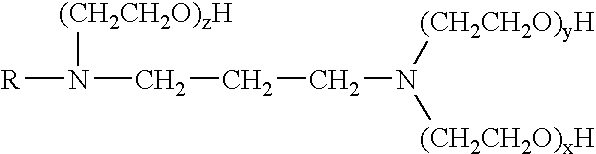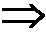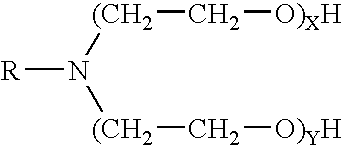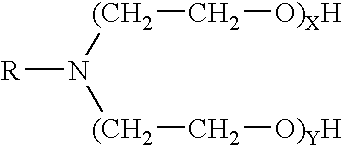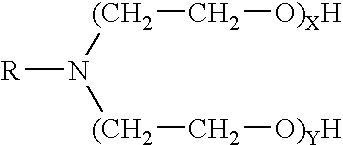Patents
Literature
214 results about "Ph dependent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
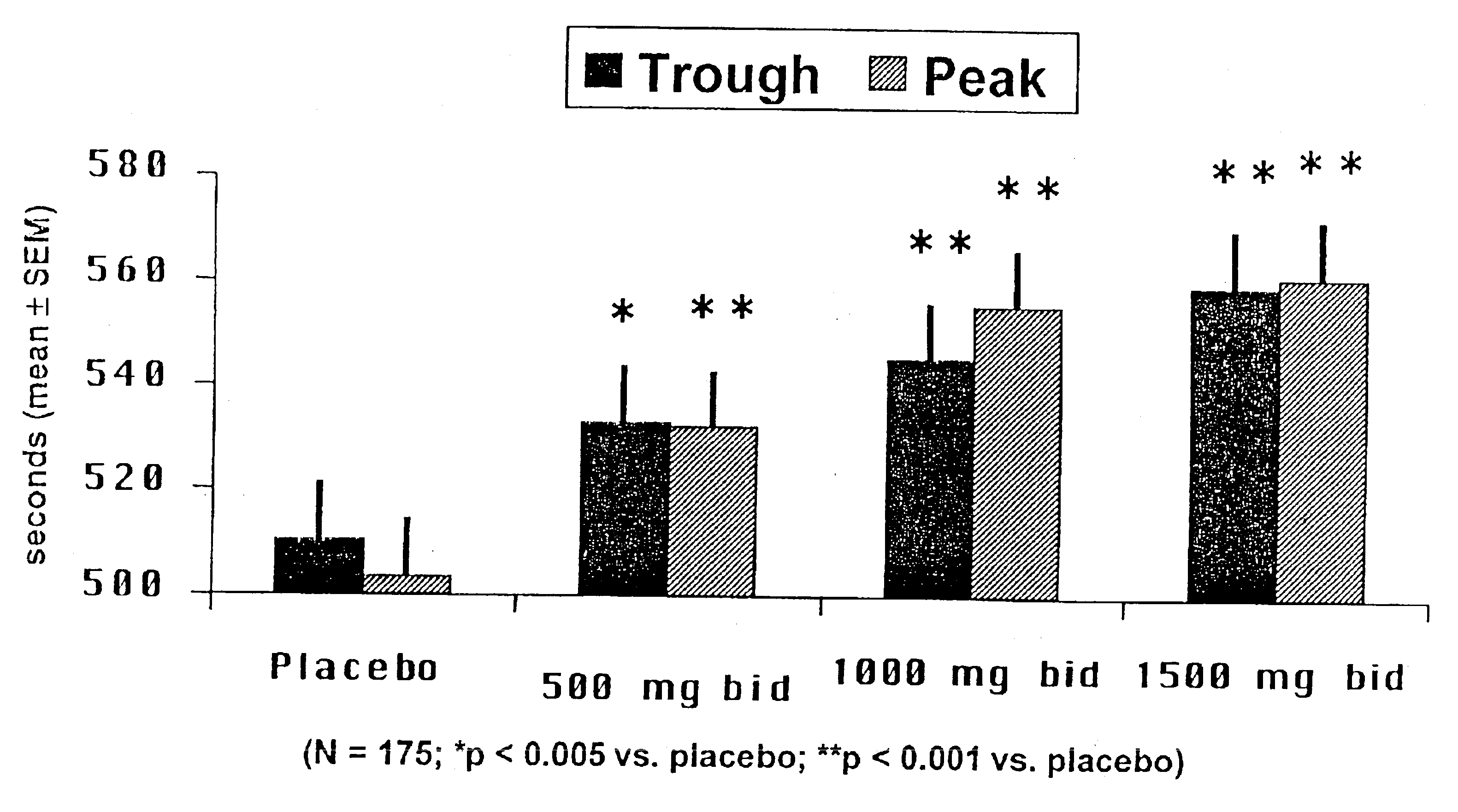
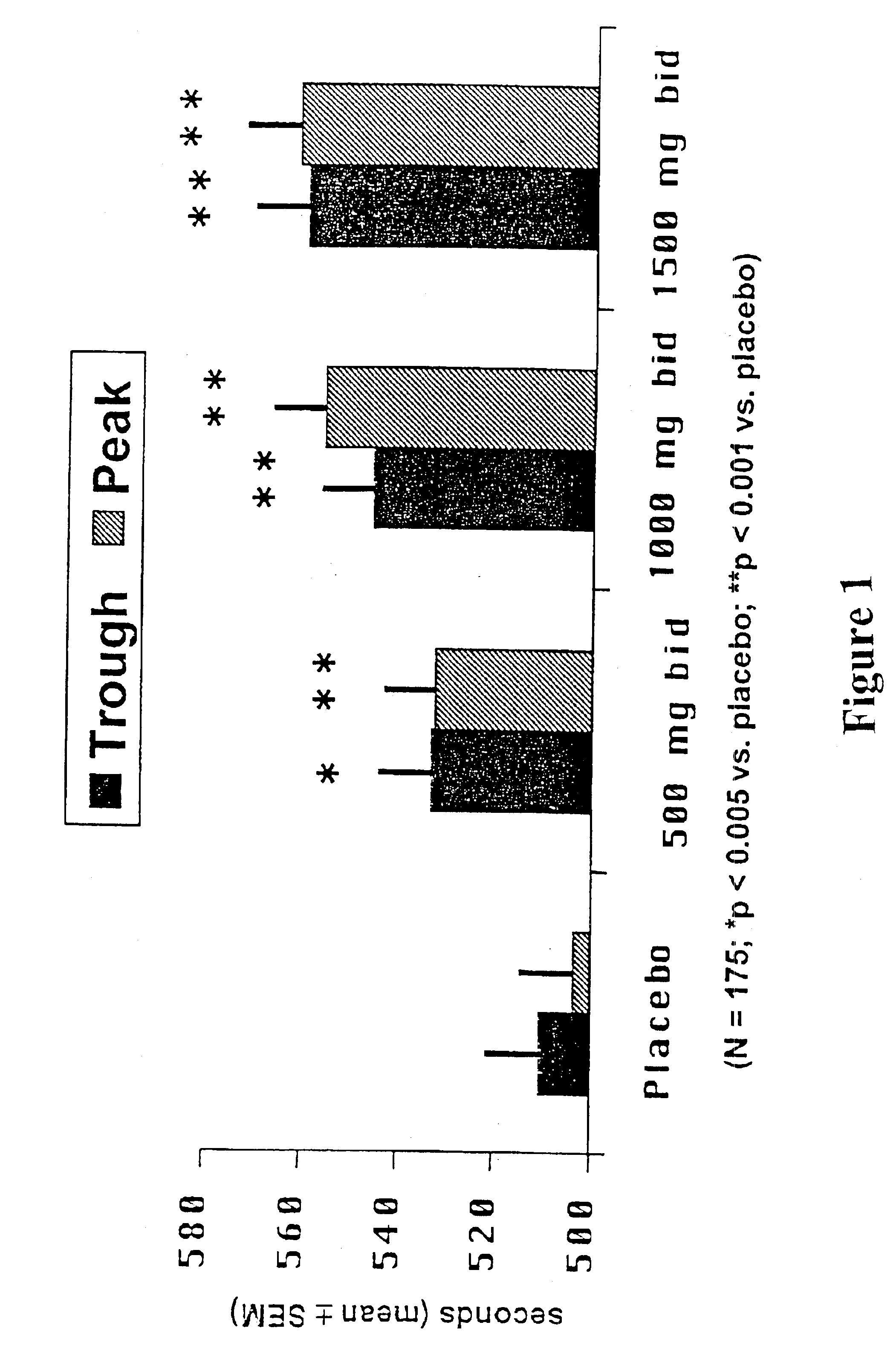
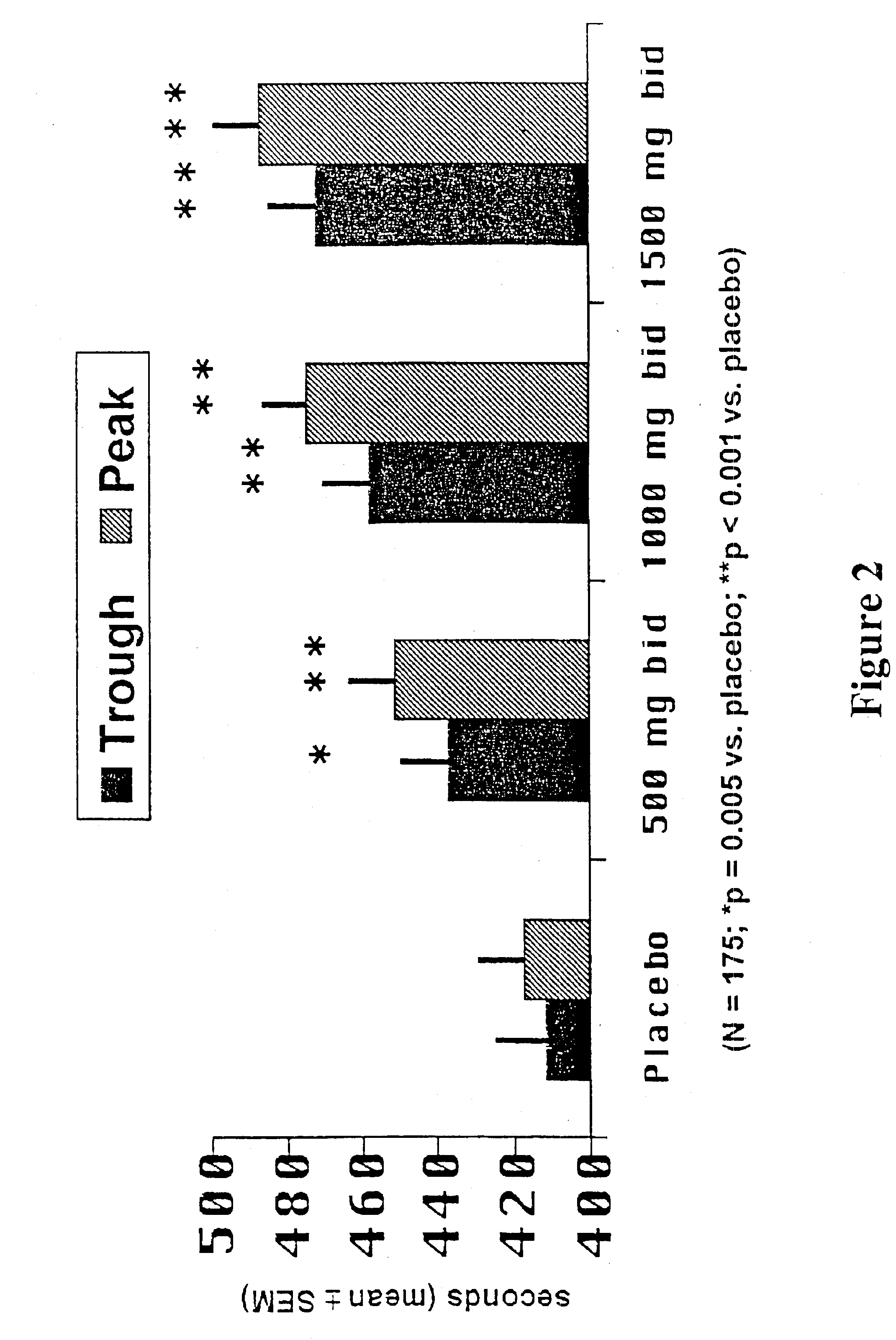
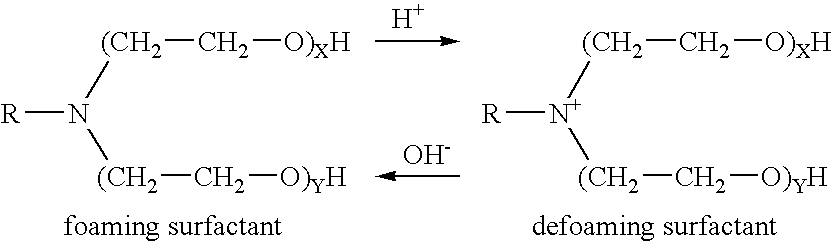
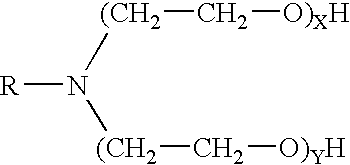
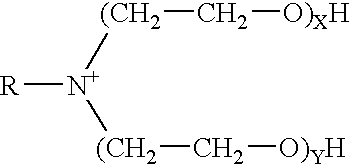
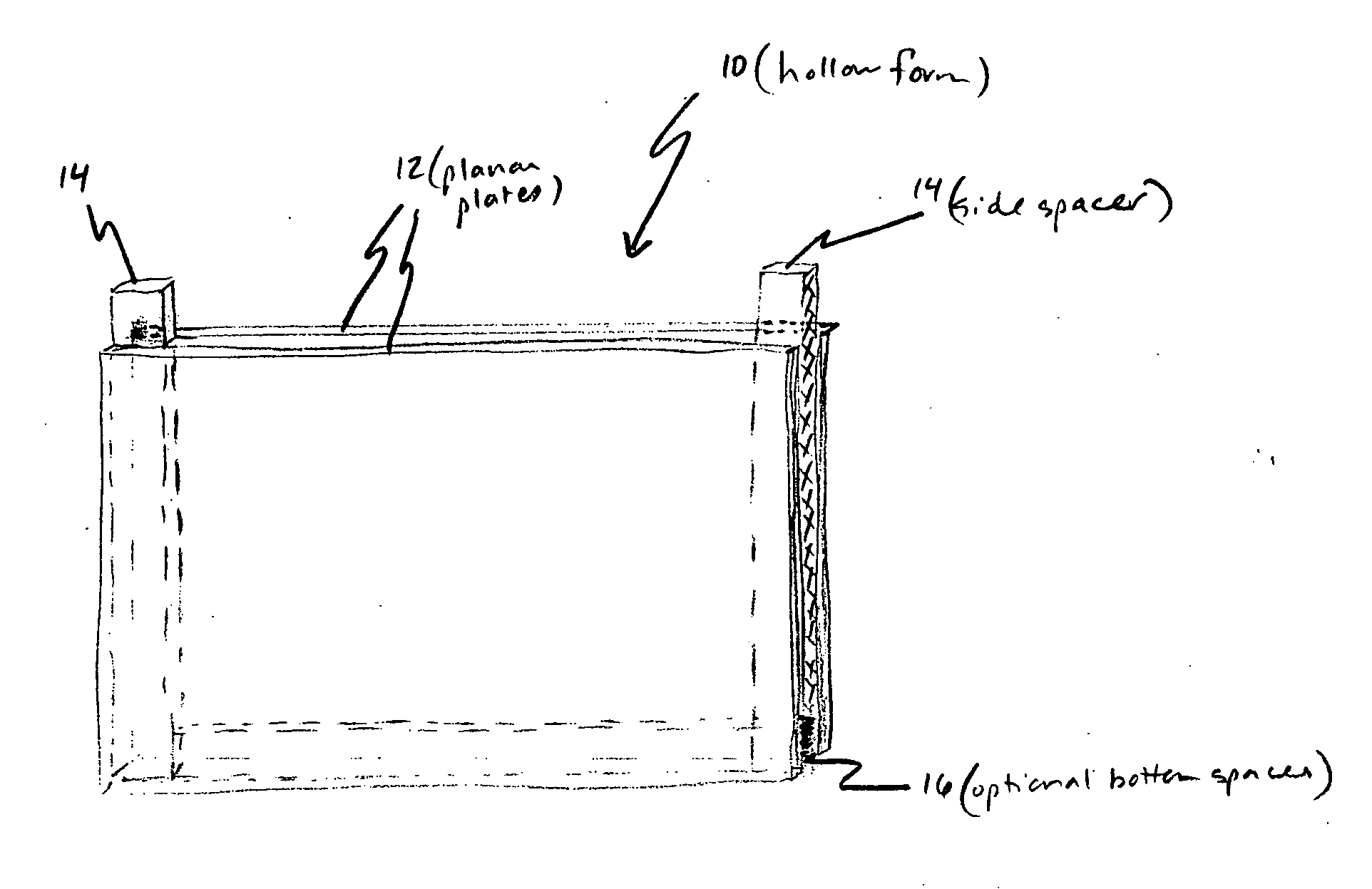
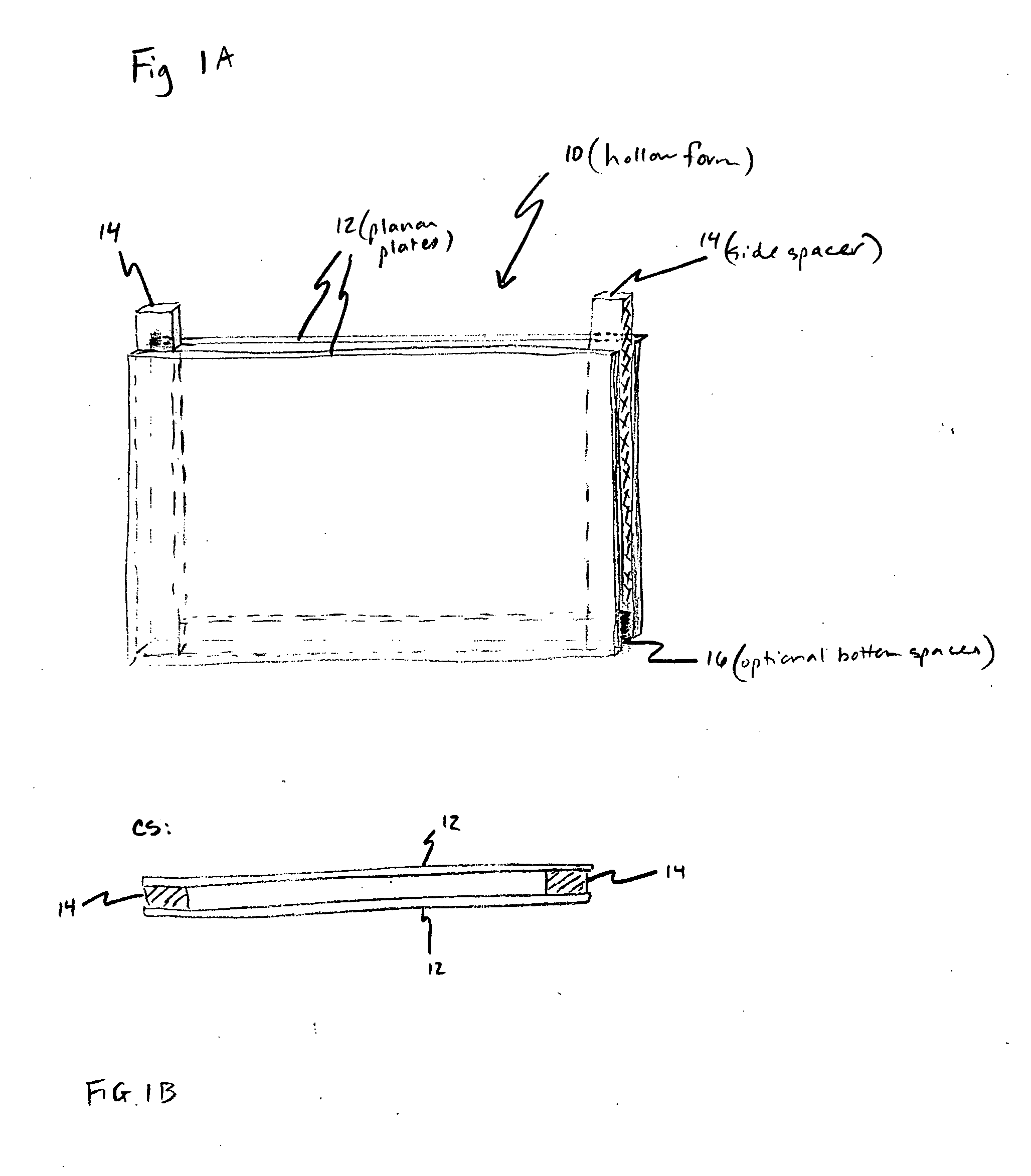
A pH-dependent foamed fracturing fluid has been described. The fracturing fluid is made by combining a gelling agent, a surfactant, and a proppant. The surfactant is capable of facilitating foaming of the fracturing fluid at the initial pH and defoaming of the fracturing fluid when its pH is changed.
