Patents
Literature
1188 results about "Viral gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The answer is the host cell translates the viral gene. The central dogma of molecular biology explains that the sequence of DNA specifies the sequence of mRNA, which, further, specifies the sequence of proteins. The same can be used for viral genes. Inside the host cell, the viral gene undergoes transcription.
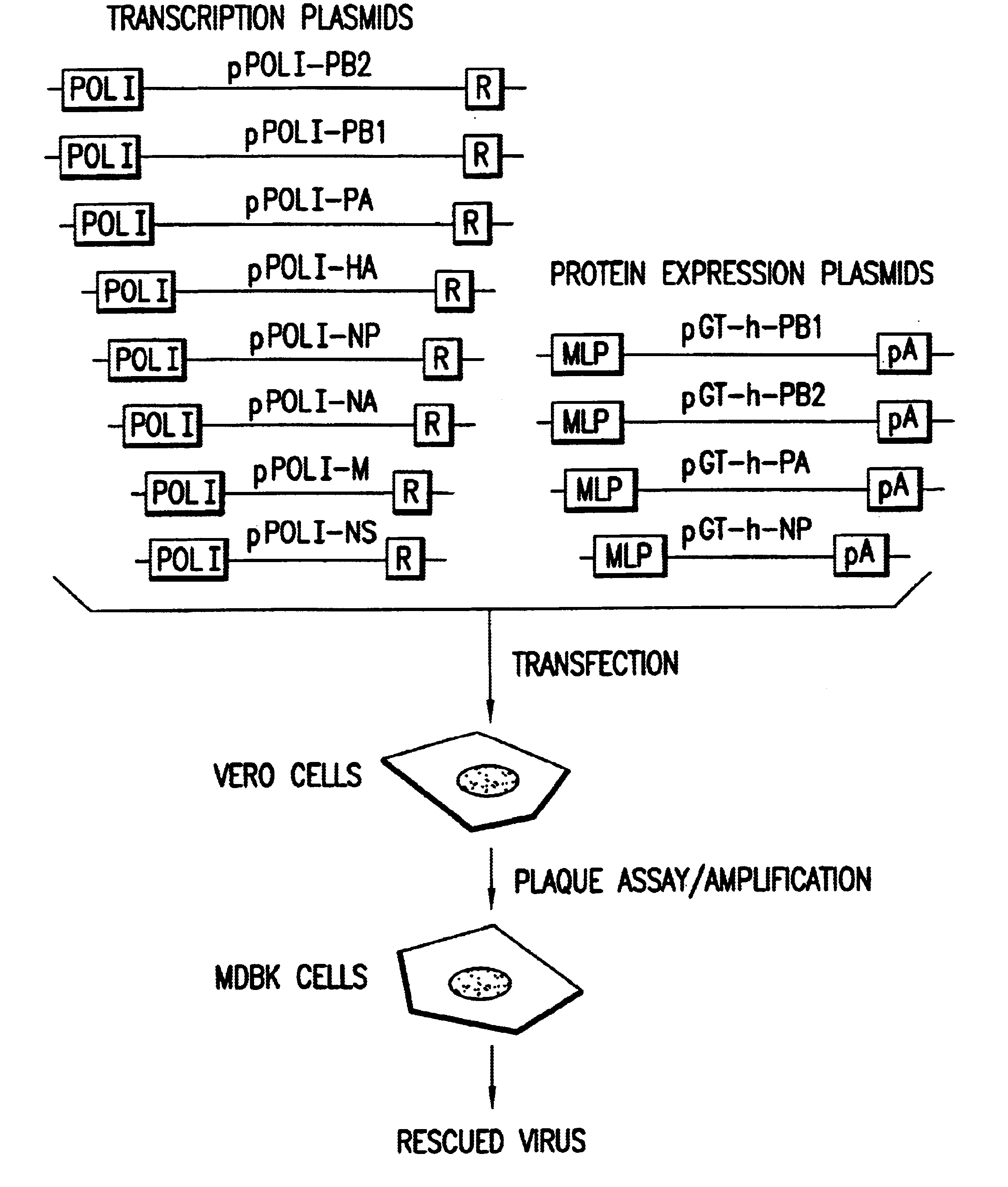
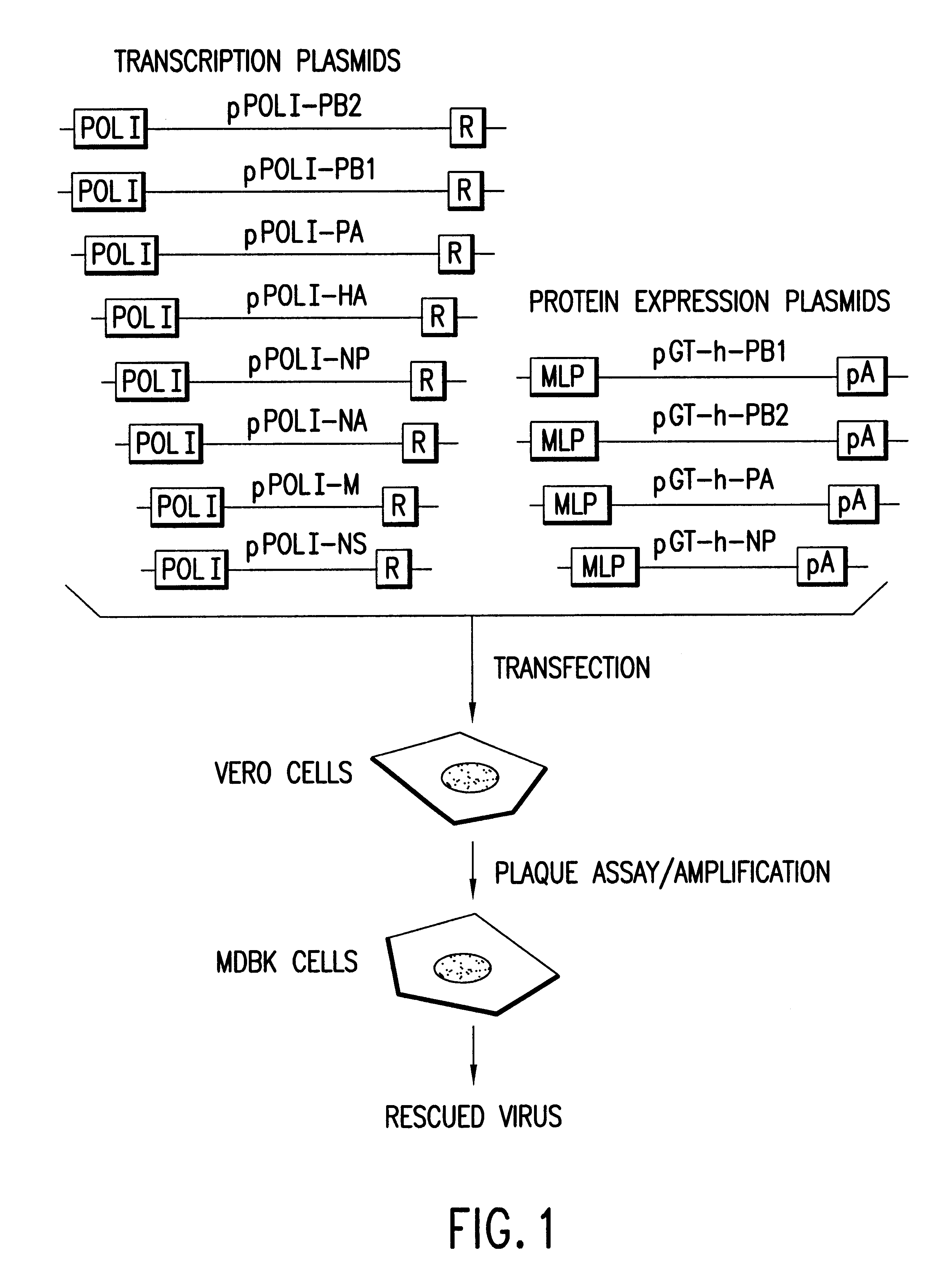
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Interferon inducing genetically engineered attenuated viruses
InactiveUS6468544B1Reduce in quantityReduced characteristicsSsRNA viruses negative-senseVectorsGenetic engineeringRecombinant DNA
The present invention relates to genetically engineered attenuated viruses and methods for their production. In particular, the present invention relates to engineering live attenuated viruses which contain a modified NS gene segment. Recombinant DNA techniques can be utilized to engineer site specific mutations into one or more noncoding regions of the viral genome which result in the down-regulation of one or more viral genes. Alternatively, recombinant DNA techniques can be used to engineer a mutation, including but not limited to an insertion, deletion, or substitution of an amino acid residue(s) or an epitope(s) into a coding region of the viral genome so that altered or chimeric viral proteins are expressed by the engineered virus.
Owner:MT SINAI SCHOOL OF MEDICINE +1
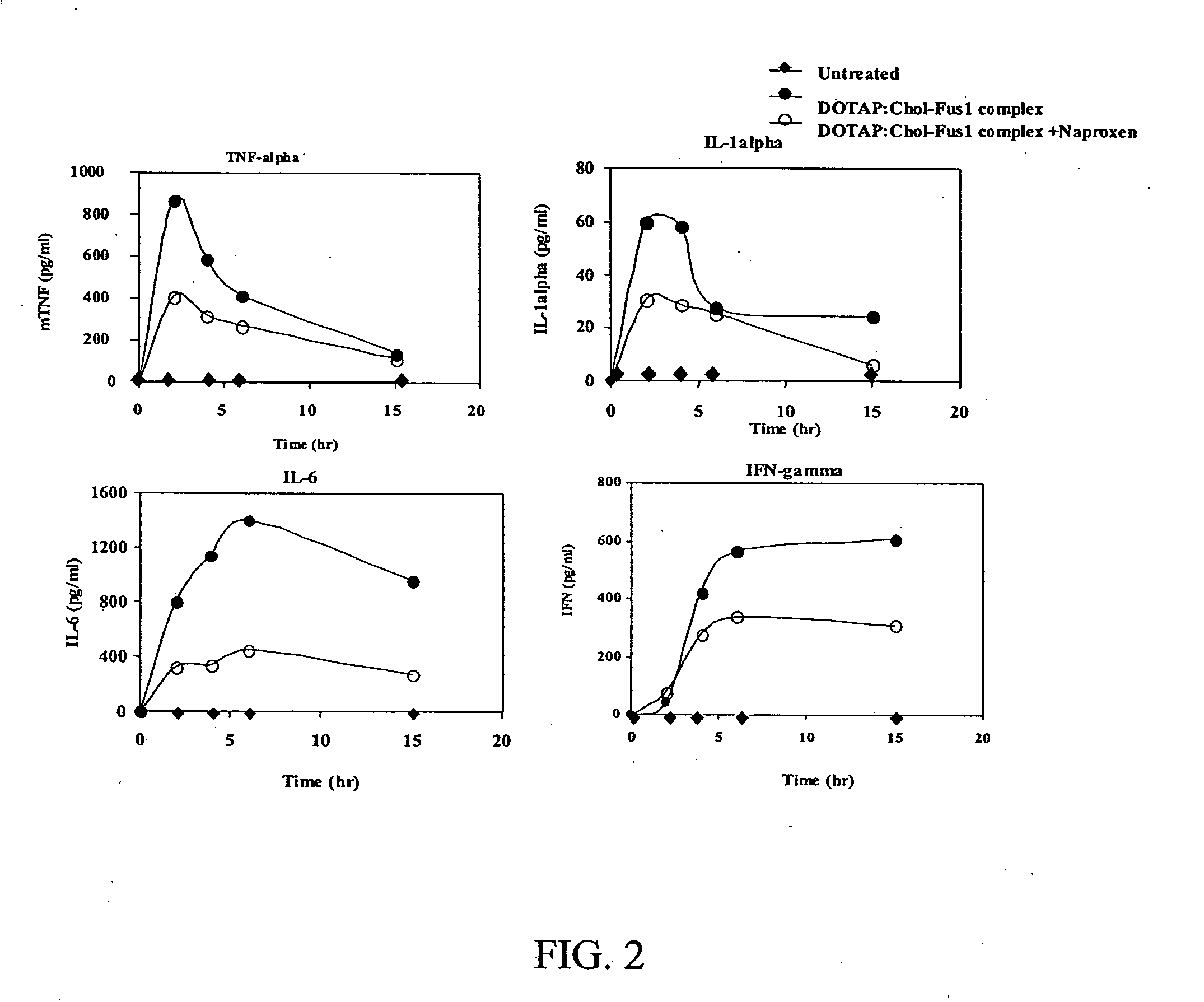
Methods and compositions for improved non-viral gene therapy
Methods to prevent or reduce inflammation secondary to administration of a lipid-nucleic acid complex in a subject, that include administering to the subject a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunsuppressive agent with the lipid-nucleic acid complex are disclosed. Also disclosed are methods of screening for inhibitors of the inflammatory response associated with administration of a lipid-nucleic acid complex to a subject, including providing a candidate substance suspected of preventing or inhibiting the inflammation associated with administration of a lipid-nucleic acid complex to the subject. Also disclosed are compositions that include a lipid, a nucleic acid, and a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunosuppressive agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for specifically knocking out hepatitis B virus by CRISPR/Cas9 and gRNA applied to specific targeting HBV DNA
ActiveCN104498493AEfficient removalReduce escape from treatmentGenetic material ingredientsAntiviralsDrugRNA
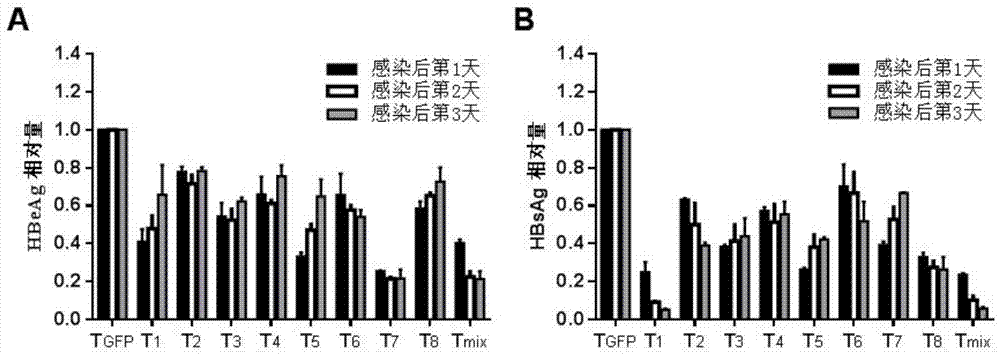
The invention relates to the technical field of molecular biology and biological medicines, and particularly relates to application of gRNA sequences and combination thereof based on a CRISPR system in treatment on hepatitis B virus. According to the method disclosed by the invention, eight types of guidance RNAs (gRNA) are designed according to design rules of CRISPR gRNA and a conservative region of different genotypes of HBV sequences, and the eight types of guidance RNAs are structured on a PX330 expression vector. By utilizing the eight gRNAs in a cell model, a mouse model and a CRISPR / Cas9 system guided by combination of the cell model and the mouse model, the expression and replication of the hepatitis B virus can be effectively inhibited. By united application of a plurality of gRNAs, a better effect can be achieved, and different genotypes of HBV replication can be better inhibited. The system has the characteristics of being easy to operate, high in inhibition efficiency on HBV replication and applicable to various genotypes. Therefore, the gRNA and the combination thereof related to the invention are expected to be applied in preparation of a novel drug for treating the hepatitis B virus.
