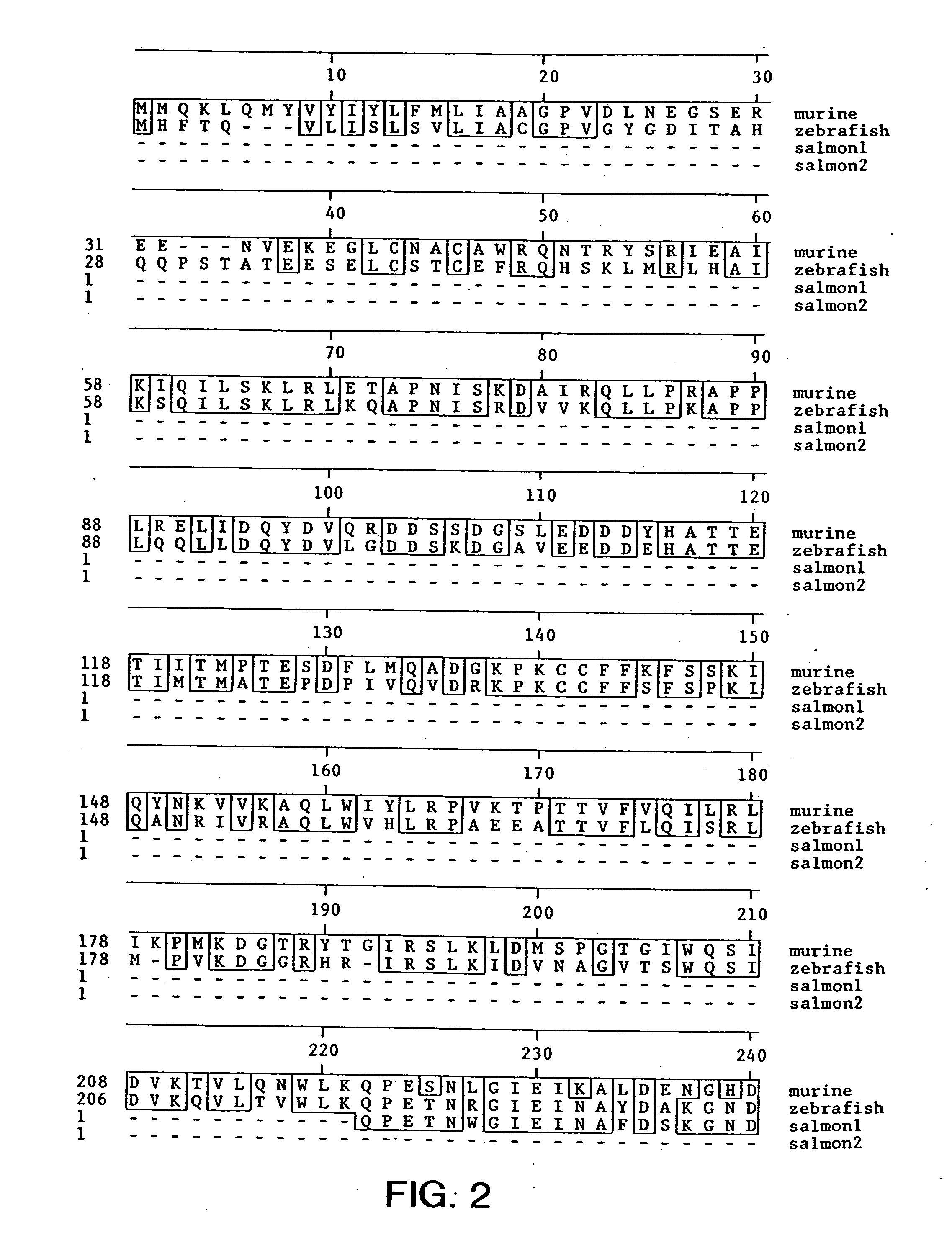
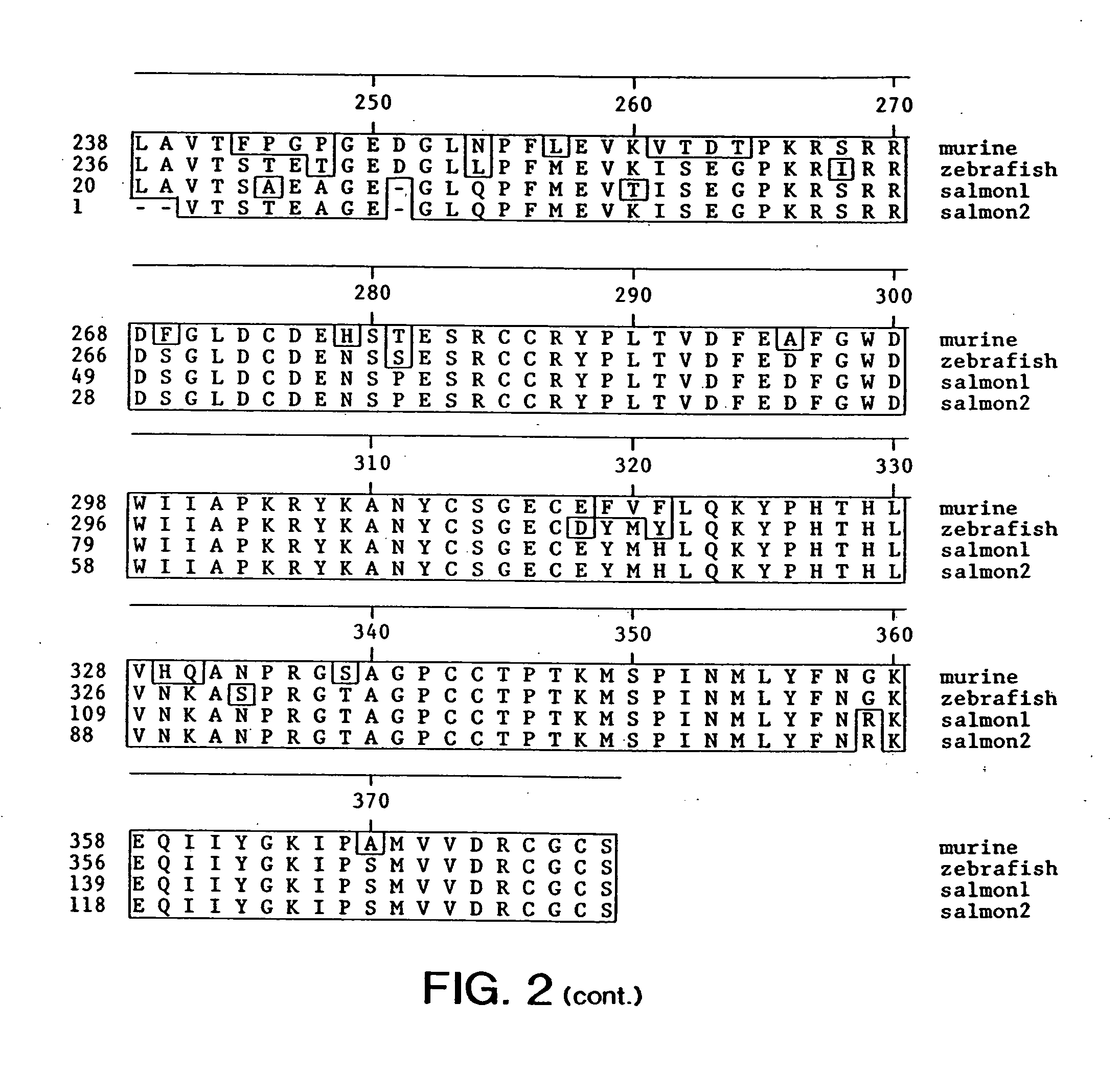
Transgenic non-human animals expressing a truncated activin type II receptor
a technology of truncated activin and non-human animals, which is applied in the field of gdf8 (myostatin) receptors, can solve the problems of morbidity and mortality, increasing the amount of time, effort and money spent in the united states by individuals intent on losing weight, and becoming a major problem among the pediatric population. it can increase myostatin signal transduction, reduce or inhibit myostatin signal transduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Myostatin Acts in a Dose Dependent Manner
[0248] This example demonstrates that the activity of myostatin in inhibiting muscle growth is dependent on the level of myostatin expression in vivo.
[0249] Myostatin is a negative regulator of skeletal muscle mass (McPherron et al., supra, 1997; McPherron and Lee, supra, 1997). Myostatin knock-out mice that were homozygous for a deletion of the myostatin gene had a 25-30% increase in total body mass. An examination of the homozygous knock-out mice revealed that the increased muscle mass was due to about a 100-200% increase in skeletal muscle mass throughout the body.
[0250] Mice that were heterozygous for the myostatin mutation also had an increase in total body mass. However, the increase mass of the heterozygotes was less than that of the homozygotes, and was statistically significant in only one age and sex group among the many examined. In order to determine whether heterozygous mice have an intermediate phenotype between that of wild ...
example 2
Myostatin Effect Decreases with Age in Knock-Out Mice
[0252] This example demonstrates that a decreased difference in body weight between wild type mice and homozygous myostatin knock-out mice is associated with a decline in muscle weight of the mutant mice.
