Transgenic animal with enhanced immune response and method for the preparation thereof
A genetically modified, non-transgenic technology, applied in the field of immunology, can solve the problem of failing to point out the advantages of immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
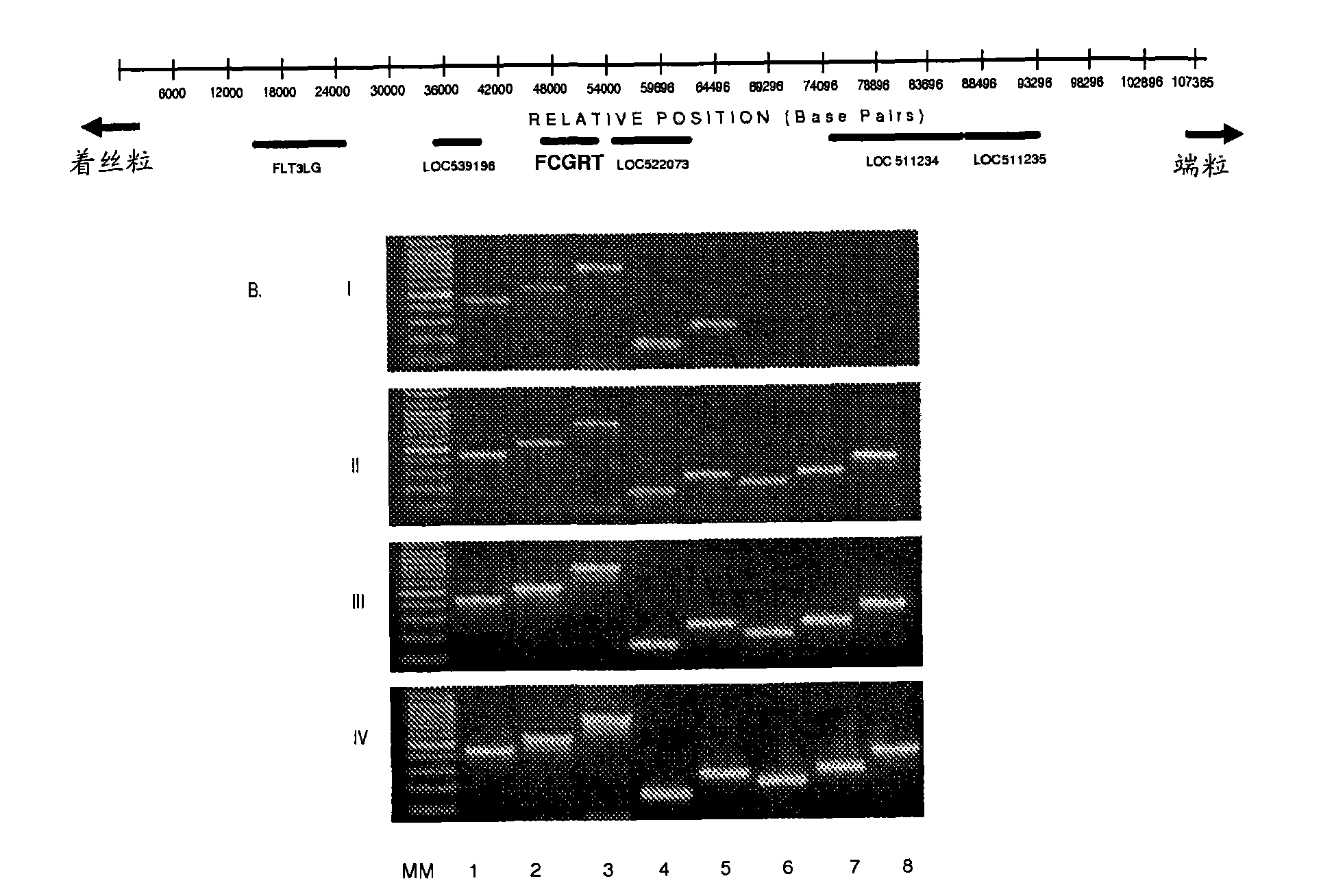
[0173] Example 1 - Isolation and characterization of a BAC clone carrying the bFcRn alpha chain gene (bFCGRT)
[0174] Isolation of BAC clones carrying bFCGRT
[0175] The 90αBAC was isolated from a bovine BAC library obtained from an adult (2-year-old) male New Jersey cattle (acquired from the ResourceCenter / Primary Database of the German Genome Project (RZDP), Max Planck Institute of Molecular Genetics, Berlin, Germany; http: / / www.rzdp.de / ) preparation. bFCGRT positive BAC clones were identified by PCR screening with primers specific for bFcRn alpha chain mRNA, [206-425bp; GenBank AF139106) with BFclS: 5'-CAGTACCACTTCACCGCCGTGT-3' (SEQ.ID.NO.: 1) and BFclas : 5'-CTTGGAGCGCTTCGAGGAAGAG-3' (SEQ.ID.NO.: 2) amplification], next, bFCGRT DNA was sequenced with primers that can anneal to the exons of the gene (Kacskovics et al., 2000). To analyze the flanking region upstream of bFCGRT, the BAC
[0176] The DNA was digested with BamHI and the digested DNA was separated on an aga...