Owner:浙江安维珞诊断技术有限公司
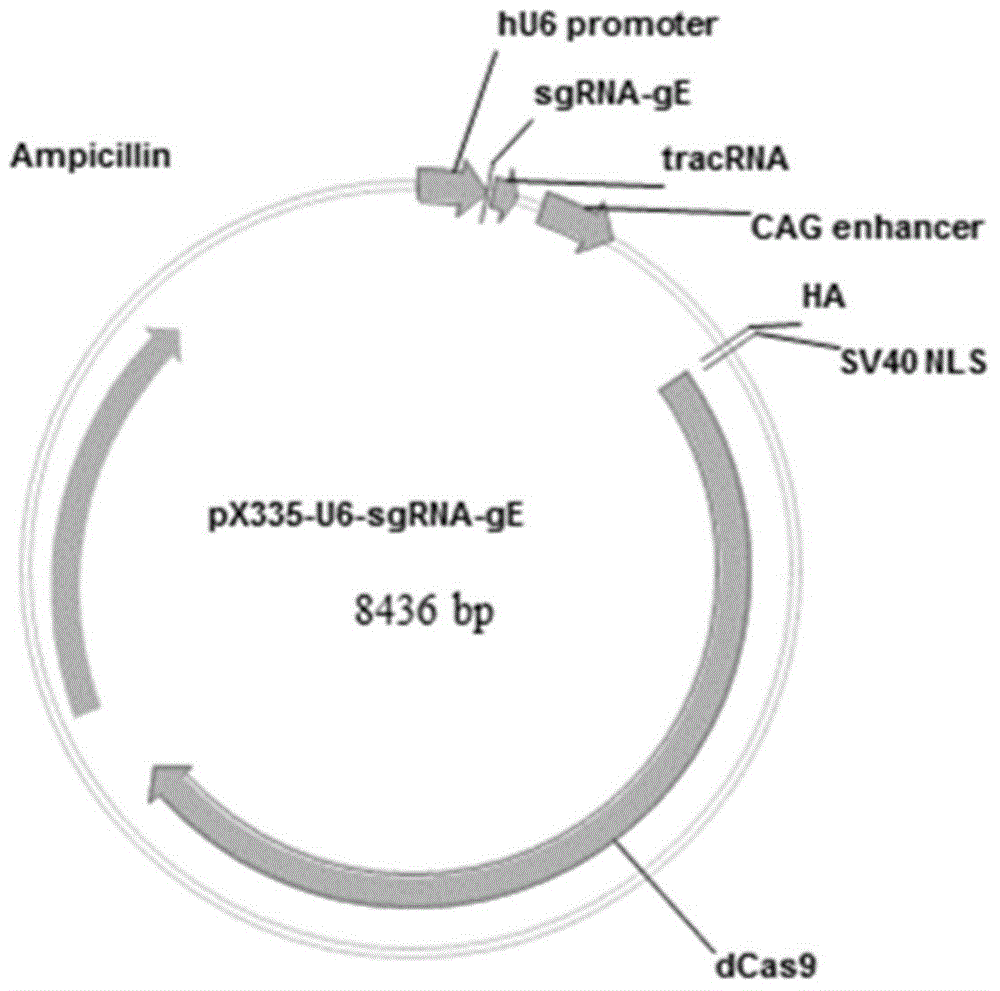
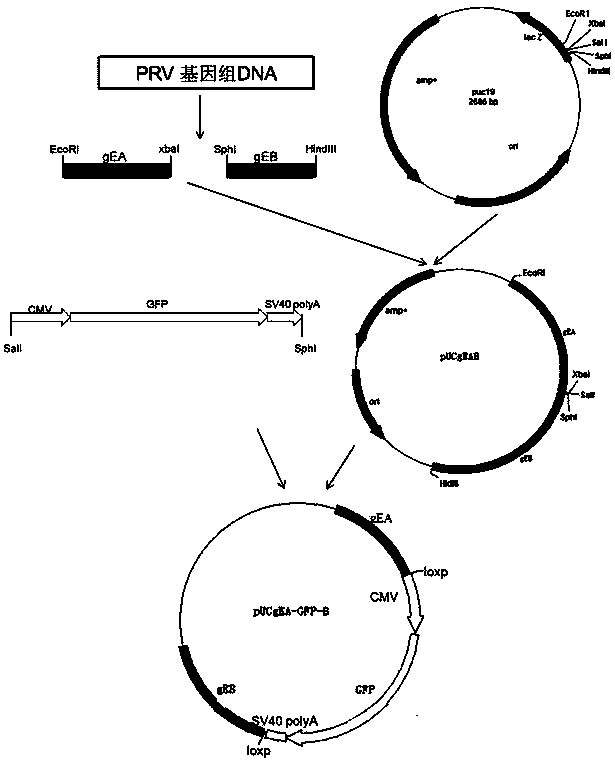
Method for preparing vaccine by editing pseudorabies virus genomes based on CRISPR/Cas9 and Cre/lox systems and application of method
ActiveCN104894075AReduce disease lossEasy to operateAntiviralsViruses/bacteriophagesMCherry fluorescent proteinBiology
The invention discloses a method for quickly preparing a vaccine by editing pseudorabies virus genomes based on a CRISPR / Cas9 gene editing system and a Cre / lox recombination system and an application of the method. According to the method, the CRISPR / Cas9 gene editing system is used for synchronously and efficiently recombining a GFP gene and a mCherry gene to a pseudorabies virus gE gene site and a TK gene site respectively to obtain conditional deletion strains of a gE gene and a TK gene; after purification, the Cre / lox system is used for cutting off extraneous GFP and mCherry genes in the pseudorabies virus recombinant virus genome so as to perform purification quickly to obtain a live pseudorabies virus vaccine lack of gE / TK genes; multiple genes are operated at the same time, so that multiple rounds of flows for knocking out multiple genes in the conventional method are reduced to one round; and meanwhile, the efficient edition of virus genes by using the CRISPR / Cas9 and Cre / lox systems simplifies about thirty generations of plaque purification processes into 3-4 generations, so that the preparation efficiency of the virus vaccine is greatly improved, and the method provides a strong guarantee for effectively preventing and controlling the larger-range popularization of variant pseudorabies viruses and reducing heavy economic losses.
Owner:武汉都为康生物科技有限公司
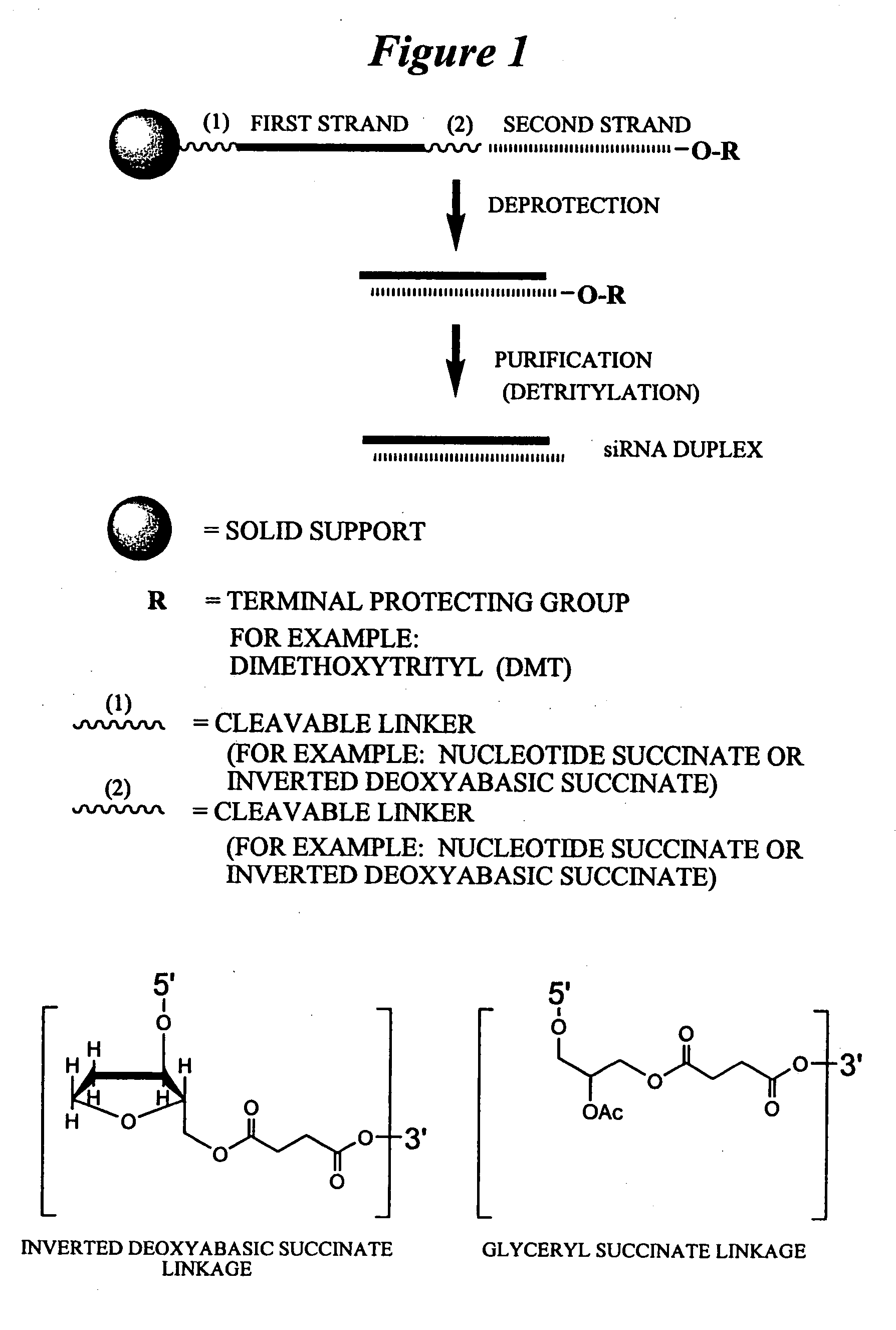
RNAi Agents Comprising Universal Nucleobases
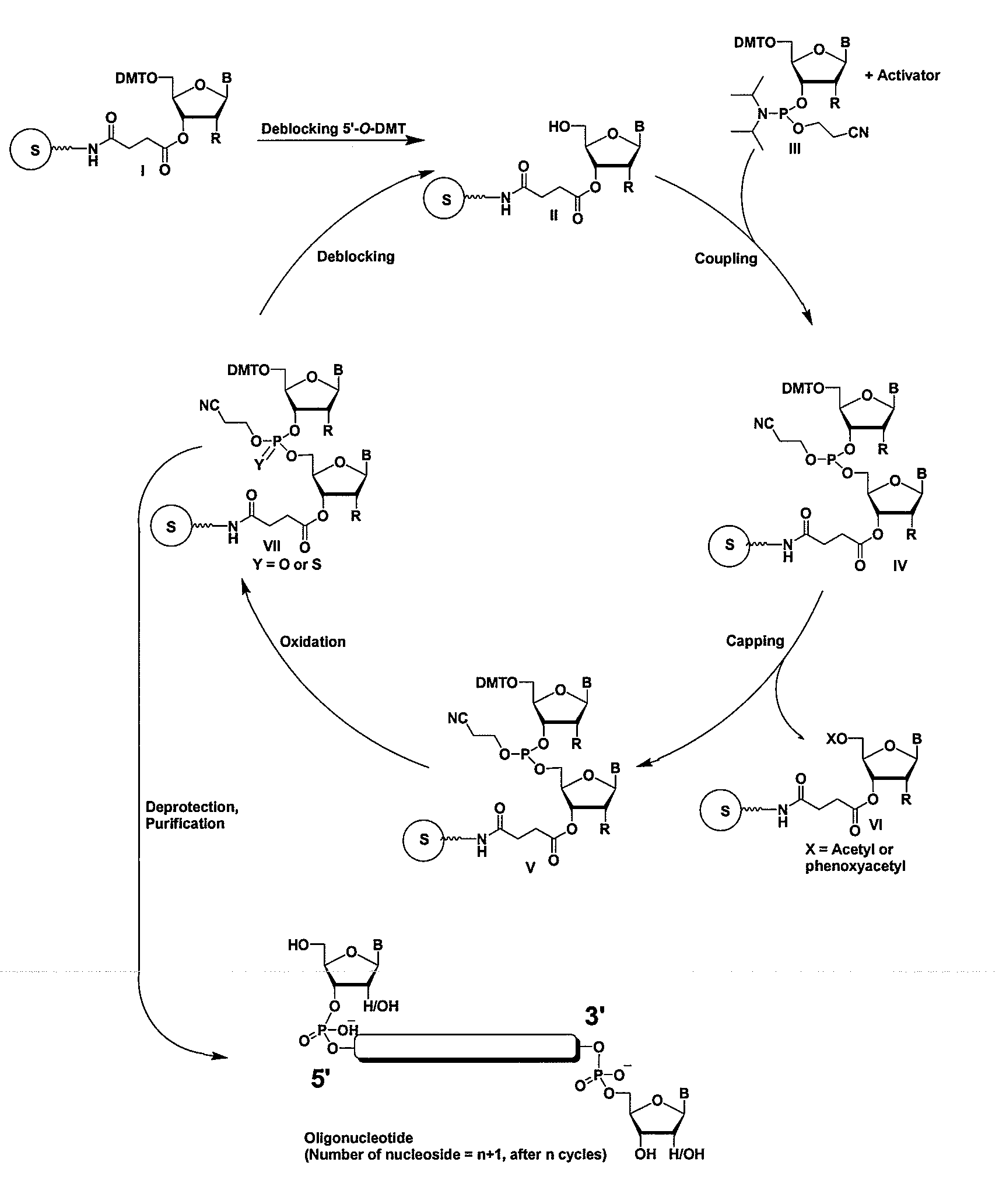
InactiveUS20080213891A1Broad scopeReduce needSugar derivativesPolymorphism usesNitroimidazoleViral Genes
