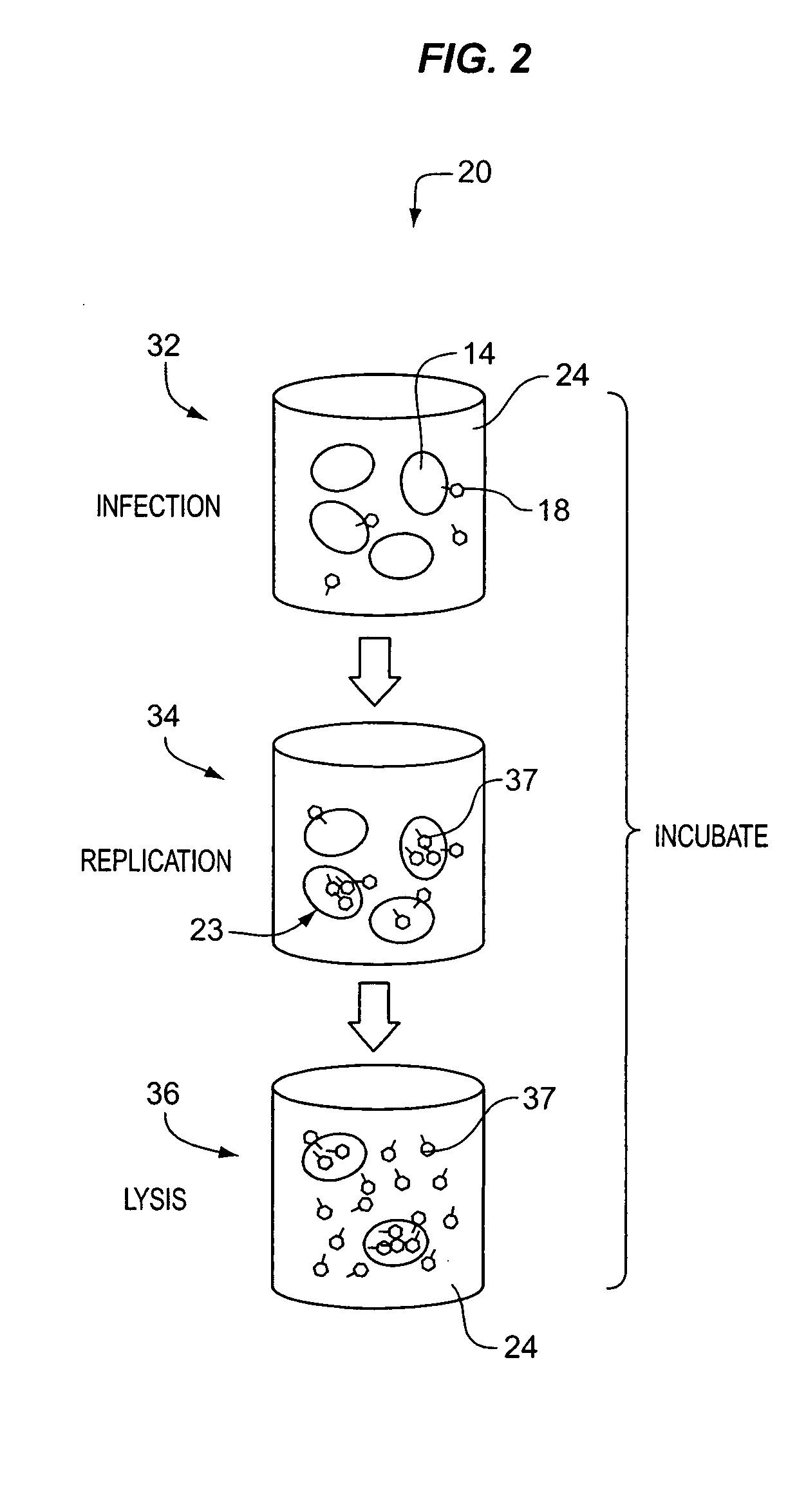
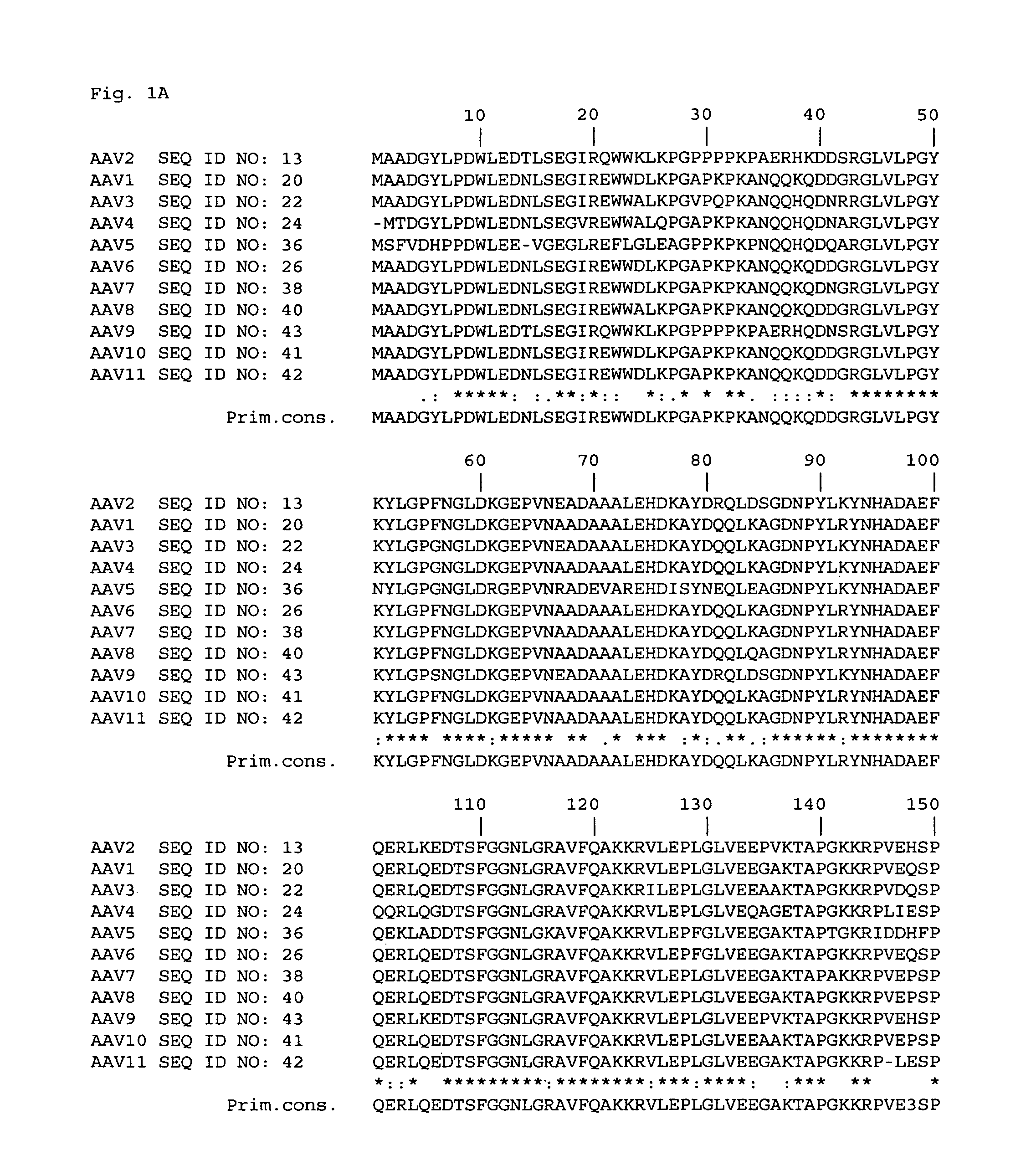
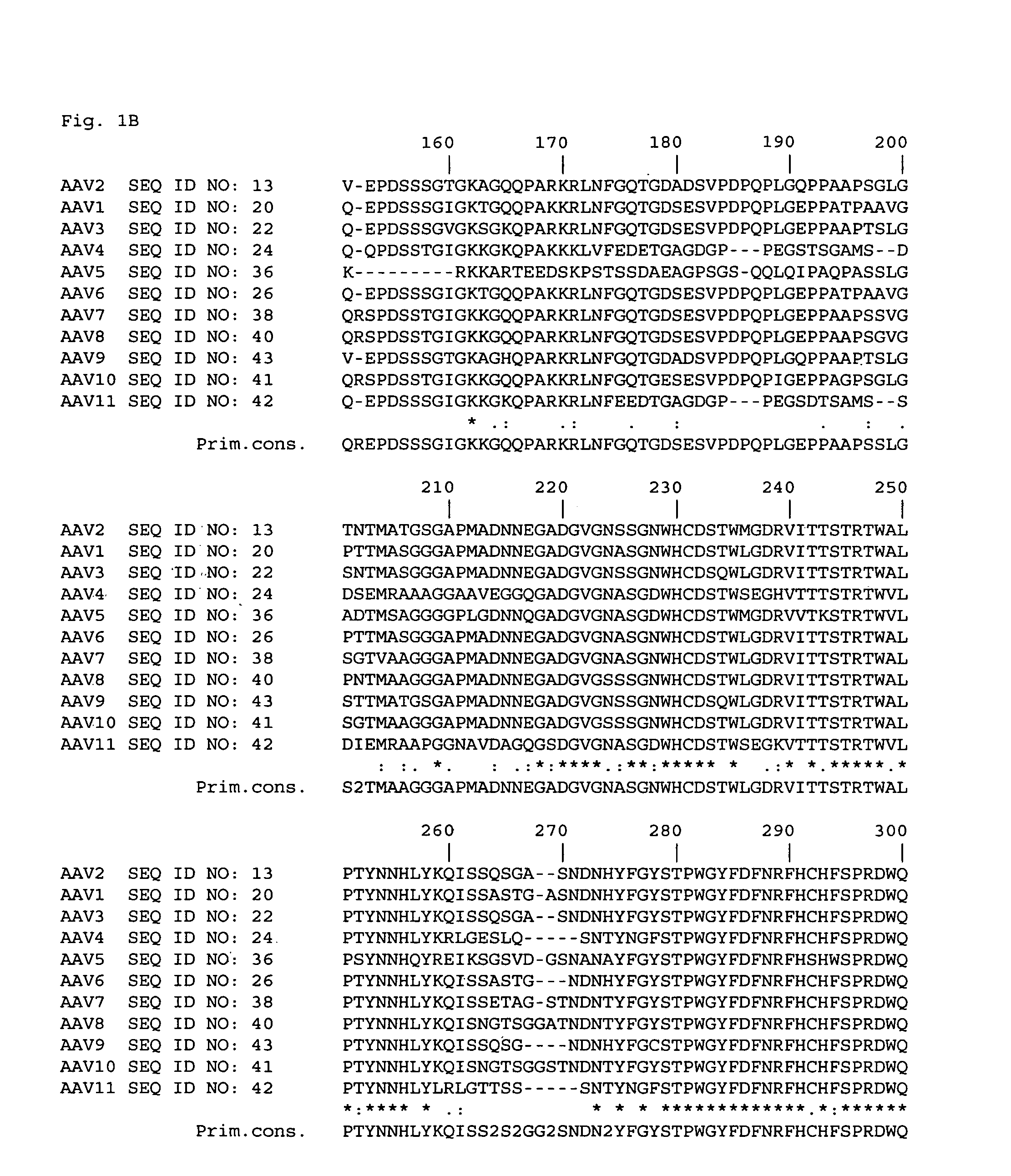
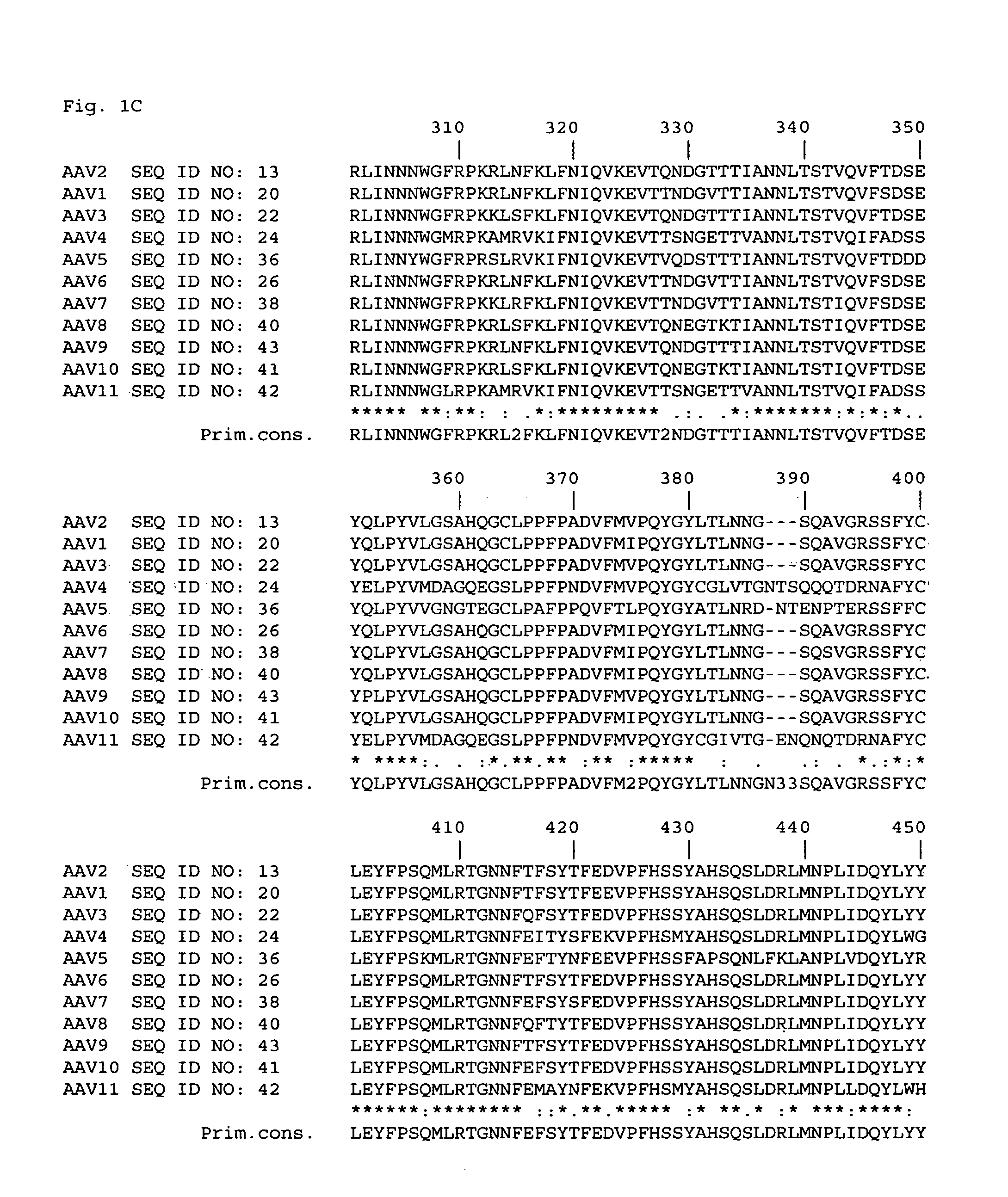
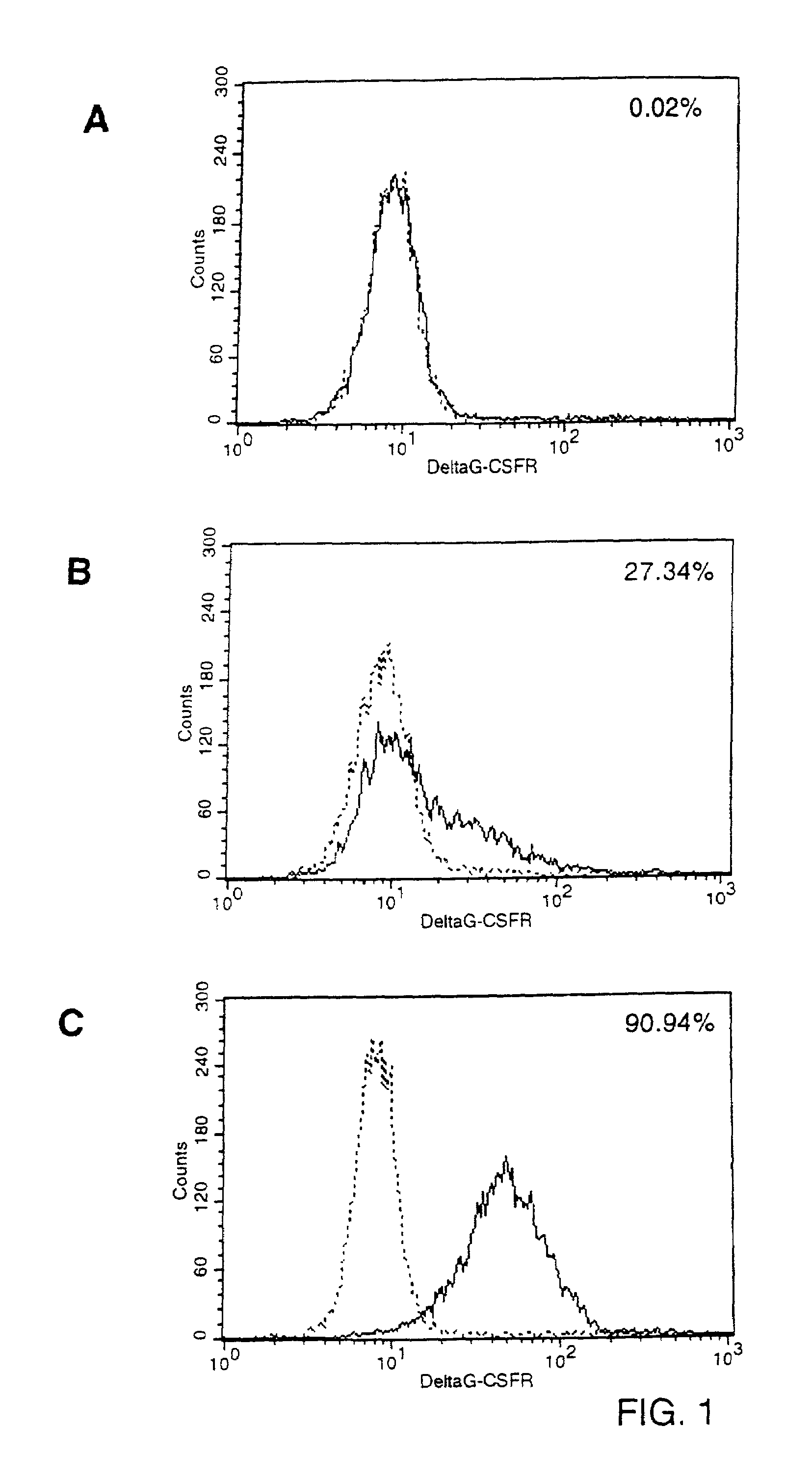
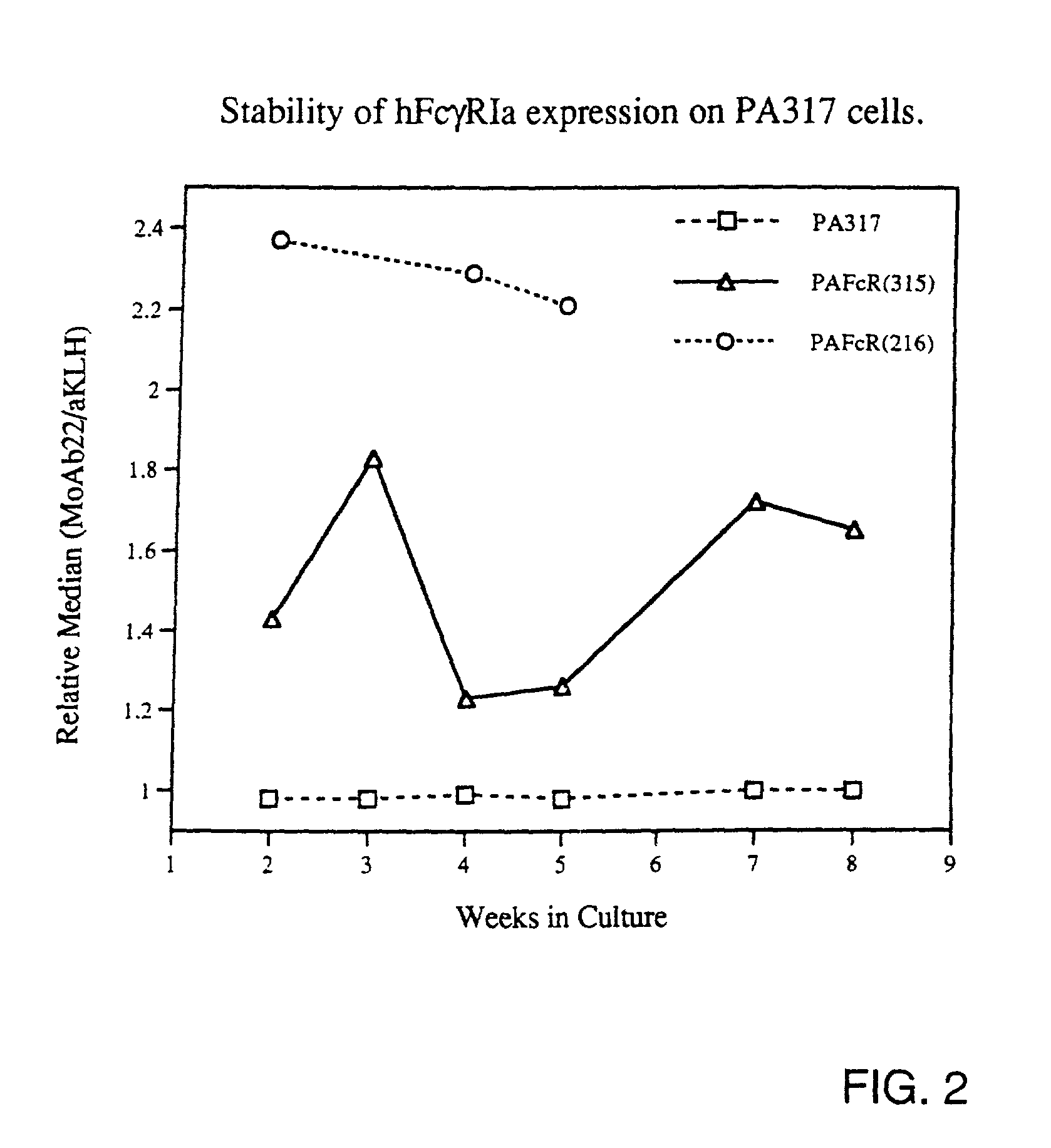
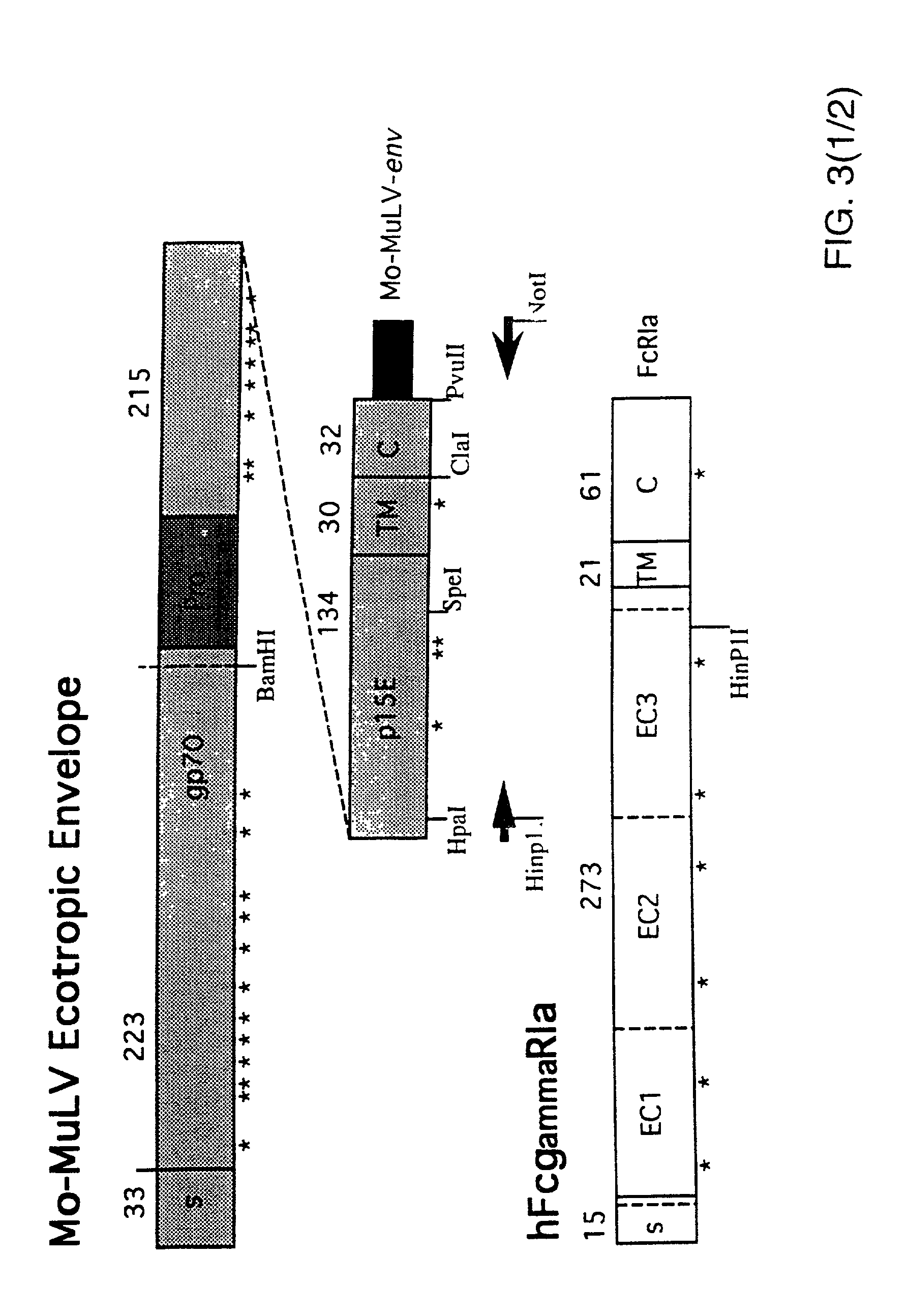
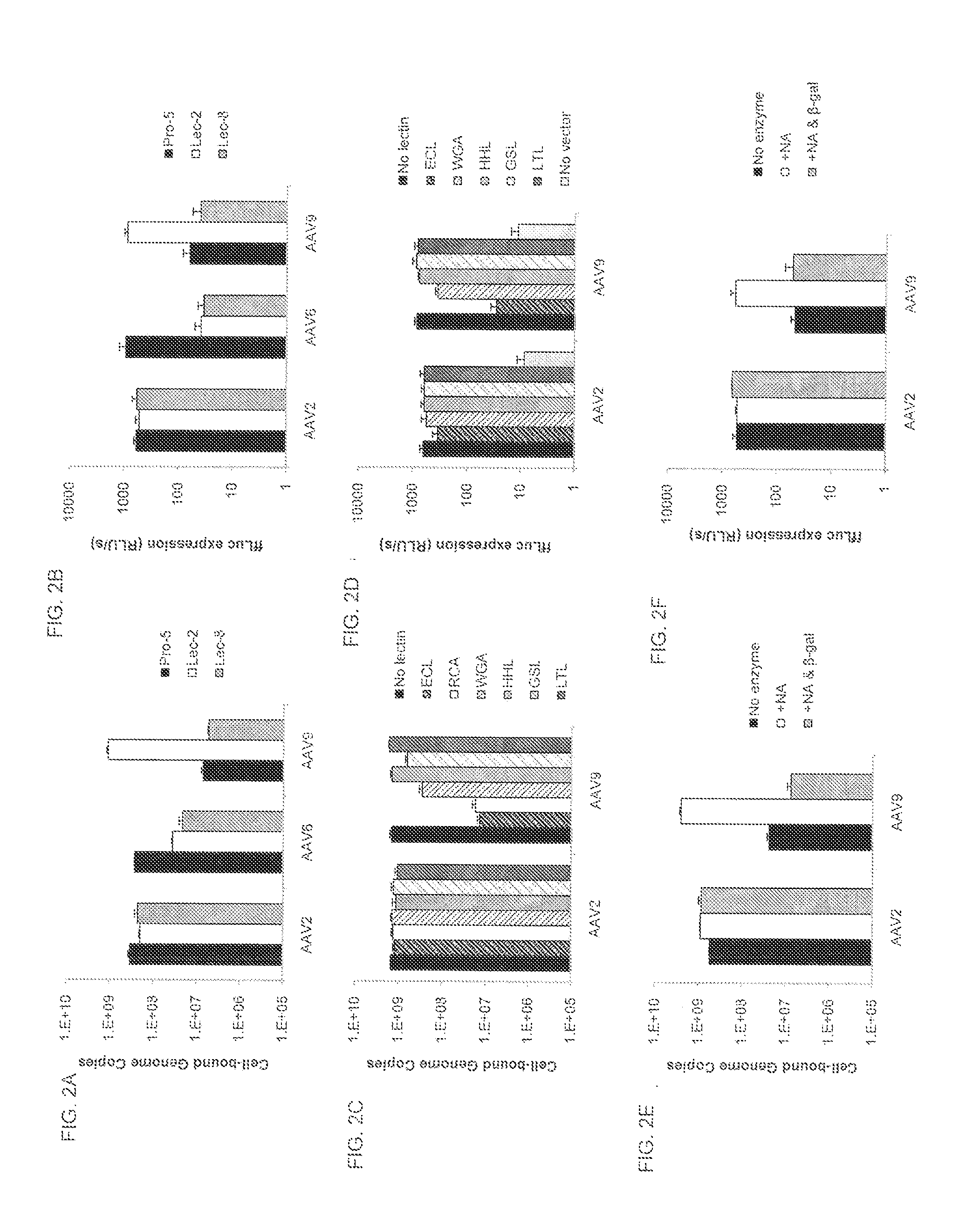Patents
Literature
1086 results about "Capsid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A capsid is the protein shell of a virus. It consists of several oligomeric structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may not correspond to individual proteins, are called capsomeres. The capsid encloses the genetic material of the virus.
