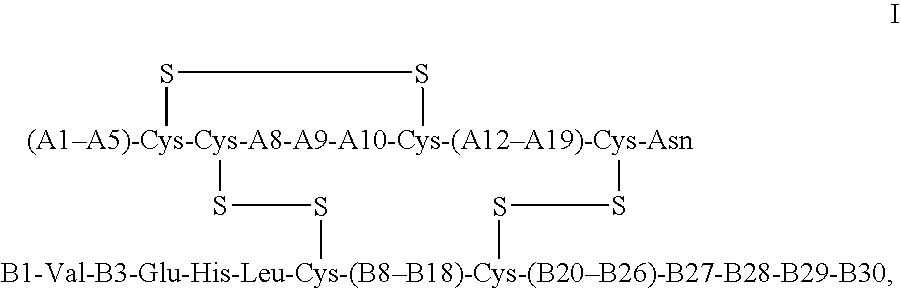Patents
Literature
800 results about "Basic amino acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell penetrating peptides for intracellular delivery
ActiveUS8372951B2In-vivo radioactive preparationsPeptide/protein ingredientsCrystallographySide chain
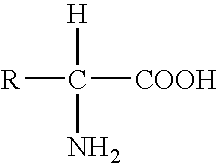
A cell penetrating peptide which has following sequence: NYBX1BX2BNQX3, wherein B represents a basic amino acid, X1 represents an amino acid with an aromatic, a hydrophobic or an uncharged side chain, X2 represents any amino acid, and X3 represents N or none is described. A method for delivering a cargo into a subject by administrating a complex comprising the cell penetrating peptide and the desired cargo to the subject is also described.
Owner:NATIONAL TSING HUA UNIVERSITY
Oral care product and methods of use and manufacture thereof
ActiveUS20090202451A1BenefitLower potentialAntibacterial agentsCosmetic preparationsDentistrySmall particles
This invention relates to oral care compositions comprising a basic amino acid or salt thereof, and a small particle fraction: and to methods of using and of making these compositions.
Owner:COLGATE PALMOLIVE CO
Method for the production of polypeptides
The present invention relates to a novel method for the production of short chain polypeptides, including polypeptides having up to 3 disulfide bonds and / or structures rich in basic amino acid residues, and open structured short chain polypeptides, e.g. glucagon, glucagon like peptides and their functional analogues, in genetically modified yeast cells, said genetically modified yeast cells, and a method for the preparation of said yeast cells.
Owner:NOVO NORDISK AS
Oral care product and methods of use and manufacture thereof
InactiveUS20090202450A1Promotes Oral HealthReduce adhesionAntibacterial agentsOrganic active ingredientsMedicineSURFACTANT BLEND
This invention relates to oral care compositions comprising an effective amount of a basic amino acid in free or salt form, together with an anionic surfactant, and to methods of using and of making such compositions.
Owner:COLGATE PALMOLIVE CO
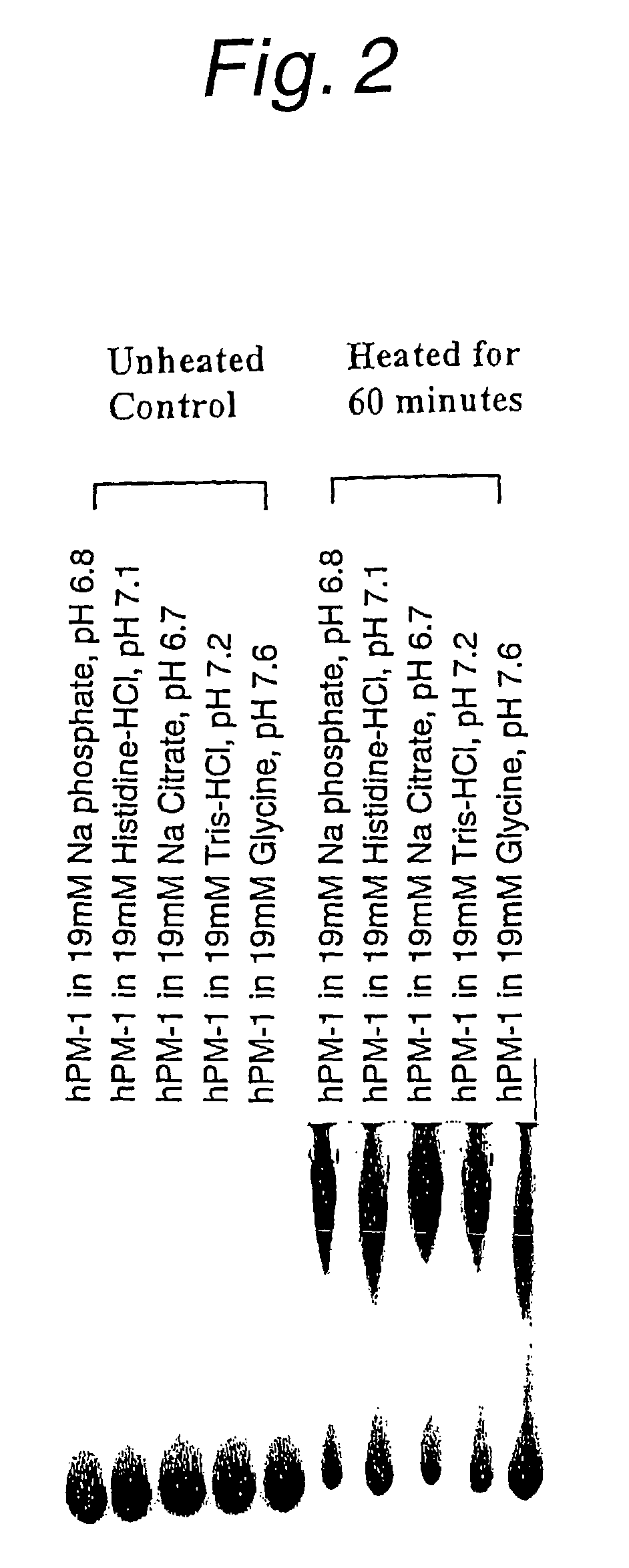
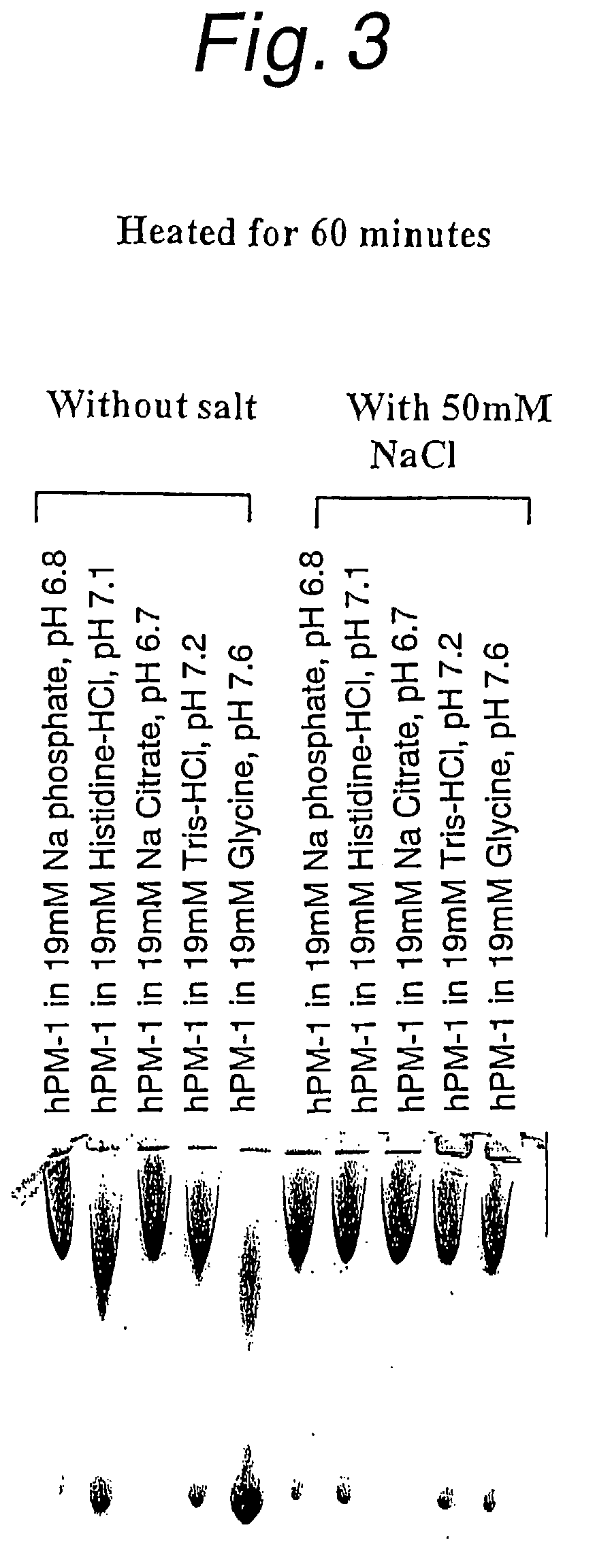
Stabilized anti-interleukin-6 antibody-containing preparations
InactiveUS8632778B2Reduce aggregationImprove stabilityOrganic active ingredientsBiocideCrystallographyInterleukin 6
The present invention provides stabilized preparations containing an antibody in a glycine buffer and / or a histidine buffer and also provides processes for preparing a protein-containing stabilized preparation, comprising adjusting the pH with a basic amino acid or a basic amino acid derivative or a salt thereof.
