Patents
Literature
5996 results about "Amino acid residue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
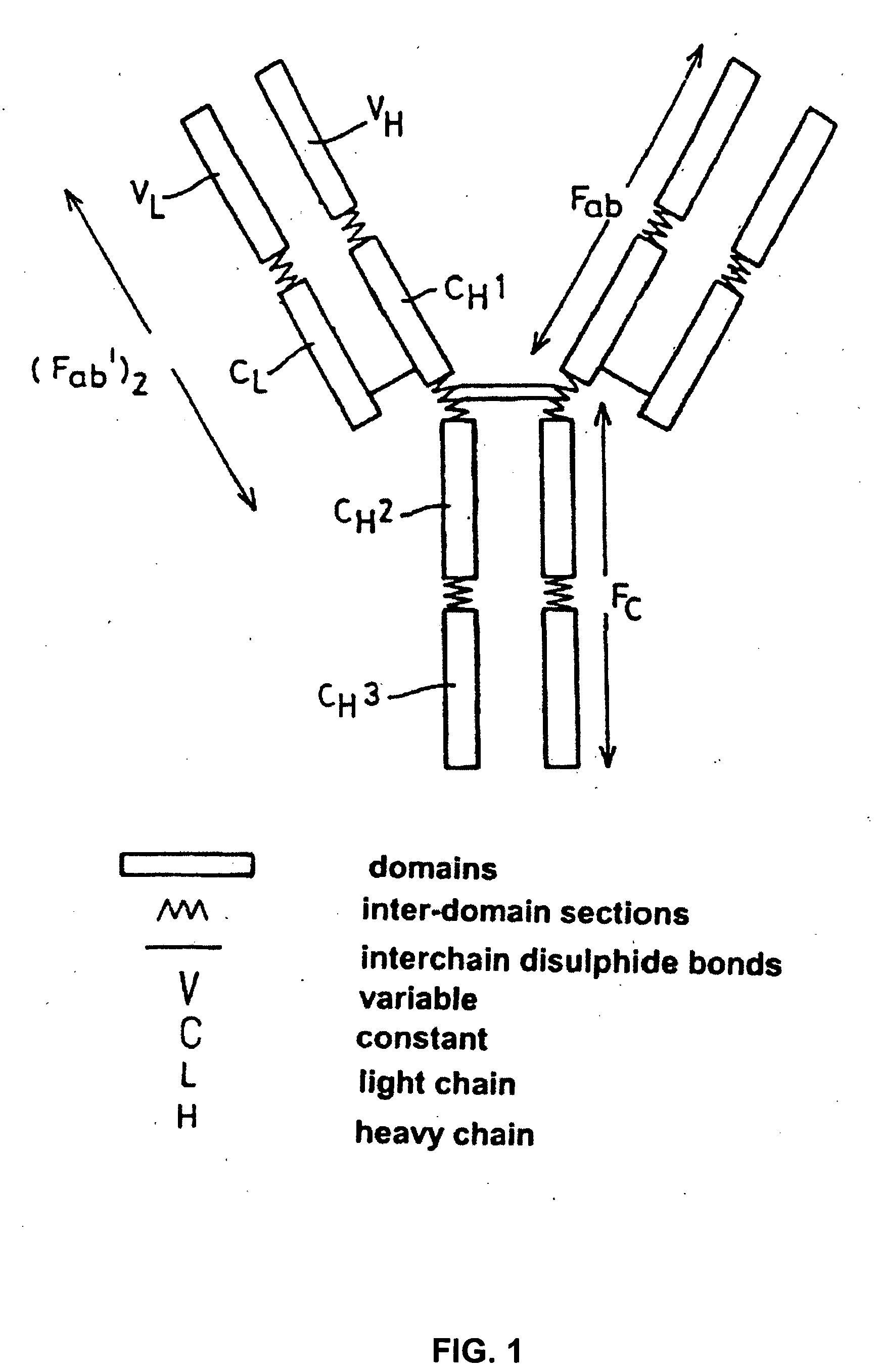
Amino acid residue is the part of an amino acid that makes it unique from all the others. Its features, such as how it interacts with water, help guide the structure of a finished protein.
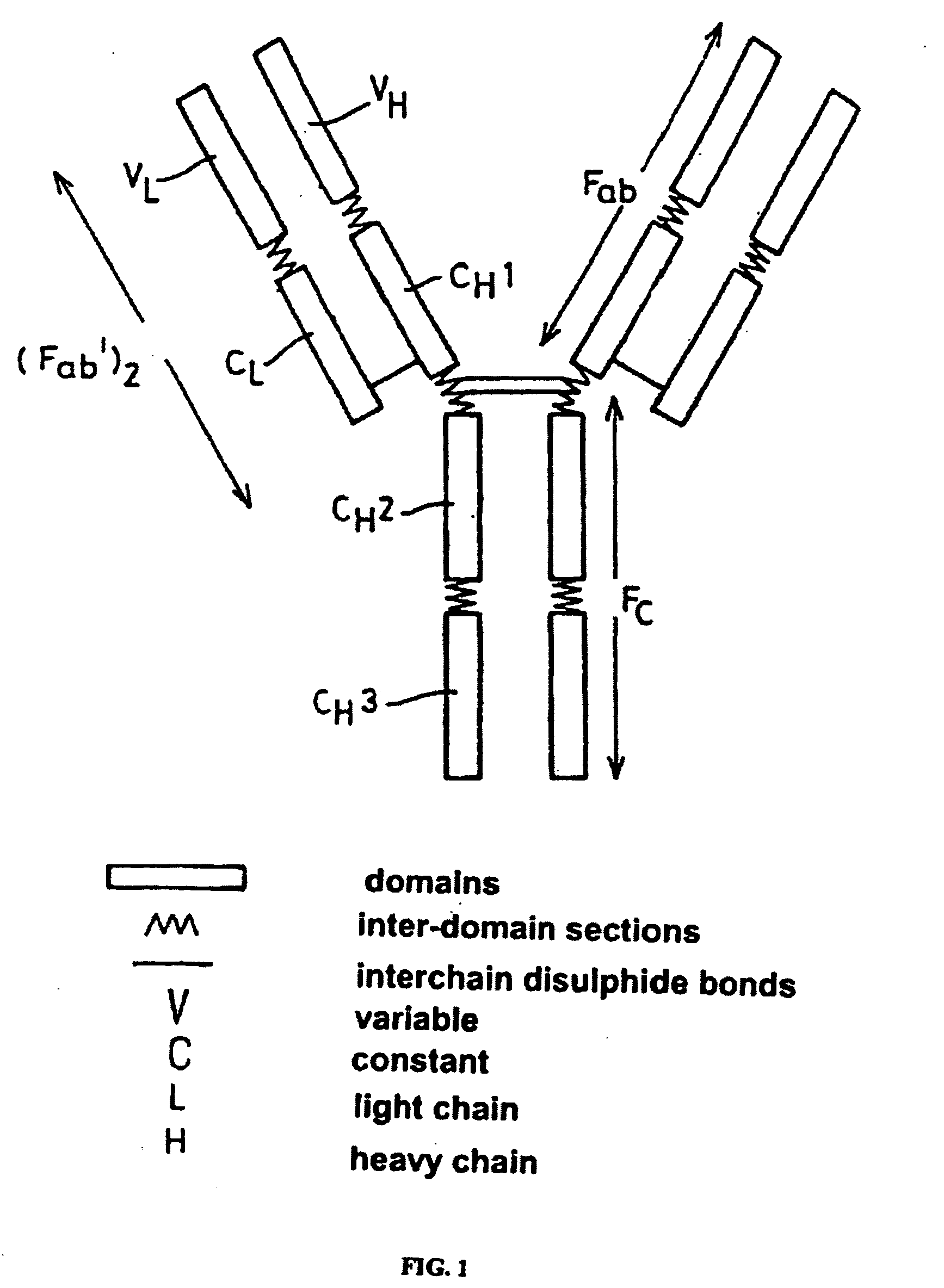
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050014934A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
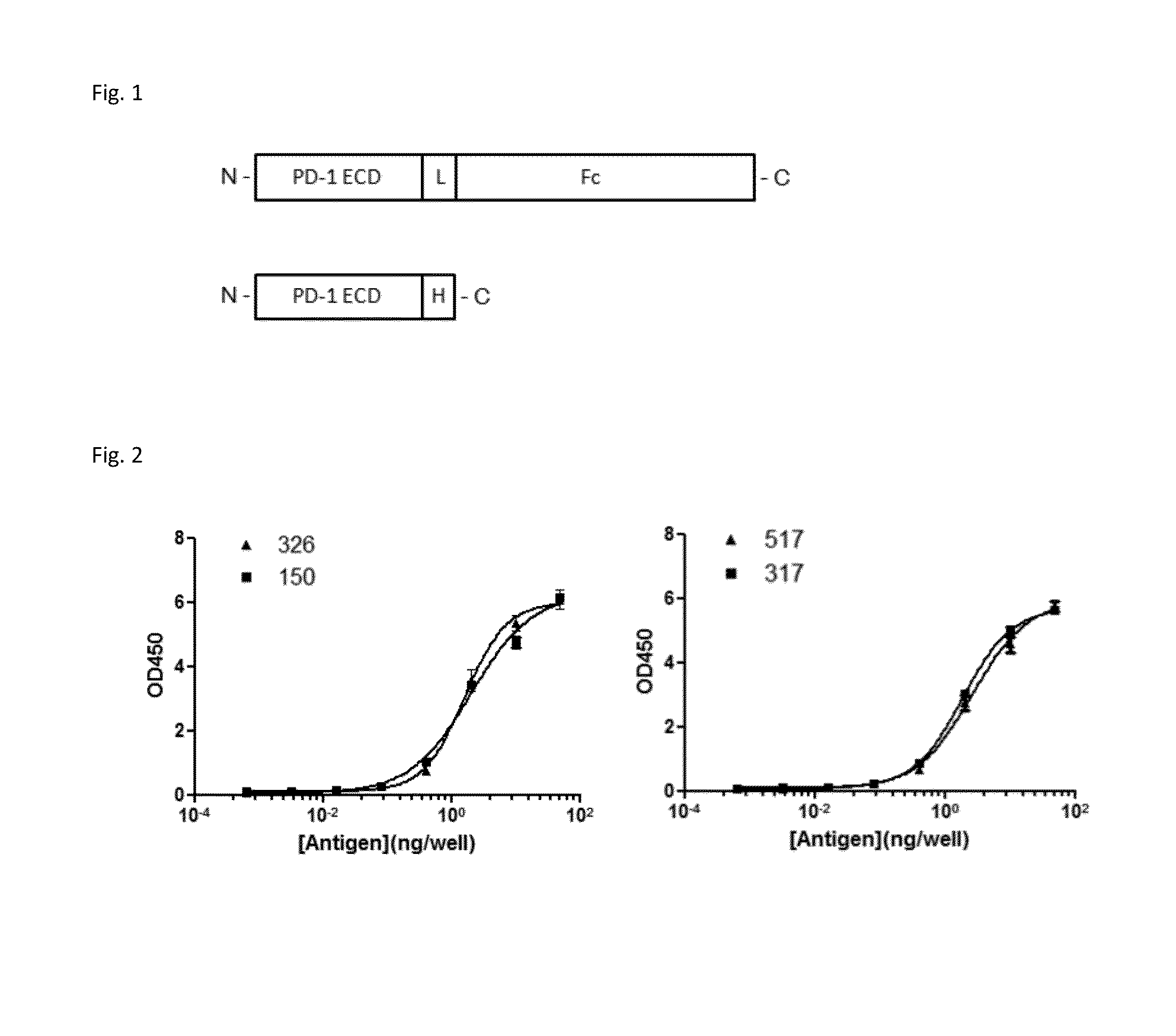
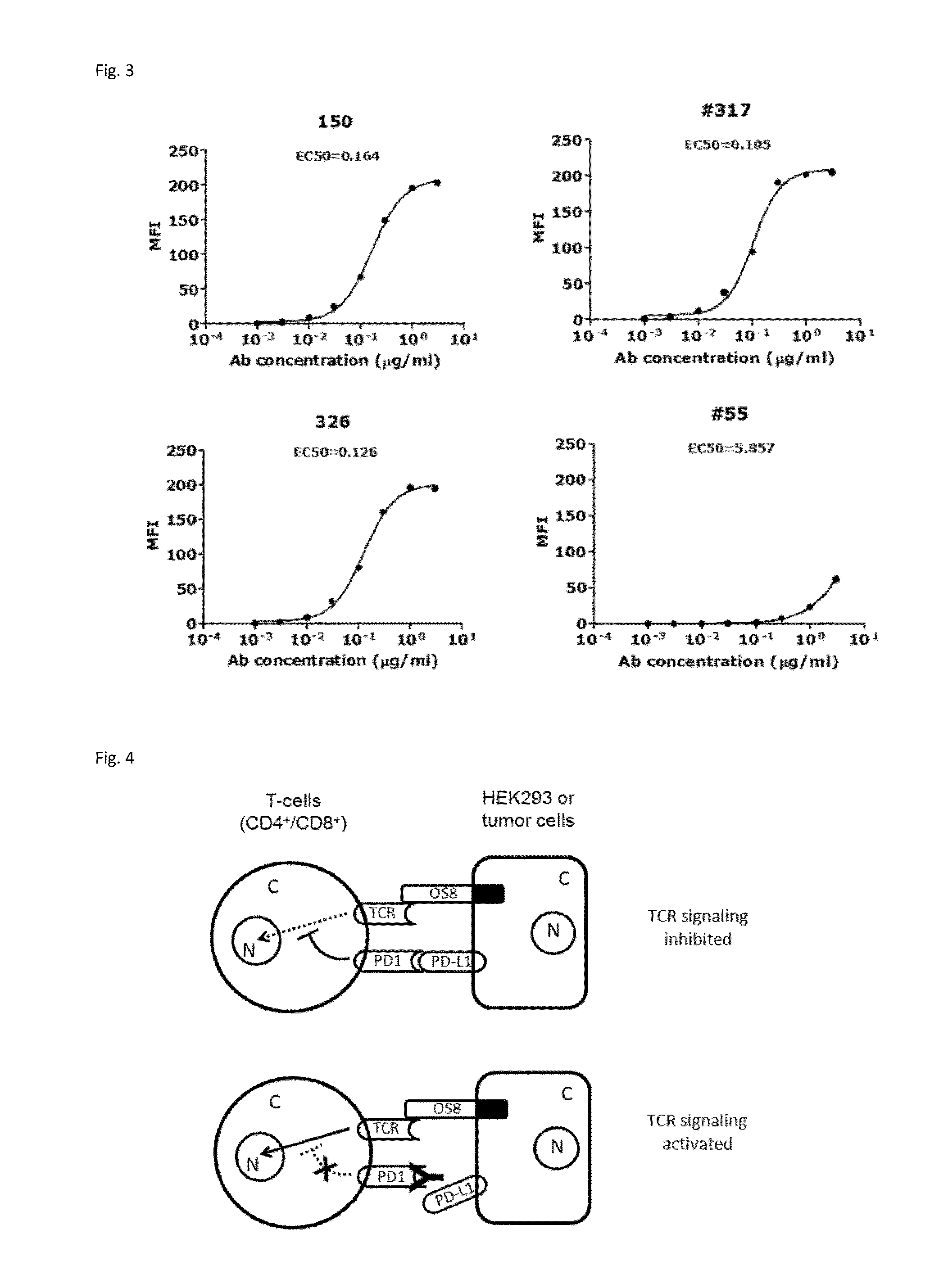
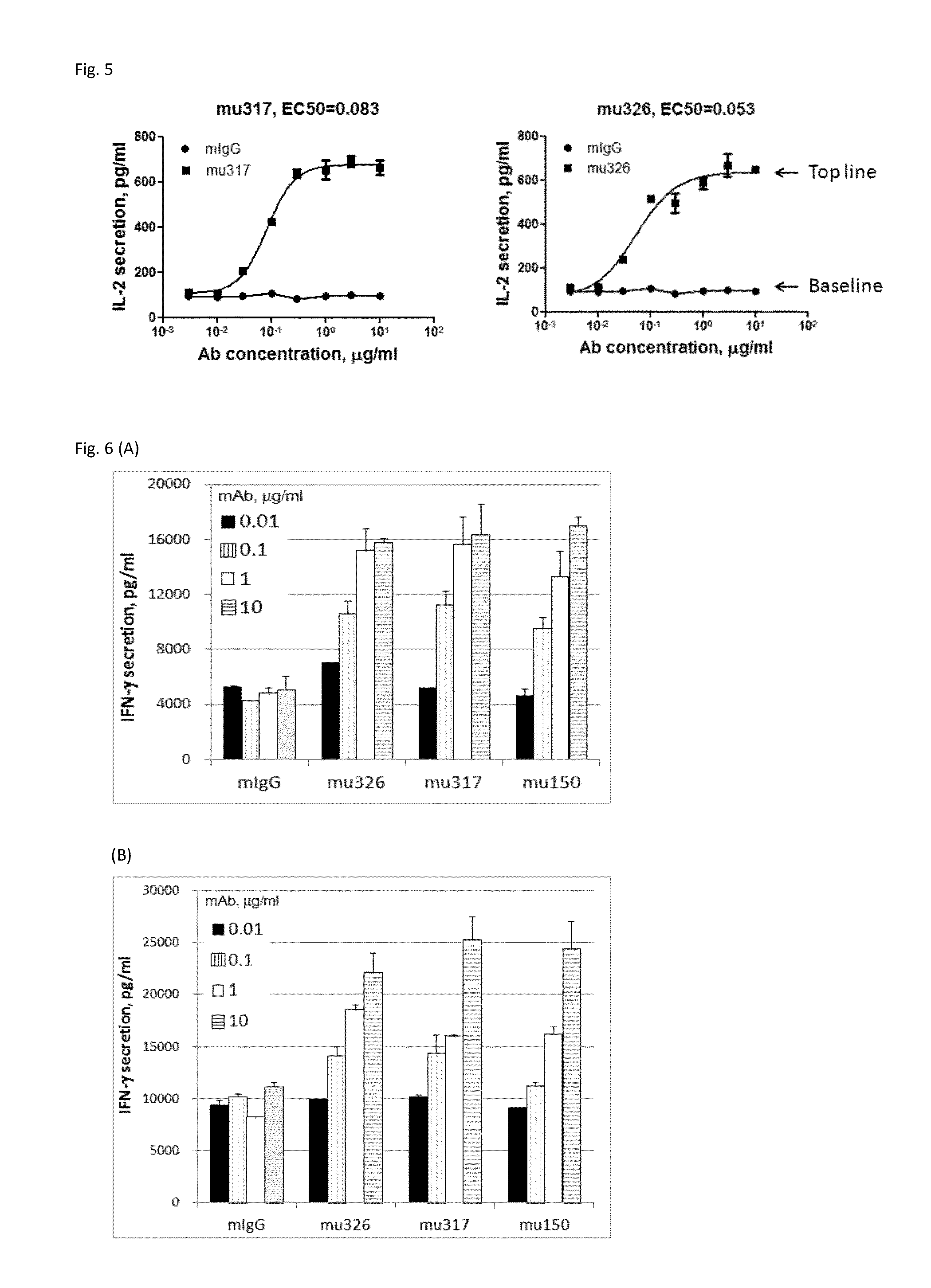
Anti-PD1 antibodies and their use as therapeutics and diagnostics
ActiveUS8735553B1Inhibition of secretionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathAnti pd1
Owner:BEIGENE SWITZERLAND GMBH
Methods for Producing Polypeptides by Regulating Polypeptide Association
ActiveUS20100015133A1Efficient productionEfficient formationAnimal cellsSugar derivativesHeterologousAntiendomysial antibodies
In the course of the present invention, it was discovered that one could regulate association between polypeptides by modifying amino acid residues that form the interface during the association to amino acids carrying the same type of charge. In this context, the present invention enables efficient formation of heterologous molecules. For example, the present invention can be suitably applied to the preparation of bispecific antibodies.
