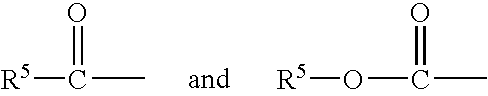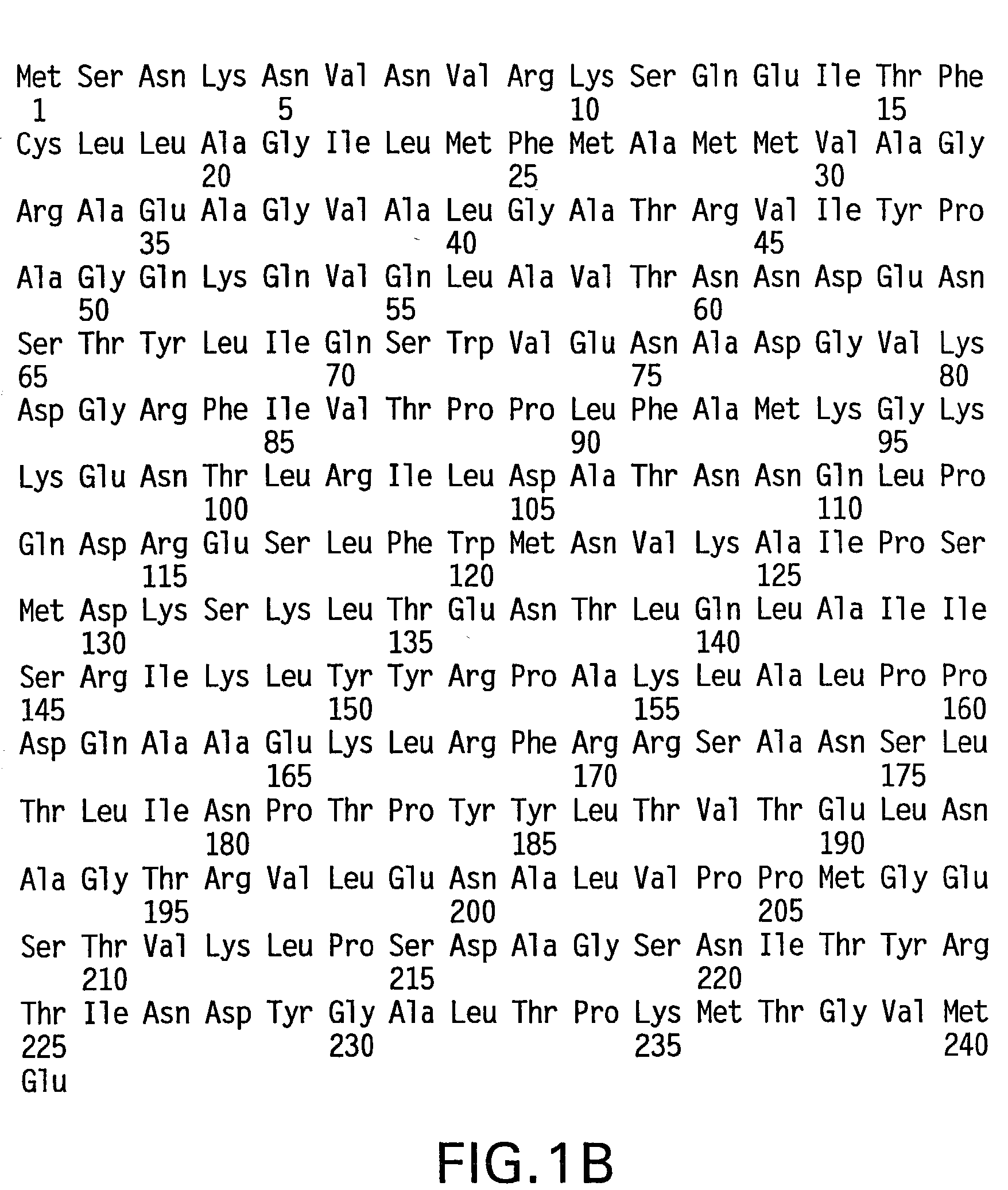Patents
Literature
434 results about "Human animal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Human Animal: A Personal View of the Human Species is a BBC nature documentary series written and presented by Desmond Morris, first transmitted in the United Kingdom from 27 July 1994. The series was produced in association with Discovery Channel.
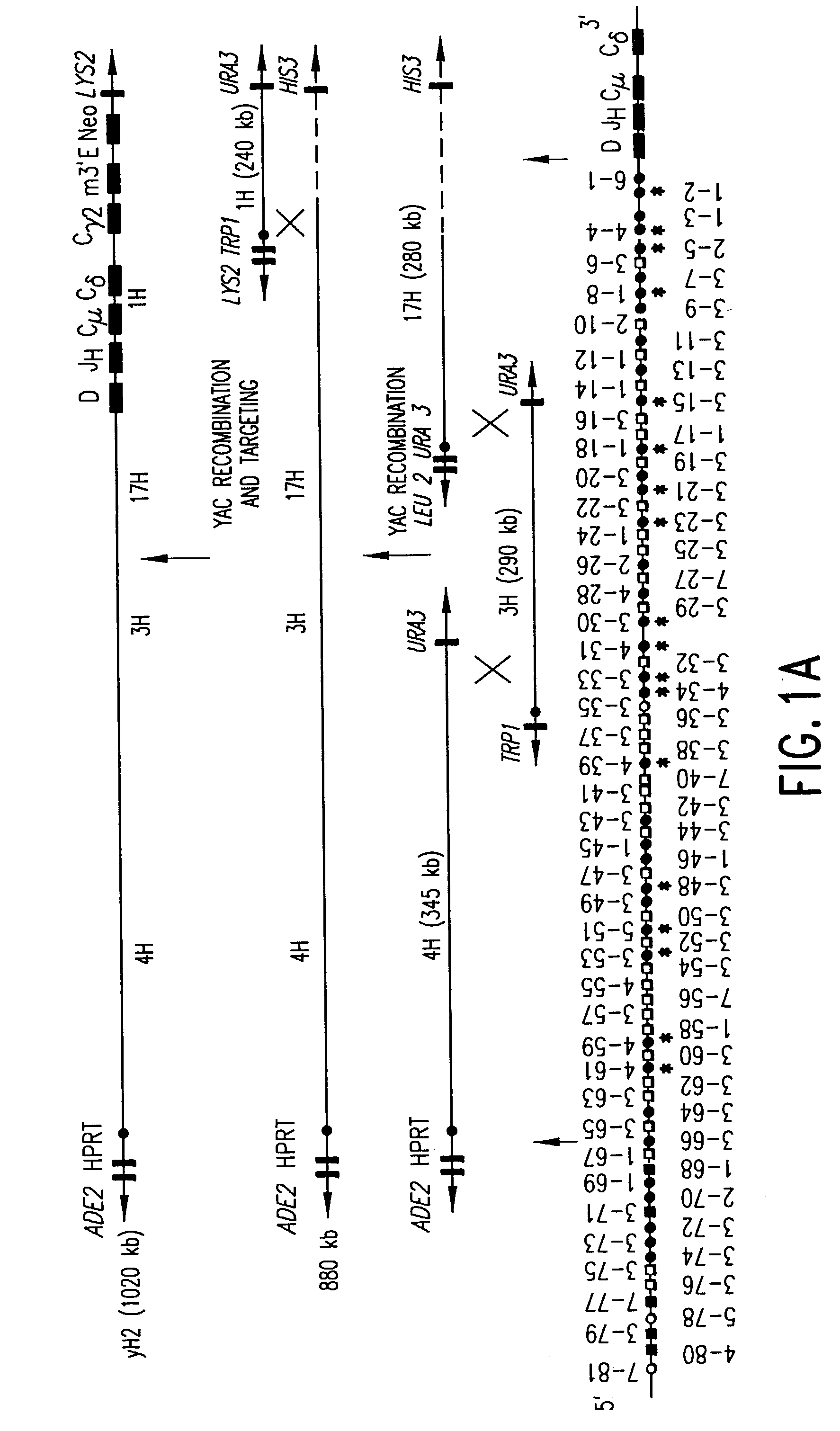
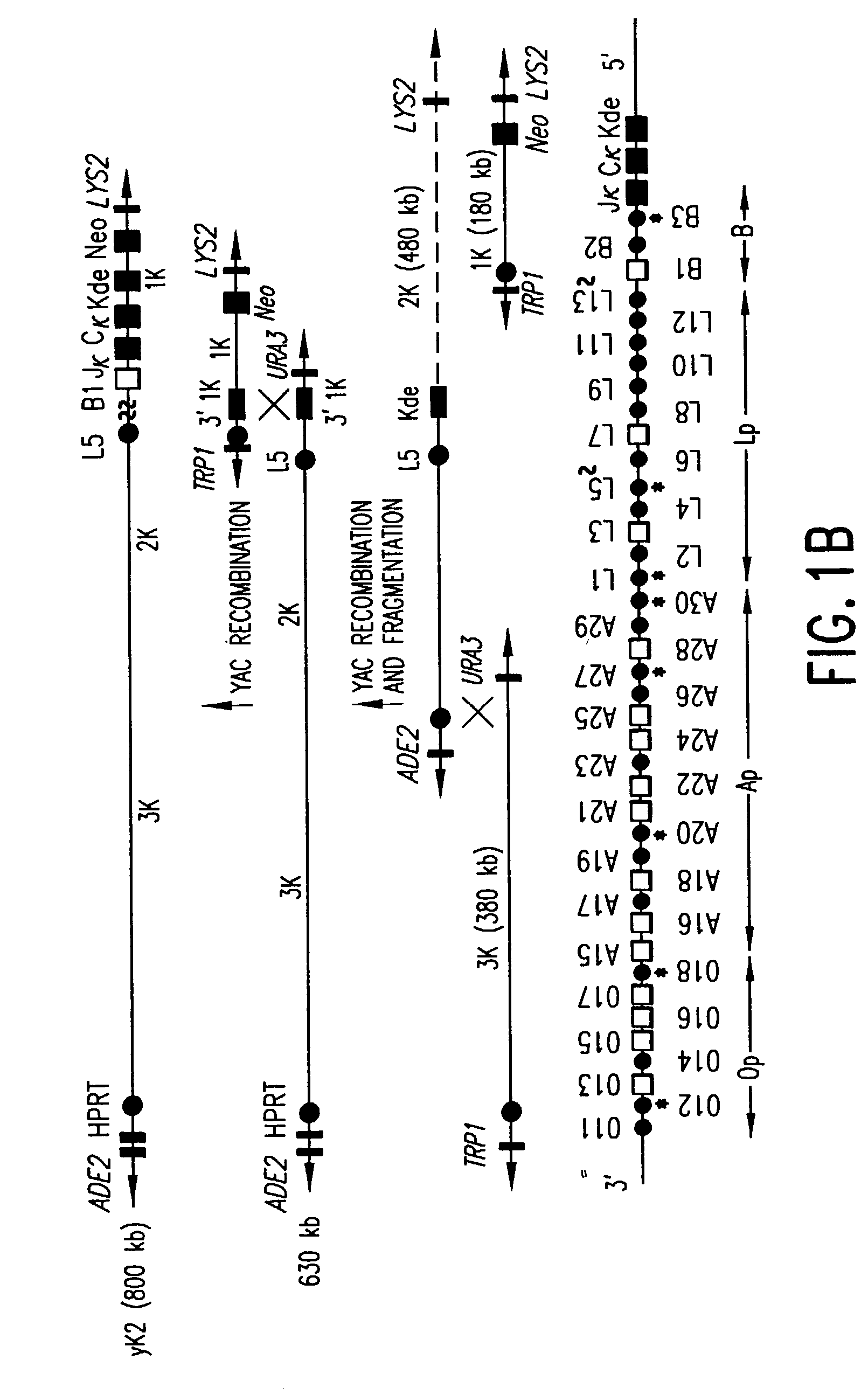
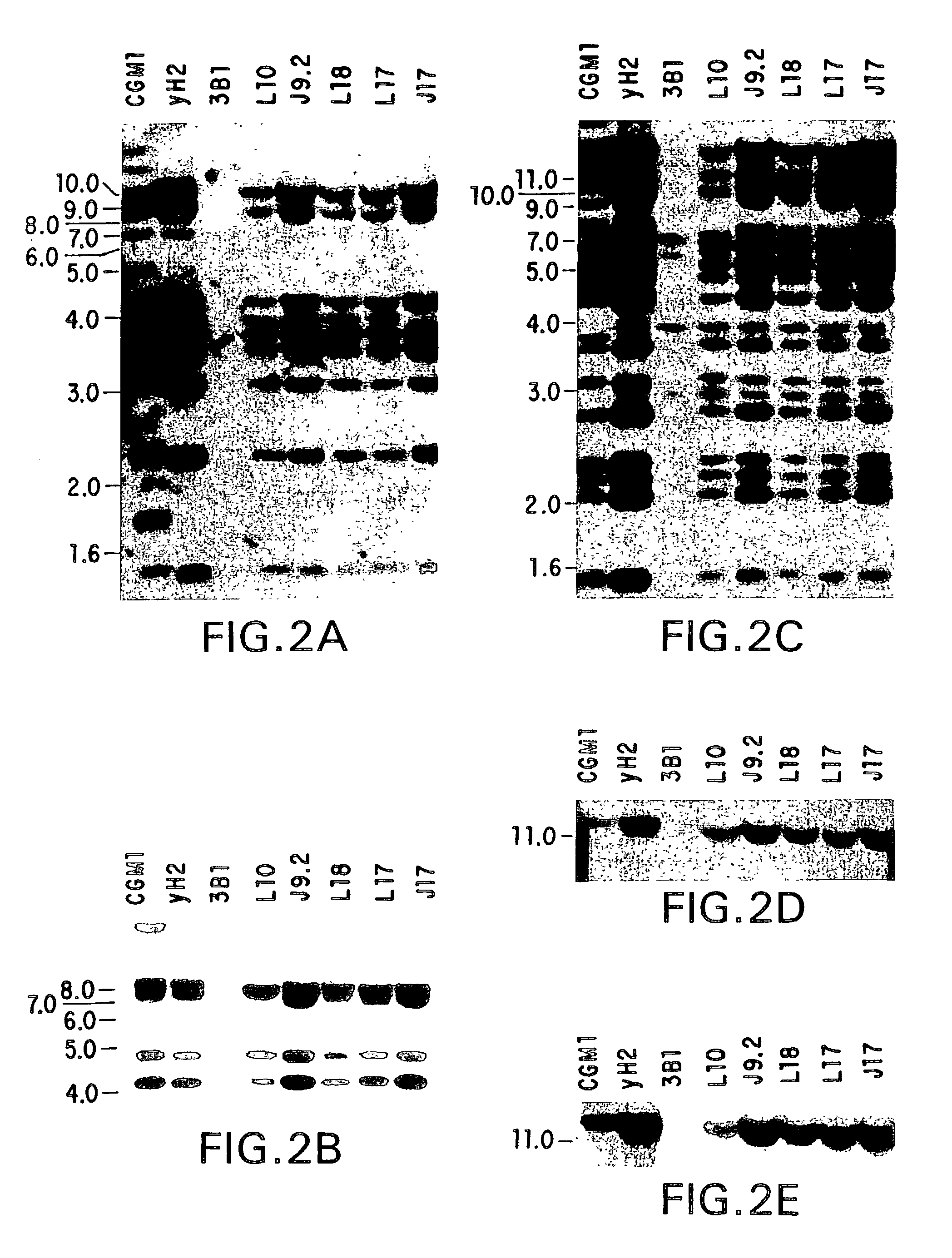
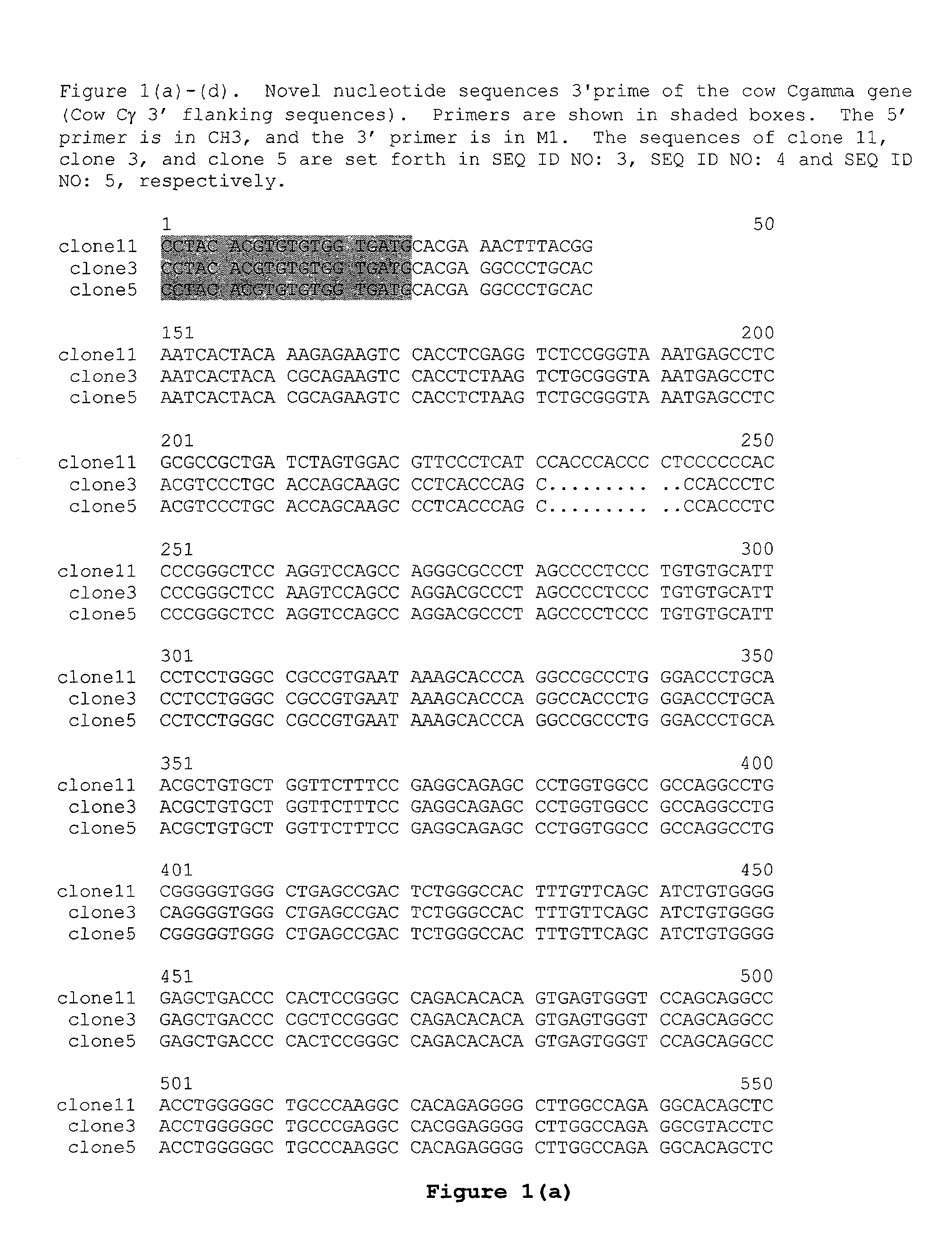
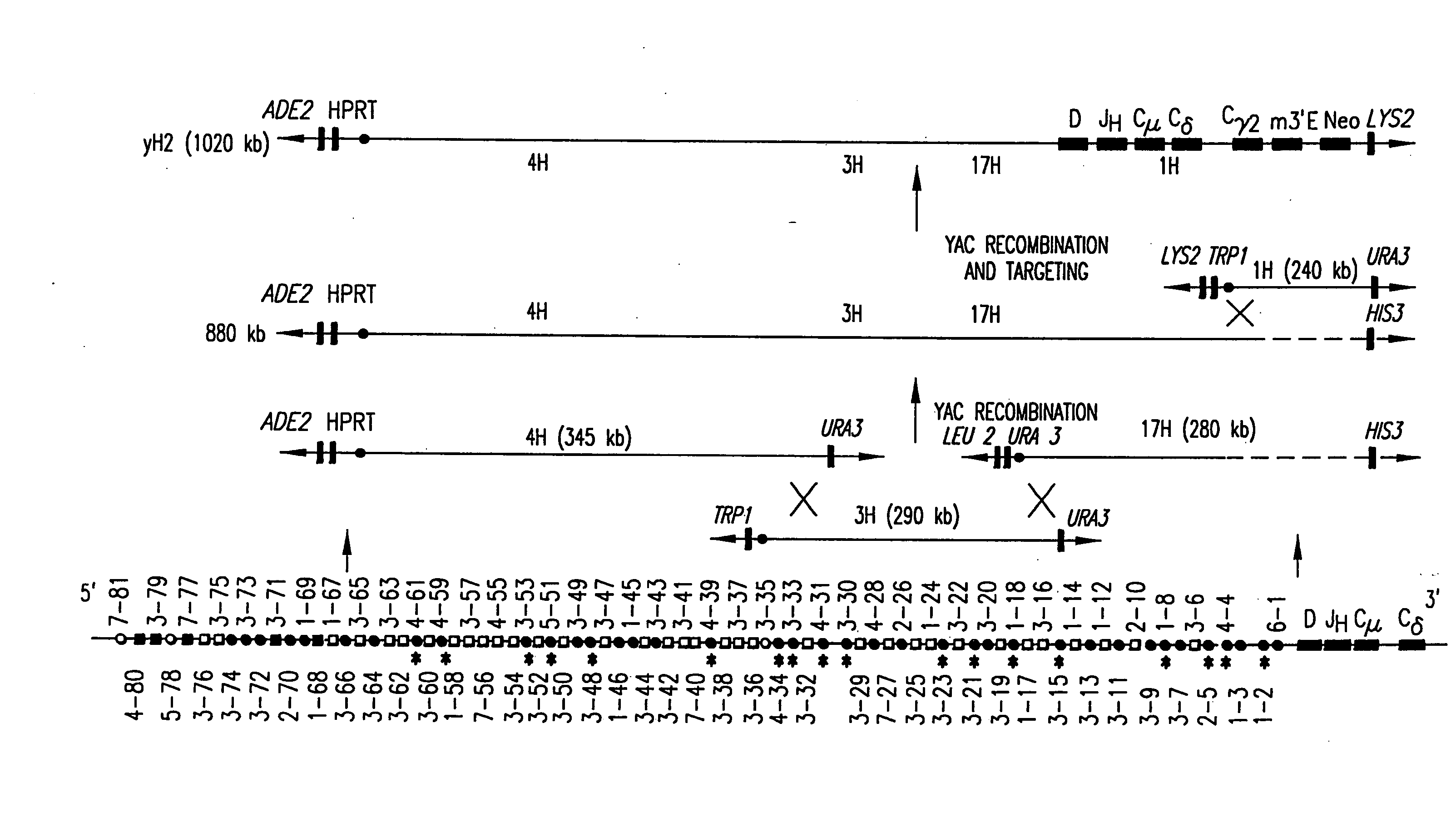
