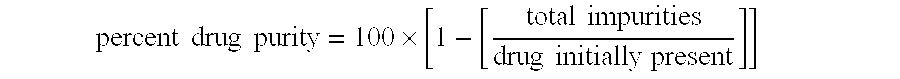Patents
Literature
551 results about "Physical stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
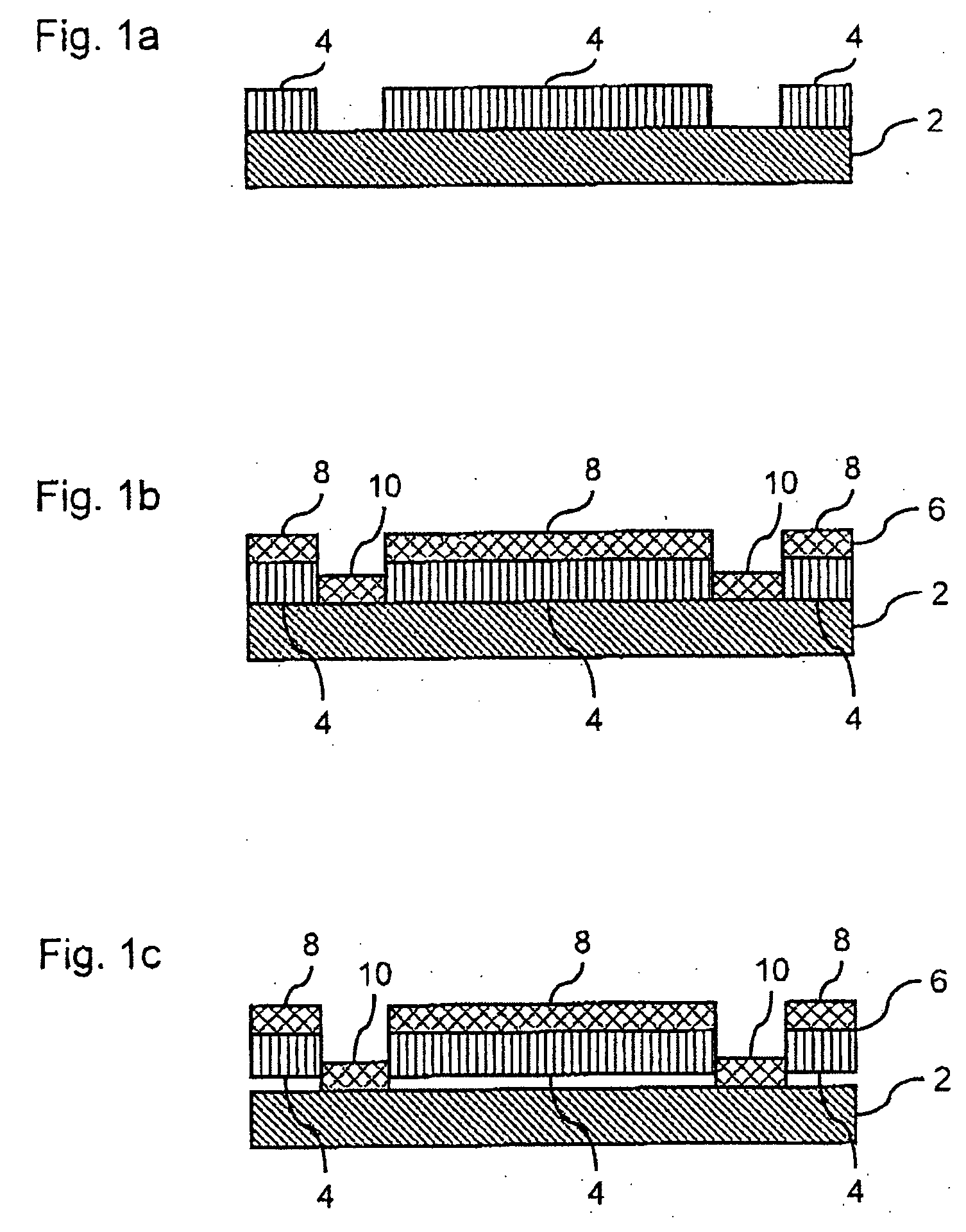
Physical stability. The ability of a product to maintain its physical dimensions and properties when exposed to conditions normally encountered in its service environment.
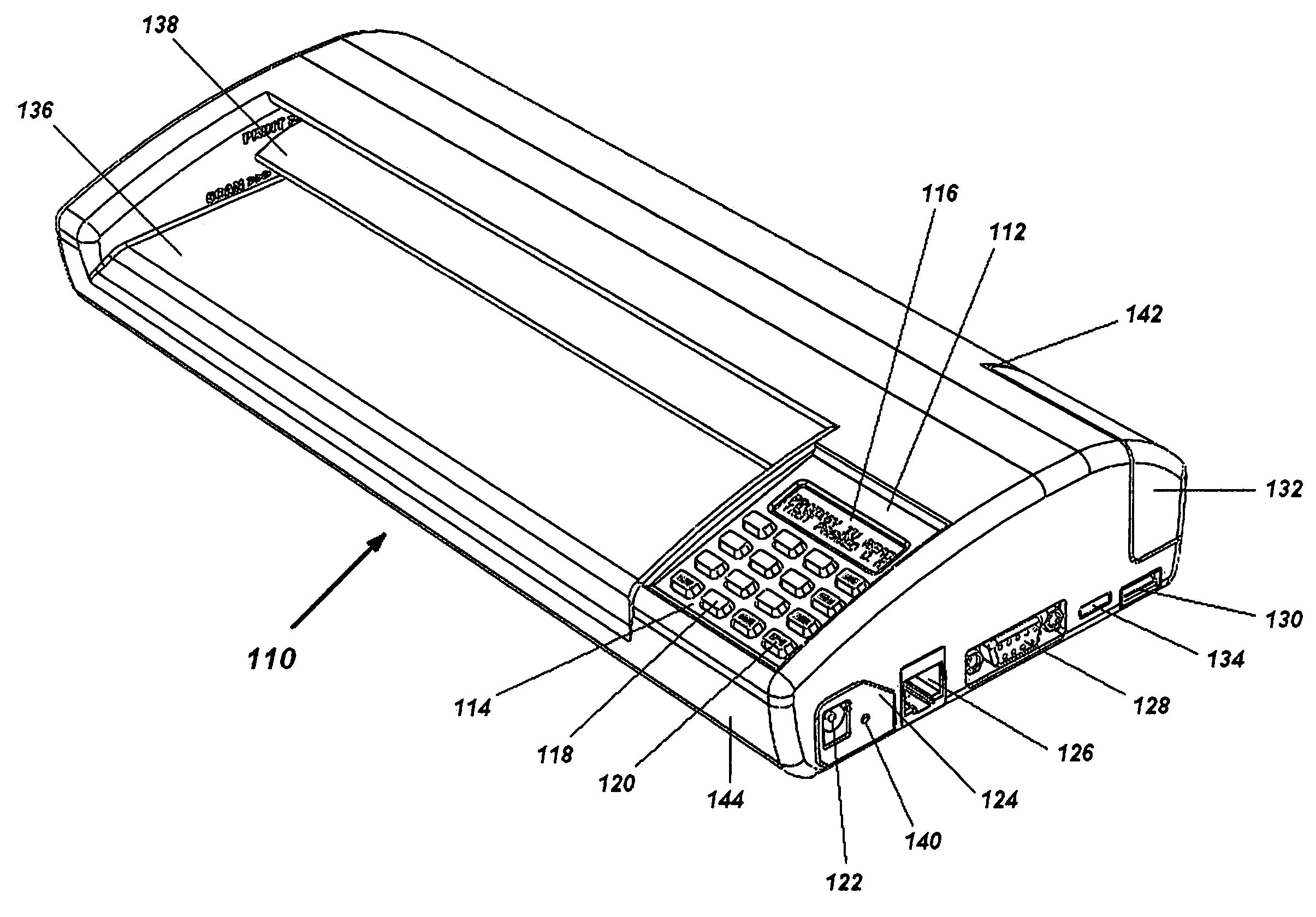
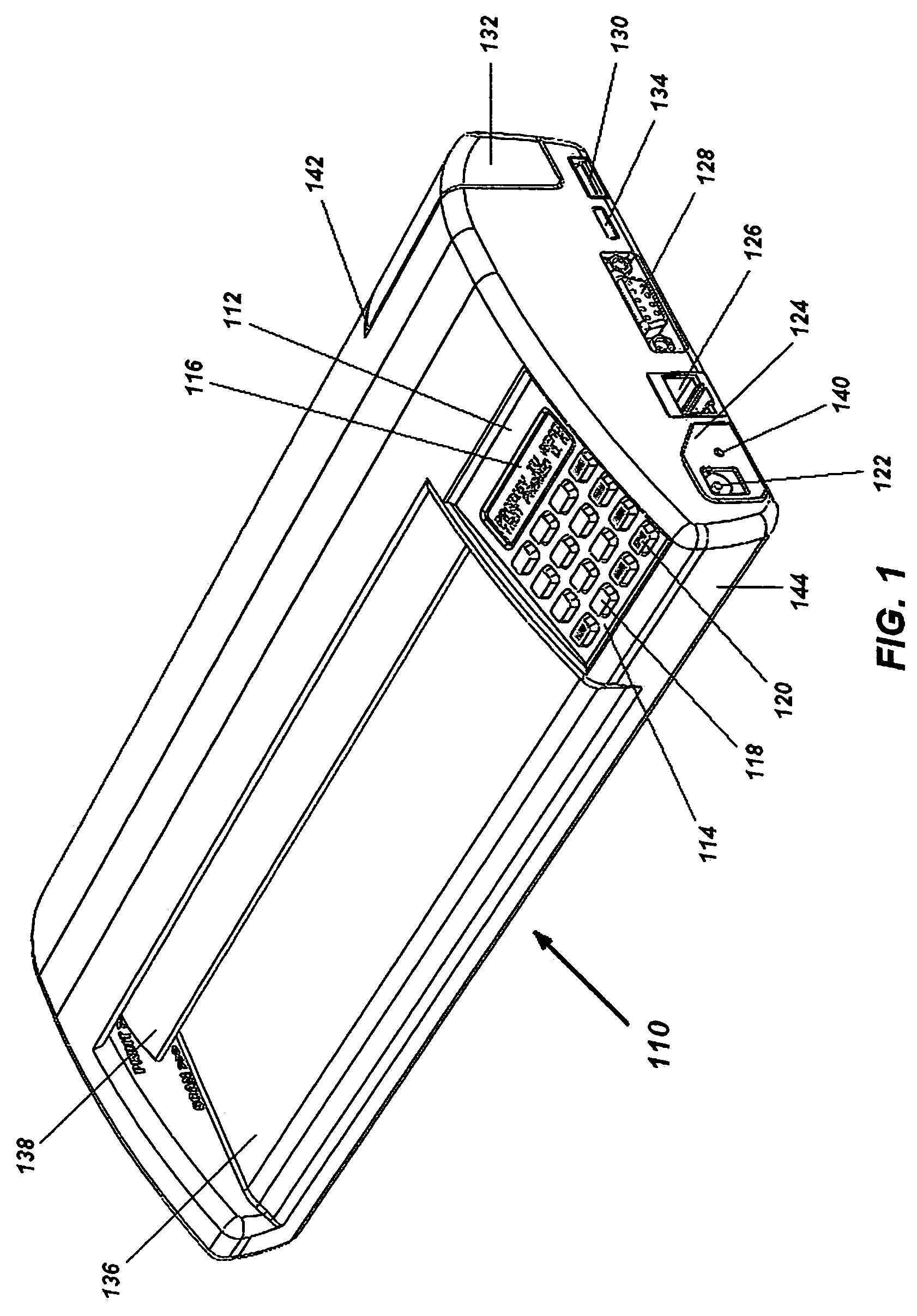
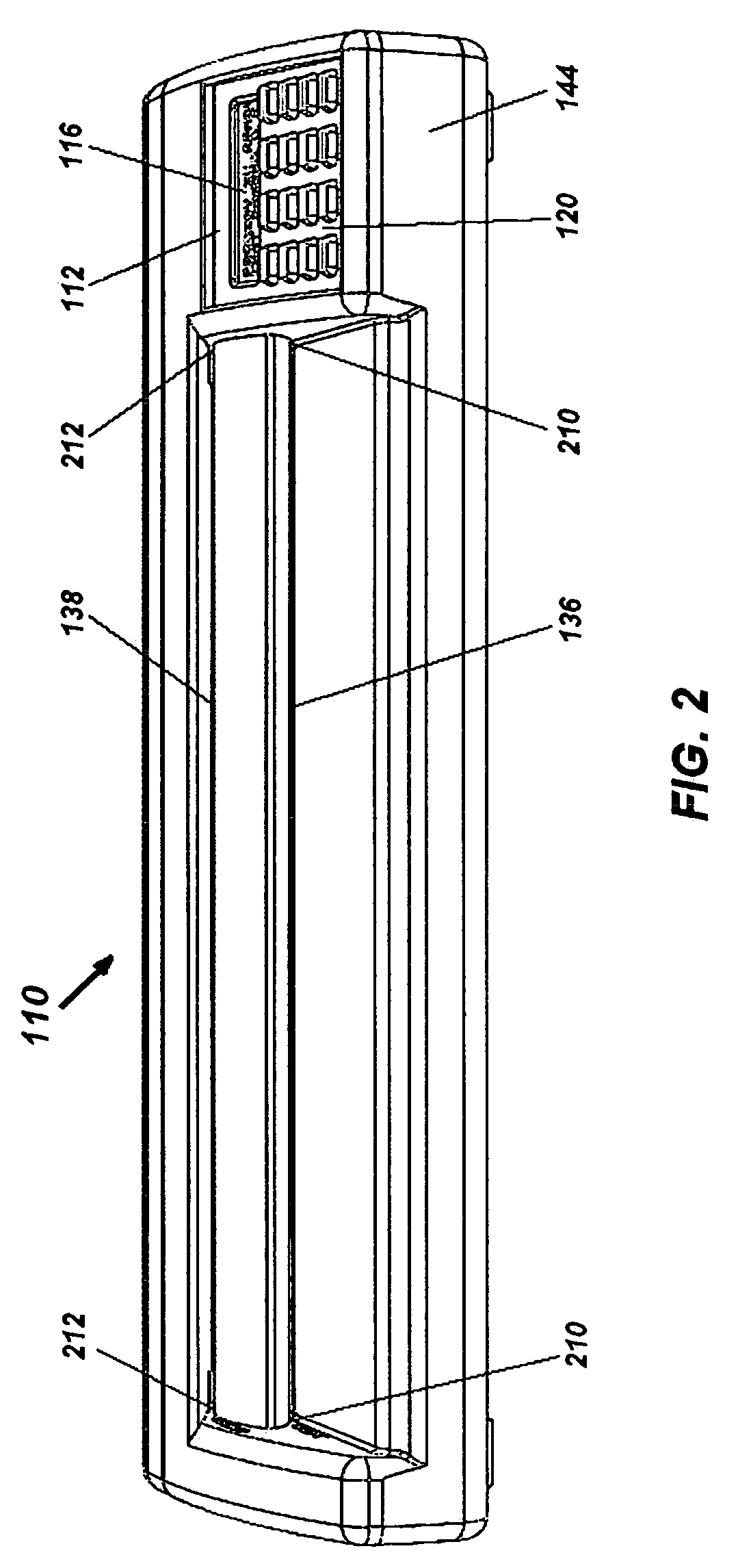
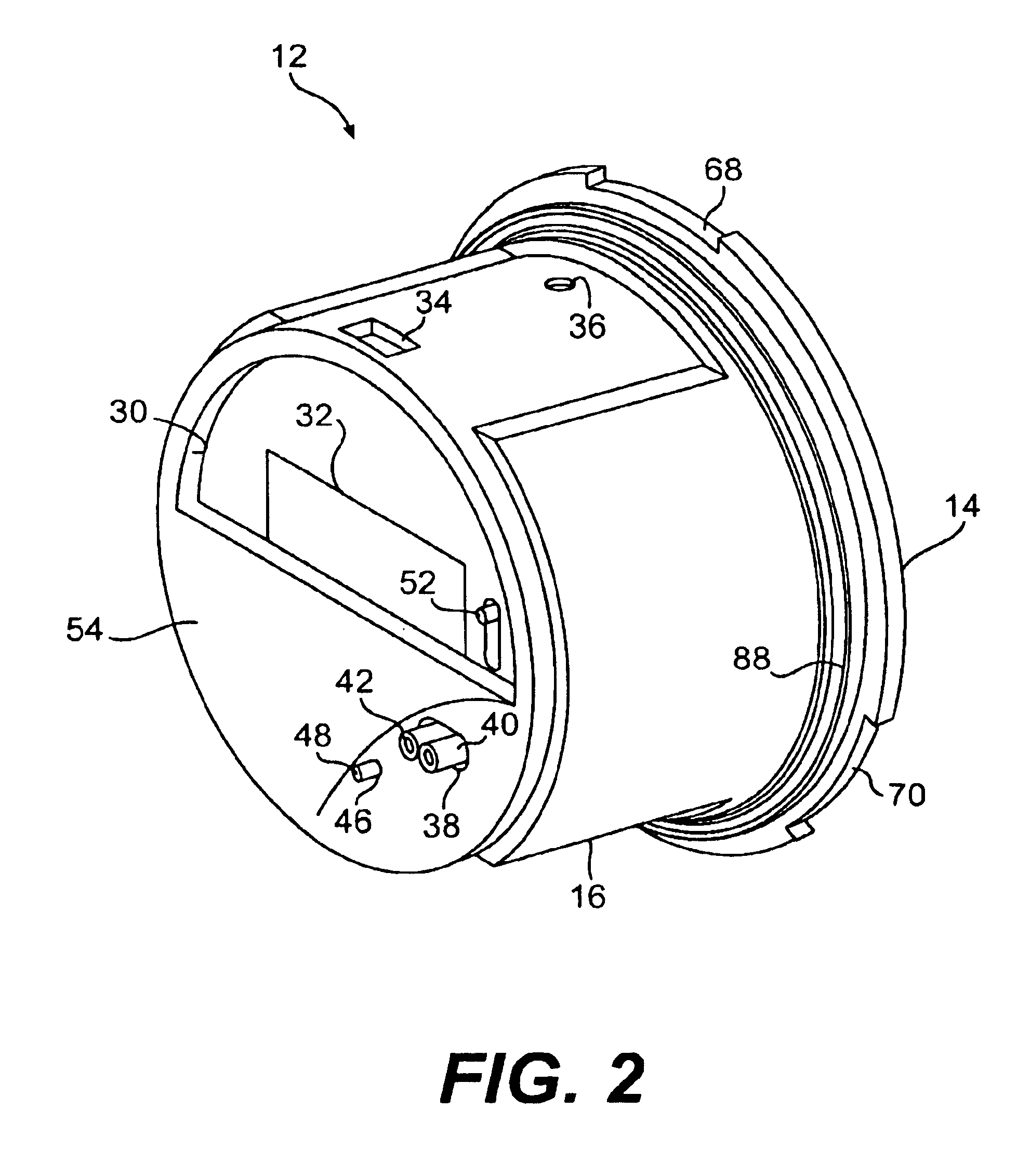
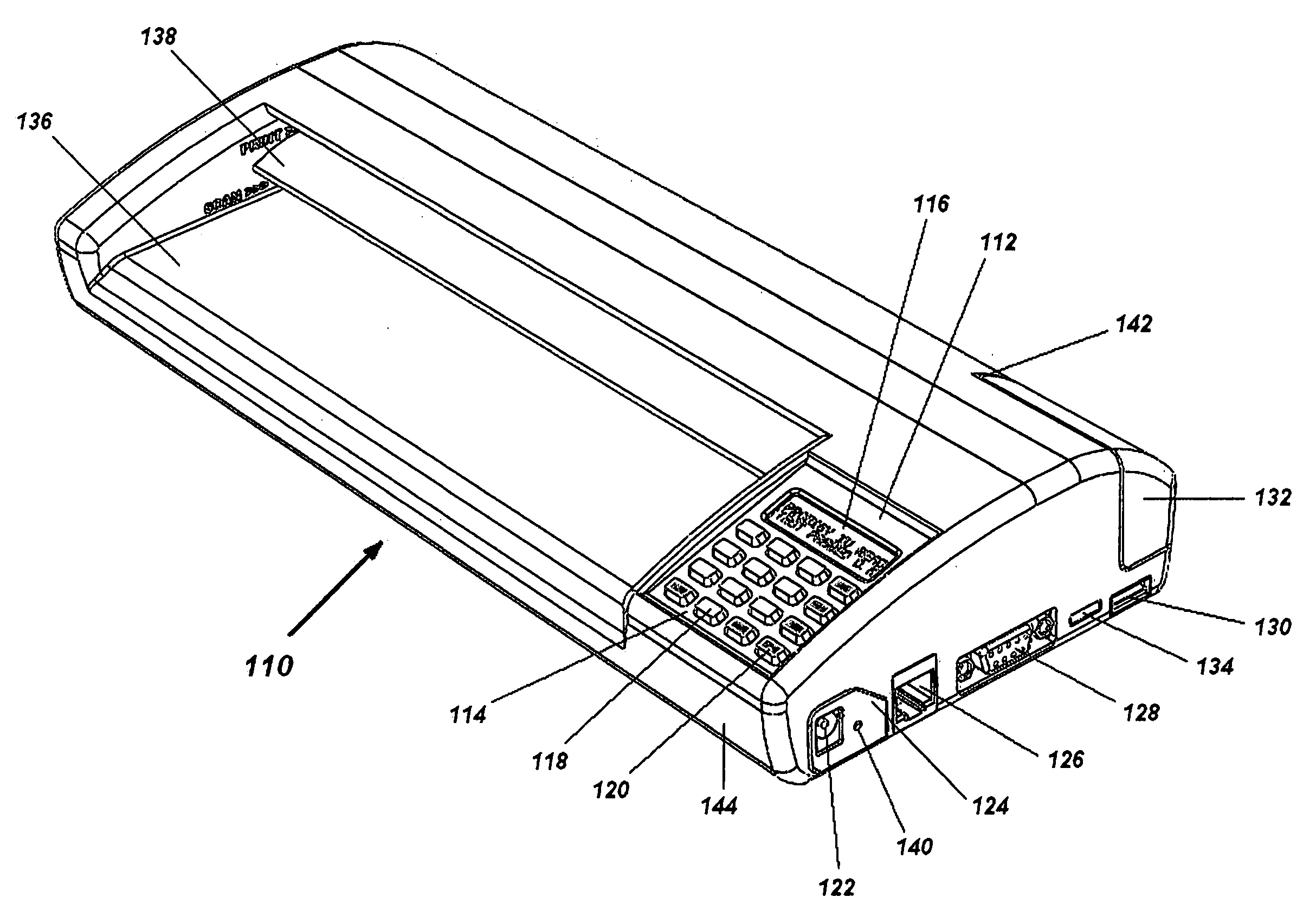
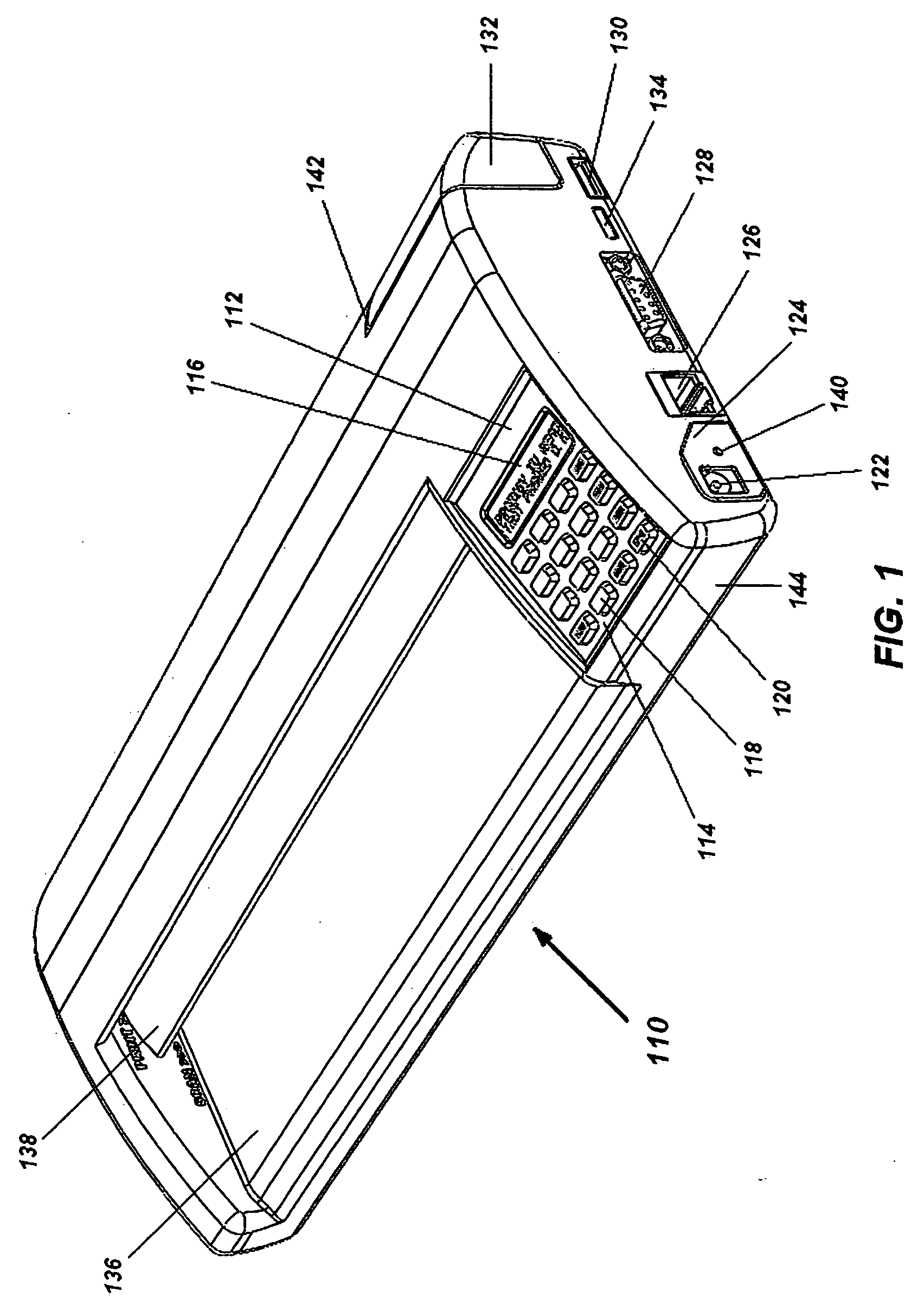
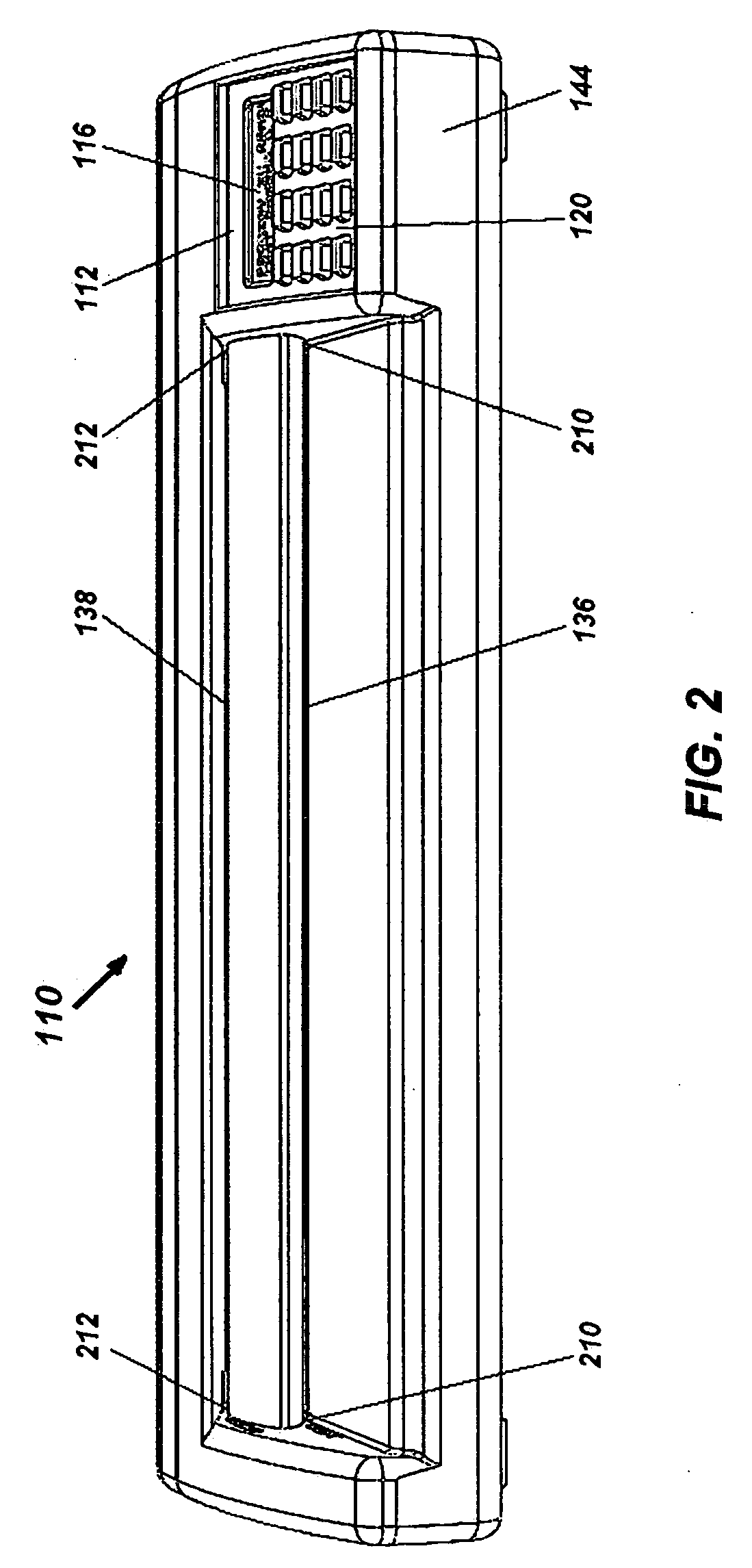
Portable electronic faxing, scanning, copying, and printing device
A lightweight, battery operated, portable, personal electronic device capable of faxing, scanning, printing and copying media as a standalone device or in cooperation with other electronic devices including PCs, mobile telephones, PDAs, etc. is provided. The device automatically detects the presence of fax-capable devices and reconfigures the software for compatibility with the fax-capable device eliminating the need for user programming. The device's ergonomic design, intrinsic physical stability, and same side paper feeds and user interface provide use in work areas having limited space. The device includes unidirectional, independent pathways for original and recording media such that paper jams are minimized. Portability is maximized through innovative power management software and hardware.
Owner:SCI FORGE
Methods of evaluating protein formulation stability and surfactant-stabilized insulin formulations derived therefrom