Owner:CHUGAI PHARMA CO LTD
Oral Care Composition Comprising Arginine and Calcium Carbonate
InactiveUS20130224270A1Inhibiting and reducing accumulationDiminishing acid levelCosmetic preparationsToilet preparationsPrecipitated calcium carbonateMedicine
This invention relates to oral care compositions comprising a basic amino acid or salt thereof, and an abrasive system comprising natural calcium carbonate and precipitated calcium carbonate; and to methods of using and of making these compositions.
Owner:COLGATE PALMOLIVE CO
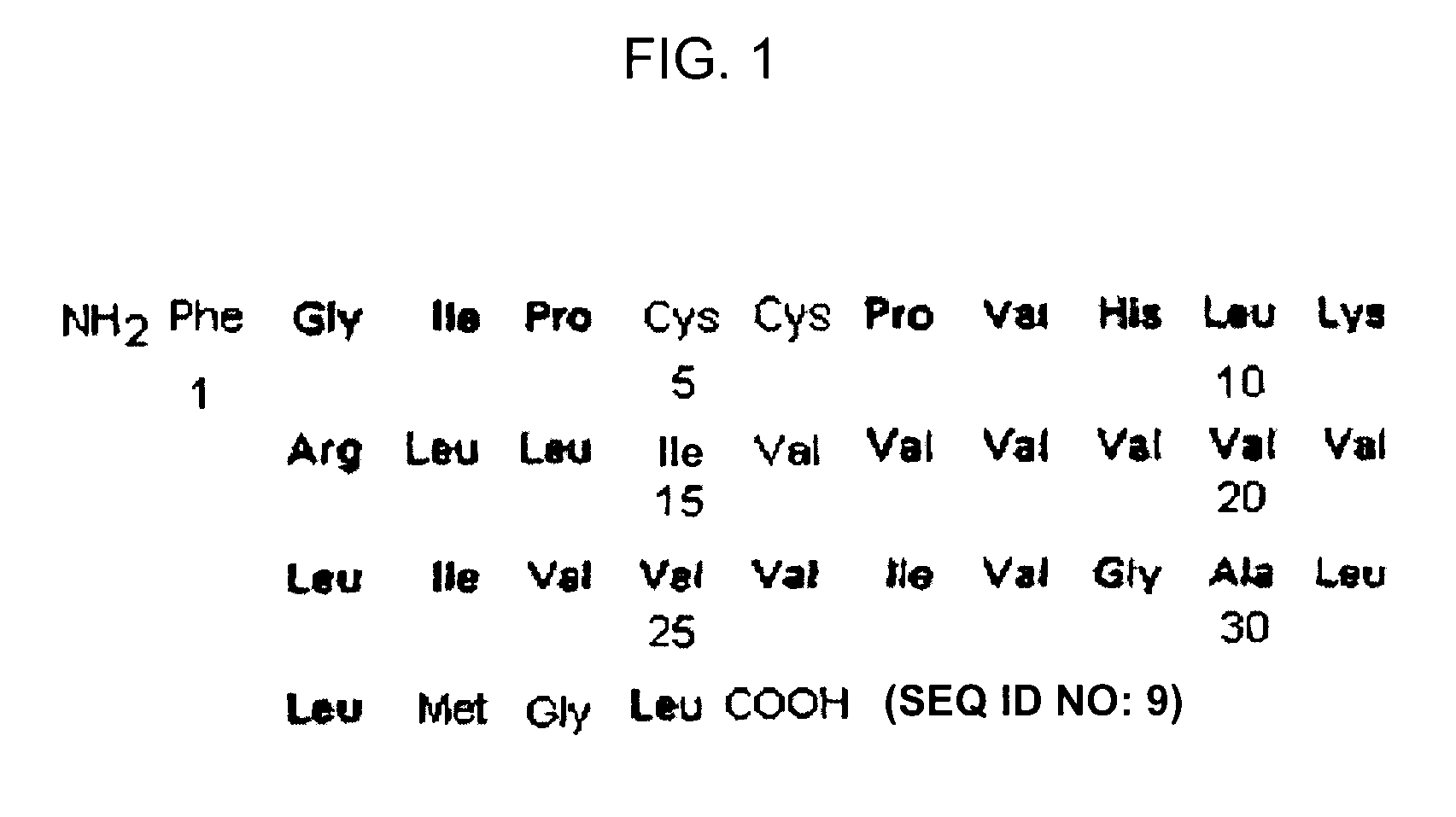
Reconstituted surfactants having improved properties
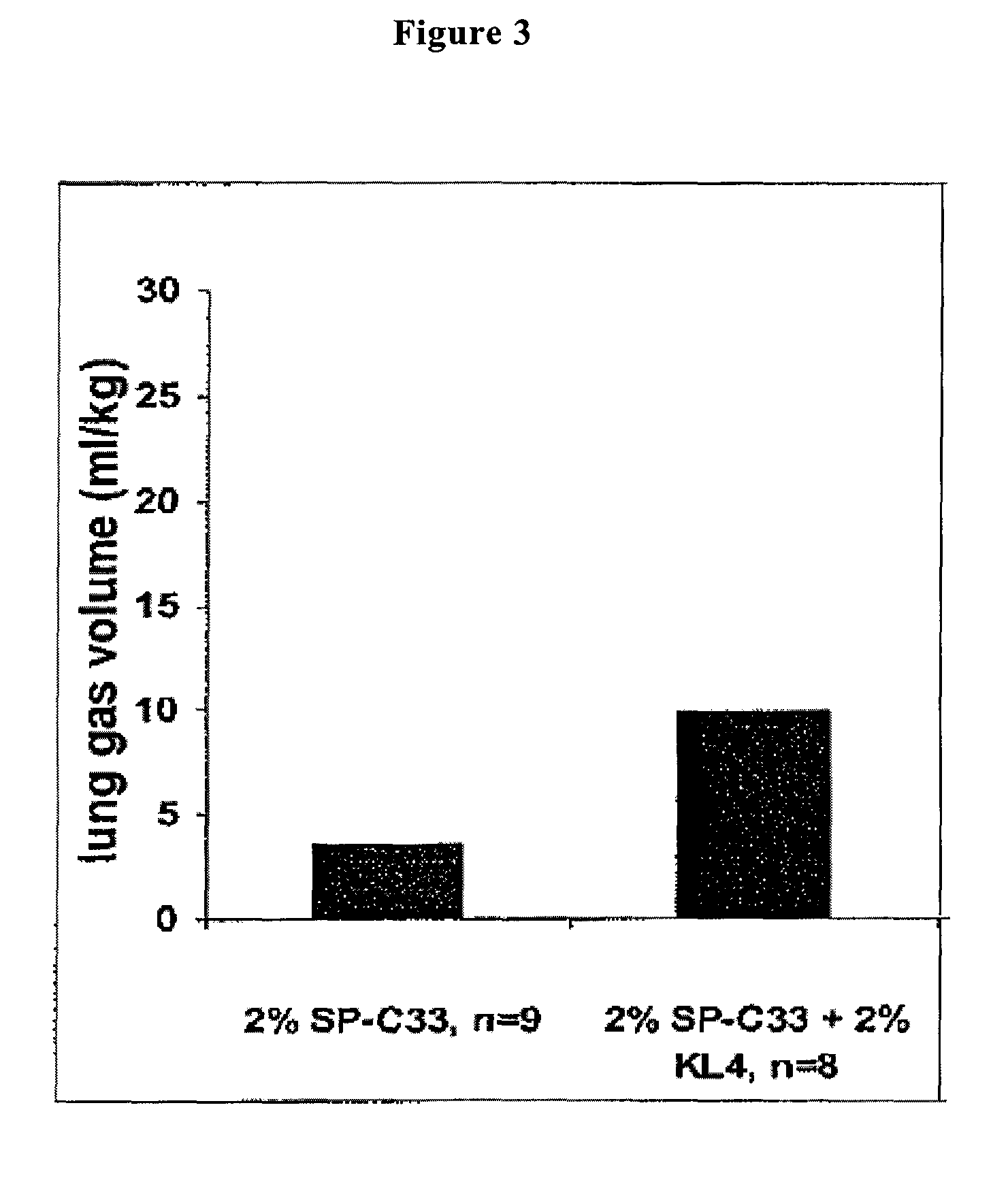
ActiveUS7897577B2Peptide/protein ingredientsAlveolar/pulmonary surfactant peptidesSURFACTANT BLENDRepeat unit
The present invention is directed to a reconstituted surfactant containing a lipid carrier, a polypeptide analog of the native surfactant protein SP-C, and a polypeptide comprising a sequence comprised of repeated units where each unit contains between 3 and 8 hydrophobic amino acid residues and one basic amino acid residue. The invention is also directed to the pharmaceutical compositions thereof and to its use for the prophylaxis and / or treatment of RDS and other respiratory disorders.