Chia seed beverage and related method
InactiveUS20090181114A1Enhancing heart healthImprove regularityBiocideDigestive systemFruit juiceFlavored water
A beverage is disclosed that is effective for enhancing gastrointestinal regularity and heart health. It is formed by a liquid comprising fruit derived juices, water or naturally or artificially flavored water. A composition of matter is mixed within the liquid in a shelf stable pasteurized beverage form and formed from sterilized whole seed extracted from Salvia hispanica L. The resulting beverage exhibits a pH dependent viscosity requiring no additional thickening agents and suitable as a beverage for human consumption.
Owner:US NUTRACEUTICALS LLC
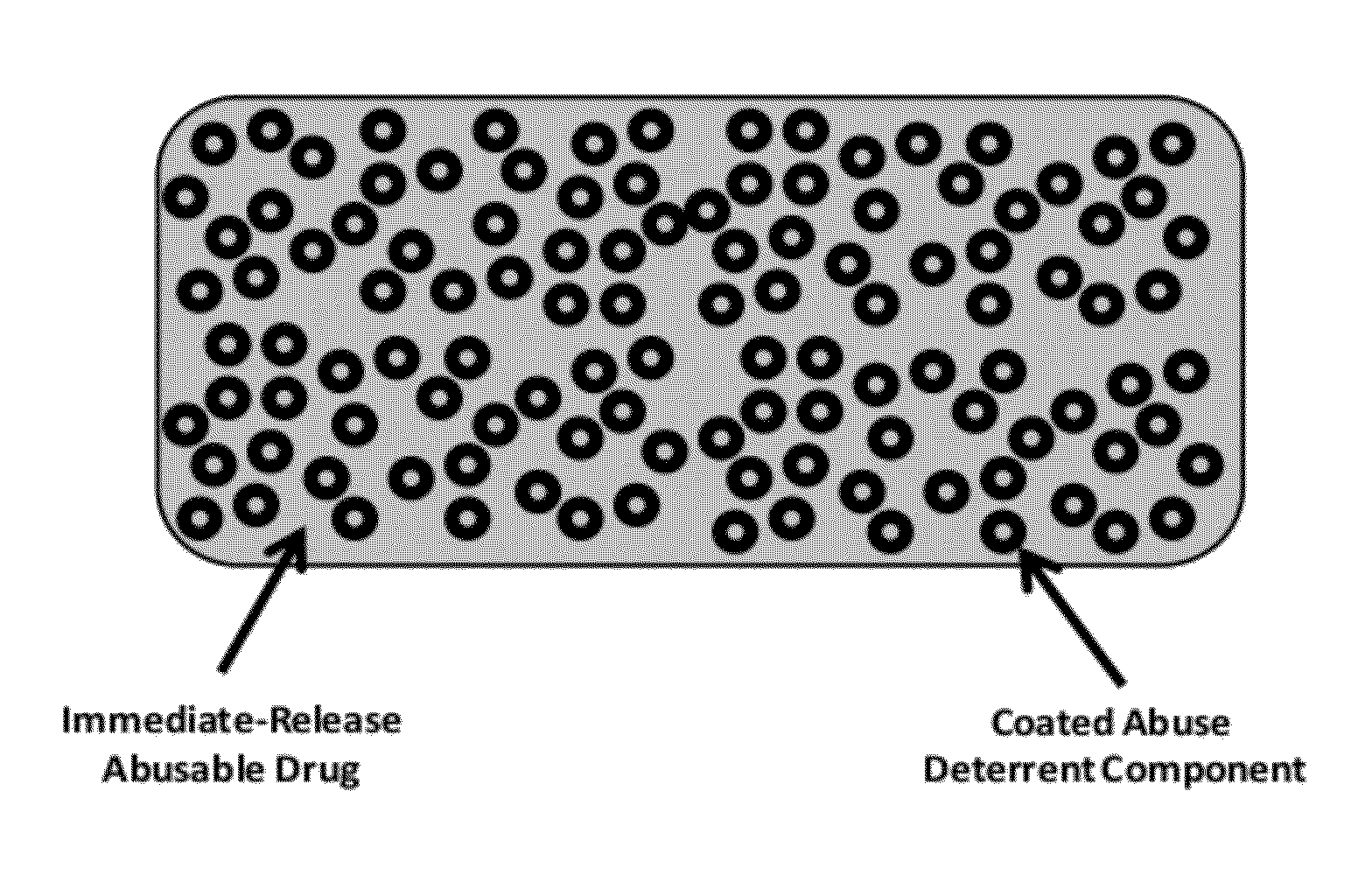
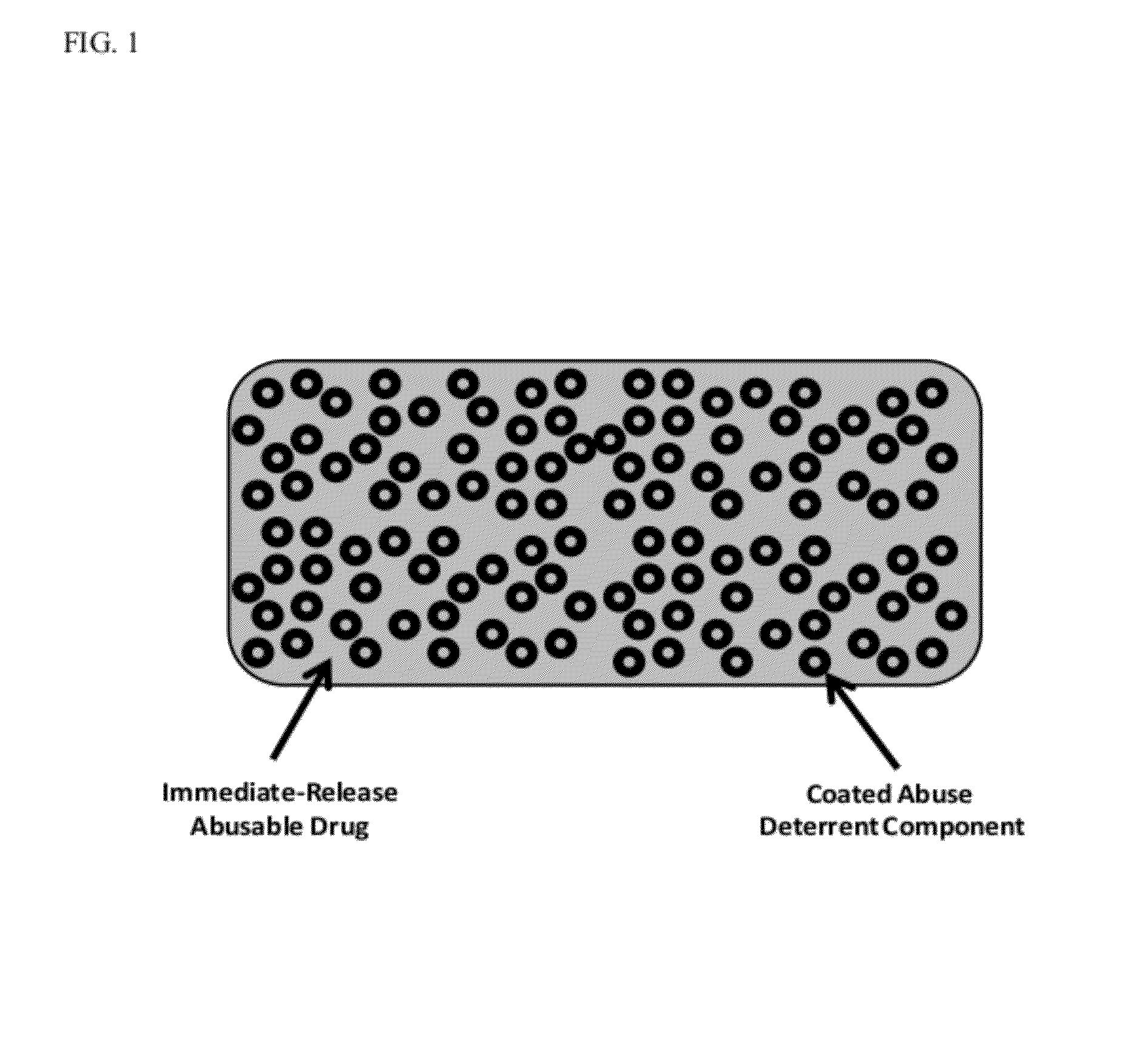
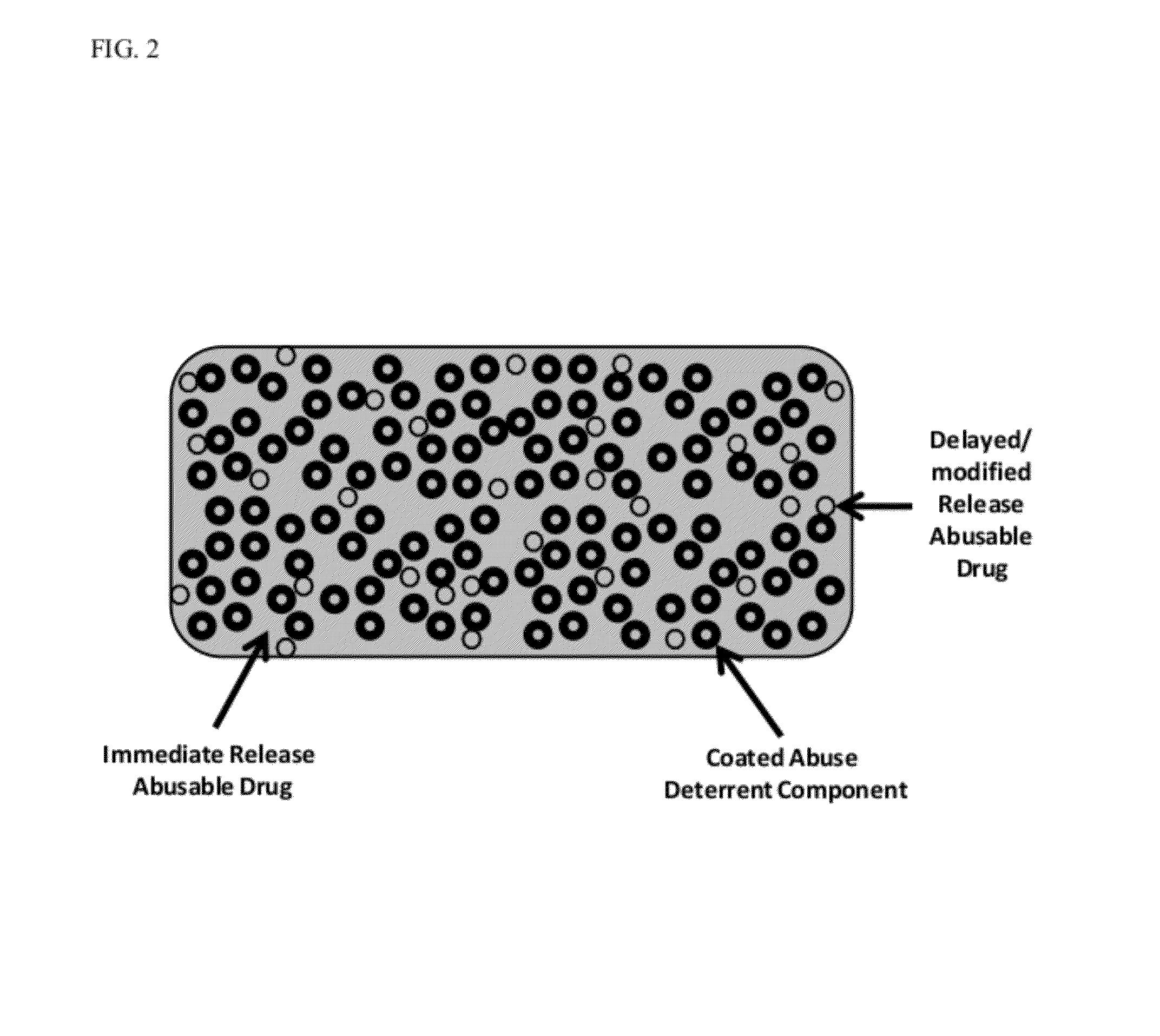
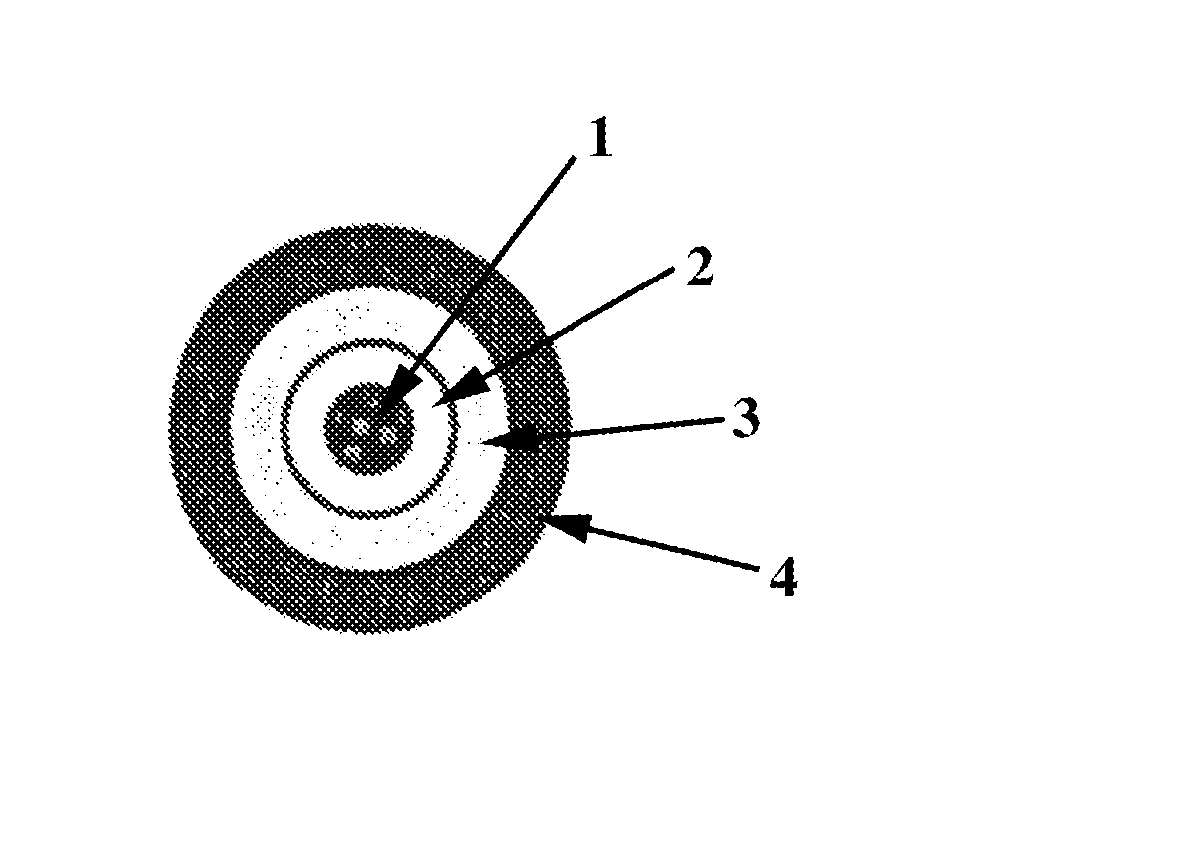
Technology for preventing abuse of solid dosage forms
InactiveUS20120321716A1Excessive amountReduce probabilityBiocidePowder deliveryAbuse deterrentPharmaceutical formulation
Abuse resistant pharmaceutical formulations are provided that contain one or more abusable drugs and one or more abuse deterrent components. The abuse deterrent component(s) prevent the abusable drug(s) from being removed / extracted to an appreciable extent and / or rate. The abuse deterrent component(s) may be in the form of pellets, beads, beadlets, granules, powders, or the like, and may comprise a core that contains a material that is both hydrophilic and hydrophobic, and optionally a pH-dependent coating.