Owner:CHUGAI PHARMA CO LTD
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS7217797B2Function increaseExtended half-lifeAnimal cellsSugar derivativesAntiendomysial antibodiesBiochemistry
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Method for reducing the immunogenicity of antibody variable domains
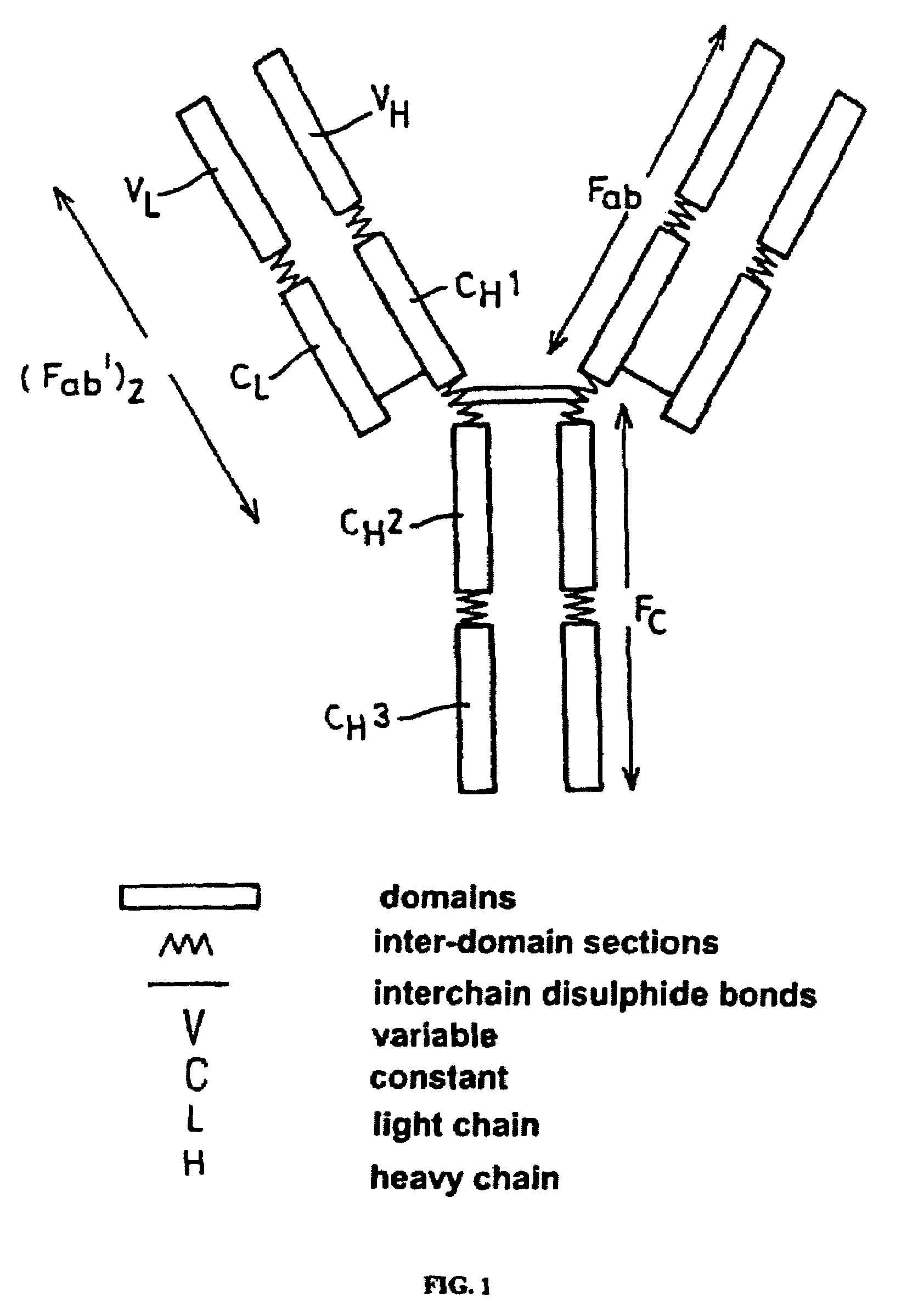
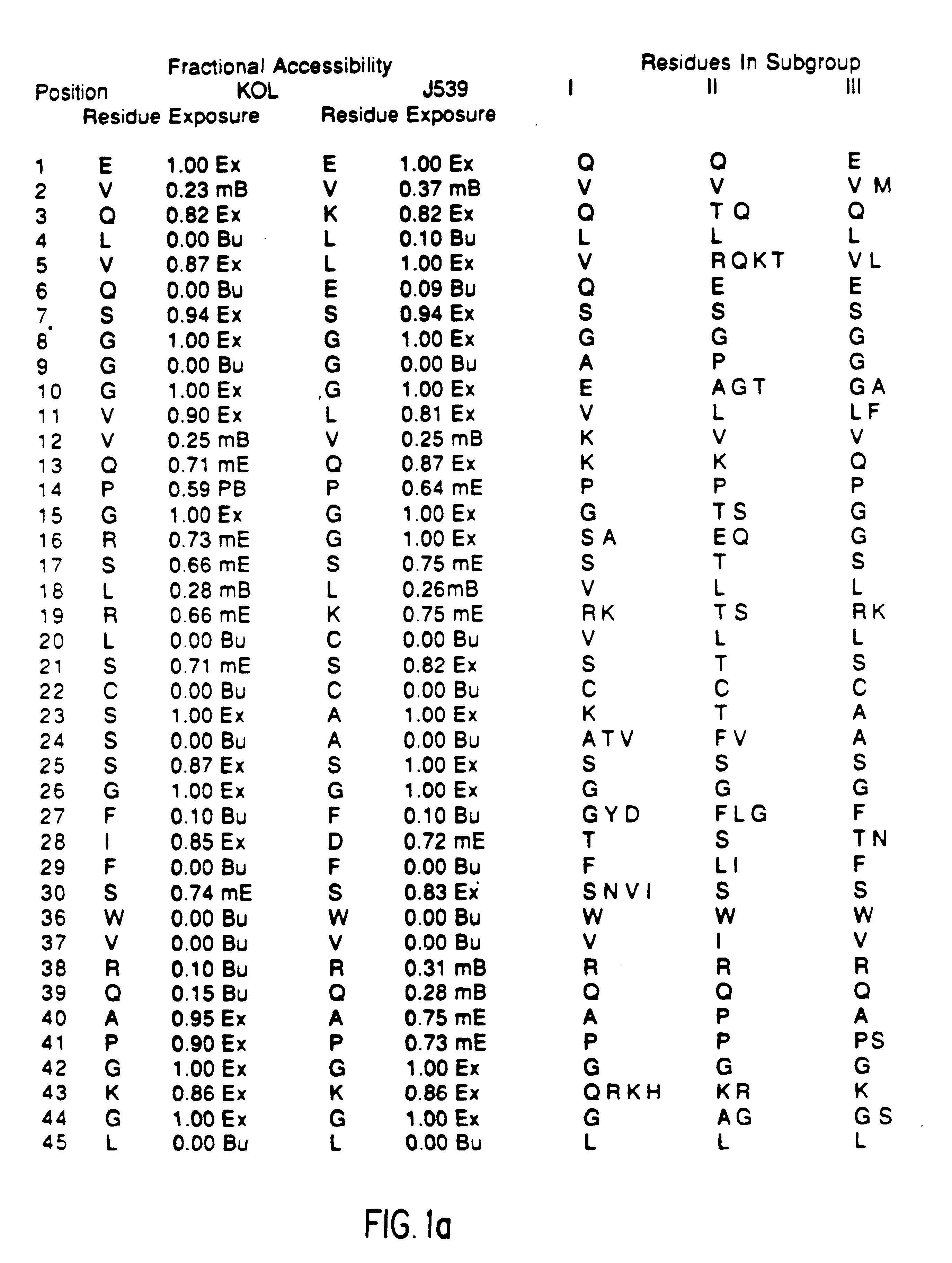
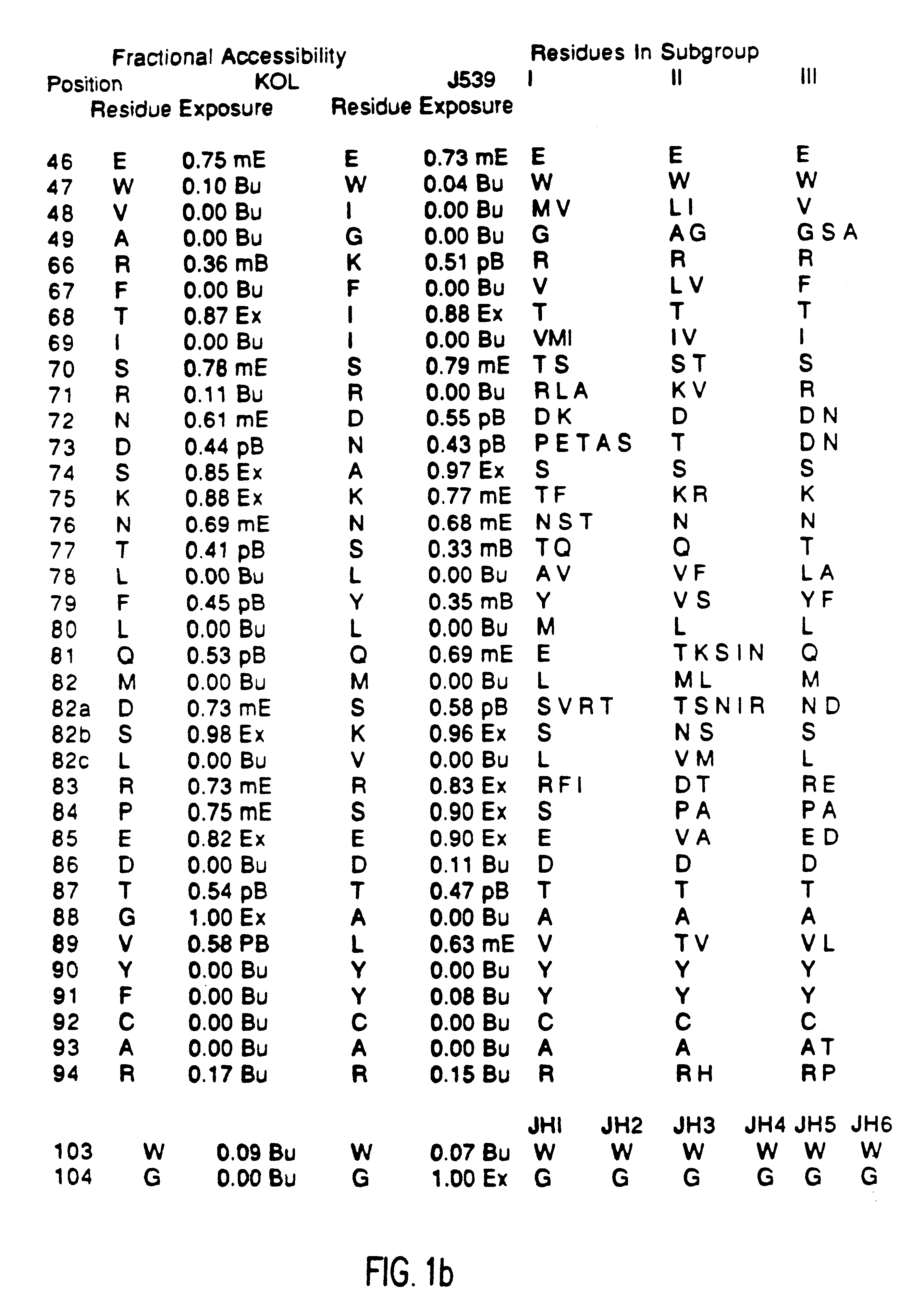
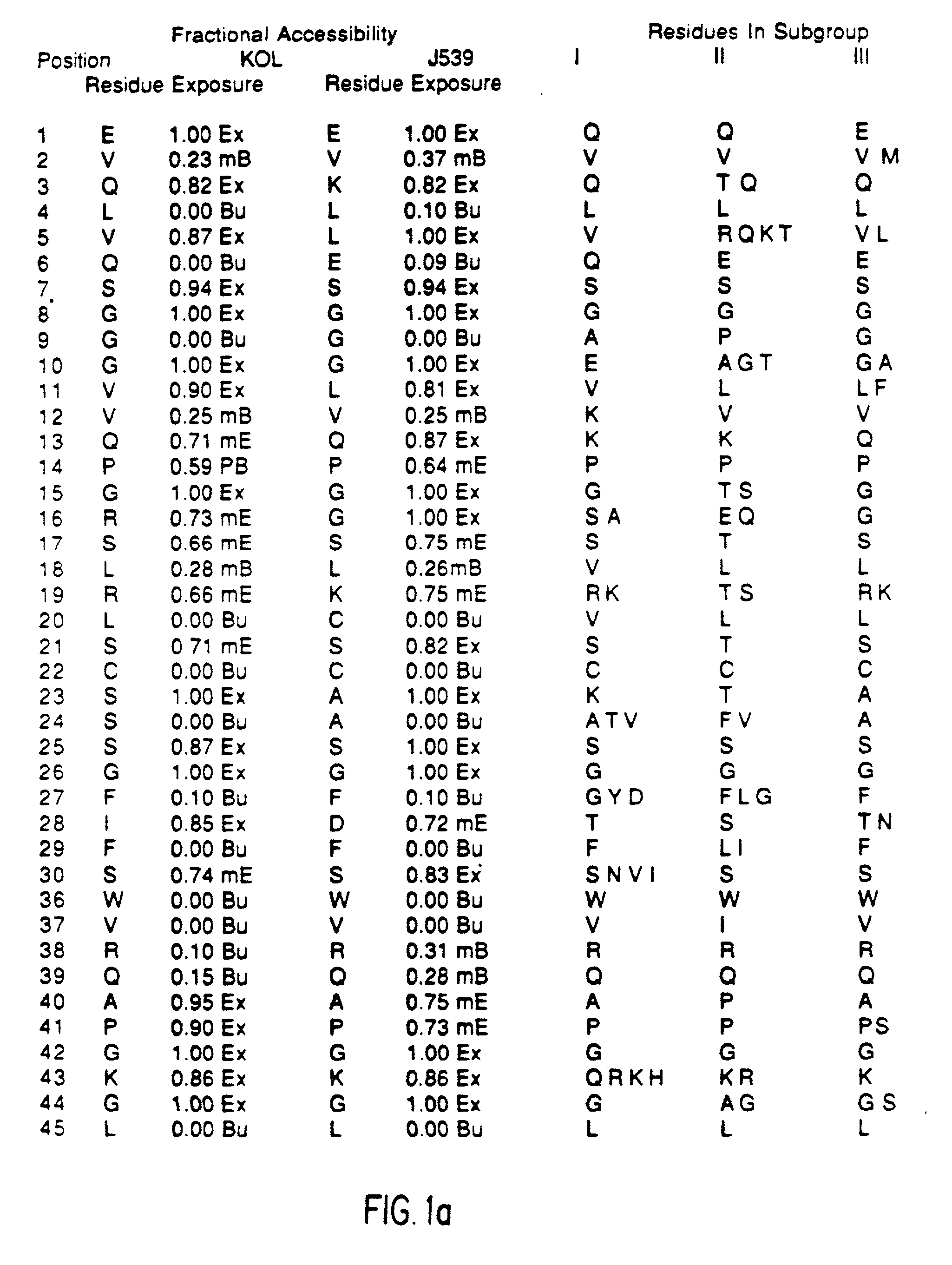
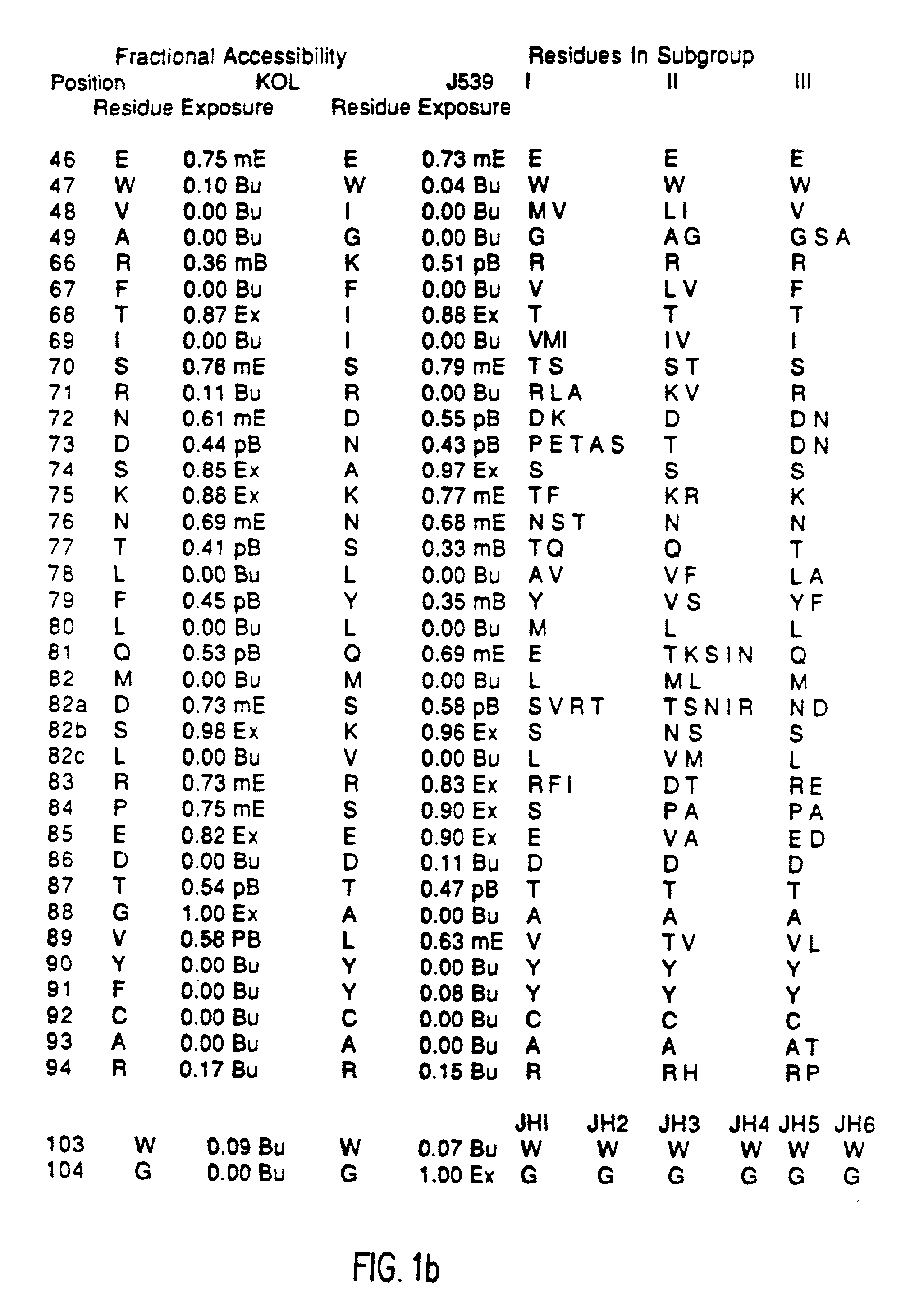
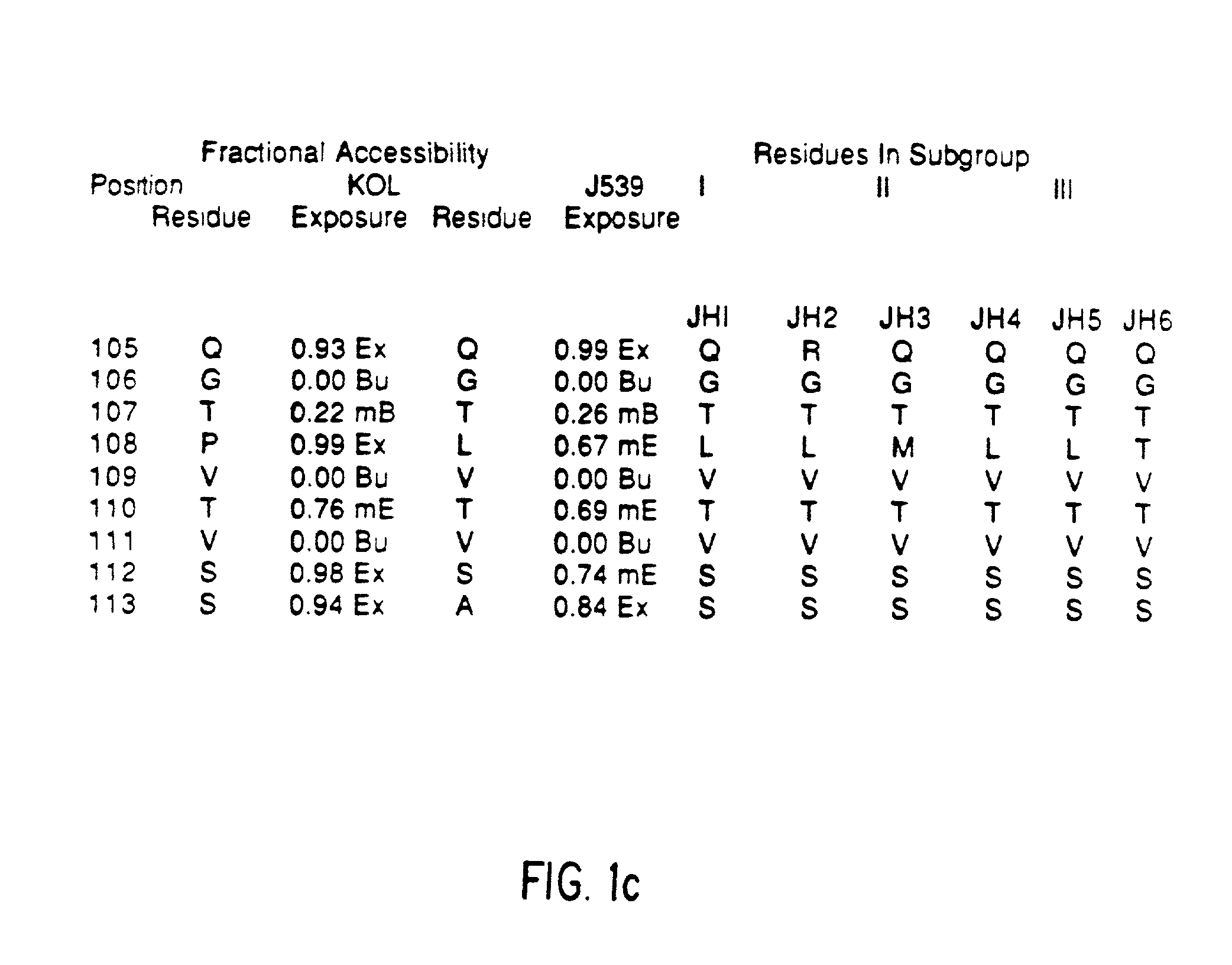
A unique method is disclosed for identifying and replacing immunoglobulin surface amino acid residues which converts the antigenicity of a first mammalian species to that of a second mammalian species. The method will simultaneously change immunogenicity and strictly preserve ligind binding properties. The judicious replacement of exterior amino acid residues has no effect on the ligind binding properties but greatly alters immunogenicity.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE +1
Neonatal Fc receptor (FcRn)-binding polypeptide variants, dimeric Fc binding proteins and methods related thereto
InactiveUS20070148164A1Increase free energyReduced binding affinityAnimal cellsSugar derivativesFc bindingNeonatal Fc receptor
The compositions and methods of the present invention are based, in part, on our discovery that an effector function mediated by an Fc-containing polypeptide can be altered by modifying one or more amino acid residues within the polypeptide (by, for example, electrostatic optimization). The polypeptides that can be generated according to the methods of the invention are highly variable, and they can include antibodies and fusion proteins that contain an Fc region or a biologically active portion thereof.
Owner:BIOGEN MA INC
Anti-PD1 Antibodies and their Use as Therapeutics and Diagnostics
ActiveUS20150079109A1Improve the level ofHigh expressionNervous disorderAntiviralsAntiendomysial antibodiesInfectious Disorder
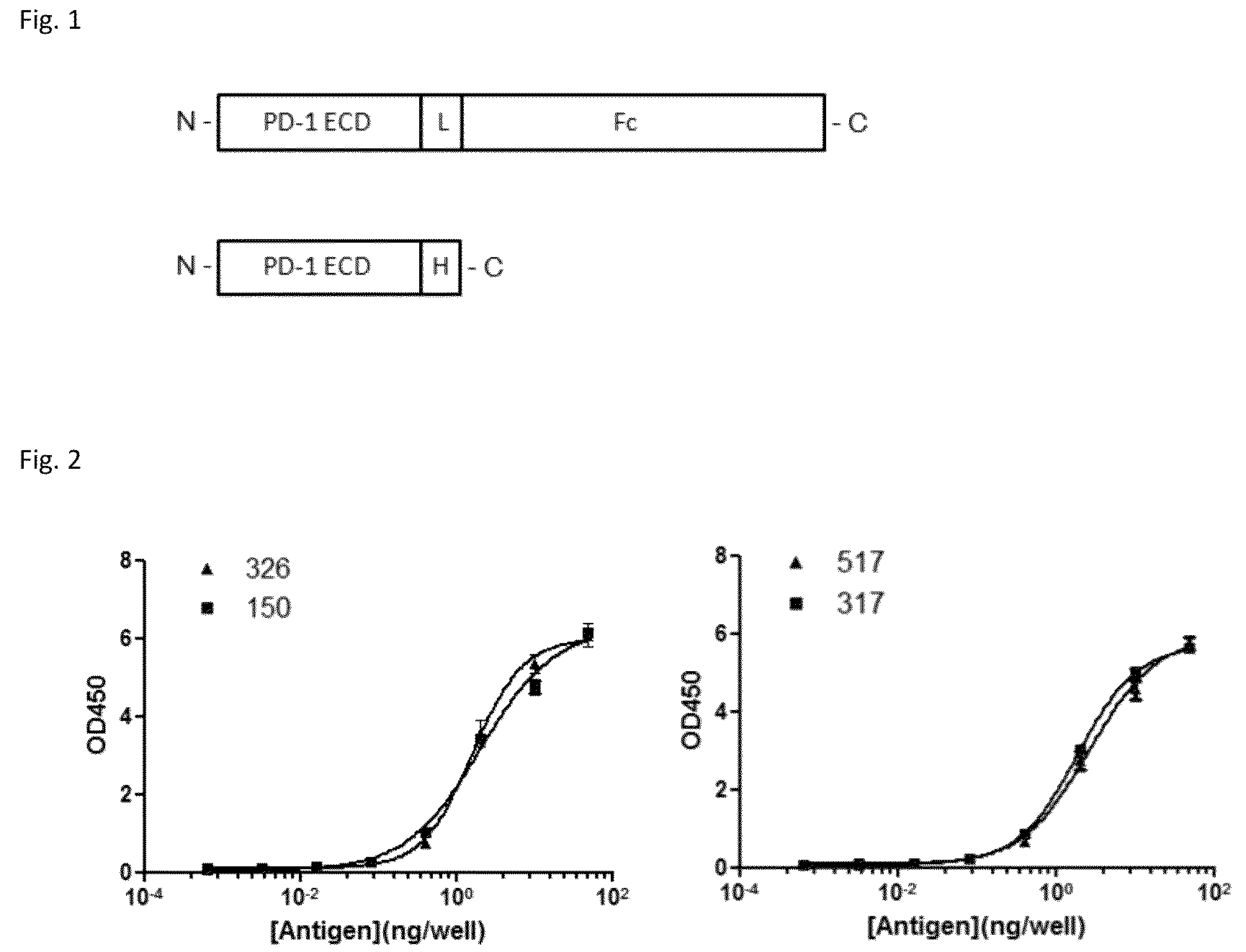
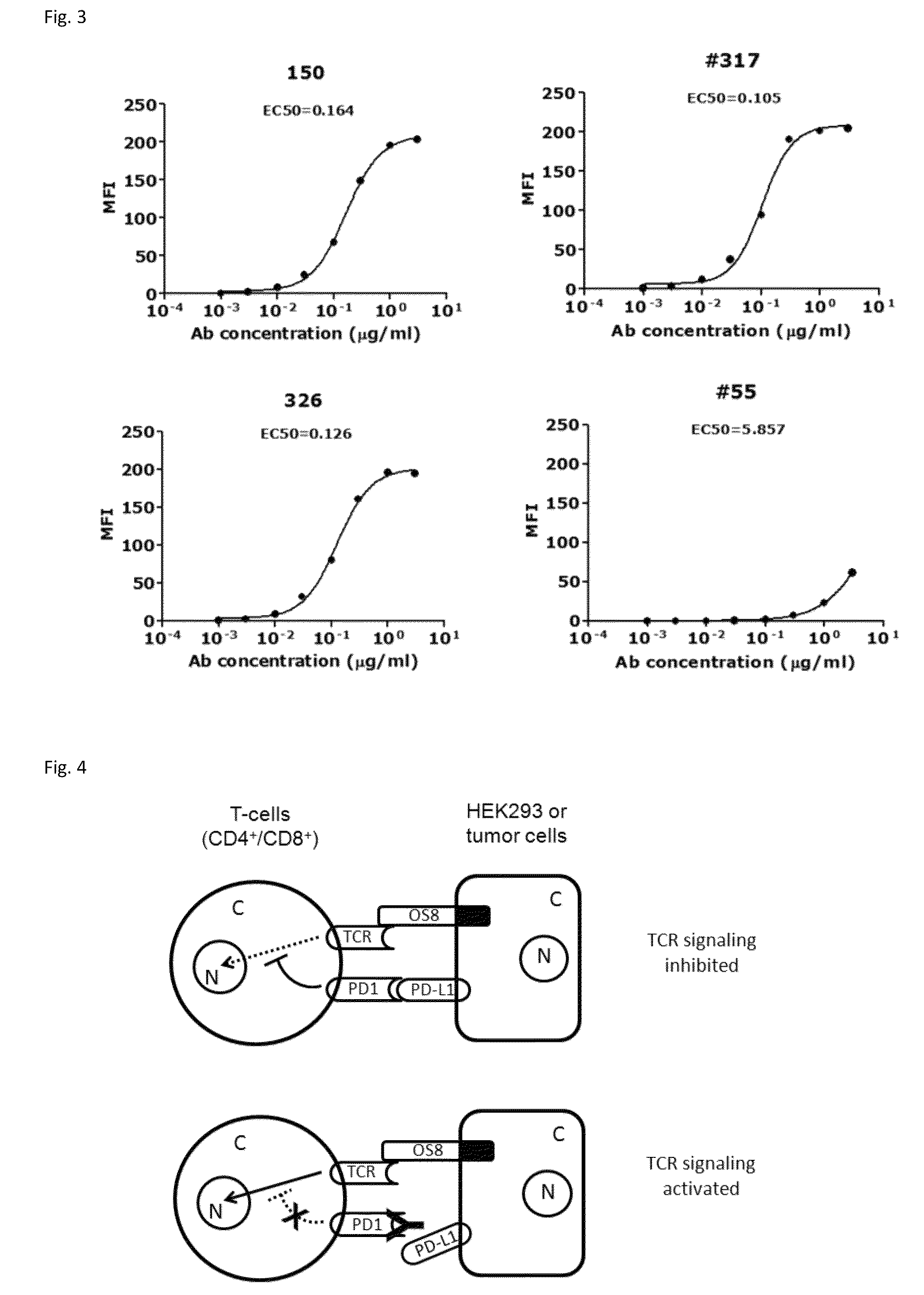
Provided are antibodies that specifically bind to Programmed Death-1 (PD1, Pdcd-1, or CD279) and inhibit PD1-mediated cellular signaling and activities in immune cells, antibodies binding to a set of amino acid residues required for its ligand binding, and uses of these antibodies to treat or diagnose cancer, infectious diseases or other pathological disorders modulated by PD1-mediated functions.
