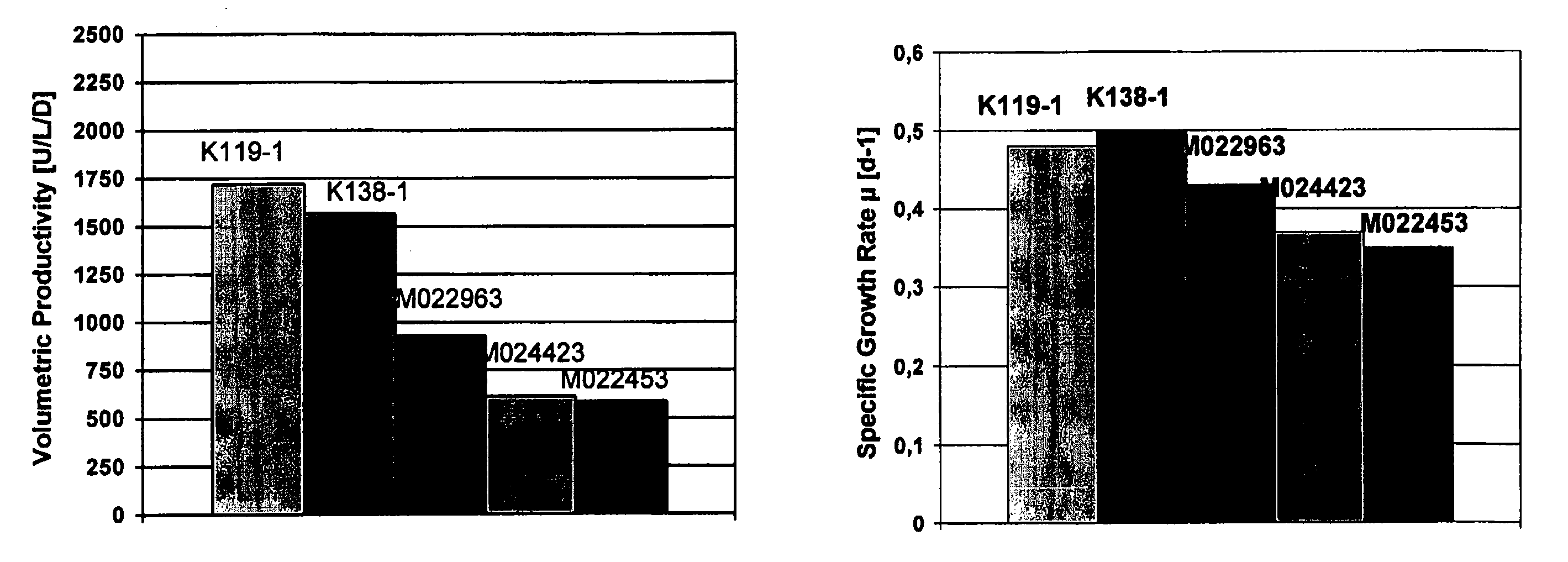
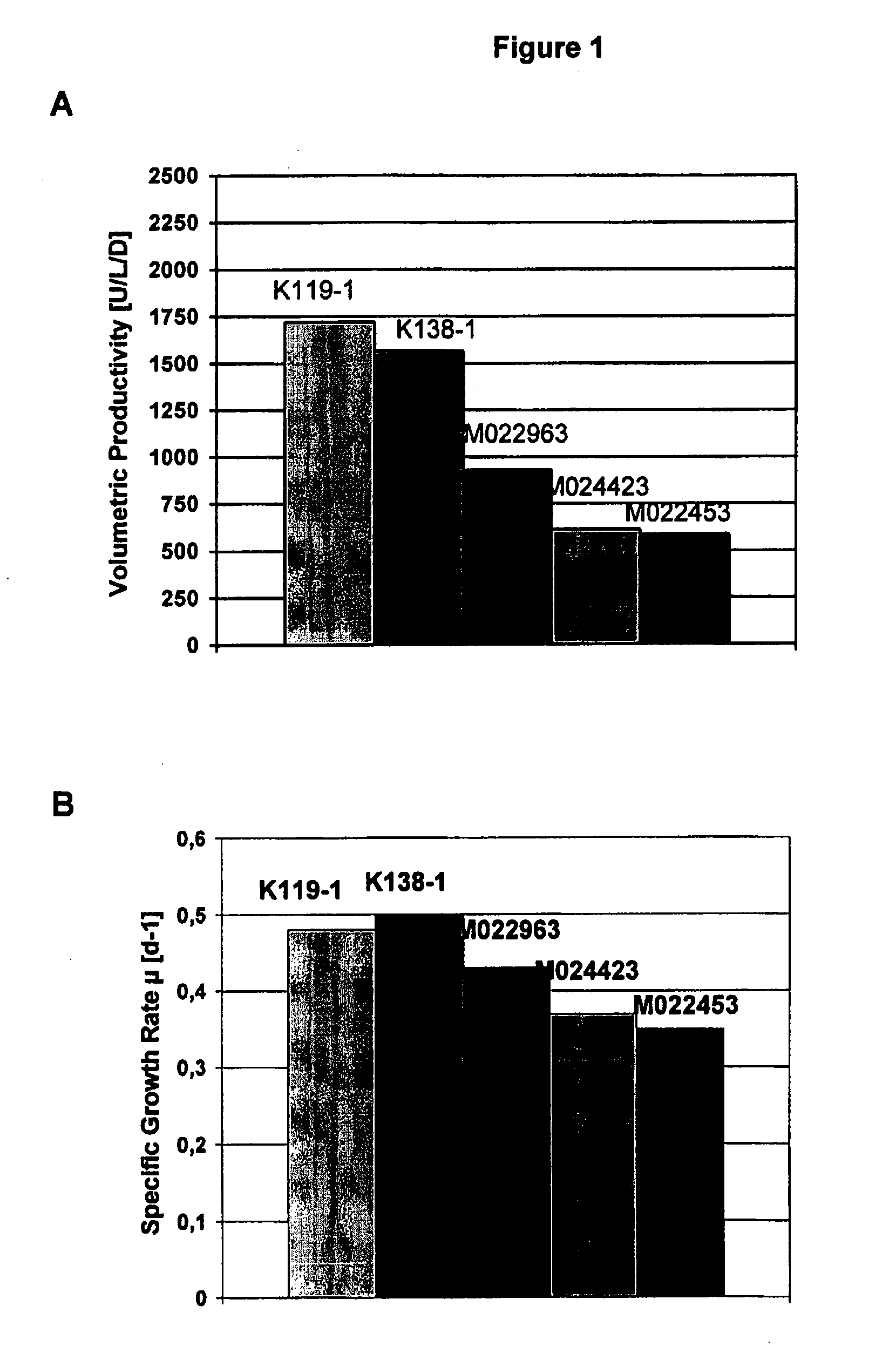
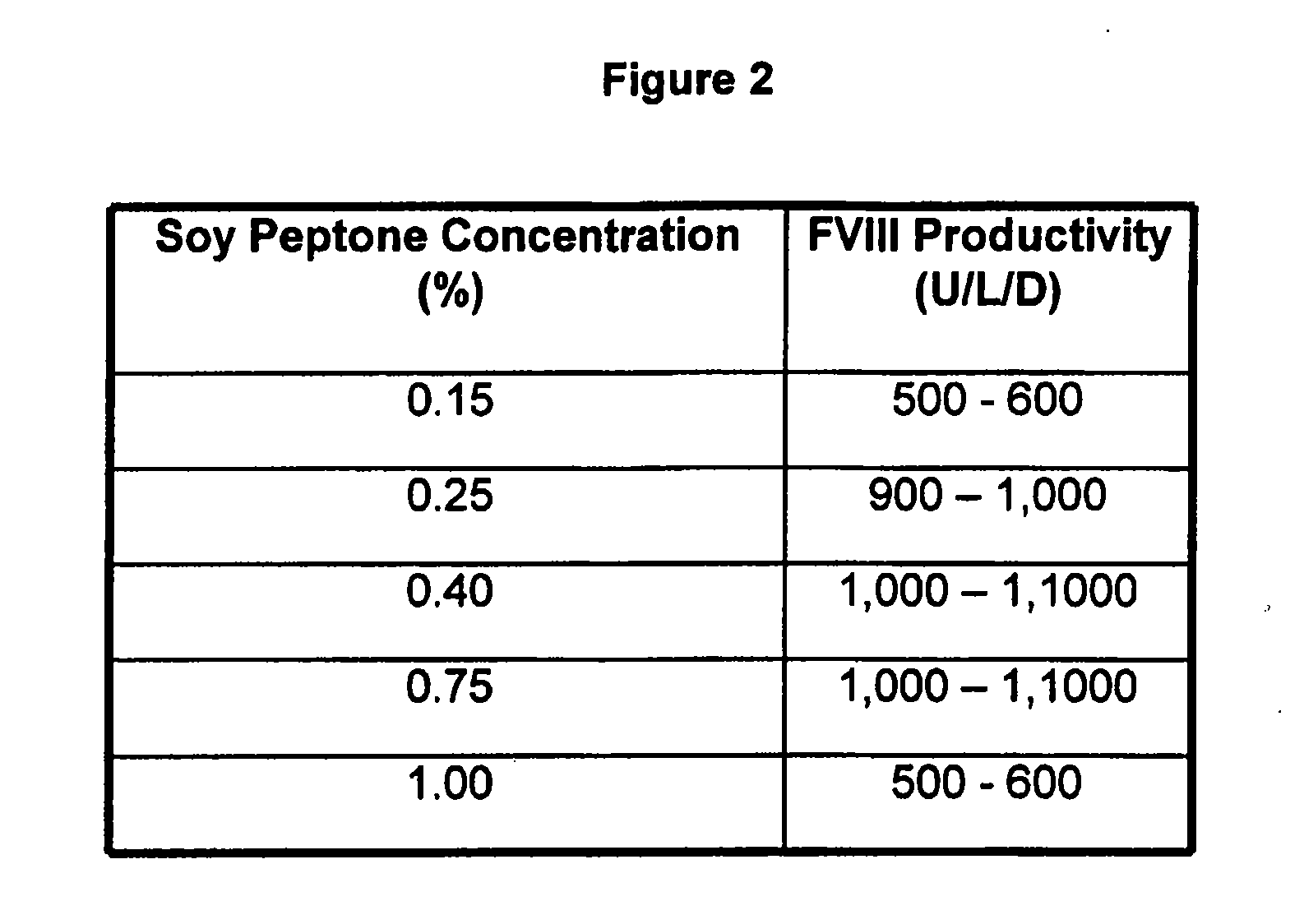
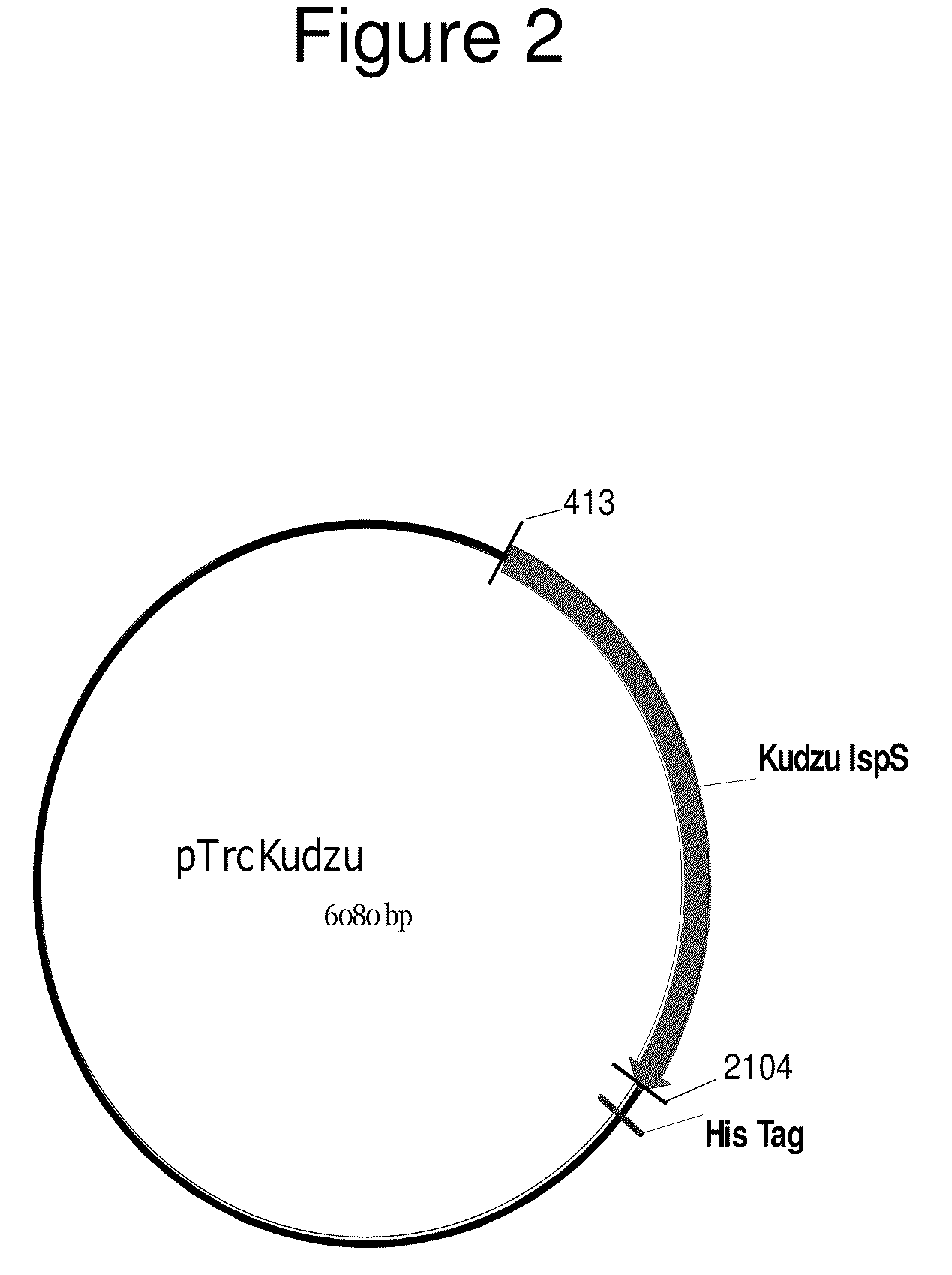
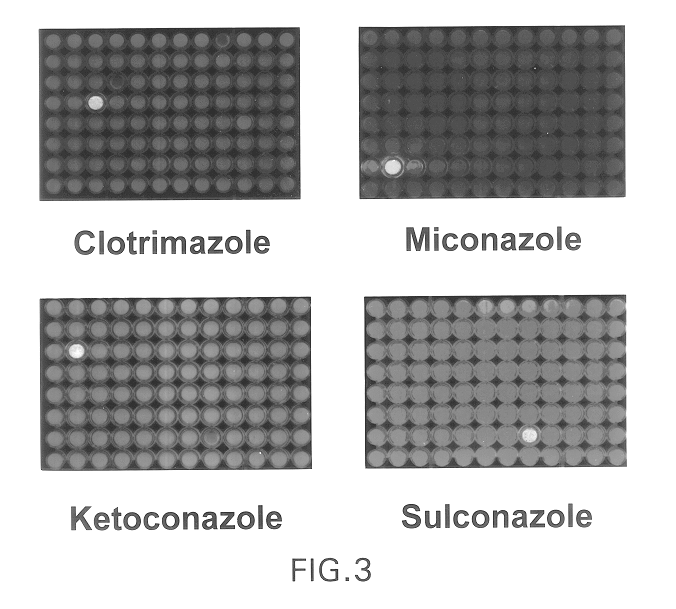
Patents
Literature
1736 results about "Cultured cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
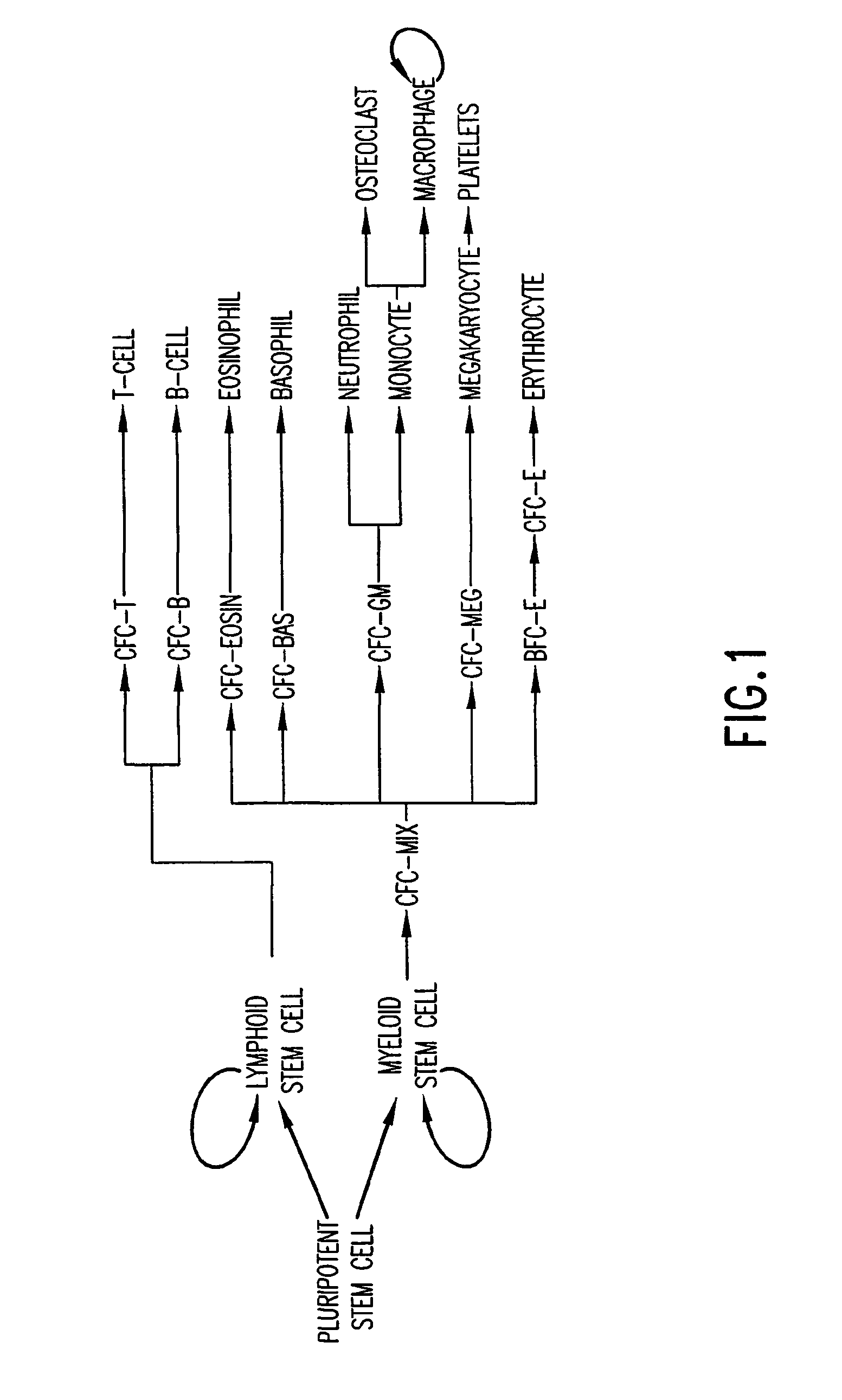
Essentially, cell culture involves the distribution of cells in an artificial environment (in vitro) which is composed of the necessary nutrients, ideal temperature, gases, pH and humidity to allow the cells to grow and proliferate. In vivo - When the study involves living biological entities within the organism.
Nucleic acids and polypeptides of invertebrate TWIK channels and methods of use
Tandem pore domain weak inward rectifying K+ (TWIK) channel nucleic acids and proteins that have been isolated from Drosophila melanogaster and Leptinotarsa are described. The TWIK channel nucleic acids and proteins can be used to genetically modify metazoan invertebrate organisms, such as insects, coelomates, and pseudocoelomates, or cultured cells, resulting in TWIK channel expression or mis-expression. The genetically modified organisms or cells can be used in screening assays to identify candidate compounds which are potential pesticidal agents or therapeutics that interact with TWIK channel proteins. They can also be used in methods for studying TWIK channel activity and identifying other genes that modulate the function of, or interact with, the TWIK channel gene.