Owner:VACHON MICHAEL +1
Controlled release system and method for manufacturing the same
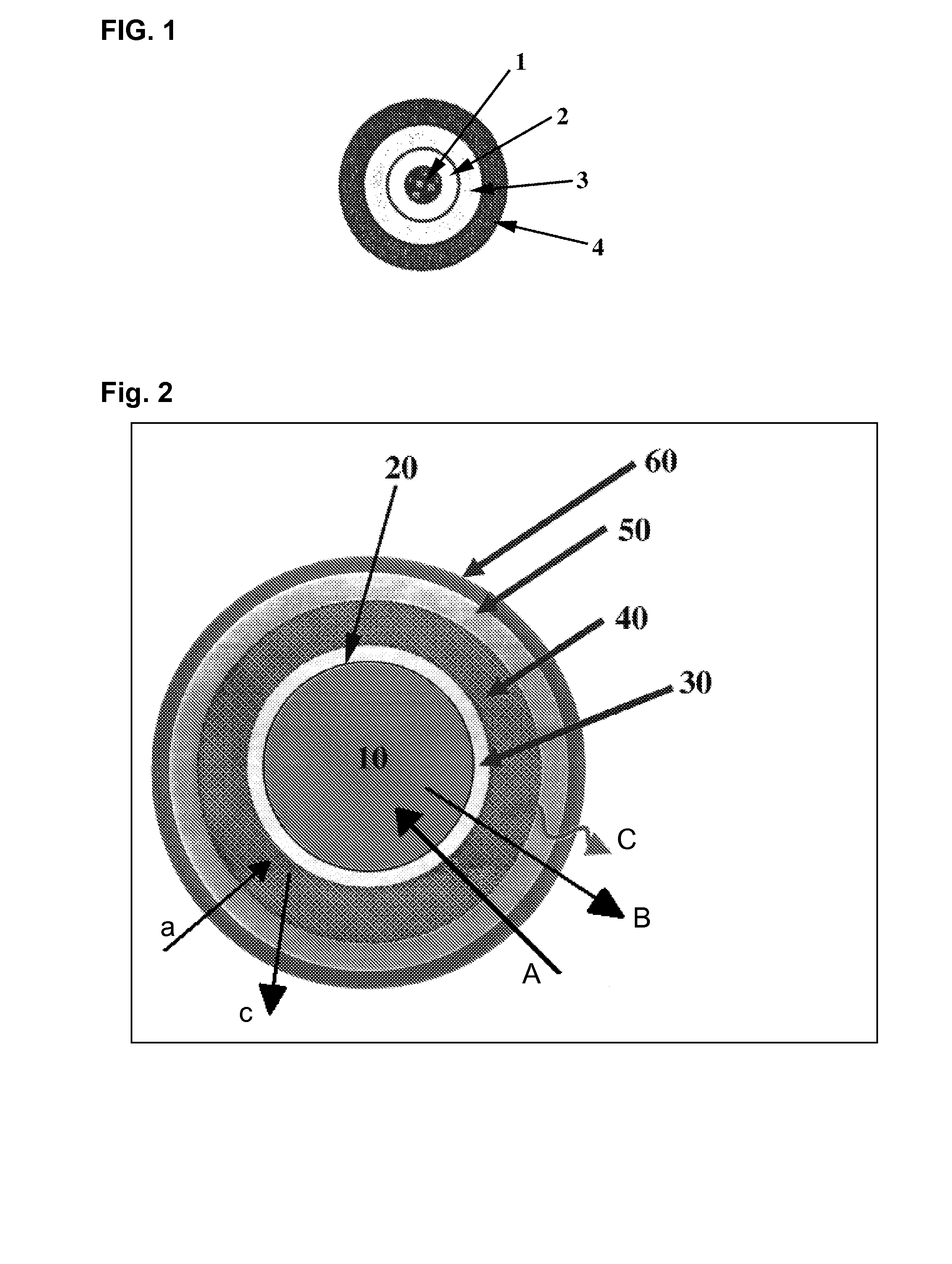
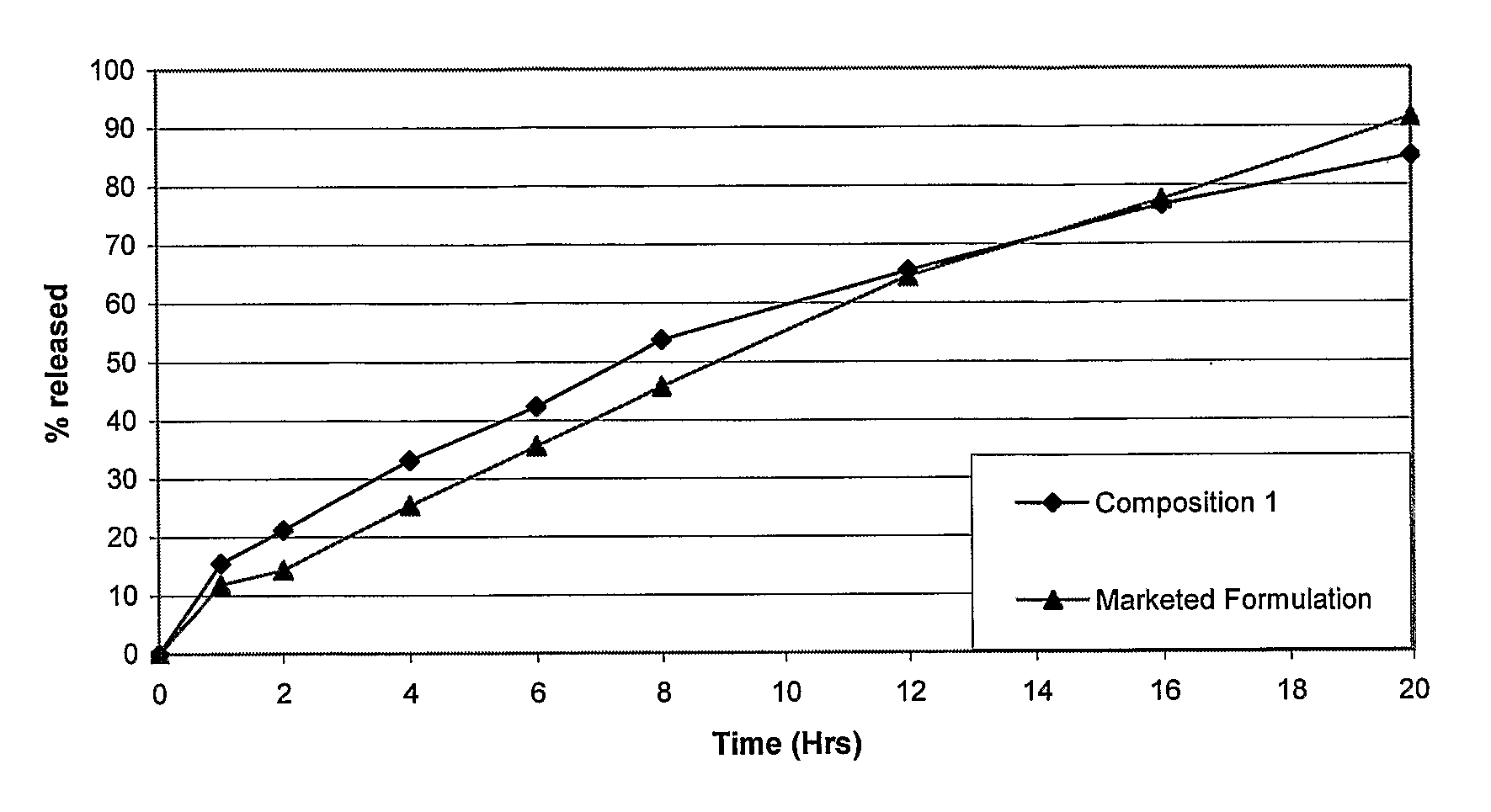
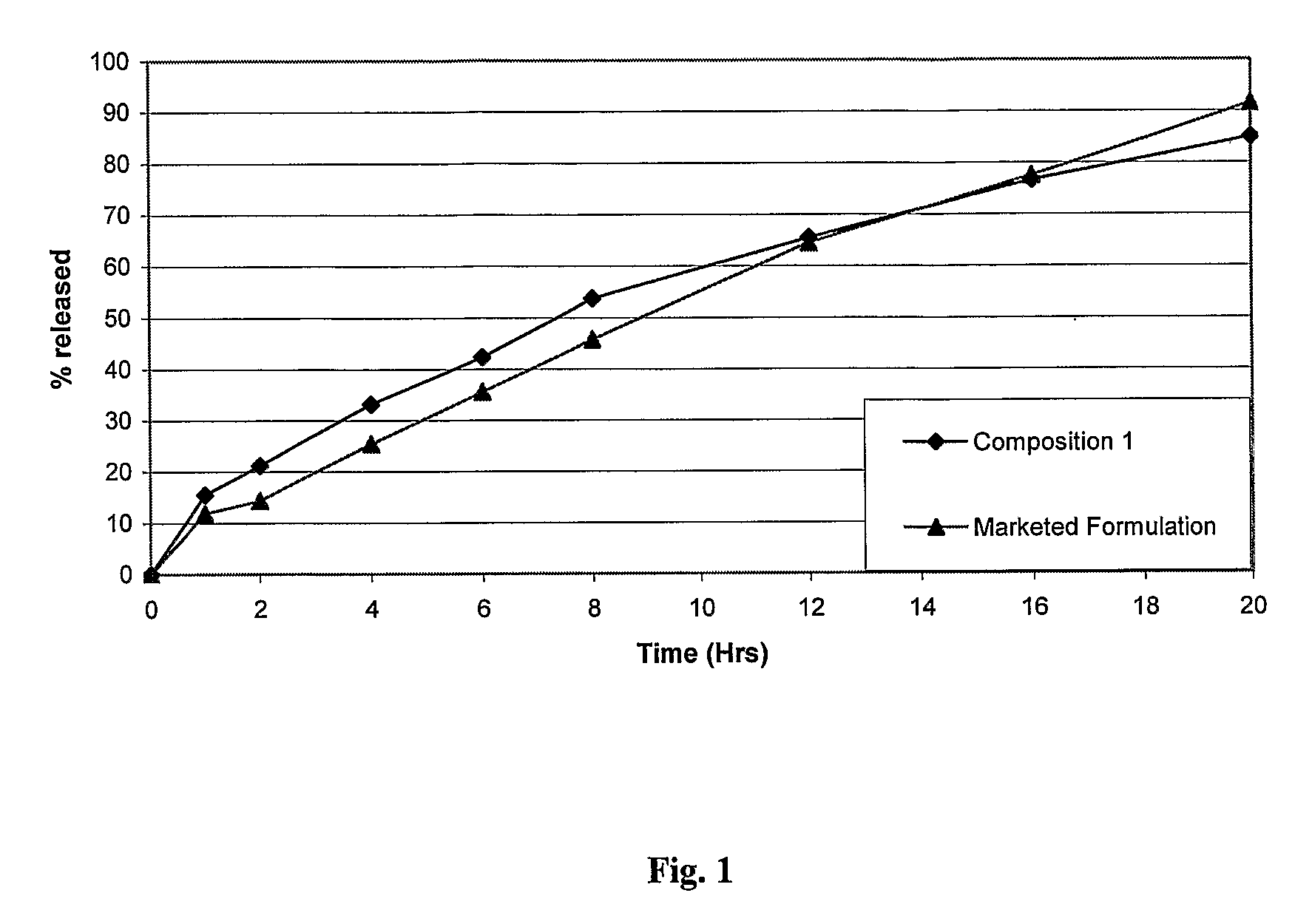
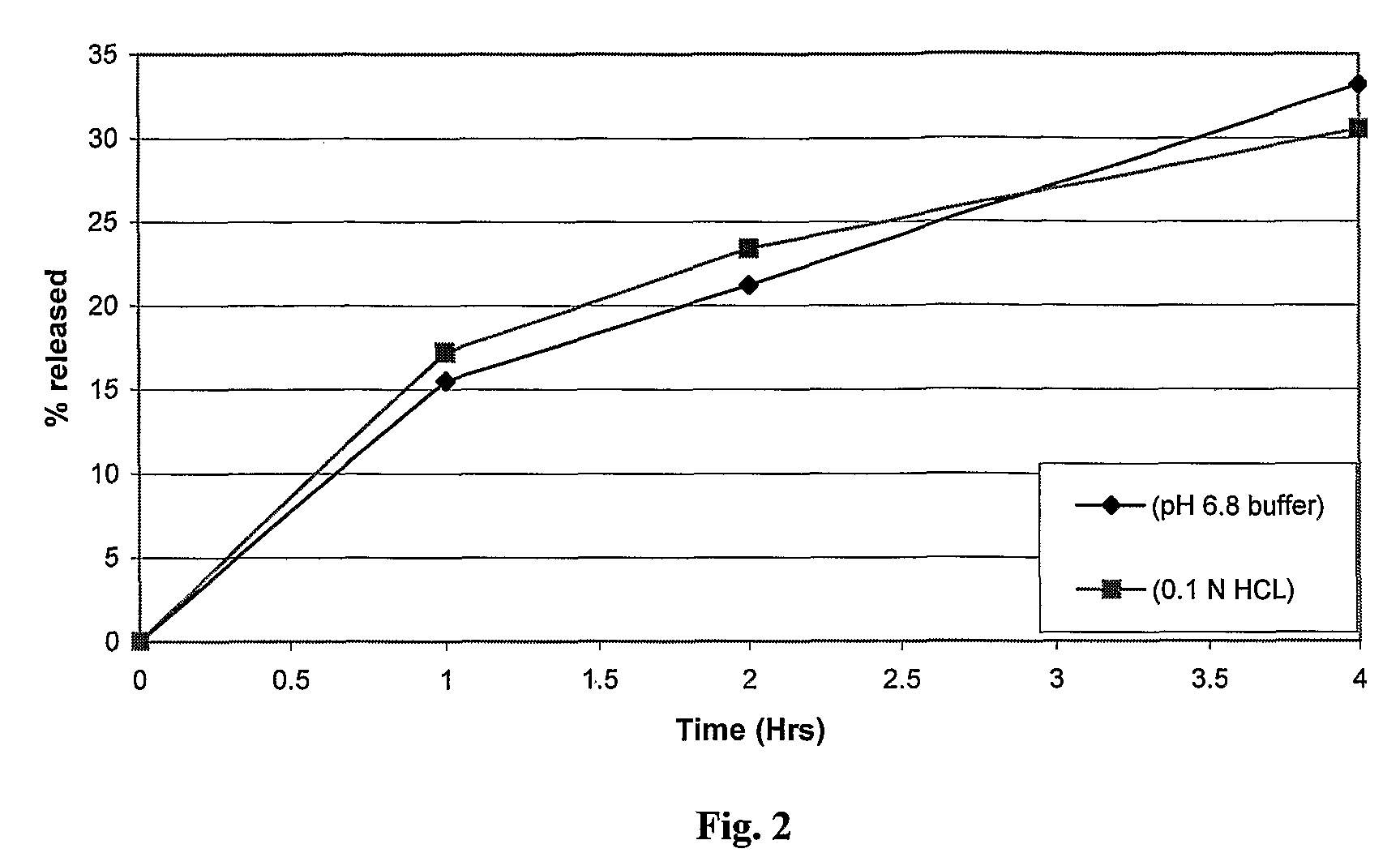
The invention is directed to a pharmaceutical controlled release system for administration, particularly oral administration, of active substances with pH-dependent solubilities, comprising a) a core material containing or consisting of one or more pharmaceutically acceptable pH modifiers; b) optionally an insulating layer, c) a first layer containing or consisting of one or more pharmaceutically acceptable water-insoluble polymers; d) a second layer containing or consisting of at least one active substance having a pH-dependent solubility; e) a third layer containing or consisting of one or more pharmaceutically acceptable polymers having anionic or no ionic groups; and f) optionally a fourth layer, preferably in form of an outer coating layer. It is provided a pH-independent release profile of active substances having pH-dependent solubilities in vitro and vivo.
Owner:BOEHRINGER INGELHEIM INT GMBH
Abuse resistant opioid drug-ion exchange resin complexes having hybrid coatings
InactiveUS20120148672A1Improved resistance characteristicsPowder deliveryNervous disorderMedicineIon-exchange resin
A sustained release formulation for opioid drugs is described. The formulation contains an opioid-ion exchange resin complex having a hybrid coating. The hybrid coating contains a cured polyvinylacetate polymer and a pH-dependent enteric coating layer mixed therein. Also provided are methods of making and using same.
Owner:TRIS PHARMA
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038346A1Reduce solubilitySufficiently slow releasePowder deliveryOrganic active ingredientsOral medicationExtended release tablets
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting of a) flibanserin or a pharmaceutically acceptable derivative thereof as active substance; b) one or more pharmaceutically acceptable pH-dependent polymers; c) one or more pharmaceutically acceptable pH-independent polymers; d) one or more pharmaceutically acceptable acids; and e) optionally one or more additives. The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
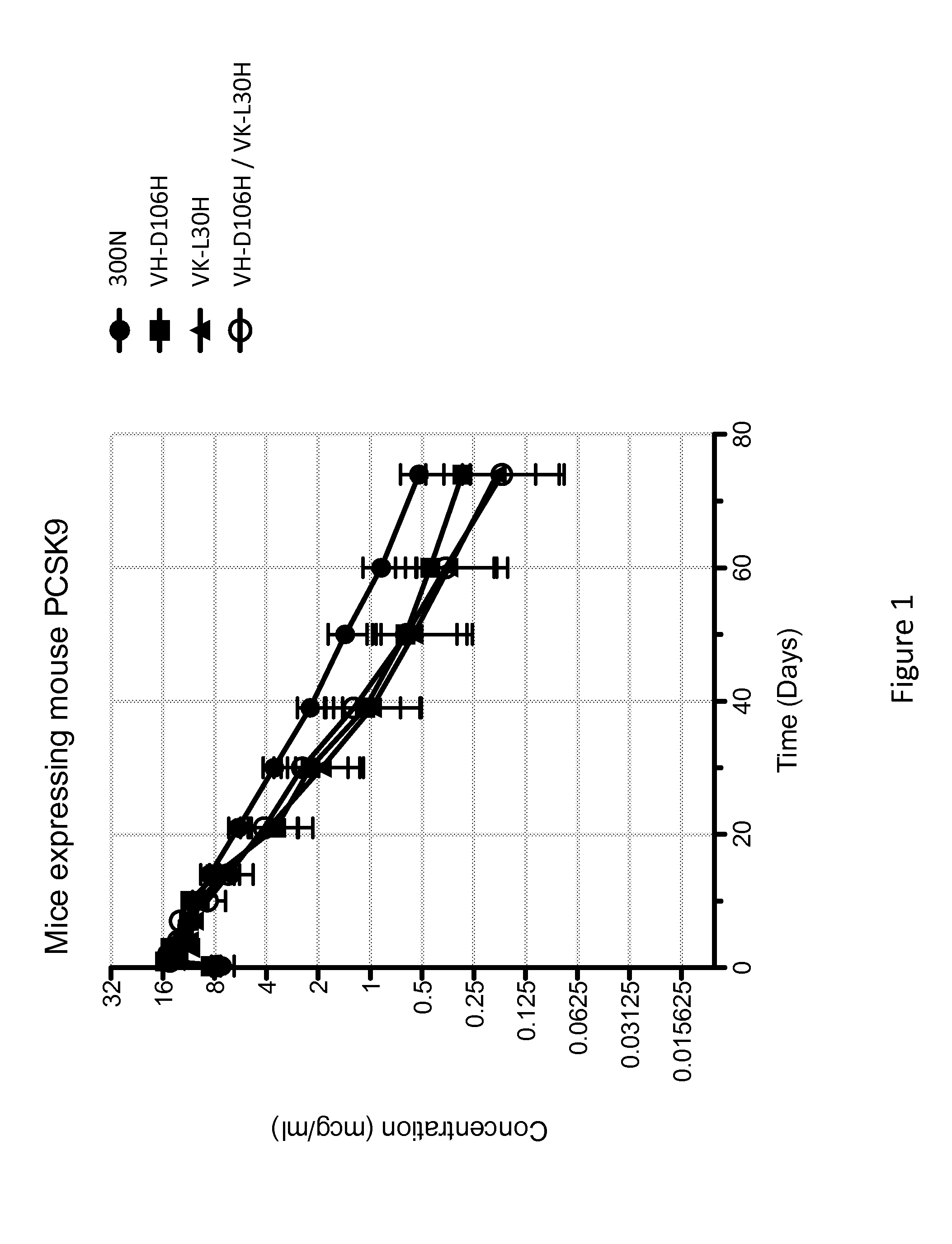
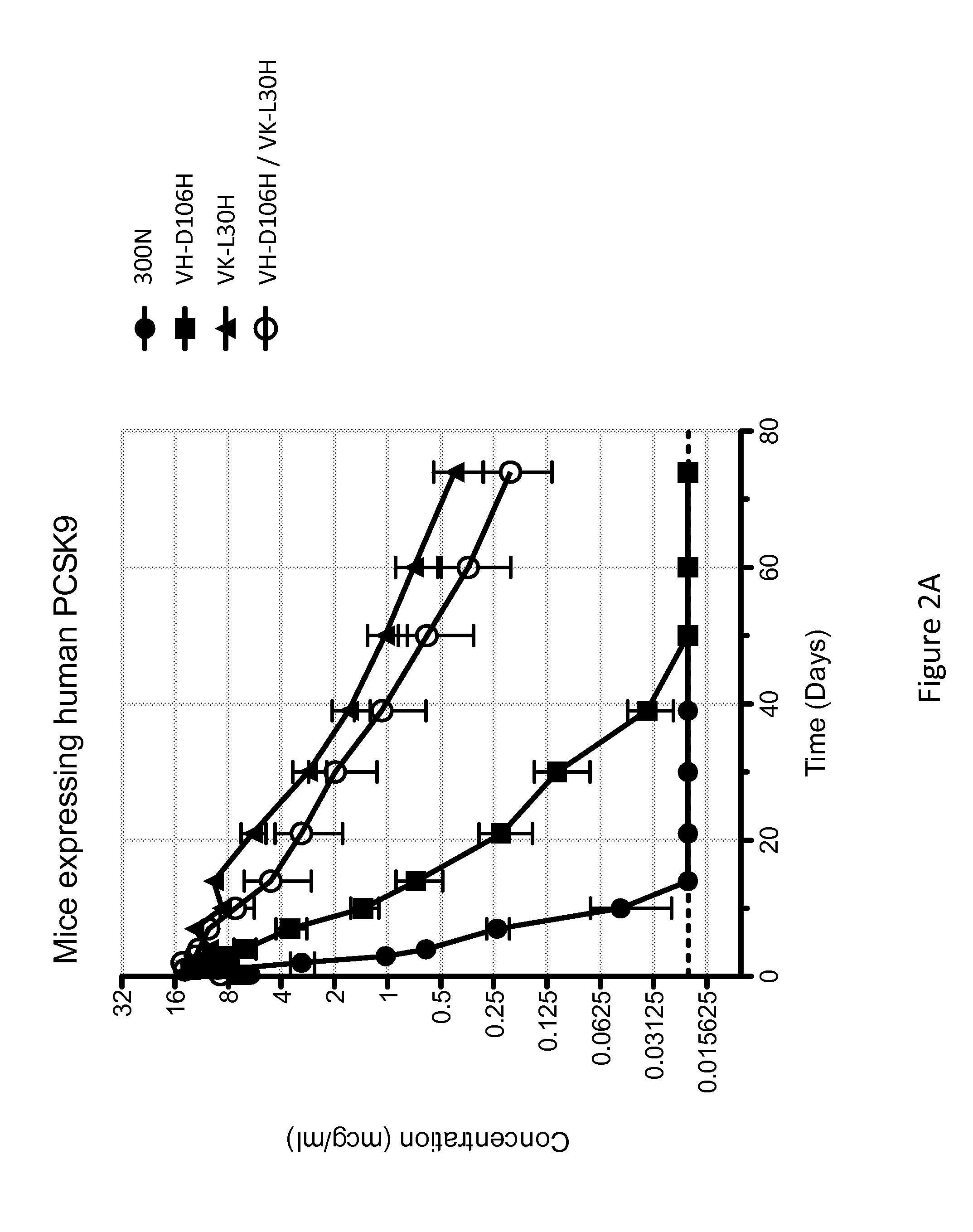
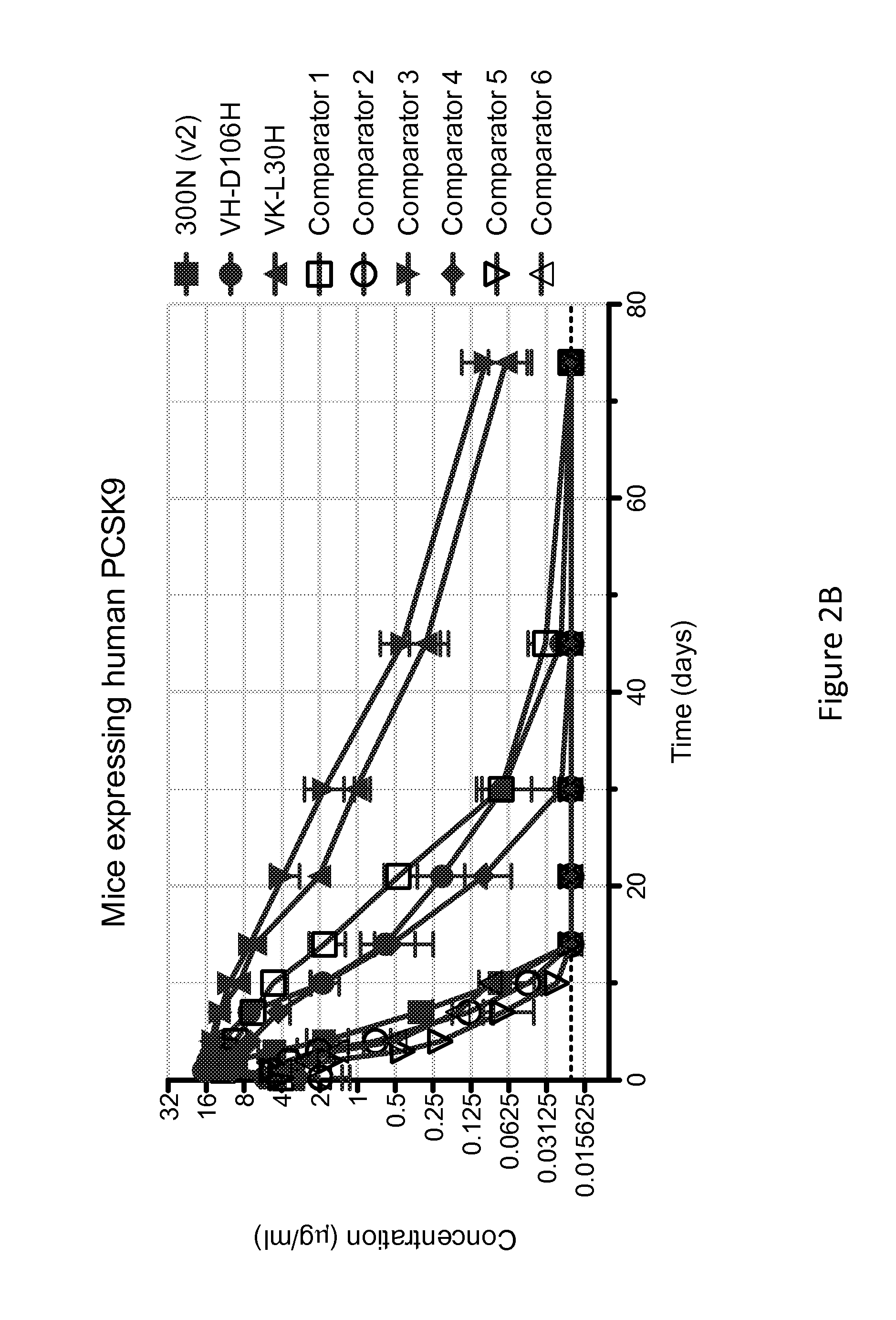
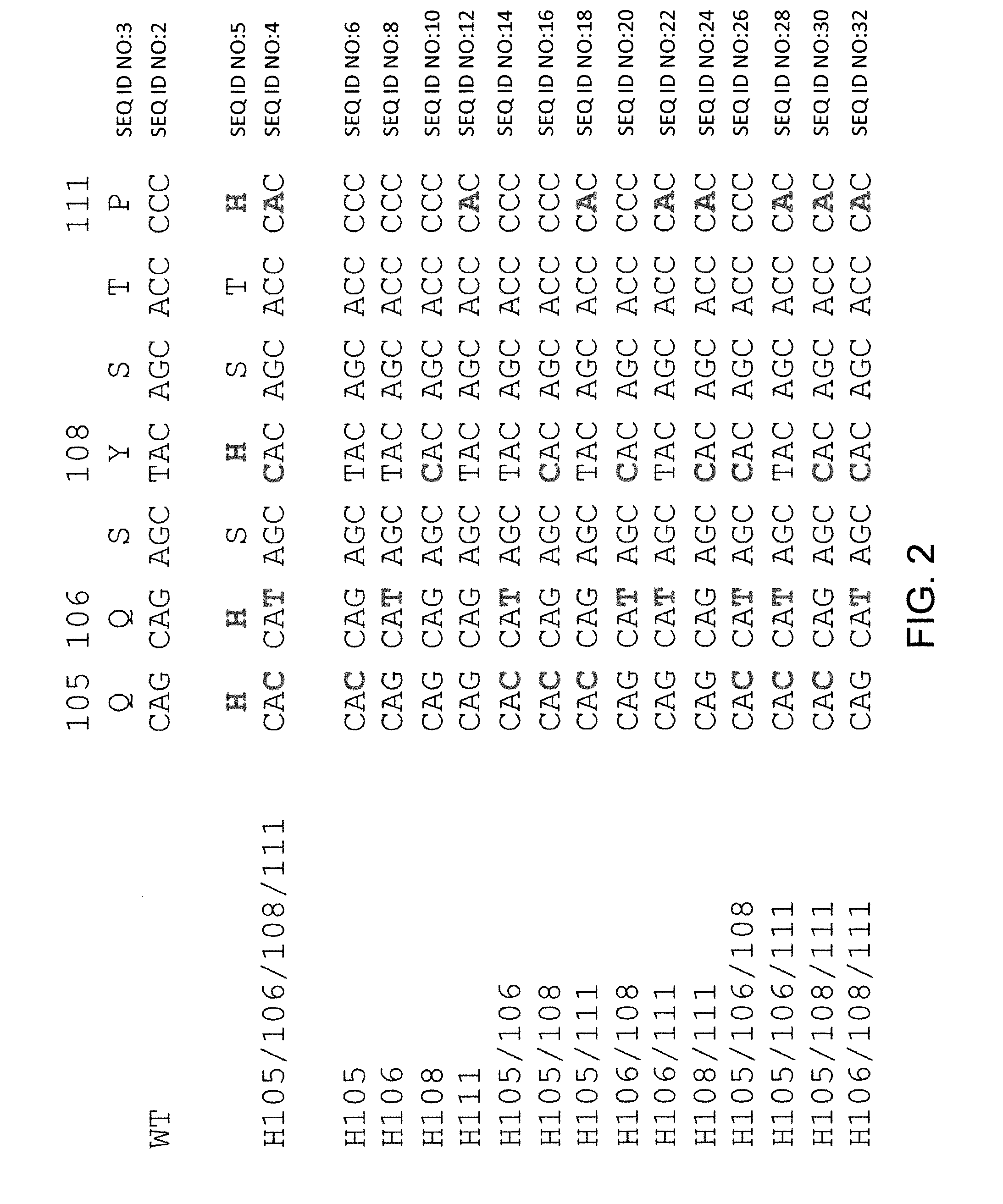
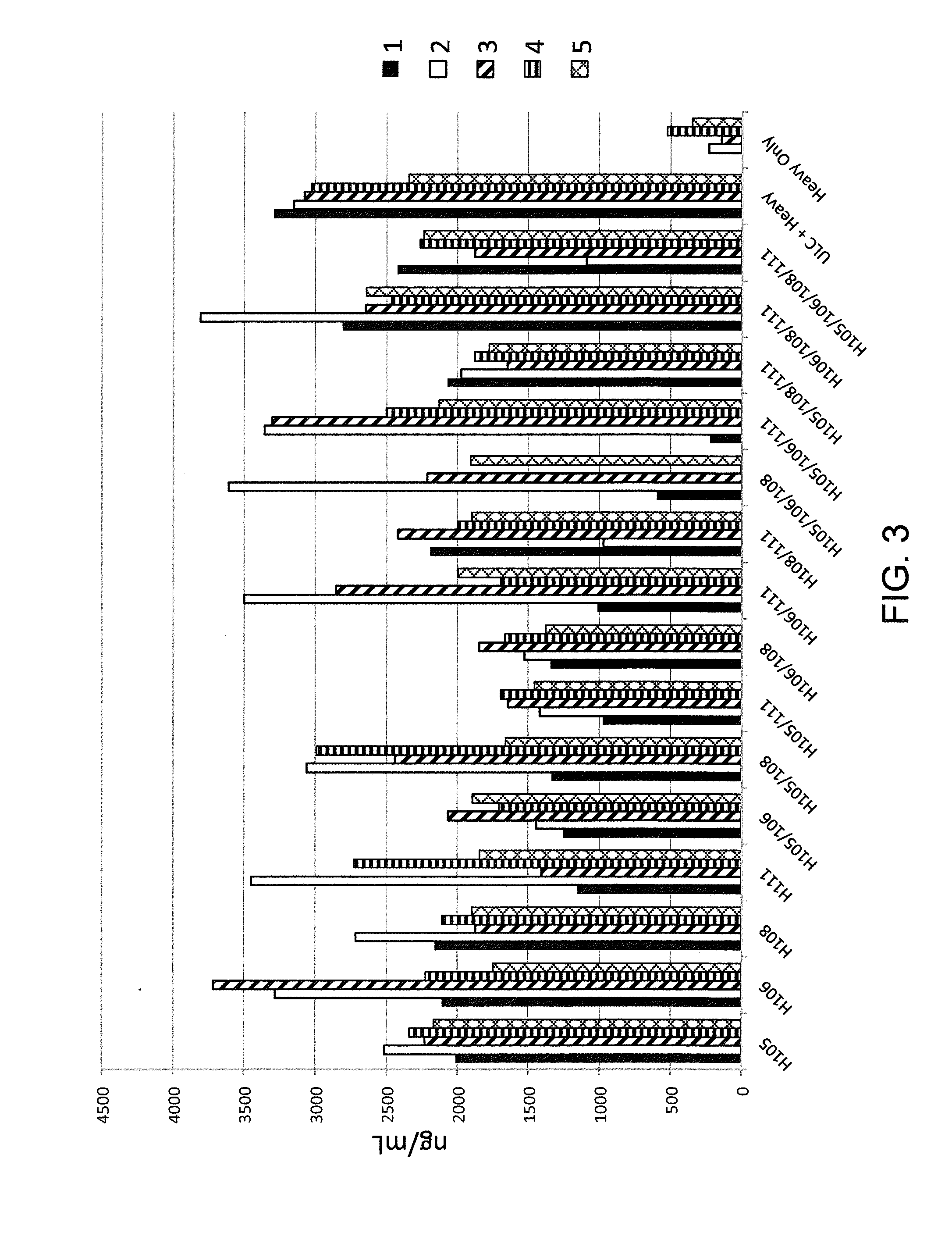
ANTI-PCSK9 ANTIBODIES WITH pH-DEPENDENT BINDING CHARACTERISTICS
ActiveUS20140044730A1High affinityReduced binding affinityMetabolism disorderAntibody ingredientsDiseaseKexin
