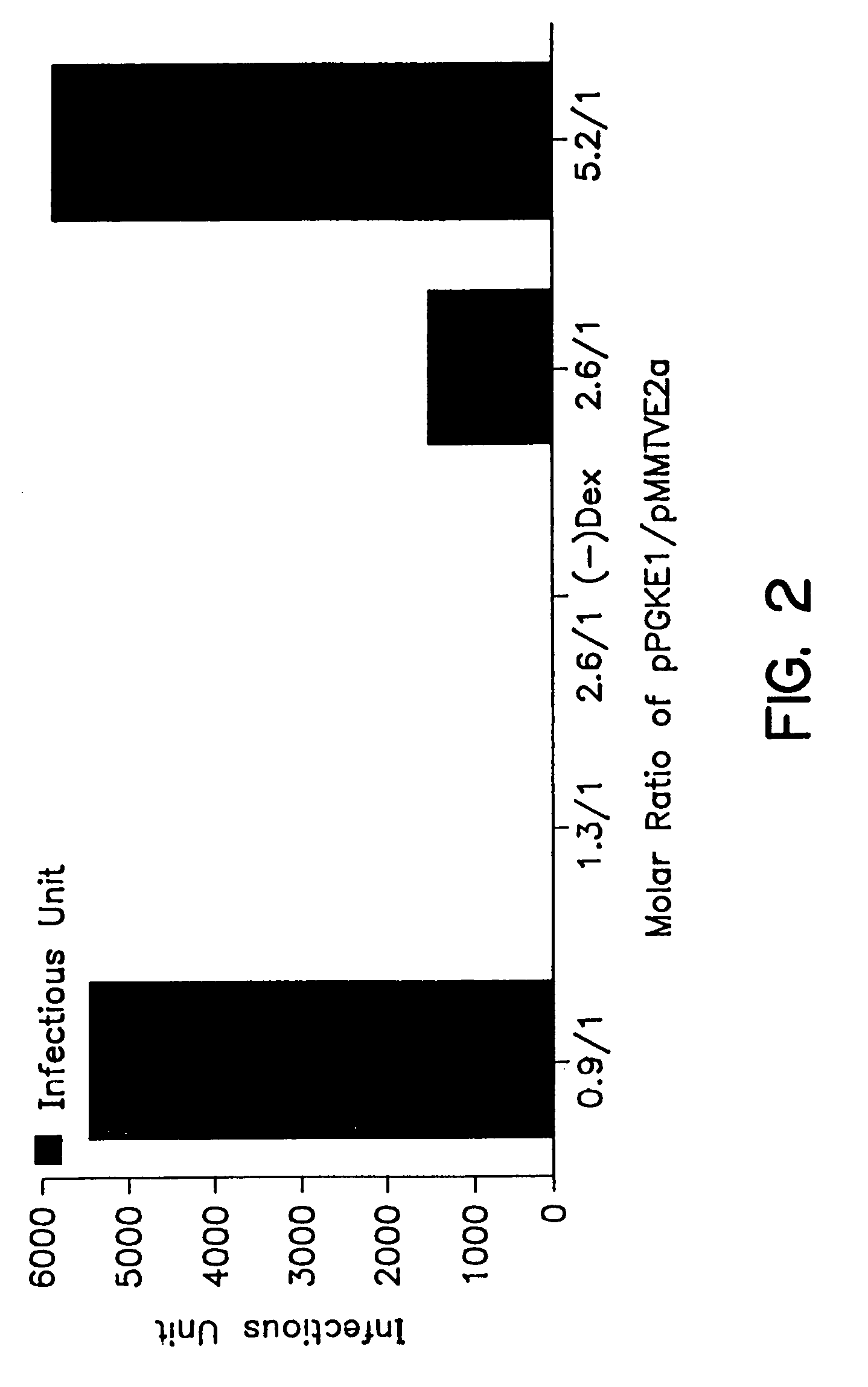
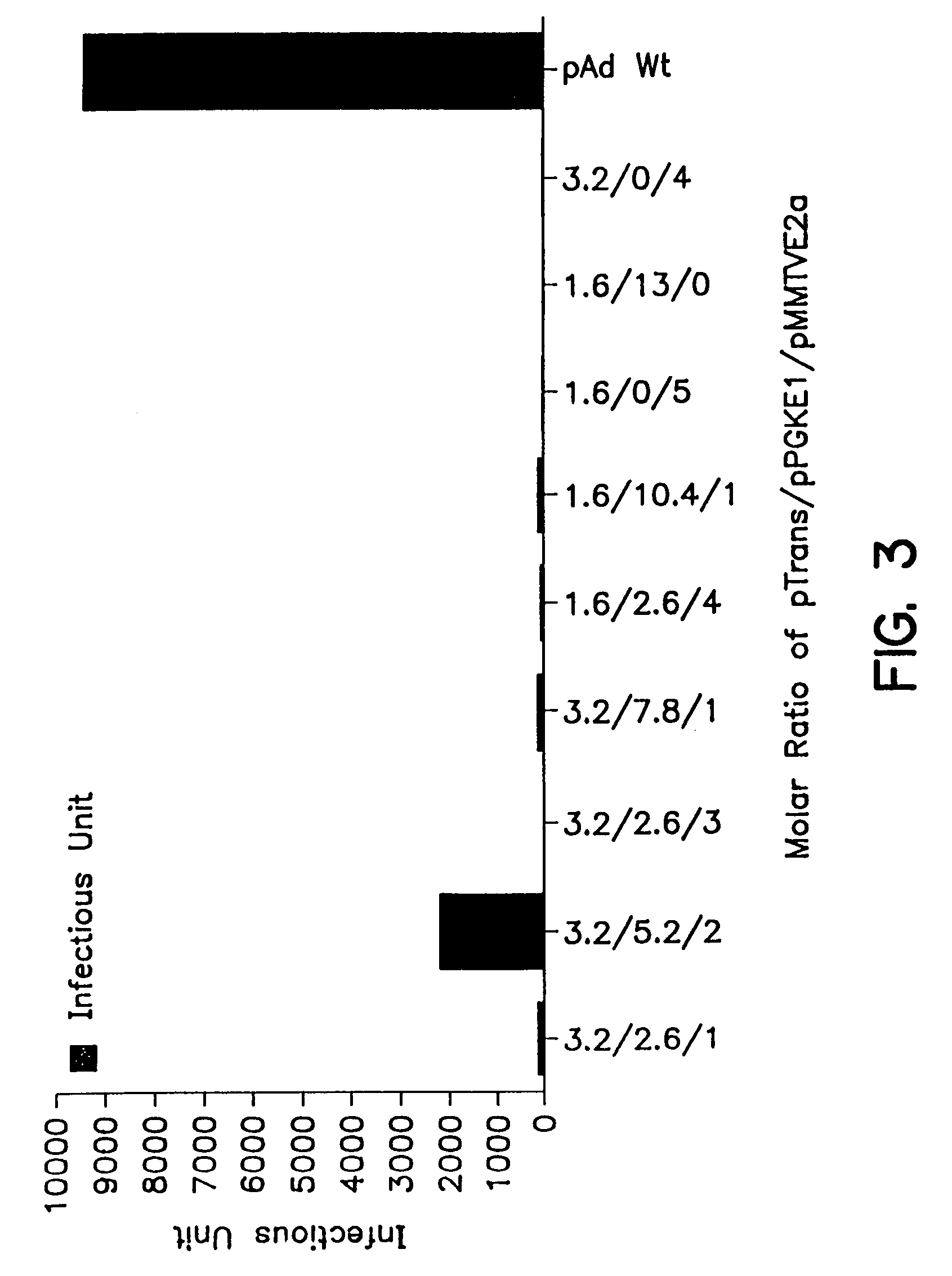
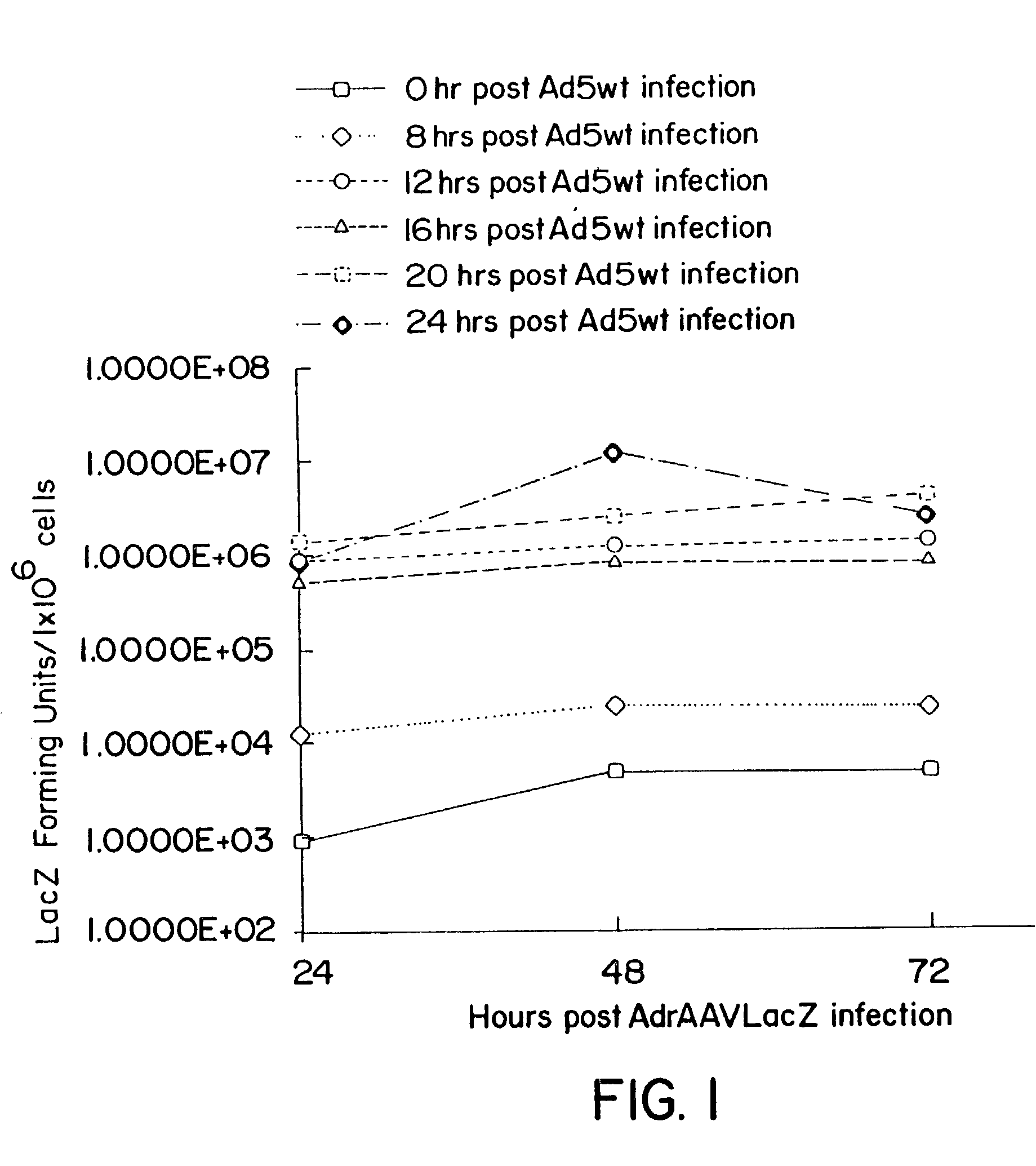
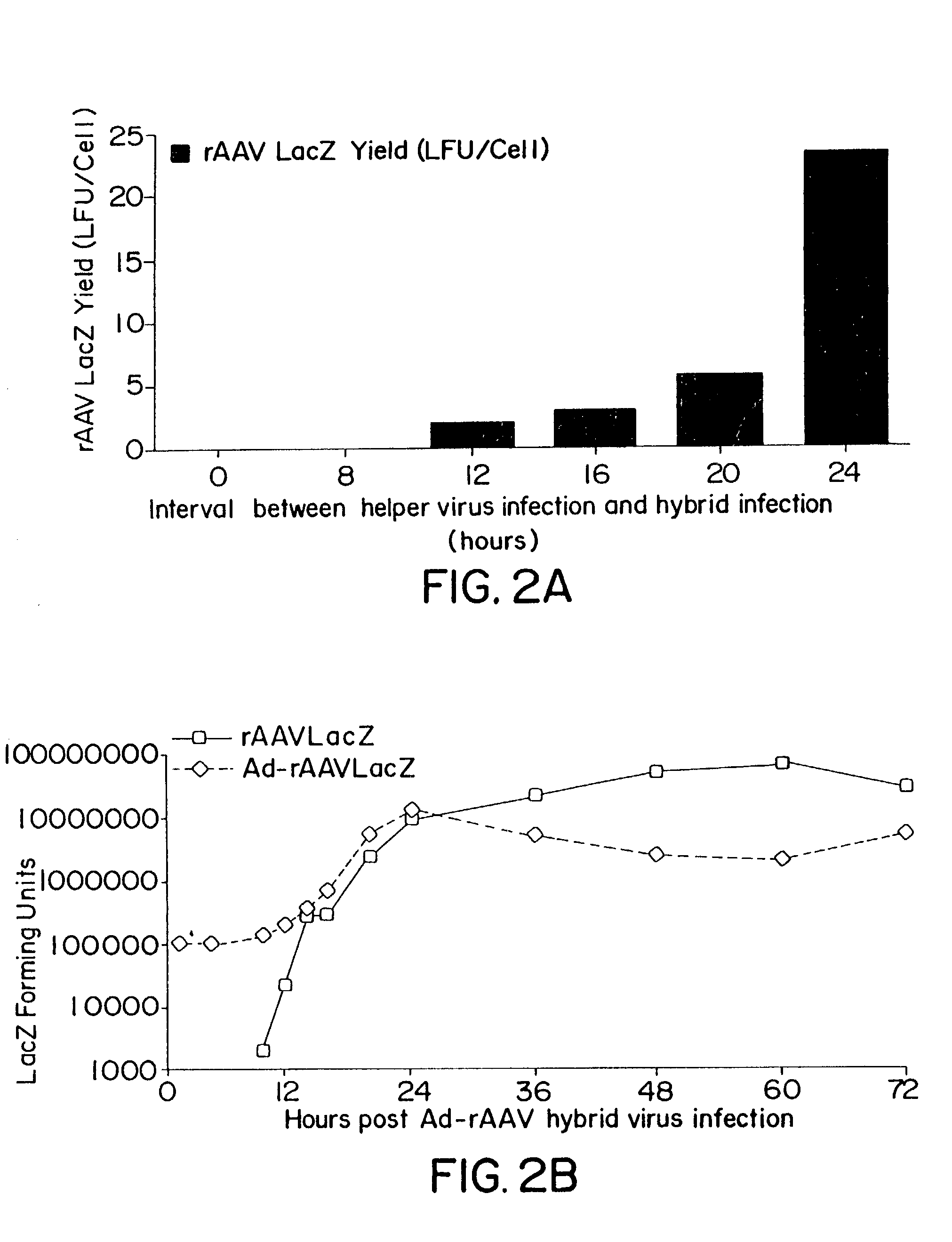
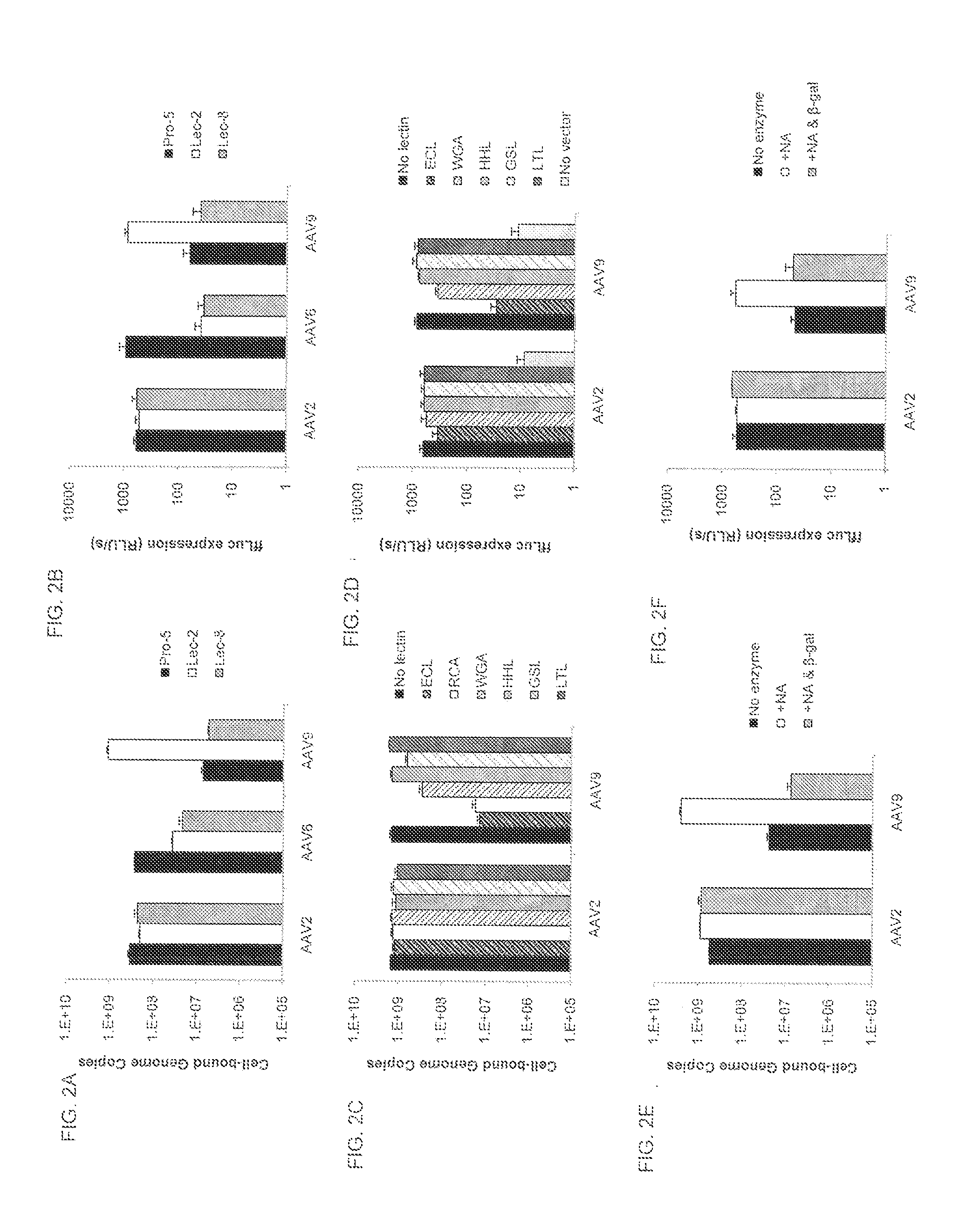Patents
Literature
228 results about "Adeno associate virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
AAV's and uses thereof
The invention in some aspects relates to recombinant adeno-associated viruses having distinct tissue targeting capabilities. In some aspects, the invention relates to gene transfer methods using the recombinant adeno-associate viruses. In some aspects, the invention relates to isolated AAV capsid proteins and isolated nucleic acids encoding the same.