InactiveUS6737401B2Improve physical stabilityReliable timePeptide/protein ingredientsMicrobiological testing/measurementCell AggregationsProtein aggregation
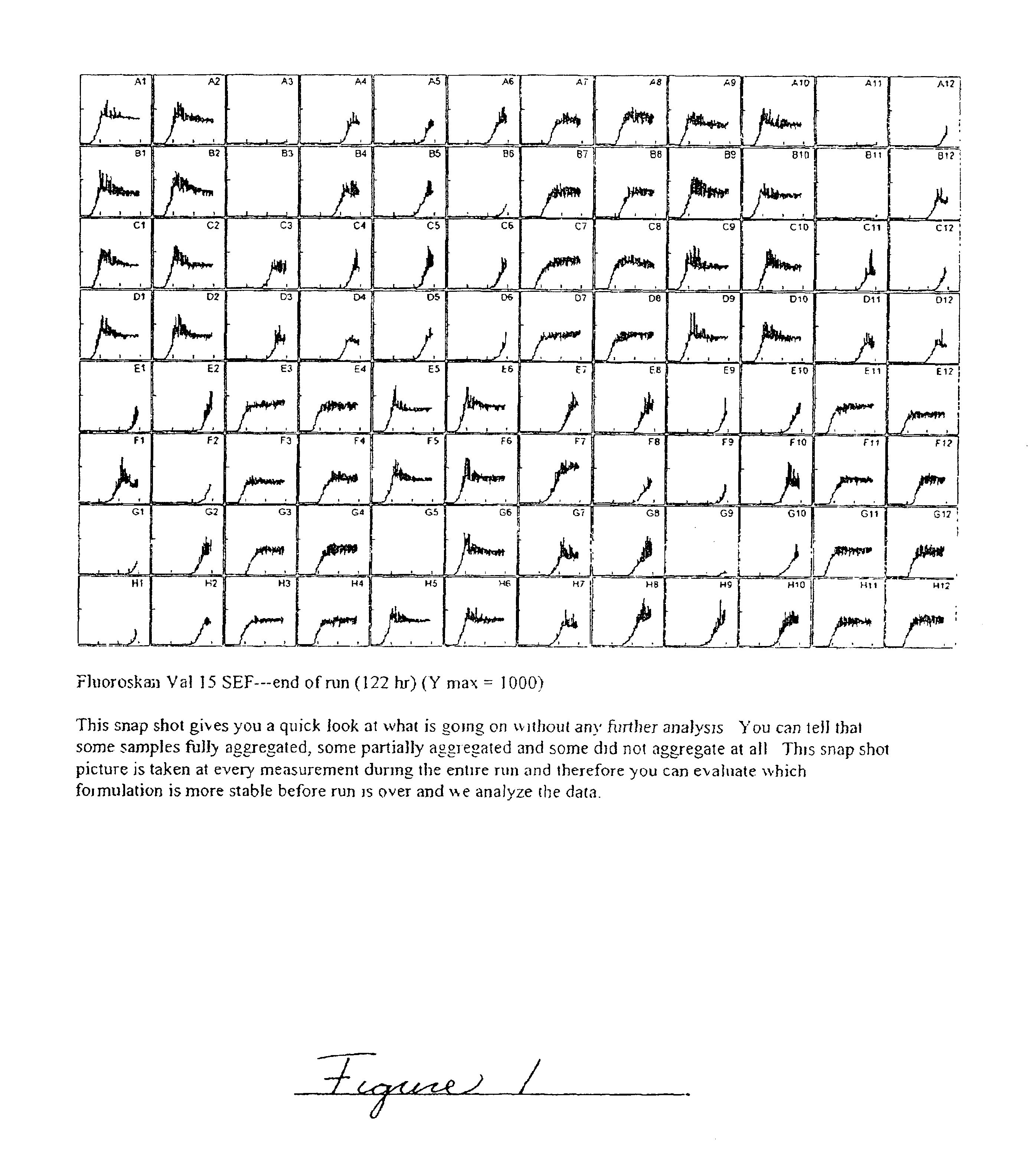
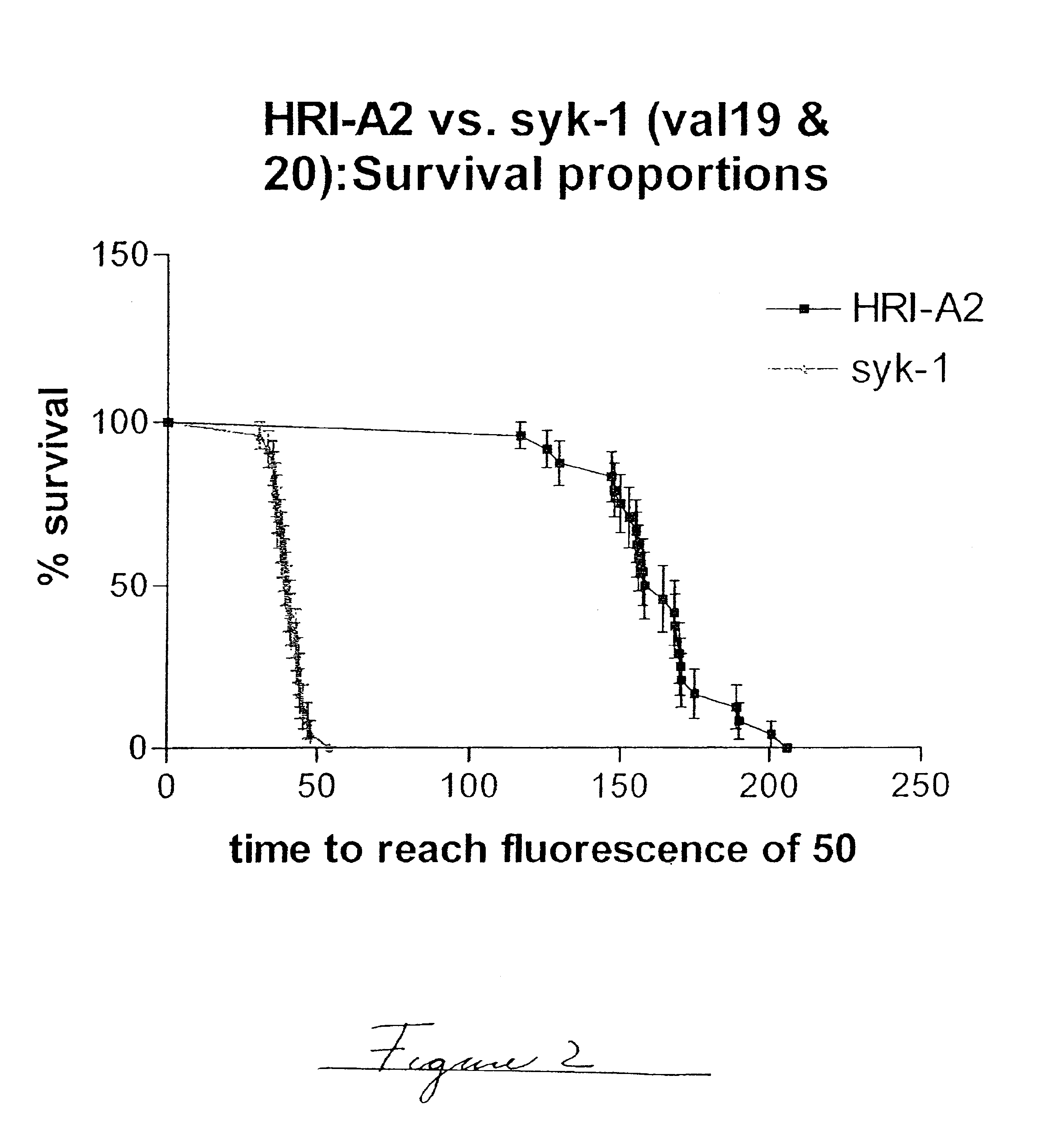
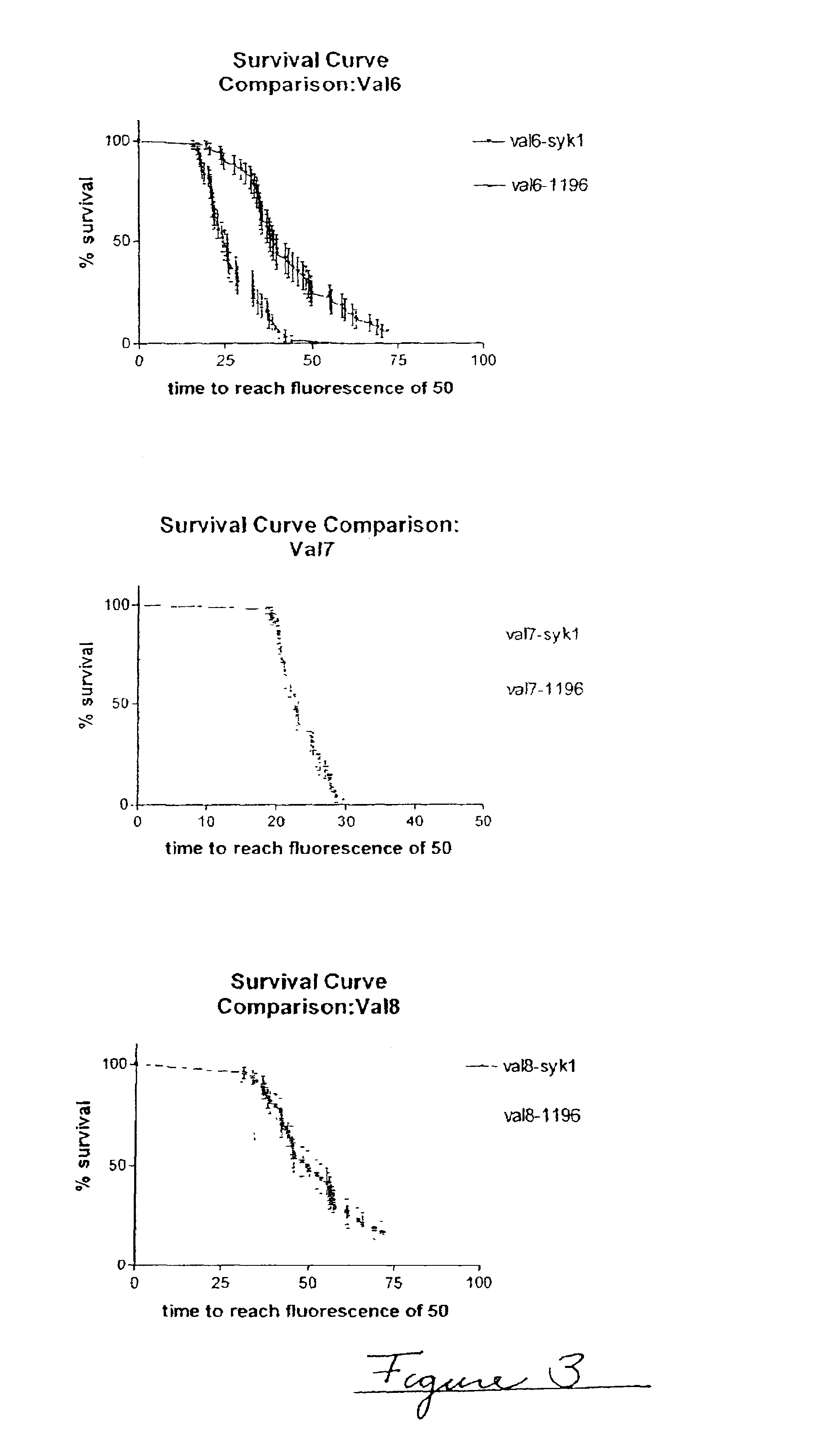
Embodiments of the invention are directed to a method of estimating the physical stability of a protein formulation. A particular embodiment of the invention places the protein formulation under an agitational stress that causes the protein to aggregate at an accelerated rate. In one embodiment, the change in protein aggregation is monitored spectroscopically using Thioflavin-T. Embodiments of the invention then utilize a survival curve analysis to ascertain the relative physical stability of the different protein formulations under study. This method was used to develop novel surfactant-stabilized insulin formulations in a rapid, cost efficient manner, thus illustrating the utility of the inventive method to the discovery and development of pharmaceutical protein formulations.
Owner:MEDTRONIC MIMIMED INC
Modular meter configuration and methodology
InactiveUS6885185B1Improve simplicityCost efficiencyDynamo-electric motor metersMeasurement through mechanical displacementCustomer requirementsMetrology
A modular electricity meter configuration and corresponding methodology permits use of certain common components in combination with either a variety of mechanical displays or electronic displays. In electricity meter arrangements making use of printed circuit board or solid