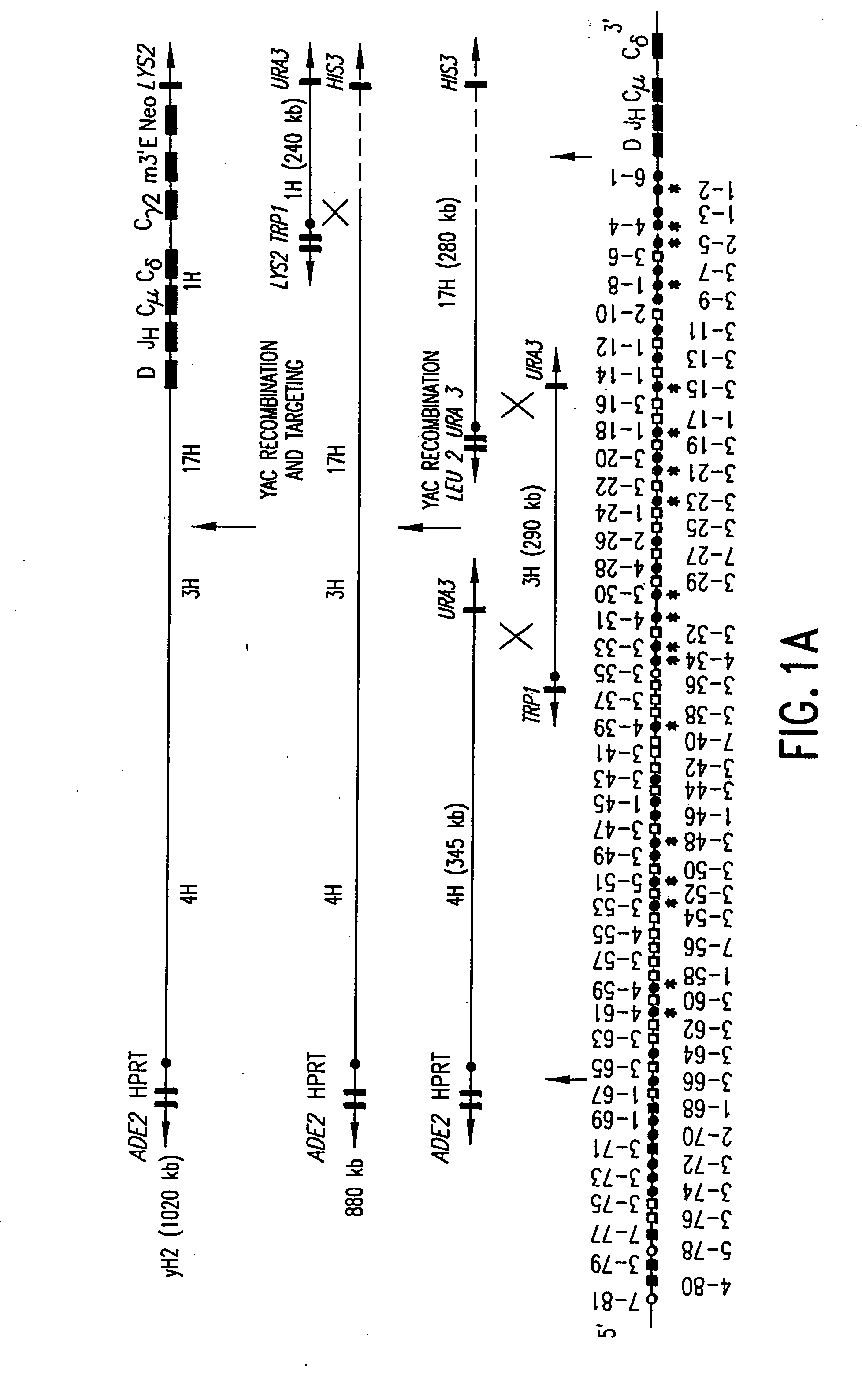
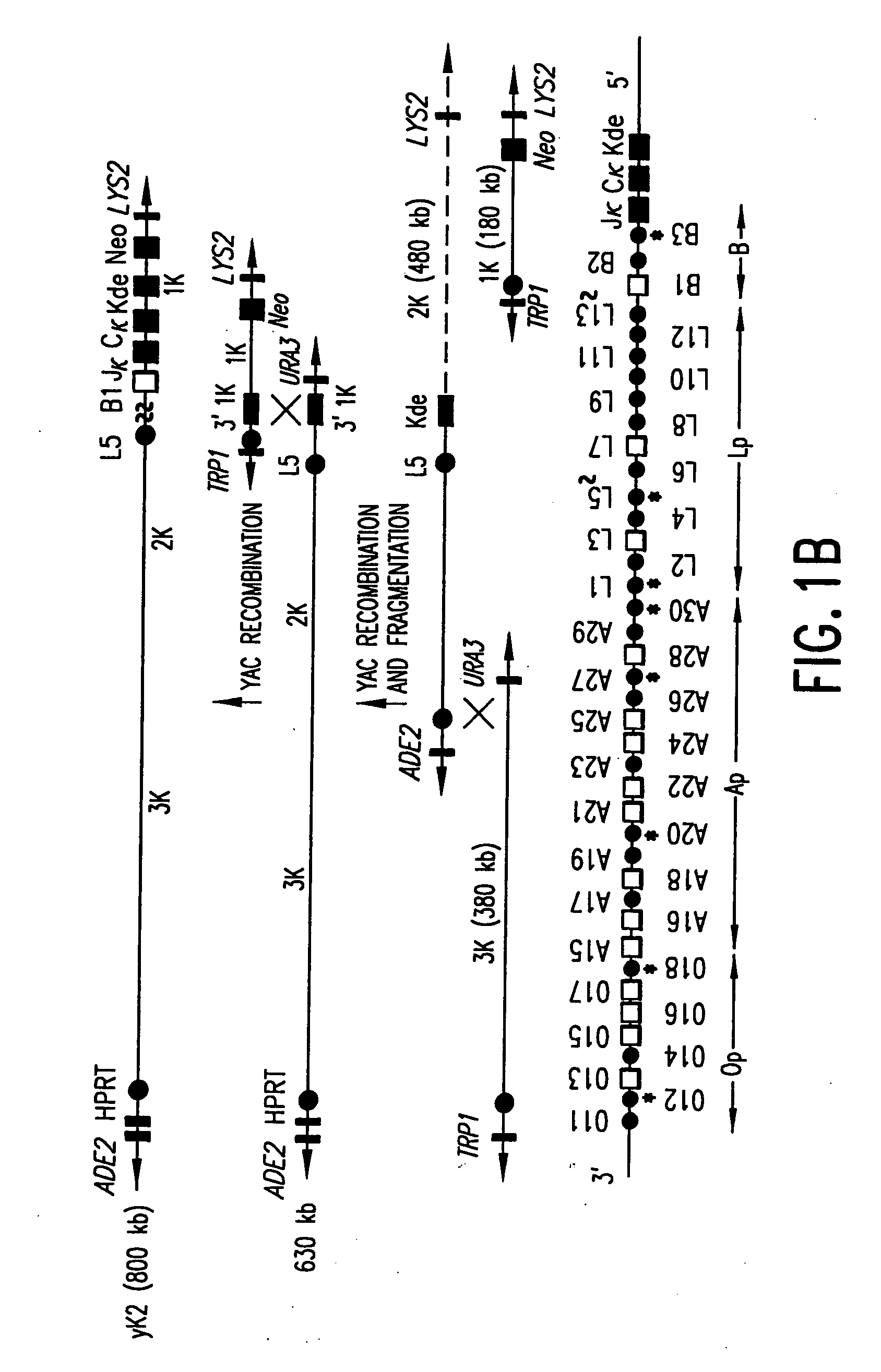
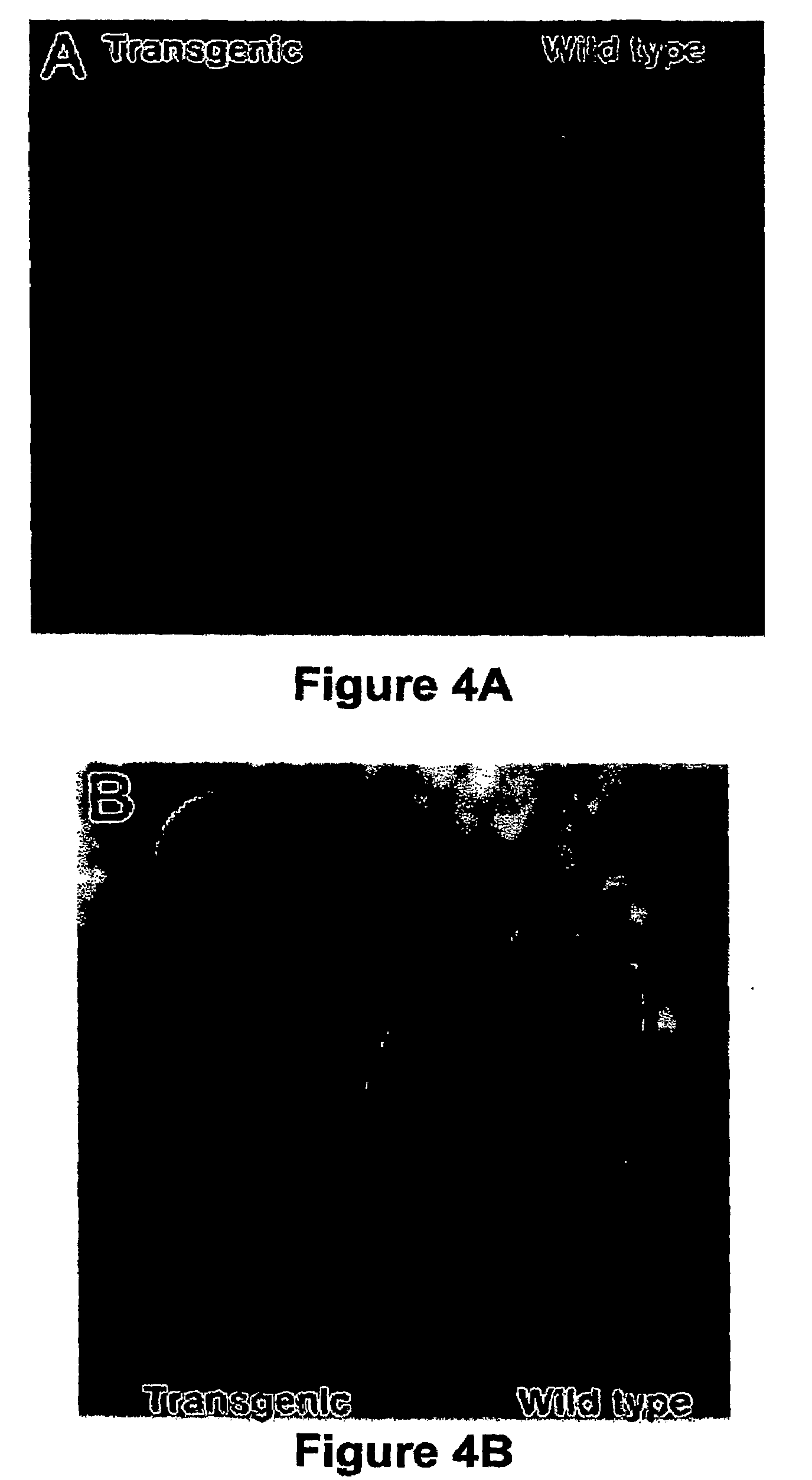
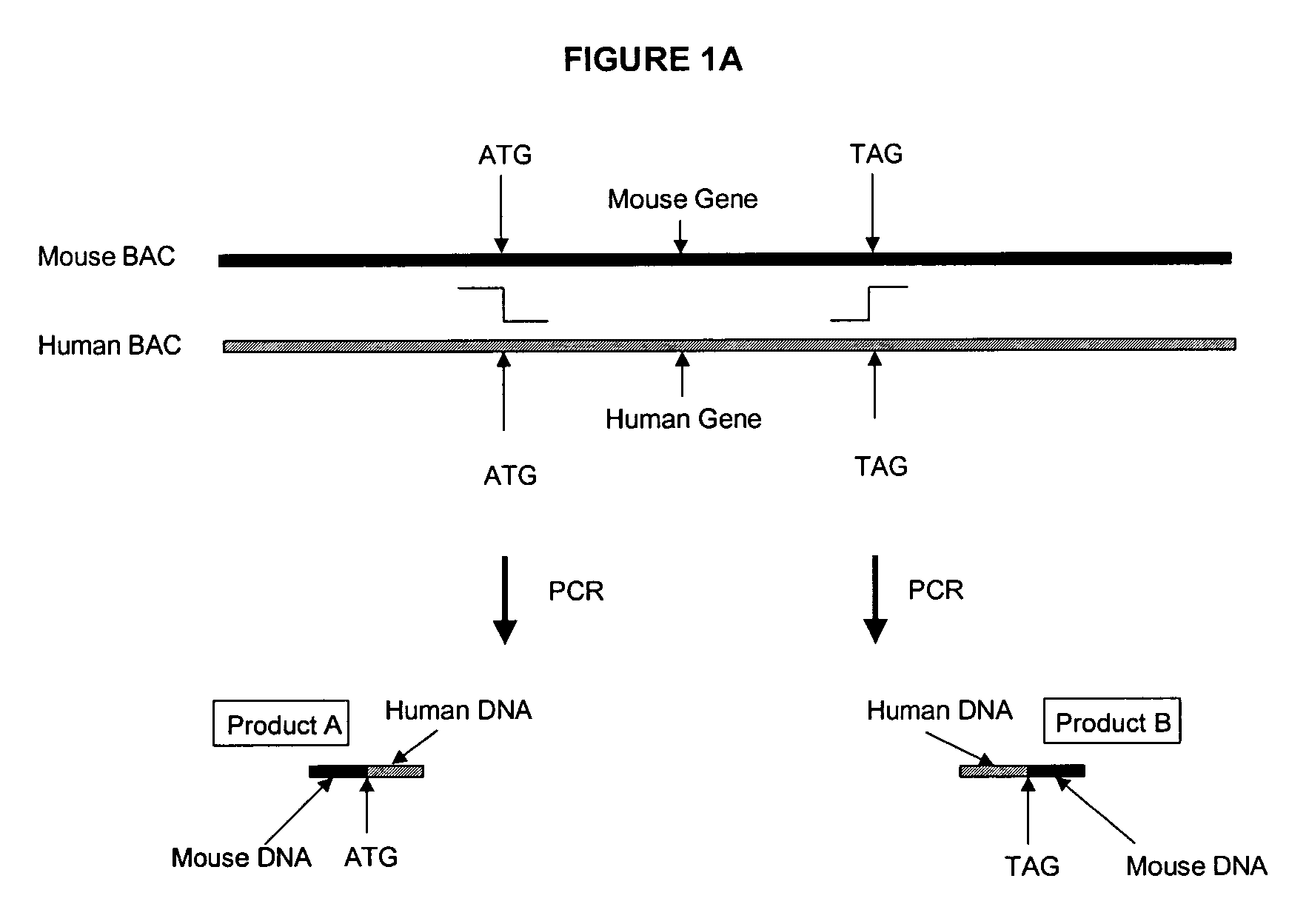
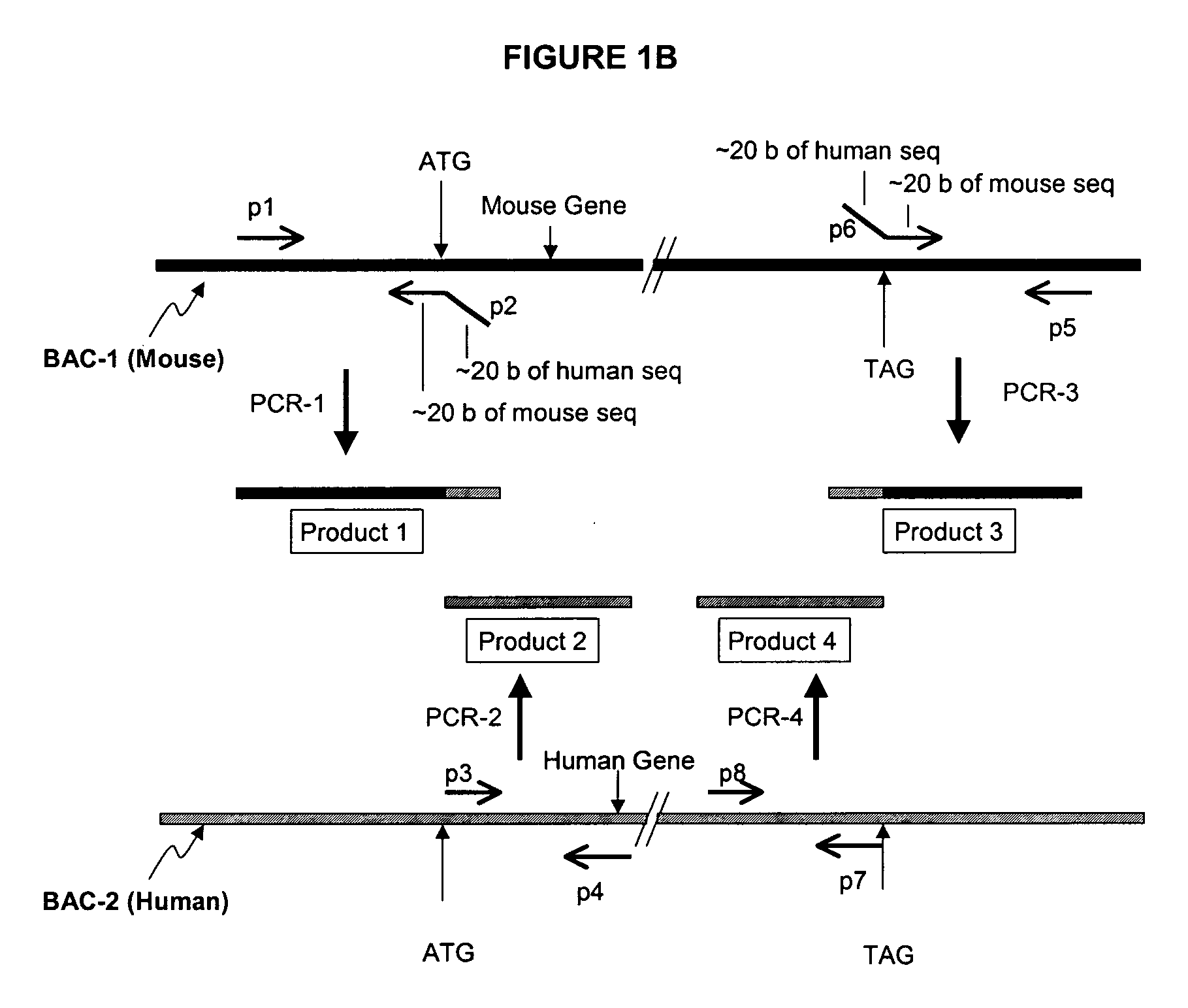
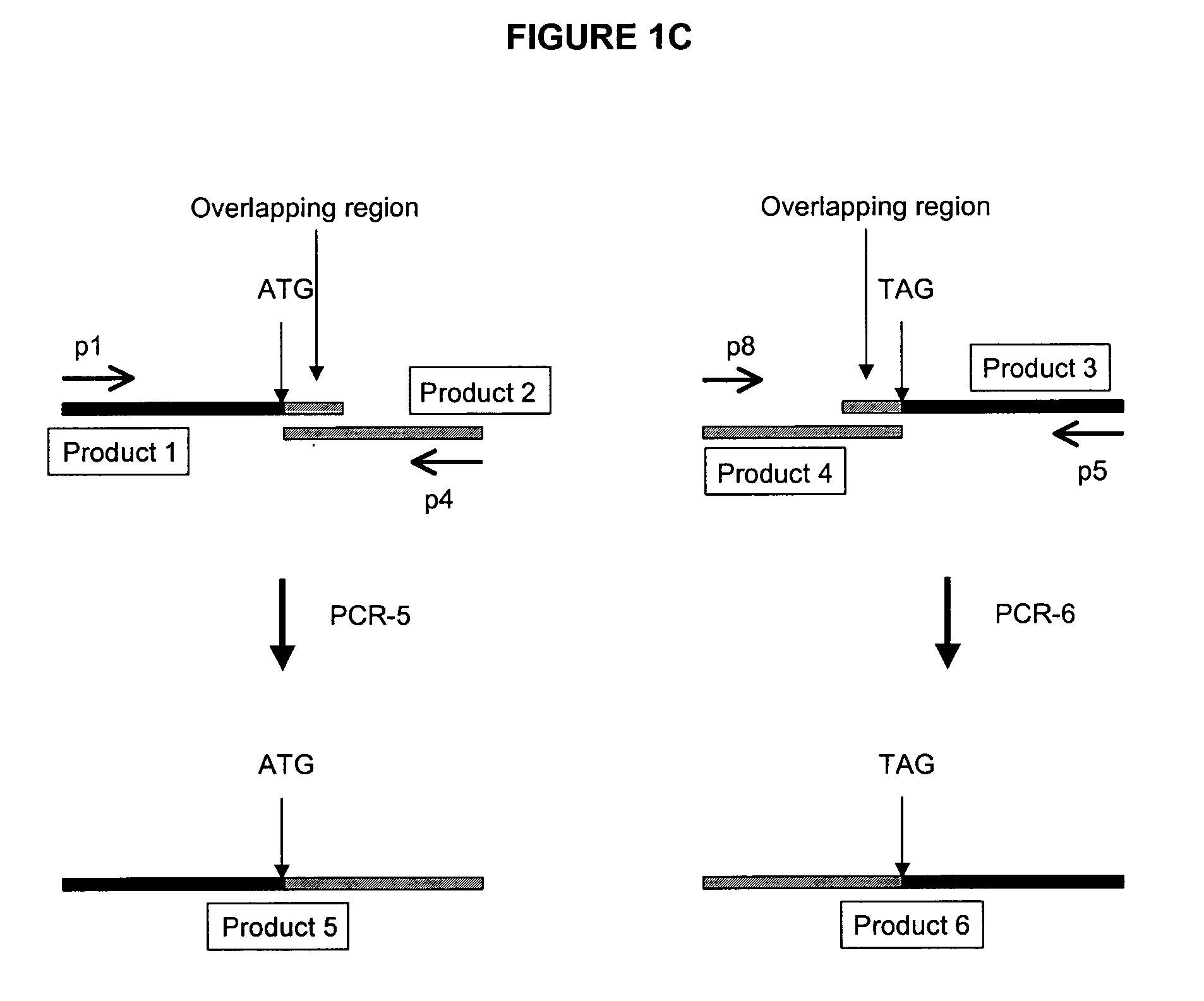
Transgenic non-human animals for producing chimeric antibodies
InactiveUS20060015957A1Inhibit expressionEasy to switchImmunoglobulinsGenetic engineeringAntigenHuman animal
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
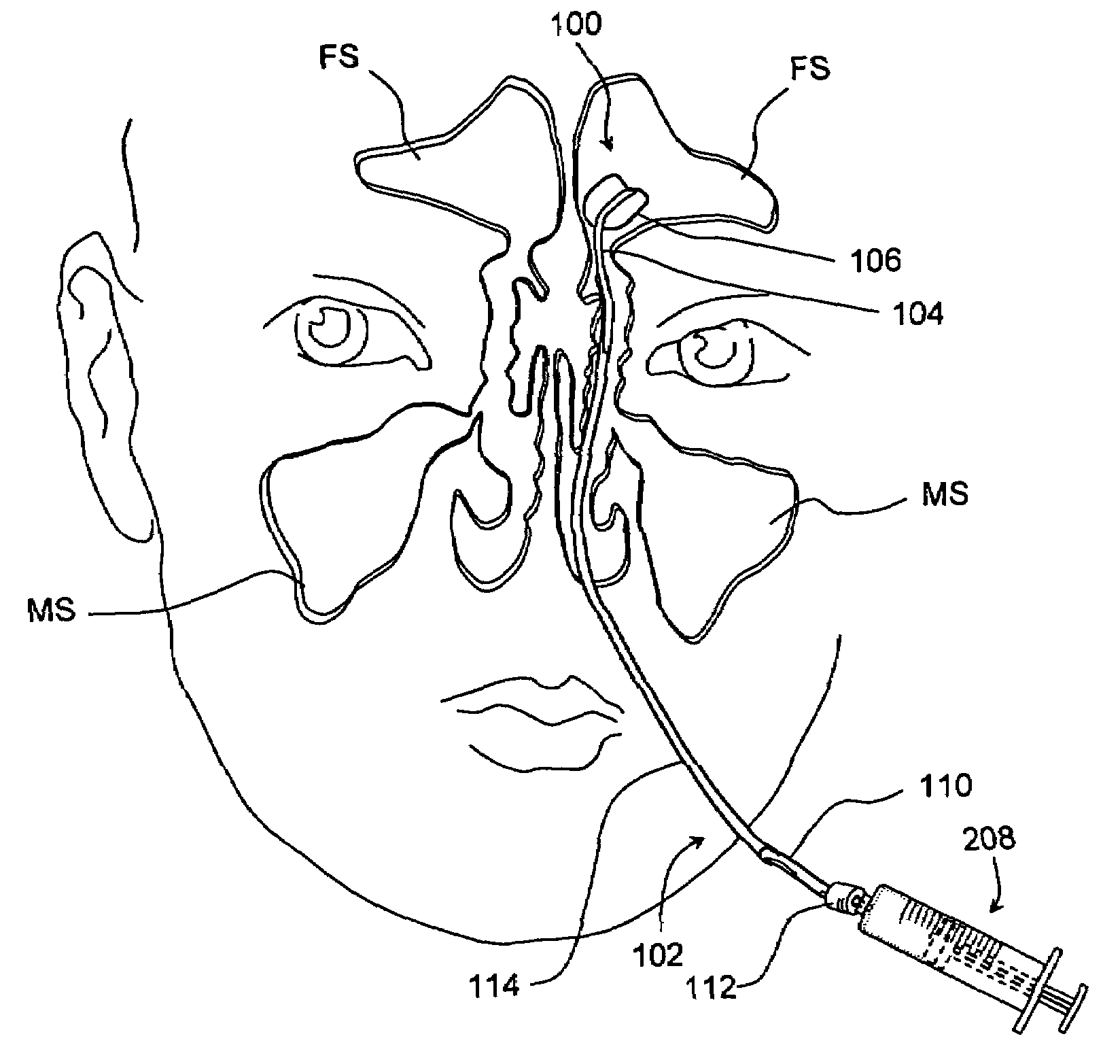
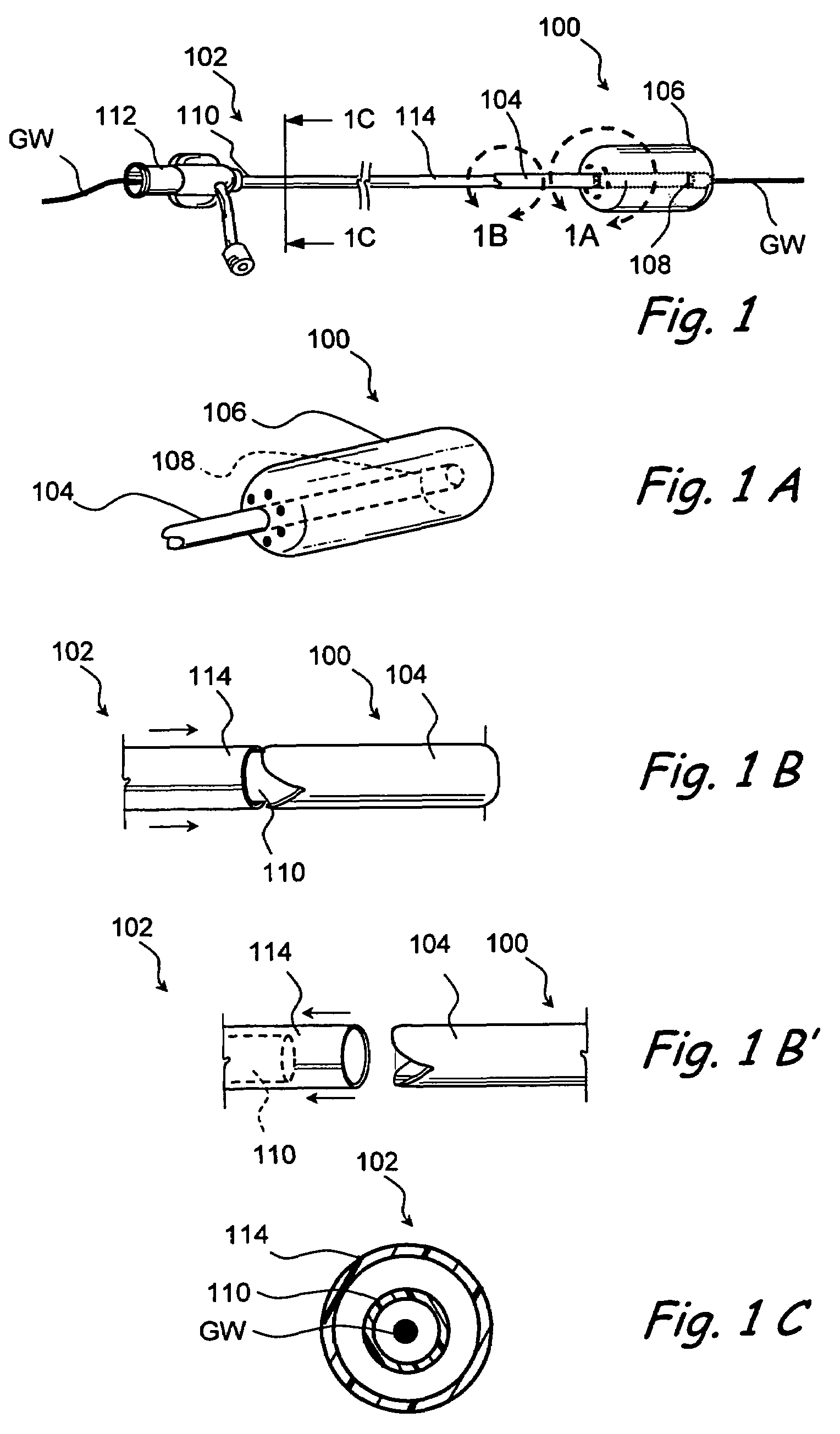
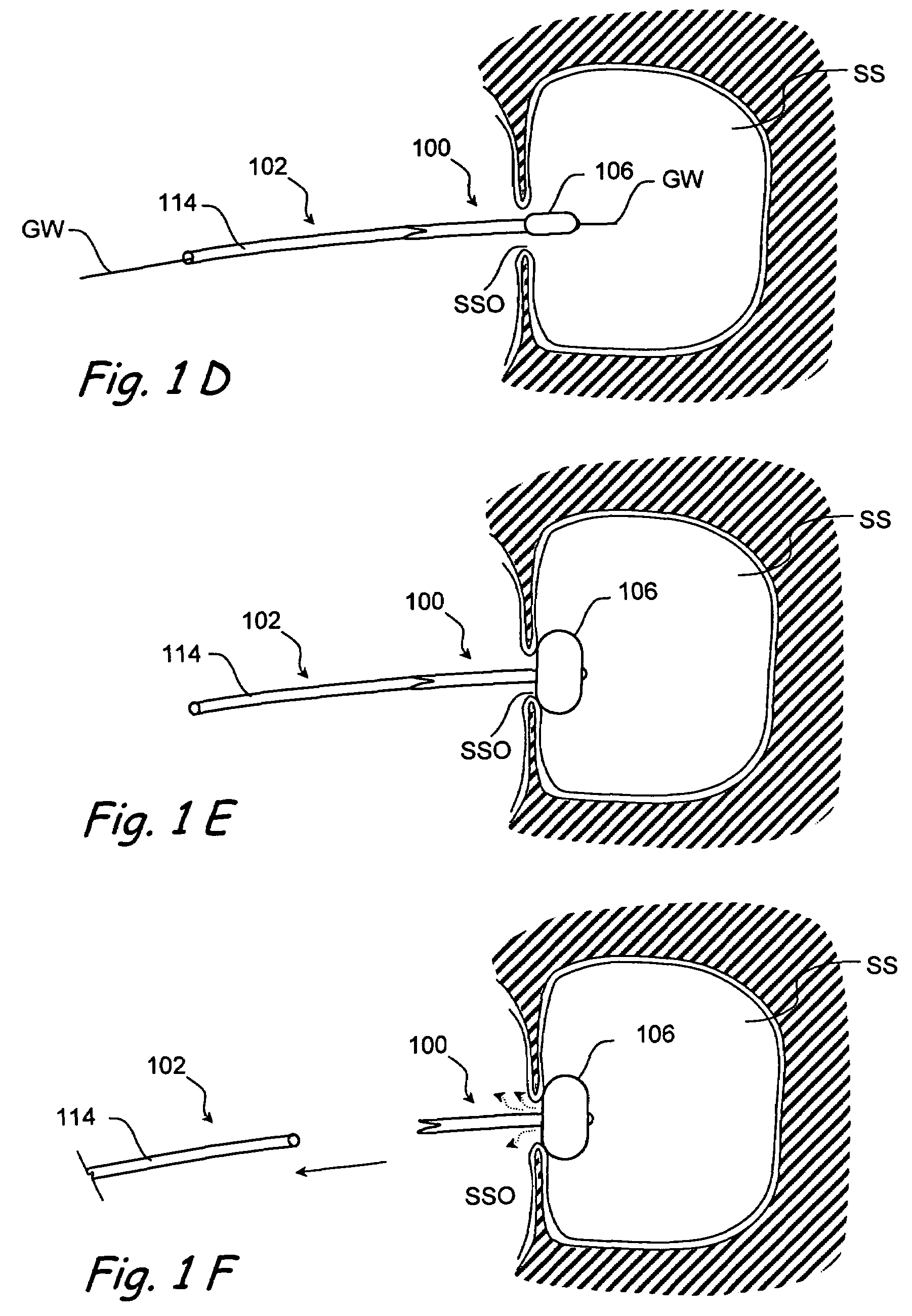
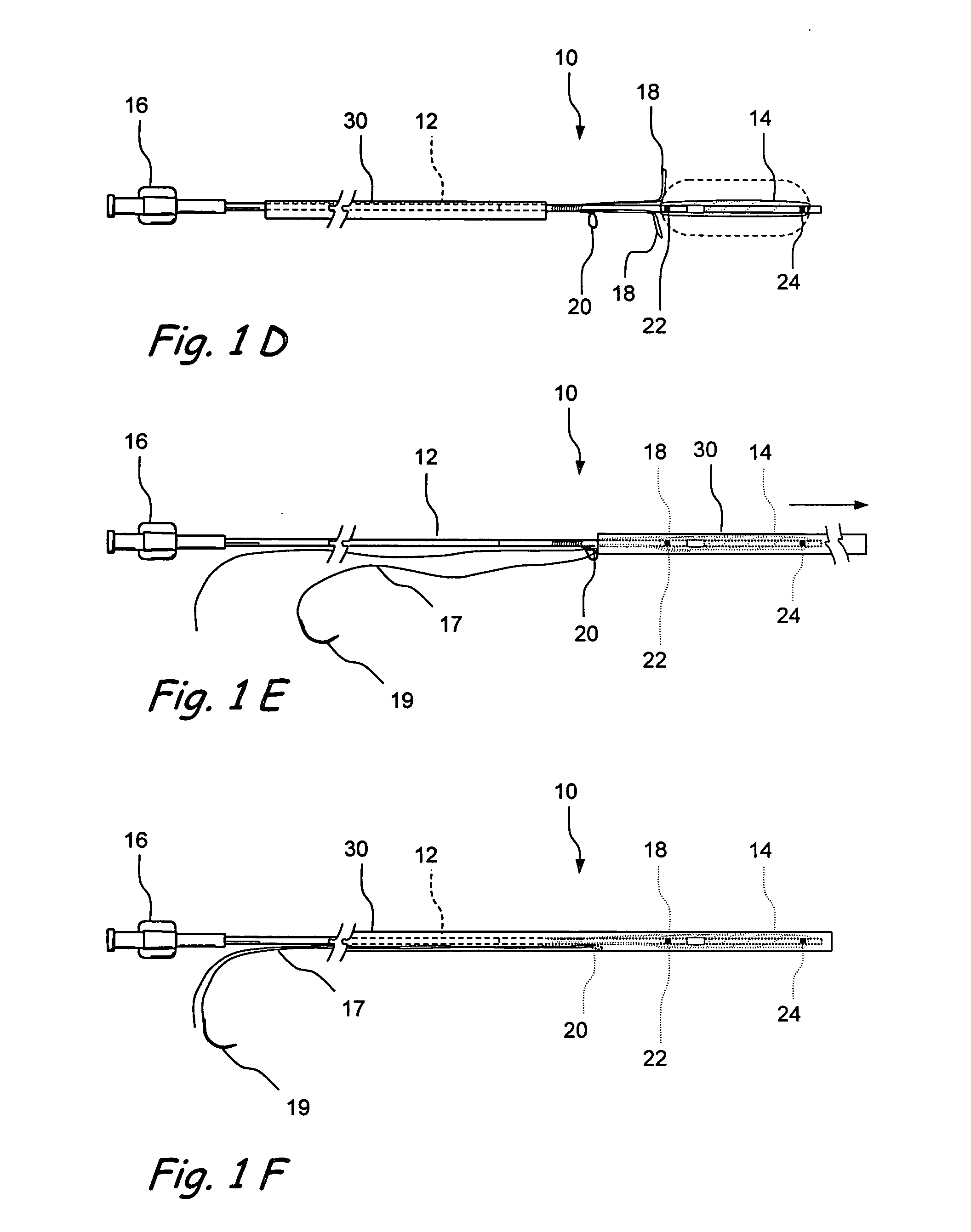