Owner:EXELIXIS PHARMA
Method for the evolutionary design of biochemical reaction networks
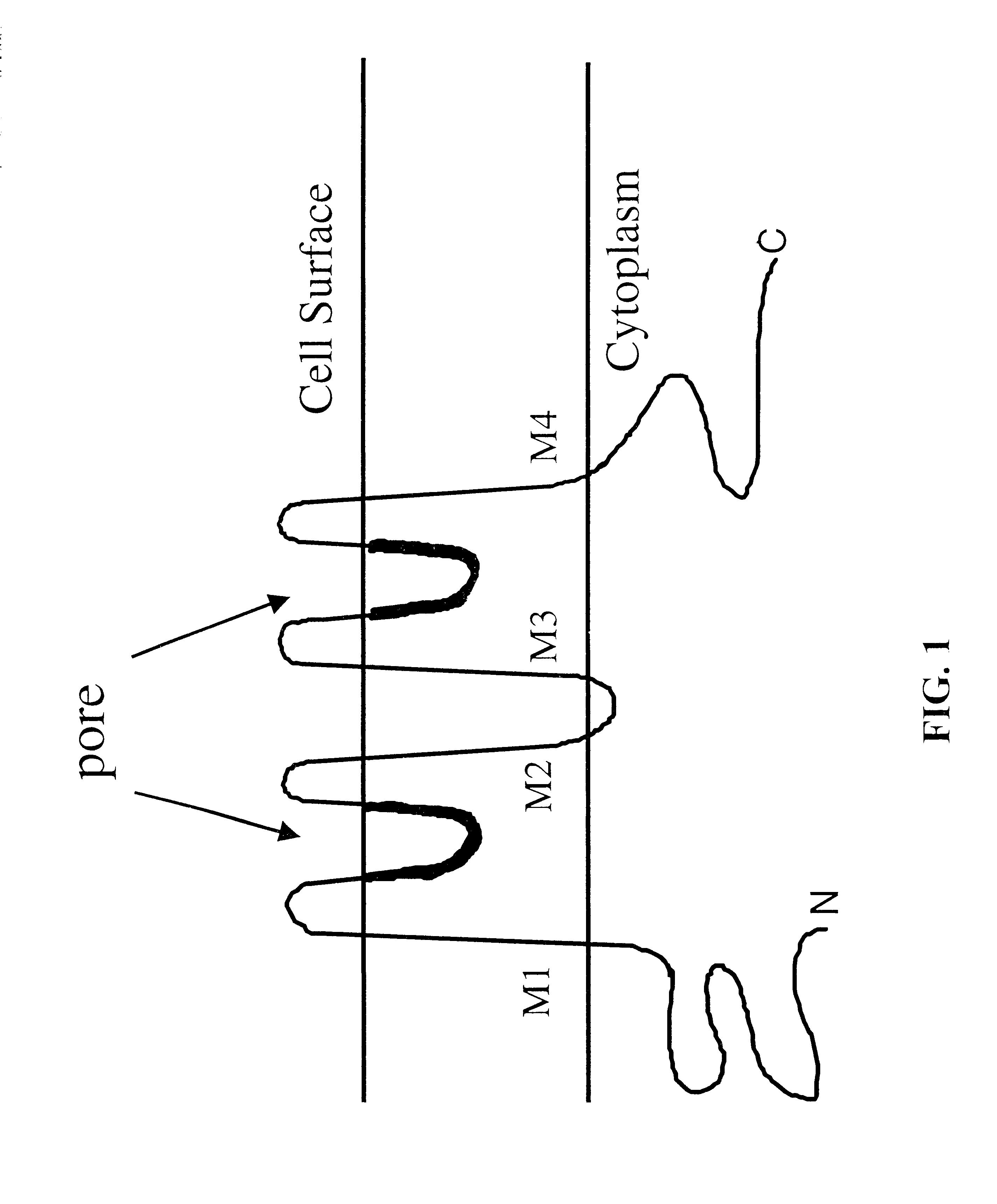
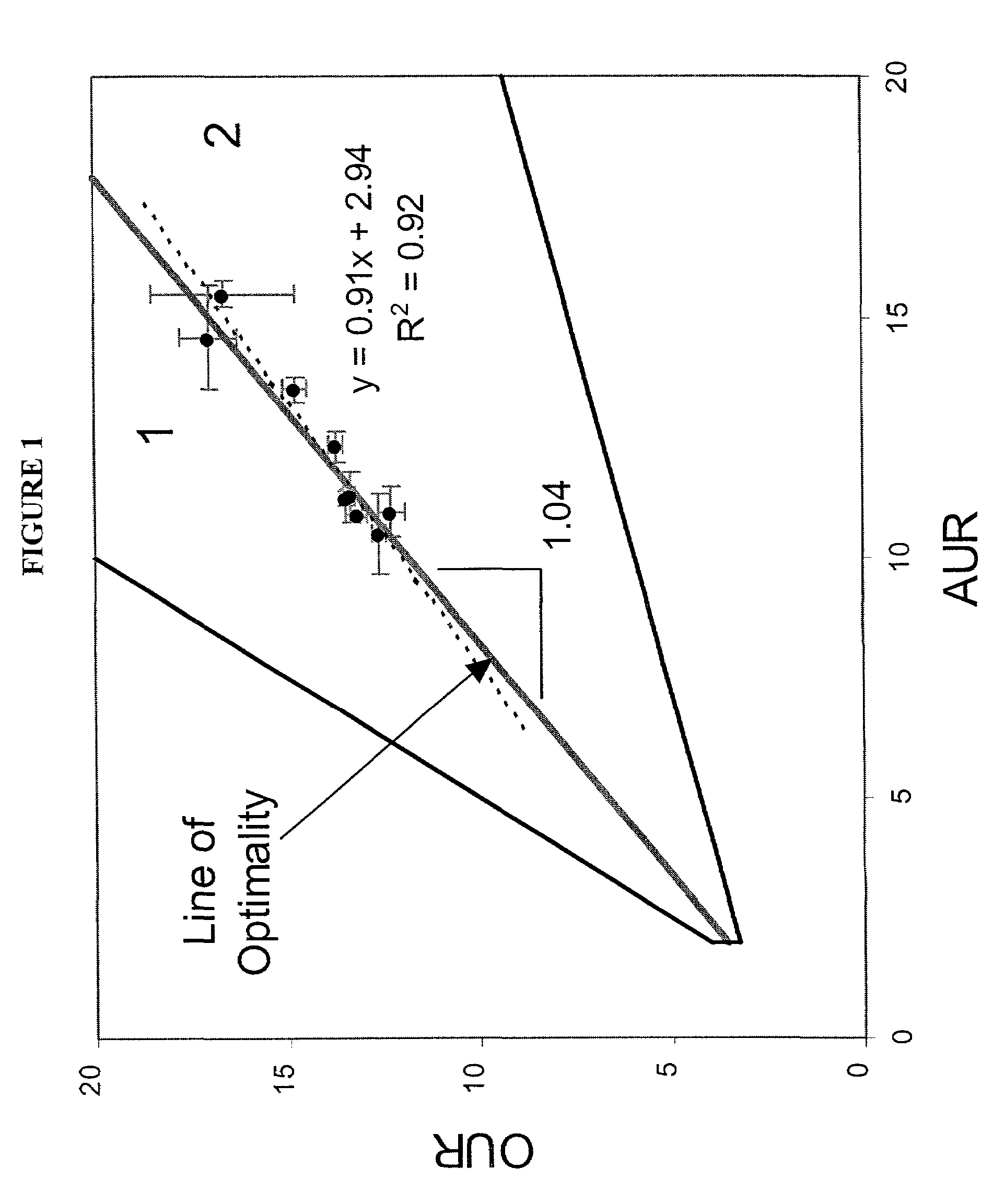
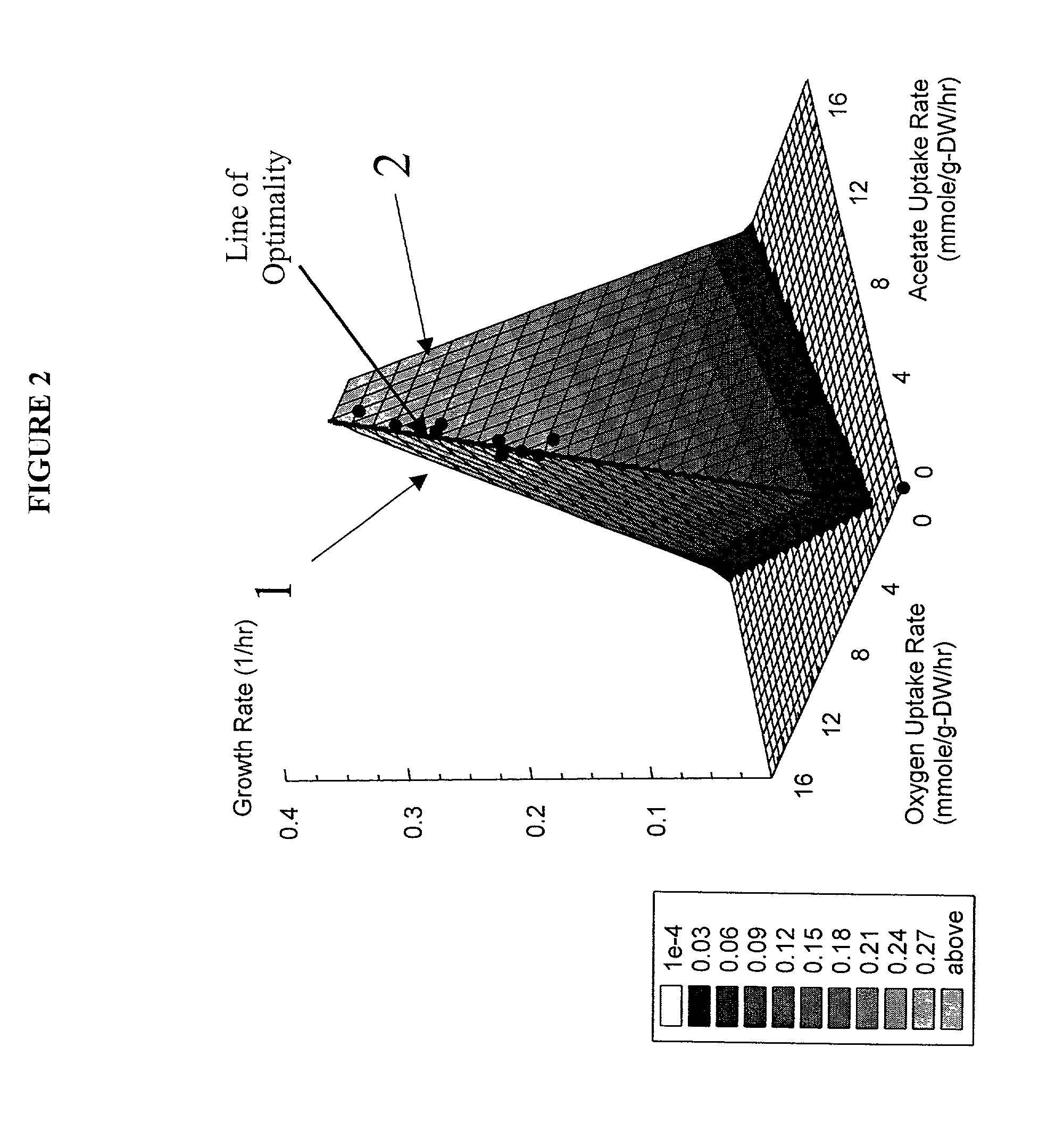
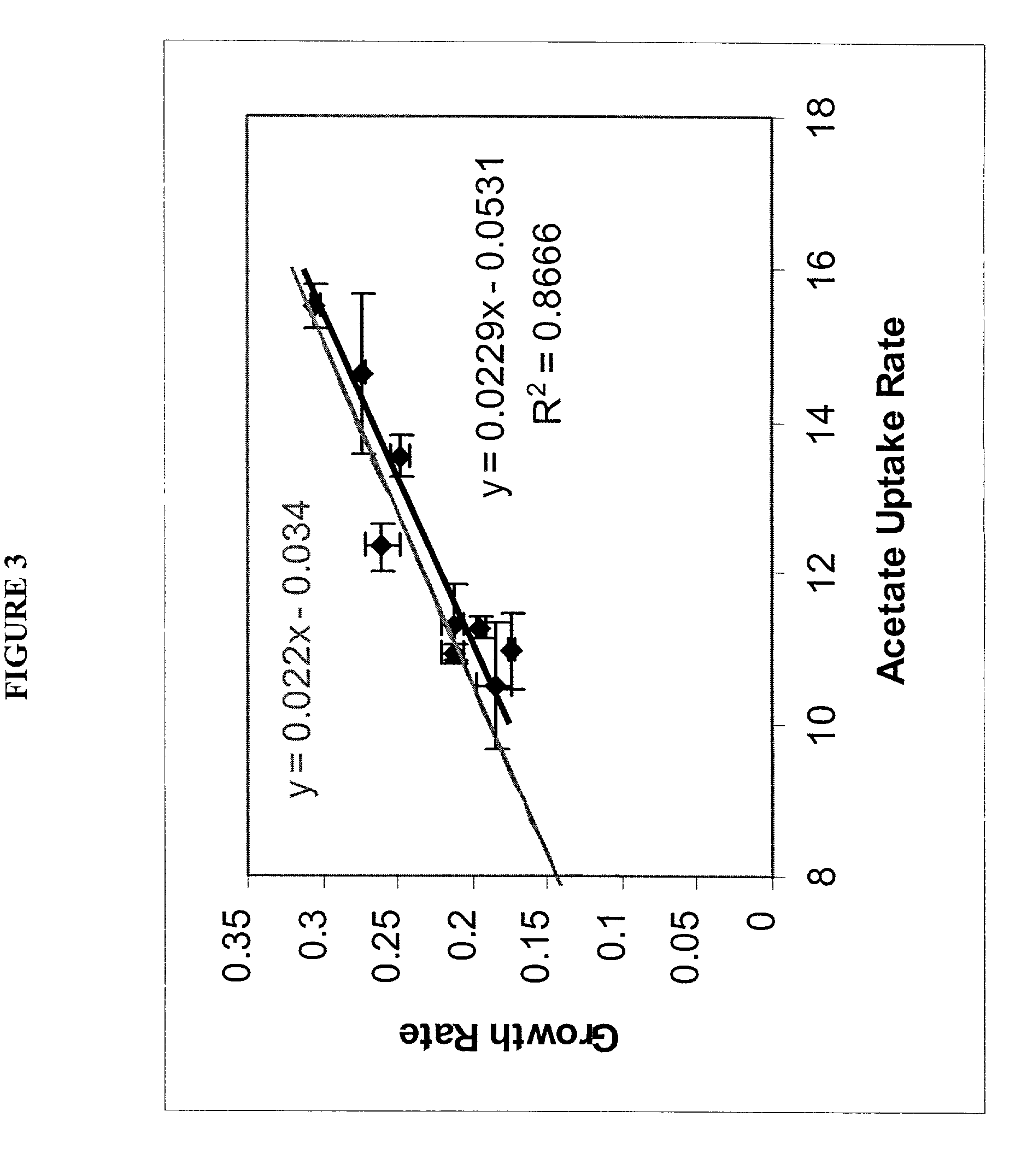
The present invention relates to methods for achieving an optimal function of a biochemical reaction network. The methods can be performed in silico using a reconstruction of a biochemical reaction network of a cell and iterative optimization procedures. The methods can further include laboratory culturing steps to confirm and possibly expand the determinations made using the in silico methods, and to produce a cultured cell, or population of cells, with optimal functions. The current invention includes computer systems and computer products including computer-readable program code for performing the in silico steps of the invention.
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for the augmentation and repair of defects in tissue
InactiveUS20070065415A1Improve successful adaptationBiocidePeptide/protein ingredientsRepair tissueCultured cell
Compositions and methods of treating a defect in a patient are disclosed, including expanding a culture of autologous cells in vitro to form cultured cells, collecting the cultured cells for introduction into the patient, and depositing the cultured cells with ancillary proteins.
Owner:DASK TECH
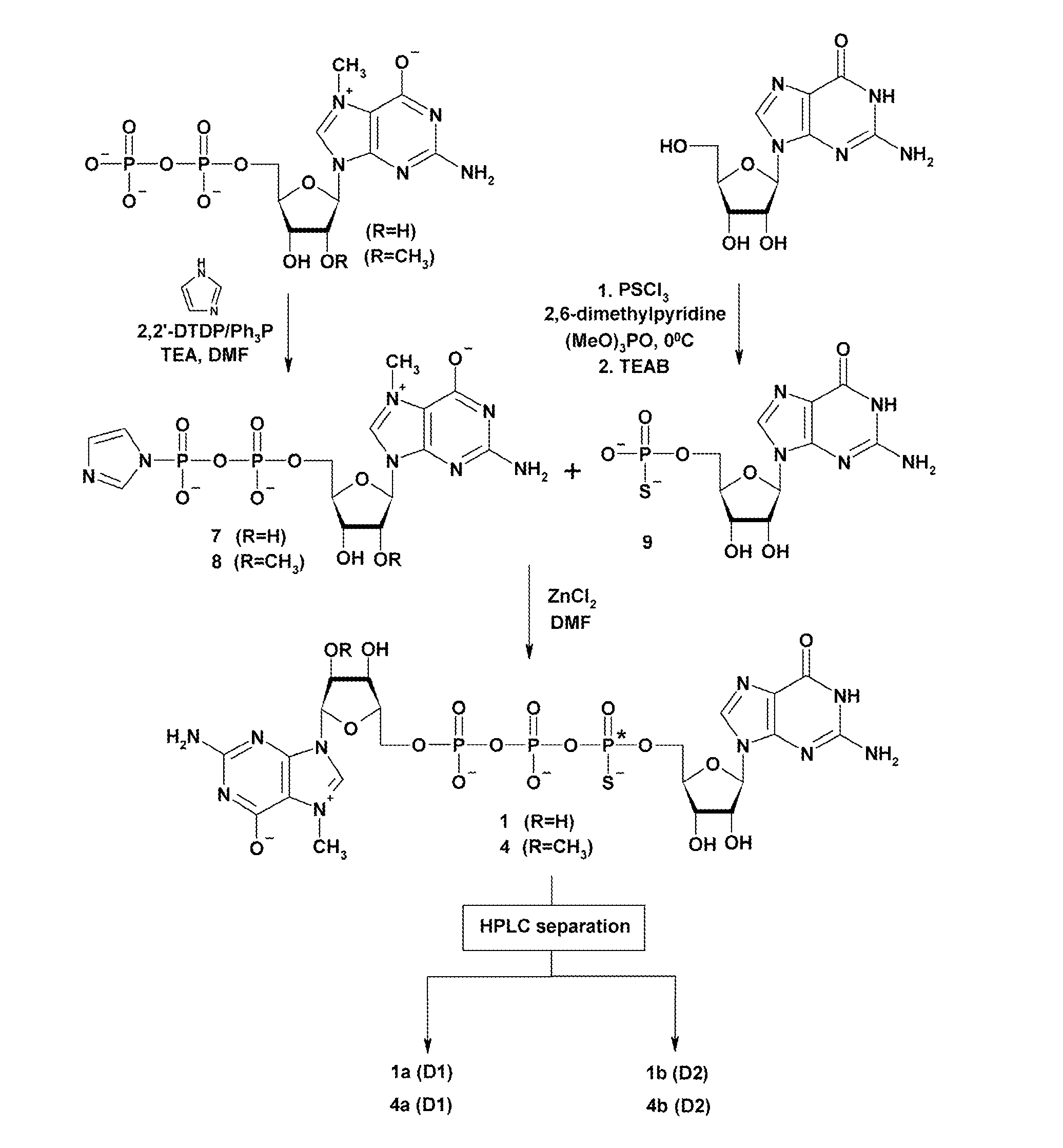
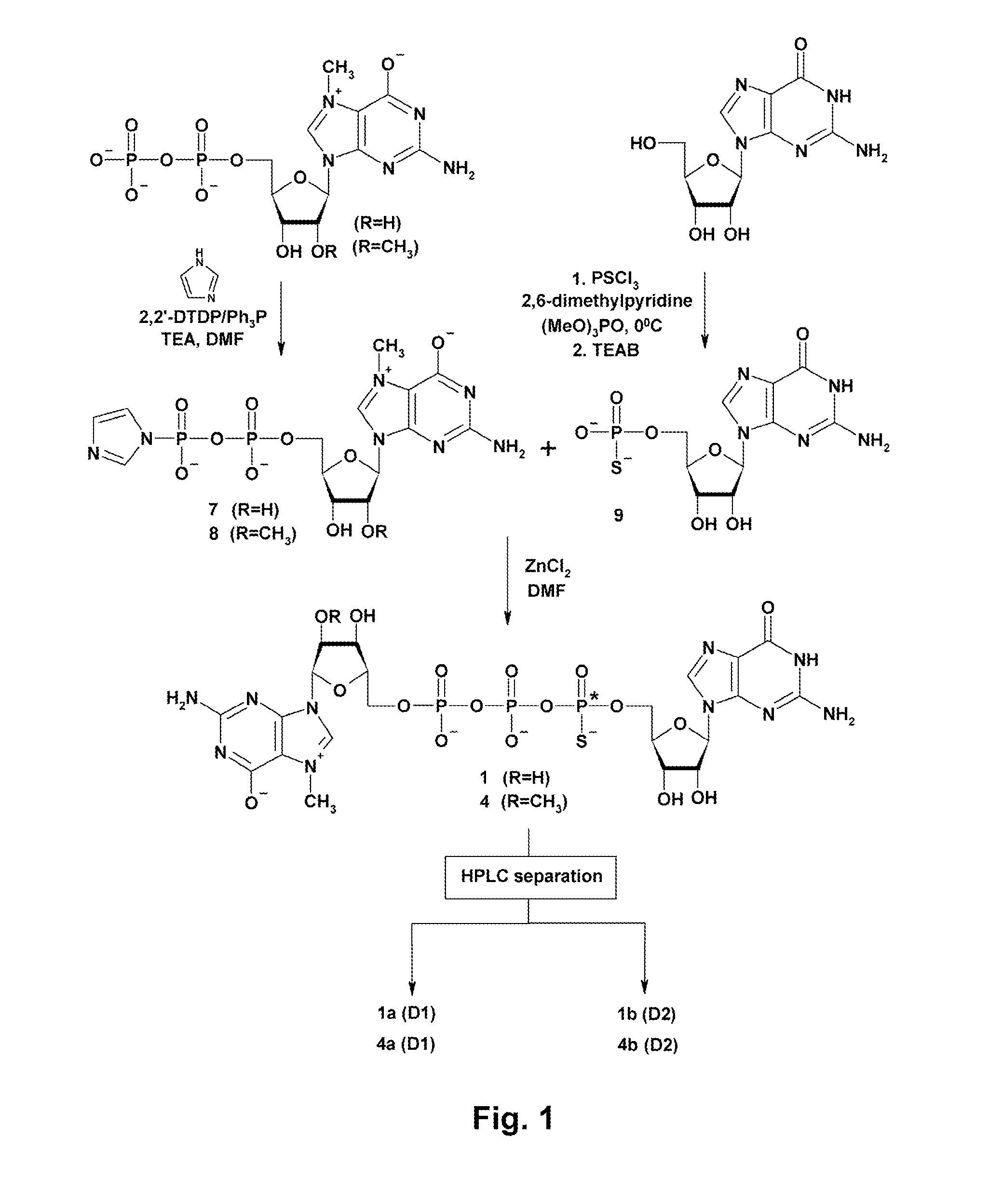
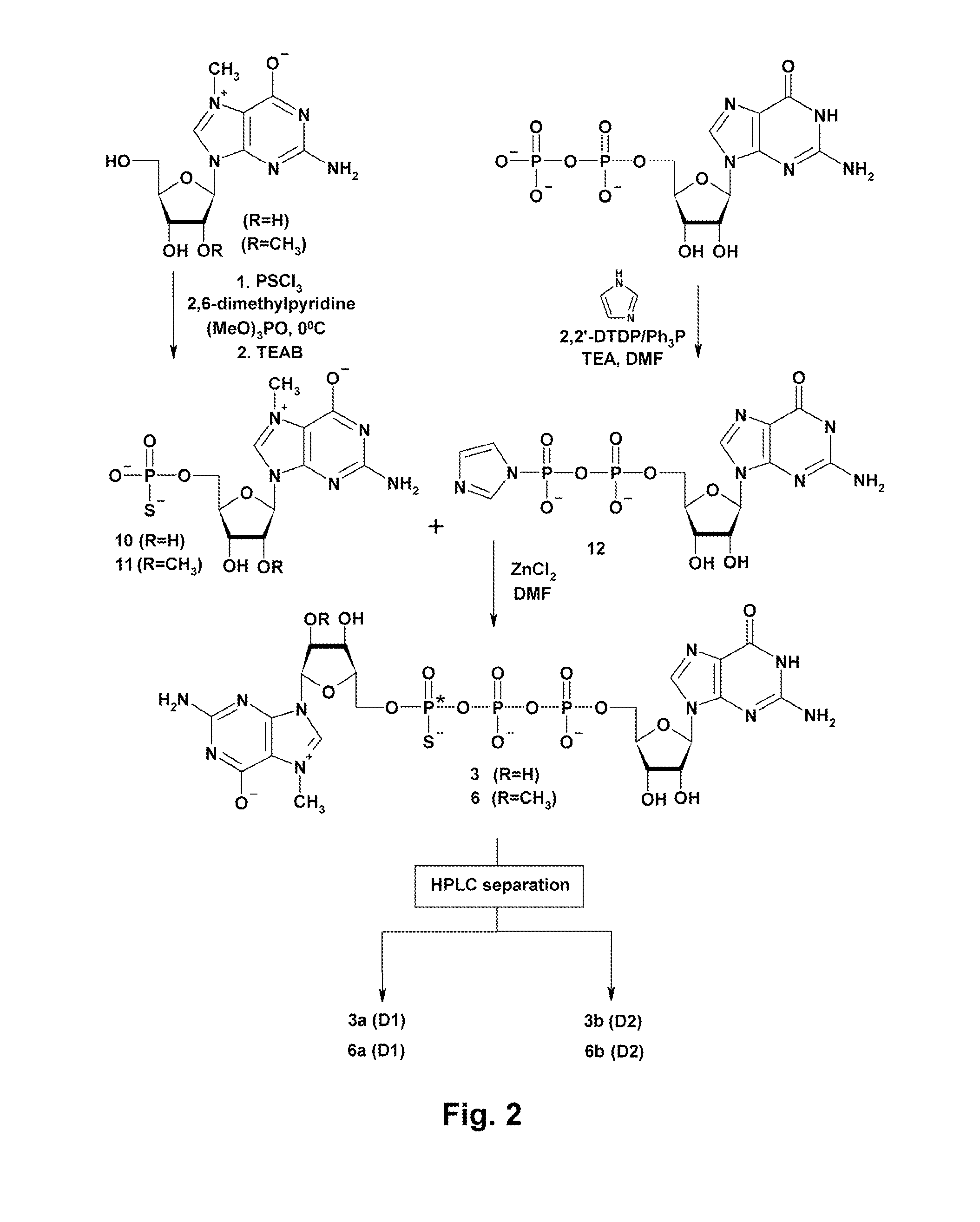
Synthesis and use of anti-reverse phosphorothioate analogs of the messenger RNA cap
ActiveUS8153773B2High affinityHigh productSugar derivativesPeptide/protein ingredients7-MethylguanosineEIF4E
New RNA cap analogs are disclosed containing one or more phosphorothioates groups. The analogs also contain modifications at the 2′-O position of 7-methylguanosine that prevent them from being incorporated in the reverse orientation during in vitro synthesis of mRNA and that hence are “anti-reverse cap analogs” (ARCAs). The ARCA modification ensures that the S atom is precisely positioned within the active sites of cap-binding proteins in both the translational and decapping machinery. The new S-ARCA analogs are resistant to in vivo decapping enzymes. Some S-ARCAs have a higher affinity for eIF4E than the corresponding analogs not containing a phosphorothioate group. When mRNAs containing the various S-ARCAs are introduced into cultured cells, some are translated as much as five-fold more efficiently than mRNAs synthesized with the conventional analog m7GpppG.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
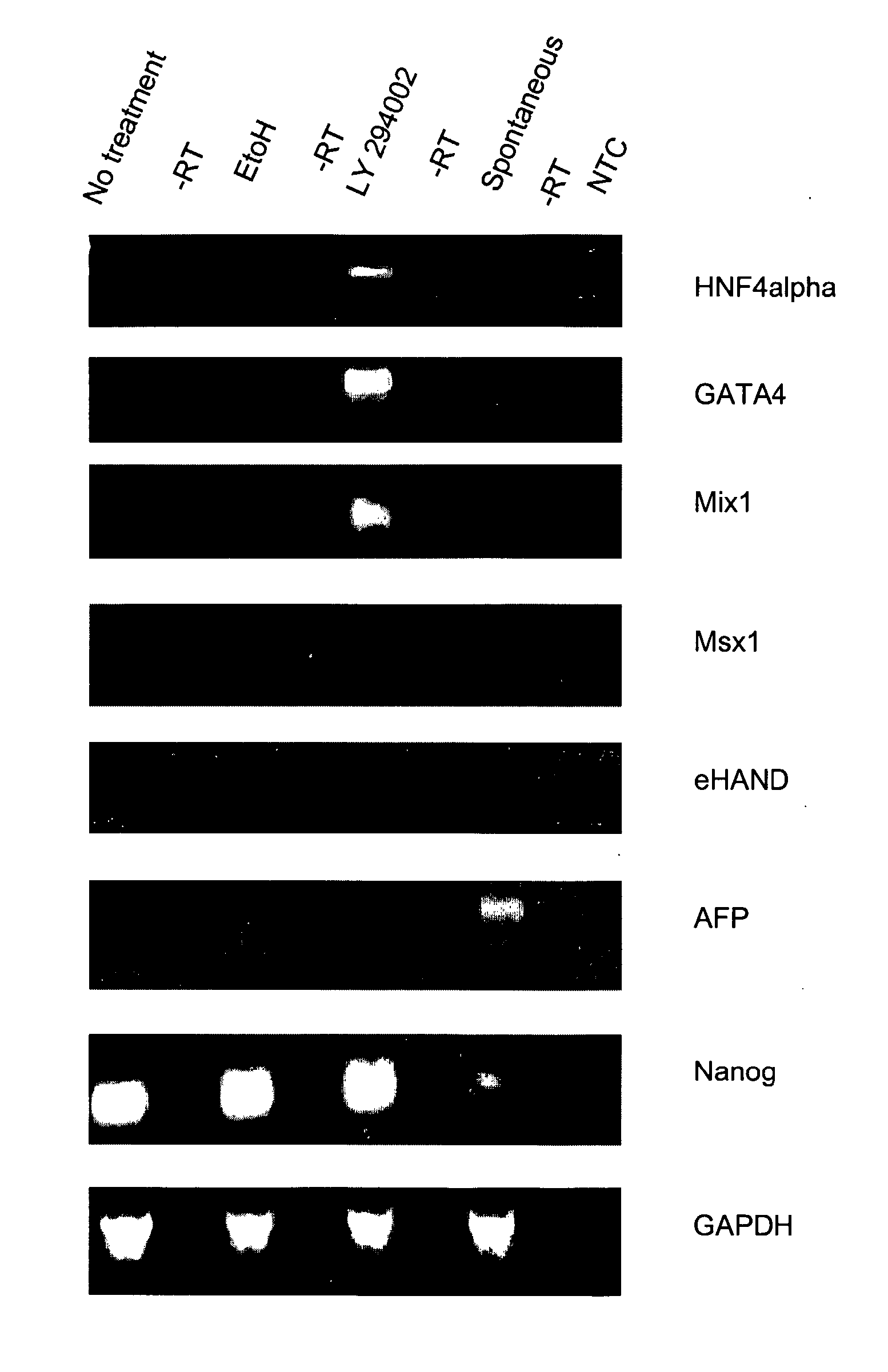
Compositions And Methods For Self-Renewal And Differentiation In Human Embryonic Stem Cells
ActiveUS20070281355A1Efficient productionEffectively lead to differentiationPeptide/protein ingredientsMetabolism disorderGerm layerFeeder Layer
The present invention provides compositions and methods for the production of differentiated mammalian cells. More particularly, the present invention provides cellular differentiation methods employing culturing the cells on a feeder layer or under feeder-free conditions in cell culture and further contacting the cells with an inhibitor of the PI3-kinase pathway for the generation of differentiated mammalian cells from pluripotent mammalian stem cells. Preferably, the differentiated cell is selected from the group consisting of a mesendodermal cell, a mesodermal cell, and an endodermal cell.
Owner:UNIV OF GEORGIA RES FOUND INC +1
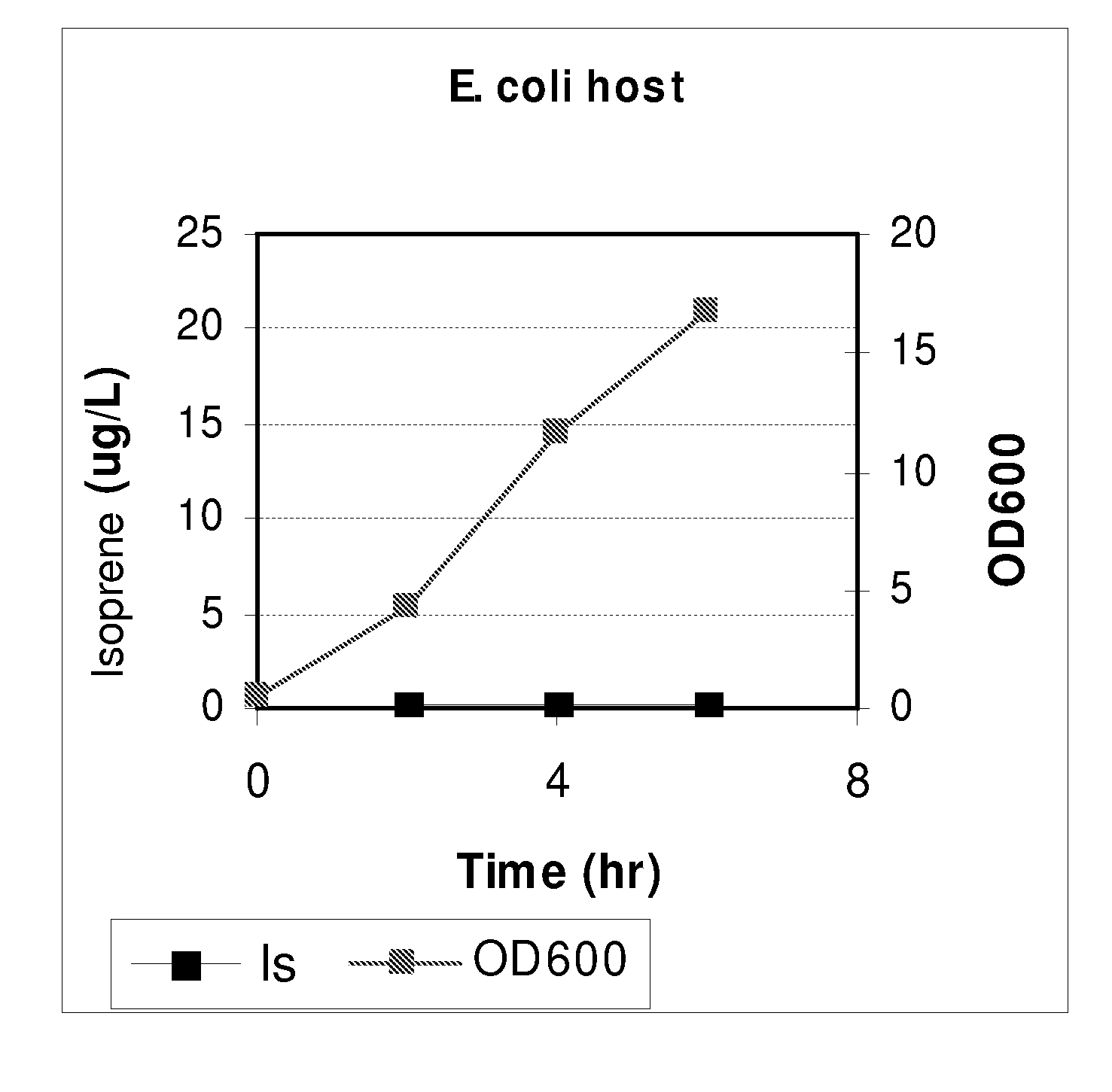
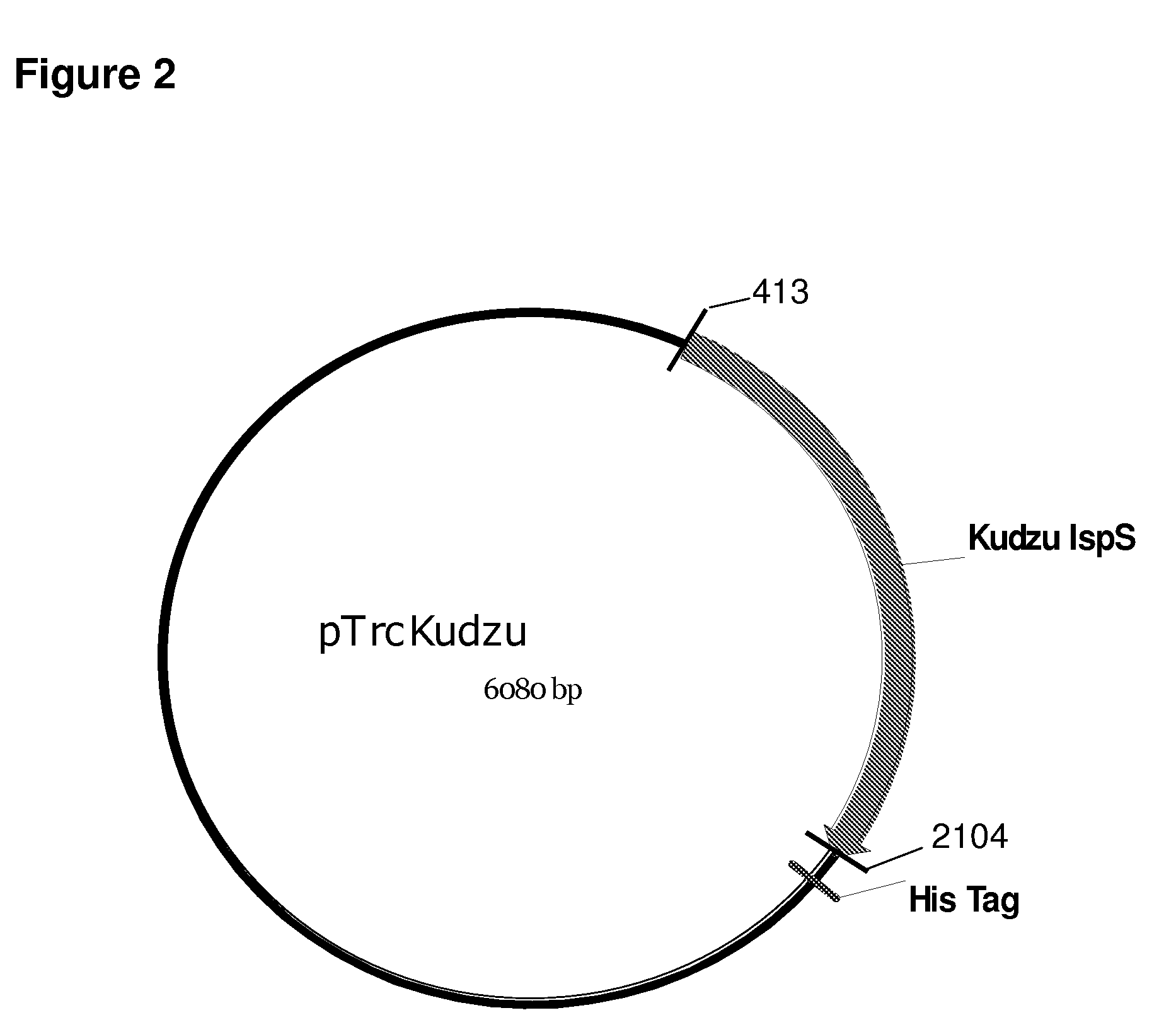