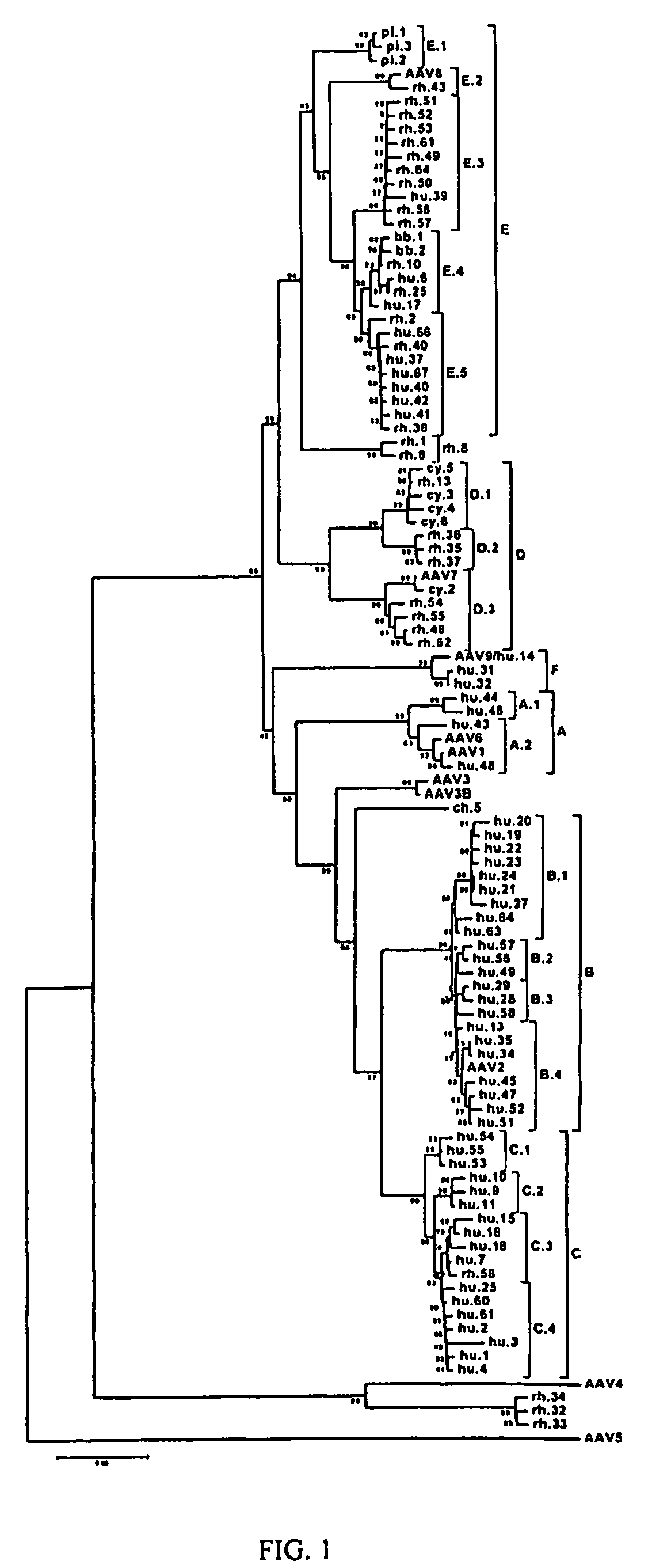
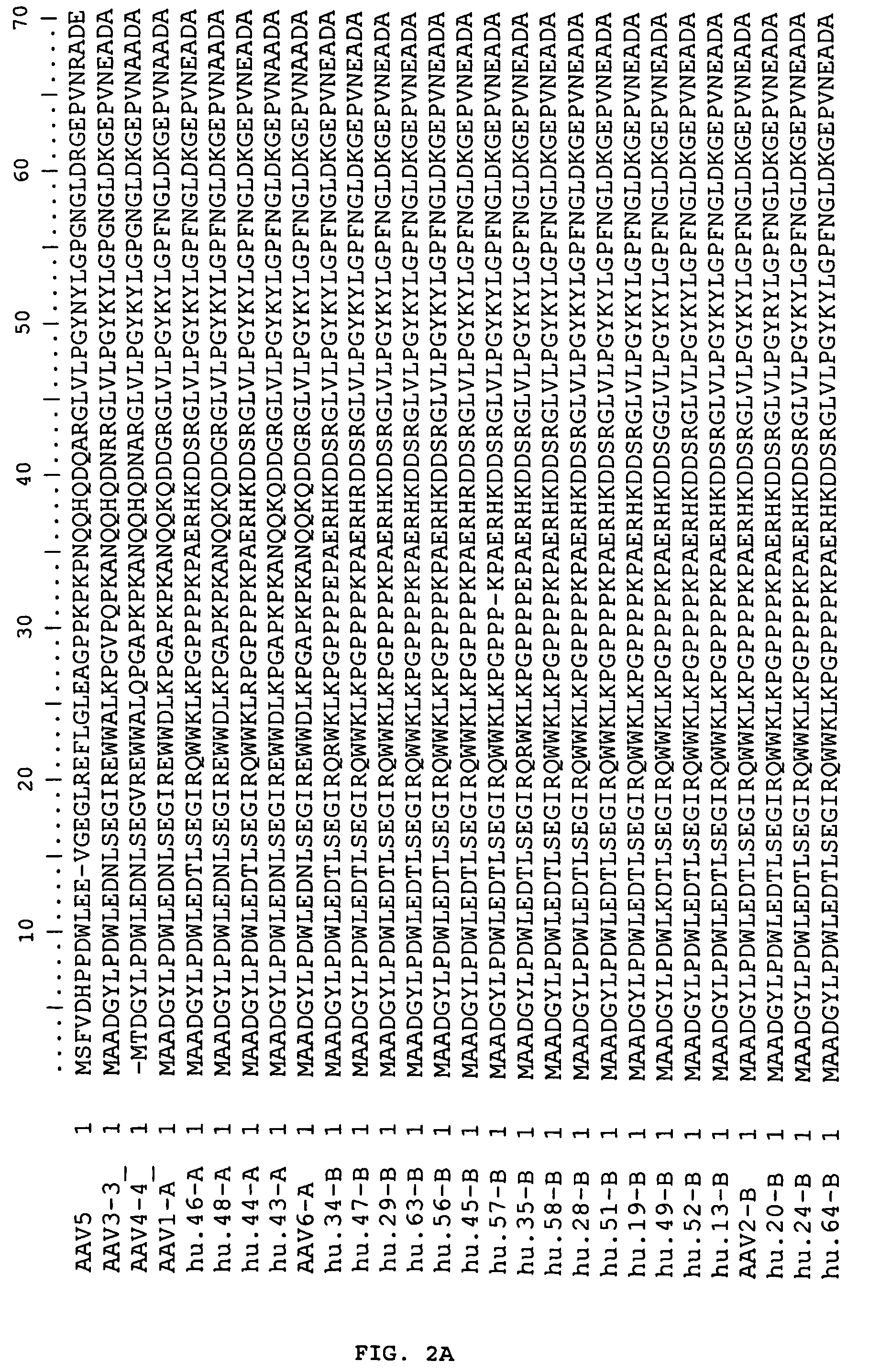
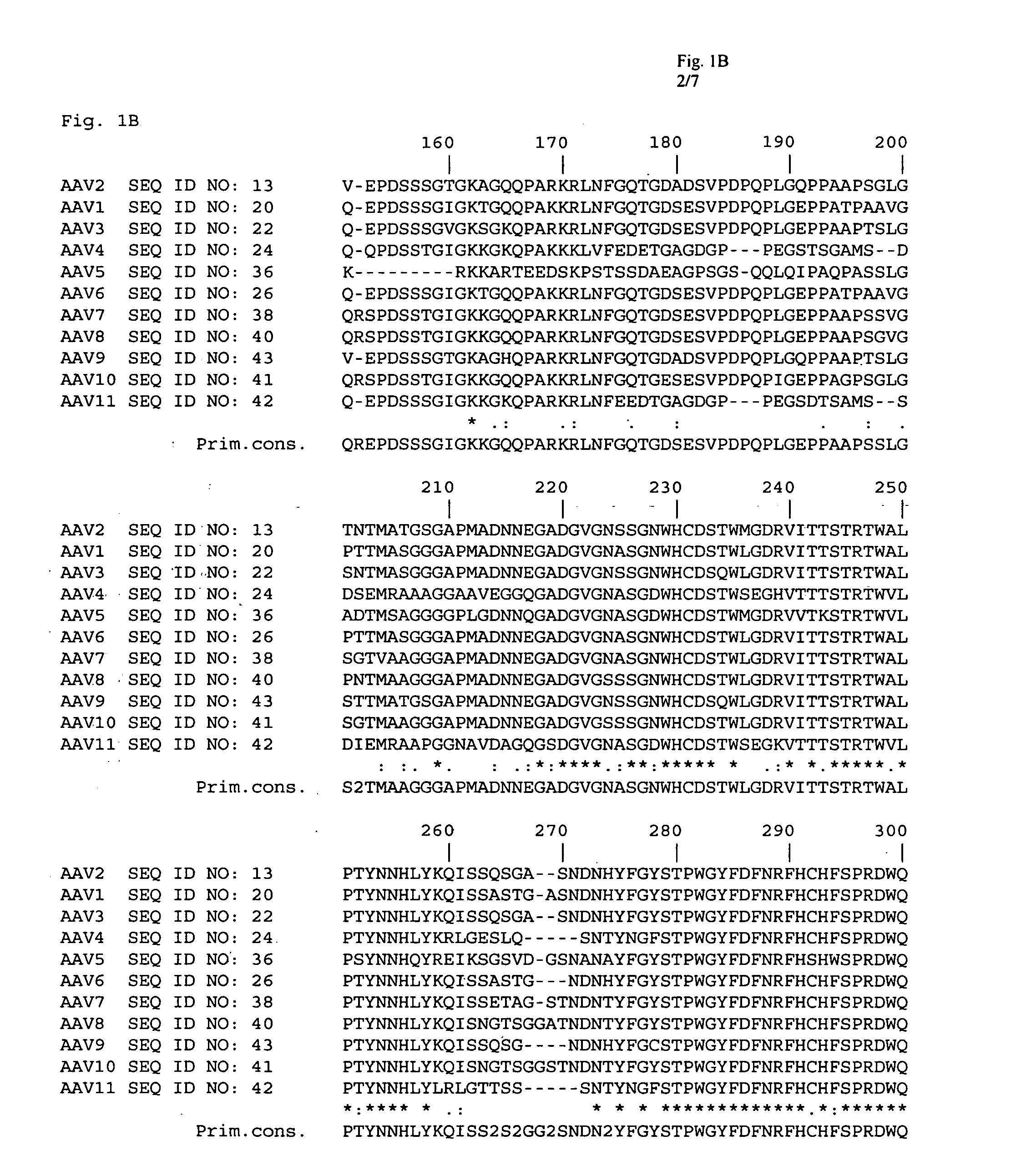
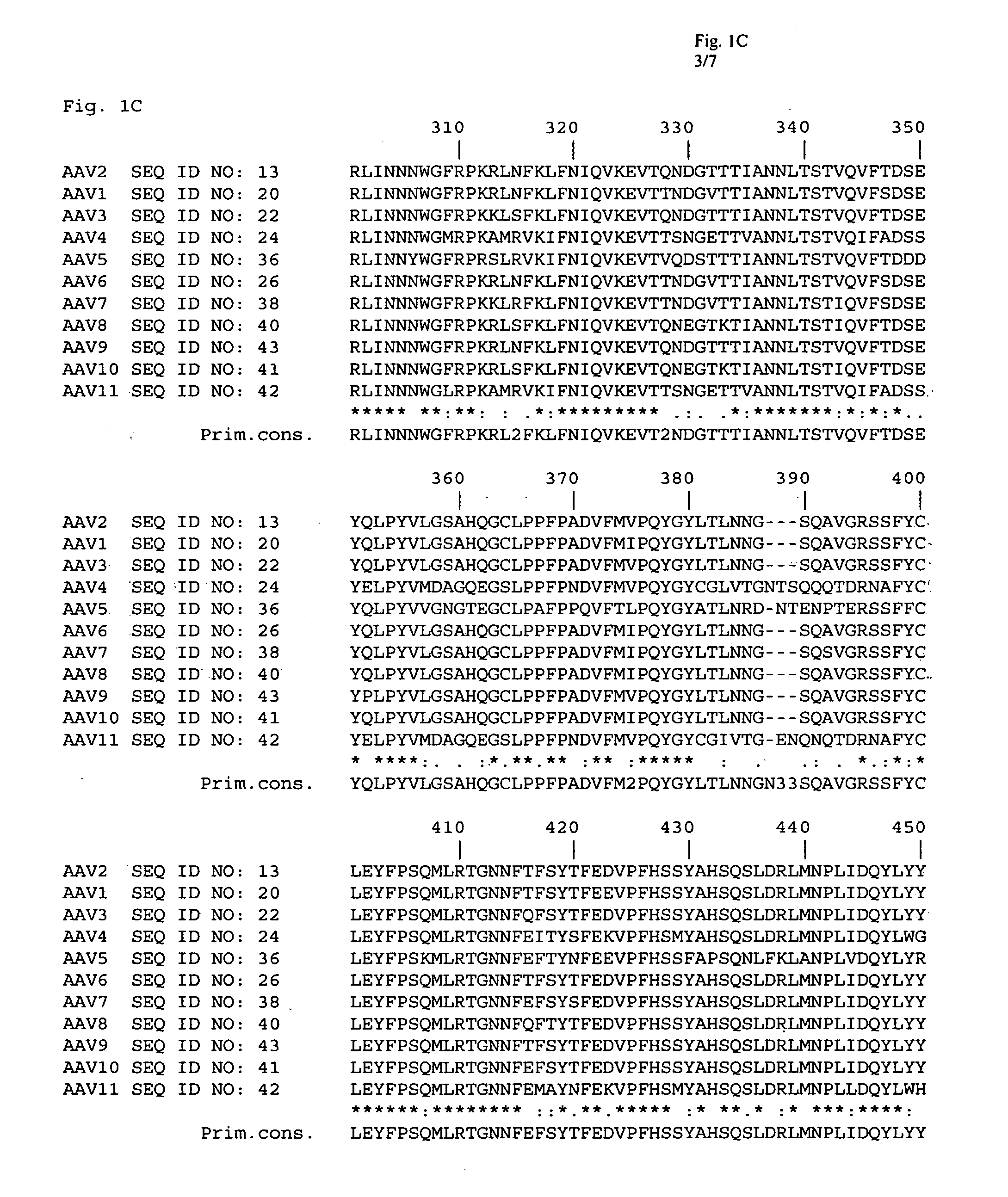
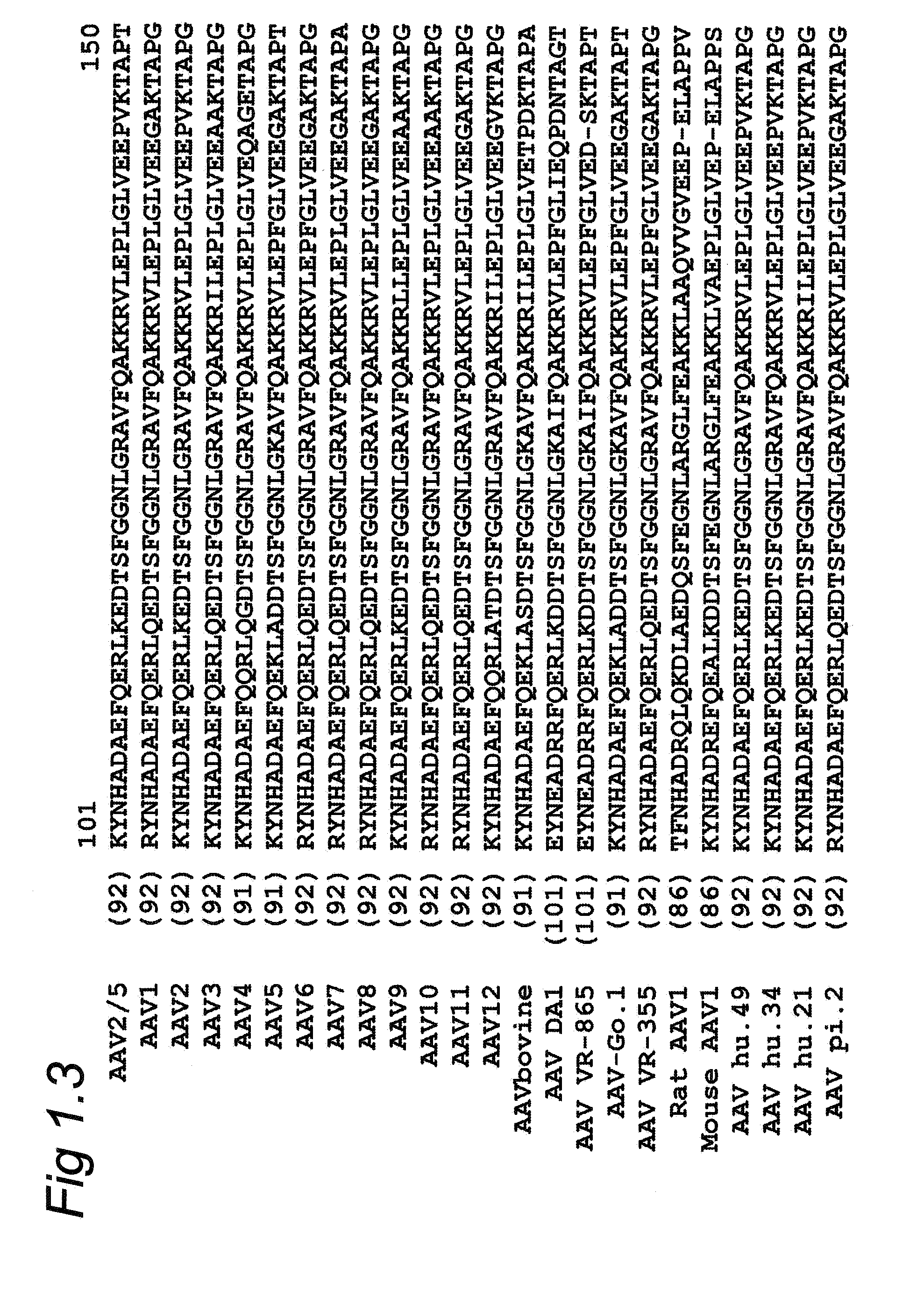
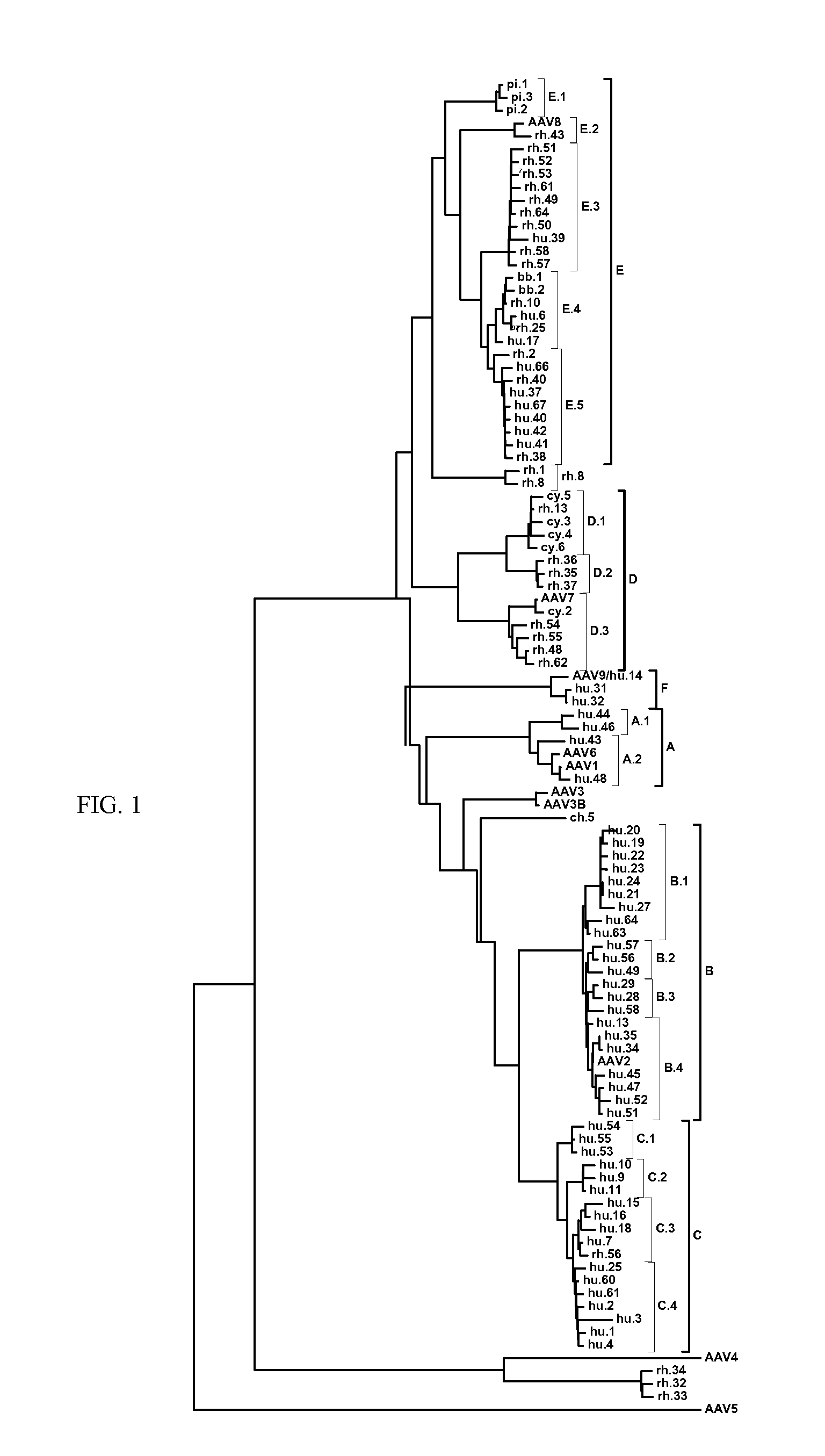
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Virus vectors and methods of making and administering the same
The present invention provides genetically-engineered parvovirus capsids and viruses designed to introduce a heterologous gene into a target cell. The parvoviruses of the invention provide a repertoire of vectors with altered antigenic properties, packaging capabilities, and / or cellular tropisms as compared with current AAV vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Small Molecule Modulators of HIV-1 Capsid Stability and Methods Thereof
InactiveUS20130165489A1Inhibiting and suppressing and preventing viral infectionBiocideOrganic chemistryViral infectionVirology
The present invention includes a method of inhibiting, suppressing or preventing a viral infection in a subject, comprising administering to the subject a pharmaceutical composition comprising one or more of the compounds useful within the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20090202490A1Reduce the binding forceAltered infectivityOrganic active ingredientsBiocideCell type specificNeutralizing antibody
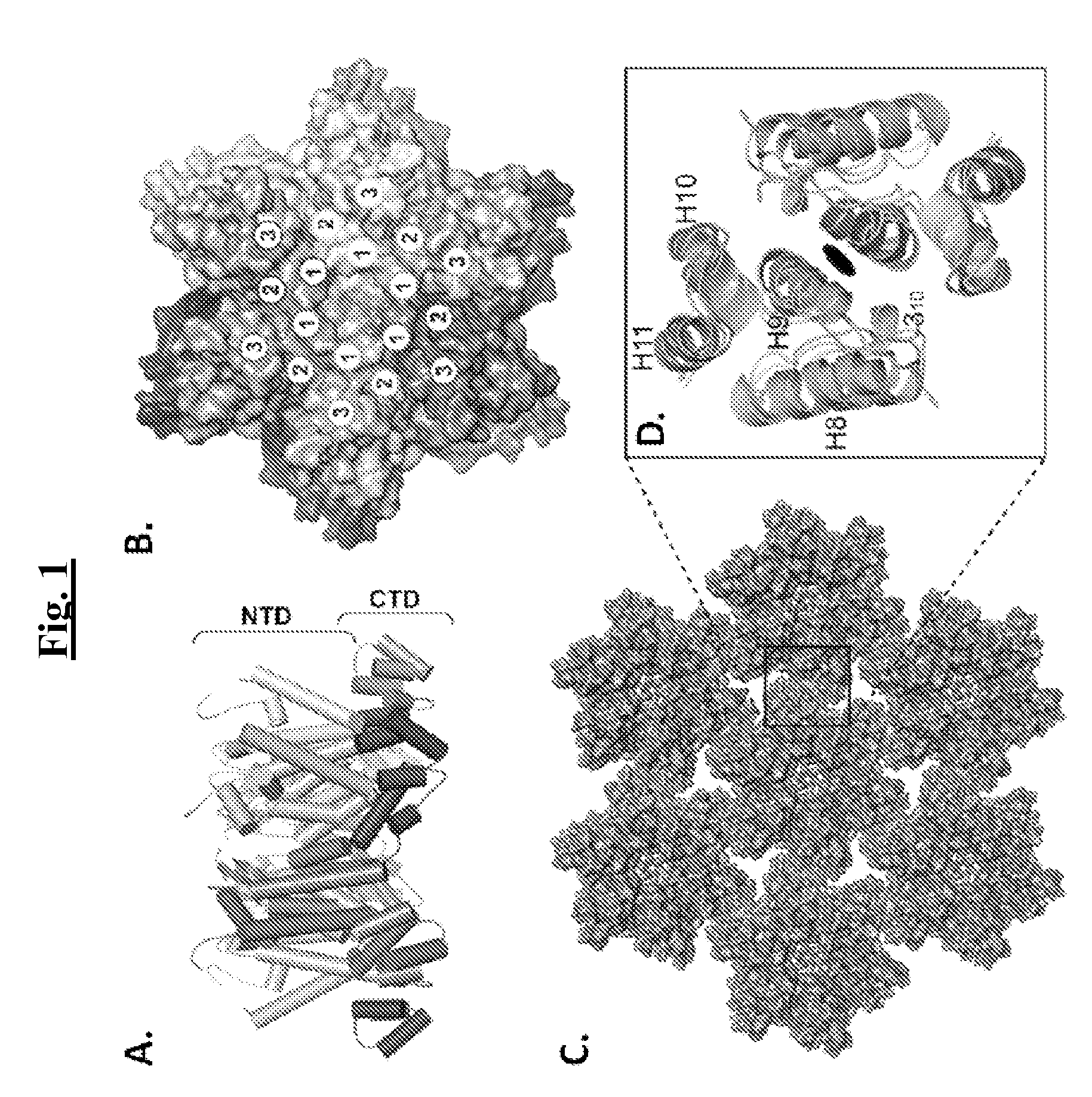
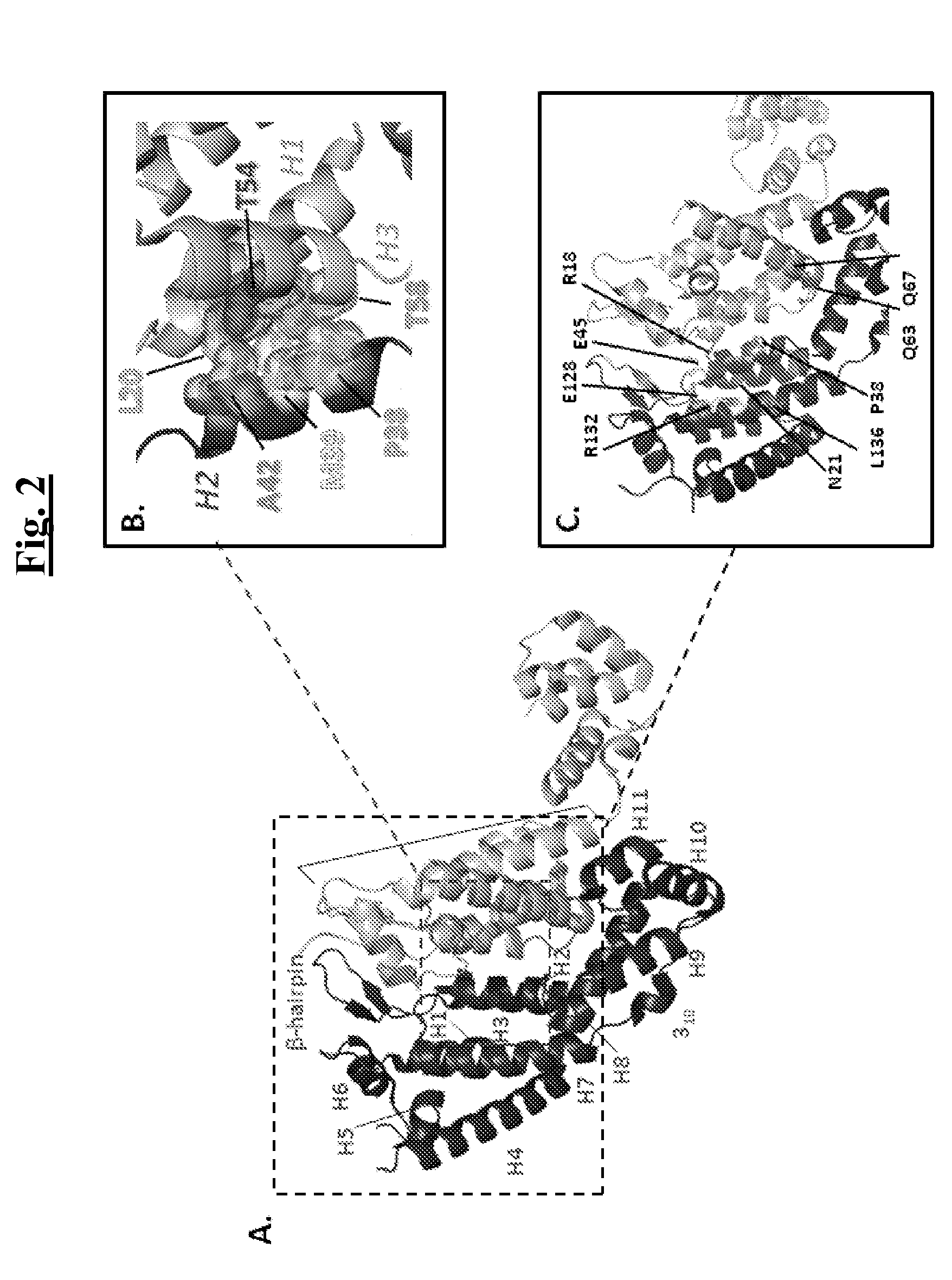
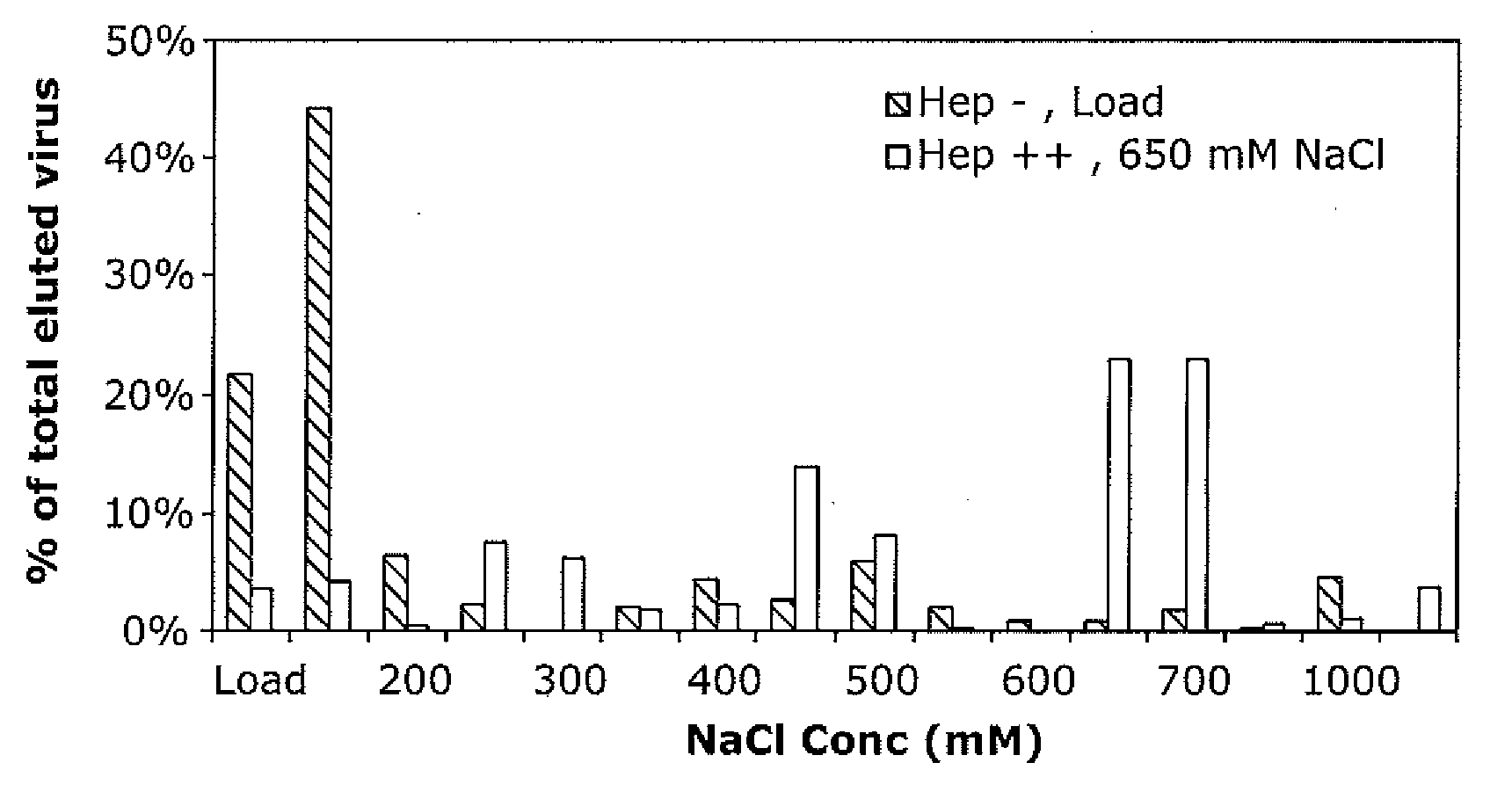
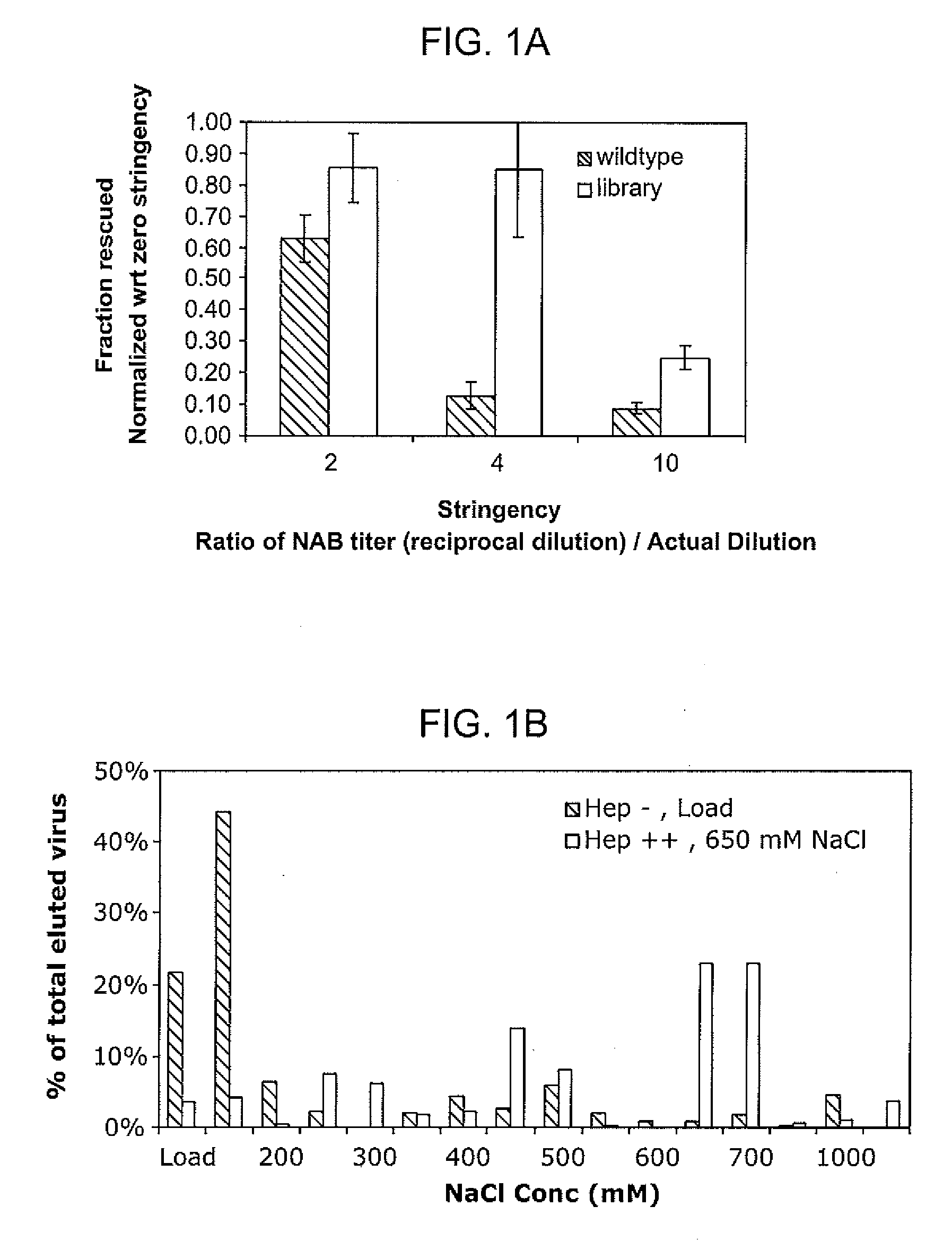
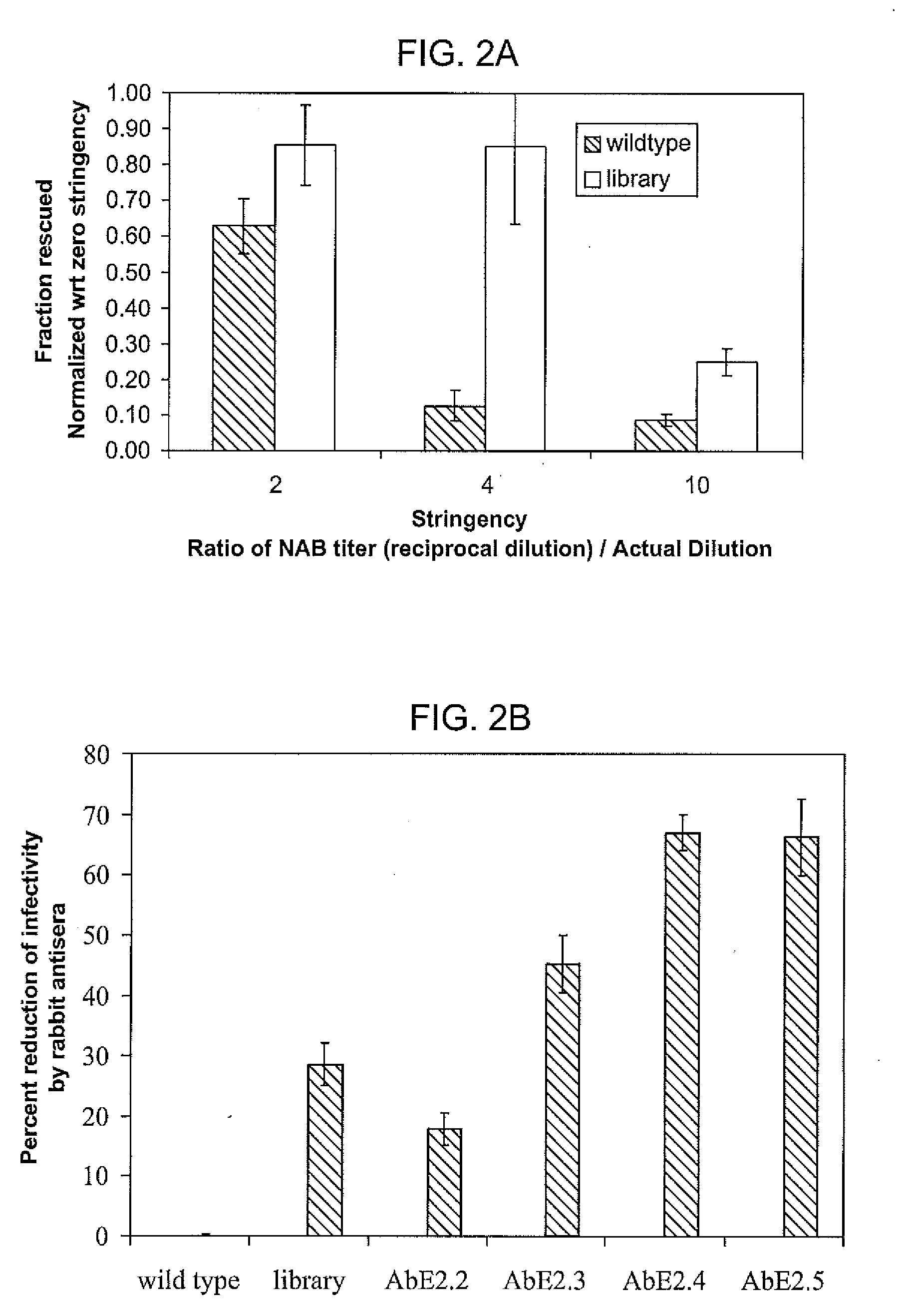
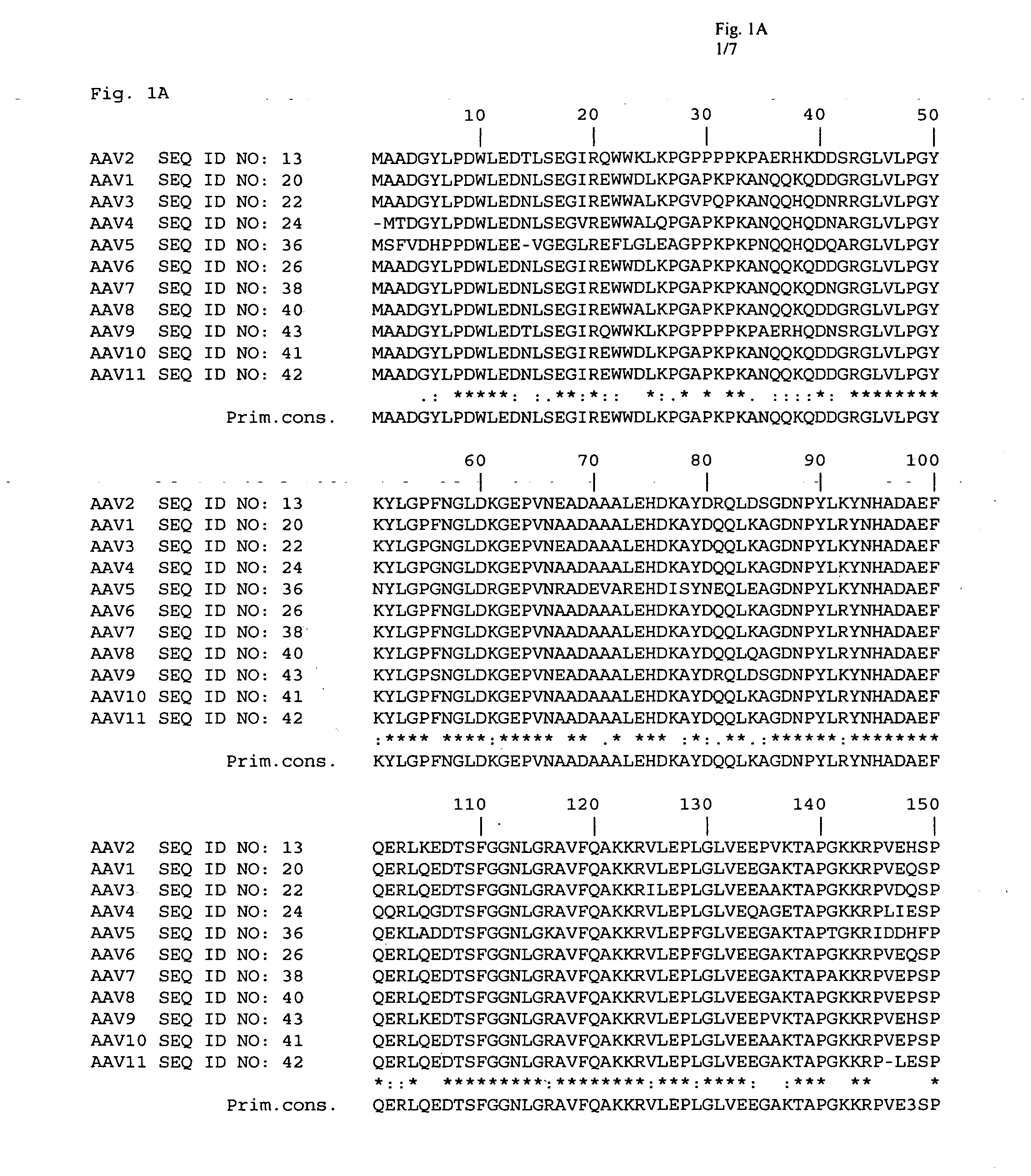
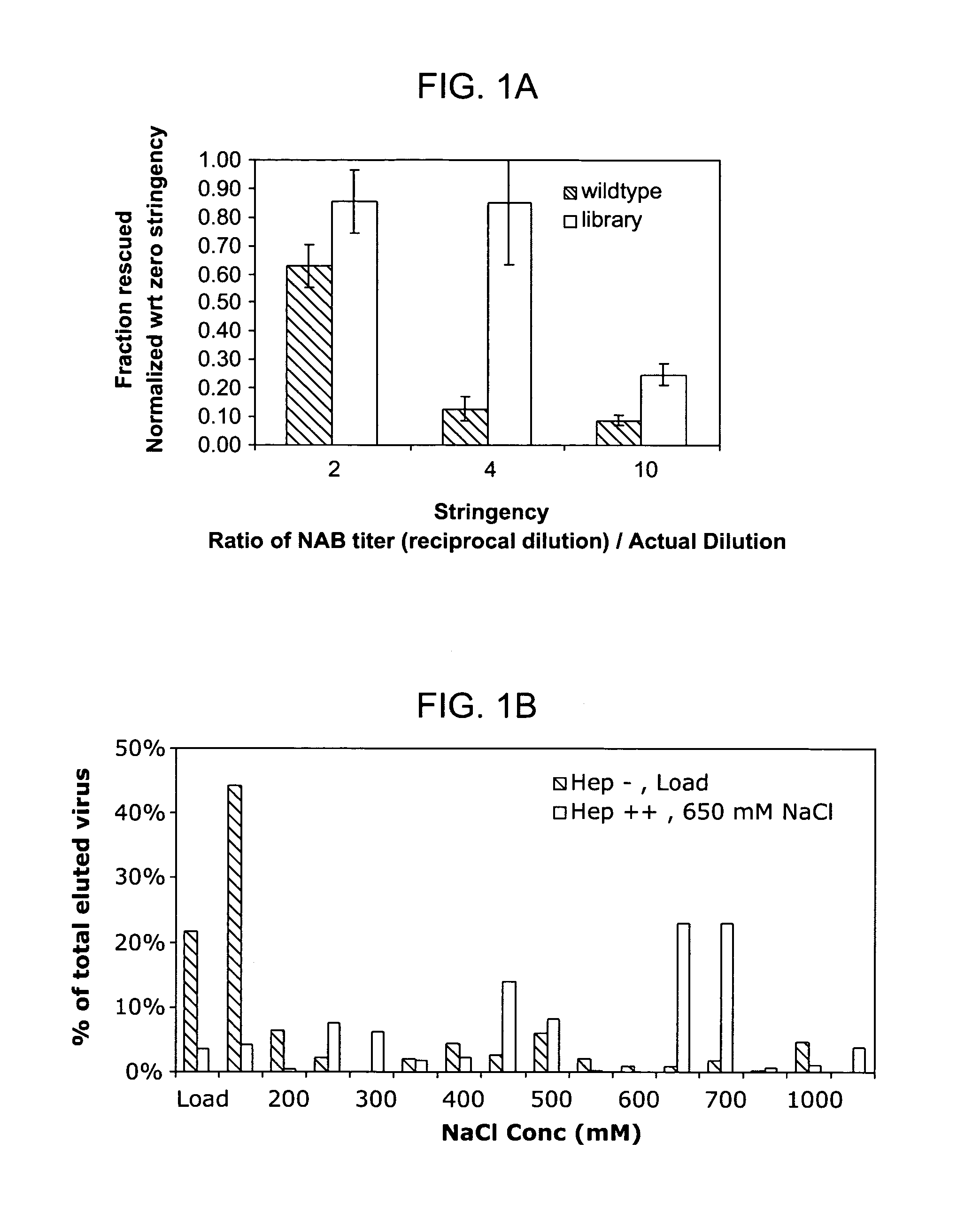
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:RGT UNIV OF CALIFORNIA +1
AAV vectors and methods
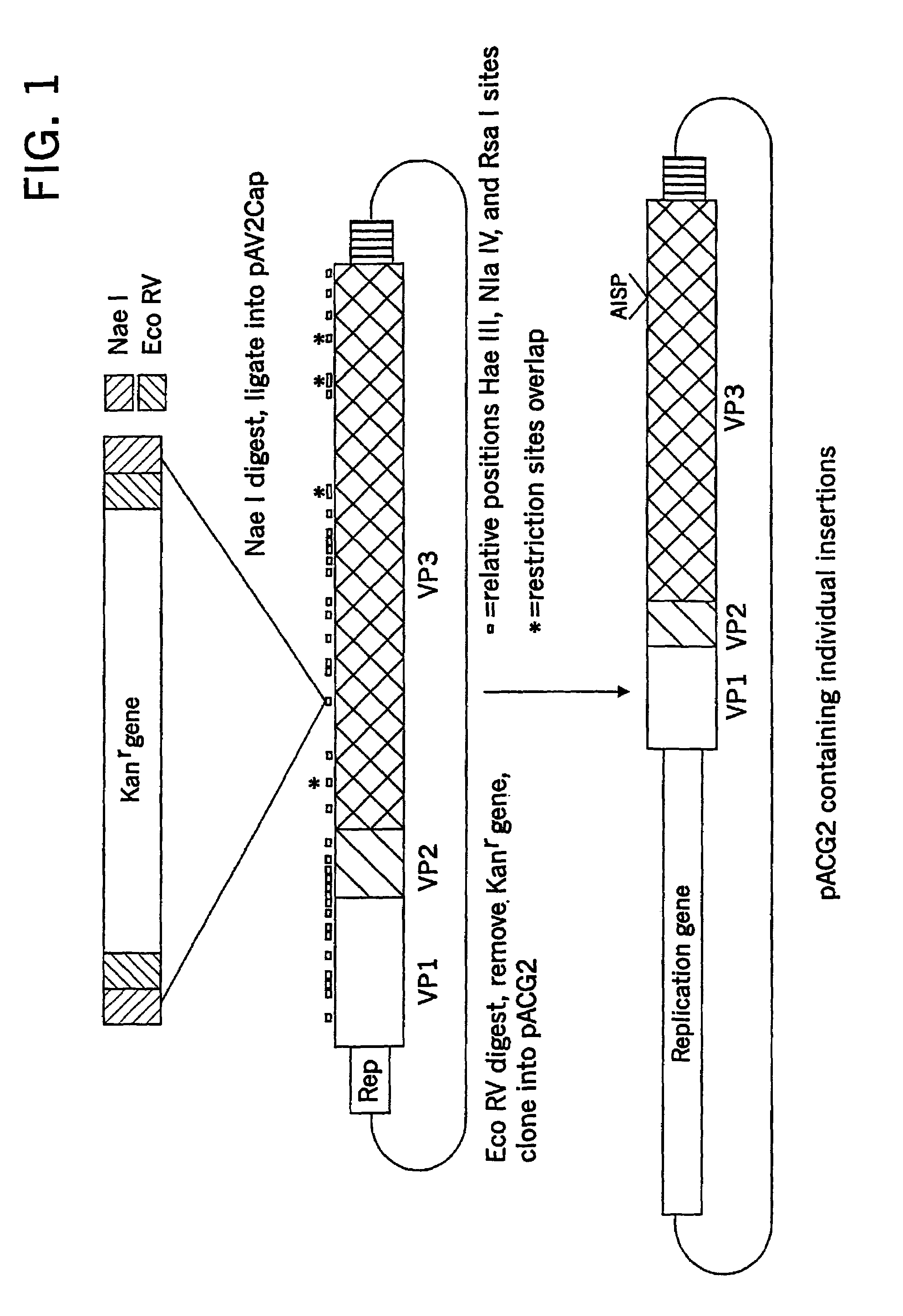
InactiveUS20050287122A1Easy to adaptSuitable for mass productionBiocidePeptide/protein ingredientsCapsidAdeno-associated virus
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20050053922A1Reduce the binding forceAltered infectivityAntibacterial agentsVirusesReassortant VirusesNeutralizing antibody
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
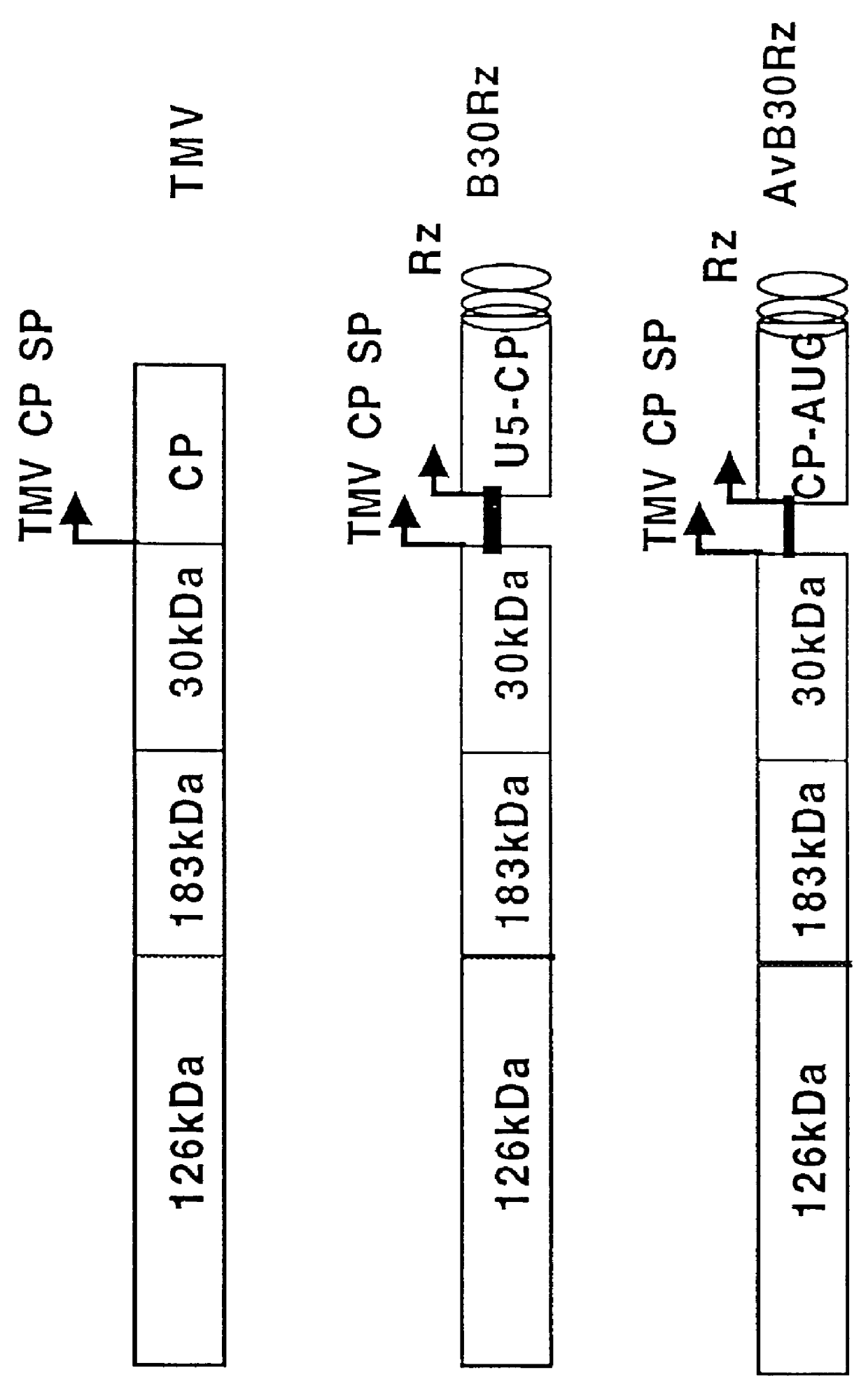
Duplexed parvovirus vectors
InactiveUS7465583B2High transduction efficiencyRapid onsetBiocidePeptide/protein ingredientsGeneticsViral vector