Owner:CHIESI FARM SPA
Production process of cross-linked polysuccinimide resin
Owner:MITSUI CHEM INC
Hair treatment compositions
The invention provides a hair treatment composition such as a shampoo or conditioner suitable for topical application to hair for the repair and prevention of damage, comprising (i) cholesterol, and (ii) a hair benefit agent which is a mixture of a basic amino acid and a fatty acid. Preferably the cholesterol and hair benefit agent are compounded into a dispersion of multilamellar vesicles, stabilised with nonionic surfactant.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Slurry for CMP of Cu film, polishing method and method for manufacturing semiconductor device
A slurry for CMP of Cu film is provided, which includes water, peroxosulfuric acid or a salt thereof, basic amino acid, a complexing agent which forms a water-insoluble metal complex, a surfactant, and colloidal silica having a primary diameter ranging from 5 to 50 nm. The basic amino acid is included at a content of 0.05 to 0.5 wt %.
Owner:KK TOSHIBA
Dentifrice compositions containing calcium silicate and a basic amino acid
ActiveUS20120141588A1Reduce and inhibit formationPromote remineralizationAntibacterial agentsCosmetic preparationsCalcium silicateMedicine
An oral care composition includes an effective amount of a basic amino acid in free or salt form; and an effective amount of calcium silicate particles. The calcium silicate particles have an average diameter of less than about 5 microns, such that they can occlude dentinal tubules of the teeth. An oral care method includes applying the composition to an oral cavity of a subject to reduce or inhibit hypersensitivity of the teeth and to achieve other benefits.
Owner:COLGATE PALMOLIVE CO
Peptide having an extending action for half-life of object peptide in plasma
ActiveUS20100305031A1Improve securityLow costPeptide/protein ingredientsAntipyreticHalf-lifeBlood plasma
A peptide of the following (I) or (II).(I) a peptide represented by the formula B, A-B, B-C or A-B-C in which A, B and C each is represented by the following (1), (2) and (3) and, when it is bonded to other object peptide, it is able to extent the half-life in plasma as compared with the object peptide where the physiological activity of the object peptide is still retained.(II) a peptide comprising a reversed sequence of the peptide of (I); a sequence which is represented by A-B in (I) and A or B is reversed; a sequence which is represented by B-C in (I) and B or C is reversed; or a sequence which is represented by A-B-C in (I) and A, B, C, A and B, B and C or A and C is reserved.(1) A is a peptide comprising 1 to 14 of any amino acid(s)(2) B is a peptide represented by the formula 1:(Wk-Xl-Y-Zm-Wn)-(Wo-Xp-Y-Zq-Wr)s(In the formula 1, W is a basic amino acid; X and Z are any amino acids; Y is an acidic amino acid; k is 1 or 2; l is an integer of 4≧l≧0; m is an integer of 2≧m≧0; 4≧l+m≧0; n is 1 or 2; o is 1 or 2; p is an integer of 4≧p≧0; q is an integer of 2≧q≧0; 4≧p+q≧0; r is 1 or 2; and s is 0 or 1.)(3) C is a peptide comprising 2 to 14 of any amino acids.
Owner:DAIICHI SANKYO CO LTD
Cell-penetrating peptides
ActiveUS20120065124A1Simple and patient-oriented pharmacotherapyEfficient transferOrganic active ingredientsBiocideCell membraneCell membrane permeability
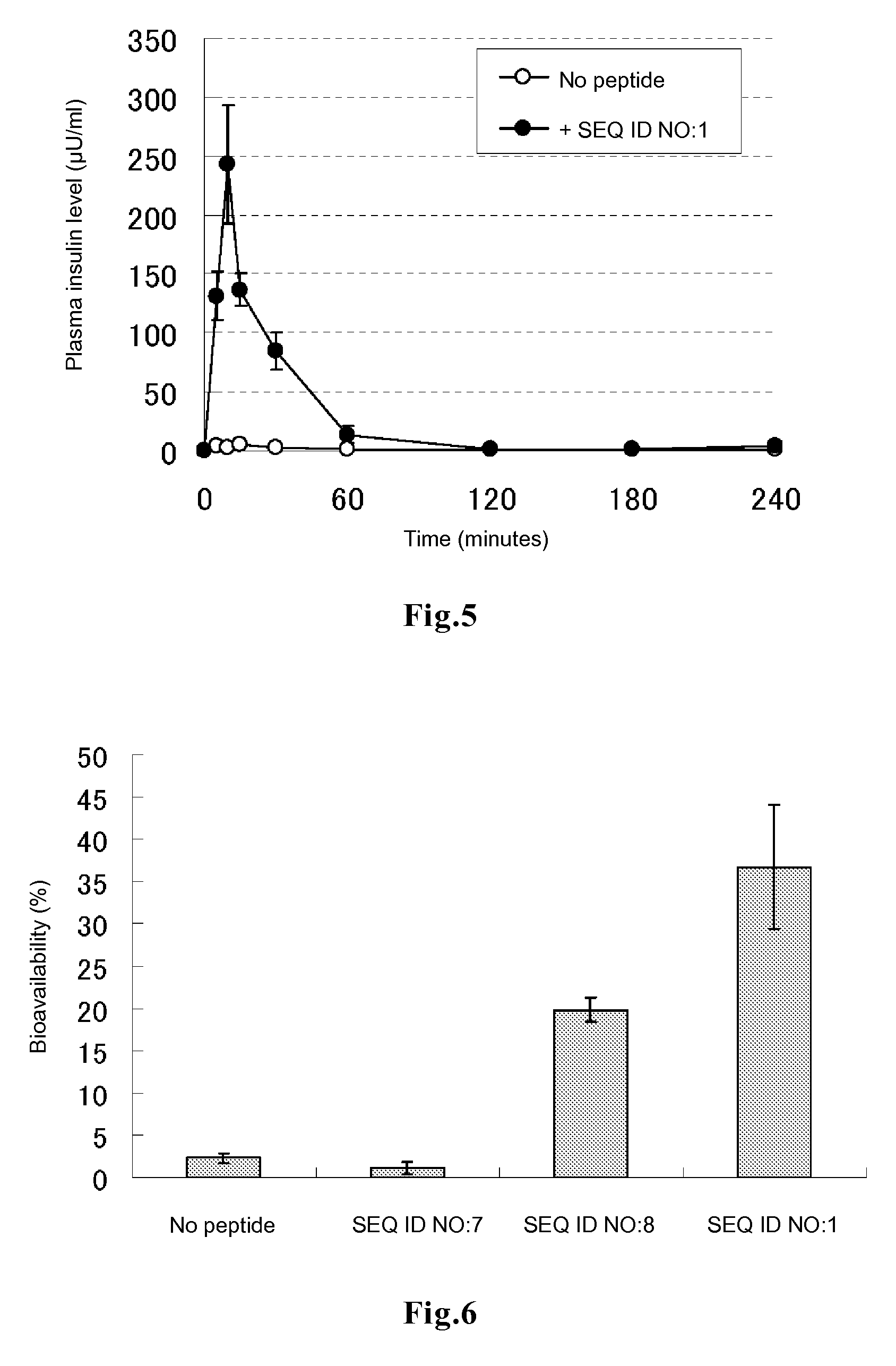
A cell-penetrating peptide of (A) to (D) below gives cell membrane permeability and transmucosal absorbability to a physiologically active substance:(A) a peptide having the amino acid sequence of SEQ ID NO:1;(B) a peptide represented by SEQ ID NO:1 except that one or several basic amino acids are changed;(C) a peptide represented by SEQ ID NO:1 except that 1 to 5 amino acids are changed;(D) a peptide having: the reverse sequence of any of (A) to (C); an amino acid sequence which is the same as the reverse sequence of (A) except that one or several basic amino acids are changed; or an amino acid sequence which is the same as the reverse sequence of (A) except that 1 to 5 amino acids are changed.