Compositions and methods for producing isoprene free of c5 hydrocarbons under decoupling conditions and/or safe operating ranges
The invention features methods for producing isoprene from cultured cells wherein the cells in the stationary phase. The invention also provides compositions that include these cultured cells and / or increased amount of isoprene. The invention also provides for systems that include a non-flammable concentration of isoprene in the gas phase. Additionally, the invention provides isoprene compositions, such as compositions with increased amount of isoprene or increased purity.
Owner:DANISCO US INC +1
Animal protein-free media for cultivation of cells
InactiveUS20080009040A1Efficient expressionGuaranteed efficient growthMicroorganismsCulture processHydrolysateCell culture media
The present invention relates to animal protein-free cell culture media comprising polyamines and a plant- and / or yeast-derived hydrolysate. The invention also relates to animal protein-free culturing processes, wherein cells can be cultivated, propagated and passaged without adding supplementary animal proteins in the culture medium. These processes are useful in cultivating cells, such as recombinant cells or cells infected with a virus, and for producing biological products by cell culture processes.
Owner:BAXTER INT INC +1
Adipose-derived stem cells and lattices
InactiveUS20050282275A1Promote growthFacilitate differentiationGenetic material ingredientsMammal material medical ingredientsCell-Extracellular MatrixConnective tissue fiber
The present invention provides adipose-derived stem cells and lattices. In one aspect, the present invention provides a lipo-derived stem cell substantially free of adipocytes and red blood cells and clonal populations of connective tissue stem cells. The invention also provides a method of isolating stem cells from adipose tissues. The cells can be employed, alone or within biologically-compatible compositions, to generate differentiated tissues and structures, both in vivo and in vitro. Additionally, the cells can be expanded and cultured to produce hormones and to provide conditioned culture media for supporting the growth and expansion of other cell populations. In another aspect, the present invention provides a lipo-derived lattice substantially devoid of cells, which includes extracellular matrix material from adipose tissue. The lattice can be used as a substrate to facilitate the growth and differentiation of cells, whether in vivo or in vitro, into anlagen or even mature tissues or structures.
Owner:UNIVERSITY OF PITTSBURGH +1
Increased isoprene production using the archaeal lower mevalonate pathway
Owner:THE GOODYEAR TIRE & RUBBER CO
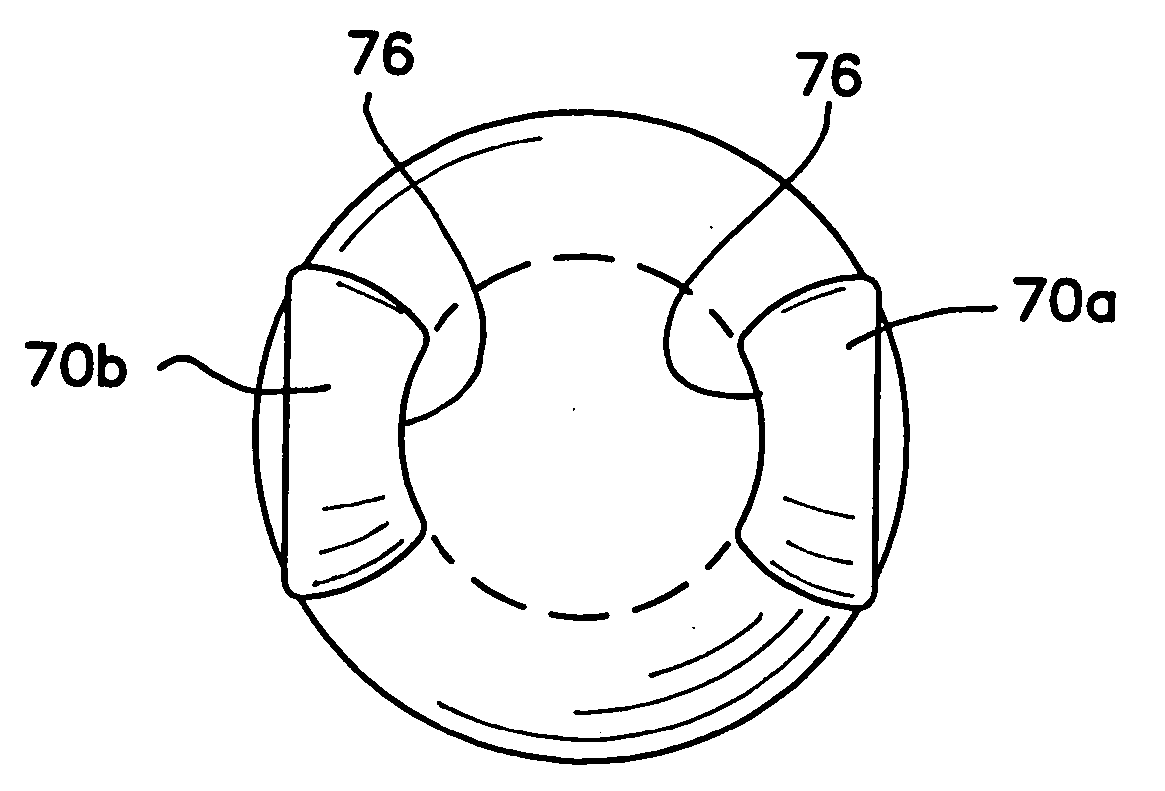
Devices and methods for improving vision
InactiveUS20050080484A1Improve eyesightCorrect and improve visionEye implantsEye surgeryEpitheliumLens plate
A corneal appliance that is placed over an eye has a lens body and epithelial cells secured over the lens body. The epithelial cells of the appliance may be derived from cultured cells, including stem cells, such as limbal stem cells, or epithelial cell lines, or may include at least a portion of the epithelium of the eye on which the appliance is placed. The corneal appliance may have a cellular attachment element between the lens body and the epithelial cells to facilitate attachment of the epithelial cells over the lens body. The corneal appliance is intended to be used on a deepithelialized eye, which may be an eye that has had the epithelium fully or partially removed. The corneal appliance may be used to improve vision. Methods of producing the corneal appliance and of improving vision are also disclosed.
