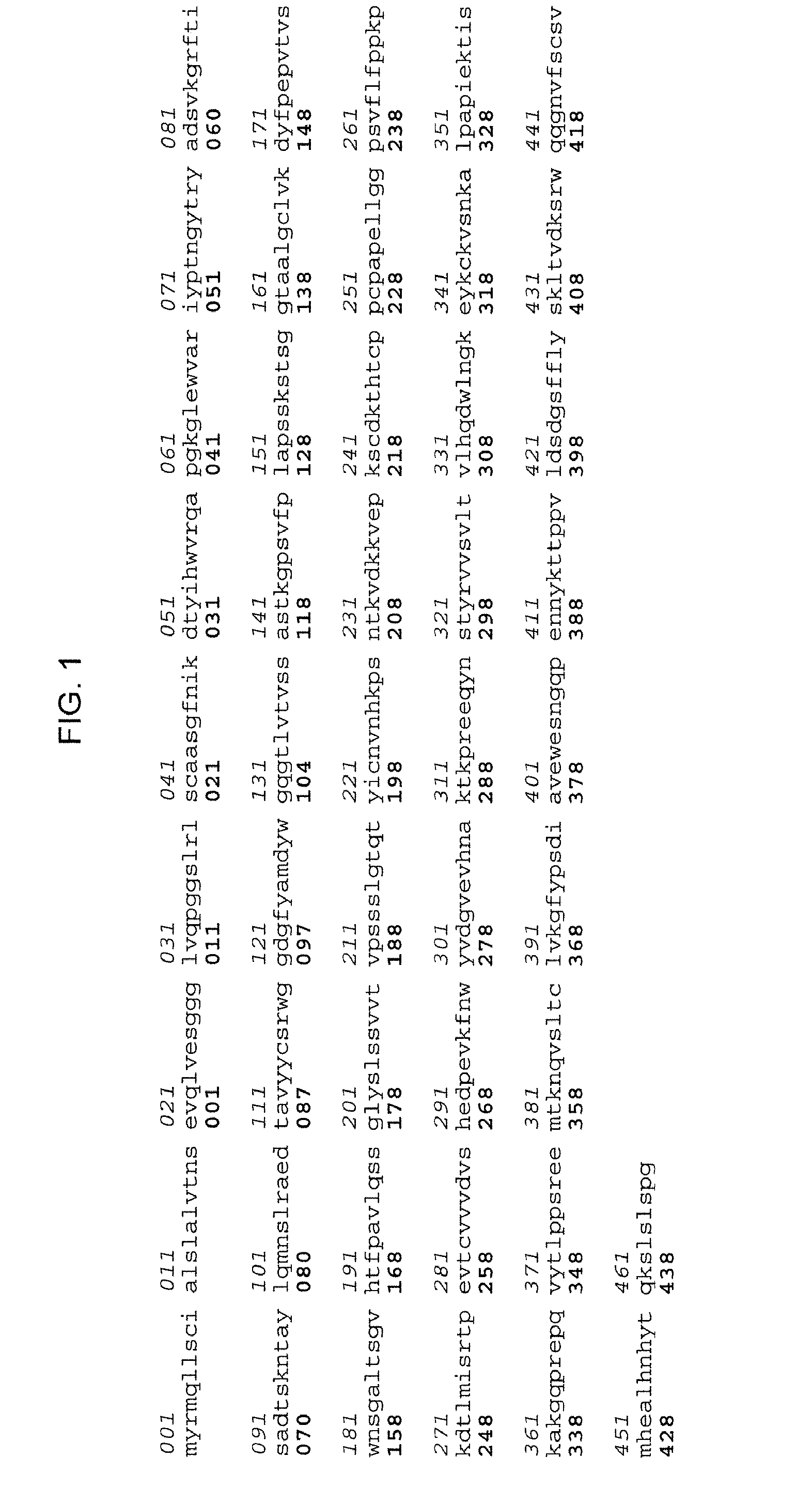
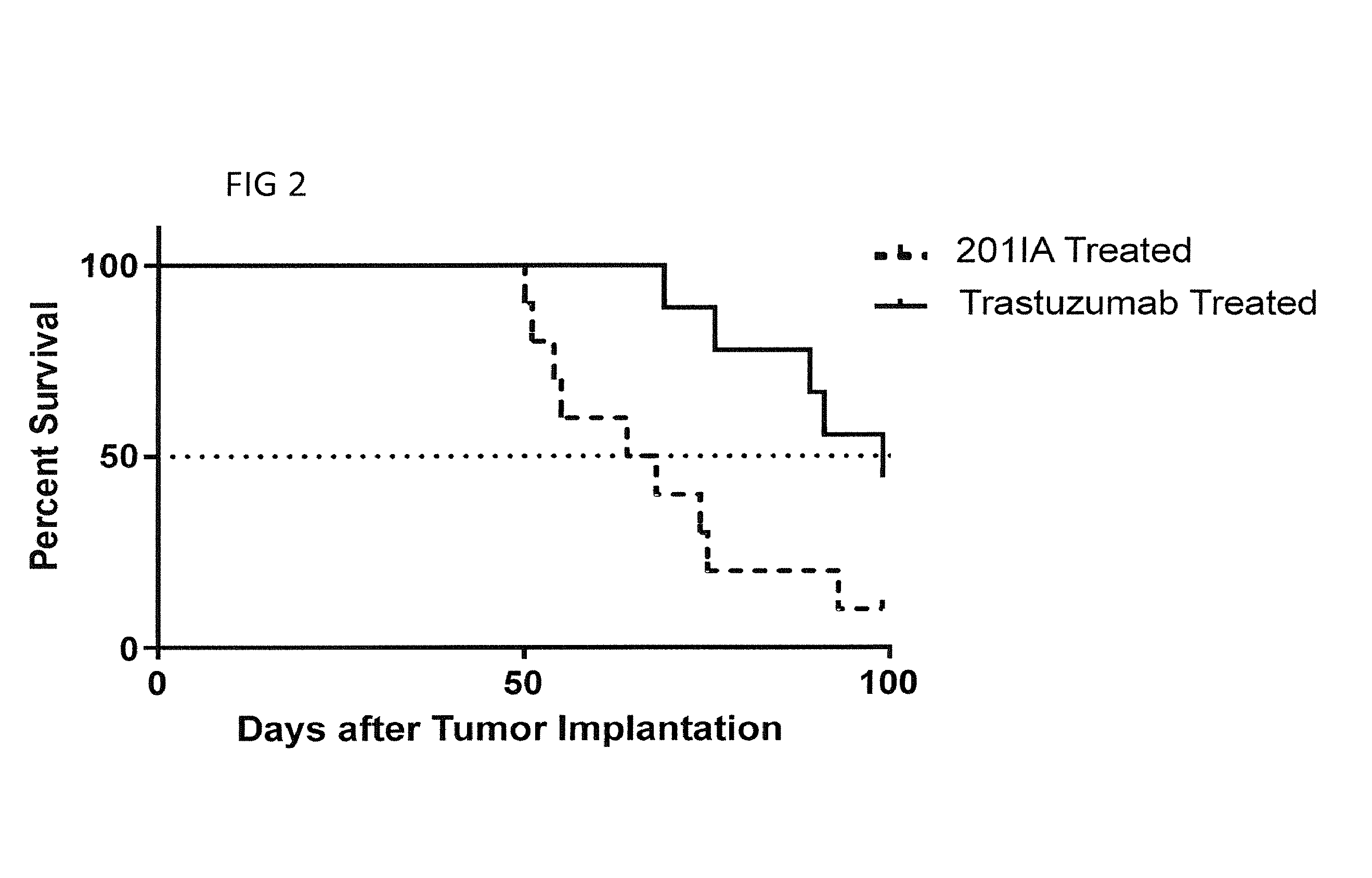
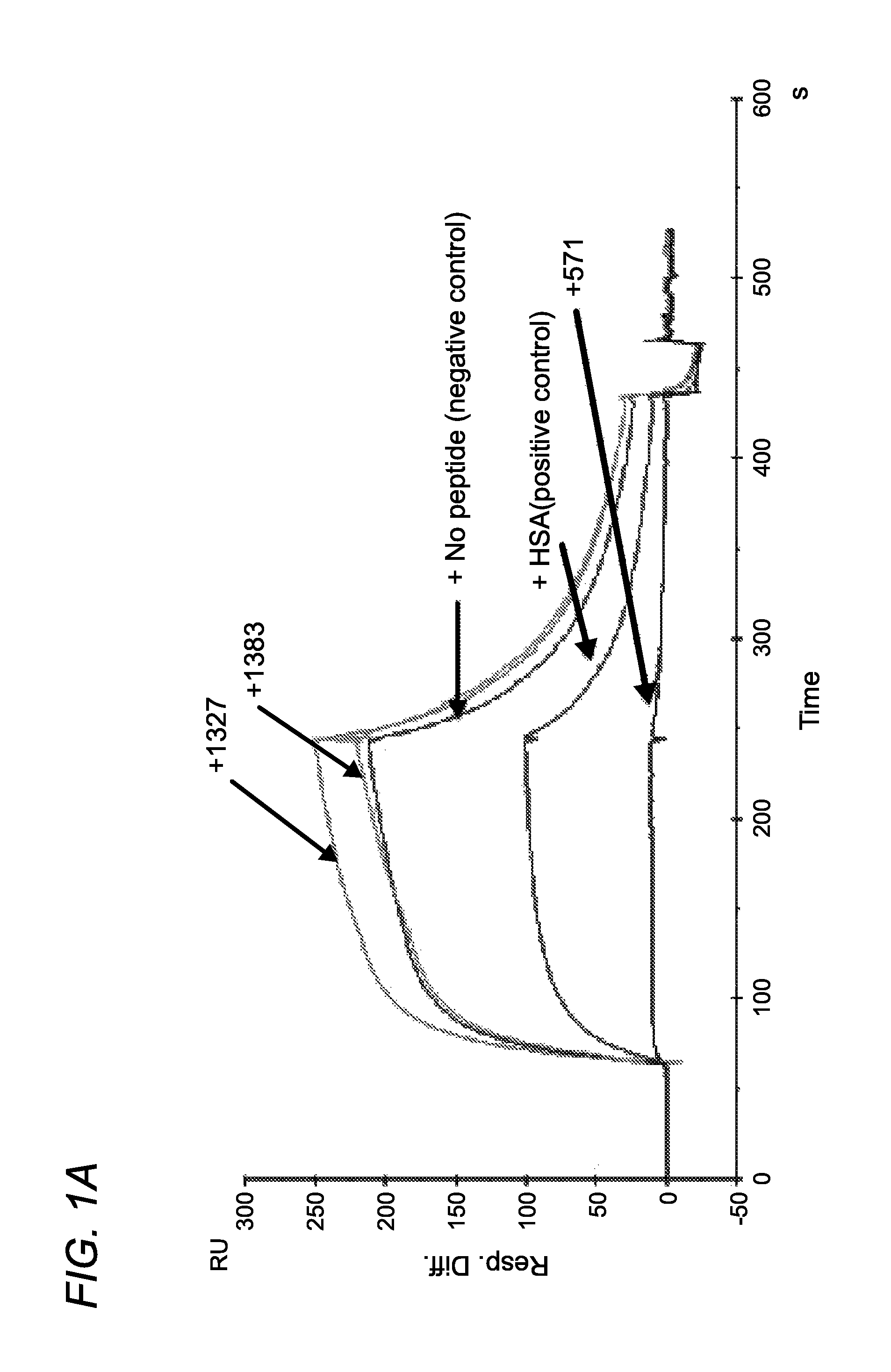
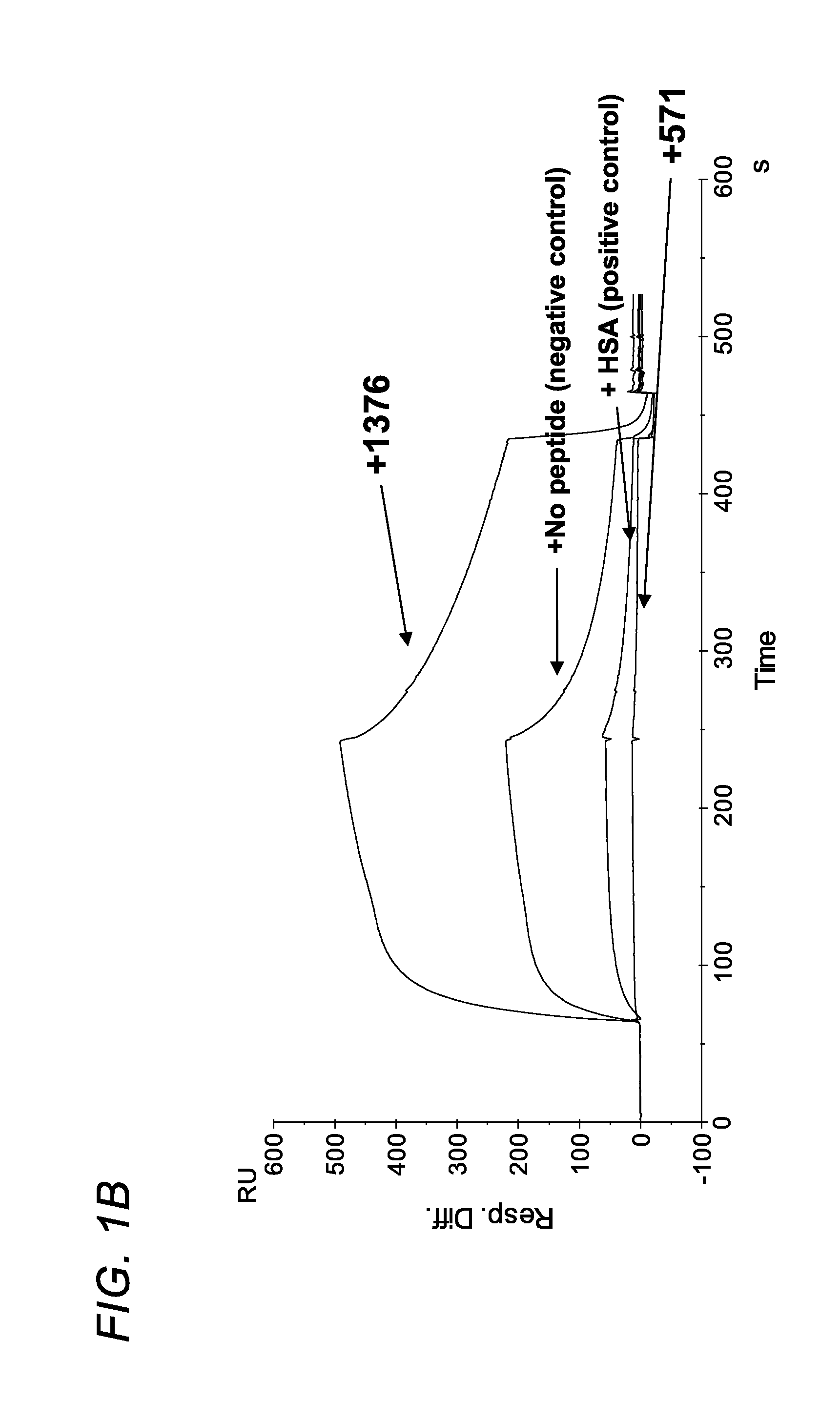
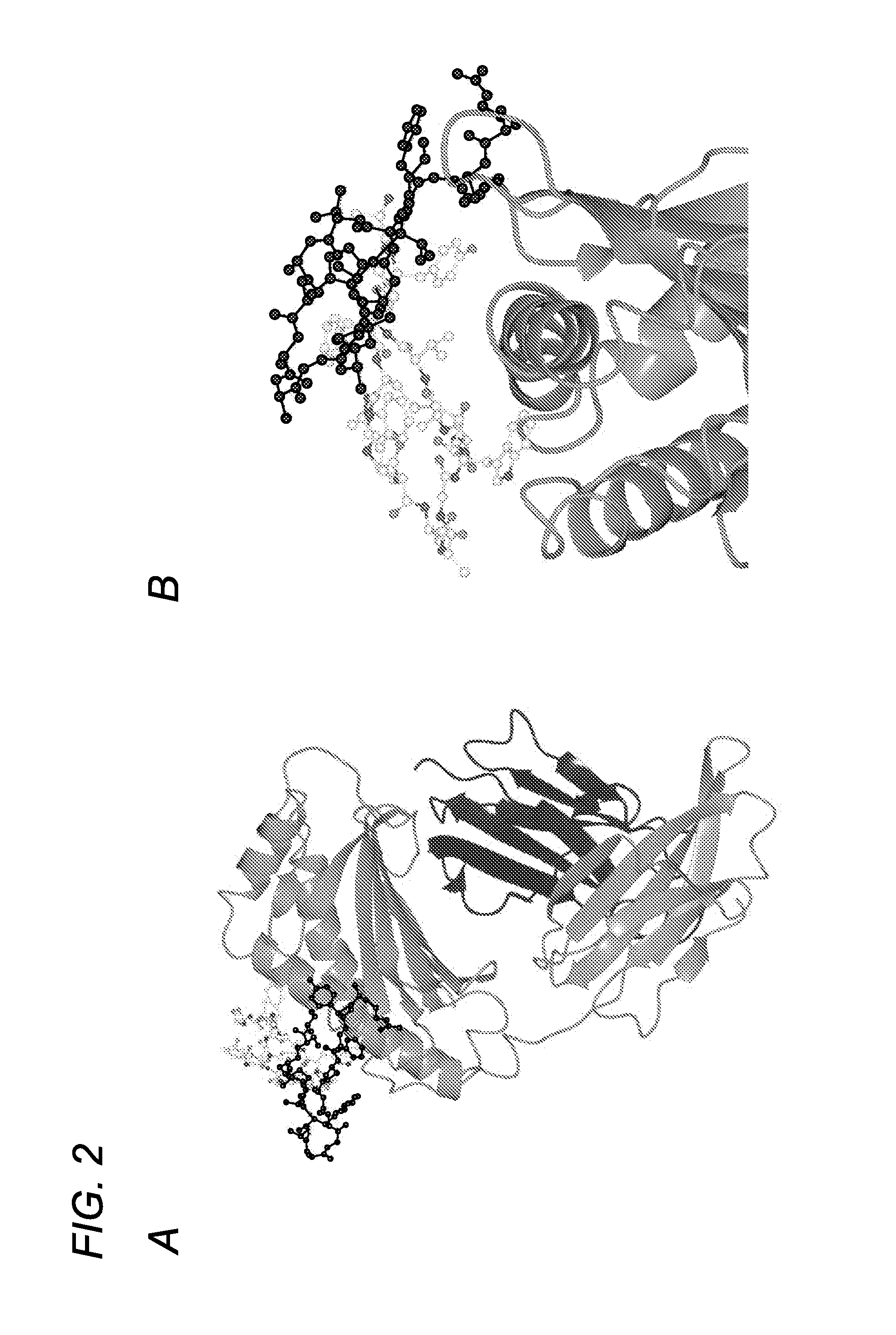
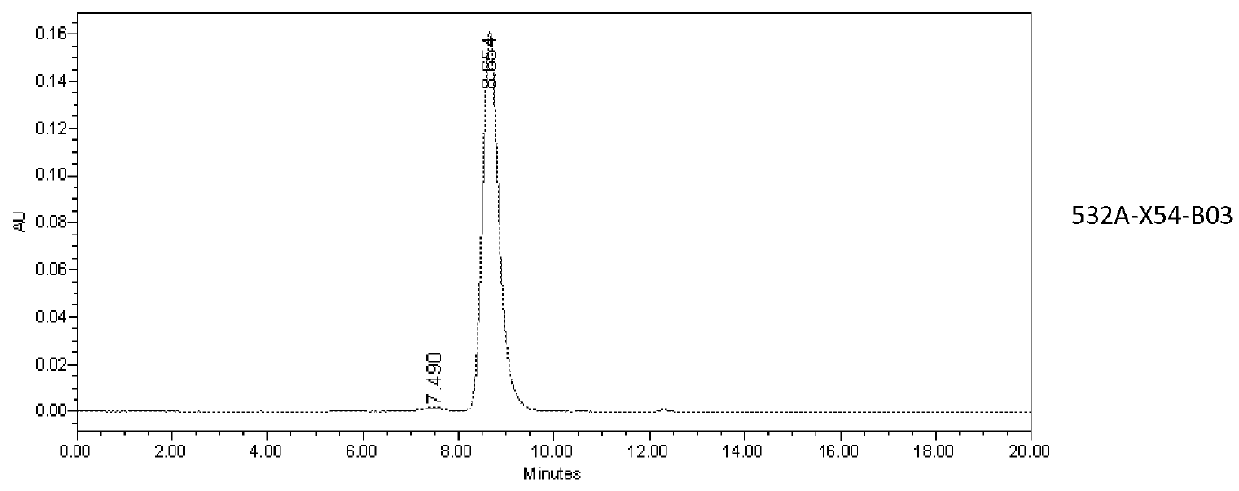
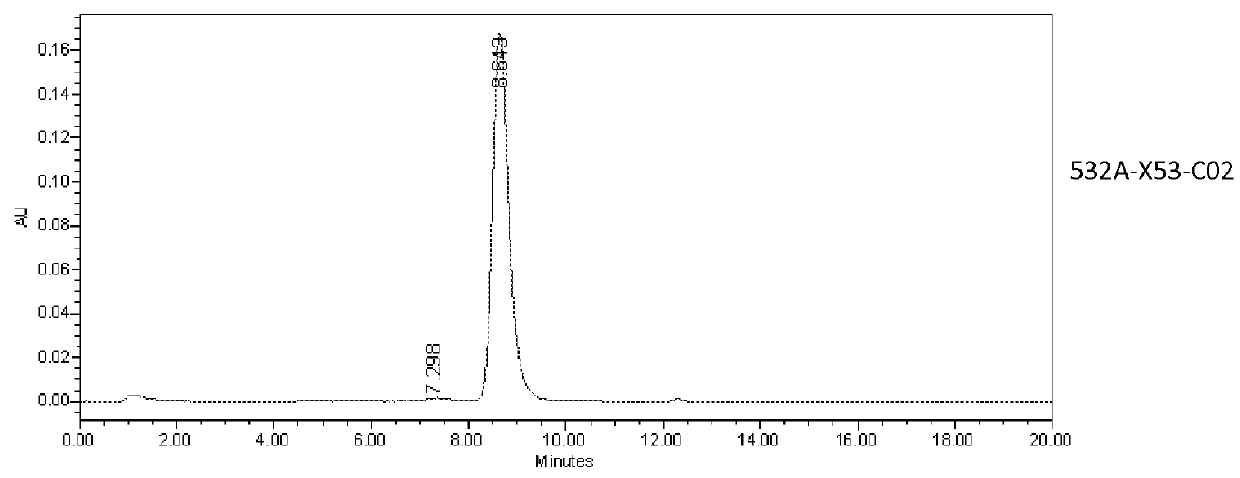
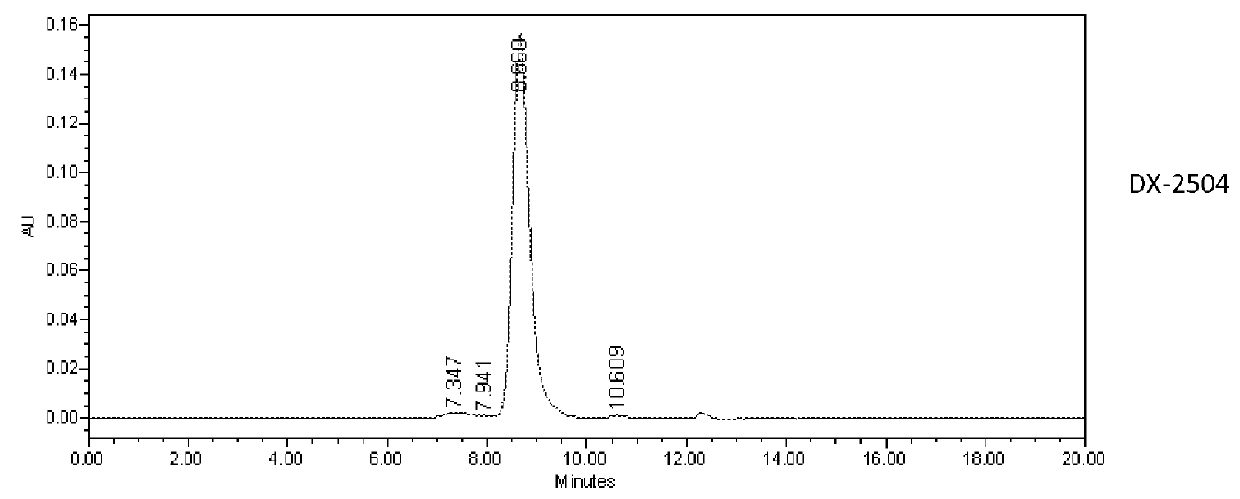
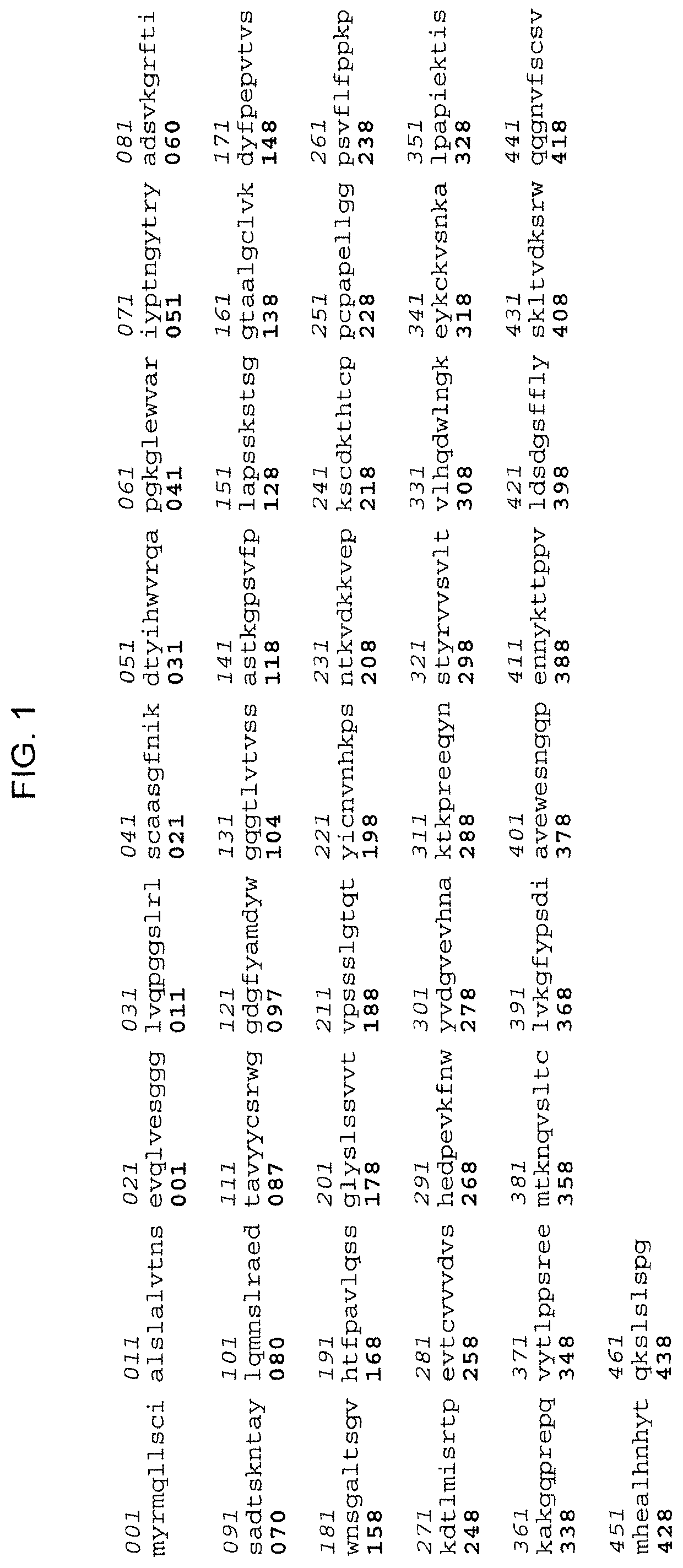
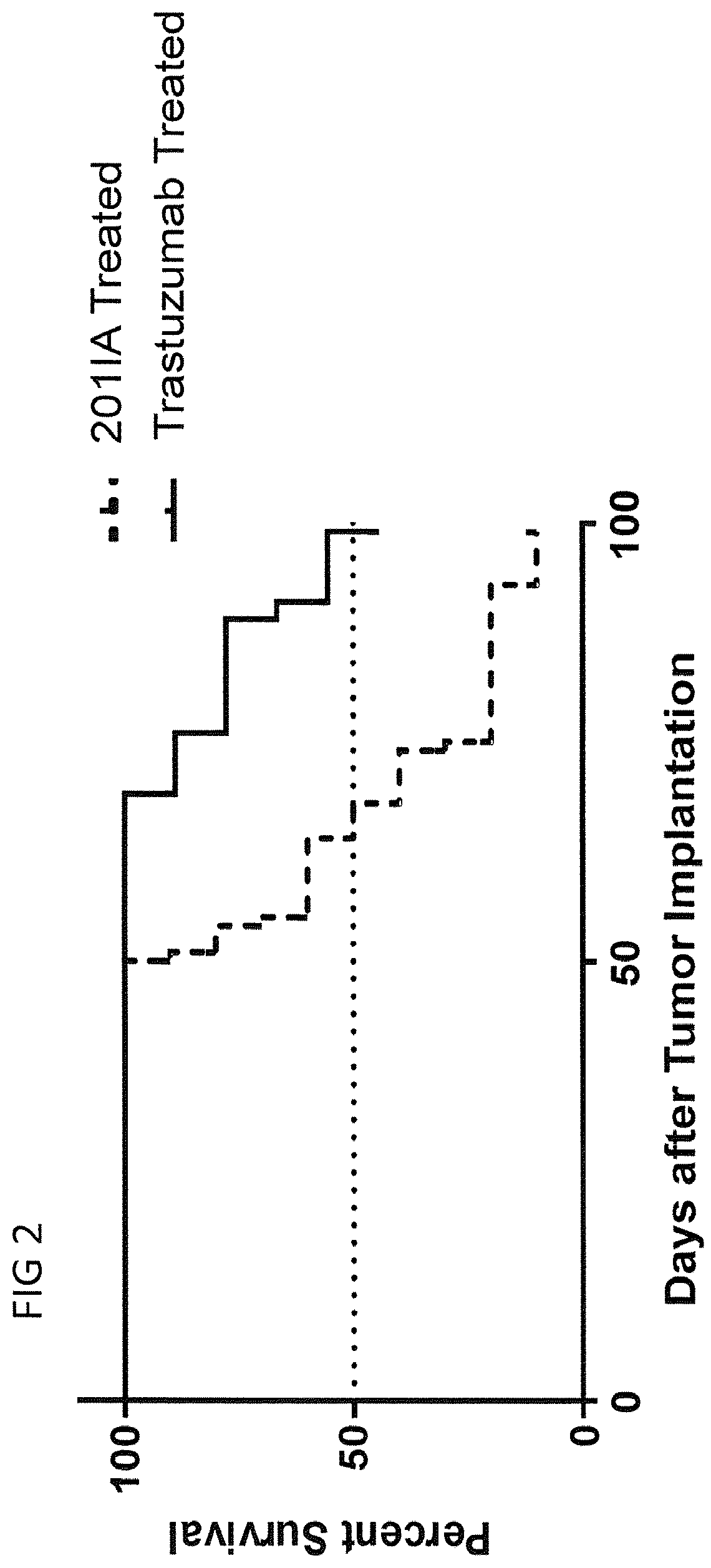
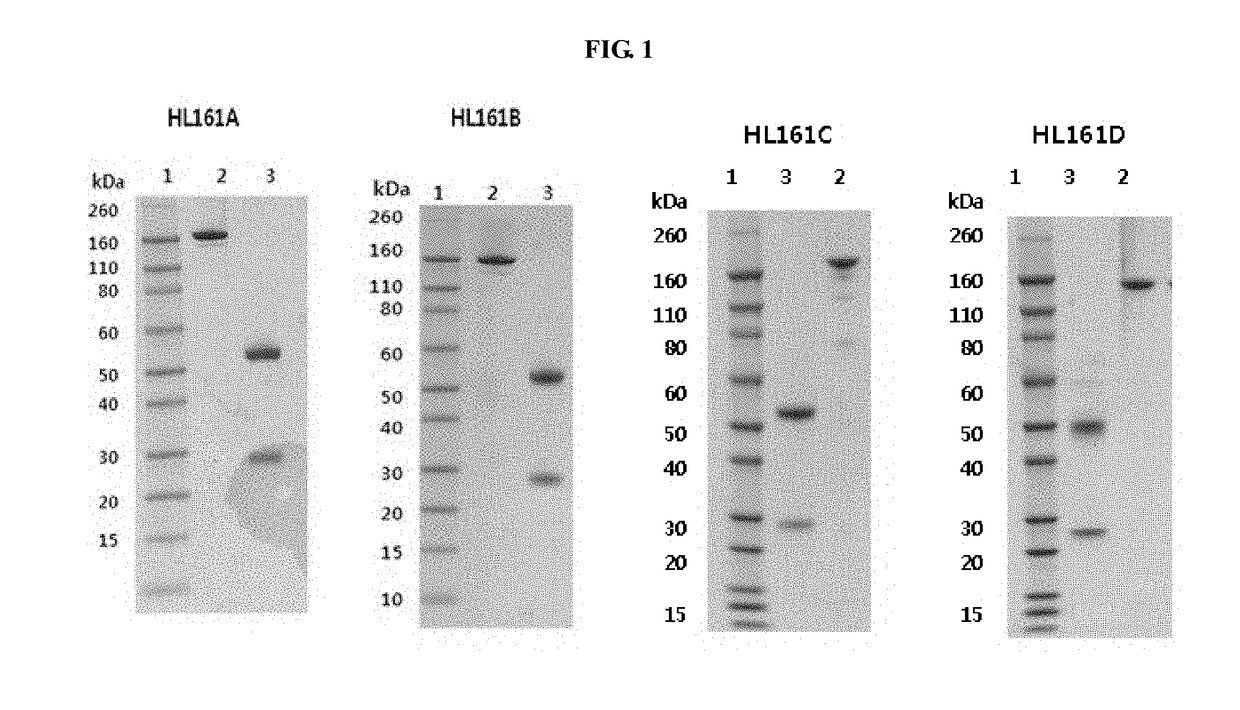
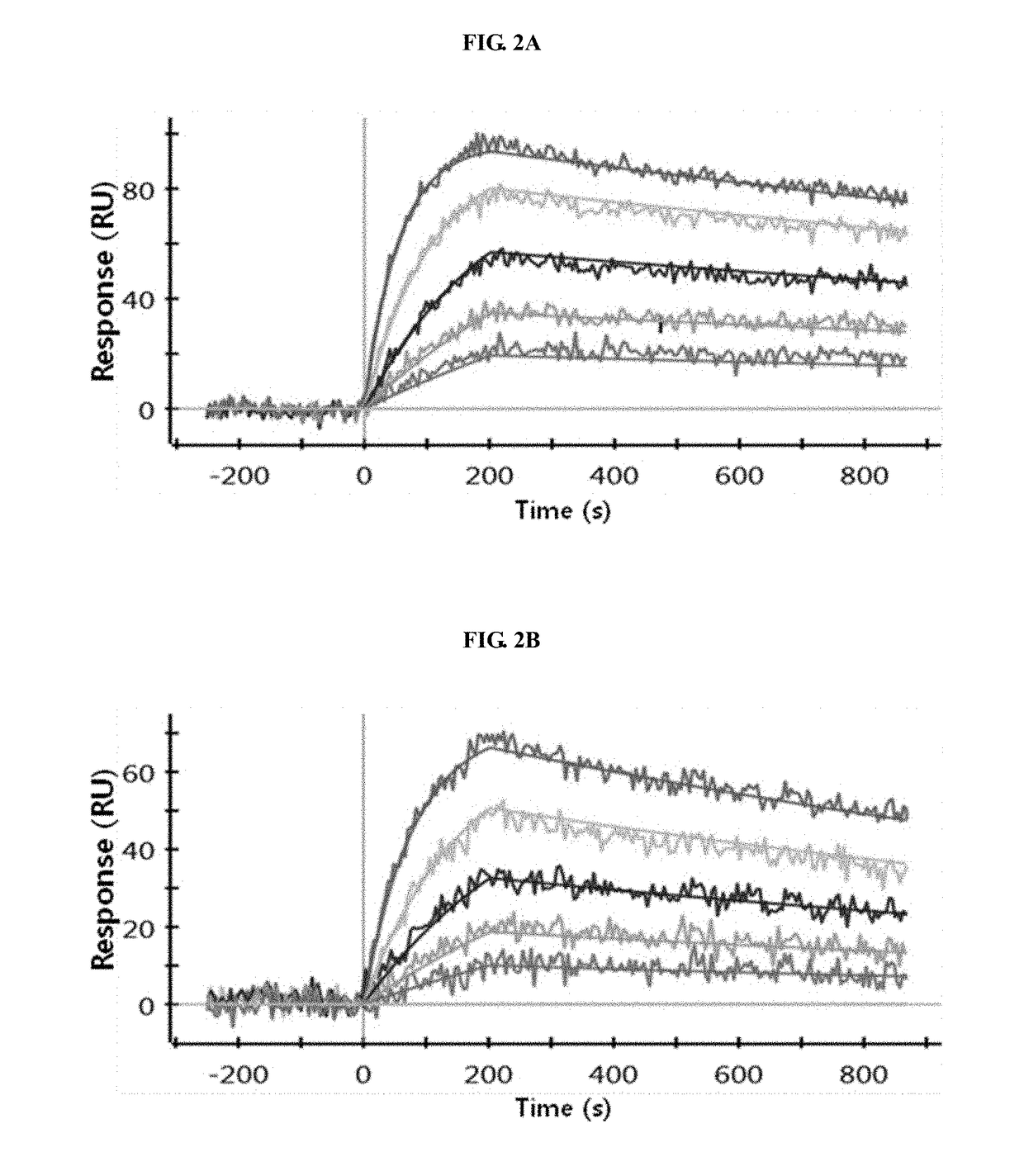
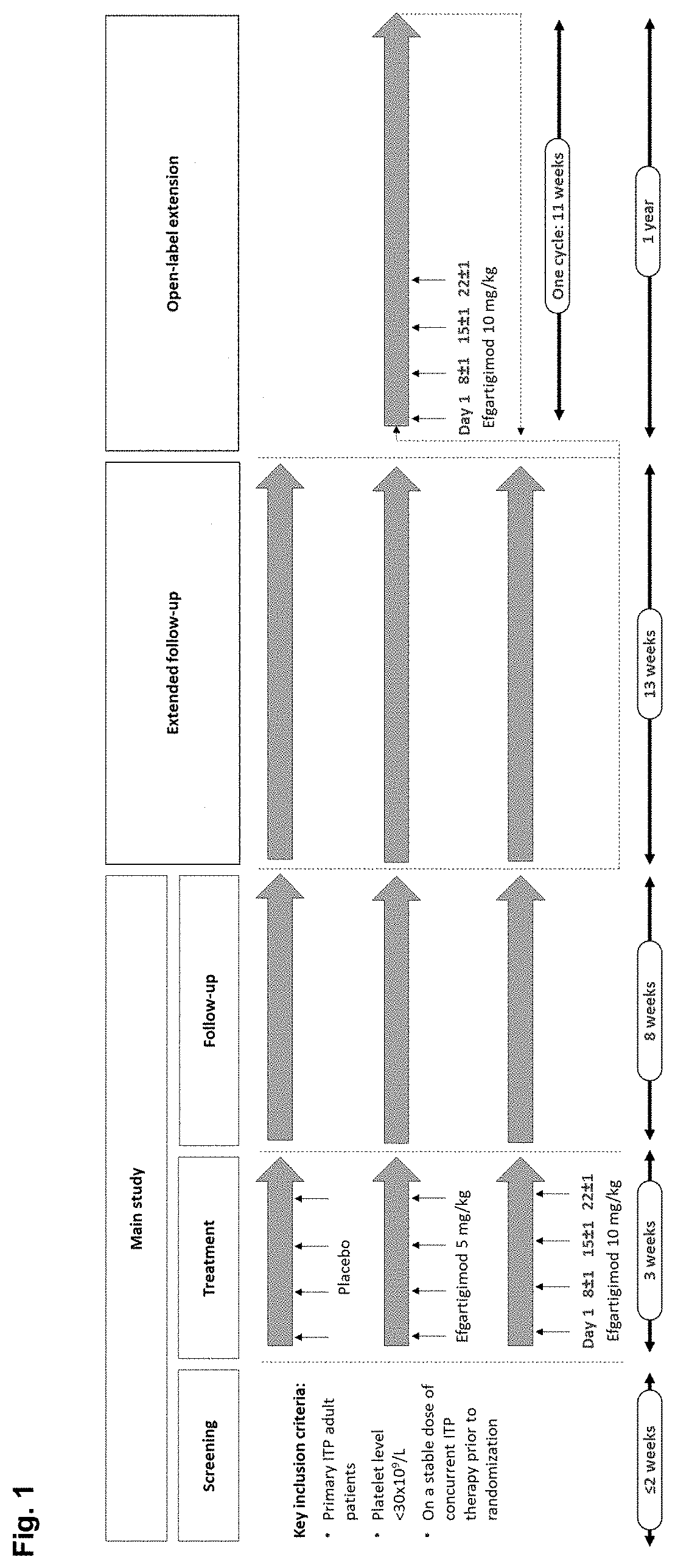
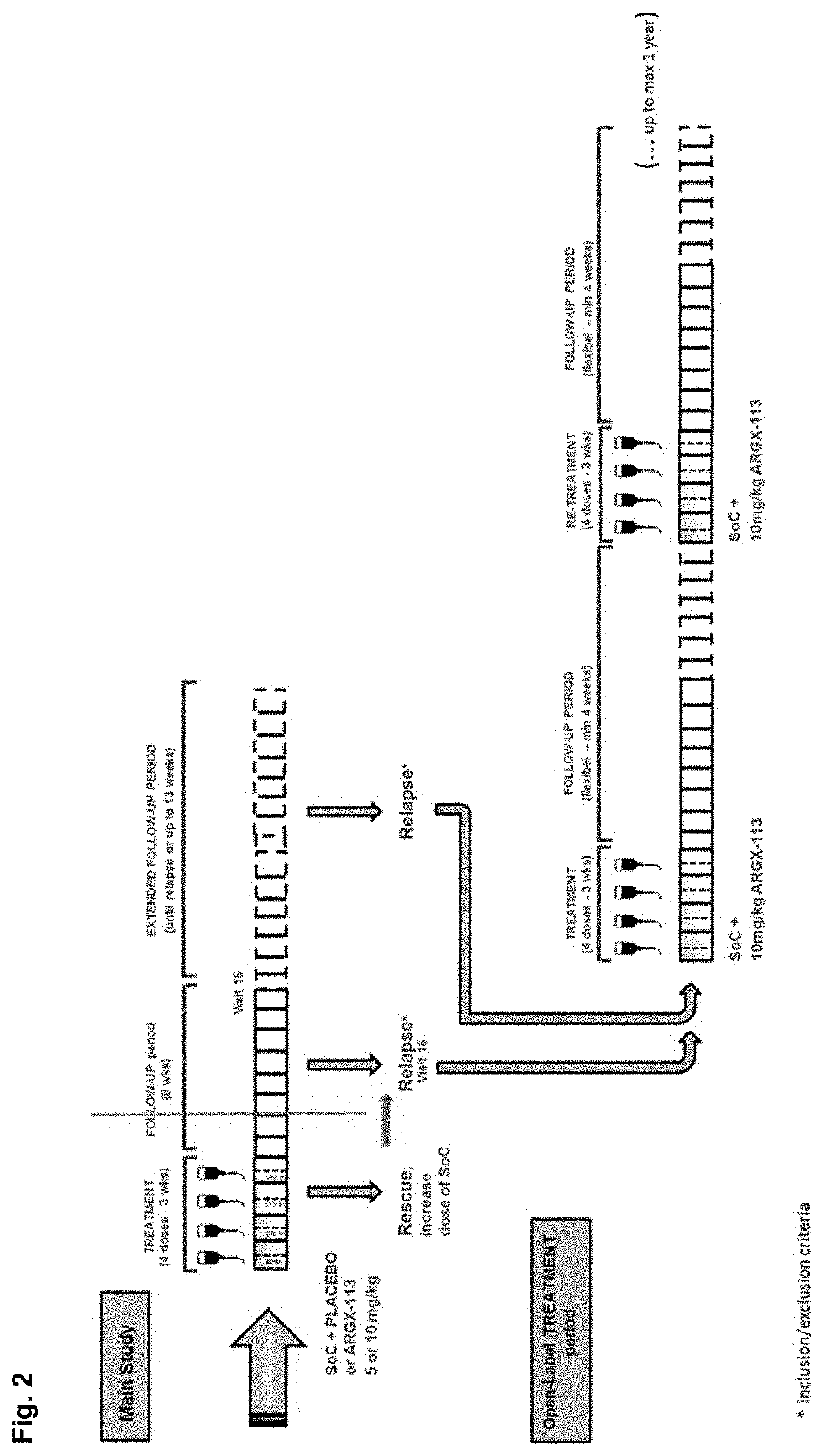
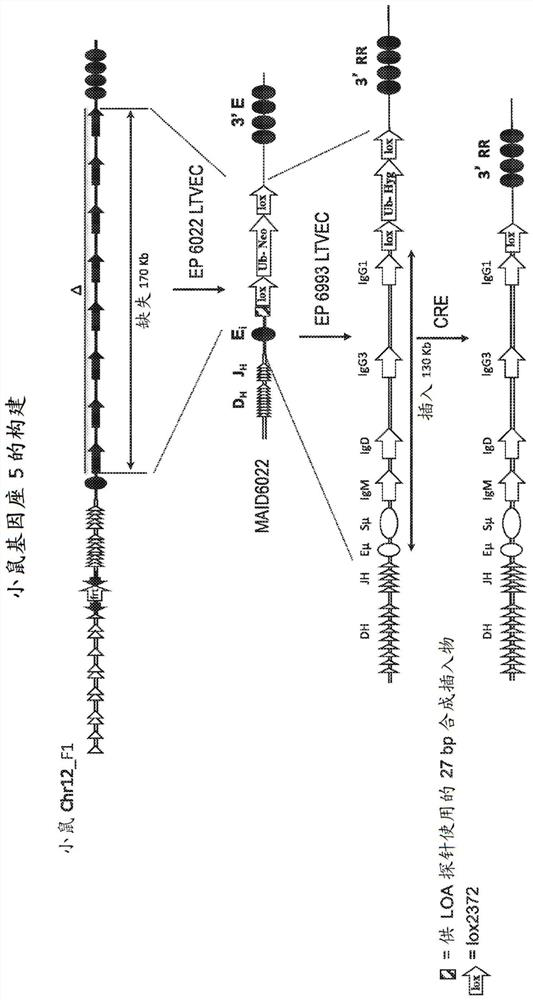