[0253] Myostatin knock-out mice weighed approximately 25-30% more than wild type mice at five months of age (McPherron et al., supra, 1997). However, this difference in total body weights became significantly smaller or disappeared altogether as the animals aged. In order to determine whether this effect was due to a relative loss of weight in the knock-out mice due, for example, to muscle degeneration, or to a relatively greater weight gain by wild type mice, a detailed analysis of muscle weights was made as a function of age.
[0254] At all ages examined from 2 months to 17 months, the pectoralis muscle weighed significantly more in homozygous mutant mice than in wild type littermates. The most dramatic difference was observed ...
example 3
Myostatin Affects Fat Accumulation in a Dose Dependent Manner
[0255] This example demonstrates that myostatin knock-out mice fail to accumulate fat, and that the decrease in fat accumulation is associated with the level of myostatin expression in vivo.
[0256] Since the decline in muscle weights in myostatin mutants, as demonstrated in Example 2, did not fully account for the observation that the wild type animals eventually weighed about the same as the mutant mice, the amount of fat accumulation in wild type and mutant mice was examined. The inguinal, epididymal and retroperitoneal fat pads in male mice were examined. There was no difference in the weights of any of these fat pads between wild type and mutant mice at two months of age. By 5 to 6 months of age, wild type and heterozygous knock-out mice both exhibited a large range of fat pad weights, and, on average, fad pad weights increased by approximately 3-fold to 5-fold by the time the animals reached 9 to 10 months of age. Du...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com