The present invention provides antibodies and antigen-binding fragments thereof that specifically bind proprotein convertase subtilisin / kexin-9 (PCSK9) with greater affinity at neutral pH than at acidic pH. The antibodies of the invention may possess one or more amino acid changes as compared to antibodies that do not exhibit pH-dependent binding properties. For example, the present invention includes anti-PCSK9 antibodies which possess one or more histidine substitutions in one or more complementarity determining regions. The antibodies of the invention, with pH-dependent binding properties, remain in circulation and exhibit cholesterol lowering activity for prolonged periods of time in animal subjects as compared to anti-PCSK9 antibodies that do not exhibit pH-dependent binding properties. The antibodies of the invention are therefore useful for treating diseases and disorders related to elevated HDL cholesterol, wherein the antibodies of the invention can be administered to a patient at a lower dose and / or with less frequent dosing as compared to antibodies that do not exhibit pH-dependent binding properties.
Owner:REGENERON PHARM INC
Sustained release ranolazine formulations
InactiveUS6852724B2Without fluctuationBiocidePharmaceutical non-active ingredientsRanolazineDissolution
A sustained release ranolazine formulation contains an intimate mixture of ranolazine and a partially neutralized pH-dependent binder to form a film that is mostly insoluble in aqueous media below pH 4.5 and soluble in aqueous media above pH 4.5. The formulation is suitable for twice daily administration of ranolazine and is useful for controlling the rate of dissolution of ranolazine, and to maintain human plasma ranolazine levels at between 850 and 4000 ng base / mL.
Owner:GILEAD SCI INC
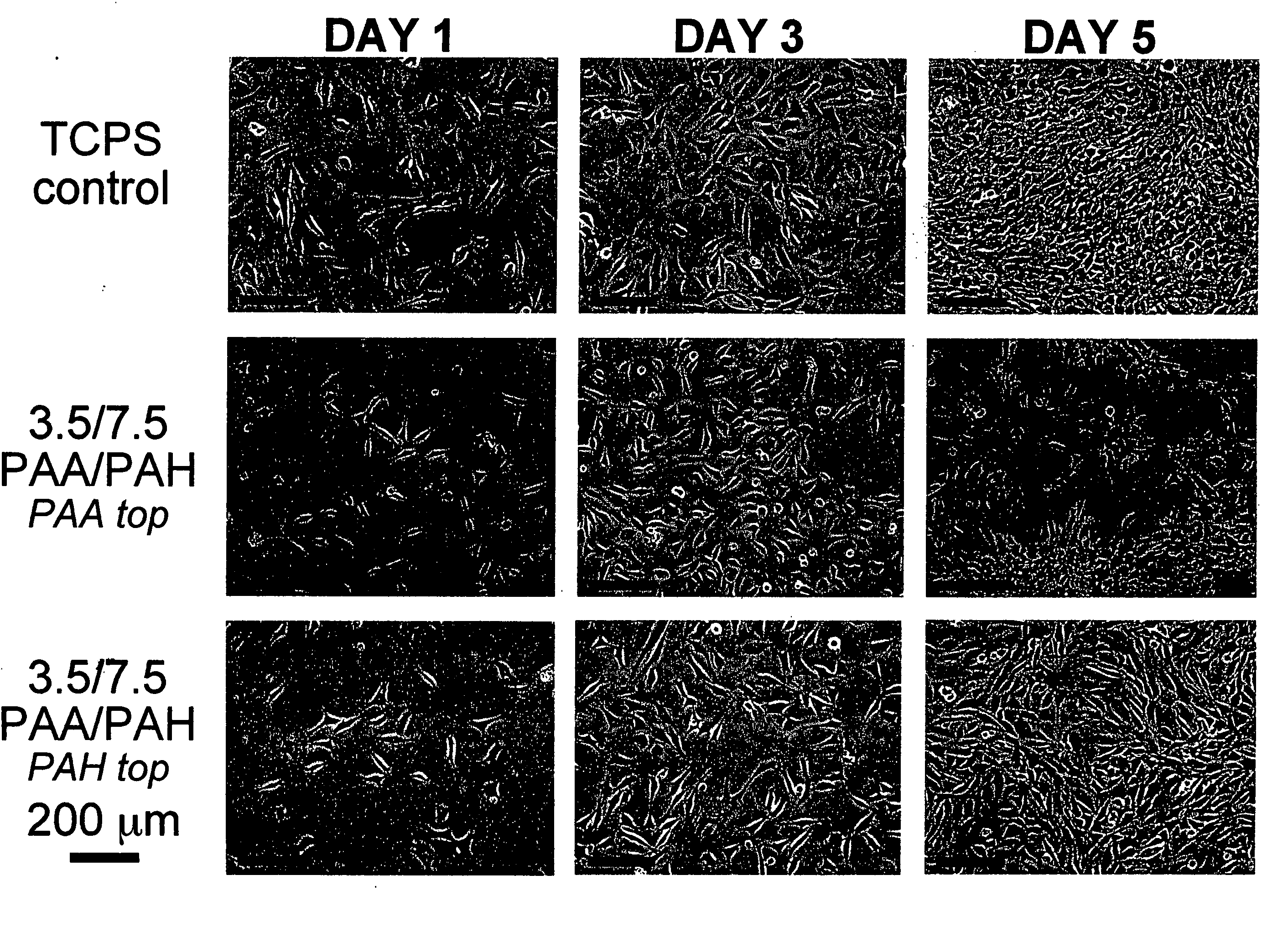
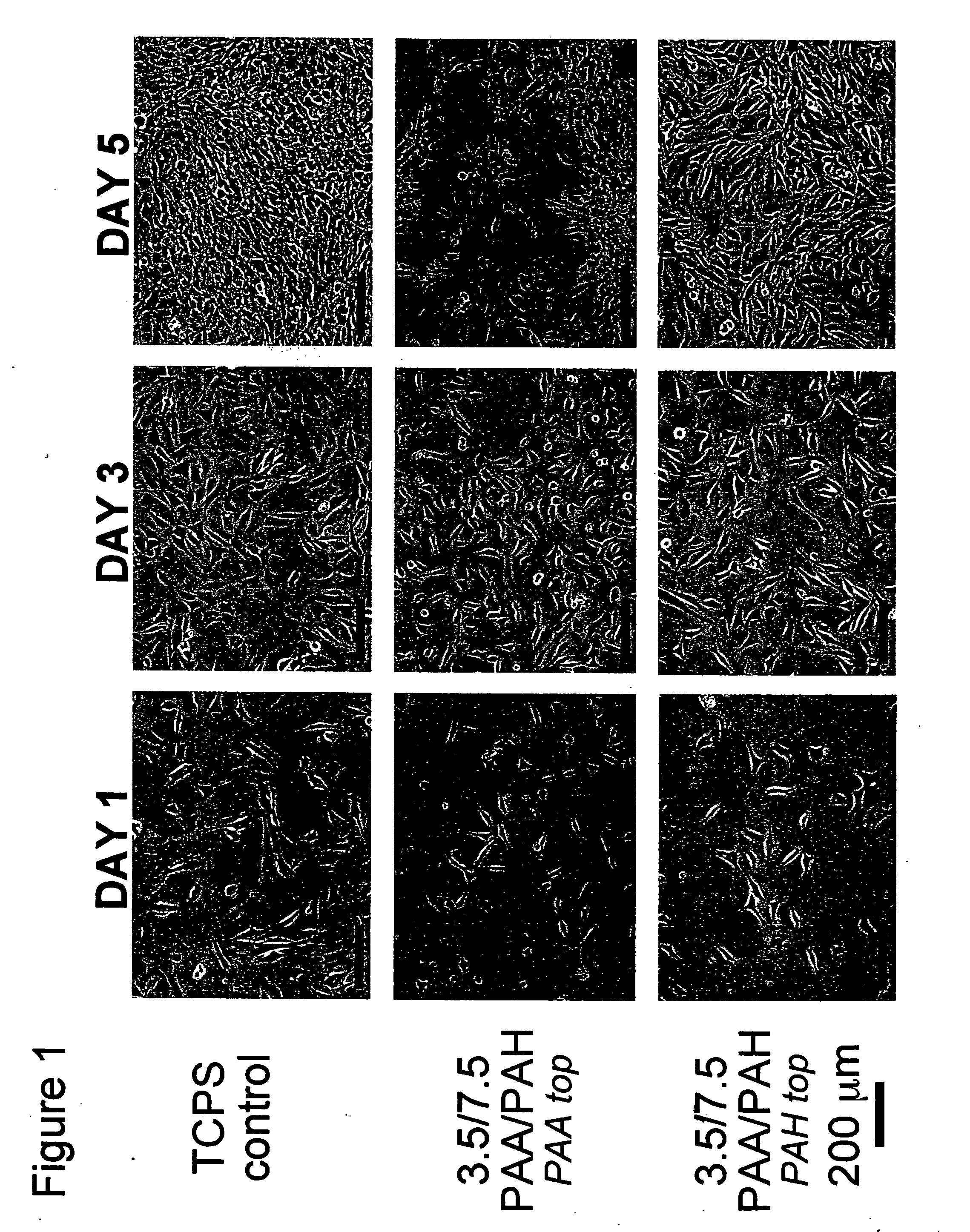
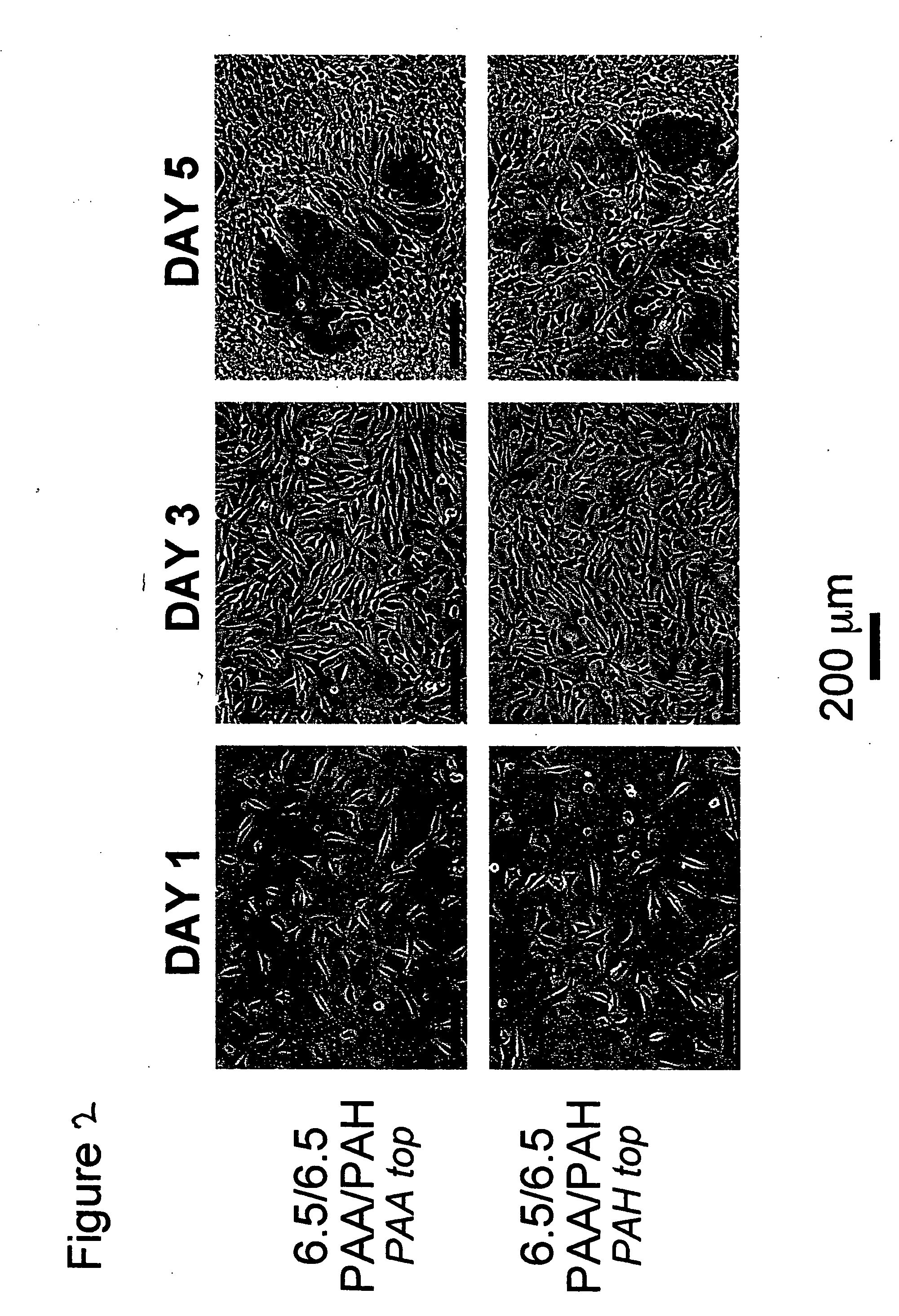
Polyelectrolyte multilayers that influence cell growth methods of applying them, and articles coated with them
InactiveUS20050191430A1Inhibiting adhesion of cellLiquid surface applicatorsCoatingsCoated surfaceCell adhesion
One aspect of the present invention relates to a method of coating a surface, comprising sequentially depositing on a surface, under pH-controlled conditions, alternating layers of polymers to provide a coated surface, wherein a first polymer is selected from the group consisting of pH dependent cationic polyelectrolytes and neutral polymers, and a second polymer is selected from the group consisting of anionic polyelectrolytes, thereby permitting or preventing cell adhesion to said coated surface. In certain embodiments, the aforementioned method provides a coated surface to which cell adhesion is permitted. In certain embodiments, the aforementioned method provides a coated surface to which cell adhesion is prevented. Another aspect of the present invention relates to a method of rendering a surface cytophilic, comprising the step of coating a surface with a polyelectrolyte multilayer film, which film swells to less than or equal to about 150% of its original thickness when exposed to an aqueous medium. Another aspect of the present invention relates to a method of rendering a surface cytophobic, comprising the step of coating a surface with a polyelectrolyte multilayer film, which film swells to greater than or equal to about 200% of its original thickness when exposed to an aqueous medium.