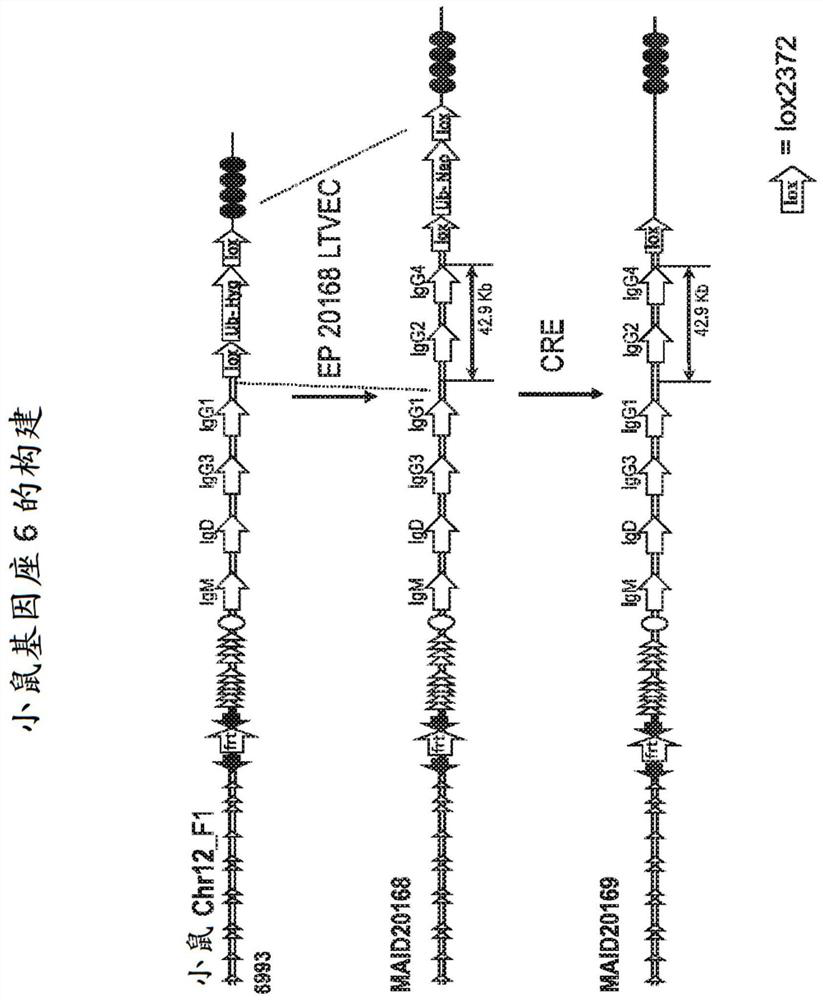
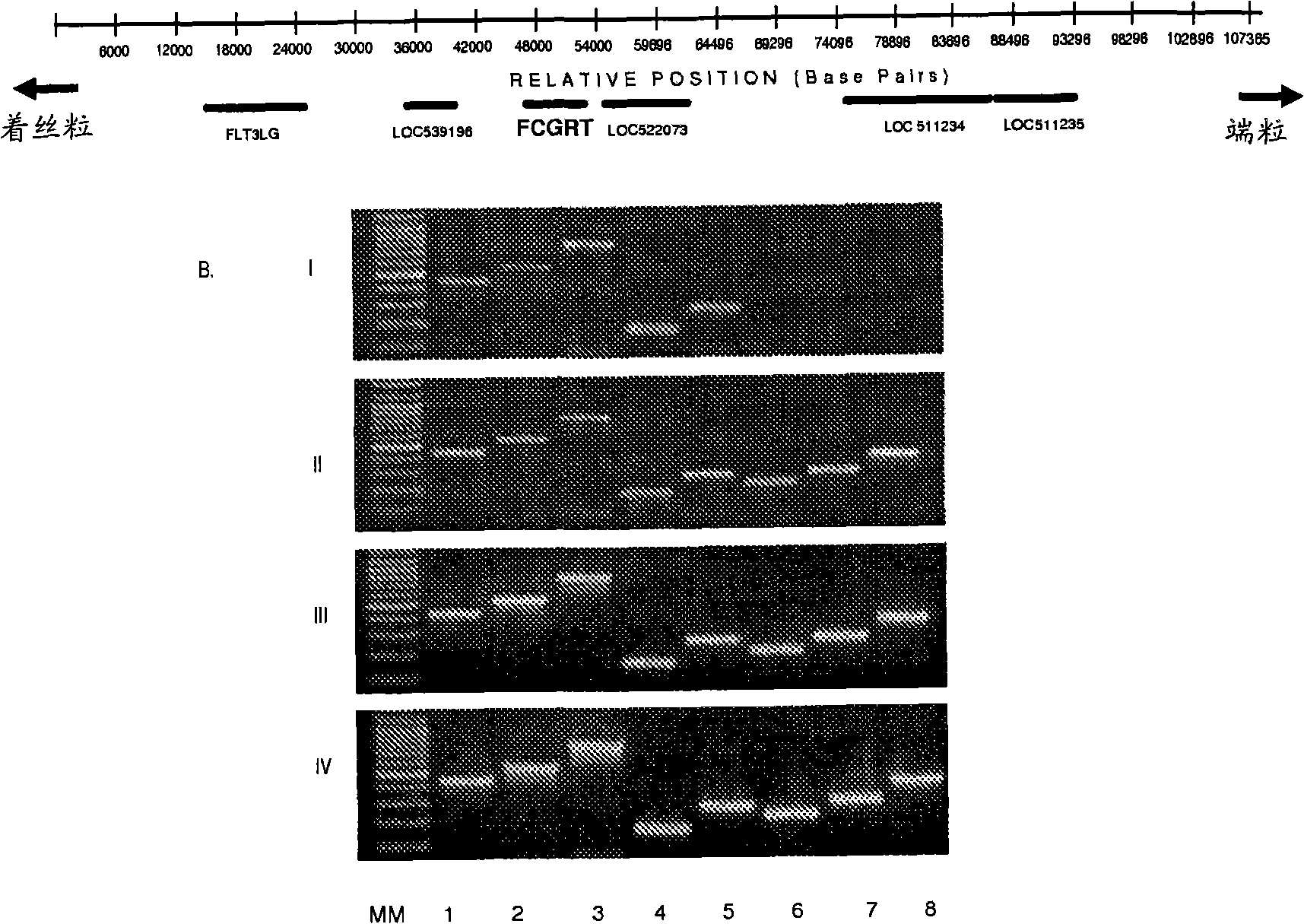
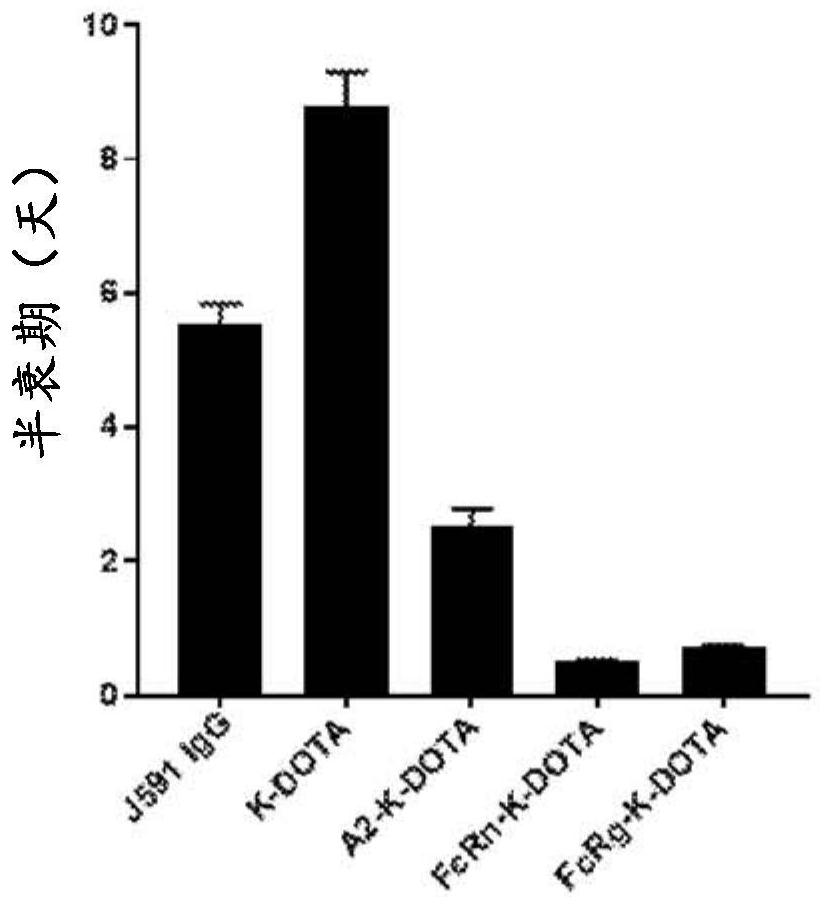
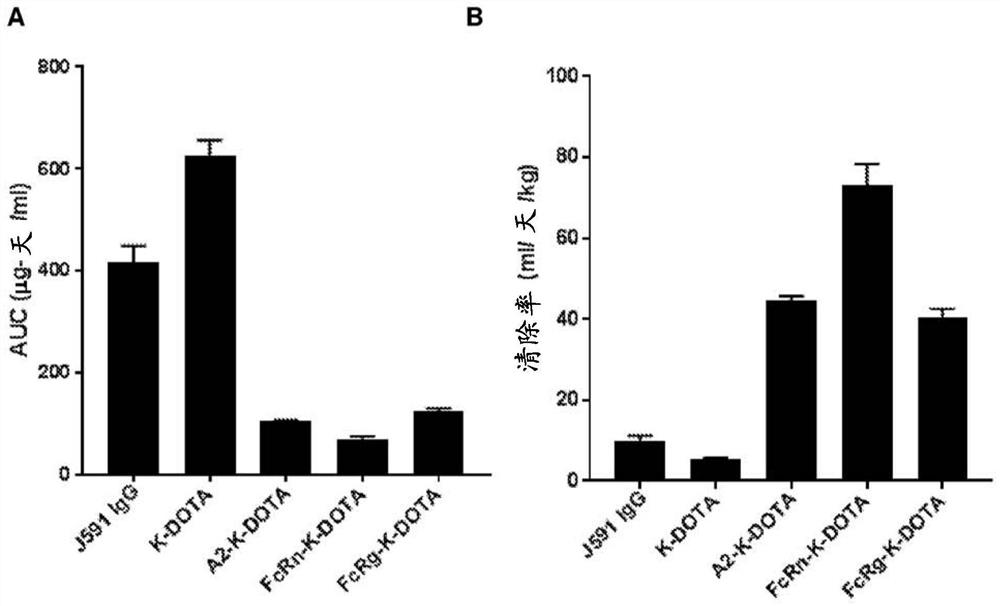
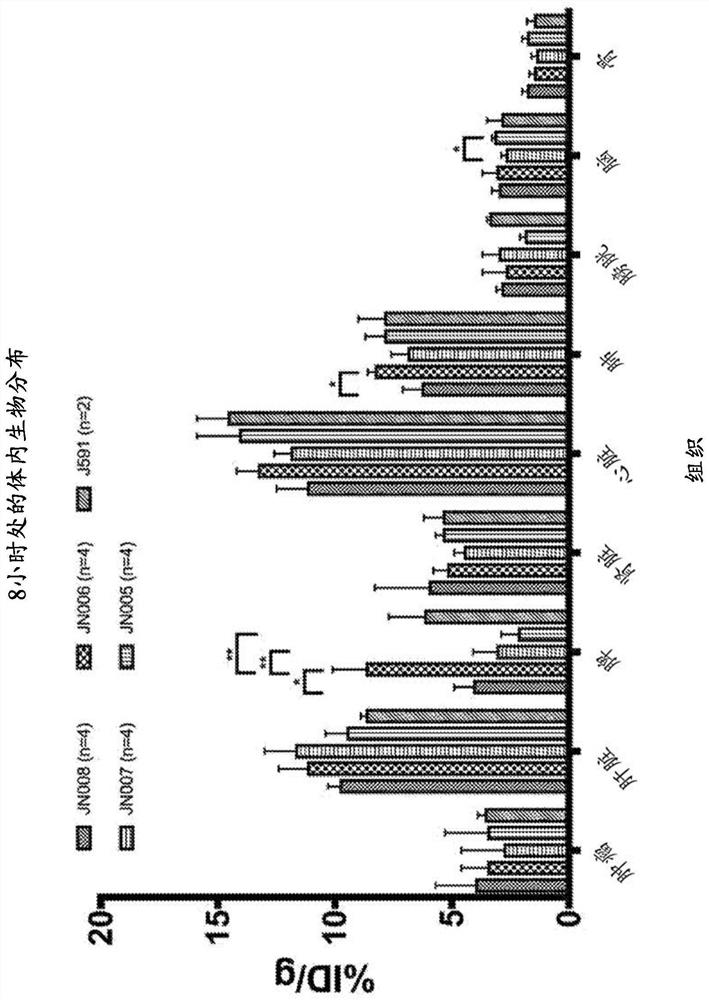
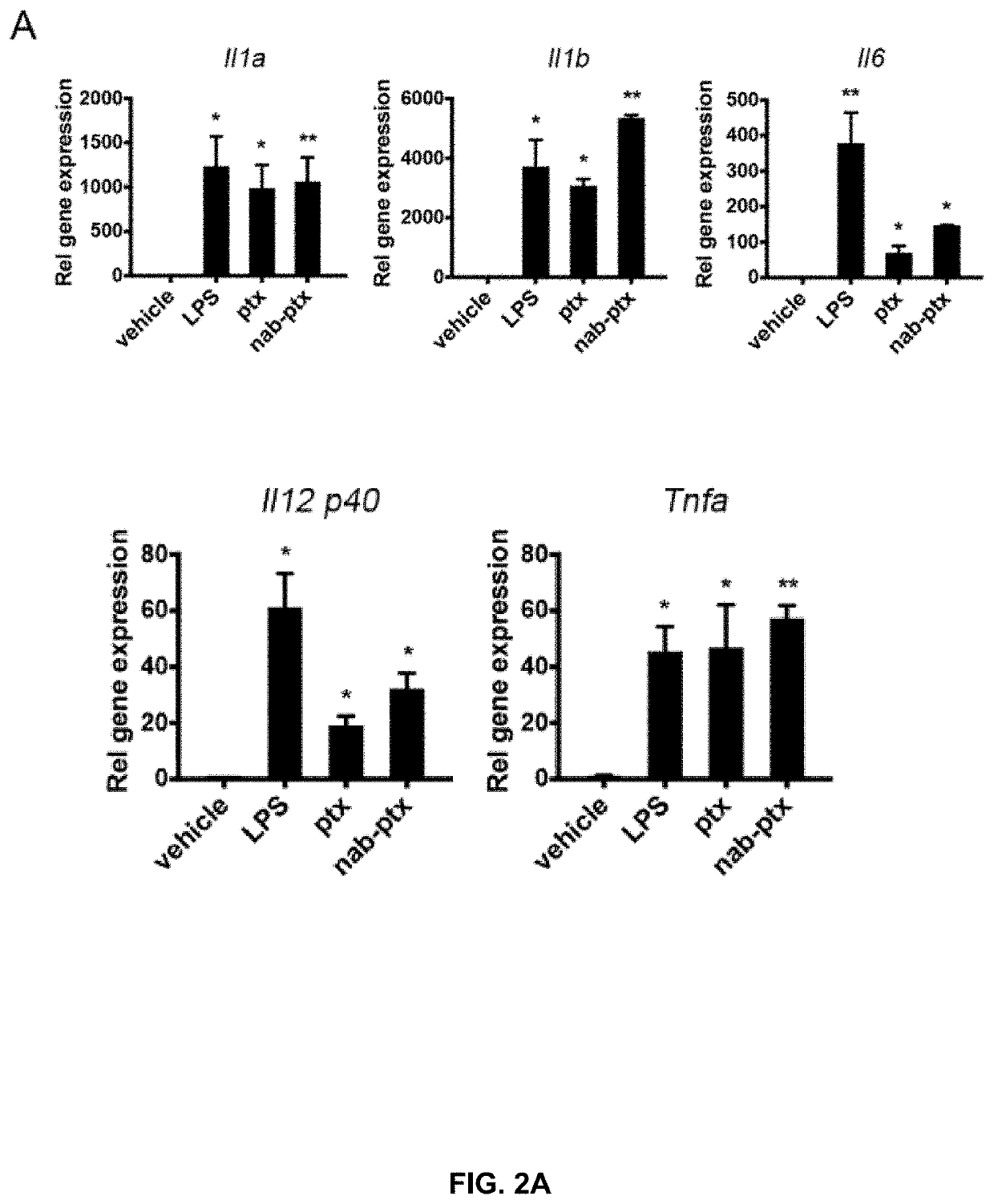
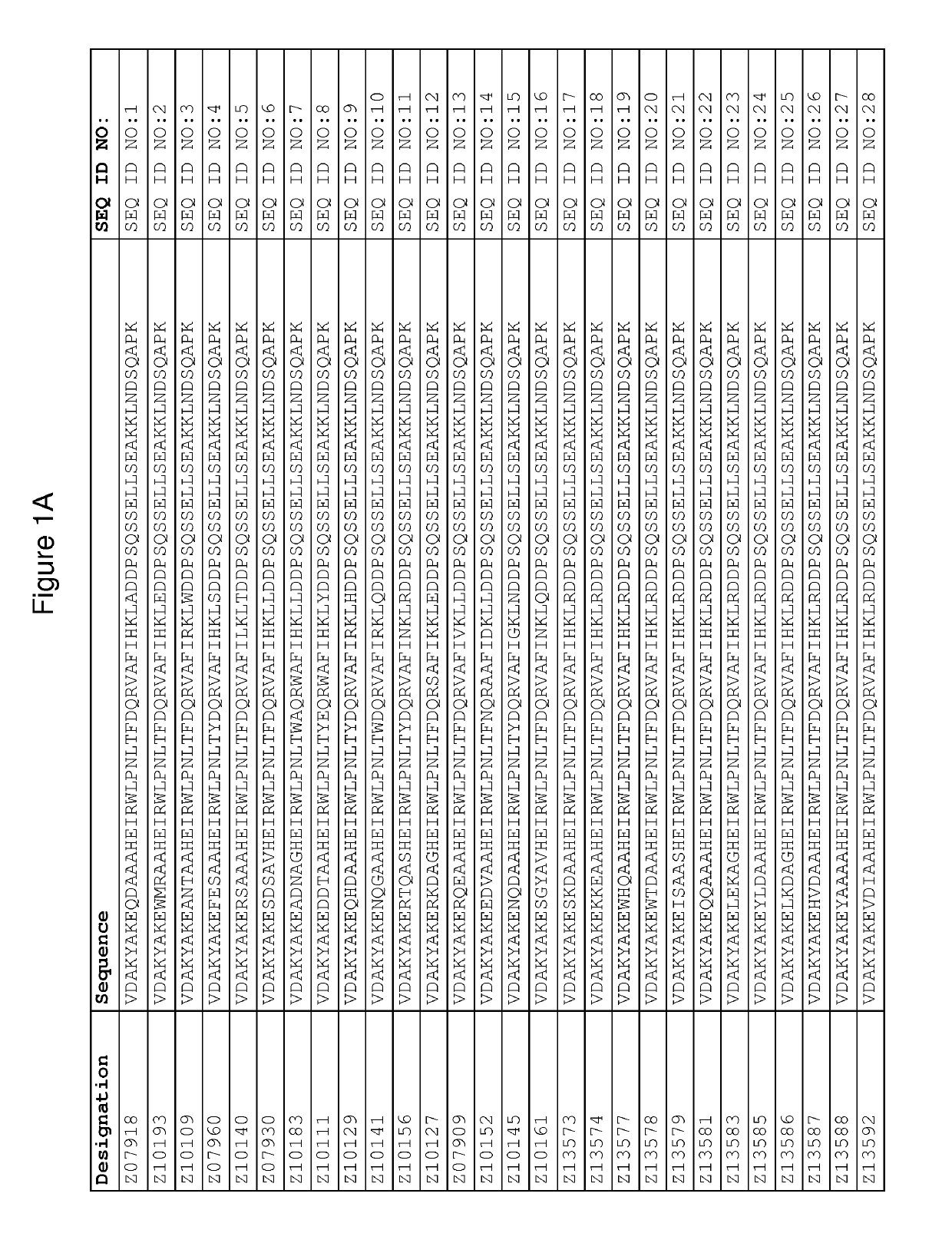
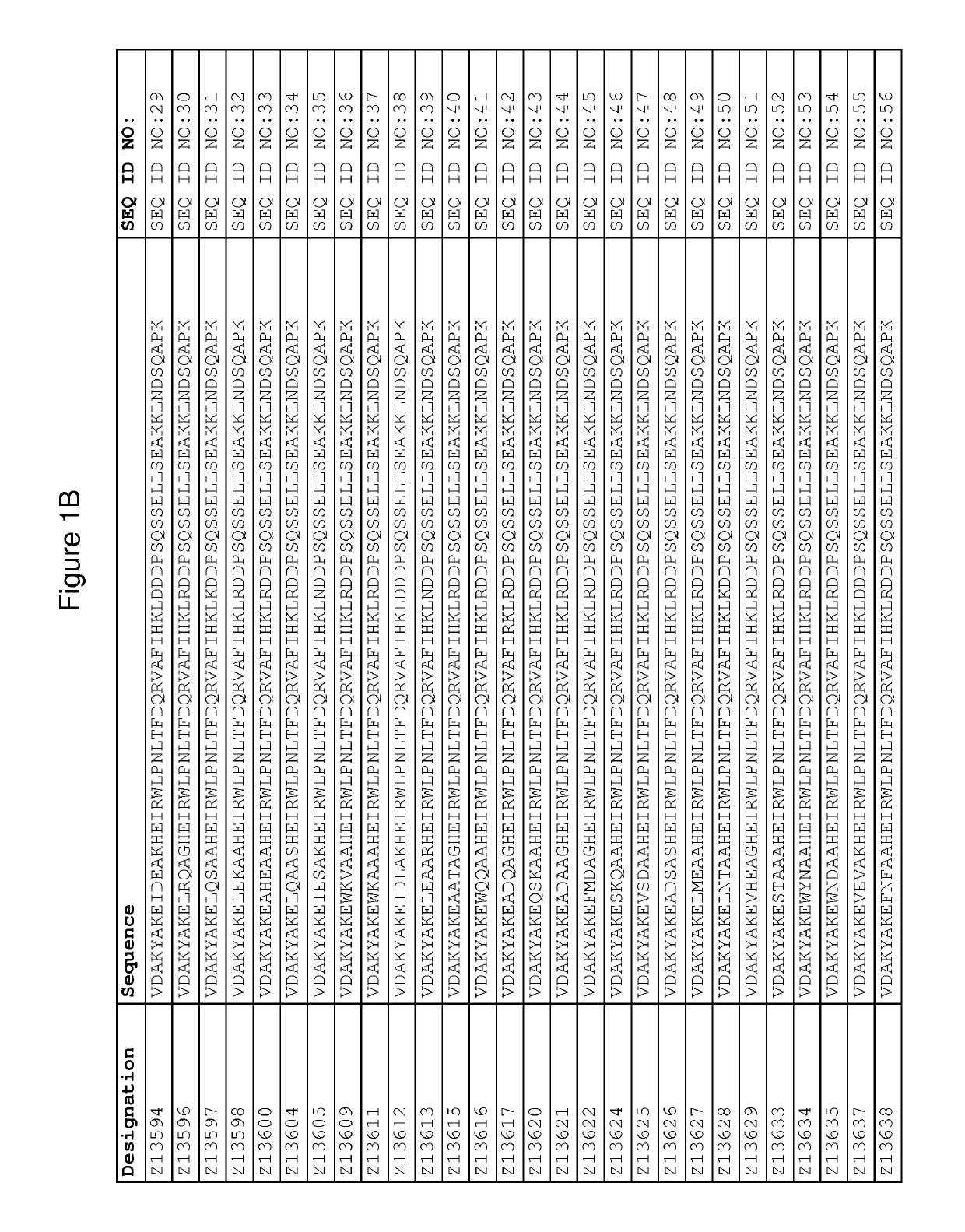
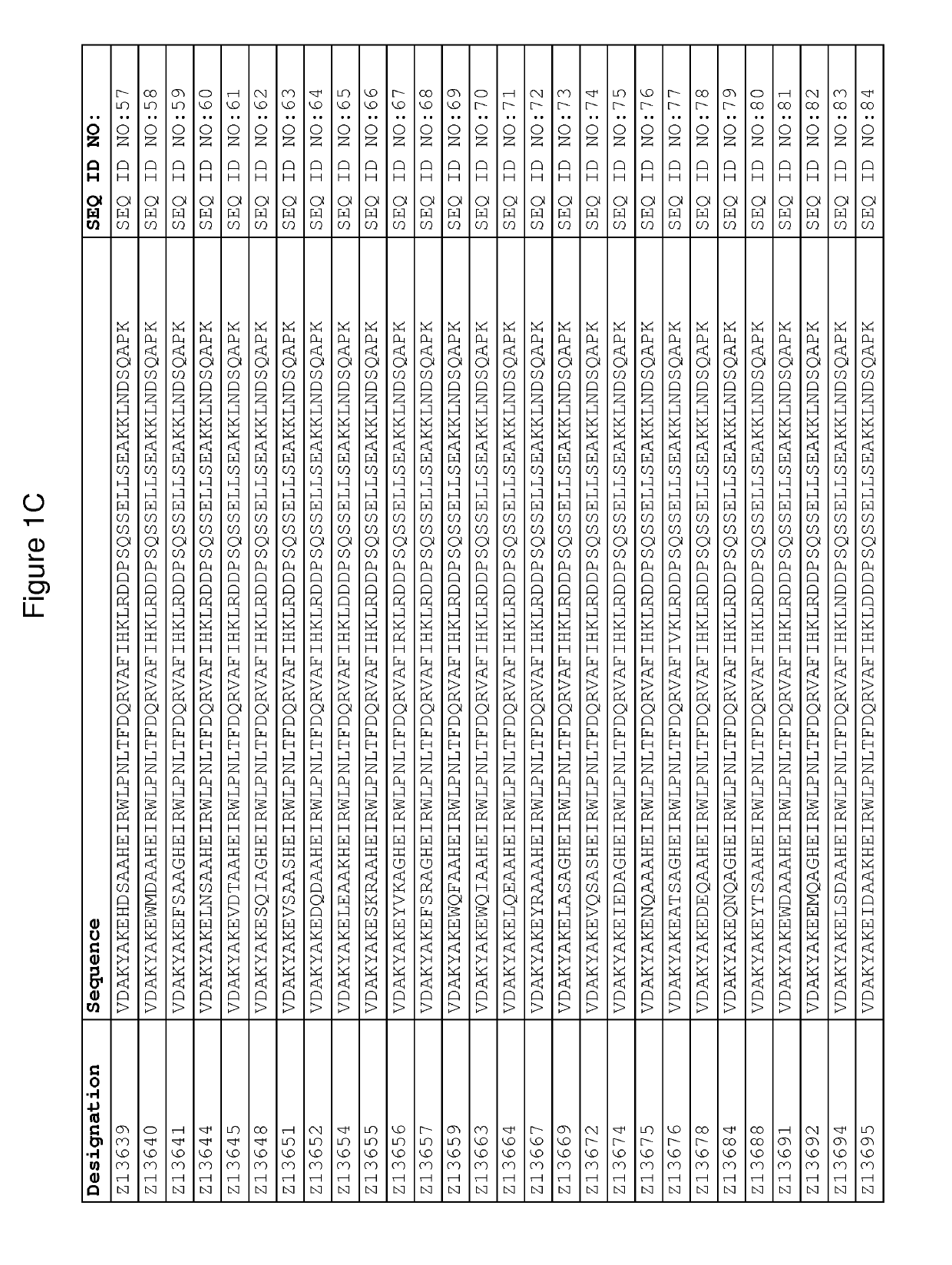
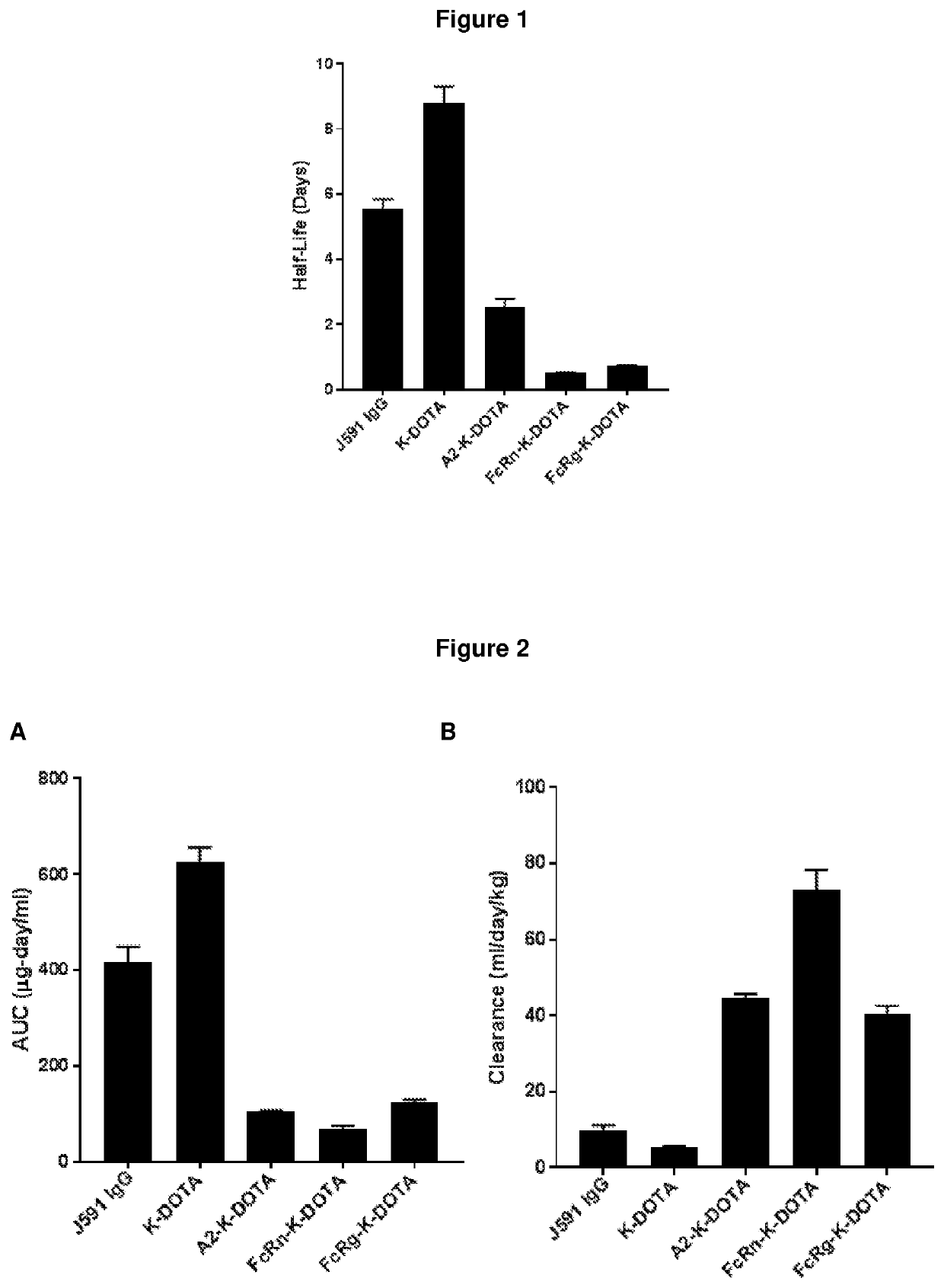
Patents
Literature
35 results about "Neonatal Fc receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The neonatal Fc receptor (FcRn), also known as the Brambell receptor, is a protein that in humans is encoded by the FCGRT gene. The neonatal Fc receptor is an Fc receptor which is similar in structure to the MHC class I molecule and also associates with beta-2-microglobulin. Further studies revealed a similar receptor in humans, leading to the naming as a neonatal Fc receptor. In humans, however, it is found in the placenta to help facilitate transport of mother's IgG to the growing fetus. It has also been shown to play a role in monitoring IgG and serum albumin turnover. Neonatal Fc receptor expression is up-regulated by the proinflammatory cytokine, TNF-α, and down-regulated by IFN-γ.