Owner:BEIGENE SWITZERLAND GMBH
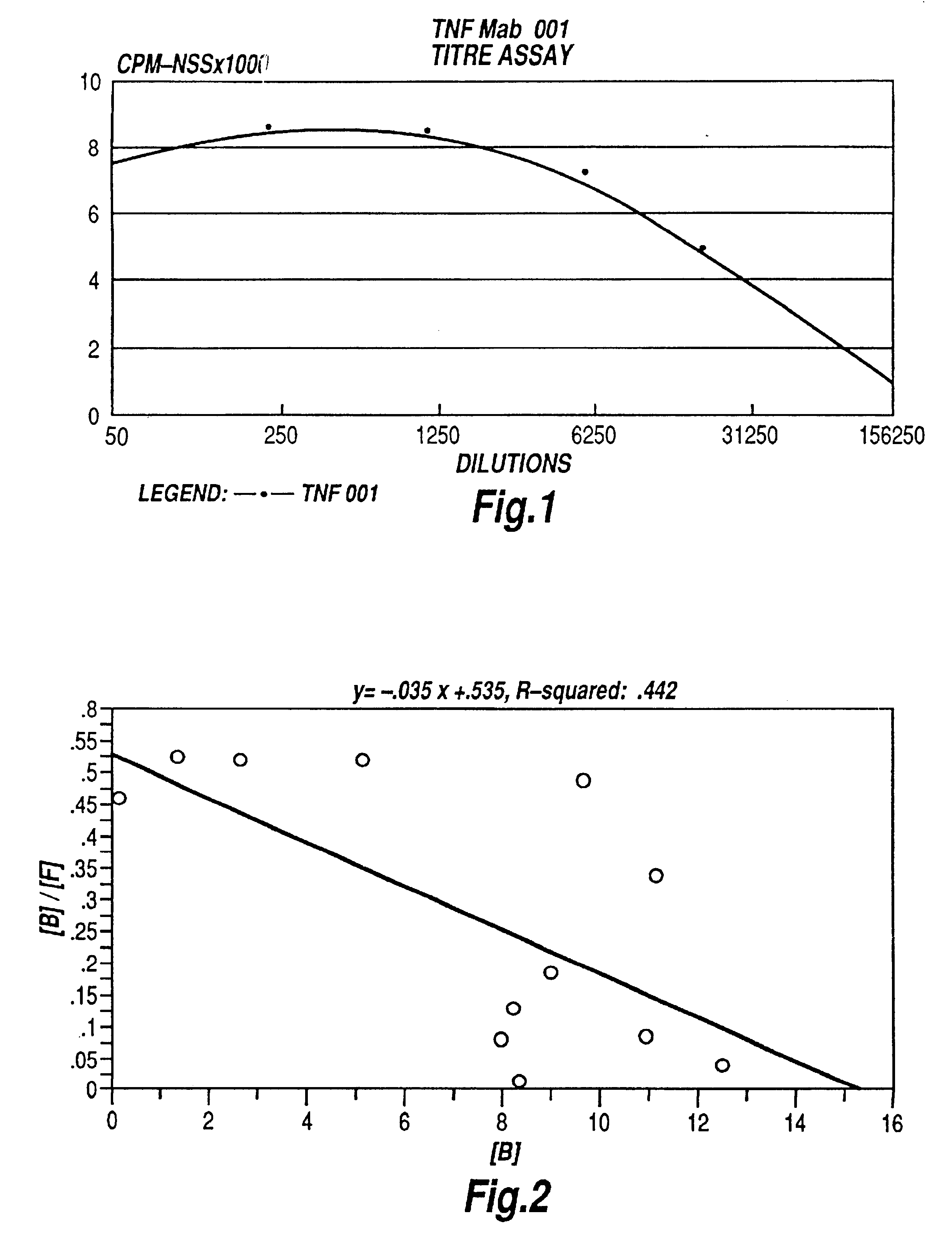
Tumor necrosis factor peptide binding antibodies
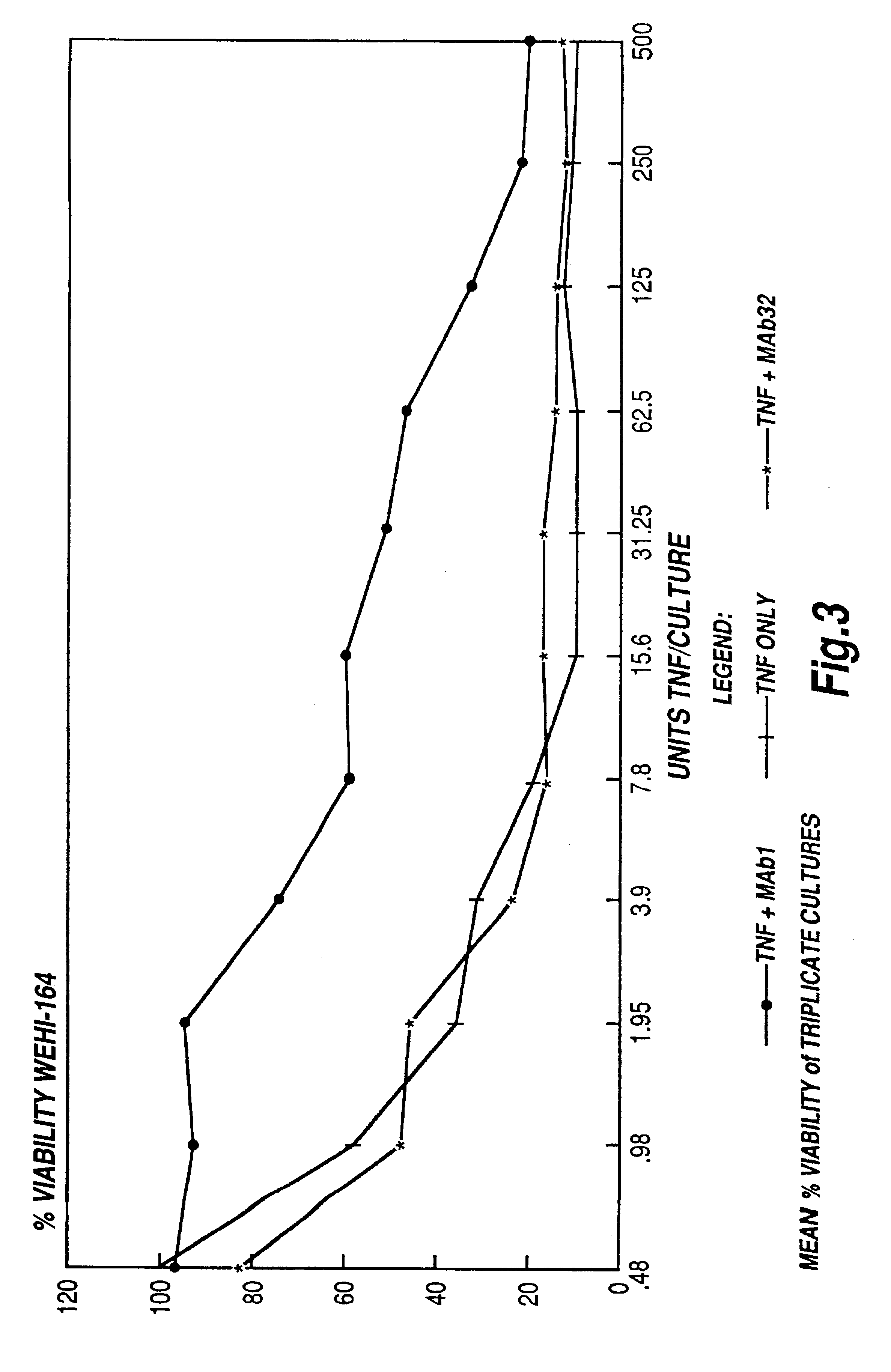
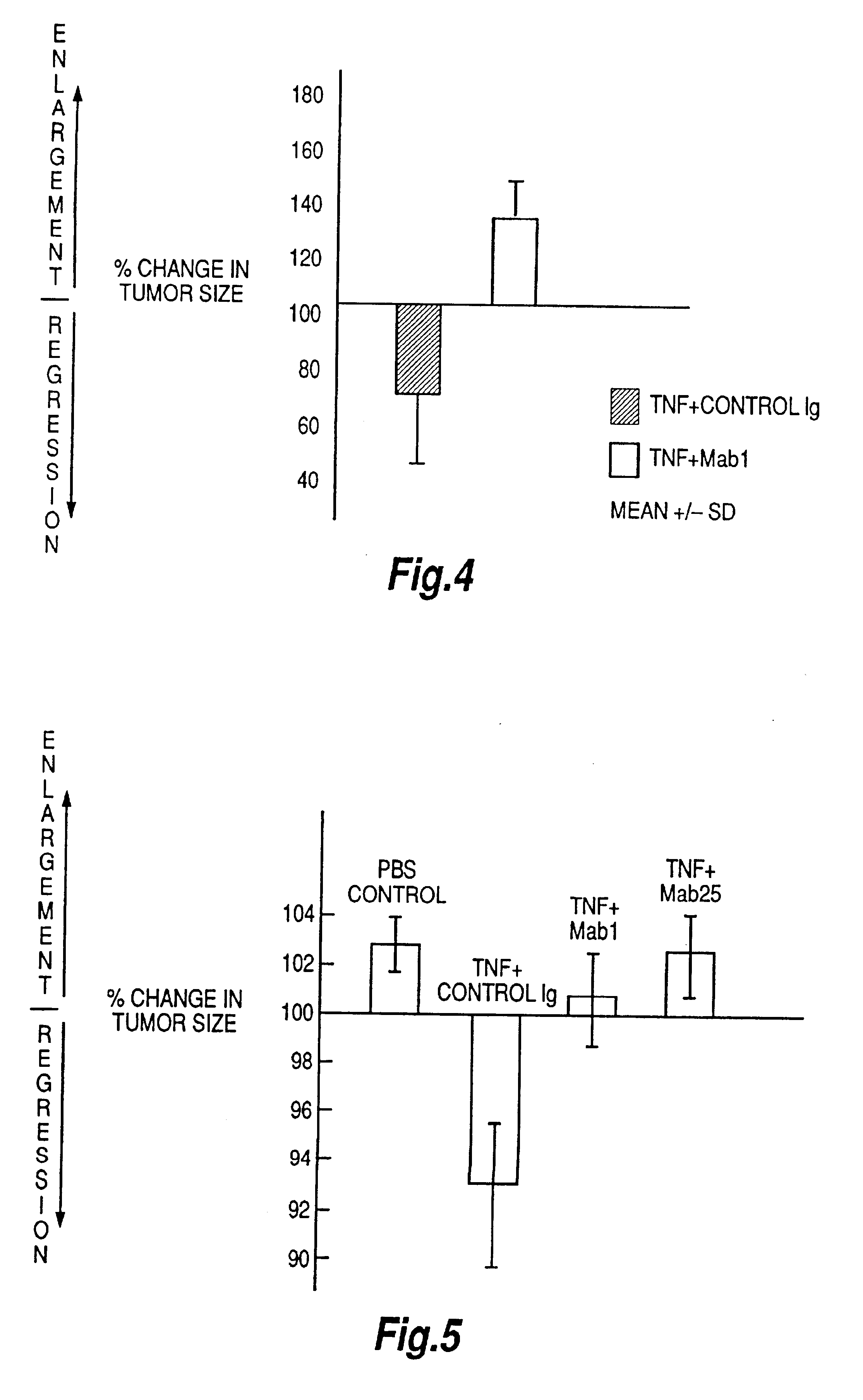
InactiveUS6593458B1Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDrug biological activityAntibody
Provided are isolated antibodies or fragments thereof which bind a peptide consisting of residues Leu63-Phe64-Lys65-Gly66-Gln67-Gly68-Cys69-Pro70-Ser71-Thr72-His73-Val74-Leu75-Leu76-Thr77-His78-Thr79-Ile80-Ser81-Arg82-Ile83 (peptide 304) of mature human TNF-alpha. The antibodies and fragments thereof may be used to identify antibodies capable of binding in the region of mature human TNF-alpha of amino acid resides 63-83. Antibodies or fragments thereof which bind particular regions of mature human TNF-alpha are shown to elicit particular biological activities dependent upon the particular region wherein binding occurs.
Owner:ARANA THERAPEUTIC LTD
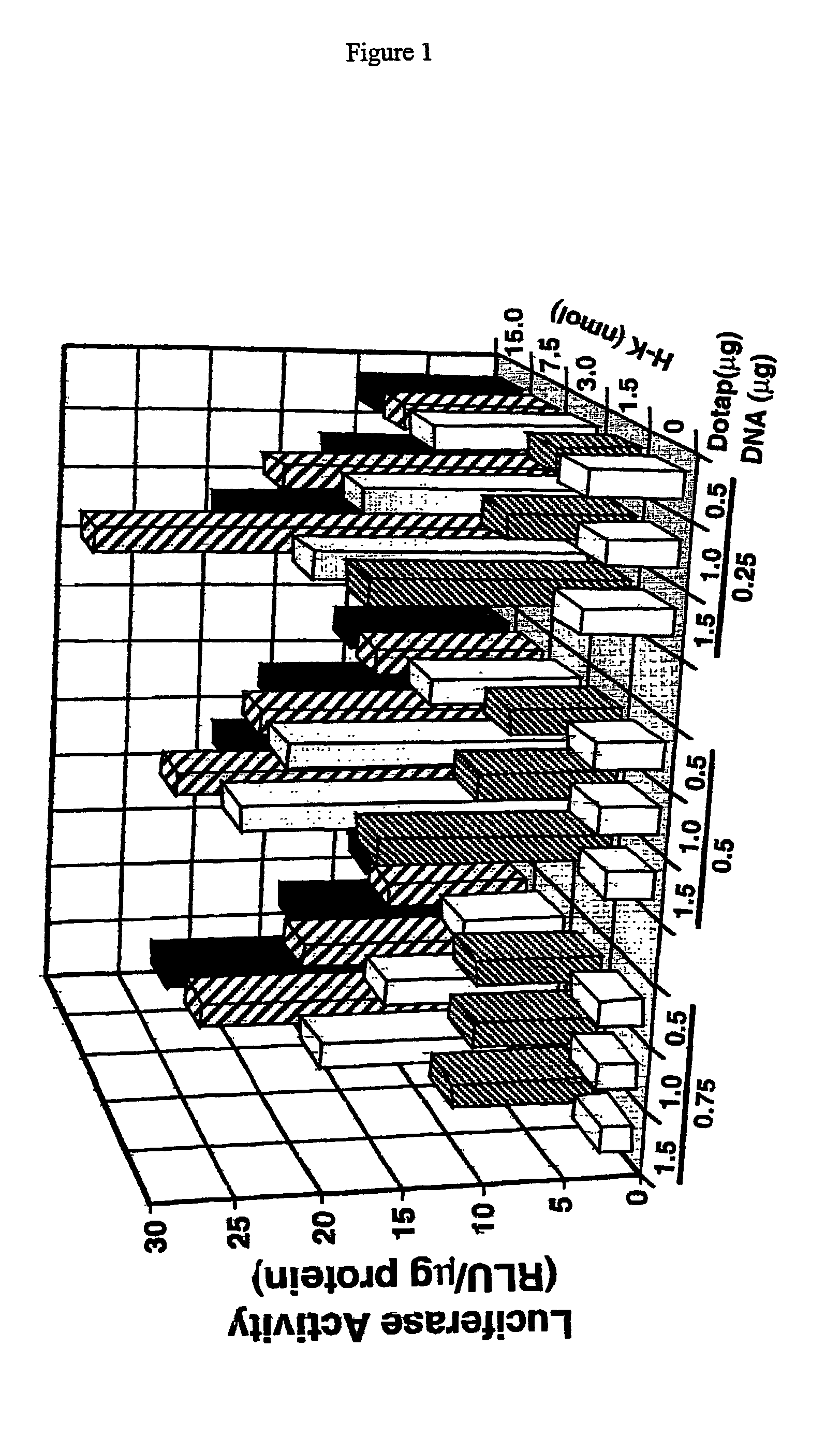
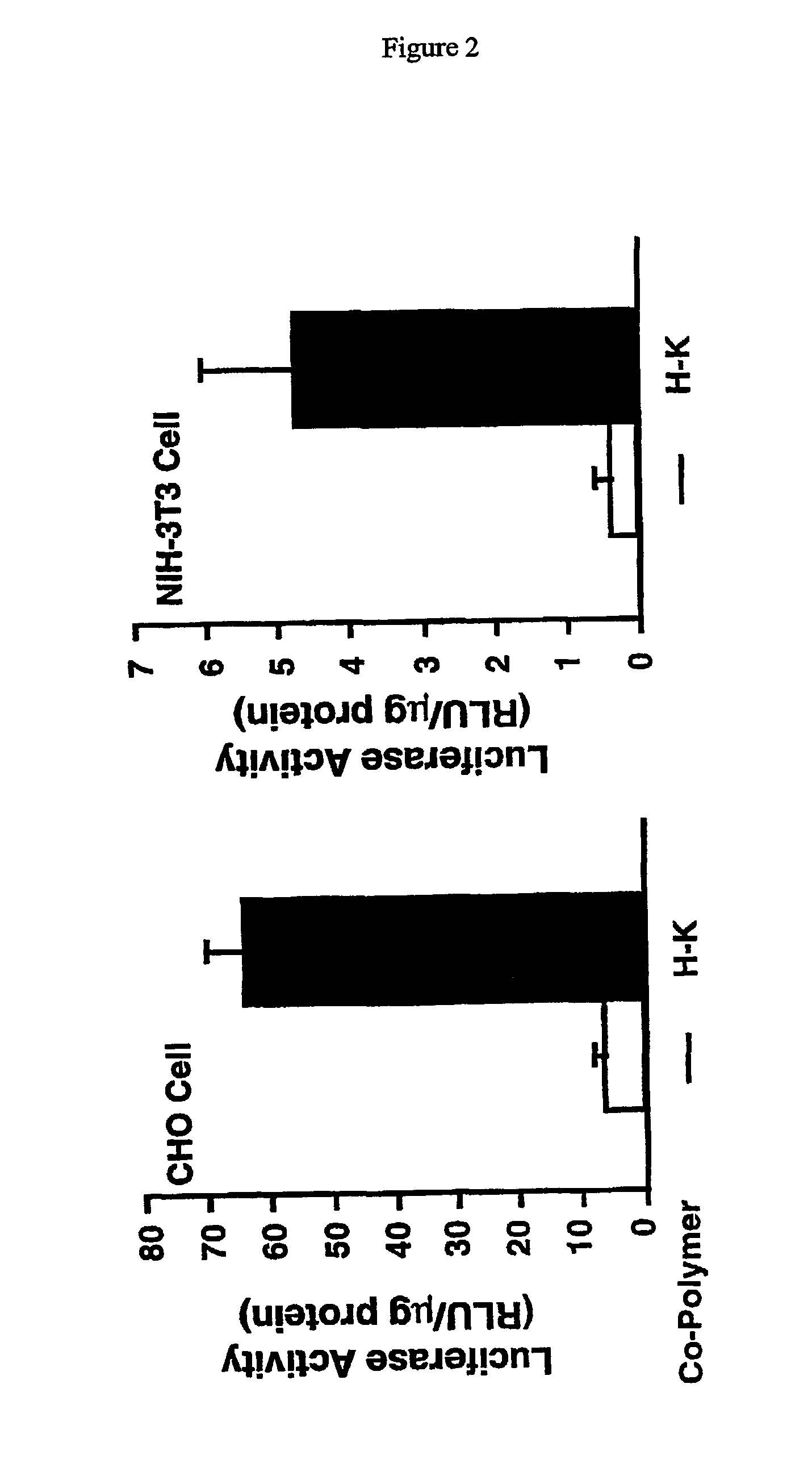
Histidine copolymer and methods for using same
InactiveUS7163695B2Prolong half-life in vivoEnhanced transfectionPowder deliveryPeptide/protein ingredientsHistidine residueCopolymer
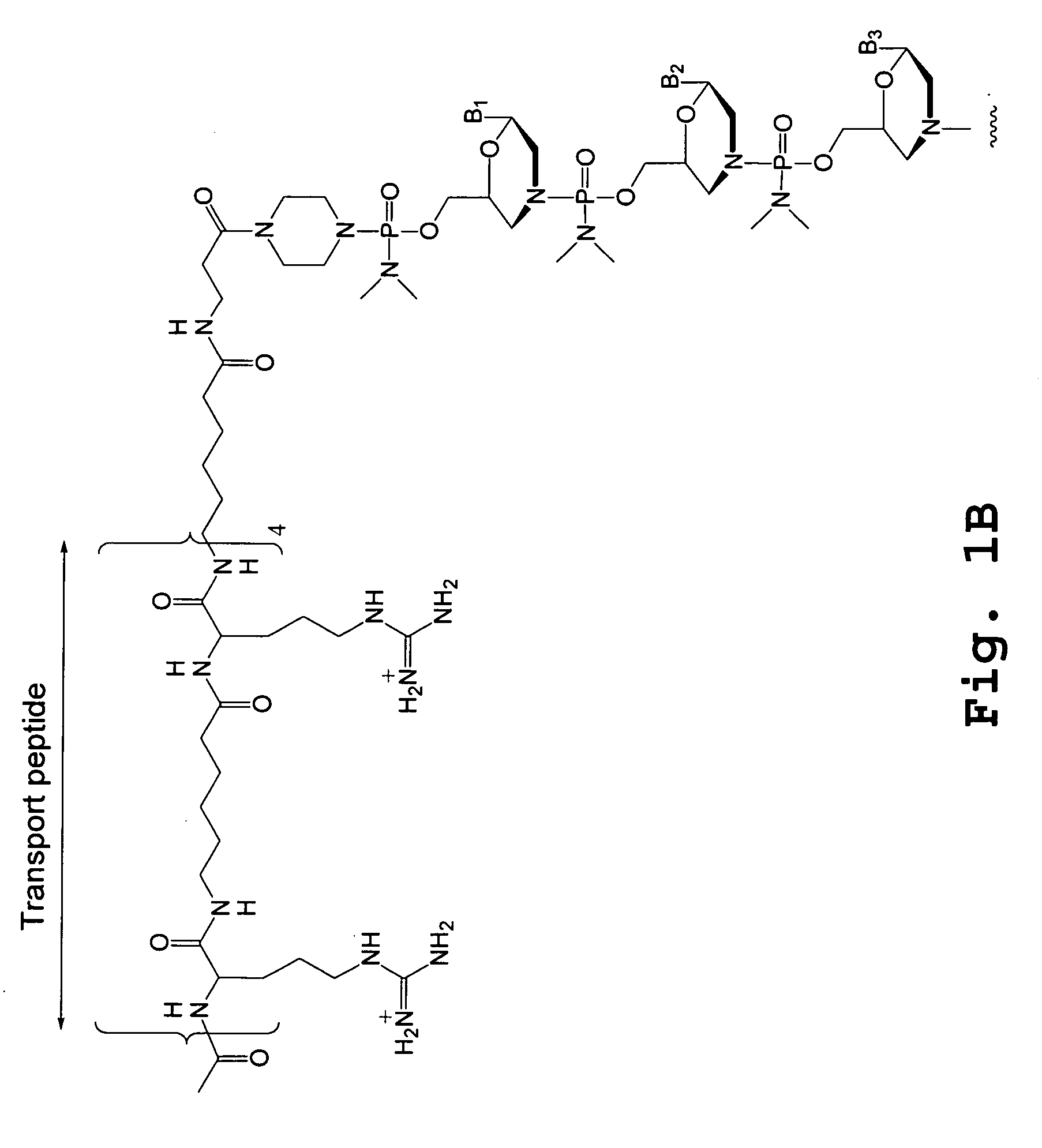
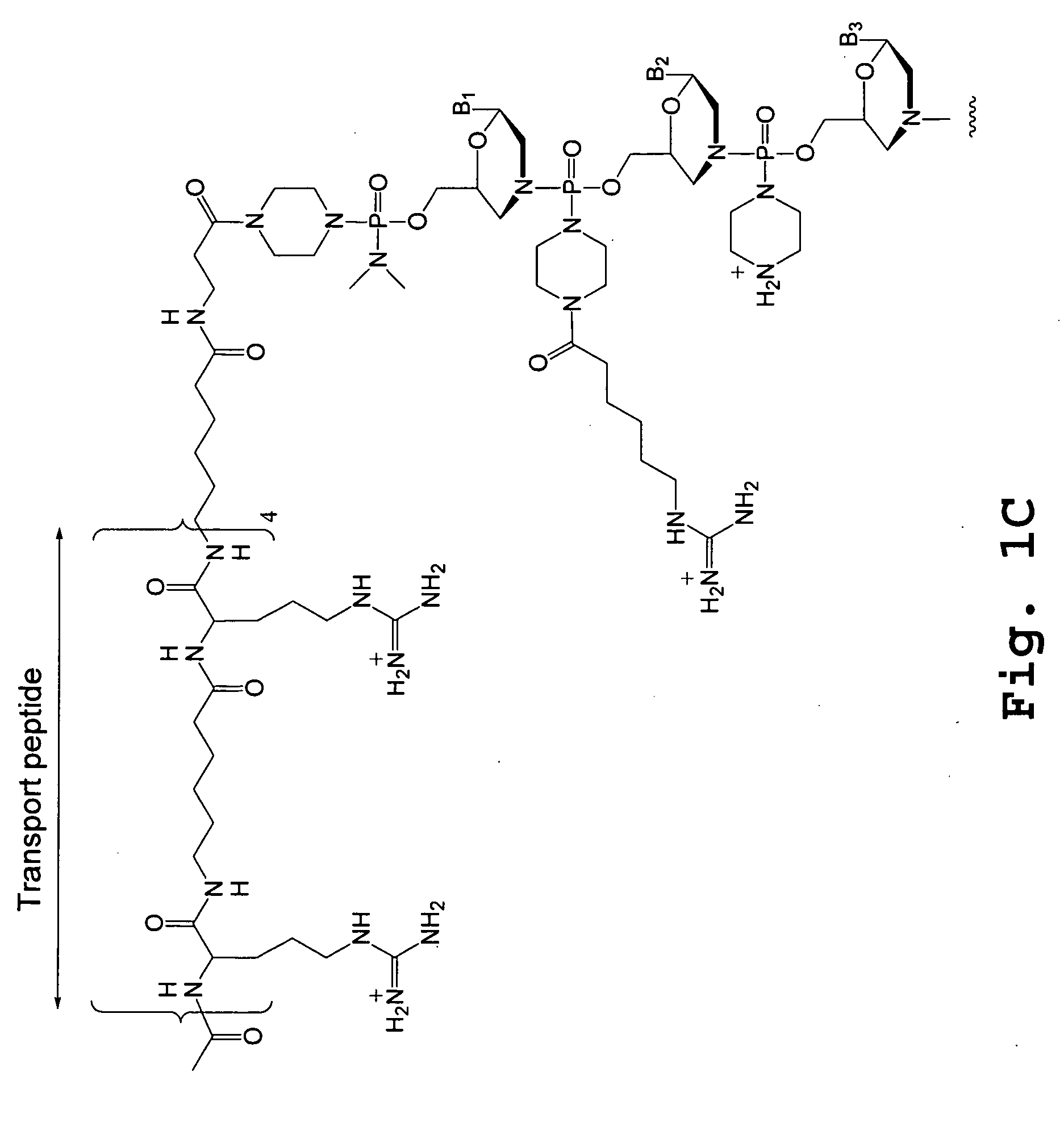
The invention provides a pharmaceutical agent delivery composition comprising: (i) a transport polymer comprising a linear or branched peptide having from about 10 to about 300 amino acid residues, having from about 5 to 100% histidine residues, and optionally having from 0 to about 95% non-histidine amino acid residues; (ii) at least one pharmaceutical agent; and optionally (iii) one or more intracellular delivery components in association with the transport polymer. The invention also provides methods for using such composition to deliver the pharmaceutical agent to the interior of cells.