Owner:RUBNER MICHAEL F +2
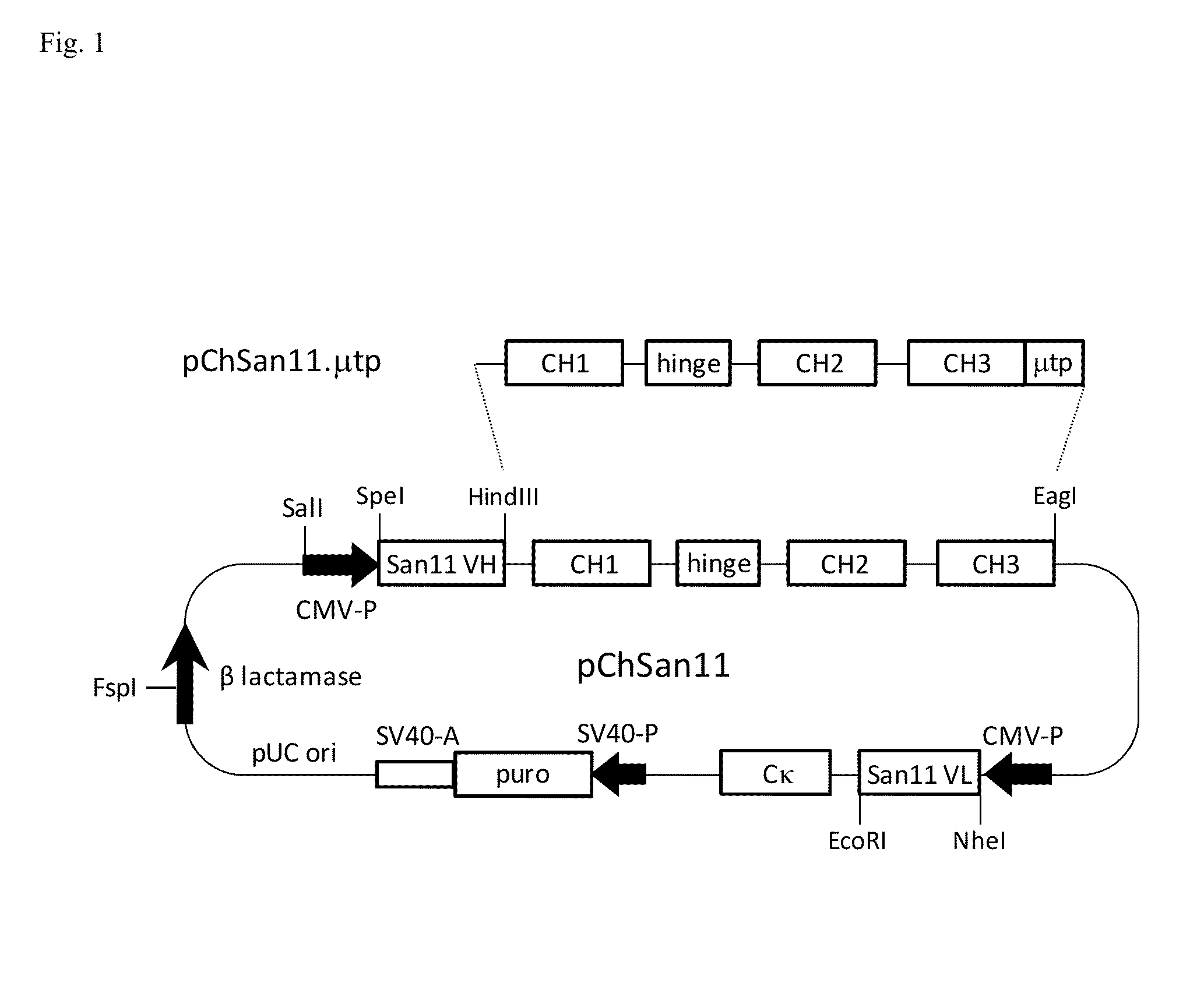
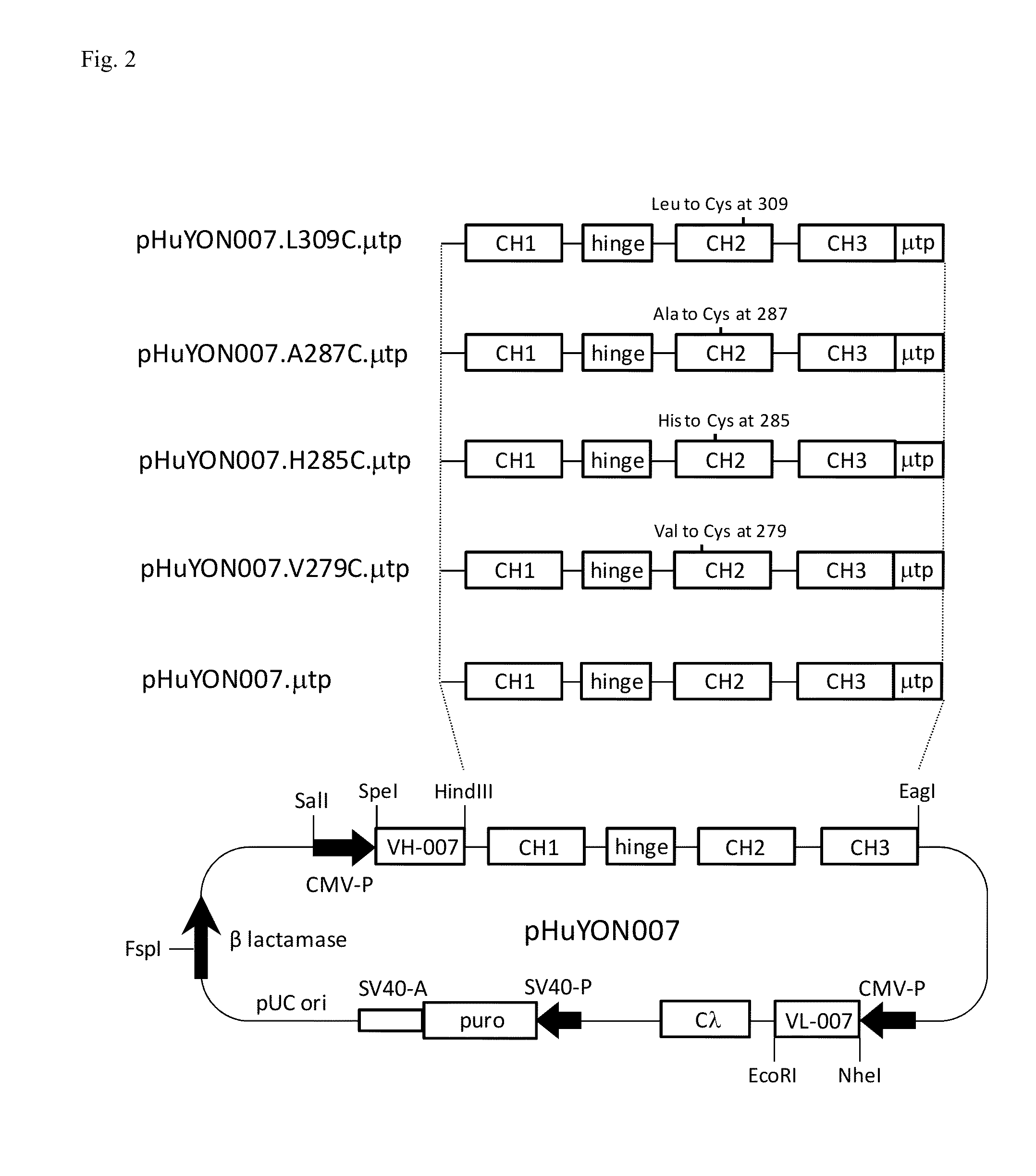
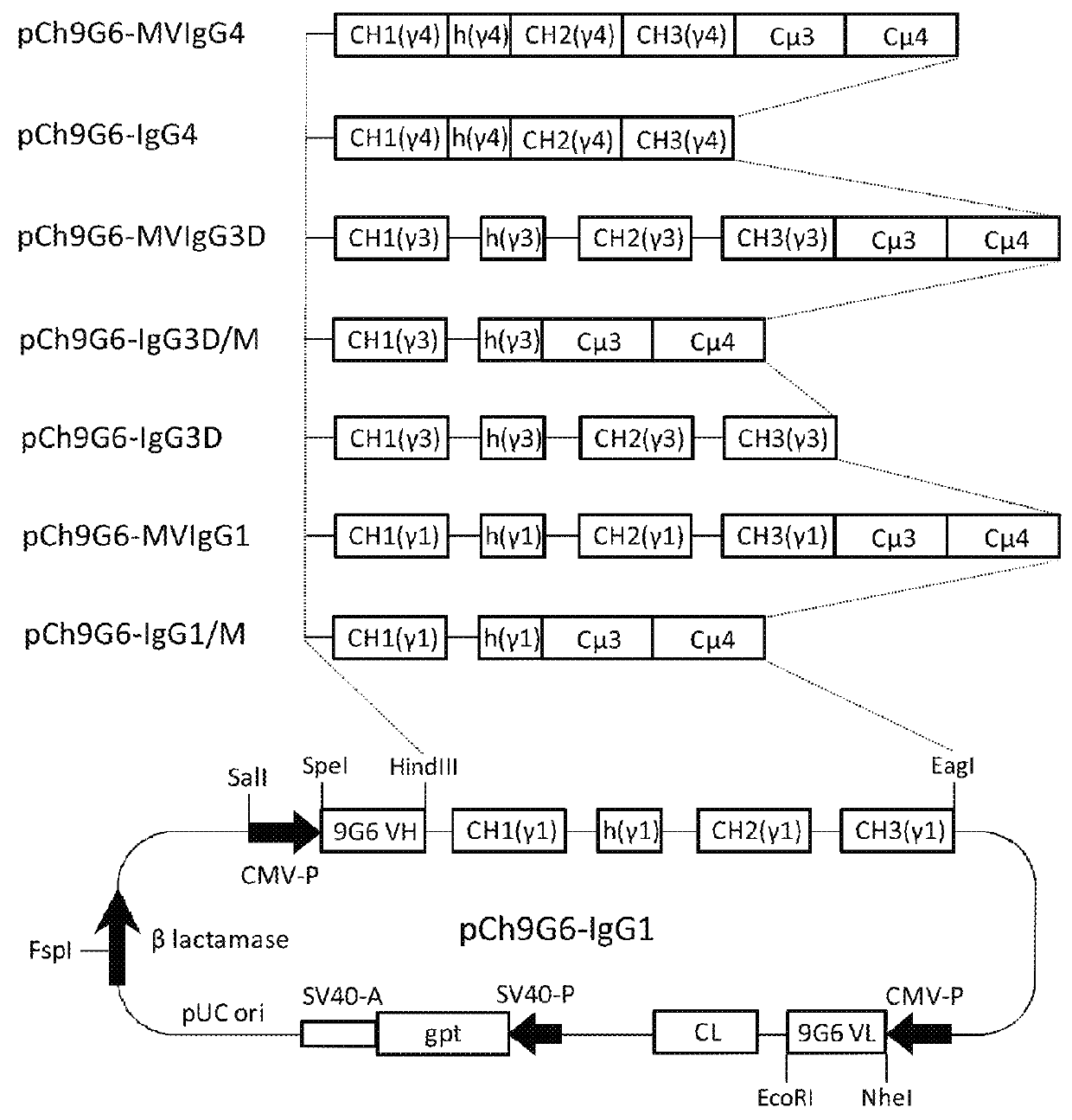
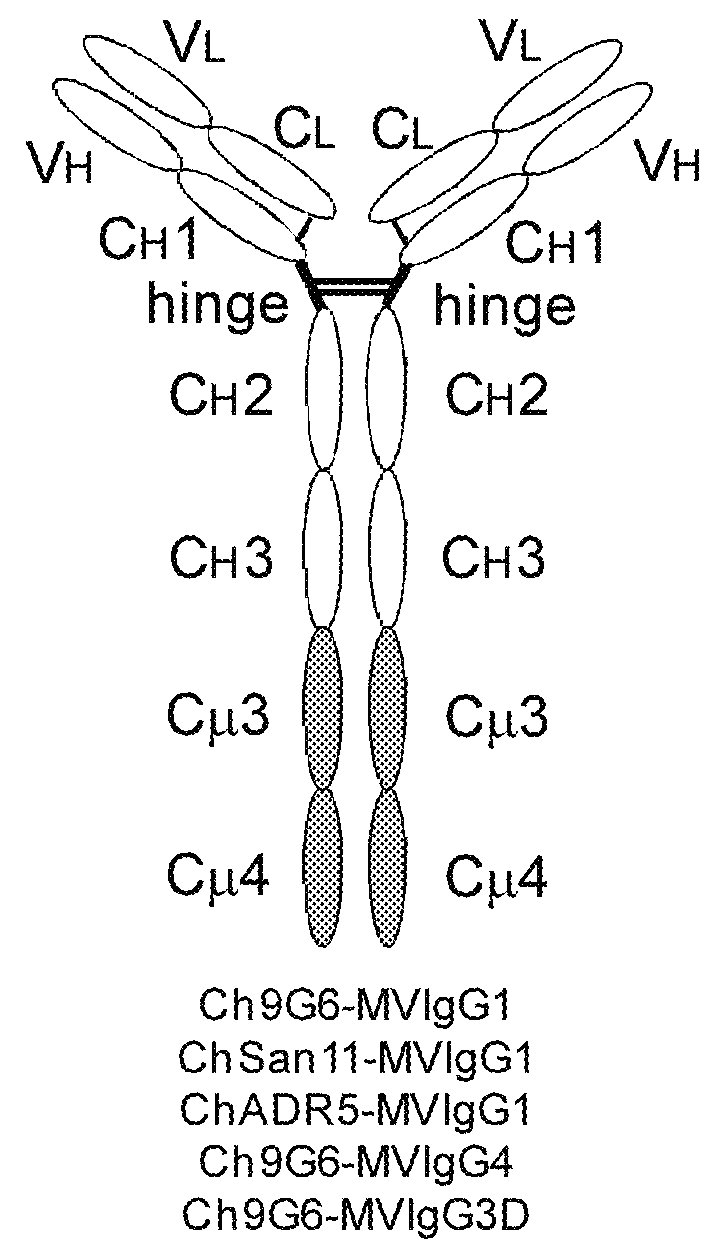
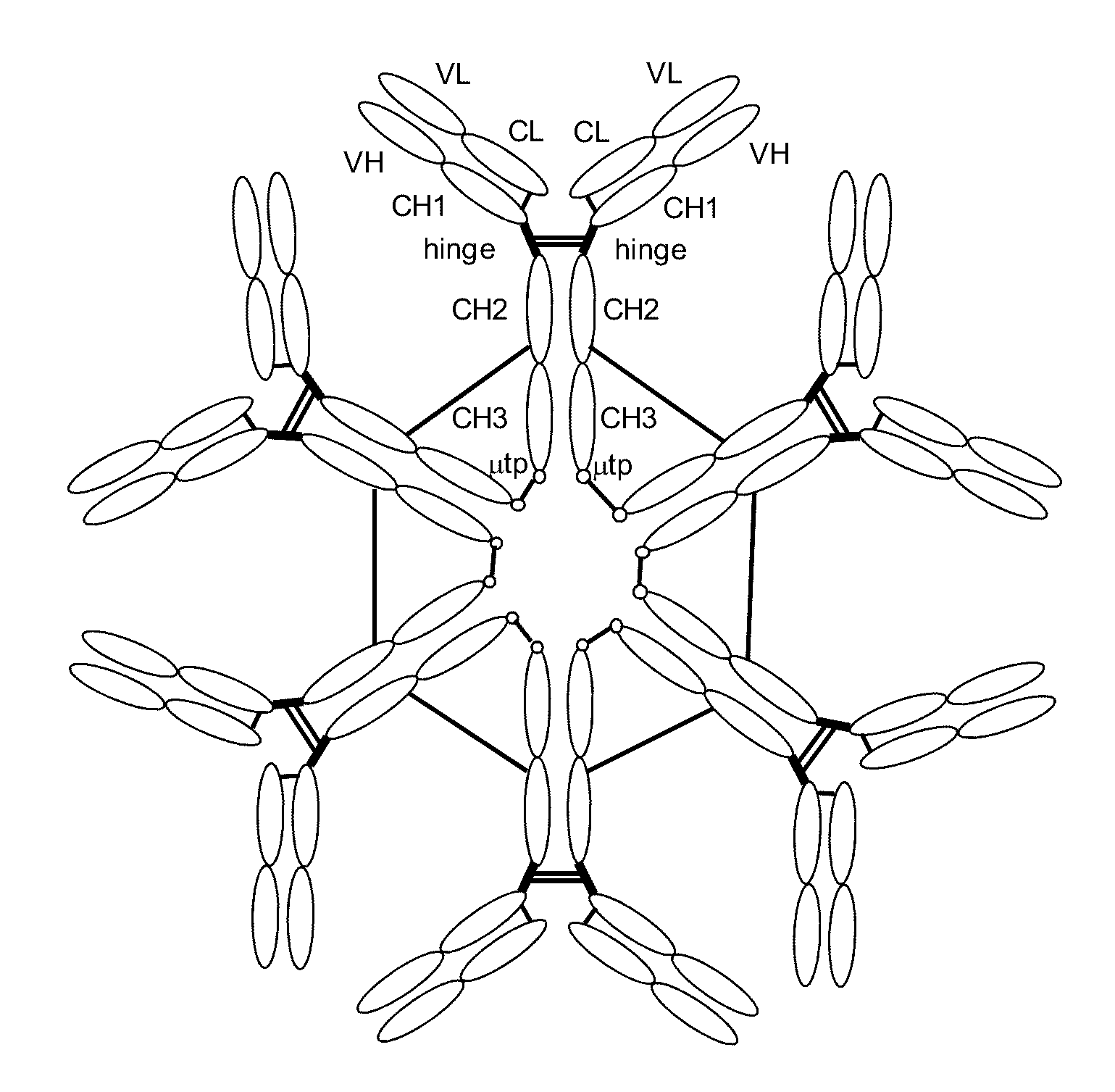
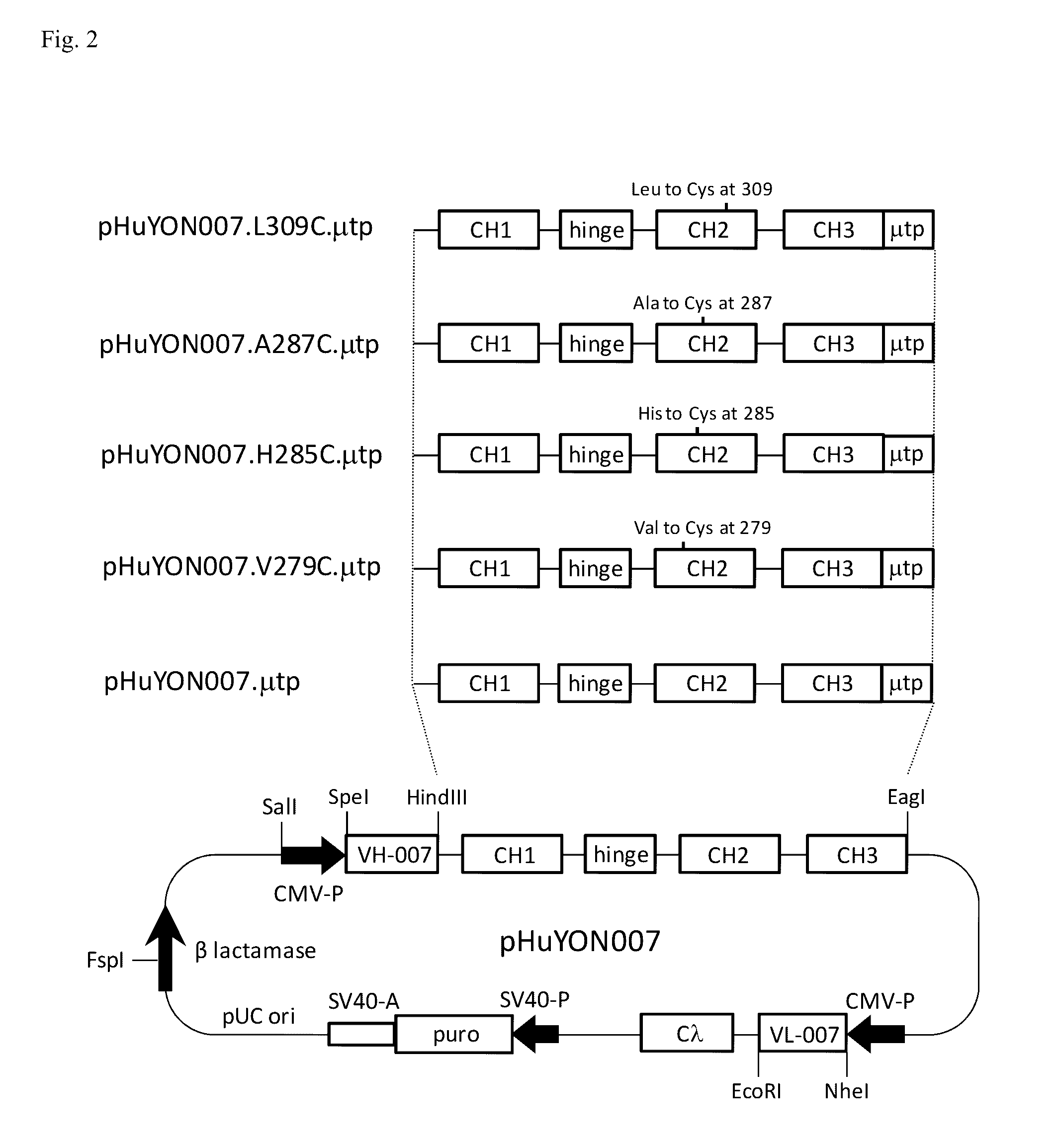
Antibodies or fusion proteins multimerized via cysteine mutation and a mu tailpiece
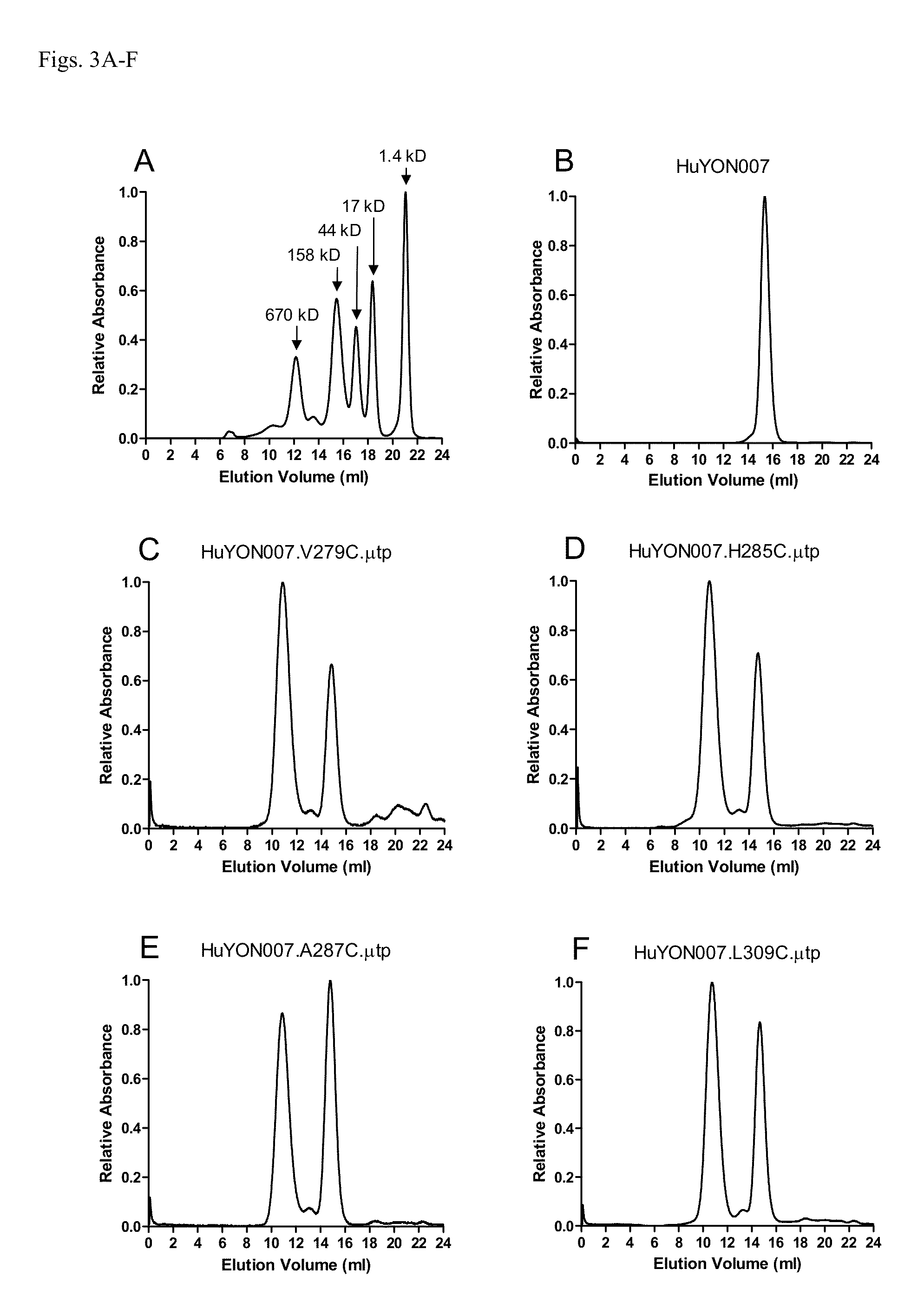
ActiveUS20140037621A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenIgG.heavy chain
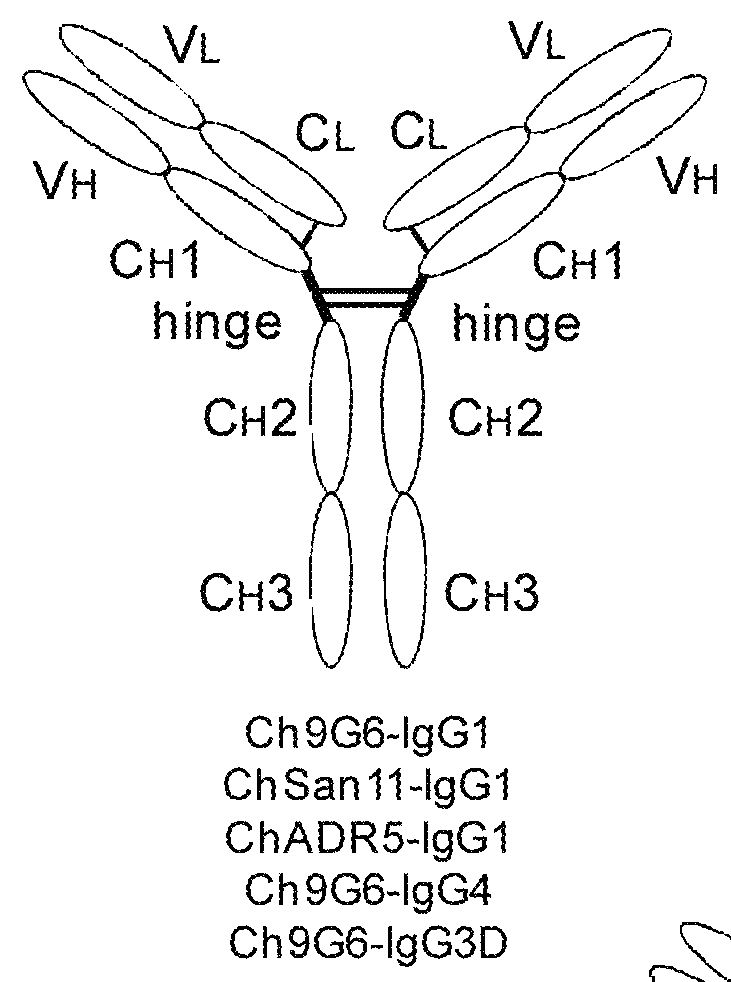
The invention provides constant regions incorporating a cysteine mutation and linked to a μ tailpiece and antibodies or fusion proteins incorporating the same. The constant regions include at least CH2 and CH3 regions of an IgG heavy chain constant region including a cysteine mutation and μ tailpiece. Antibodies or fusion proteins incorporating the constant regions gains the ability to form multivalent complexes, e.g., pentameric or hexameric structures. Antibodies or fusion proteins incorporating the constant regions also retain IgG properties including specific binding to protein G, which facilitates purification