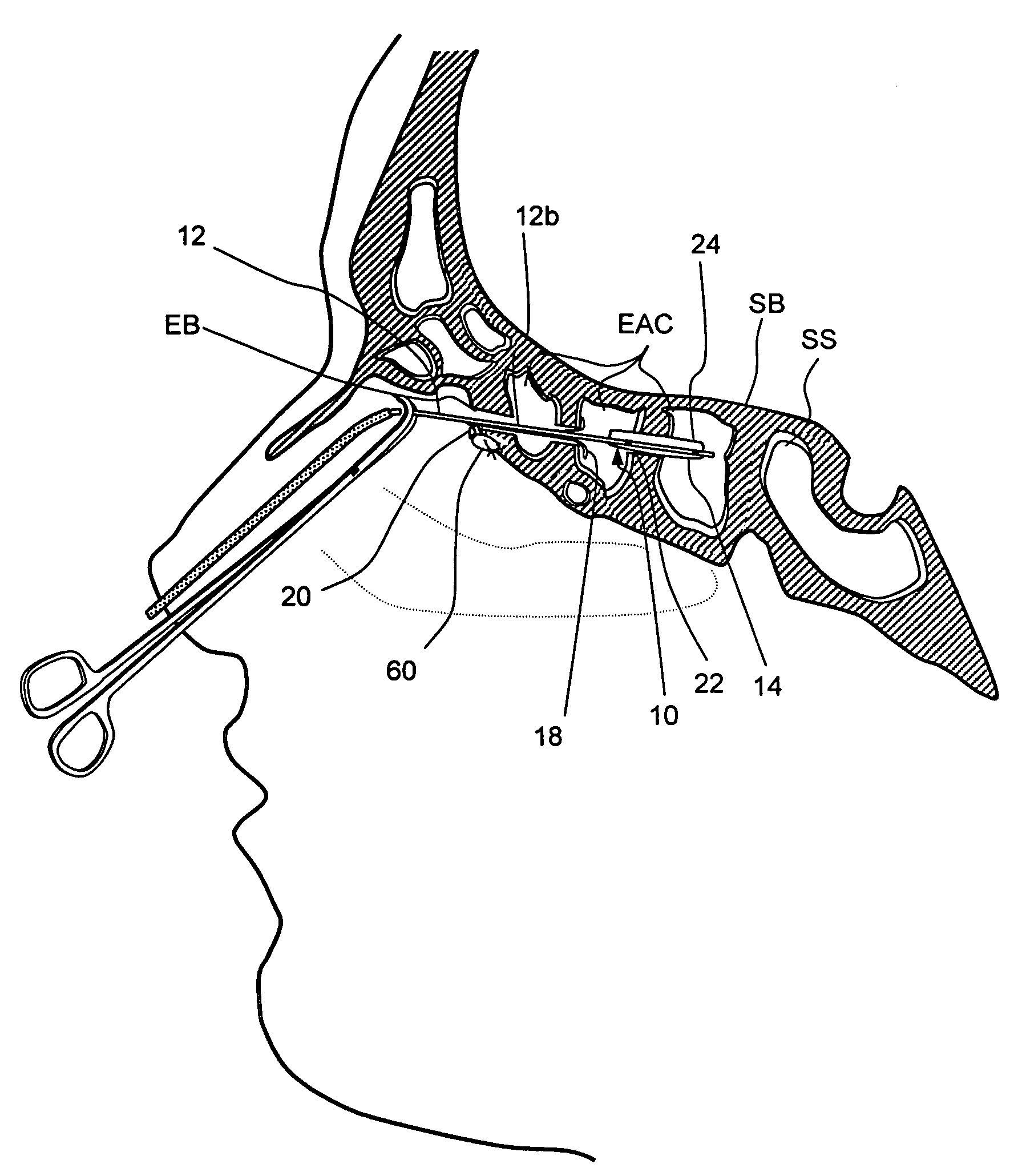
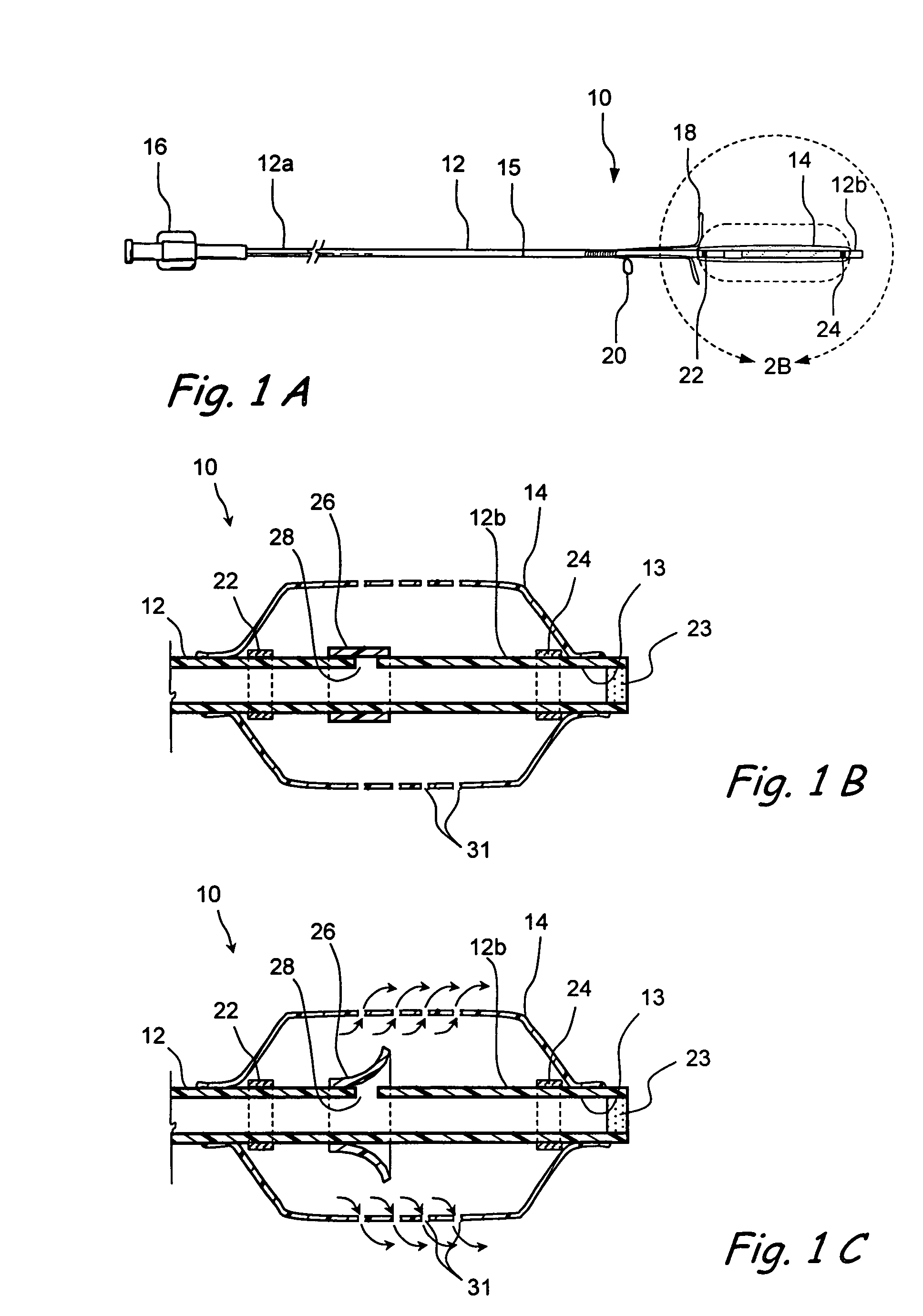
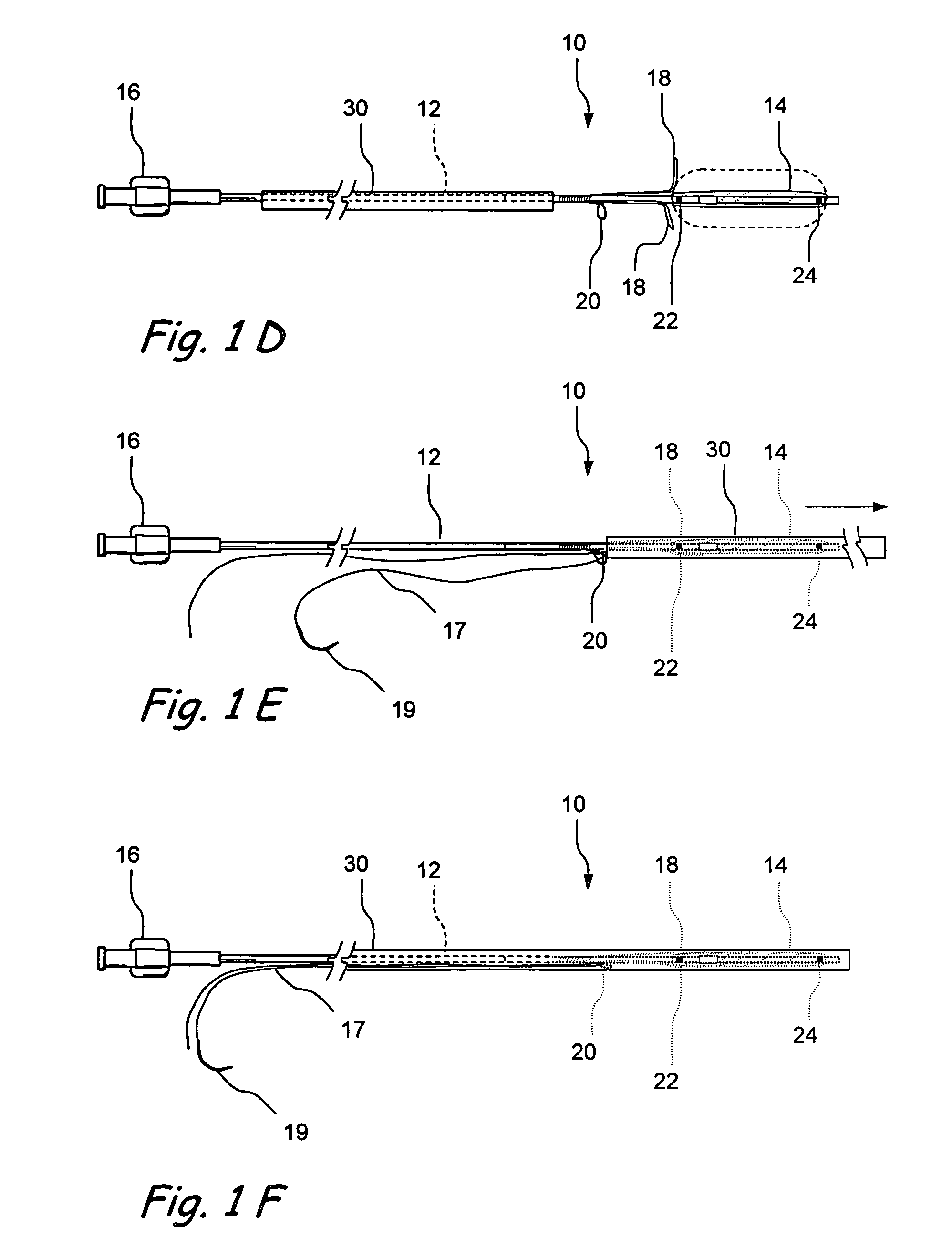
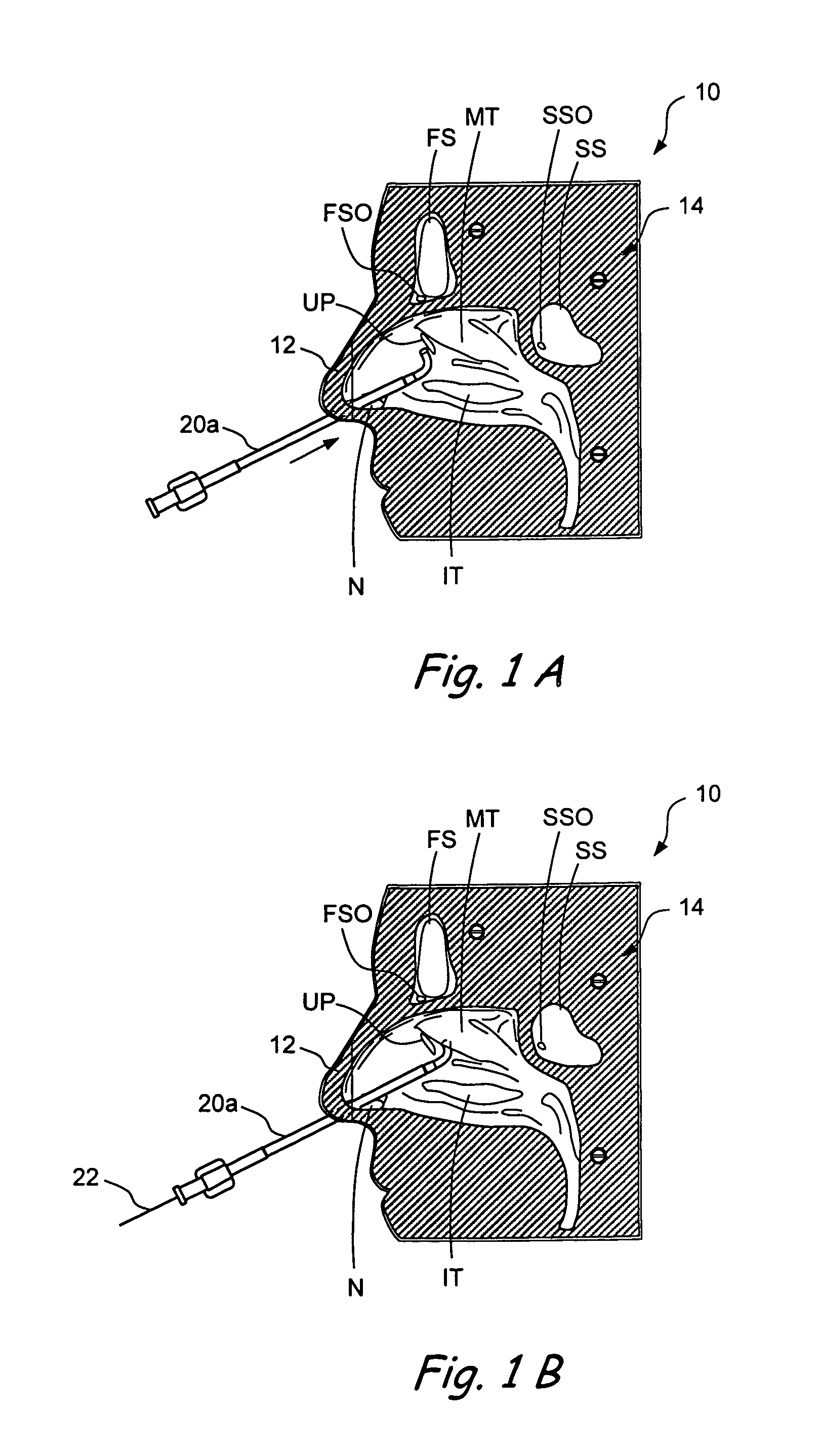
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
Implantable devices and methods for treating sinusitis and other disorders
ActiveUS20080015540A1Low profileMinimize traumaGuide needlesBalloon catheterParanasal sinusitisDisease
Devices, systems and methods for stenting, spacing, draining, ventilating and / or delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects, including methods and systems for treating paranasal sinusitis and ethmoid disease.