Owner:MIXSON A JAMES
Method of Modifying Isoelectric Point of Antibody Via Amino Acid Substitution in CDR
ActiveUS20110076275A1Enhanced antigen-neutralizing activityImprove retentionSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionAmino acid substitution
The present inventors provide methods for modifying the isoelectric point of an antibody while retaining its antigen-binding activity, comprising modifying the charge of at least one exposable amino acid residue on the surface of the complementarity determining region (CDR). The present invention also provides methods for purifying multispecific antibodies, comprising modifying isoelectric point, and methods for improving the plasma pharmacokinetics of antibodies, comprising modifying isoelectric point. The present invention further provides antibodies with a modified isoelectric point, pharmaceutical compositions comprising the antibodies as an active ingredient, and methods for producing the antibodies and compositions.
Owner:CHUGAI PHARMA CO LTD
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
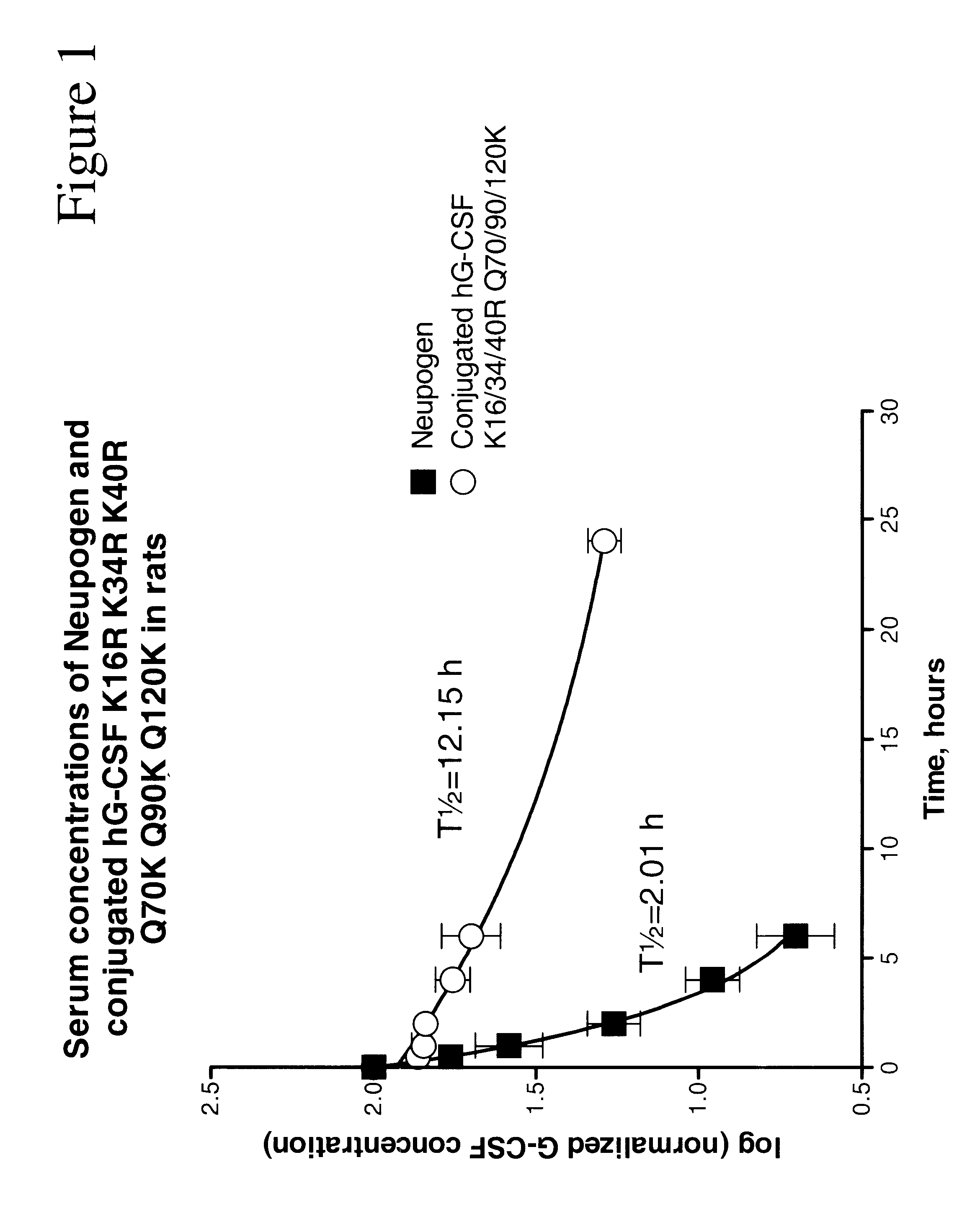
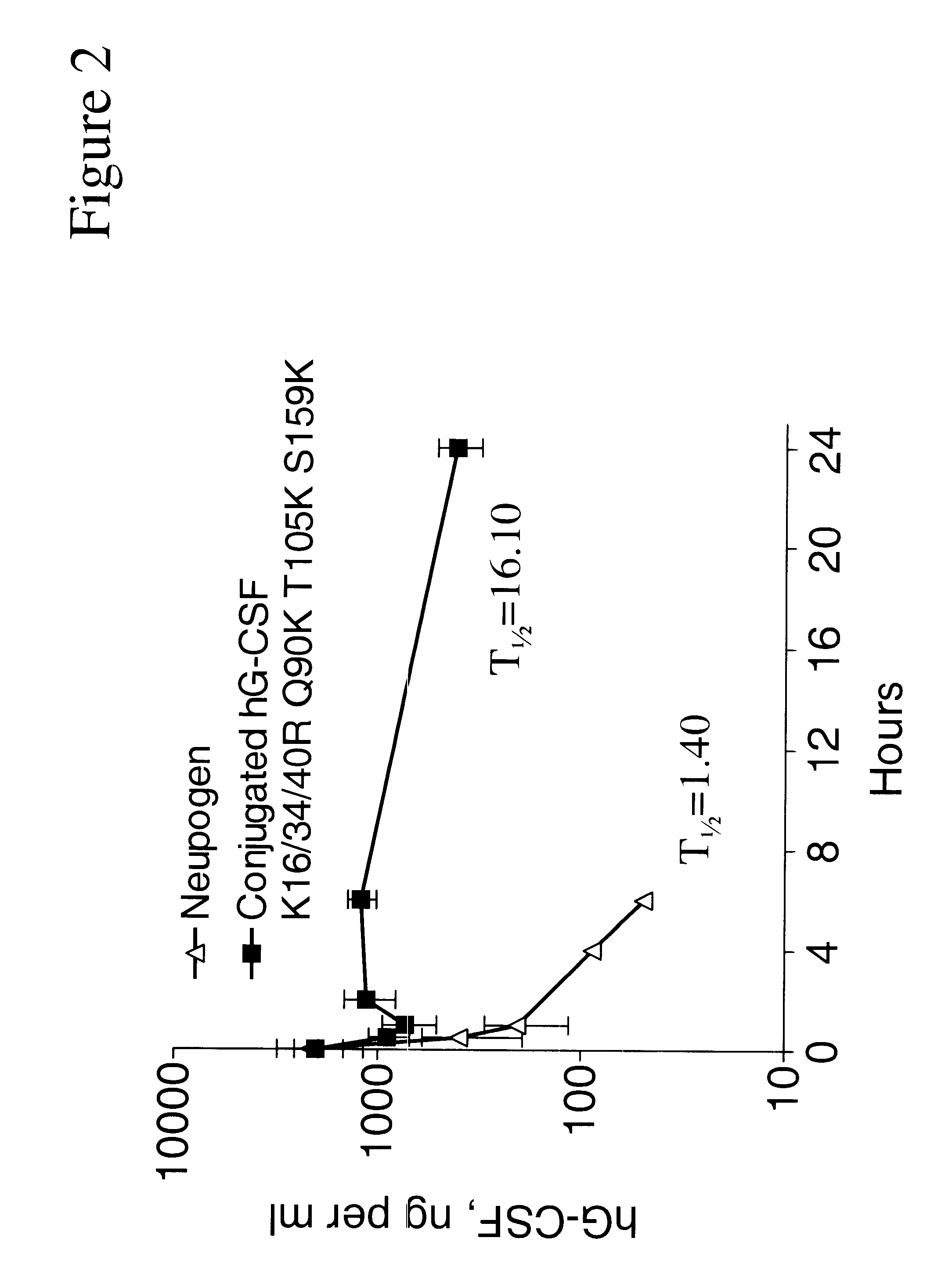
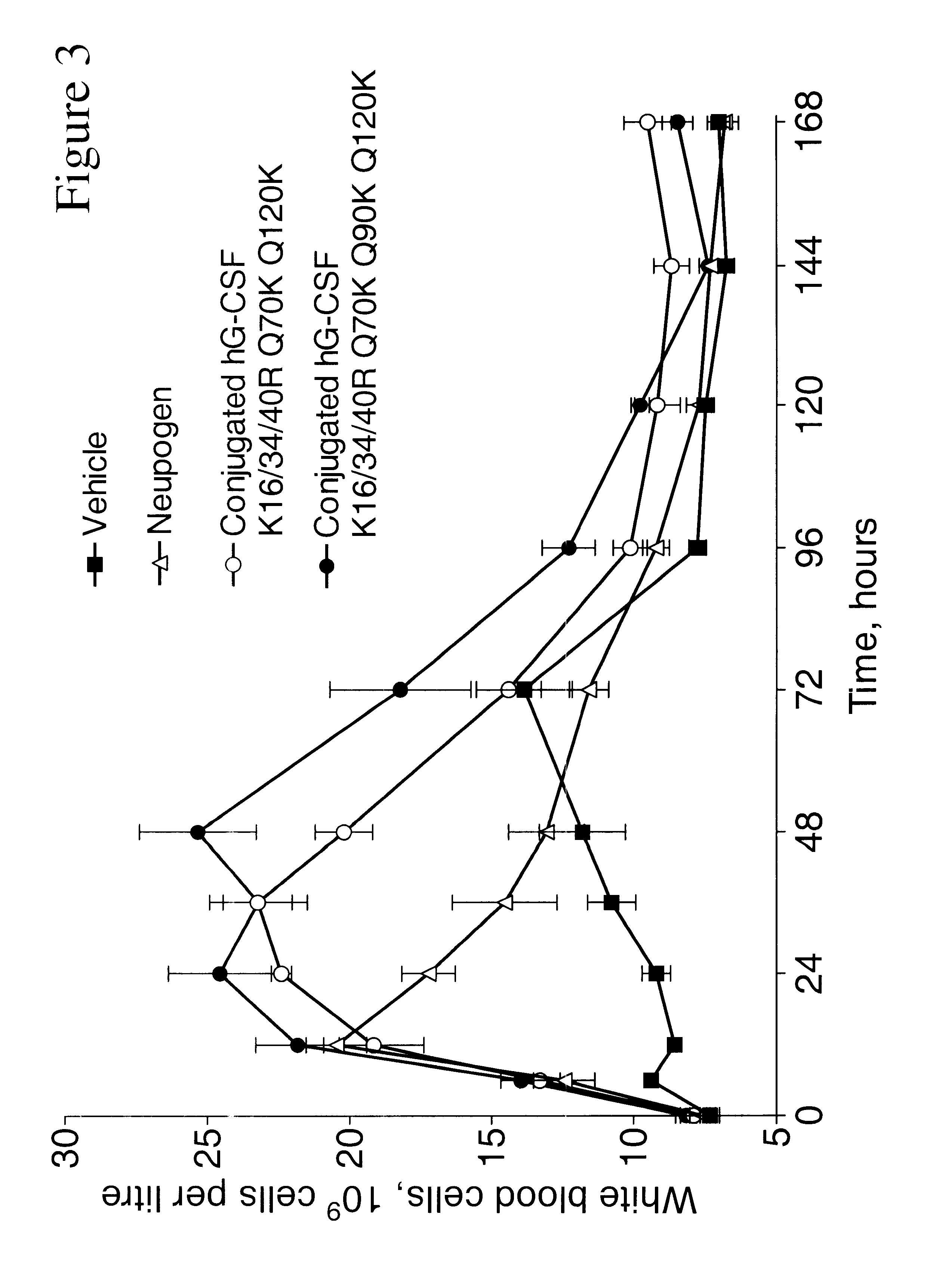
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
Tissue specific peptide conjugates and methods
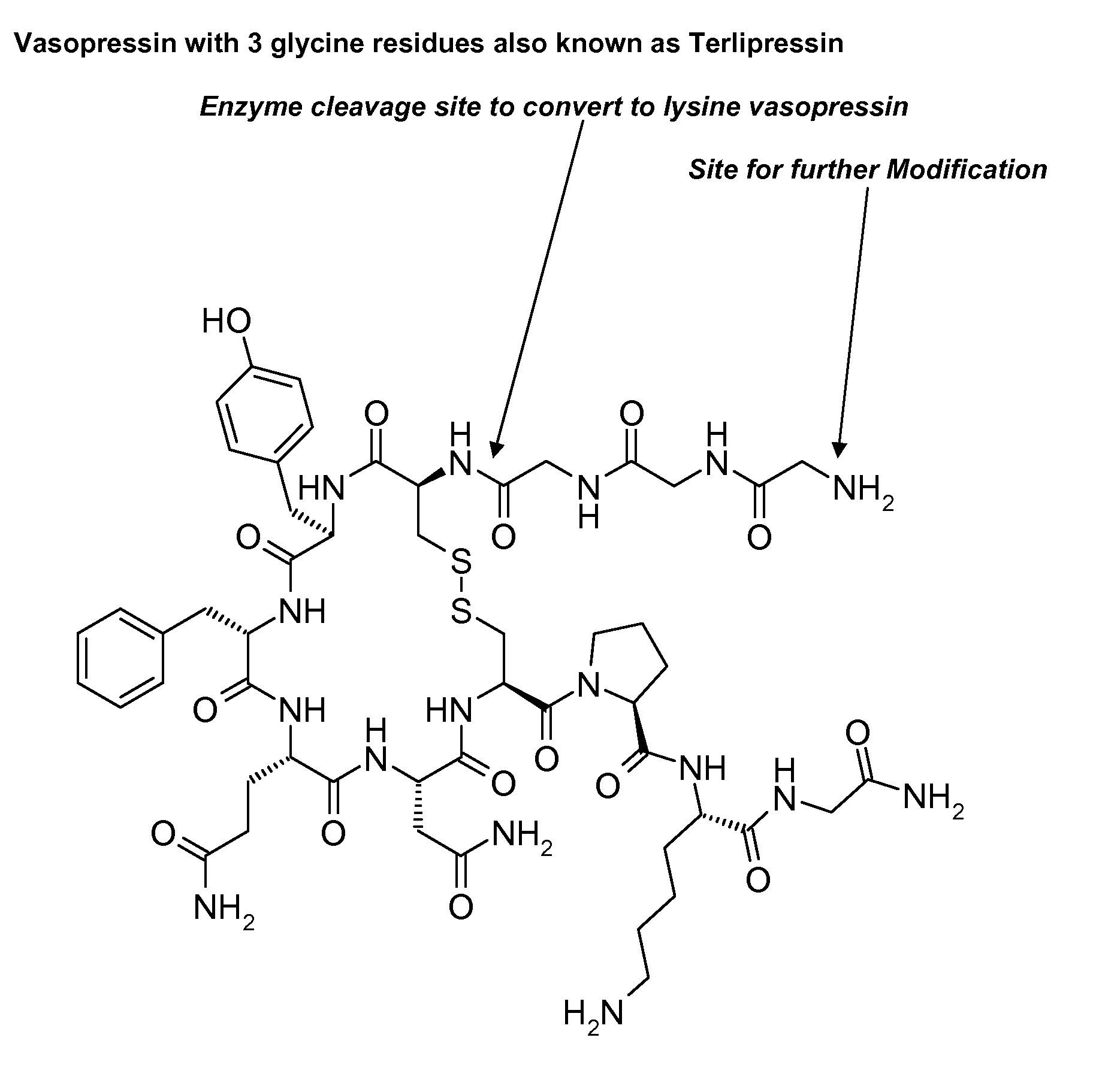
Cell-penetrating peptides useful for targeting a therapeutic compound to a selected mammalian tissue, methods for their identification, methods of forming conjugate compounds containing such peptides, and conjugates formed thereby are disclosed. The cell-penetrating peptides are 8 to 30 amino acid residues in length and consist of subsequences selected from the group consisting of RXR, RX, RB, and RBR; where R is arginine, B is β-alanine, and each X is independently —C(O)—(CHR1)n—NH—, where n is 4-6 and each R1 is independently H or methyl, such that at most two R1's are methyl. In one embodiment, X is a 6-aminohexanoic acid residue.
Owner:AVI BIOPHARMA
Modified Fc molecules
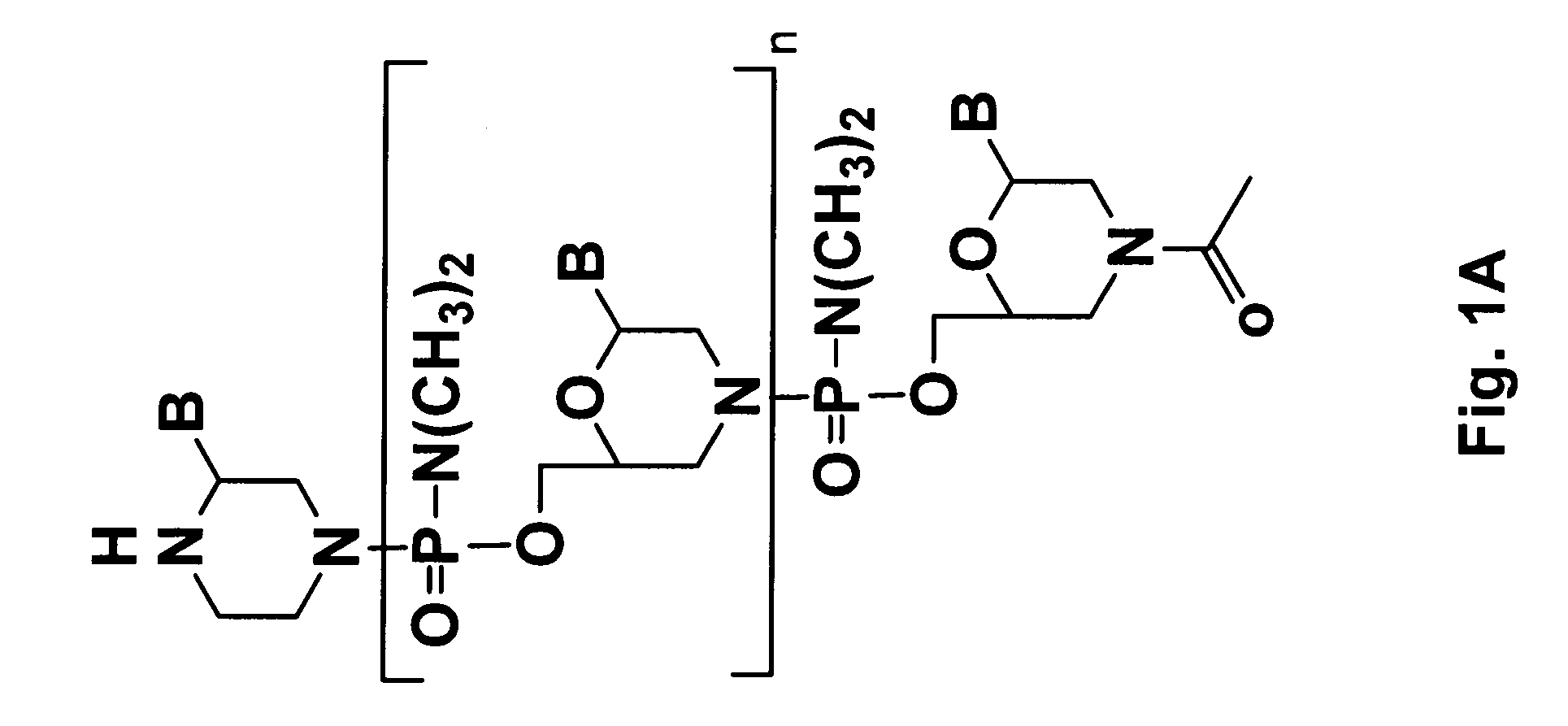
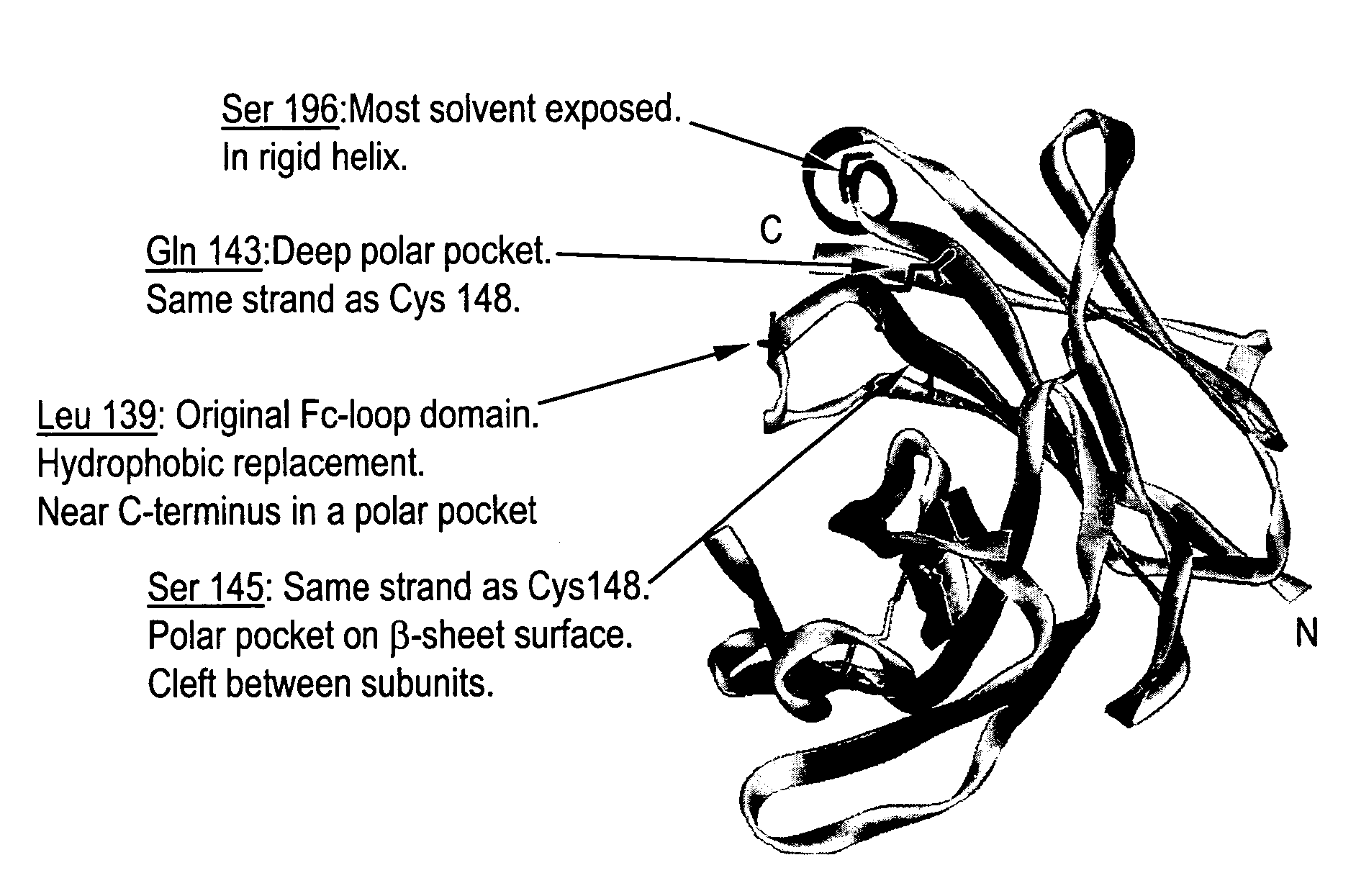
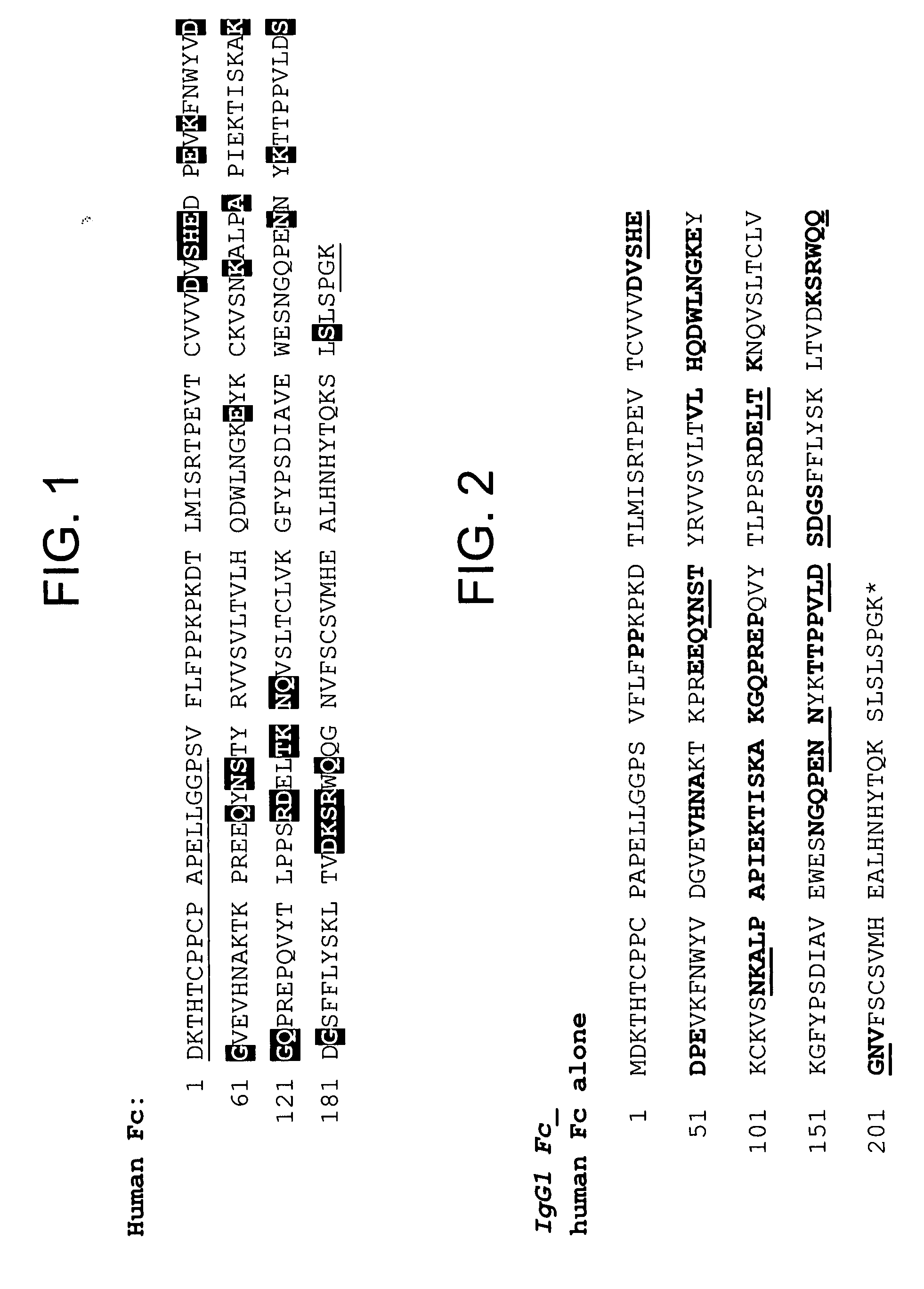
Disclosed is a process for preparing a pharmacologically active compound, in which at least one internal conjugation site of an Fc domain sequence is selected that is amenable to conjugation of an additional functional moiety by a defined conjugation chemistry through the side chain of an amino acid residue at the conjugation site. An appropriate amino acid residue for conjugation may be present in a native Fc domain at the conjugation site or may be added by insertion (i.e., between amino acids in the native Fc domain) or by replacement (i.e., removing amino acids and substituting different amino acids). In the latter case, the number of amino acids added need not correspond to the number of amino acids removed from the previously existing Fc domain. This technology may be used to produce useful compositions of matter and pharmaceutical compositions containing them. A DNA encoding the inventive composition of matter, an expression vector containing the DNA, and a host cell containing the expression vector are also disclosed.