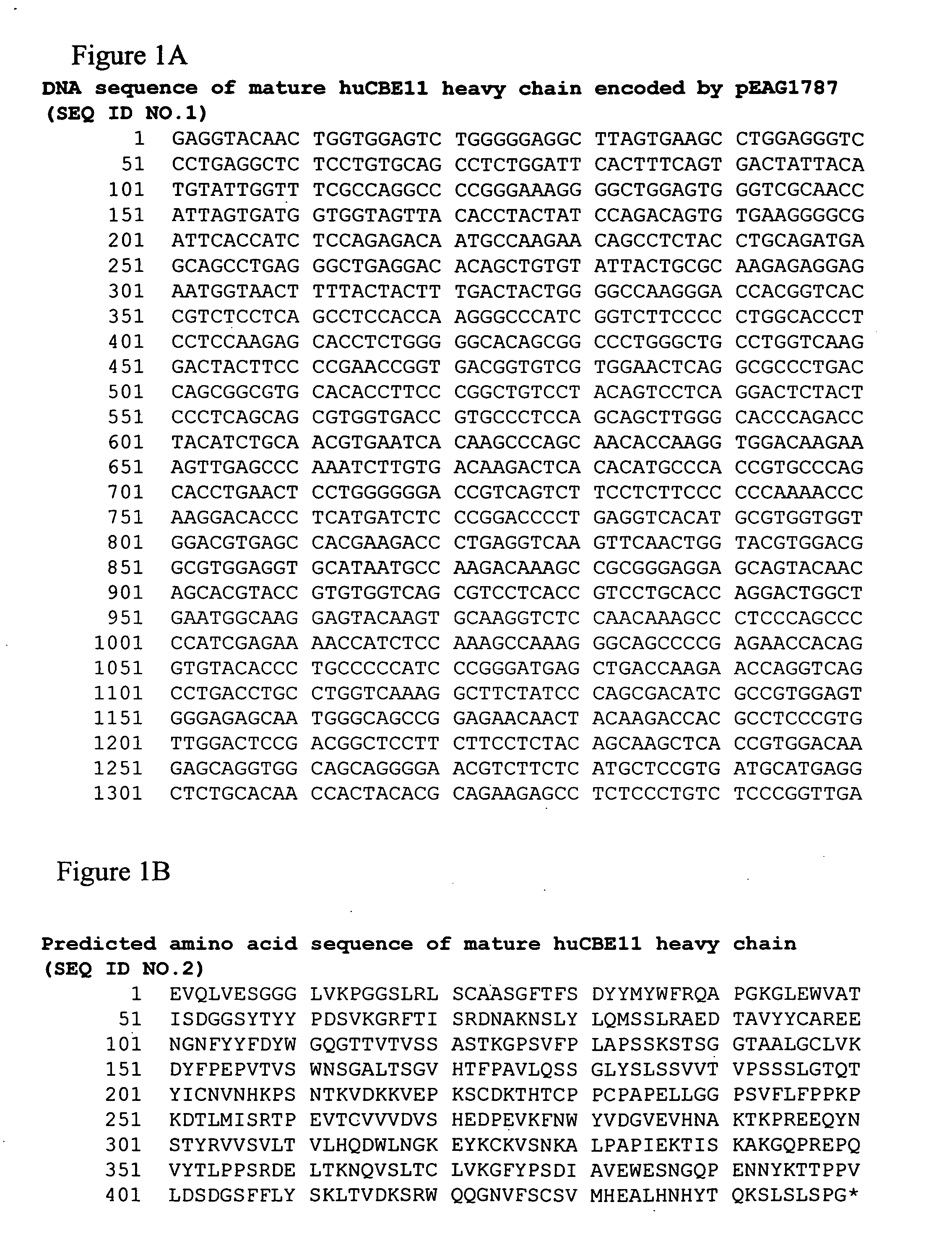
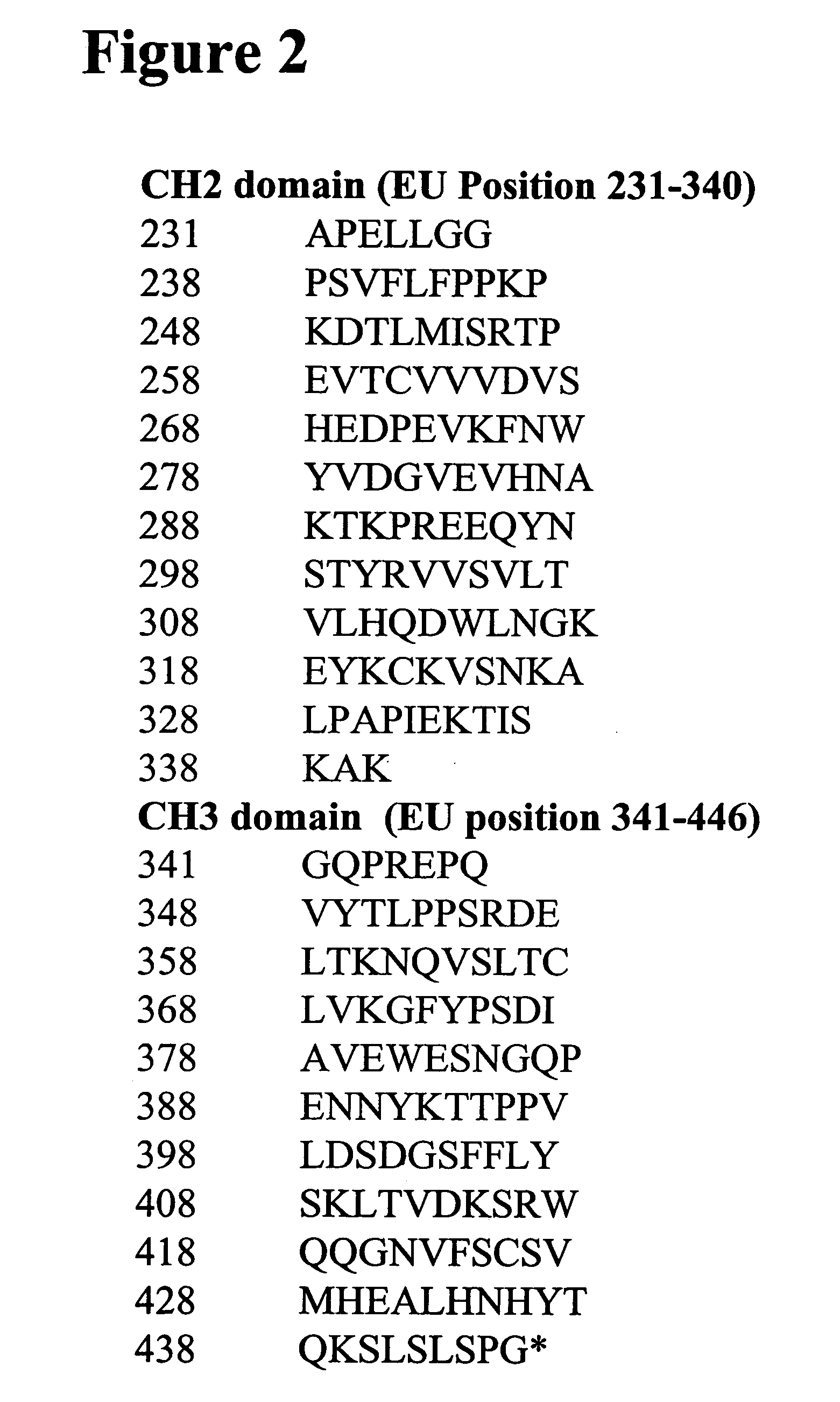
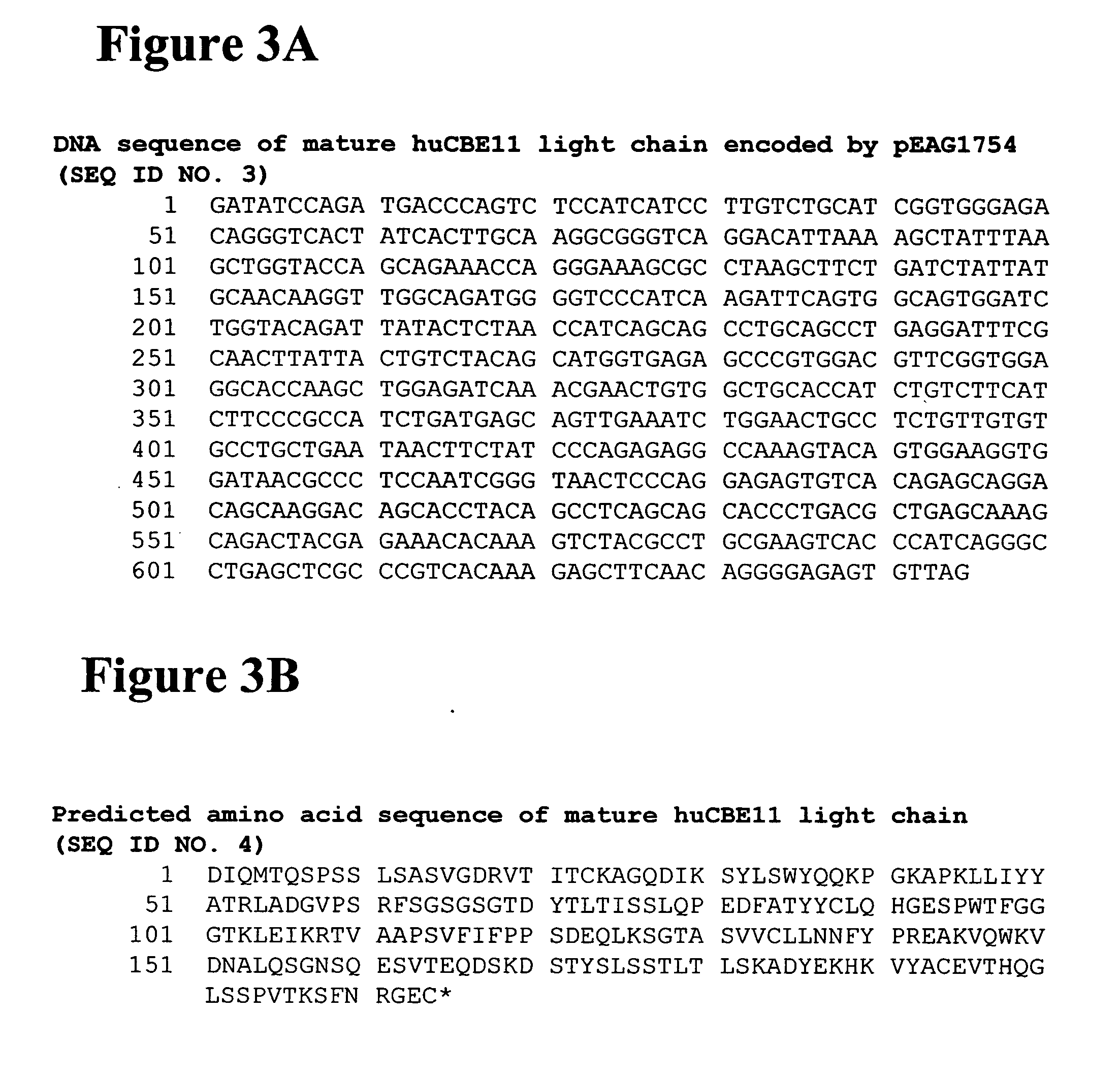
Neonatal Fc receptor (FcRn)-binding polypeptide variants, dimeric Fc binding proteins and methods related thereto
InactiveUS20070148164A1Increase free energyReduced binding affinityAnimal cellsSugar derivativesFc bindingNeonatal Fc receptor
The compositions and methods of the present invention are based, in part, on our discovery that an effector function mediated by an Fc-containing polypeptide can be altered by modifying one or more amino acid residues within the polypeptide (by, for example, electrostatic optimization). The polypeptides that can be generated according to the methods of the invention are highly variable, and they can include antibodies and fusion proteins that contain an Fc region or a biologically active portion thereof.
Owner:BIOGEN MA INC
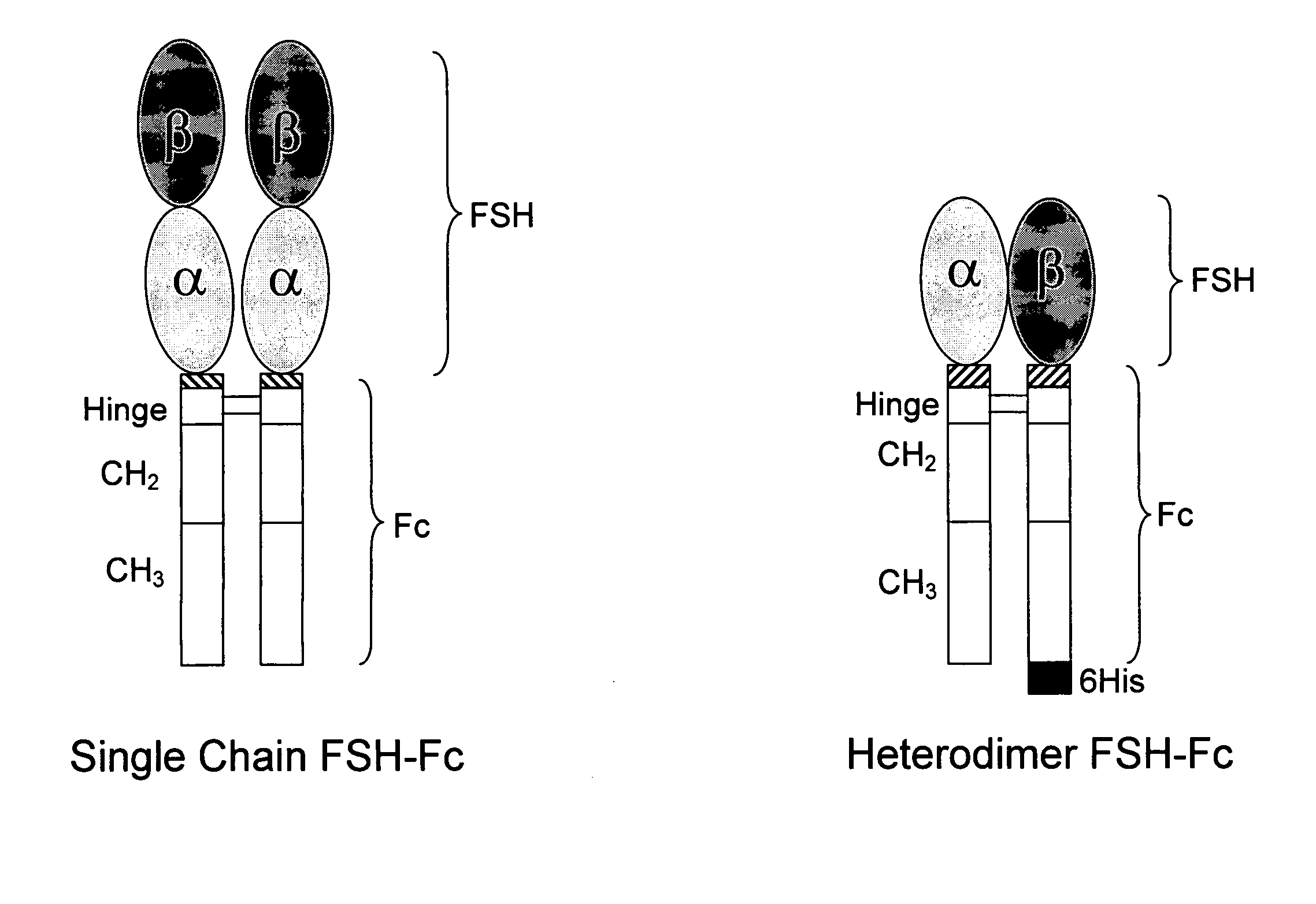
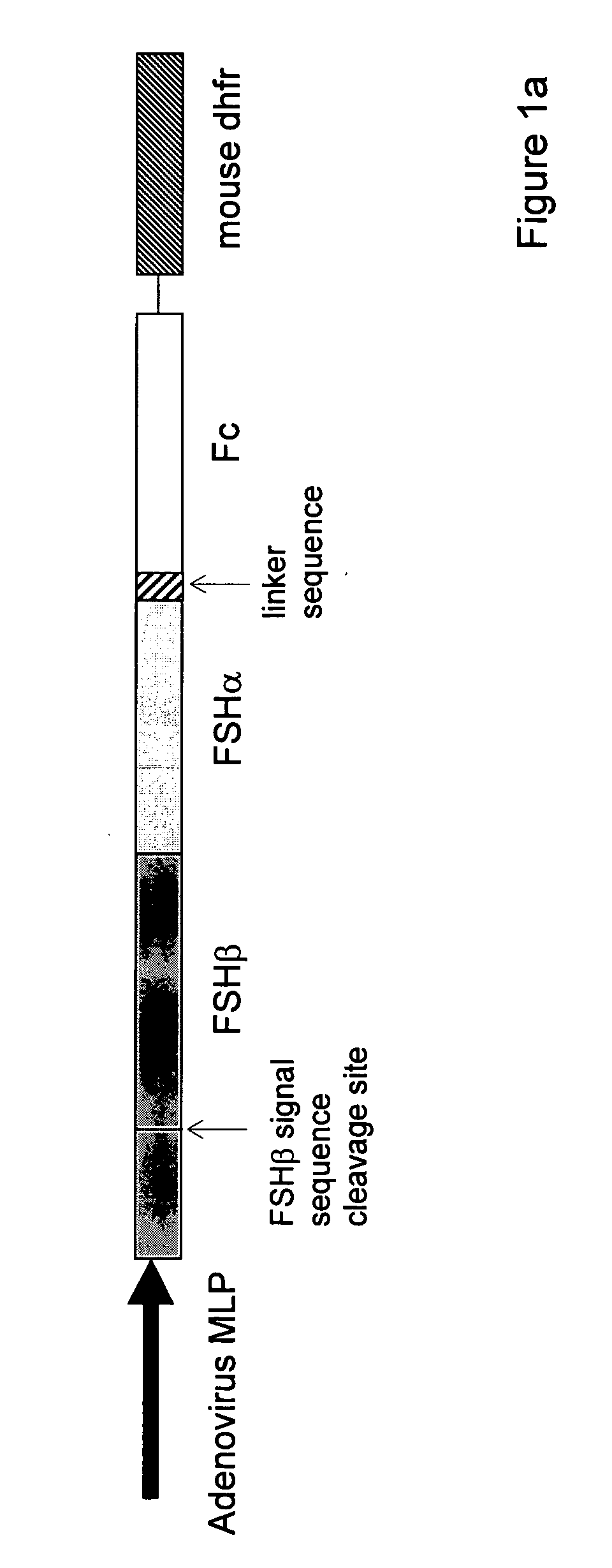
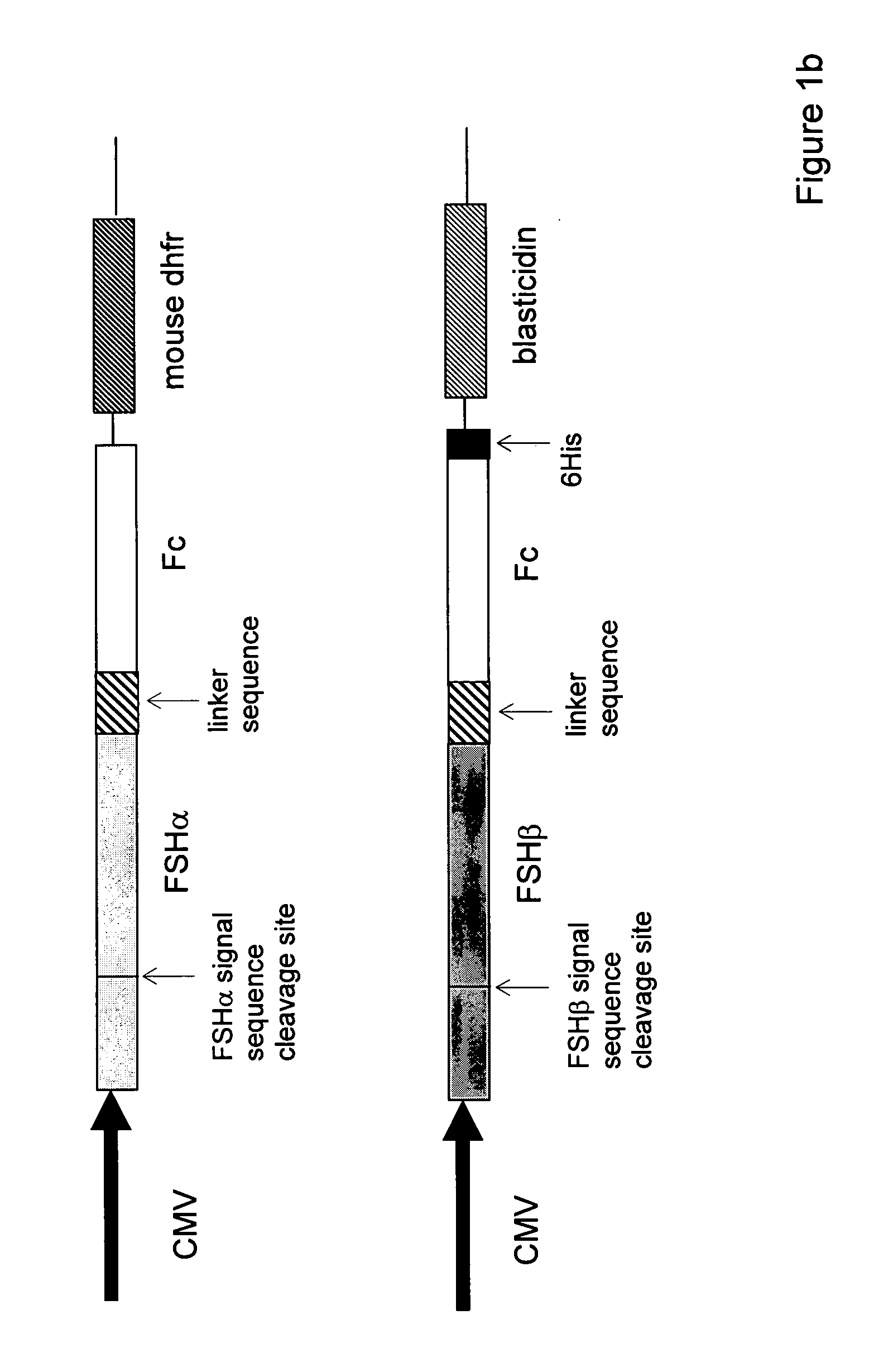
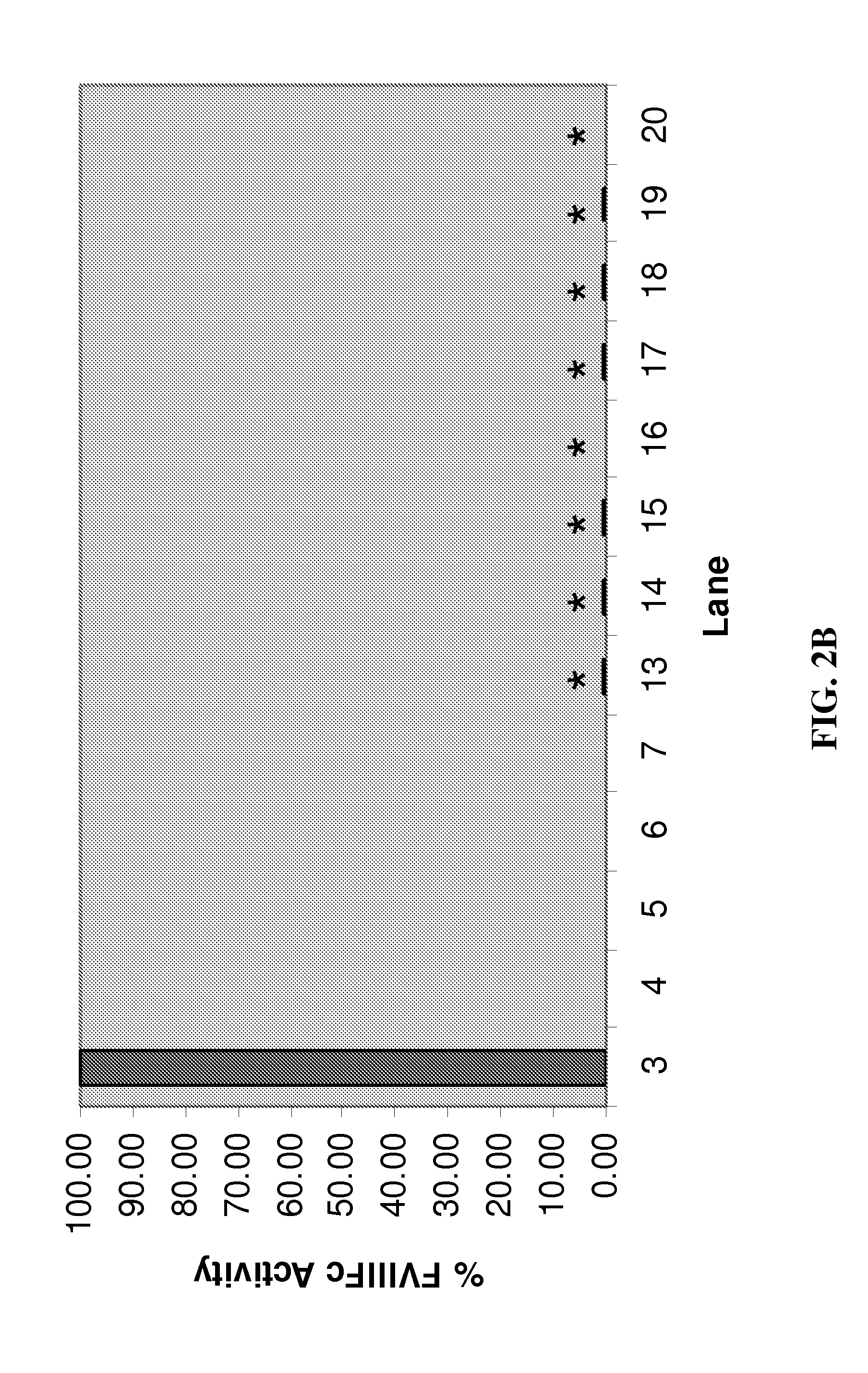
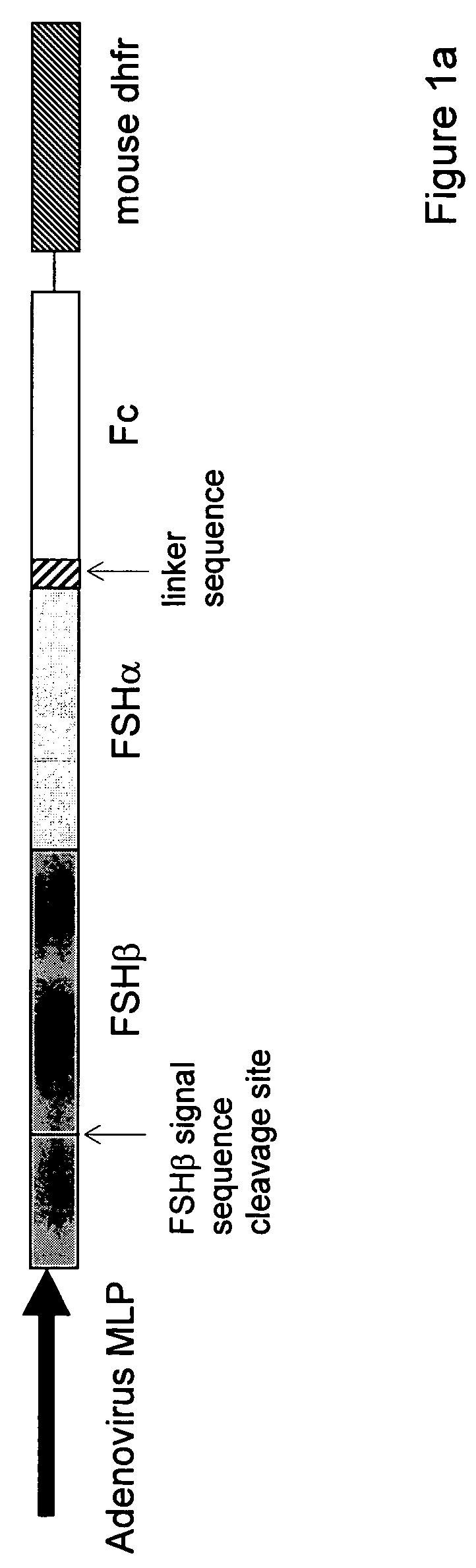
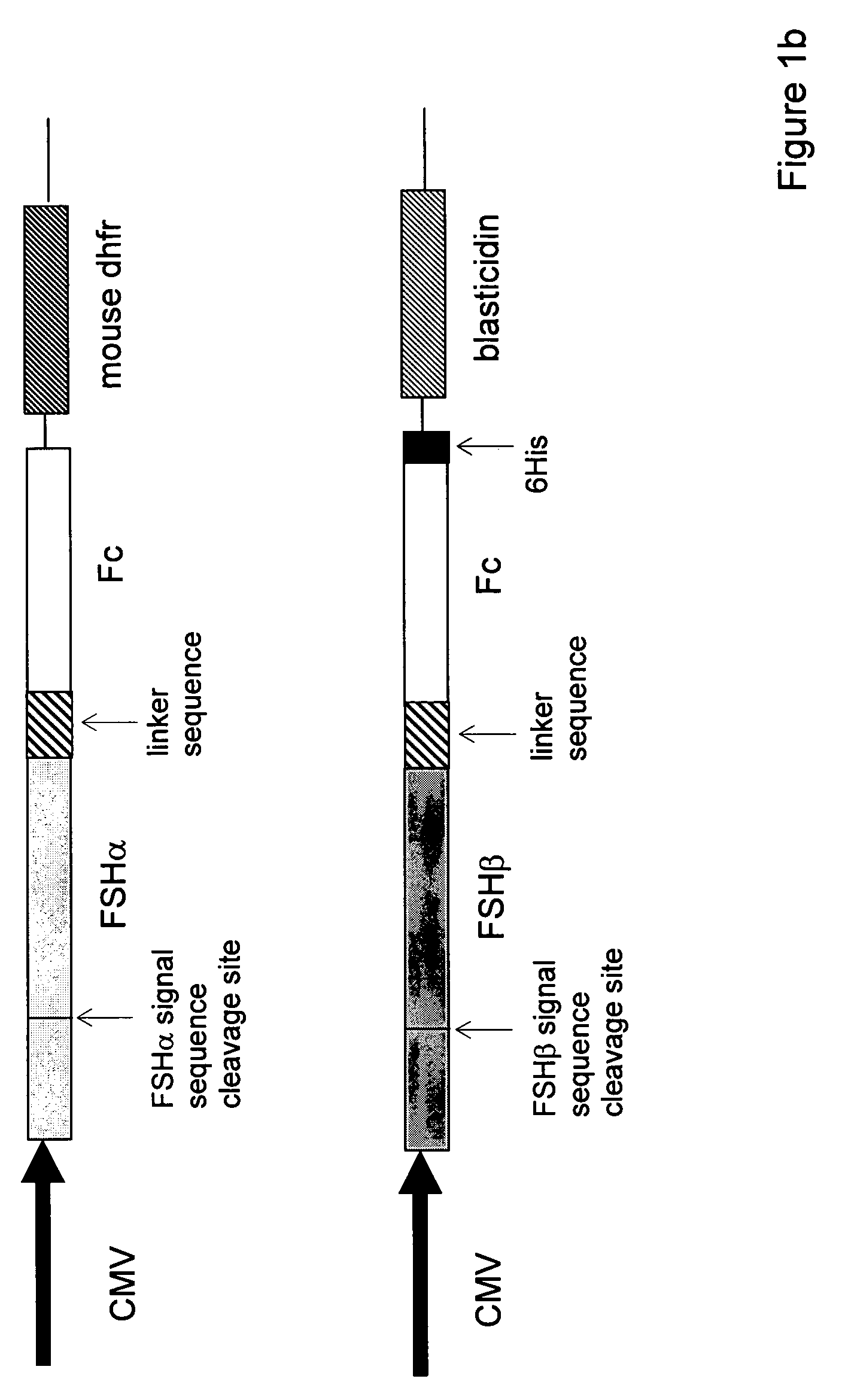
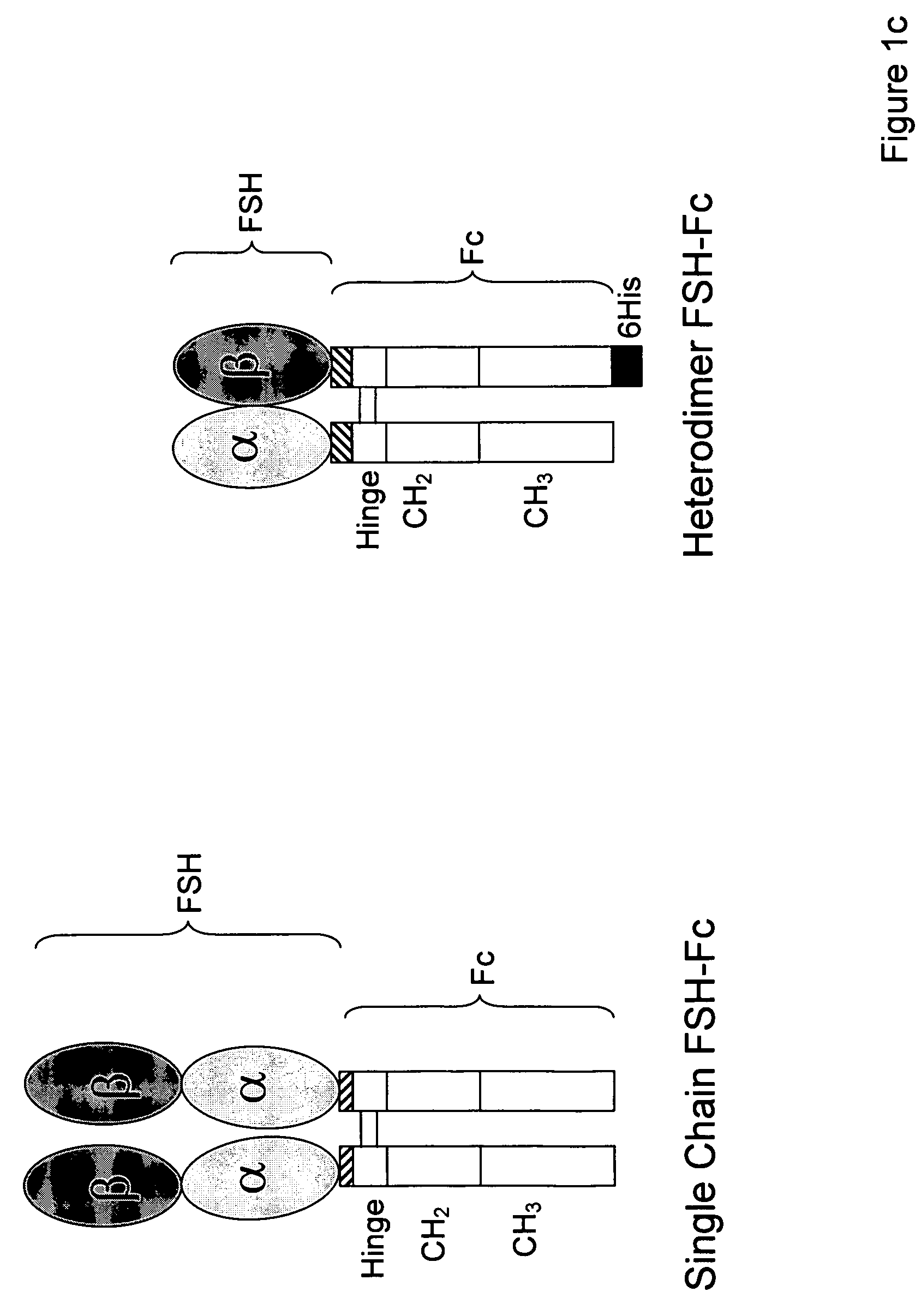
Heterodimeric follicle stimulating hormone-Fc (FSH-Fc) fusion proteins for the treatment of infertility
InactiveUS20050186662A1Extended half-lifeImprove fertilityBacteriaPeptide/protein ingredientsFollicle-stimulating hormoneIncreased fertility
The invention provides novel heterodimeric fusion proteins comprising a first polypeptide including an alpha subunit of FSH (αFSH) linked directly or indirectly to a binding partner of neonatal Fc receptor (FcRn) and a second polypeptide including a beta subunit of FSH (βFSH) linked directly or indirectly to an FcRn binding partner. In one embodiment the FcRn binding partner includes an Fc fragment of an immunoglobulin, e.g., an Fc fragment of IgG. Also provided are methods making and using the fusion proteins of the invention. The invention provides a method for increasing fertility in a subject and a method for treating a subject having a disease state responsive to treatment by FSH.
Owner:SYNTONIX PHARMA
Neonatal Fc receptor (FcRn)-binding polypeptide variants, dimeric Fc binding proteins and methods related thereto
InactiveCN101124245AImprove stabilityEnhance binding interactionSerum immunoglobulinsAntibody ingredientsFc bindingDrug biological activity
The compositions and methods of the invention are based in part on our discovery that effector functions mediated by Fc-containing polypeptides can be altered by modifying one or more amino acid residues in the polypeptide (by, eg, electrostatic optimization). Polypeptides that can be produced according to the methods of the invention are highly variable and they can include antibodies and fusion proteins comprising Fc regions or biologically active portions thereof.
Owner:BIOGEN IDEC MA INC
Intrathecal administration of adeno-associated-viral vectors for gene therapy
ActiveUS20180339065A1Readily apparentAntibacterial agentsNervous disorderIntrathecal useViral vector
A composition comprising at least one AAV vector formulated for intrathecal delivery to the central nervous system is described. The composition comprises at least one expression cassette which contains sequences encoding an immunoglobulin construct linked to expression control sequences therefor and a pharmaceutically acceptable carrier. The immunoglobulin construct may be an immunoglobulin modified to have decreased or no measurable affinity for neonatal Fc receptor (FcRn).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and reagents for modulating macrophage phenotype
ActiveUS20190290688A1Restore immune recognitionReduced polarization effectsOrganic active ingredientsPeptide/protein ingredientsP PHENOTYPENeonatal Fc receptor