One aspect of the present invention relates to an oligonucleotide agent comprising at least one universal nucleobase. In certain embodiments, the universal nucleobase is difluorotolyl, nitroindolyl, nitropyrrolyl, or nitroimidazolyl. In a preferred embodiment, the universal nucleobase is difluorotolyl. In certain embodiments, the oligonucleotide is double-stranded. In certain embodiments, the oligonucleotide is single-stranded. Another aspect of the present invention relates to a method of altering the expression level of a target in the presence of target sequence polymorphism. In a preferred embodiment, the oligonucleotide agent alters the expression of different alleles of a gene. In another preferred embodiment, the oligonucleotide agent alters the expression level of two or more genes. In another embodiment, the oligonucleotide agent alters the expression level of a viral gene from different strains of the virus. In another embodiment, the oligonucleotide agent alters the expression level of genes from different species.
Owner:ALNYLAM PHARMA INC
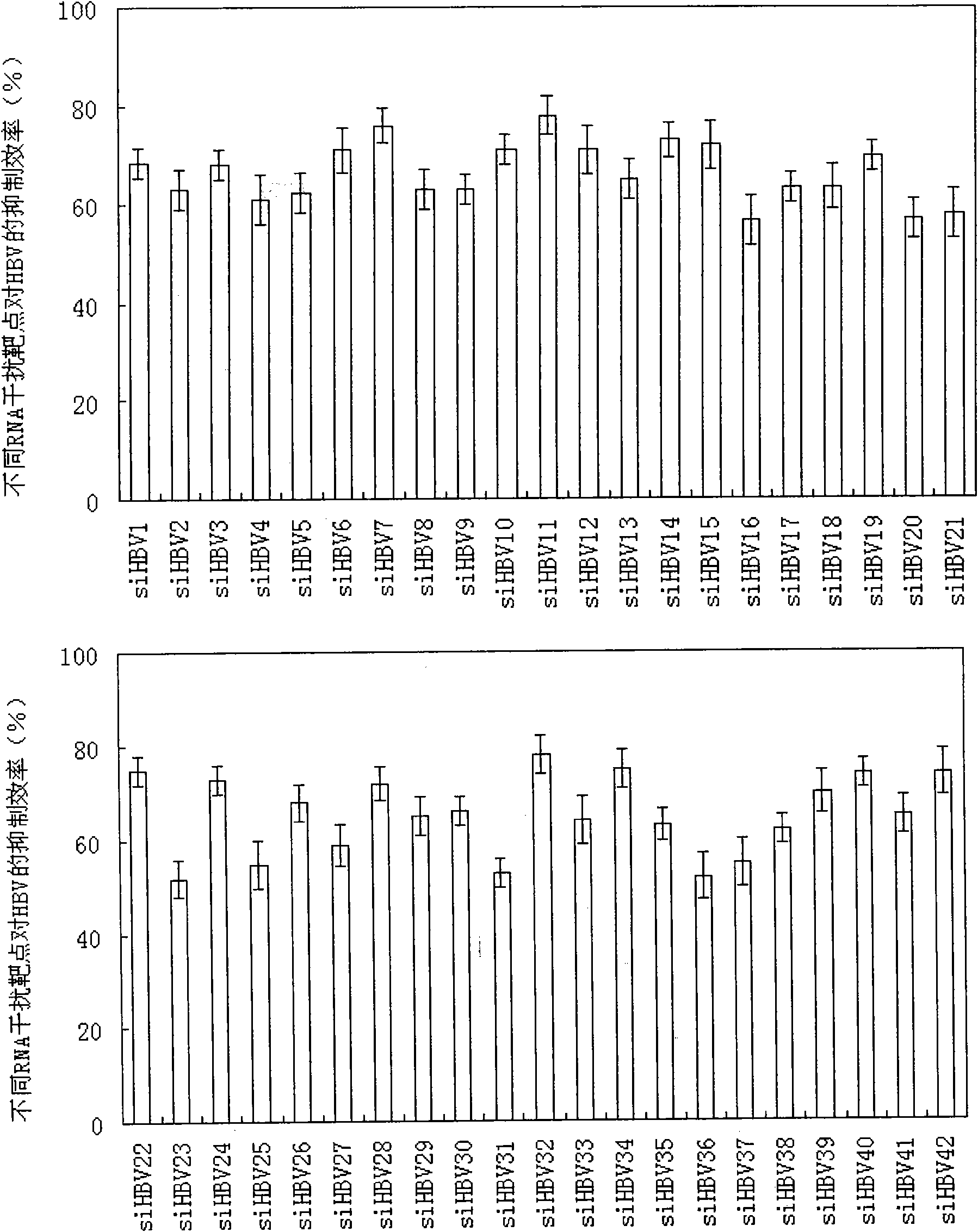
RNA interference target for treating hepatitis B virus infection
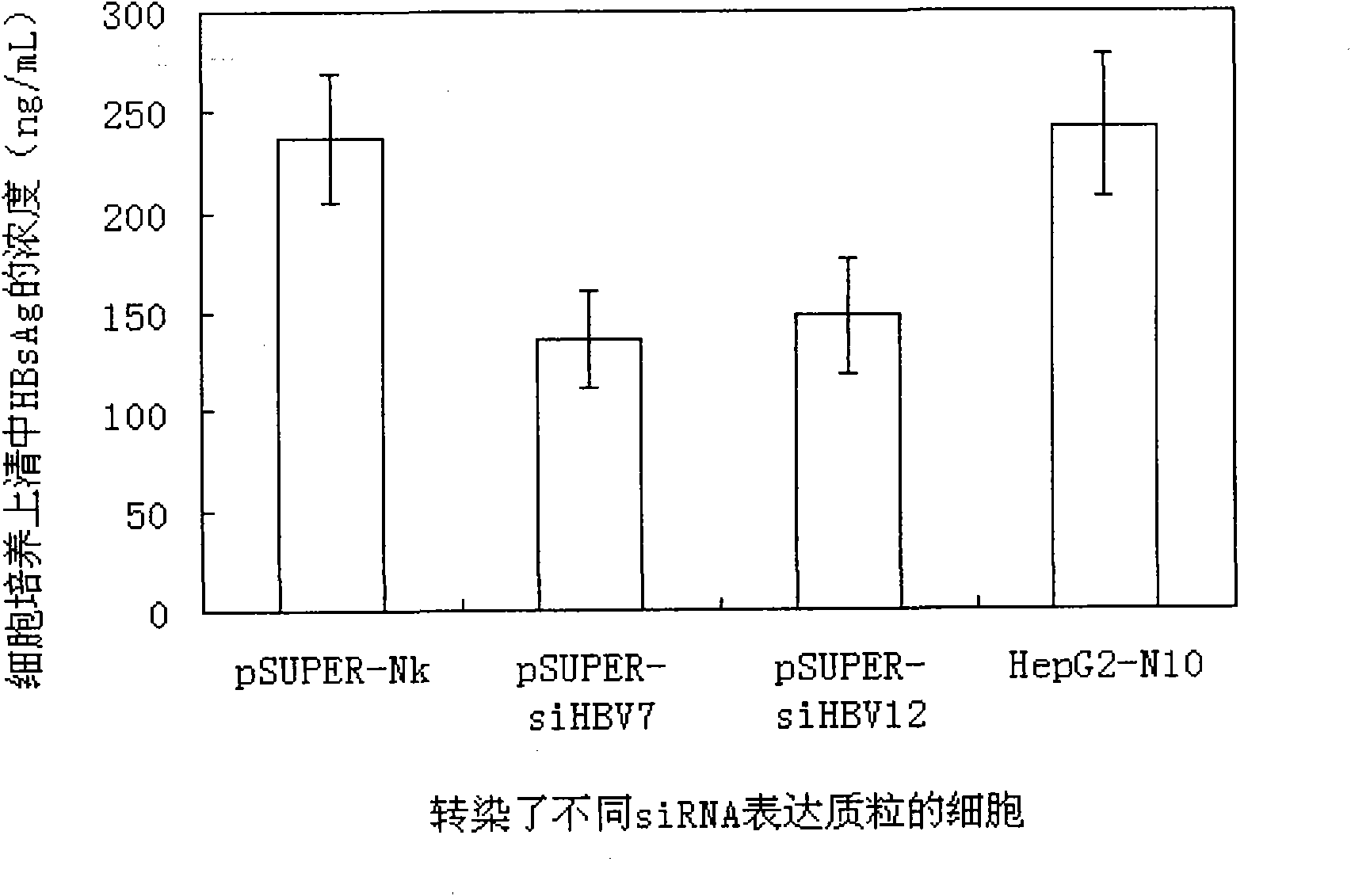
The invention relates to an RNA interference target for treating hepatitis B virus infection with 42 different targeting hepatitis B viruses (HBV), which can be used for preparing a medicament for treating the hepatitis B virus infection. The invention provides a recombinant expression vector of siRNA and / or miRNA and / or ribozyme and / or antisense oligonucleotide, which can be used for expressing targeting HBV. The invention relates to a cell which has the function of inhibiting the expression of HBV virus gene and can express and / or is induced with the siRNA and / or the miRNA and / or the ribozyme and / or the antisense oligonucleotide and / or the medication obtained according to the RNA interference target.