Owner:FORSIGHT LABS
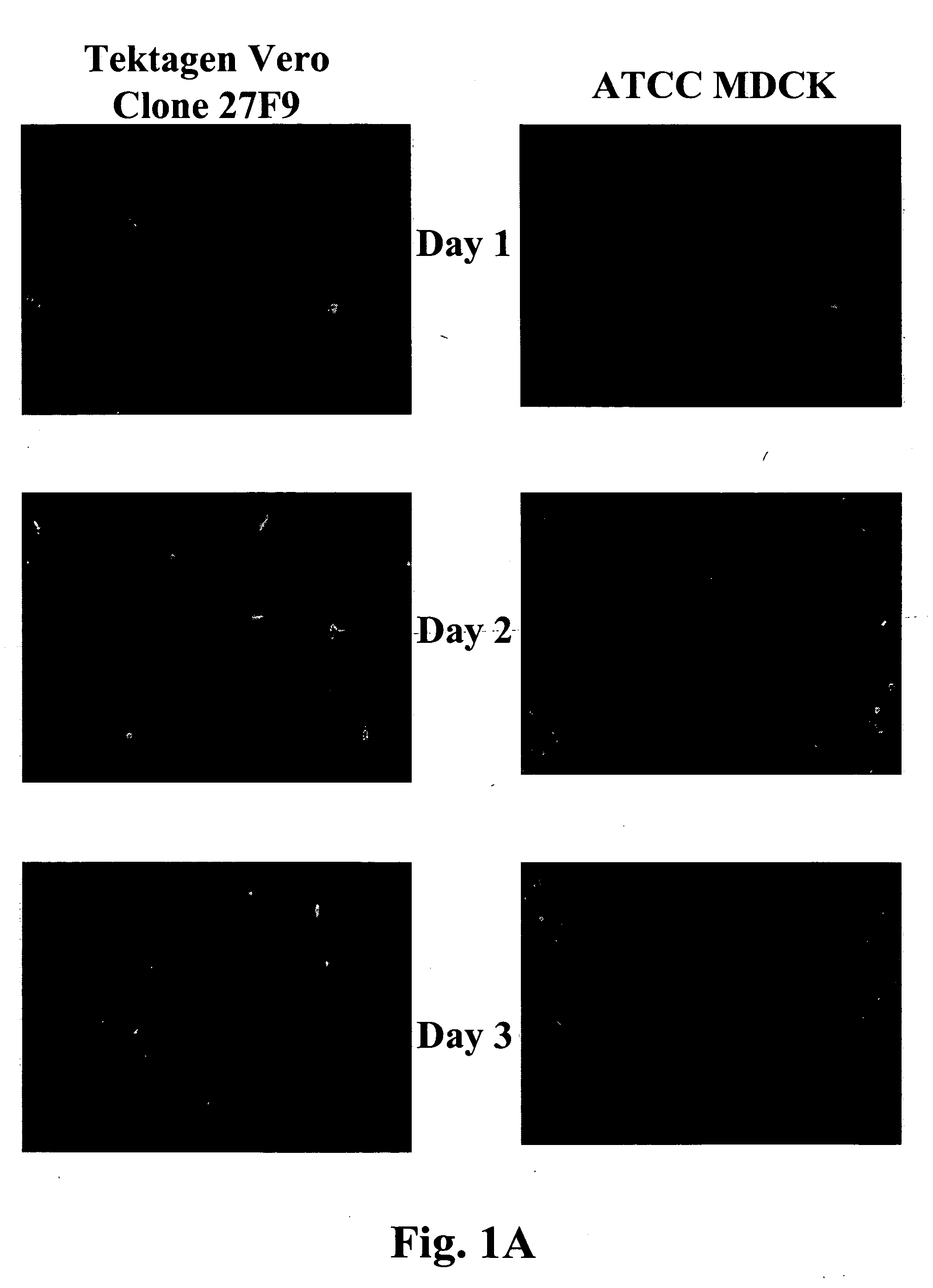
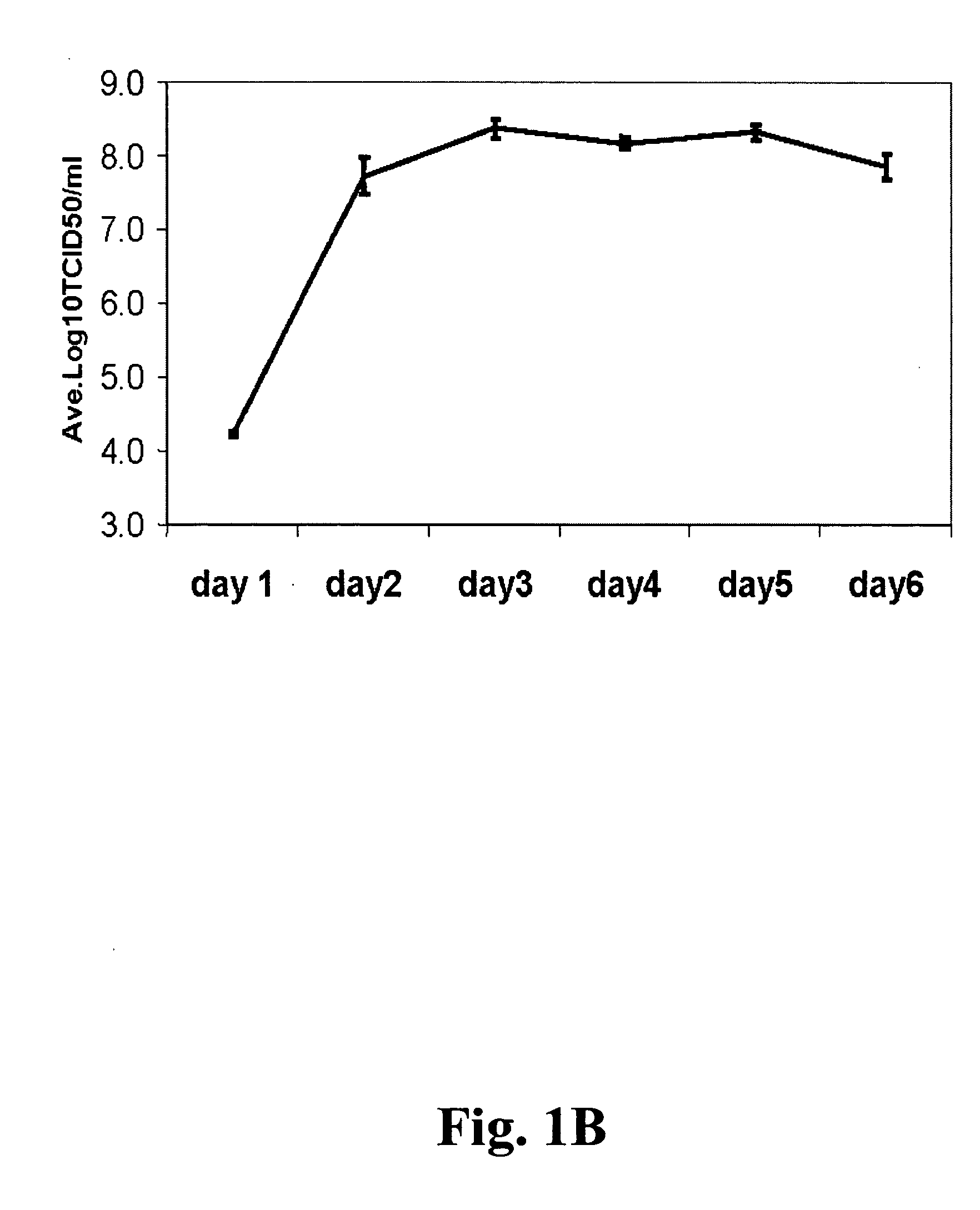
Non-tumorigenic MDCK cell line for propagating viruses
The present invention provides novel MDCK-derived adherent non-tumorigenic cell lines that can be grown in the presence or absence of serum. The cell lines of the present invention are useful for the production of vaccine material (e.g., viruses). More specifically, the cell lines of the present invention are useful for the production of influenza viruses in general and ca / ts influenza viruses in particular. The invention further provides methods and media formulations for the adaptation and cultivation of MDCK cells such that they remain non-tumorigenic. Additionally, the present invention provides methods for the production of vaccine material (e.g., influenza virus) in the novel cell lines of the invention.
Owner:MEDIMMUNE LLC
Methods for increasing definitive endoderm differentiation of pluripotent human embryonic stem cells with PI-3 kinase inhibitors
ActiveUS8187878B2Efficient productionEffectively lead to differentiationPeptide/protein ingredientsMetabolism disorderGerm layerFeeder Layer
Owner:UNIV OF GEORGIA RES FOUND INC +1
Multilayered cell culture apparatus
ActiveUS7745209B2Effective trainingProvide consistencyBioreactor/fermenter combinationsBiological substance pretreatmentsCell growthCultured cell
A multilayered cell culture apparatus for the culturing of cells is disclosed. The cell culture apparatus is defined as an integral structure having a plurality of cell culture chambers in combination with tracheal space(s). The body of the apparatus has imparted therein gas permeable membranes in combination with tracheal spaces that will allow the free flow of gases between the cell culture chambers and the external environment. The flask body. also includes an aperture that will allow access to the cell growth chambers by means of a needle or cannula. The size of the apparatus, and location of an optional neck and cap section, allows for its manipulation by standard automated assay equipment, further making the apparatus ideal for high throughput applications.
Owner:CORNING INC
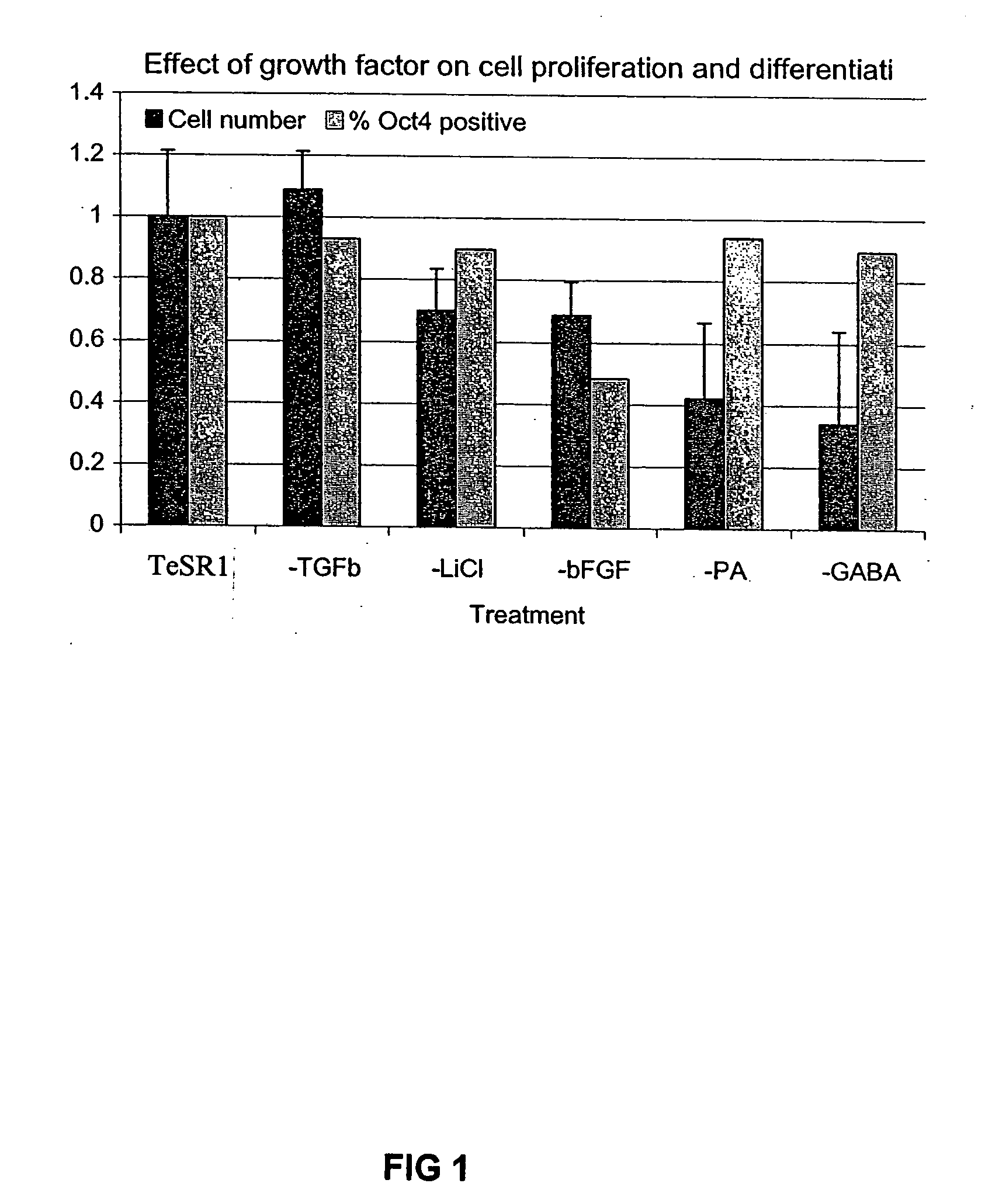
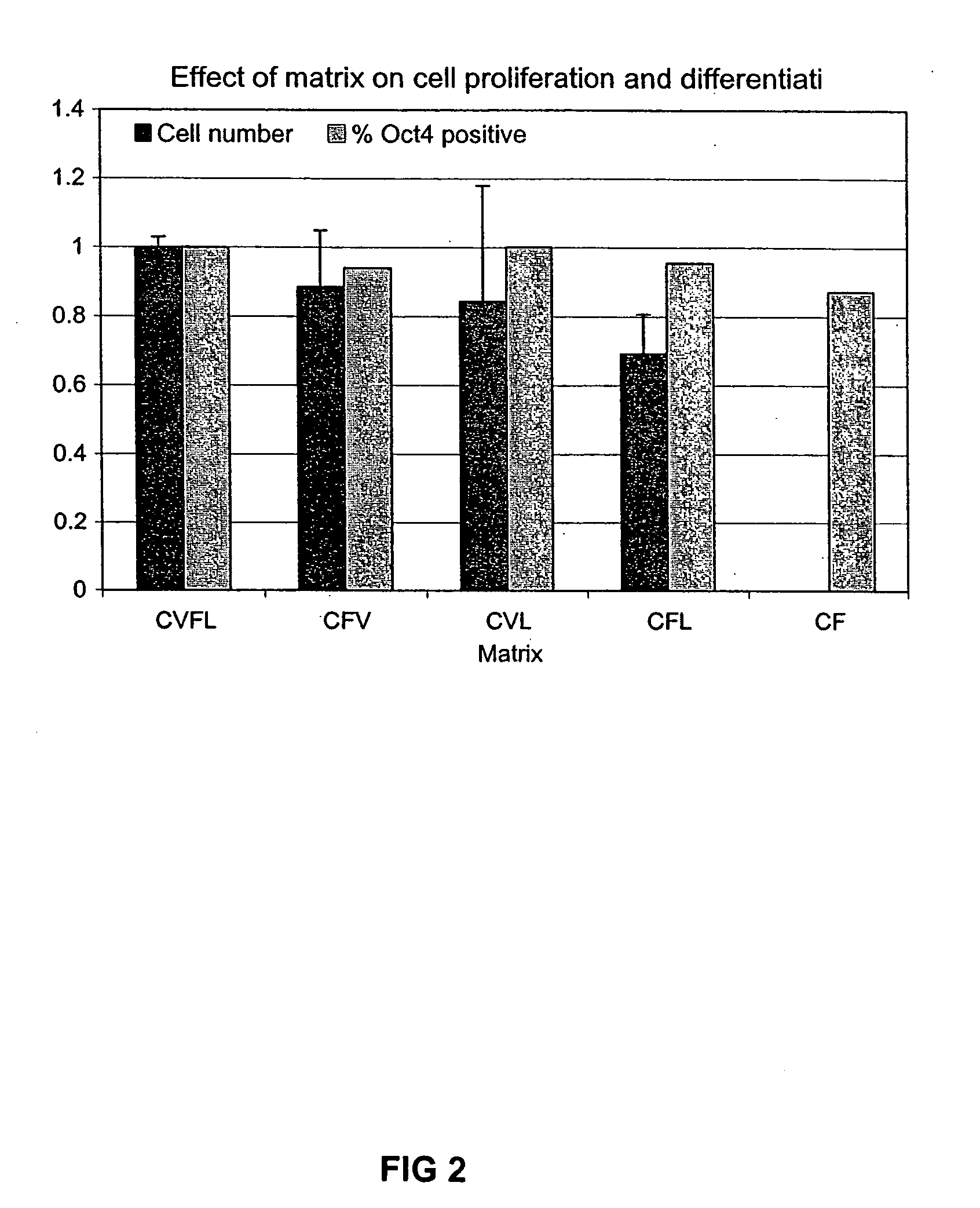
Culturing human embryonic stem cells
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor are used in a medium with gamma amino butyric acid, pipecholic acid, lithium and lipids, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium. A humanized matrix of human proteins can be used as a basement matrix to culture the cells. New lines of human embryonic stem cells made using these culture conditions, the medium and the matrix, will never have been exposed to animal cells, animal products, feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Bioreactor for growing cell or tissue cultures
ActiveUS20060019388A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMetaboliteCultured cell
The invention relates to a bioreactor comprising a chamber for containing cells or tissue cultures within a culture medium. The bioreactor also comprises a detector capable of detecting a change in one or more metabolites associated with growth of the cell or tissue cultures within the chamber and a chamber drive capable of rotating the chamber at a first speed about a first axis and a second speed about a second axis, the second axis being disposed at an angle relative to the first axis. In use, the magnitude of the first speed and the second speed are independently variable of each other.