The present invention provides duplexed parvovirus vector genomes that are capable under appropriate conditions of forming a double-stranded molecule by intrastrand base-pairing. Also provided are duplexed parvovirus particles comprising the vector genome. Further disclosed are templates and methods for producing the duplexed vector genomes and duplexed parvovirus particles of the invention. Methods of administering these reagents to a cell or subject are also described. Preferably, the parvovirus capsid is an AAV capsid. It is further preferred that the vector genome comprises AAV terminal repeat sequences.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
AAV2 vectors and methods
InactiveUS6962815B2Easy to adaptSuitable for mass productionBiocideGenetic material ingredientsViral vectorCapsid
Owner:NATIONWIDE CHILDRENS HOSPITAL
Methods for producing members of specific binding pairs
InactiveUS7063943B1Simple procedureSpeedSugar derivativesMicrobiological testing/measurementFree formGenetic diversity
A member of a specific binding pair (sbp) is identified by expressing DNA encoding a genetically diverse population of such sbp members in recombinant host cells in which the sbp members are displayed in functional form at the surface of a secreted recombinant genetic display package (rgdp) containing DNA encoding the sbp member or a polypeptide component thereof, by virtue of the sbp member or a polypeptide component thereof being expressed as a fusion with a capsid component of the rgdp. The displayed sbps may be selected by affinity with a complementary sbp member, and the DNA recovered from selected rgdps for expression of the selected sbp members. Antibody sbp members may be thus obtained, with the different chains thereof expressed, one fused to the capsid component and the other in free form for association with the fusion partner polypeptide. A phagemid may be used as an expression vector, with said capsid fusion helping to package the phagemid DNA. Using this method libraries of DNA encoding respective chains of such multimeric sbp members may be combined, thereby obtaining a much greater genetic diversity in the sbp members than could easily be obtained by conventional methods.
Owner:MEDIMMUNE LTD
Compositions and Methods for Altering Tissue Specificity and Improving AAV9-Mediated Gene Transfer
ActiveUS20130323226A1Improve efficiencyImprove usabilityOrganic active ingredientsVectorsViral vectorFhit gene
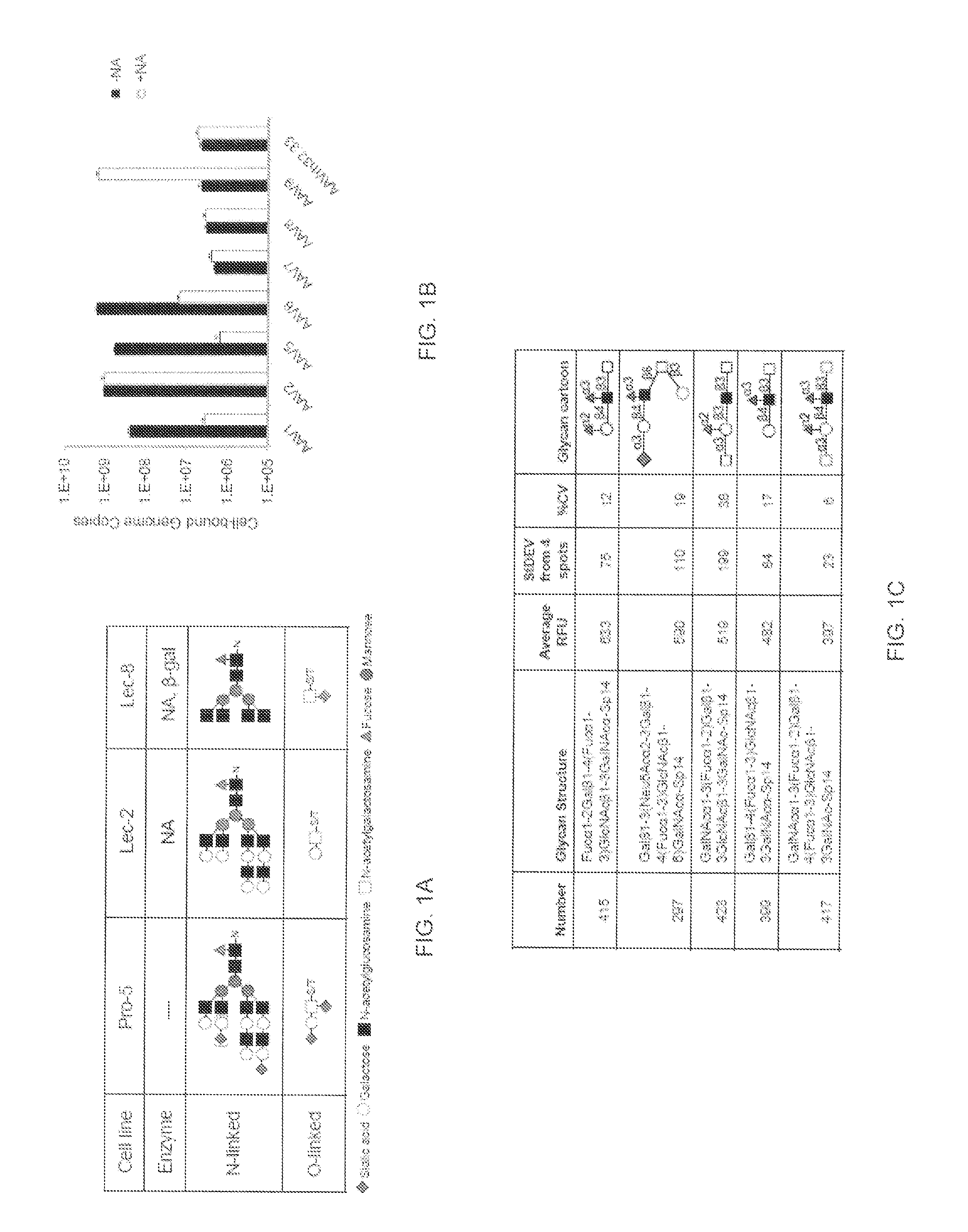
