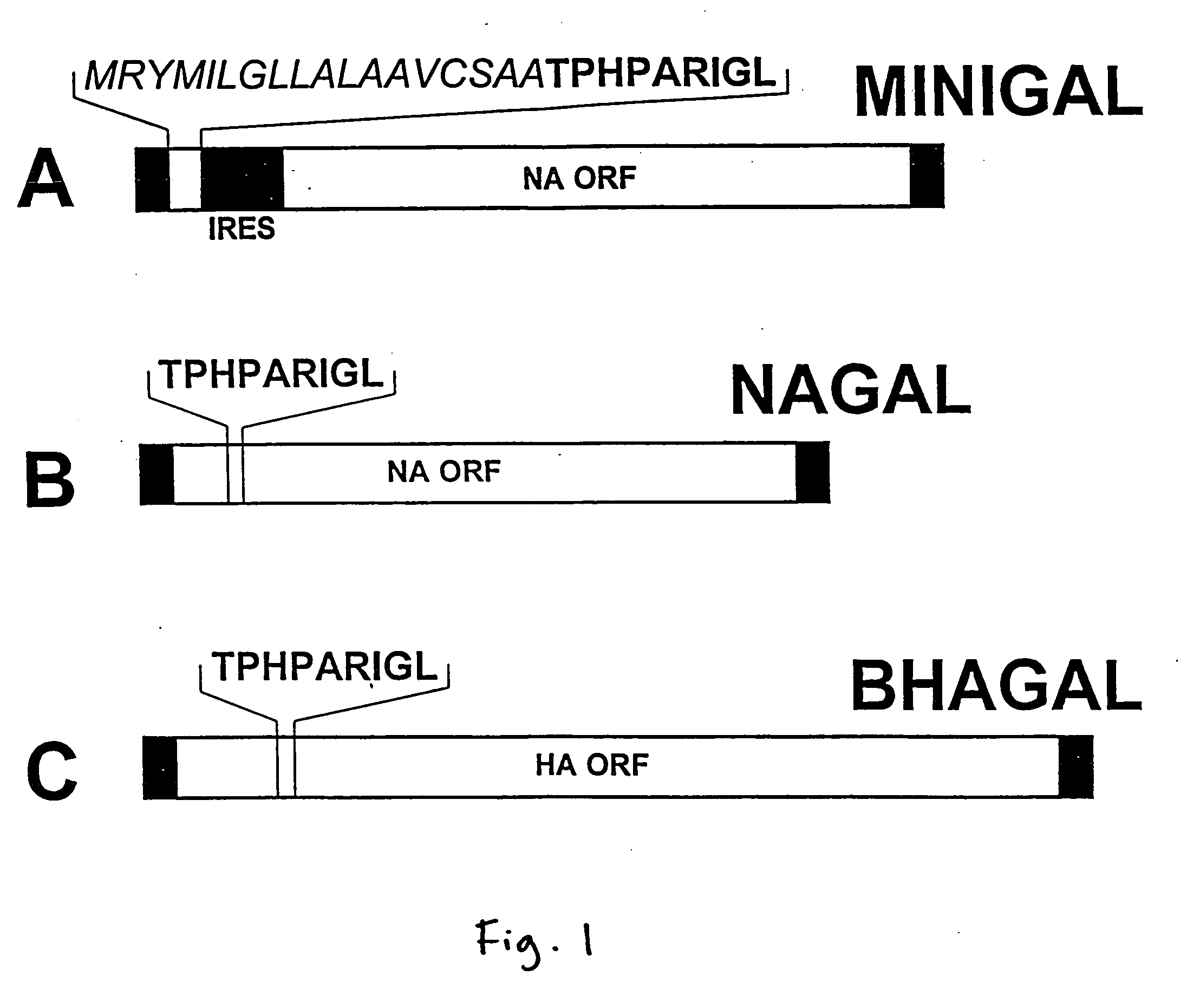
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
a technology of recombinant influenza viruses and tumor-associated antigens, which is applied in the field of engineered recombinant influenza viruses, can solve the problems of limited