The present invention is directed to methods of inducing a phenotypic change in a population of monocytes and / or macrophages. The method includes administering to the population of monocytes and / or macrophages, a macrophage stimulating agent coupled to a carrier molecule, wherein the carrier molecule facilitates macropinocytic uptake of the agent by monocytes and macrophages in the population and is defective in neonatal Fc receptor binding, wherein the administering induces a phenotypic change in the monocytes and macrophages in the population.
Owner:NEW YORK UNIV
NEONATAL Fc RECEPTOR (FcRn)- BINDING POLYPEPTIDE VARIANTS, DIMERIC Fc BINDING PROTEINS AND METHODS RELATED THERETO
InactiveUS20120003210A1Improve performanceReduce and eliminate bindingFungiBacteriaEffector functionsNeonatal Fc receptor
The compositions and methods of the present invention are based, in part, on our discovery that an effector function mediated by an Fc-containing polypeptide can be altered by modifying one or more amino acid residues within the polypeptide (by, for example, electrostatic optimization). The polypeptides that can be generated according to the methods of the invention are highly variable, and they can include antibodies and fusion proteins that contain an Fc region or a biologically active portion thereof.
Owner:BIOGEN MA INC
Methods and compositions for treating metastatic breast cancer and other cancers in the brain
ActiveUS20170043035A1Decreased and no measurable affinityShrink tumorNervous disorderPharmaceutical delivery mechanismRegimenWilms' tumor
A composition comprising at least one AAV vector formulated for central nervous system delivery is described. The composition comprises at least one expression cassette which contains sequences encoding an anti-neoplastic immunoglobulin construct for delivery to the brain operably linked to expression control sequences therefor and a pharmaceutically acceptable carrier. The anti-neoplastic immunoglobulin construct may be an immunoglobulin modified to have decreased or no measurable affinity for neonatal Fc receptor (FcRn). Also provided are methods of using these constructs in preparing pharmaceutical compositions and uses thereof in anti-neoplastic regimens, particularly for primary and / or metastatic cancers of the brain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant FcRn and Variants Thereof for Purification of Fc-Containing Fusion Proteins
InactiveUS20120021484A1Efficient couplingEasy to attachFactor VIIAntibody mimetics/scaffoldsElutionADAMTS Proteins
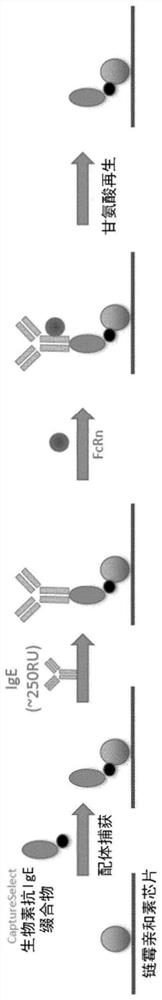
The invention is directed to methods of purifying Fc-containing molecules using a soluble neonatal Fc receptor (sFcRn). Native FcRn binds Fc-containing proteins at or below about pH 6.5 and releases them at or above about pH 7 and provides a much milder approach for capturing and purifying Fc-containing proteins, in particular, therapeutic Fc-containing proteins. Other embodiments of the invention provide modifications to alter the pH for binding and elution to the sFcRn, to modulate Fc-containing protein binding affinity, to affect sFcRn linkage to a support surface, or to improve the stability of sFcRn to conditions utilized in the methods of the invention.
Owner:BIOGEN MA INC
Heterodimeric follicle stimulating hormone-Fc (FSH-Fc) fusion proteins for the treatment of infertility
InactiveUS7601516B2Extended half-lifeImprove fertilityFungiBacteriaDiseaseFollicle-stimulating hormone
The invention provides novel heterodimeric fusion proteins comprising a first polypeptide including an alpha subunit of FSH (αFSH) linked directly or indirectly to a binding partner of neonatal Fc receptor (FcRn) and a second polypeptide including a beta subunit of FSH (βFSH) linked directly or indirectly to an FcRn binding partner. In one embodiment the FcRn binding partner includes an Fc fragment of an immunoglobulin, e.g., an Fc fragment of IgG. Also provided are methods making and using the fusion proteins of the invention. The invention provides a method for increasing fertility in a subject and a method for treating a subject having a disease state responsive to treatment by FSH.