Embodiment 2
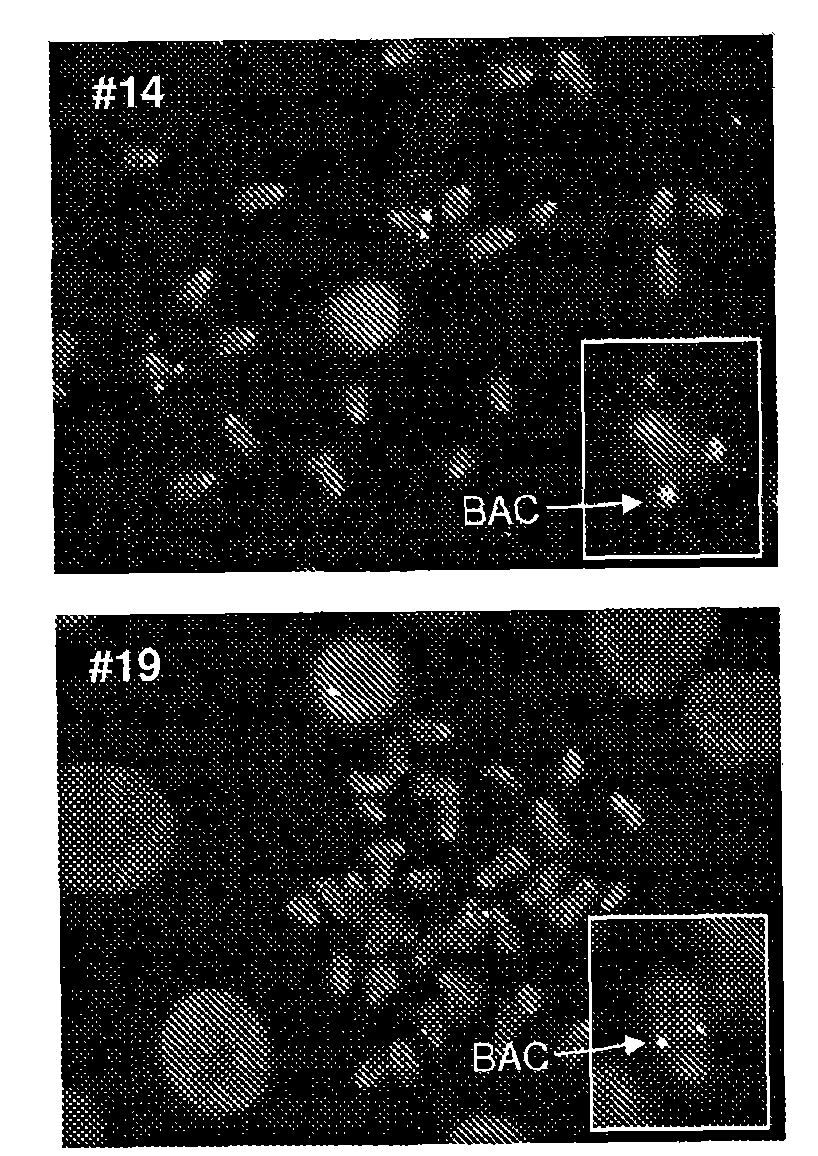
[0185]Example 2 - Generation and Genotyping of Transgenic Mice Carrying Bovine BAC 128E04
[0186] Preparation of a 102 kb genomic insert from BAC clone 128E04 for microinjection and generation of transgenic mice containing bovine BAC 128E04
[0187] BAC (clone 128E04) DNA was prepared for microinjection by slightly modifying the protocol published by Schedl et al. (Schedl et al., 1996). Purified BACs (Qiagen plasmid purification for very low copy number plasmids) were digested with Not I (Fermentas) to release the insert, which was separated on a precast pulsed field 1% agarose gel. Gel pieces containing the insert were electrophoresed into LMP (low melting point) gels which were digested with Gelase (Epicentre). A Microcon YM50 (Millipore) column was used to elute the insert from the agarose. The insert was washed in a buffer suitable for microinjection (10 mM Tris-HCl, pH 7.5, 0.1 mM EDTA, pH 8.0, 100 mM NaCl, with or without 0.03 mM spermine / 0.03 mM spermine (SIGMA)). t...