state technology, at least two separate electronics boards may be provided. One may constitute a standard board for basic metrology functions while the other may comprise selected implementation of various higher level functions for creating a custom design electricity meter to meet customer requirements. Different customers may be provided with differently outfitted meters by corresponding customization of the higher level function board. A unitary power supply may be provided for both boards through a fixed connector. A common baseplate includes a circuitry link through a nonremovable plug or clip for alternatively providing a tamper proof embodiment or one with exposed terminals for permitting customer testing. Physical stability and strength is provided by using tapered mounting posts and integrated snap fit arrangements without requiring any screws for assembly. A light pipe provides external output through an innercover to indicate correct meter operation. Meter data and other metered information may be output through different configurations optionally involving hardwired output, RF links, pulse outputs, and telephone connections via modem or wireless.
Owner:ITRON
Oral capsule formulation with increased physical stability
InactiveUS7011846B2Improve capsule stabilityImprove stabilityPharmaceutical non-active ingredientsCapsule deliveryMonoglycerideOral medication
A formulation for a stabilized capsule for oral administration of a hydrophobic pharmaceutically active agent; comprising a non-aqueous solubilizer selected from 2-pyrrolidone, N-alkylpyrrolidones and combinations thereof; and a capsule stabilizing agent selected from mono-, di- and triglycerides, mono- and di-fatty esters of polyethylene glycol, fatty acids and combinations thereof wherein capsule integrity is maintained for at least 24 hours is disclosed.
Owner:SUPERNUS PHARM INC
Single-chain insulin
InactiveUS20070129284A1Lower blood sugar levelsPeptide/protein ingredientsMetabolism disorderPeptidePhysical stability
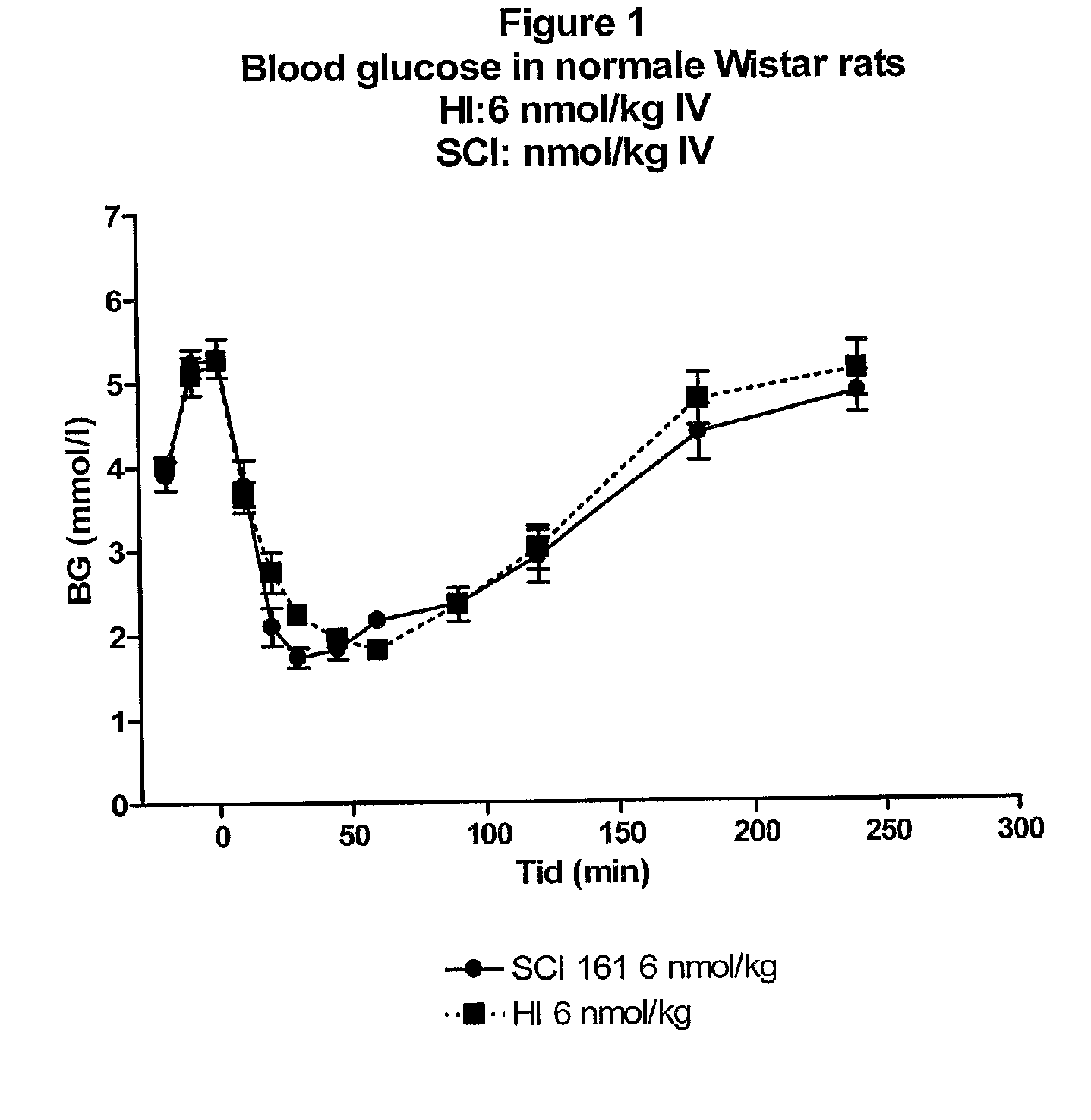
The present invention is related to single-chain insulin having insulin activity comprising a B- and an A-chain or a modified B- and A-chain connected by a connecting peptide of from 6-11 amino acids. The single-chain insulins will have biological insulin activity and an IGF-1 receptor affinity similar to or lower than that of human insulin and a high physical stability. The single-chain insulin may contain at least one basic amino acid residues in the connecting peptide. The single-chain insulins may also be acylated in one or more Lys residues.
Owner:NOVO NORDISK AS
Storage stable powder compositions of interleukin-4 receptor
InactiveUS6896906B2Good chemical stabilityImprove physical stabilityPowder deliveryPeptide/protein ingredientsWhite blood cellPhysical chemistry
The present invention provides storage stable dry powder compositions of IL-4R. The powder compositions demonstrate superior chemical and physical stability over their solution counterparts, particularly upon storage under varying conditions of temperature and humidity. Moreover, the powders, as prepared, possess good aerosol properties, which are maintained upon storage.