Owner:SYNTONIX PHARMA
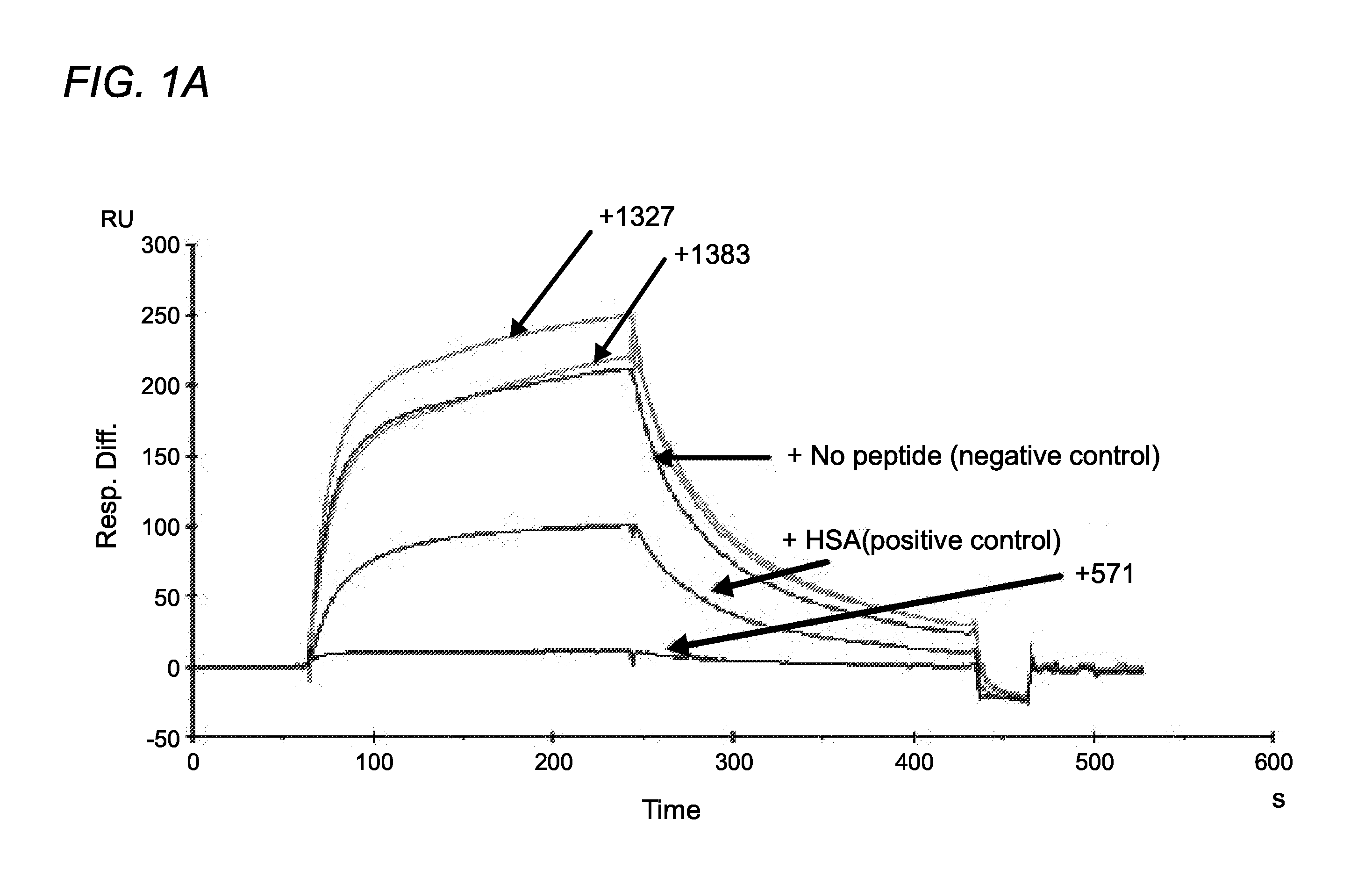
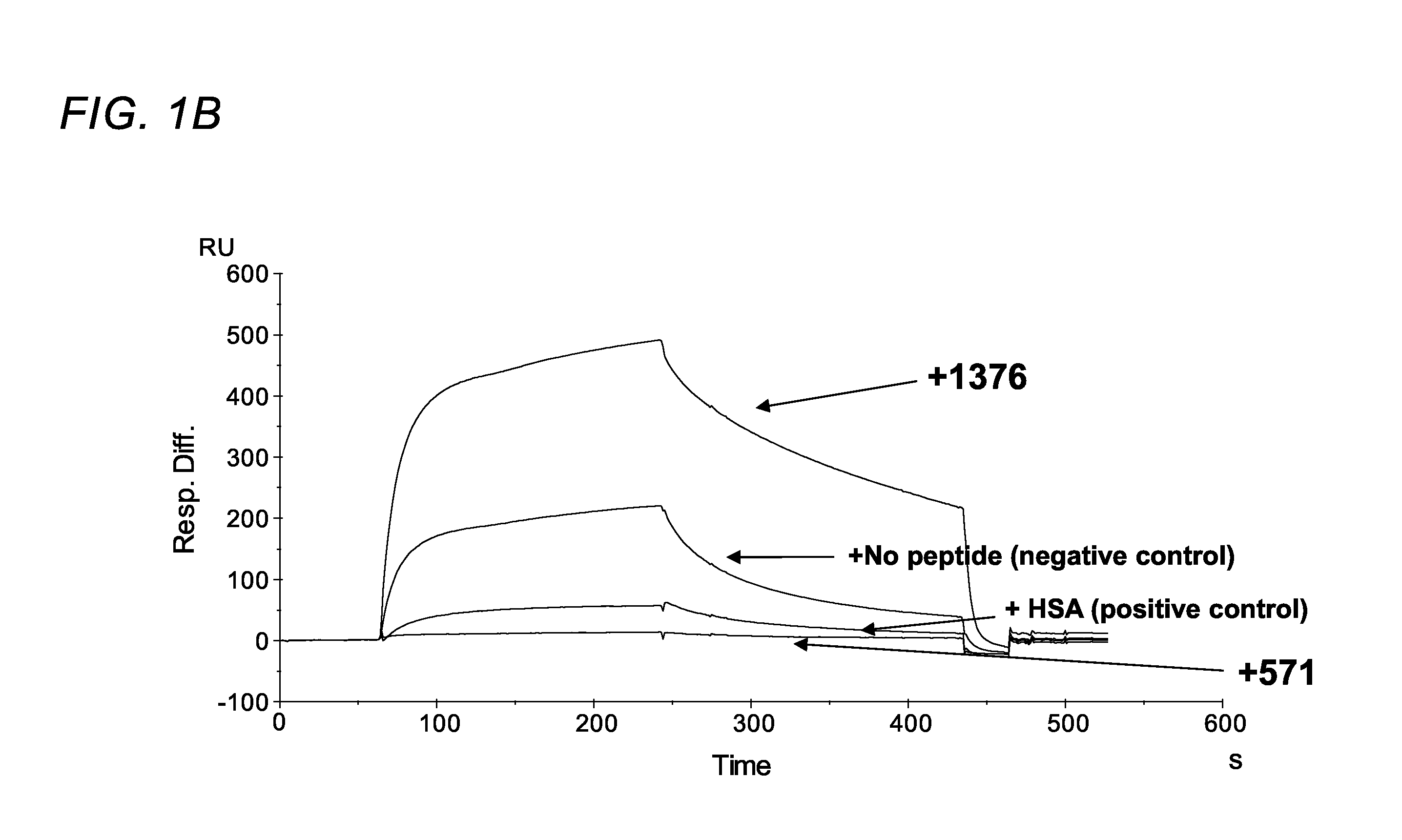
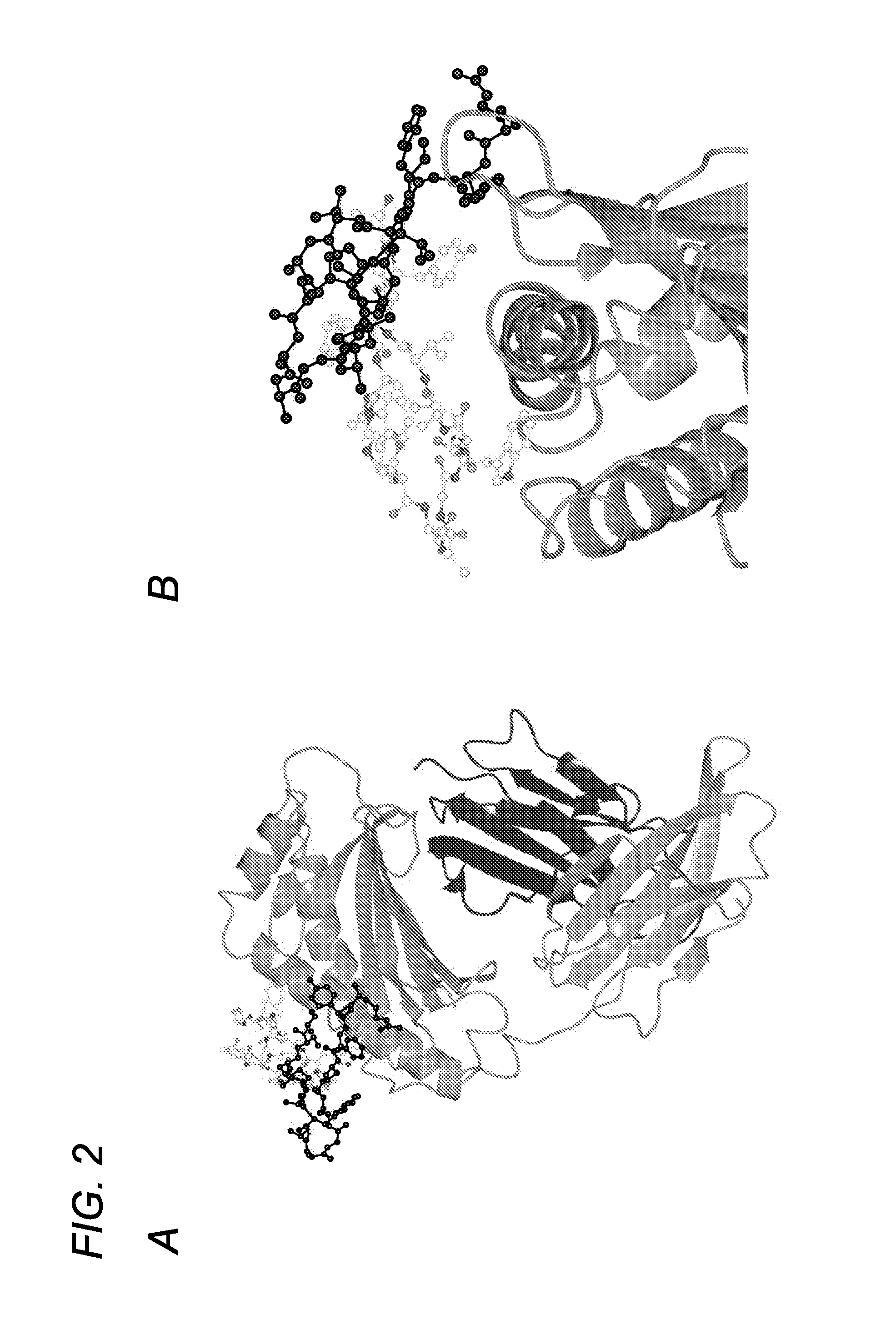
FC receptor (FcRn) binding peptides and uses thereof
ActiveUS9527890B2Reduce and inhibit and block interactionPromote degradationPeptide-nucleic acidsPeptide/protein ingredientsDiseaseFc(alpha) receptor
The invention provided herein includes isolated polypeptides that specifically block the interaction between neonatal Fc receptor (FcRn) and albumin. Blocking the interaction treats diseases and conditions caused by increased amounts of albumin or modified albumin that possesses pathogenic properties wherein it is deemed desirable to decrease albumin levels. Accordingly, also provided are methods of using these isolated polypeptides to treat various diseases and conditions caused by increased amounts of albumin or modified albumin that possesses pathogenic properties. The invention provided herein also includes isolated polypeptides capable of binding to a non-IgG and non-albumin competitive site on an FcRn alpha 3 domain. These can be useful for tracking FcRn without inhibiting IgG or albumin binding or function. Accordingly, the invention also includes methods and systems to track FcRn without inhibiting IgG or albumin binding or function.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
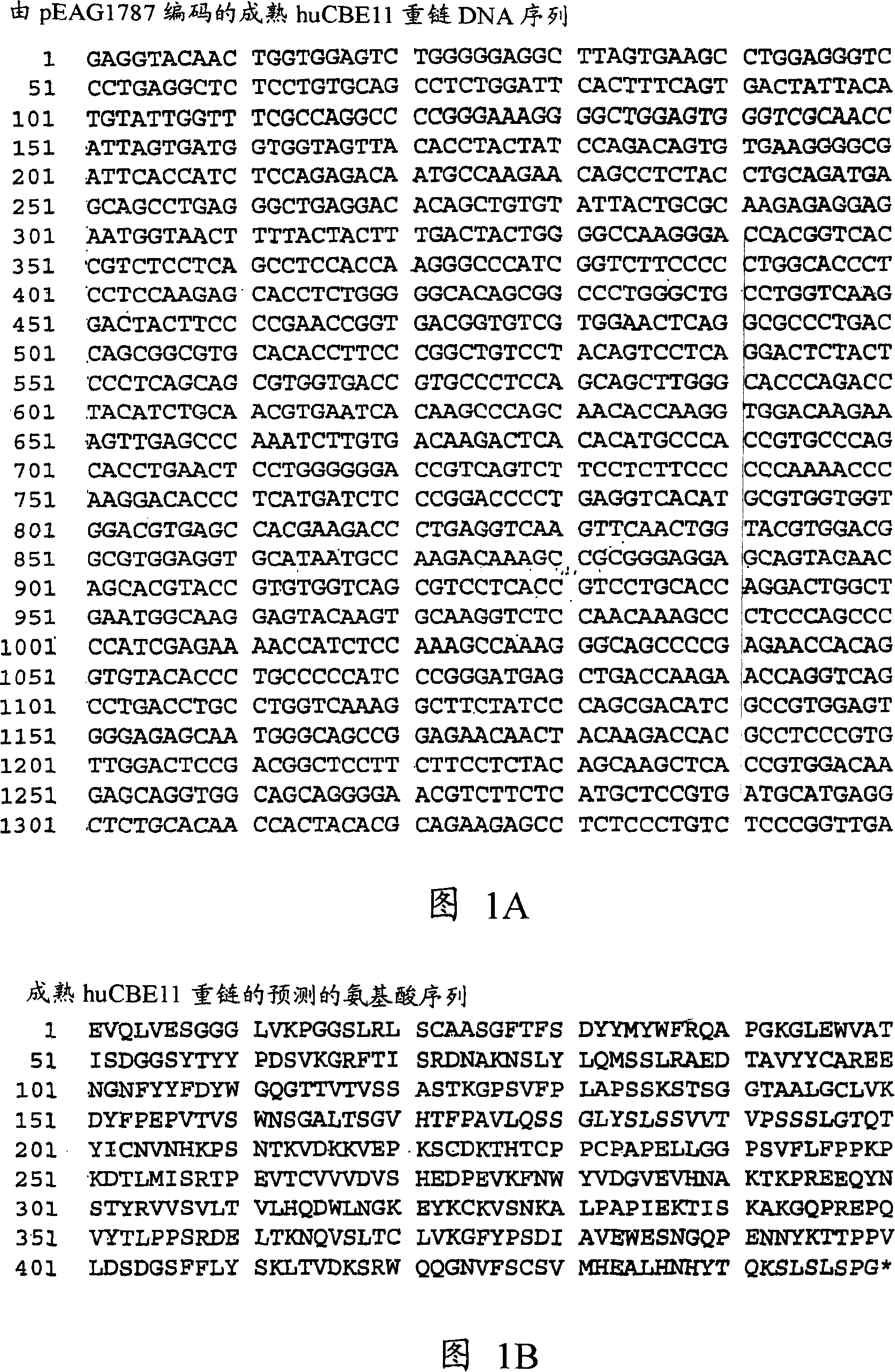
Human neonatal Fc receptor antibodies and methods of use thereof
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for treating metastatic breast cancer and other cancers in the brain
ActiveUS10780182B2Decreased and no measurable affinityConducive to survivalNervous disorderPharmaceutical delivery mechanismRegimenIntravenous gammaglobulin
A composition comprising at least one AAV vector formulated for central nervous system delivery is described. The composition comprises at least one expression cassette which contains sequences encoding an anti-neoplastic immunoglobulin construct for delivery to the brain operably linked to expression control sequences therefor and a pharmaceutically acceptable carrier. The anti-neoplastic immunoglobulin construct may be an immunoglobulin modified to have decreased or no measurable affinity for neonatal Fc receptor (FcRn). Also provided are methods of using these constructs in preparing pharmaceutical compositions and uses thereof in anti-neoplastic regimens, particularly for primary and / or metastatic cancers of the brain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Transgenic animal with enhanced immune response and method for the preparation thereof
ActiveCN101595128ACell receptors/surface-antigens/surface-determinantsGenetic material ingredientsAntigenMHC class I
The present invention provides a method for producing a transgenic (Tg) non-human animal capable of developing an enhanced humoral immune response against an antigen as compared to a non-transgenic control animal of the same species, comprising introducing into said non-human animal a genetic construct providing for enhanced MHC class I-related neonatal Fc receptor (FcRn) activity. Also provided a Tg non-human animal comprising a genetic construct providing for enhanced FcRn activity, as well as the use of such animal in a non- therapeutical method. Therapeutic genetic constructs and methods are also provided. The present invention further provides methods for producing immunoglobulins.
Owner:AGRI BIOTECH CENT +1
Antibody binding to fcrn for treating autoimmune diseases
ActiveUS20170210805A1High affinityStrong specificityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSerum igeAutoimmune disease
The present disclosure relates to an isolated anti-FcRn antibody, which is an antibody binding to FcRn (stands for neonatal Fc receptor, also called FcRP, FcRB or Brambell receptor) that is a receptor with a high affinity for IgG or a fragment thereof, a method of preparing thereof, a composition for treating autoimmune disease, which comprises the antibody, and a method of treating and diagnosing autoimmune diseases using the antibody. The FcRn-specific antibody according to the present disclosure binds to FcRn non-competitively with IgG to reduce serum pathogenic auto-antibody levels, and thus can be used for the treatment of autoimmune diseases.
Owner:HANALL PHARMA CO LTD
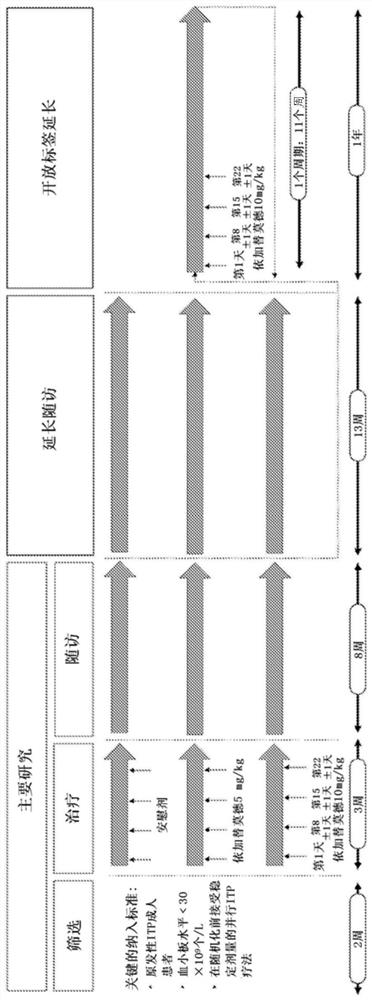
Compositions and methods for treating immune thrombocytopenia
PendingUS20200024344A1Raise countImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAgonistImmune thrombocytopenia
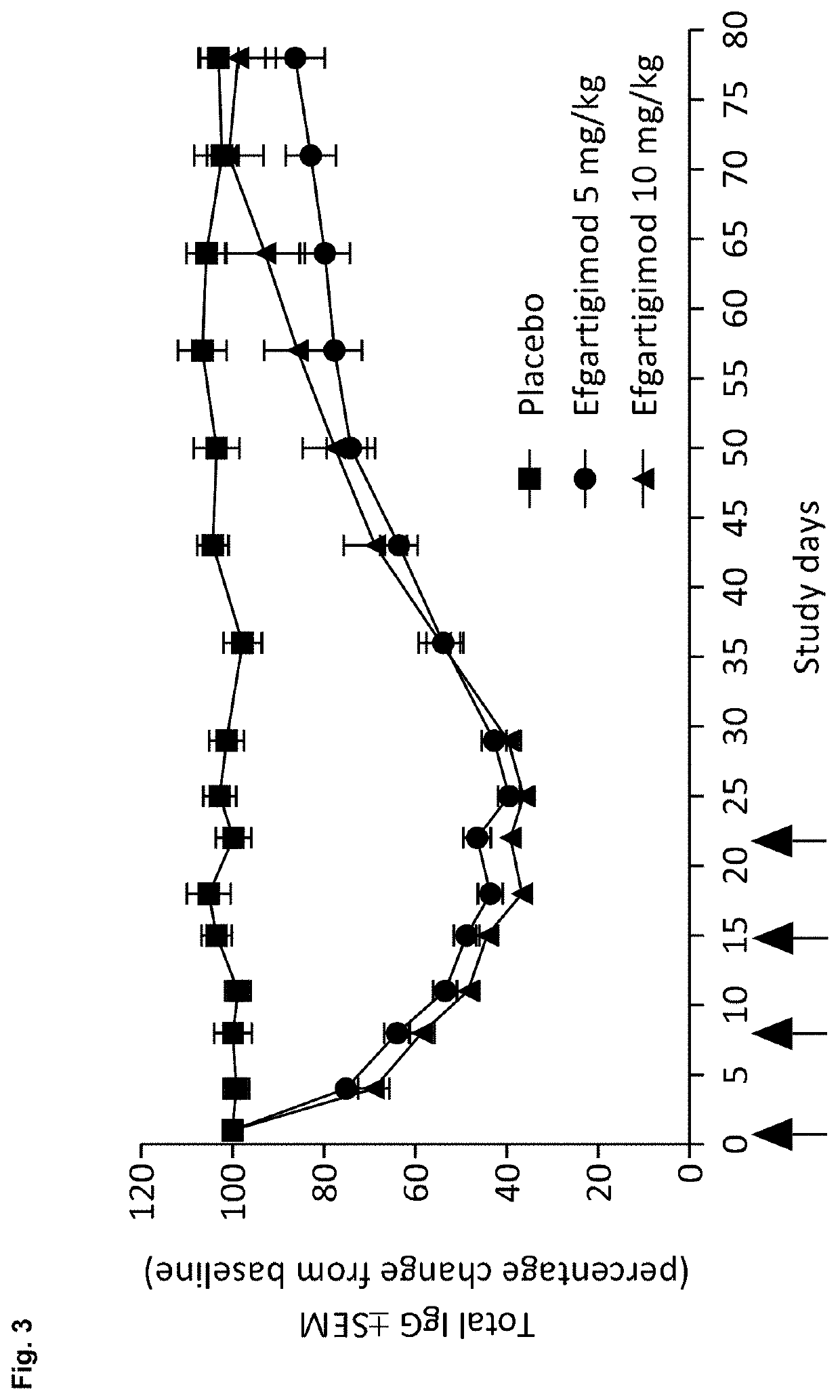
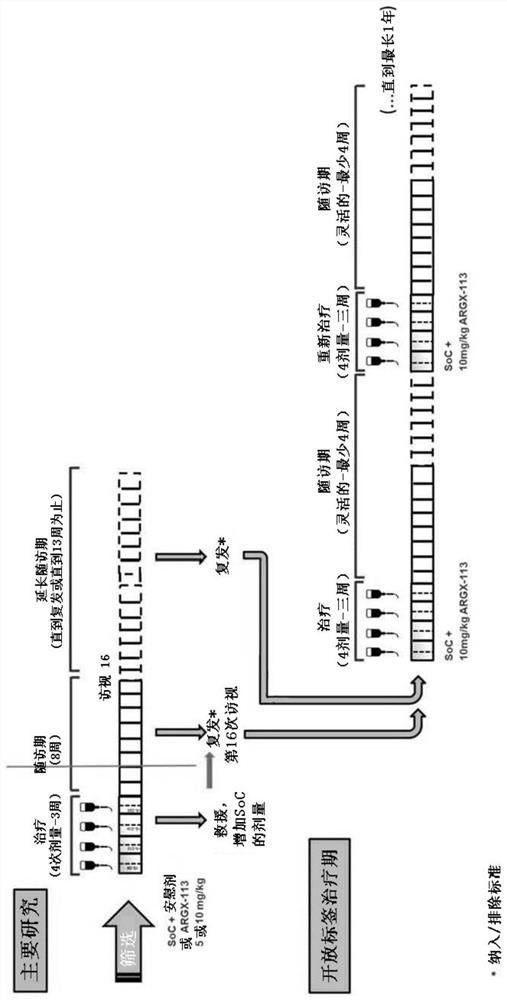
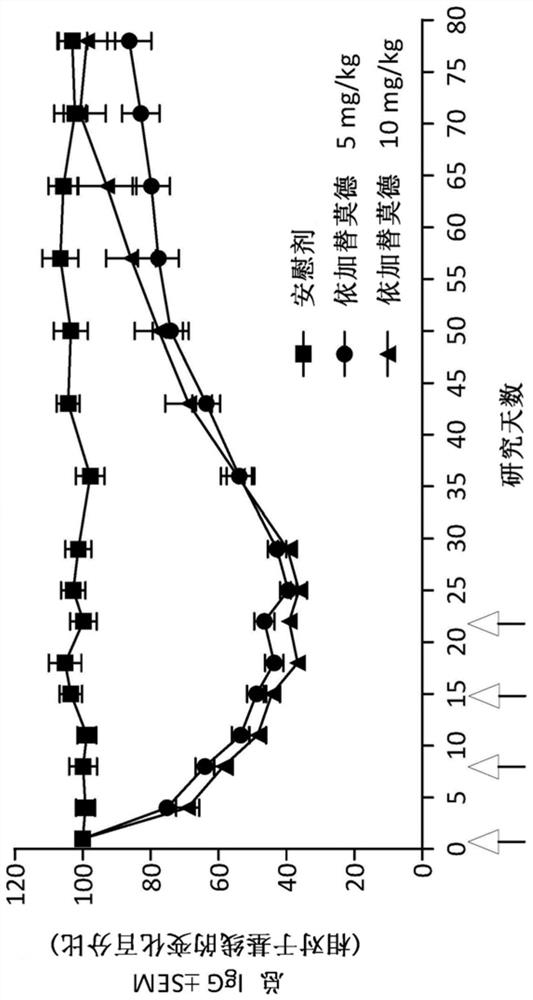
A method is disclosed for the treatment of human subjects diagnosed with immune thrombocytopenia (ITP). The method comprises administering to a human subject a human neonatal Fc receptor (hFcRn) antagonist, optionally in combination with standard-of-care ITP treatment. In certain embodiments, the hFcRn antagonist is efgartigimod (ARGX-113). Standard-of-care ITP treatment may comprise administration of corticosteroids, immunosuppressants, and / or thrombopoietin receptor (TPO-R) agonists.