Owner:TORAY IND INC
Normal temperature formaldehyde removal catalysis material
InactiveCN104741130ALow costEfficient enrichmentDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsAlkaline earth metalCatalytic oxidation
The invention relates to a formula and a corresponding preparation technology of a normal temperature formaldehyde removal catalysis material, and belongs to the technical fields of normal temperature adsorption catalysis and air pollution treatment. The catalysis material is prepared by adopting a one-step co-precipitation process or a step-by-step dipping process to load active components with granular, cylindrical, spherical or honeycomb high-specific surface area shell activated carbon as a carrier, one or more non-noble metal salts as main active components, such as nitrate, sulfate or chloride of manganese, iron, cobalt, nickel, copper, zinc, lanthanum or cerium, one or more alkali metal / alkaline earth metal salts as an inorganic additive, such as oxalate, nitrate, sulfate, acetate, carbonate and bicarbonate of sodium, potassium, magnesium, calcium, and ethylenediamine tetracetic acid / ethylenediamine tetraacetic acid salt, basic amino acid, polyamide monomer and polyurethane monomer as a nitrogen-containing organic additive. The normal temperature formaldehyde removal catalysis material has the characteristics of rapid capture, high efficiency catalytic oxidation and long working cycle, and can meet requirements of long-time effective removal of formaldehyde in indoor real environment.
Owner:江苏瑞丰科技实业有限公司
Single-Chain Insulin
InactiveUS20090170750A1Lower blood sugar levelsSugar derivativesPeptide/protein ingredientsInsulin activityPhysical stability
The present invention is related to single-chain insulin having insulin activity comprising a B- and an A-chain or a modified B- and A-chain connected by a connecting peptide of from 6-11 amino acids. The single-chain insulins will have biological insulin activity and an IGF-1 receptor affinity similar to or lower than that of human insulin and a high physical stability. The single-chain insulin may contain at least one basic amino acid residues in the connecting peptide. The single-chain insulins may also be acylated in one or more Lys residues.
Owner:NOVO NORDISK AS
Controlled release biocidal salts
InactiveUS20100173993A1Environment safetyLong-lasting lethalityBiocidePeptide/protein ingredientsControlled releaseArginine
A controlled release biocidal salt of a first component comprises a cation of a Nα—(C1-C22)alkanoyl di-basic amino acid alkyl(C1-C22)ester cationic biocidal molecule and a second component comprising an anion of a monomeric anionic molecule having insignificant biocidal activity. The salt is characterized such that when the salt is exposed to an aqueous medium, the salt partially dissolves thereby releasing biocidal ions in an amount sufficient to exceed the MIC or MBC of a target bacteria being controlled, and further characterized as leaving a residual reservoir of undissolved salt capable of releasing more biocidal ions as the salt is consumed or otherwise removed from the environment encompassing the target bacteria. The preferred cationic biocidal molecule comprises Nα-lauroyl-L-arginine ethyl ester (“LAE”).
Owner:NEVADA NATURALS
Casein-derived antioxidant peptide and preparation method thereof
ActiveCN105254714AInhibit oxidation functionPeptide/protein ingredientsPeptide preparation methodsAntioxidant capacityProteinase activity
The invention relates to the technical field of bioactive peptides, in particular to a casein-derived antioxidant peptide mixture. Casein is hydrolyzed with protease, various pre-products of the casein antioxidant peptide are obtained, then separation, desalination and concentration are performed through ion exchange column chromatography, and the casein antioxidant peptide rich in basic amino acid is obtained. The casein antioxidant peptide has higher cell antioxidant capacity and low-density lipoprotein oxidation inhibition capacity and can still keep good antioxidant capacity after being digested and absorbed by gastrointestinal tracts, so that the casein antioxidant peptide rich in basic amino acid can be applied to fields of food and healthcare products and has very high social and economic benefit.
Owner:CHINA AGRI UNIV
Production process of cross-linked polyaspartic acid resin
InactiveUS6072024AMeet high volumeImprove water absorptionLiquid surface applicatorsCoatingsCross-linkProduction rate
A process is disclosed for producing with good productivity a cross-linked polyaspartic acid resin having biodegradability and high water absorbency. The process features inclusion of one of the following steps: (a) a polysuccinimide, which has been brought into a dispersed state by a dispersant, and a cross-linking agent are reacted to produce the cross-linked polyaspartic acid resin; (b) imide rings of a cross-linked polysuccinimide are subjected to a hydrolysis reaction while controlling a swelling degree of a resulting gel, whereby the cross-linked polyaspartic acid resin is produced; and (c) a gel of a cross-linked polysuccinimide, which has been obtained by reacting a cross-linking agent to a solution of a polysuccinimide in an organic solvent, is disintegrated to subject imide rings of the cross-linked polysuccinimide to a hydrolysis reaction, so that the cross-linked polyaspartic acid resin is produced. The process may also include one or both of the following steps as needed: (d) a gel of the cross-linked polyaspartic acid resin is washed with water and / or a water-miscible organic solvent; and (e) the polysuccinimide is produced using a basic amino acid as a cross-linking agent.
Owner:MITSUI CHEM INC
Oral care product and methods of use and manufacture thereof
PendingUS20100330003A1Reducing and repairing and inhibiting early enamel lesionInhibition of lesionsAntibacterial agentsOrganic active ingredientsMedicineFluoride
This invention relates to methods of treating early enamel lesions comprising applying an effective amount of a basic amino acid in free or salt form, together with fluoride to a patient in need thereof.
Owner:COLGATE PALMOLIVE CO
Amino-modified polysaccharides and methods of generating and using same
A pharmaceutical composition is provided. The pharmaceutical composition comprises an amino-modified polysaccharide having at least one amino group linked to a peptide composed of at least two basic amino acid residues.