Owner:UNIV OF MASSACHUSETTS
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and cell line useful for production of recombinant adeno-associated viruses
InactiveUS7238526B2High yieldInduce expressionGenetically modified cellsGenetic material ingredientsPlasmid VectorAdeno associate virus
Methods for efficient production of recombinant AAV employ a host cell which comprising AAV rep and cap genes stably integrated within the cell's chromosomes, wherein the AAV rep and cap genes are each operatively linked to regulatory sequences capable of directing the expression of the rep and cap gene products upon infection of the cell with a helper virus, a helper gene, and a helper gene product. A method for producing recombinant adeno-associated virus (rAAV) involves infecting such a host cell with a helper virus, gene or gene product and infecting the infected host cell with a recombinant hybrid virus or plasmid vector containing adenovirus cis-elements necessary for replication and virion encapsidation, AAV sequences comprising the 5′ and 3′ ITRs of an AAV, and a selected gene operatively linked to regulatory sequences directing its expression, which is flanked by the above-mentioned AAV sequences.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
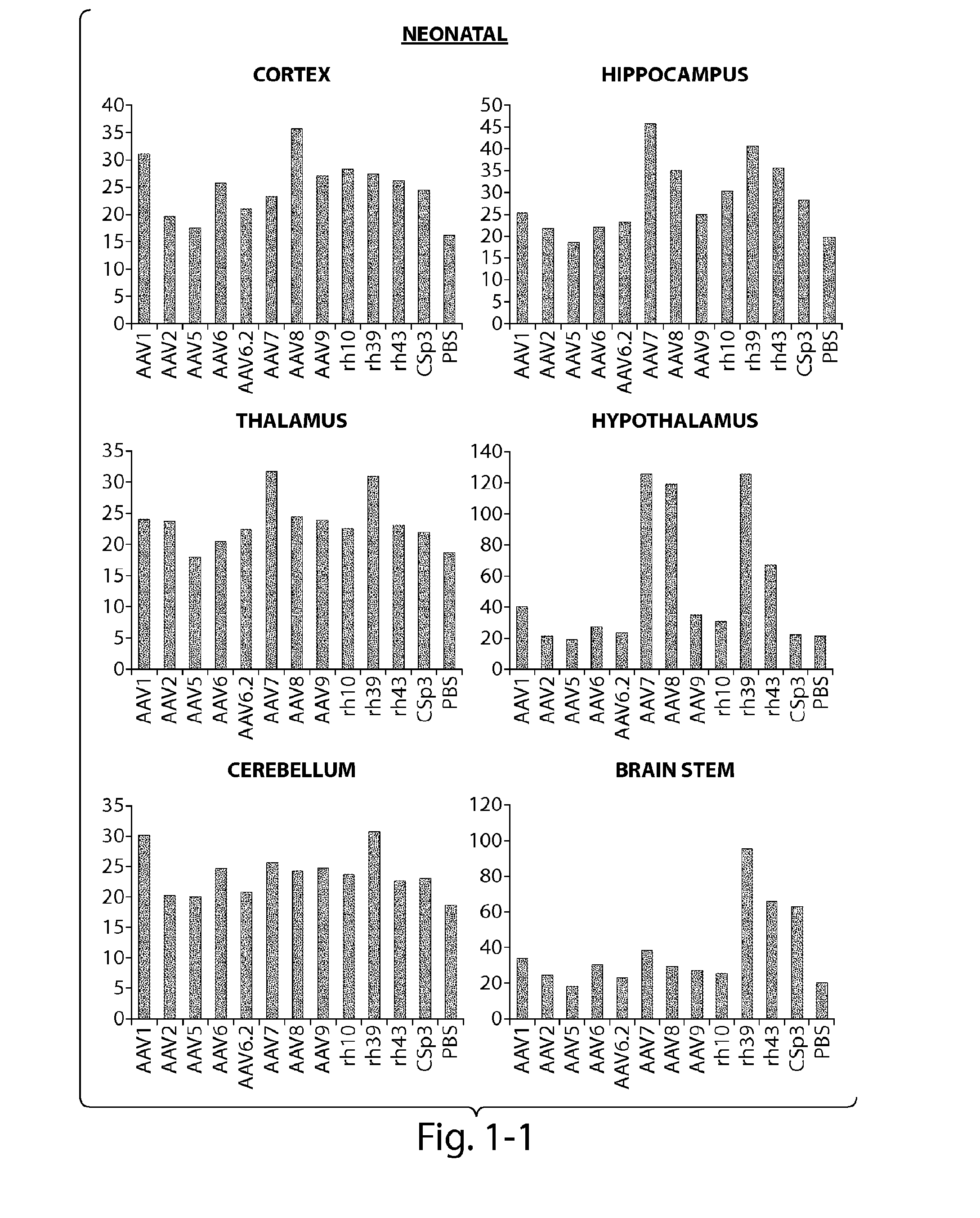
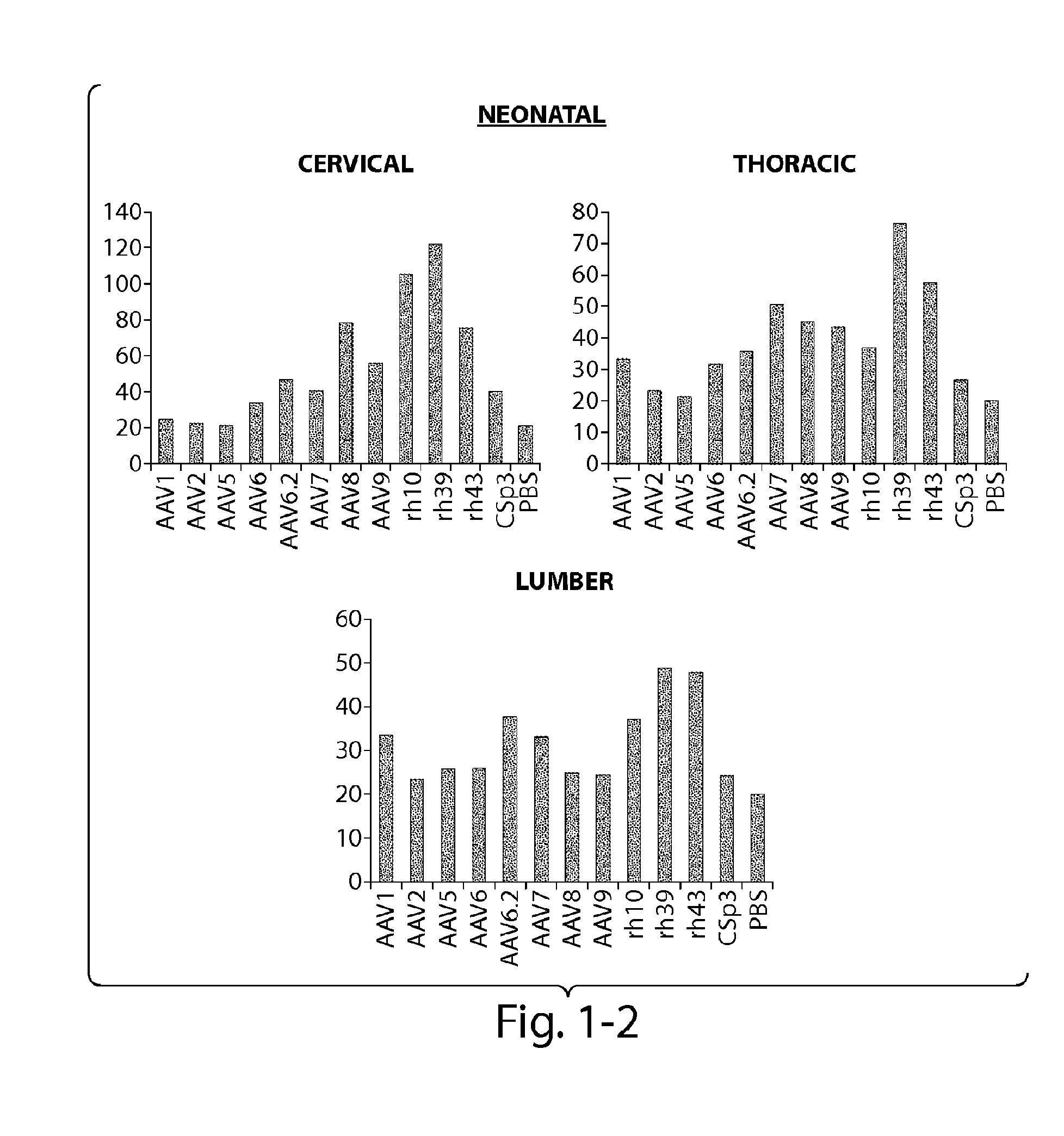
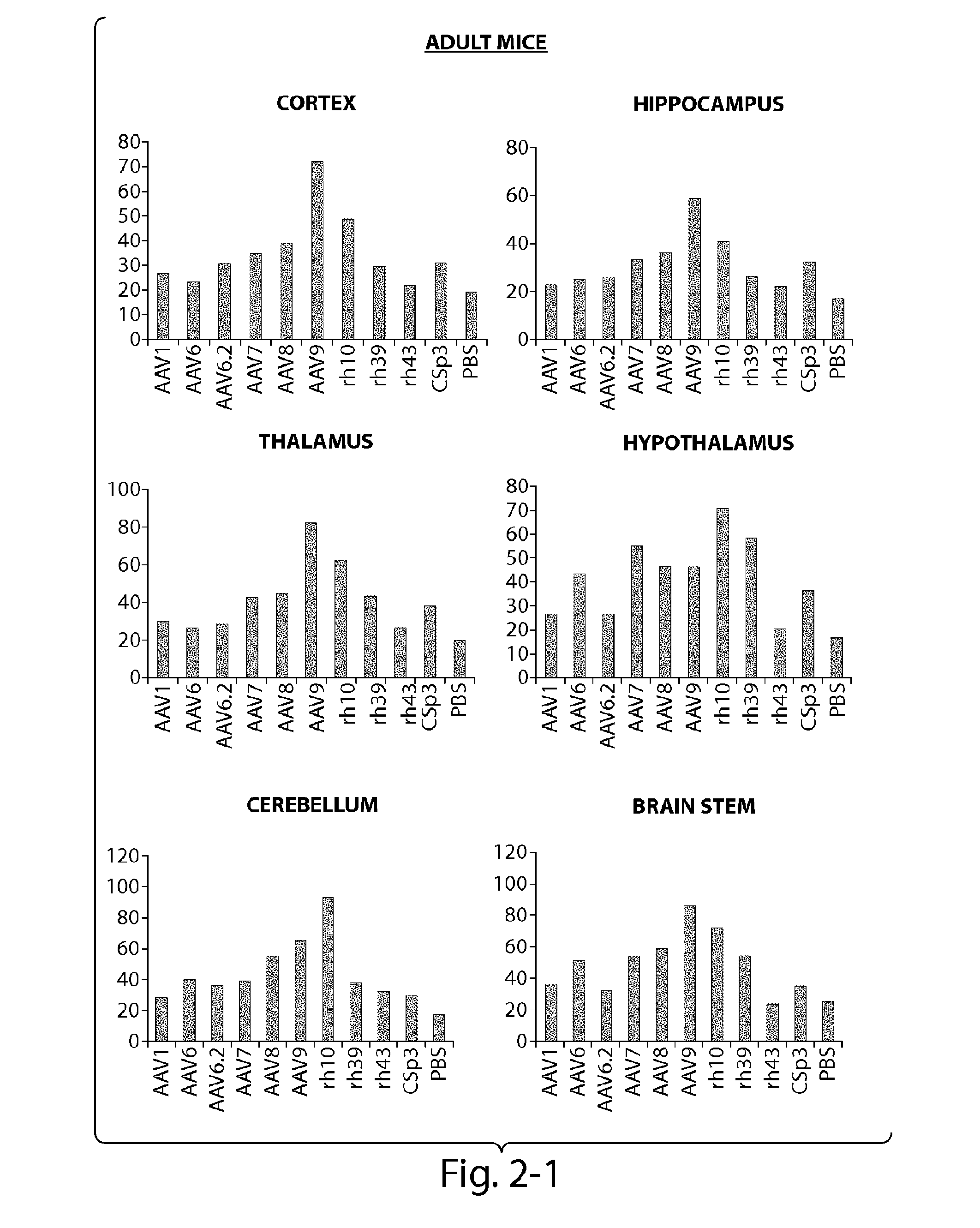
CNS targeting aav vectors and methods of use thereof
ActiveUS20130195801A1Widely distributedStable and nontoxic gene transferOrganic active ingredientsBiocideAdeno associate virusTransgene
Owner:UNIV OF MASSACHUSETTS
Efficient and stable in vivo gene transfer to cardiomyocytes using recombinant adeno-associated virus vectors
InactiveUS7078387B1The process is stable and efficientBiocideSugar derivativesCoronary sinusAdeno associate virus
This invention relates to the use of recombinant adeno-associated virus (rAAV) vectors to transduce cardiomyocytes in vivo by infusing the rAAV into a coronary artery or coronary sinus. rAAV infection is not associated with detectable myocardial inflammation or myocyte necrosis. Thus, rAAV is a useful vector for the stable expression of therapeutic genes in the myocardium and can be used to deliver genes for inducing angiogenesis, inhibiting angiogenesis, stimulating cell proliferation, inhibiting cell proliferation and / or treating or ameliorating other cardiovascular conditions.