Owner:ACCLARENT INC
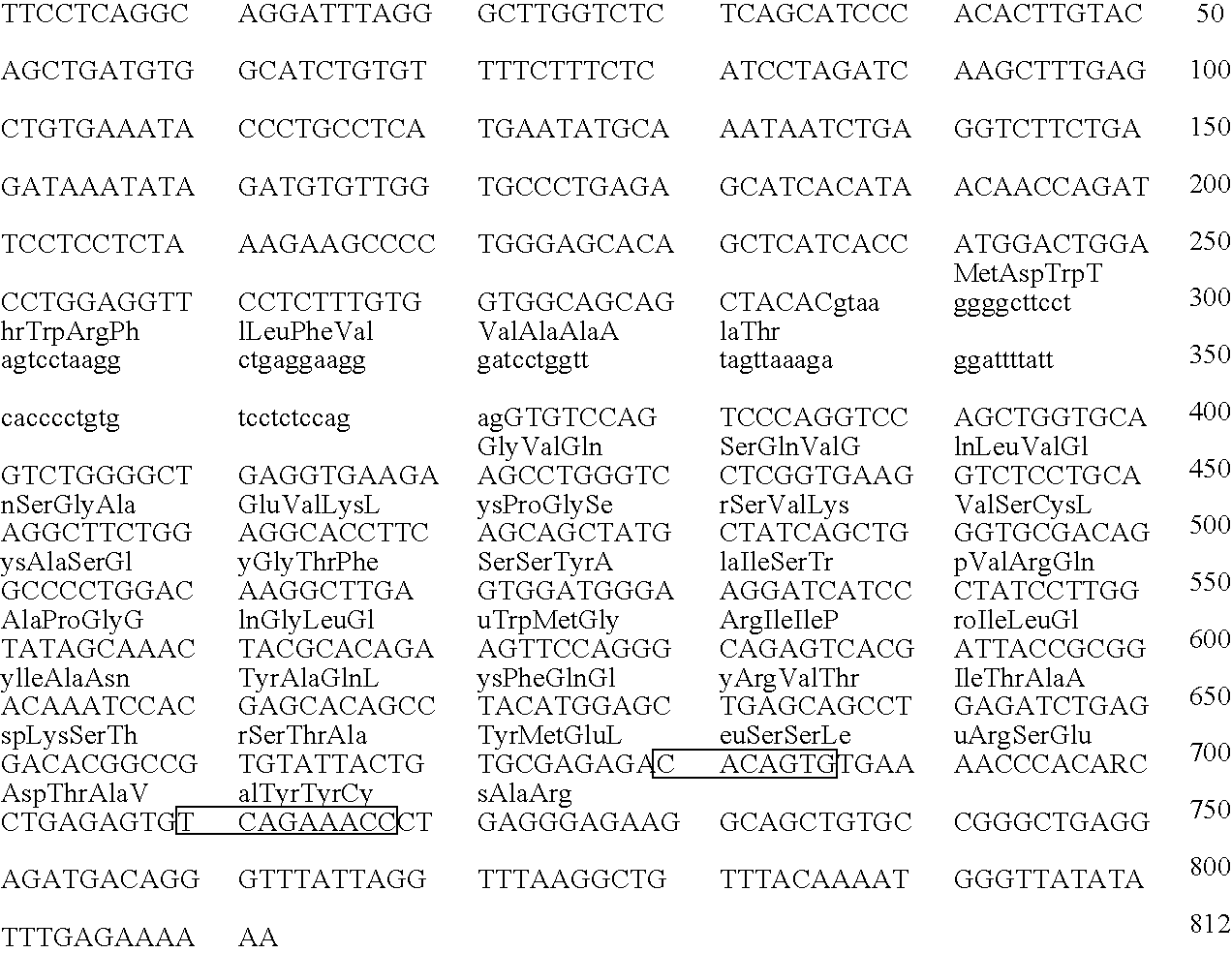
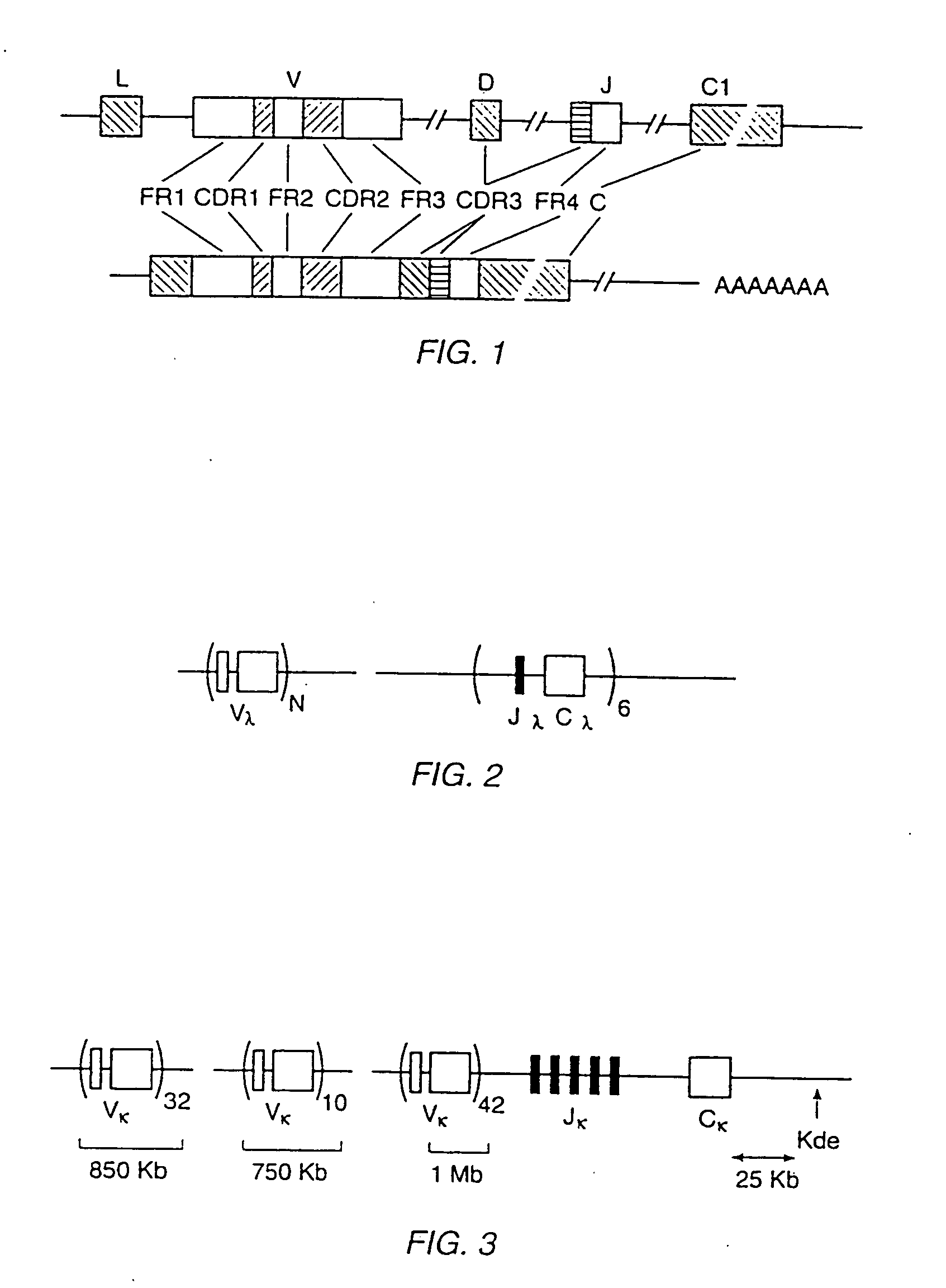
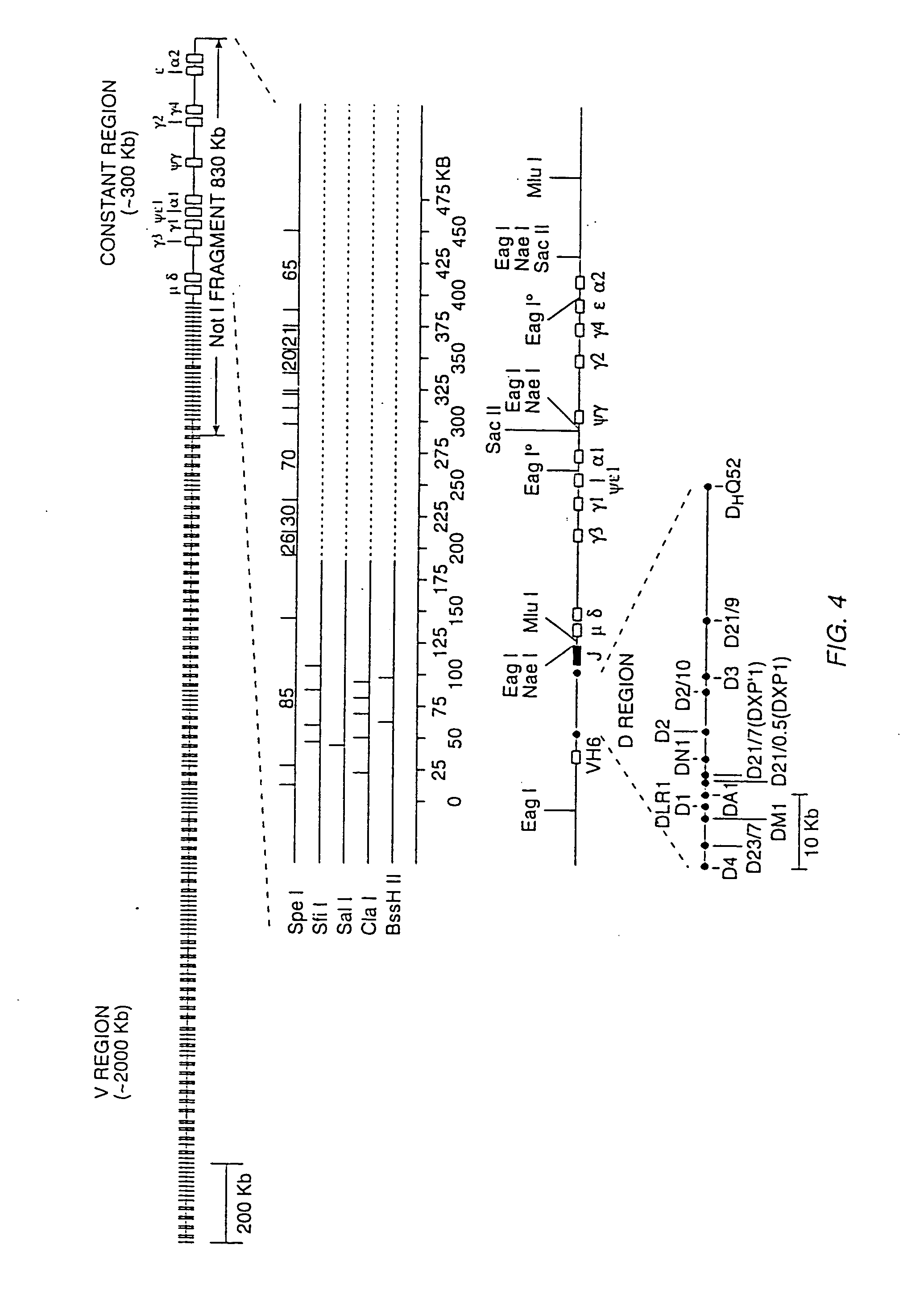
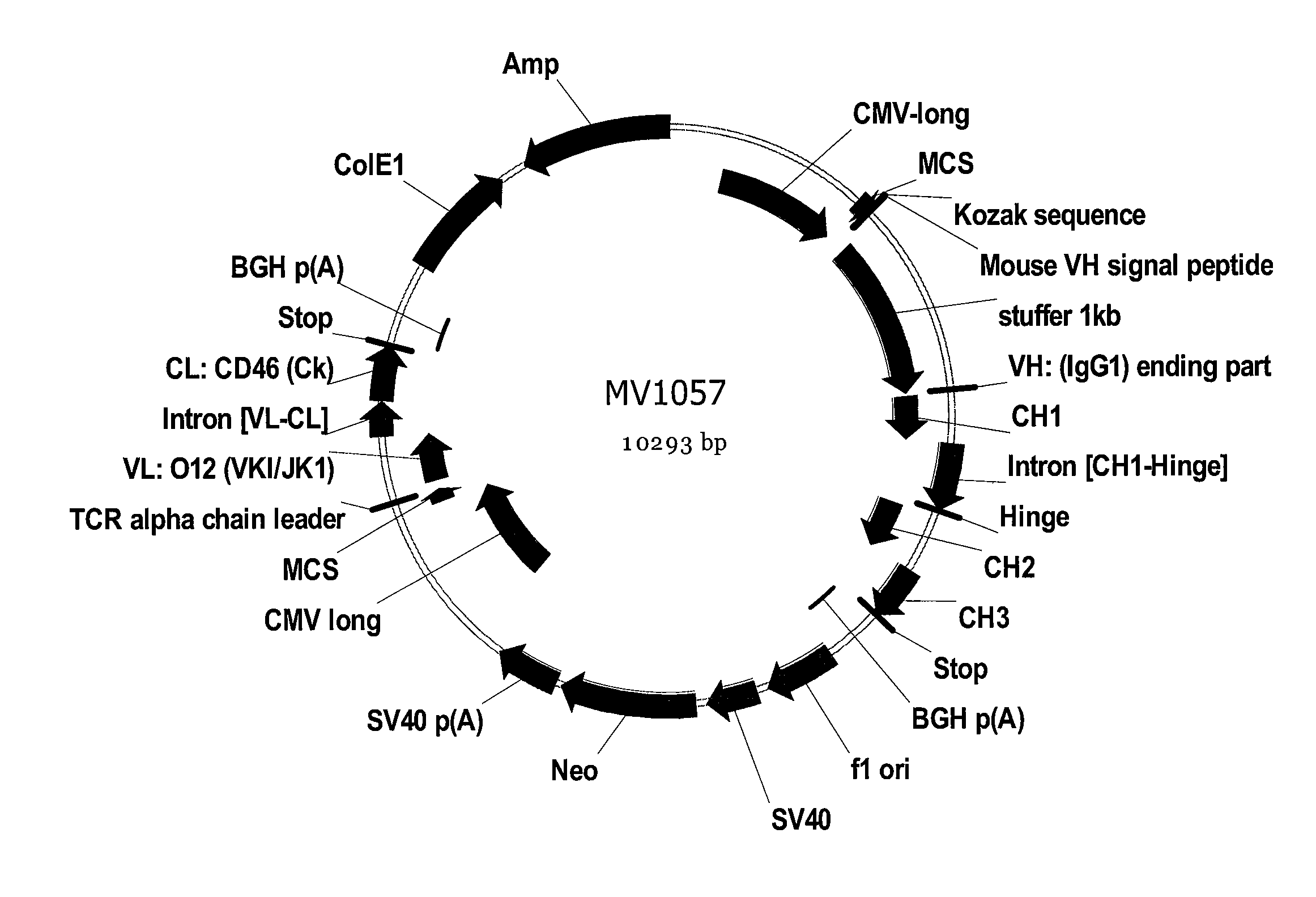
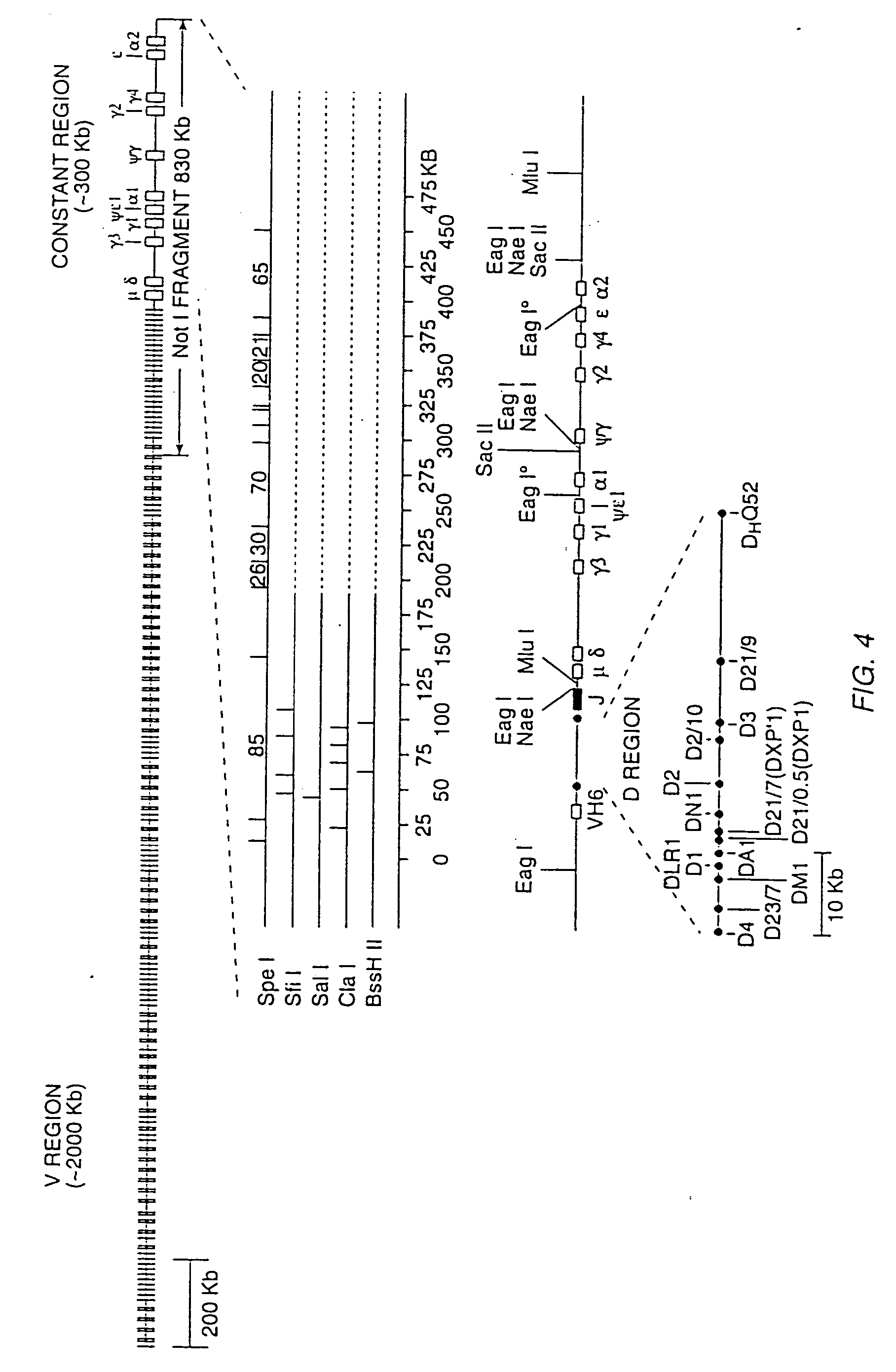
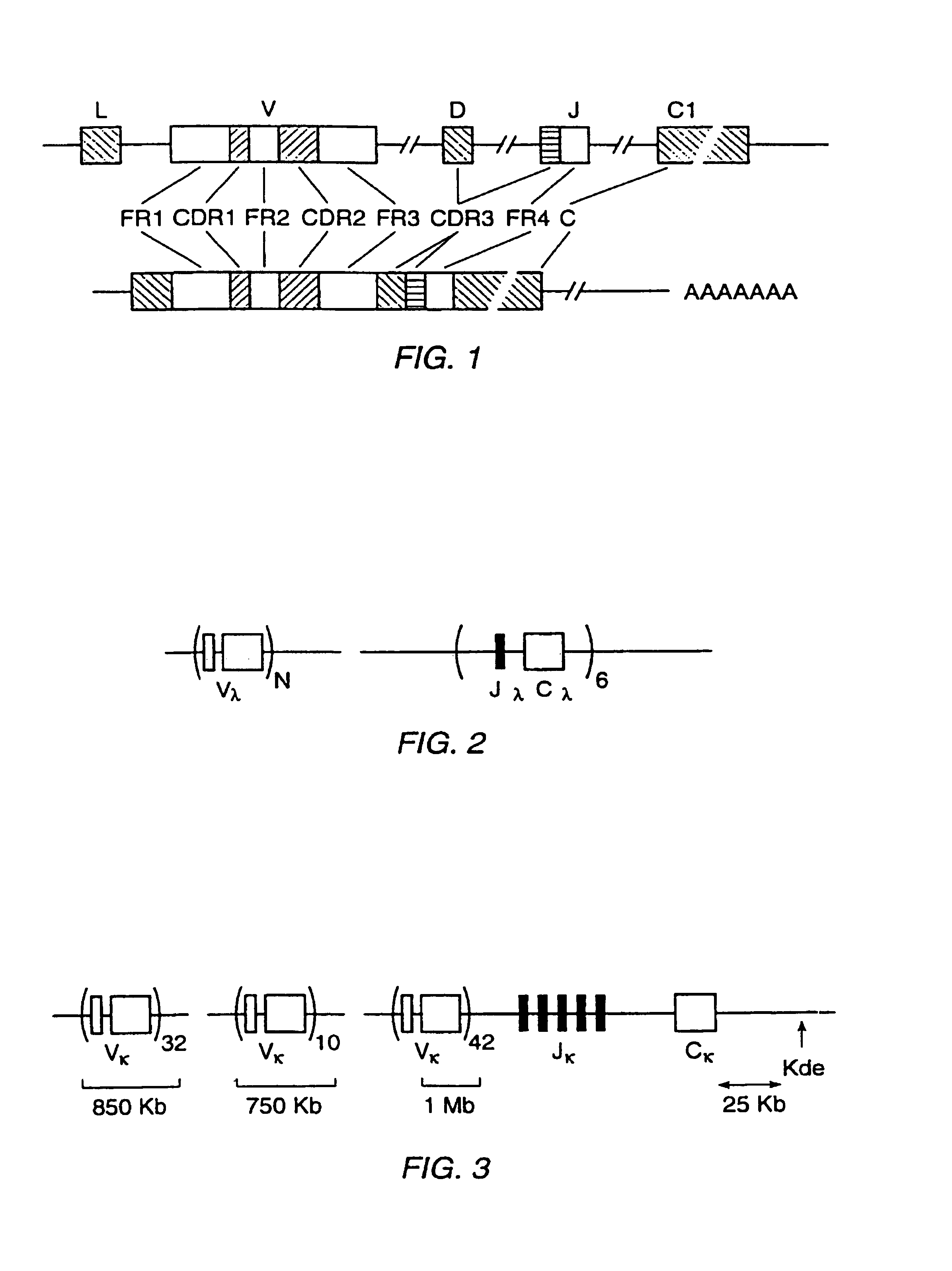
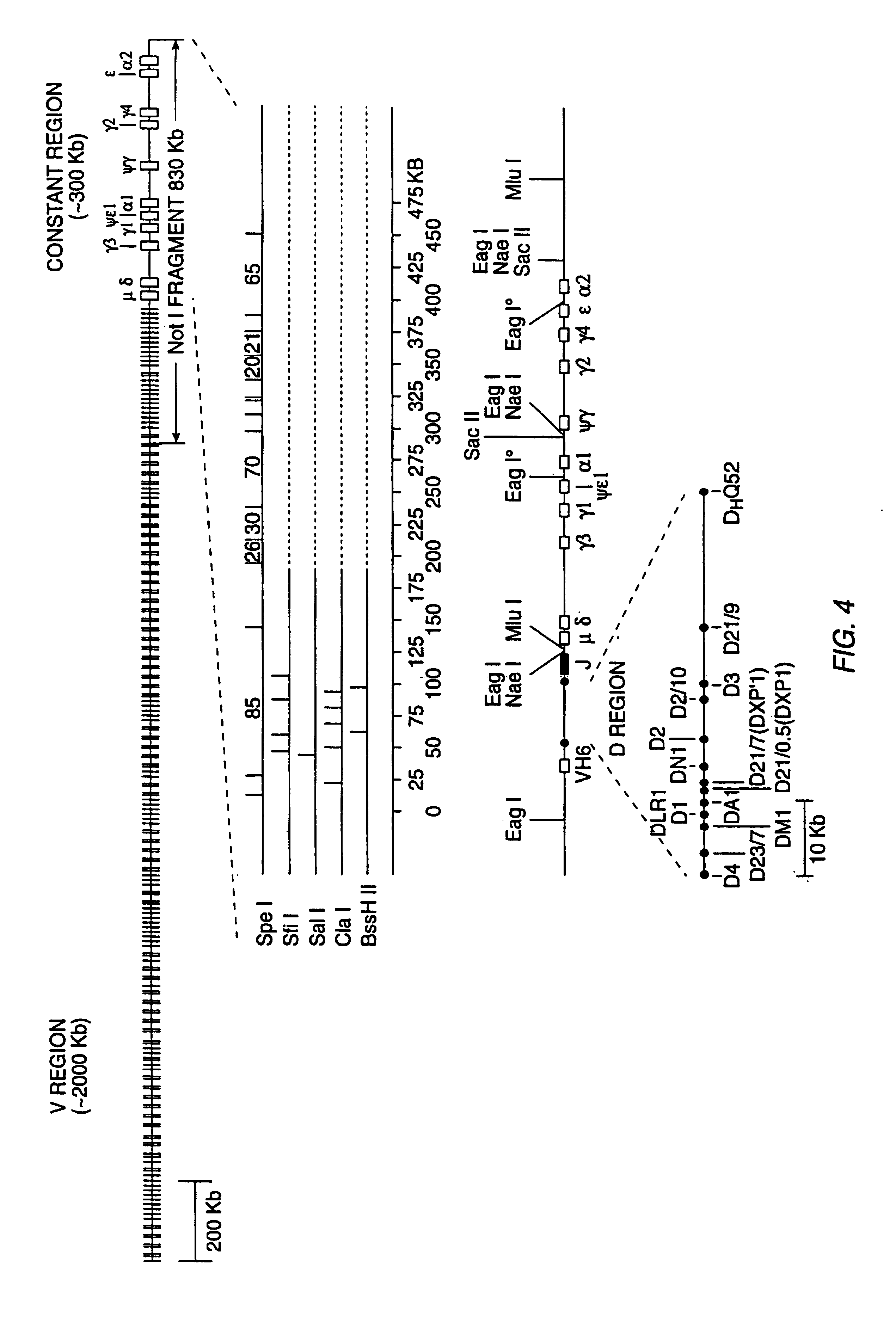
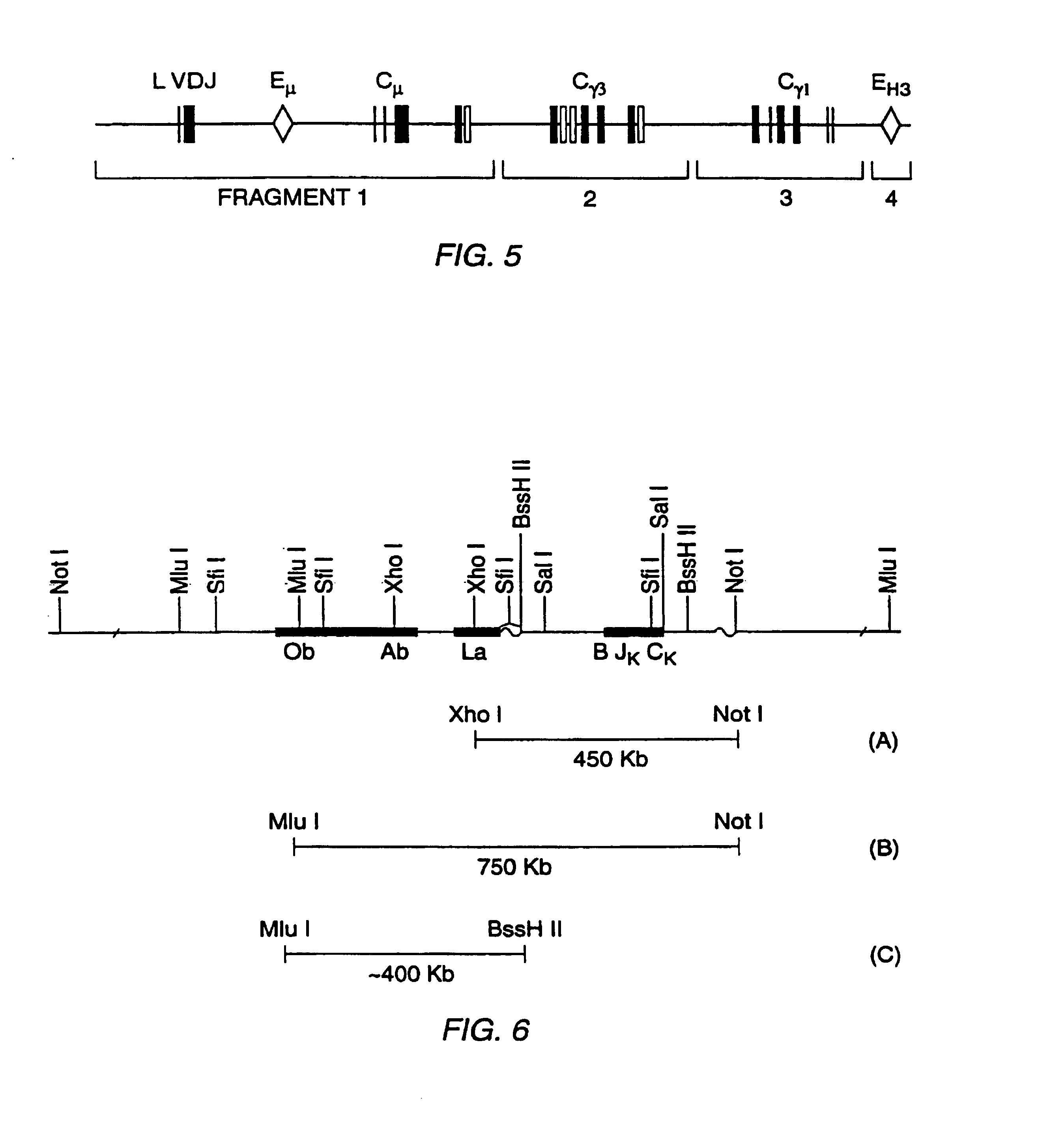
Transgenic mammals having human Ig loci including plural VH and VK regions and antibodies produced therefrom
InactiveUS7064244B2Reduced development and maturation of B-cellsEfficient productionAntipyreticAnalgesicsHuman animalMammal
The present invention relates to transgenic non-human animals that are engineered to contain human immunoglobulin gene loci. In particular, animals in accordance with the invention possess human Ig loci that include plural variable (VH and Vκ) gene regions. Advantageously, the inclusion of plural variable region genes enhances the specificity and diversity of human antibodies produced by the animal. Further, the inclusion of such regions enhances and reconstitutes B-cell development to the animals, such that the animals possess abundant mature B-cells secreting extremely high affinity antibodies.
Owner:ABQENIX INC
Methods for treating ethmoid disease
ActiveUS7419497B2Low profileMinimize traumaGuide needlesBalloon catheterParanasal sinusitisHuman animal
Devices, systems and methods for stenting, spacing, draining, ventilating and / or delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects, including methods and systems for treating paranasal sinusitis and ethmoid disease.