A method of altering the targeting and / or cellular uptake efficiency of an adeno-associated virus (AAV) viral vector having a capsid containing an AAV9 cell surface binding domain is described. The method involves modifying a clade F cell surface receptor which comprises a glycan having a terminal sialic acid residue and a penultimate β-galactose residue. The modification may involve retargeting the vector by temporarily functionally ablate AAV9 binding in a subset of cells, thereby redirecting the vector to another subset of cells. Alternatively, the modification may involve increasing cellular update efficiency by treating the cells with a neuraminidase to expose cell surface β-galactose. Also provided are compositions containing the AAV9 vector and a neuraminidase. Also provided is a method for purifying AAV9 using β-galactose linked to solid support. Also provided are mutant vectors which have been modified to alter their targeting specificity, including mutant AAV9 in which the galactose binding domain is mutated and AAV in which an AAV9 galactose binding domain is engineered.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Scalable Production Method for AAV
ActiveUS20090275107A1Reduce and eliminate bindingHigh yieldGenetic therapy composition manufactureVirus peptidesLysisBinding site
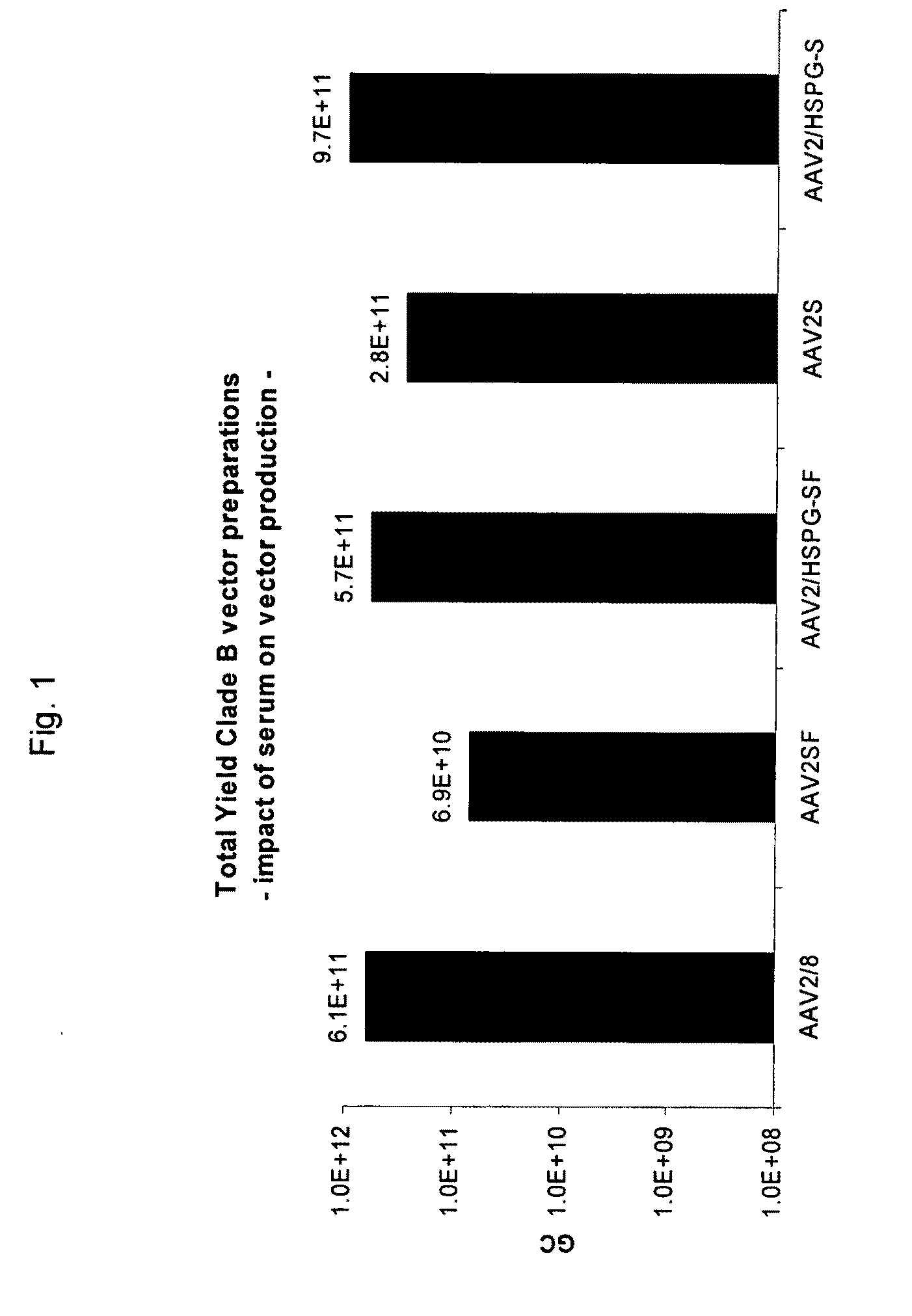
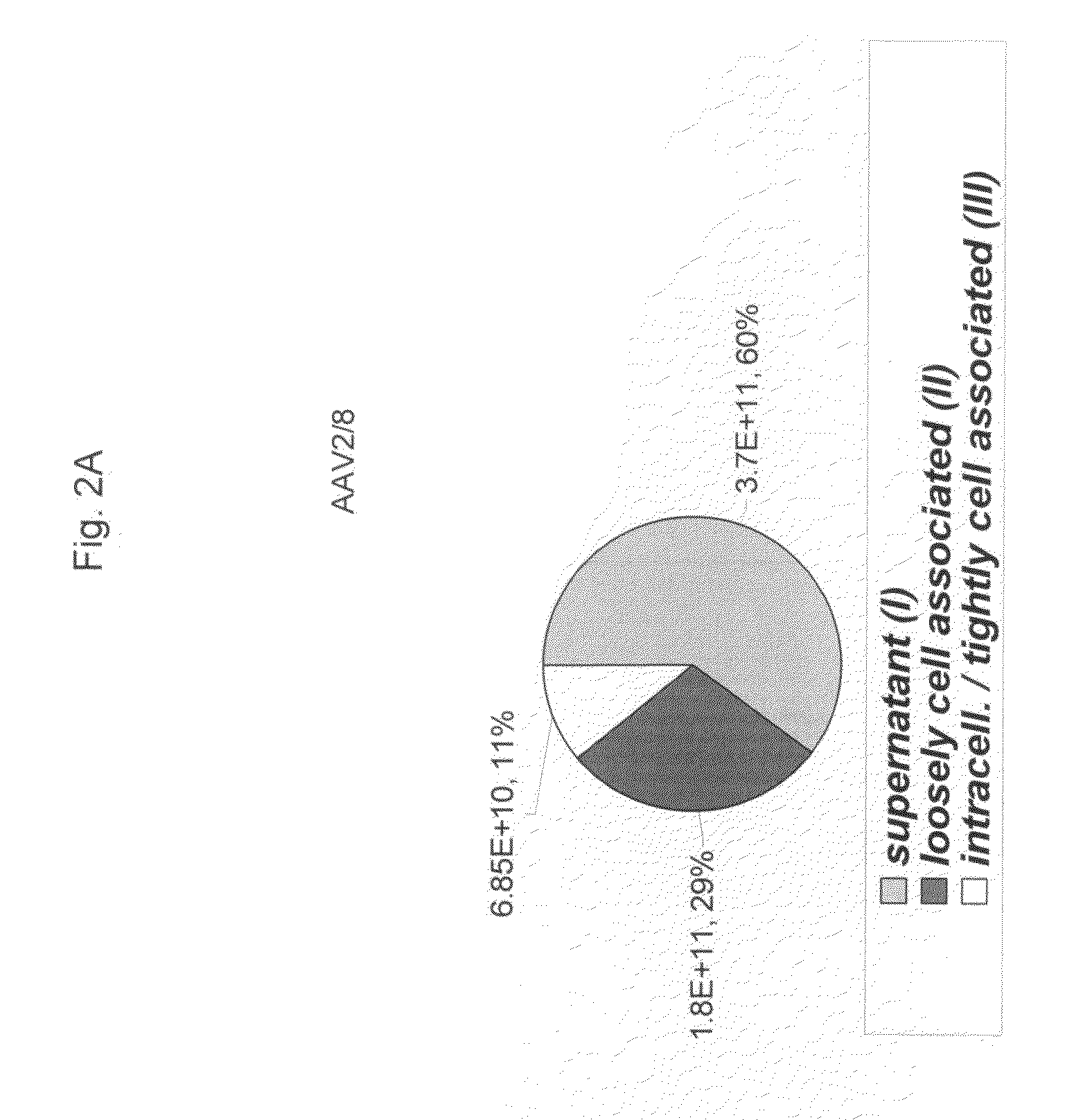
A method for producing AAV, without requiring cell lysis, is described. The method involves harvesting AAV from the supernatant. For AAV having capsids with a heparin binding site, the method involves modifying the AAV capsids and / or the culture conditions to ablate the binding between the AAV heparin binding site and the cells, thereby allowing the AAV to pass into the supernatant, i.e., media. Thus, the method of the invention provides supernatant containing high yields of AAV which have a higher degree of purity from cell membranes and intracellular materials, as compared to AAV produced using methods using a cell lysis step.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Parvoviral capsid with incorporated gly-ala repeat region
InactiveUS20110171262A1Improve stabilityReduced expression levelBiocideAntibody mimetics/scaffoldsNucleic acid sequencingCapsid