Owner:ARCH DEVMENT
Compositions and Methods for Altering Tissue Specificity and Improving AAV9-Mediated Gene Transfer
ActiveUS20130323226A1Improve efficiencyImprove usabilityOrganic active ingredientsVectorsViral vectorFhit gene
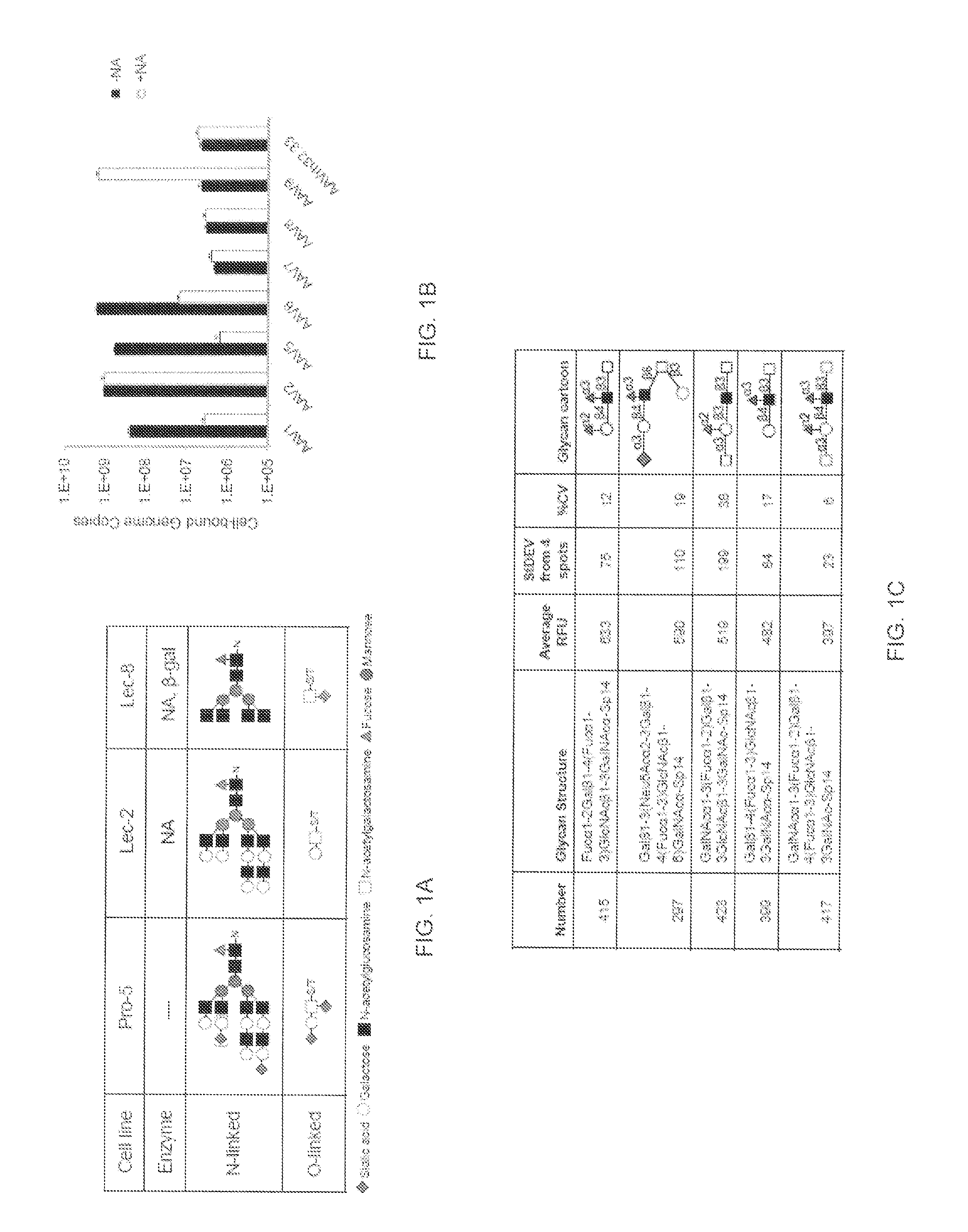
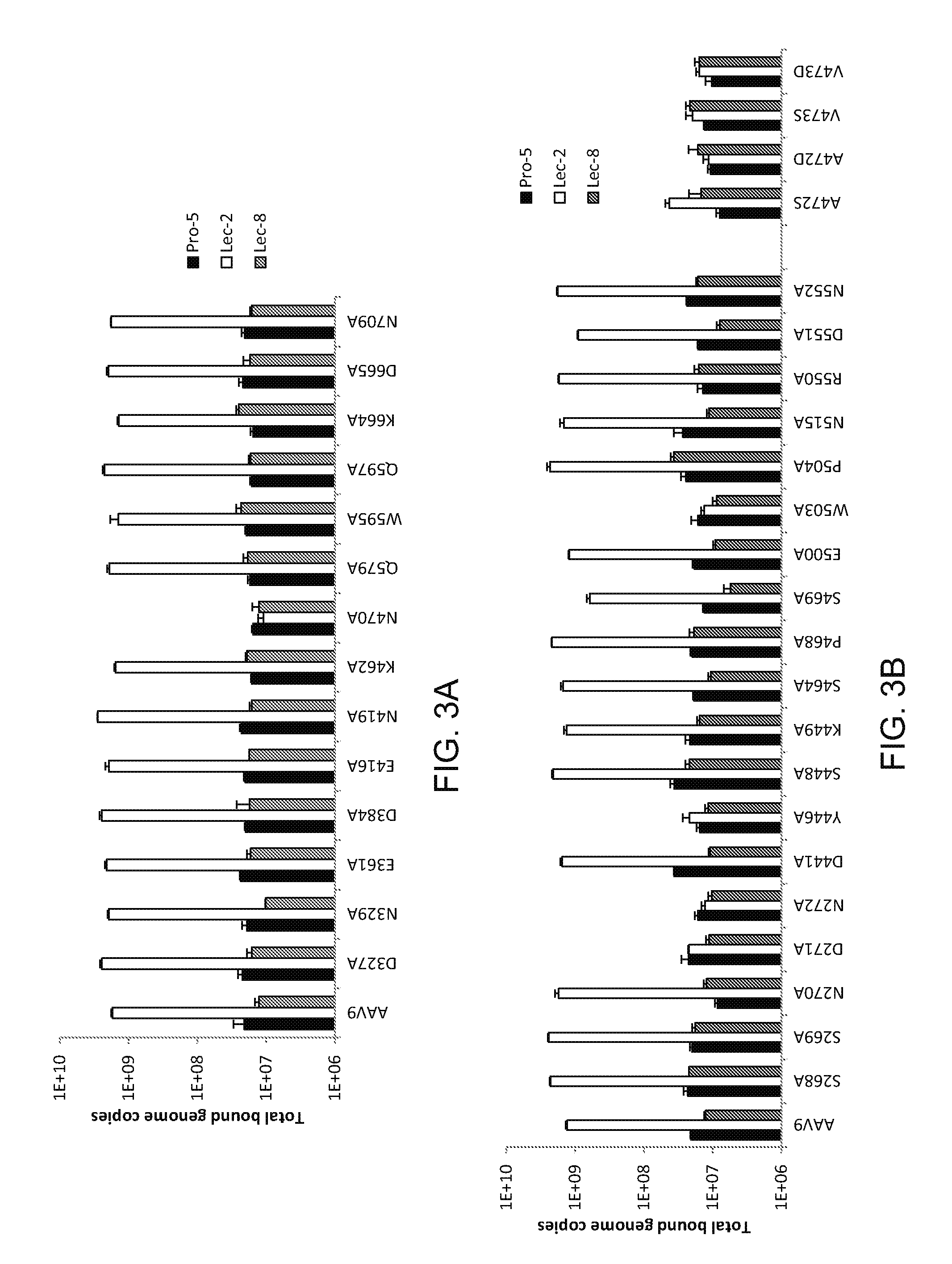
A method of altering the targeting and / or cellular uptake efficiency of an adeno-associated virus (AAV) viral vector having a capsid containing an AAV9 cell surface binding domain is described. The method involves modifying a clade F cell surface receptor which comprises a glycan having a terminal sialic acid residue and a penultimate β-galactose residue. The modification may involve retargeting the vector by temporarily functionally ablate AAV9 binding in a subset of cells, thereby redirecting the vector to another subset of cells. Alternatively, the modification may involve increasing cellular update efficiency by treating the cells with a neuraminidase to expose cell surface β-galactose. Also provided are compositions containing the AAV9 vector and a neuraminidase. Also provided is a method for purifying AAV9 using β-galactose linked to solid support. Also provided are mutant vectors which have been modified to alter their targeting specificity, including mutant AAV9 in which the galactose binding domain is mutated and AAV in which an AAV9 galactose binding domain is engineered.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
AAV5 vector and uses thereof
The present invention provides an adeno-associated virus 5 (AAV5) virus and vectors and particles derived therefrom. In addition, the present invention provides methods of delivering a nucleic acid to a cell using the AAV5 vectors and particles.
Owner:DEPT OF HEALTH & HUMAN SERVICE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE
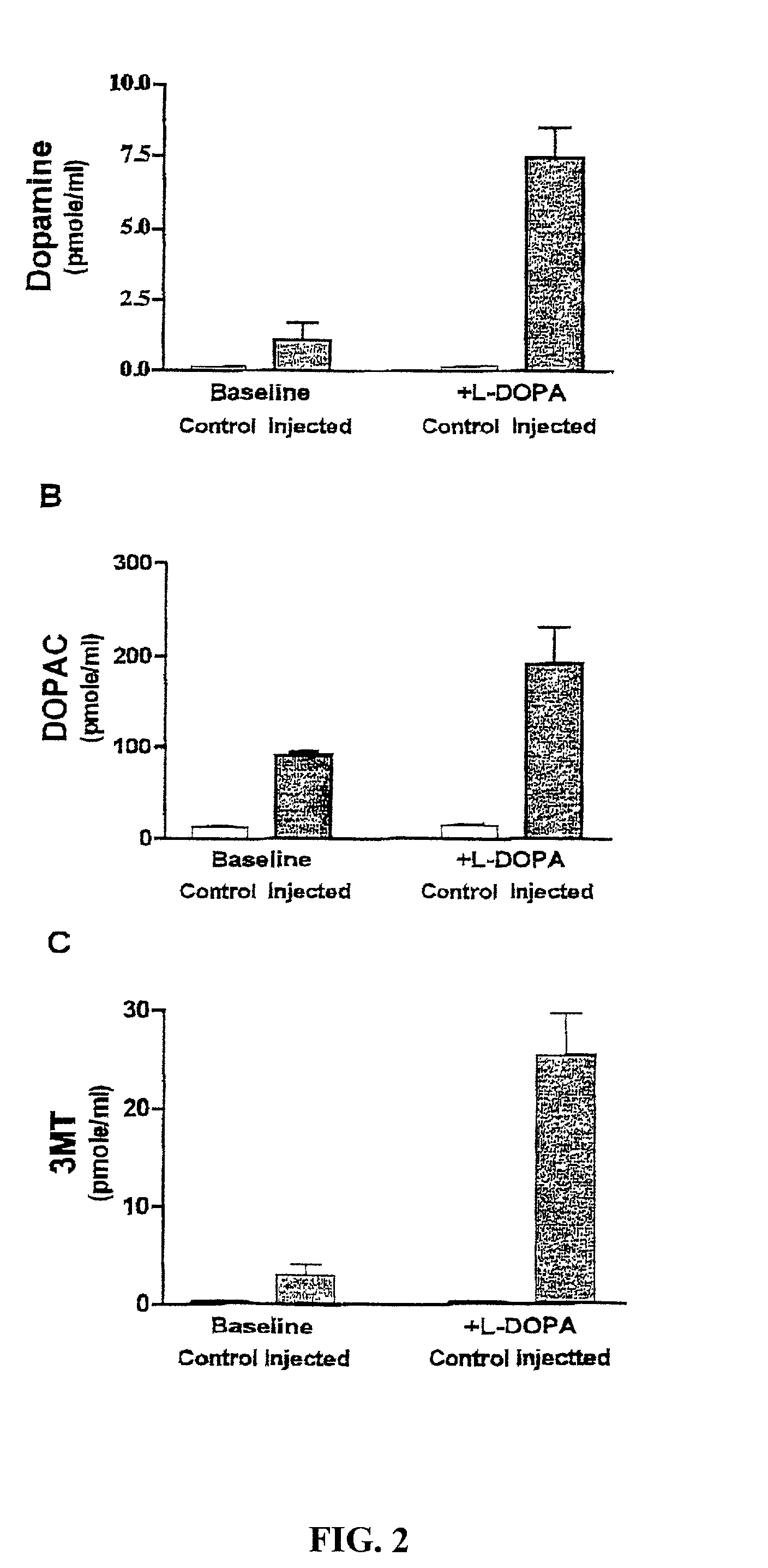
Methods of treating Parkinson's disease using recombinant adeno-associated virus virions
ActiveUS7588757B2Reduce deliveryIncrease in fine motor taskingBiocidePeptide/protein ingredientsGene deliveryDisease
Methods for treating Parkinson's disease (PD) are provided. Recombinant adeno-associated virus (rAAV) virions are used to deliver genes encoding dopamine-synthesizing enzymes to the central nervous system of a primate. Once delivered, the genes are expressed, which then results in dopamine synthesis and amelioration in the clinical signs and symptoms of PD. The methods of the present invention can be used to deliver the three central dopamine synthesizing enzymes: tyrosine hydroxylase, aromatic L-amino acid decarboxylase, and guanosine triphosphate cyclohydrolase I thereby enhancing dopamine biosynthesis and providing for enhanced therapeutic efficacy.
