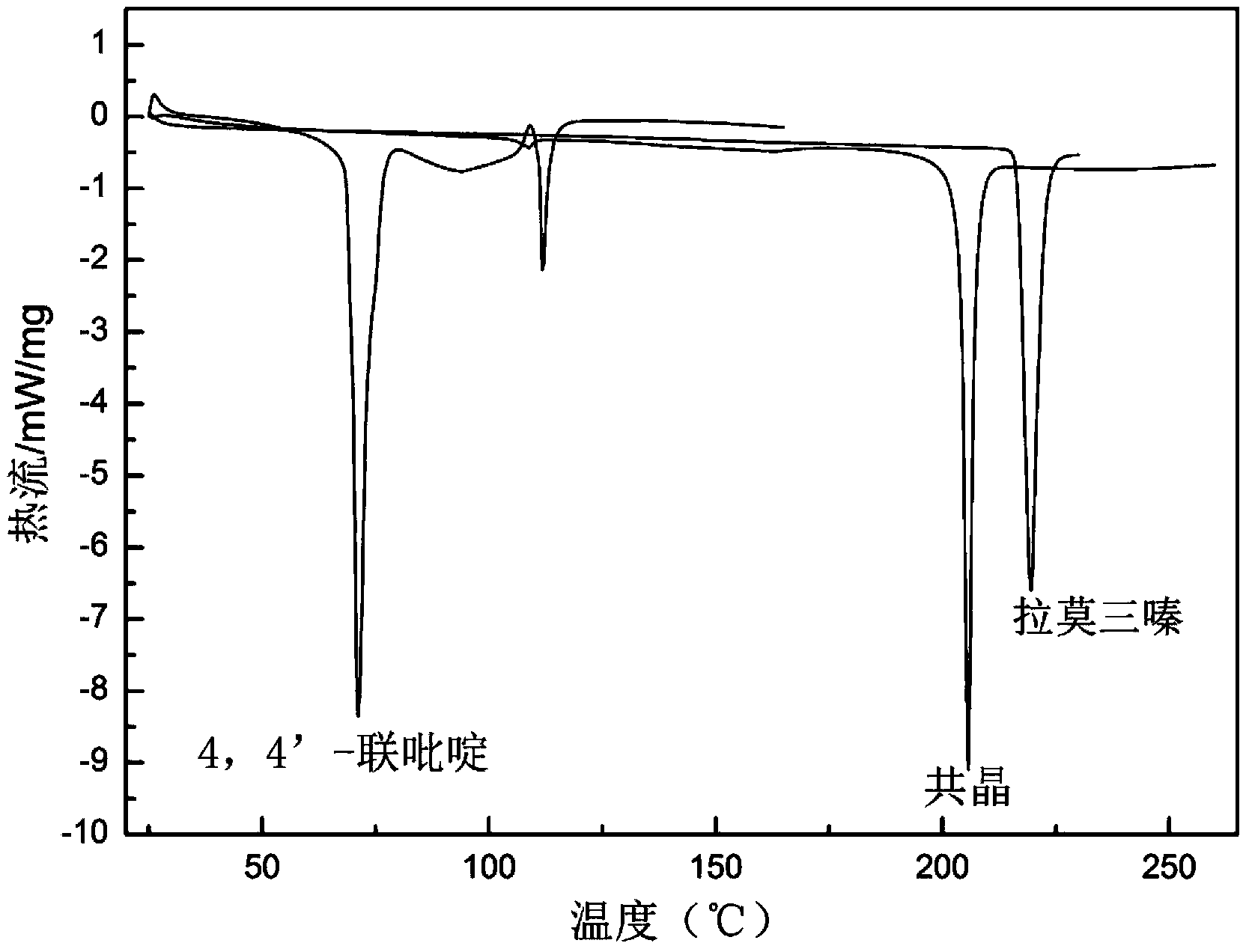
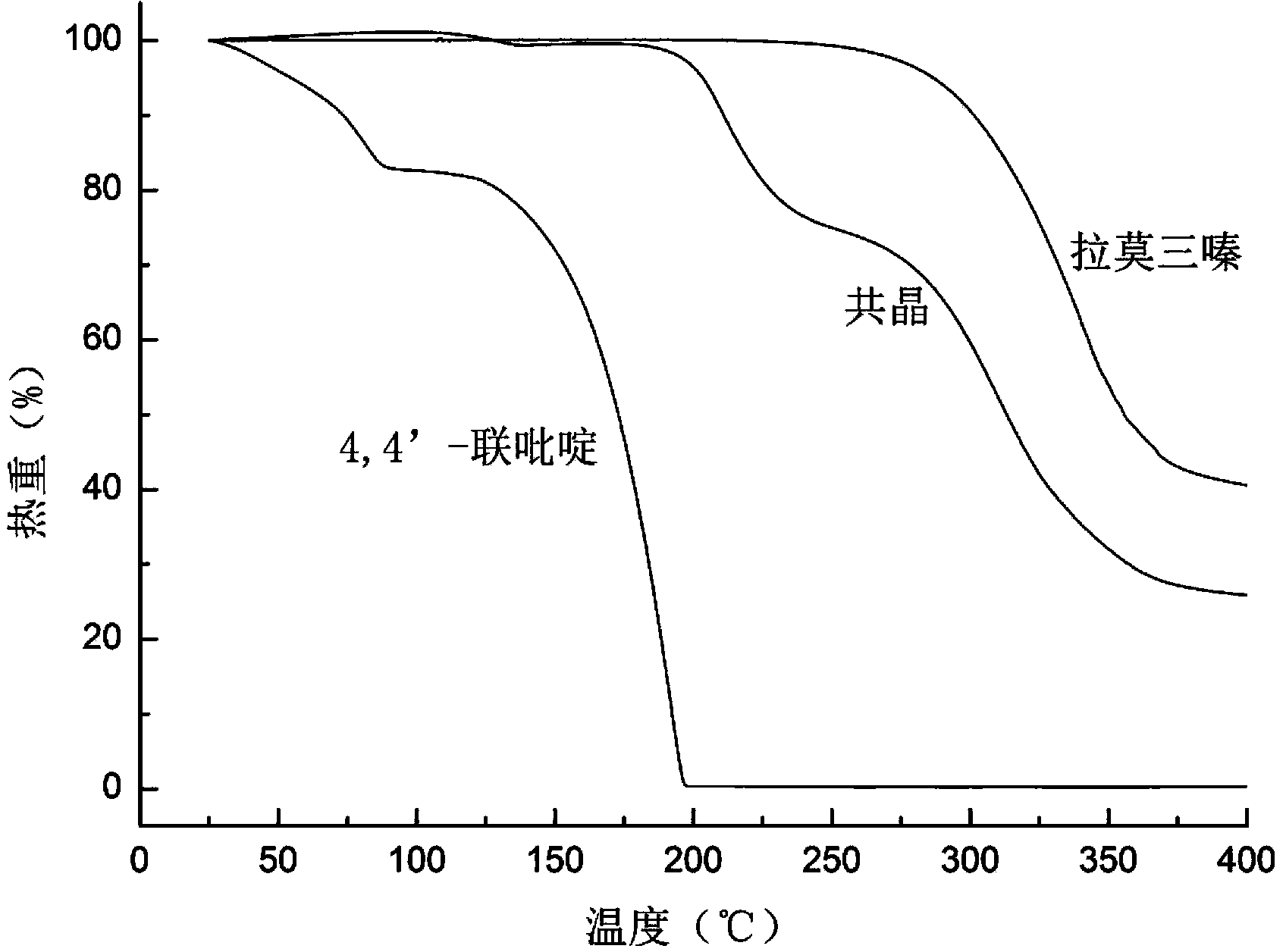
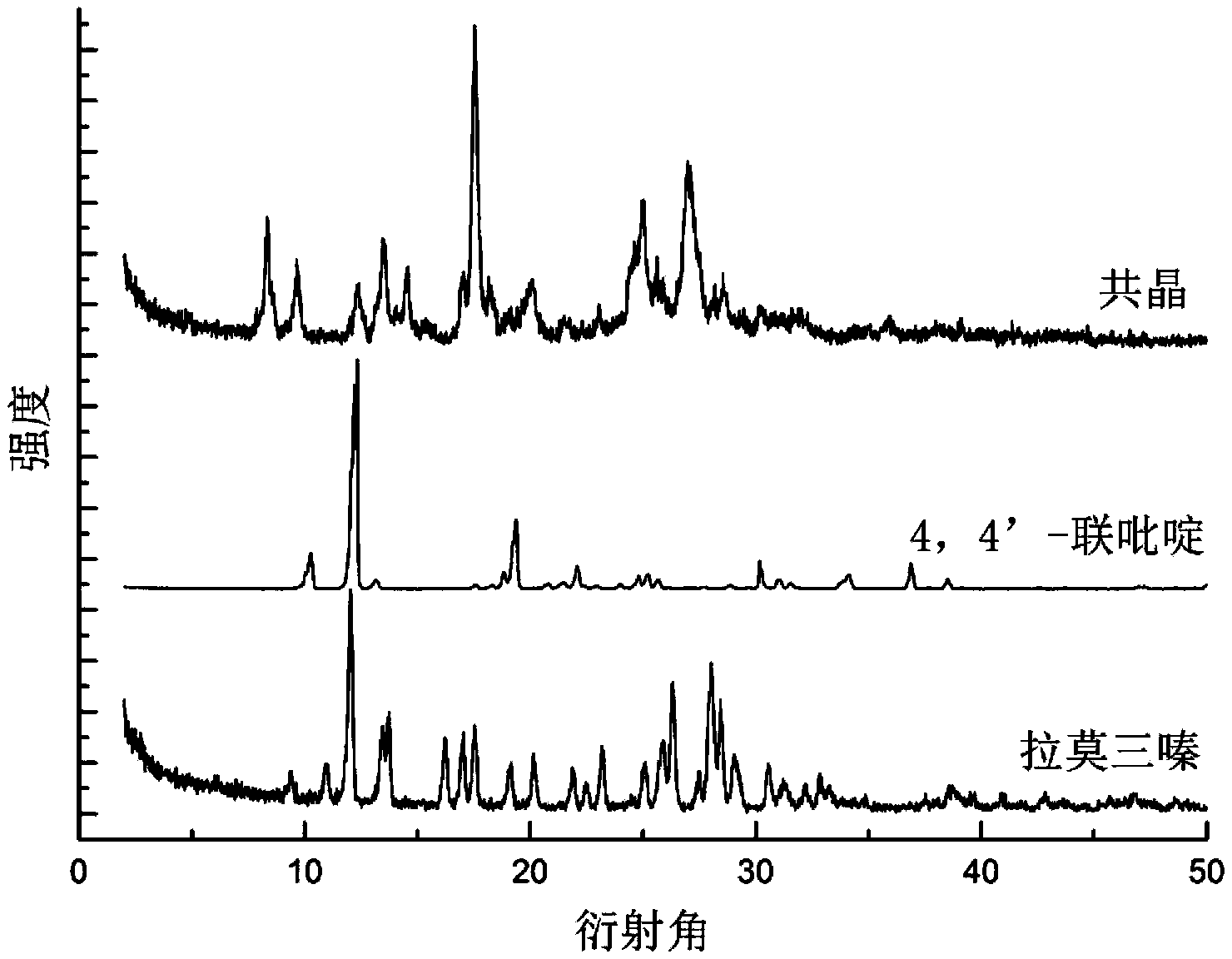
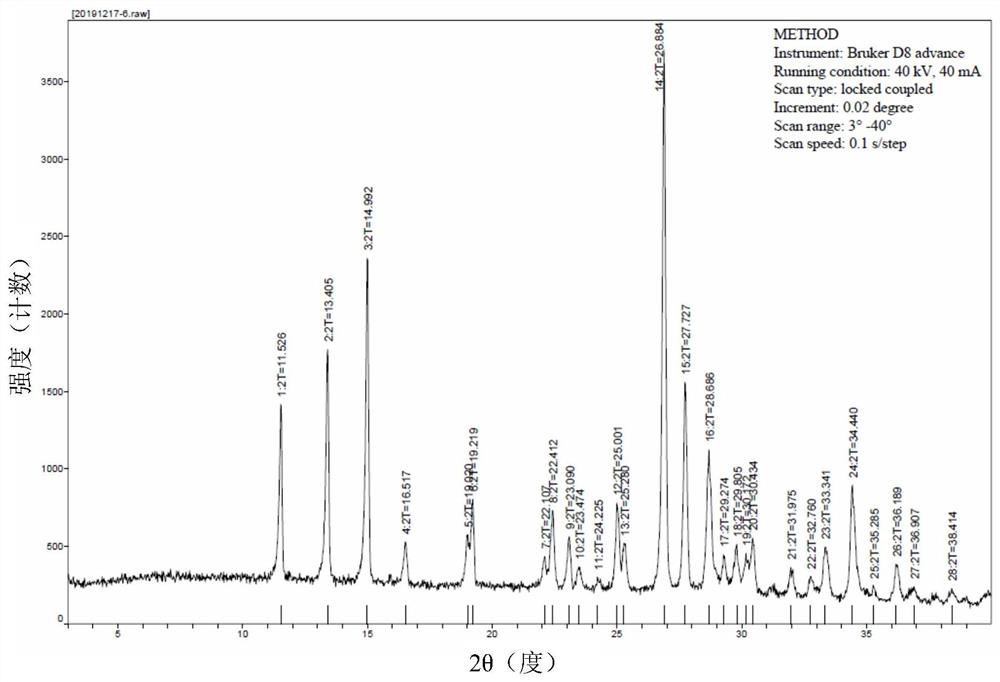
Novel lamotrigine pharmaceutical co-crystal and preparation method thereof
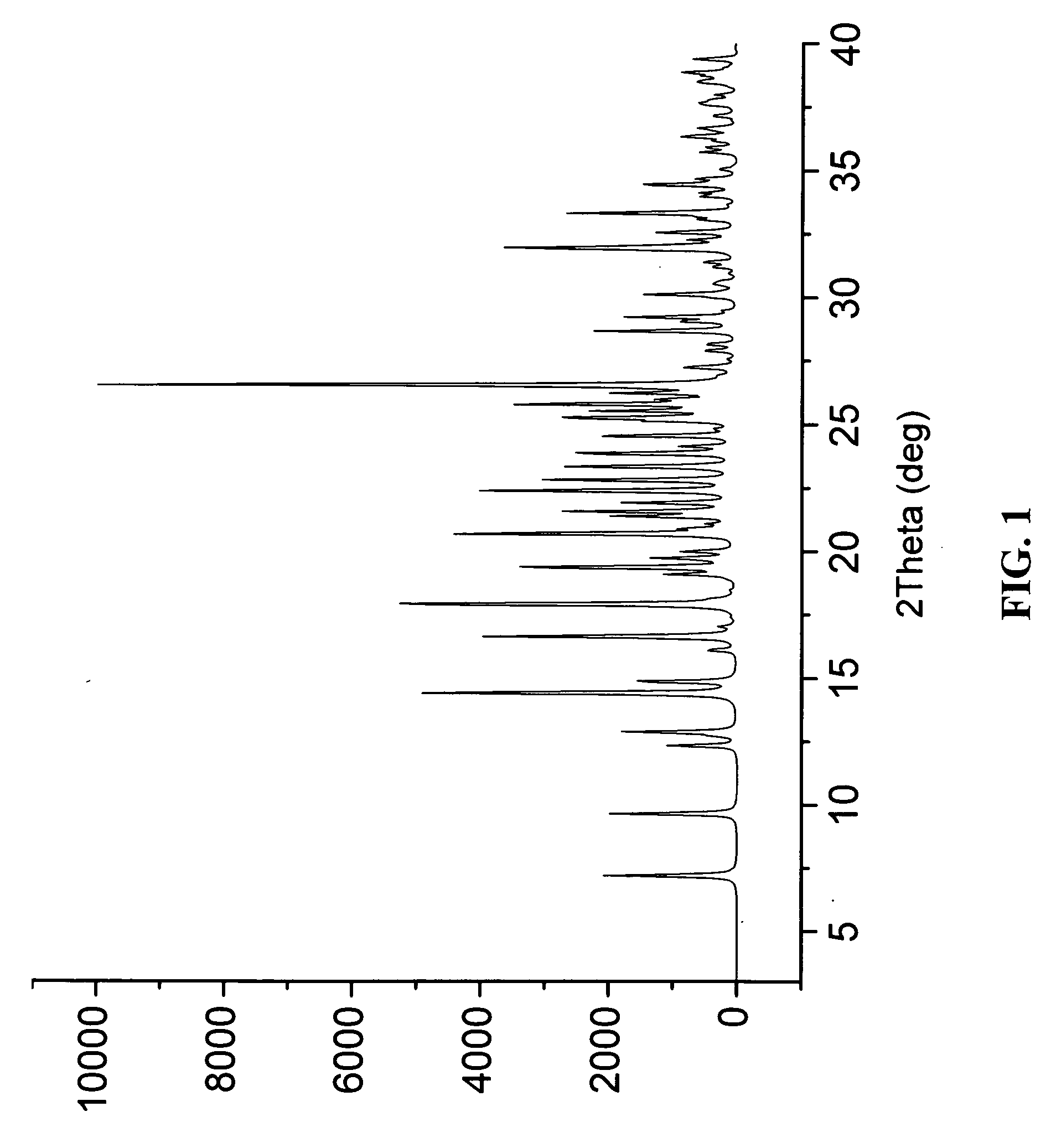
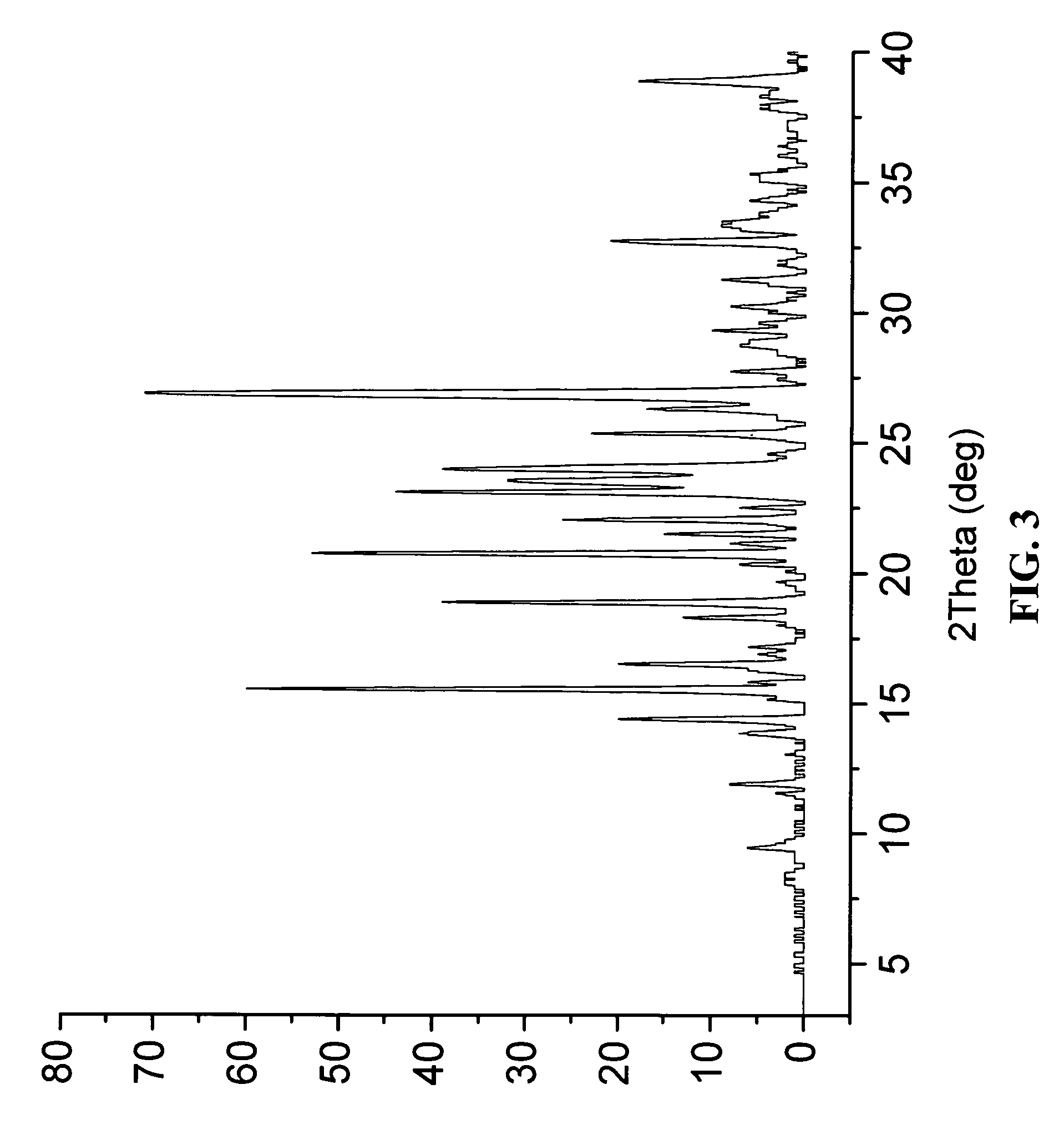
The invention relates to a novel lamotrigine pharmaceutical co-crystal and a preparation method thereof. A PXRD (Powder X Ray Diffractio) of the lamotrigine pharmaceutical co-crystal shows a series of characteristic peaks in 8.3+ / -0.2, 9.7+ / -0.2, 12.4+ / -0.2, 13.5+ / -0.2, 14.0+ / -0.2, 14.5+ / -0.2, 17.0+ / -0.2, 17.5+ / -0.2, 18.2+ / -0.2, 20.0+ / -0.2, 23.0+ / -0.2, 24.6+ / -0.2, 25.0+ / -0.2, 25.6+ / -0.2, 27.0+ / -0.2, and 28.5+ / -0.2. The lamotrigine pharmaceutical co-crystal is prepared through a solution mediate transformation or a grinding method. The prepared pharmaceutical co-crystal remains the characteristic of the traditional raw medicine on treating refractory epilepsy and also shows remarkable improvement on solubility, stability and bioavailability.
Owner:TIANJIN UNIV
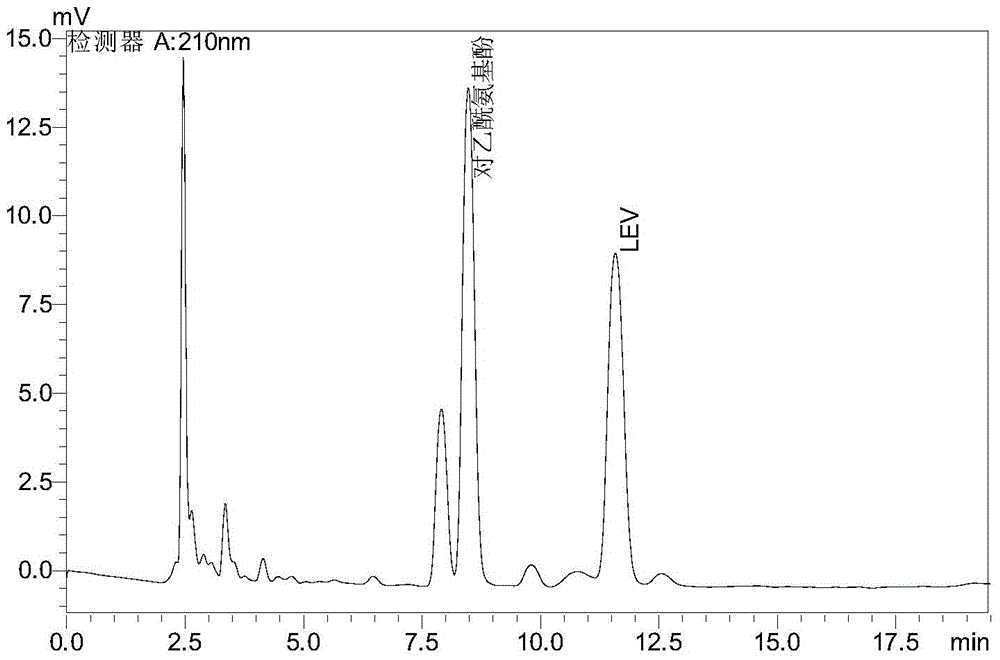
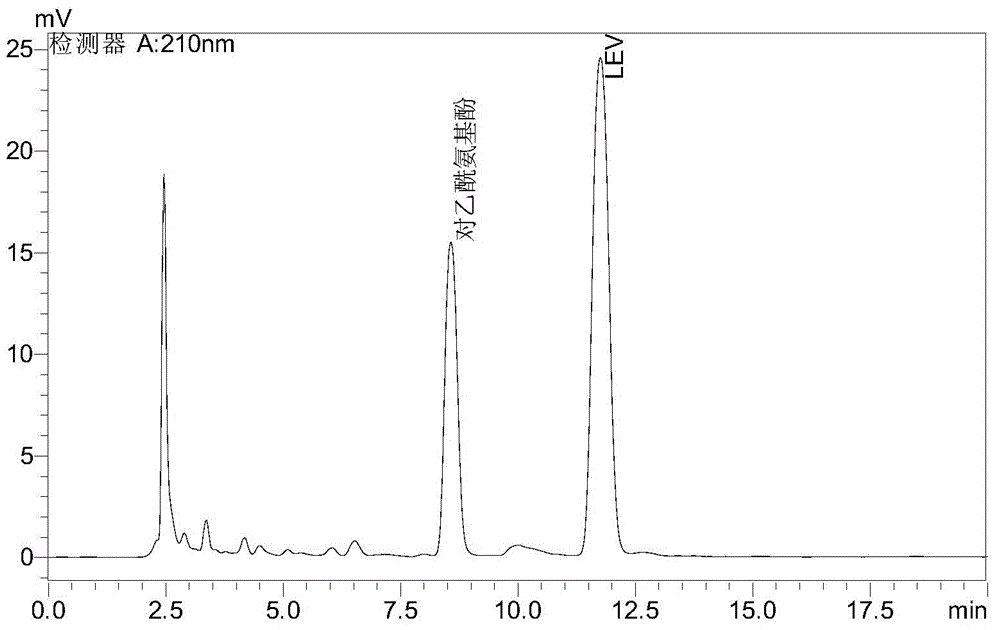
Method for detecting levetiracetam in breast milk
The invention provides a method for detecting lamotrigine in breast milk. The method comprises the following steps: 1) preparation of a tested object: taking the test breast milk, adding ethyl acetate with five times of volume and uniformly mixing the materials, performing centrifugation, taking