Crystal form of lamotrigine hydrate, preparation method thereof and composition containing same
A technology of lamotrigine and hydrate, applied in the field of crystal form, its preparation method and composition comprising the same, can solve the problems of poor solubility, low drug loading in solution dosage form, unable to meet clinical needs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Example 1 - Preparation of a Suspension Containing Lamotrigine Hydrate Form A Using Lamotrigine Particles of Different Particle Sizes
[0158] The particle size (D90) of 10 parts by weight is respectively the lamotrigine particle (purchased from Indian Arabindu Pharmaceutical Co., Ltd.) of 8 μm, 12 μm and 60 μm, and the xanthan gum of 3 parts by weight (purchased from U.S. Co., Ltd.), added to 1000 parts by weight of purified water to disperse evenly, and placed at 4°C for 24 hours to prepare a suspension. The obtained suspension was placed at normal temperature for 1 month, and the suspension and the crystal form in the suspension were checked at different time points, and the results are shown in Table 1:
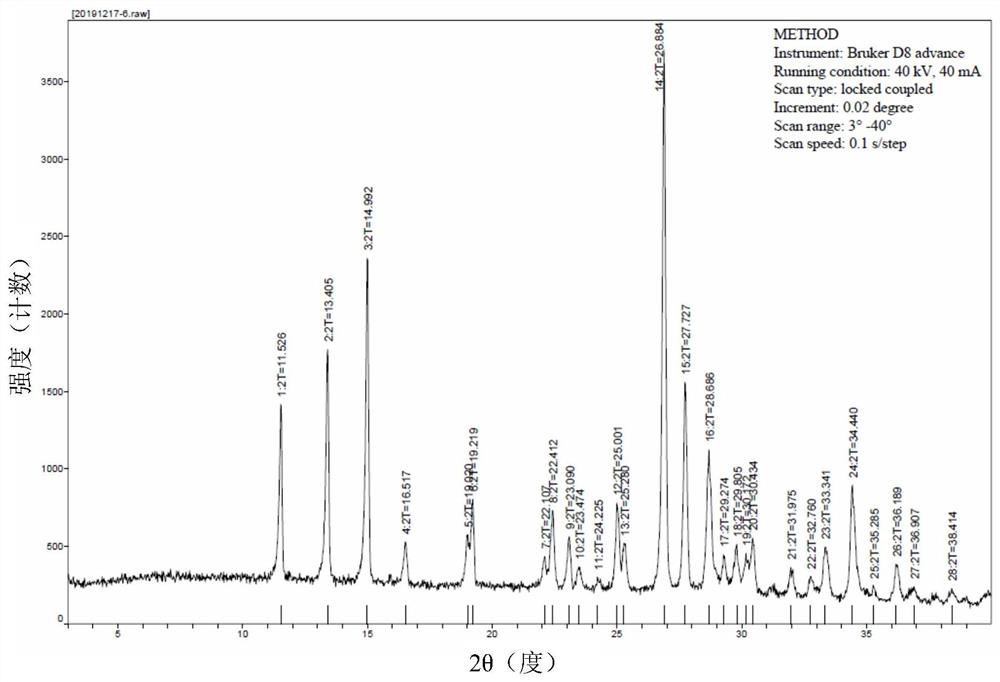
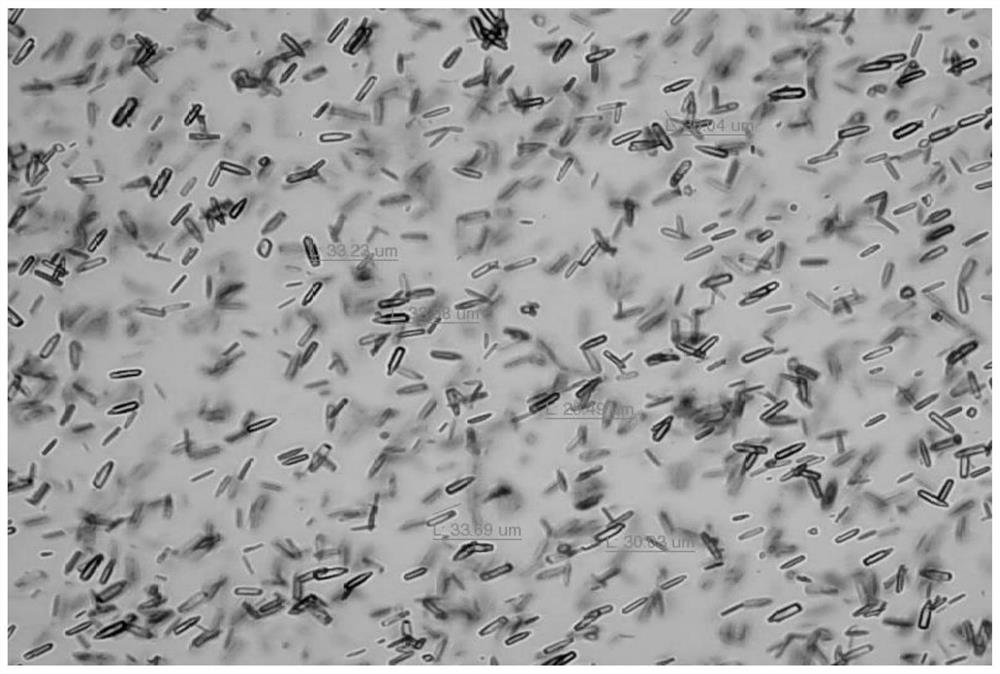
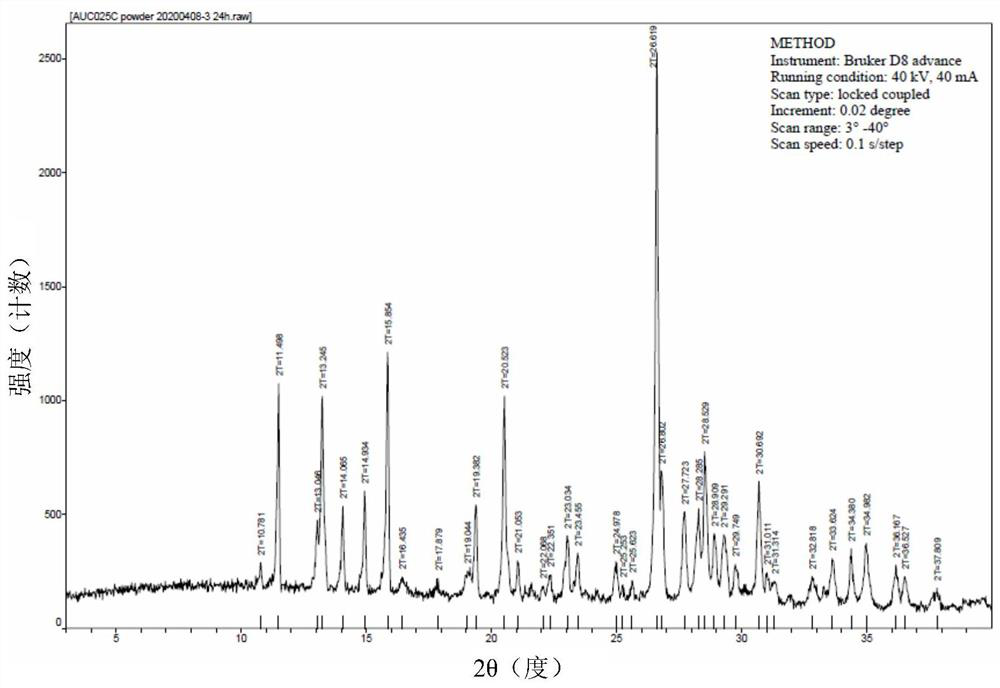
[0159] Unless otherwise specified, the XRPD results in the examples were all measured by a German Bruker D8 advance X-ray diffractometer, and the microscope results were all measured by a Nanjing Nanpai CM2000S microscope.
[0160] Table 1
[0161]
[0162]
...
Embodiment 2
[0164] Example 2 - Preparation of Suspensions Containing Lamotrigine Hydrate Form A Using Different Thickeners