Owner:GENZYME CORP
CNS targeting aav vectors and methods of use thereof
Owner:UNIV OF MASSACHUSETTS
Novel aav's and uses thereof
ActiveUS20120137379A1Determining effectOrganic active ingredientsVirusesTissue targetingAdeno associate virus
The invention in some aspects relates to recombinant adeno-associated viruses having distinct tissue targeting capabilities. In some aspects, the invention relates to gene transfer methods using the recombinant adeno-associate viruses. In some aspects, the invention relates to isolated AAV capsid proteins and isolated nucleic acids encoding the same.
Owner:UNIV OF MASSACHUSETTS
Adeno-Associated Virus Virion for Gene Transfer to Nervous System Cells
ActiveUS20130224836A1Improve efficiencyLower Level RequirementsNervous disorderSpecial deliveryNervous systemAdeno associate virus
The present invention provides a means for transferring a therapeutic gene of interest into a nervous system cell by a highly-efficient and simpler means. More specifically, the present invention provides a recombinant vector that uses an adeno-associated virus (AAV), a method for manufacturing the recombinant vector, and a method for using the recombinant vector. More specifically, recombinant adeno-associated virus virions, which are capable of passing through the brain-brain barrier, for transferring a therapeutic genes of interest into a nervous system cell in a highly-efficient manner, a drug composition containing the recombinant adeno-associated virus virions, a method for manufacturing the recombinant adeno-associated virus virions, and a kit or the like are provided.
Owner:JICHI MEDICAL UNIVERSITY
Isolation Of Novel AAV'S And Uses Thereof
ActiveUS20100186103A1Determine effectAvoid time-consume and costly processVectorsSpecial deliveryAdeno associate virusBinding site
The invention in some aspects relates to isolated nucleic acids, compositions, and kits useful for identifying adeno-associated viruses in cells. In some aspects, the invention provides kits and methods for producing somatic transgenic animal models using recombinant AAV (rAAV) to an animal having at least one transgene that expresses a small interfering nucleic acid or at least one binding site for a miRNA.
Owner:UNIV OF MASSACHUSETTS
Adeno-associated virus vectors and uses thereof
InactiveUS7241447B1Improve stabilityIncreased episomal stabilityBiocideViral antigen ingredientsAdeno associate virusAdeno-associated virus
The invention provides an isolated and purified DNA molecule comprising at least one DNA segment, a biologically active subunit or variant thereof, of a circular intermediate of adeno-associated virus, which DNA segment confers increased episomal stability, persistence or abundance of the isolated DNA molecule in a host cell. The invention also provides a composition comprising at least two adeno-associated virus vectors.
Owner:RES FOUND UNIV OF IOWA
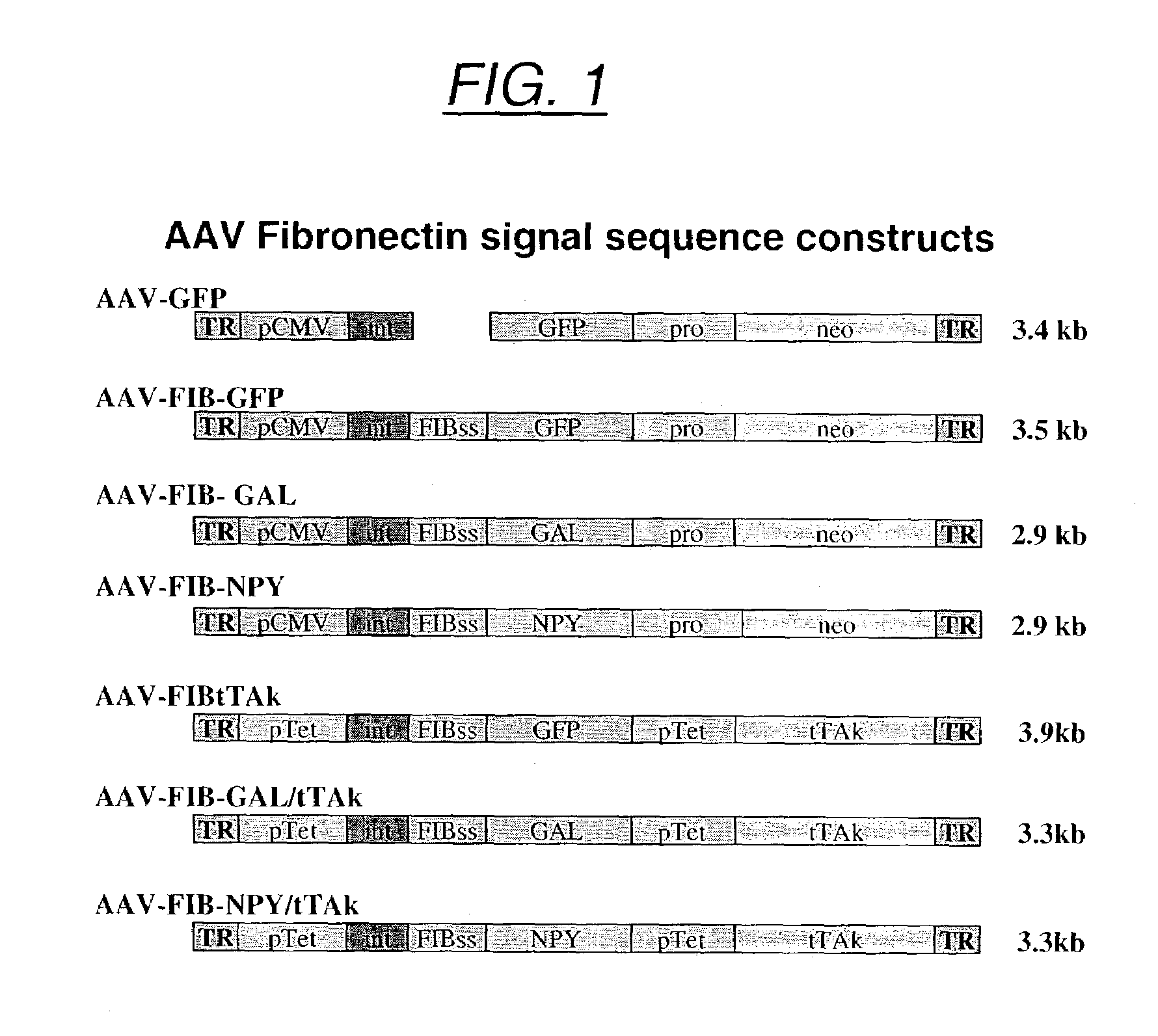
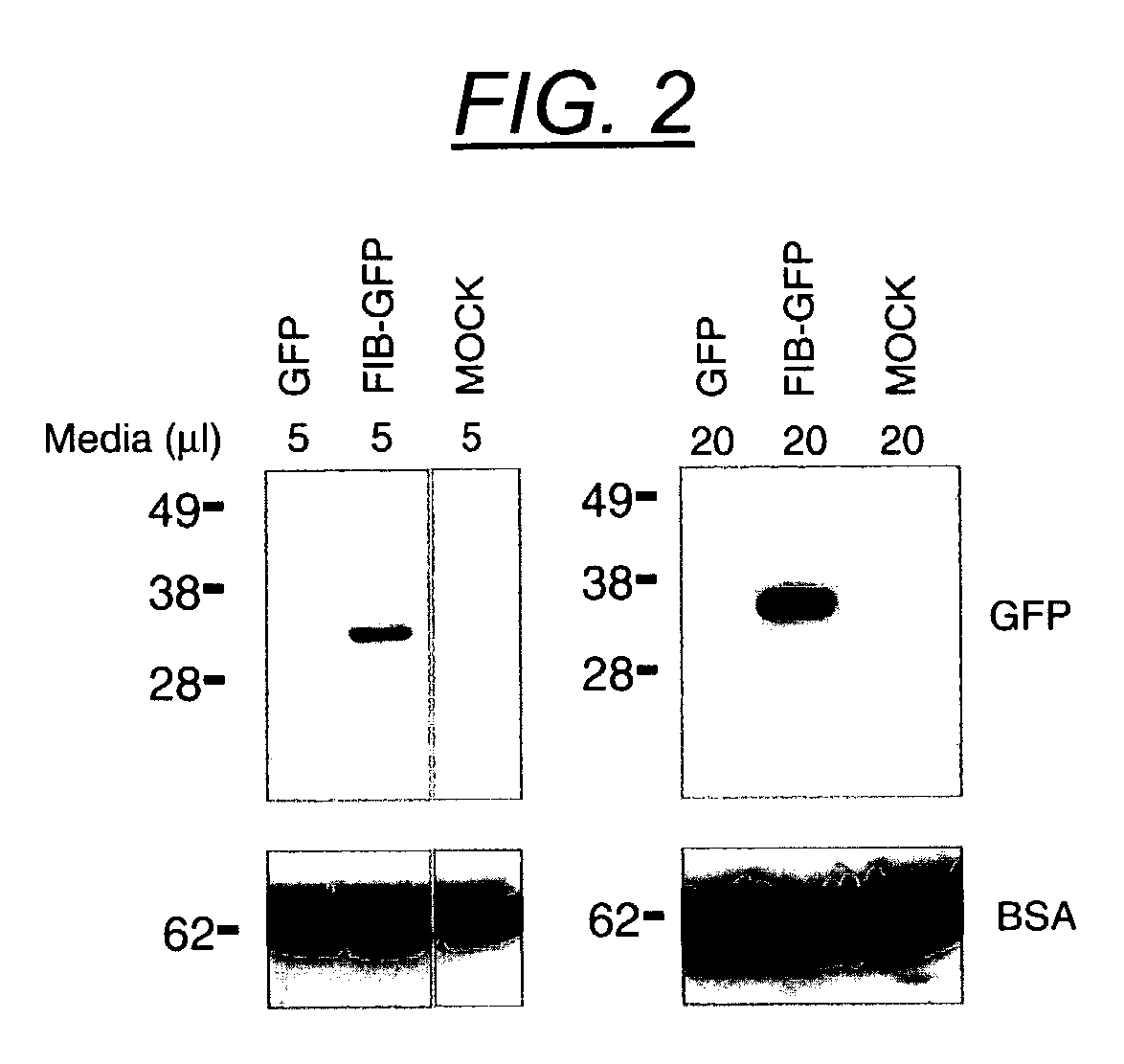
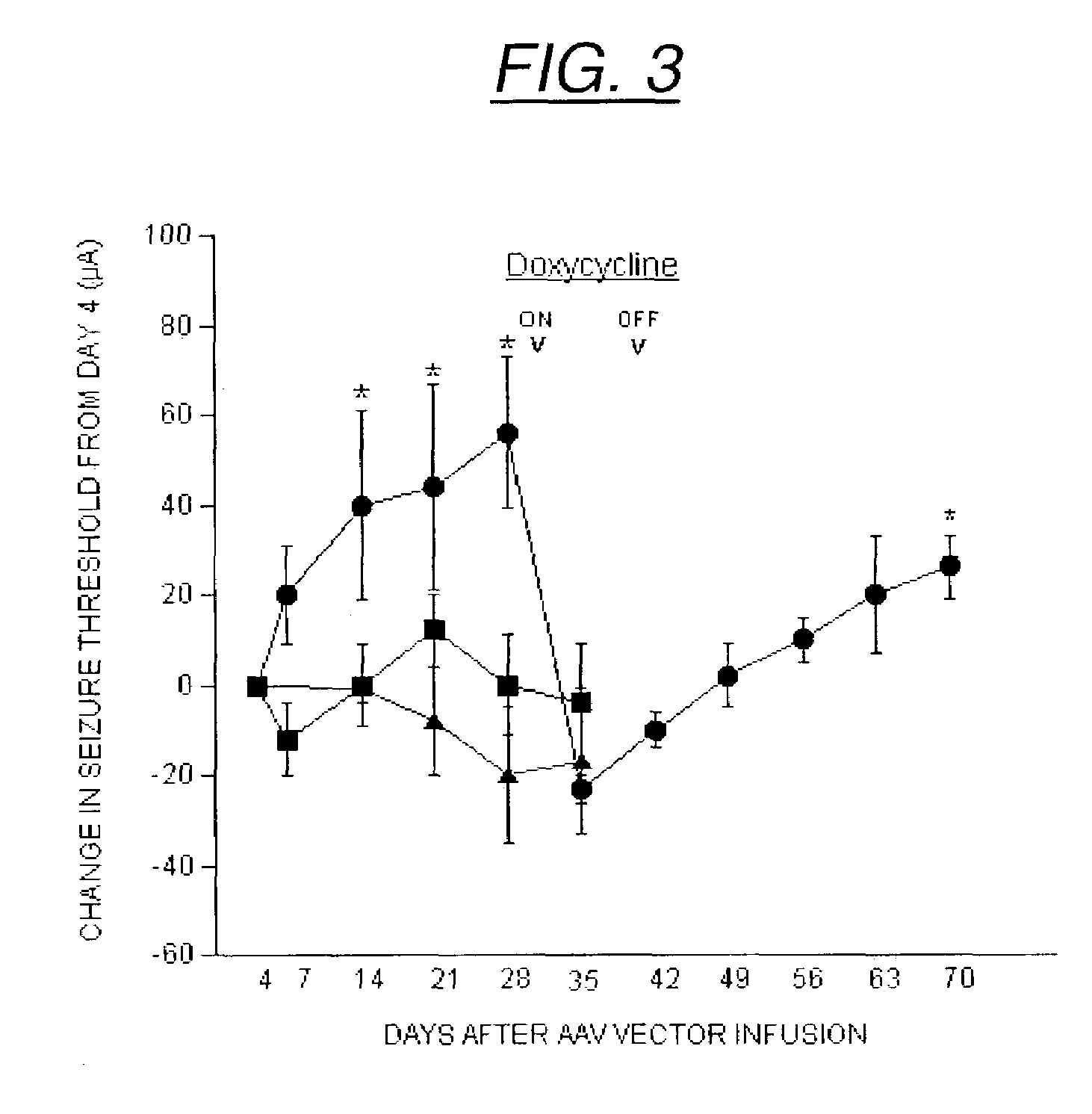
Secretion signal vectors
InactiveUS7071172B2Minimize potential deleterious side effectAttenuate seizure activityBiocideAnimal repellantsHeterologousAdeno associate virus
The present invention provides delivery vectors for transferring a nucleic acid sequence to a cell in vitro, ex vivo or in vivo. The delivery vector comprises a segment encoding a secretory signal peptide. In embodiments of the invention, the delivery vector is an adeno-associated virus (AAV) vector. In other embodiments, the secretory signal peptide is a fibronectin secretory signal peptide (including variations and modifications, thereof). The delivery vectors of the invention may further comprise a heterologous nucleic acid sequence encoding a polypeptide of interest for transfer to a target cell, where the polypeptide of interest is operably associated with the secretory signal. Also disclosed are methods of transferring a nucleic acid of interest to a cell using the delivery vectors of the invention.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
AAV5 nucleic acids
The present invention provides an adeno-associated virus 5 (AAV5) virus and vectors and particles derived therefrom. In addition, the present invention provides methods of delivering a nucleic acid to a cell using the AAV5 vectors and particles.