an ethyl acetate layer, drying the ethyl acetate layer, redissolving a mobile phase as the tested object; and 2)detection: taking the tested object in the step 1) and detecting by high performance liquid chromatography, wherein the detection condition is characterized in that a stationary phase is C18 chromatographic column; a mobile phase is a methanol-inorganic salting solution, the volume ratio of the stationary phase to the mobile phase is 17:83, the pH value of the inorganic salting liquid is 4.5, and the detection wavelength is 210 nm. The method provided by the invention can accurately detect levetiracetam in breast milk, has the advantages of rapid and simple process, strong specialization, high sensitivity and good stability, can be used for clinically detecting the concentration of levetiracetam, and provides guidance for woman with breast nursing who administrates levetiracetam.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Kit and detection method for accurately determining blood concentration of multiple antiepileptic drugs in human serum
The invention discloses a kit and a detection method for accurately determining the blood concentration of multiple antiepileptic drugs in human serum. The kit mainly comprises a calibration product mother liquor, methanol or acetonitrile is used as a diluent to prepare the calibration product mother liquor comprising six concentration points, the calibration product mother liquor includes lamotrigine, phenobarbital, oxcarbazepine, carbamazepine and phenytoin in order; the kit also comprises an internal standard, a quality control material, a matrix correction solution and an extract, whereinthe extract is a tert-butyl methyl ether solution, a reconstitution solution and a mobile phase. Complex purification steps are not needed, and the required analysis time is short; and the detection speed is increased.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Composition and method for compounded therapy
The present embodiments relate to topically delivered compounded medications. A transdermal cream may provide the effective topical administration of multiple medications simultaneously. The transdermal cream may include low concentrations of local anesthetics, a NSAID, an anticonvulsant, and / or other active ingredients. For instance, the transdermal cream may include lidocaine, prilocaine, meloxicam, and lamotrigine and / or topiramate. Alternatively, the transdermal cream may include a lidocaine / prilocaine base cream to which is added a fine powder of one or more ground up medications to form a compounded medication. The compounded medication in powder form may be generated from grinding up tablets of NSAIDs, anticonvulsants, nerve depressants, antidepressants, muscle relaxants, NMDA receptor antagonists, opiate or opioid agonists, and / or other agents. The compounded medication in powder form may include meloxicam, lamotrigine, topiramate, and / or other active ingredients. In another aspect, the present embodiments relate to methods of compounding medications and transdermal creams or gels.