Parvoviral capsid with incorporated Gly-Ala repeat region The present invention provides a nucleic acid construct comprising a nucleic acid sequence encoding a parvoviral VP1, VP2 and VP3 capsid proteins comprising an immuno evasion repeat sequence. In addition, the present invention provides a cell comprising such construct, a parvoviral virion comprising a capsid protein that comprises an immune evasion repeat sequence, use of that parvoviral virion in gene therapy and a pharmaceutical composition comprising such parvoviral virion.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS
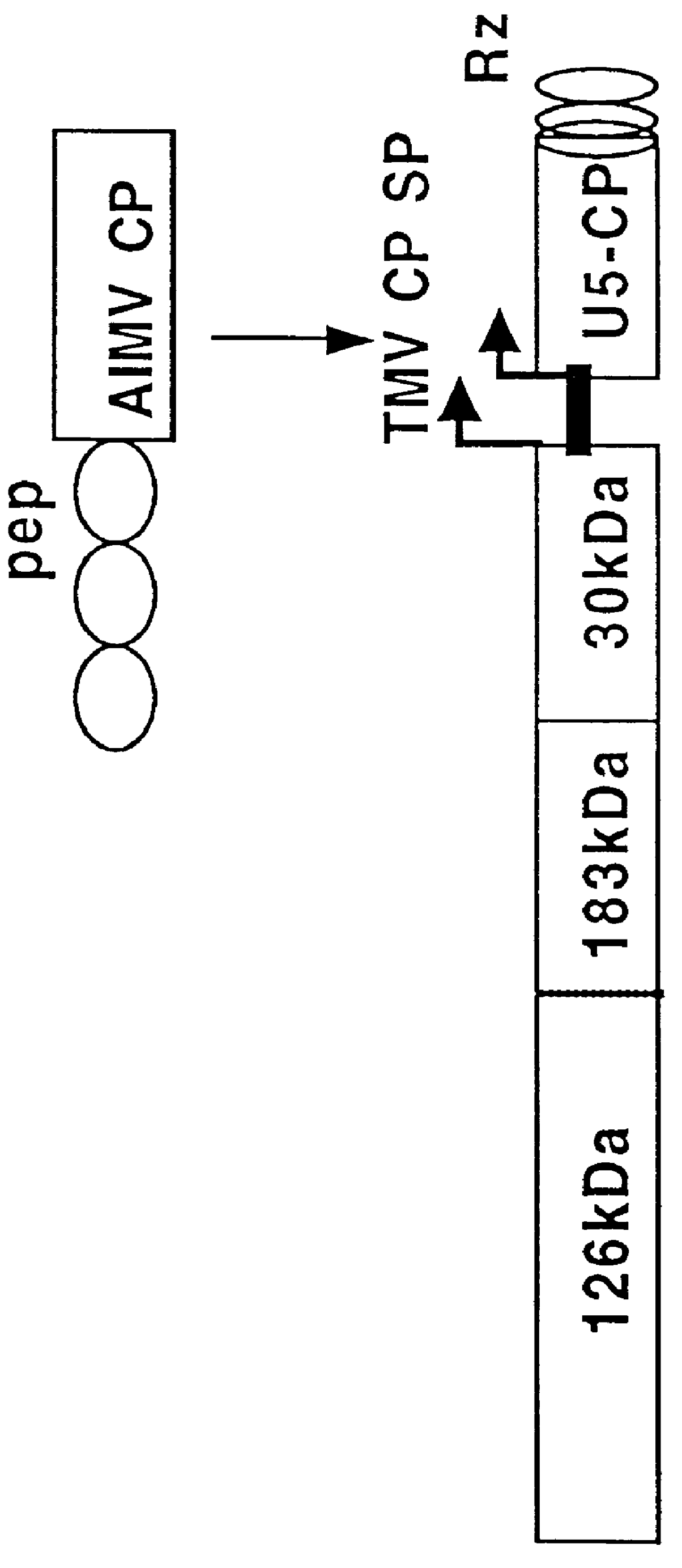
Polypeptides fused with alfalfa mosaic virus or ilarvirus capsid proteins
InactiveUS6042832AEfficient presentation of antigenImprove protectionSsRNA viruses negative-senseSsRNA viruses positive-senseAntigenIlarvirus
Owner:THOMAS JEFFERSON UNIV
AAV capsid proteins for nucleic acid transfer
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
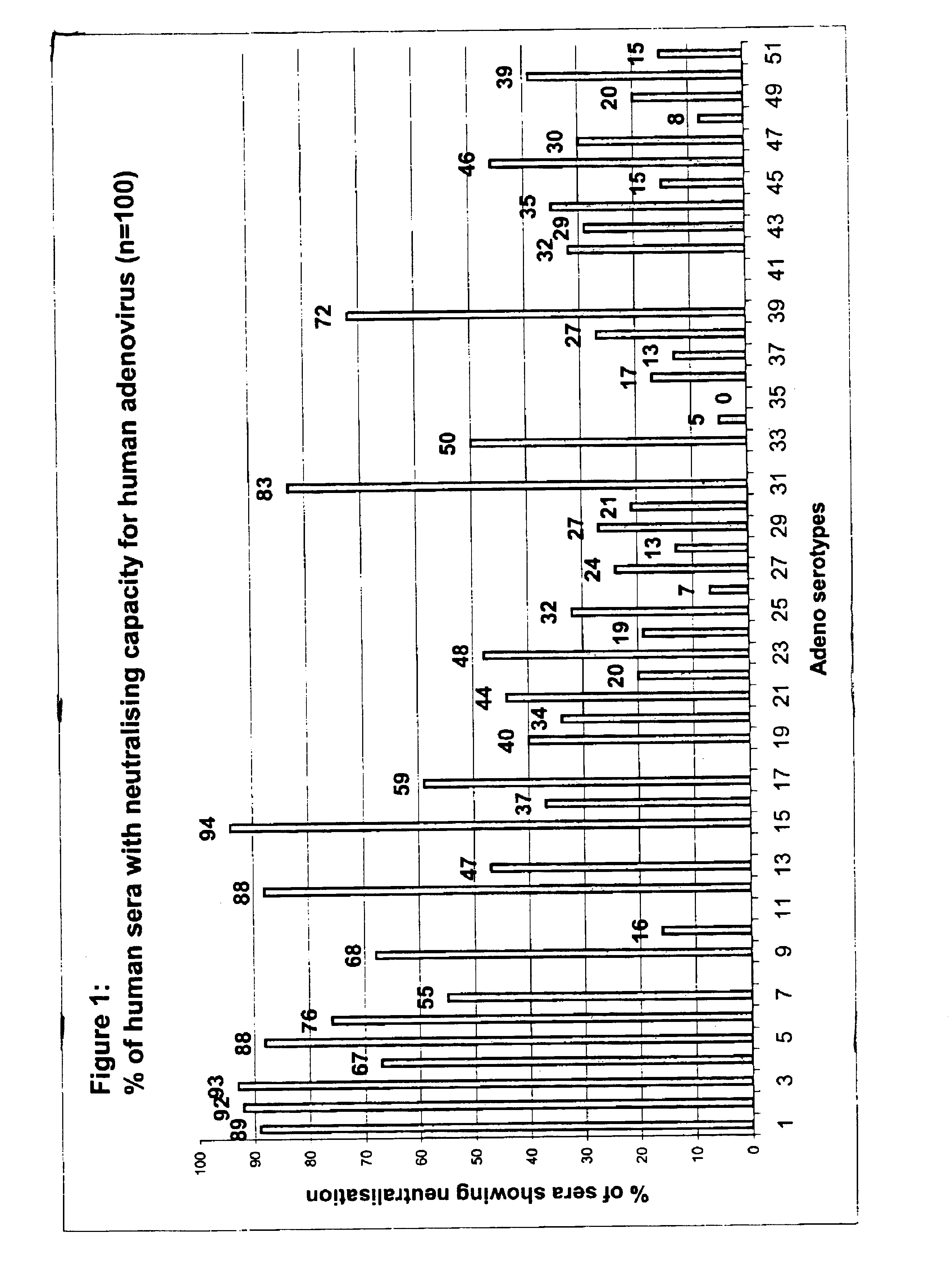
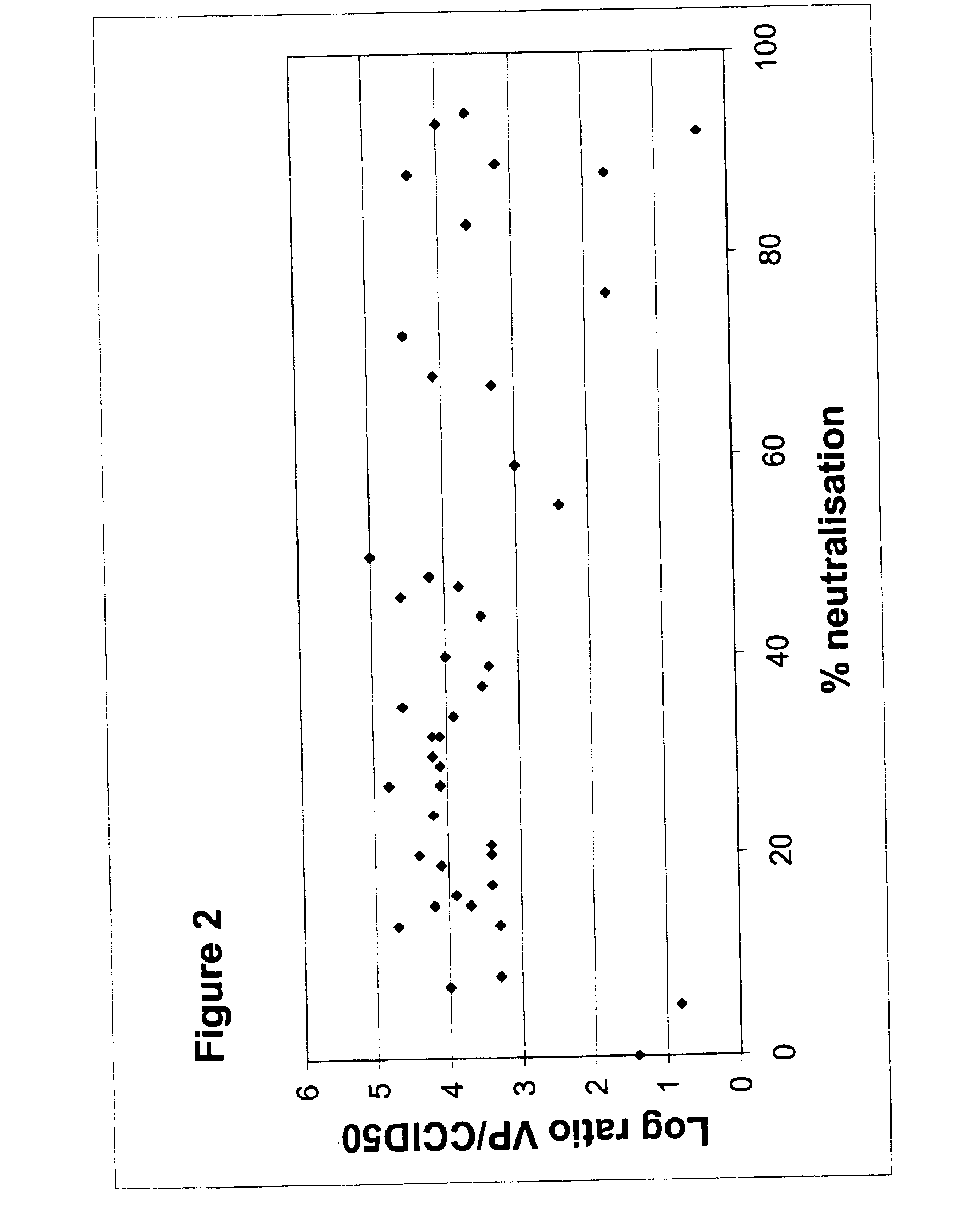
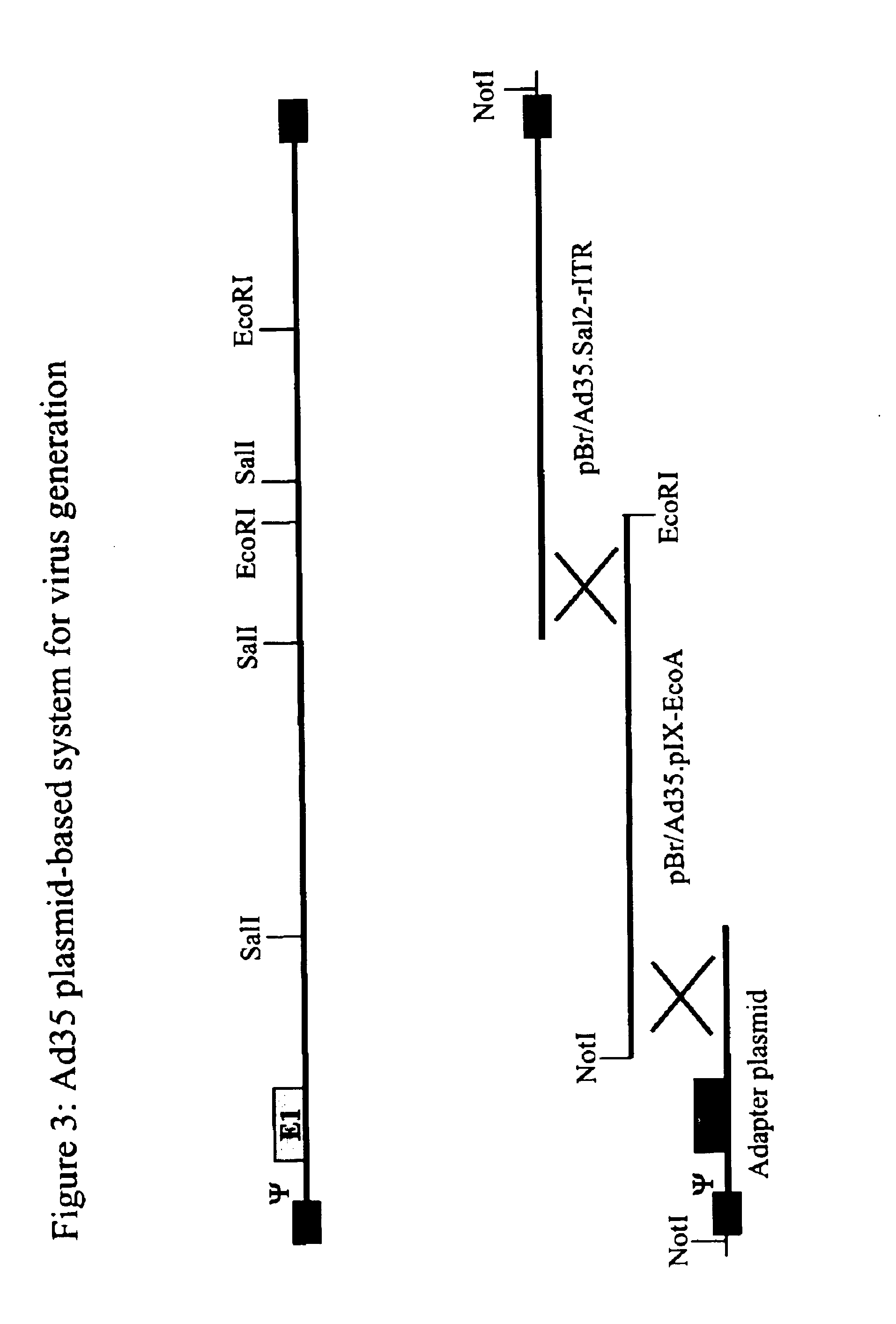
Serotype of adenovirus and uses thereof
InactiveUS6913922B1High infection efficiencyEasy to copyBiocideGenetic material ingredientsCell bindingSerotype
Adenovirus serotypes differ in their natural tropism. The adenovirus serotypes 2, 4, 5, and 7 all have a natural affiliation towards lung epithelia and other respiratory tissues. In contrast, serotypes 40 and 41 have a natural affiliation towards the gastrointestinal tract. The serotypes described, differ in at least capsid proteins (penton-base, hexon), proteins responsible for cell binding (fiber protein), and proteins involved in adenovirus replication. This difference in tropism and capsid protein among serotypes has led to the many research efforts aimed at redirecting the adenovirus tropism by modification of the capsid proteins.
Owner:JANSSEN VACCINES & PREVENTION BV
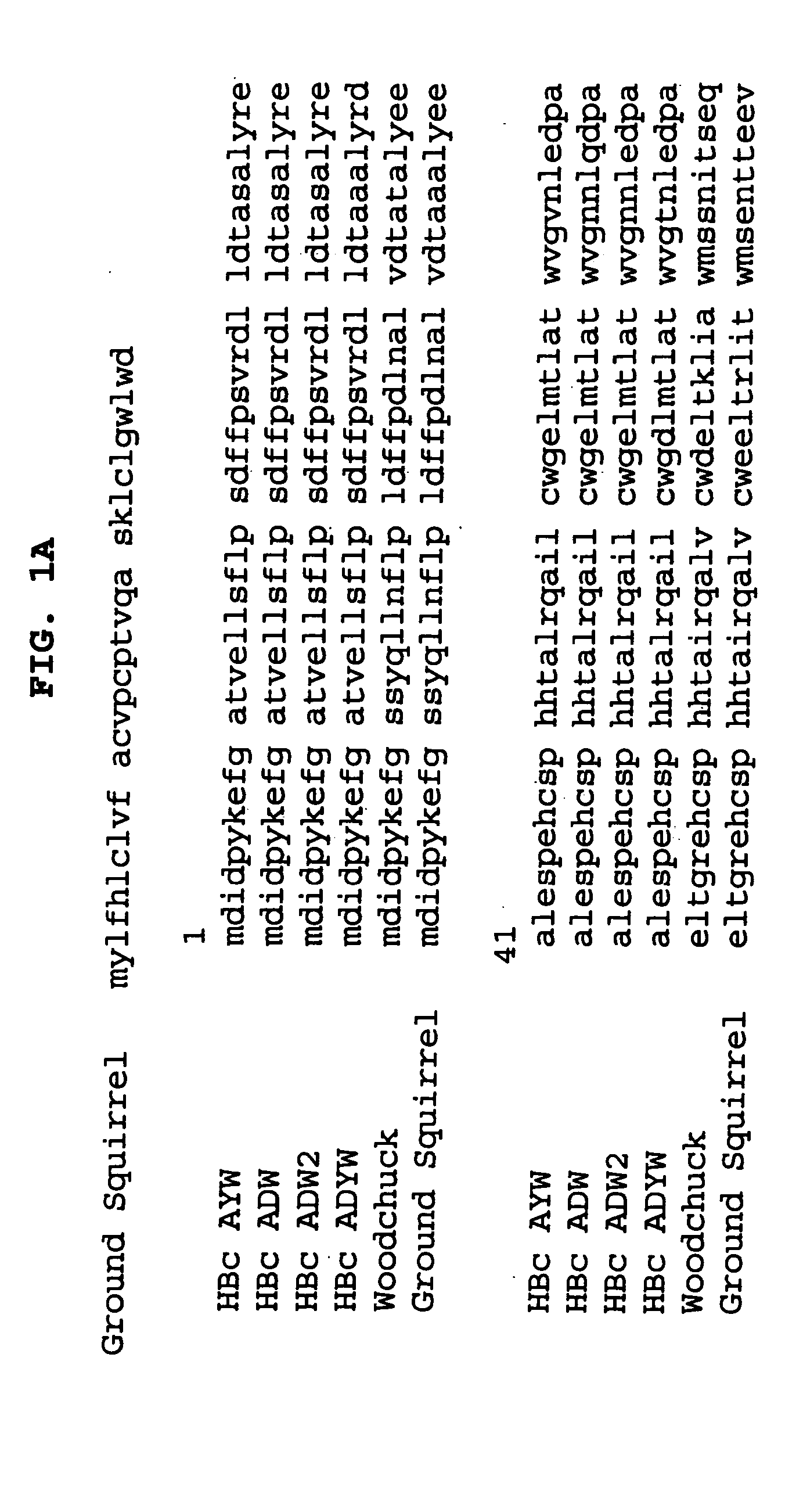
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
Adeno-associated virus virions with variant capsid and methods of use thereof
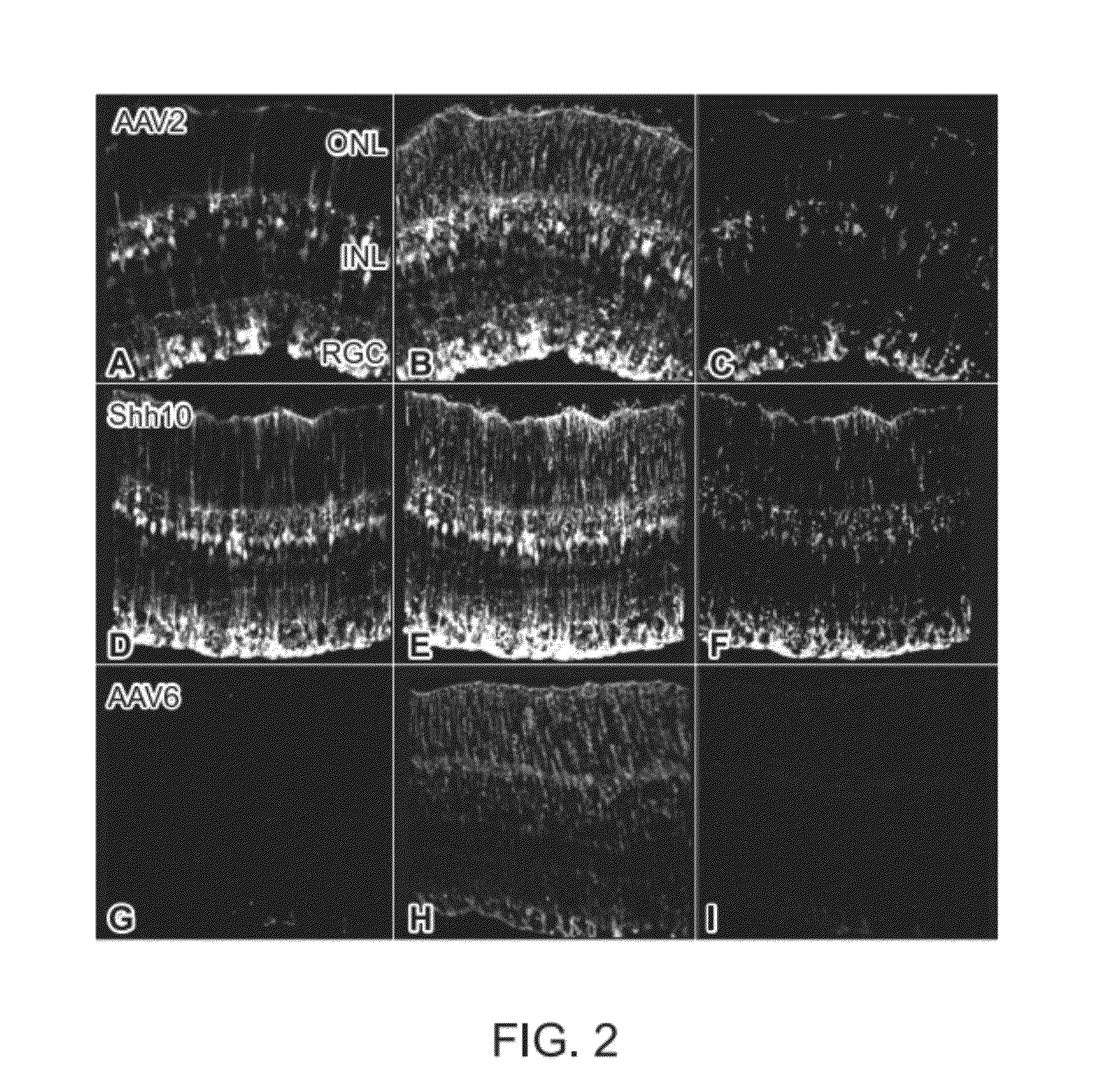
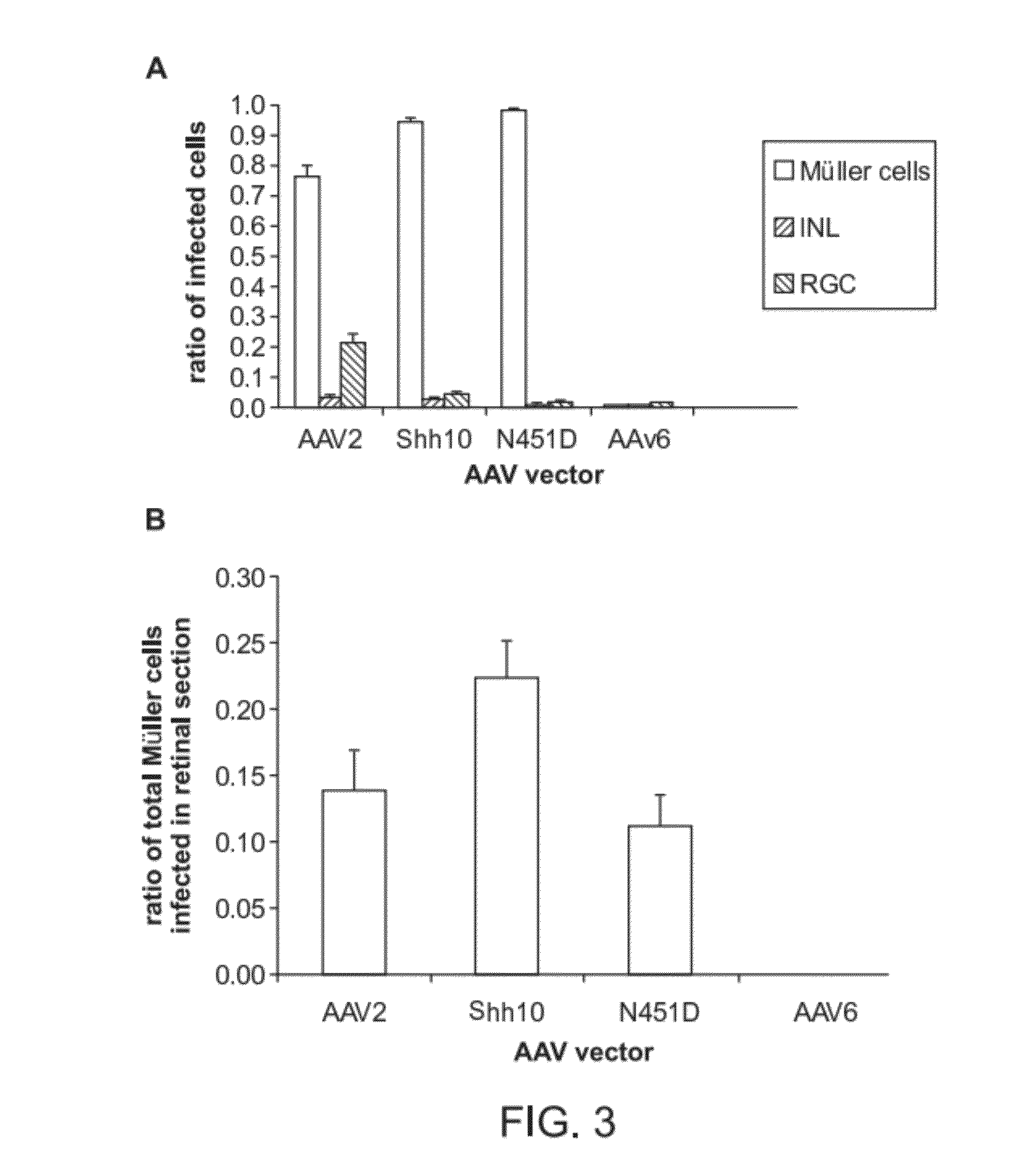
The present disclosure provides adeno-associated virus (AAV) virions with altered capsid protein, where the AAV virions exhibit greater infectivity of retinal cells compared to wild-type AAV. The present disclosure further provides methods of delivering a gene product to a retinal cell in an individual, and methods of treating ocular disease.
