Synthetic method of drug lamotrigine for curing bipolar disorder and epilepsy
A technology of bipolar affective disorder and lamotrigine, which is applied in the direction of organic chemistry, can solve the problems of complex production process, unsuitable for large-scale production, harsh reaction conditions, etc., and achieve high raw material utilization rate, high social and economic benefits , the effect of fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
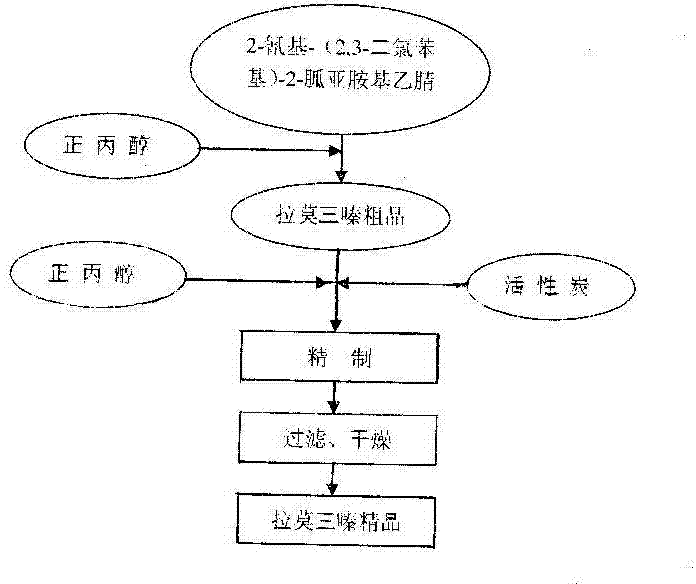
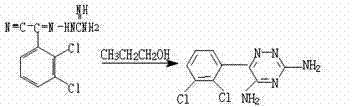
[0019] Example 1: First put 2-cyano-(2,3-dichlorophenyl)-2-guanidine iminoacetonitrile and n-propanol into the reaction tank at a ratio of 1:5, heat up and stir, and the reaction temperature reaches 65 0 C. After all the reactants are dissolved, time the reaction for 5 hours. After the reaction is completed, the solution is cooled to 5 0 8 hours of crystallization at C, filtered to obtain the crude product of lamotrigine, about 40% of moisture; then the crude product of lamotrigine was dropped into the refining tank, added 10 times the amount of n-propanol of the crude product of lamotrigine, stirred and heated up to 65 0 Above C, the crude product of lamotrigine is completely dissolved, and the temperature is lowered to 50 0 After C, add gac, add-on is 5% of lamotrigine crude product weight, stirring and reflux decolorization 30 minutes, filter while hot then, when filtrate is cooled to normal temperature, in 5 0 Crystallize at C for 10 hours; finally filter to obtain the w...
Embodiment 2
[0020] Example 2: Put 2-cyano-(2,3-dichlorophenyl)-2-guaniminoacetonitrile and n-propanol into the reaction tank at a ratio of 1:20, heat up and stir, and the reaction temperature reaches 60 0 C, reflux timing reaction for 8 hours, the reaction is completed, filtered while hot, and the filtrate is cooled to 10 0 C crystallized for 2 hours, filtered to obtain the wet crude product of lamotrigine, with a moisture content of about 40%; put the wet crude product of lamotrigine into the refining tank, add 5 times the amount of n-propanol solution of the crude wet product, and stir to heat up to 70 0 C reflux to dissolve all the crude product of lamotrigine and cool to 50 0 C, adding activated carbon, the addition is 10% of the crude product weight of lamotrigine, stirred and refluxed for decolorization for 0.5 hour, filtered while hot, and the filtrate was cooled at 10 0 C for 0.5 hours of crystallization, and finally filtered to obtain the wet product of lamotrigine, which was dr...
Embodiment 3
[0021] Example 3: First put 2-cyano-(2,3-dichlorophenyl)-2-guanidine iminoacetonitrile and n-propanol into the reaction tank at a ratio of 1:10, heat up and stir, and the reaction temperature reaches 90 0 C. After all the reactants are dissolved, time the reaction for 0.5 hours. After the reaction is completed, the solution is cooled to 20 0 0.5 hours of crystallization at C, filtered to obtain the crude product of lamotrigine, about 40% of moisture; then the crude product of lamotrigine was dropped into the refining tank, added 20 times the amount of n-propanol of the crude product of lamotrigine, stirred and heated up to 60 0 C, the crude product of lamotrigine is completely dissolved, and the temperature is lowered to 40 0 After C, add gac, add-on is 8% of lamotrigine crude product weight, stirring and reflux decolouring 30 minutes, filter while hot then, when filtrate is cooled to normal temperature, at 8 0 Crystallize at C for 3 hours; finally filter to obtain the wet r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com