Owner:THE GOVERNMENT OF THE US SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER
Isolation of novel AAV'S and uses thereof
ActiveUS9217155B2Avoid time-consume and costly processVectorsSpecial deliveryBinding siteAdeno associate virus
The invention in some aspects relates to isolated nucleic acids, compositions, and kits useful for identifying adeno-associated viruses in cells. In some aspects, the invention provides kits and methods for producing somatic transgenic animal models using recombinant AAV (rAAV) to an animal having at least one transgene that expresses a small interfering nucleic acid or at least one binding site for a miRNA.
Owner:UNIV OF MASSACHUSETTS
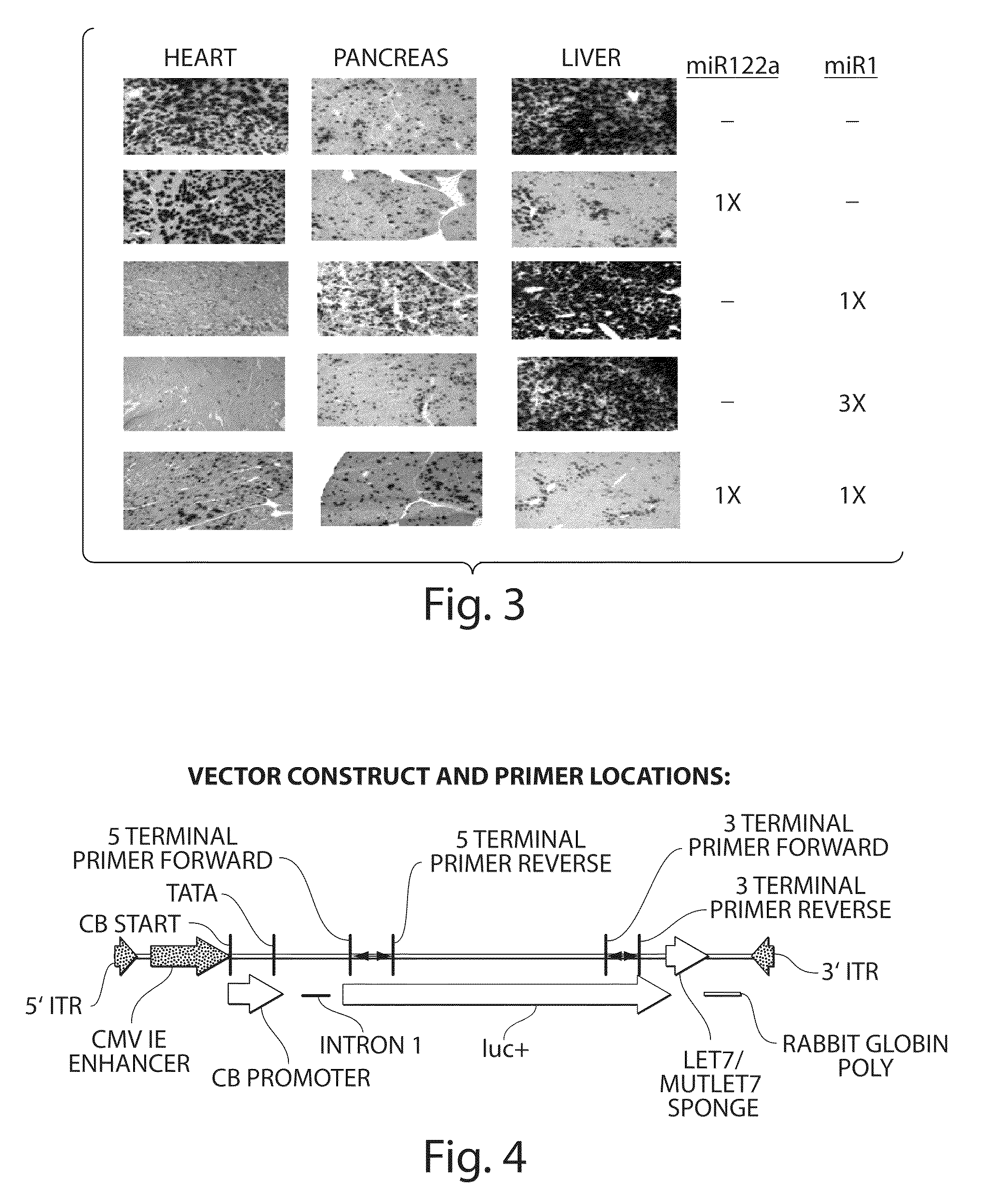
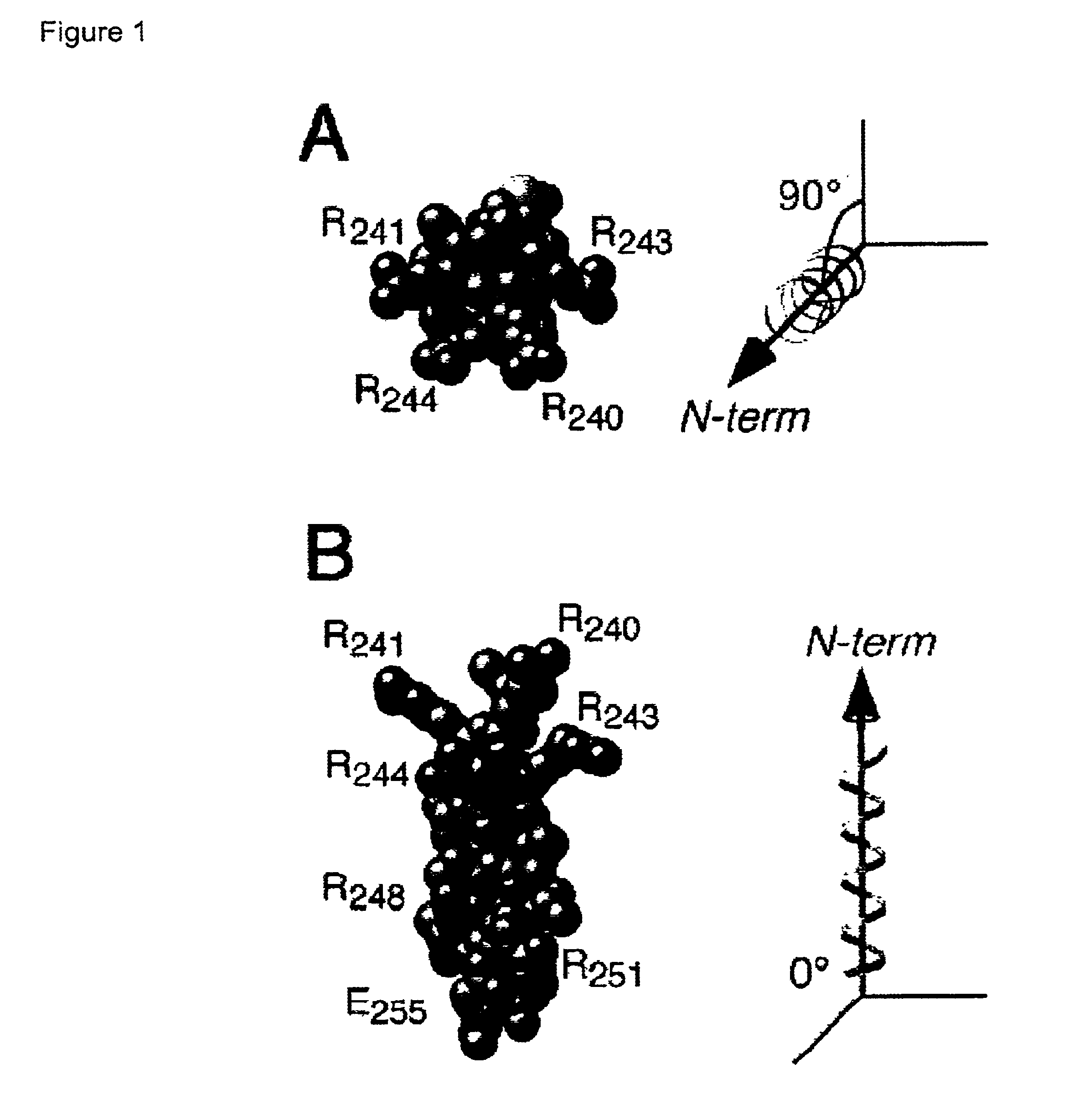
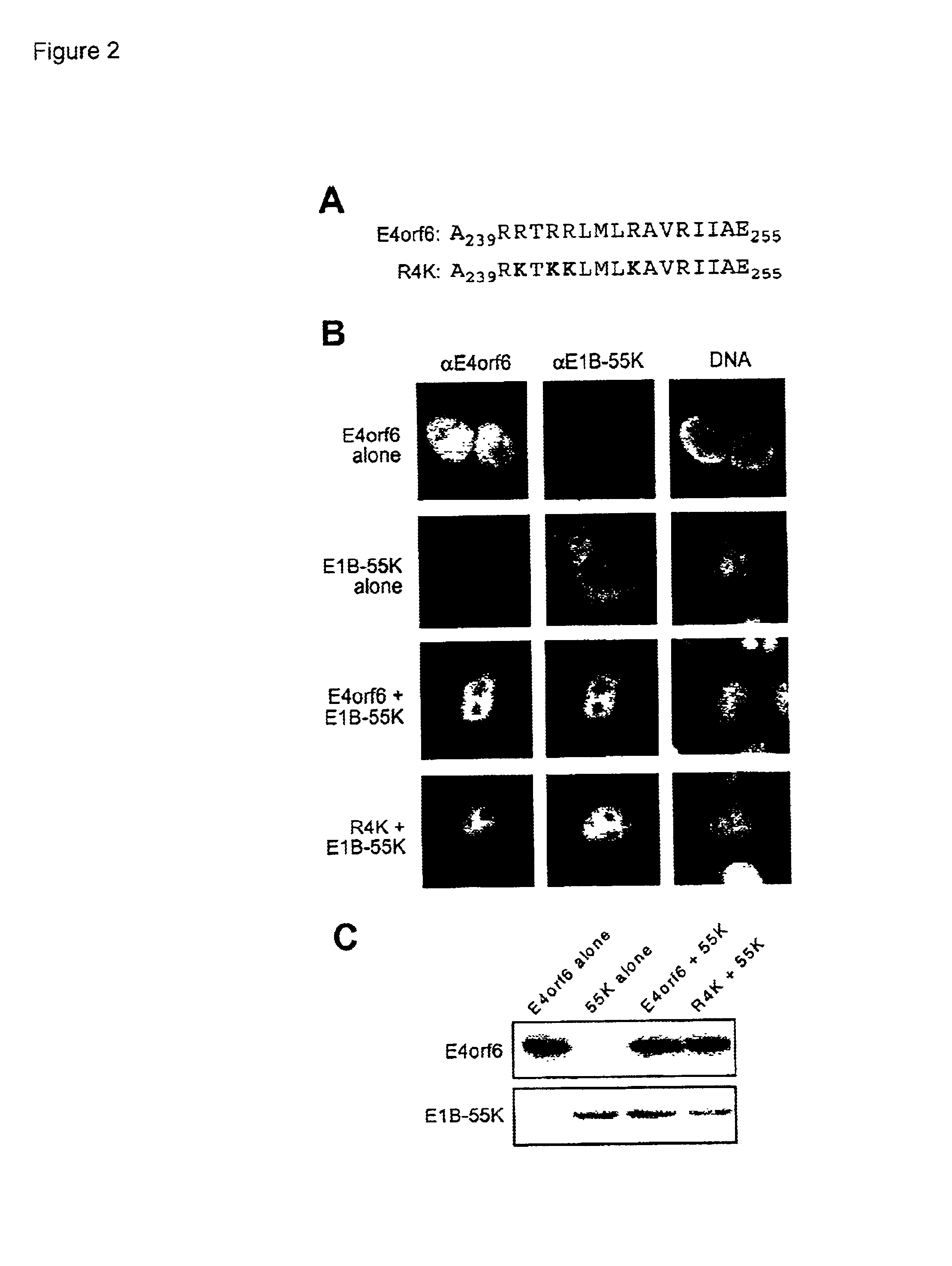
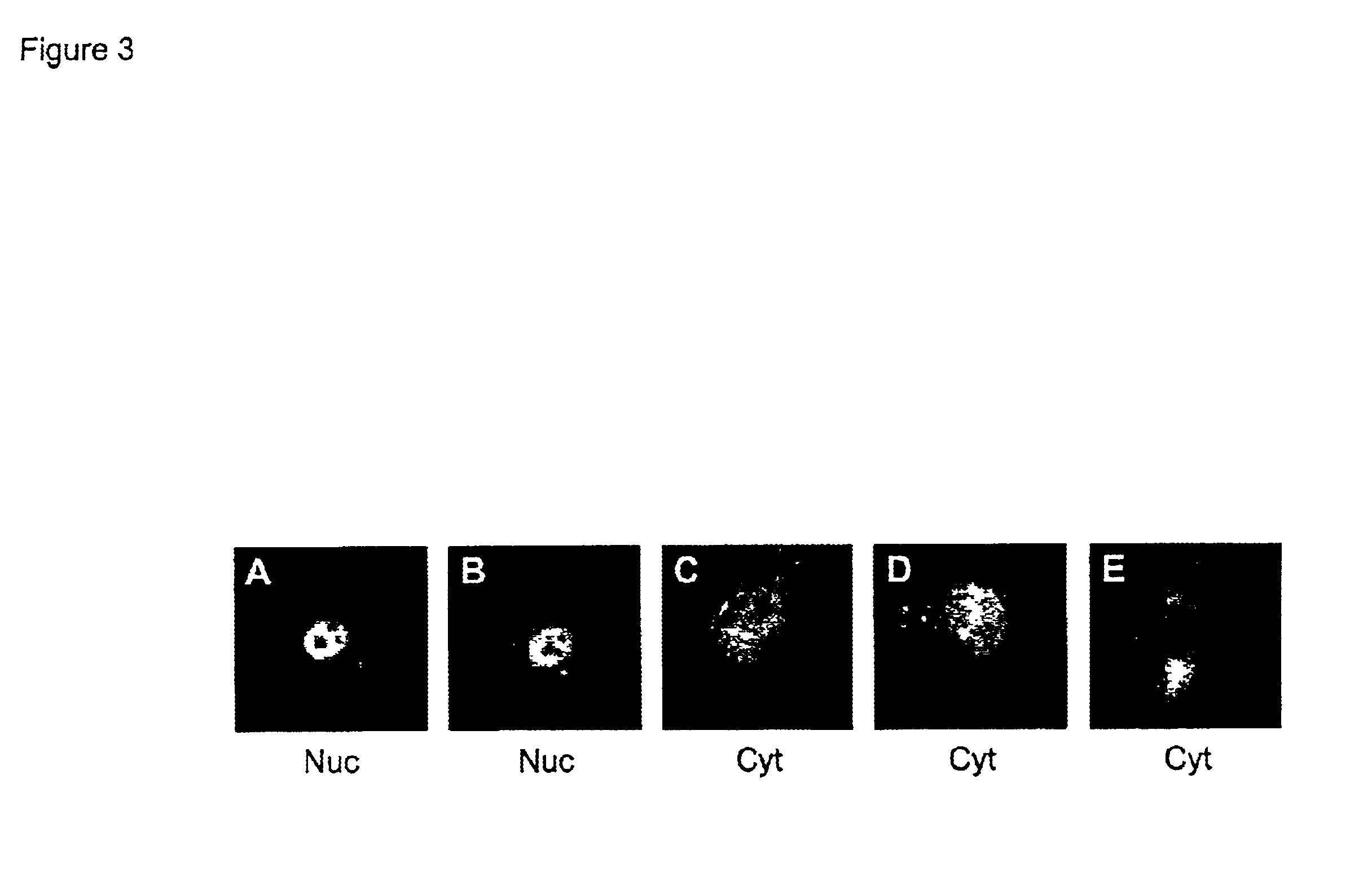
Adenovirus E4 protein variants for virus production
A method of packaging a recombinant viral vector is carried out by: (a) providing a packaging cell, the packaging cell containing and expressing a nucleic acid encoding a mutant adenovirus E4orf6 protein, the E4orf6 protein containing at least one mutation that renders the protein non-toxic to the host cell; (b) transfecting or infecting the packaging cell with a nucleic acid that encodes a recombinant viral vector (e.g., an adenovirus vector or an adeno-associated virus vector), where the vector lacks a functional gene encoding E4orf6 protein; (c) culturing the transfected cells; and then (d) collecting packaged recombinant viral vector from the cultured cells. Nucleic acids, vectors and packaging cells used for carrying out the methods, as well as proteins utilized in the methods, are also described.