Owner:NOVARTIS FARMA
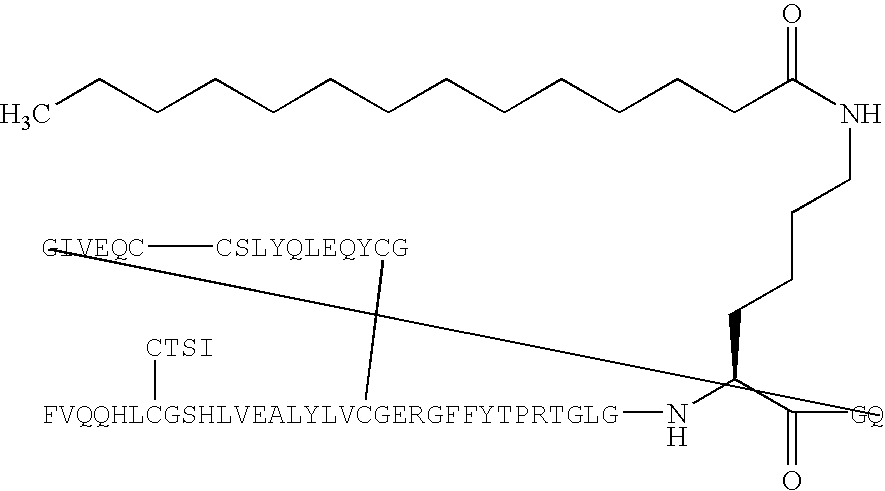
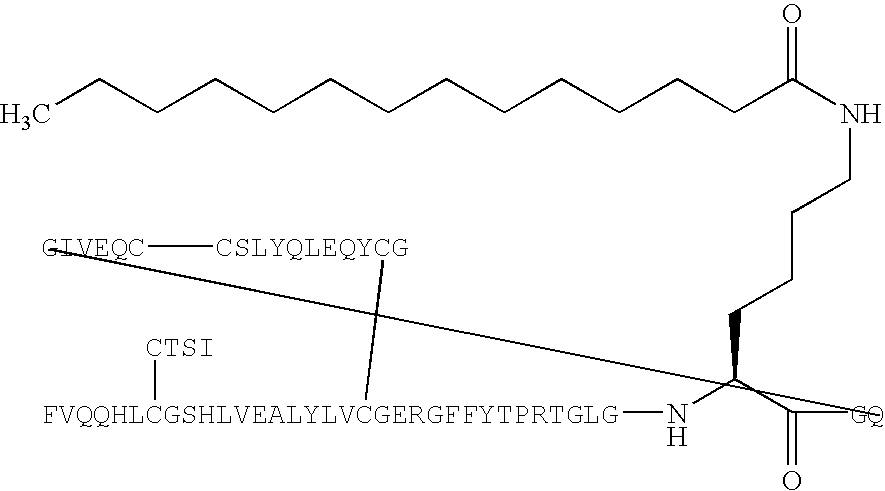
Modifications of peptide compositions to increase stability and delivery efficiency
ActiveUS8067532B2Increased stability and potencyReduce probabilityPeptide/protein ingredientsTransferasesPhysical stabilityDisulfide bond
The disclosed invention relates to methods of modifying peptide compositions to increase stability and delivery efficiency. Specifically, the disclosed invention relates to methods to increase the stability and delivery efficiency of protein kinase C (PKC) modulatory peptide compositions. A “therapeutic peptide composition” comprises a “carrier peptide” and a “cargo peptide.” A “carrier peptide” is a peptide or amino acid sequence within a peptide that facilitates the cellular uptake of the therapeutic peptide composition. The “cargo peptide” is a PKC modulatory peptide. Peptide modifications to either the carrier peptide, the cargo peptide, or both, which are described herein increase the stability and delivery efficiency of therapeutic peptide compositions by reducing disulfide bond exchange, physical stability, reducing proteolytic degradation, and increasing efficiency of cellular uptake.
Owner:KAI PHARMA
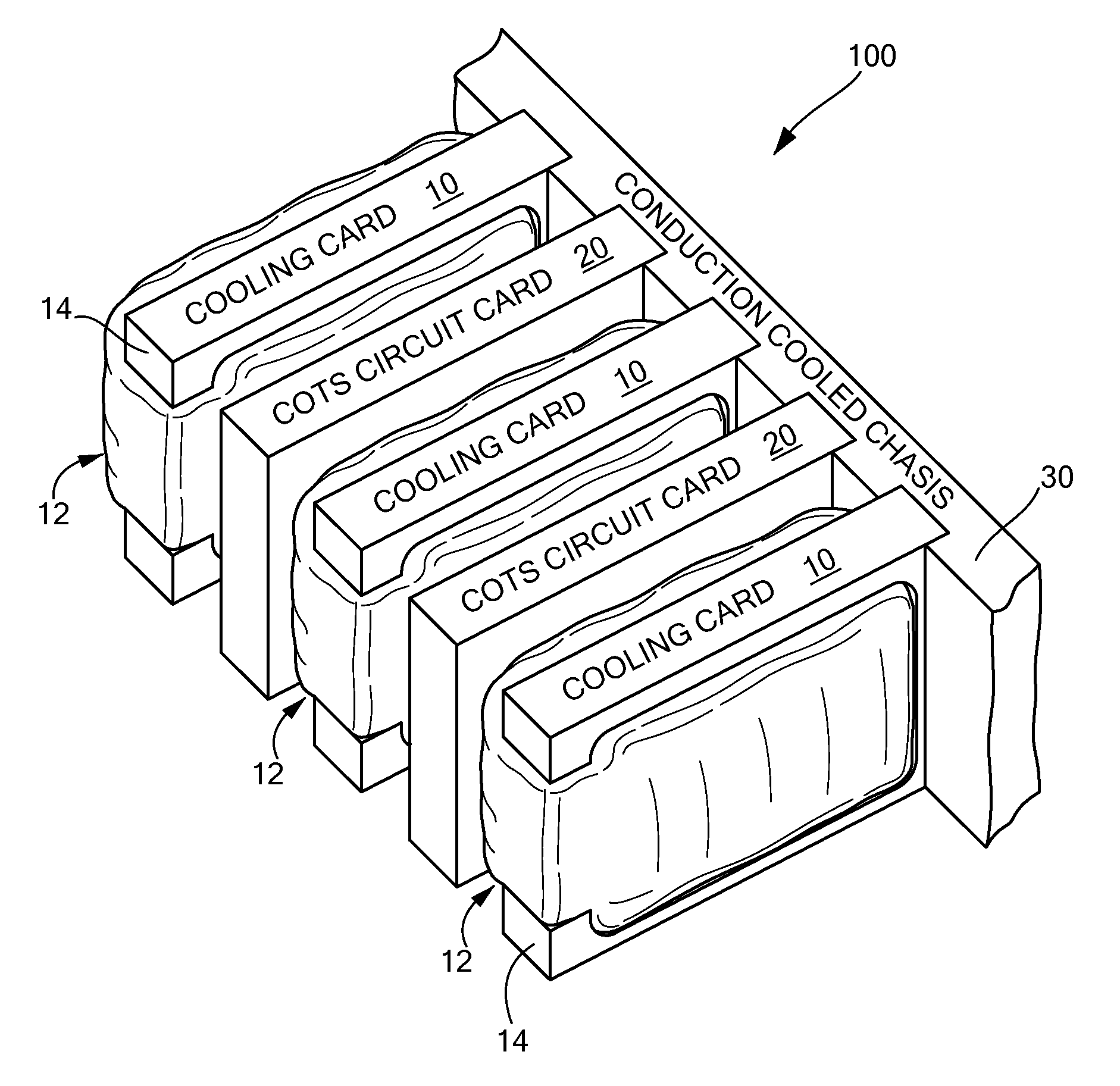
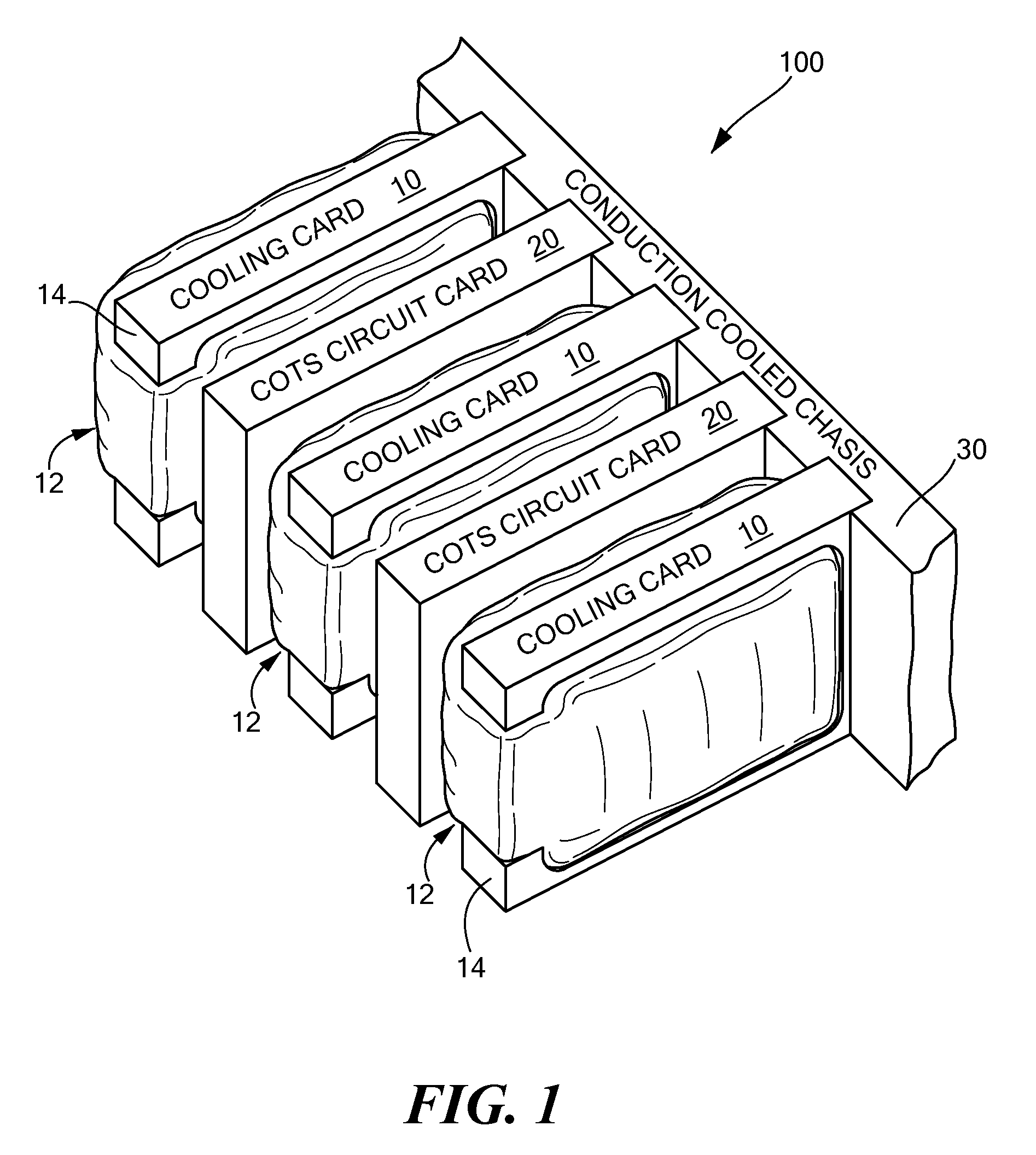
Passive conductive cooling module
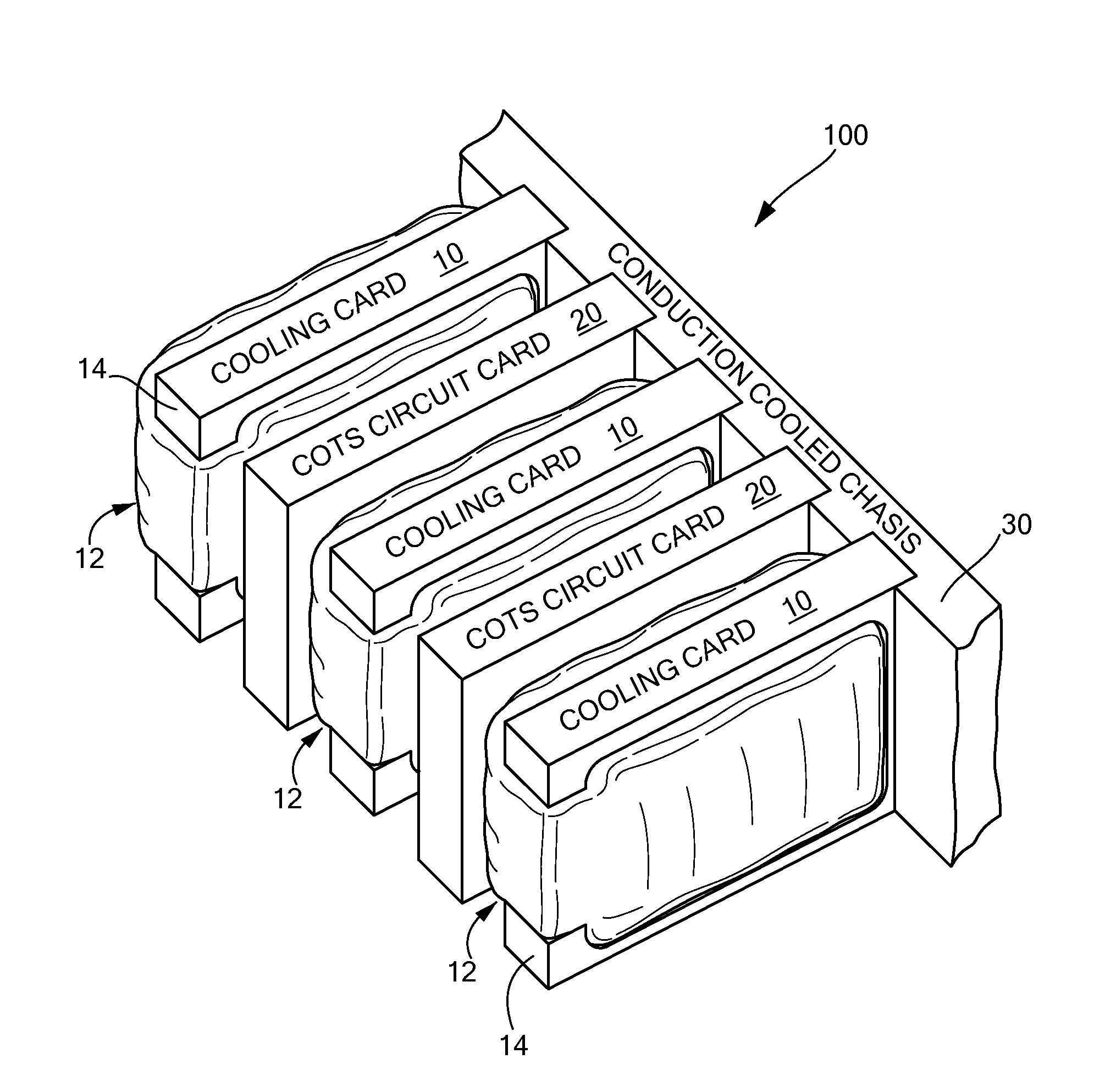
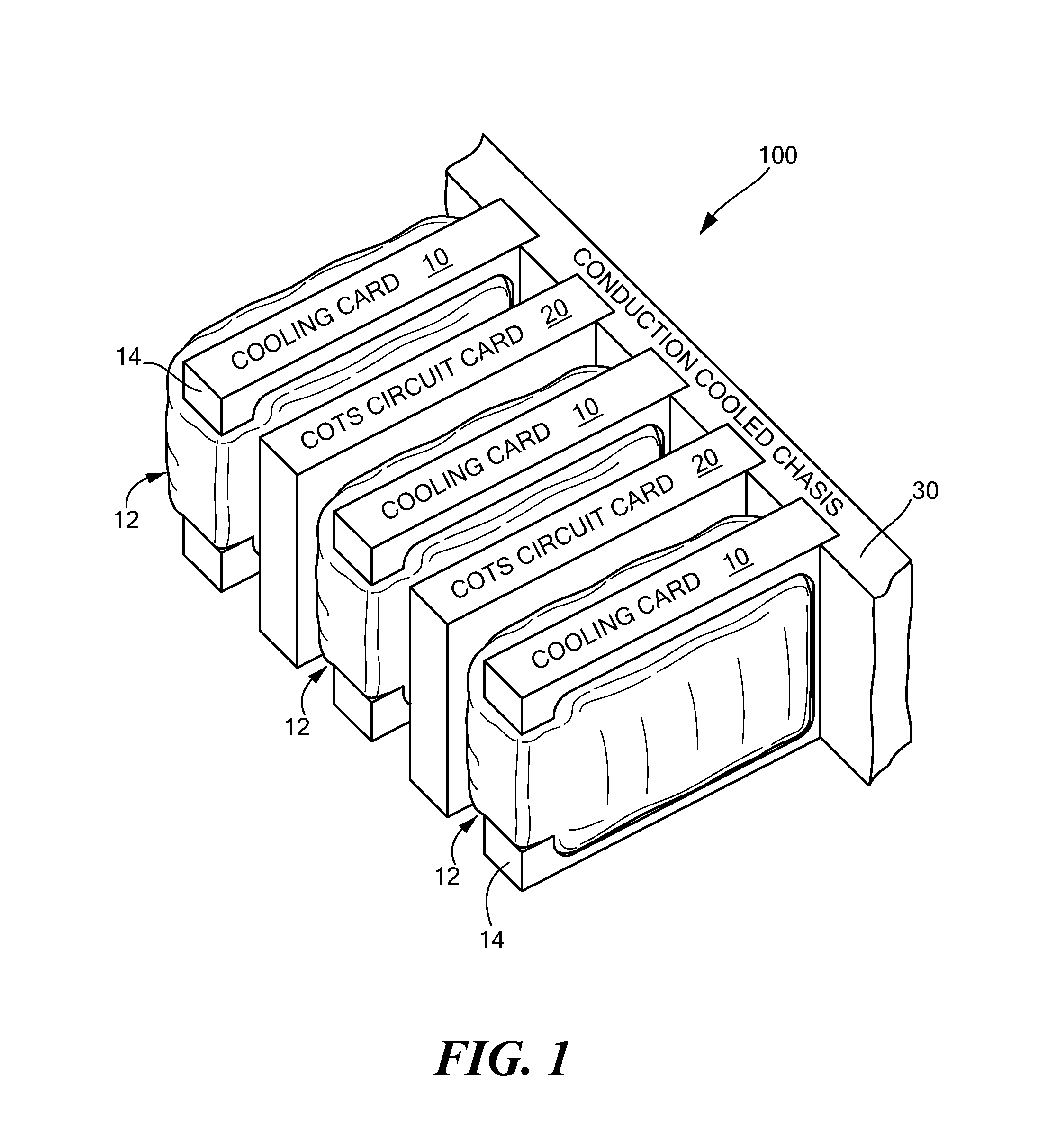
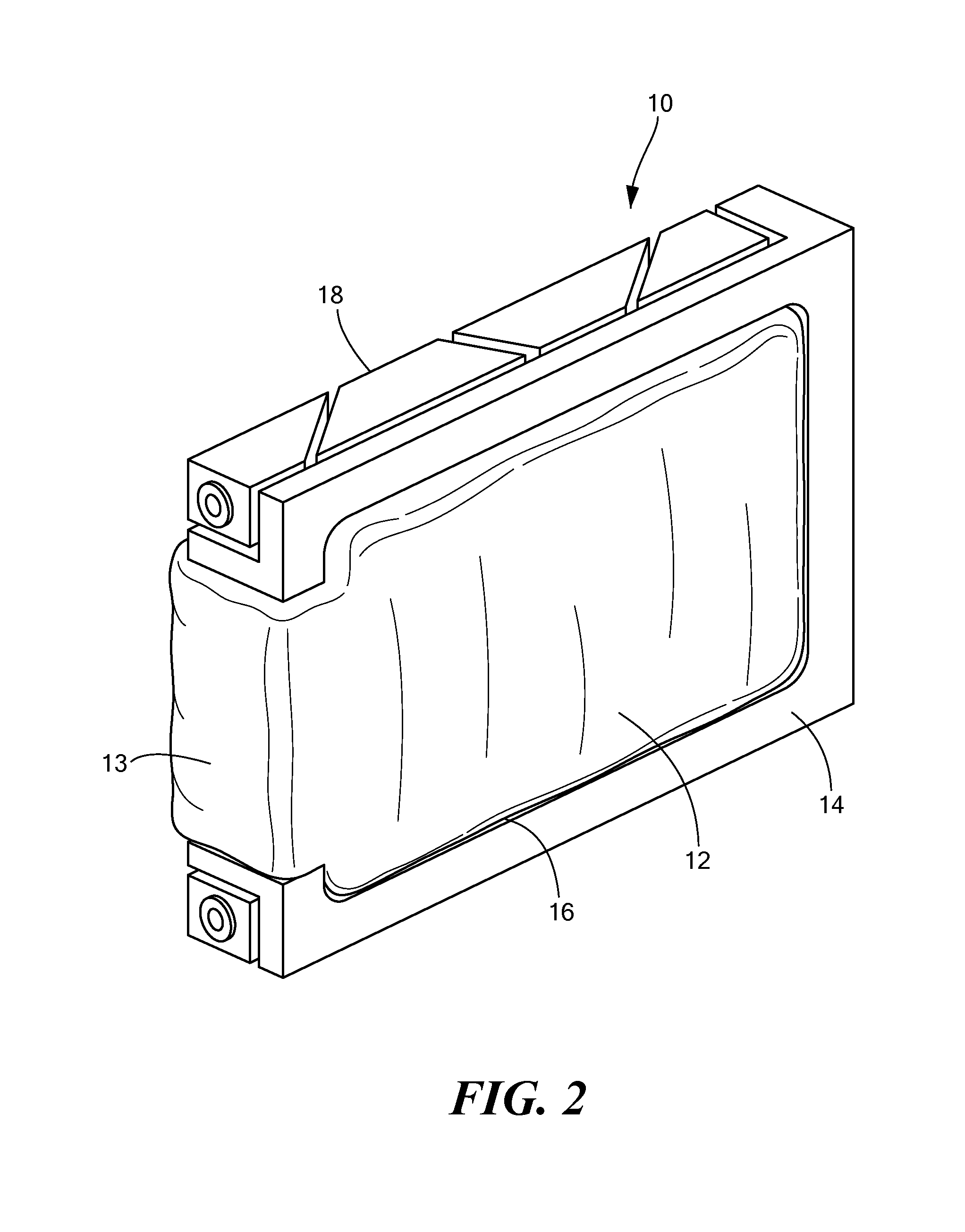
InactiveUS7952873B2Improve physical stabilityCounteract vibrationSemiconductor/solid-state device detailsSolid-state devicesComputer modulePhysical stability
A cooling module includes a thermally conductive plate, a bladder disposed on at least one side of the plate, the bladder have a chamber, and fluid disposed in the chamber of the bladder wherein the bladder in an inflated state impresses the cooling module against an adjacent electronic circuit card. where the cooling module is forcibly pressed against adjacent electronic circuit card providing increased physical stability to the electronic circuit card as well as provide a cooling technique for the circuit card.
Owner:RAYTHEON CO
