Method for preparing lamotrigine
The technology of lamotrigine and dichlorobenzoyl cyanide is applied in the field of preparation of epilepsy drug lamotrigine, can solve the problems of difficulty in realizing industrialized production, long reaction steps, unfriendly environment, etc. The effect of fewer steps and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
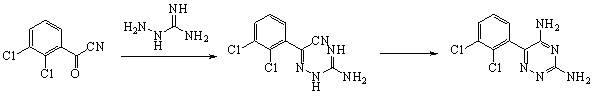
[0016] Slowly mix concentrated sulfuric acid (114g, 1.015mol) with 50ml of water, then add 2 drops of methanesulfonic acid, add aminoguanidine bicarbonate (10.2g, 0.075mol) under stirring at 0°C, and then add dropwise 2,3- Dichlorobenzoyl cyanide (10g, 0.05mol) in 20ml of acetonitrile solution, stirred at 28°C for 0.5h, heated to 50°C for 2h, cooled, filtered, washed with water, and drained, the resulting solid was added to 10g of silica gel, and added 2.5 g powdered NaOH, stir well, then heat in microwave for 1 minute, the maximum temperature is 110°C, the solid mixture is cooled, extracted (residual silica gel is recovered and used repeatedly), most of the solvent is evaporated, crystallized, and finally dried to obtain 10.8 g of white solid, the total yield is 78% based on 2,3-dichlorobenzoyl cyanide. Melting point: 216-217°C, HPLC: >99.9%.
Embodiment 2
[0018] Slowly mix concentrated sulfuric acid (57g, 0.501mol) with 50ml of water, add 2 drops of methanesulfonic acid, add aminoguanidine bicarbonate (10.2g, 0.075mol) under stirring at 0°C, and then add dropwise 2,3- Dichlorobenzoyl cyanide (10g, 0.05mol) in 20ml of acetonitrile solution, stirred at 28°C for 0.5h, heated to 50°C for 2h, cooled, filtered, washed with water, and drained, the resulting solid was added to 10g of silica gel, and added 2.5 g powdered NaOH, stir well, then heat in microwave for 1 minute, the maximum temperature is 110°C, the solid mixture is cooled, extracted (residual silica gel is recovered and used repeatedly), most of the solvent is evaporated, crystallized, and finally dried to obtain 8.0 g of white solid, the total yield is 58% based on 2,3-dichlorobenzoyl cyanide.
Embodiment 3
[0020] Slowly mix concentrated sulfuric acid (114g, 1.015mol) with 50ml of water, add aminoguanidine bicarbonate (10.2g, 0.075mol) and 2 drops of methanesulfonic acid under stirring at 0°C, and then add 2,3-dichloro Benzoyl cyanide (10g, 0.05mol) in 20ml of acetonitrile solution, stirred at 25-28°C for 0.5h, heated to 50°C for 2h, cooled, filtered, washed with water, and drained, the obtained solid was added to 10g of aluminum oxide, And add 2.5g of powdered NaOH, stir well, then heat in microwave for 1 minute, the maximum temperature is 110°C, the solid mixture is cooled, extracted, solvent evaporated, washed with water, recrystallized, and finally dried to obtain 9.7g of white solid , The total yield is 70% based on 2,3-dichlorobenzoyl cyanide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com