Owner:ACCLARENT INC
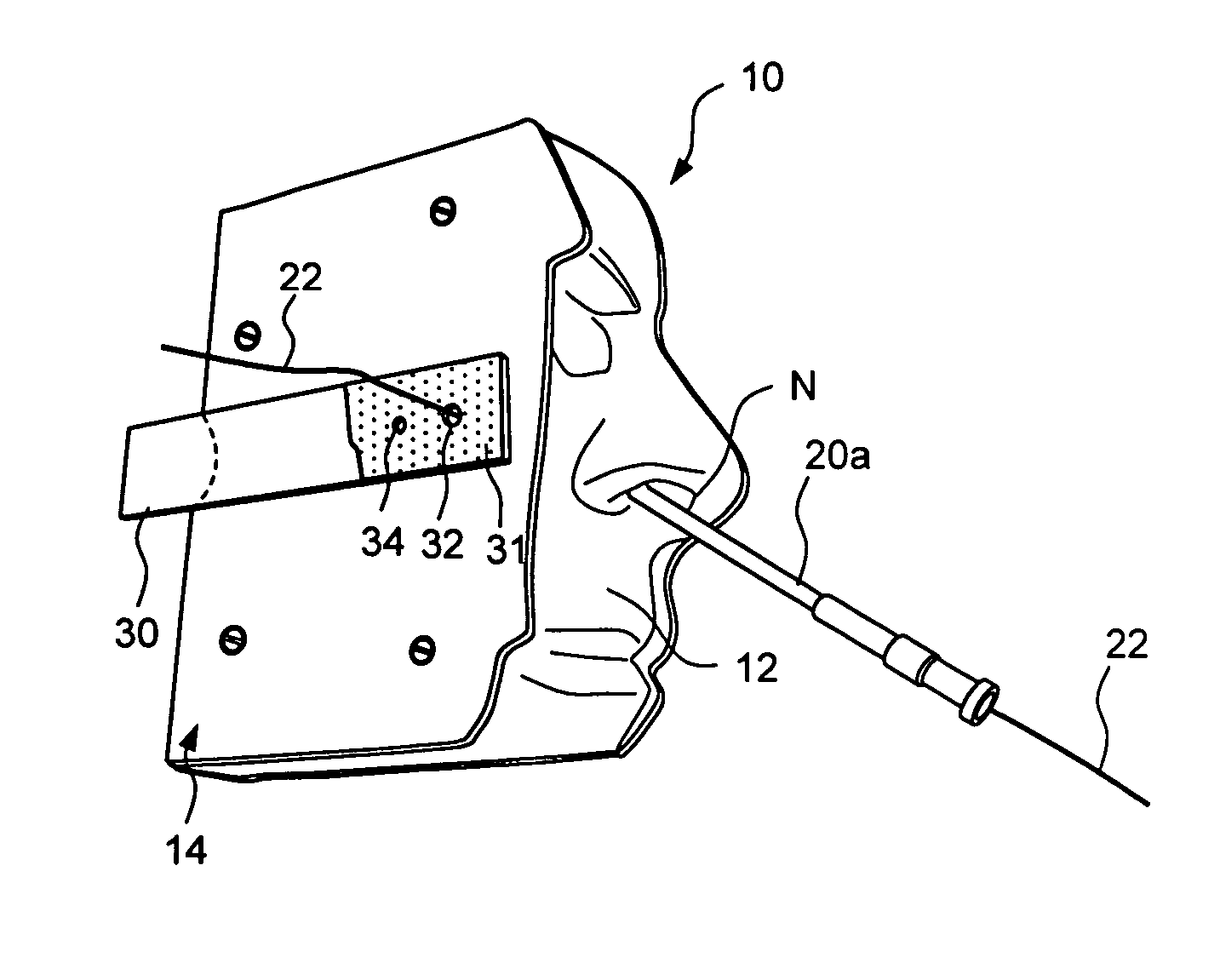
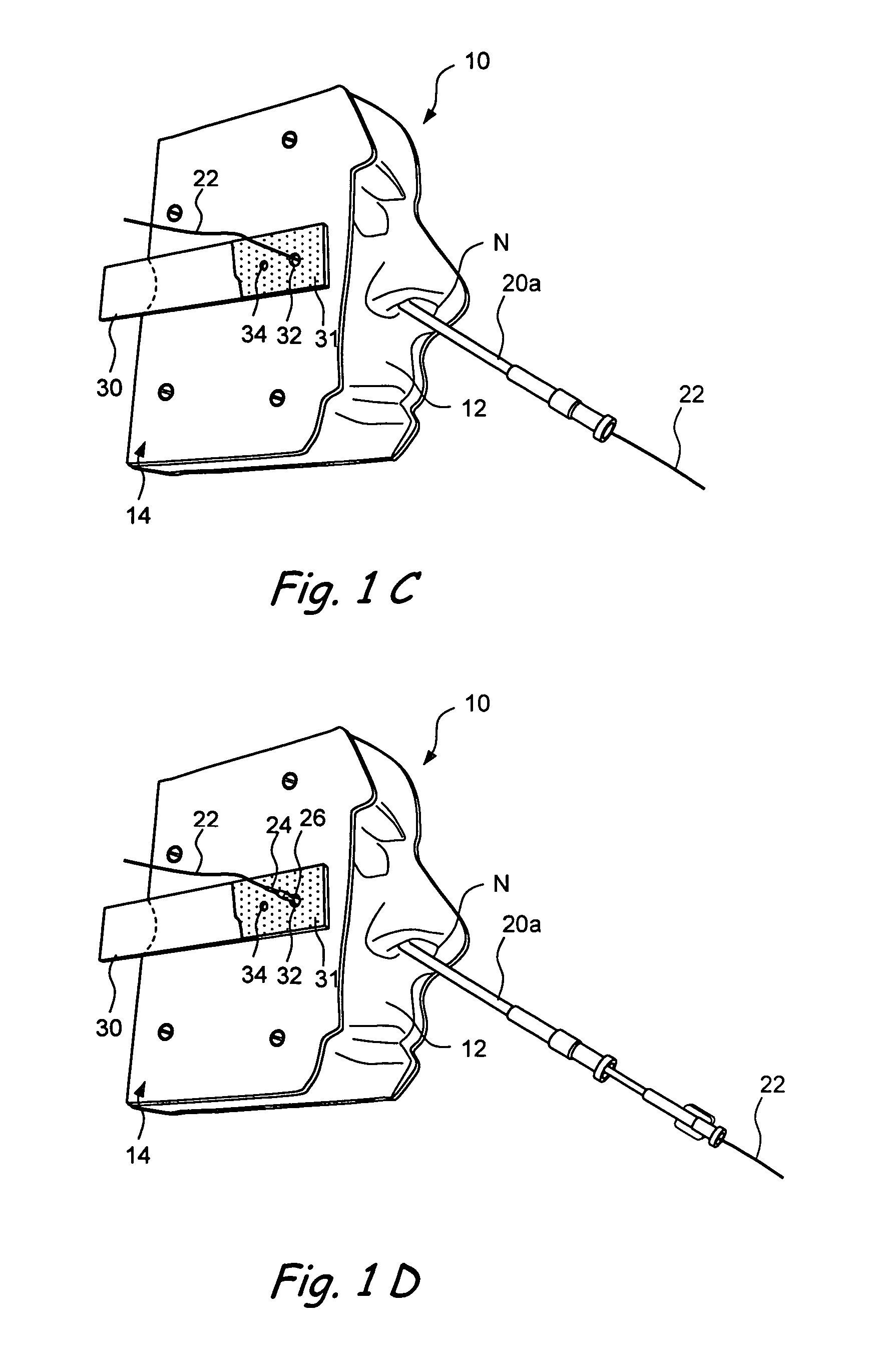
Anatomical models and methods for training and demonstration of medical procedures
Anatomical models representing one or more anatomical structures of a human or non-human animal and related methods for demonstrating, training, promotion and sale of medical, cosmetic and surgical products or procedures. A working device is inserted into the anatomical model and used to perform a simulated procedure, thereby causing an indicator apparatus (e.g., a removable business card) to become perceptibly altered. The indicator apparatus may then be removed and provided a potential user, consumer or purchaser of the medical, surgical or cosmetic product.
Owner:ACCLARENT INC
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Antibody producing non-human mammals
ActiveUS20100146647A1Low variabilityLow immunogenicityAnimal cellsAntibody mimetics/scaffoldsHuman animalDNA rearrangement
Owner:MERUS NV
Antibody producing non-human mammals
InactiveUS20100069614A1Easy to openEasy maintenanceAntibody mimetics/scaffoldsImmunoglobulins against virusesHuman animalDNA rearrangement
Described are transgenic, non-human animals comprising a nucleic acid encoding an immunoglobulin light chain, whereby the immunoglobulin light chain is human, human-like, or humanized. The nucleic acid is provided with a means that renders it resistant to DNA rearrangements and / or somatic hypermutations. In one embodiment, the nucleic acid comprises an expression cassette for the expression of a desired molecule in cells during a certain stage of development in cells developing into mature B cells. Further provided is methods for producing an immunoglobulin from the transgenic, non-human animal.
Owner:MERUS NV
Production of humanized antibodies in transgenic animals
InactiveUS7129084B2Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Transgenic non-human animals capable of producing heterologous antibodies
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
Transgenic non-human animals expressing a truncated activin type II receptor
The present invention provides a substantially purified growth differentiation factor (GDF) receptor, including a GDF-8 (myostatin) receptor, as well as functional peptide portions thereof. In addition, the invention provides a virtual representation of a GDF receptor or a functional peptide portion thereof. The present invention also provides a method of modulating an effect of myostatin on a cell by contacting the cell with an agent that affects myostatin signal transduction in the cell. In addition, the invention provides a method of ameliorating the severity of a pathologic condition, which is characterized, at least in part, by an abnormal amount, development or metabolic activity of muscle or adipose tissue in a subject, by modulating myostatin signal transduction in a muscle cell or an adipose tissue cell in the subject. The invention also provides a method of modulating the growth of muscle tissue or adipose tissue in a eukaryotic organism by administering an agent that affects myostatin signal transduction to the organism.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Human antipneumococcal antibodies from non-human animals
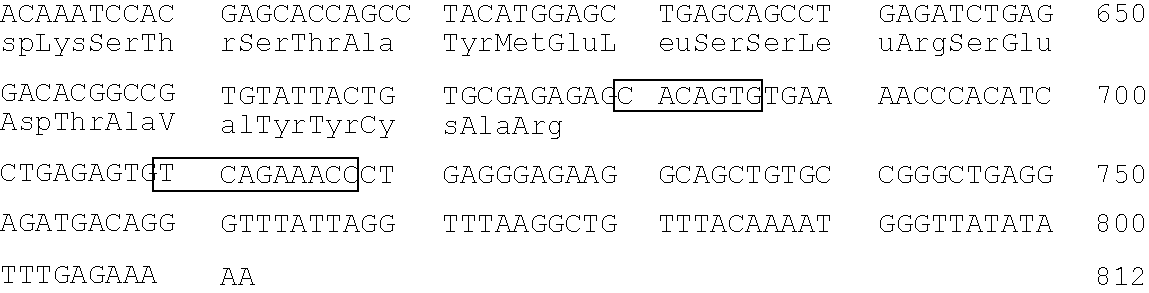
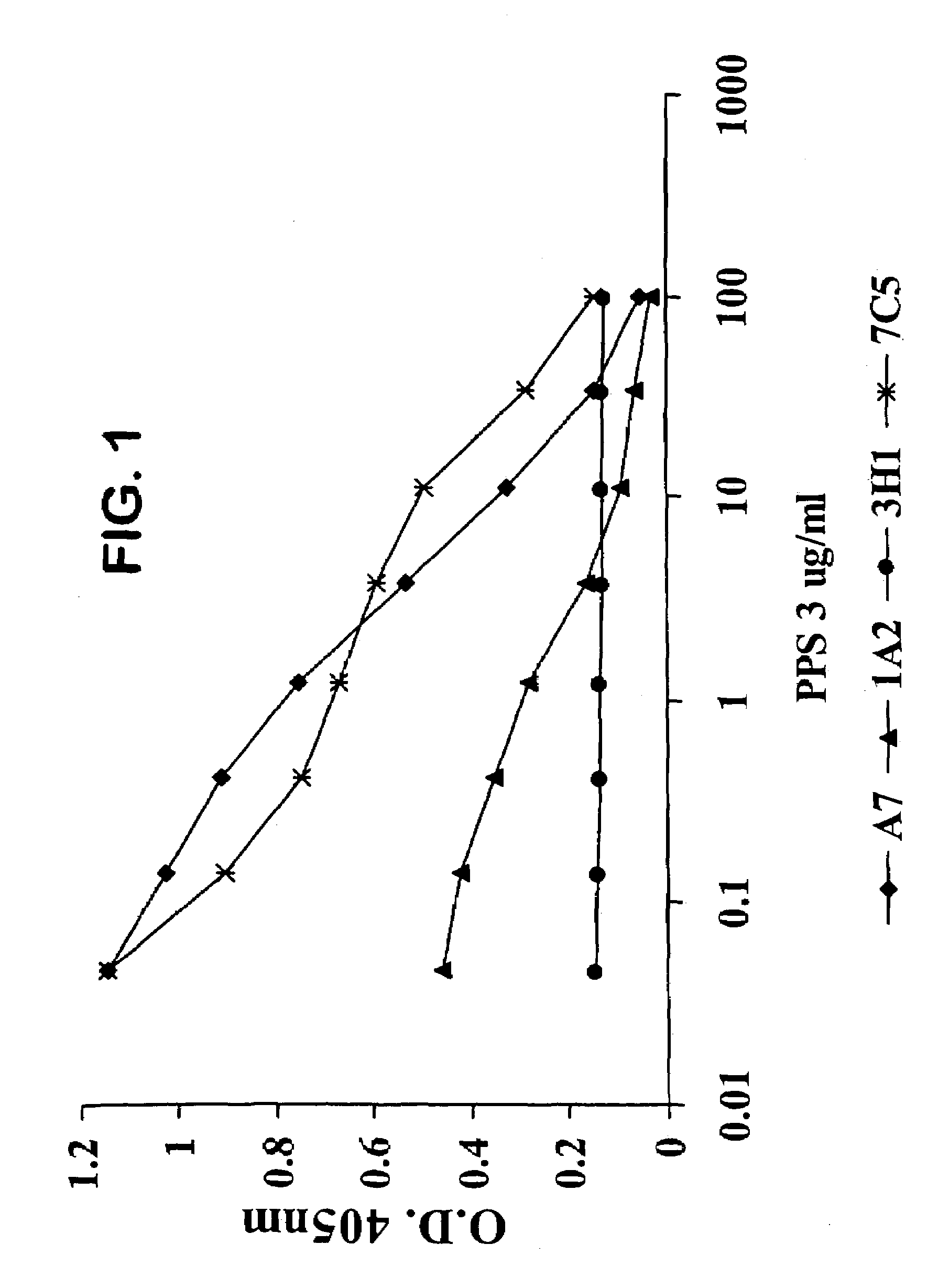
The invention described herein provides human antibodies produced in non-human animals that specifically bind to Streptococcus pneumoniae capsular polysaccharide (PPS-3). The invention further provides methods for making the antibodies in a non-human animal and for expressing the antibodies in cells including hybridomas and recombinant host cell systems. Kits and pharmaceutical compositions comprising the antibodies are also provided in addition to methods of treating, inhibitng or preventing S. pneumoniae infection or conditions or disorders caused by such infection by administering to a patient the pharmaceutical compositions described herein.
Owner:ABQENIX INC
Transgenic non-human animals for producing heterologous and chimeric antibodies
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
High affinity human antibodies and human antibodies against human antigens
The invention relates to transgenic non-human animals capable of producing high affinity human sequence antibodies. The invention is also directed to human sequence antibodies specific for human antigens, such as, human CD4. The invention also is directed to methods for producing human sequence antibodies.
Owner:GENPHARM INT INC
Method of magnetic resonance imaging of a sample with ex vivo polarization of an MR imaging agent
The present invention provides a method of magnetic resonance investigation of a sample, preferably of a human or non-human animal body, said method comprising the step of ex vivo polarisation of a high T1 agent and wherein the polarising agent is optionally seperated from the high T1 agent before the high T1 agent is administered to the sample.
Owner:OXFORD INSTR MOLECULAR BIOTOOLS
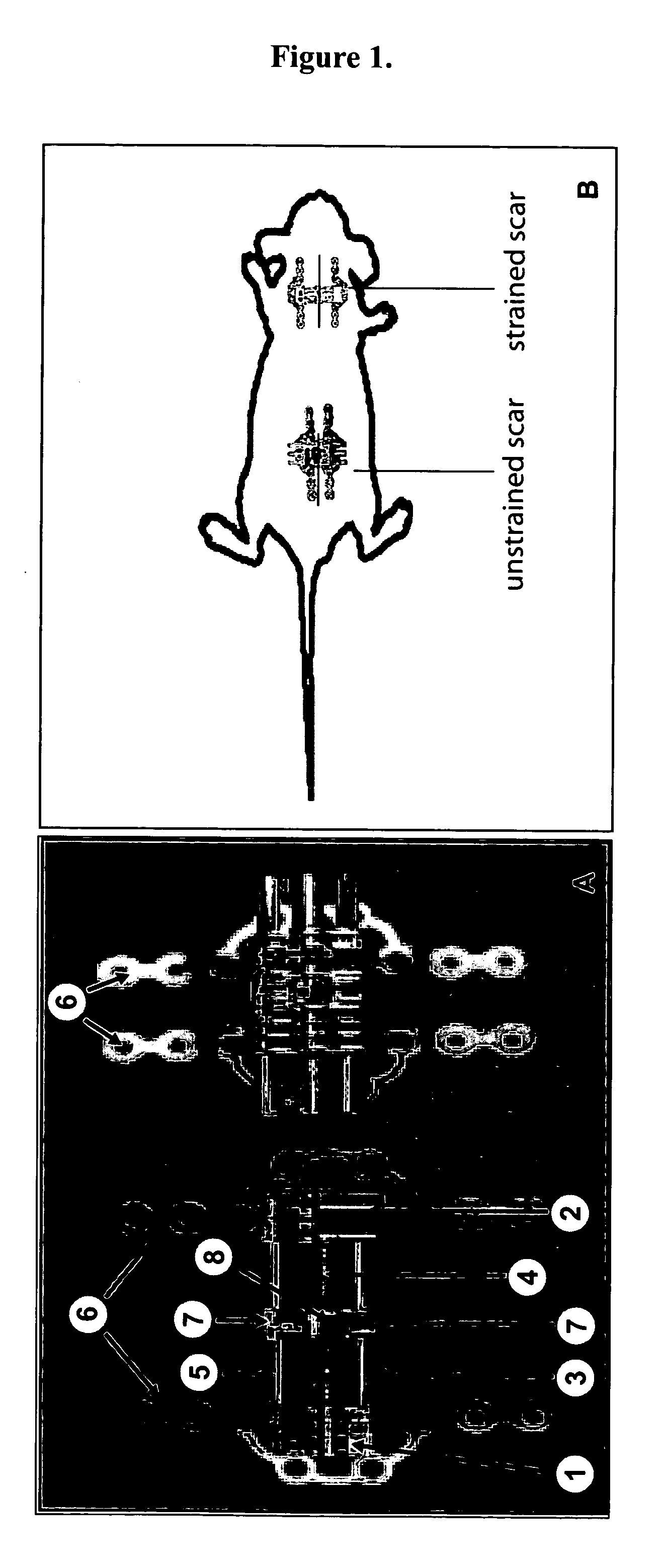
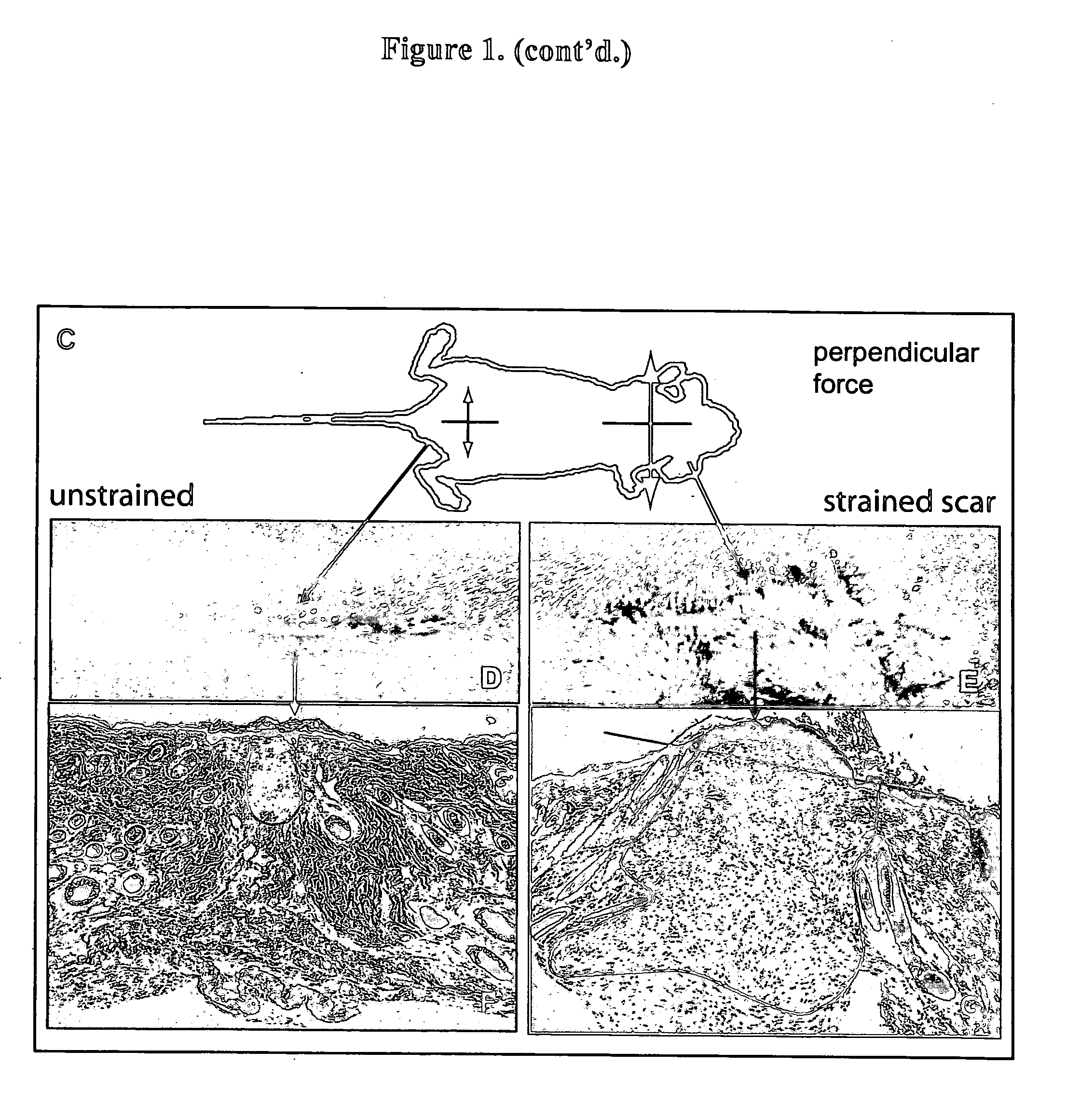
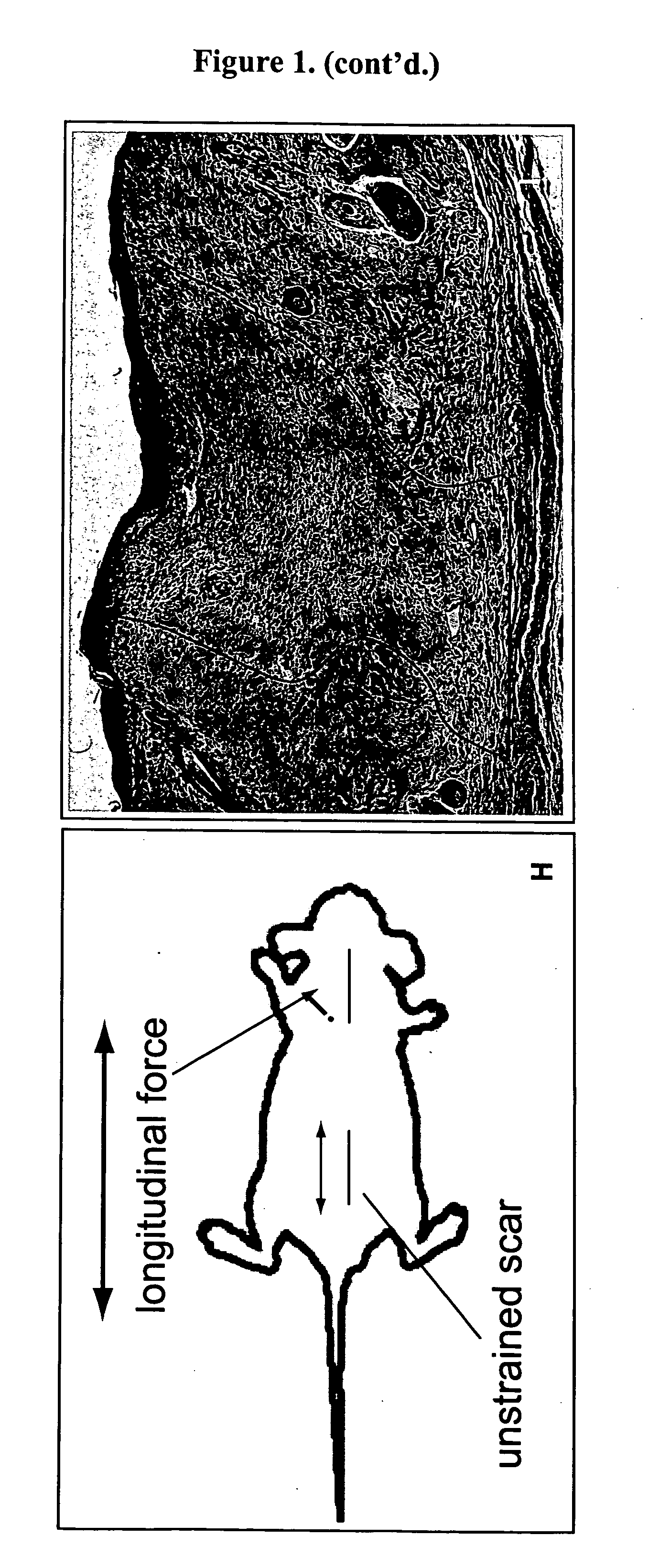
Method for producing hypertrophic scarring animal model for identification of agents for prevention and treatment of human hypertrophic scarring
InactiveUS20060037091A1Risk minimizationMinimal scarringPeptide/protein ingredientsDepsipeptidesDiseaseHuman animal
The present invention relates to a method of producing a non-human animal model of hypertrophic scarring. This involves producing an incision in a non-human animal and applying mechanical strain over the incision under conditions effective to produce hypertrophic scarring, thereby producing a non-human animal model of hypertrophic scarring. The present invention also relates to a method of determining the efficacy of an agent for prevention or treatment of a disease condition. This method involves providing a non-human animal having an incision over which mechanical strain is applied under conditions effective to produce hypertrophic scarring, administering an agent to the incision, and determining whether the agent is efficacious for prevention or treatment of a disease condition. Also provided is a non-human animal model of hypertrophic scarring. This involves a non-human animal having an incision over which mechanical strain has been applied under conditions effective to produce hypertrophic scarring.