Therapeutic peptide formulations with improved stability
InactiveUS20060188555A1Improved and optimal physical stabilityExtended shelf lifePeptide/protein ingredientsAntipyreticBiocompatible coatingStratum corneum
Compositions of and methods for formulating and delivering peptide, polypeptide and protein therapeutic agent formulations having enhanced physical stability, and wherein fibril formation is minimized and / or controlled, to yield a consistent and predictable composition viscosity. The compositions of and methods for formulating and delivering peptide, polypeptide and protein therapeutic agents of the present invention further facilitate their incorporation into a biocompatible coating which can be employed to coat a stratum-corneum piercing microprojection, or a plurality of stratum-corneum piercing microprojections of a delivery device, for delivery of the biocompatible coating through the skin of a subject, thus providing an effective means of delivering the peptide therapeutic agents.
Owner:ALZA CORP
Non-woven, uni-directional multi-axial reinforcement fabric and composite article
InactiveUS20060121805A1Negligible effectInexpensive, dependableAdhesive processes with surface pretreatmentSynthetic resin layered productsAdhesiveNonwoven fabric
A non-woven, unidirectional, multi-axial layered fabric for reinforcement of composite structures provides for holding the non-woven yarns as laid-out by adhesion of polymeric adhesive applied to the non-woven yarns. The adhesive layer on the yarns, dissolves as liquid resin is applied to form a composite structure, the polymeric coating dissolving in the liquid resin. The polymeric adhesive dissolves to allow liquid resin to wet the yarns. Curing creates the desired composite structure. Filament yarns useful in the present invention include but are not limited to those made of aramid, boron, carbon, fiberglass, nylon, PBO, PEN, polyester, and polyethylene. A preferred adhesive is low molecular weight polyester and a preferred liquid resin is polyester resin. A web of netting material is applied in a similar manner for added physical stability of the inventive reinforcement fabric.