Owner:RGT UNIV OF CALIFORNIA
Inorganic nanowires
InactiveUS20050221083A1Good monodispersityHigh degreeMaterial nanotechnologyNanoinformaticsNanowireSingle crystal
An inorganic nanowire having an organic scaffold substantially removed from the inorganic nanowire, the inorganic nanowire consisting essentially of fused inorganic nanoparticles substantially free of the organic scaffold, and methods of making same. For example, a virus-based scaffold for the synthesis of single crystal ZnS, CdS and free-standing L10 CoPt and FePt nanowires can be used, with the means of modifying substrate specificity through standard biological methods. Peptides can be selected through an evolutionary screening process that exhibit control of composition, size, and phase during nanoparticle nucleation have been expressed on the highly ordered filamentous capsid of the M13 bacteriophage. The incorporation of specific, nucleating peptides into the generic scaffold of the M13 coat structure can provide a viable template for the directed synthesis of a variety of materials including semiconducting and magnetic materials. Removal of the viral template via annealing can promote oriented aggregation-based crystal growth, forming individual crystalline nanowires. The unique ability to interchange substrate specific peptides into the linear self-assembled filamentous construct of the M13 virus introduces a material tunability not seen in previous synthetic routes. Therefore, this system provides a genetic tool kit for growing and organizing nanowires from various materials including semiconducting and magnetic materials.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Virus vectors for highly efficient transgene delivery
ActiveUS20140336245A1Enhanced transductionPeptide/protein ingredientsGenetic material ingredientsGene transferTransgene
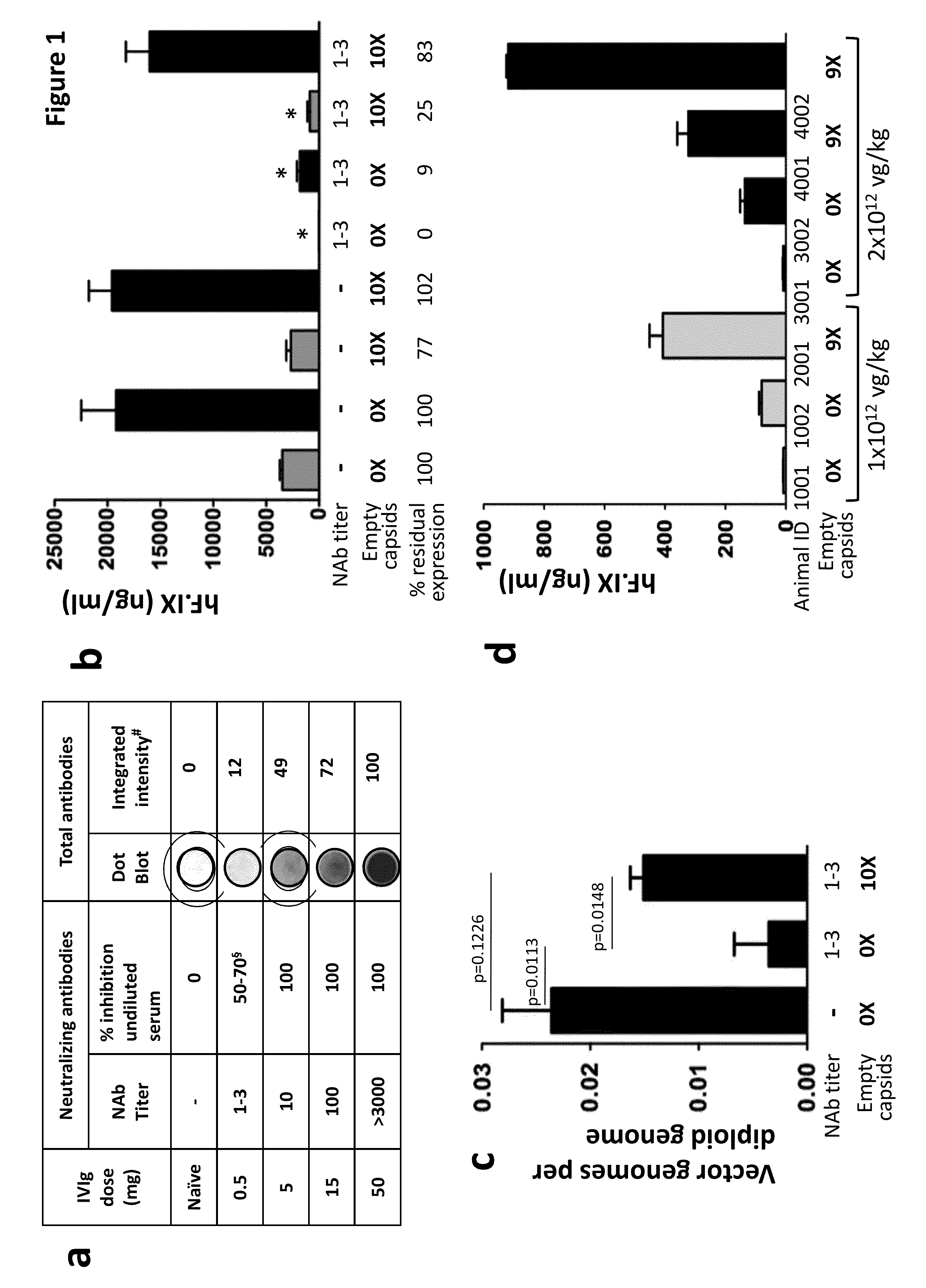
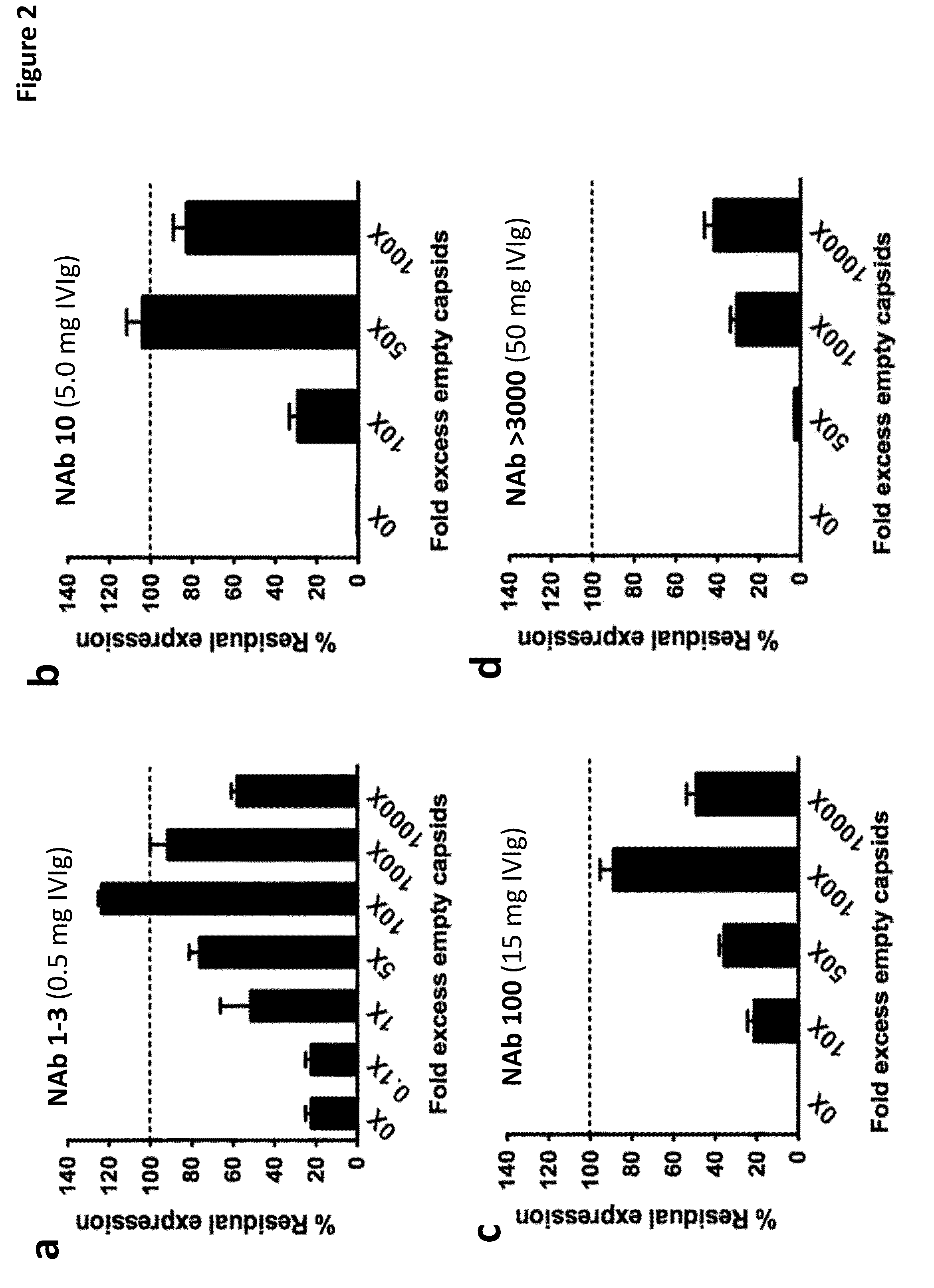
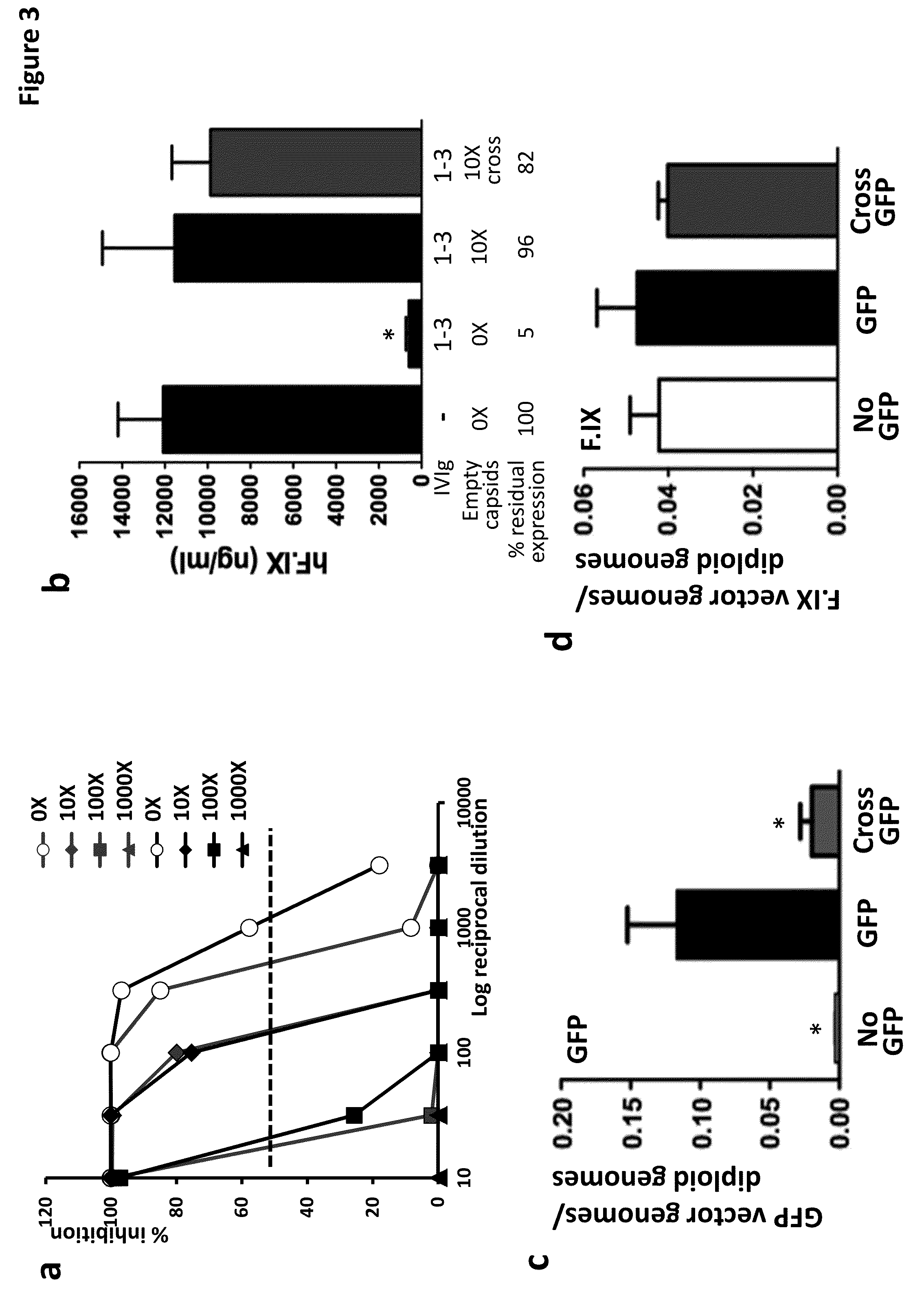
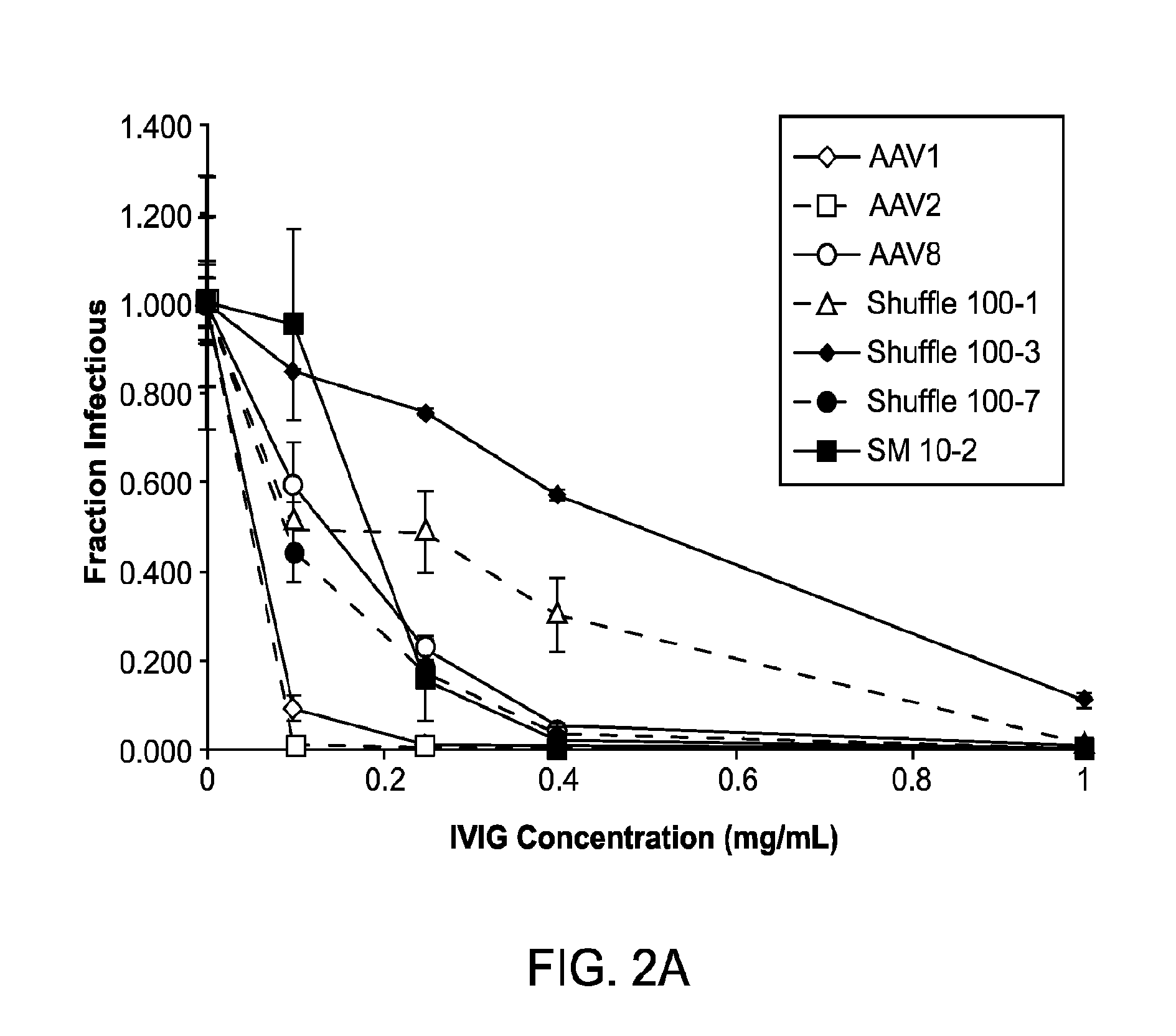
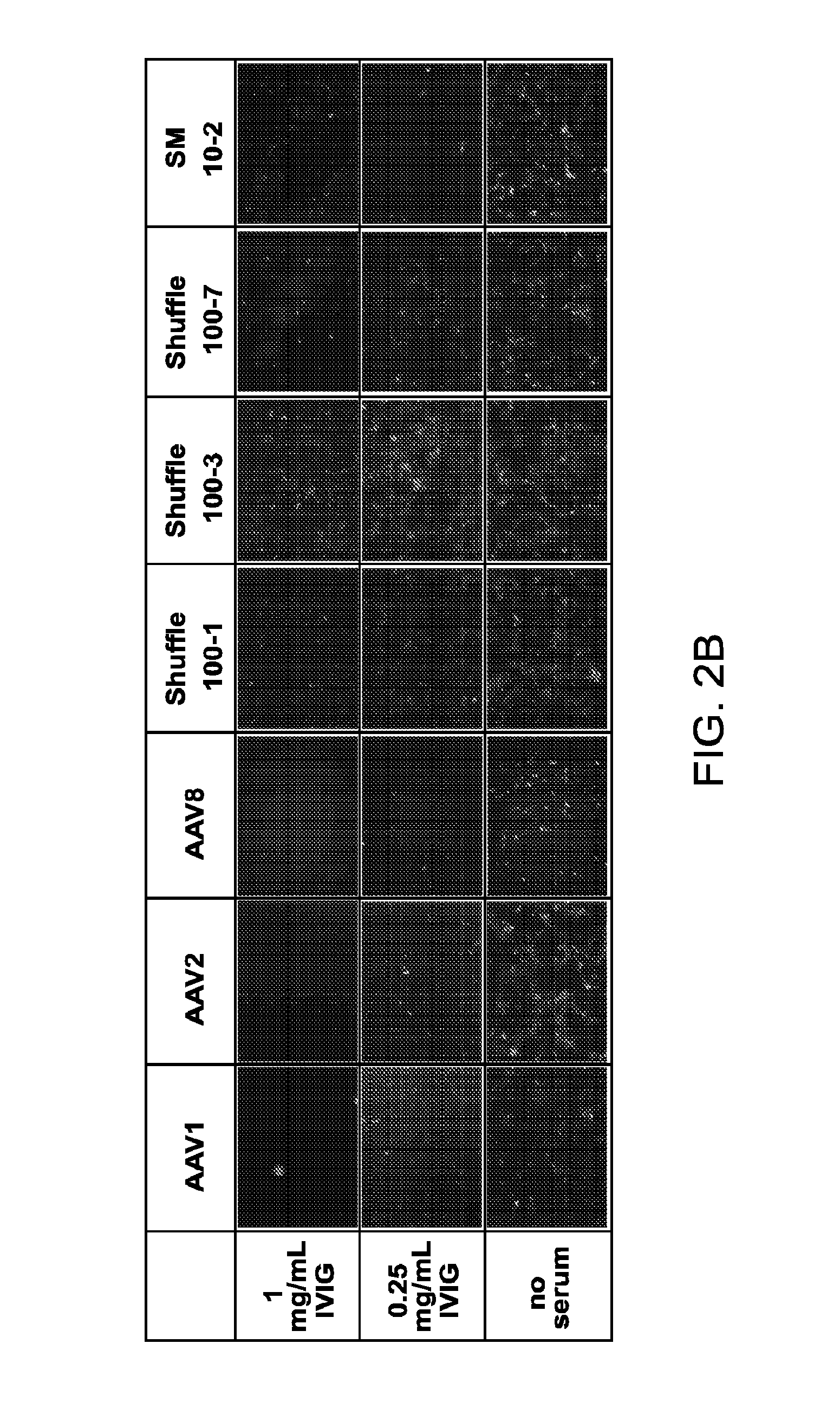
The invention provides viral vector formulations and methods of uses thereof for delivery of transgenes or therapeutic nucleic acids to human subjects. The formulations include a vector and suitable amounts of empty capsids, viral genome-containing capsids, or viral capsid proteins which are optionally chemically or structurally modified and which bind to neutralizing anti-AAV antibodies thereby reducing or preventing antibody-mediated clearance of the vector, but still allowing the genome-containing (therapeutic) vector to transduce target cells and achieve therapeutic gene transfer.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Apparatus and method for detecting microscopic living organisms using bacteriophage
InactiveUS20050003346A1Wide concentration rangeFast resultsMicrobiological testing/measurementMaterial analysisBacteroidesAntibiotic resistance
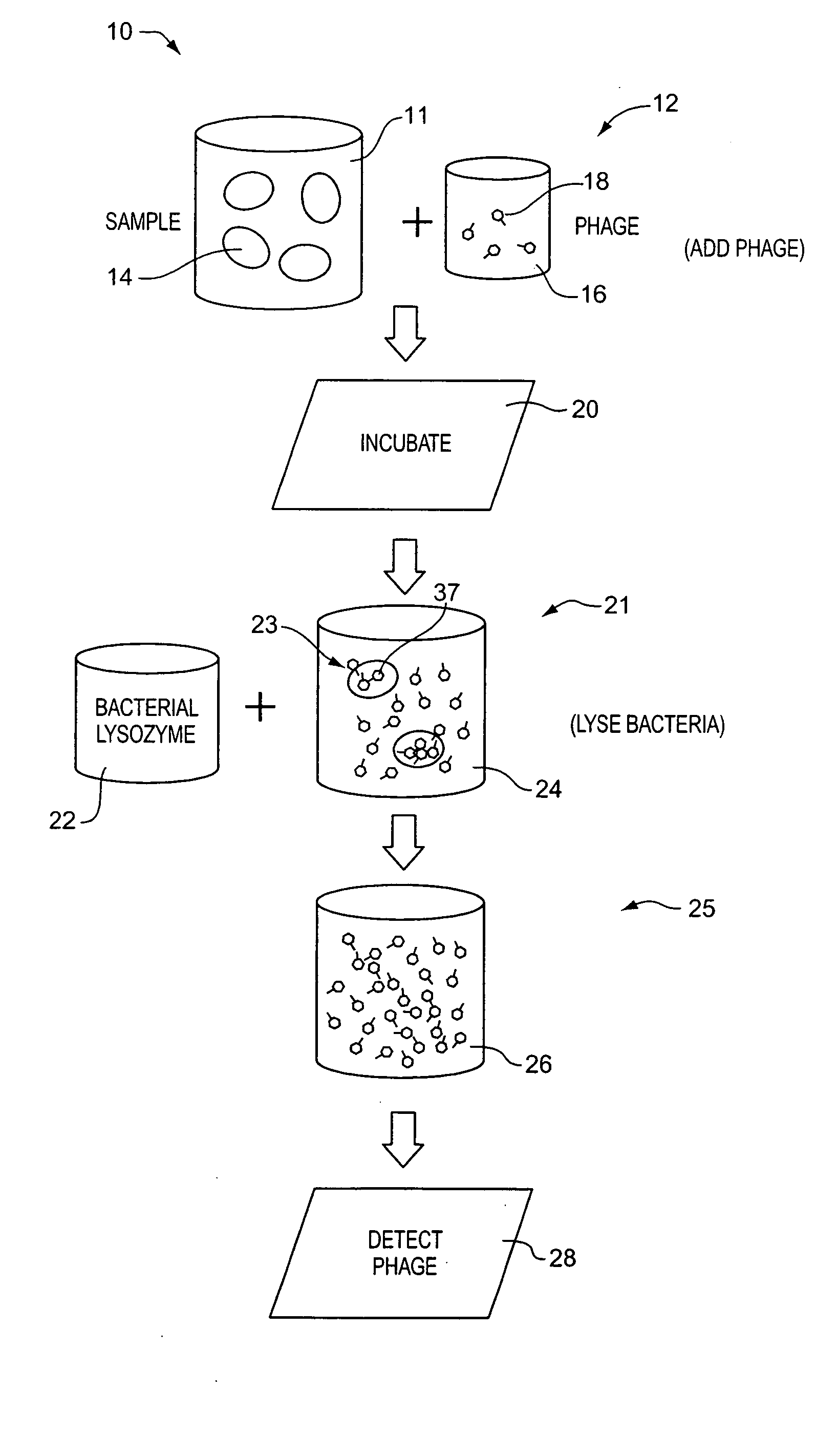
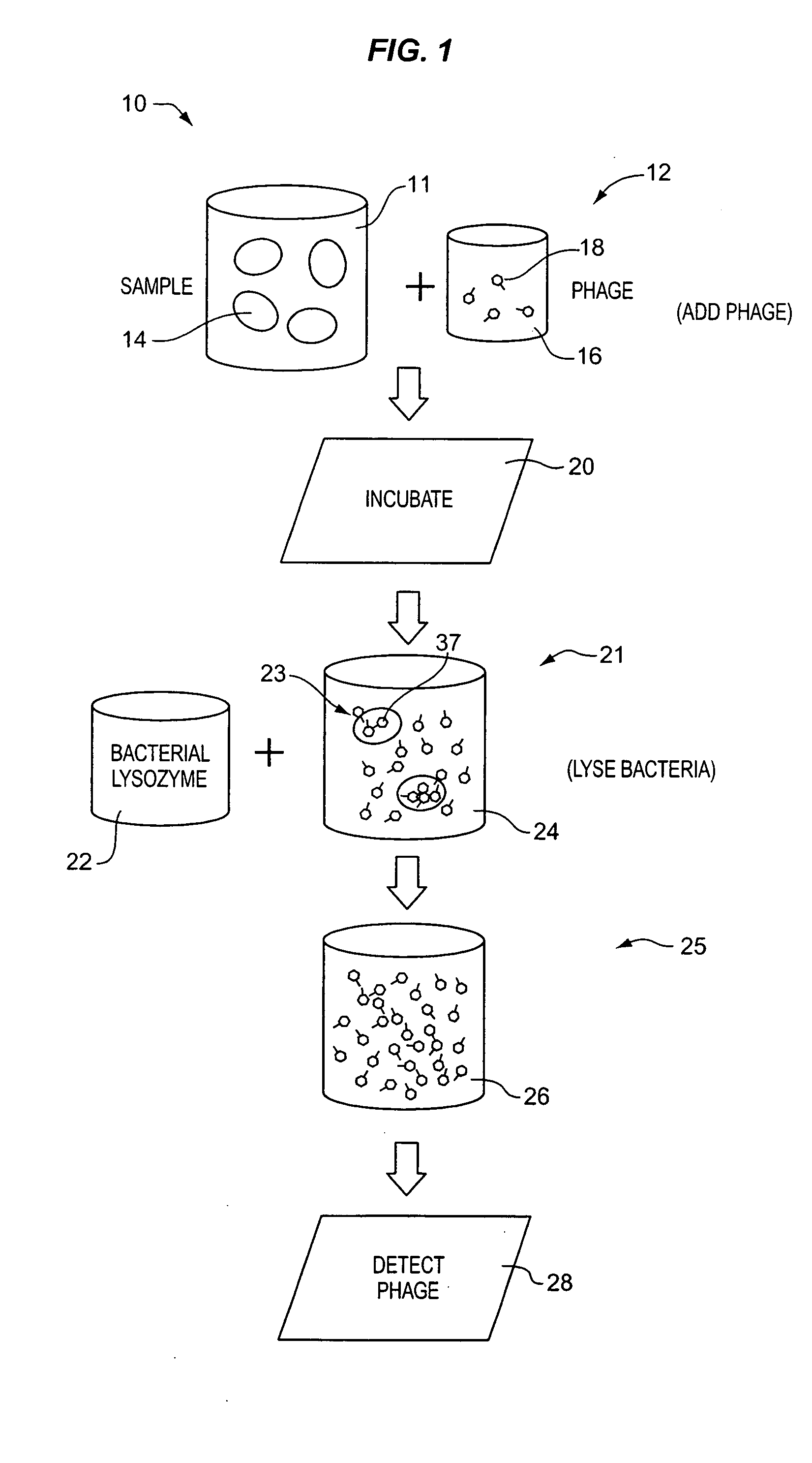