Owner:SINGAPORE POLYTECHNIC +1
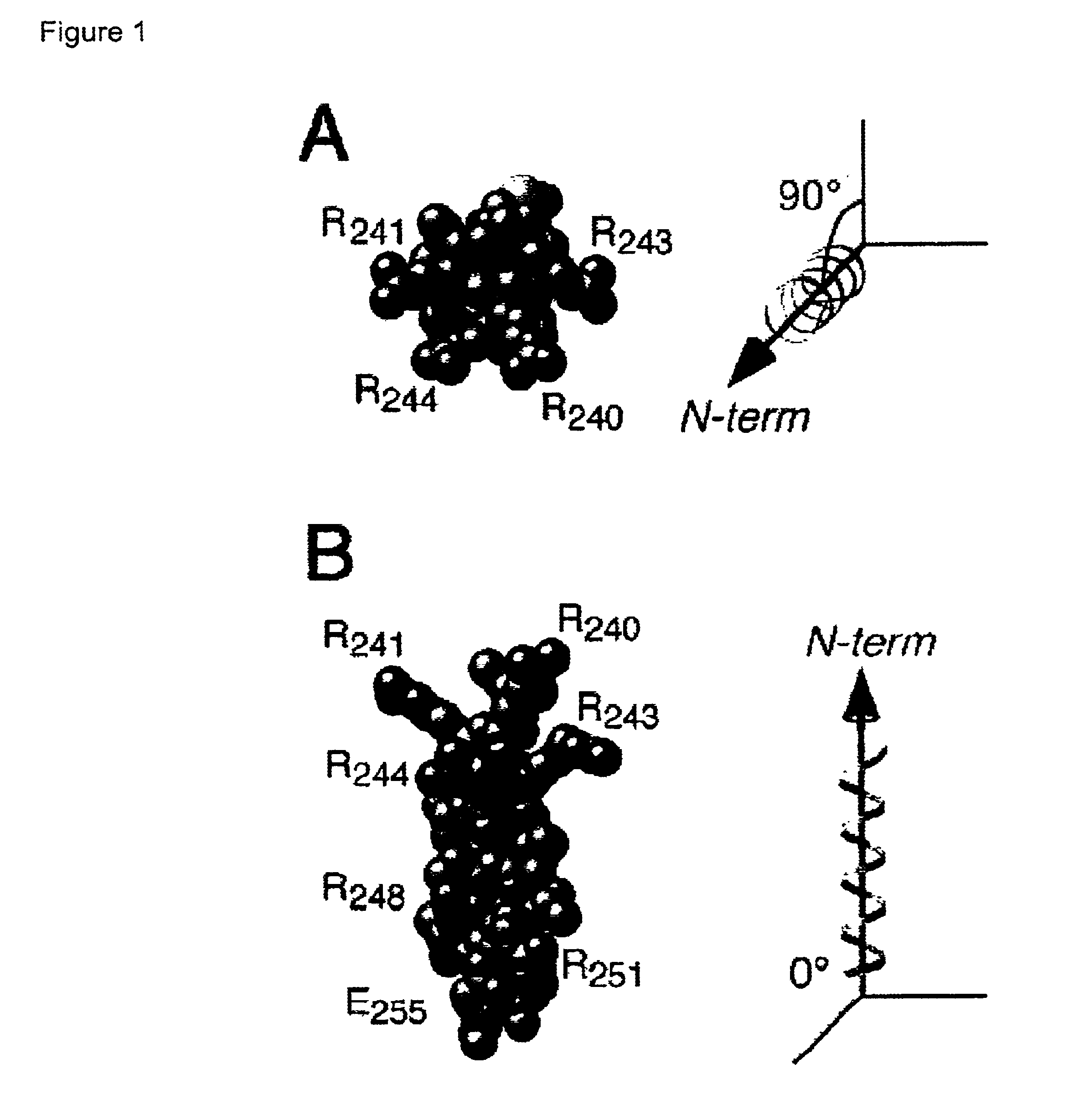
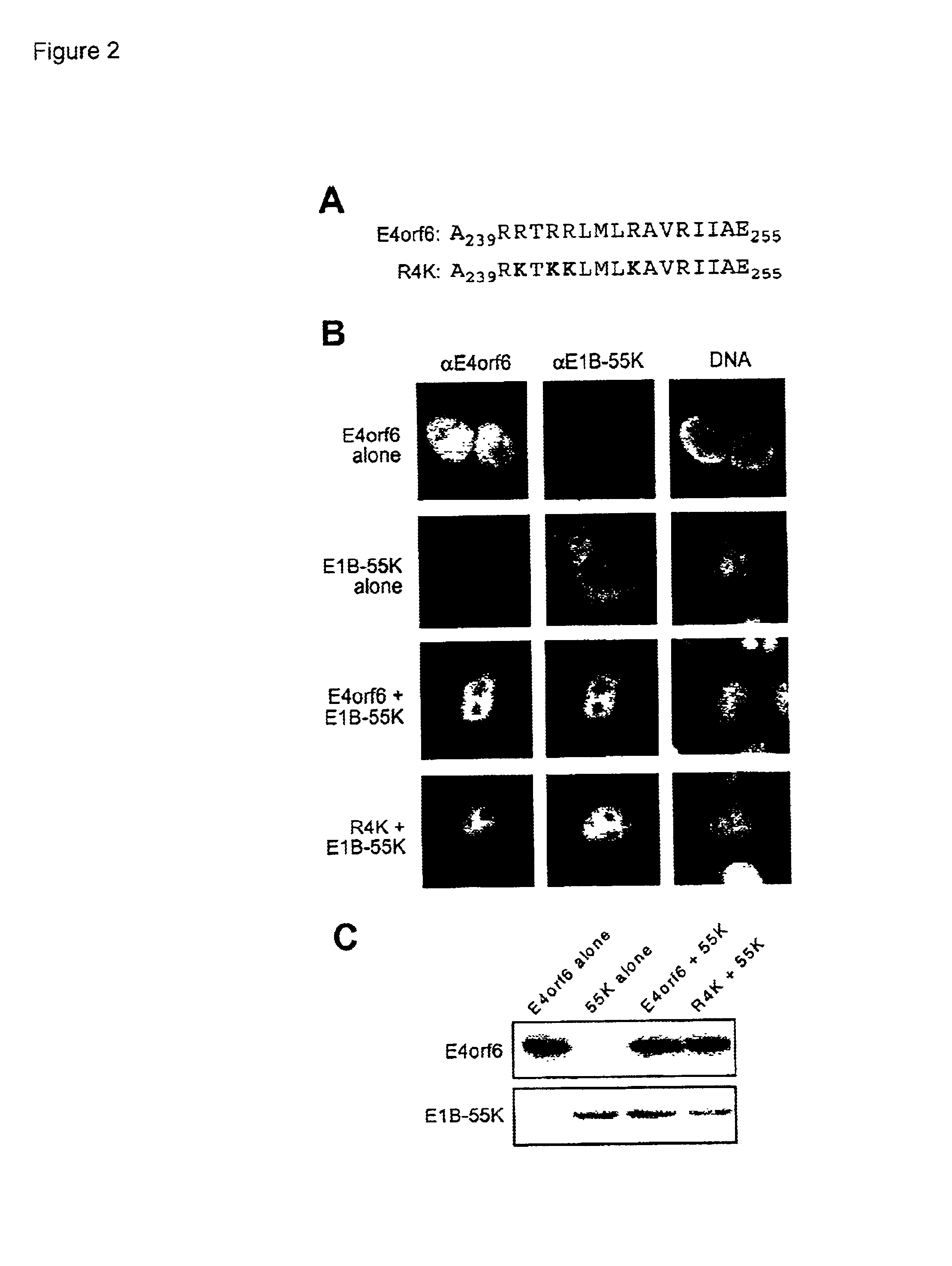
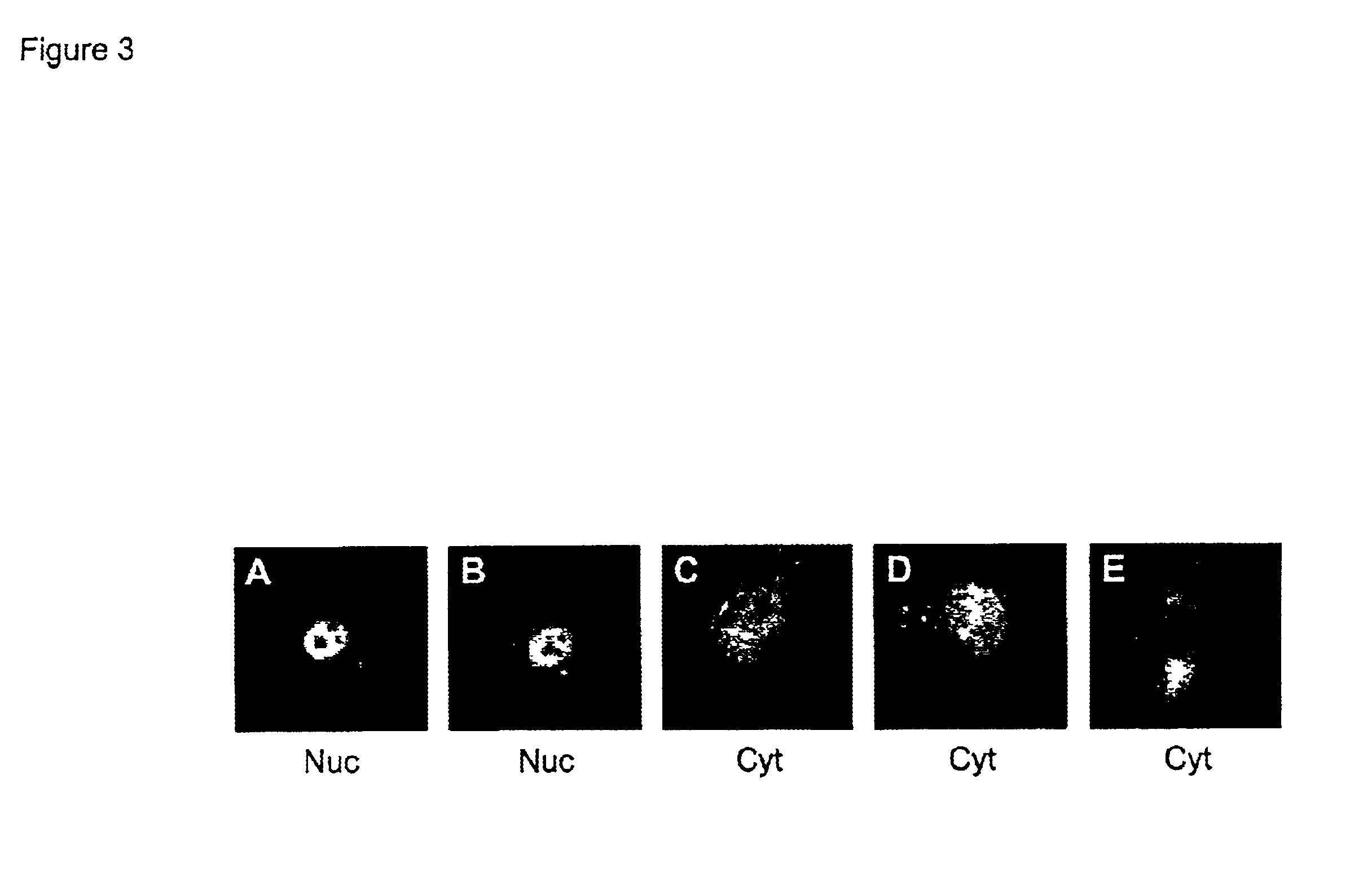
Adenovirus E4 protein variants for virus production
A method of packaging a recombinant viral vector is carried out by: (a) providing a packaging cell, the packaging cell containing and expressing a nucleic acid encoding a mutant adenovirus E4orf6 protein, the E4orf6 protein containing at least one mutation that renders the protein non-toxic to the host cell; (b) transfecting or infecting the packaging cell with a nucleic acid that encodes a recombinant viral vector (e.g., an adenovirus vector or an adeno-associated virus vector), where the vector lacks a functional gene encoding E4orf6 protein; (c) culturing the transfected cells; and then (d) collecting packaged recombinant viral vector from the cultured cells. Nucleic acids, vectors and packaging cells used for carrying out the methods, as well as proteins utilized in the methods, are also described.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Mammalian cell culture processes for protein production
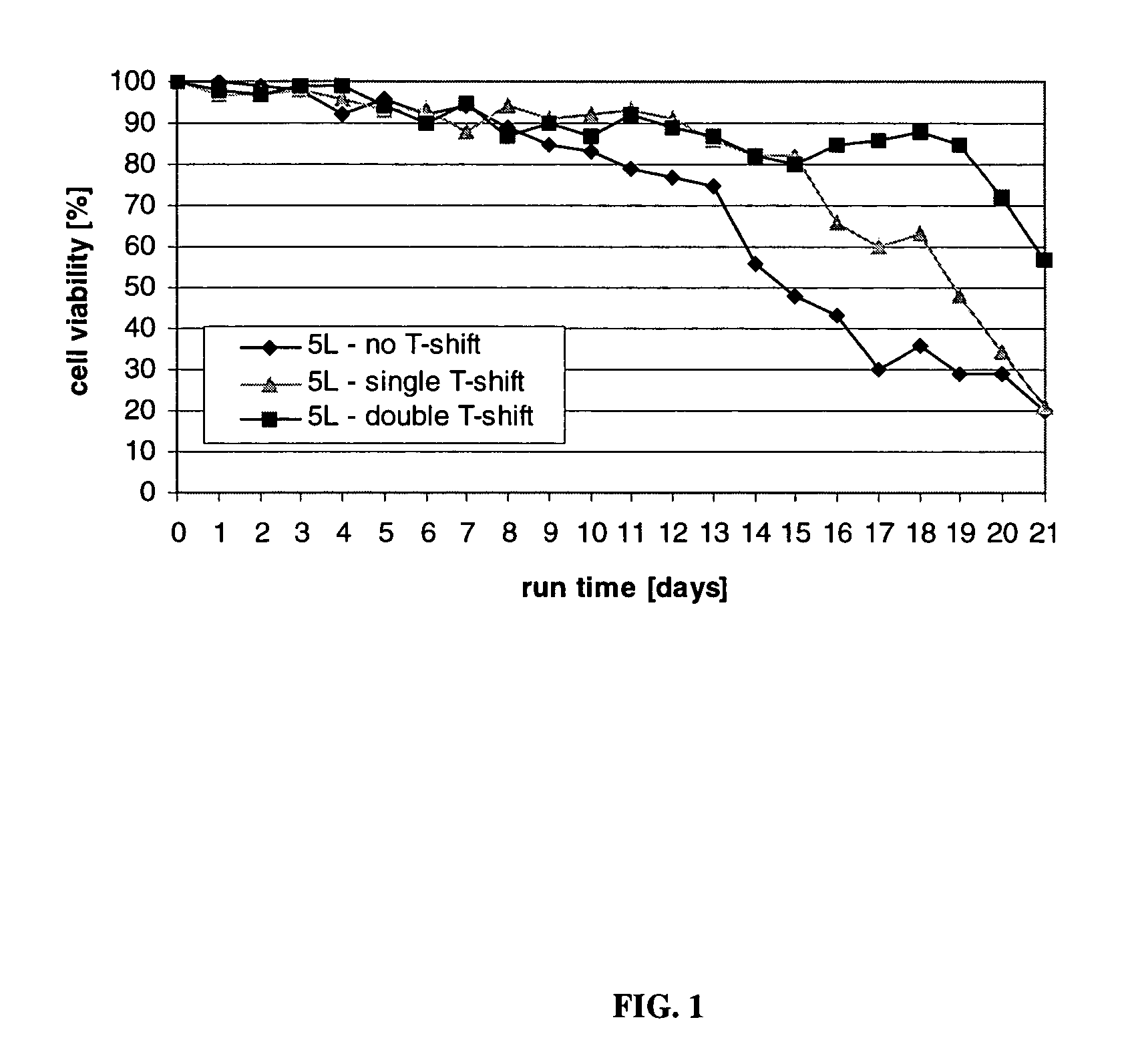
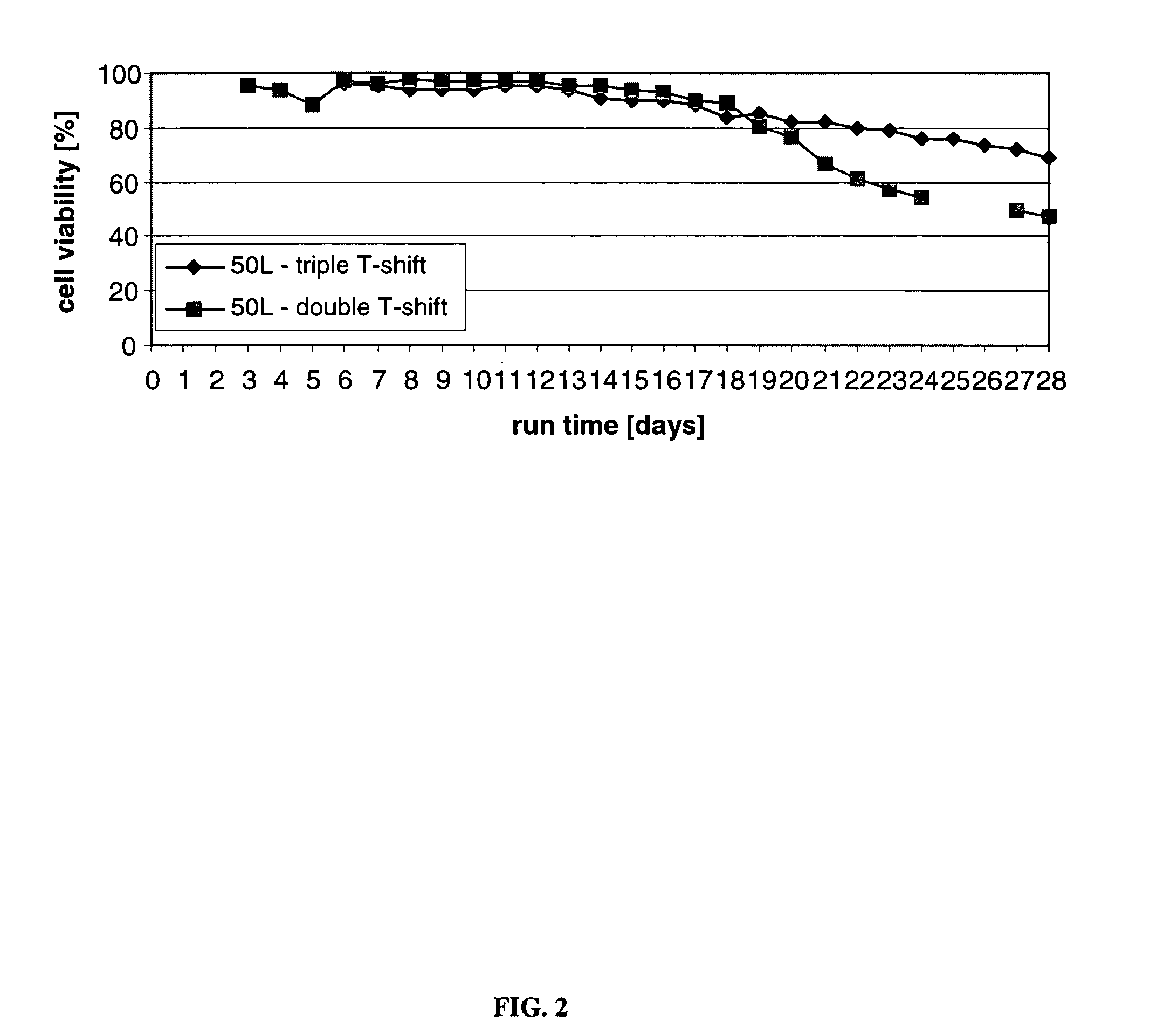
ActiveUS7541164B2Enhance cell viabilityPeptide/protein ingredientsImmunoglobulinsGrowth phaseTwo temperature
Owner:BRISTOL MYERS SQUIBB CO
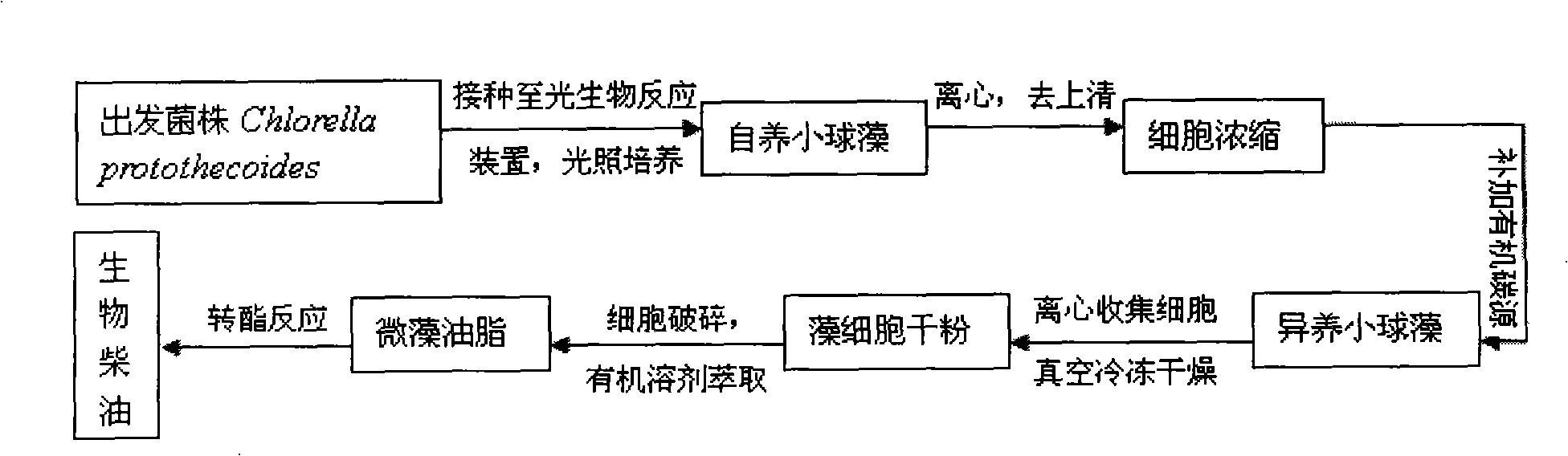
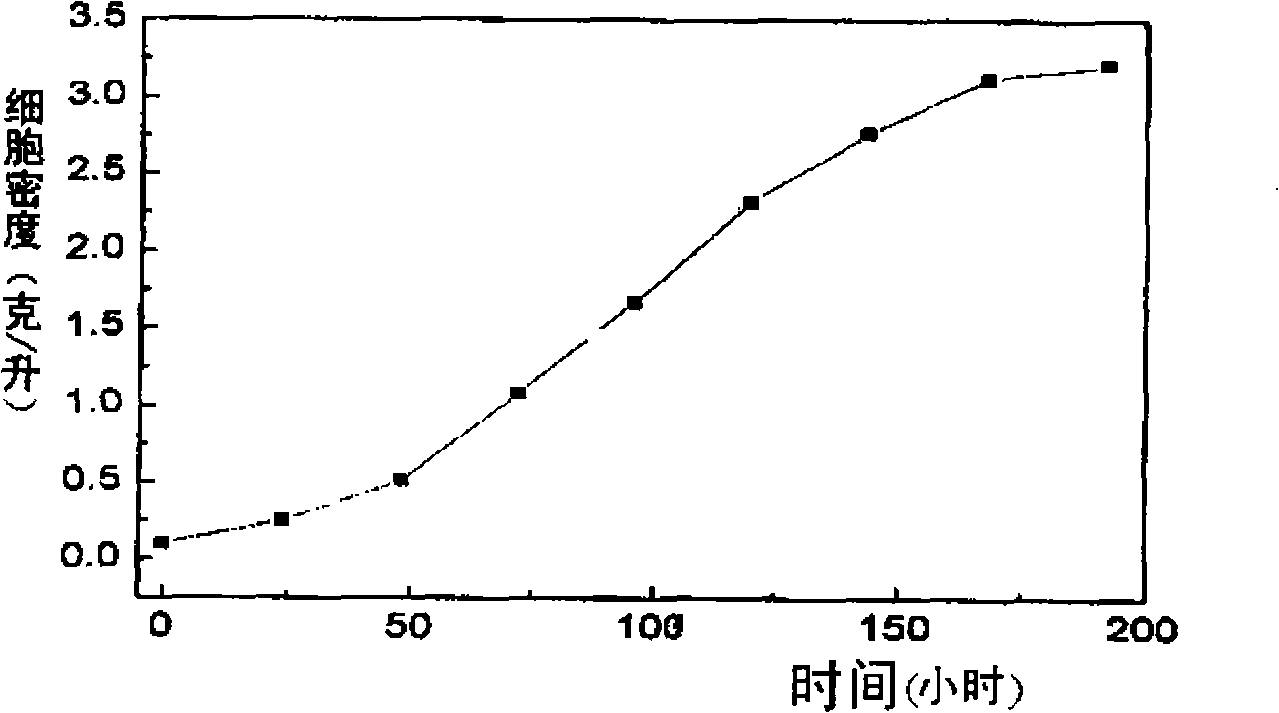
Method for producing biodiesel by autotrophic culture and heterotrophic culture of chlorella
InactiveCN101280328AReduce consumptionReduce releaseFatty acid esterificationUnicellular algaeBiodiesel feedstockOil and grease
The invention discloses a method for producing biological diesel oil by two steps of culture of chlorellas, namely, autotrophy and heterotrophy, which belongs to the renewable biological energy field. Concentrated autotrophic algae is put into a fermentation tank to perform heterotrophic growth from the processes of autotrophic culture, cell concentration and fermentation pollution controlling of the chlorellas, to ensure the chlorellas to be synthesized into neutral fat. Effective feeding strategy is established to ensure the fat to be synthesized into optimal after the optimization of the fermentation conditions and the process control. The biomass can reach 108g.L-1, and the grease content can reach 52 percent of cell dry weight. After the culturing is finished, biological diesel oil can be prepared after extracting algal oil and transesterification reaction. The cell concentration technology adopted by the invention can effectively avoid the problem of light restriction during the high density culture process Not only is the carbon dioxide emission reduced, but also the organic carbon source consumption is reduced, thus the preparation cost of the biological diesel oil material is saved. The whole technical line is environmental friendly, high efficient, and can meet the industrial application requirements of the microalgae biological diesel oil.