Owner:XIAMEN UNIV +1
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
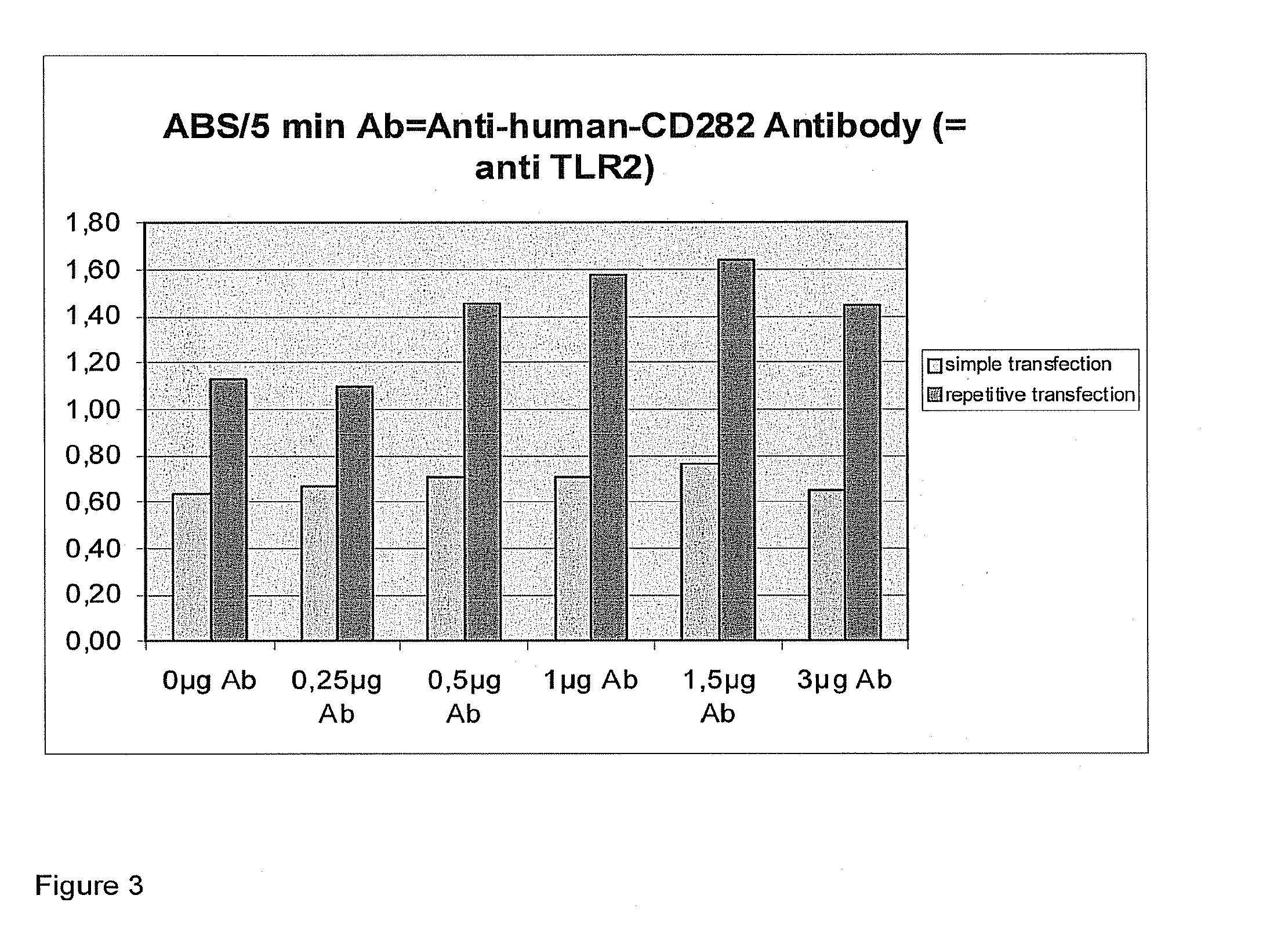
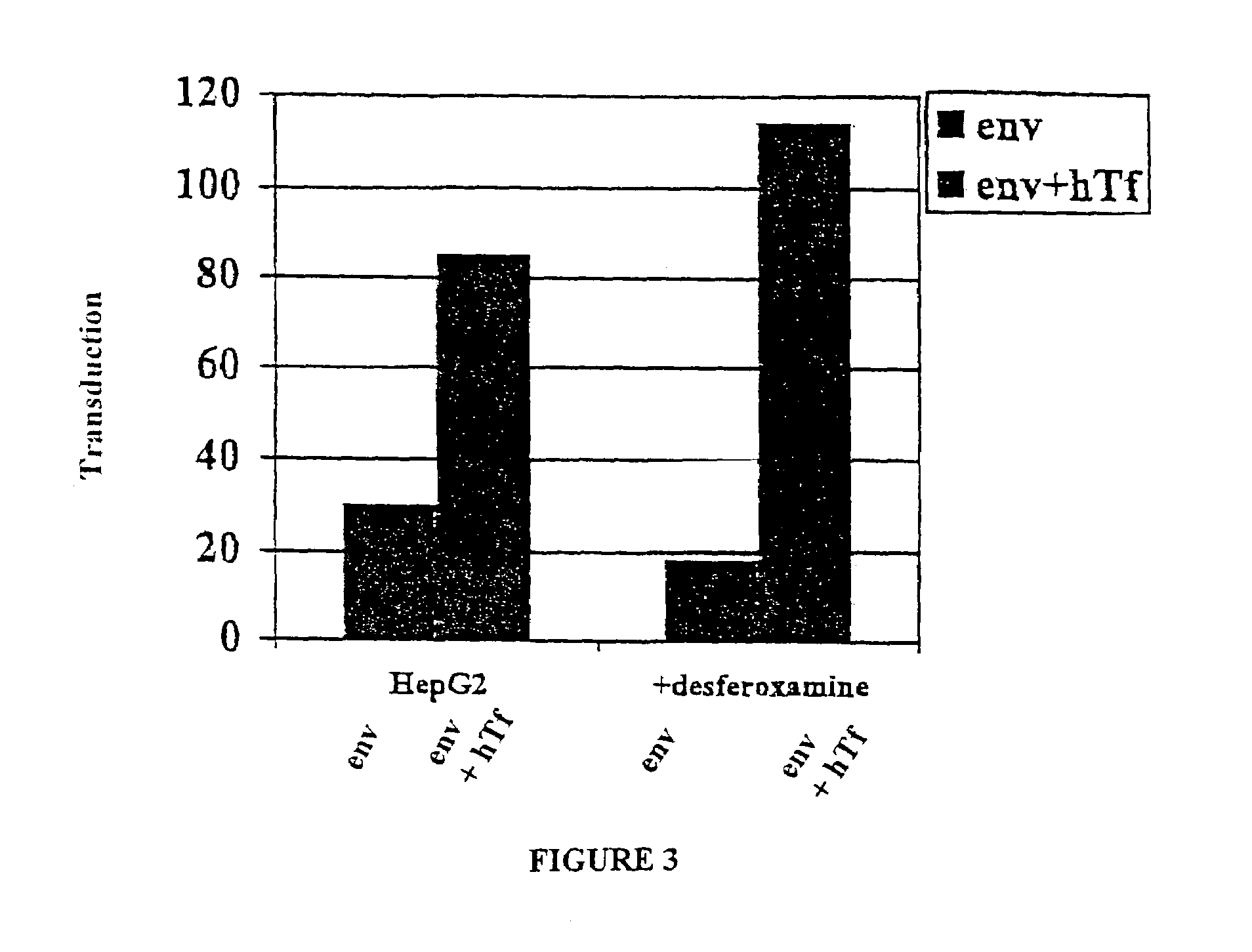
Transfection results of non-viral gene delivery systems by influencing of the innate immune system
The innate immune system of eukaryotes is able to recognise foreign genetic material by means of Toll-like receptors and to initiate signal transduction cascades that trigger an antiviral state of cell populations by way of an interferon response. That antiviral state is also a barrier for non-viral gene delivery systems. If the signal transduction cascade is interrupted intracellularly or intercellularly, transfection efficiencies of non-viral gene delivery systems can be increased and undesirable changes in the expression profile can be avoided. Since RNA-interference is to be attributed to the antiviral state, the RNAi machinery is likewise activated after activation of the innate immune system. In that way, knock-down efficiencies on transfection with siRNA can be increased.
Owner:BIONTEX LAB
Methods and means for targeted gene delivery
InactiveUS7033834B2Efficient introductionEfficiently introducedBiocideGenetic material ingredientsGene deliveryDelivery vehicle
A method for producing viral gene delivery vehicles which can be transferred to pre-selected cell types by using targeting conjugates. The gene delivery vehicles comprise: 1) the gene of interest; and 2) a viral capsid or envelope carrying a member of a specific binding pair, the counterpart of which is not directly associated with the surface of the target cell. These vehicles can be rendered unable to bind to their natural cell receptor. The targeting conjugates include the counterpart member of the specific binding pair, linked to a targeting moiety which is a cell-type specific ligand (or fragments thereof). The number of the specific binding pair present on the viral vehicles can be, for example, an immunoglobulin binding moiety (e.g., capable of binding to a Fc fragment, protein A, protein G, FcR or an anti-Ig antibody), or biotin, avidin or streptavidin. The virus' outer membrane or capsid may contain a substance which mediates entrance of the gene delivery vehicle into the target cell. Due to the specificity of the ligand, the binding pair's high affinity, and the gene delivery vehicle's inability to be targeted when used alone, the universality of the method for gene delivery, together with its high cell type selectively can be achieved by using various targeting conjugates.
Owner:JANSSEN VACCINES & PREVENTION BV
Compositions and Methods for Inhibiting Gene Expression of Hepatitis B Virus
ActiveUS20130005793A1Inhibit expressionReduce expressionOrganic active ingredientsAntipyreticDrugDouble stranded
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of a Hepatitis B Virus gene. The invention also relates to a pharmaceutical composition comprising the dsRNA or nucleic acid molecules or vectors encoding the same together with a pharmaceutically acceptable carrier; methods for treating diseases caused by Hepatitis B Virus infection using said pharmaceutical composition; and methods for inhibiting the expression of a Hepatitis B Virus gene in a cell.
Owner:ARROWHEAD PHARMA INC
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
InactiveUS6884414B1Quick changeAvoid problemsSsRNA viruses negative-senseBiocideTumor reductionIn vivo
The present invention relates to the engineering of recombinant influenza viruses that express tumor-associated antigens. Expression of tumor-associated antigens by these viruses can be achieved by engineering specific epitopes into influenza virus proteins, or by engineering viral genes that encode a viral protein and the specific antigen as independent polypeptides. Tumor-bearing patients can be immunized with the recombinant influenza viruses alone, or in combination with another treatment, to induce an immune response that leads to tumor reduction. The recombinant viruses can also be used to vaccinate high risk tumor-free patients to prevent tumor formation in vivo.