Owner:GURTNER GEOFFREY C +1
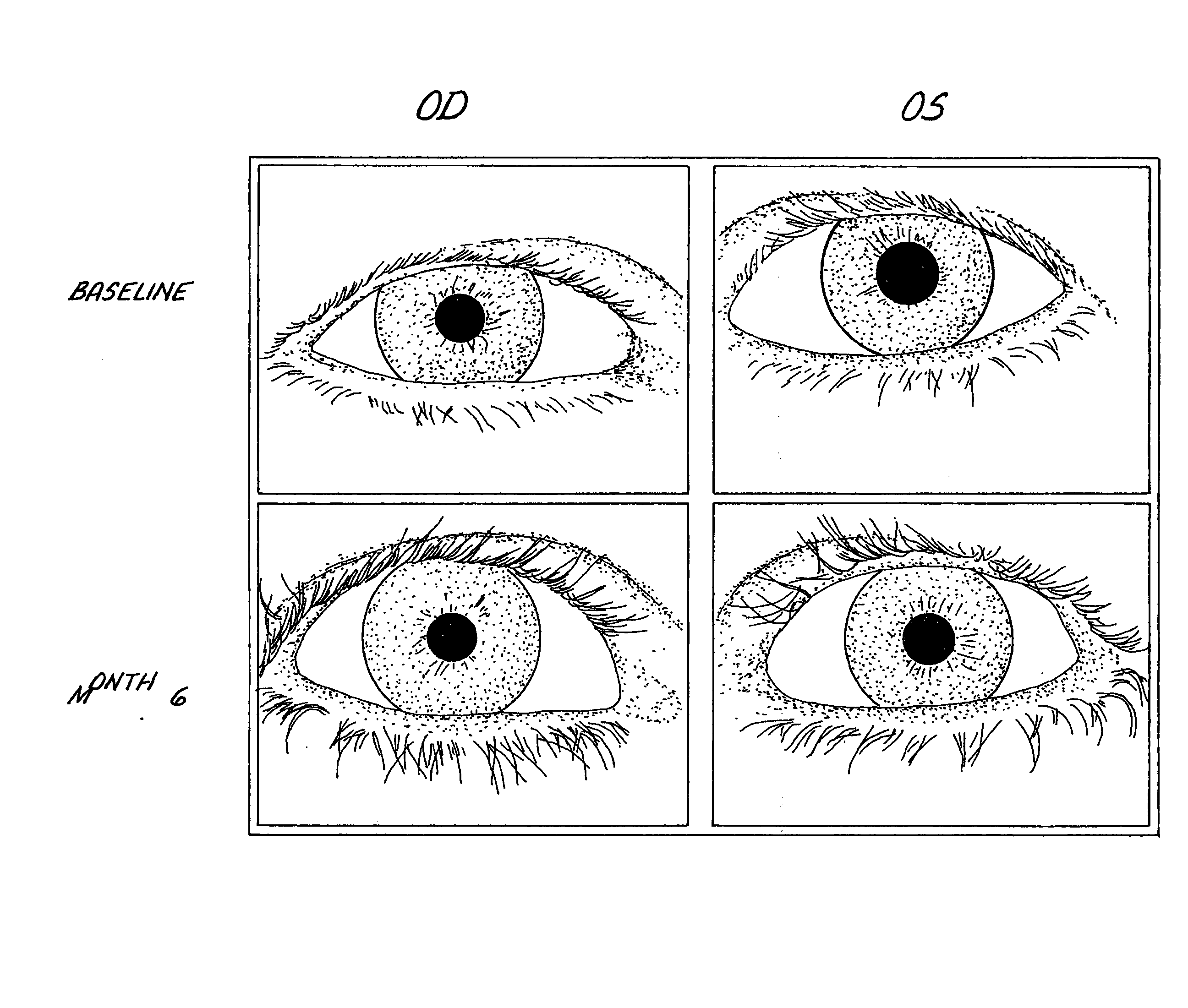
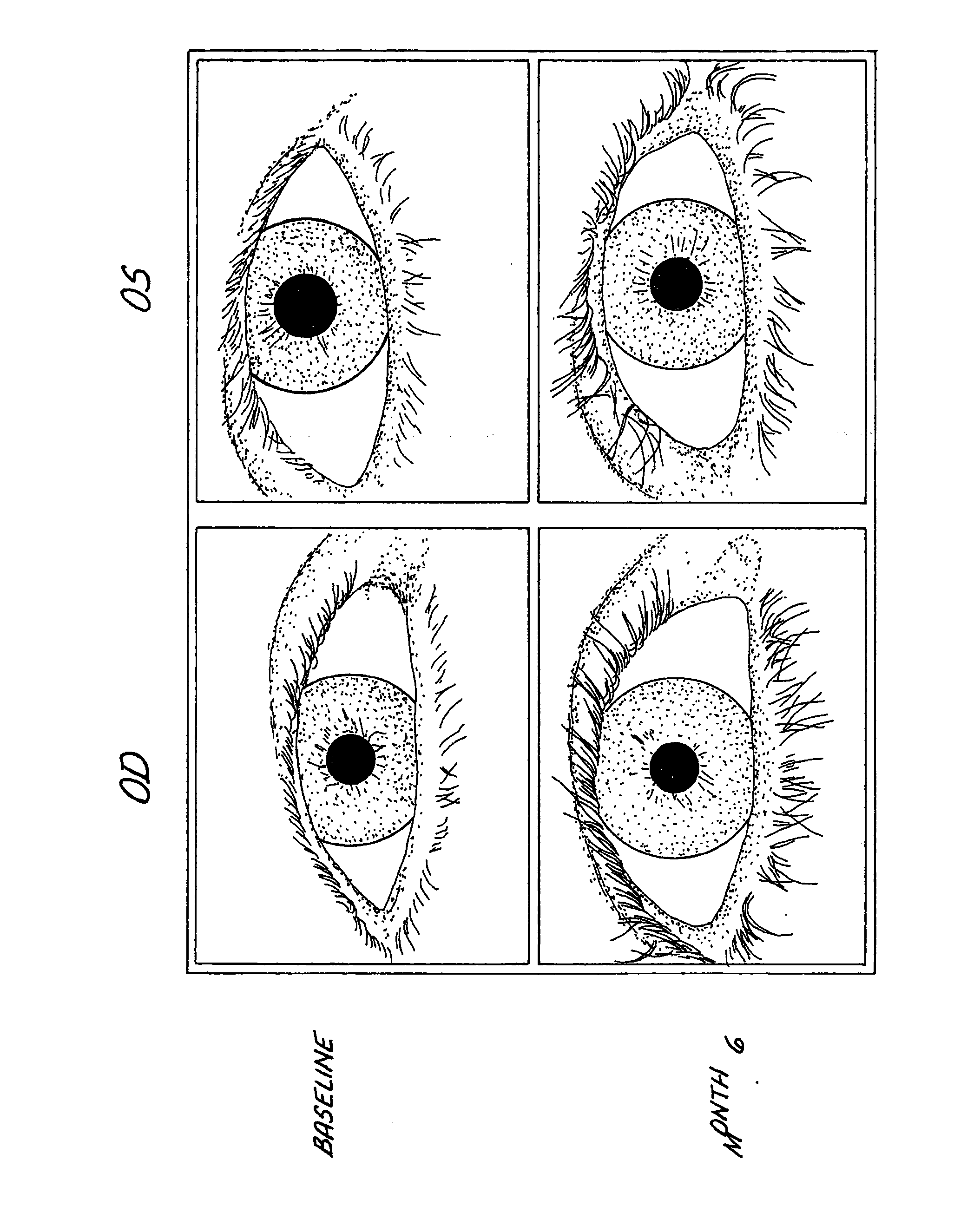
Method of enhancing hair growth
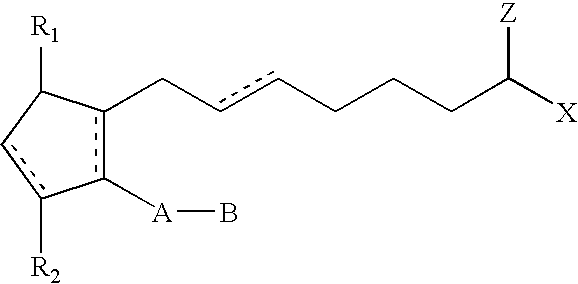
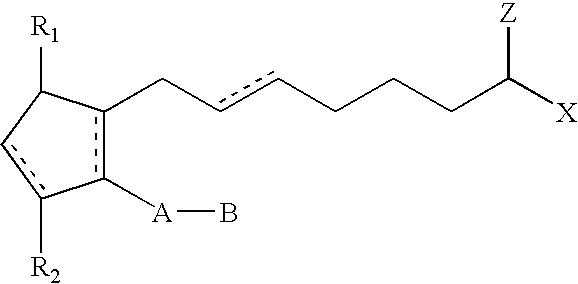
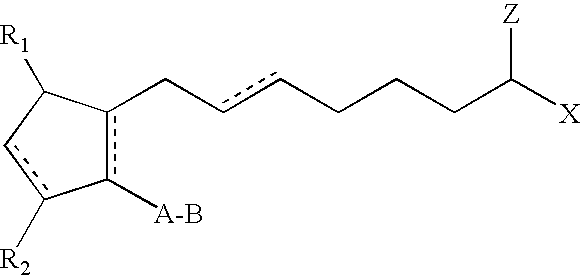
ActiveUS20030147823A1Efficient use ofSimple and safe processBiocideCosmetic preparationsHuman animalDouble bond
Methods and compositions for stimulating the growth of hair are disclosed wherein said compositions include a cyclopentane heptanoic acid, 2-cycloalkyl or arylalkyl compound represented by the formula I wherein the dashed bonds represent a single or double bond which can be in the cis or trans configuration, A, B, Z, X, R1 and R2 are as defined in the specification. Such compositions are used in treating the skin or scalp of a human or non-human animal. Bimatoprost is preferred for this treatment.
Owner:ALLERGAN INC
Transgenic mammals having human Ig loci including plural Vh and Vk regions and antibodies produced therefrom
InactiveUS20080098490A1Reduced development and maturation of B-cellsEfficient productionAntipyreticAnalgesicsHuman animalMammal
Owner:AMGEN FREMONT INC
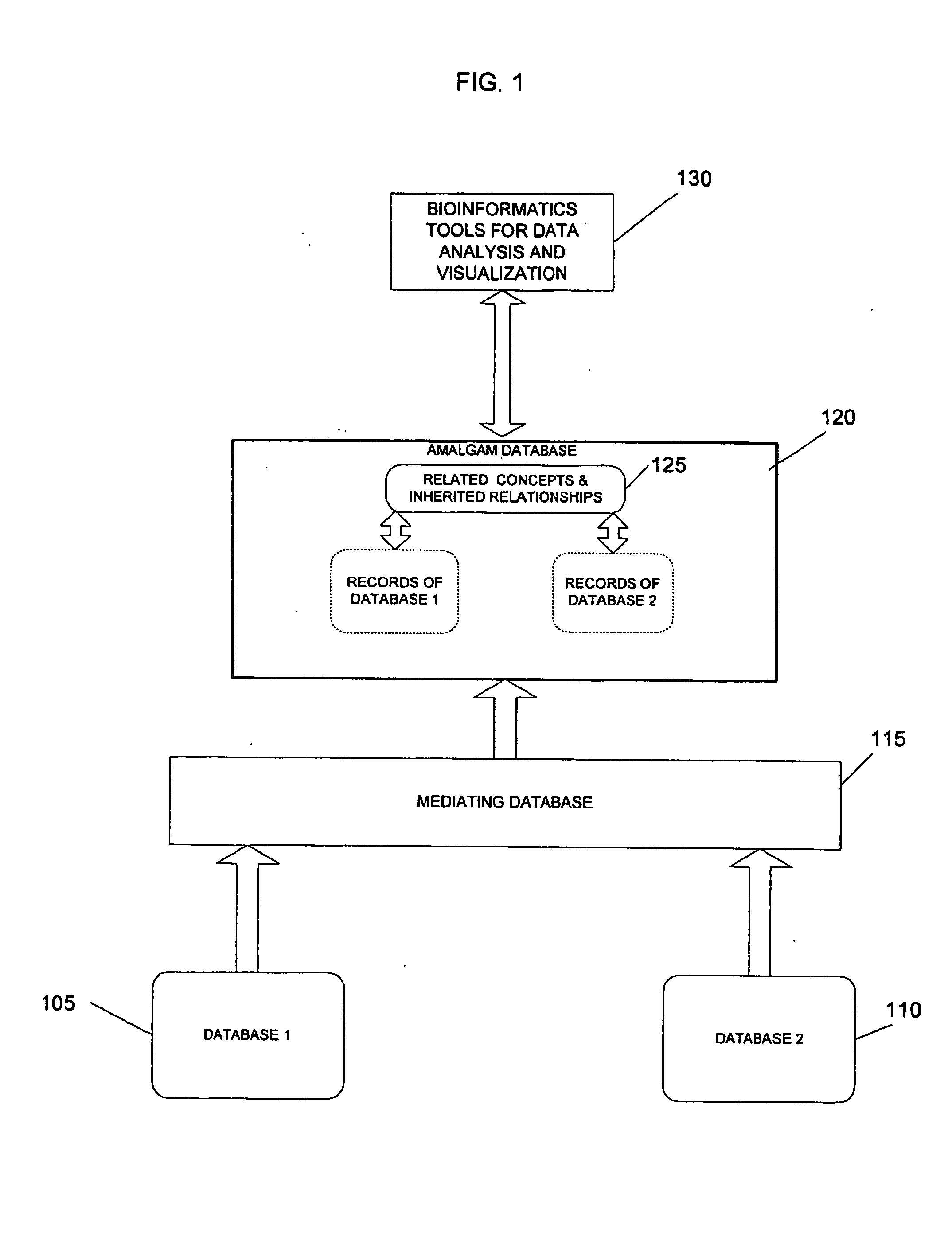
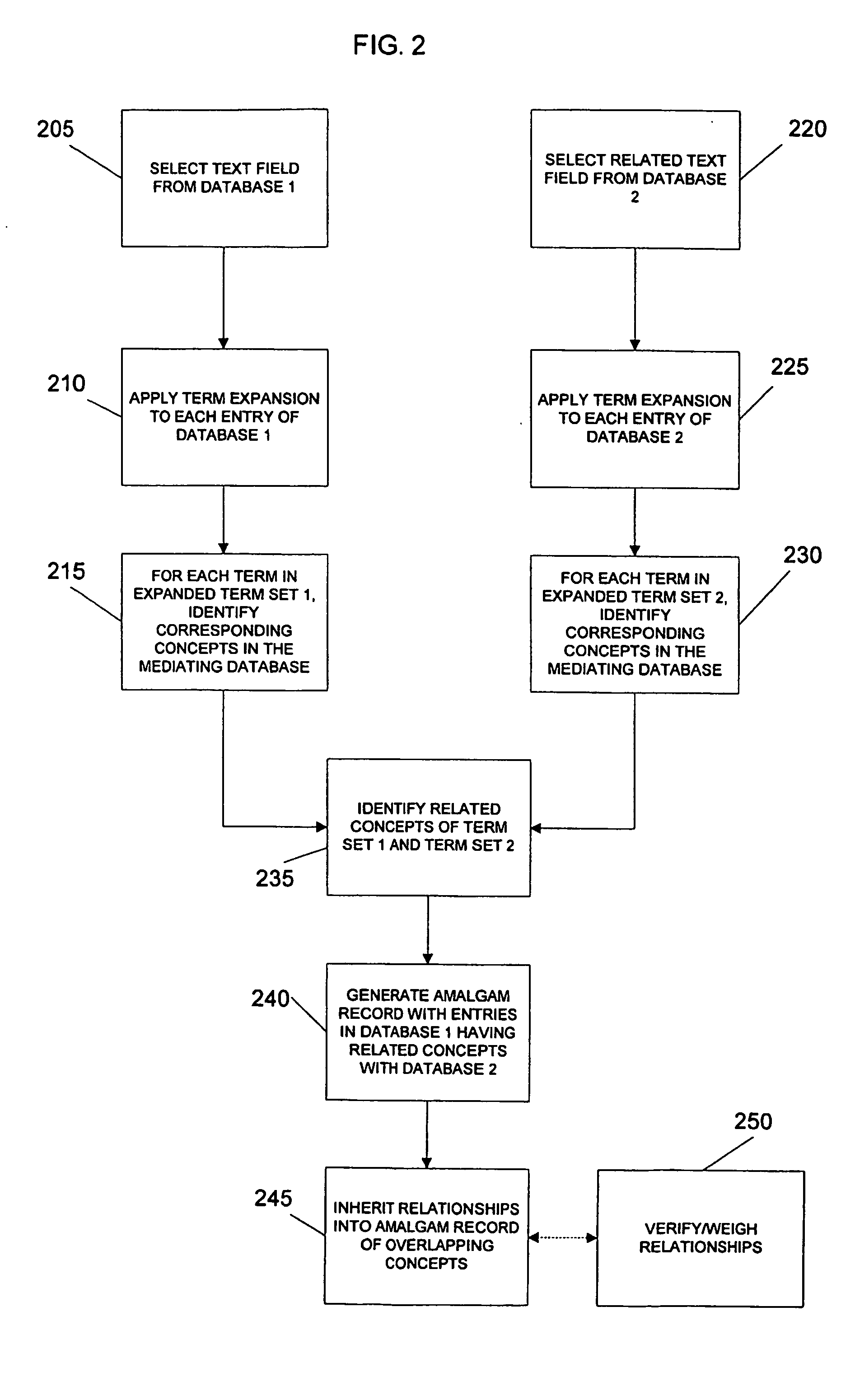
Terminological mapping
InactiveUS20050097628A1Improve interoperabilityClarify problemOrganic active ingredientsNervous disorderHuman animalData mining
The present invention relates to the systematic use of terminology and knowledge based technologies to enable high-throughput mapping between databases having different vocabularies. In particular embodiments, it may be used to map between a database having a phenotypic terminology descriptive of non-human animals and a database having a broad-coverage clinical (anthropocentric) terminology.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Pharmaceutical compositions comprising an oligonucleotide as an active agent
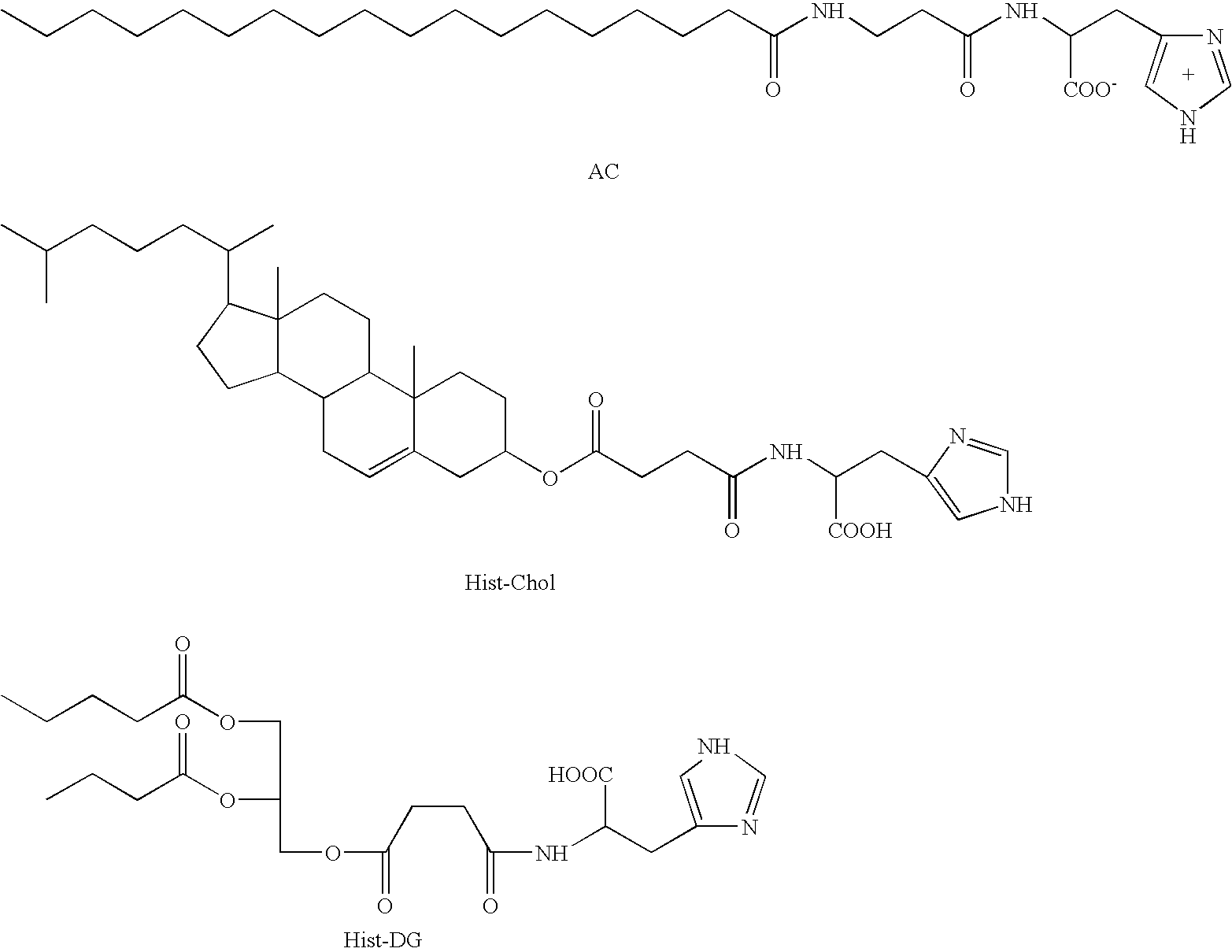
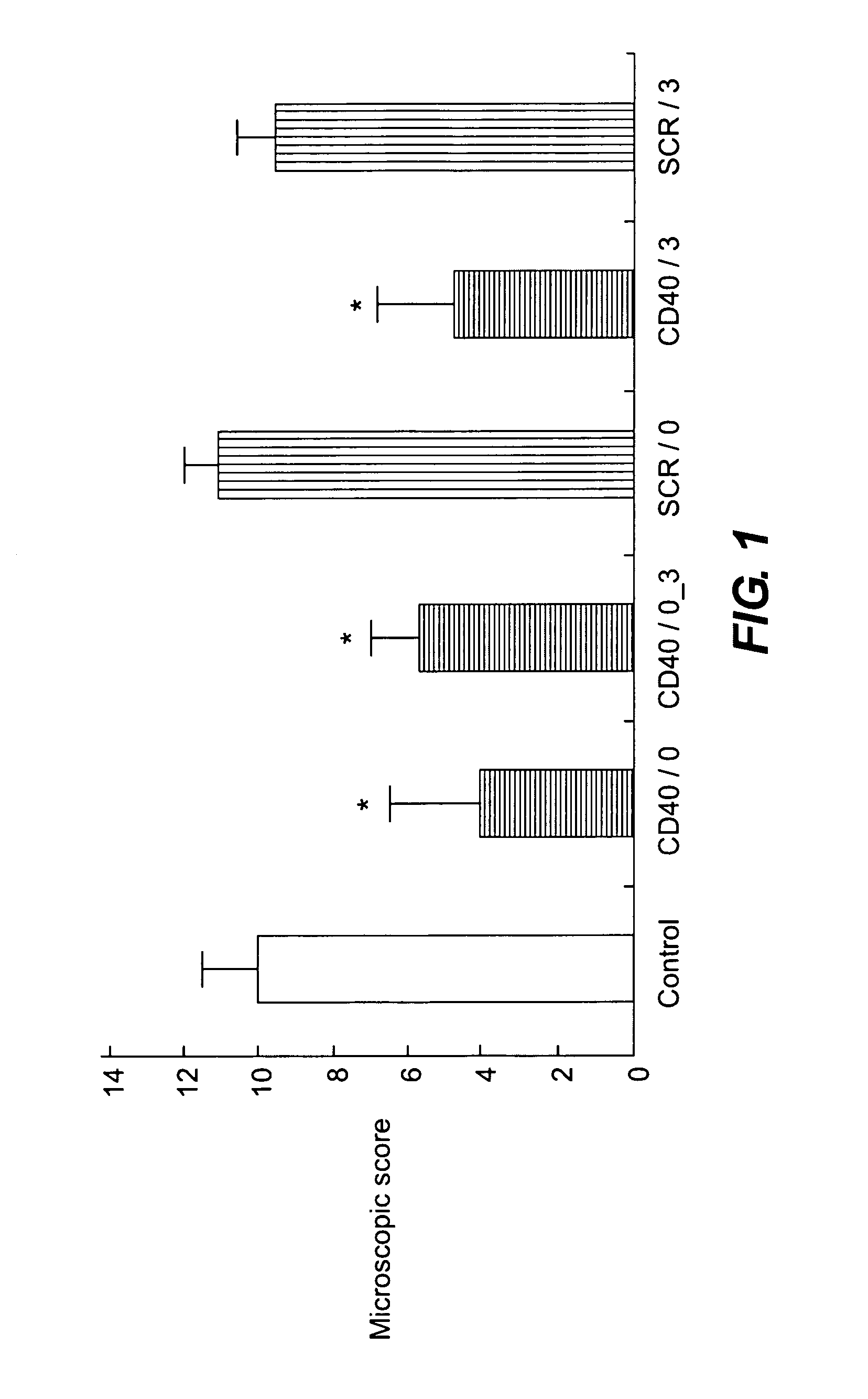
A pharmaceutical composition is disclosed, which composition comprises an oligonucleotide as an active agent, the oligonucleotide being adapted to target nucleic acids encoding CD40 thereby to modulate the expression of CD40 in mammalian cells, and a liposome as an excipient. Said liposome is an amphoteric liposome. Also disclosed is a method for the treatment or prophylaxis of a disease or condition associated with the expression of CD40 in a human or non-human animal patient by administering to said patient a therapeutically or prophylactically effective amount of such a composition.
Owner:NOVOSOM
Growth differentiation factor-8
InactiveUS6858208B2Improved muscle contentPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman animalMuscle tissue
A transgenic non-human animal of the species selected from the group consisting of avian, bovine, ovine and porcine having a transgene which results in disrupting the production of and / or activity of growth differentiation factor-8 (GDF-8) chromosomally integrated into the germ cells of the animal is disclosed. Also disclosed are methods for making such animals, and methods of treating animals with antibodies or antisense directed to GDF-8. The animals so treated are characterized by increased muscle tissue.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Method of Enhancing Hair Growth
Methods and compositions for stimulating the growth of hair are disclosed wherein said compositions include a cyclopentane heptanoic acid, 2-cycloalkyl or arylalkyl compound represented by the formula I wherein the dashed bonds represent a single or double bond which can be in the cis or trans configuration, A, B, Z, X, R1 and R2 are as defined in the specification. Such compositions are used in treating the skin or scalp of a human or non-human animal. Bimatoprost is preferred for this treatment.