Owner:YEDA RES & DEV CO LTD
Peptides whose uptake by cells is controllable
ActiveUS8642561B2Improve efficiencyDecrease possible side effect of treatmentUltrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsImmunogenicityChemo therapy
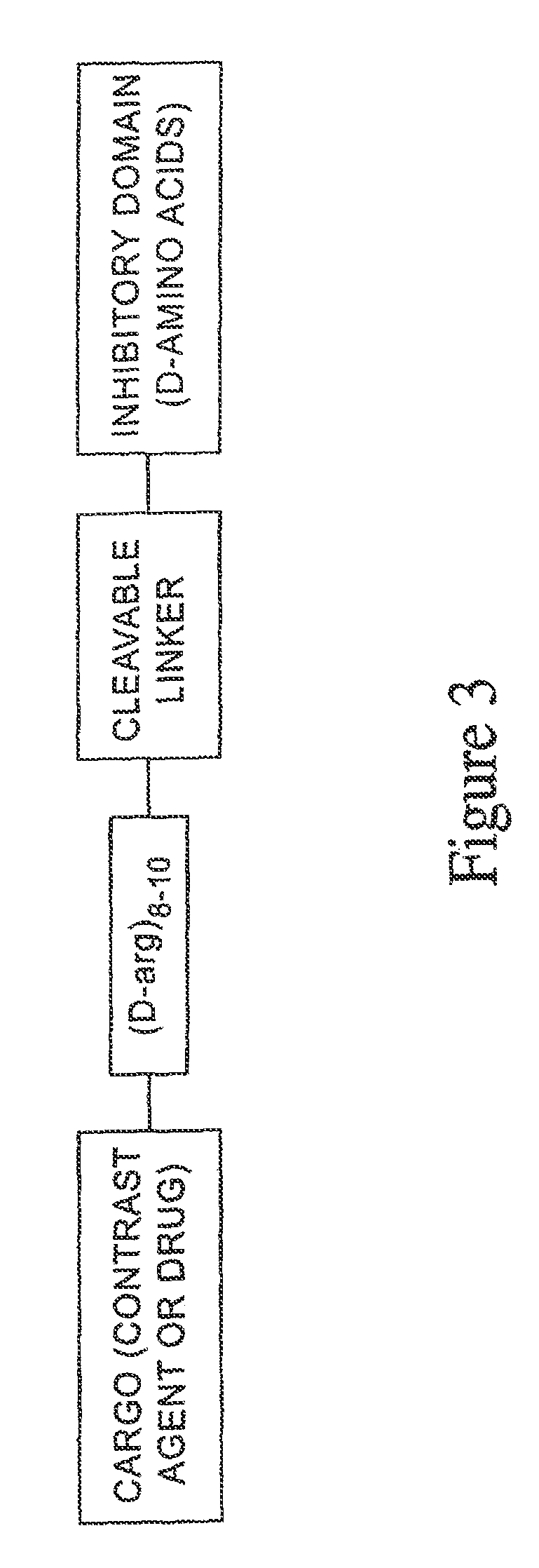
A generic structure for the peptides of the present invention includes A-X-B-C, where C is a cargo moiety, the B portion includes basic amino acids, X is a cleavable linker sequence, and the A portion includes acidic amino acids. The intact structure is not significantly taken up by cells; however, upon extracellular cleavage of X, the B-C portion is taken up, delivering the cargo to targeted cells. Cargo may be, for example, a contrast agent for diagnostic imaging, a chemotherapeutic drug, or a radiation-sensitizer for therapy. Cleavage of X allows separation of A from B, unmasking the normal ability of the basic amino acids in B to drag cargo C into cells near the cleavage event. X is cleaved extracellularly, preferably under physiological conditions. D-amino acids are preferred for the A and B portions, to minimize immunogenicity and nonspecific cleavage by background peptidases or proteases.
Owner:RGT UNIV OF CALIFORNIA
Preservative compositions
Green and naturally derived biocides such as Nα—(C1-C22) alkanoyl di basic amino acid alkyl (C1 to C22) ester salt cationic molecules can be combined with an anionic molecule not generally considered as having significant biocidal activity to provide antimicrobial, antibacterial, and / or antifungal properties with multifunctional benefits including preservative or self-preserving activity.
Owner:STOCKEL RICHARD F +1
Synthetic peptides with antimicrobial and endotoxin neutralizing properties for management of the sepsis syndrome
