Single-chain insulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0163] A number of single-chain insulins were produced as described above and isolated from the culture medium and purified for further testing. The single-chain insulins were tested for biological insulin activity as measured by binding affinity to the human insulin receptor relative to that of human insulin as described below.
[0164] Furthermore, the affinity to the IGF-1 was tested as described below. The results are shown Table 1 where “IR” means human insulin receptor binding relative to that of human insulin.
TABLE 1Human IGF-1 receptorSingle-chainbinding relative toinsulin (PAK)Connecting peptideAmino acid substitutionsIRhuman insulin1606RSFDGK41%(SEQ ID NO: 34)1663TVGSSRGK46%(SEQ ID NO: 35)1664TGSSRGK43%(SEQ ID NO: 36)1735VGRSSGK[A21G]143%(SEQ ID NO: 31)1754AGRGSGP53%(SEQ ID NO: 18)1767AGRGSGP[A18Q_A21G]28%(SEQ ID NO: 18)1801AGRGSGK129%(SEQ ID NO: 15)1817AGRGSGK[A21G]63%(SEQ ID NO: 15)1800AGRGSGK[A18Q_A21G]175%(SEQ ID NO: 15)1805AGRGSGK[B3Q_A18Q_A21G]83%(SEQ ID NO: 15)1808A...
example 2
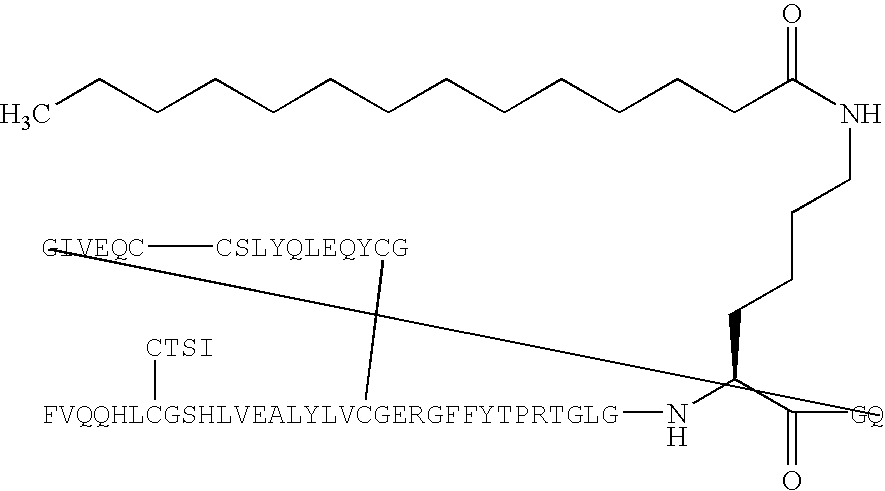
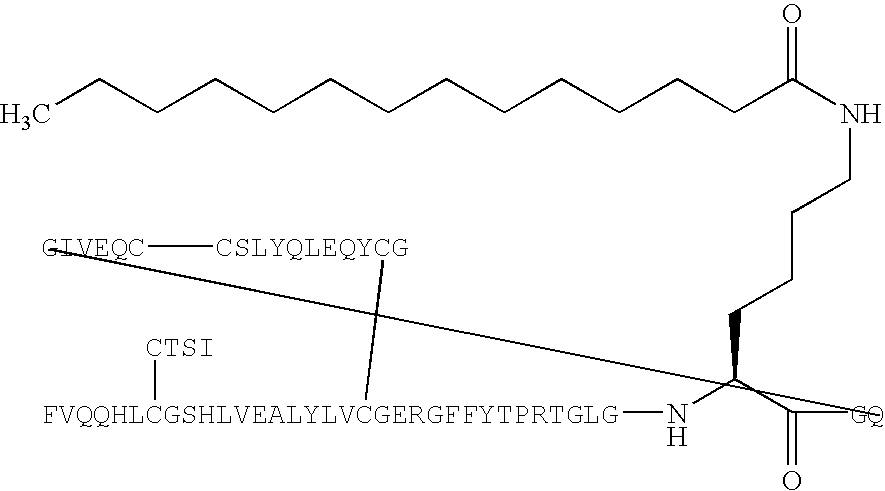
[0166] B(1-29)-B3Q-B29R-TGLGK((eps)myristoyl)GQ-A(1-21)-A18Q-A21 G Human Insulin (SEQ ID NO:133) (PAK1820)
[0167] B(1-29)-B30-B29R-TGLGKGQ-A(1-21)-A18Q-A21G human insulin (SEQ ID NO:133) (150 mg, 24 μmol) was dissolved in aqueous sodium carbonate (100 mM, 2.8 mL) and added a solution of myristic acid N-hydroxysuccinimide ester (7.7 mg, 24 μmol, may be prepared according to B. Faroux-Corlay et al., J. Med. Chem. 2001, 44, 2188-2203) in N-methylpyrrolidin-2-one (0.5 