Owner:WAKE FOREST UNIV HEALTH SCI INC
AAV5 nucleic acids
The present invention provides an adeno-associated virus 5 (AAV5) virus and vectors and particles derived therefrom. In addition, the present invention provides methods of delivering a nucleic acid to a cell using the AAV5 vectors and particles.
Owner:THE GOVERNMENT OF THE US SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER
Compositions and methods for helper-free production of recombinant adeno-associated viruses
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Adeno-associated virus vector for boosting immunogenicity of cells
InactiveUS7001765B2Improving immunogenicityBiocideGenetic material ingredientsAdeno associate virusVaccine Immunogenicity
The present invention provides an Adeno-Associated Virus (AAV) vector having a foreign DNA coding for a protein that boosts immunogenicity of cells. The invention also provides a vaccine containing such a vector and the use of both.
Owner:MEDIGENE
Methods for purifying adeno-associated virus
InactiveUS7625570B1Peptide/protein ingredientsViral antigen ingredientsAdeno associate virusHydrostatic pressure
The present invention provides methods of purifying adeno-associated virus (AAV) from compositions comprising AAV and at least a second, non-AAV; and methods for selectively inactivating a non-adeno-associated virus (non-AAV) in a liquid composition comprising AAV and the non-AAV. The methods generally involve subjecting the composition to hydrostatic pressure such that the non-AAV is selectively inactivated.
Owner:RGT UNIV OF CALIFORNIA
Enhanced aav-mediated gene transfer for retinal therapies
ActiveUS20150376240A1Preventing and arresting progressionPreventing and arresting of and vision lossBiocideSenses disorderAdeno associate virusRetinal treatments
Described herein are capsid proteins and adeno-associated viruses capable of targeting various types of ocular cells including bipolar and horizontal cells. Also described herein are methods of treating various ocular disorders in a subject in need thereof by administering to the subject an effective concentration of a composition comprising the recombinant adeno-associated virus (AAV) of the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
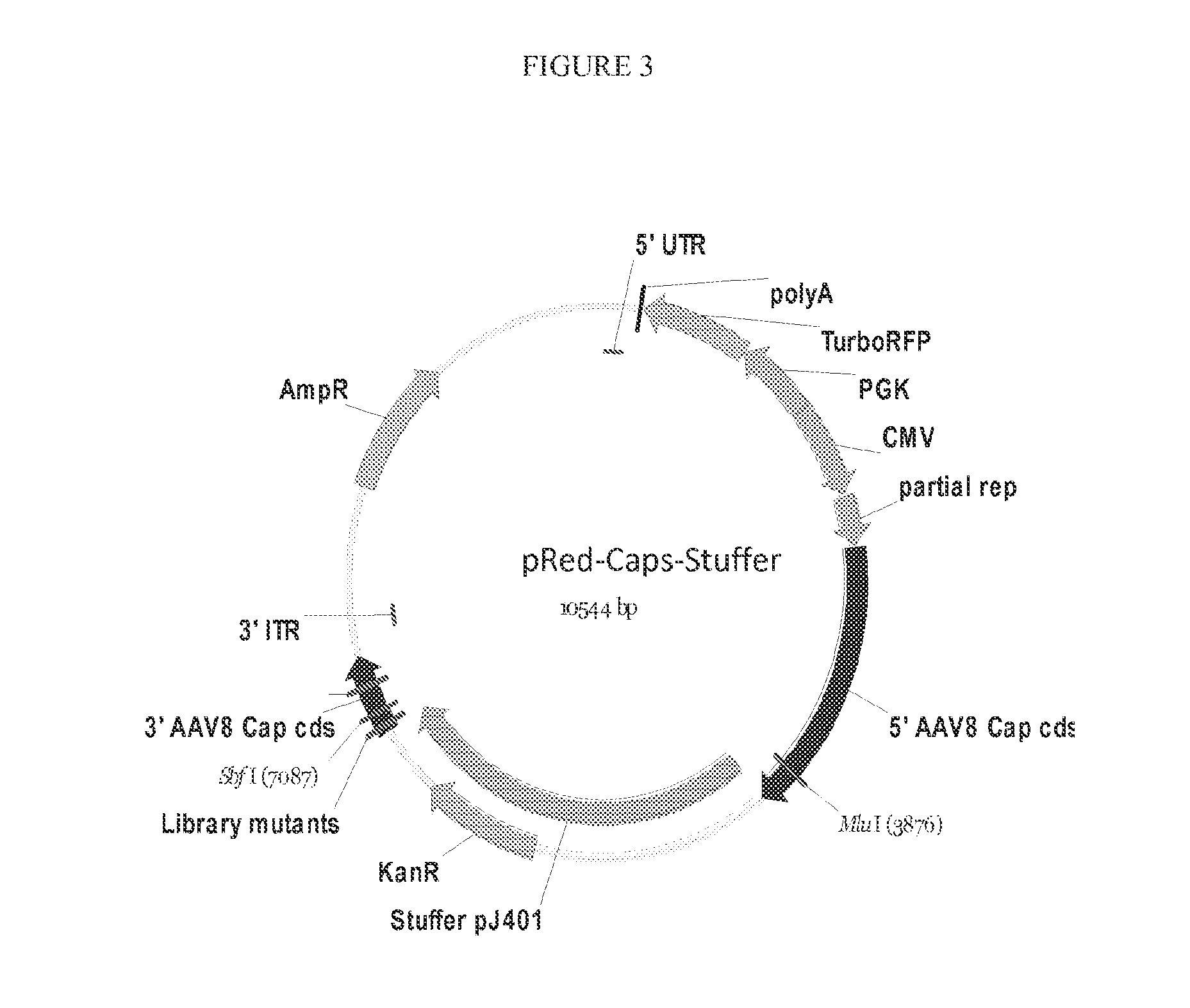
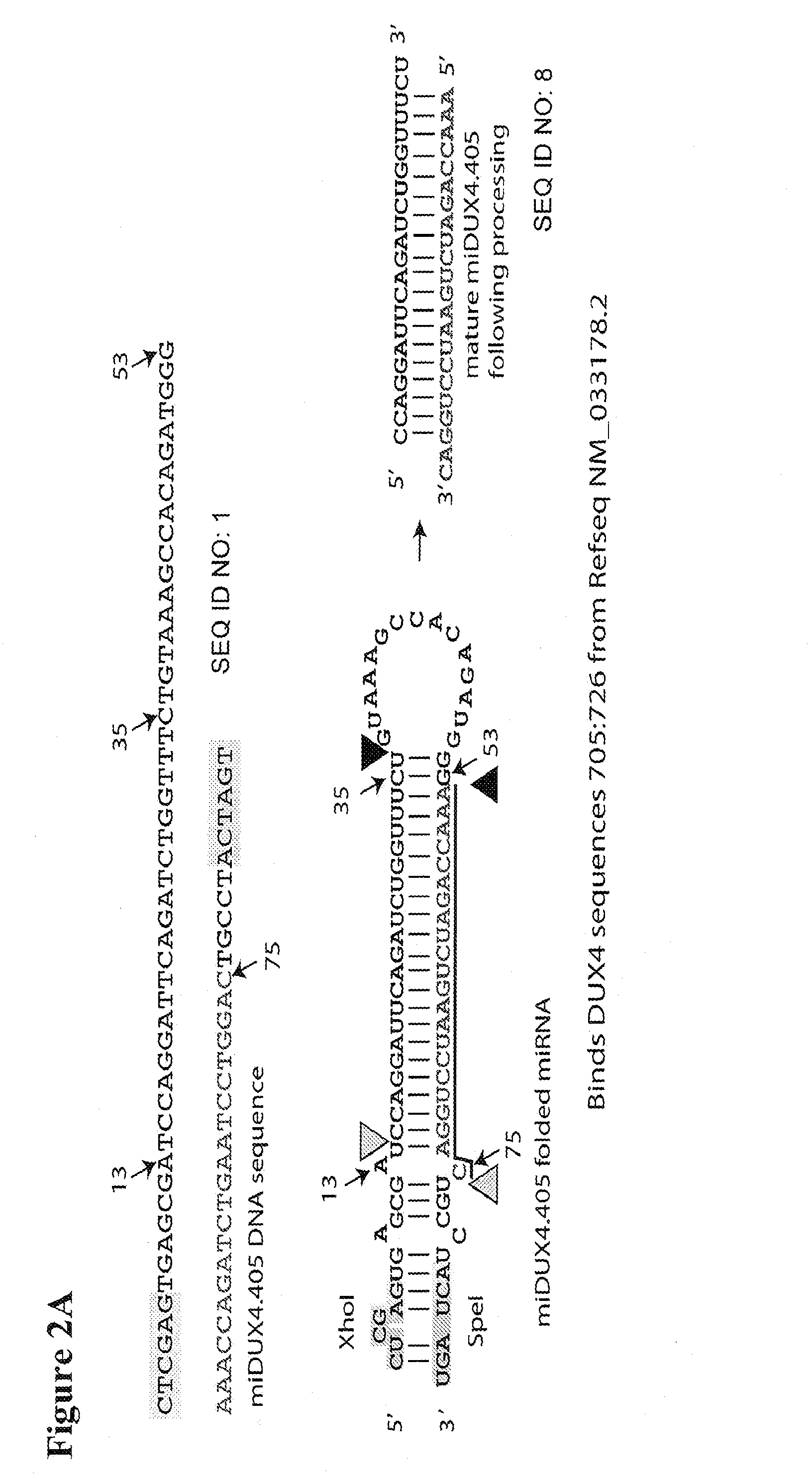
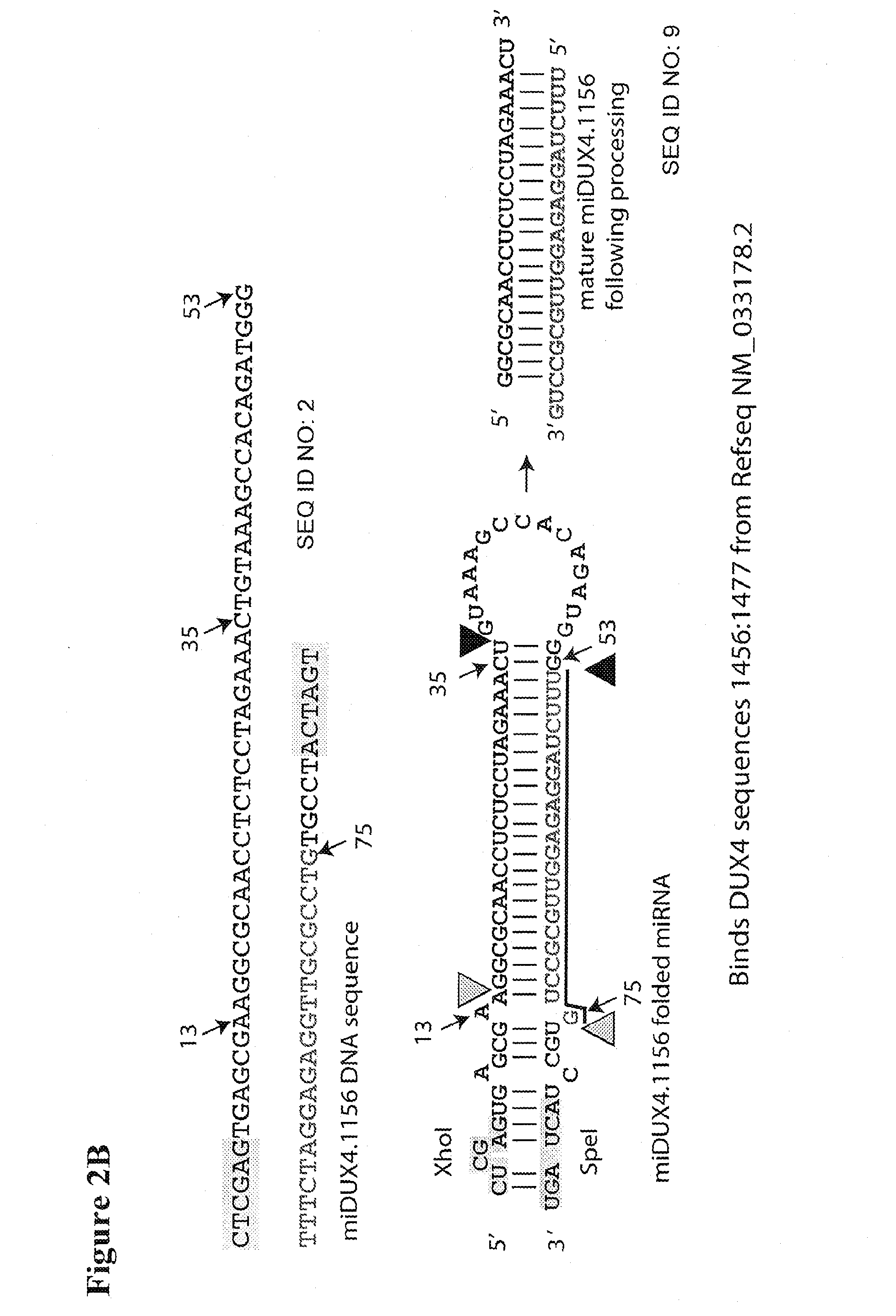
Recombinant Virus Products and Methods for Inhibition of Expression of DUX4
ActiveUS20140322169A1Ease of administrationEase of handlingBiocideSugar derivativesAdeno associate virusDna encoding
The present invention relates to RNA interference-based methods for inhibiting the expression of the DUX4 gene, a double homeobox gene on human chromosome 4q35. Recombinant adeno-associated viruses of the invention deliver DNAs encoding microRNAs that knock down the expression of DUX4. The methods have application in the treatment of muscular dystrophies such as facioscapulohumeral muscular dystrophy.