A peptide with an amino acid composition such that the peptide is amphipathic, cationic and forms a stable alpha-helix and has the following structure comprising at least 12 amino acidsA=an amino acid selected from the basic amino acids Lys,Arg or HisB=an amino acid selected from the aromatic amino acids Phe, Trp or TyrC=an amino acid selected from the group comprising the hydrophobic amino acids Leu, Ile, Val or Ala, andsaid peptide has either the orientation according to the formula or the retro orientation thereof, wherein at least 0-m of the repetitive sequence motifs (A2-B2-C1-A3) have the retro orientation and the remaining repetitive motifs (A2-B2-C1-A3) have the orientation as presented in the formula and wherein,R1-R2- and R3 are a number of amino acids, and whereinm=1-10, preferably 2-8, more preferably 2-5 andn=1-3, a pharmaceutical composition comprising such a peptide application thereof in treatment or diagnosis related to i.a. parasite infection topical and systemic tumors and septic shock.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT +1
Salty taste enhancer and food or drink containing the same
ActiveUS20110064861A1Enhanced salty tasteReduce in quantityMeat/fish preservationSolid waste disposalSalty tasteDecomposition
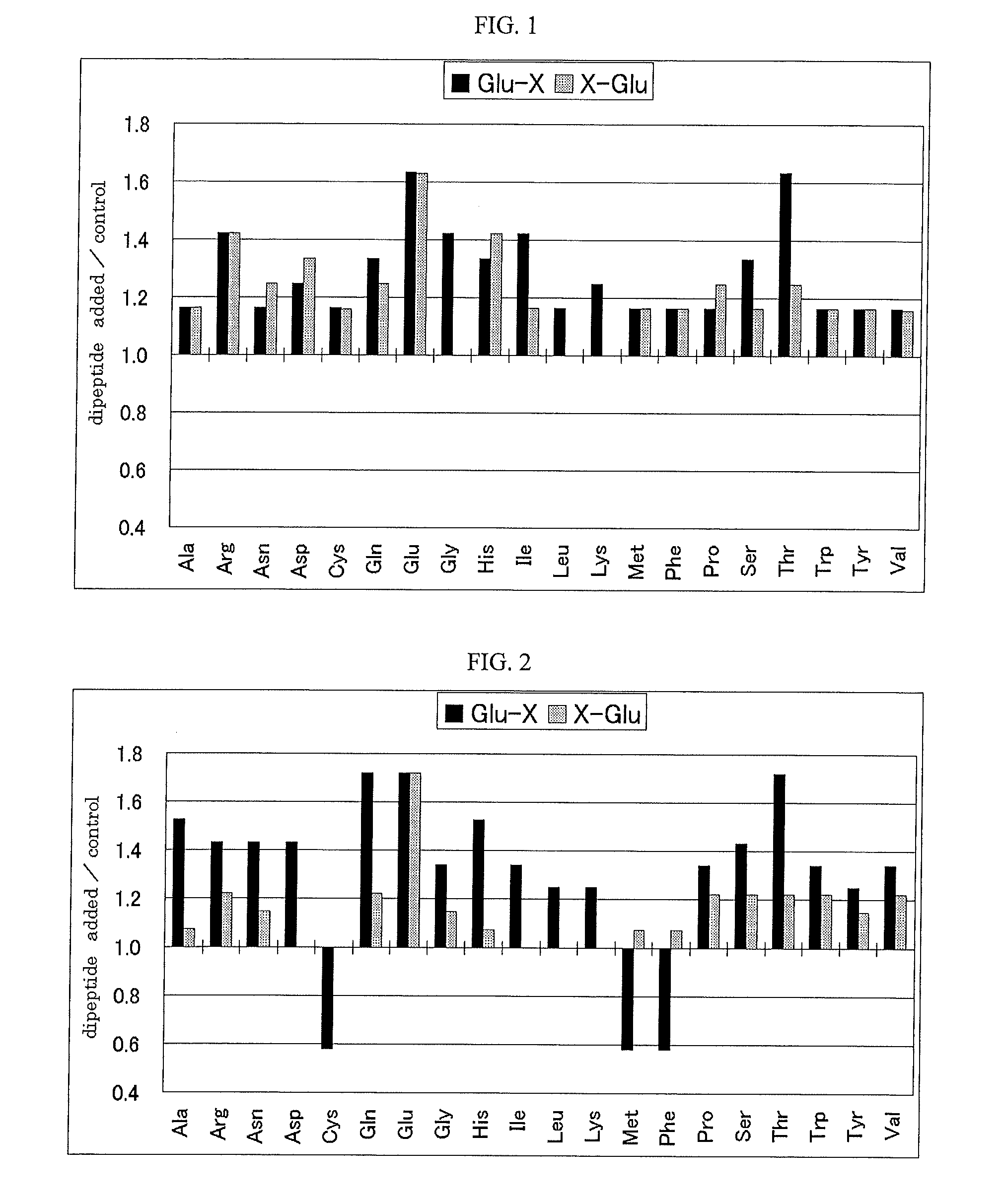
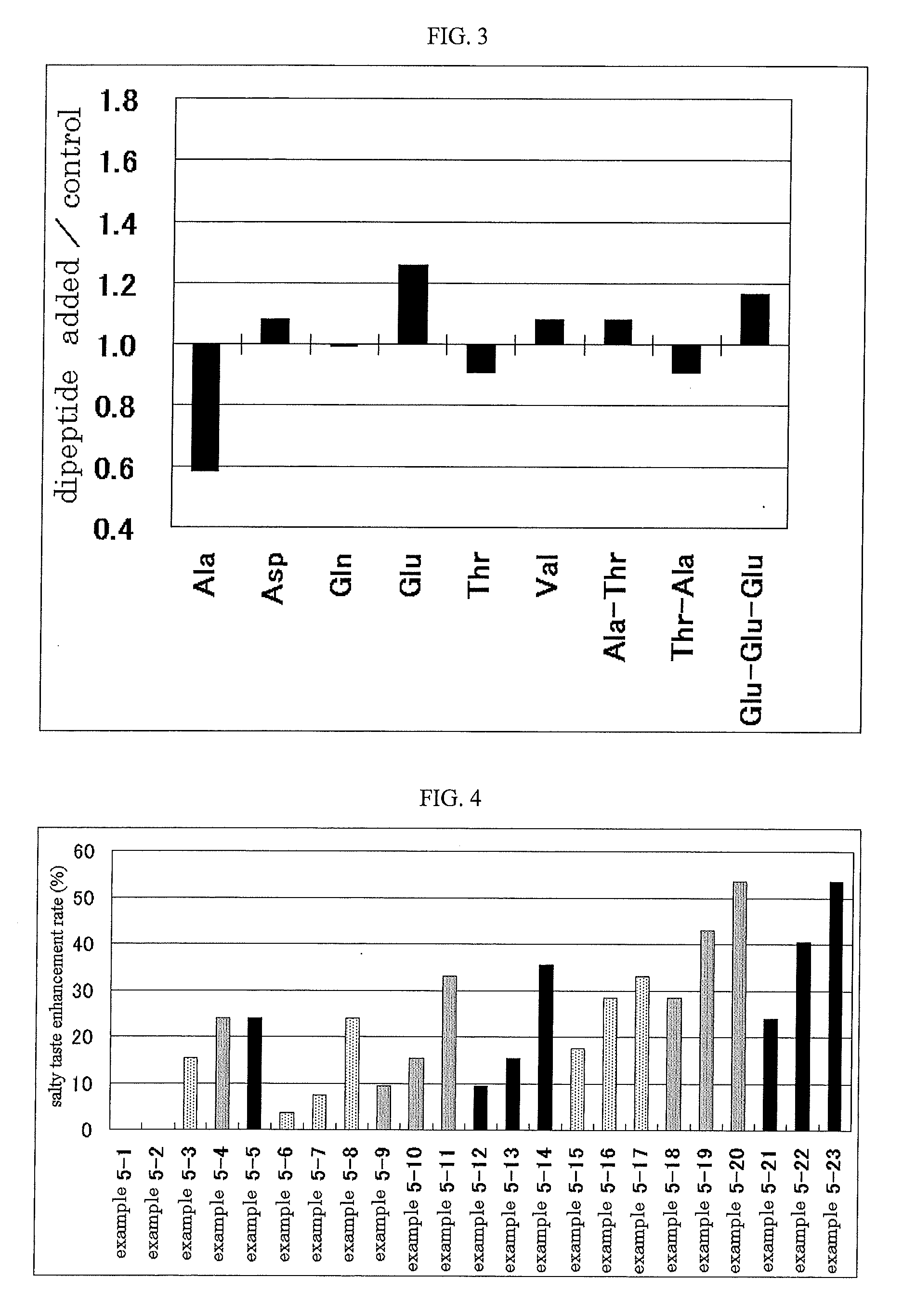
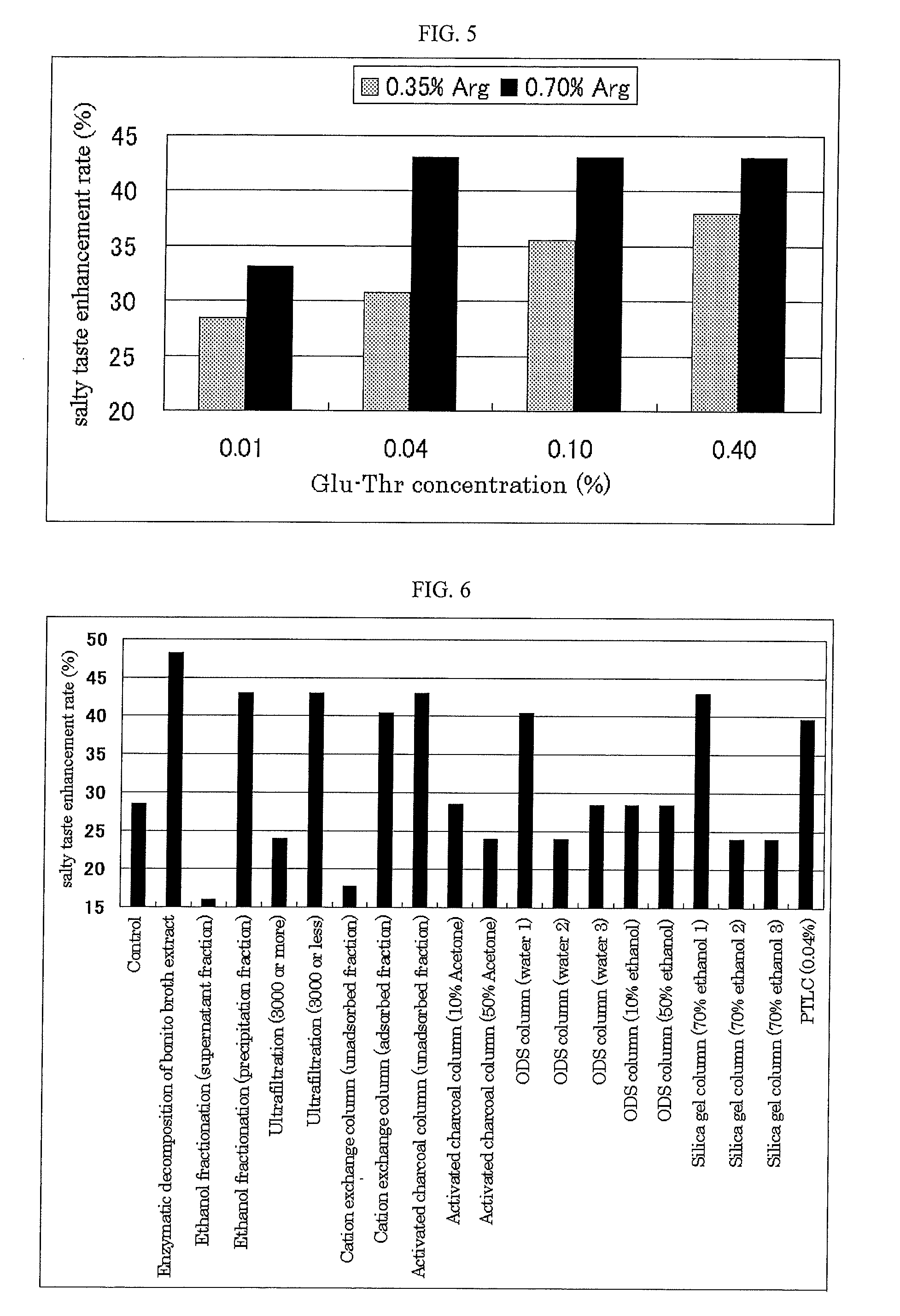
It is possible to provide an excellent salty taste enhancer that can compensate for insufficient salty taste when attempting to reduce the salt content of food. A salty taste enhancer obtained by adding a glutamic acid, containing dipeptide, specifically, Glu-Ala, Glu-Arg, Glu-Asn, Glu-Asp, Glu-Gln, Glu-Glu, Glu-Gly, Glu-His, Glu-Ile, Glu-Leu, Glu-Lys, Glu-Pro, Glu-Ser, Glu-Thr, Glu-Trp, Glu-Tyr, Glu-Val, Arg-Glu, Asn-Glu, Asp-Glu, Gln-Glu, His-Glu, Pro-Glu, Ser-Glu, Thr-Glu or Trp-Glu to an enzymatic decomposition product of a protein material and / or a basic amino acid, especially arginine. A method for producing these salty taste enhancers, a method for enhancing a salty taste by using these salty taste enhancers, and a food or drink that contains these salty taste enhancers.