Owner:MT SINAI SCHOOL OF MEDICINE +1
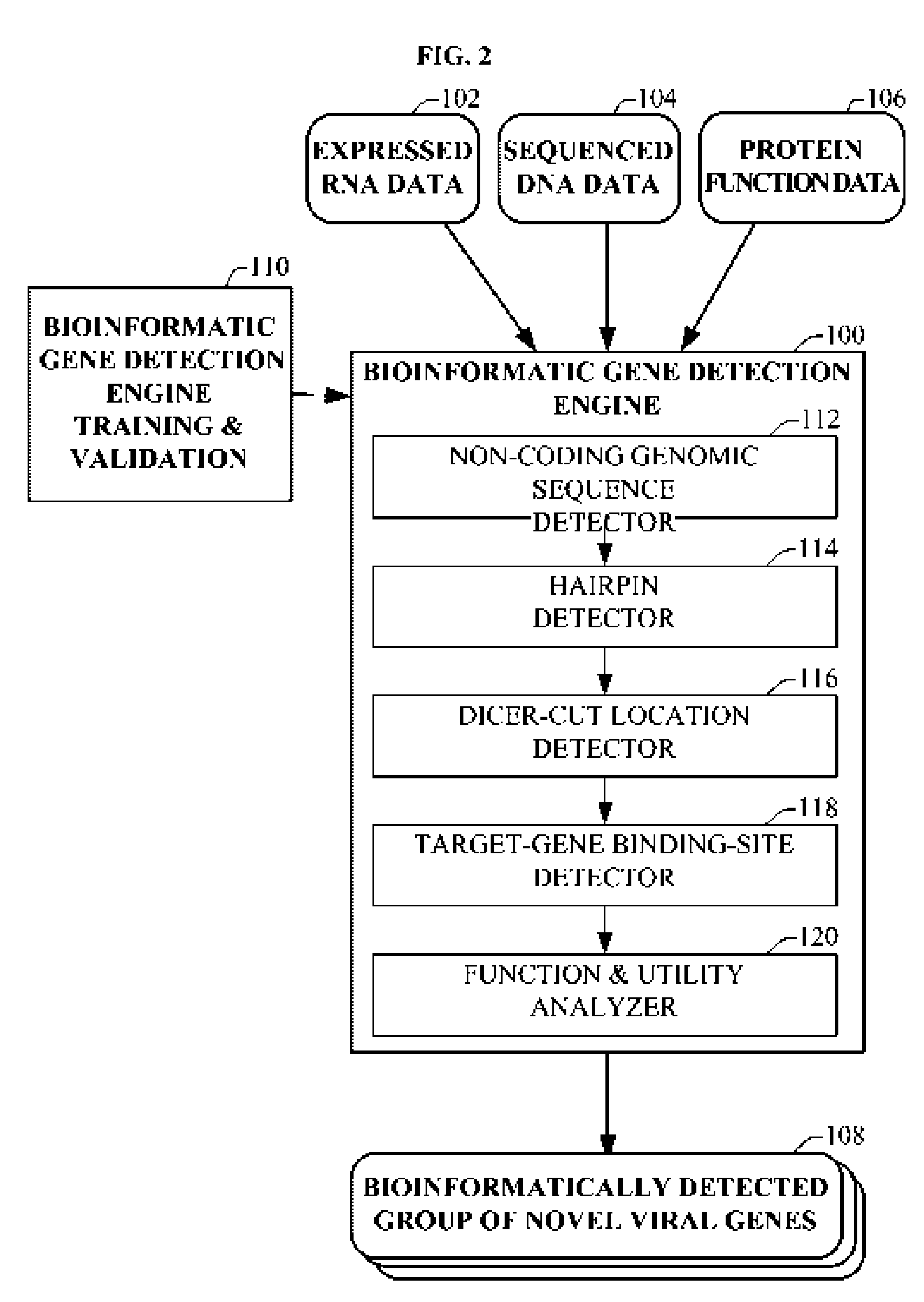
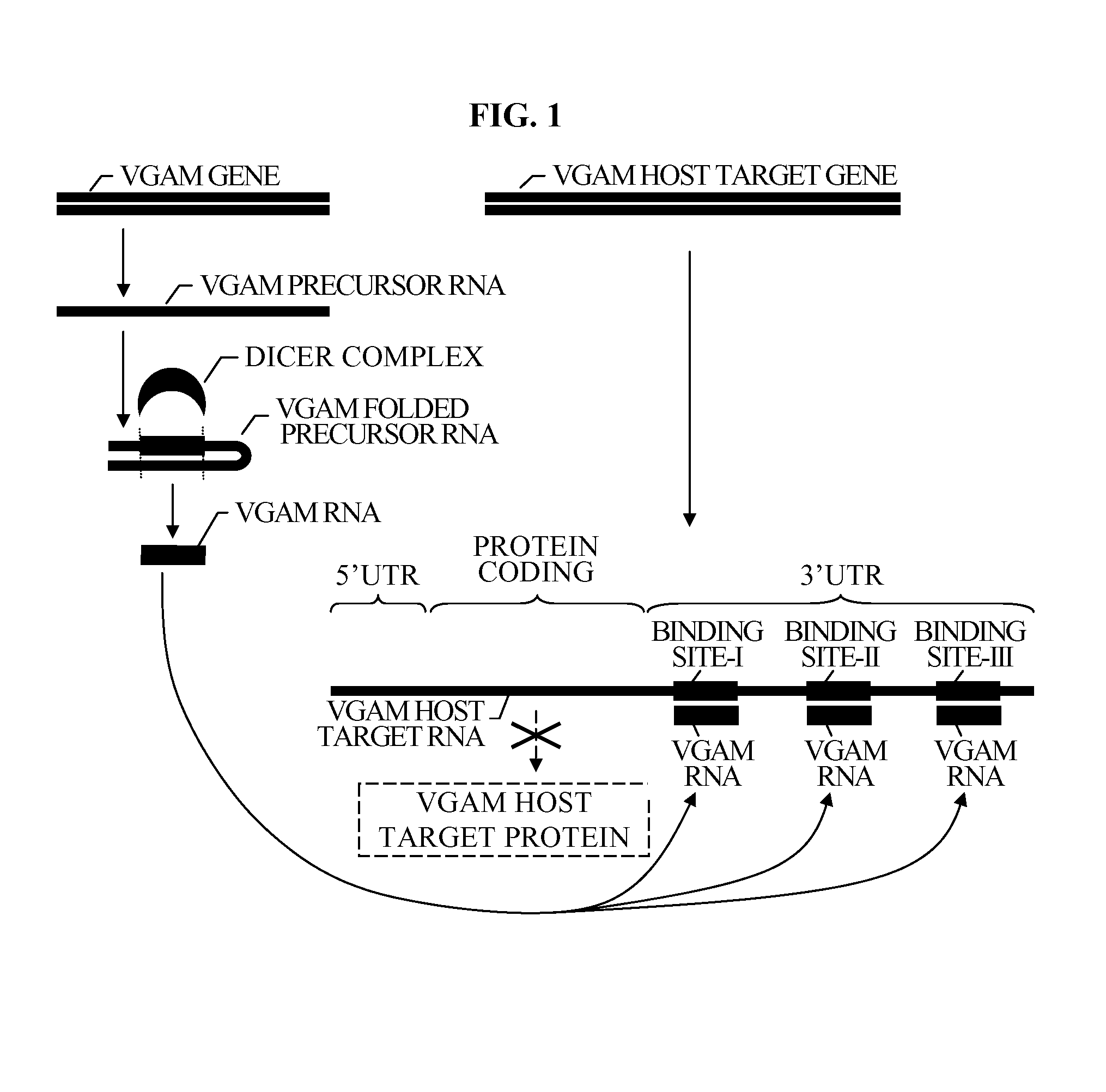
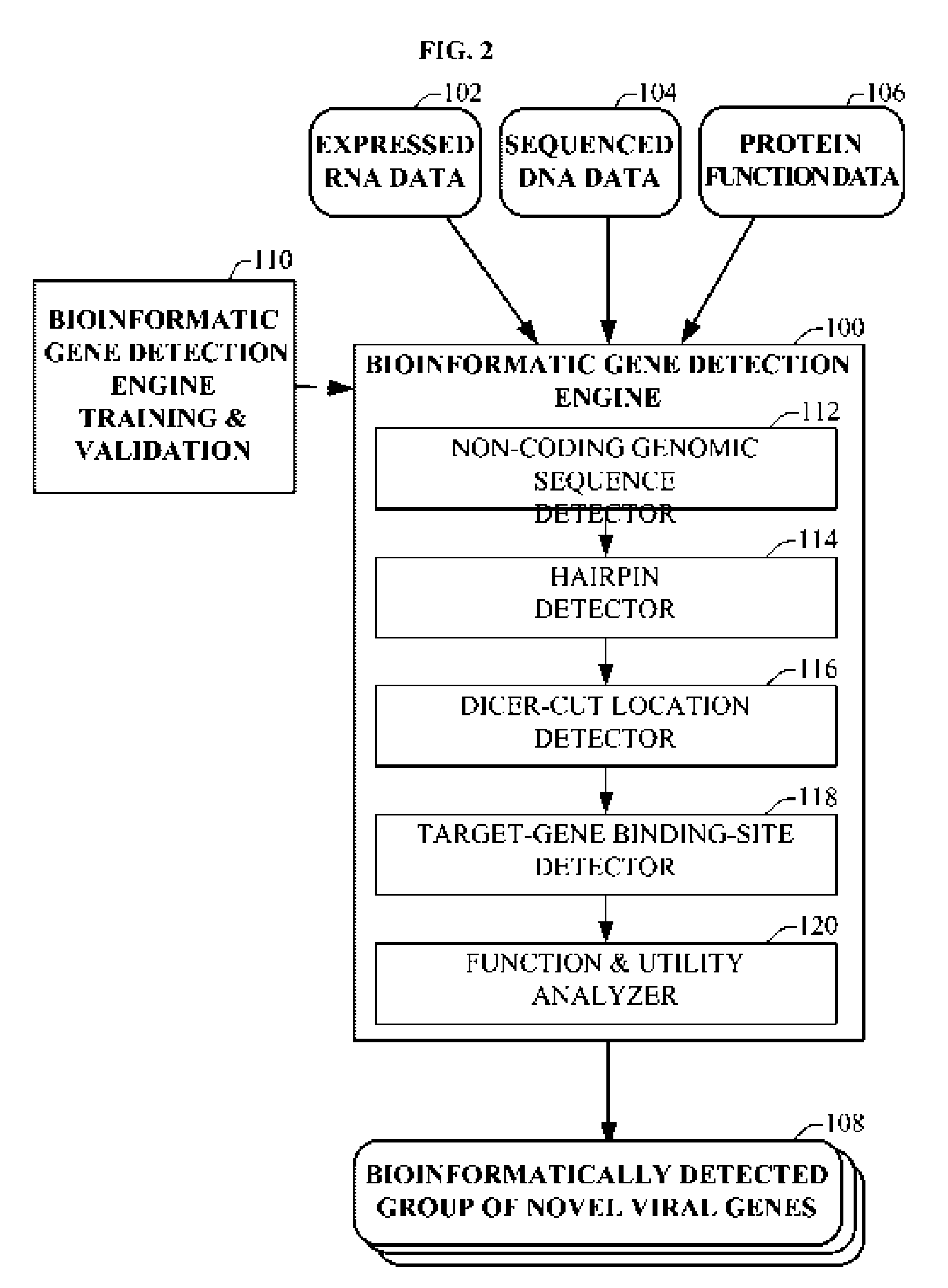
Bioinformatically detectable group of novel HIV regulatory genes and uses thereof
InactiveUS7217807B2Preventing and treating viral diseasesSugar derivativesMicrobiological testing/measurementViral diseaseOperon
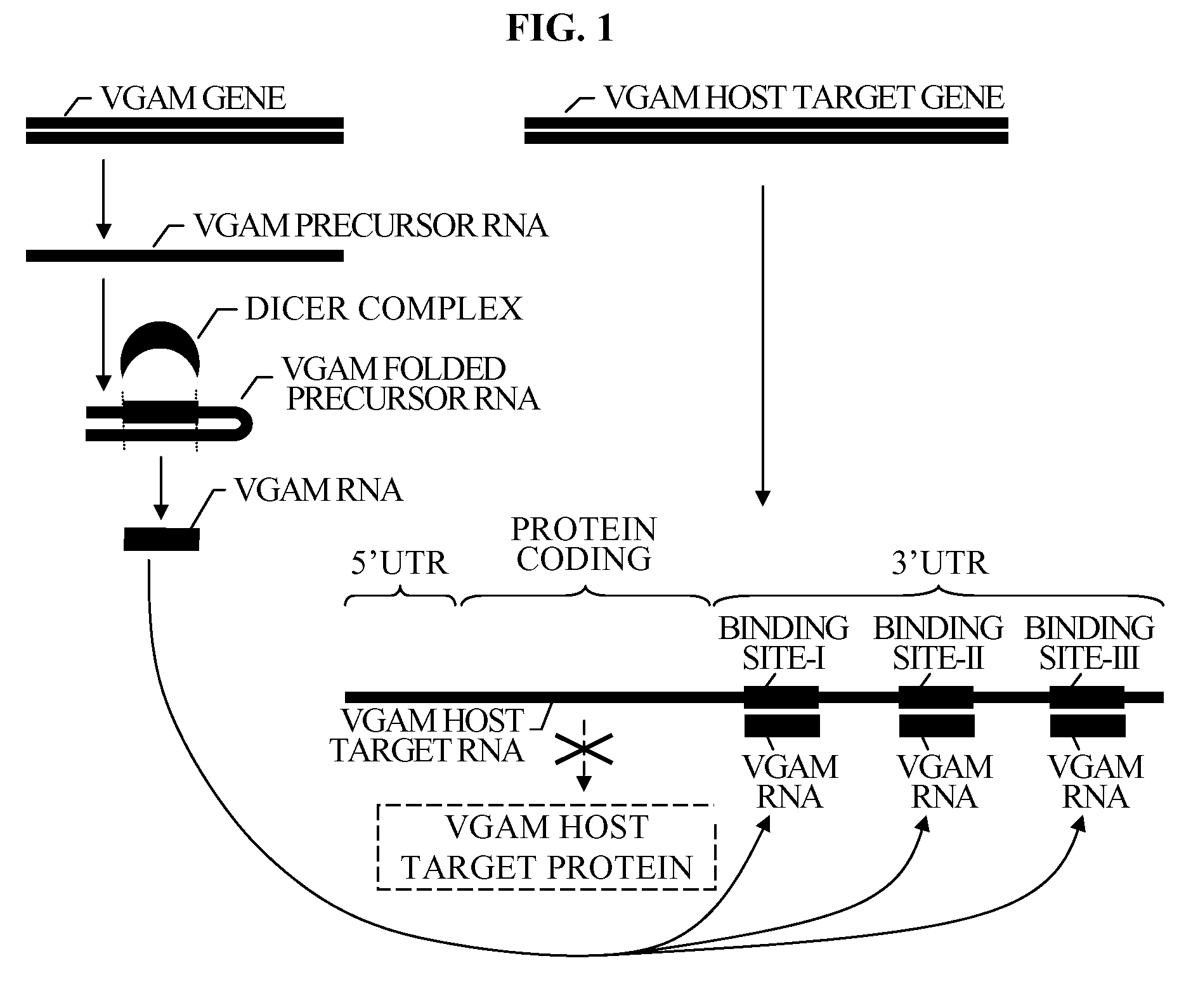
The present invention relates to a group of novel viral RNA regulatory genes, here identified as “viral genomic address messenger genes” or “VGAM genes”, and as “genomic record” or “GR” genes. VGAM genes selectively inhibit translation of known host target genes, and are believed to represent a novel pervasive viral attack mechanism. GR genes encode an operon-like cluster of VGAM genes. VGAM and viral GR genes may therefore be useful in diagnosing, preventing and treating viral disease. Several nucleic acid molecules are provided respectively encoding several VGAM genes, as are vectors and probes, both comprising the nucleic acid molecules, and methods and systems for detecting VGAM genes, and for counteracting their activity.