Owner:ARGENX BV
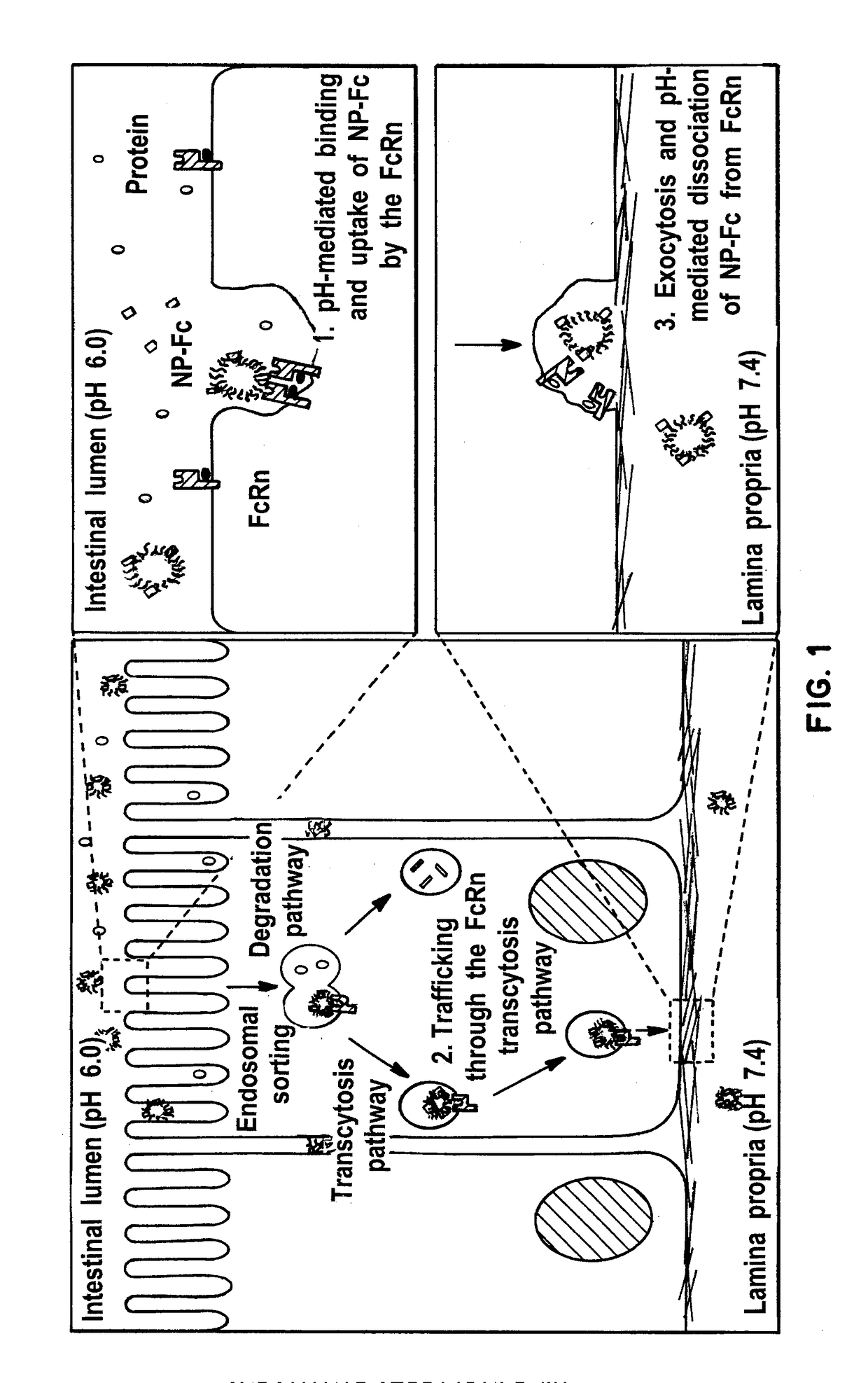
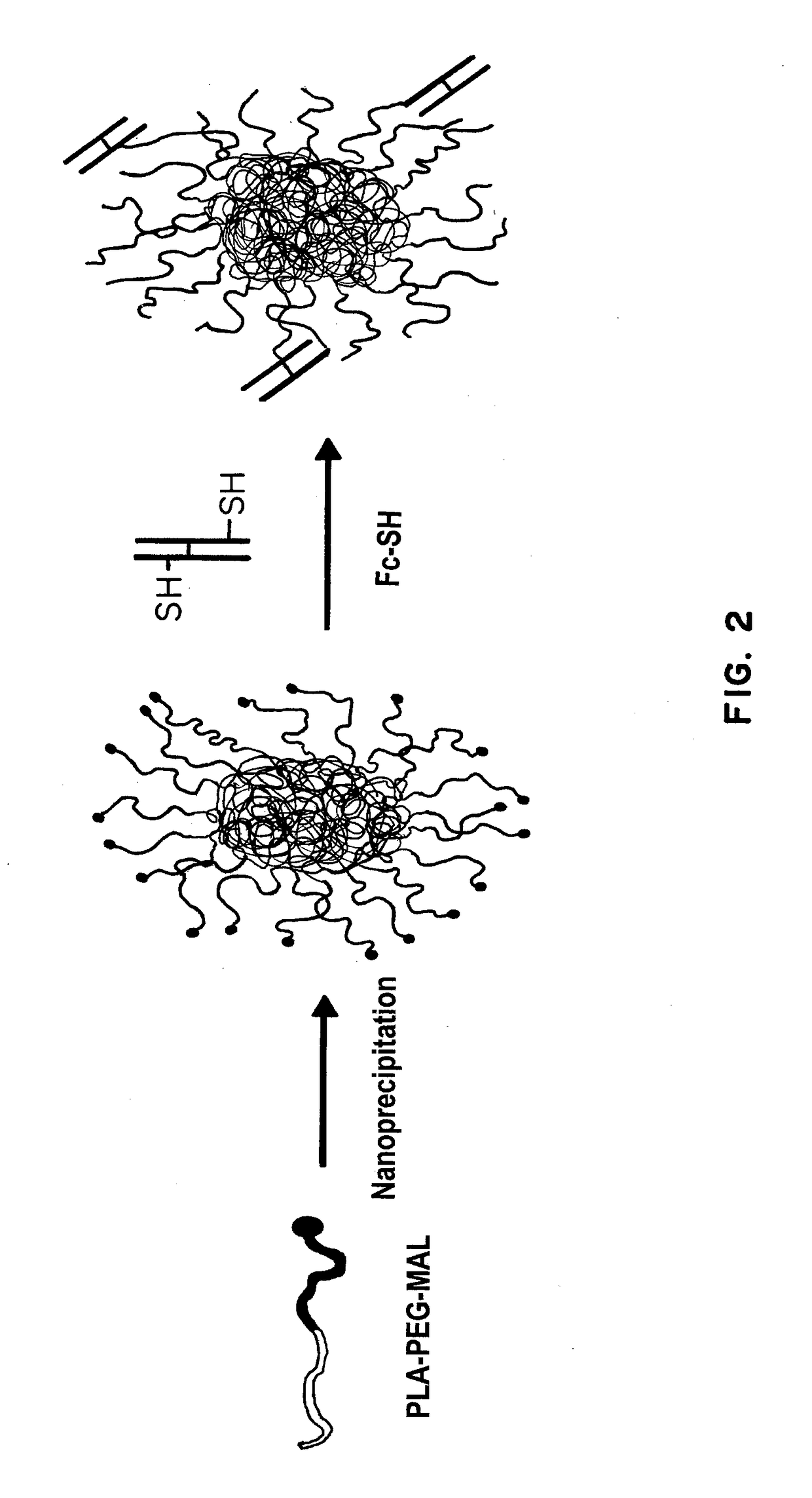
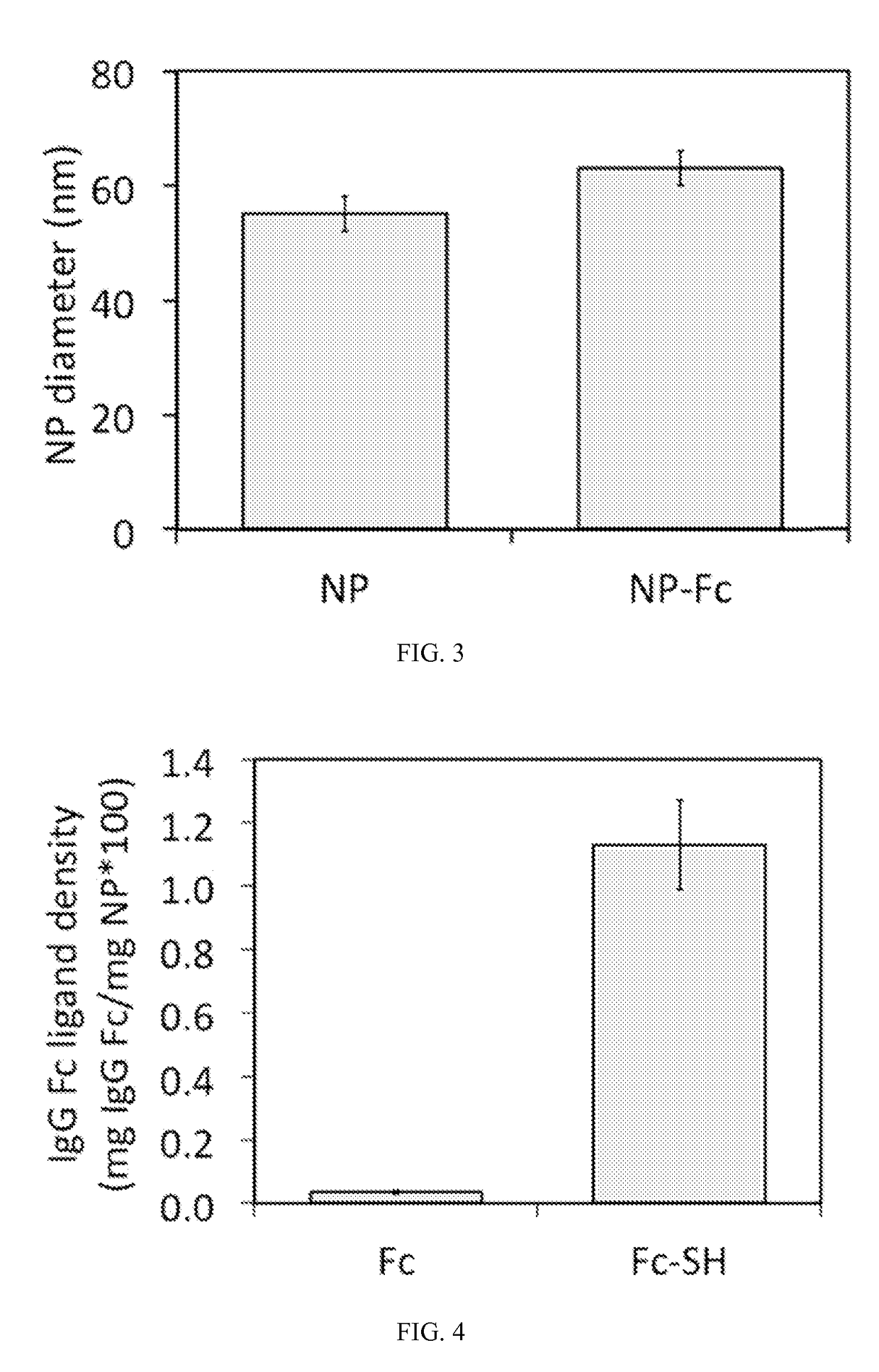
Receptor-targeted nanoparticles for enhanced transcytosis mediated drug delivery
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
FC RECEPTOR (FcRn) BINDING PEPTIDES AND USES THEREOF
ActiveUS20160122394A1Reduce and inhibit and block interactionPromote degradationAntibacterial agentsPeptide/protein ingredientsDiseaseFc receptor
The invention provided herein includes isolated polypeptides that specifically block the interaction between neonatal Fc receptor (FcRn) and albumin. Blocking the interaction treats diseases and conditions caused by increased amounts of albumin or modified albumin that possesses pathogenic properties wherein it is deemed desirable to decrease albumin levels. Accordingly, also provided are methods of using these isolated polypeptides to treat various diseases and conditions caused by increased amounts of albumin or modified albumin that possesses pathogenic properties. The invention provided herein also includes isolated polypeptides capable of binding to a non-IgG and non-albumin competitive site on an FcRn alpha 3 domain. These can be useful for tracking FcRn without inhibiting IgG or albumin binding or function. Accordingly, the invention also includes methods and systems to track FcRn without inhibiting IgG or albumin binding or function.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Compositions and methods for treating immune thrombocytopenia
Owner:ARGENX BVBA
Hybrid antibodies
PendingCN114761087AAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAmino acid replacement
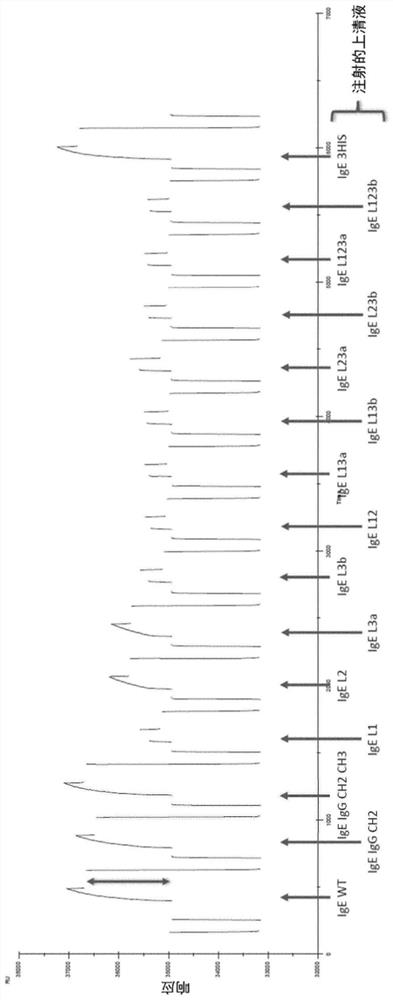
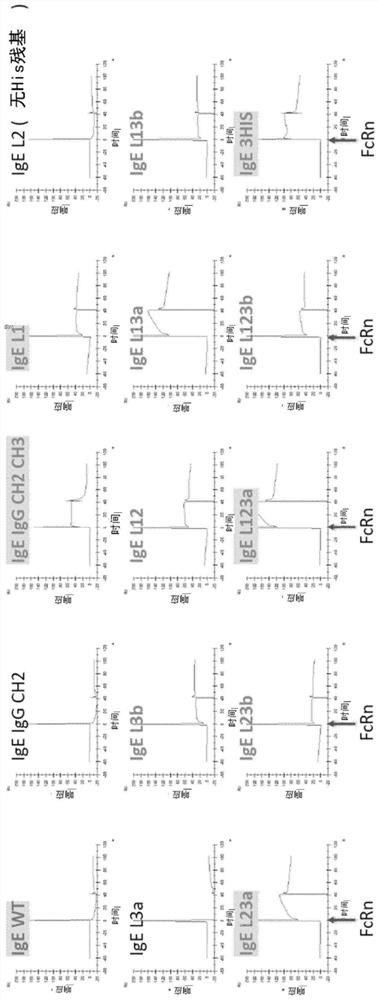
Described herein are hybrid antibodies targeted for the treatment of cancer. The antibodies have binding ability to Fc epsilon receptor and neonatal Fc receptor, which can be achieved, for example, by replacing the sequence or amino acid in the IgE constant domain with the corresponding sequence and amino acid derived from IgG.
Owner:艾普西洛根有限公司
Antibody binding to FcRn for treating autoimmune diseases
ActiveUS10336825B2High affinityStrong specificityImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsSerum igeAutoimmune disease
Owner:HANALL PHARMA CO LTD
Methods and reagents for modulating macrophage phenotype
ActiveUS11110122B2Restore immune recognitionEasy to keepOrganic active ingredientsPeptide/protein ingredientsCell phenotypePotocytosis
The present invention is directed to methods of inducing a phenotypic change in a population of monocytes and / or macrophages. The method includes administering to the population of monocytes and / or macrophages, a macrophage stimulating agent coupled to a carrier molecule, wherein the carrier molecule facilitates macropinocytic uptake of the agent by monocytes and macrophages in the population and is defective in neonatal Fc receptor binding, wherein the administering induces a phenotypic change in the monocytes and macrophages in the population.
Owner:NEW YORK UNIV
Humanized rodents for testing therapeutic agents
ActiveCN112105260APolypeptide with localisation/targeting motifCompounds screening/testingFc-Gamma ReceptorPharmacodynamic Study
Provided herein are methods and compositions related to the in vivo testing of therapeutic agents comprising a human Fc in genetically modified rodents (e.g., the testing of the pharmacokinetic and / orpharmacodynamic properties of such a therapeutic agent in genetically modified rodents). In some embodiments the genetically modified rodents express antibodies comprising a human Fc (e.g., a human IgG1 Fc, a human IgG4 Fc). In some embodiments, the rodents express fully human antibodies (i.e., antibodies having human heavy chains and human light (gamma or kappa) chains). In certain embodiments the genetically modified rodents comprise one or more Fc receptors with a human extracellular domain (e.g., a Neonatal Fc Receptor (FcRn), a beta-2-microglobulin polypeptide (beta 2Mu), a Fc epsilon receptor 1 alpha (Fc[epsilon]R1[alpha]), a Fc gamma receptor 1 alpha (Fc[gamma]R1a), a Fc gamma receptor 2a (Fc[gamma]R2a), a Fc gamma receptor 2b (Fc[gamma]R2b), a Fc gamma receptor 3a (Fc[gamma]R3a),a Fc gamma receptor 3b (Fc[gamma]R3b), a Fc gamma receptor 2c (Fc[gamma]R2c)). The transmembrane and cytoplasmic domain of such receptors can be human or non-human (e.g., rodent).
Owner:REGENERON PHARM INC
Transgenic animal with enhanced immune response and method for the preparation thereof
InactiveCN101595128BCell receptors/surface-antigens/surface-determinantsGenetic material ingredientsFc receptorAntigen
The present invention provides a method for producing a transgenic (Tg) non-human animal capable of developing an enhanced humoral immune response against an antigen as compared to a non-transgenic control animal of the same species, comprising introducing into said non-human animal a genetic construct providing for enhanced MHC class I-related neonatal Fc receptor (FcRn) activity. Also provided a Tg non-human animal comprising a genetic construct providing for enhanced FcRn activity, as well as the use of such animal in a non- therapeutical method. Therapeutic genetic constructs and methods are also provided. The present invention further provides methods for producing immunoglobulins.
Owner:AGRI BIOTECH CENT +1
Method for selecting antibodies with modified fcrn interaction
PendingUS20210041163A1Easy to recycleReduce gapLighting and heating apparatusImmunoglobulins against cytokines/lymphokines/interferonsHeavy chainAntibody production
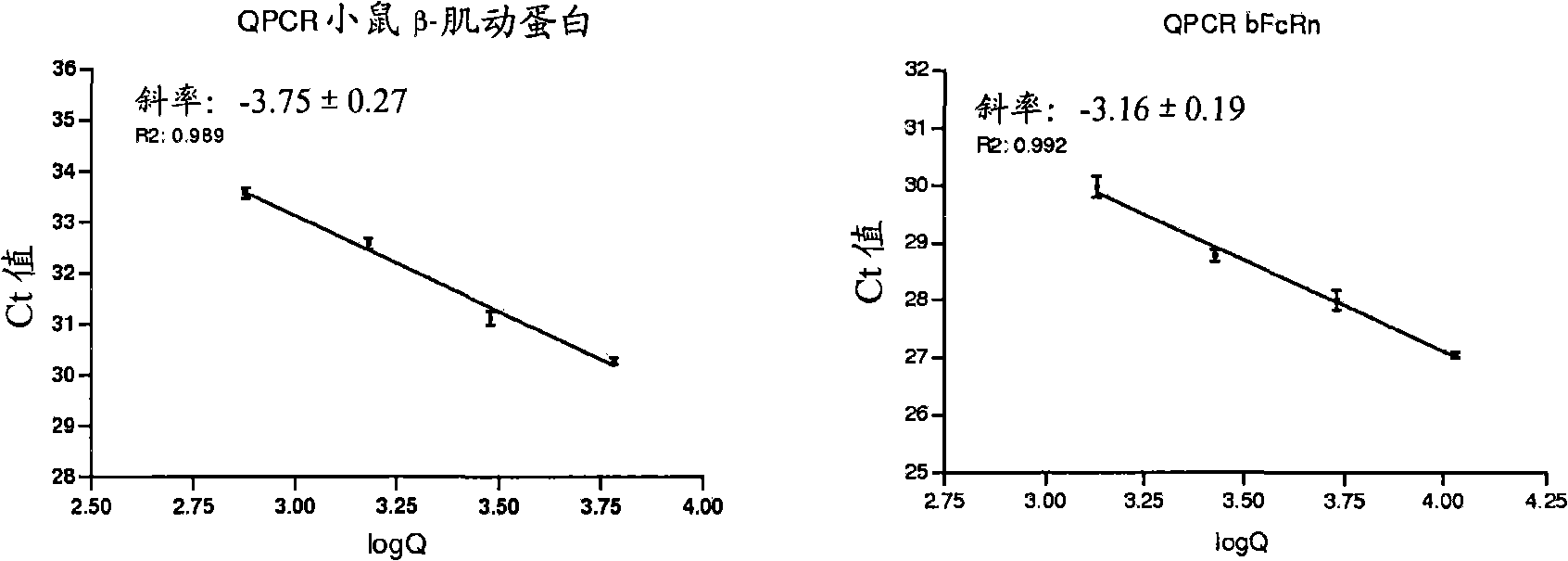
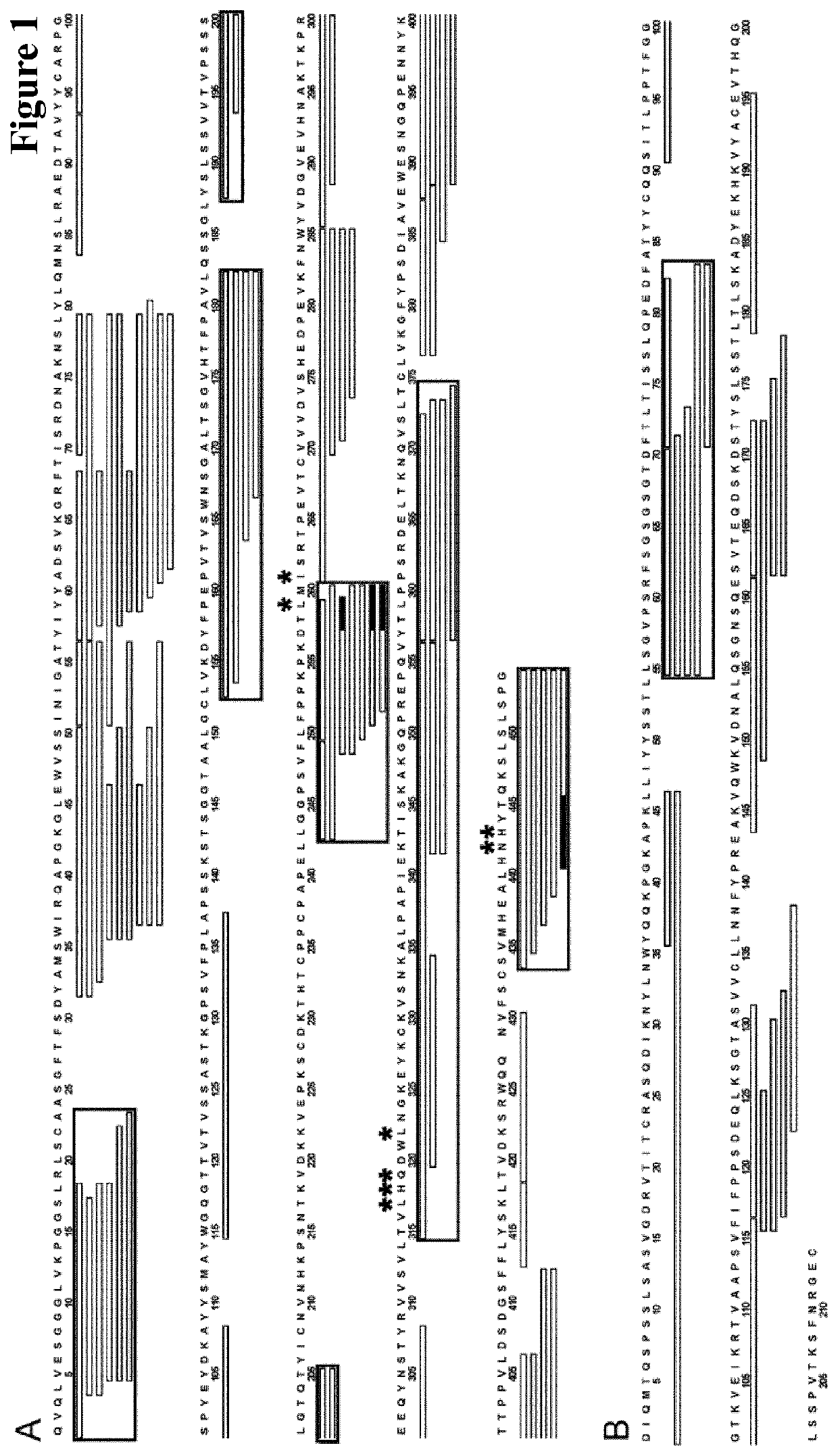
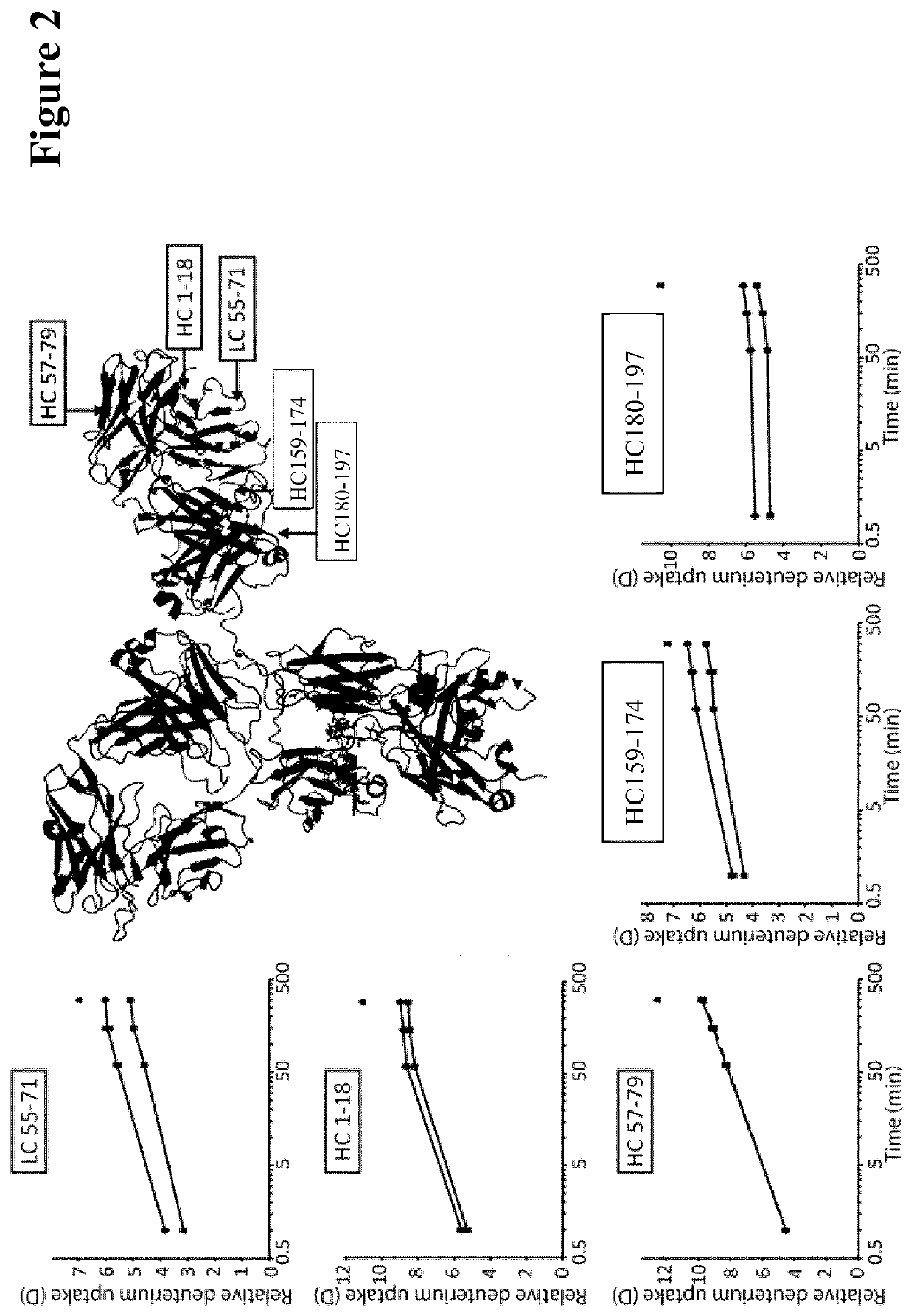
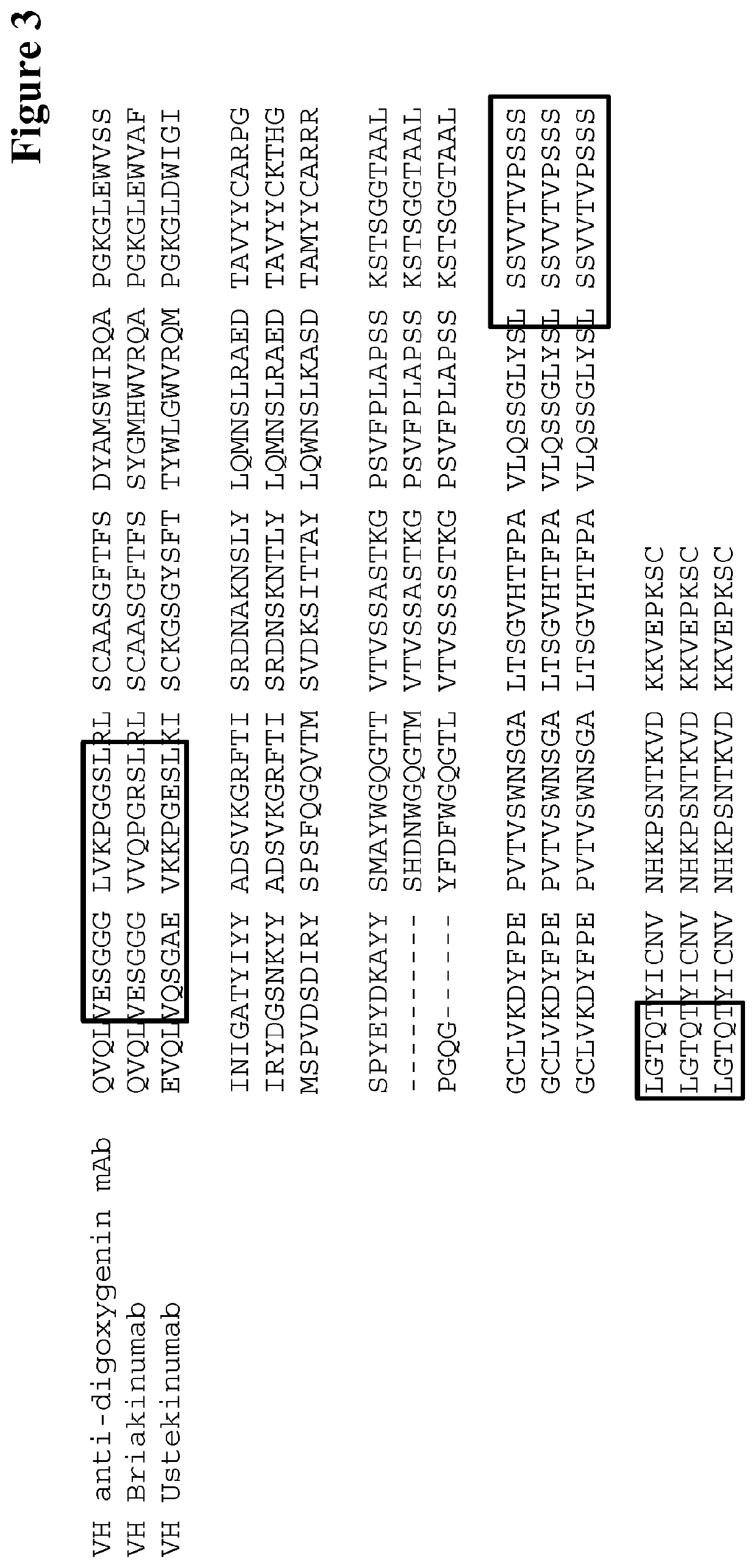
Herein is reported a method for selecting a full length antibody comprising the steps of a) generating from a parent full length antibody a plurality of full length antibodies by randomizing one or more amino acid residues selected from the amino acid residues at positions 1-23 in the heavy chain variable domain (numbering according to Kabat), at positions 55-83 in the light chain variable domain (numbering according to Kabat), at positions 145-174 in the first heavy chain constant domain (numbering according to EU index) and at positions 180-97 in the first heavy chain constant domain (numbering according to EU index), b) determining the binding strength of each of the full length antibodies from the 10 plurality of antibodies to the human neonatal Fc receptor (FcRn), and c) selecting a full length antibody from the plurality of full length antibodies that has a different binding strength to the FcRn than the parent full length antibody.