Owner:AMGEN INC
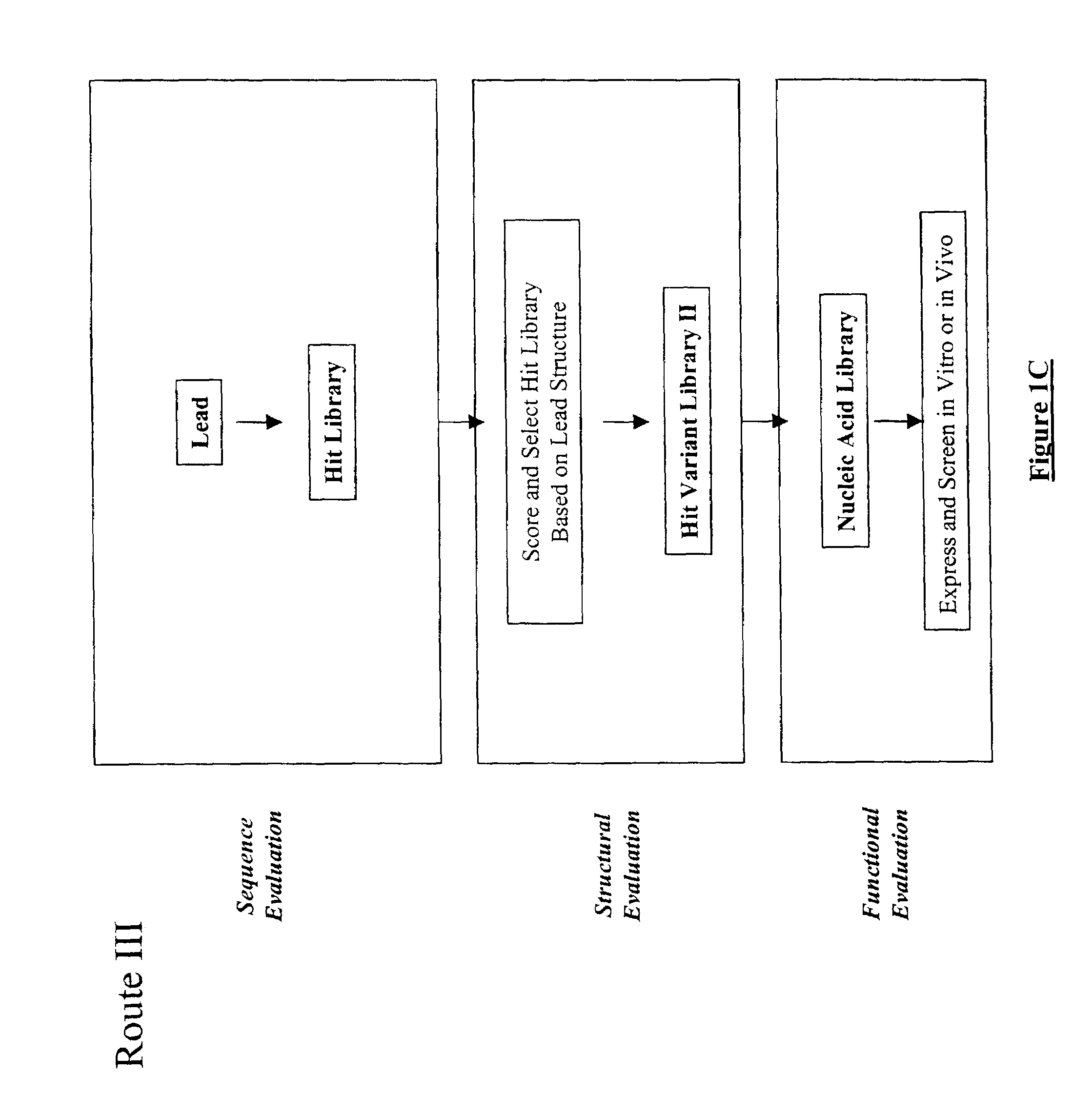
Structure-based selection and affinity maturation of antibody library
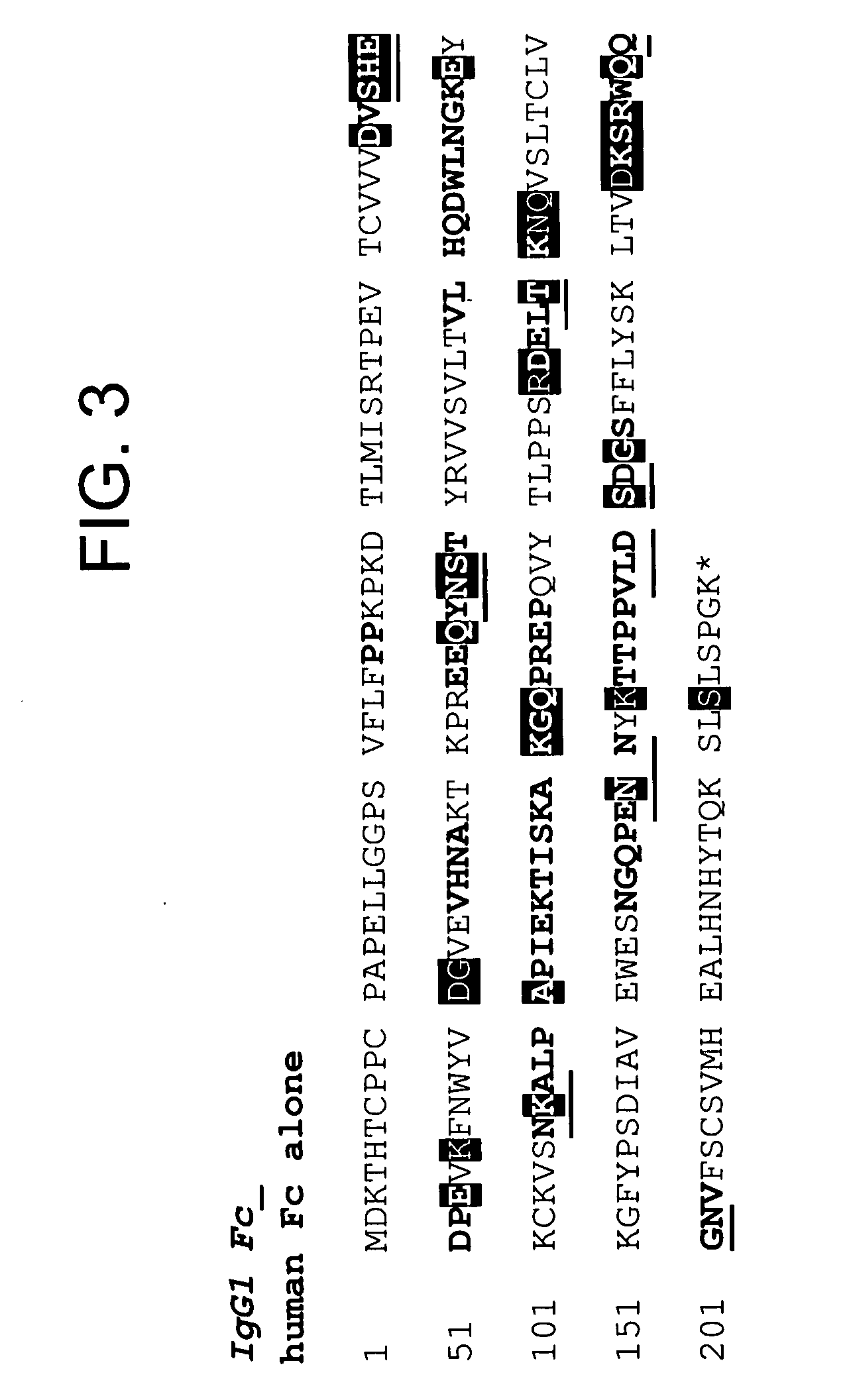
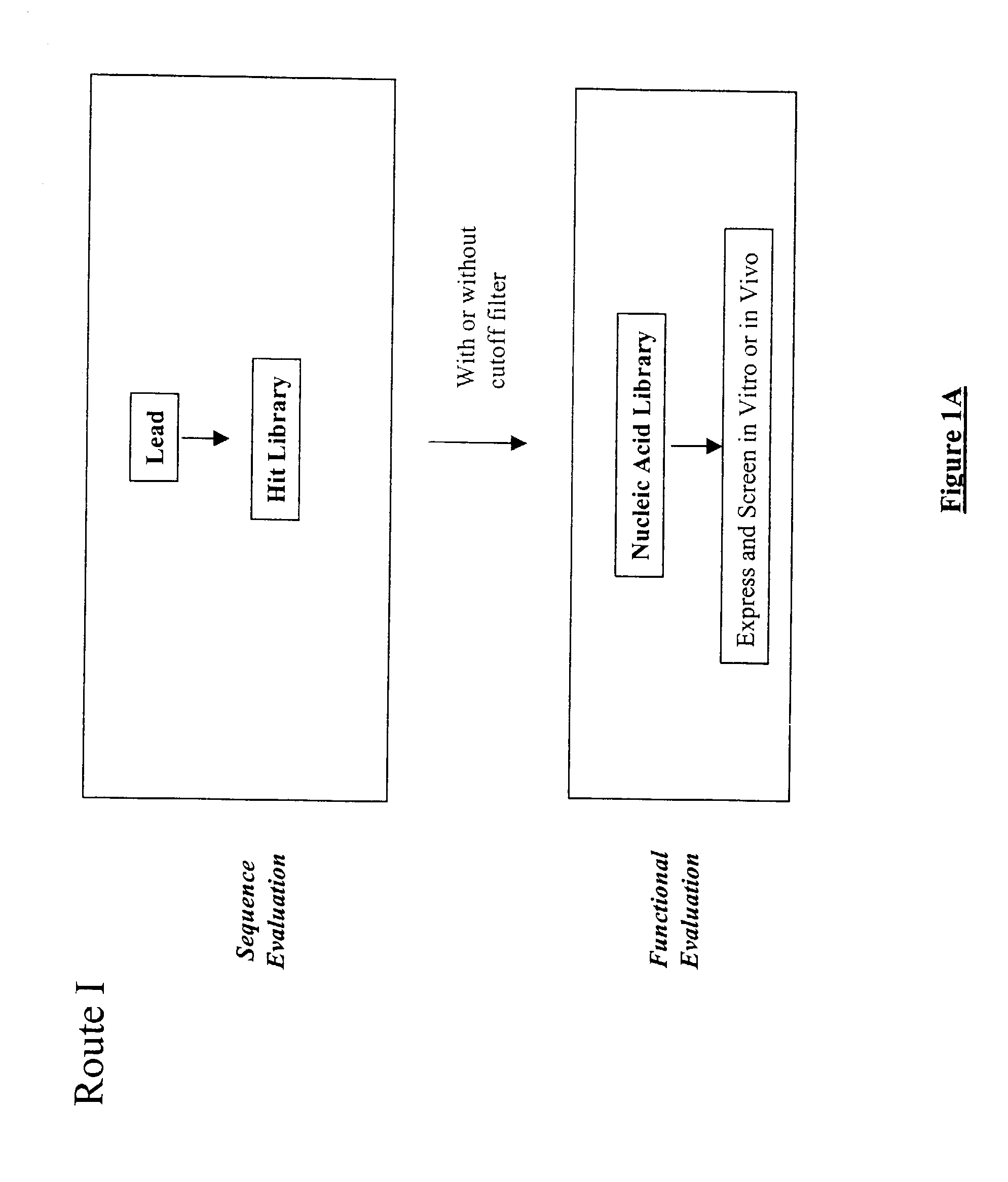
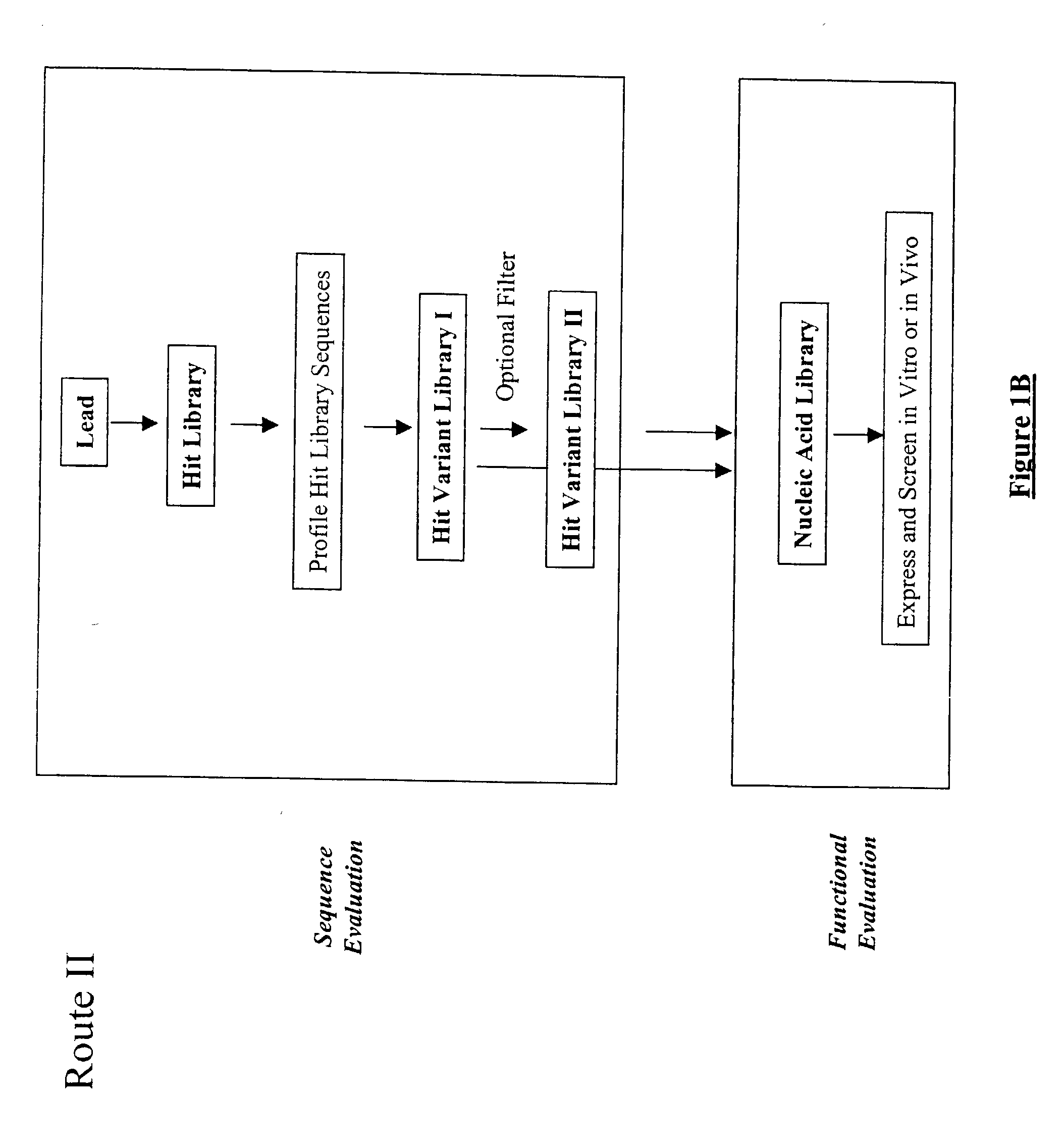
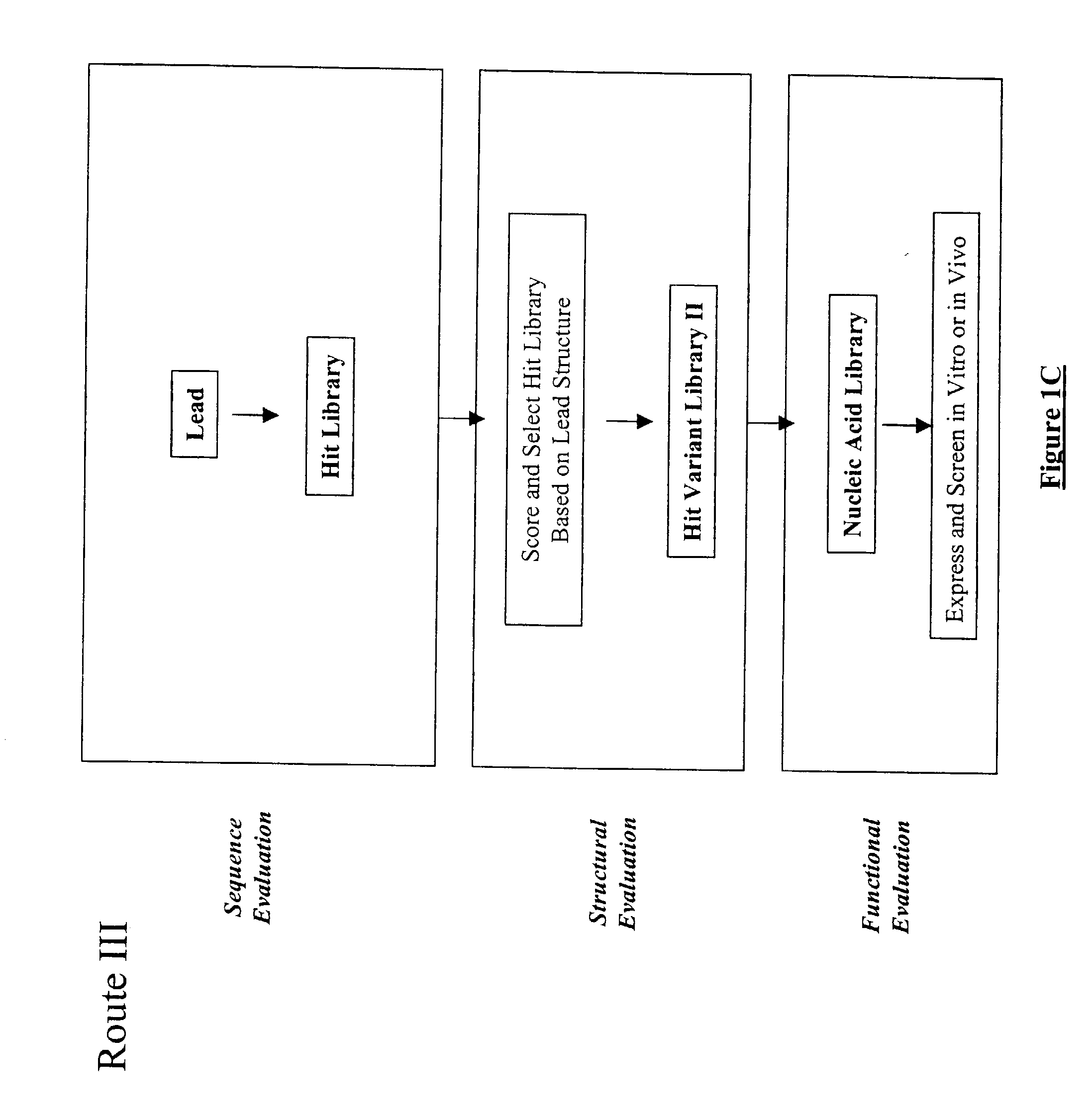
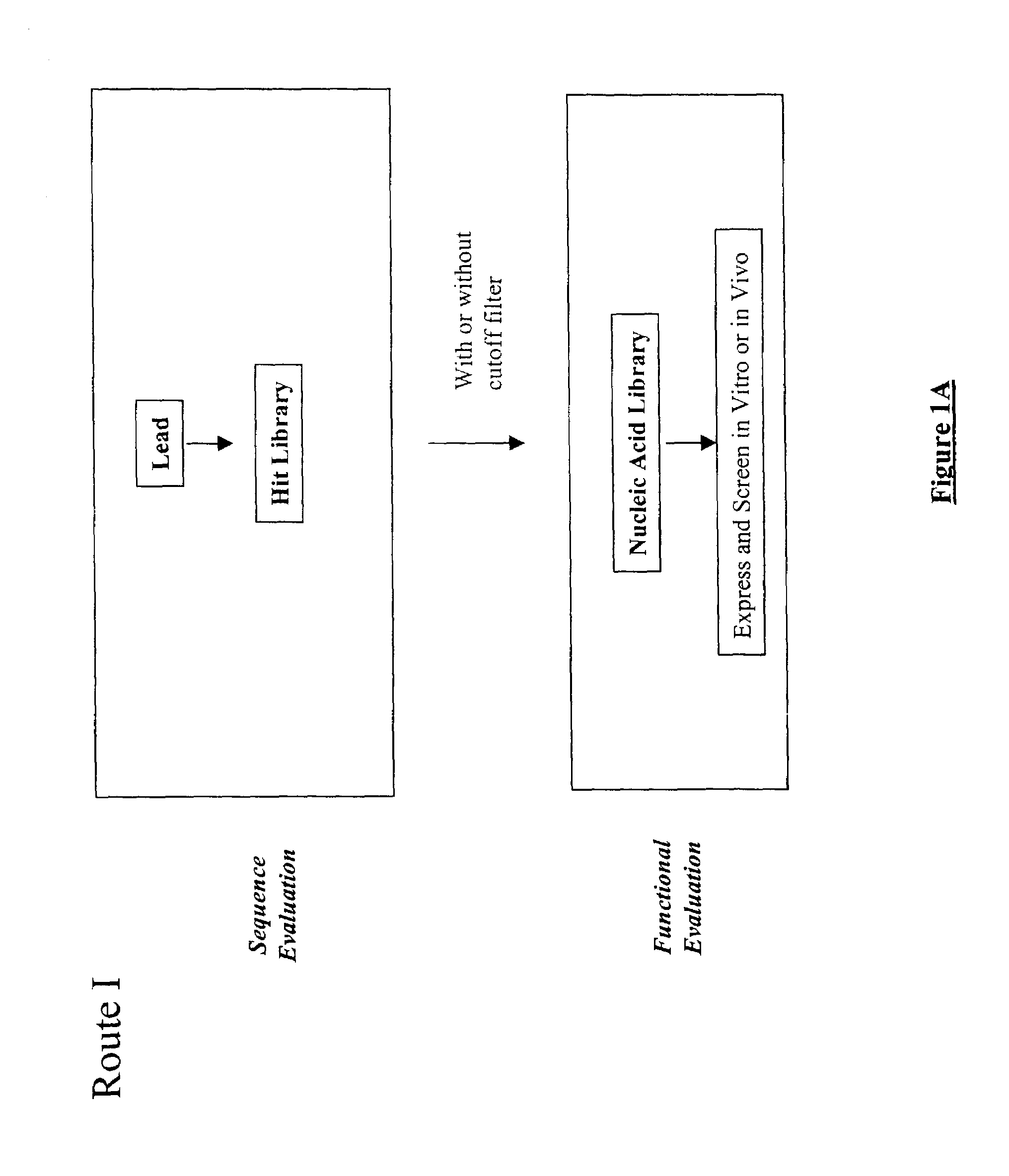
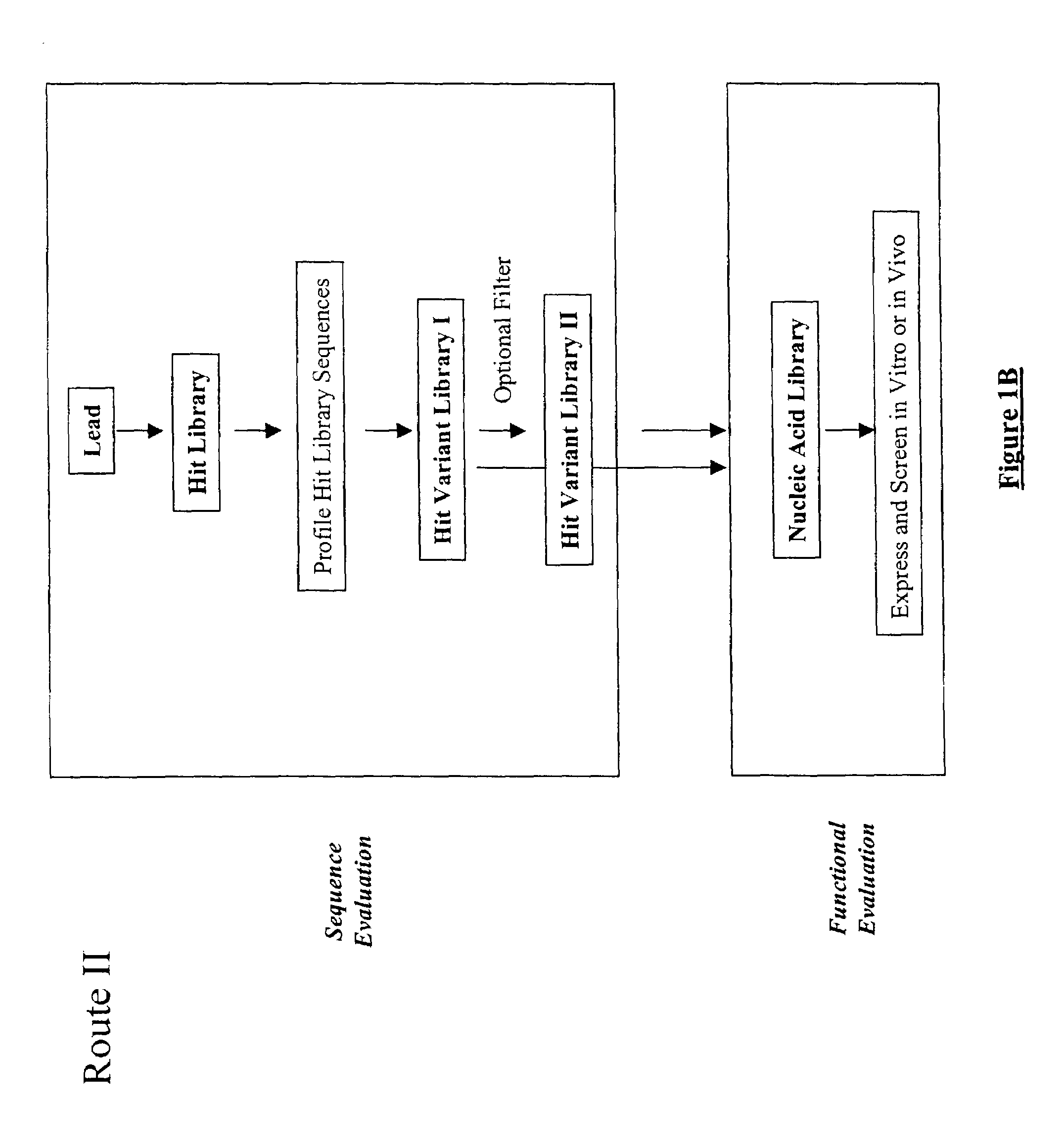
The present invention provides a structure-based methodology for efficiently generating and screening protein libraries for optimized proteins with desirable biological functions, such as antibodies with high binding affinity and low immunogenicity in humans. In one embodiment, a method is provided for constructing a library of antibody sequences based on a three dimensional structure of a lead antibody. The method comprises: providing an amino acid sequence of the variable region of the heavy chain (VH) or light chain (VL) of a lead antibody, the lead antibody having a known three dimensional structure which is defined as a lead structural template; identifying the amino acid sequences in the CDRs of the lead antibody; selecting one of the CDRs in the VH or VL region of the lead antibody; providing an amino acid sequence that comprises at least 3 consecutive amino acid residues in the selected CDR, the selected amino acid sequence being a lead sequence; comparing the lead sequence profile with a plurality of tester protein sequences; selecting from the plurality of tester protein sequences at least two peptide segments that have at least 10% sequence identity with lead sequence, the selected peptide segments forming a hit library; determining if a member of the hit library is structurally compatible with the lead structural template using a scoring function; and selecting the members of the hit library that score equal to or better than or equal to the lead sequence. The selected members of the hit library can be expressed in vitro or in vivo to produce a library of recombinant antibodies that can be screened for novel or improved function(s) over the lead antibody.