Owner:ROSETTA GENOMICS
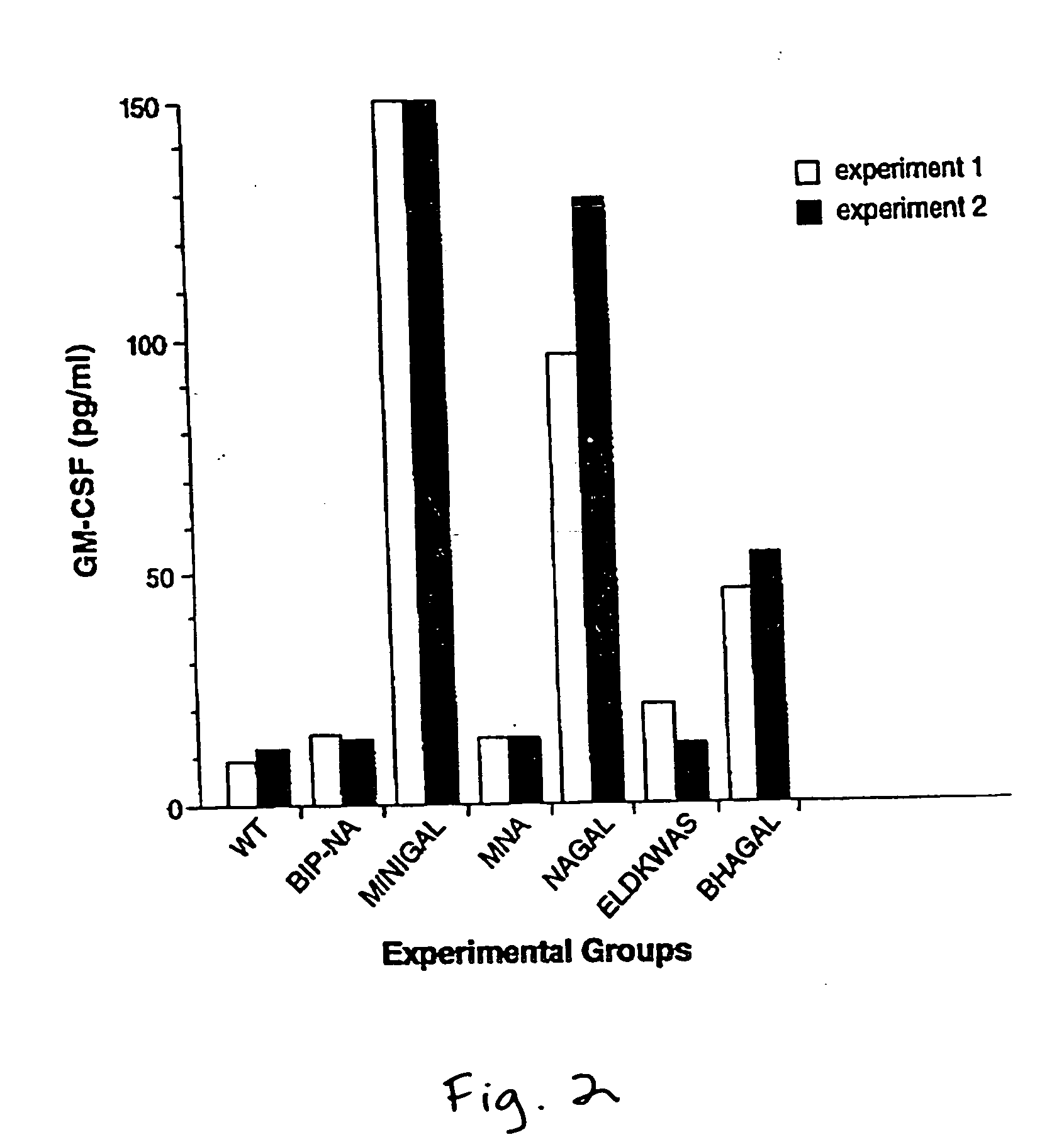
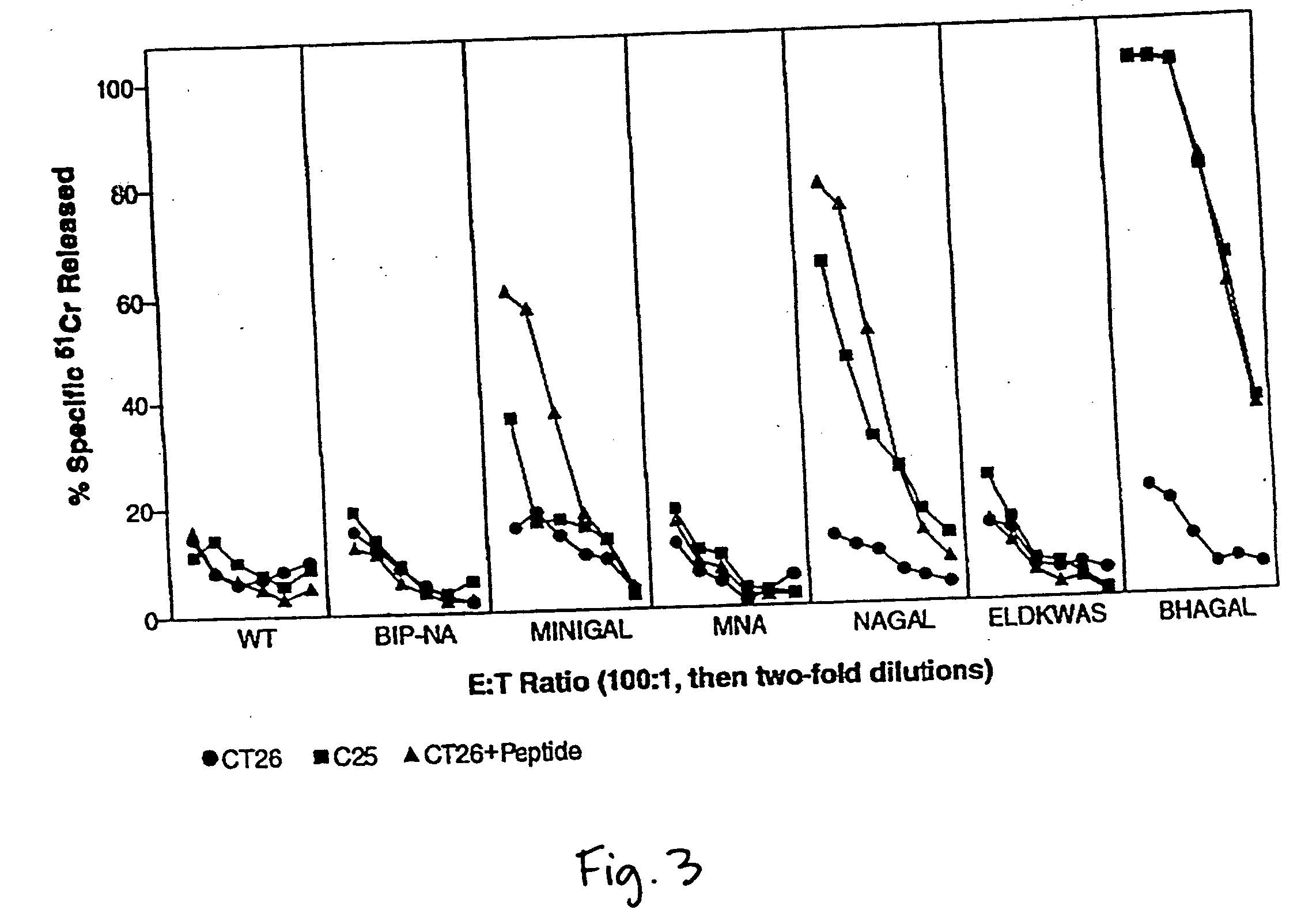
Methods of gene therapy using herpes viral vectors expressing GM-CSF
A genetically disabled mutant virus has a genome which is defective in respect of a selected gene that is essential for the production of infectious new virus particles, and which carries heterologous genetic material encoding an immunomodulatory protein such as GM-CSF, IL-2, or others, such that the mutant virus can infect normal host cells and cause expression of immunomodulatory protein, but the mutant virus cannot cause production of infectious new virus particles except when the virus infects recombinant complementing host cells expressing a gene that provides the function of the essential viral gene; the site of insertion of the heterologous genetic material encoding the immunomodulatory protein preferably being at the site of the defect in the selected essential viral gene. Uses include prophylactic and therapeutic use in generating an immune response in a subject treated therewith; use in the preparation of an immunogen such as a vaccine for use in tumor therapy; use in the in-vitro expansion of (e.g. virus-specific) cytotoxic T cells; and therapeutic or prophylactic use in corrective gene therapy.
