Lamotrigine dry suspension and preparation method thereof
A technology of dry lamotrigine and lamotrigine, which is applied in the field of dry suspension of lamotrigine and its preparation, and can solve problems such as difficulty in accurate dosage of patients, difficulty in separating crystals, and difficulty in dispersing drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
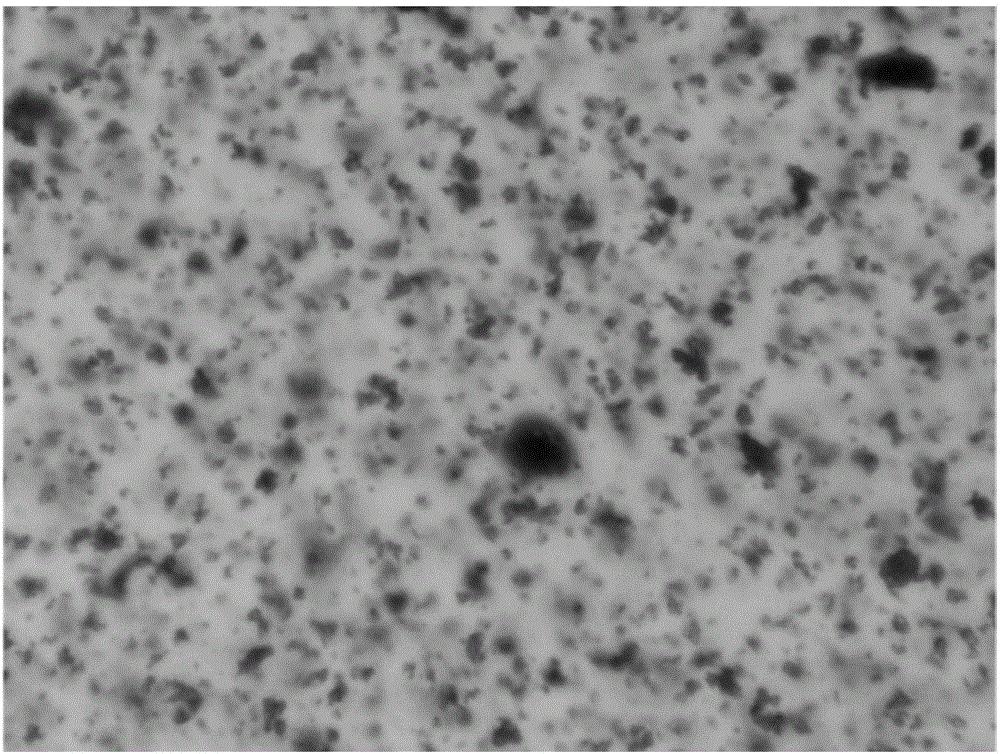
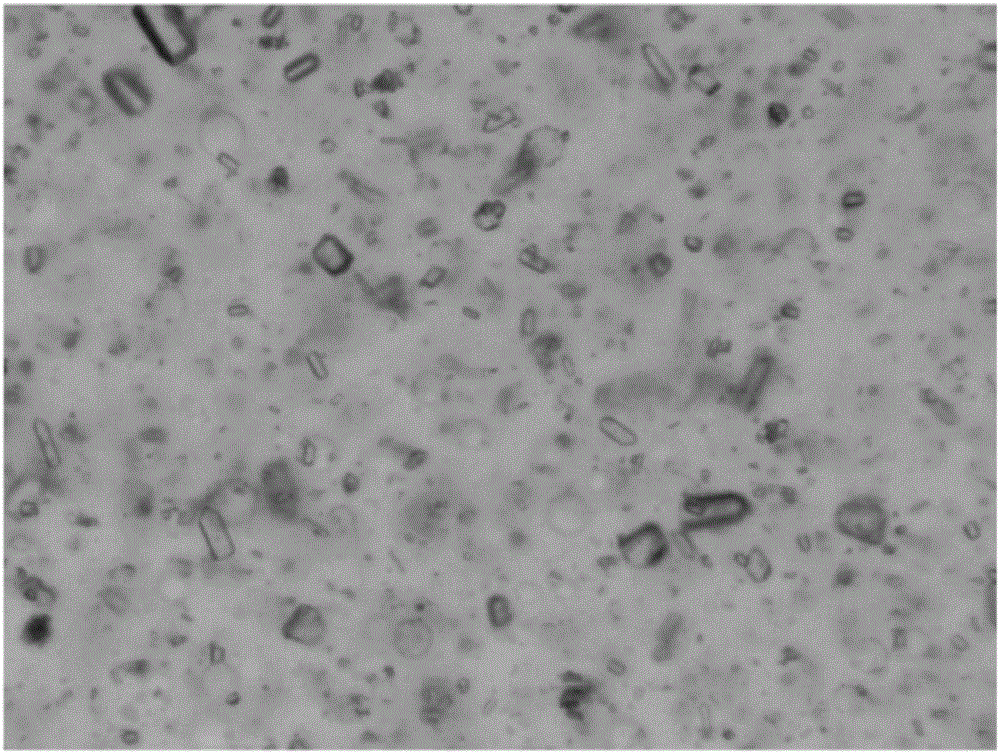
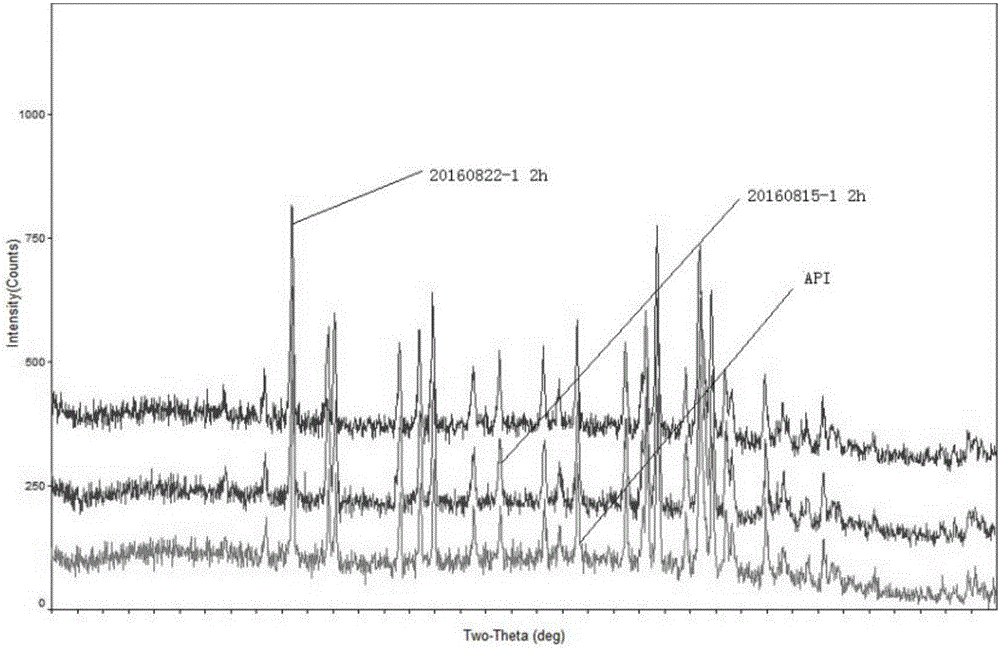
Image
Examples
Embodiment 1
[0087] Prescription 1
[0088]
[0089]
[0090] Dry powder mixing, the specific steps are:
[0091] 1. Pass the lamotrigine bulk drug and all auxiliary materials in the prescription through a 1016 micron sieve respectively.
[0092] 2. Weigh the prescribed amount of lamotrigine bulk drug and all auxiliary materials, put them in a mixer with a suitable size, and mix them for 10 minutes at a speed of 20 rpm.
[0093] 3. Use an automatic packaging machine to pack the prepared lamotrigine dry suspension. Obtain described lamotrigine dry suspension.
Embodiment 2
[0095] Adopt the prescription 1 of embodiment 1, use wet granulation.
[0096] The specific steps are:
[0097] 1. Pass the lamotrigine bulk drug and all auxiliary materials in the prescription through a 1016 micron sieve respectively.
[0098] 2. Weigh the prescribed amount of lamotrigine raw material and introverted excipients, mix them in a wet granulator with a suitable volume for 5 minutes, and then add an appropriate amount of water to granulate. The stirring shear rate of the wet granulator is 300rpm, and the granulation time is 5 minutes.
[0099] 3. After wet sizing with a sieve of 6350 microns, dry at 50°C in a fluidized bed until LOD<2%.
[0100] 4. Use a 1016um sieve to dry the granules, and place the prescribed amount of external excipients in a mixer with a suitable volume, and mix for 10 minutes at a speed of 20 rpm.
[0101] 5. Use an automatic packaging machine to pack the prepared lamotrigine dry suspension. Obtain described lamotrigine dry suspension.
Embodiment 3
[0103] Prescription 2
[0104]
[0105]
[0106] Prepared by the dry powder mixing method of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com