Owner:KRULIC CHARLIE B
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
InactiveUS20140296191A1Less viscousLess denseBiocideOrganic chemistryDiethylene glycol monoethyl etherNasal spray
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Method for forming high performance surface coatings and compositions of same
InactiveUS6410086B1Improve performanceHigh mechanical strengthPhotography auxillary processesElectrolysis componentsGas phaseCompound (substance)
A method of forming on an object a surface coating having a high mechanical strength and chemical and physical stability above 700° C. is disclosed. The method comprises (a) electrophoretically depositing at least one surface coating material on a surface of the object for obtaining a green coating on the surface; and (b) infiltrating into and depositing onto the green coating at least one additional surface coating material by a gas-phase infiltration / deposition method, thereby forming a high performance surface coating, wherein, the at least one surface coating material and the at least one additional surface coating material are chemically and physically stable above 700° C.
Owner:CEREL CERAMIC TECH +1
High-pressure fluidized bed reactor for preparing granular polycrystalline silicon
ActiveUS20100047136A1Reduce the possibilitySafely heldPressurized chemical processSiliconFluidized bedHigh pressure
The present invention relates to a high-pressure fluidized bed reactor for preparing granular polycrystalline silicon, comprising (a) a reactor tube, (b) a reactor shell encompassing the reactor tube, (c) an inner zone formed within the reactor tube, where a silicon particle bed is formed and silicon deposition occurs, and an outer zone formed in between the reactor shell and the reactor tube, which is maintained under the inert gas atmosphere, and (d) a controlling means to keep the difference between pressures in the inner zone and the outer zone being maintained within the range of 0 to 1 bar, thereby enabling to maintain physical stability of the reactor tube and efficiently prepare granular polycrystalline silicon even at relatively high reaction pressure.
Owner:KOREA RES INST OF CHEM TECH
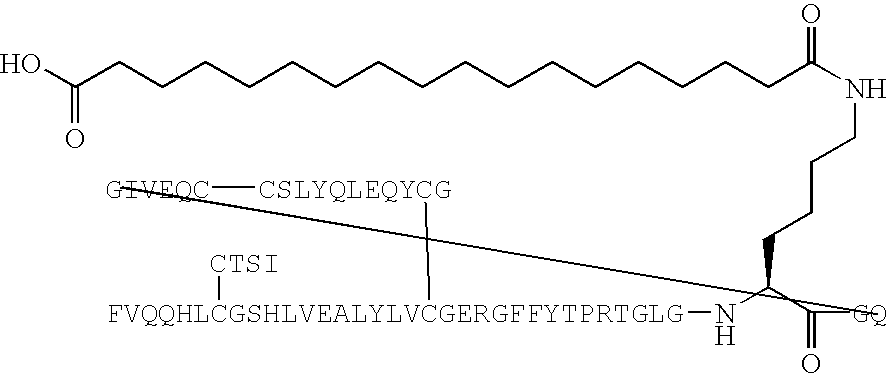
Stable Therapeutic Formulations
ActiveUS20070184096A1Improve physical stabilityReduces and eliminates undesirable deteriorationBiocideOrganic active ingredientsBiocompatible coatingActive agent
Compositions of and methods for formulating and delivering biologically active agent formulations having enhanced physical stability, and wherein deterioration from the presence of oxygen and / or water is minimized and / or controlled, to yield a stable formulation. The compositions of and methods for formulating and delivering biologically active agents of the present invention further facilitate their incorporation into a biocompatible coating which can be employed to coat a stratum-corneum piercing microprojection, or a plurality of stratum-corneum piercing microprojections of a delivery device, for delivery of the biocompatible coating through the skin of a subject, thus providing an effective means of delivering the biologically active agents.
Owner:ALZA CORP
Stable insulin formulations
InactiveUS6906028B2Improve stabilityEasy to separatePeptide/protein ingredientsMetabolism disorderArginineBuffering agent
The present invention provides a monomeric insulin analog formulation stabilized against aggregation in which the buffering agent is either TRIS or arginine. The stable formulations of the present invention are useful for treating diabetes, and are particularly advantageous in treatment regimes requiring lengthy chemical and physical stability, such as, in continuous infusion systems.
Owner:ELI LILLY & CO
Aqueous compositions comprising a lipid and a lanolin-derived surfactant, and their use
InactiveUS6224853B1High viscosityReduce dragCosmetic preparationsHair cosmeticsLipid formationLANOLIN DERIVATIVES
An aqueous composition comprising, in addition to water: (a) one or more surfactant materials selected from polyoxyalkylene condensate derivatives of lanolin or a lanolin derivative; and (b) a lipid component comprising one or more lipid materials, especially lanolin or a lanolin derivative, present as particles emulsified by the said one or more lanolin-derived surfactant materials and having a median particle size of less than about 5 mum, especially from 0.01 to 1 mum. The compositions have very good physical stability and are particularly useful as carriers for transdermal delivery of pharmaceutical actives to the human skin.
Owner:CRODA CHEM INT
Proliposomal and liposomal compositions of poorly water soluble drugs
InactiveUS20090017105A1Good storage stabilityGood potencyOrganic active ingredientsAntineoplastic agentsLipid formationAntioxidant
Concentrates or proliposomal compositions of poorly water-soluble drugs and compounds, comprising of one or more membrane forming lipids, a membrane stabilizing agent, in a suitable vehicle, and optionally containing a Polyethylene Glycol (PEG)-coupled phospholipid or a mixture thereof and further, optionally containing pharmaceutically acceptable excipients such as antioxidants, buffering agents, acidifying agents etc. are provided, which have superior long term stability. The concentrates of proliposomal compositions instantly form liposomes of the said poorly water-soluble drugs and compounds on rapid injection to a diluting fluid, the liposomal composition so obtained, characterized by a physical stability more than 24 hours, ≧95% drug encapsulation and having a particle size diameter of less than 100 nm. The liposomal compositions so obtained can further be directly administered to patients in need of treatment of the poorly water-soluble drugs and compounds.
Owner:FRESENIUS KABI ONCOLOGY LTD
Method for connecting substrate and composite element
ActiveUS20060030074A1OpenSemiconductor/solid-state device detailsElectric lighting sourcesEvaporationCompound (substance)
The invention relates to a process for joining substrates having electrical, semiconducting, mechanical and / or optical components, and to a composite element. The process is to be suitable for the substrates which are to be joined substantially irrespective of material and in particular also for sensitive substrates, is to have a high chemical and physical stability and / or is to produce a hermetic cavity. According to the invention, a raised frame, in particular formed from anodically bondable glass, is applied by evaporation coating to one of the two substrates in order to serve as a joining element.
Owner:SCHOTT AG
Portable electronic device
InactiveUS20060001920A1Lower levelMultiple functionsPictoral communicationEngineeringPhysical stability
A lightweight, battery operated, portable, personal electronic device capable of faxing, scanning, printing and copying media as a standalone device or in cooperation with other electronic devices including PCs, mobile telephones, PDAs, etc. is provided. The device automatically detects the presence of fax-capable devices and reconfigures the software for compatibility with the fax-capable device eliminating the need for user programming. The device's ergonomic design, intrinsic physical stability, and same side paper feeds and user interface provide use in work areas having limited space. The device includes unidirectional, independent pathways for original and recording media such that paper jams are minimized. Portability is maximized through innovative power management software and hardware.
Owner:SCI FORGE
Microcapsule insecticide-fertilizer preparation, preparation method and application thereof
InactiveCN101857482ANo pollutionSlow release rateBiocidePlant growth regulatorsChemical compatibilitySolvent
The invention discloses a microcapsule insecticide-fertilizer preparation, a preparation method and application thereof. The preparation consists of the following components in part by weight: 1 to 30 parts of pesticide microcapsules and 70 to 99 parts of nutrient substance. The preparation method mainly comprises the following steps of: after fully dissolving a pesticide with a solvent, adding a wall material monomer into solution and mixing uniformly; transporting a mixture into a shear dispersion reaction kettle which is filled with smashed capsule bodies; stirring the mixture and the capsule bodies so that the capsules fully absorb the solution; performing a shear dispersion reaction to form capsules; performing solid-liquid separation and drying to prepare the pesticide microcapsules; adding the nutrient substances into the pesticide microcapsules; and fully stirring and uniformly mixing to prepare the microcapsule insecticide-fertilizer preparation. By using the microcapsule insecticide-fertilizer preparation and the preparation method, the problems of the chemical compatibility and the physical stability of the conventional insecticide-fertilizer preparation in the same system are solved effectively. A product of the invention has the advantages of: shielding and slow-releasing functions, high chemical compatibility and physical stability, lasting pesticide effect, natural degradability, no environment pollution, low use cost and simple production process.
Owner:侯金荣 +1
Coenzyme Q10 injection emulsion and its preparing process
InactiveCN1857239AEasy to manufactureEasy to implementOrganic active ingredientsPowder deliveryVegetable oilEmulsion
The coenzyme Q10 injection emulsion has coenzyme Q10 as the effective medicine component, and each 1000 ml emulsion contains coenzyme Q10 1-10g, vegetable oil for injection 0-200g, emulsifier 1-50g, isoosmotic regulator 5-50g, antioxidant 0.05-5g, pH regulator in the quantity of regulating pH value to 3.0-9.0, co-emulsifier 10-500g and water for injection for the rest. It provides the patient with treating medicine and essential nourishing matter. It has high physical stability and may be prepared into freeze dried preparation for further raised stability and convenient storing and transportation. The coenzyme Q10 injection emulsion has certain targeting effect, so that it has raised bioavailability and lowered toxic side effect.
Owner:YUTAI MEDICINE SCI TECH HANZHOU
Single-Chain Insulin
InactiveUS20090170750A1Lower blood sugar levelsSugar derivativesPeptide/protein ingredientsInsulin activityPhysical stability
The present invention is related to single-chain insulin having insulin activity comprising a B- and an A-chain or a modified B- and A-chain connected by a connecting peptide of from 6-11 amino acids. The single-chain insulins will have biological insulin activity and an IGF-1 receptor affinity similar to or lower than that of human insulin and a high physical stability. The single-chain insulin may contain at least one basic amino acid residues in the connecting peptide. The single-chain insulins may also be acylated in one or more Lys residues.
Owner:NOVO NORDISK AS
Multiparticulate compositions with improved stability
ActiveUS20050186285A1Increase in drug crystallinityGood chemical stabilityPowder deliveryGranular deliveryParticle compositionChemical stability
A process is described for producing drug-containing multiparticulates with improved stability, characterized by an improvement in one or more of chemical stability, physical stability, or dissolution stability.