Owner:CANTAB PHARMA RES
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
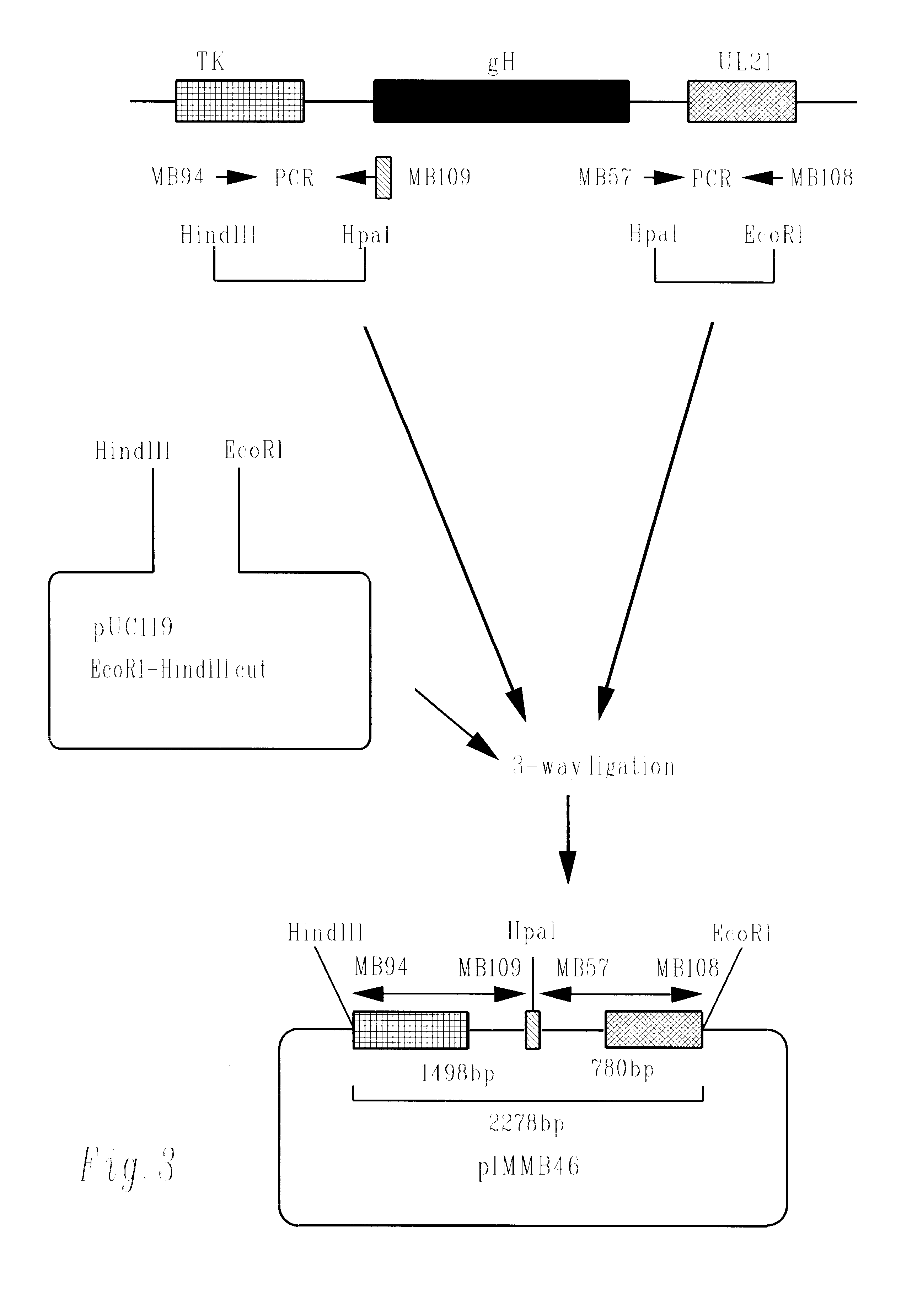
Cytomegalovirus gene function and methods for developing antivirals, anti-CMV vaccines, and CMV-based vectors
ActiveUS20050064394A1Enhance their long-term survivabilitySugar derivativesMicrobiological testing/measurementSurvivabilityORFS
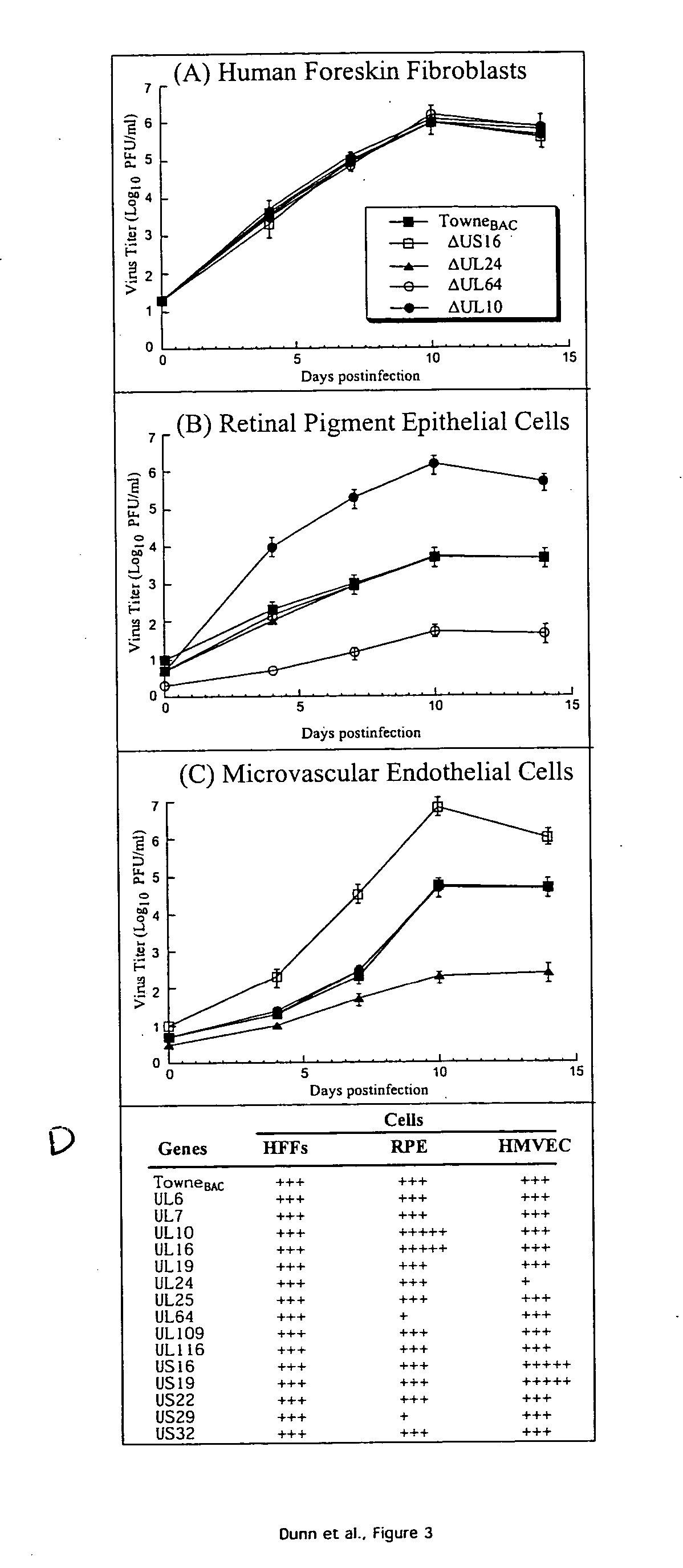
A global functional analysis of HCMV genes is performed by constructing virus gene-deletion mutants and examining their growth phenotypes in different natural HCMV host cells. This systematic analysis of the HCMV genome identified 45 viral ORFs essential for viral replication and characterizes of 115 growth-dispensable viral genes. Of particular interest is the finding that HCMV encodes genes (temperance factors) that repress its own replication on a cell type-specific basis. In addition to HCMV, pathogen temperance may be a strategy employed by other infectious agents to enhance their long-term survivability within their respective host population.
Owner:RGT UNIV OF CALIFORNIA
Recombinant Newcastle disease virus RNA expression systems and vaccines
This invention relates to genetically engineered Newcastle disease viruses and viral vectors which express heterologous genes or mutated Newcastle disease viral genes or a combination of viral genes derived from different strains of Newcastle disease virus. The invention relates to the construction and use of recombinant negative strand NDV viral RNA templates which may be used with viral RNA-directed RNA polymerase to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. In a specific embodiment of the invention, the heterologous gene product is a peptide or protein derived from the genome of a human immunodeficiency virus. The RNA templates of the present invention may be prepared by transcription of appropriate DNA sequences using any DNA-directed RNA polymerase such as bacteriophage T7, T3, SP6 polymerase, or eukaryotic polymerase I.
Owner:MT SINAI SCHOOL OF MEDICINE
Veterinary pharmaceutical formulacion that comprises an RNA recombinant particle that encodes for a cu/zn superoxide dismutase protein of ruminant pathogenic bacteria and at least one RNA alphavirus belonging to the semliki forest virus family
InactiveUS20110200667A1Improve efficacyProtective efficacyAntibacterial agentsOrganic active ingredientsBrucella abortusZn superoxide dismutase
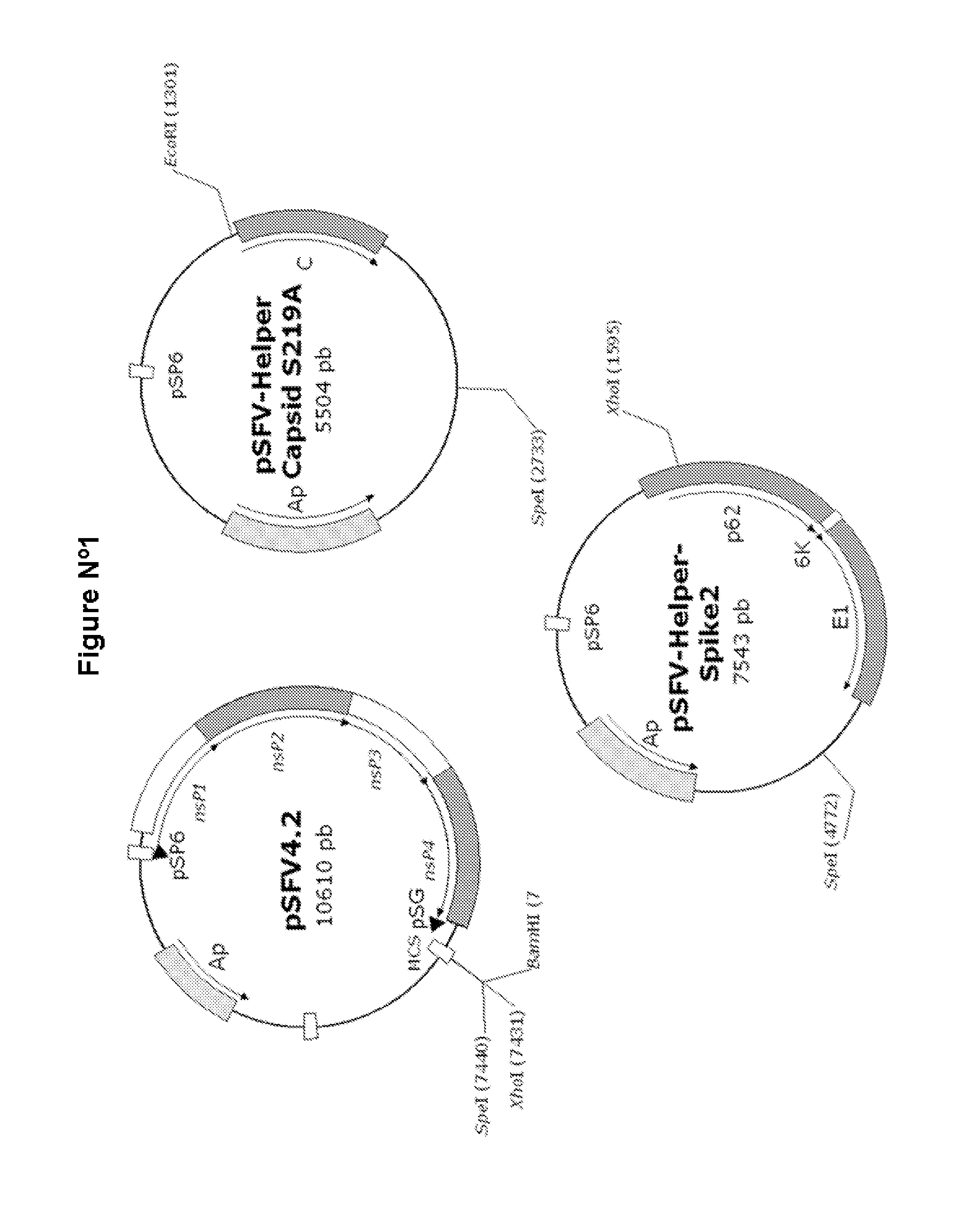
The technology is a veterinary pharmaceutical formulation of two vaccines, one from an RNA viral vector system constituted by an RNA recombinant particle that codifies for a Cu / Zn superoxide dismutase protein of Brucella abortus, and the other based on naked RNA constituted by a recombinant molecule of naked RNA that carries a sequence for the synthesis of at least one recombinant Cu / Zn superoxide dismutase protein of Brucella abortus and some Semliki Forest virus genes. An expression system based on the Semliki Forest virus and a use of this system, in addition to a method for the preparation of the pharmaceutical formulations.
Owner:UNIV DE CONCEPCION
Avipox recombinants expressing foot and mouth disease virus genes
ActiveUS20050287672A1SsRNA viruses positive-senseViral antigen ingredientsGene productProtective immunity
The present invention relates to modified poxviral vectors and to methods of making and using the same. In particular, the invention relates to recombinant avipox that expresses gene products of foot and mouth disease virus (FMDV), and to compositions or vaccines that elicit immune responses directed to FMDV gene products and which can confer protective immunity against infection by FMDV.
Owner:MERIAL INC
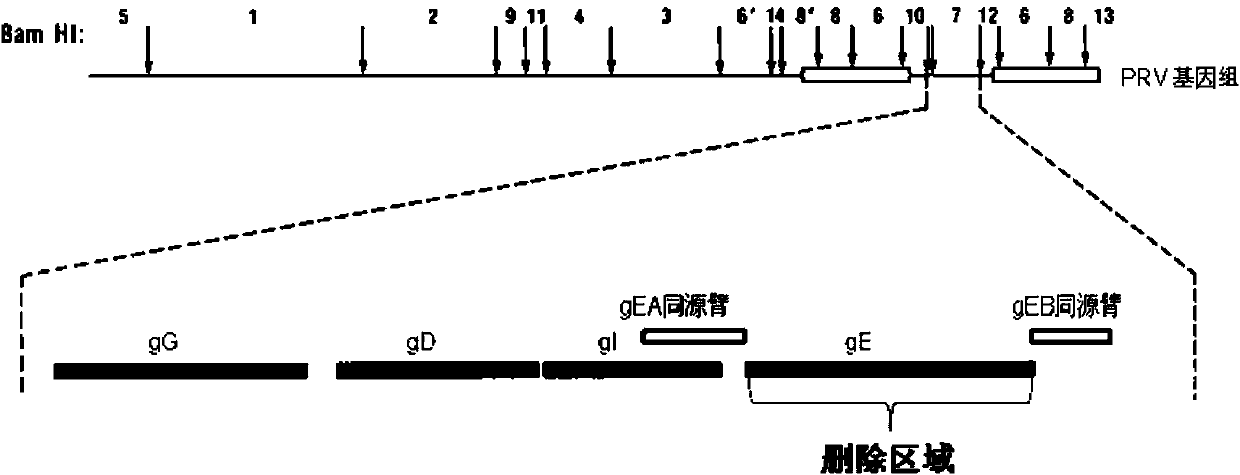
Porcine pseudorabies virus gene deletion strain, vaccine composition, and preparation method and application of vaccine composition
ActiveCN103923884ASymptoms relieved or improvedMicroorganism based processesAntiviralsVirus antigenTGE VACCINE
The invention provides a porcine pseudorabies virus gene deletion strain, a vaccine composition, and a preparation method and an application of the vaccine composition. The vaccine composition comprises an immunizing dose of an attenuated livetotivirus antigen and an inactivated totivirus antigen of the porcine pseudorabies virus gene deletion strain or its culture. The vaccine composition can effectively induce the antibody production, can effectively protect pigs, and can be used as a marking vaccine to effectively differentiate wild strains and vaccine strains.