Owner:CMPD LICENSING
Water Dispersible Pharmaceutical Formulation and Process for Preparing The Same
Water dispersible compressed tablets and a process for preparing the same. The tablet comprising about 0.1 to 50% w / w of lamotrigine or its pharmaceutically acceptable salts, solvates, hydrates or polymorphs, about 5 to about 50% w / w of a water-soluble diluent(s), about 15 to about 70% w / w of a water swellable diluent(s), optionally one or more pharmaceutically acceptable adjuvants, wherein the ratio of water-soluble diluent(s) to water swellable diluents(s) is from about 0.6 to about 0.9 and said composition is essentially free of disintegrant, superdisintegrant and swellable clay.
Owner:JUBILANT ORGANOSYS LTD
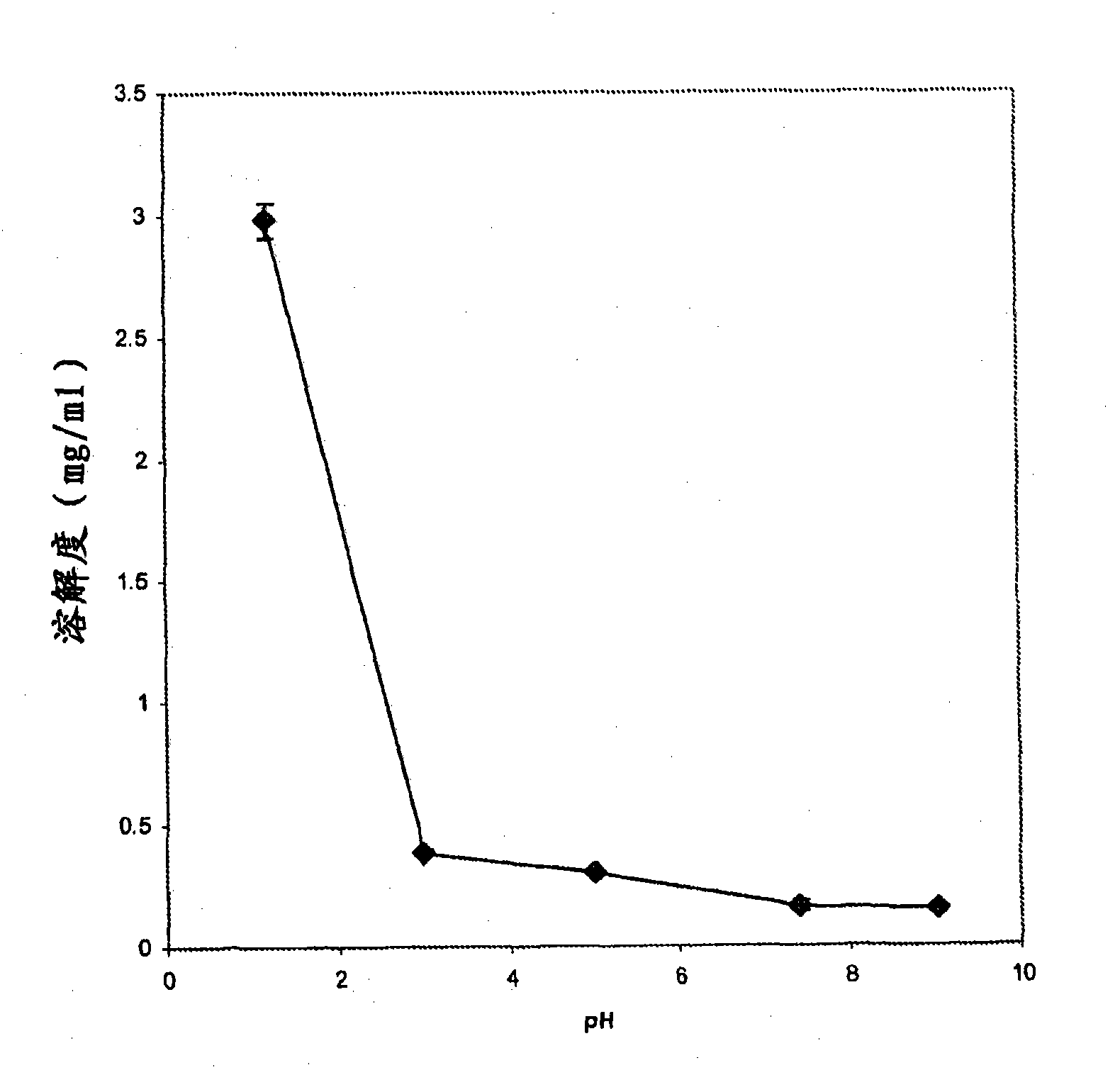
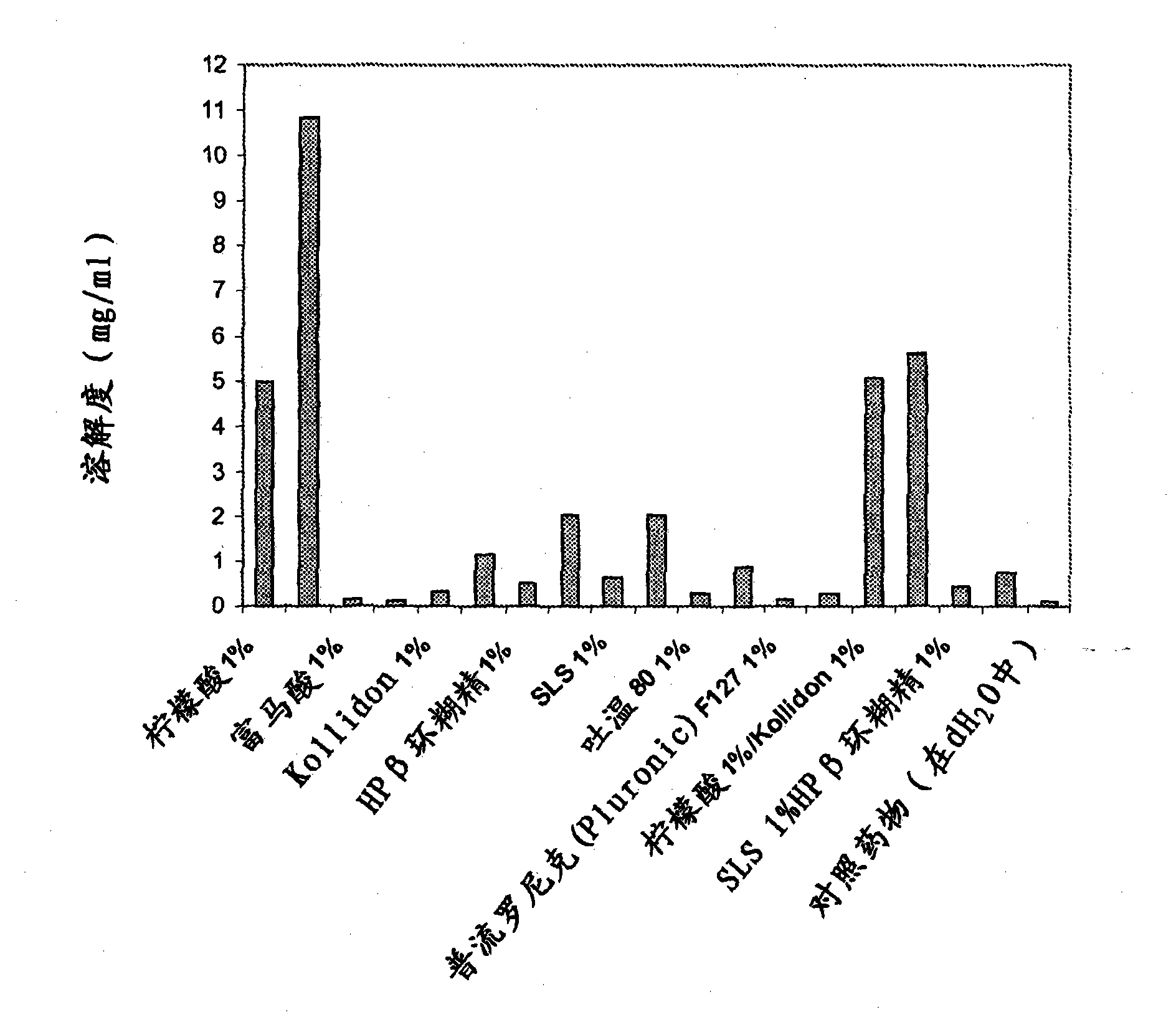
Crystalline Forms of lamotrigine
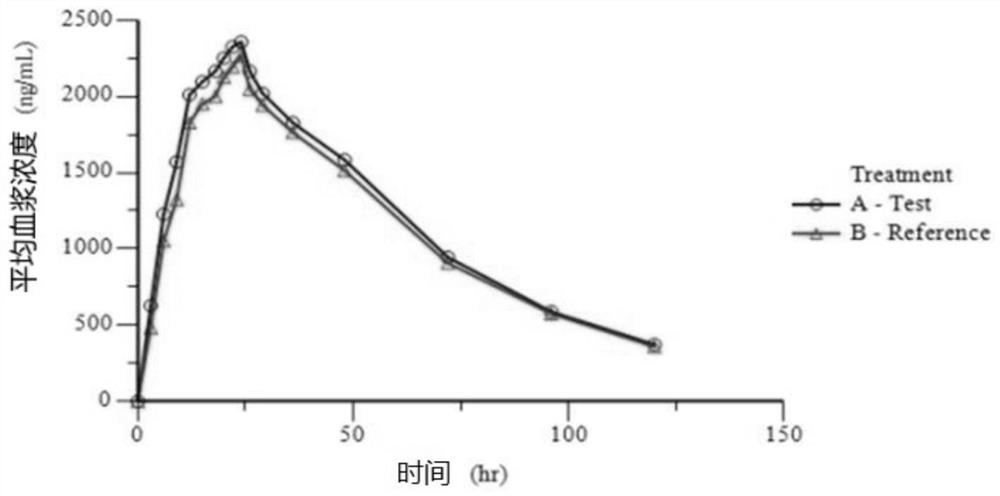
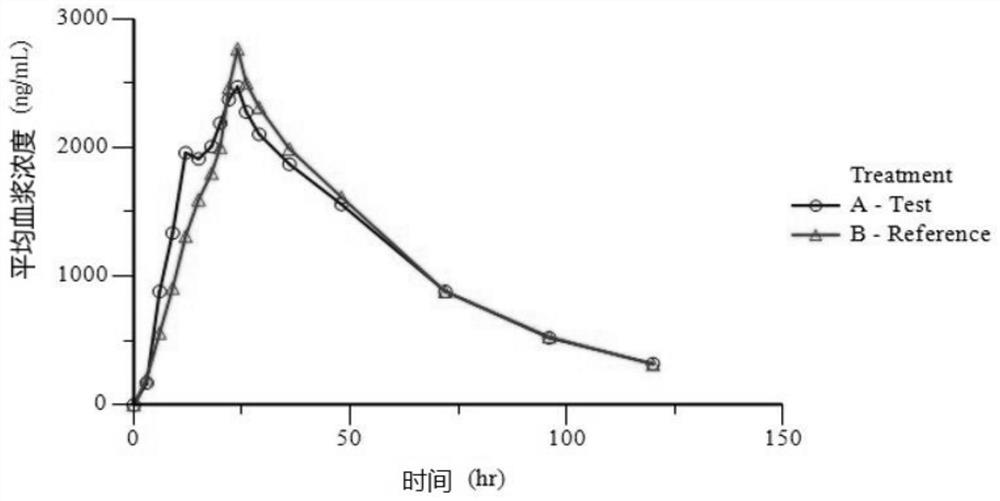
InactiveUS20090176787A1Improved in-vivo absorption profileImproved profileBiocideOrganic chemistryBlood concentrationMedicine
The invention is directed to novel crystalline forms of lamotrigine. These novel crystalline forms of lamotrigine have improved dissolution and in-vivo absorption profile, as compared to pure lamotrigine. These novel crystalline forms of lamotrigine provide a substantial increase in the blood concentration of lamotrigine, as compared to pure lamotrigine when administered to a subject. These novel crystalline forms of lamotrigine also provide a slower, steady build up of lamotrigine blood concentration suitable for sustained release of lamotrigine in-vivo, as compared to pure lamotrigine.
Owner:THAR PHARMA
Determination of lamotrigine by mass spectrometry