Owner:RES INST AT NATIONWIDE CHILDRENS HOSPITAL
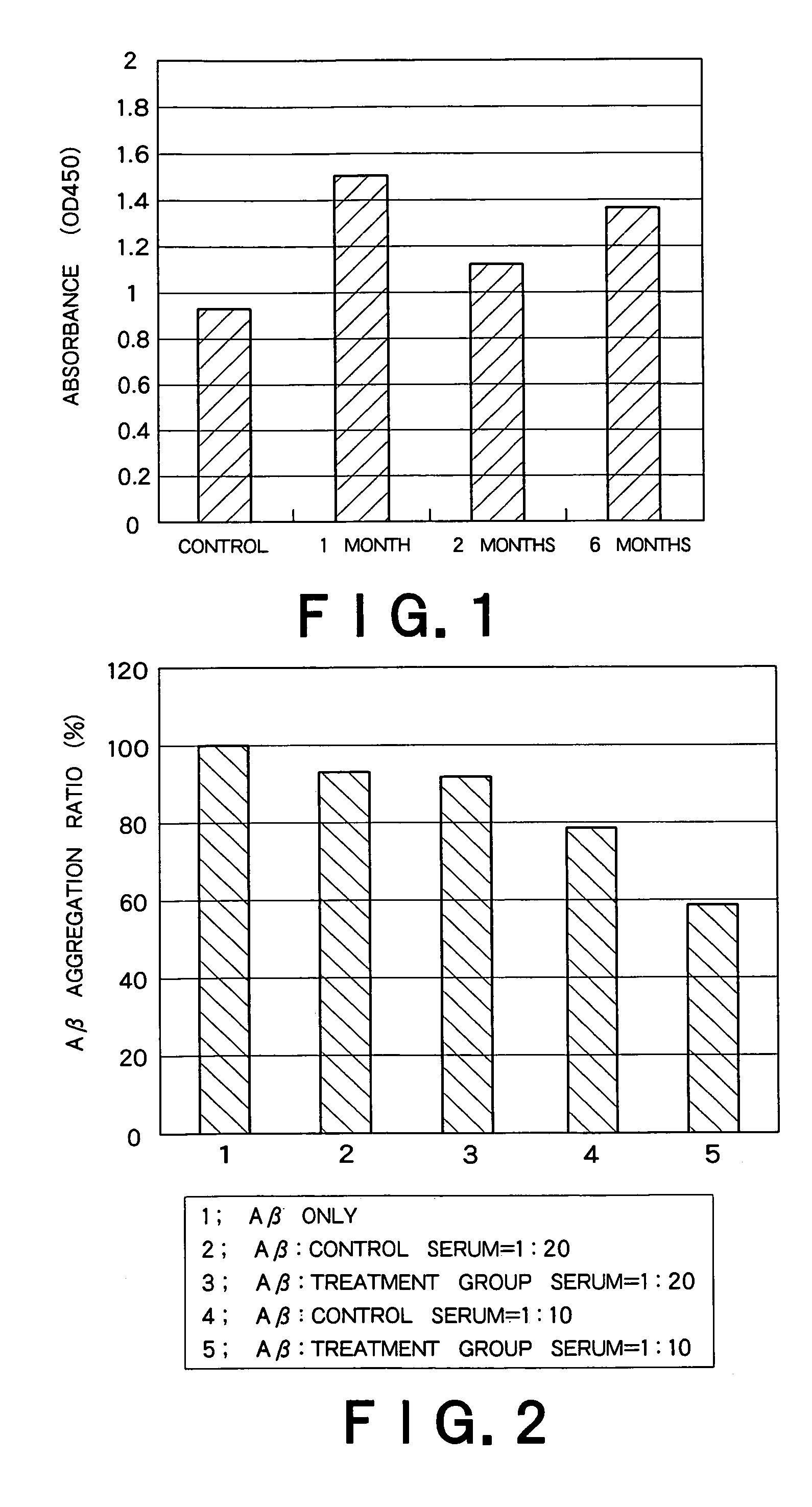
Recombinant adeno-associated virus vector for treatment of Alzheimer disease
ActiveUS8318687B2Highly safe treatmentReduce concentrationNervous disorderVirusesAdeno associate virusDna encoding
Owner:TABIRA TAKESHI +1
Use of apotosis inhibiting compounds in degenerative neurological disorders
The invention provides methods and compositions for localized delivery of a vector comprising a therapeutic agent to a specific region of the brain associated with a neurodegenerative diseases that is characterized by an excess buildup of buildup of intracellular protein aggregates. In particular, the invention provides methods and compositions used to deliver an adeno-associated virus vector (AAV) comprising a nucleotide sequence encoding an inhibitor of apoptosis protein (IAP) to cells in the region.
Owner:MEIRAGTX UK II LTD
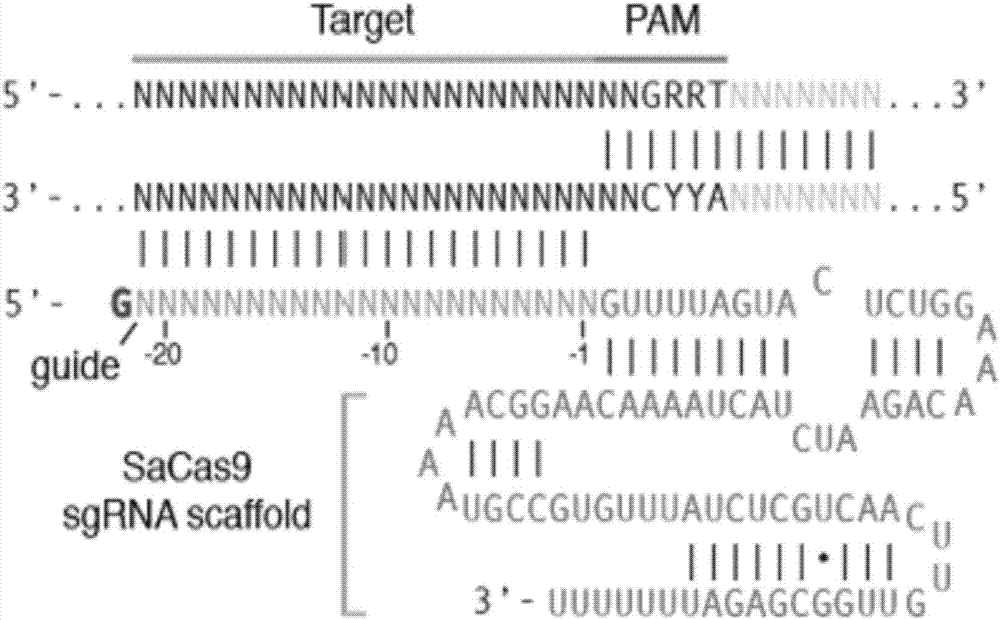
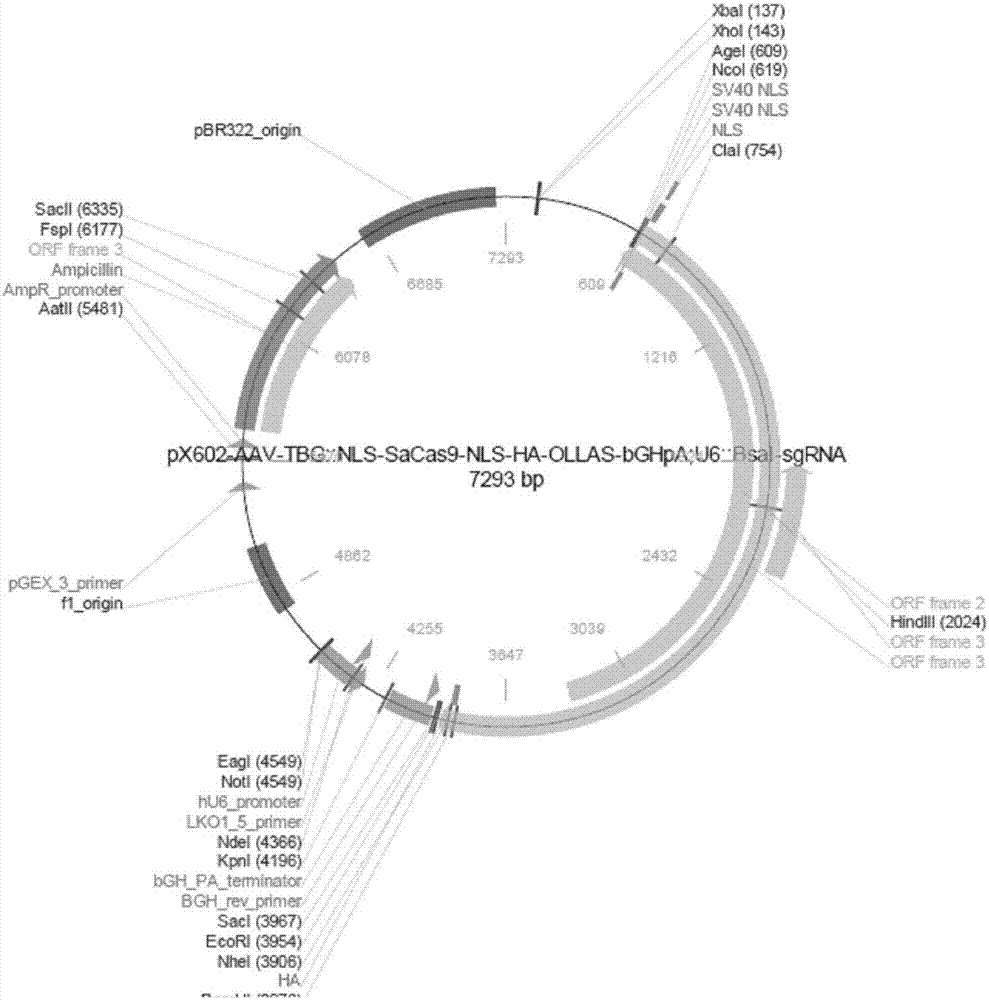
rAAV8-CRISPR-SaCas9 system and application of system in preparing hepatitis B therapeutics
The invention discloses a gRNA sequence. The sequence is capable of editing a DNA sequence in the manner of taking a hepatitis B viral genome specific locus as a target sequence. The invention also discloses a CRISPR-SaCas9 system containing the gRNA sequence and a recombinant adeno-associated virus packaged with the system. The system and the packaging virus show higher HBV scavenging activity in cells and in transgenic mice body. On the 38th day after twice continuous high dose injection, the contents of HBsAg and HBeAg in experimental group mice serum, compared with the control group, are respectively reduced by 62.96+ / -7.59% and 65.18+ / -3.08%; the HBV DNA content in the serum, compared with the control group, is reduced by 92.82+ / -3.67%; the liver and other visceral organs of the experimental mice are all free from any pathologic change, the off-target effect is also not detected and the application prospects of the gRNA sequence and the corresponding CRISPR-SaCas9 system provided by the invention in preparing the hepatitis B therapeutics are shown.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Methods of increasing efficiency of vector penetration of target tissue
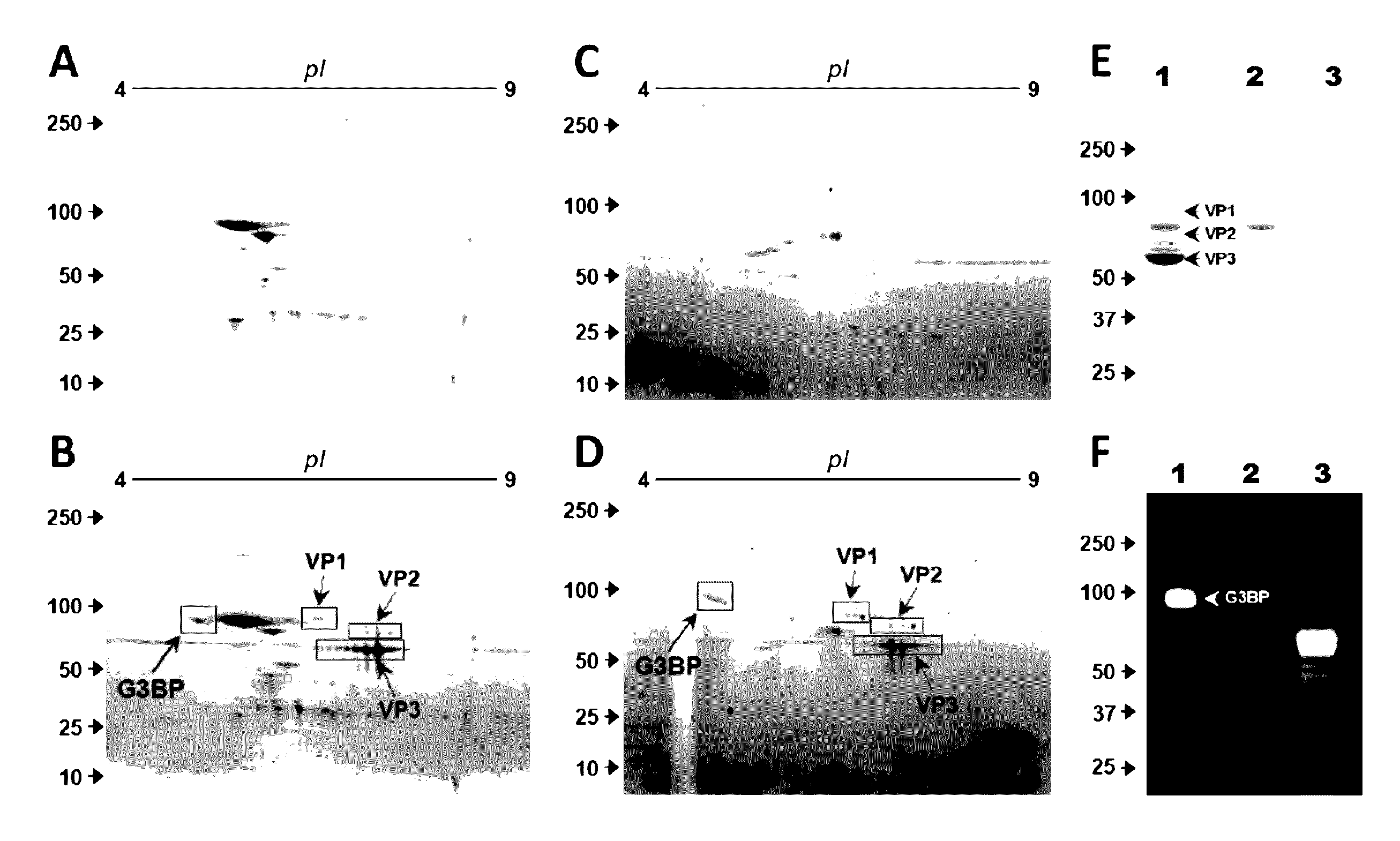
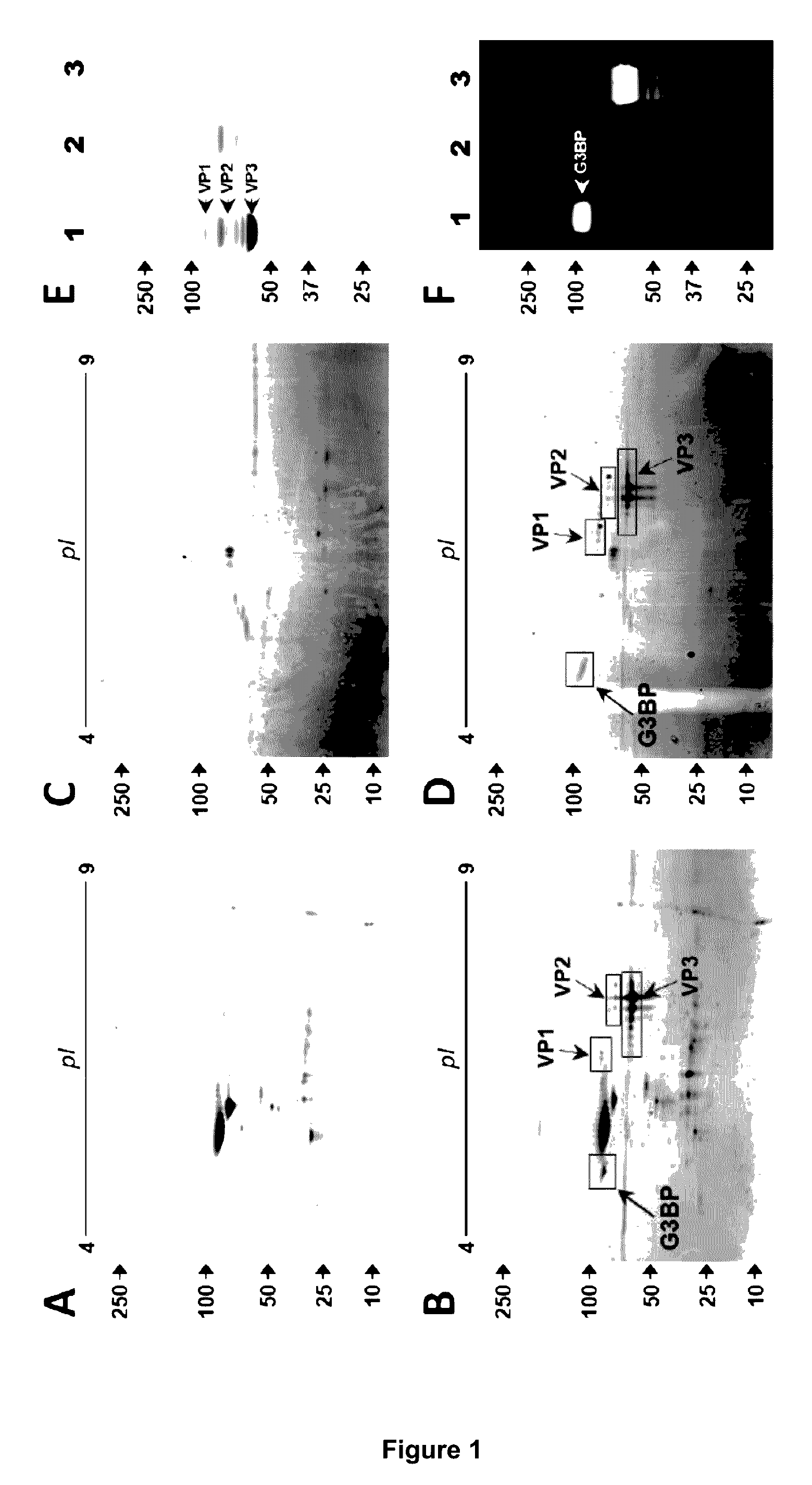
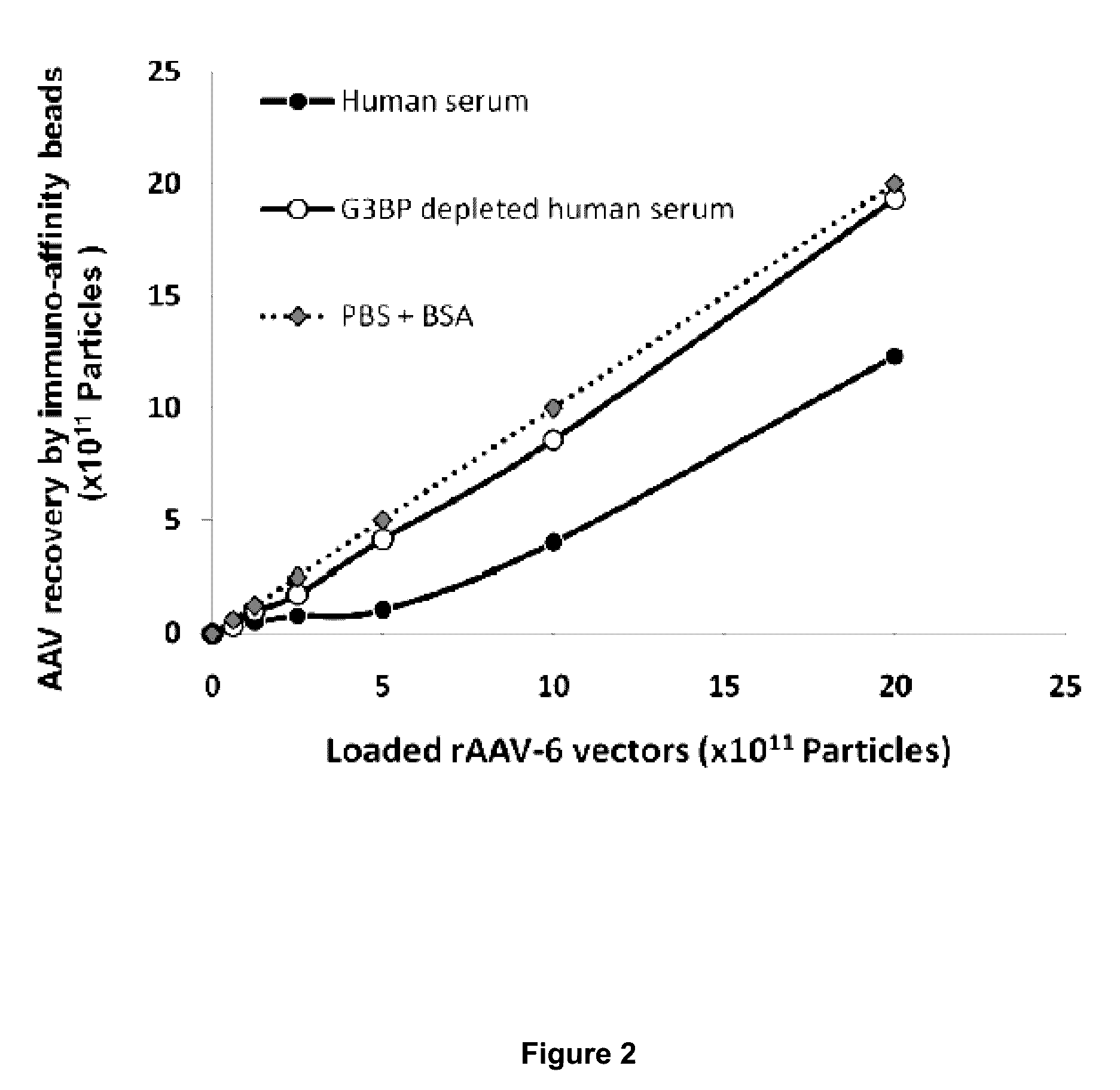
InactiveUS9546112B2Improve efficiencyReduce the binding forceOrganic active ingredientsElectrotherapyGalectin 3 binding proteinAdeno associate virus