and may exhibit pH-dependent FcRn binding, which is associated with a relatively long in vivo half-life. Depending on the isotype and subtype, the nature of the antigen and presence of an additional IgG hinge domain, such antibodies or fusion proteins may also have properties of specific binding to protein A, and effector functions such as ADCC, CDC and opsonization.
Owner:JN BIOSCI
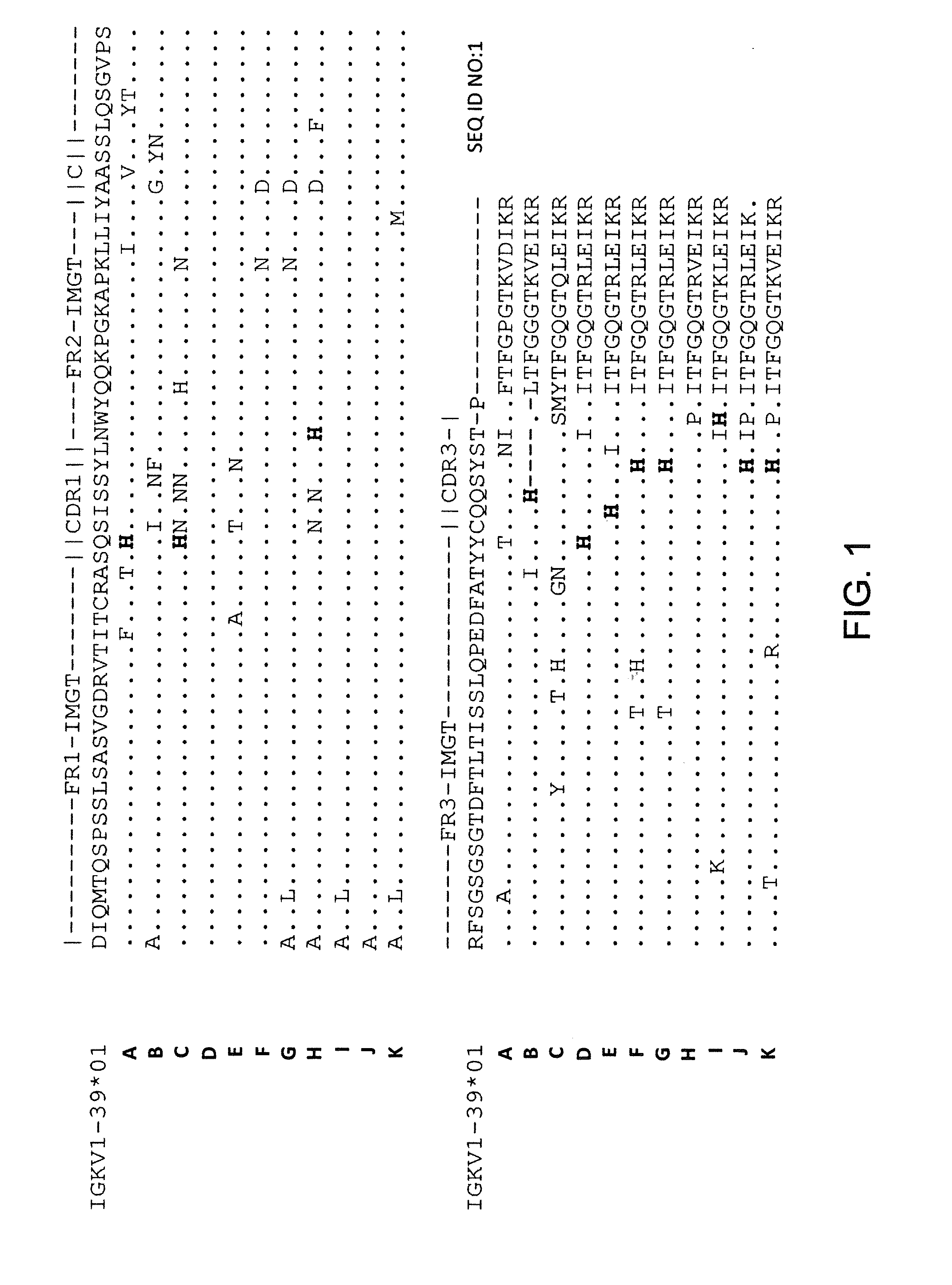
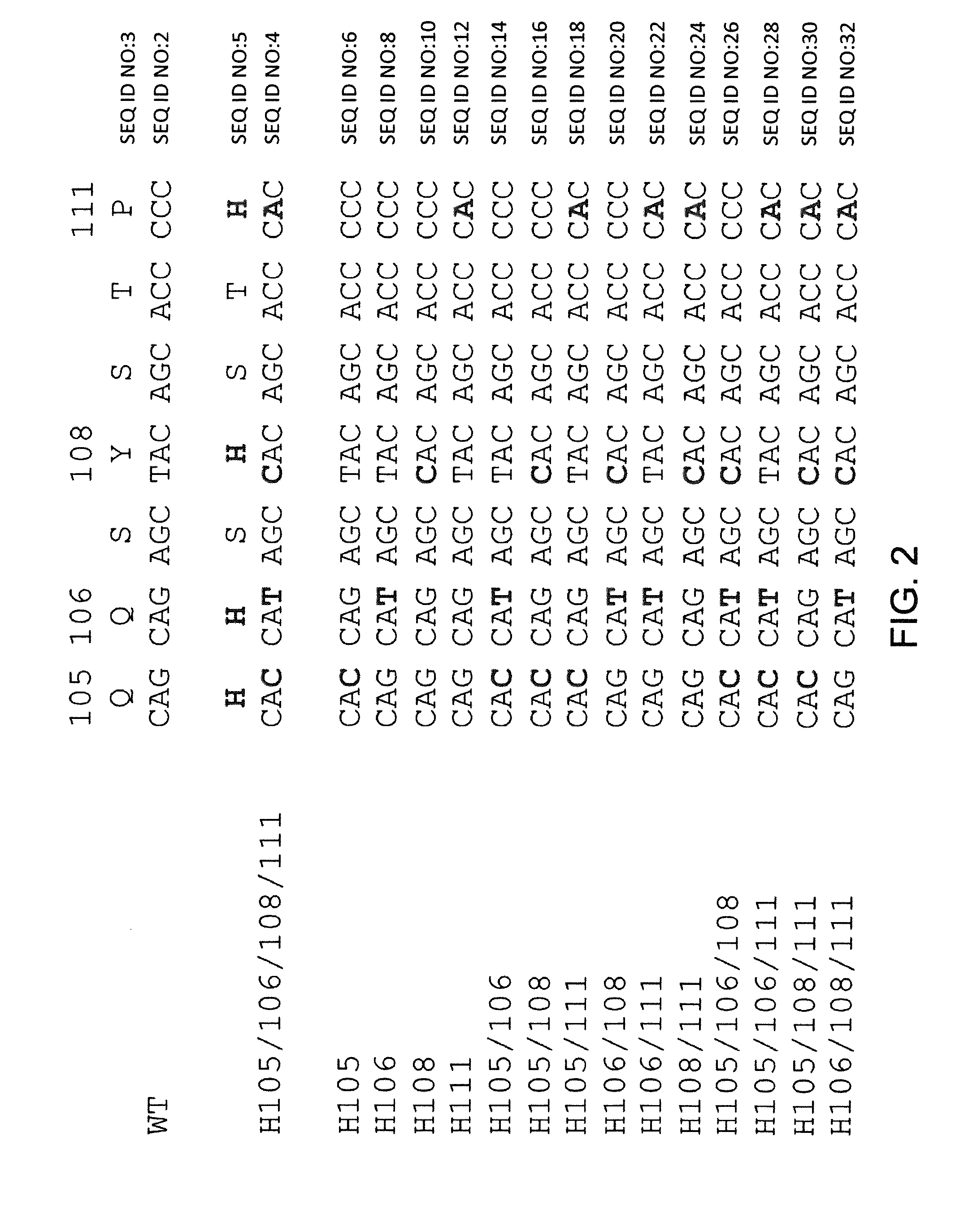
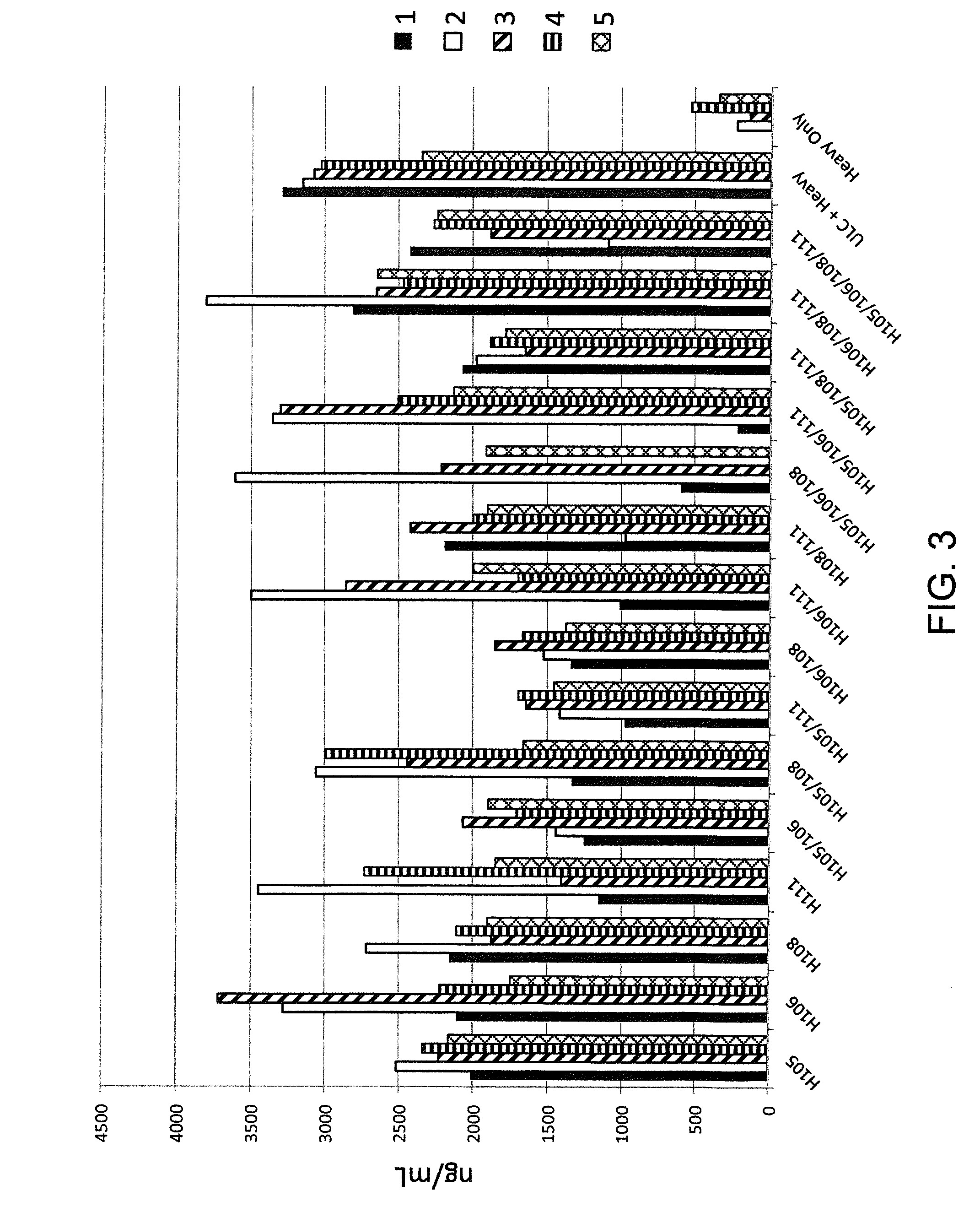
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
InactiveUS20140013456A1Reduce the binding forceNucleic acid vectorImmunoglobulinsNucleotideGenetically modified crops
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses human immunoglobulin light chain variable domains derived from a limited repertoire of human immunoglobulin light chain variable gene segments that comprise histidine modifications in their germline sequence. Methods of making non-human animals that express antibodies comprising histidine residues encoded by histidine codons introduced into immunoglobulin light chain nucleotide sequences are provided.