InactiveUS20110306080A1Low costRapid and cost-effective platformMicrobiological testing/measurementIsotope separationPresent methodMass spectrometry imaging
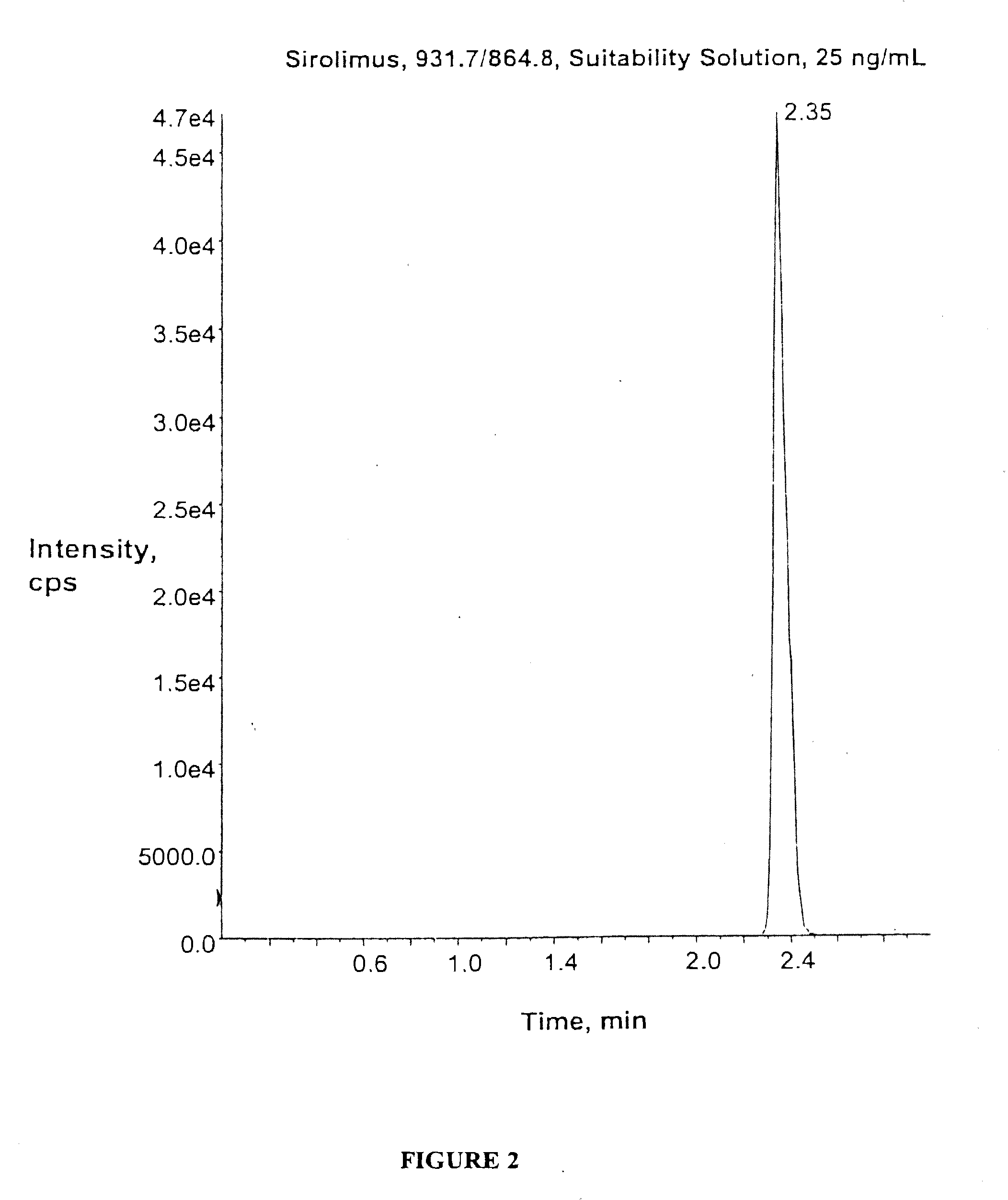
The present invention relates to compositions and methods for analyzing analytes of interest in liquid samples by mass spectrometry, and preferably in patient samples. Preferred analytes of interest include sirolimus (rapamycin), corticosteroids, bile acids and lamotrigine (lamictal). In one embodiment, by careful selection of target ions, a number of corticosteroids can be analyzed simultaneously and without interference from closely related molecules. In another embodiment, the present methods combine high turbulence liquid chromatography with mass spectrometry performed in positive and negative mode in a single assay to enable the detection and quantification of the composition of bile acid pools. By combining mass spectrometry and high-throughput chromatography, the methods and compositions described herein can provide a rapid, sensitive, and accurate assay for use in large clinical laboratories.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
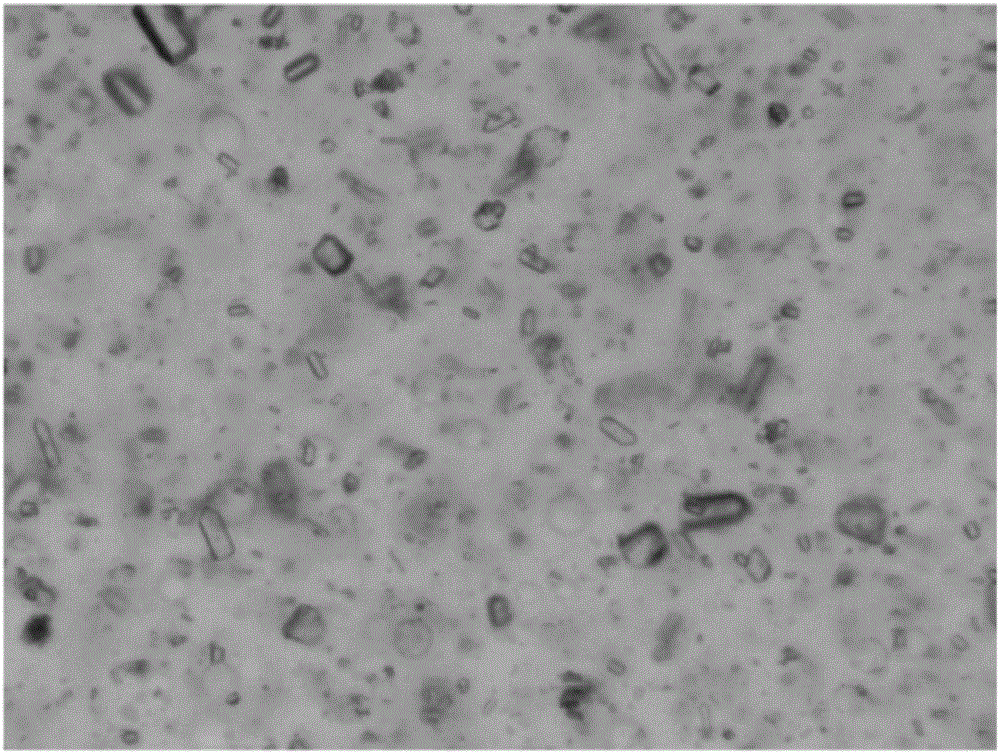
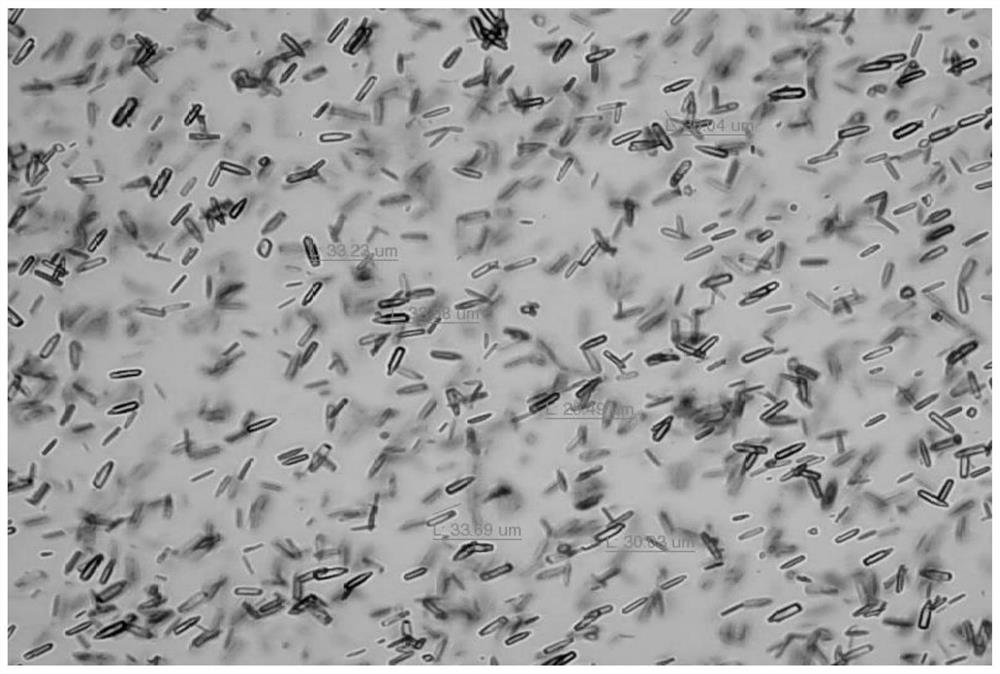
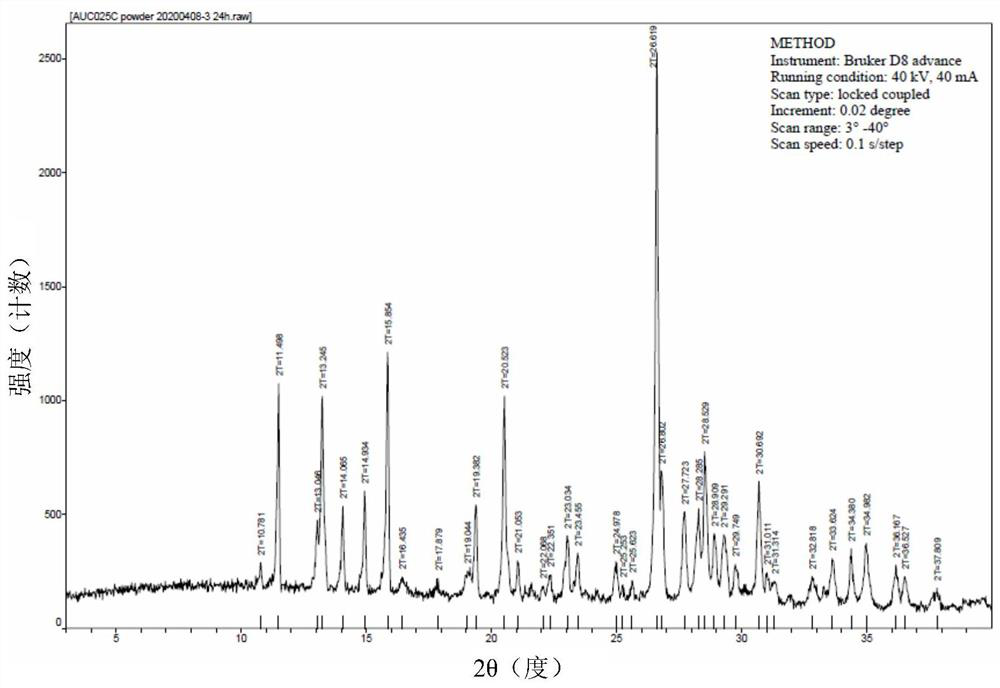
Crystal form of lamotrigine hydrate, preparation method thereof and composition containing same
ActiveCN113214177AOrganic compounds purification/separation/stabilisationNervous disorderTriazineLamotrigine
Owner:SHANGHAI AUCTA PHARMA CO LTD
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
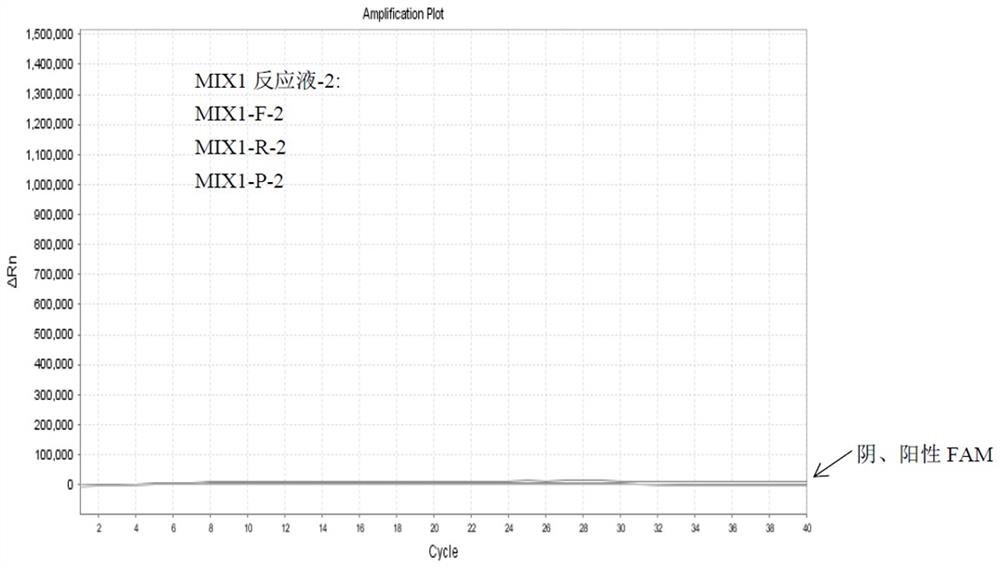
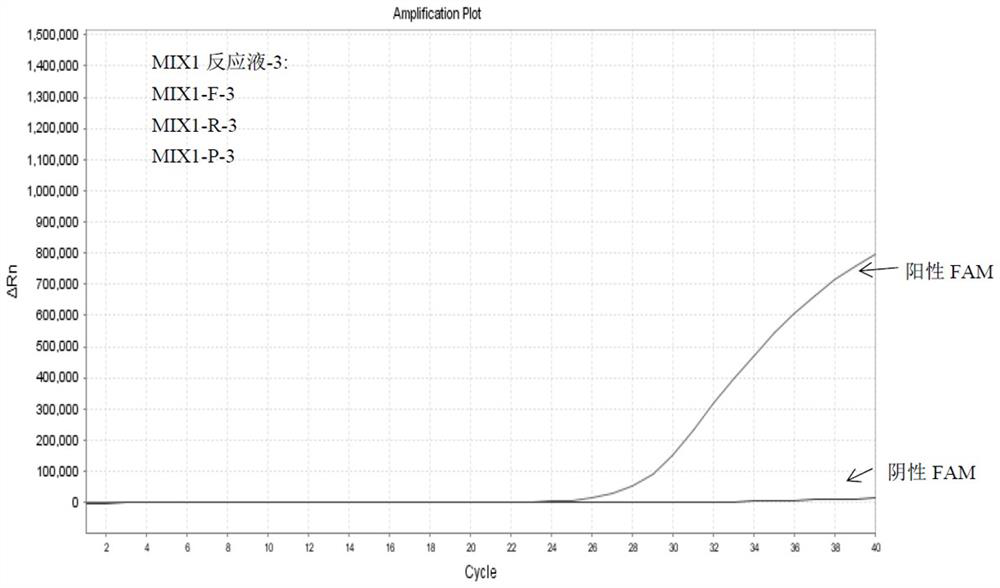
