Enhanced formulations of lamotrigine
A technology for lamotrigine preparations, applied in the field of enhanced lamotrigine preparations, capable of solving problems such as inability to enhance the release of lamotrigine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0039] Example 1. Lamotrigine Immediate Release ("IR") Granules for Control
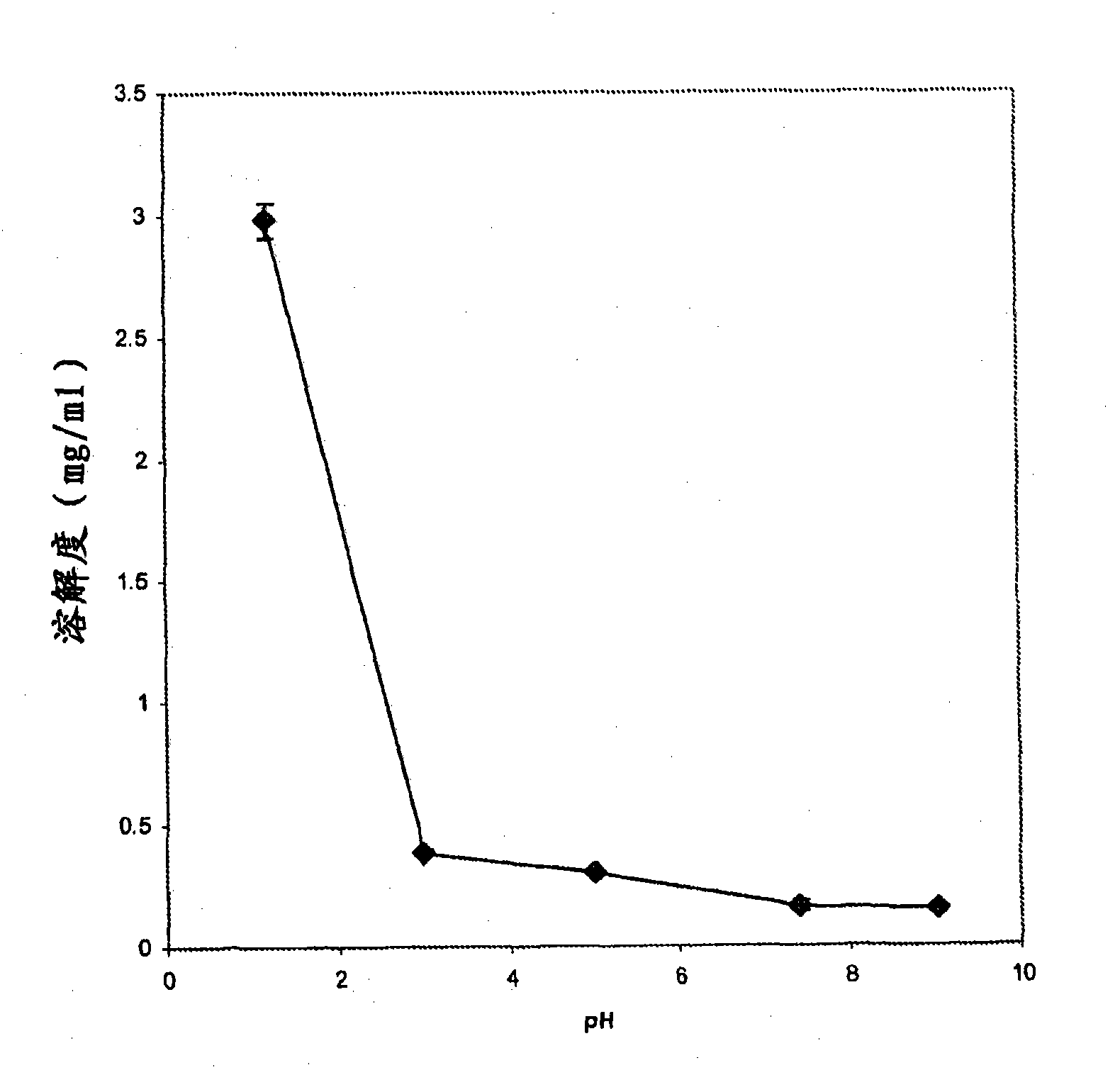
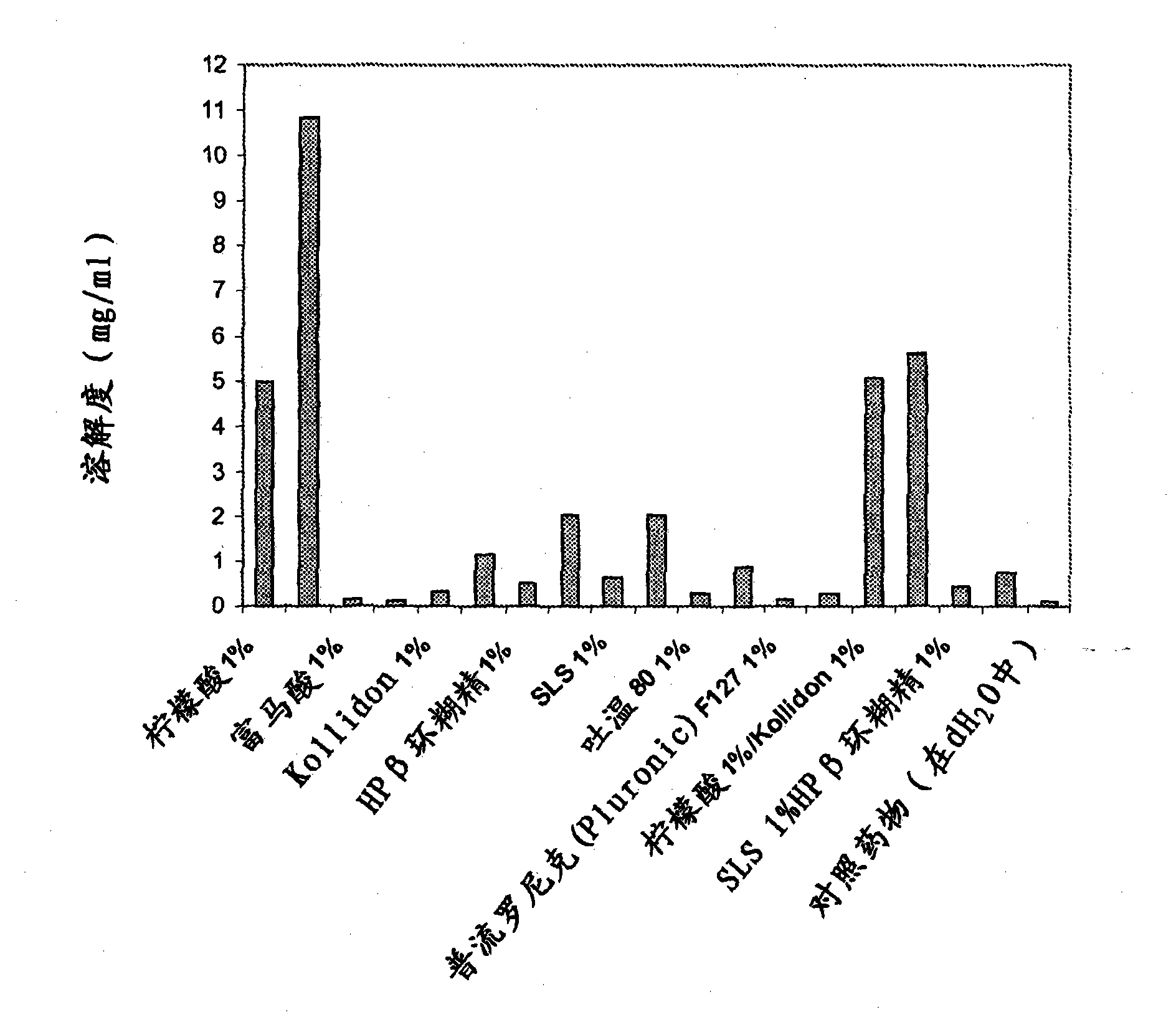
[0040] Table 1 provides the composition of two control formulations of lamotrigine IR particles (Formulation A and Formulation B). Formulations A and B were prepared as follows: The dry ingredients were mixed in a KG-5 high shear granulator (Key International, Englishtown, NJ). The granules were then extruded using a DG-L2Dome granulator (LCI Corporation, Charlotte, NC). The extrudate was pelletized using a QJ-400G pelletizer (LCI Corporation, Charlotte, NC). The granules were dried overnight in an oven at 40°C. Dissolution tests were carried out in acidic (pH 1.1) and phosphate buffer (pH 6.8) media. The dissolution profile is shown in image 3 with Figure 4 middle. Both formulations exhibited a pH-dependent release profile.