Owner:ABMAXIS
CD20-Binding polypeptide compositions and methods
ActiveUS20050069545A1Low level of immunogenicityReduce capacityAntipyreticAntibody mimetics/scaffoldsCD20Immunologic disorders
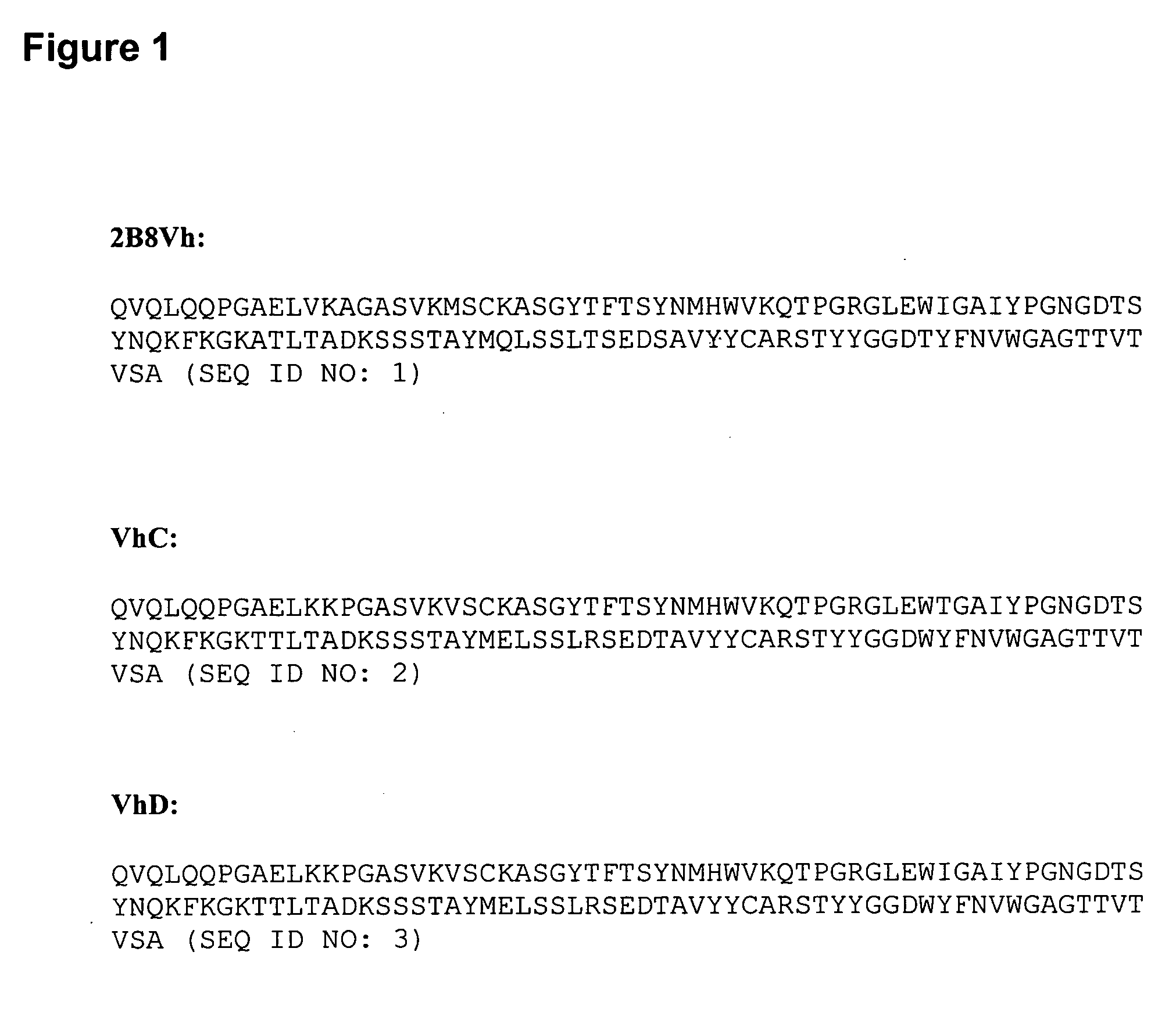
A CD20-binding polypeptide composition of the invention comprises at least one polypeptide selected from the group consisting of a polypeptide having the amino acid residue sequence of SEQ ID NO: 1 (Vh of 2B8 antibody), but which includes at least one amino acid residue substitution in SEQ ID NO: 1 selected from the group consisting of V12K, A14P, M20V, I48T, A68T, Q82E, T87R, S91T, and T106W; a polypeptide having the amino acid residue sequence of SEQ ID NO: 4 (Vk of 2B8 antibody), but which includes at least one amino acid residue substitution in SEQ ID NO: 4 selected from the group consisting of L11I, S12T, S27T, V29A, G40T, V59S, S69T, L72M, R76S, and V77L; a polypeptide having the amino acid residue sequence of SEQ ID NO: 9 (Vh of Leu16 antibody), but which includes at least one amino acid residue substitution in SEQ ID NO: 9 selected from the group consisting of V12K, M20V, A68T, Q82E, T87R, S91T, D93V, and A114T; and a polypeptide having the amino acid residue sequence of SEQ ID NO: 11 (Vk of Leu16 antibody), but which includes at least one amino acid residue substitution in SEQ ID NO: 11 selected from the group consisting of L11I, S12T, A59S, S69T, L72M, R76S, and V77L. The compositions are useful for diagnosis and treatment of autoimmune diseases.
Owner:MERCK PATENT GMBH
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050032114A1Reducing FcRn binding affinityReduced half-lifeAnimal cellsSugar derivativesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050276799A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesSerum igeHalf-life
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Structure-based selection and affinity maturation of antibody library
InactiveUS7117096B2High affinityImprove throughputPeptide librariesPeptide/protein ingredientsProtein insertionIn vivo
The present invention provides a structure-based methodology for efficiently generating and screening protein libraries for optimized proteins with desirable biological functions, such as antibodies with high binding affinity and low immunogenicity in humans. In one embodiment, a method is provided for constructing a library of antibody sequences based on a three dimensional structure of a lead antibody. The method comprises: providing an amino acid sequence of the variable region of the heavy chain (VH) or light chain (VL) of a lead antibody, the lead antibody having a known three dimensional structure which is defined as a lead structural template; identifying the amino acid sequences in the CDRs of the lead antibody; selecting one of the CDRs in the VH or VL region of the lead antibody; providing an amino acid sequence that comprises at least 3 consecutive amino acid residues in the selected CDR, the selected amino acid sequence being a lead sequence; comparing the lead sequence profile with a plurality of tester protein sequences; selecting from the plurality of tester protein sequences at least two peptide segments that have at least 10% sequence identity with lead sequence, the selected peptide segments forming a hit library; determining if a member of the hit library is structurally compatible with the lead structural template using a scoring function; and selecting the members of the hit library that score equal to or better than or equal to the lead sequence. The selected members of the hit library can be expressed in vitro or in vivo to produce a library of recombinant antibodies that can be screened for novel or improved function(s) over the lead antibody.
Owner:ABMAXIS
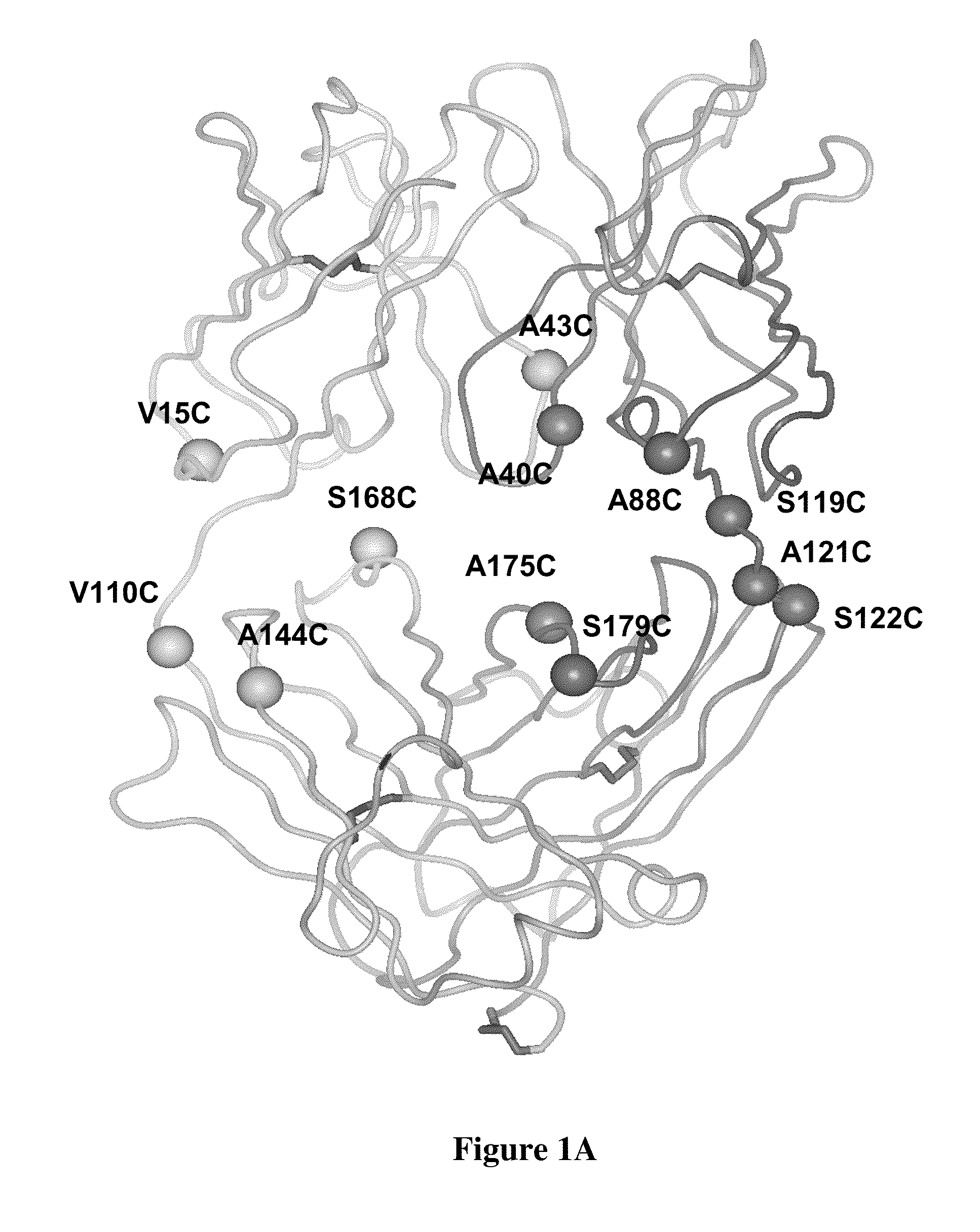
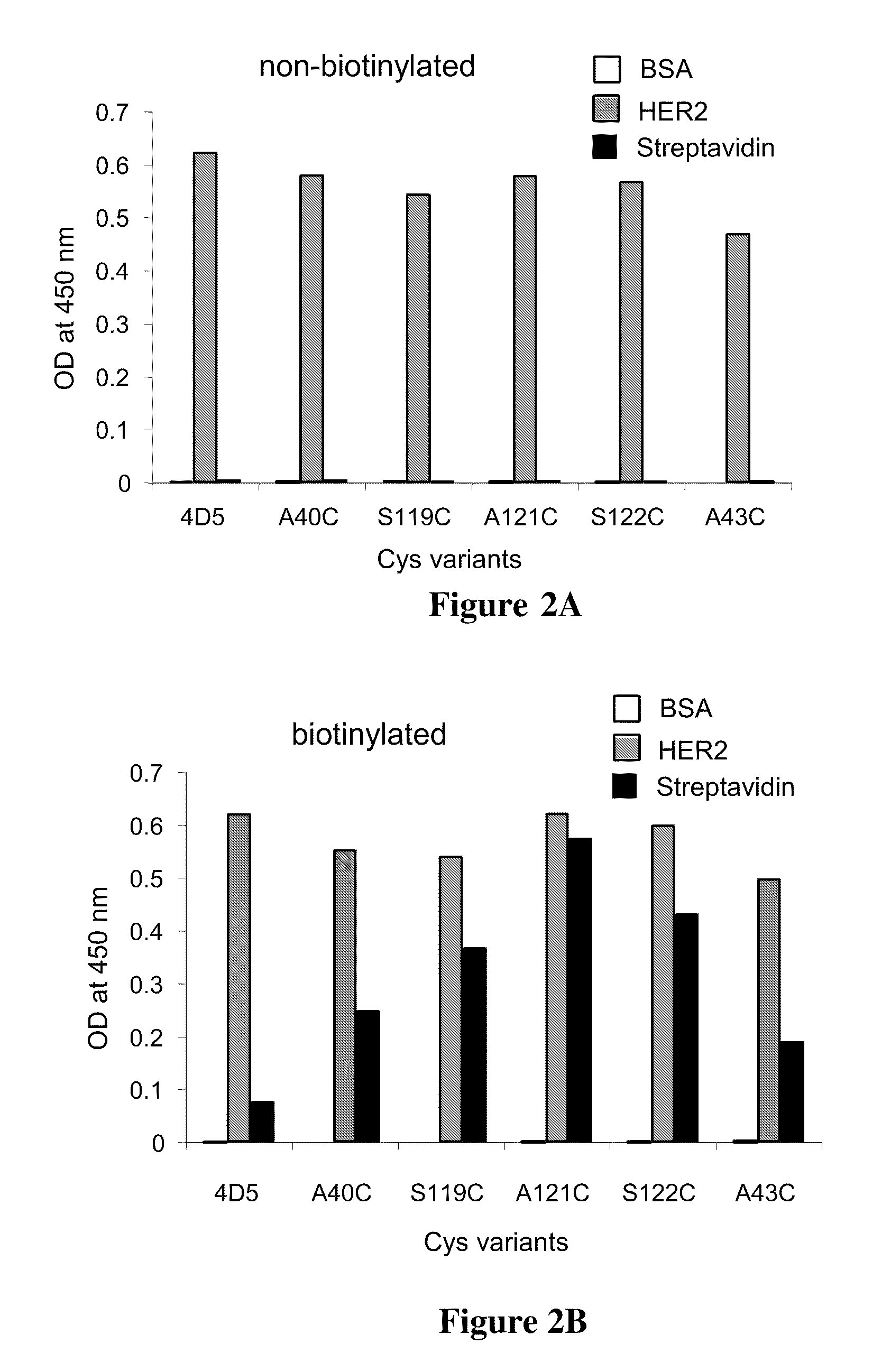
Cysteine engineered antibodies and conjugates
Cysteine engineered antibodies comprising a free cysteine amino acid in the heavy chain or light chain are prepared by mutagenizing a nucleic acid sequence of a parent antibody and replacing one or more amino acid residues by cysteine to encode the cysteine engineered antibody; expressing the cysteine engineered antibody; and isolating the cysteine engineered antibody. Certain highly reactive cysteine engineered antibodies were identified by the PHESELECTOR assay. Isolated cysteine engineered antibodies may be covalently attached to a capture label, a detection label, a drug moiety, or a solid support.