Owner:TSINGHUA UNIV
Method for preparing cell cultures from biological specimens for chemotherapeutic and other assays
InactiveUS6887680B2Promote growthMicrobiological testing/measurementMicroorganism separationParticulatesAnticarcinogen
An improved system for screening a multiple of candidate therapeutic or chemotherapeutic agents for efficacy as to a specific patient, in which a tissue sample from the patient is harvested, cultured and separately exposed to a plurality of treatments and / or therapeutic agents for the purpose of objectively identifying the best treatment or agent for the particular patient. Specific method innovations such as tissue sample preparation techniques render this method practically as well as theoretically useful. One particularly important tissue sample preparation technique is the initial preparation of cohesive multicellular particulates of the tissue sample, rather than enzymatically dissociated cell suspensions or preparations, for initial tissue culture monolayer preparation. With respect to the culturing of malignant cells, for example, it is believed (without any intention of being bound by the theory) that by maintaining the malignant cells within a multicellular particulate of the originating tissue, growth of the malignant cells themselves is facilitated versus the overgrowth of fibroblasts or other cells which tends to occur when suspended tumor cells are grown in culture. Practical monolayers of cells may thus be formed to enable meaningful screening of a plurality of treatments and / or agents. Growth of cells is monitored to ascertain the time to initiate the assay and to determine the growth rate of the cultured cells; sequence and timing of drug addition is also monitored and optimized. By subjecting uniform samples of cells to a wide variety of active agents (and concentrations thereof), the most promising agent and concentration for treatment of a particular patient can be determined. For assays concerning cancer treatment, a two-stage evaluation is contemplated in which both acute cytotoxic and longer term inhibitory effect of a given anti-cancer agent are investigated.
Owner:PRECISION THERAPEUTICS
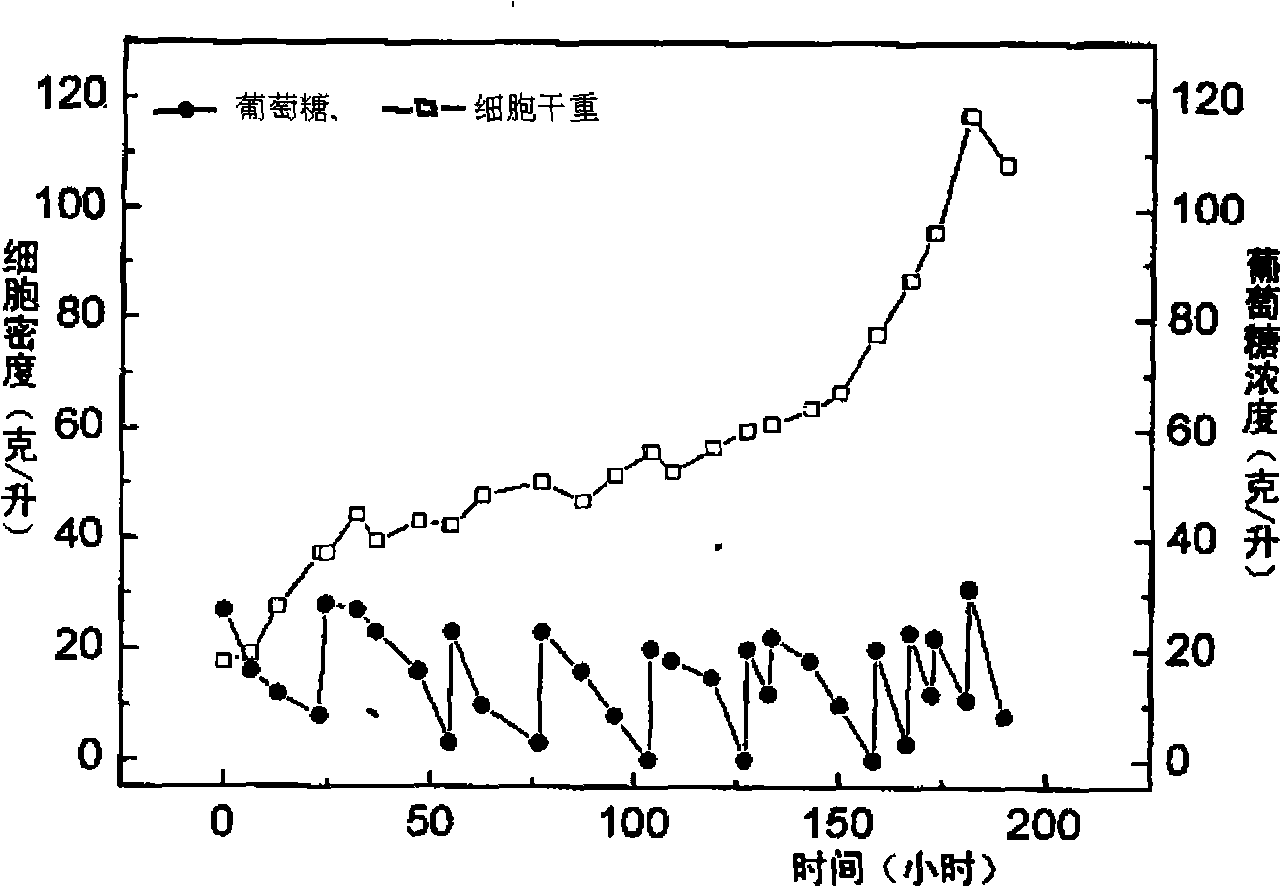
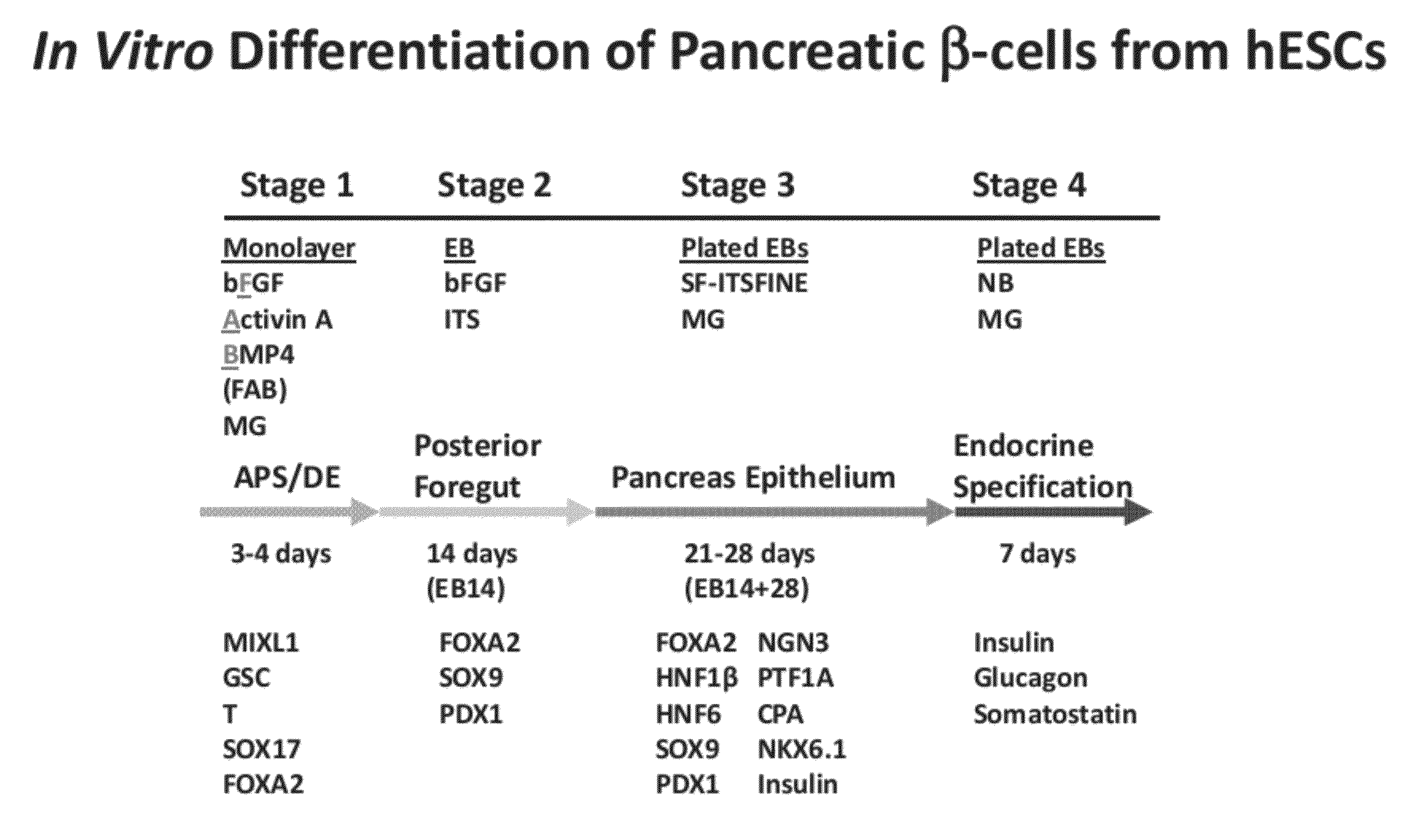
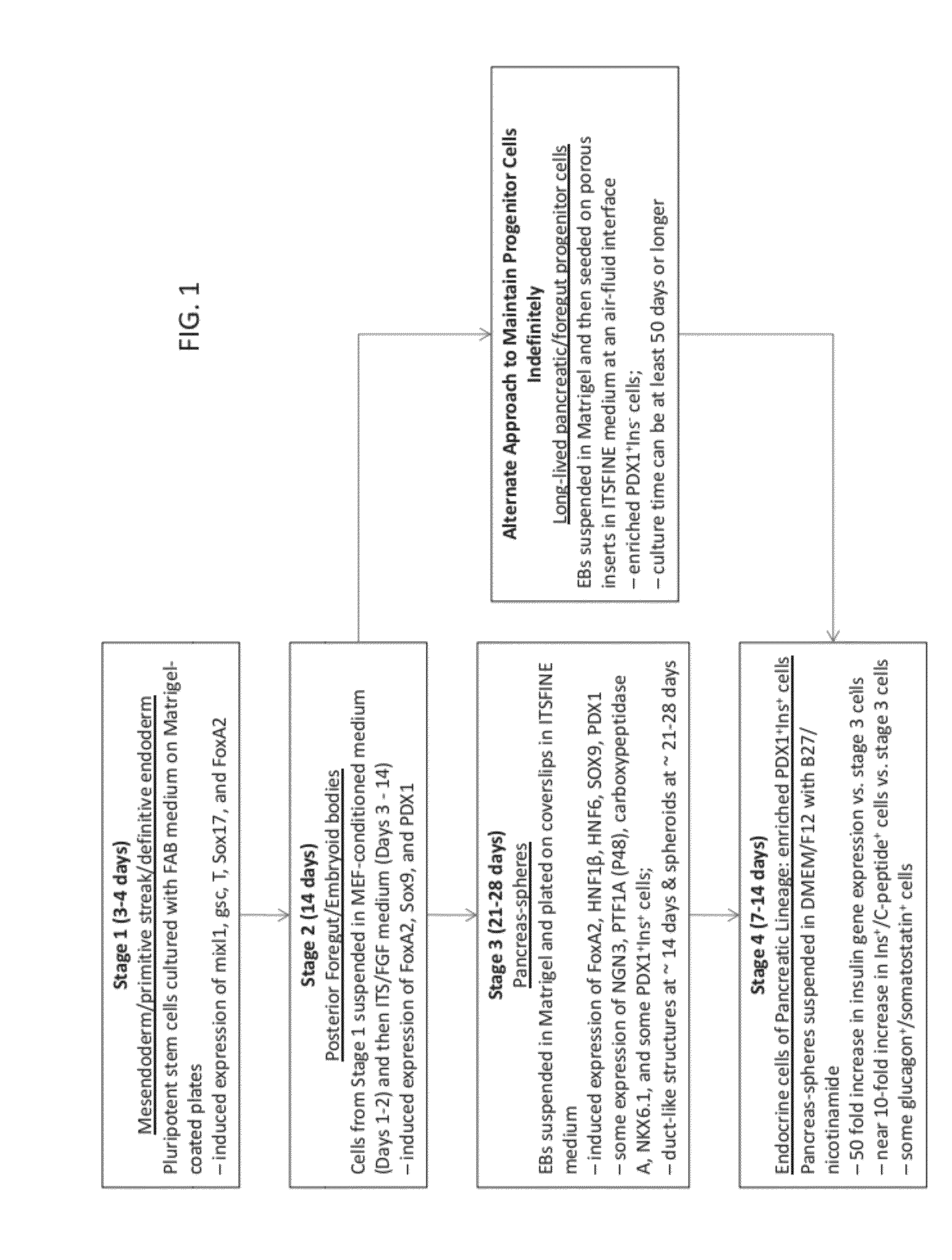
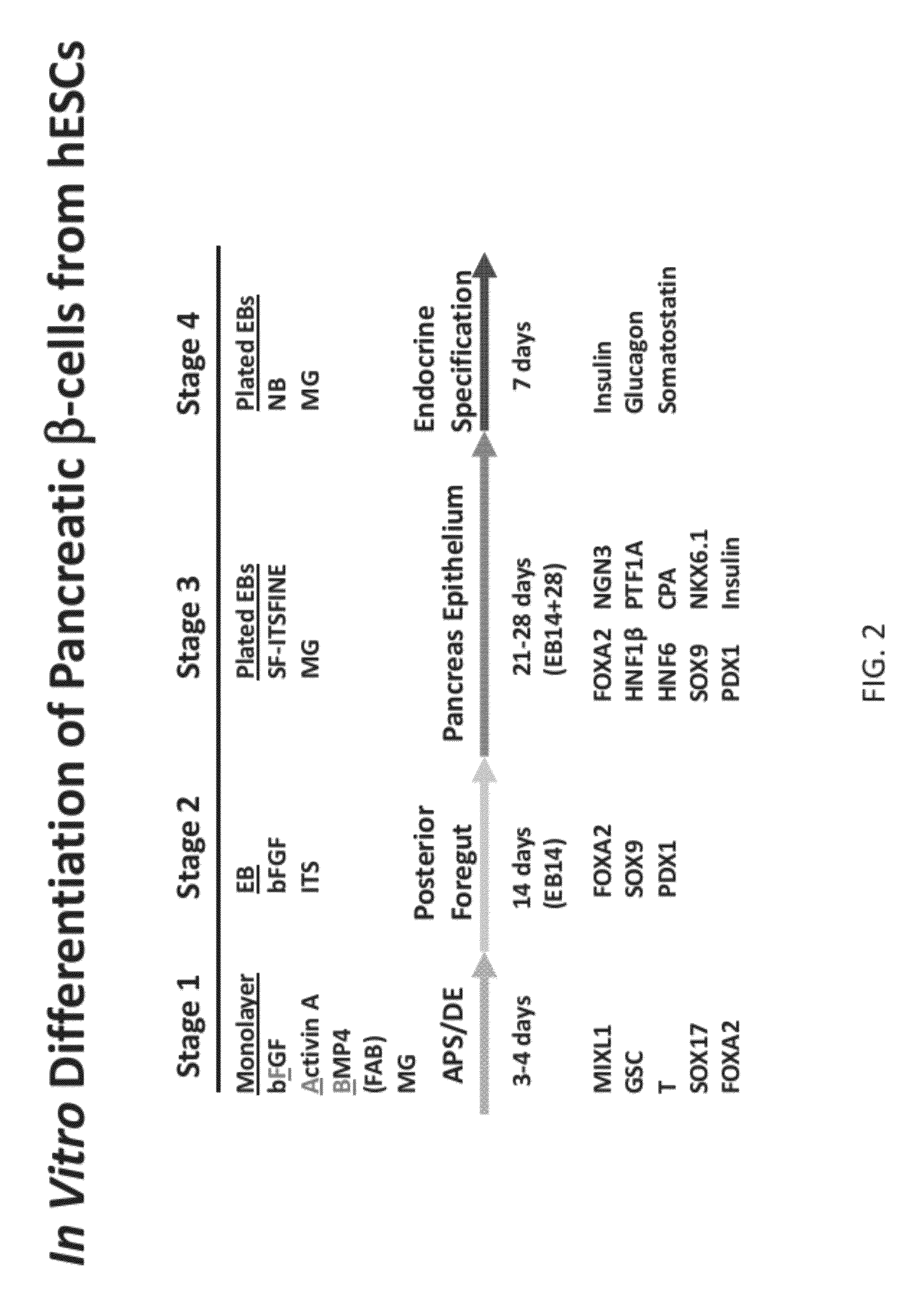
Methods and devices for differentiating pluripotent stem cells into cells of the pancreatic lineage
Methods and devices for culturing human pluripotent stem cells to produce cells of the pancreatic lineage are disclosed. The methods include steps of culturing the stem cells under conditions that induce the expression of mesendoderm / primitive streak and definitive endoderm markers in a chemically defined medium including an effective amount of i) fibroblast growth factor, ii) Activin A, and iii) bone morphogenetic protein. The methods further include the steps of culturing cells under conditions favoring the formation of at least one of intact embryoid bodies and pancreatic progenitor PDX1+ Ins− cells.