A method for detecting one or more target bacteria in a raw sample where: 1) bacteriophage(s) specific to each target bacterium are added to the raw sample, 2) the test sample is incubated, and 3) the test sample is tested for the presence of each phage in sufficient numbers to indicate the presence of the associated target bacteria in the raw sample. In one embodiment, each phage is initially added to the raw sample in concentrations below the detection limit of the final phage detection process. In another embodiment, the parent phages are tagged in such a way that they can be separated from the progeny phage prior to the detection process. Preferred phage detection processes are immunoassay methods utilizing antibodies that bind specifically to each phage. Antibodies can be used that bind to the protein capsid of the phage. Alternatively, the phage can by dissociated after the incubation process and the sample tested for the presence of individual capsid proteins or phage nucleic acids. The invention can be used to test target bacteria for antibiotic resistance.
Owner:MICROPHAGE +1
AAV vectors and methods
InactiveUS7749492B2Easy to adaptSuitable for mass productionBiocidePeptide/protein ingredientsViral vectorCapsid
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
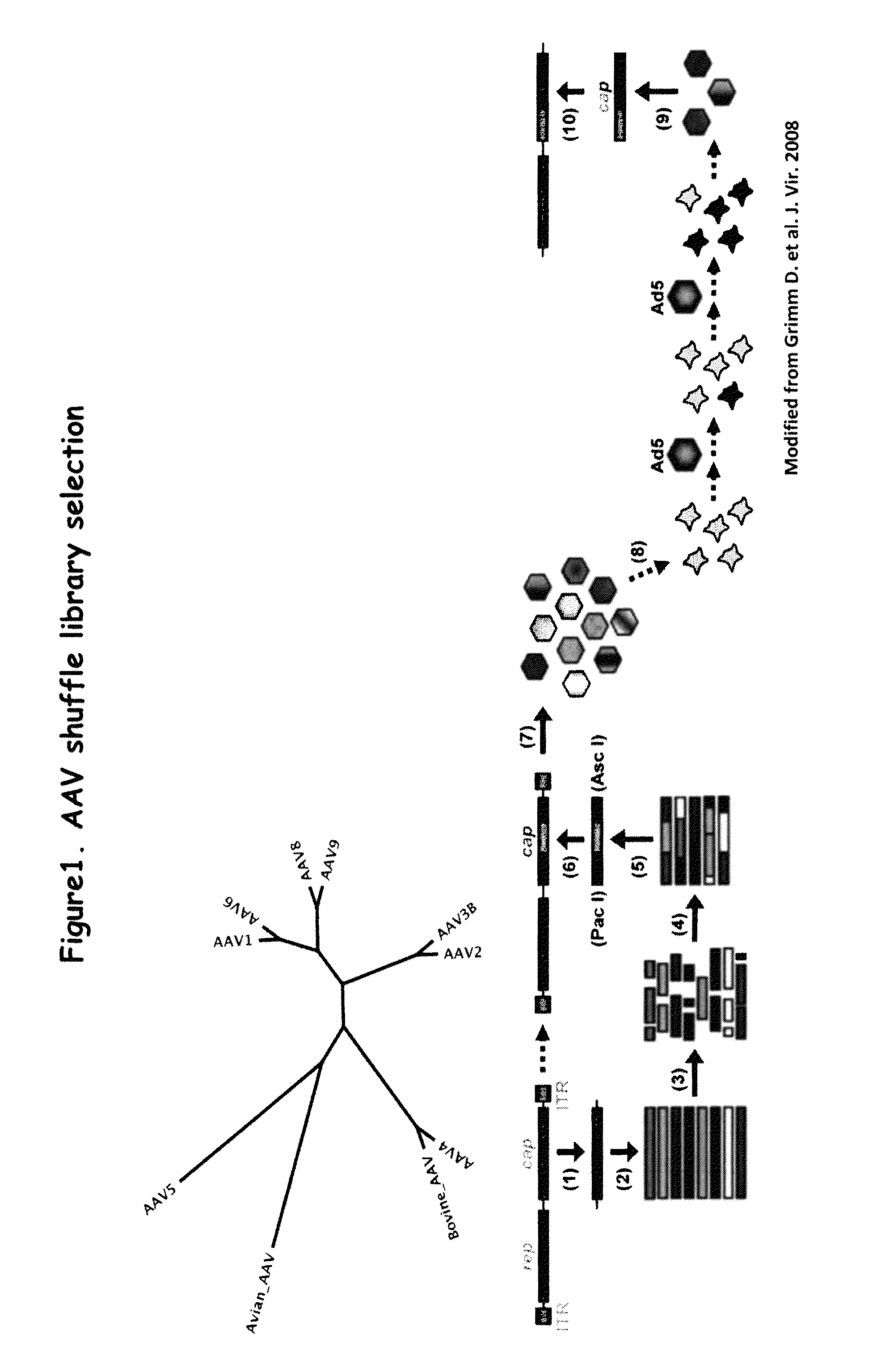
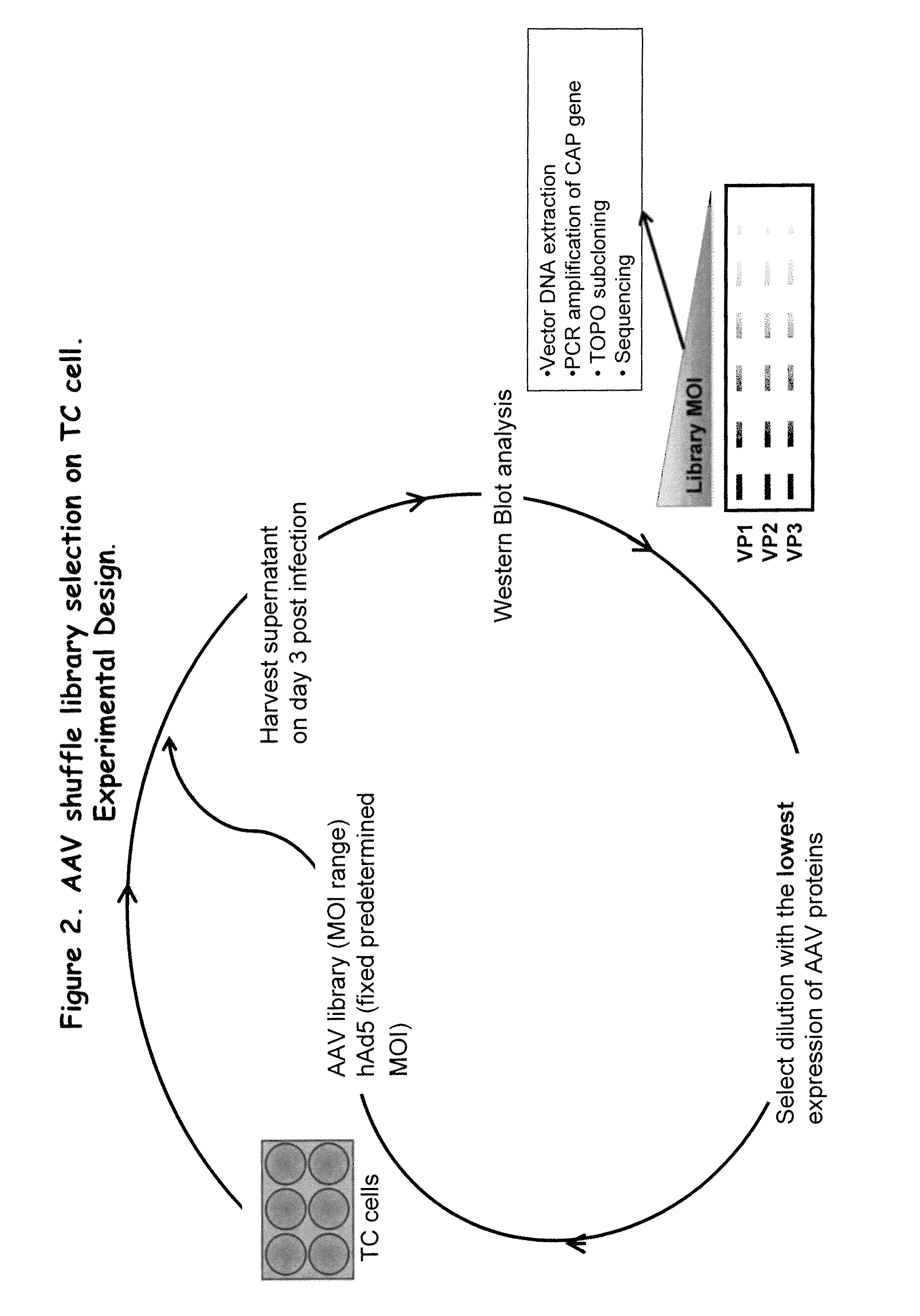
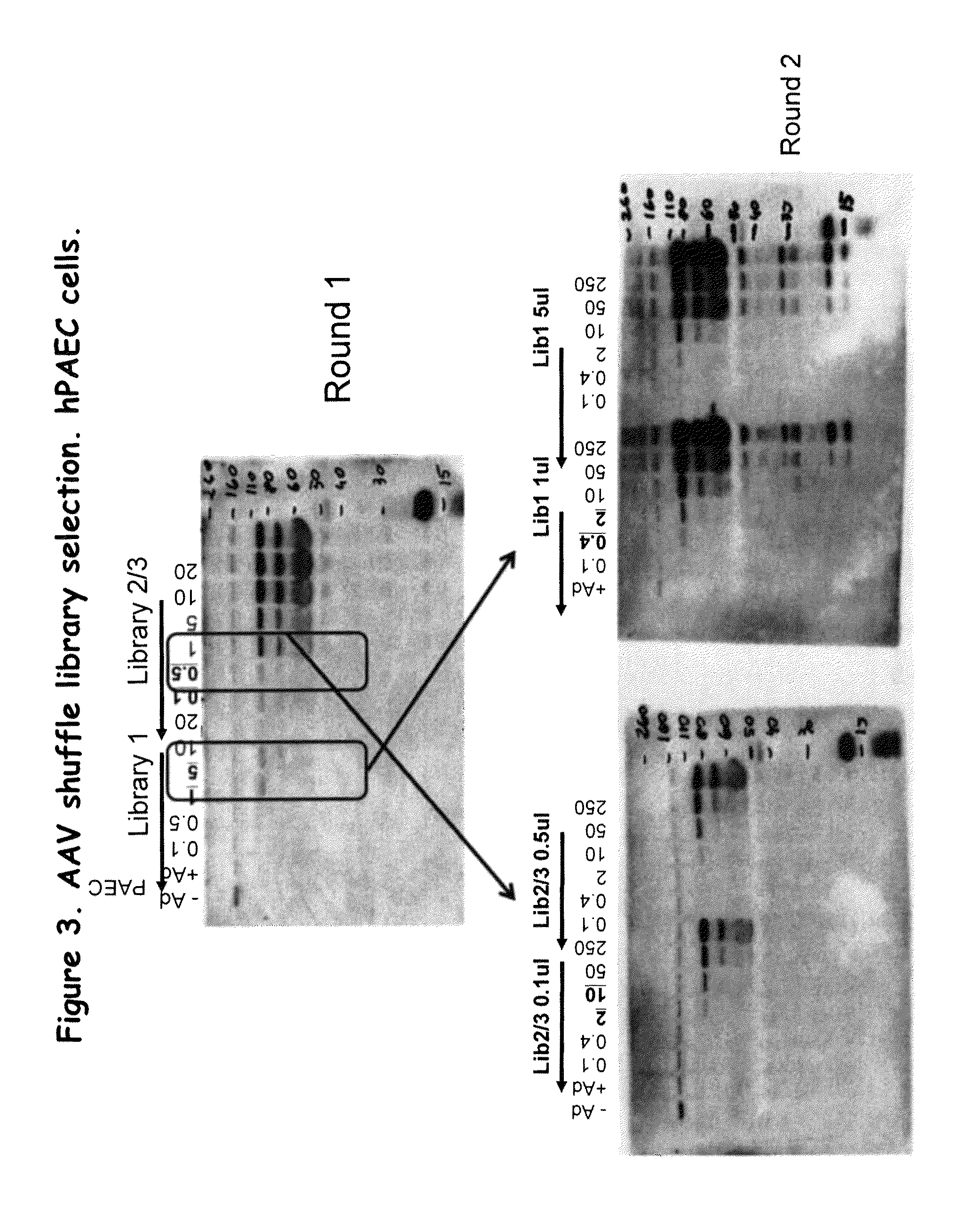
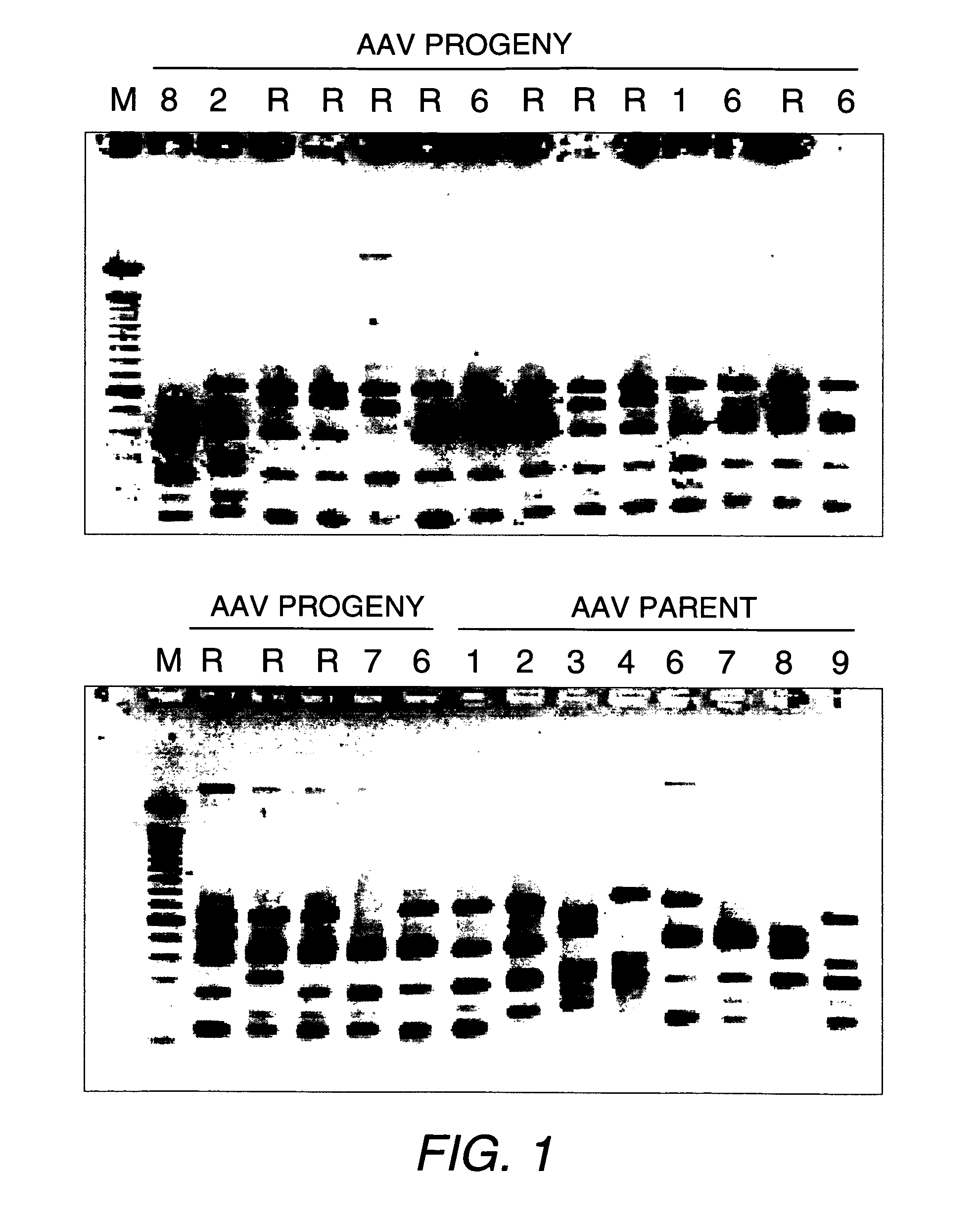
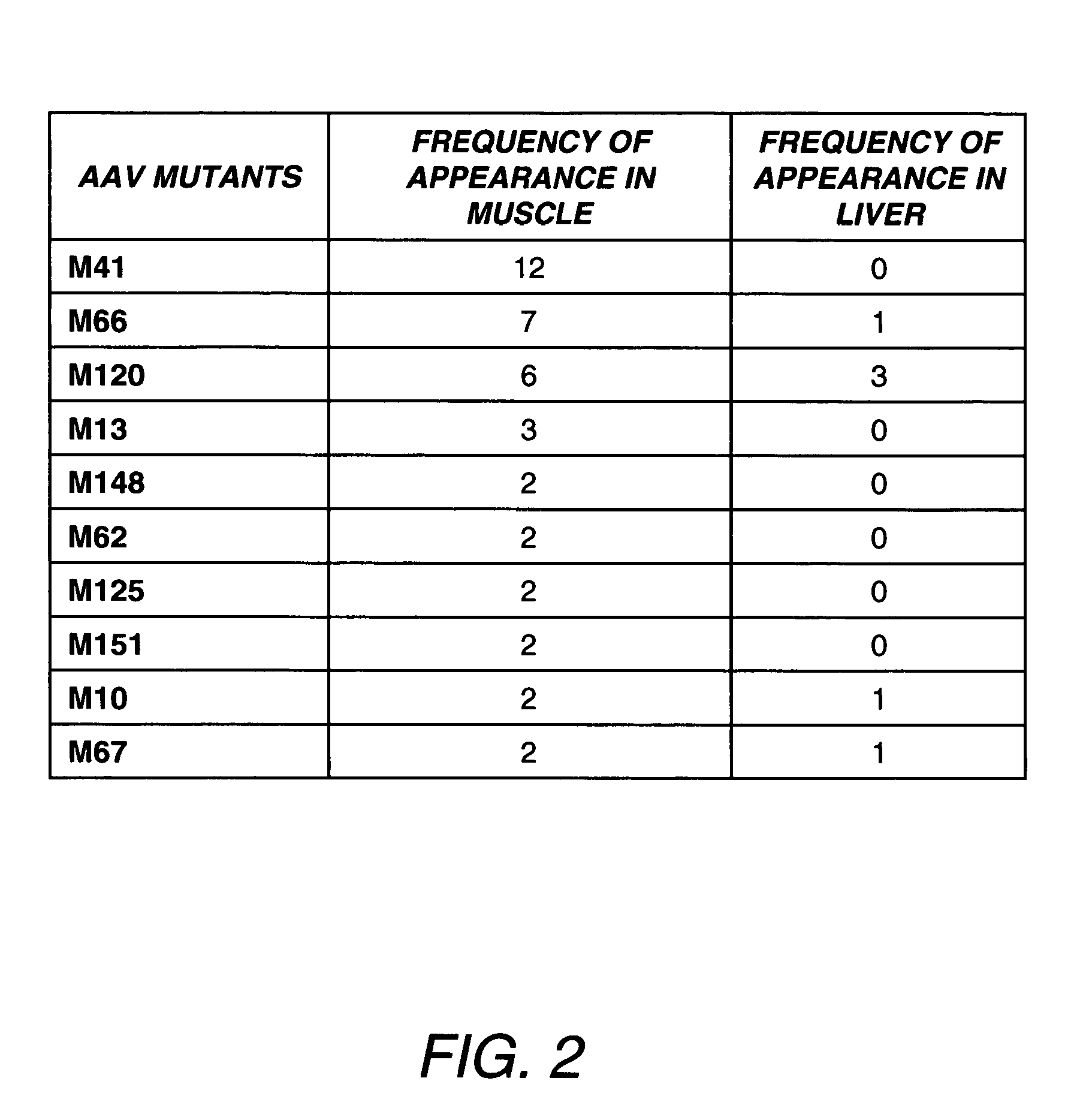
Directed evolution and in vivo panning of virus vectors
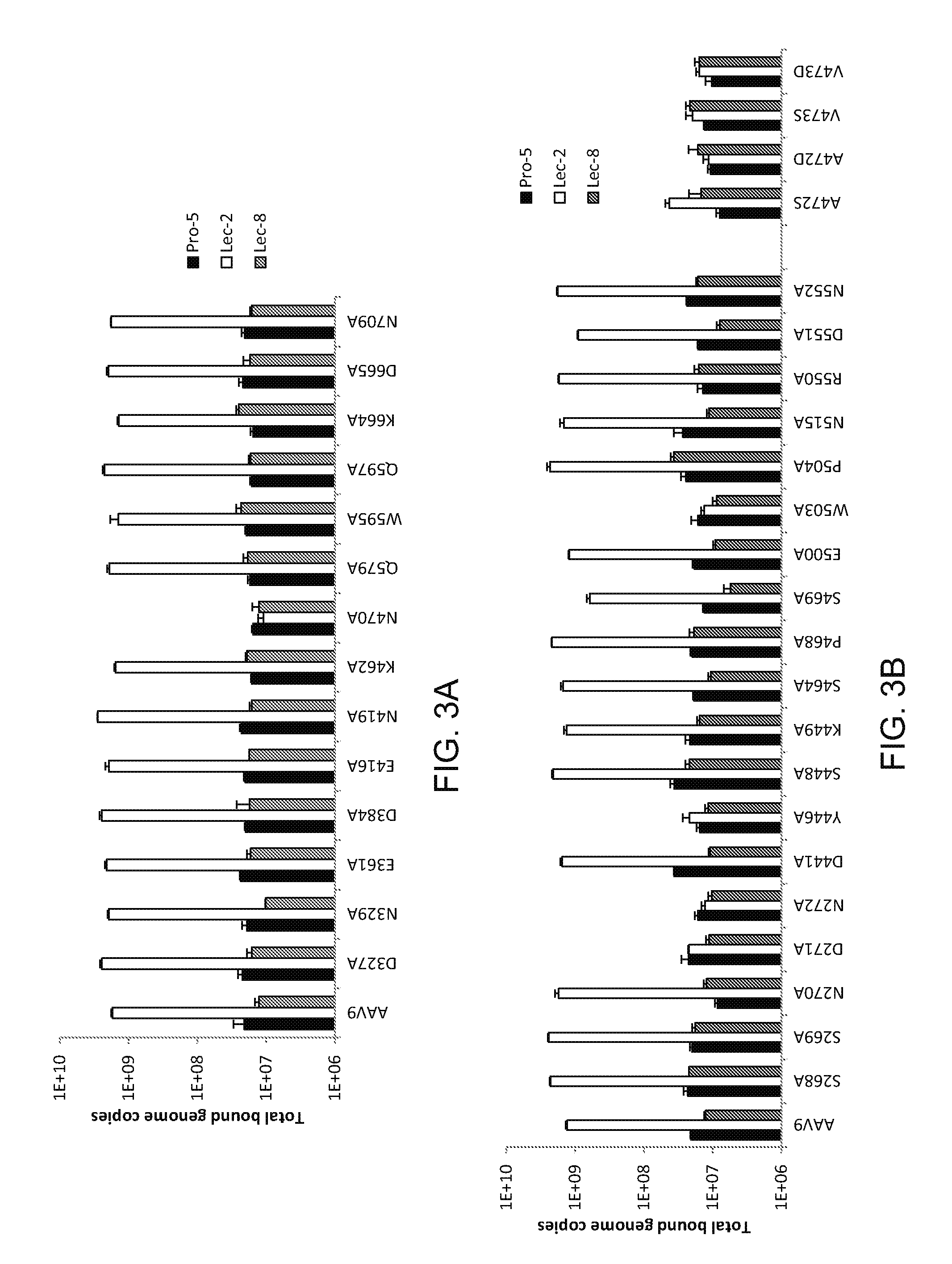
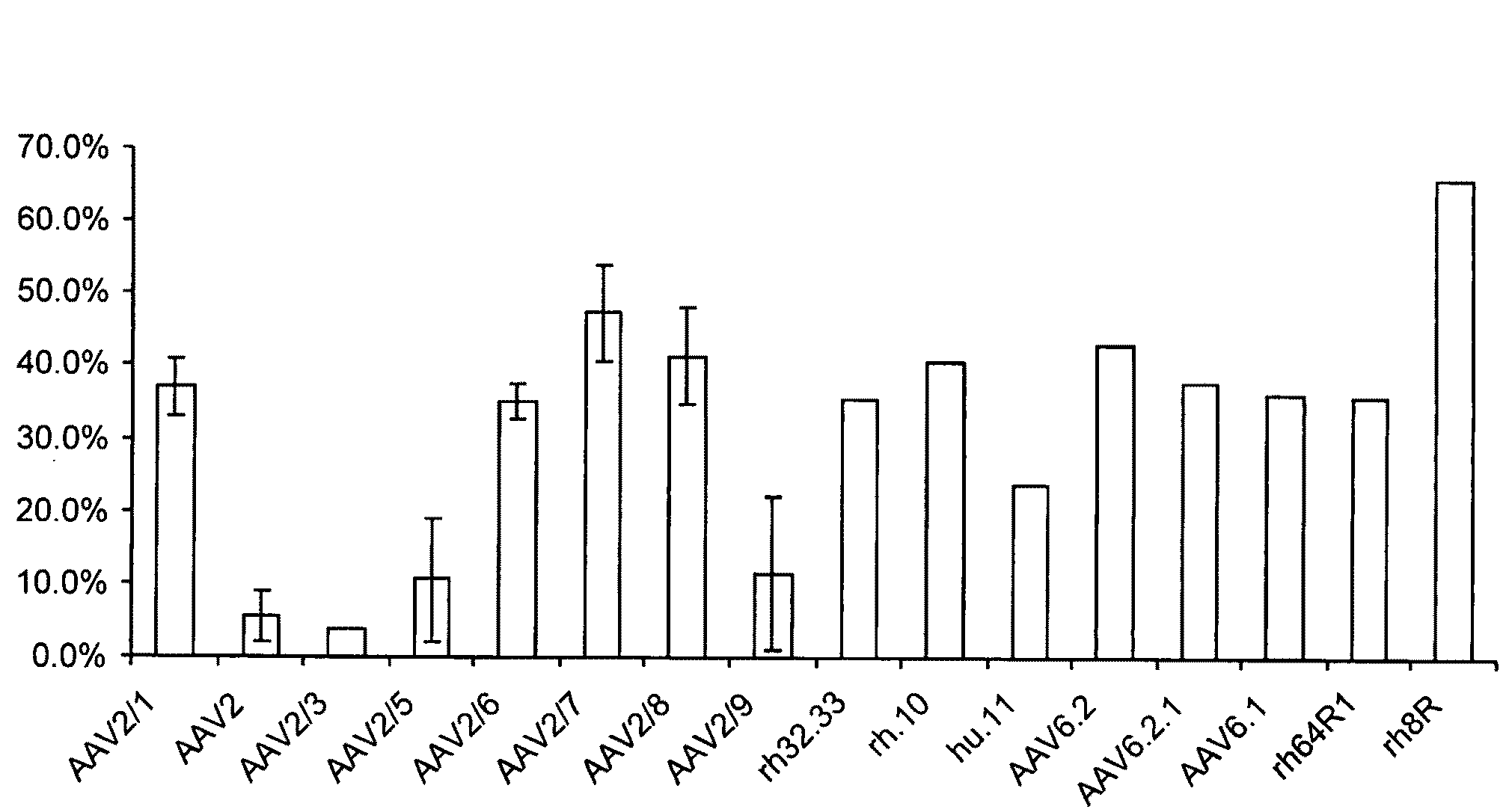
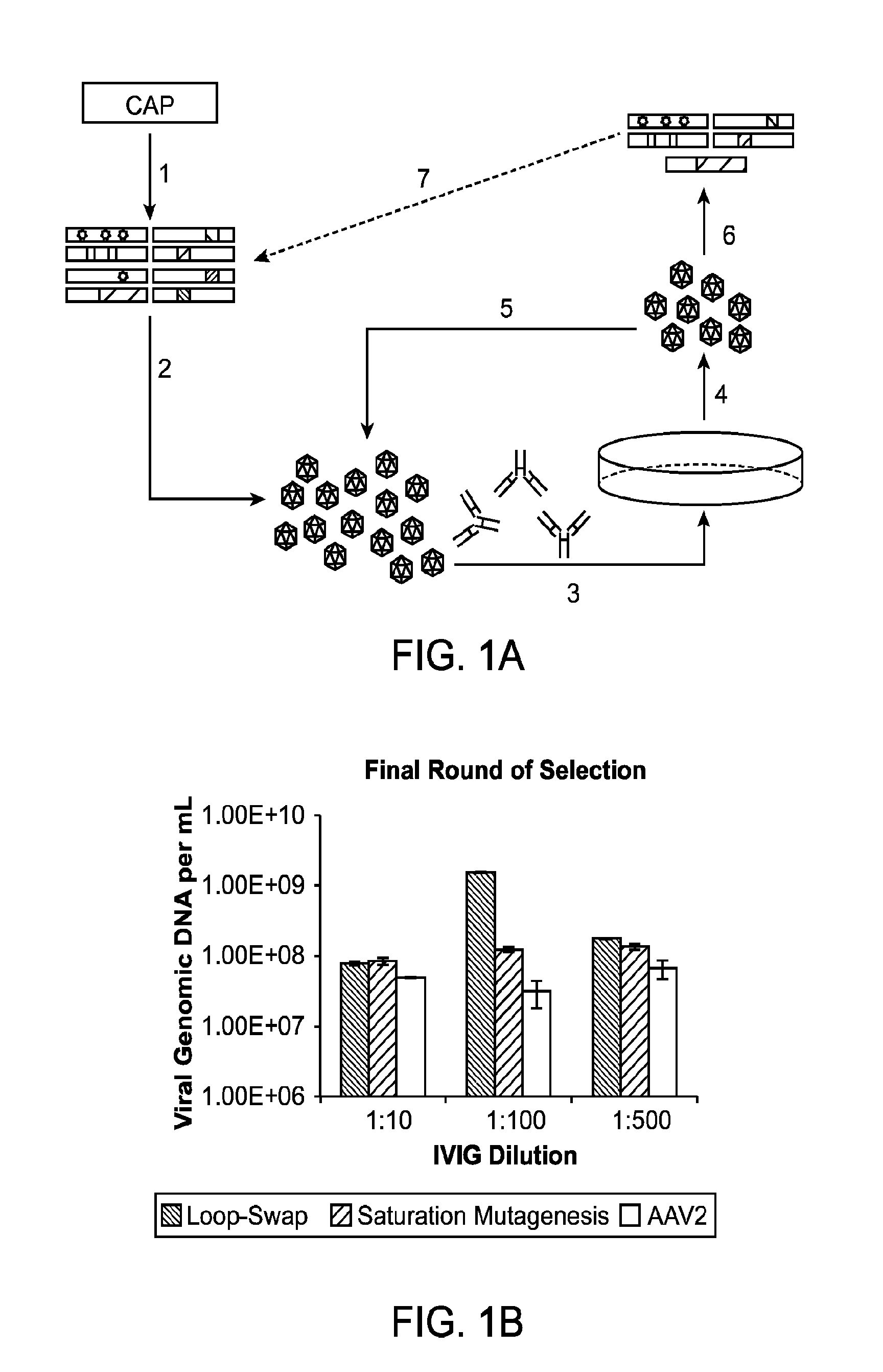
The present invention provides methods of achieving directed evolution of viruses by in vivo screening or “panning” to identify viruses comprising scrambled AAV capsids having characteristics of interest, e.g., tropism profile and / or neutralization profile (e.g., ability to evade neutralizing antibodies). The invention also provides scrambled AAV capsids and virus particles comprising the same.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
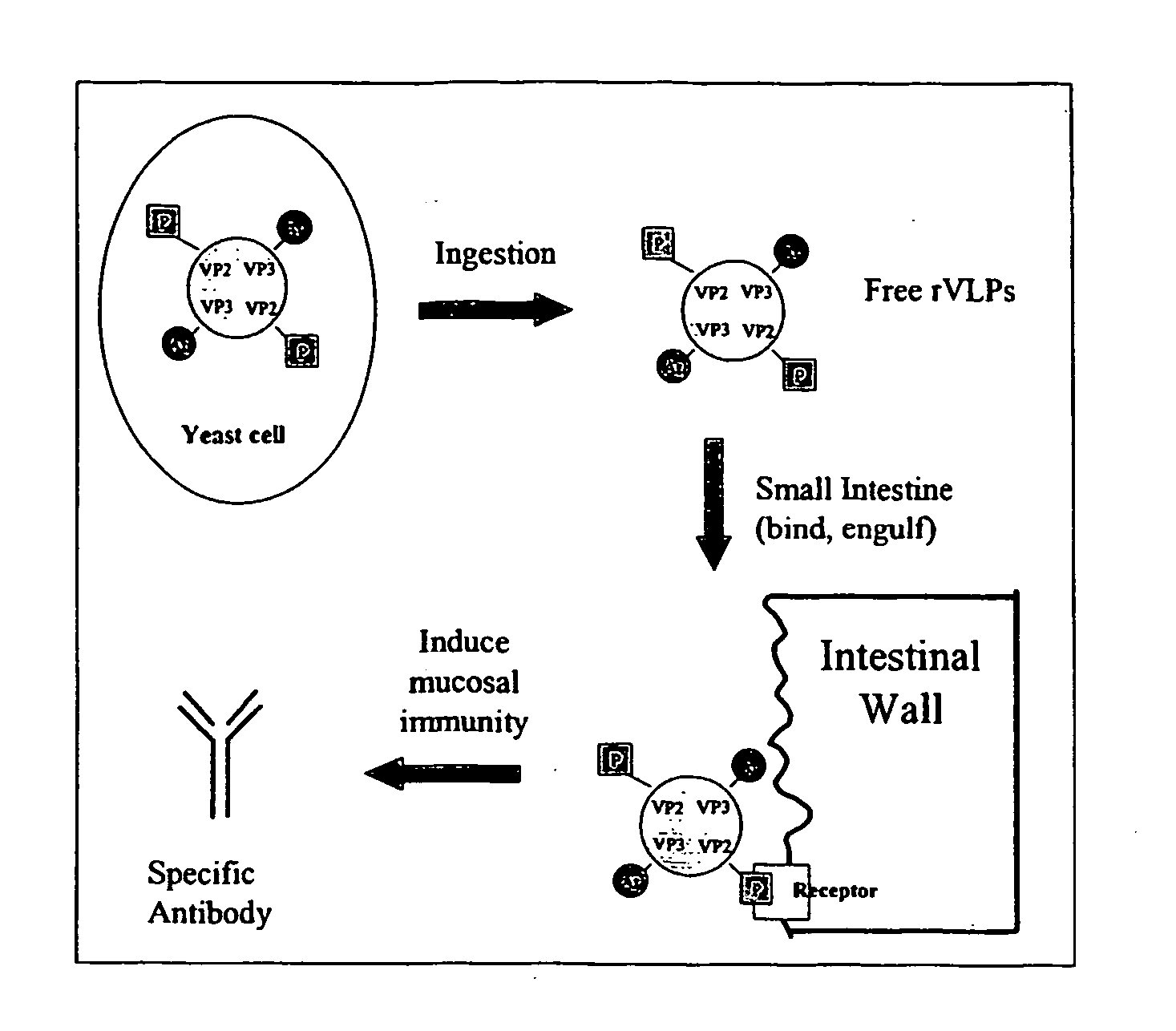
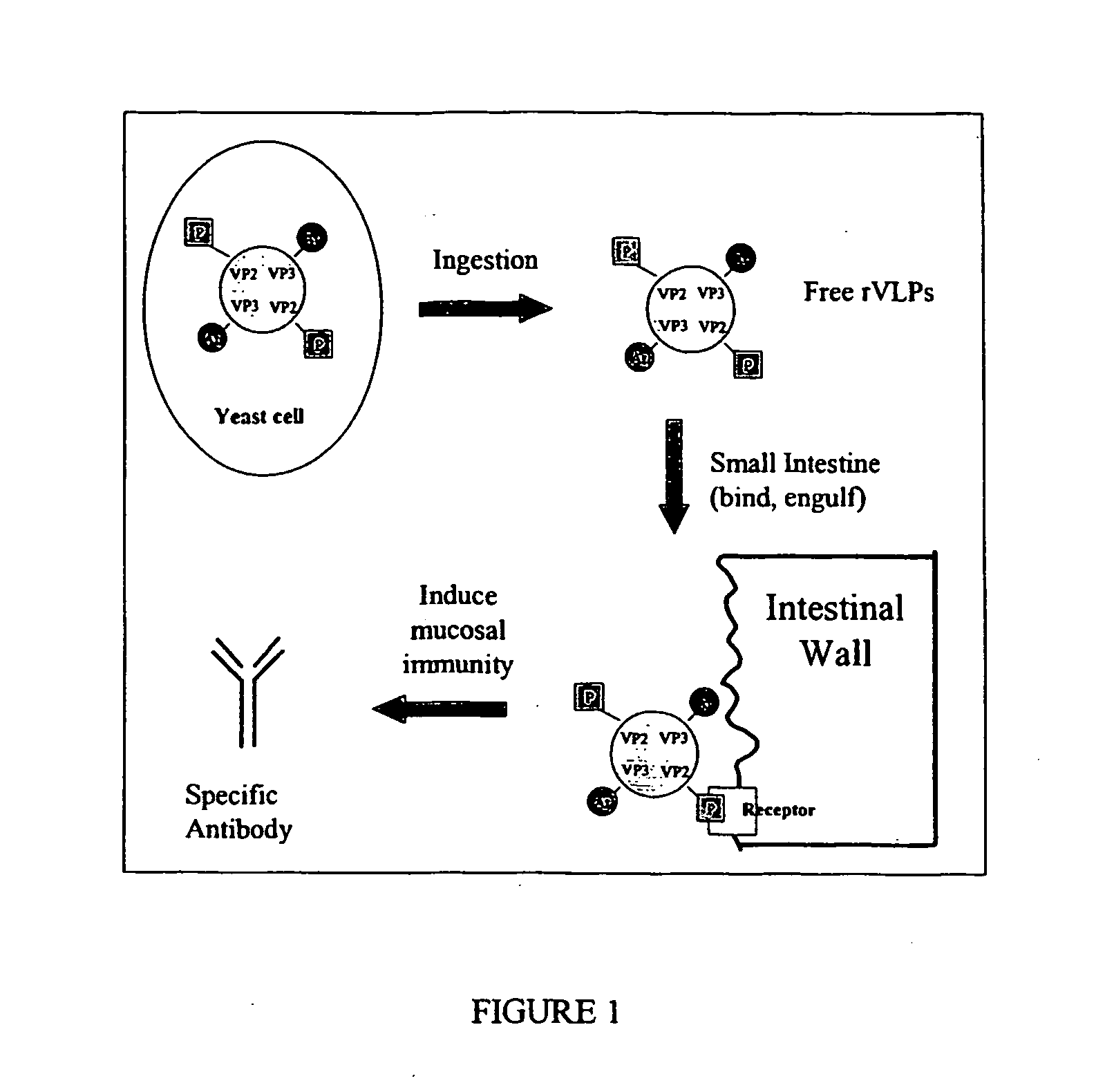
Viruses and virus-like particles for multiple antigen and target display
InactiveUS20060121468A1Improve effectivenessImprove responseSsRNA viruses positive-senseVectorsTissue targetingVaccine Immunogenicity
The present invention relates to the display of antigenic or allergenic components along with a tissue-targeting component on viruses or virus-like particles. Capsid protein genes are recombinantly modified to contain the specified components then expressed within a host organism, such as yeasts, bacteria, or algae, and allowed to spontaneously form active virus particles or virus-like particles. The recombinant complexes (virus or virus-like particle) can then be purified or used in situ as a therapeutic tool for disease or allergy prevention. The expression of multivalent and multifunctional components to increase the immunogenicity of the recombinant complexes, especially on oral administration, is provided.
