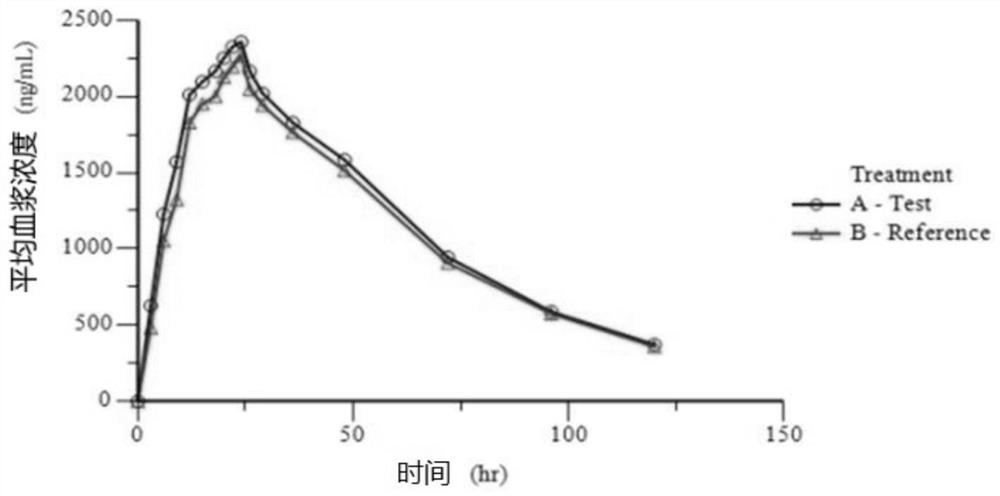
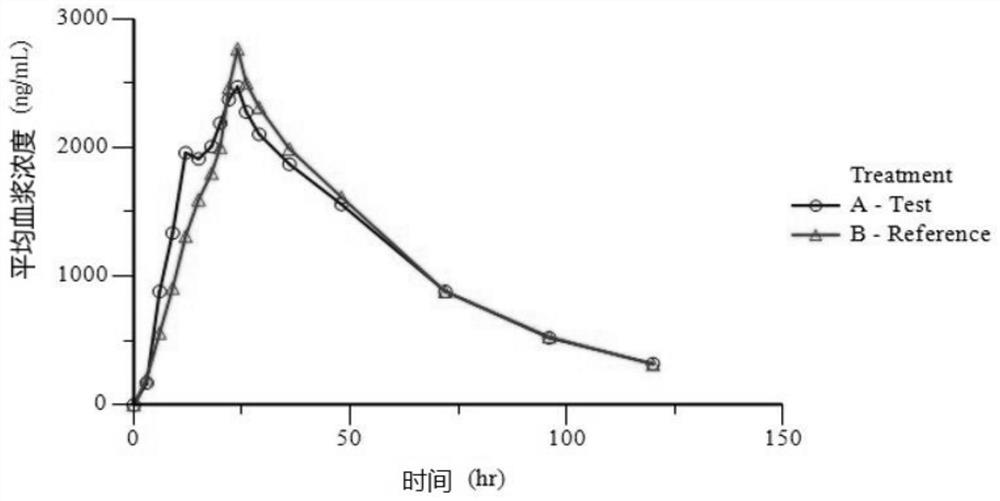
Preparation composition containing lamotrigine and preparation method thereof
The technology of lamotrigine and composition is applied in the field of preparation compositions containing lamotrigine, and can solve the problems of expensive laser drilling equipment, high production threshold and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] This embodiment provides a sustained-release tablet containing lamotrigine, wherein the proportion of the coloring agent accounts for 7.08% of the dry material weight of the outer layer coating; the specific composition of its raw materials is as follows:
[0051]
[0052]
[0053] Remarks: In the Eudragit L30D-55 aqueous dispersion, the solid content is 30% (w / w).
[0054] The present embodiment further provides the preparation method of the sustained-release tablet, specifically:
[0055] (1) Preparation of tablet core:
[0056] 1) Take by weighing lamotrigine, hypromellose, purified water and magnesium stearate according to the batch dosage in the above table;
[0057] 2) Lamotrigine and hydroxypropyl methylcellulose were passed through a 20-mesh sieve in turn, and then placed in a 4L high-speed shear wet granulator, and the stirring blade was turned on at a speed of 300 rpm, and mixed for 10 minutes;
[0058] 3) The rotation speed of the stirring paddle is s...
Embodiment 2
[0079] This embodiment provides a sustained-release tablet containing lamotrigine, wherein the proportion of the coloring agent accounts for 3.04% of the dry material weight of the outer layer coating; the specific composition of the raw materials is as follows:
[0080]
[0081] The preparation method is the same as in Example 1.
Embodiment 3
[0083] This embodiment provides a sustained-release tablet containing lamotrigine, wherein the proportion of the coloring agent accounts for 0.43% of the weight of the dry material of the outer layer coating; the specific composition of the raw materials is as follows:
[0084]
[0085]
[0086] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com