Imine coupled covalent organic framework material and preparation method and application thereof
A technology of covalent organic framework and imine is applied in the field of porous materials to achieve good industrial application prospects, high crystallinity and high specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
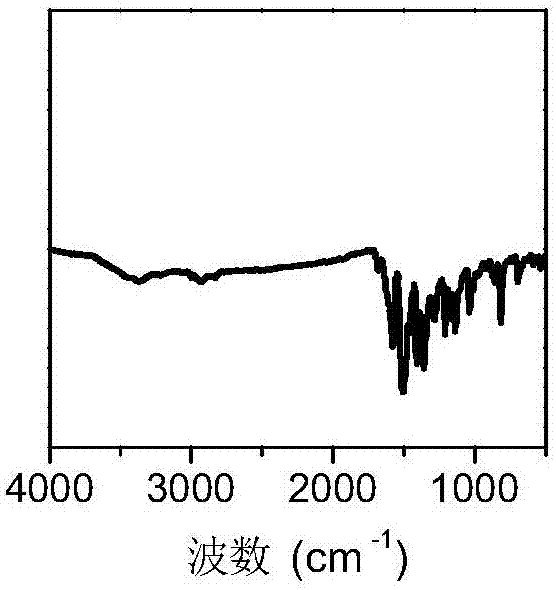
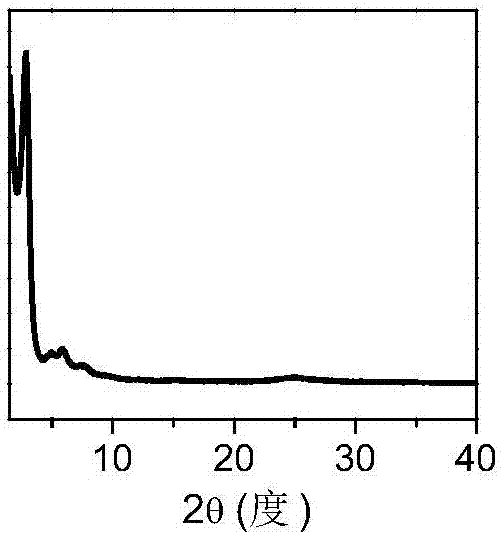
[0046] Dioxane (2.36mmol) and mesitylene (5.74mmol) glacial acetic acid (0.60mmol) were dispersed in distilled water (5.56mmol) to form a mixed solution; 2,4,6-tri(4-aminophenyl )-1,3,5-triazine (0.08 mmol, 28.3 mg) and 2,5-dimethoxy-terephthalaldehyde (0.12 mmol, 23.3 mg) were dispersed in the mixed solution. Under a nitrogen atmosphere, the resulting mixture was reacted at 120°C for 72 hours, filtered, and washed three times with dry tetrahydrofuran and acetone to obtain a solid powder; the solid powder was vacuum-dried at 80°C for 12 hours to obtain Highly crystalline, high specific surface area and pore volume imine-linked covalent organic framework material COF-1 with a yield of 88%.
[0047] The response is as follows
[0048]
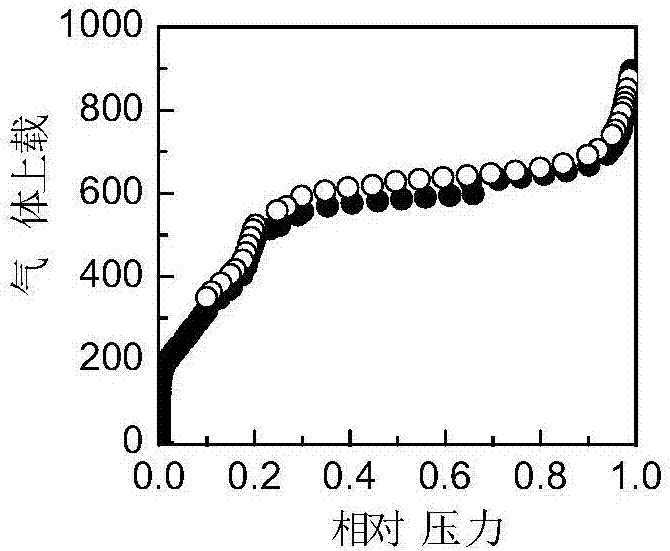
[0049] The specific surface area and pore size distribution of COF-1 were tested with a specific surface area and pore size analyzer (JW-BK 132F), and the measured specific surface area was 1630m 2 g -1 . The pore volume is 1.59cm 3 g -1...
Embodiment 2
[0051] Disperse 2.36mmol of dioxane, 5.74mmol of mesitylene and 0.60mmol of glacial acetic acid in 5.56mmol of distilled water to form a mixed solution; Hazine (0.08 mmol, 28.3 mg) and 2,5-dimethoxy-terephthalaldehyde (0.12 mmol, 23.3 mg) were dispersed in the mixed solution. Under the protection of nitrogen atmosphere, the obtained mixed solution was stirred and reacted at 120°C for 72 hours, filtered, washed with dry tetrahydrofuran and acetone three times respectively to obtain a solid powder; the solid powder was vacuum-dried at 80°C for 12 hours to obtain Imine-linked covalent organic framework material COF-1 with high crystallinity, high specific surface area and pore volume in 85% yield.
[0052] The specific surface area and pore size distribution of the obtained COF-1 were tested with a specific surface area and pore size analyzer (JW-BK 132F), and the measured specific surface area was 1357m 2 g -1 . The pore volume is 1.38cm 3 g -1 . The pore size is mainly di...
Embodiment 3
[0054] Disperse 2.36mmol of dioxane, 5.74mmol of mesitylene and 0.60mmol of glacial acetic acid in 5.56mmol of distilled water to form a mixed solution; Hazine (0.08 mmol, 28.3 mg) and 2,5-dimethoxy-terephthalaldehyde (0.12 mmol, 23.3 mg) were dispersed in the mixed solution. The mixture was sealed in a 10 mL microwave tube under nitrogen and heated with stirring at 100 °C for 1 h under microwave irradiation using a CEM Explorer microwave synthesizer. Filter, wash with dry tetrahydrofuran and acetone three times respectively to obtain a solid powder; dry the solid powder at 80°C for 12 hours in vacuum to obtain an imine-linked covalent organic compound with high crystallinity, high specific surface area and pore volume. The skeleton material, coded as COF-1, has a yield of 86%.
[0055] The specific surface area and pore size distribution of the obtained COF-1 were tested with a specific surface area and pore size analyzer (JW-BK 132F), and the measured specific surface area ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore volume | aaaaa | aaaaa |
| pore volume | aaaaa | aaaaa |
| pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com