Owner:WISCONSIN ALUMNI RES FOUND
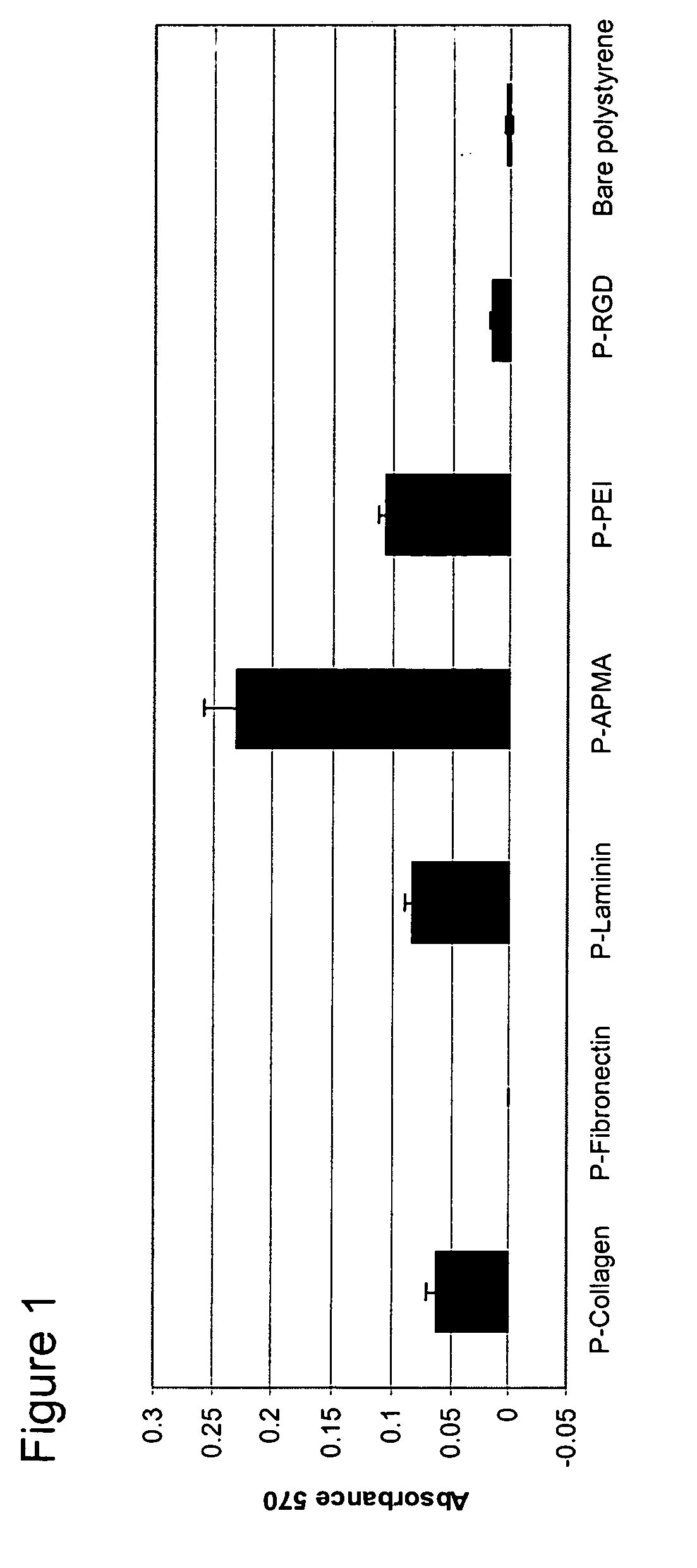
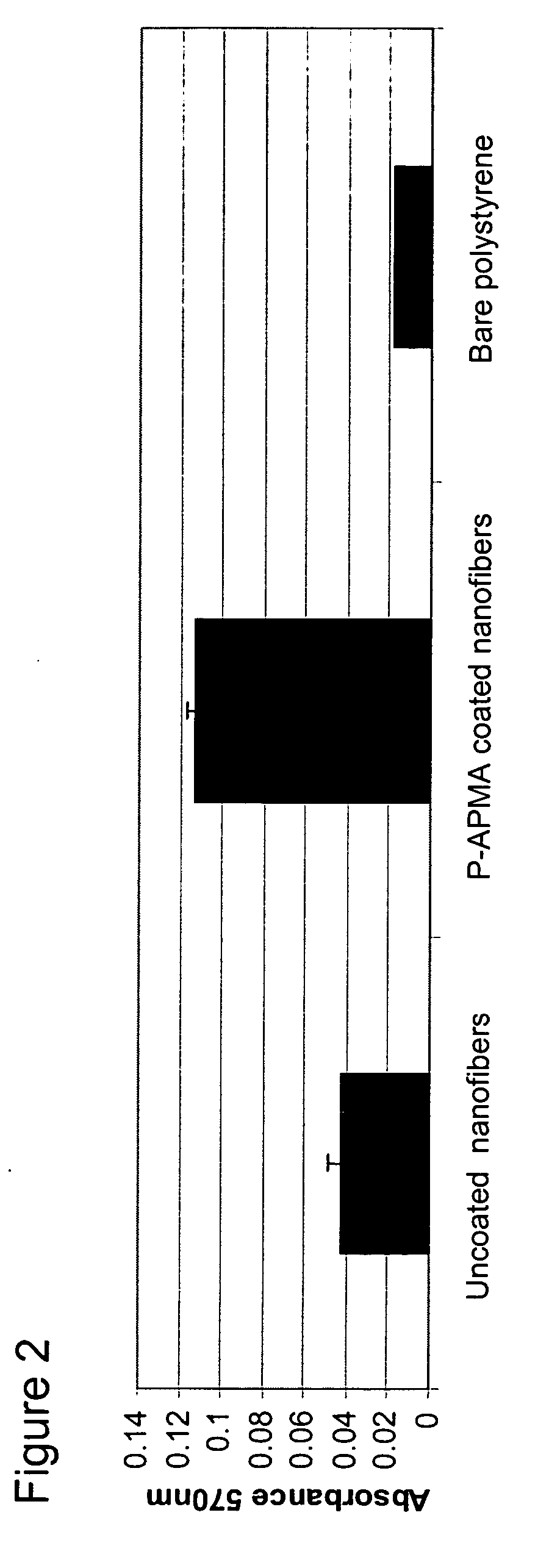
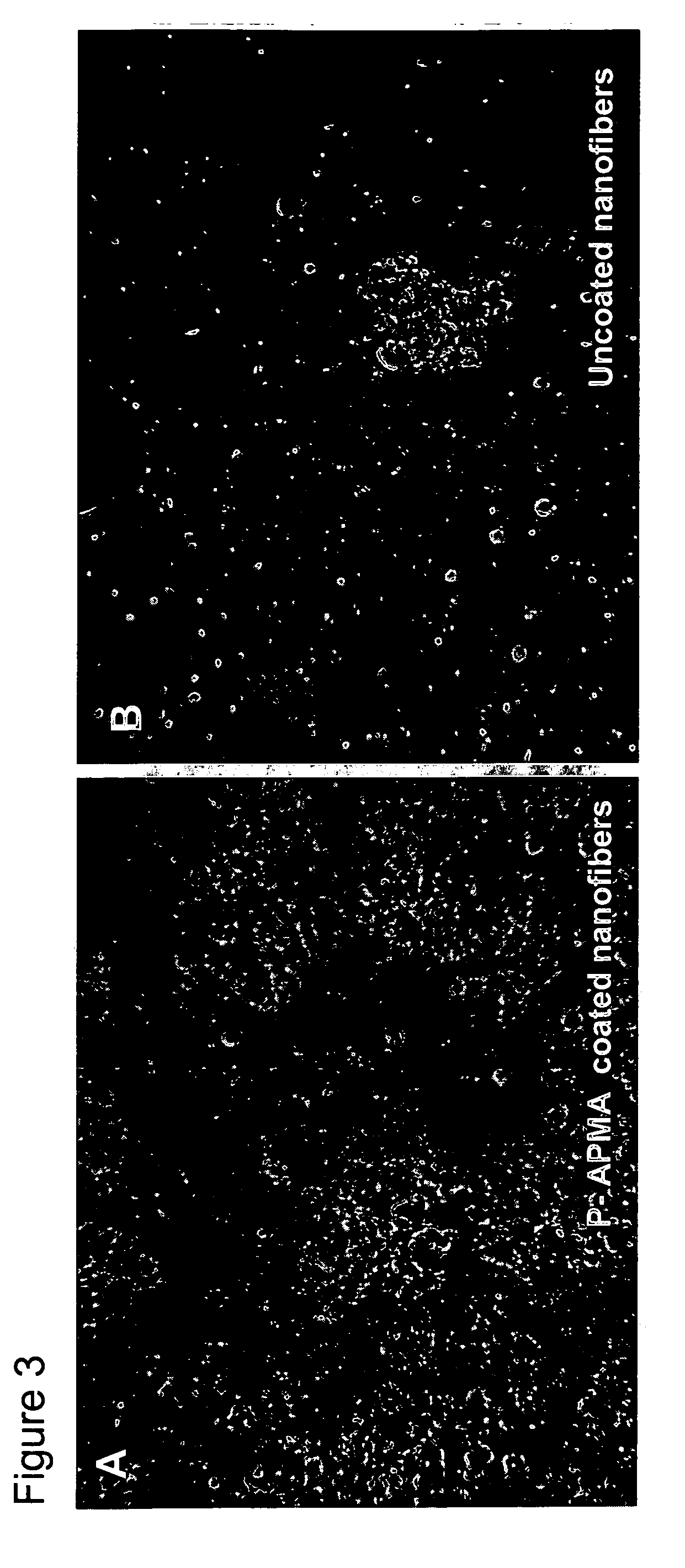
Polymer coated nanofibrillar structures and methods for cell maintenance and differentiation
InactiveUS20070082393A1Improve adhesionReduced metabolic activityBioreactor/fermenter combinationsBiological substance pretreatmentsCell adhesionPolymer chemistry
The invention provides cell-adherent polymeric coatings for articles having a nanofibrillar structure. The coatings include a synthetic, non-biodegradable polymer having at least one pendent amine group, wherein the polymer is covalently immobilized on the article via latent reactive groups. The invention also provides methods for the long term culturing of cells using the polymer coated nanofibrillar structures. The polymer coated nanofibrillar structures of the invention have been found to be particularly useful for the growth and differentiation of cells, including neural precursors.
Owner:LODHI MUHAMMAD +4
Methods for immortalizing cells
InactiveUS7399633B2Antibody mimetics/scaffoldsGenetically modified cellsCell Differentiation processCultured cell
The present invention provides methods for immortalizing precursor cells that are non-terminally differentiated cells such as stem cells, said methods comprising culturing the precursor cells in the presence of a Notch agonist and one or more growth factors that support the proliferation but not differentiation of the non-terminally differentiated cells. The present invention further provides methods to induce the differentiation of immortalized cells, comprising growing the cells in the presence of a Notch agonist and at least one growth factor which supports the differentiation of the cell into a more specialized cell type. The immortalized and / or differentiated cells of the invention can be used to repopulate cell populations that have been diminished, for example as a result of infection or exposure to certain drugs. The invention further provides a cell culture comprising a population of non-terminally differentiated cells immortalized by the methods of the present invention and kits comprising reagents that promote the immortalization of precursor cells. The invention further provides methods for screening for Notch modulators and for identifying genes involved in processes of cellular differentiation.
Owner:FRED HUTCHINSON CANCER CENT
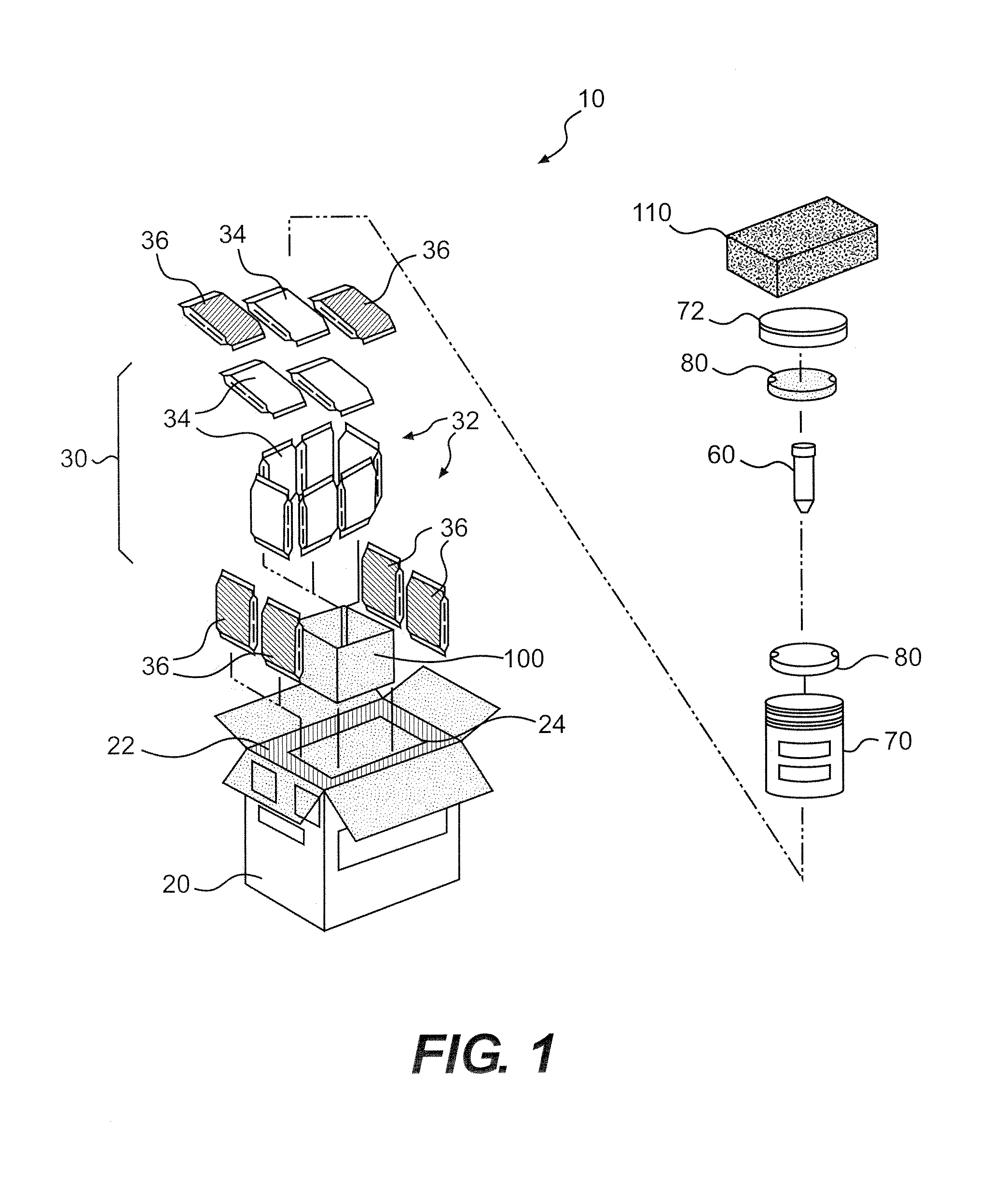
Thermally Insulated Transport Container For Cell-Based Products and Related Methods
ActiveUS20080276643A1Adequate cell viability of cellAdequate therapeutic propertyLighting and heating apparatusDead animal preservationEngineeringCultured cell
The present invention provides a temperature sensitive transport container (20) for packaging and shipping cell-based products such as cultured cells intended for transplantation. In particular, the present disclosure provides a thermally insulated transport container system (10) for storing and transporting cells for transplantation which is capable of maintaining the cells at a desirable temperature range for a sufficient period of time to ensure adequate cell viability and therapeutic properties.
Owner:GENZYME CORP
Conversion of prenyl derivatives to isoprene
InactiveUS20100113846A1HydrolasesHydrocarbon from oxygen organic compoundsPetrochemicalCultured cell
The present invention provides methods for producing derivatives from cultured cells. In addition, the present invention provides methods for conversion of prenyl deerivatives, obtained from biological or petrochemical sources, to isoprene by employing chemical or biological catalysts. The present invention also provides compositions that include the cultured cells or isoprene or prenyl derivatives produced there from.
Owner:THE GOODYEAR TIRE & RUBBER CO
Swellable (METH)acrylate surfaces for culturing cells in chemically defined media
InactiveUS20090191632A1Reduce Potential ContaminationExtended shelf lifeCell culture supports/coatingEmbryonic cellsSerum free mediaPolymer science
Synthetic surfaces capable of supporting culture of undifferentiated human embryonic stem cells in a chemically defined medium include a swellable (meth)acrylate layer and a peptide conjugated to the swellable (meth)acrylate layer. The swellable (meth)acrylate layer may be formed by polymerizing monomers in a composition that includes hydroxyethyl methacrylate, 2-carboxyehylacrylate, and tetra(ethylene glycol) dimethacrylate. The conjugated peptide may include an amino acid sequence of XaanProGlnValThrArgGlyAspValPheThrMetPro, where n is an integer from 0 to 3 and where Xaa is any amino acid. Further, disclosed herein is a swellable (meth)acrylate synthetic surface which can be sterilized by gamma irradiation.
Owner:GERON CORPORATION
Targeted methods of drug screening using co-culture methods
The present invention provides methods of screening for a molecule that inhibits the expression or activity of a protein encoded by a target gene which affects the fitness of a cell. The methods are based on a co-culture assay, and entail culturing together two cell populations, each of which is a population of identical cells, of the same species that differs substantially only in the expression or activity of the gene to be targeted or its encoded protein and the presence or absence of a reporter gene. The screen can be applied to cultured cells, unicellular and multicellular organisms. Manipulating the expression or activity of the target gene sensitizes the host to a molecule which inhibits the target gene or its encoded protein such that the cell or organism comprising the manipulated target gene grows at a different rate from the cell or organism comprising the unmanipulated gene in response to exposure to the molecule. The methods of the invention can be used for identifying drugs, proteins or any other molecules that inhibit the function of proteins encoded by target genes.
Owner:ROSETTA INPHARMATICS LLC
Devices and methods to immobilize analytes of interest
InactiveUS20050254995A1Rapid and efficient immobilizationSimple processAnalysis using chemical indicatorsComponent separationTarget analysisDiffusion
The invention provides devices and methods using assemblies of shaped inserts coated with diverse binder materials optionally positioned inside conformal housings. The devices are capable of immobilizing target analytes of interest (e.g., specific or groups of biomolecules) and are optionally usable as single units, linear strips or array formats. The coatings on the inserts and, optionally also on the inside walls of the housings, create variable gaps with narrow fluid paths resulting in enhanced diffusion and absorption of target analytes from transiting fluid samples while exhibiting minimal resistance to flow or back pressure commonly seen in conventional plug-type packings. The devices are intended for sample preparations requiring: filtering, enriching, separating, purifying of target analytes by selective absorption / elution as part of desalting, buffer exchange and / or enrichment applications in analyses by mass spectrometry and / or electrophoresis; purifying biomolecules; culturing cells; analytical separation processes, and general chemical, biological and / or biochemical separations in manual or automated systems.