Owner:NIPPON SUISAN KAISHA LTD
Heparin-binding peptides and uses thereof
InactiveUS20060172931A1Reduce anticoagulant effectReducing anticoagulant effectPeptide/protein ingredientsImmunoglobulinsDiseaseCysteine thiolate
Heparin-binding peptides are provided of the formula R1(X1B1B2X2B3X3Y1R2)nR3, R1(X1B1B2B3X2X3B4X4Y1R2)nR3, and C(X1B1B2B3X2X3B4X4)nC; wherein X1, X2, X3, and X4 are independently selected from the group consisting of hydropathic amino acids; B1, B2, B3, and B4 are independently selected from the group consisting of basic amino acids; C is cysteine; Y1 is zero or one to ten amino acid residues, wherein at least one amino acid residue is proline; n is an integer from one to ten; and R1, R2, and R3 are independently selected segments containing from zero to twenty amino acid residues, provided, at least one of the segments R1, R2, and R3 comprises at least one hydrophobic amino acid residue. The peptide C(X1B1B2B3X2X3B4X4)nC is optionally cyclized via a disulfide bond formed between cysteine residues. The peptides are administered to reduce plasma LMWH and heparin levels and to reduce the anticoagulant effects of heparin and LMWH. The peptides are also administered to inhibit microbial growth and to inhibit mast cell serine proteases involved in various diseases and disorders. The peptides are also administered as carriers to deliver active agents.
Owner:THOMAS JEFFERSON UNIV
Method for removing viruses from high concentration monoclonal antibody solution
ActiveUS20120077963A1High yieldHigh removal ratePeptide preparation methodsImmunoglobulinsHigh concentrationMonoclonal antibody
An object of the present invention is to provide a method for removing even small viruses from a high concentration monoclonal antibody solution using a membrane, and thus for recovering the antibody within a short time at high yield in the form of a filtrate. The present invention provides a method for producing a preparation containing a monoclonal antibody, which comprises a step of removing viruses by filtering viruses in a monoclonal antibody solution using a virus-removing membrane, wherein(1) the monomer content of the monoclonal antibody accounts for 90% or more;(2) the monoclonal antibody concentration in the monoclonal antibody solution ranges from 20 mg / ml to 100 mg / ml;(3) the monoclonal antibody solution contains at least a basic amino acid; and(4) the parvovirus removal rate of the virus-removing membrane satisfies the following conditions:LRV (Log Reduction Value: logarithmic reduction value) ≧4.
Owner:ASAHI KASEI MEDICAL CO LTD
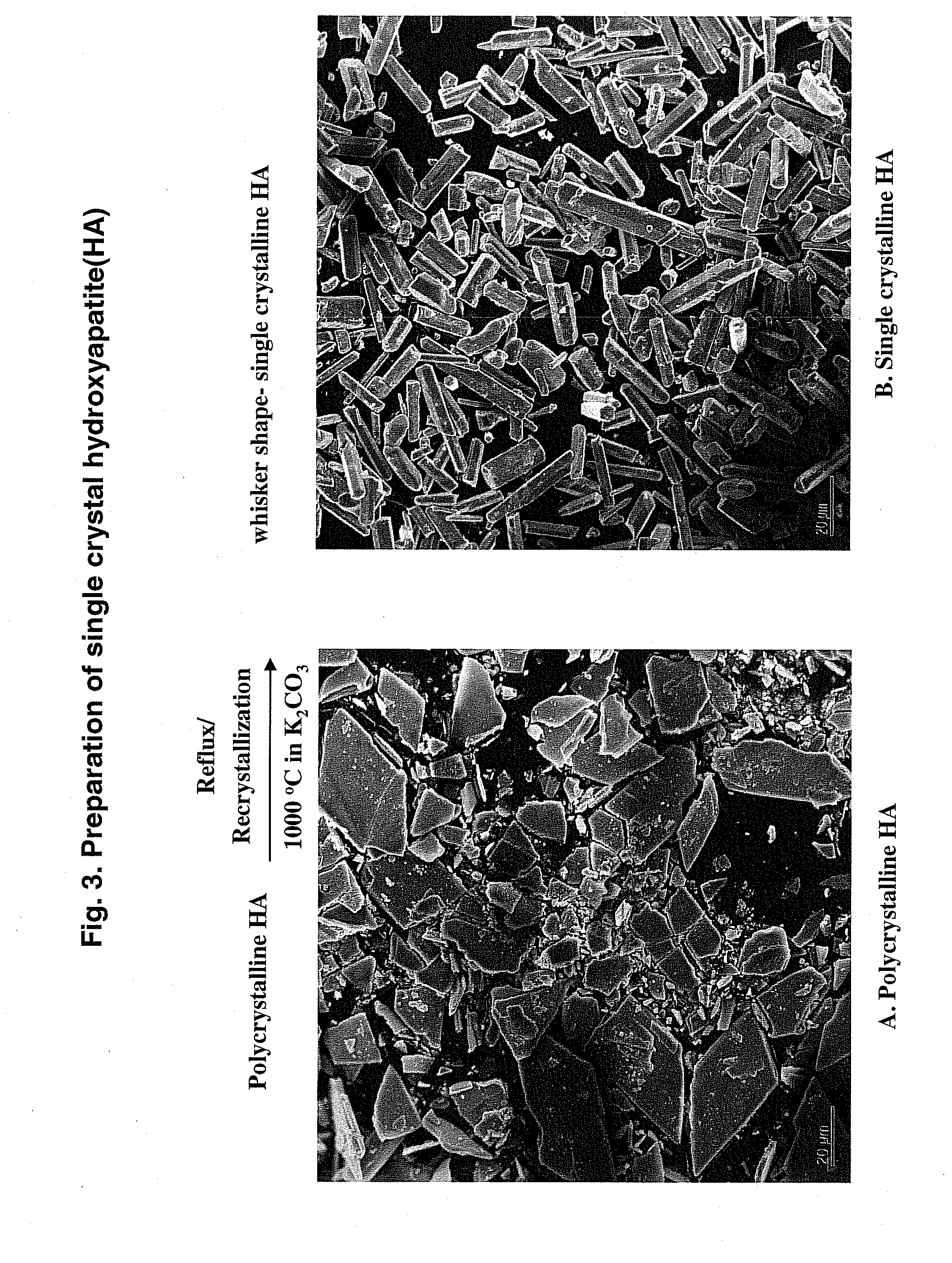
Hydroxyapatite-Binding Peptides for Bone Growth and Inhibition
ActiveUS20080279908A1Inhibiting mineral growthPhosphatesPeptide/protein ingredientsCell-Extracellular MatrixApatite
Hydroxyapatite (HA)-binding peptides are selected using combinatorial phage library display. Pseudo-repetitive consensus amino acid sequences possessing periodic hydroxyl side chains in every two or three amino acid sequences are obtained. These sequences resemble the (Gly-Pro-Hyp)x repeat of human type I collagen, a major component of extracellular matrices of natural bone. A consistent presence of basic amino acid residues is also observed. The peptides are synthesized by the solid-phase synthetic method and then used for template-driven HA-mineralization. Microscopy reveal that the peptides template the growth of polycrystalline HA crystals ˜40 nm in size.