Owner:GENENTECH INC
Immunobiologically-active linear peptides and method of identification
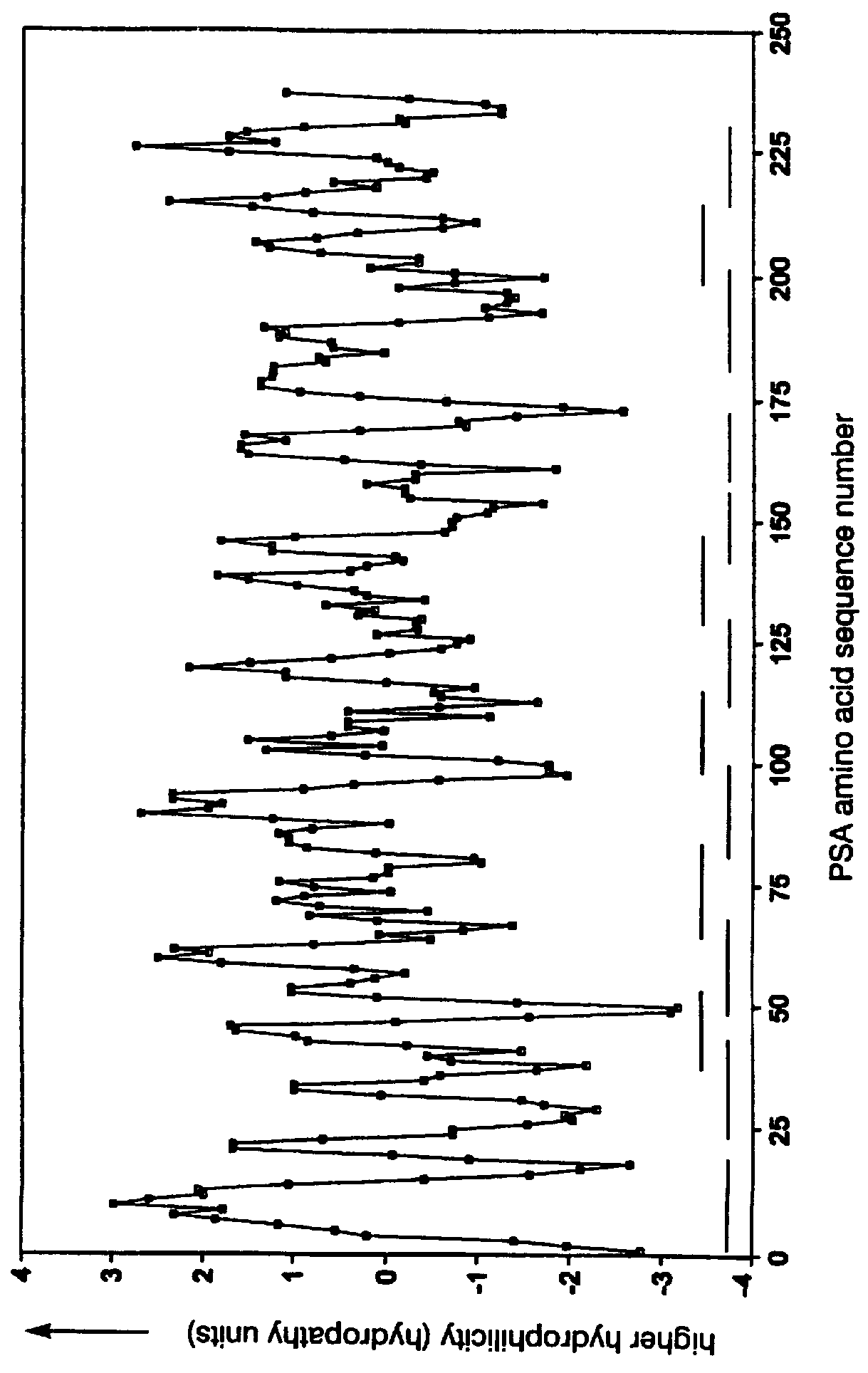
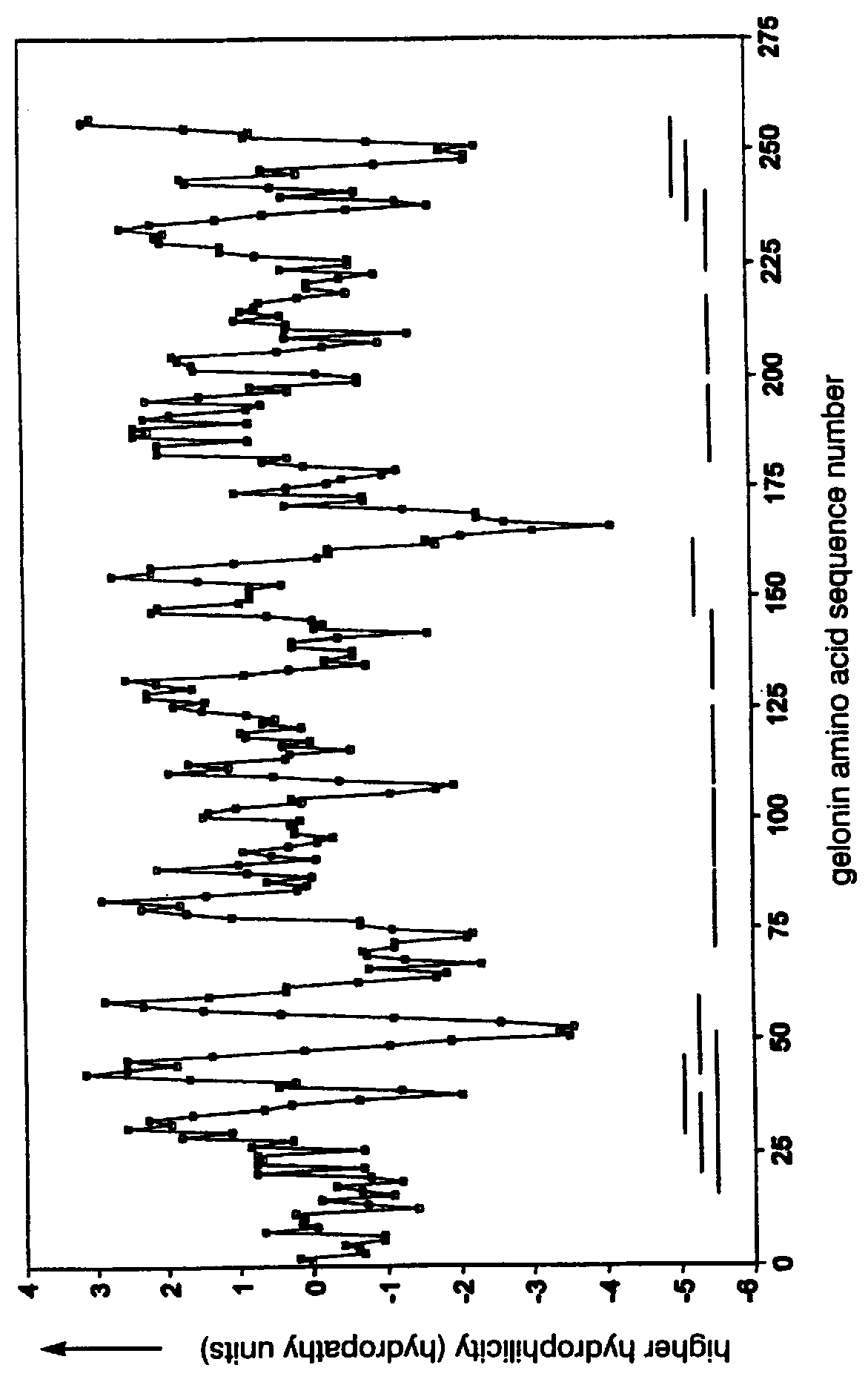
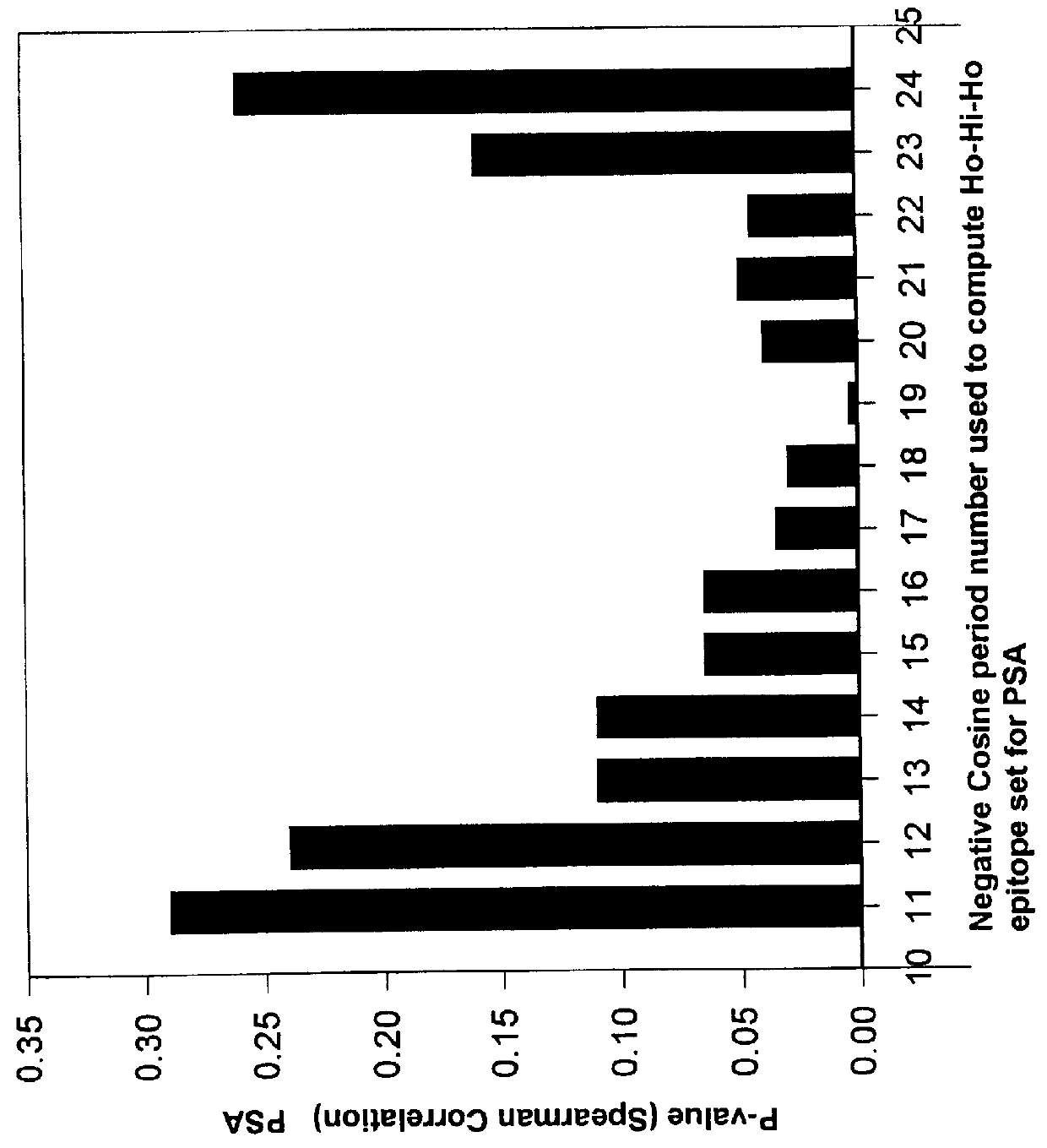
The present invention relates to identifying protein epitopes and more particularly to a novel method for identifying, determining the location, optimal length of amino acid residues and immunobiological potency of protein epitopes by applying a custom negative cosine function fit algorithm to a protein hydropathy scale. This fit analysis is supplemented with experimental immunobiological data. The amino acid sequence of the protein epitopes of the present invention exhibit a hydrophobic-hydrophilic-hydrophobic hydropathy pattern of an approximately fixed length in a given protein.
Owner:KOKOLUS WILLIAM J
Thermostable protease
InactiveUS6358726B1Improve efficiencyImprove expression levelPolypeptide with localisation/targeting motifHydrolasesProteinase activityProtease
A hyperthermostable protease having the amino acid sequence represented by the SEQ ID NO:1 of the Sequence Listing or a sequence derived therefrom by deletion, substitution, insertion or addition of one to several amino acid residues, a gene encoding the hyperthermostable protease, and a process for preparing the protease, aiming at providing by genetic engineering techniques a hyperthermophile protease which is advantageous for industrial use.
Owner:TAKARA HOLDINGS
Fcgamma receptor-binding polypeptide variants and methods related thereto
InactiveUS20060275283A1Increase free energyReduced binding affinityFermentationPlant genotype modificationAntibodyMolecular biology
The compositions and methods of the present invention are based, in part, on our discovery that an effector function mediated by an Fc-containing polypeptide can be altered by modifying one or more amino acid residues within the polypeptide (by, for example, electrostatic optimization). The polypeptides that can be generated according to the methods of the invention are highly variable, and they can include antibodies and fusion proteins that contain an Fc region or a biologically active portion thereof.
Owner:BIOGEN IDEC MA INC
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
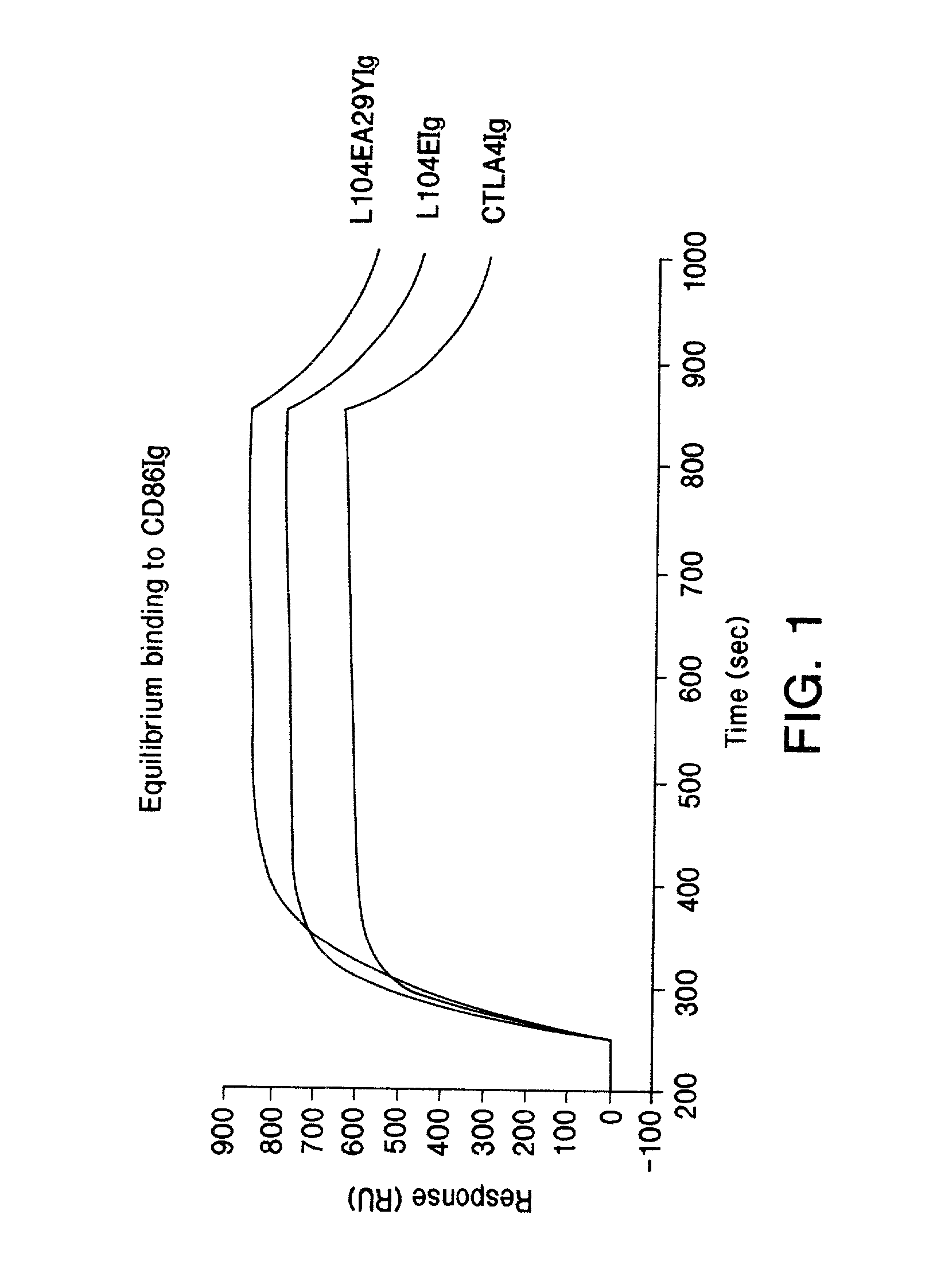
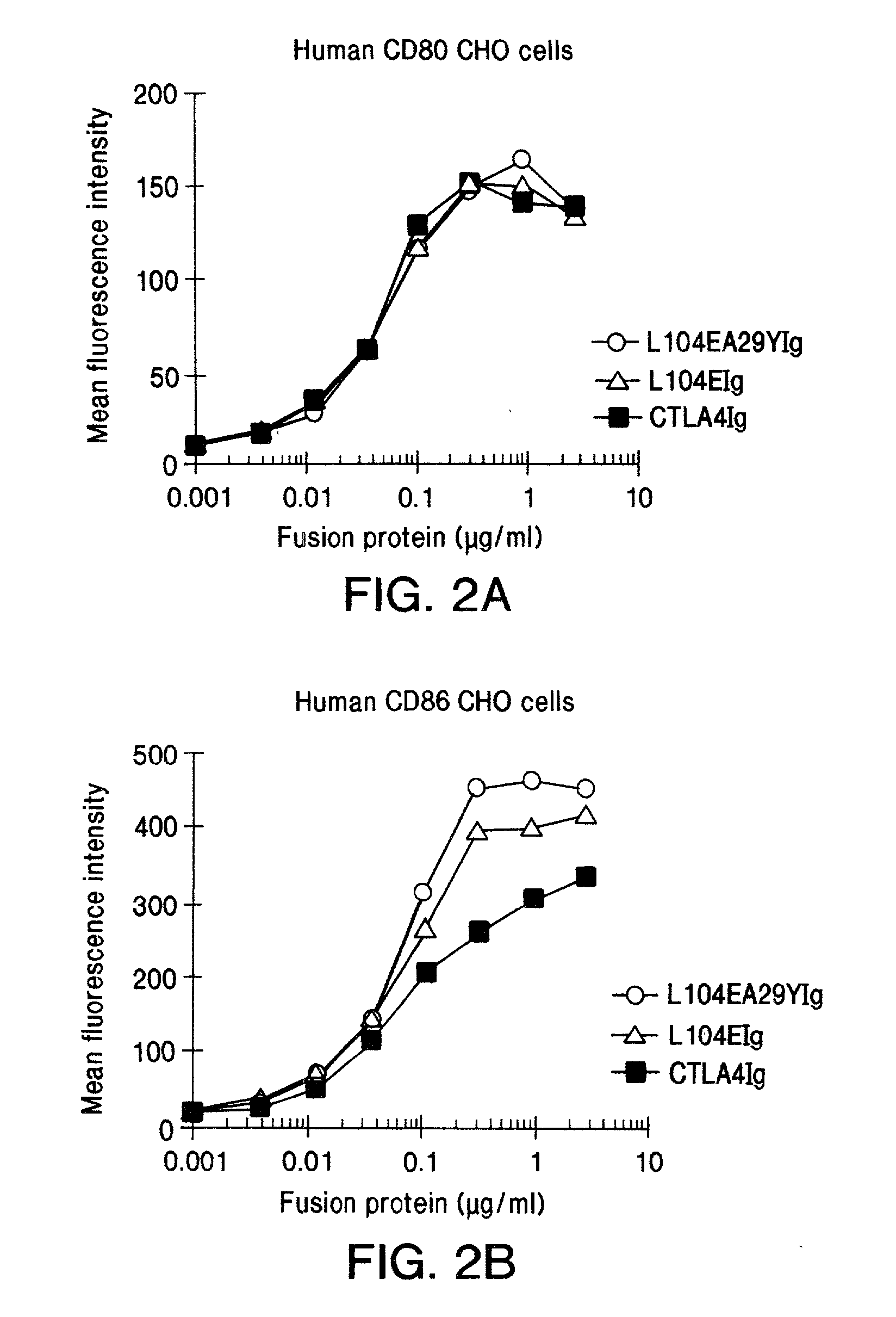
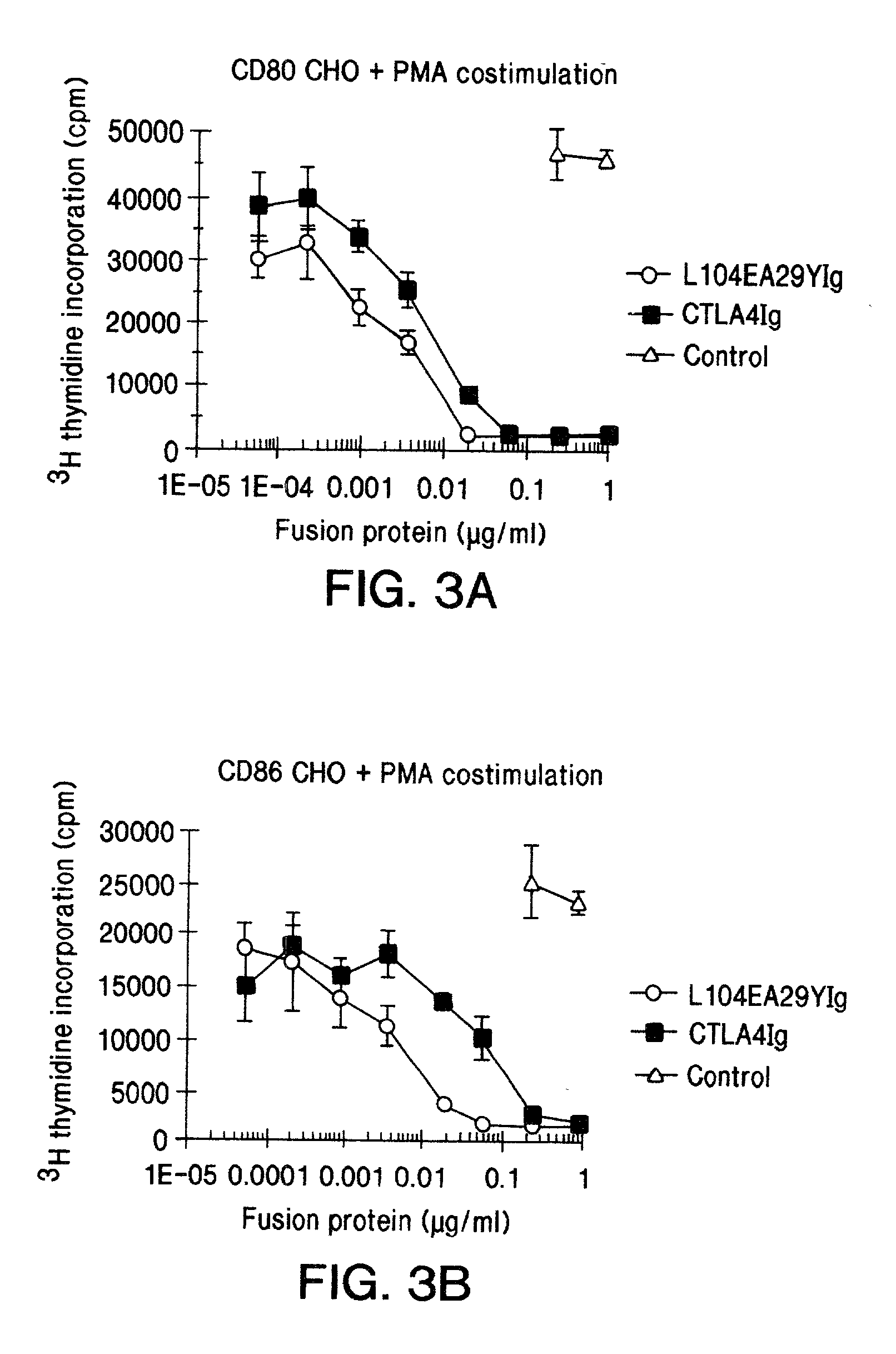
Soluble CTLA4 mutant molecules
Owner:BRISTOL MYERS SQUIBB CO
Pegylated interleukin-10
InactiveUS7052686B2Minimize disruptionPeptide/protein ingredientsAntipyreticInterleukin 10White blood cell
Interleukin-10 (IL-10) conjugated via a linker to one or more polyethylene glycol (PEG) molecules at a single amino acid residue of the IL-10, and a method for preparing the same, are provided. The method produces a stable mono-pegylated IL-10, which retains IL-10 activity, where pegylation is selective for the N-terminus on one subunit of IL-10 with little or no formation of monomeric IL-10. The method also provides a substantially homogenous population of mono-PEG-IL-10.