Owner:HARVARD APPARATUS
Apparatus and method for preparing and culturing cells
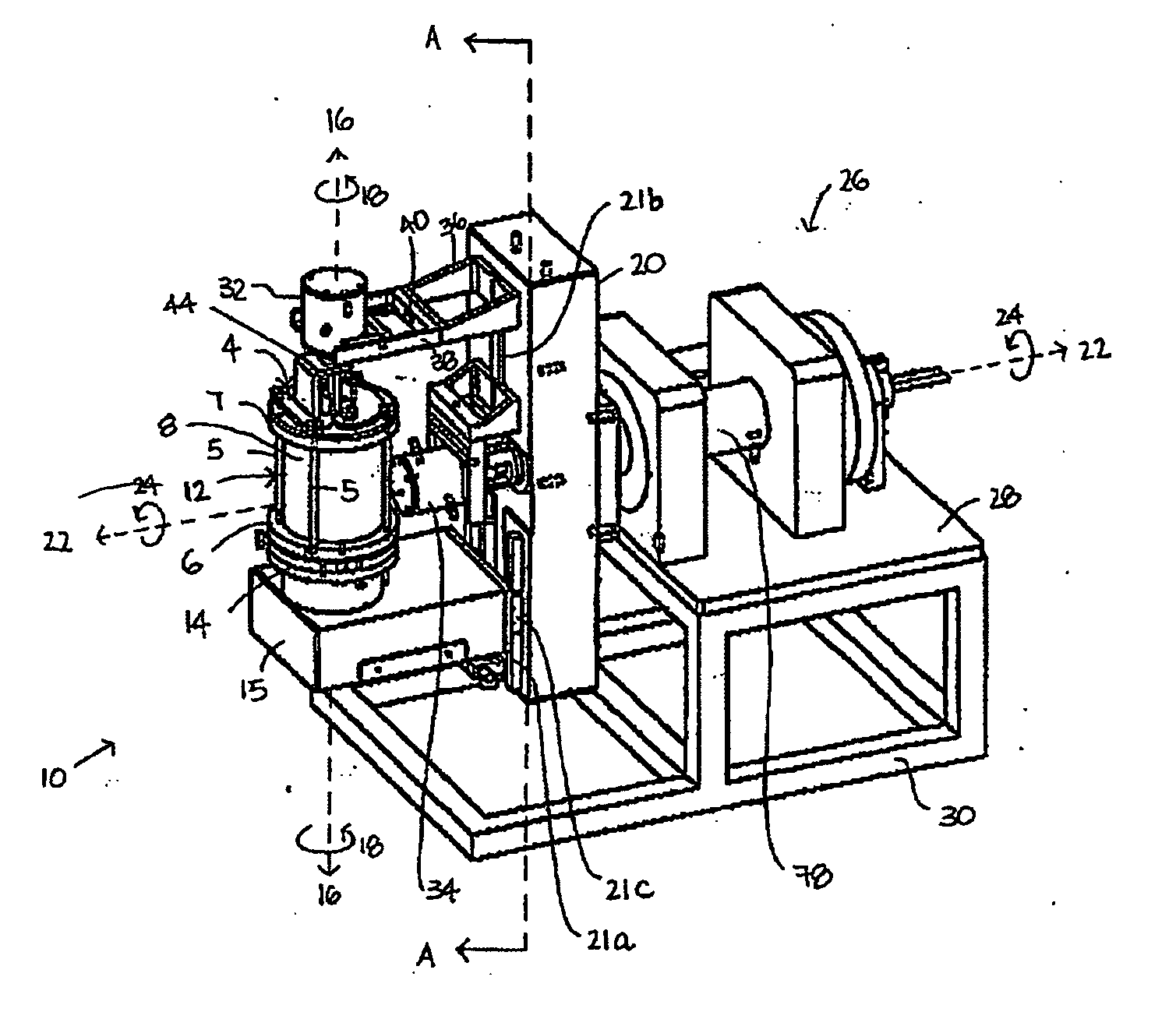
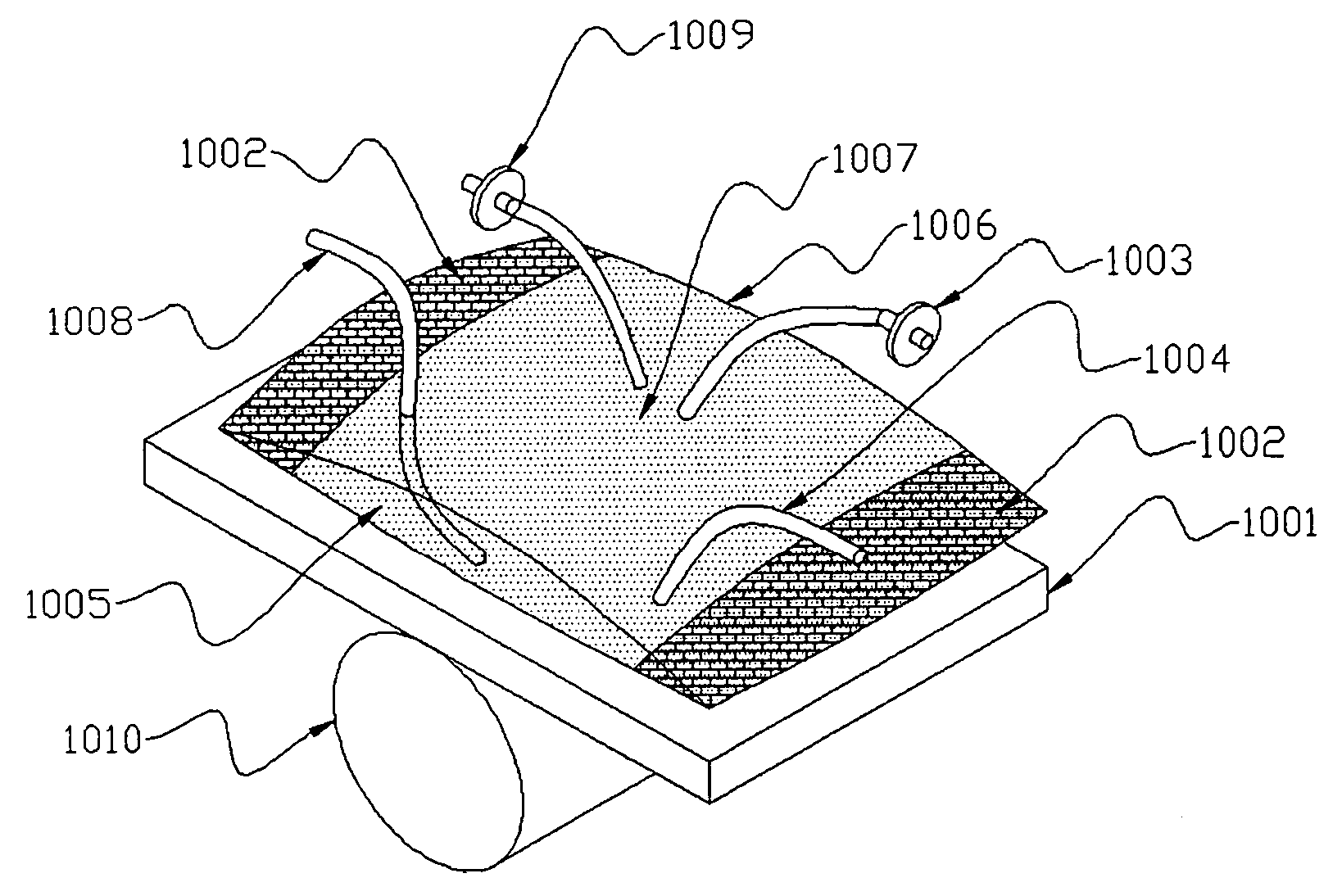
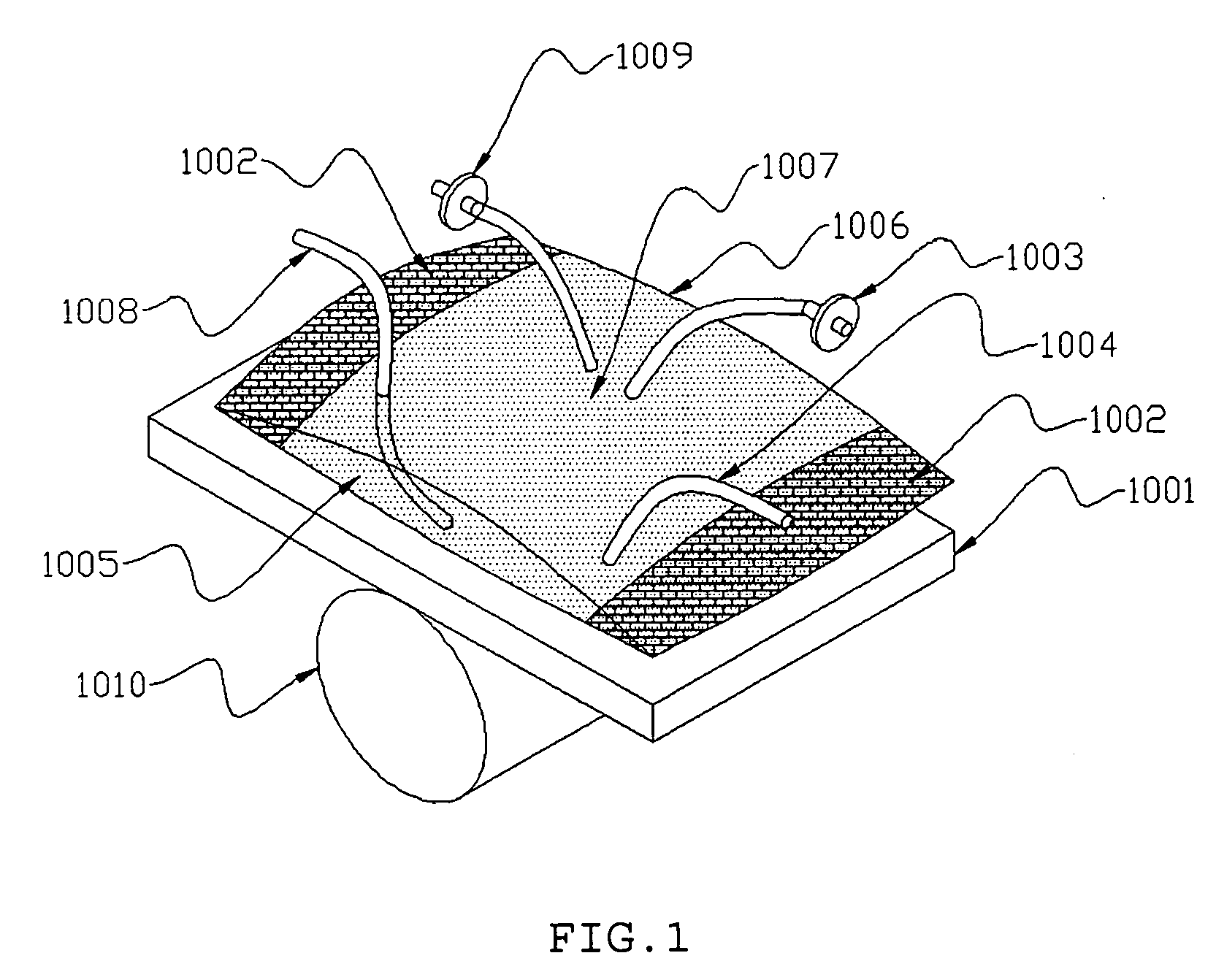
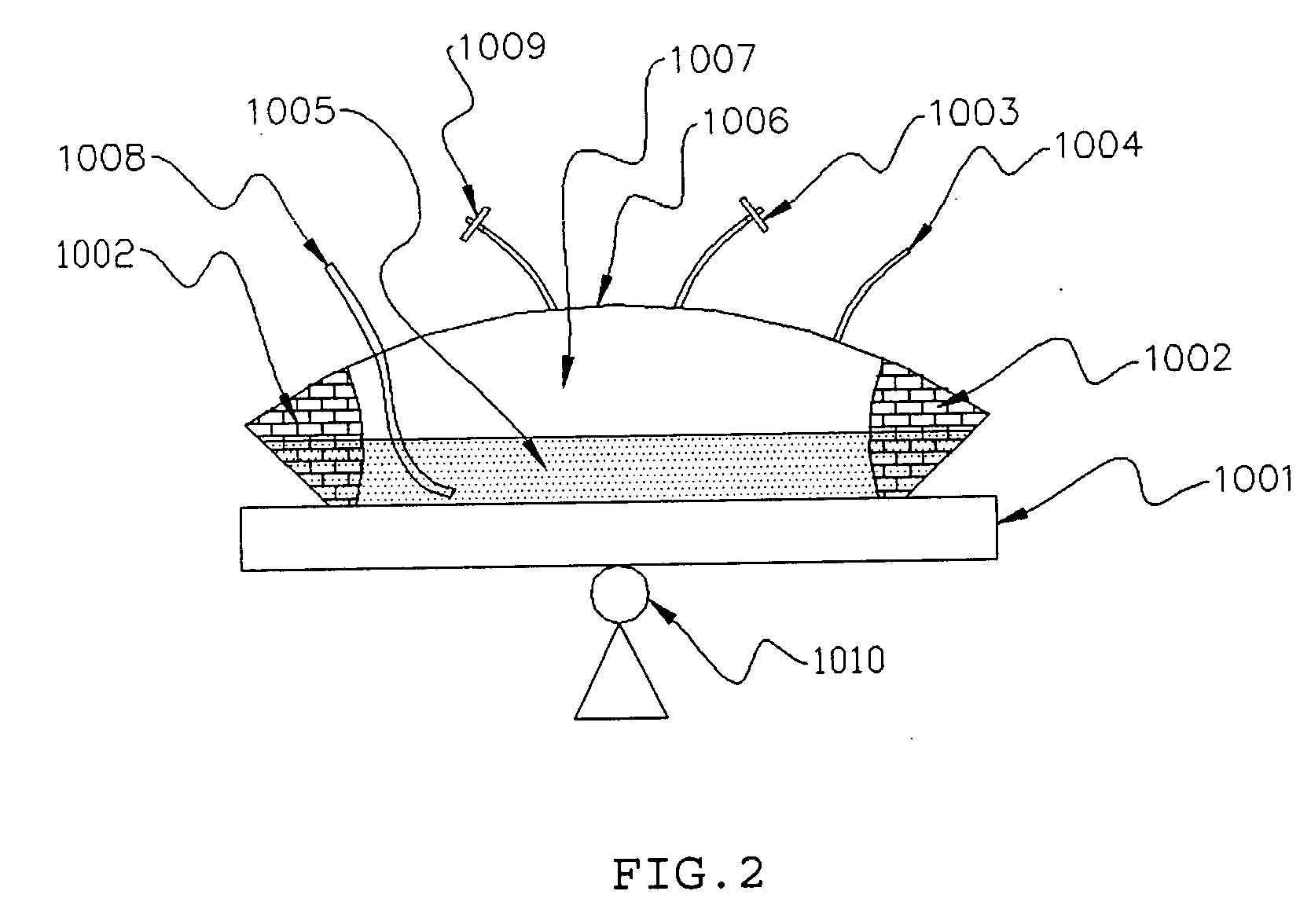
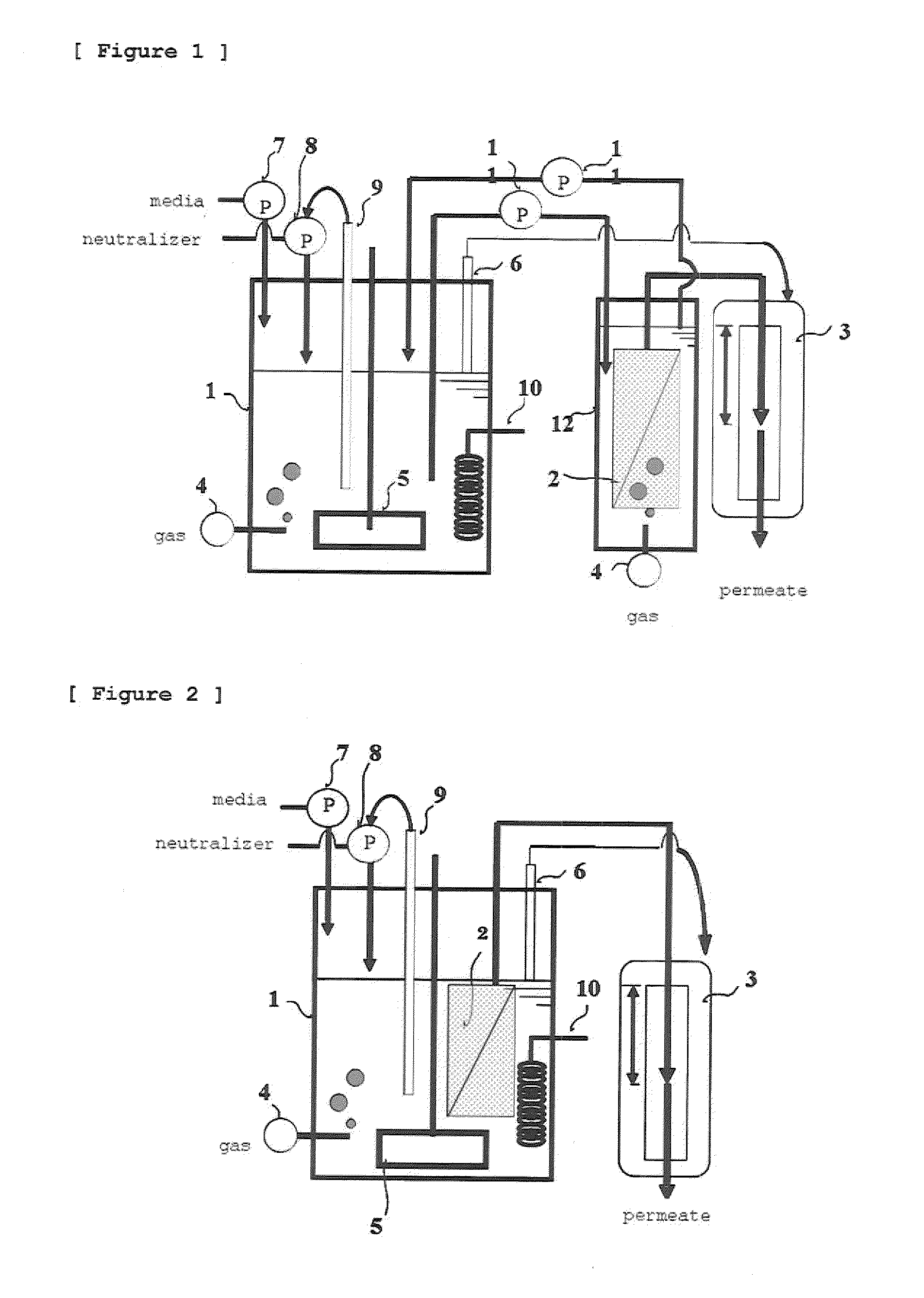
InactiveUS20050186669A1Maximizes cell adhesionIncrease surface areaBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyCulture cell
An apparatus and method for preparing and culturing cells includes a chamber having an inlet and an outlet for introducing culture medium and air. A cell growth substrate placed in the chamber for cells anchored or embedded. The preferred embodiment of invention claims a novel process for controlling the platform form to tilt at one end, to maintain the seesaw and rocking movement for cultured process. The seesaw movement or rocking movement efficiently the nutrition, carbon dioxide and oxygen transfer during cultured process for production of cellular protein products or harvesting the cells.
Owner:CESCO BIOENGINEERING CO LTD
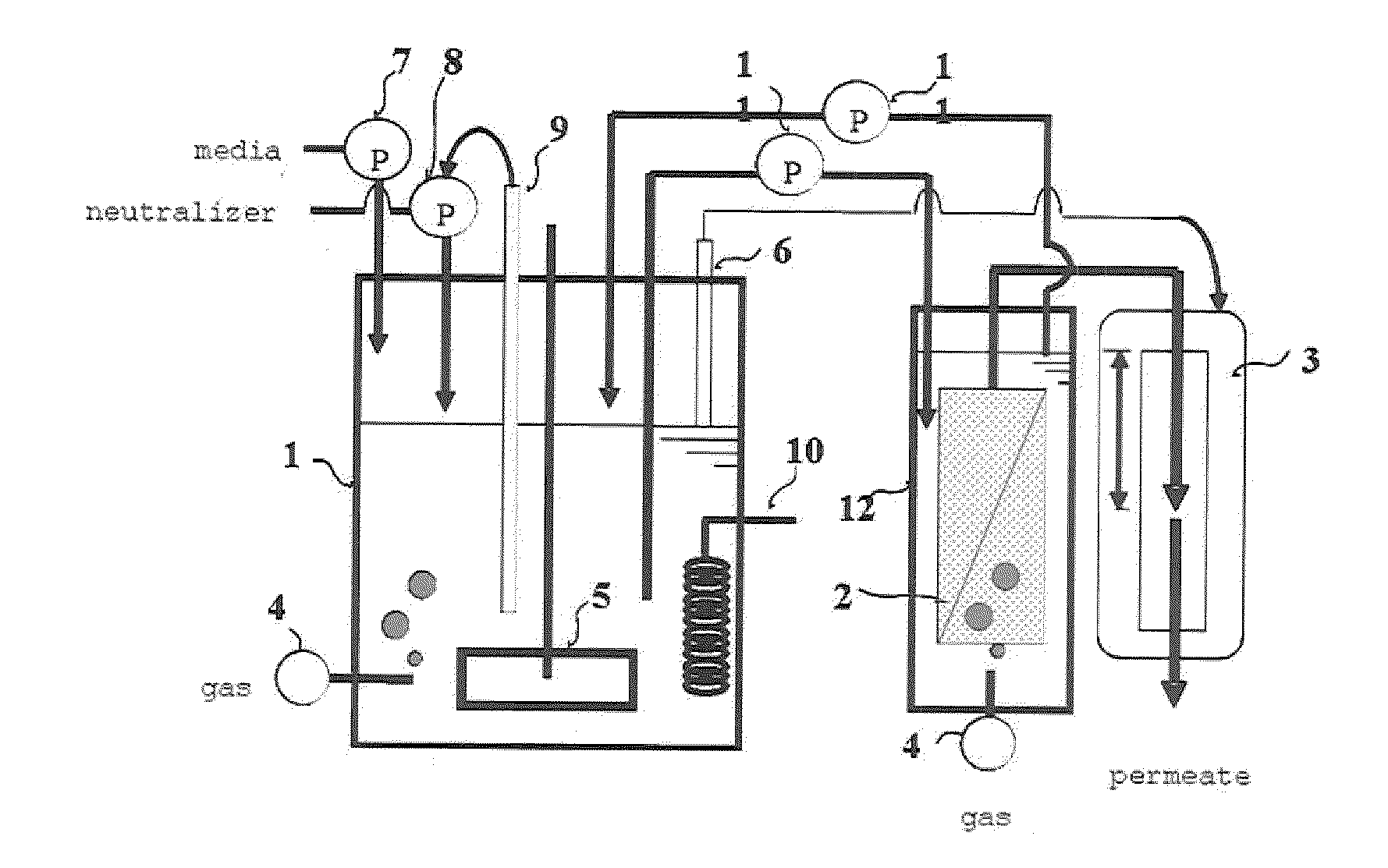
Method of producing chemical product and continuous fermentation apparatus
ActiveUS20090269812A1Bioreactor/fermenter combinationsBiological substance pretreatmentsContinuous fermentationMicroorganism
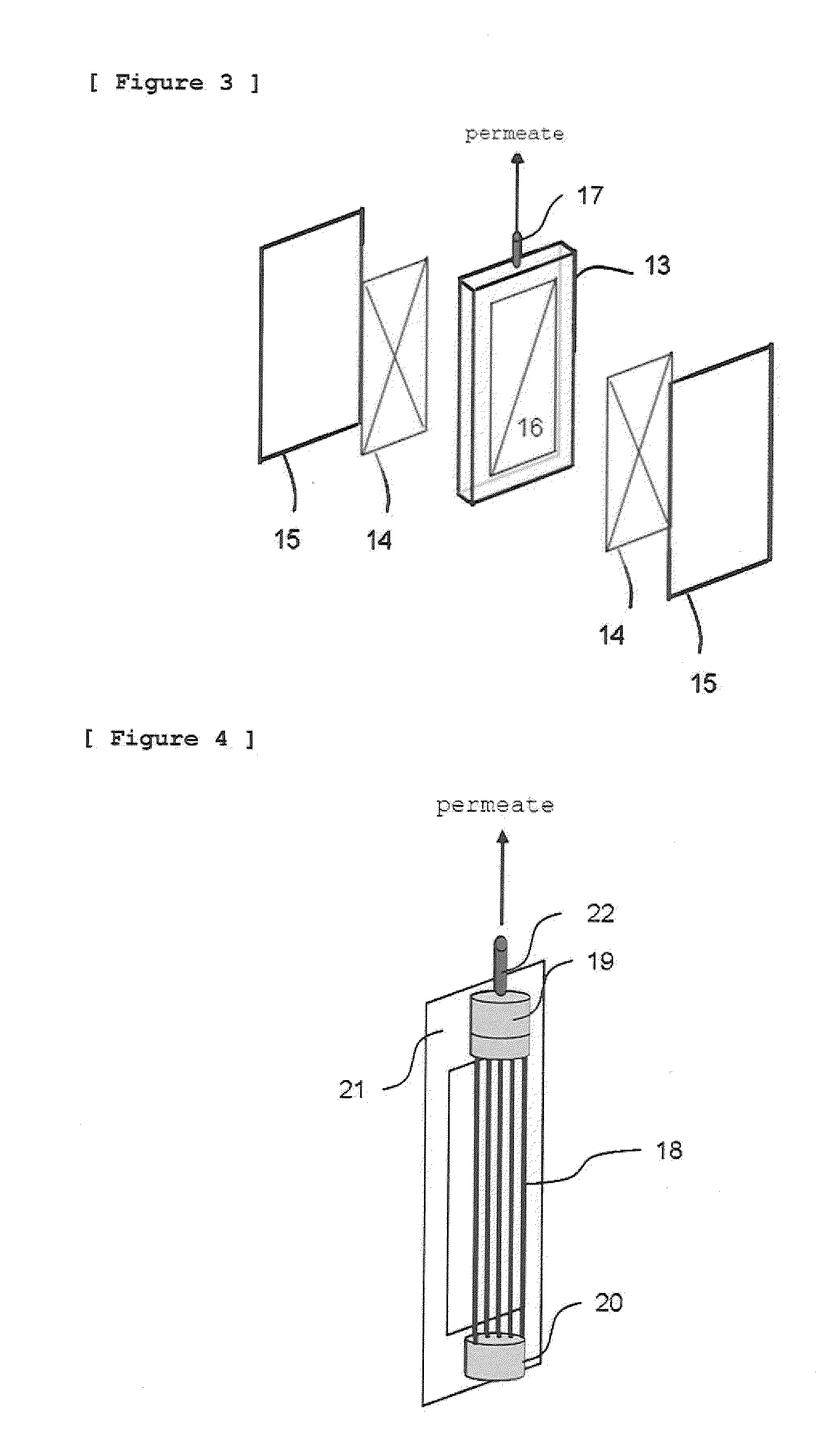
The invention provides a method of producing a chemical product through continuous fermentation which includes filtering a culture of a microorganism or cultured cells with a separation membrane to recover a product from a filtrate and simultaneously retaining a nonfiltered fluid in, or refluxing it to, the culture, and adding fermentation materials to the culture, wherein a porous membrane having an average pore size of 0.01 μm or more to less than 1 μm is used as the separation membrane and the filtration is conducted with a transmembrane pressure difference in the range of 0.1 to 20 kPa. According to this method, the fermentation productivity of the chemical product can be largely elevated at high stability and a low cost.
Owner:TORAY IND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com