Methods for increasing the efficiency of target tissue penetration of an adeno-associated virus (AAV) vector in a patient are provided. In some aspects, the methods involve inhibiting the interaction of the serum protein galectin 3 binding protein (G3BP) with AAV vector. Further provided are methods for reducing tissue distribution of a virus or for neutralizing a virus harbored by an organ destined for transplant, or newly transplanted, by administering a composition comprising G3BP.
Owner:UNIV PIERRE & MARIE CURIE +4
Recombinant human NADH (nicotinamide-adenine dinucleotide) dehydrogenase subunit-4 gene and constructing method of expression vector thereof
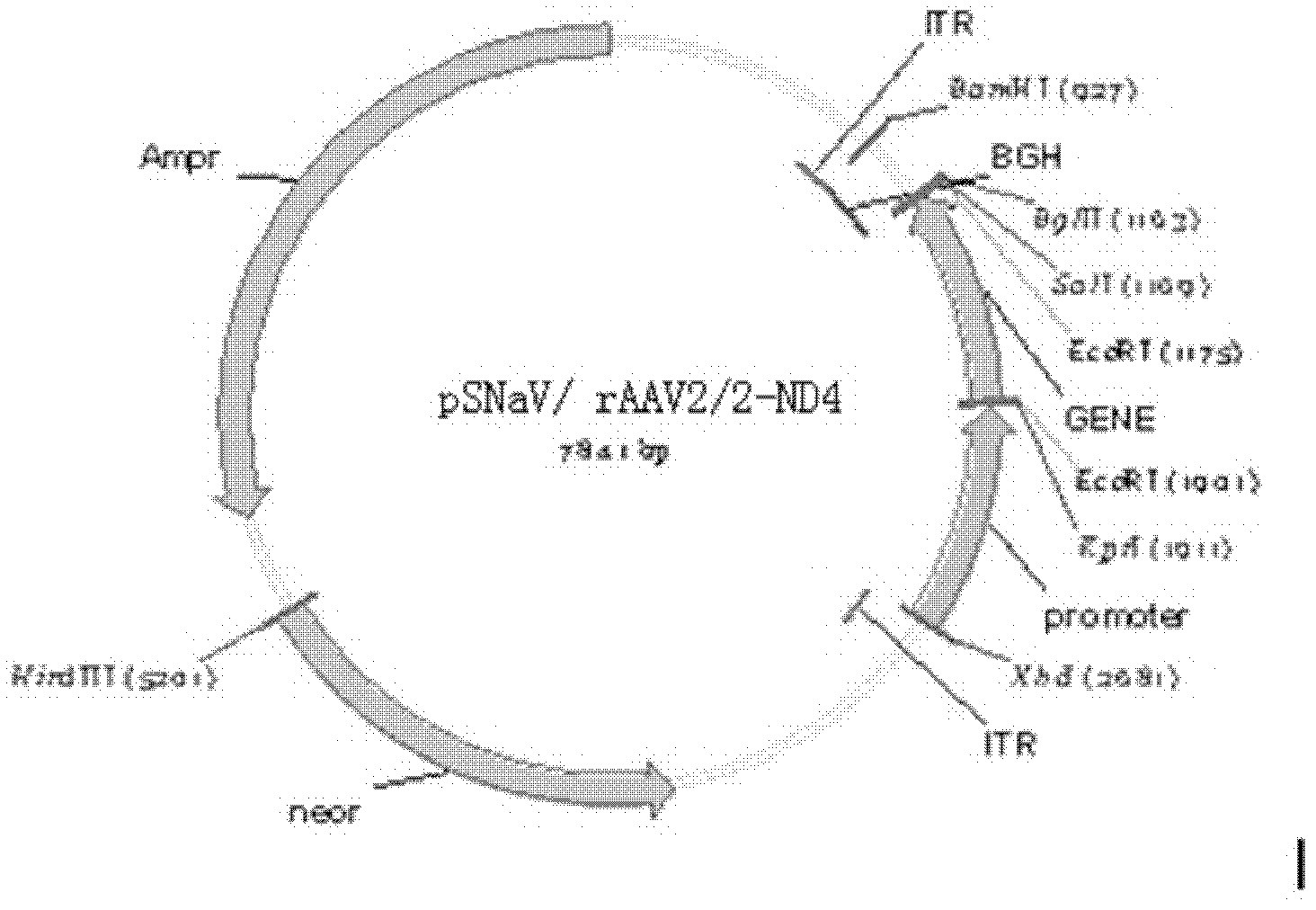
The invention discloses a recombinant human NADH (nicotinamide-adenine dinucleotid) dehydrogenase subunit-4 gene and a constructing method of an expression vector thereof. The nucleotide sequence of the gene is shown in SEQ ID NO:1, and the size of the nucleotide sequence is 2889 bp. The constructing steps of adeno-associate virus vectors are as follows: firstly constructing a recombinant adeno-associated virus vector containing the human NADH dehydrogenase subunit-4 gene, and then coating, infecting, purifying, concentrating and identifying the recombinant adeno-associated virus. According to the method, the recombinant adeno-associated virus vector with the recombinant human NADH dehydrogenase subunit-4 gene can be quickly and simply constructed, and is packaged to obtain the adeno-associated virus vector with complex defects. The gene can be used for gene therapy of LHON (leber's hereditary optic neuropathy).
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Delivery of therapeutic agents to the bone
This invention relates to compositions and methods of delivering therapeutic agents to bone. More specifically, the invention relates to endowing a large molecule vectors i.e., adeno virus, retrovirus, liposomes, micelles, natural and synthetic polymers, or combinations thereof, with the ability to target bone tissue in vivo and with improved stability in the blood, by attaching multiple copies of acid amino acid peptides. One preferred embodiment of the invention relates to endowing an adeno-associated virus (AAV) vector with the ability to target bone-tissue in vivo and improve its stability, by the addition of multiple acidic amino acid peptides attached to the capsid of the viral vector.
Owner:SAINT LOUIS UNIVERSITY
Enhanced AAV-mediated gene transfer for retinal therapies
Described herein are capsid proteins and adeno-associated viruses capable of targeting various types of ocular cells including bipolar and horizontal cells. Also described herein are methods of treating various ocular disorders in a subject in need thereof by administering to the subject an effective concentration of a composition comprising the recombinant adeno-associated virus (AAV) of the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com