Owner:RGT UNIV OF CALIFORNIA
Feed additive composition for ruminants and method of producing the same
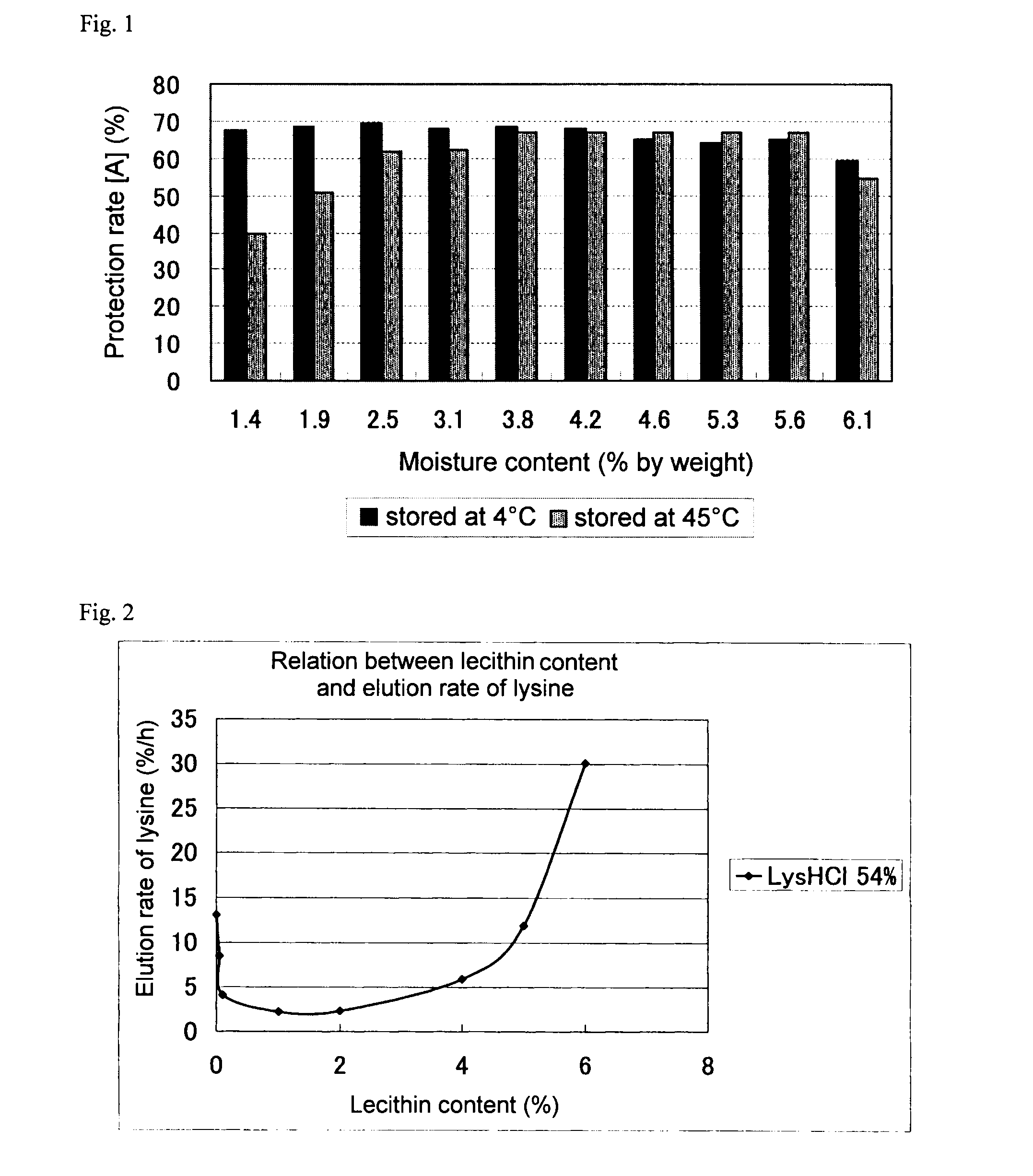
InactiveUS20090232933A1Enhancement of milk yieldIncrease productionAnimal feeding stuffAccessory food factorsBasic amino acidsChemistry
A food additive composition for ruminants of the dispersion type and a method of continuously producing the same. This food additive composition for ruminants, which contains 40% by weight or more but less than 65% by weight of a basic amino acid and has rumen bypass properties, is formulated into granules in an arbitrary shape which are scarcely classified when added to a silage or another feed. Thus, attempts have been made to develop a method of producing granules by which the milk yield of a lactation cow can be increased. Namely, it is intended to provide a food additive composition for ruminants which contains at least one protecting agent selected from among a hardened vegetable oil and a hardened animal oil having a melting point higher than 50° C. but lower than 90° C., 0.05 to 6% by weight of lecithin, water and 40% by weight or more but less than 65% by weight of a basic amino acid. It is also intended to provide a method of producing a food additive composition for ruminants characterized by comprising solidifying a molten mixture, which comprises at least one protecting agent selected from among a hardened vegetable oil and a hardened animal oil having a melting point higher than 50° C. but lower than 90° C., lecithin and a basic amino acid, by dipping in water.
Owner:AJINOMOTO CO INC
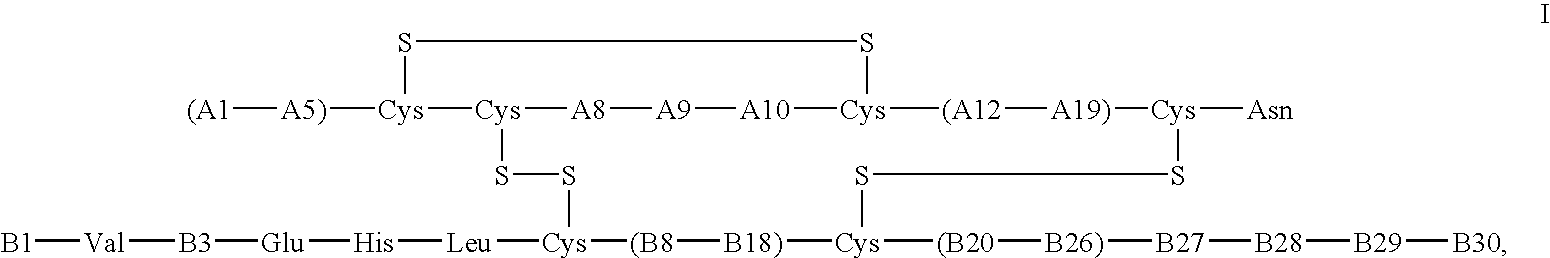
Crystals of insulin analogs and processes for their preparation
The invention relates to crystals of an insulin analog in which asparagine (Asn) in position B3 of the B chain is replaced by a naturally occurring basic amino acid residue and at least one amino acid residue in the positions B27, B28 or B29 of the B chain is replaced by another naturally occurring neutral or acidic amino acid residue, where phenylalanine (Phe) in position B1 of the B chain can optionally be absent, the crystals being present in the space group R3 (No. 146) with the cell axes A=81.5 ű1 Å and C=33.3 ű1 Å, their preparation and use, and a pharmaceutical composition comprising these crystals.
Owner:SANOFI AVENTIS DEUT GMBH
Support having protein immobilized thereon and method of producing the same
InactiveUS20100105879A1Reduce reaction efficiencyHigh in proteinPeptide preparation methodsDepsipeptidesChemistryBasic amino acids
A method of producing a protein-immobilized support includes immobilizing a protein having a tag sequence on a support, the tag sequence including a sequence that includes three or more consecutive basic amino acids.
Owner:JSR CORPORATIOON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com