[0165] 10 parts by weight of lamotrigine particles with a particle size (D90) of 8 μm, and 3 parts by weight of thickeners (xanthan gum, povidone, colloidal microcrystalline cellulose and sodium alginate) (where xanthan Glue was purchased from CP Kelco in the United States, povidone was purchased from BASF in Germany, colloidal microcrystalline cellulose was purchased from FMC in the United States, and sodium alginate was purchased from Qingdao Mingyue Seaweed Group) and added to 1000 parts by weight of purified water to disperse evenly , placed at 4°C for 24 hours to prepare a suspension. The obtained suspension was placed at normal temperature for 1 month, and the suspension and the crystal form in the suspension were checked at different time points, and the results are shown in Table 2:
[0166] Table 2
[0167]
[0168]
[0169] As shown in Table 2, usi...
Embodiment 3
[0170] Example 3 - Preparation of Suspensions Containing Lamotrigine Hydrate Form A Using Different Amounts of Thickening Agents
[0171] The particle diameter (D90) of 10 parts by weight is the lamotrigine particle of 8 μ m, and the xanthan gum that is respectively 0 weight part, 1 weight part and 5 parts by weight, joins the purified water of 1000 parts by weight and disperses evenly, puts A suspension was prepared by standing at 4°C for 24 hours. The obtained suspension was placed at normal temperature for 1 month, and the suspension and the crystal form in the suspension were checked at different time points, and the results are shown in Table 3:
[0172] table 3
[0173]
[0174] As shown in Table 3, using 1 and 5 parts by weight of thickener, the crystalline form in the suspension was Lamotrigine Hydrate Form A. No thickener is used, and the crystal form in the suspension contains unknown crystals after standing at room temperature for one week, and the stability of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com