Owner:REGENERON PHARM INC
Method for stimulating angiogenesis and wound healing
InactiveUS20070141101A1Effective combinationPromote angiogenesisPowder deliveryPeptide/protein ingredientsWound healingCell-Extracellular Matrix
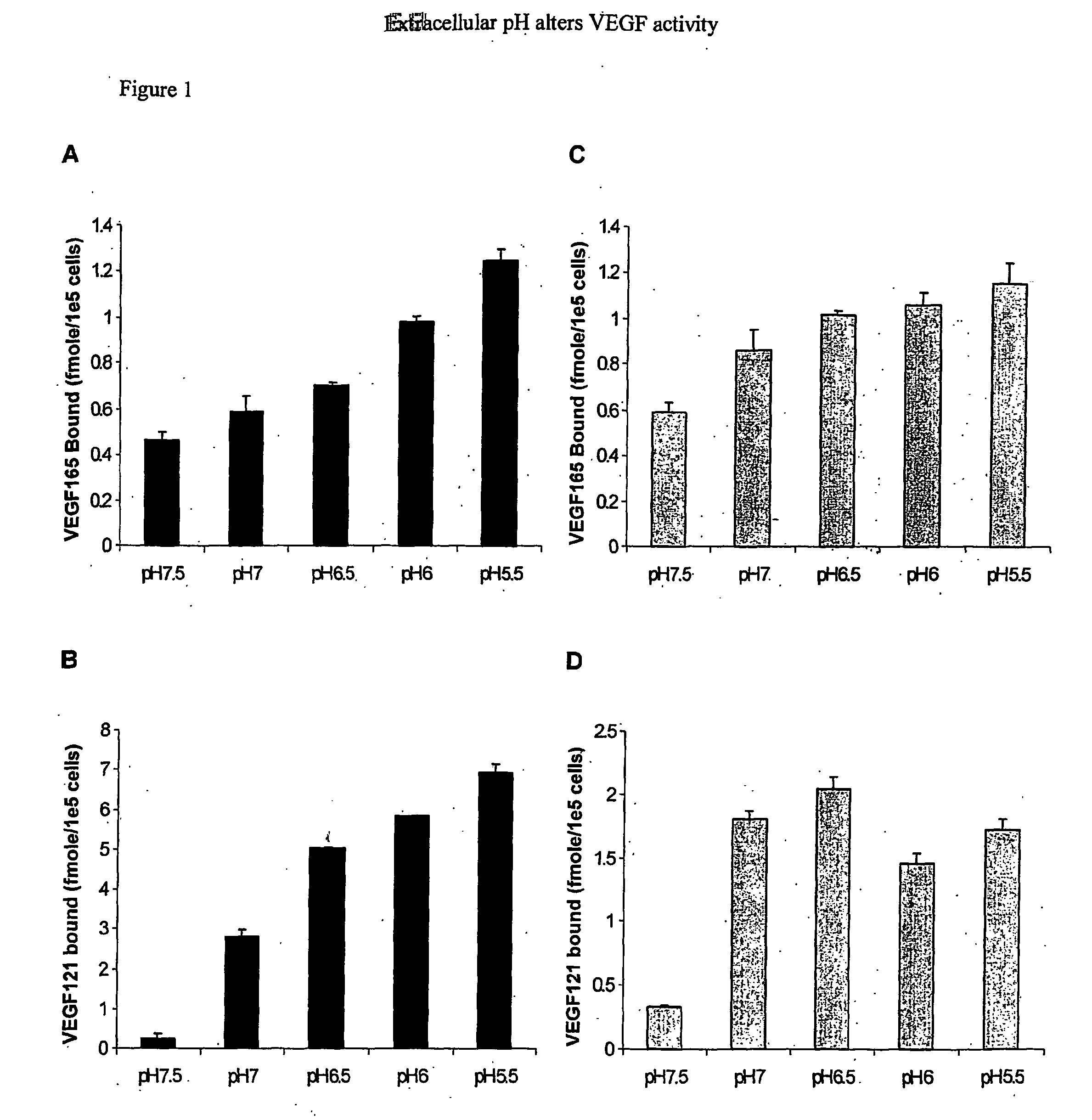
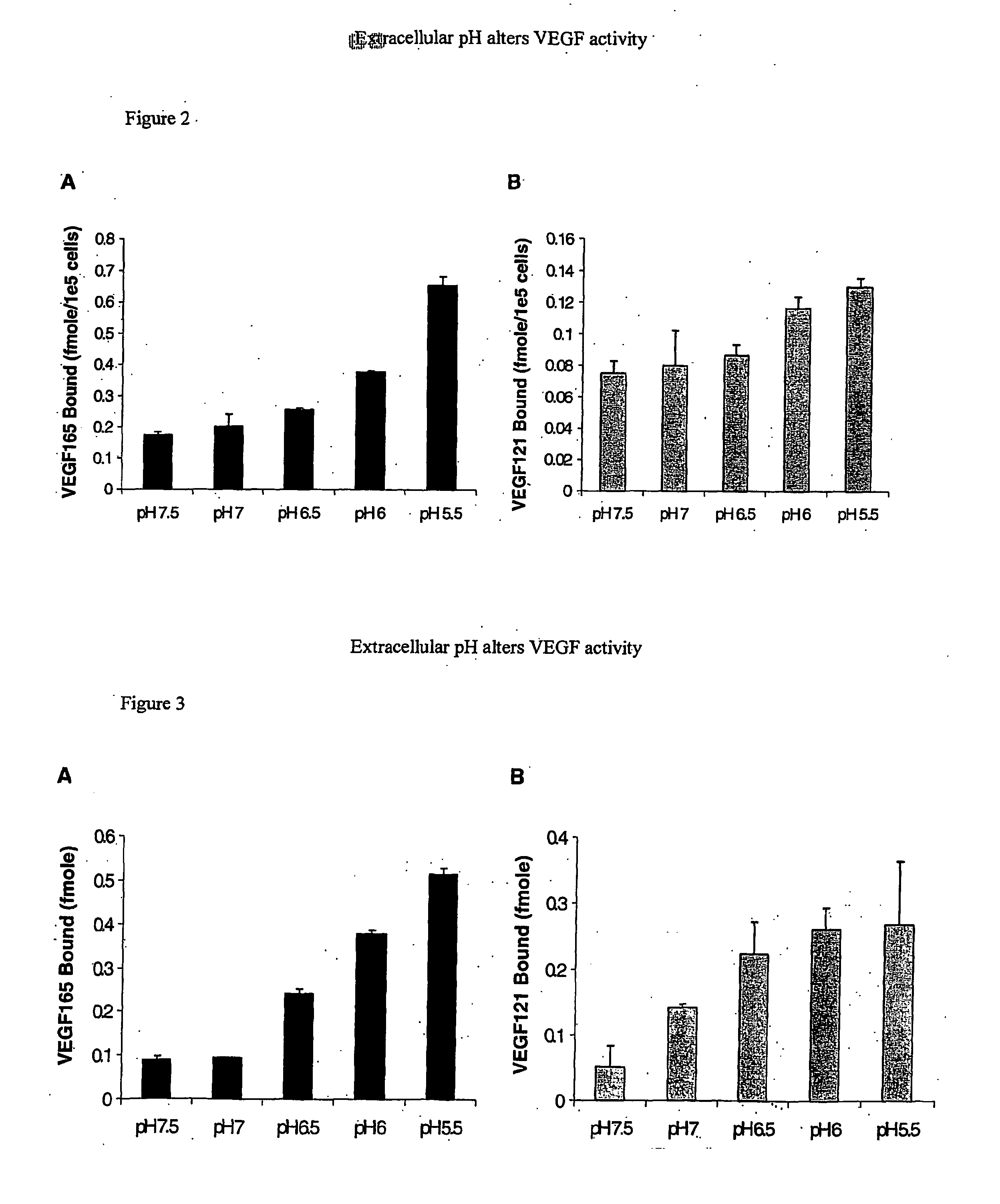
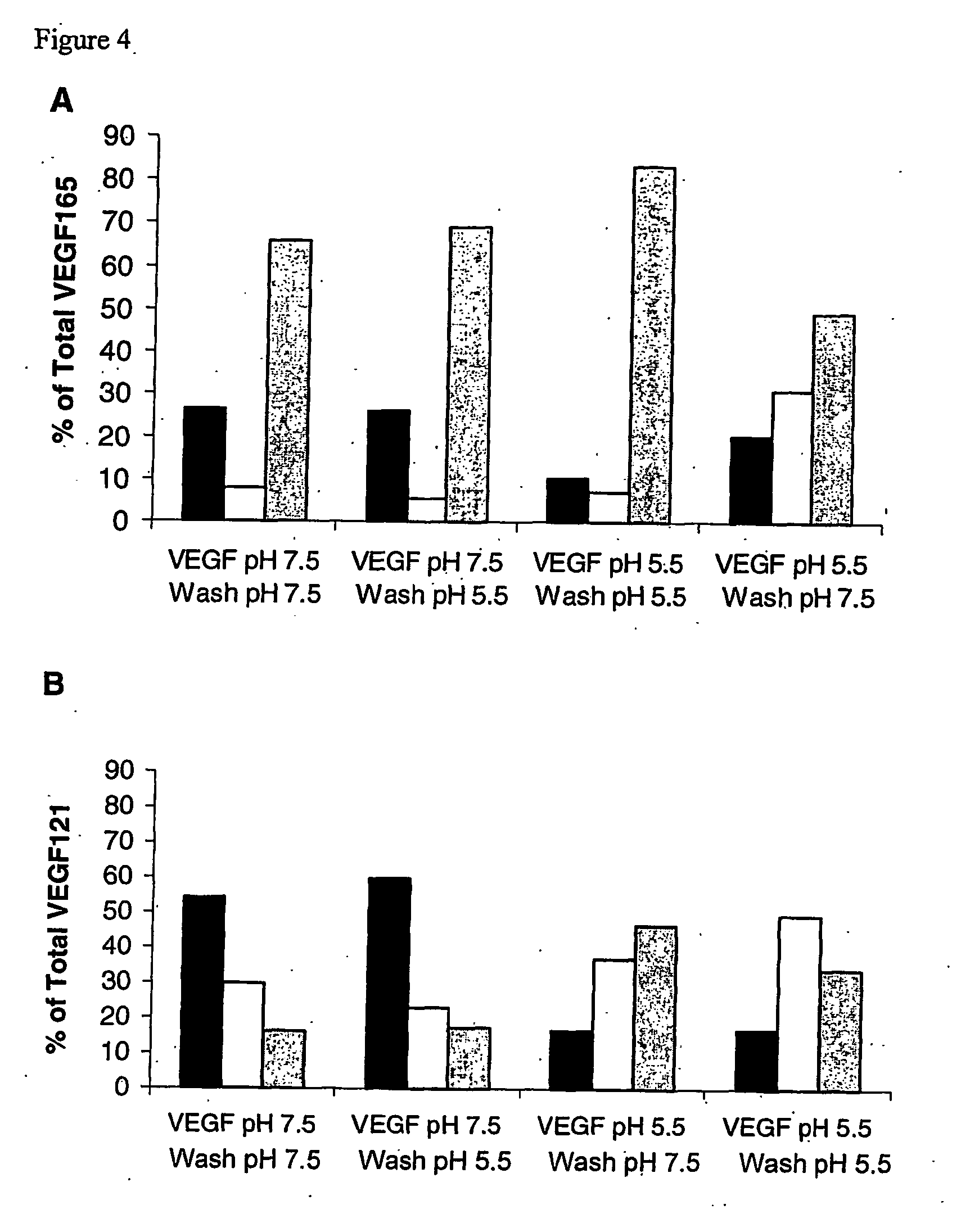
A device comprising an extracellular matrix having an internal pH between 4.0 and 6.0 is discussed. This matrix contains heparin or a heparin-related compound such as heparan sulfate. Preferably the matrix also contains fibronectin or a fragment thereof. The matrix will bind to a protein having a pH dependent binding to heparin such as VEGF, preferably VEGF 121 or VEGF 165. The device will release the protein as the pH increases to physiological pH, such as 7.0 to 7.5. The device can be used to deliver a drug to a specific site. For example, with VEGF to a site in need of angiogenesis.
Owner:TRUSTEES OF BOSTON UNIV
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
ActiveUS20130247234A1Reduce the binding forceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman animalVariable domain
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses a single light chain variable domain derived from a single rearranged light chain variable region gene in the germline of the non-human animal, wherein the single rearranged light chain variable region gene comprises a substitution of at least one non-histidine encoding codon with a histidine encoding codon. Methods of making non-human animals that express antibodies comprising a histidine-containing universal light chain are provided.
Owner:REGENERON PHARM INC
Delayed release film coatings containing calcium silicate and substrates coated therewith
ActiveUS9233074B2Reduces caking tendencyResistant to agglomerationCoatingsDrageesCalcium silicatePh dependent
The present invention includes pH dependent, dry film coating compositions containing calcium silicate for use on orally-ingestible substrates such as tablets and the like. The film coating compositions can be applied as an aqueous suspension either directly to a substrate or after the substrate has been coated with a subcoat. In preferred aspects, the polymer is either an enteric or reverse-enteric polymer. Methods of preparing the dry film coatings, methods of preparing corresponding aqueous suspensions, methods of applying the coatings to substrates and the coated substrates themselves are also disclosed.
Owner:BPSI HLDG LLC
Abuse resistant opioid drug-ion exchange resin complexes having hybrid coatings
ActiveUS20120135077A1Favorable abuse resistance propertyImproved resistance characteristicsPowder deliveryNervous disorderMedicineIon-exchange resin
A sustained release formulation for opioid drugs is described. The formulation contains an opioid-ion exchange resin complex having a hybrid coating. The hybrid coating contains a cured polyvinylacetate polymer and a pH-dependent enteric coating layer mixed therein. Also provided are methods of making and using same.
Owner:TRIS PHARMA
Hybrid constant regions
Owner:JN BIOSCI
Self-emulsifying drug delivery system for improving bioavailability of insoluble medicine, and application thereof
ActiveCN105535979AGood water solubilityIncrease dissolution rateOrganic active ingredientsCapsule deliverySolubilityOil phase
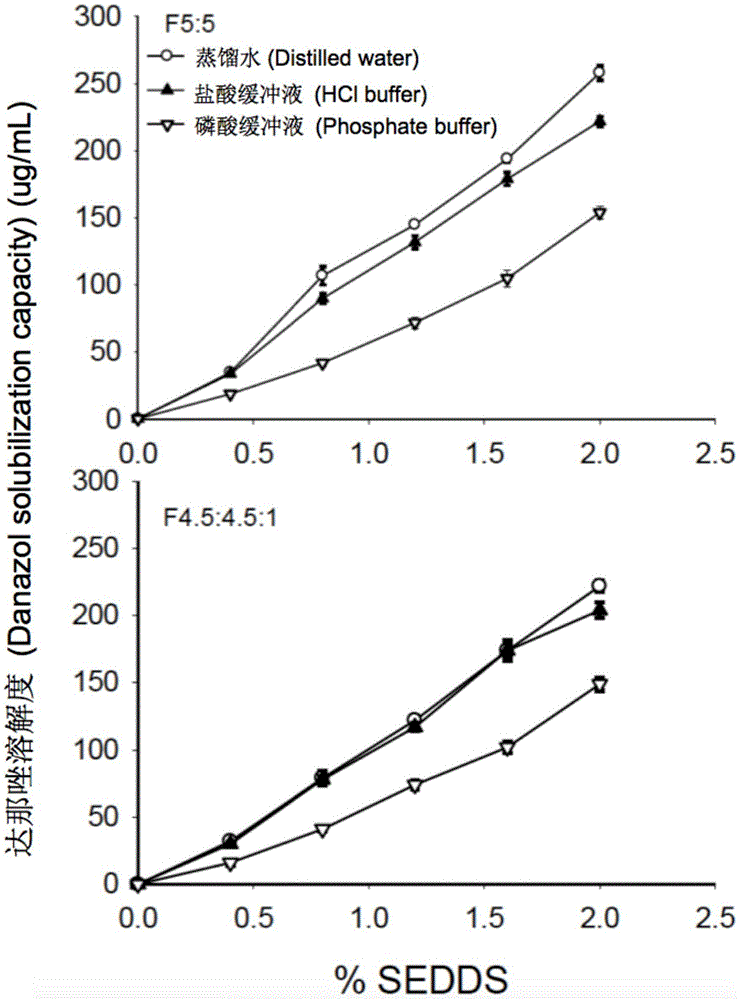
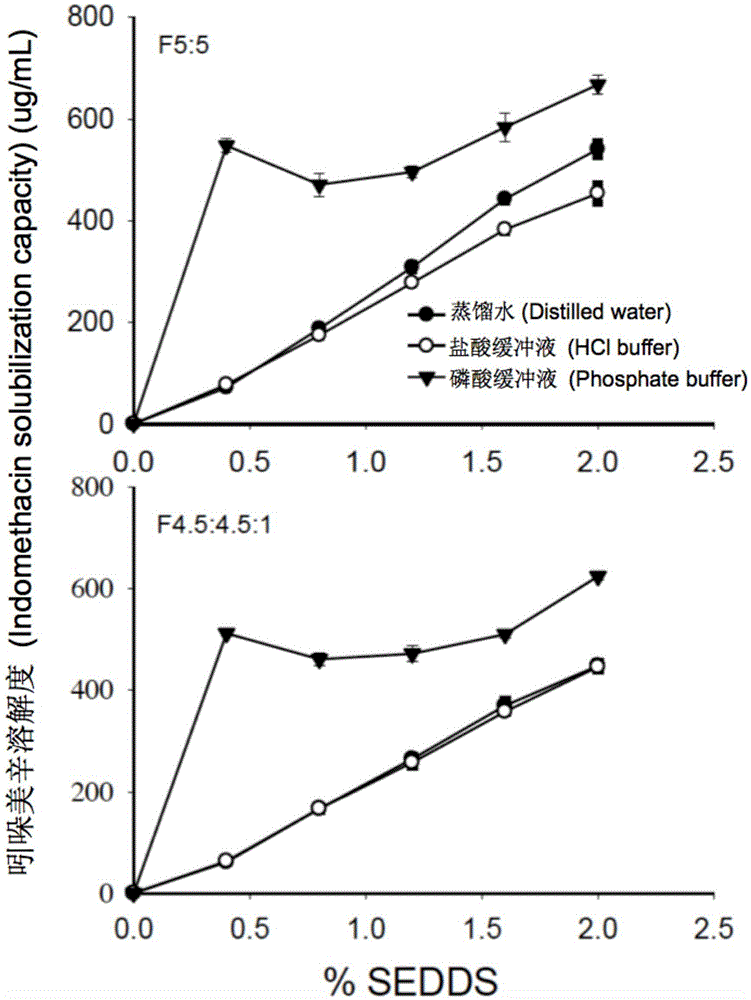
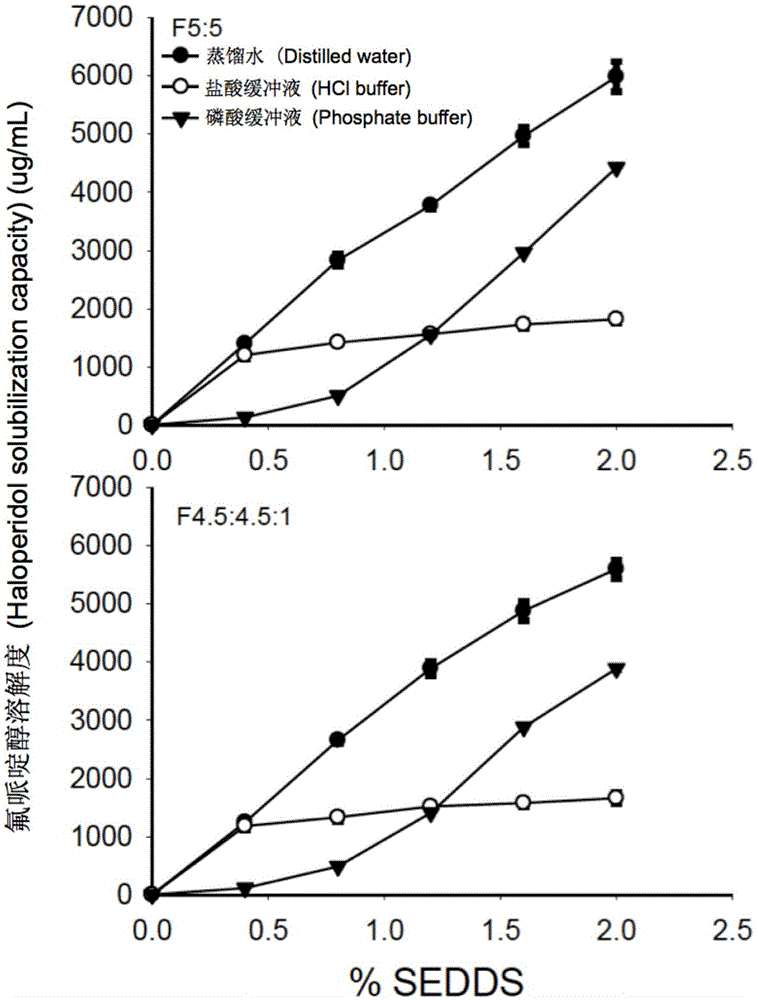
The invention provides a novel self-emulsifying drug delivery system (SEDDS). An SEDDS carrier material comprises a surfactant and an oil phase containing Capmul MCMs and medium chain fatty acids, is suitable for loading pH-dependent (weakly acidic and weakly alkaline) and a pH-independent (neutral) insoluble medicines, greatly improves the solubility of the medicines to realize optimum bioavailability, and has important application values in the development of preparations of the insoluble medicines.