Owner:PU LIKE BIO ENG
RNA interference mediated inhibition of human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050191618A1Improves various propertyImprove the immunitySugar derivativesMicrobiological testing/measurementDiseaseBiological body
This invention relates to compounds, compositions, and methods useful for modulating human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of human immunodeficiency virus (HIV) gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of HIV genes. The small nucleic acid molecules are useful in the treatment of HIV infection, AIDS, and / or diseaes and conditions related to HIV infection and / or AIDS in a subject or organism.
Owner:SIRNA THERAPEUTICS INC
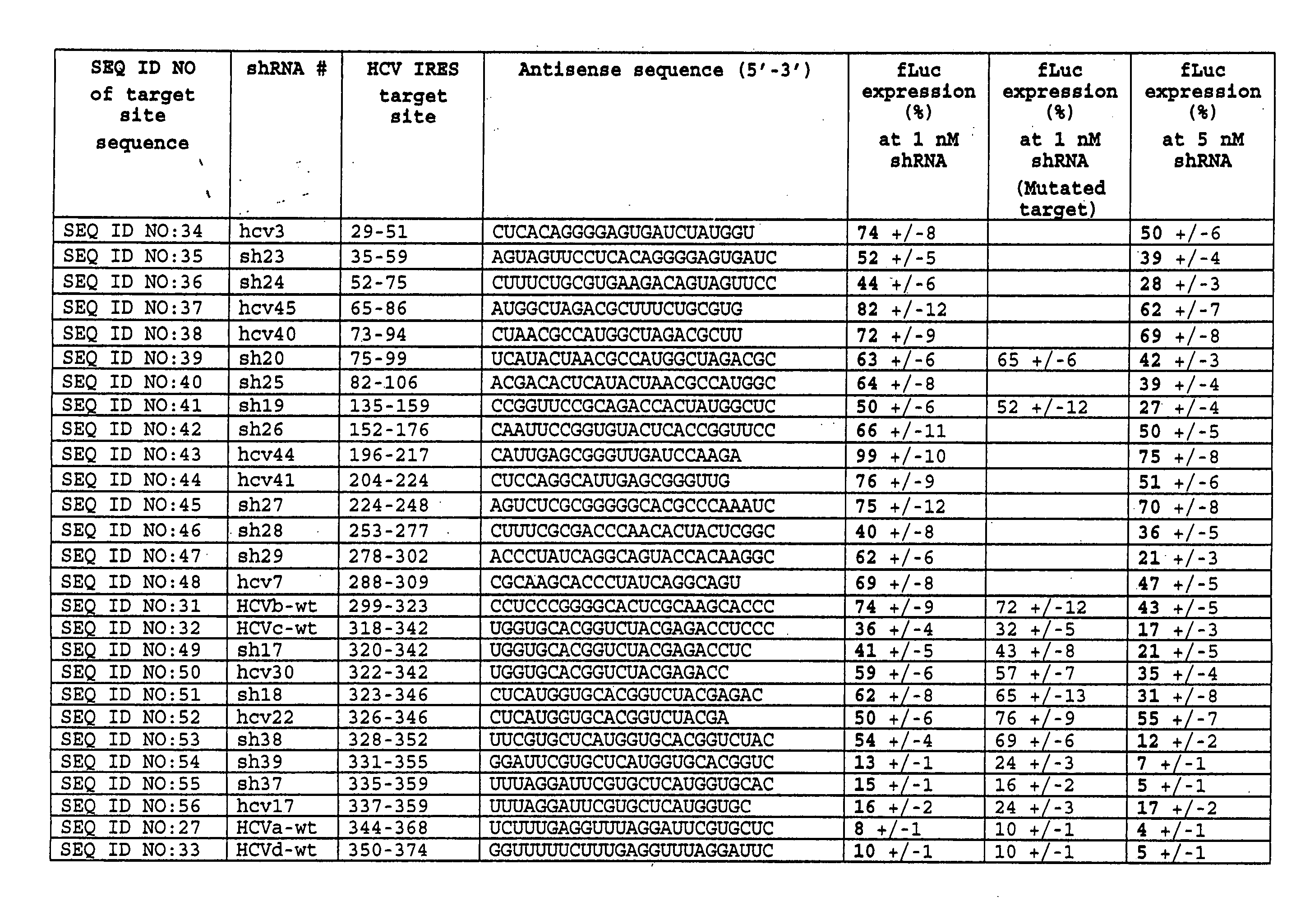
Inhibition of viral gene expression using small interfering RNA
InactiveUS20070149470A1Enhanced inhibitory effectBiocideSsRNA viruses positive-senseSmall interfering RNAViral gene
The invention provides methods, compositions, and kits comprising small interfering RNA (shRNA or siRNA) that are useful for inhibition of viral-mediated gene expression. Small interfering RNAs as described herein can be used in methods of treatment of HCV infection. ShRNA and siRNA constructs targetING the internal ribosome entry site (IRES) sequence of HCV are described.
Owner:SOMAGENICS INC
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
InactiveUS20040253273A1Prevent tumor formationQuick changeSsRNA viruses negative-senseAntibody mimetics/scaffoldsTumor reductionEpitope
The present invention relates to the engineering of recombinant influenza viruses that express tumor-associated antigens. Expression of tumor-associated antigens by these viruses can be achieved by engineering specific epitopes into influenza virus proteins, or by engineering viral genes that encode a viral protein and the specific antigen as independent polypeptides. Tumor-bearing patients can be immunized with the recombinant influenza viruses alone, or in combination with another treatment, to induce an immune response that leads to tumor reduction. The recombinant viruses can also be used to vaccinate high risk tumor-free patients to prevent tumor formation in vivo.
Owner:PALESO PETER +2
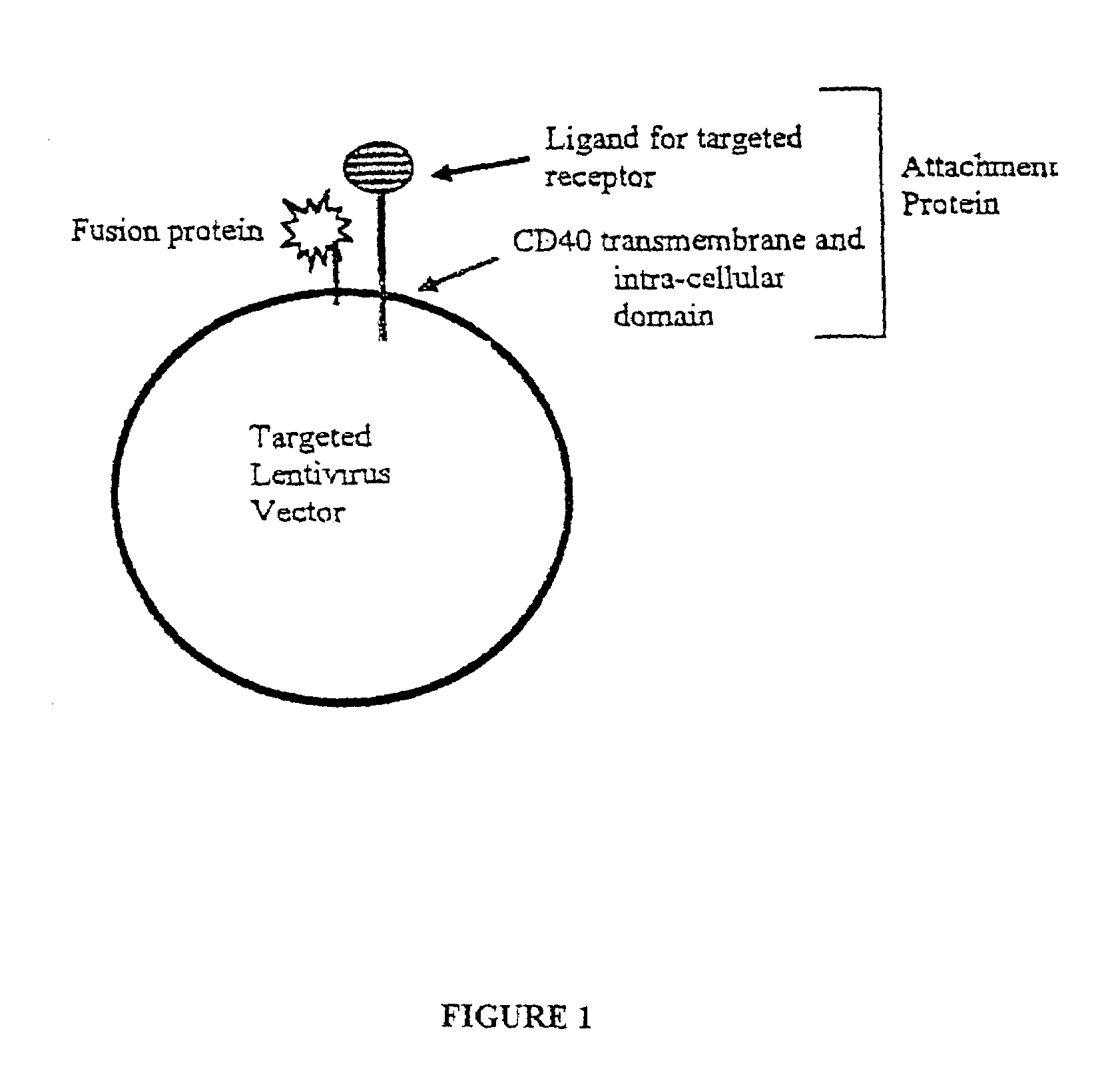
Compositions and methods for tissue specific targeting of lentivirus vectors
This disclosure provides lentiviral vectors containing an attachment incompetent fusogenic polypeptide and a heterologous targeting polypeptide. Also provided are lentiviral packaging constructs, lentiviral packaging systems, and lentiviral gene delivery systems. Finally, methods of transducing a cell and methods of targeting a gene to a cell or tissue using the disclosed lentiviral vectors and systems are also provided.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Synthetic human papillomavirus genes
InactiveUS7001995B1Easy to insertEasy to removeBiocideGenetic material ingredientsPolynucleotide VaccinesHuman papillomavirus
Synthetic DNA molecules encoding papillomavirus proteins are provided. The codons of the synthetic molecules are codons preferred by the projected host cell. The synthetic molecules may be used as a polynucleotide vaccine which provides effective immunoprophylaxis against papillomavirus infection through stimulation of neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME CORP
Bioinformatically detectable human herpesvirus 5 regulatory gene
ActiveUS7696334B1Preventing and treating viral diseasesSugar derivativesMicrobiological testing/measurementOperonVirus attack
The present invention relates to a group of novel viral RNA regulatory genes, here identified as “viral genomic address messenger genes” or “VGAM genes”, and as “genomic record” or “GR” genes. VGAM genes selectively inhibit translation of known host target genes, and are believed to represent a novel pervasive viral attack mechanism. GR genes encode an operon-like cluster of VGAM genes. VGAM and viral GR genes may therefore be useful in diagnosing, preventing and treating viral disease. Several nucleic acid molecules are provided respectively encoding several VGAM genes, as are vectors and probes, both comprising the nucleic acid molecules, and methods and systems for detecting VGAM genes, and for counteracting their activity.
Owner:ROSETTA GENOMICS
Therapeutic use of cis-element decoys in vivo
The invention provides for the use of oligodeoxynucleotide decoys for the prophylactic or therapeutic treatment of diseases associated with the binding of endogenous transcription factors to genes involved in cell growth, differentiation and signalling or to viral genes. By inhibiting endogenous trans-activating factors from binding transcription regulatory regions, the decoys modulate gene expression and thereby regulating pathological processes including inflammation, intimal hyperplasia, angiogenesis, neoplasia, immune responses and viral infection. The decoys are administered in amounts and under conditions whereby binding of the endogenous transcription factor to the endogenous gene is effectively competitively inhibited without significant host toxicity. The subject compositions comprise the decoy molecules in a context which provides for pharmacokinetics sufficient for effective therapeutic use.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Preparation of PCV2 ORF2 capsid protein virus-like particles derived from escherichia coli
InactiveCN103173470AKeep natural activityHigh activityBacteriaViral antigen ingredientsEscherichia coliOrf2 gene
The invention relates to porcine circovirus PCV2 ORF2 gene optimized by using escherichia coli expression codon and a preparation method of PCV2 ORF2 capsid protein virus-like particles derived from the escherichia coli.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com