Owner:PFIZER INC
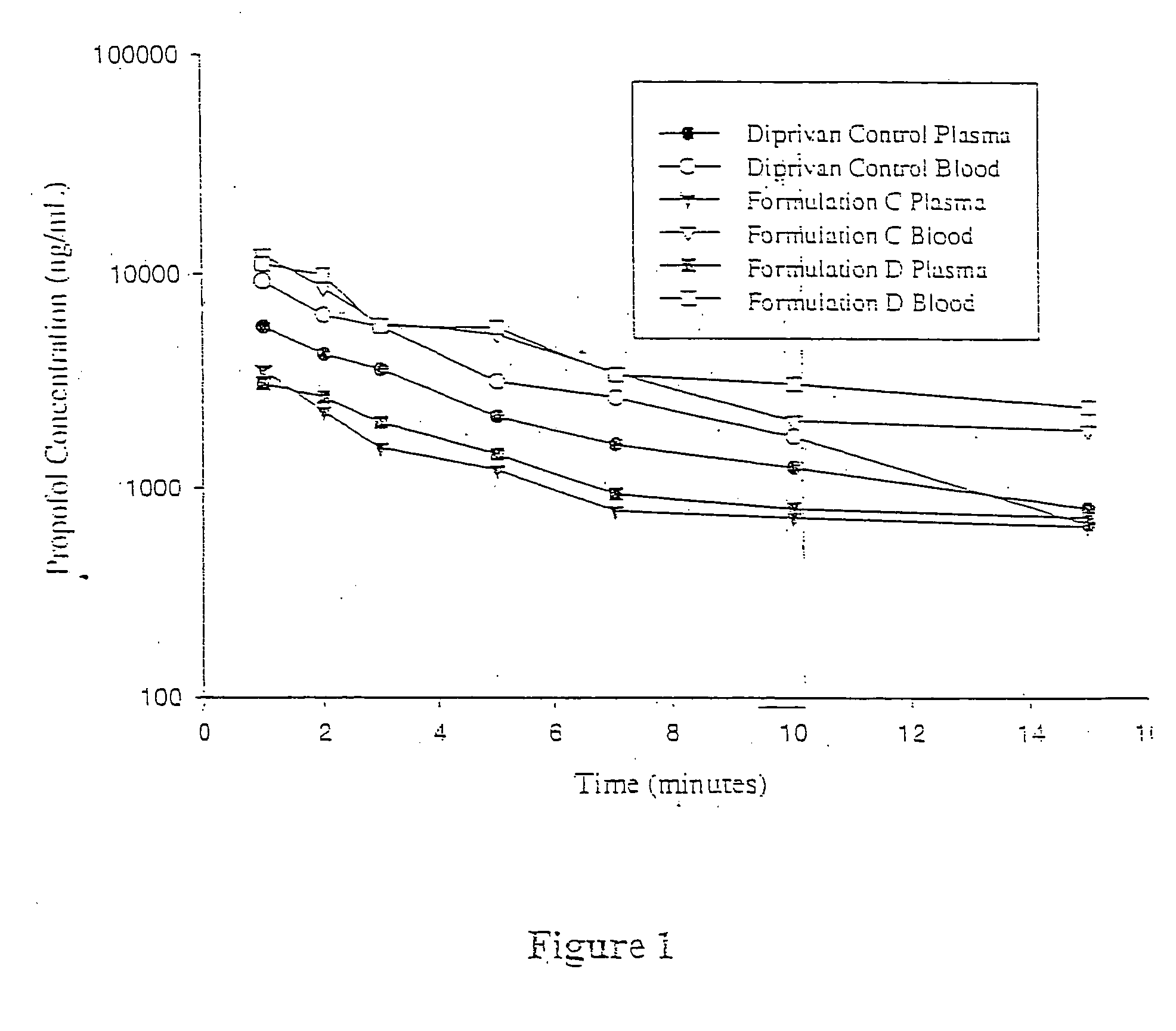
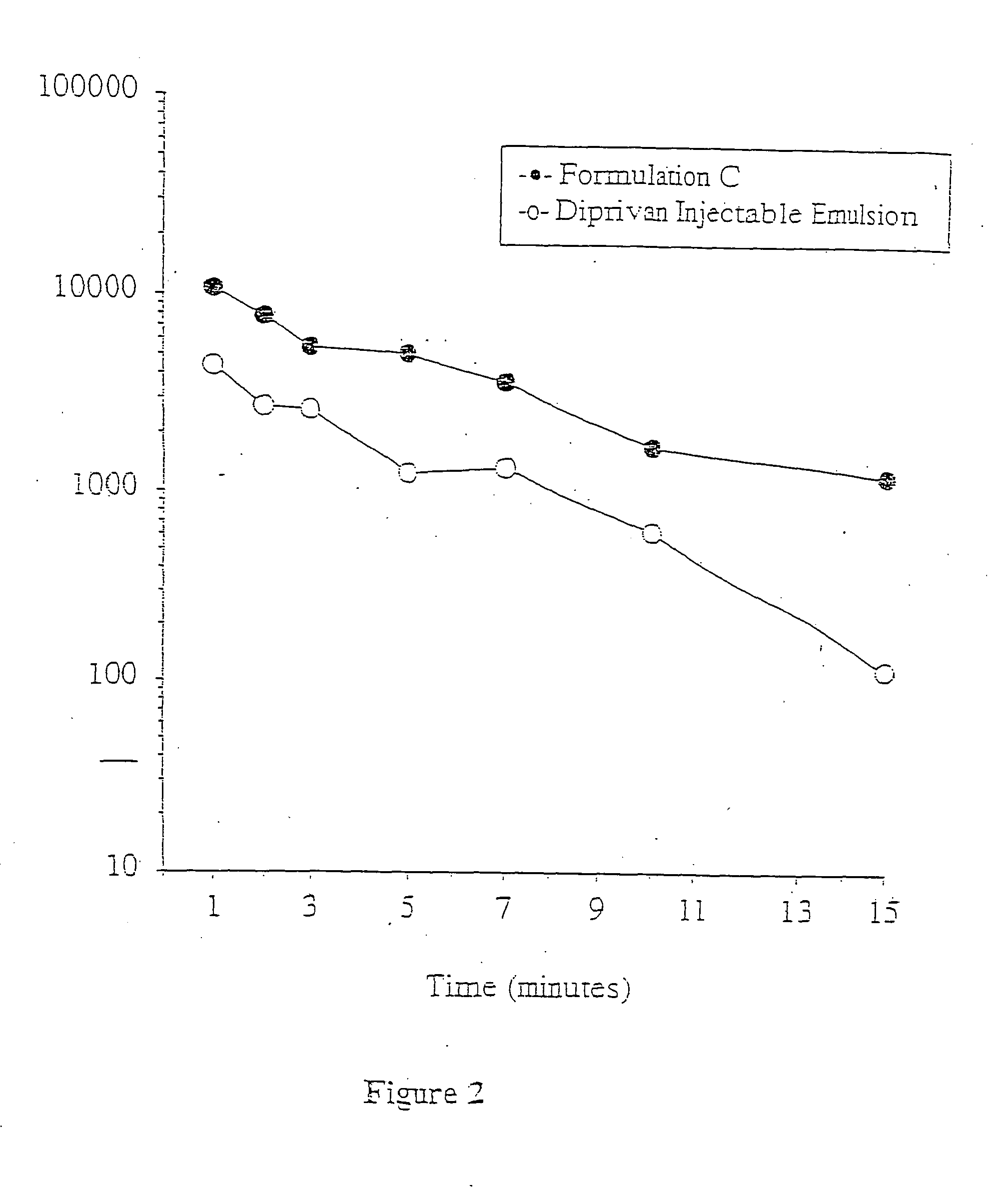
Aqueous 2,6-diisopropylphenol pharmaceutical compositions
InactiveUS20050027019A1Good chemical stabilityImprove physical stabilityBiocideHydroxy compound active ingredientsAqueous solutionExcipient
The present invention relates to aqueous pharmaceutical compositions comprising 2,6-diisopropylphenol (propofol). A composition of the present invention can comprise propofol and two or more excipients as an aqueous mixture. The propofol containing compositions are preferably sterile and are parenterally administered to any animal, including humans. The compositions are also chemically and physically stable over a wide range of environmental conditions.
Owner:JANSSEN BIOTECH INC
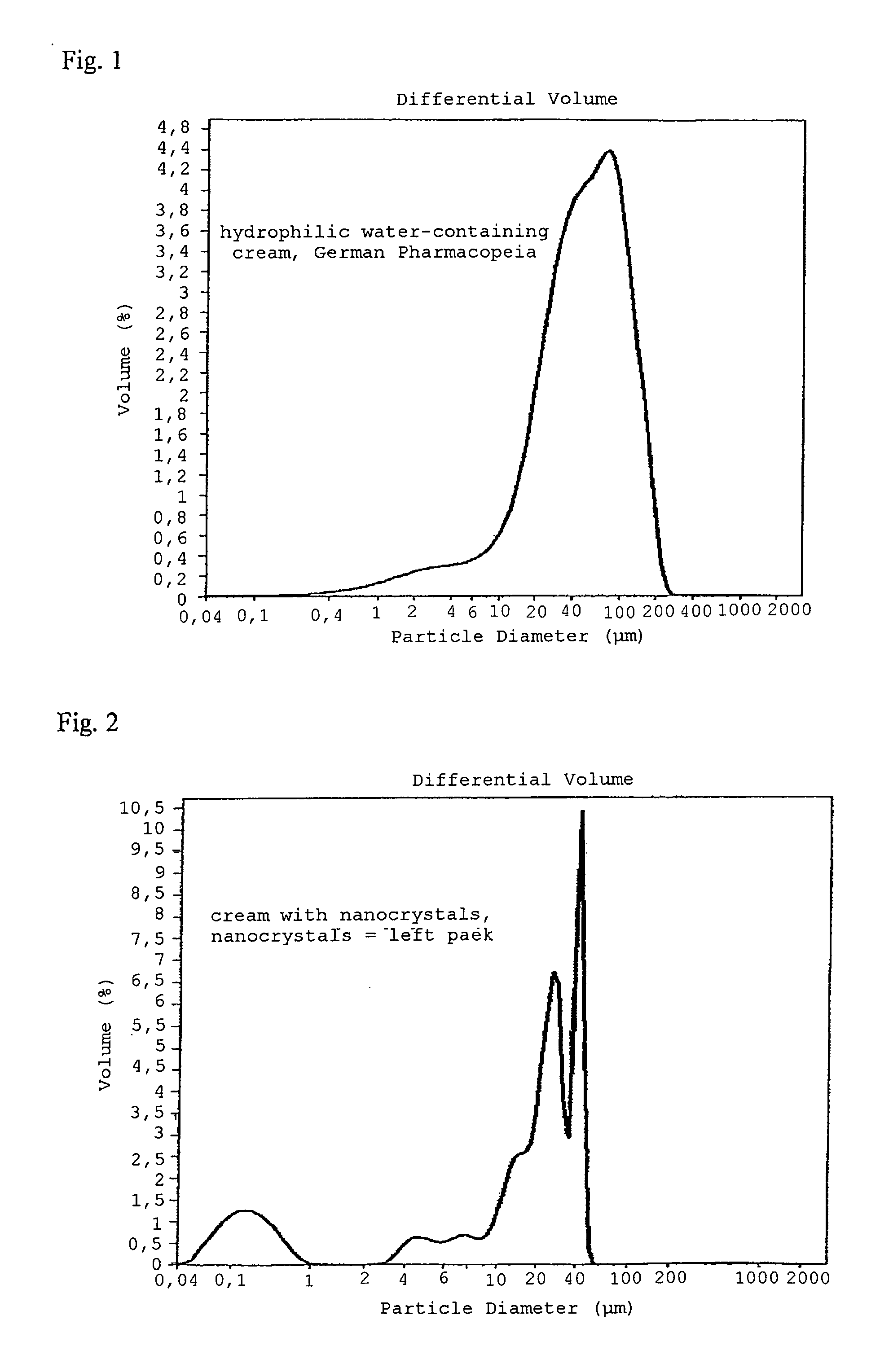
Nanocrystals for use in topical cosmetic formulations and method of production thereof
ActiveUS20100047297A1Improve skinSignificant positive effectBiocideCosmetic preparationsHigh energyLotion
Provided are cosmetic preparations for topical application containing nanocrystals of cosmetic actives leading to an increased bioactivity of the molecules in the skin and methods of making the cosmetic preparations. The nanocrystals can be added to any cosmetic topical formulation, e. g. creams, lotions and liposomal dispersions. The drug nanocrystals are produced by a combination process of low energy pearl milling followed by a high energy high-pressure homogenization leading to nanocrystal suspensions (nanosuspensions) of improved physical stability.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Method for preparing a photochromic nanoparticle and nanoparticle prepared therefrom
InactiveUS20080026217A1Maintain transparencyEasy production processMaterial nanotechnologyLiquid surface applicatorsMean diameterCore shell
The present invention provides a photochromic nanoparticle having a core-shell structure comprising (a) a polymer nano particle having a mean diameter controlled in a range of 10˜150 nm and containing a photochromic dye; and (b) a silicate inorganic polymer layer enveloping the polymer nanoparticle, and a method for preparing the same. The photochromic nanoparticle according to the present invention has structural and physical stability continuously in a long term and has transparency because of low light scattering, so that it can be applied to optical products.
Owner:LG CHEM LTD
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
ActiveUS20180071390A1Less viscousLess denseOrganic active ingredientsAerosol deliveryUse medicationDiethylene glycol monoethyl ether
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Passive conductive cooling module
InactiveUS20070297137A1Improve physical stabilityAdditional stability against vibrationSemiconductor/solid-state device detailsSolid-state devicesEngineeringPhysical stability
A cooling module includes a thermally conductive plate, a bladder disposed on at least one side of the plate, the bladder have a chamber, and fluid disposed in the chamber of the bladder wherein the bladder in an inflated state impresses the cooling module against an adjacent electronic circuit card. where the cooling module is forcibly pressed against adjacent electronic circuit card providing increased physical stability to the electronic circuit card as well as provide a cooling technique for the circuit card.
Owner:RAYTHEON CO
Dermatological/cosmetic gels comprising at least one retinoid and benzoyl peroxide
ActiveUS20080181963A1Improve physical stabilityGood chemical stabilityBiocideCosmetic preparationsRetinoidBenzoyl peroxide
Owner:GALDERMA RES & DEV SNC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com