Owner:李素华
Sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
PROCESS FOR REMOVING FLUORINATED EMULSIFIER FROM FLUOROPOLMER DISPERSIONS USING AN ANION-EXCHANGE RESIN AND A pH-DEPENDENT SURFACTANT AND FLUOROPOLYMER DISPERSIONS CONTAINING A pH-DEPENDENT SURFACTANT
InactiveUS20080264864A1Reduce the amount requiredGood dispersionIon-exchange process apparatusWater treatment parameter controlPolymer scienceFluoropolymer
A process of reducing the amount of fluorinated emulsifiers in fluoropolymer dispersions by contacting the fluoropolymer dispersion with an anion exchange resin in the presence of a pH-dependent surfactant, and fluoropolymer dispersions containing the pH-dependent surfactant and uses thereof.
Owner:3M INNOVATIVE PROPERTIES CO
Methods of fracturing a subterranean formation using a pH dependent foamed fracturing fluid
InactiveUS6966379B2Lower Level RequirementsFine foamFluid removalFlushingFracturing fluidSURFACTANT BLEND
Methods of fracturing a subterranean formation include providing a fracturing fluid having a first pH. The fracturing fluid may be made by combining a gelling agent, a surfactant, and a proppant. The surfactant is capable of facilitating foaming of the fracturing fluid at the first pH and defoaming of the fracturing fluid when its pH is changed to a second pH. The methods of fracturing the subterranean formation further include foaming the fracturing fluid having the first pH and subsequently pumping it to the subterranean formation to fracture the formation. The pH of the fracturing fluid changes to a second pH, for example via in situ contact with an acidic material, causing the level of foam in the fracturing fluid to be reduced. As a result of the reduction of the foam, the fracturing fluid deposits the proppant into the fractures formed in the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Novel sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
Delayed release pharmaceutical oral dosage form and method of making same
InactiveUS20070190139A1Inhibition of agglomerationBiocideElcosanoid active ingredientsVegetable oilImmediate release
The present invention relates to a multi layer pharmaceutical oral dosage form having delayed release and immediate release properties and method of making same. The delayed release formulation substantially behaves as an enterically coated dosage form but without the formulation and the application of an enteric coating. The delayed release formulation is characterized by a mixture of one or more active ingredients and one or more excipients selected from the group of solid aliphatic alcohols, mixtures of esters of saturated fatty alcohols and saturated fatty acids, natural or synthetic waxes, hydrogenated castor oil, hydrogenated vegetable oil, gums, and mixtures thereof; pH dependent soluble polymers; and optionally an opacifying agent.
Owner:INTELGENX CORP
Antibodies or fusion proteins multimerized via cysteine mutation and a mu tailpiece
ActiveUS9540442B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenPentamer
The invention provides constant regions incorporating a cysteine mutation and linked to a μ tailpiece and antibodies or fusion proteins incorporating the same. The constant regions include at least CH2 and CH3 regions of an IgG heavy chain constant region including a cysteine mutation and μ tailpiece. Antibodies or fusion proteins incorporating the constant regions gains the ability to form multivalent complexes, e.g., pentameric or hexameric structures. Antibodies or fusion proteins incorporating the constant regions also retain IgG properties including specific binding to protein G, which facilitates purification and may exhibit pH-dependent FcRn binding, which is associated with a relatively long in vivo half-life. Depending on the isotype and subtype, the nature of the antigen and presence of an additional IgG hinge domain, such antibodies or fusion proteins may also have properties of specific binding to protein A, and effector functions such as ADCC, CDC and opsonization.
Owner:JN BIOSCI
Oral solid pharmaceutical formulations with pH-dependent multiphasic release
InactiveUS7022345B2Smooth releaseOrganic active ingredientsPowder deliveryAdditive ingredientBULK ACTIVE INGREDIENT
Oral solid pharmaceutical compositions with pH-dependent multiphasic release, containing, as active ingredient, a molecule useful in the inflammatory bowel disease therapy, are described, being such compositions suitable to the release of the active ingredient in the intestinal tract.
Owner:VALDUCCI ROBERTO
Methods of fracturing a subterranean formation using a pH dependent foamed fracturing fluid
InactiveUS20050077047A1Level of foaming is reducedFine foamFluid removalFlushingFracturing fluidSURFACTANT BLEND
Methods of fracturing a subterranean formation include providing a fracturing fluid having a first pH. The fracturing fluid may be made by combining a gelling agent, a surfactant, and a proppant. The surfactant is capable of facilitating foaming of the fracturing fluid at the first pH and defoaming of the fracturing fluid when its pH is changed to a second pH. The methods of fracturing the subterranean formation further include foaming the fracturing fluid having the first pH and subsequently pumping it to the subterranean formation to fracture the formation. The pH of the fracturing fluid changes to a second pH, for example via in situ contact with an acidic material, causing the level of foam in the fracturing fluid to be reduced. As a result of the reduction of the foam, the fracturing fluid deposits the proppant into the fractures formed in the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Preparation method of (Lactobacillus planetarium subsp. plantarum)Zhang-LL and its listeria monocytogene-resistant bacteriocin
ActiveCN104531562ASource securityStable sourceBacteriaMicroorganism based processesFood borneListeria murrayi
The invention relates to a preparation method of (Lactobacillus planetarium subsp.plantarum)Zhang-LL and its listeria monocytogene-resistant bacteriocin. The preparation method is suitable for preservation and fresh-keeping of meat and meat products, milk and milk products, fruits and vegetable, and instant foods. The (Lactobacillus planetarium subsp.plantarum)Zhang-LL (CGMCC No.6936) is selected from bacon on the Fujian farmer's market. A bacteriocin production broth is obtained by fermentation of a Zhang-LL strain, and the Zhang-LL strain bacteriocin is extracted and purified by a pH-dependent adsorption-desorption method, a cation exchange chromatography and a reversed phase high-performance liquid chromatography so that titer is improved by 32 times and purity is improved by 36.65 times. The bacteriocin can inhibit a plurality of food-borne pathogenic bacteria such as listeria monocytogenes, has high bacteriostatic activity, good heat, acid and base stability, can be degraded by human protease and is a natural and safe biological preservative. The preparation method has the advantages of simple processes, stability, high efficiency, source convenience, low cost and industrial production feasibility.
Owner:BEIJING BEINONG HONGZE BIOTECH CO LTD
Pharmaceutical composition for improving palatability of drugs and process for preparation thereof
InactiveUS20060141053A1Reduce the amount requiredSuppress bitternessBiocidePowder deliveryLipid formationGastric ph
The present invention discloses compositions, comprising a lipid-polymer matrix to mask the bitter or unpleasant taste of the medicament. The lipid or a blend of lipids, are used in combination with the pH dependent polymer where the said polymer is acid soluble or swellable The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said lipid-polymer compositions are disclosed. The concomitant use of the acid soluble polymer, which remains collapsed at the pH of saliva, inhibits the release of drug at that pH and hence they further help in bitterness inhibition. The said compositions deliver substantial amount of the bitter drug immediately at the gastric pH with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Capsule for holding liquid-containing compositions and method for making the same
The present invention provides a capsule which comprises (1) a capsule shell, and (2) a liquid core composition. The capsule shell comprises a pH-dependent polymer and optionally a plasticizer. The liquid core composition contains liquid up to 70% by volume. The pH of the liquid core composition is adjusted to or at a pH in which the pH-dependent polymer is insoluble. The liquid core composition is preferably a decoction or condensate of the decoction containing herbal extract. The present invention further provides methods for making the capsule.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Method for rapid detection and evaluation of cultured cell growth
Provided is a method and system for the rapid and accurate detection of growth and metabolism of a cellular microorganism in a population of microorganisms in a non-liquid, culture medium. Further provided is a gelled culture medium containing a non-toxic, water-soluble, phosphorescent compound which measures oxygen content (partial pressure) of an microorganism also contained therein, by oxygen-dependent quenching of phosphorescence; or the gel contains a fluorescent pH indicator that demonstrates growth of the microorganism by pH-dependent intensity change or wavelength shift in the emission spectrum. Further provided is a system and method for killing undesirable microorganisms or colonies in the culture medium without harming the surrounding microorganisms.
Owner:OXYGEN ENTERPRISES
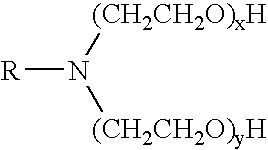
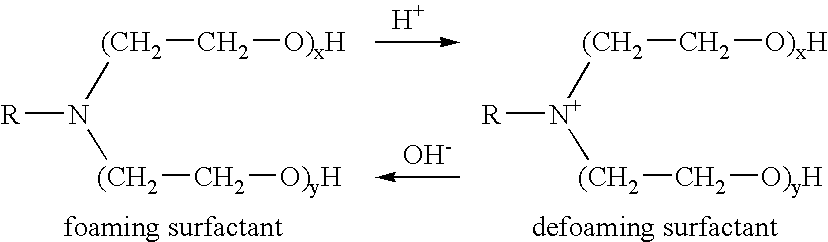
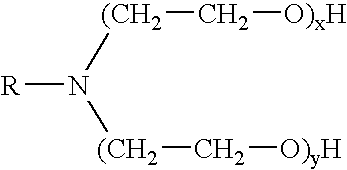
Methods for effecting controlled break in pH dependent foamed fracturing fluid
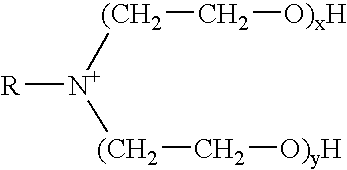
The invention provides a fluid for use in a subterranean formation penetrated by a wellbore, the fluid comprising: (a) water; (b) an orthoester; and (c) a surfactant comprising a tertiary alkyl amine ethoxylate generally represented by the following formula: wherein R is an alkyl group or aryl group, and wherein X and Y are each independently at least one. The invention also provides a method of fracturing a subterranean formation, comprising the step of forming a foamed fracturing fluid comprising water; an orthoester; a surfactant comprising a tertiary alkyl amine ethoxylate generally represented by the formula above; and a gas. The method also provides the step of introducing the foamed fracturing fluid into a subterranean formation at a pressure sufficient to create a fracture in the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
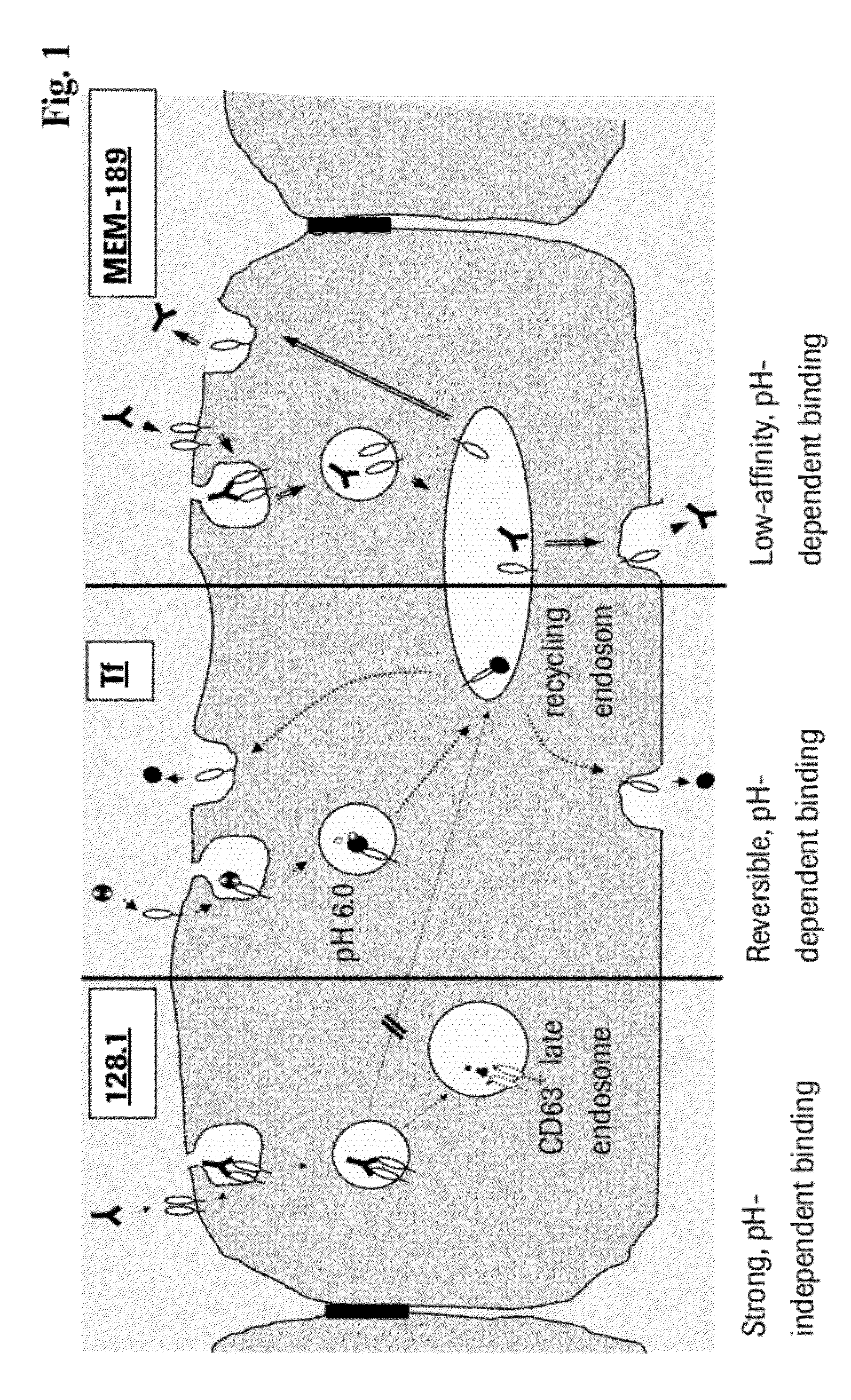
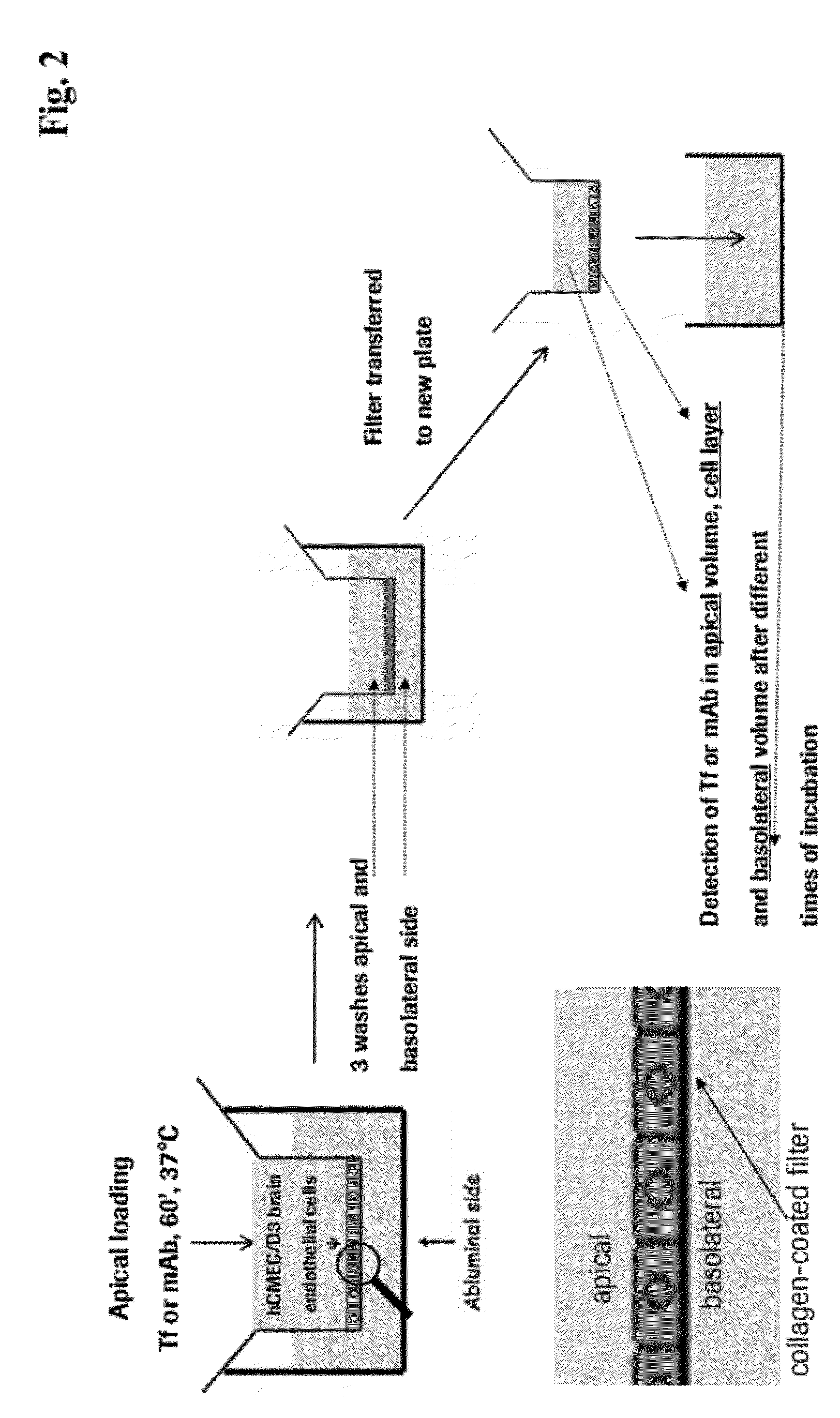
Method and Constructs for the pH Dependent Passage of the Blood-brain-barrier
InactiveUS20120282176A1Effectively crossDiminishment of any direct or indirect pathological consequencesAntibacterial agentsNervous disorderBinding siteVariable domain
Herein is reported a fusion polypeptide comprising i) at least one binding site, e.g. which comprises an antibody heavy chain variable domain and an antibody light chain variable domain, and which binds to an internalizing cell surface receptor, and ii) at least one pharmaceutically active compound, whereby the EC50-value of the binding pair that binds to an internalizing cell surface receptor determined at pH 5.5 is higher than the EC50-value of the same binding pair determined at pH 7.4 and its use for delivering a pharmaceutically active compound across the blood-brain-barrier.
Owner:ROCHE GLYCART AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com