Embodiment 3
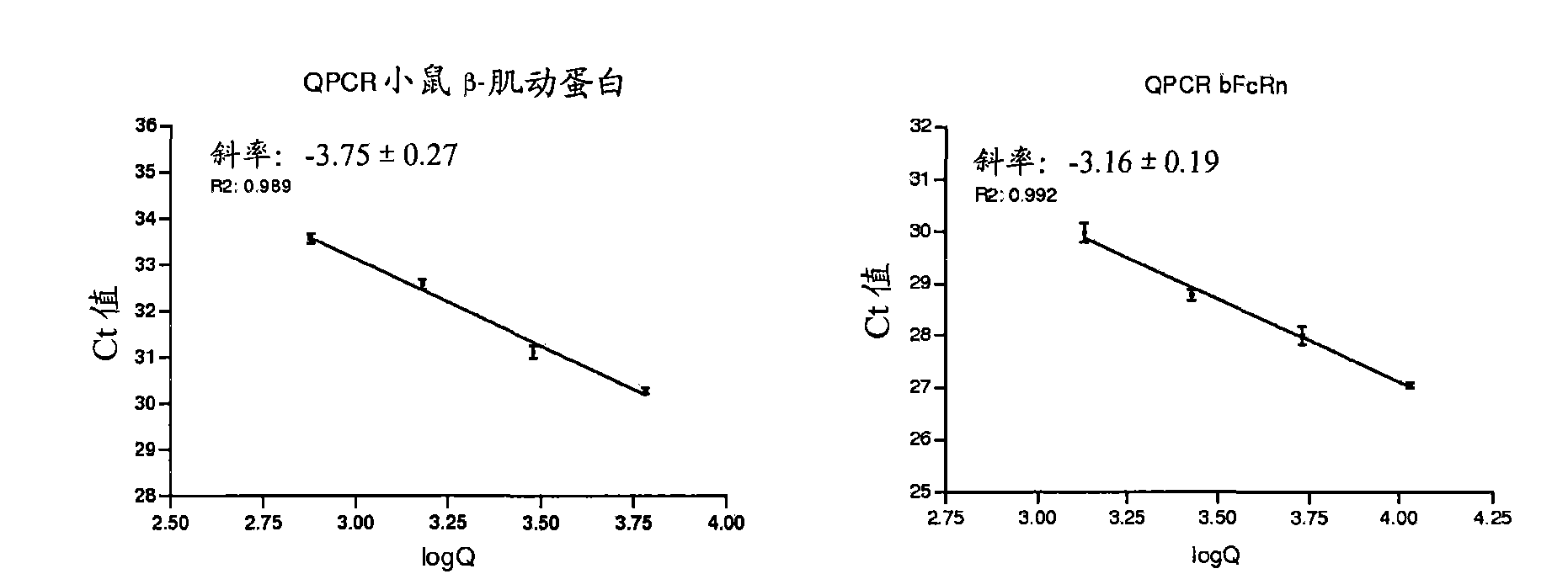
[0203] Example 3 - Effect of bovine FCGRT (bFCGRT) gene copy number on FcRn expression
[0204] Determination of Transgene Copy Number Using Real-time Quantitative Polymerase Chain Reaction
[0205] Real-time PCR is a quantitative and precise method for determining transgene copy number and zygosity in Tg animals (Tesson et al., 2002). The copy number of the 128E04 BAC transgene was determined by absolute quantification of bFCGRT and the internal standard mouse β-actin gene, as follows.
[0206] The copy number of the 128E04 BAC transgene was determined by TaqMan method using ABI Prism7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Primers and probe oligonucleotide sequences were designed by using default parameters (primers and probes shown in Table 3). The traditional phenol / chloroform method was used for DNA extraction from tail samples of hemizygous animals, which required an additional chloroform extraction step.
[0207] Table 3. Primers and prob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com