mL). The resulting mixture was added more N-methylpyrrolidin-2-one (3 mL) and aqueous sodium carbonate (100 mM, 0.8 mL), to pH 10-11. The resulting mixture was stirred at room temperature for 50 minutes. pH was adjusted to 5.5 with 1 N hydrochloric acid. The solid formed was isolated by centrifugation and decantation. The residue was purified by preparative HPLC in two runs on a Jones Kromasil RP18 5 μm, 15×225 mm column, using a flow of 8 mL / min with the following gradient:
[0168] 0.00-5.00 min: 10% CH3CN+0.1% TFA,
[0169...
example 3
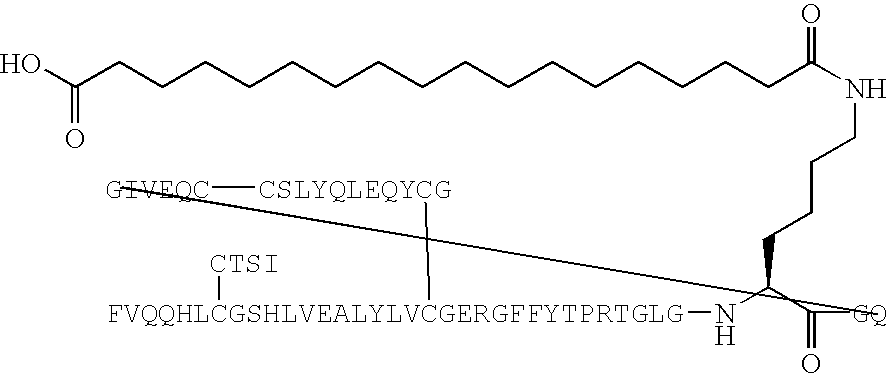
[0186] B(1-29)-B3Q-B29R-TGLGK((eps)octadecandioyl)GQ-A(1-21)-A18Q-A21 G Human Insulin (SEQ ID NO:133) (PAK1820)
[0187] B(1-29)-B30-B29R-TGLGKGQ-A(1-21)-A18Q-A21G human insulin (SEQ ID NO:133) (150 mg, 24 μmol) was dissolved in aqueous sodium carbonate (100 mM, 2.8 mL) and added a solution of succinimidyl tert-butyl octadecandioate (prepared in analogy with the method described in example 4 (11 mg, 24 μmol) in acetonitrile (2 mL). The resulting mixture was added more acetonitrile (2 mL). pH of the mixture was 10-11. The resulting mixture was stirred at room temperature for 1 hour. pH was adjusted to 5.86 with 1N hydrochloric acid. The solid formed was isolated by centrifugation and decantation. The residue was purified by preparative HPLC in two runs on a Jones Kromasil RP18 5 μm, 15×225 mm column, using a flow of 8 mL / min with the following gradient:
[0188] 0.00-5.00 min: 10% CH3CN,
[0189] 5.00-35.0 min: 10%-50% CH3CN,
[0190] 35.0-45.0 min: 50%-90% CH3CN.
[0191] Pure fractions were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com