Owner:ALLERGAN INC
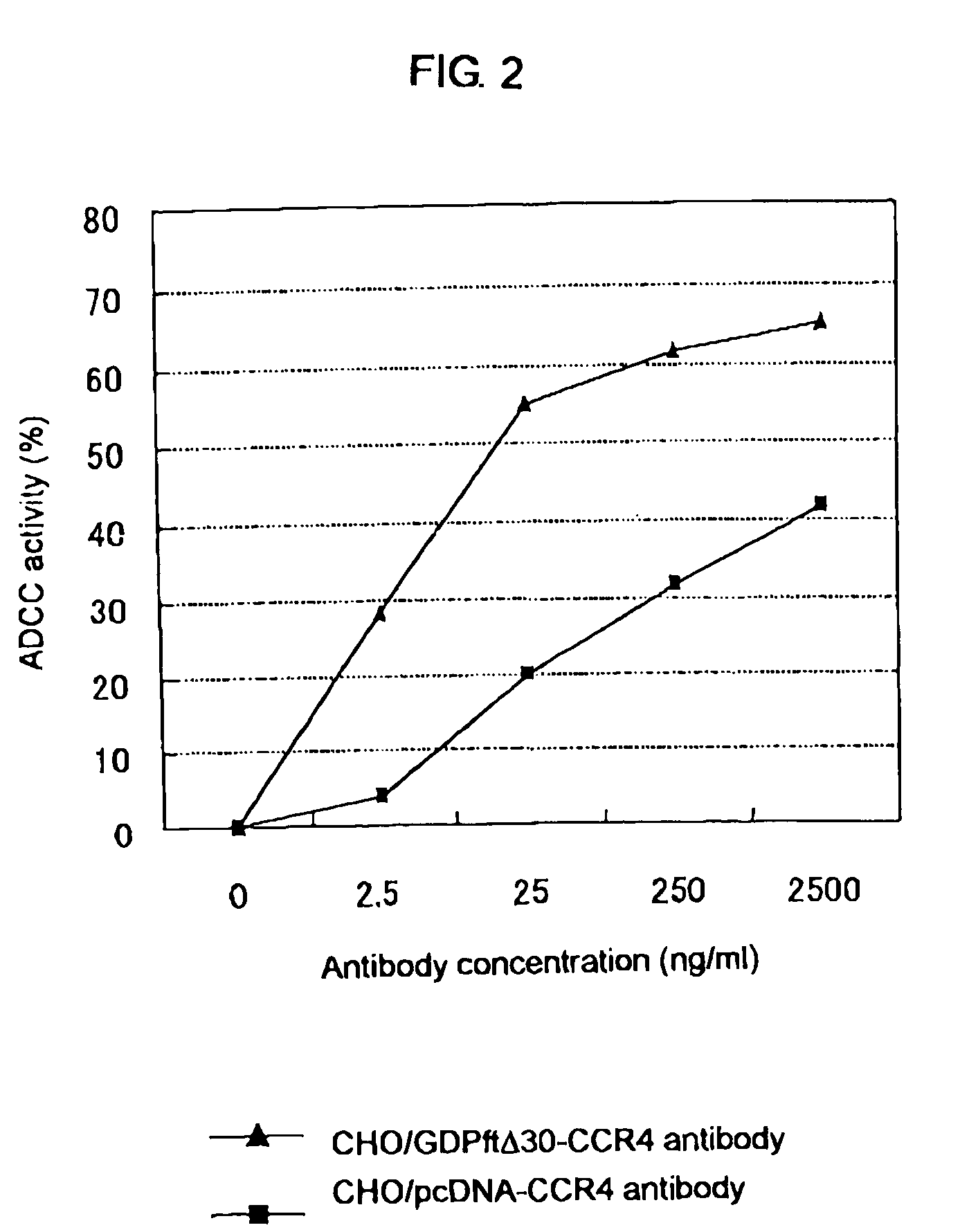
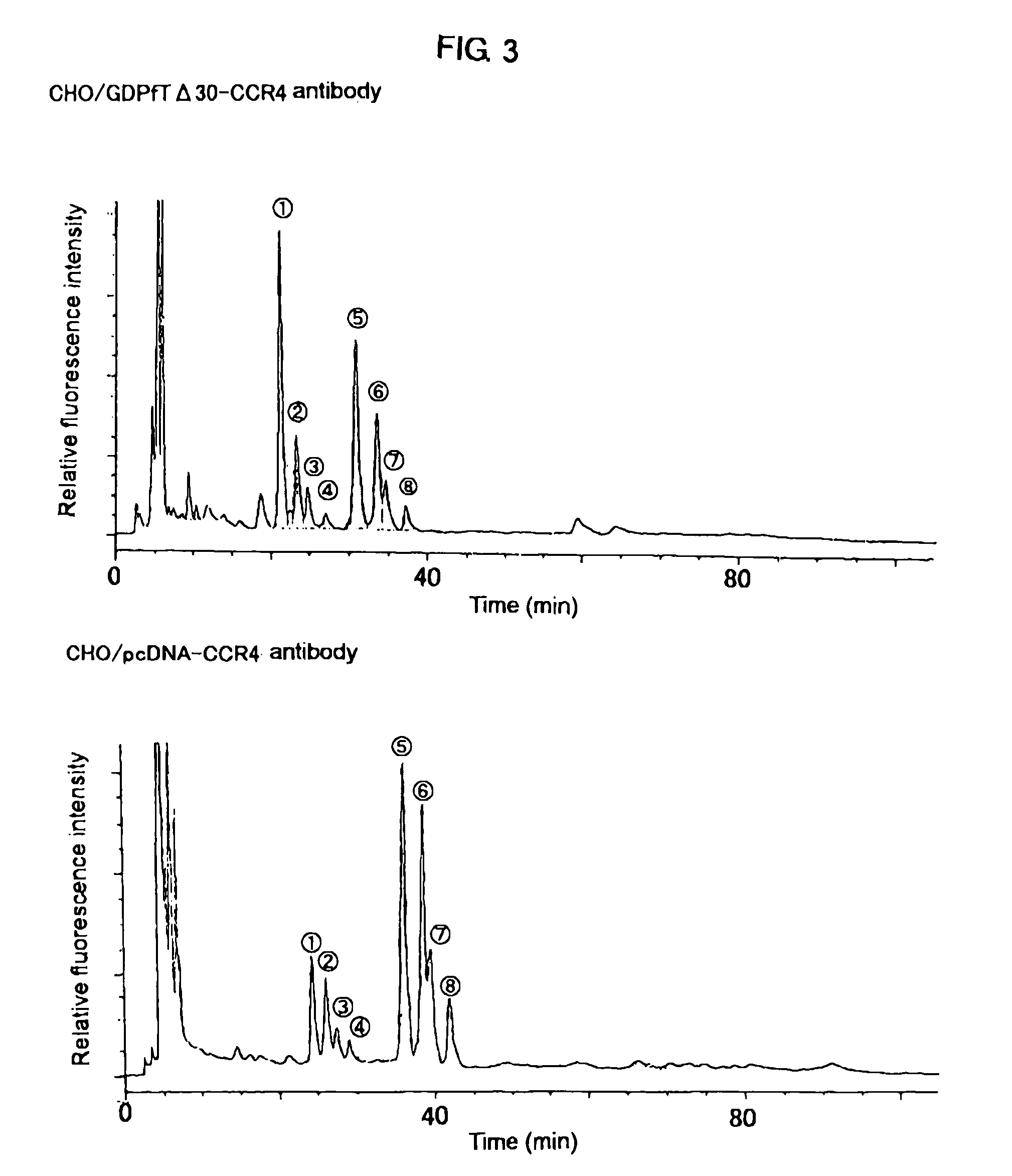
Cells in which activity of the protein involved in transportation of GDP-fucose is reduced or lost
A cell in which the activity of a protein relating to transport of an intracellular sugar nucleotide, GDP-fucose, to the Golgi body is more decreased or deleted than its parent cell; a process for producing an antibody composition using the cell; a transgenic non-human animal or plant or the progenies thereof, in which genome is modified so as to have a decreased or deleted activity of a protein relating to transport of an intracellular sugar nucleotide, GDP-fucose, to the Golgi body; a process for producing an antibody composition from the animal or plant; and a medicament comprising the antibody composition.
Owner:KYOWA HAKKO KIRIN CO LTD
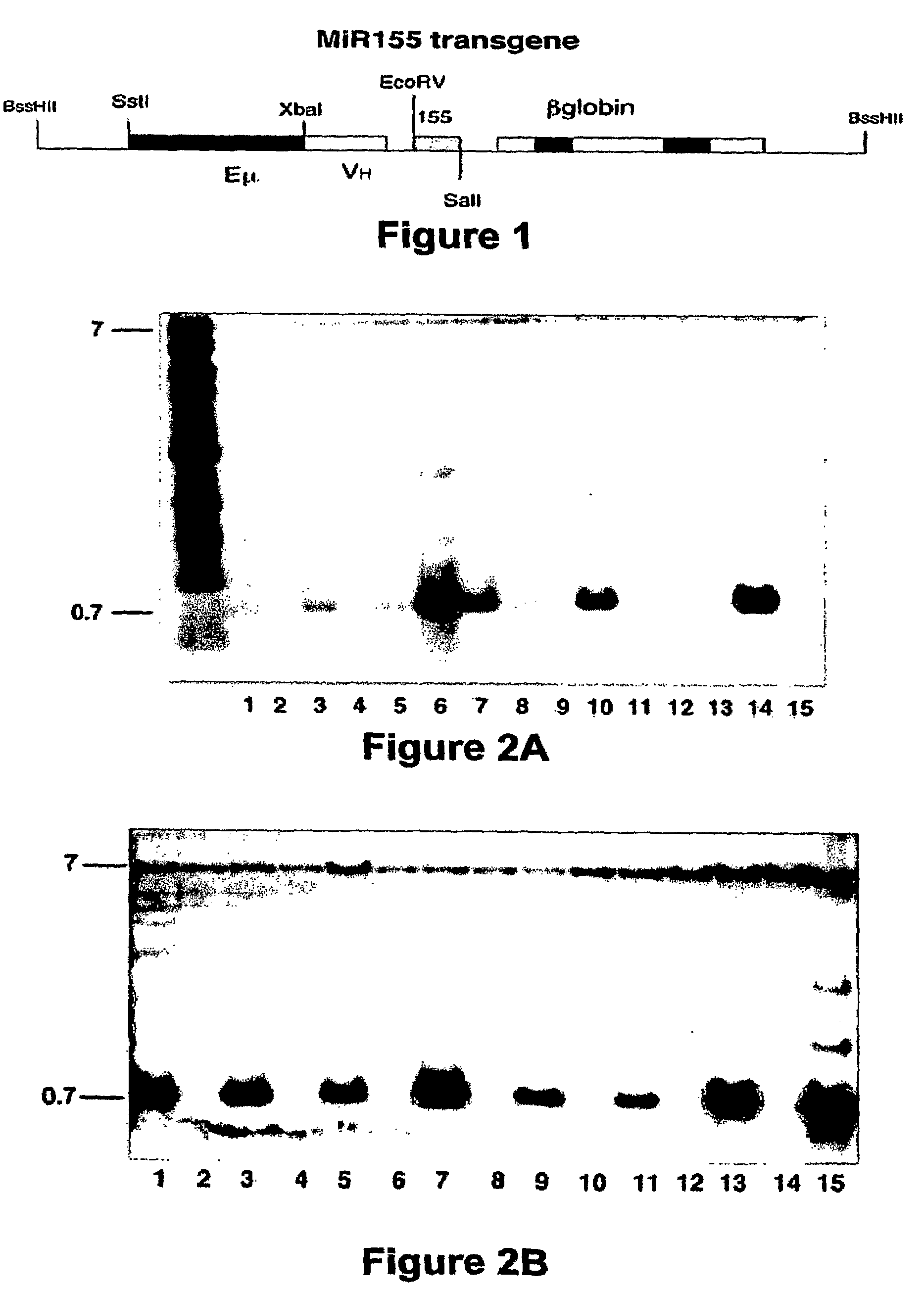
Transgenic mouse model of B cell malignancy
A transgenic non-human animal, such as a mouse, has a genome that include a nucleic acid construct having at least one transcriptional regulatory sequence capable of directing expression in B cells of the animal, wherein the transcriptional regulatory sequence is operably linked to a nucleic acid encoding a miR155 gene product. A method of testing the therapeutic efficacy of an agent in treating or preventing a lymphoproliferative condition includes assessing the effect(s) of the agent on a transgenic non-human animal.
Owner:THE OHIO STATE UNIV RES FOUND
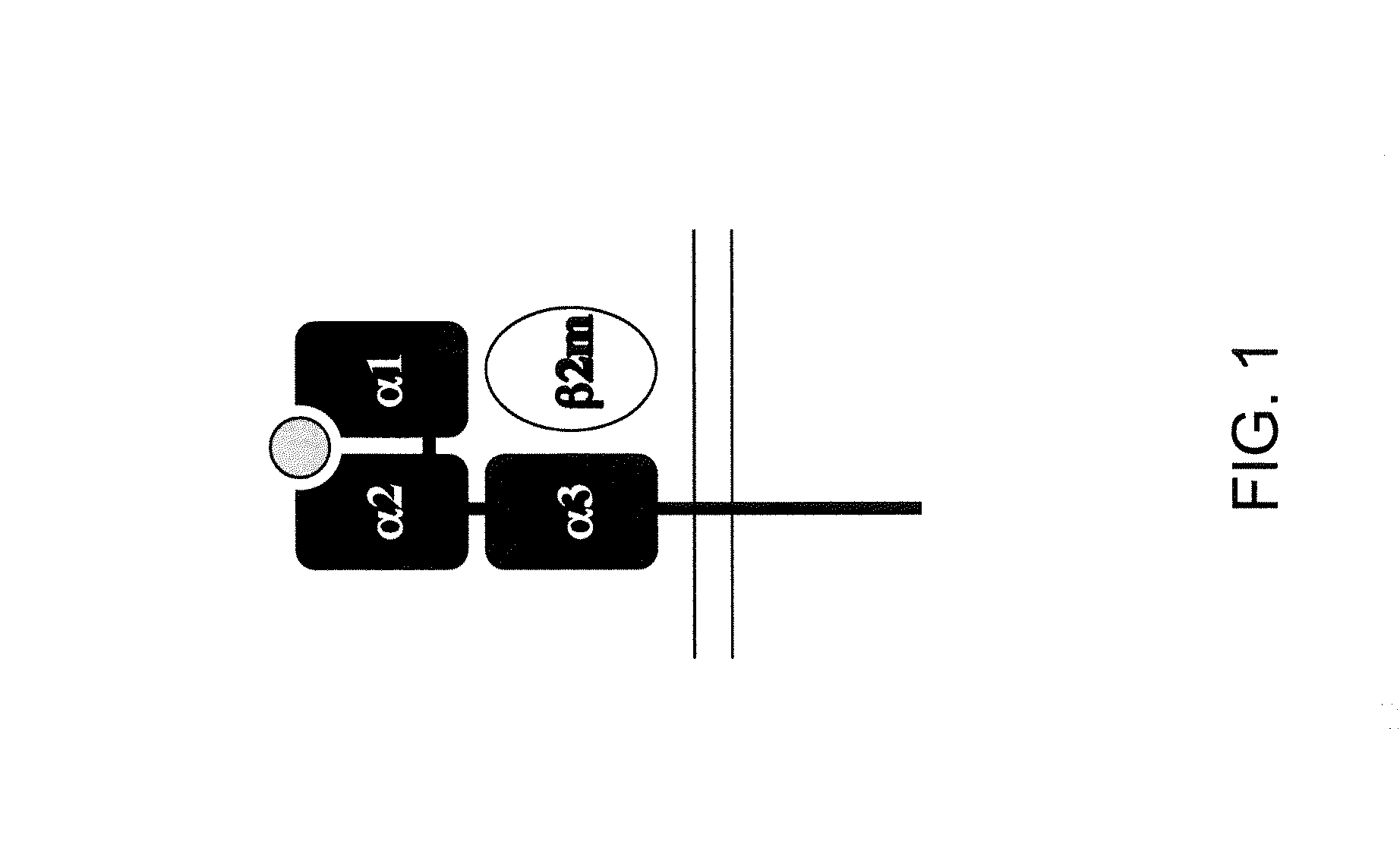
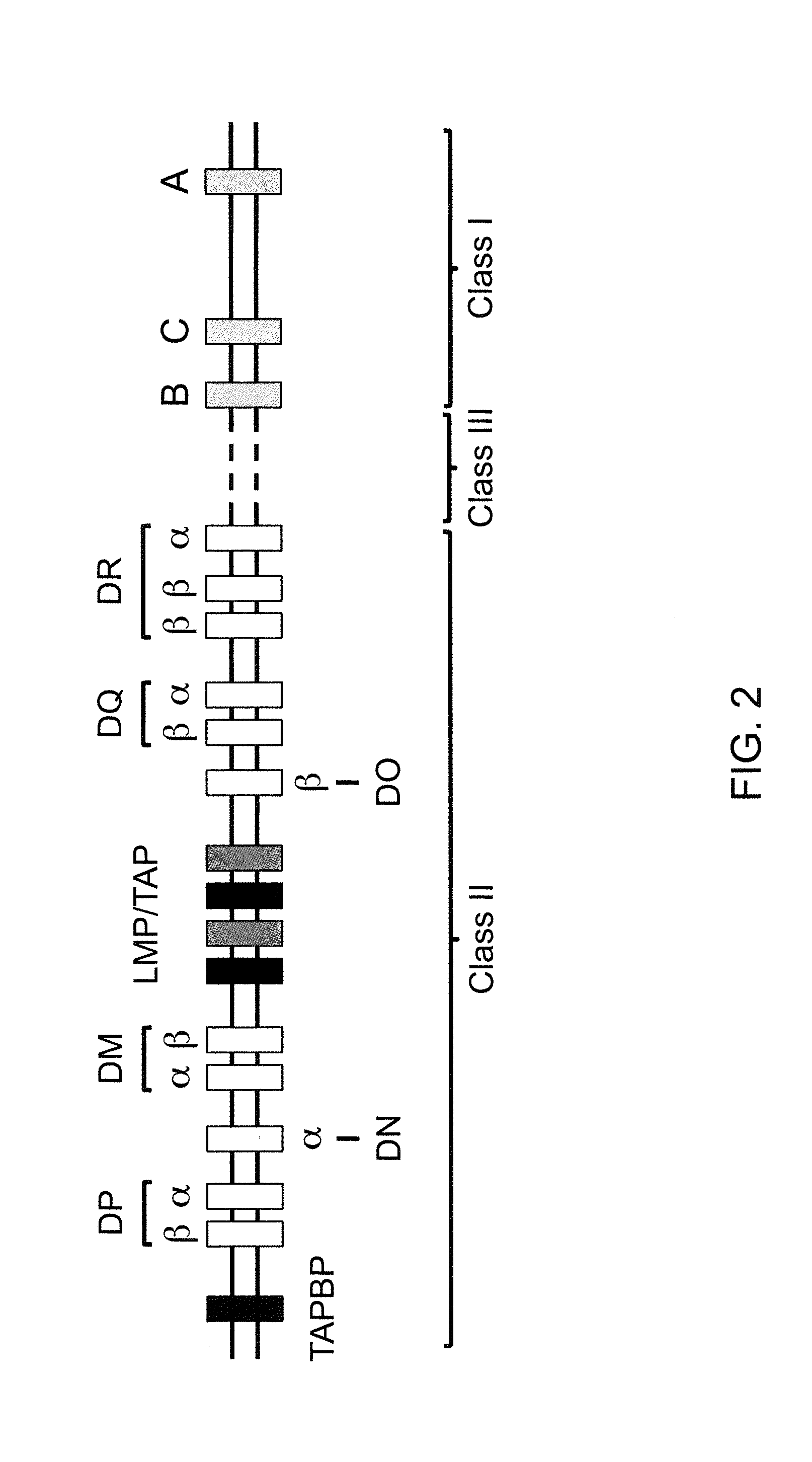
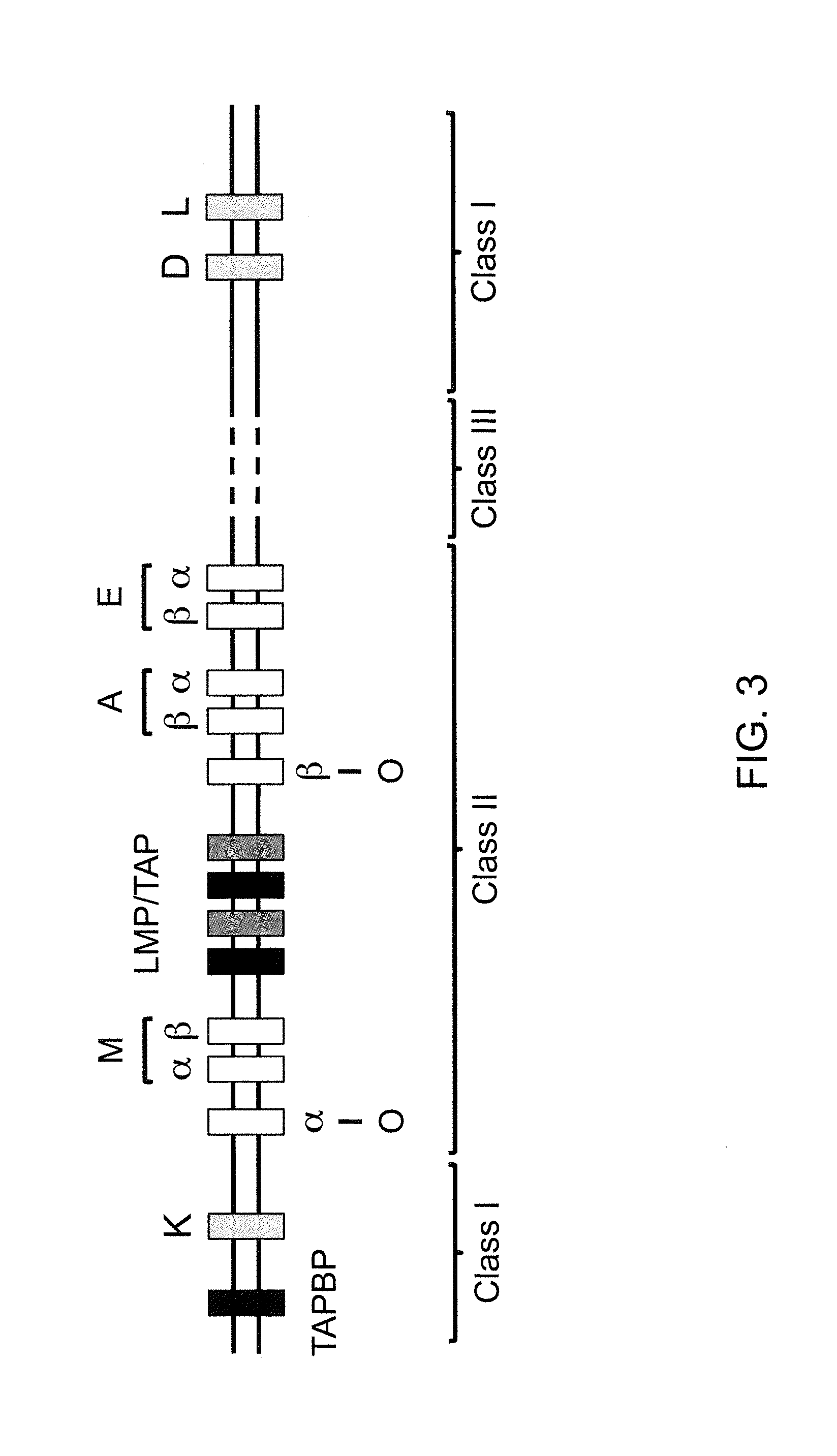
Genetically Modified Major Histocompatibility Complex Animals
The invention provides genetically modified non-human animals that express chimeric human / non-human MHC I polypeptide and / or human or humanized β2 microglobulin polypeptide, as well as embryos, cells, and tissues comprising the same. Also provided are constructs for making said genetically modified animals and methods of making the same. Methods of using the genetically modified animals to study various aspects of human immune system are provided.
Owner:REGENERON PHARM INC
Methods and compositions for the generation of humanized mice
The invention provides methods and compositions for generating non-human transgenic animals that are humanized at one or more gene sequences. According to the methods of the invention, a DNA construct containing a human DNA sequence flanked by sequences from the non-human animal is generated by recombination in a bacterial cell, for example, in E. coli. The DNA construct that is produced can then be introduced into a non-human embryogenic stem cell where it can recombine with the genomic DNA of the non-human animal.