Owner:MERCK SHARP & DOHME CORP
Protein surface remodeling
ActiveUS20120129759A1Improve thermodynamic performanceImprove solubilityPeptide/protein ingredientsDepsipeptidesBiotin-streptavidin complexBiochemistry
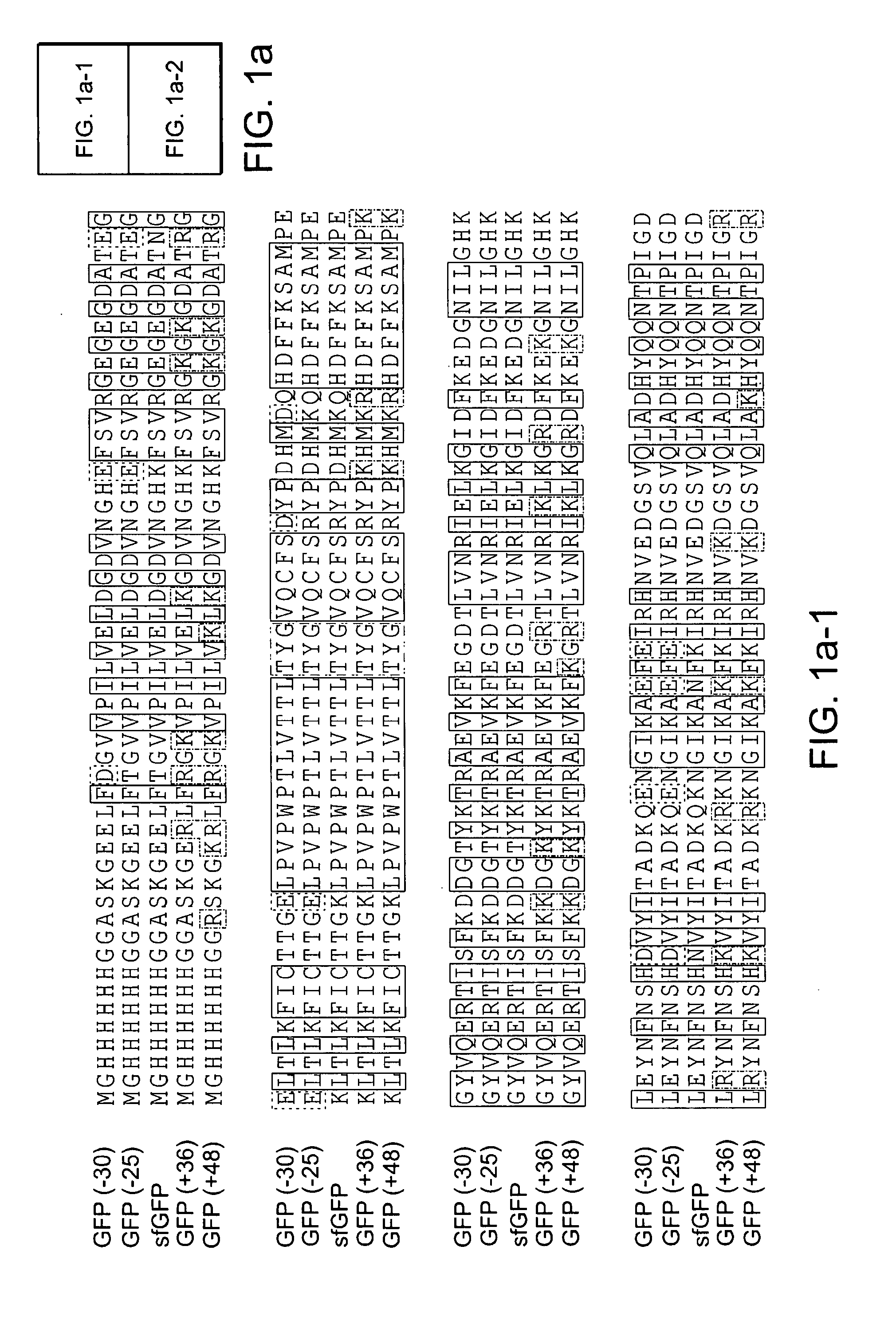
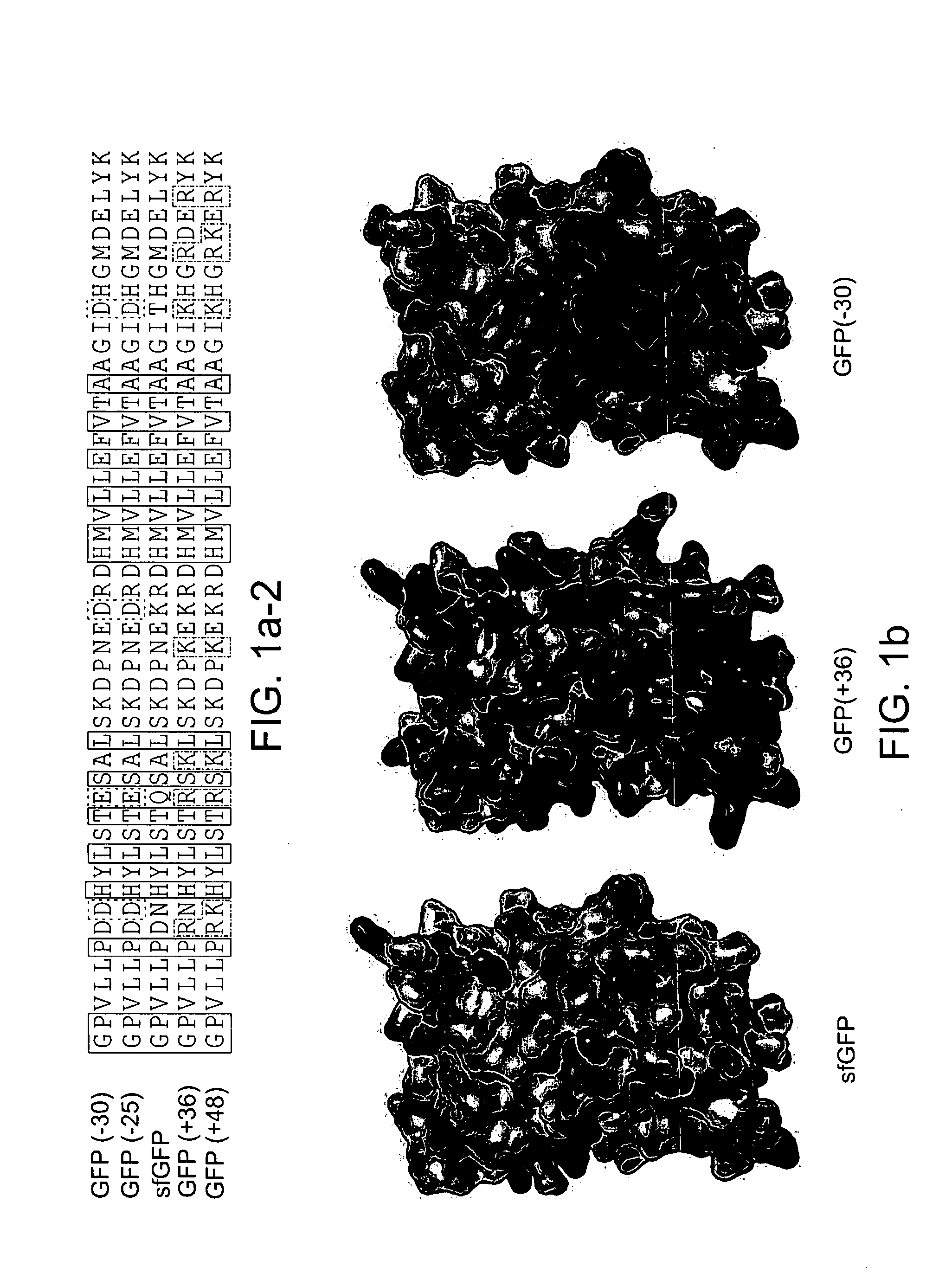
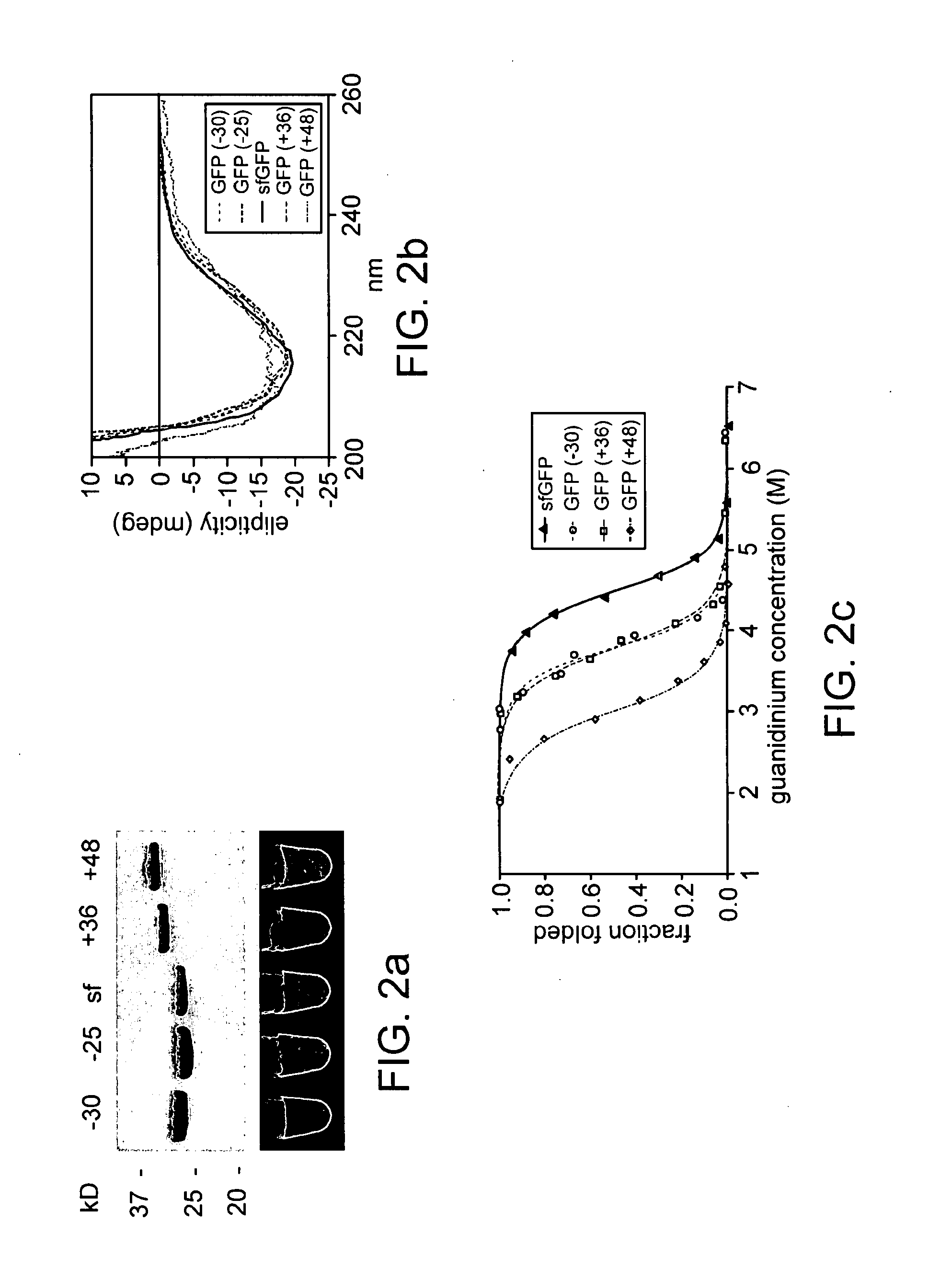
Aggregation is a major cause of the misbehavior of proteins. A system for modifying a protein to create a more stable variant is provided. The method involves identifying non-conserved hydrophobic amino acid residues on the surface of a protein, suitable for mutating to more hydrophilic residues (e.g., charged amino acids). Any number of residues on the surface may be changed to create a variant that is more soluble, resistant to aggregation, has a greater ability to re-fold, and / or is more stable under a variety of conditions. The invention also provides GFP, streptavidin, and GST variants with an increased theoretical net charge created by the inventive technology. Kits are also provided for carrying out such modifications on any protein of interest.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Composition for long-acting peptide analogs
ActiveUS20090088387A1Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
The invention describes compositions of peptide analogs that are active in blood or cleavable in blood to release an active peptide. The peptide analogs have a general formula: A-(Cm)x-Peptide, wherein A is hydrophobic moiety or a metal binding moiety, e.g., a chemical group or moiety containing 1) an alkyl group having 6 to 36 carbon units, 2) a nitrilotriacetic acid group, 3) an imidodiacetic acid group, or 4) a moiety of formula (ZyHisw)p, wherein Z is any amino acid residue other than histidine, His is histidine, y is an integer from 0-6; w is an integer from 1-6; and p is an integer from 1-6; wherein if A has alkyl group with 6 to 36 carbon units x is greater than 0; and Cm is a cleavable moiety consisting of glycine or alanine or lysine or arginine or N-Arginine or N-lysine, wherein x is an integer between 0-6 and N may be any amino acid or none. The peptide analogs are complexed with polymeric carrier to provide enhanced half-life.
Owner:PHARMAIN CORP
Interferon inducing genetically engineered attenuated viruses
InactiveUS6468544B1Reduce in quantityReduced characteristicsSsRNA viruses negative-senseVectorsGenetic engineeringRecombinant DNA
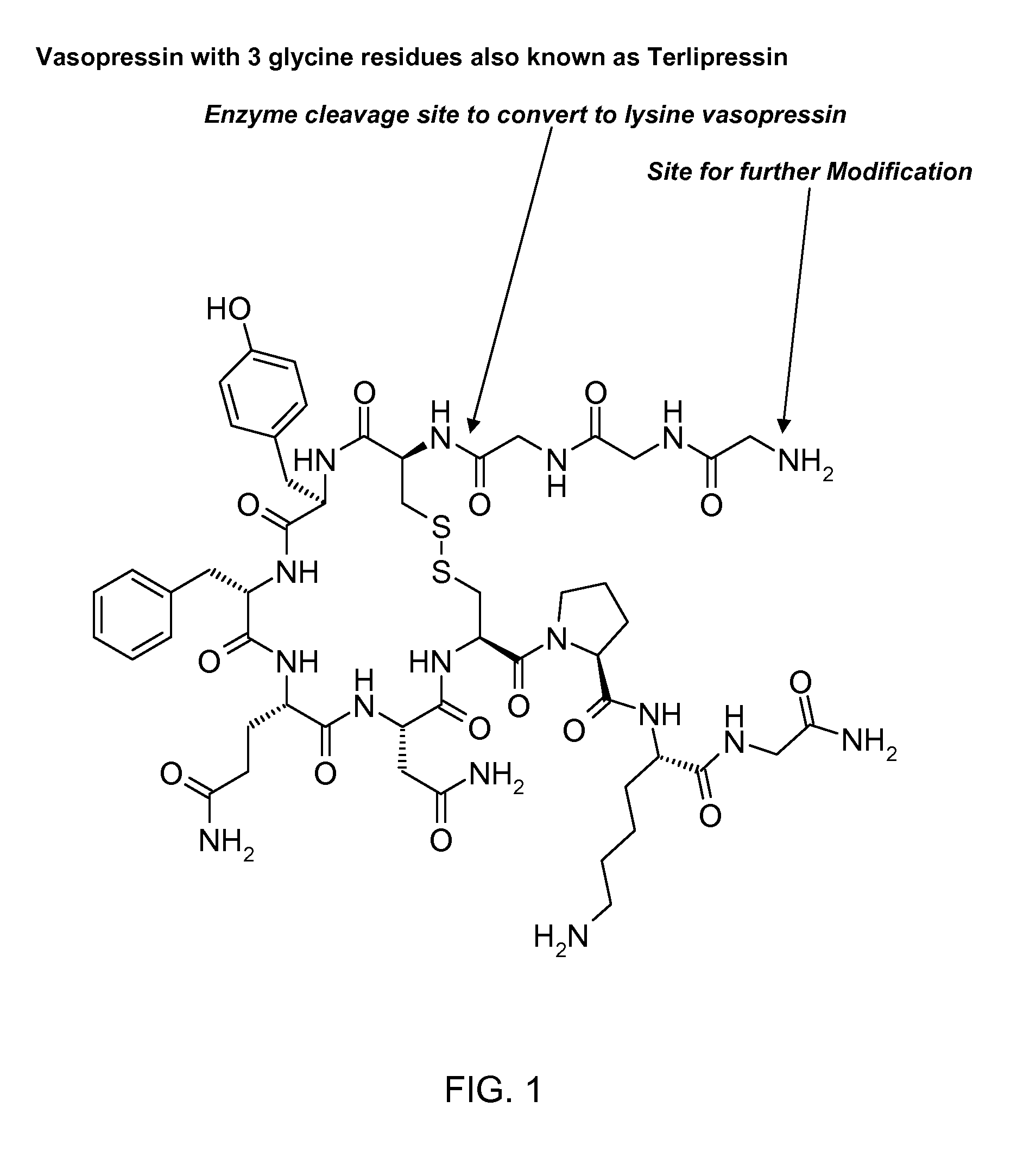
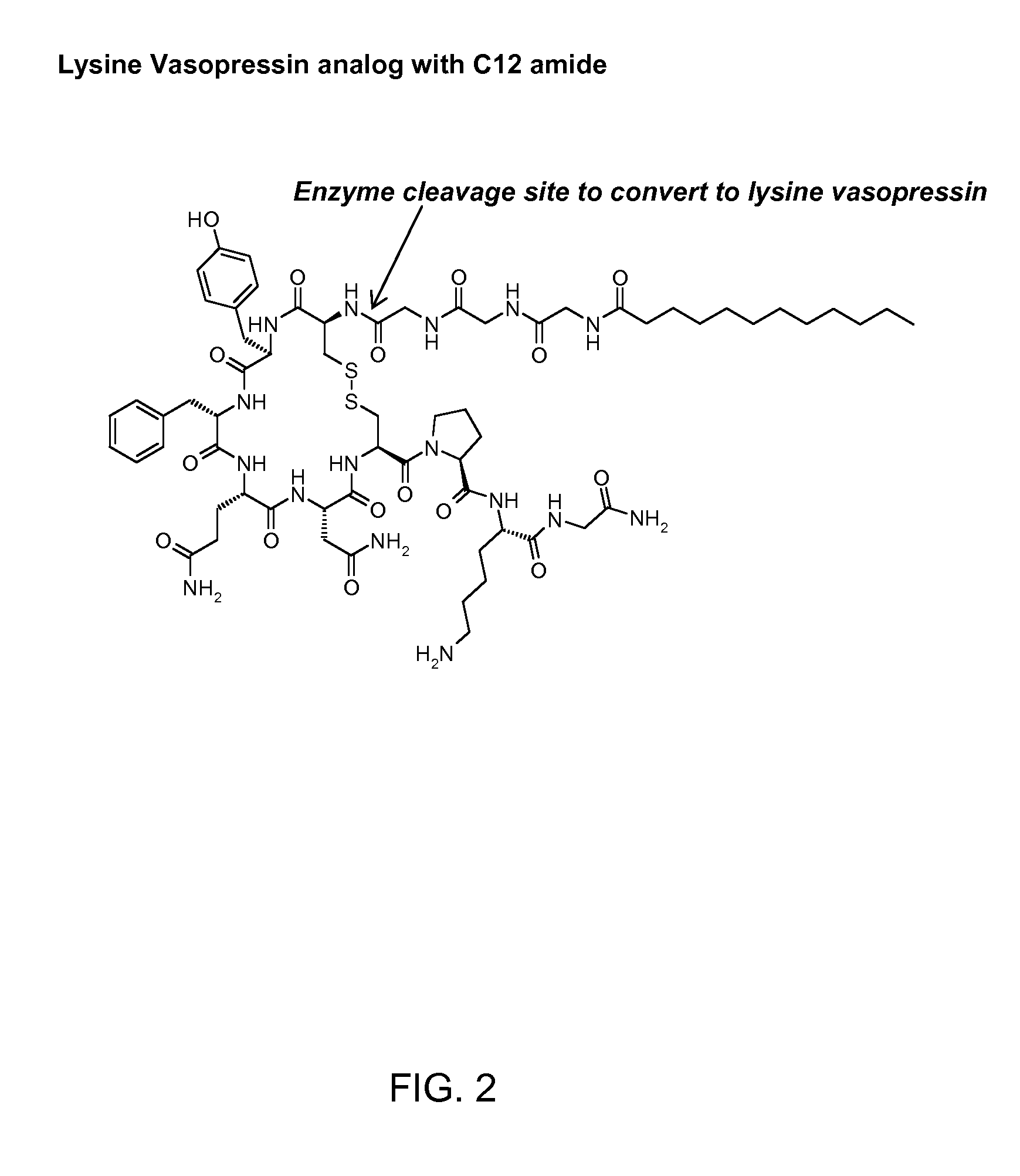
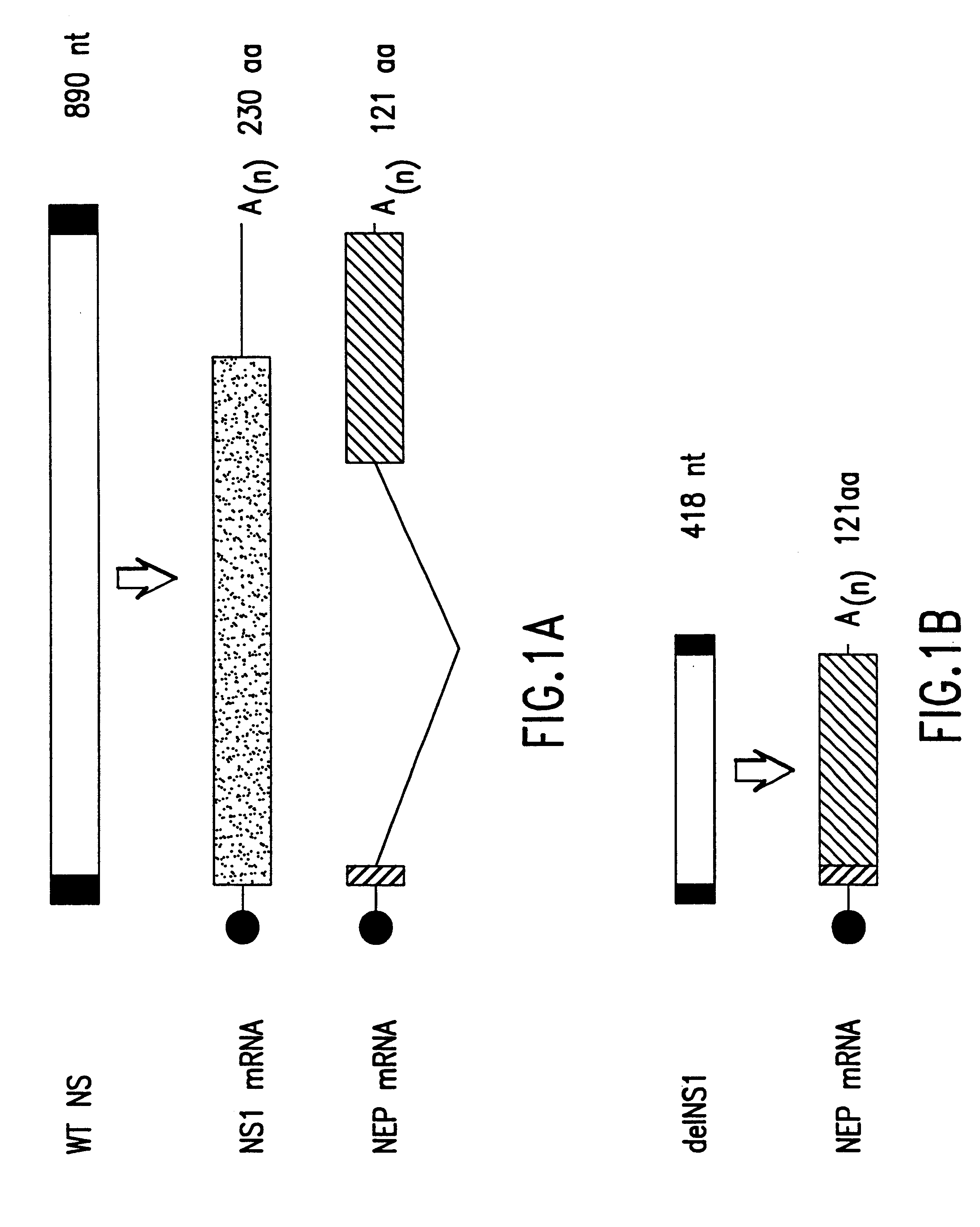
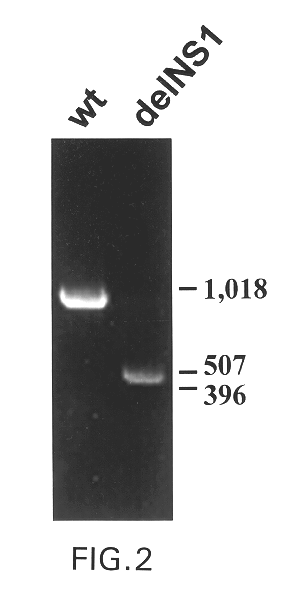
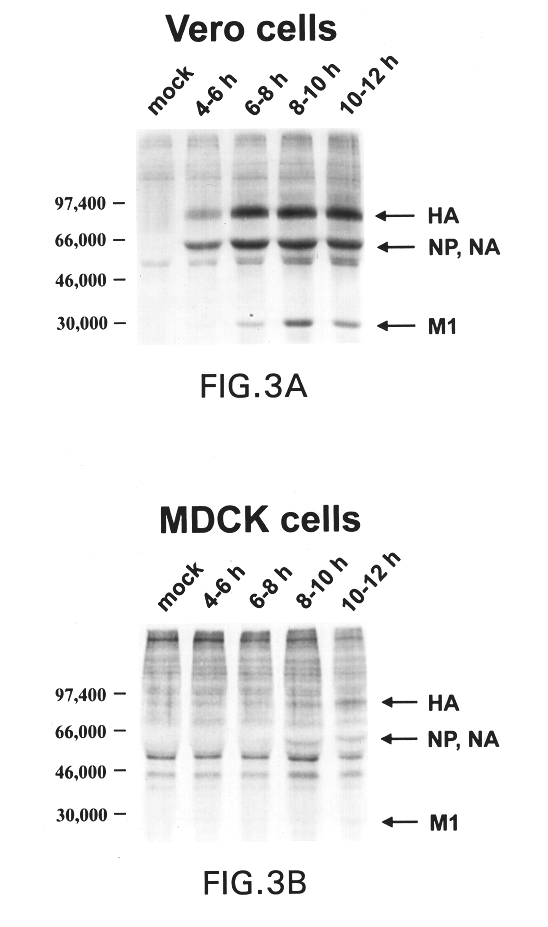
The present invention relates to genetically engineered attenuated viruses and methods for their production. In particular, the present invention relates to engineering live attenuated viruses which contain a modified NS gene segment. Recombinant DNA techniques can be utilized to engineer site specific mutations into one or more noncoding regions of the viral genome which result in the down-regulation of one or more viral genes. Alternatively, recombinant DNA techniques can be used to engineer a mutation, including but not limited to an insertion, deletion, or substitution of an amino acid residue(s) or an epitope(s) into a coding region of the viral genome so that altered or chimeric viral proteins are expressed by the engineered virus.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Compositions for prevention of adhesions and other barrier applications
ActiveUS20080032934A1Minimizing contaminationMinimizing infectionBiocideNervous disorderNucleotideMedicinal chemistry
A method has been developed of preventing or limiting formation of adhesions by administering to a site in need thereof, in the absence of or after bleeding or leakage of fluid has been substantially stopped, a self-assembling material which forms a barrier to formation of adhesions. In one embodiment, the self-assembling material comprises peptides having a sequence of amino acid residues conforming to one or more of Formulas I-IV: ((Xaa°′−Xaa+)x(Xaane1_Xaa−)y)n (I); ((Xaa′_Xaa)x(Xaa°e°−Xaa+)y)n (II); ((Xaa+−Xaa″e1)x(Xaa−Xaa″)y)n (III); and ((Xaa−XaaQe7)x(Xaa+Xaa1e″)y)n (IV), where Xaan′ represents an amino acid residue having a neutral charge; Xaa+ represents an amino acid residue having a positive charge; Xaa represents an amino acid residue having a negative charge; x and y are integers having a value of 1, 2 or 4, independently; and n is an integer having a value of 1-5. In another embodiment, the self assembling materials are peptidomimetics, nucleotidomimetics, di- and triblock copolymers, N-alkylacrylamides, or dendimers. These materials are also useful in a method for regeneration or repair of tissue or cells forming tissue.
Owner:ARCH BIOSURGERY
Method for reducing the immunogenicity of antibody variable domains
InactiveUS20020034765A1Altered immunogenicityLow immunogenicityHybrid immunoglobulinsBiological testingBound propertyVariable domain
A unique method is disclosed for identifying and replacing immunoglobulin surface amino acid residues which converts the antigenicity of a first mammalian species to that of a second mammalian species. The method will simultaneously change immunogenicity and strictly preserve ligind binding properties. The judicious replacement of exterior amino acid residues has no effect on the ligind binding properties but greatly alters immunogenicity.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com