Detection method of HLA-B*1502 gene, detection kit and application thereof
The invention belongs to the technical field of biology, and provides a reagent for detecting human leukocyte antigen B site 1502 genotype (HLA-B*1502), application of the reagent in preparation of akit for guiding administration of antiepileptic drugs such as carbamazepine, oxcarbazepine, phenytoin and lamotrigine, a corresponding kit and a detection method of the kit. According to the HLA-B*1502 genotyping, three groups of MIX reaction solutions are detected by adopting a PCR-fluorescent probe method, and target nucleic acid molecules are circularly amplified through an amplification reaction, so that a fluorescence generation group is indirectly combined with an amplified target nucleotide sequence; the amount of fluorescence generated by the fluorescence generation group is determined, and the existence of the target nucleotide is determined. The detection reagent comprises a nucleic acid amplification system of three groups of MIX reaction solutions, wherein the nucleic acid amplification system comprises an upstream primer 1 and a downstream primer 1 which can be combined with target nucleotide, and an upstream primer 2 and a downstream primer 2 which can be combined with the target nucleotide; and three groups of probes 1 and probes 4 of a fluorescence detection system matched with the nucleic acid amplification system.
Owner:上海恩元生物科技有限公司
Enhanced formulations of lamotrigine
A once-a-day, extended-release formulation of lamothgine, exhibiting a significantly similar release rate throughout the Gl tract irrespective of the pH of the environment, is provided. The formulation comprises lamothgine, an organic acid, a release enhancing polymer and a release controlling polymer. The use of the formulation for the treatment of the neurological disorders is also disclosed.
Owner:SUPERNUS PHARM INC
Controlled release lamotrigine formulations
The present invention relates to controlled release formulations comprising lamotrigine and process for preparation thereof.
Owner:RANBAXY LAB LTD
Preparation method of compound lamotrigine subcutaneous implantable controlled-release glue rod
InactiveCN103989685AEasy to useImprove quality controlNervous disorderPharmaceutical delivery mechanismControlled releaseCurative effect
The invention relates to a compound lamotrigine subcutaneous implantable controlled-release glue rod, which contains lamotrigine, alprazolam and a controlled-release agent. The compound lamotrigine subcutaneous implantable controlled-release glue rod can be implanted into a loose part of the lower abdominal wall subcutaneous tissues of a patient by using small incision and trocar introduction, after the patient obtains satisfactory curative effect from acute treatment; the controlled-release glue rod is lasting in curative effect, economical and practical, and the curative effect can be controlled at about 1 year for each implantation; and the drug is guided by the controlled-release agent and releases slowly at a uniform speed through a medical silica gel film wall, so as to guarantee stable and effective blood drug level and achieve the purpose of therapy maintenance. The controlled-release glue rod is applicable to patients with all types of epilepsy (induced mental disorders), mood disorders and other indications for lamotrigine treatment.