[0041]
Embodiment II
[0042] Embodiment II. Lamotrigine tablet composition of the present invention
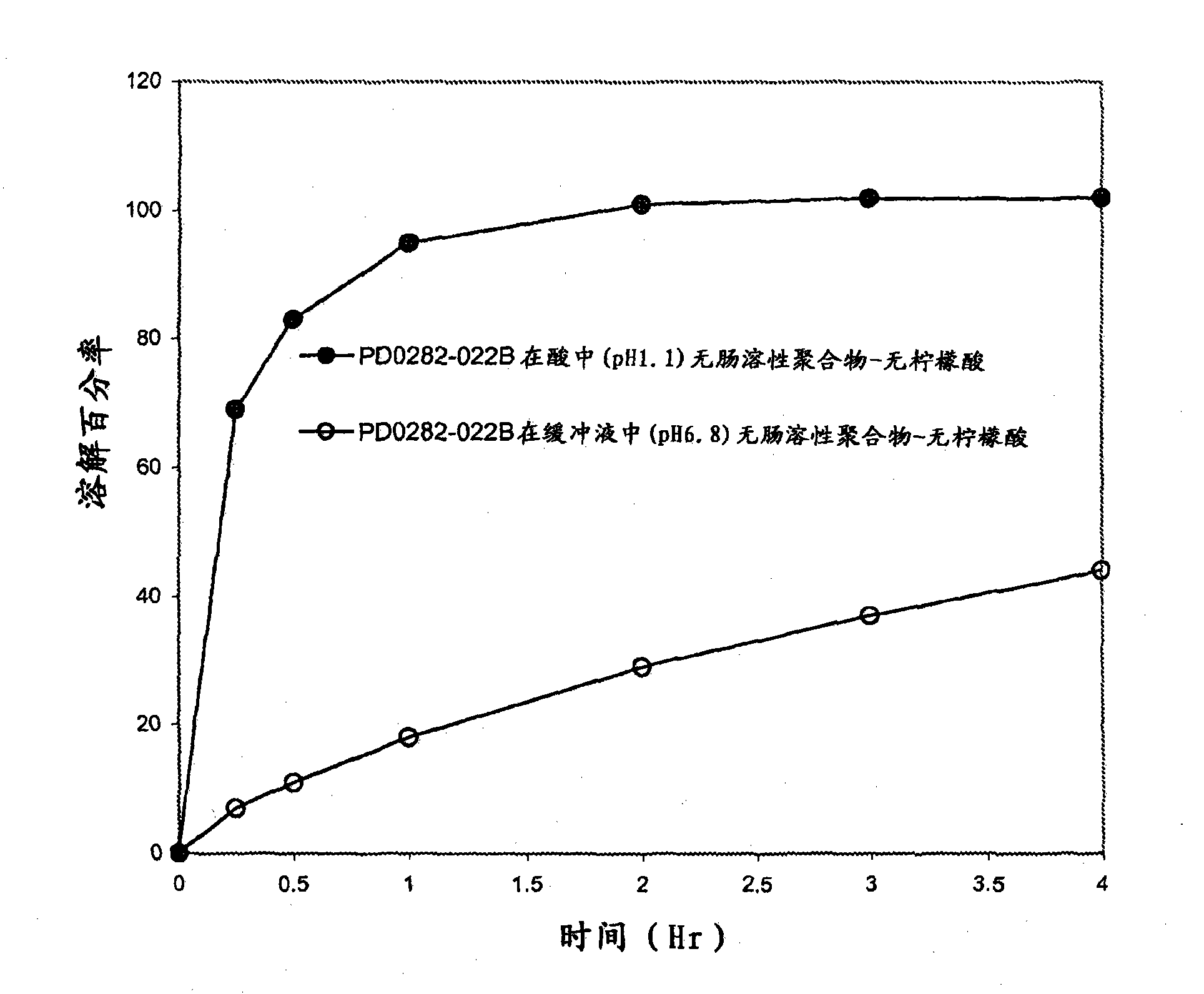
[0043] Table 2 provides the composition of Formulation C according to the invention. The formulation was granulated using a Key high shear granulator with water as the granulation liquid. Drug and excipients were added to the bowl and dry mixed at a paddle speed of ~260 RPM for approximately 1 minute. A total of 112 g of deionized water was added to achieve granulation. The batch was dried in a fluidized bed dryer (GPCG-1) with a final product temperature not exceeding 50°C. The moisture content of the granules was measured using a moisture analyzer and was 1.53% by weight. The dried granules are screened through a No. 18 mesh screen, blended with magnesium stearate and compressed on a rotary tablet machine. The target tablet weight and hardness were 333 mg and 8-10 kp, respectively. Tablet dissolution was tested in acidic (pH 1.1), phosphate buffered saline (pH 6.8), and media switch in which...
Embodiment III
[0045] Example III. Lamotrigine extended release (CR-F, CR-M, and CR-S) clinical batches.
[0046] Three inventive samples were produced to provide fast, medium, and slow sustained release profiles (lamotrigine CR-F, CR-M, and CR-S, respectively). The compositions of these samples are given in Table 3. These samples were produced according to the same processing steps as provided in Example II. The product was used in human pharmacokinetic (PK) studies.
[0047]
[0048] Image 6 The dissolution profile of these samples in phosphate buffered saline medium (pH 6.8) is shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com