Owner:F HOFFMANN LA ROCHE & CO AG
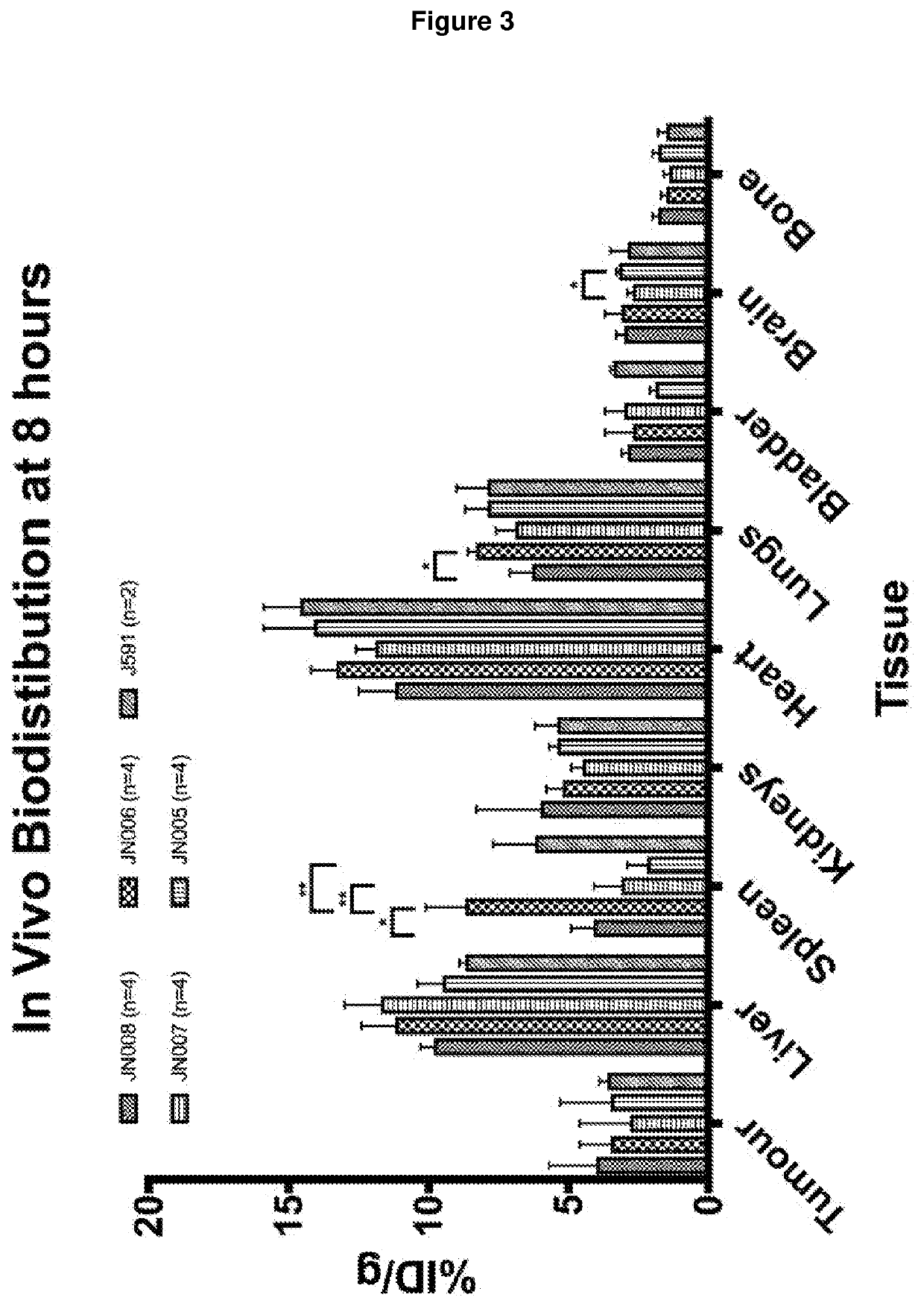
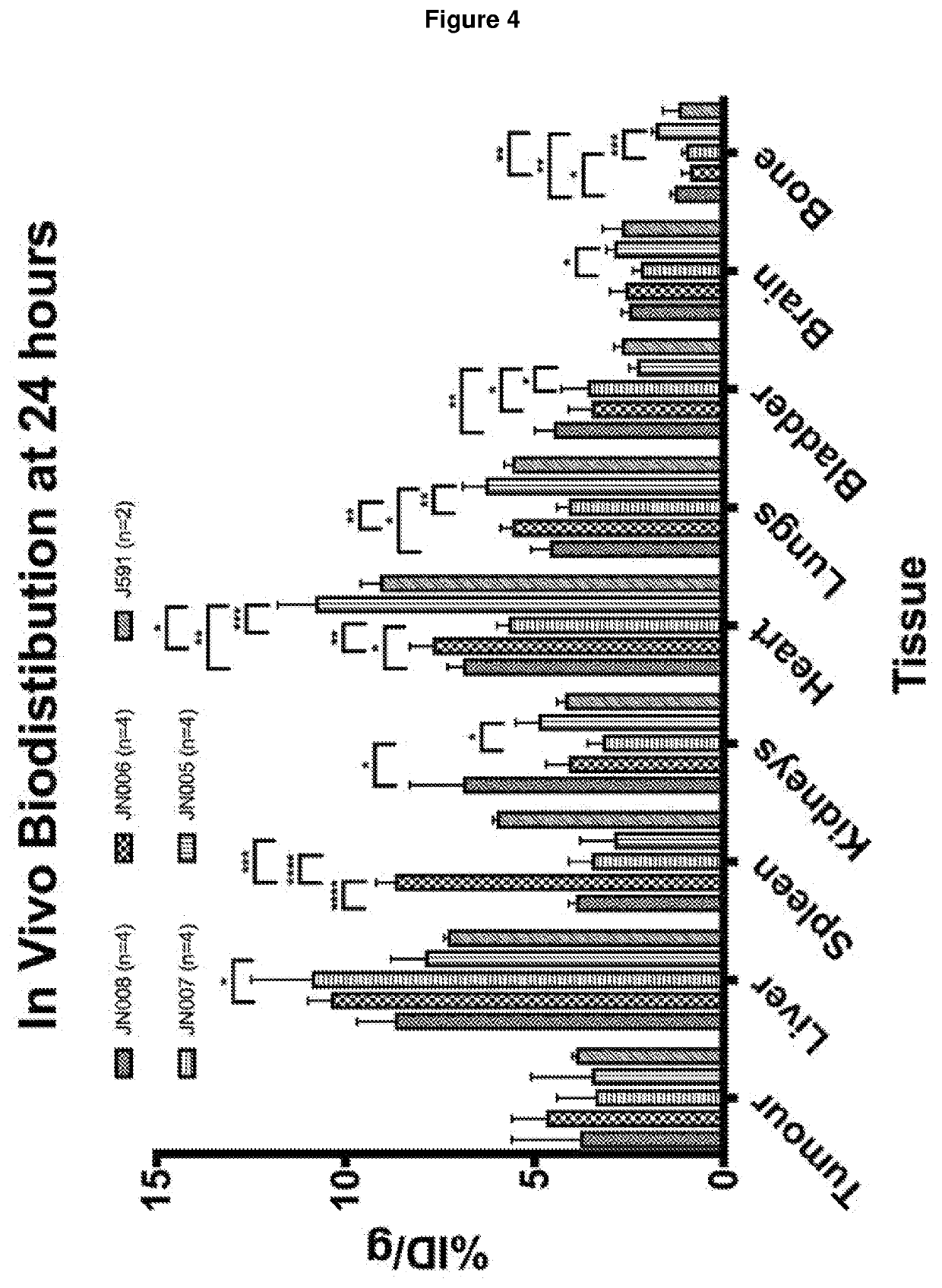
Antibodies for binding to PSMA with reduced affinity for neonatal Fc receptor
PendingCN114555641AReduced serum half-lifeQuick clearImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntiendomysial antibodiesAmino acid substitution
The present invention relates to anti-PSMA antibodies comprising a heavy chain constant region containing one or more amino acid substitutions as compared to wild-type IgG wherein the one or more amino acid substitutions reduce the affinity of the antibody for neonatal Fc receptor (FcRn) as compared to an IgG-based wild-type antibody, thereby reducing the serum half-life of the modified antibody. The one or more amino acid modifications, which have the effect of reducing FcRn binding, are selected from the group consisting of positions His310, His433, His435, His436, Ile253. The antibodies of the invention are particularly suitable for use in radioimmunotherapy.
Owner:泰利斯制药(创新)有限公司
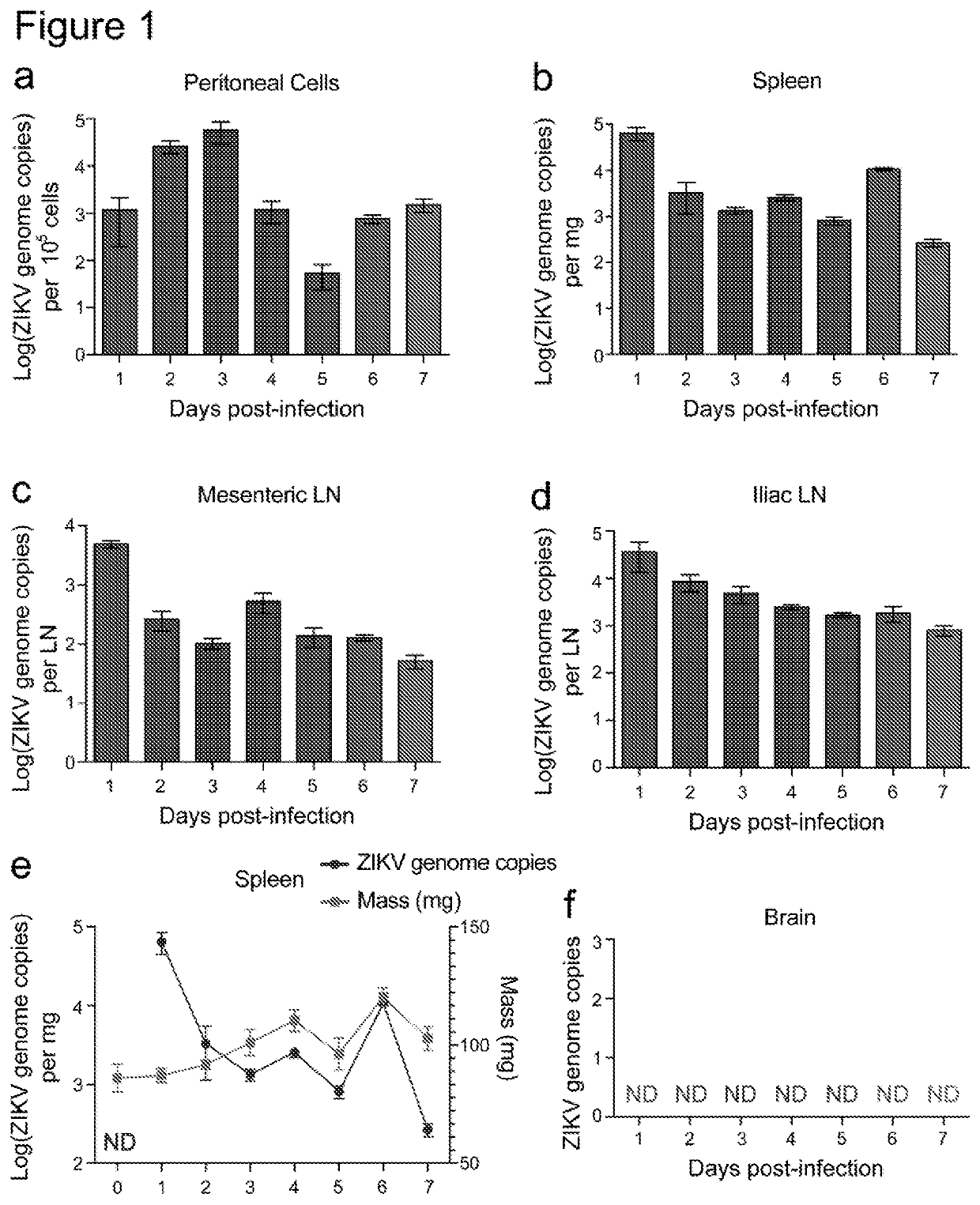
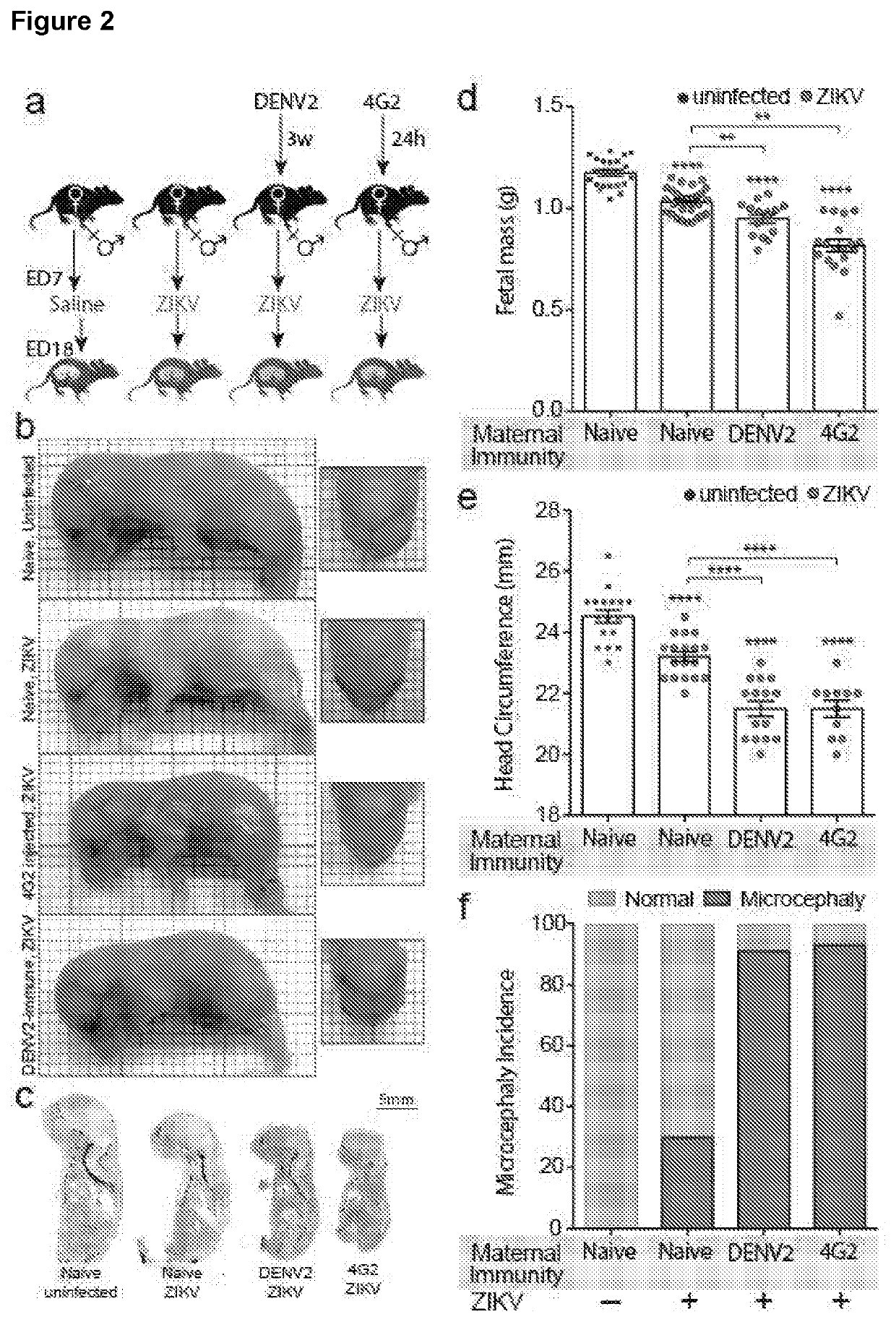
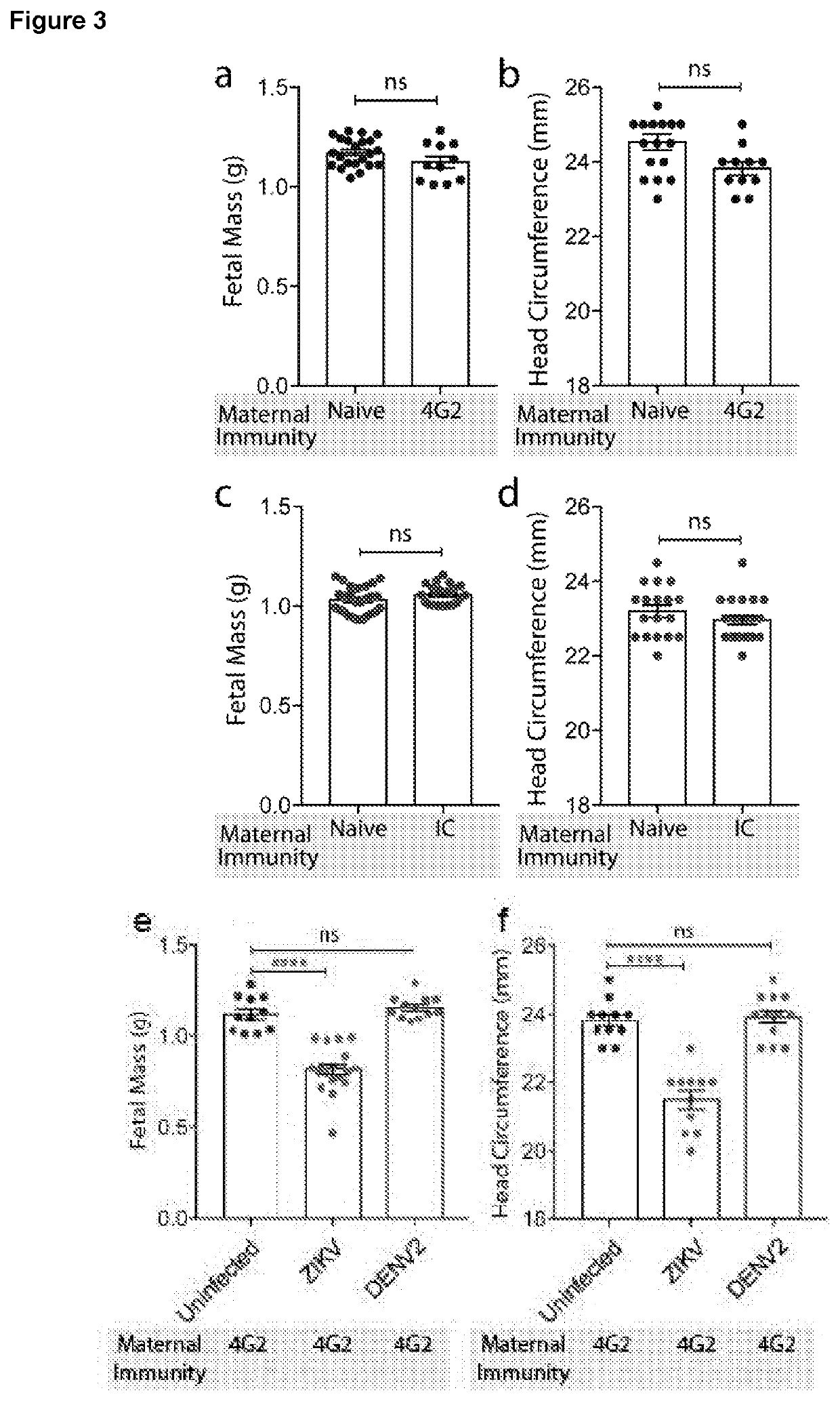
Targeted prevention of maternal to foetal vertical transmission of infection
ActiveUS20210115136A1Reducing vertical transmissionInhibitory activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsZika virusFetus fetus
The present invention relates to targeted prevention of vertical transmission of infection between mother and foetus. More particularly, the invention relates to methods of preventing neonatal Fc receptor (FcRn)-mediated transmission of virus, preferably flavivirus, more preferably Zika virus (ZIKV), from a mother immune to a cross-reactive virus e.g. Dengue virus (DENY) to her foetus, and to reagents that block or inhibit said FcRN-mediated transmission of virus.
Owner:NAT UNIV OF SINGAPORE
Methods and reagents for modulating macrophage phenotype
PendingUS20220031741A1Restore immune recognitionEasy to keepOrganic active ingredientsPeptide/protein ingredientsCell phenotypePotocytosis
The present invention is directed to methods of inducing a phenotypic change in a population of monocytes and; or macrophages. The method includes administering to the population of monocytes and / or macrophages, a macrophage stimulating agent coupled to a carrier molecule, wherein the carrier molecule facilitates macropinocytic uptake of the agent by monocytes and macrophages in the population and is defective in neonatal Fc receptor binding, wherein the administering induces a phenotypic change in the monocytes and macrophages in the population.
Owner:NEW YORK UNIV
Method for selecting antibodies with modified fcrn interaction
InactiveUS20170211876A1Clean and crisp-tasting waterLighting and heating apparatusImmunoglobulinsVariable domainReceptor for activated C kinase 1
Herein is reported a method for selecting a full length antibody comprising the steps of a) generating from a parent full length antibody a plurality of full length antibodies by randomizing one or more amino acid residues selected from the amino acid residues at positions 1-23 in the heavy chain variable domain (numbering according to Kabat), at positions 55-83 in the light chain variable domain (numbering according to Kabat), at positions 145-174 in the first heavy chain constant domain (numbering according to EU index), and at positions 180-97 in the first heavy chain constant domain (numbering according to EU index), b) determining the binding strength of each of the full length antibodies from the 10 plurality of antibodies to the human neonatal Fc receptor (FcRn), and c) selecting a full length antibody from the plurality of full length antibodies that has a different binding strength to the FcRn than the parent full length antibody.
Neonatal Fc receptor binding dimer and methods of use
The present disclosure relates to dimers of engineered polypeptides having a binding affinity for the neonatal Fc receptor FcRn, and provides an FcRn binding dimer, comprising a first monomer unit, a second monomer unit and an amino acid linker, wherein the first and second monomer units each comprises an FcRn binding motif. The FcRn binding dimer binds FcRn with higher capacity compared to the first monomer unit or second monomer unit alone. The present disclosure also relates to the use of the FcRn binding dimer as an agent for modifying pharmacokinetic and pharmacodynamic properties and as a therapeutic agent.
Owner:AFFIBODY TECH AB
Antibodies for binding psma with reduced affinity for the neonatal fc receptor
PendingUS20220323619A1Reducing serum half-lifeLow affinityImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntiendomysial antibodiesHeavy chain
The invention relates to anti-PSMA antibodies comprising a heavy chain constant region comprising one or more amino acid substitutions compared to a wild-type IgG, wherein the one or more amino acid substitutions reduce the affinity of the antibody for the neonatal Fc receptor (FcRn), thereby reducing the serum half-life of the modified antibody compared to a wild-type antibody of class IgG. The one or more amino acid modification having the effect of reducing FcRn binding is selected from positions His310, His433, His435, His436, Ile253. Antibodies of the present invention are particularly suited for use in radioimmunotherapy.
Owner:TELIX INT PTY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com