Owner:郑玉华 +2
Application of composition of ginsenoside C-K and lamotrigine in preparation of anti-epilepsy medicines
InactiveCN105963311ALower doseGood effectNervous disorderHeterocyclic compound active ingredientsAntiepileptic drugLamotrigine
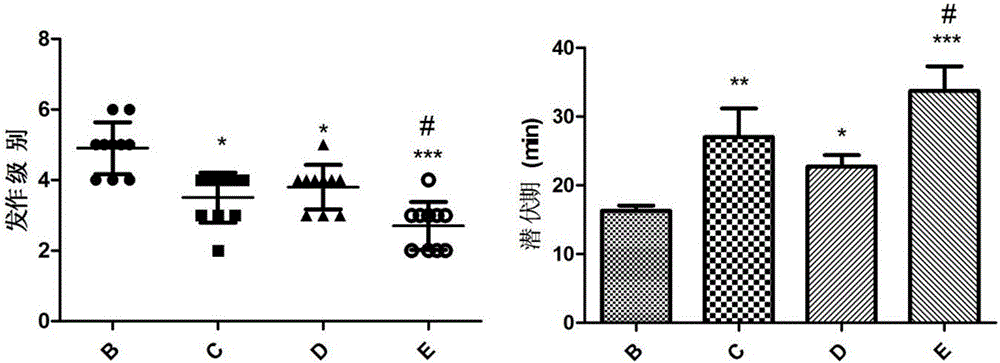
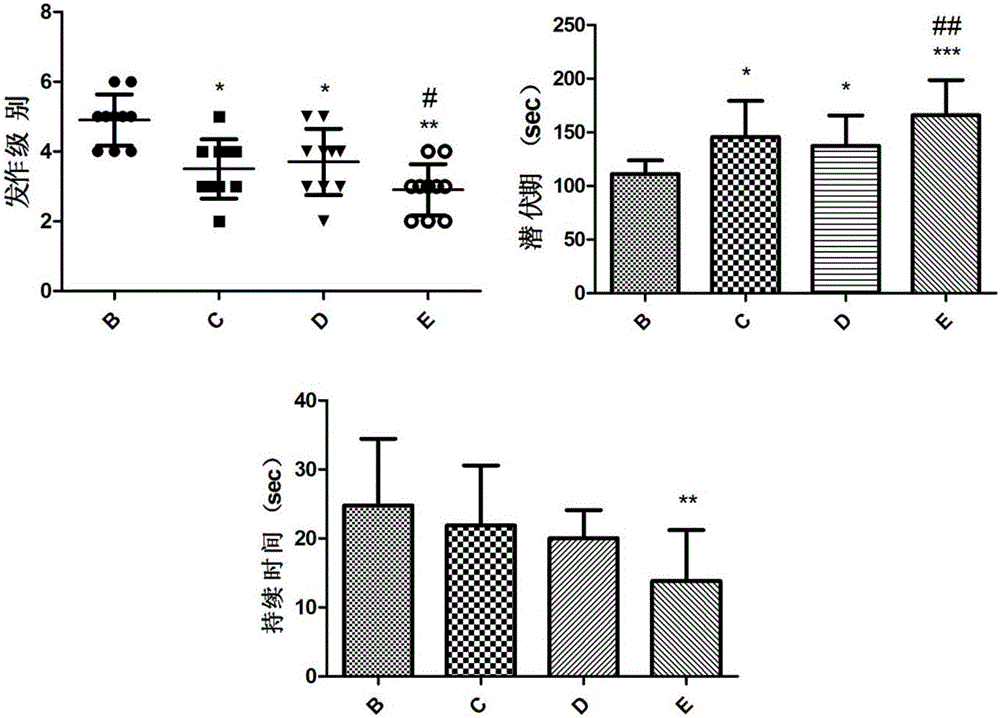
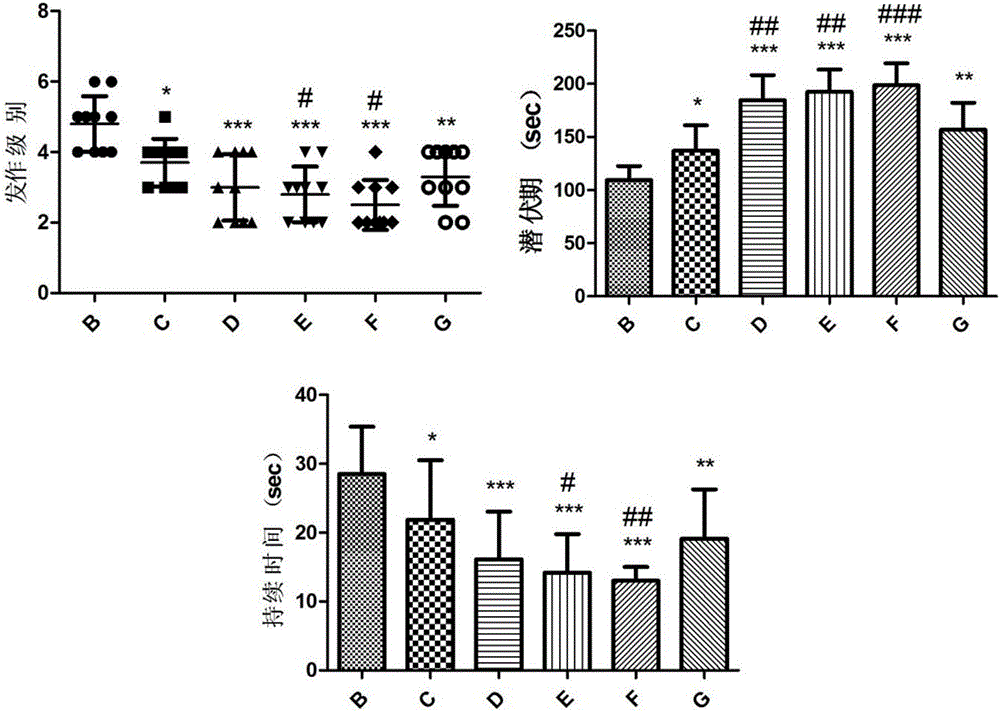
The invention relates to novel medicines and application, and particularly provides application of a composition of ginsenoside C-K and lamotrigine in preparation of anti-epilepsy medicines. Animal experiments prove that the composition of ginsenoside C-K and lamotrigine can be used for remarkably prolonging the incubation period of epilepsy, reducing the attack strength of epilepsy, shortening the duration of epilepsy and reducing the weight loss after attack, and indicate that the composition of ginsenoside C-K and lamotrigine can be used for relieving or controlling attack of epilepsy and inhibiting occurrence and development of epilepsy, and adverse medicine response caused by lamotrigine can be alleviated by reducing the dosage of lamotrigine.
Owner:欧阳冬生 +1
Stable lamotrigine pharmaceutical compositions and processes for their preparation
InactiveUS20070129549A1High yieldHigh purityOrganic active ingredientsOrganic chemistryDonepezilLamotrigine
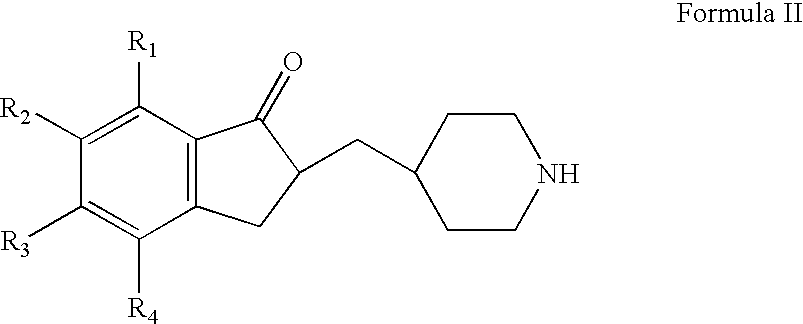
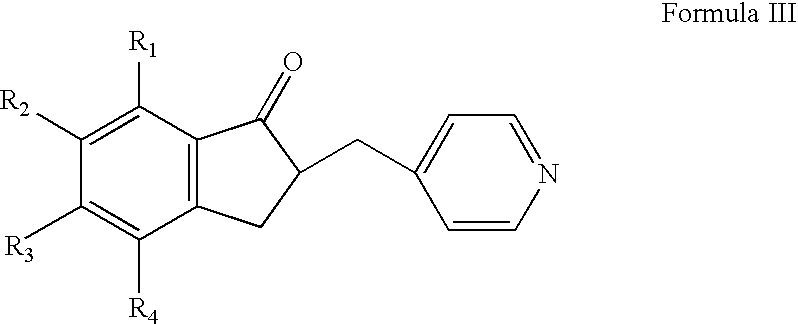
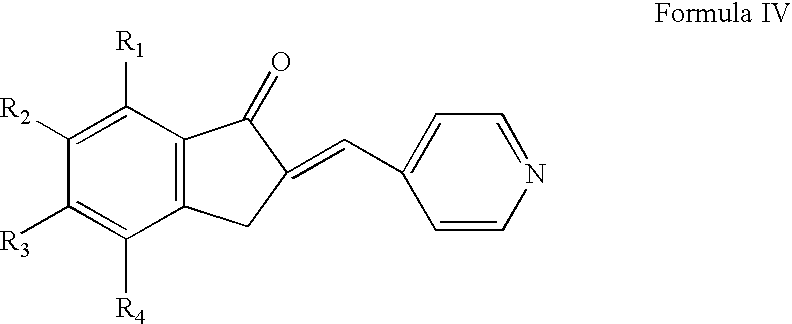
The invention relates to processes for the preparation of piperidylmethyl-indanones, and to the use of these compounds as intermediates for the preparation of benzyl-piperidylmethyl-indanones which are active compounds for the treatment of CNS disorders. The invention also relates to a process for the preparation of donepezil or a pharmaceutically acceptable salt thereof, and pharmaceutical compositions that include the donepezil or a pharmaceutically acceptable salt thereof.
Owner:YASKAWA DENKI KK +1
Preparation composition containing lamotrigine and preparation method thereof
ActiveCN112843012AReduce production capacityReduce manufacturing costNervous disorderPharmaceutical non-active ingredientsHuman bodyDrug release
The invention relates to a preparation composition containing lamotrigine. The preparation composition comprises a tablet core and an outer coating, wherein the outer coating contains a coloring agent with a mass percent of 0.1-15%. According to the invention, the coloring agent with a specific proportion is added into the outer coating, so a drug release curve can be adjusted, the in-vitro release curve of the preparation composition is similar to the in-vitro release curve of a commercially available product without punching or addition of any pore-foaming agent, and the in-vivo bioequivalence of a human body is ensured, so the requirement of drug replacement in clinical treatment is met, and production cost is greatly reduced.
Owner:SHIJIAZHUANG YILING PHARMA
Method for preparing lamotrigine
The invention discloses a method for preparing lamotrigine. Under the acid condition, aminoguanidine bicarbonate reacts with 2,3-dichlorobenzoylcyanide to generate an intermediate compound Schiff base. The method is characterized in that the intermediate compound Schiff base is directly heated in an alkaline carrier through microwaves to carry out cyclization reaction to obtain a lamotrigine product. The method disclosed by the invention has the advantages of high yield, few steps and high utilization rate of raw materials, and is simple and convenient in operation; by adopting a microwave-promoted solid-phase reaction to carry out cyclization, materials which are not environment-friendly are prevented from being used widely, thereby being more beneficial to environment protection, obviously lowering the production cost, and improving the production efficiency; and the invention has obvious social and economic benefits.
Owner:蒋勇
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com