Owner:CALIFORNIA INST OF TECH
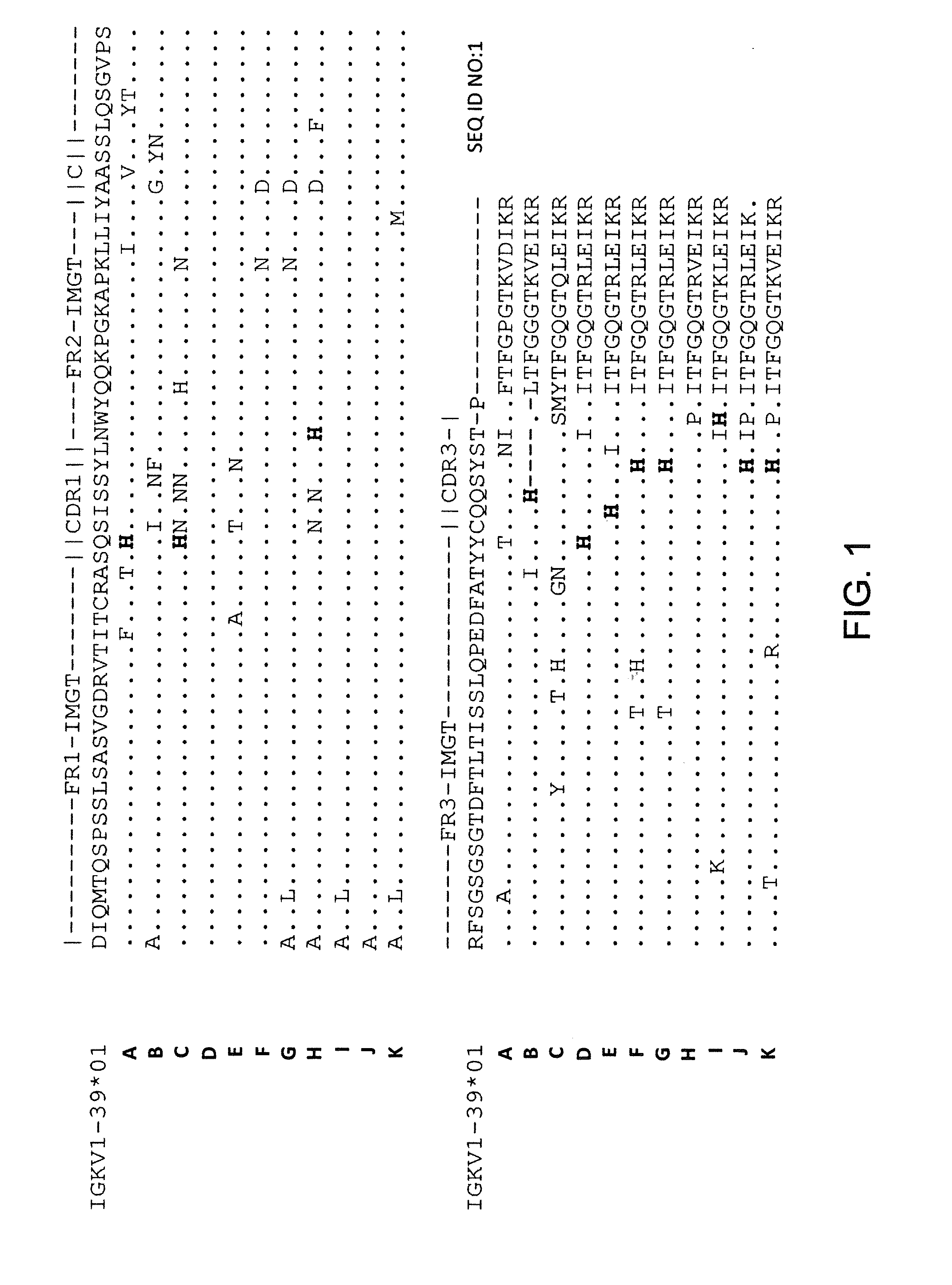
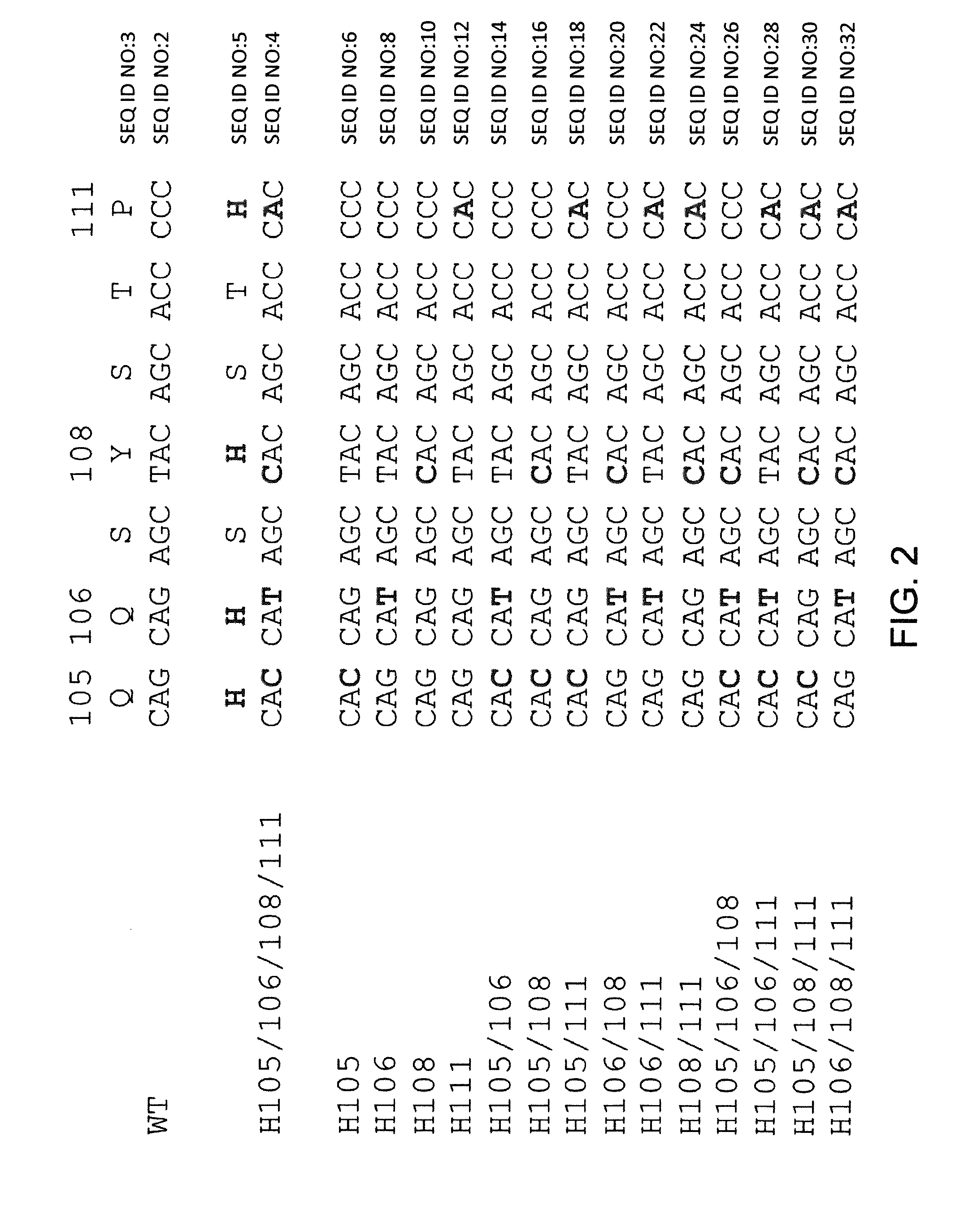
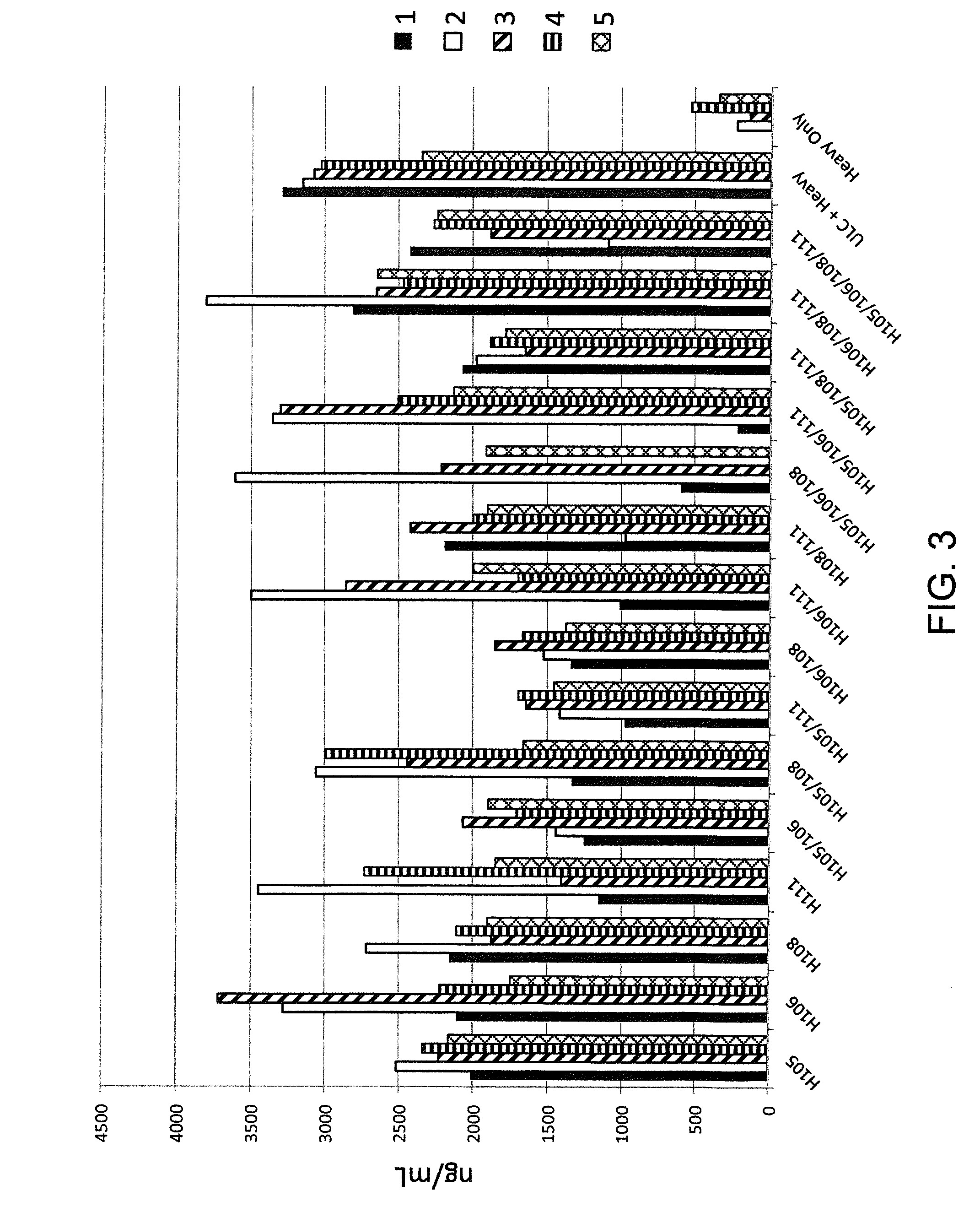
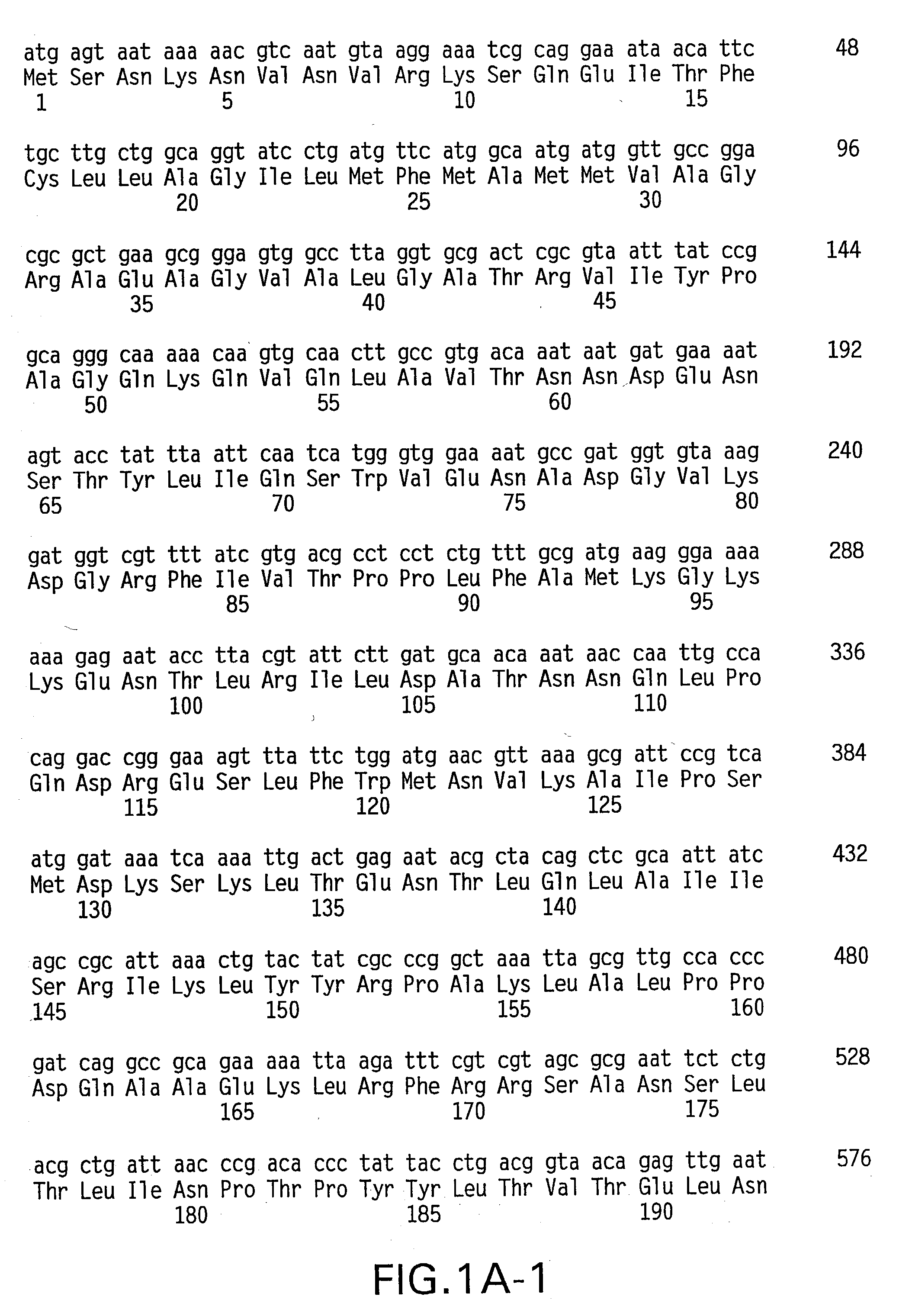
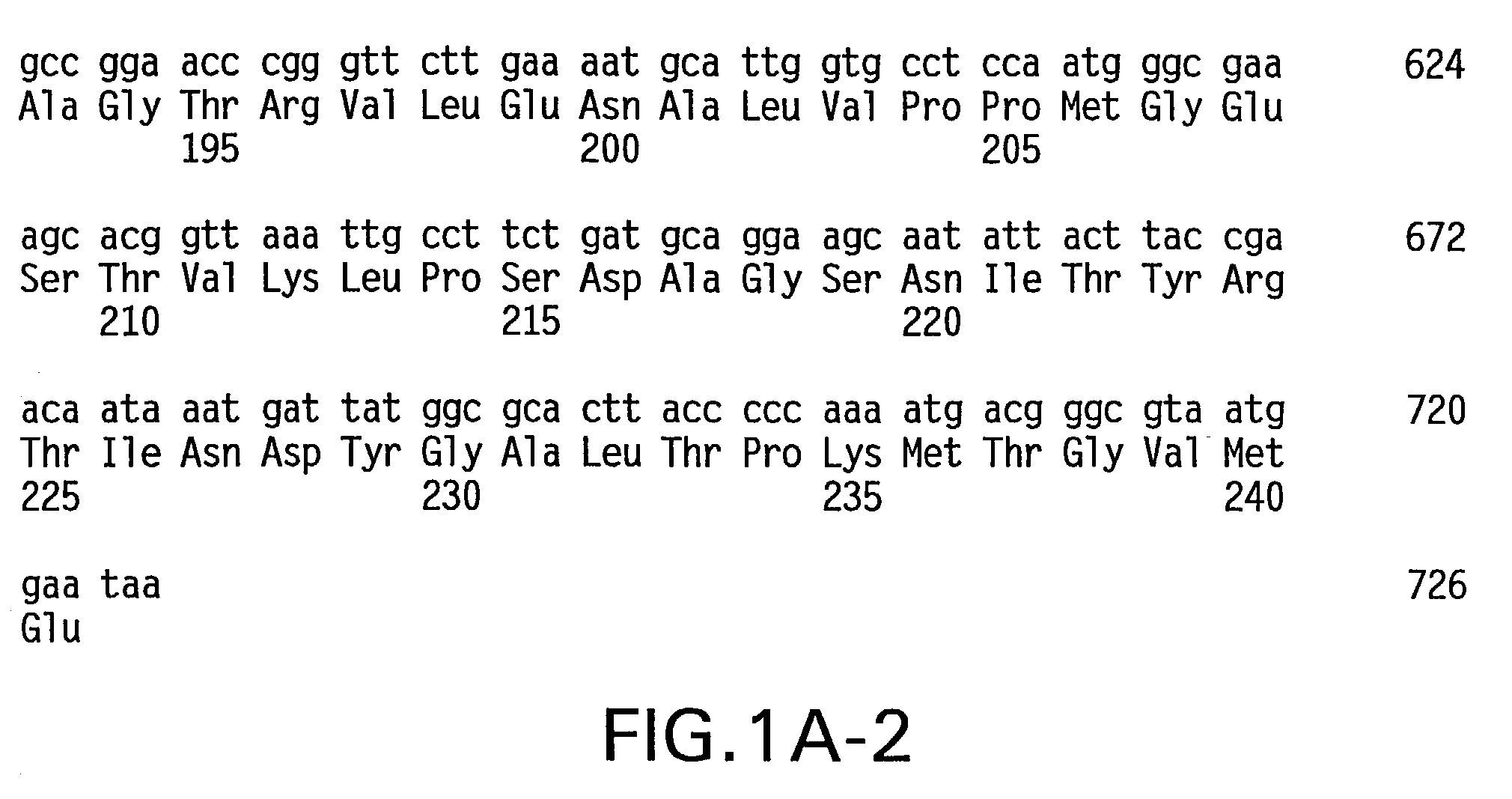
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
InactiveUS20140013456A1Reduce the binding forceNucleic acid vectorImmunoglobulinsNucleotideGenetically modified crops
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses human immunoglobulin light chain variable domains derived from a limited repertoire of human immunoglobulin light chain variable gene segments that comprise histidine modifications in their germline sequence. Methods of making non-human animals that express antibodies comprising histidine residues encoded by histidine codons introduced into immunoglobulin light chain nucleotide sequences are provided.
Owner:REGENERON PHARM INC
Mutant proteins, high potency inhibitory antibodies and fimch crystal structure
InactiveUS20030199071A1Great functional inhibitory activityStrong inhibitory activityHydrolasesImmunoglobulins against bacteriaPassive ImmunizationsMutated protein
The present invention provides bacterial immunogenic agents for administration to humans and non-human animals to stimulate an immune response. It particularly relates to the vaccination of mammalian species, especially human patients, with variants of the E. coli FimCH protein that elicit antibodies that have better functional inhibitory activity than antibodies raised against wild type protein. In particular, such variants include mutations that promote a more open confirmation of the FimH protein, particularly in regions involved in mannose binding, to expose regions previously poorly exposed and mutations that abolish a significantly reduce mannose binding. In another aspect, the invention provides antibodies against such proteins and protein complexes that may be used in passive immunization to protect or treat pathogenic bacterial infections. The present invention also provides machine readable media embedded with the three-dimensional atomic structure coordinates of FimCH bound to mannose, and subsets thereof, and methods of using the crystal structure to provide candidate amino acid residues for mutation.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Protein and lipid sources for use in aquafeeds and animal feeds and a process for their preparation
InactiveUS6955831B2Increased digestible energy contentAdditional componentBioloigcal waste fertilisersEdible oils/fatsFiberHuman animal
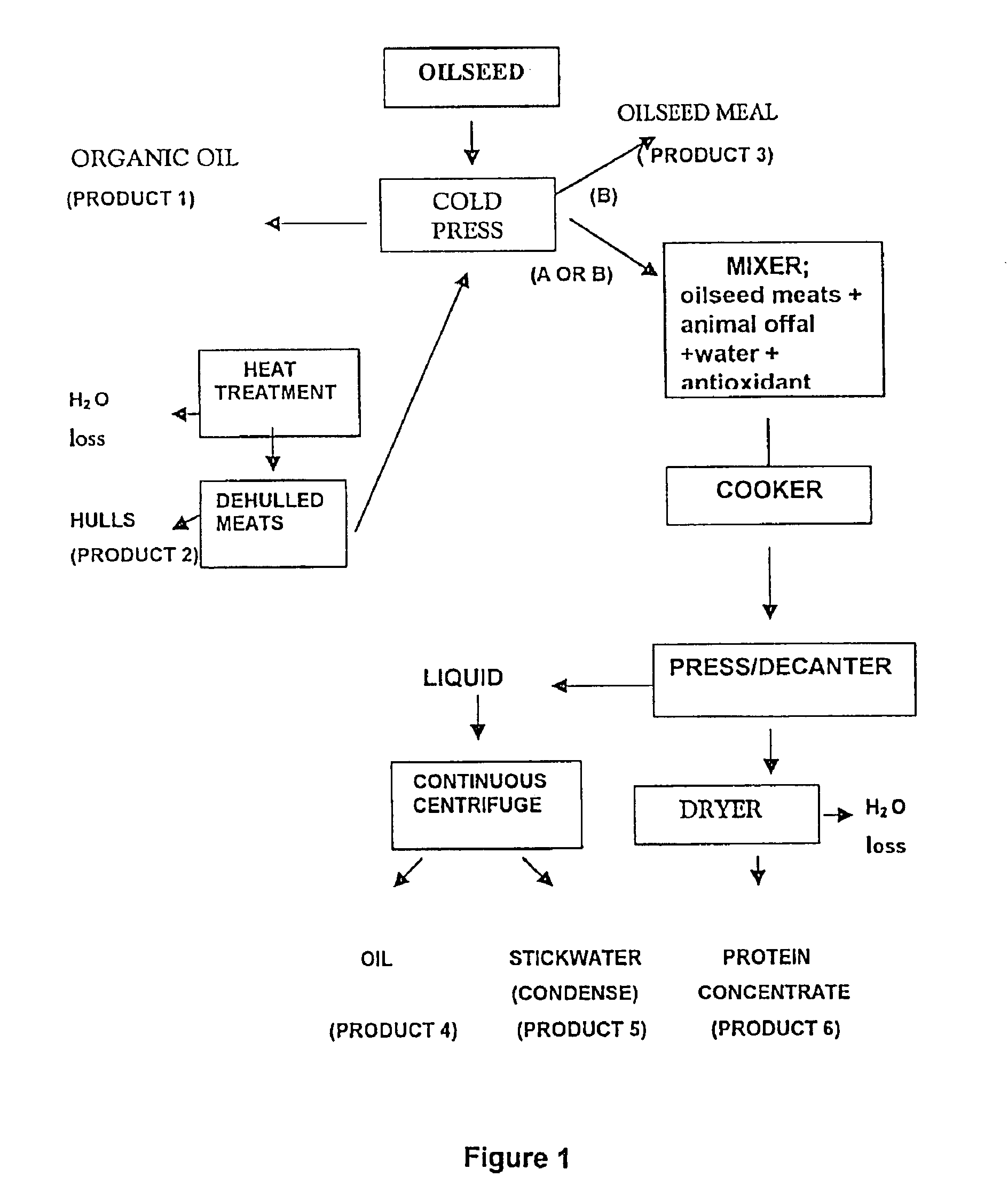
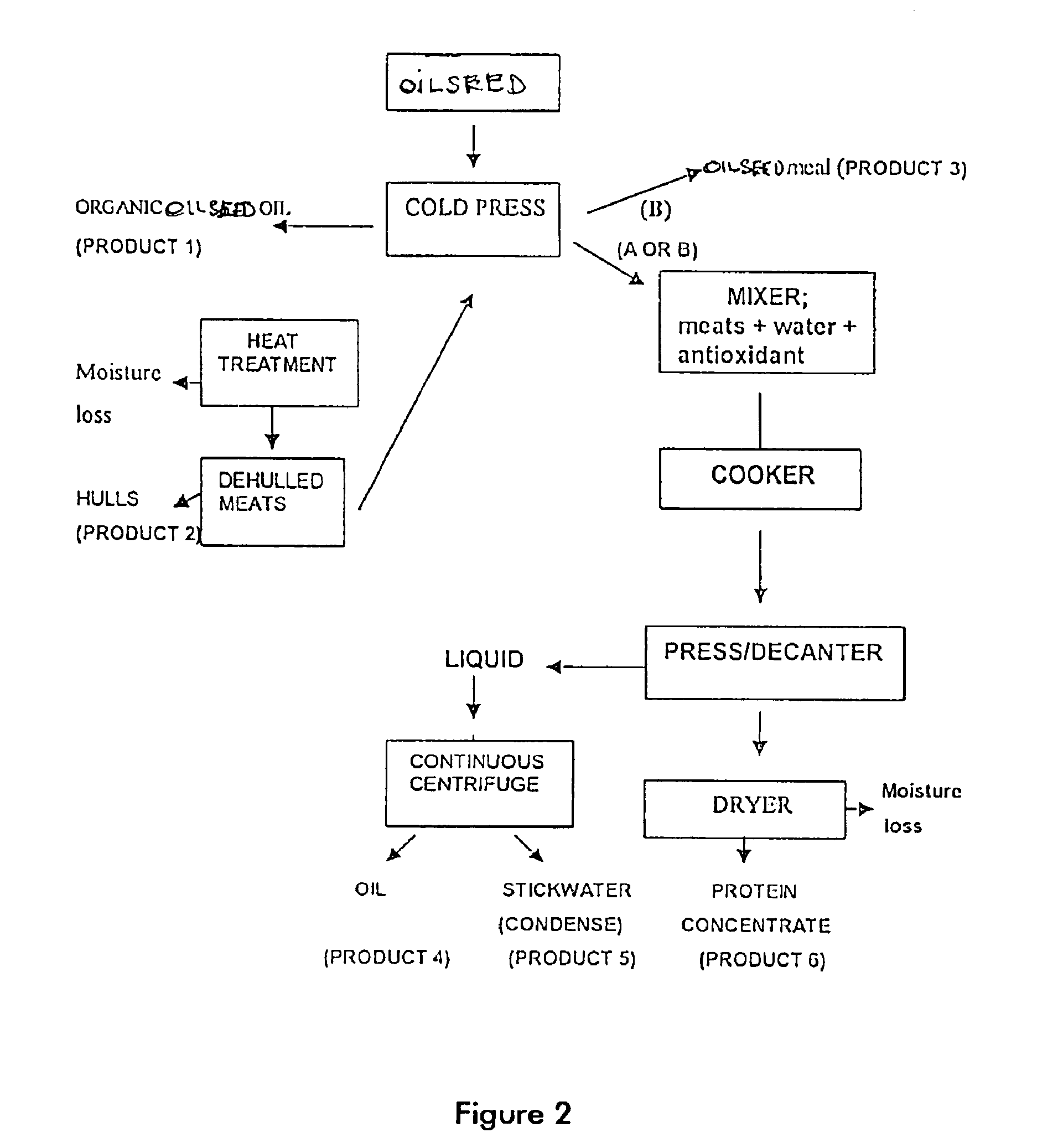
A process for preparation of nutritionally upgraded oilseed meals which are protein and lipid-rich and have a reduced fiber content, and plant oils from oilseeds for use in fish or other non-human animal diets or human foods comprising the steps of: providing a source of oilseed; subjecting the oilseed to heat treatment to substantially reduce the concentration of at least some antinutritional components normally present in the oilseed to obtain heat-treated seed; dehulling the heat-treated seed to produce a meat fraction, a hull fraction or a mixture thereof; and cold pressing the meat fraction or the mixture to yeild the plant oils and the protein and lipid-rich meals.
Owner:HER MAJESTY THE QUEEN IN RIGHT OF CANADA AS REPRRESENTED BY THE MINIST OF FISHERIES & OCEANS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com