Owner:ADVANCED BIONUTRITION CORP
Adeno-associated virus variants and methods of use thereof
ActiveUS20160017295A1Improve the immunityEfficient deliveryAntibacterial agentsVirusesHeterologousGene product
The present disclosure provides infectious recombinant adeno-associated virus (rAAV) virions that comprise a variant capsid protein and a heterologous nucleic acid. The present disclosure further provides the variant adeno-associated virus (AAV) capsid proteins (and / or a nucleic acid encoding the variant AAV capsid proteins), which confer to an infectious rAAV virion an increased resistance to human AAV neutralizing antibodies. The present disclosure further provides host cells comprising an infectious rAAV virion and / or a nucleic acid encoding a subject variant AAV capsid protein. The present disclosure further provides methods of delivering a heterologous nucleic acid to a target cell where the target cell is contacted with a subject infectious rAAV virion. The present disclosure further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:RGT UNIV OF CALIFORNIA
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS9441244B2Altered capsid propertiesReduce the binding forceAntibacterial agentsVirusesNucleotideCell type specific
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
Methods and means for targeted gene delivery
InactiveUS7033834B2Efficient introductionEfficiently introducedBiocideGenetic material ingredientsGene deliveryDelivery vehicle
A method for producing viral gene delivery vehicles which can be transferred to pre-selected cell types by using targeting conjugates. The gene delivery vehicles comprise: 1) the gene of interest; and 2) a viral capsid or envelope carrying a member of a specific binding pair, the counterpart of which is not directly associated with the surface of the target cell. These vehicles can be rendered unable to bind to their natural cell receptor. The targeting conjugates include the counterpart member of the specific binding pair, linked to a targeting moiety which is a cell-type specific ligand (or fragments thereof). The number of the specific binding pair present on the viral vehicles can be, for example, an immunoglobulin binding moiety (e.g., capable of binding to a Fc fragment, protein A, protein G, FcR or an anti-Ig antibody), or biotin, avidin or streptavidin. The virus' outer membrane or capsid may contain a substance which mediates entrance of the gene delivery vehicle into the target cell. Due to the specificity of the ligand, the binding pair's high affinity, and the gene delivery vehicle's inability to be targeted when used alone, the universality of the method for gene delivery, together with its high cell type selectively can be achieved by using various targeting conjugates.
Owner:JANSSEN VACCINES & PREVENTION BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com