success of immunotherapeutic approaches proposed for the treatment of tumors, relative inaccessibility, and hampered treatment success, and achieve the effect of preventing tumor formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
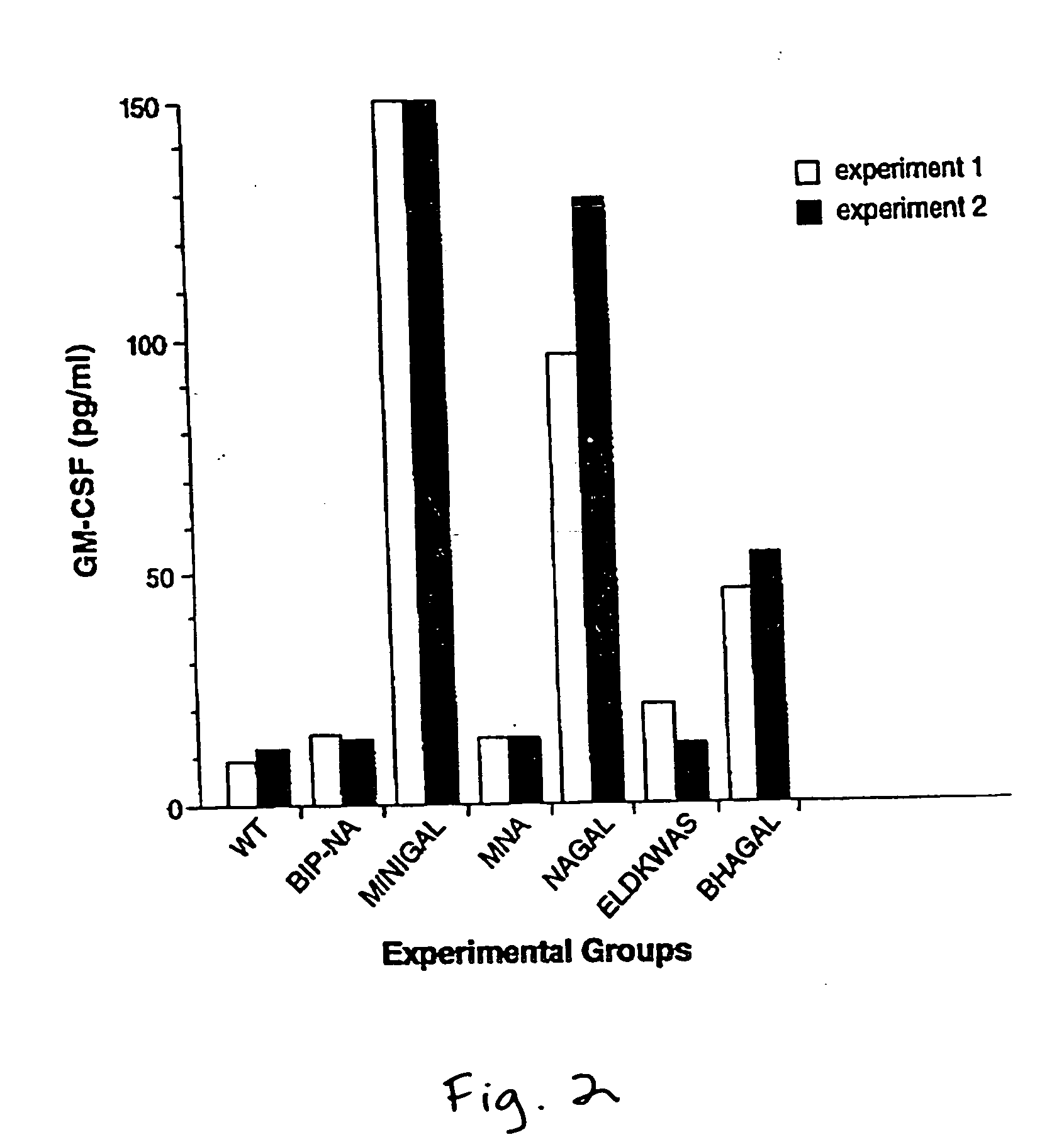
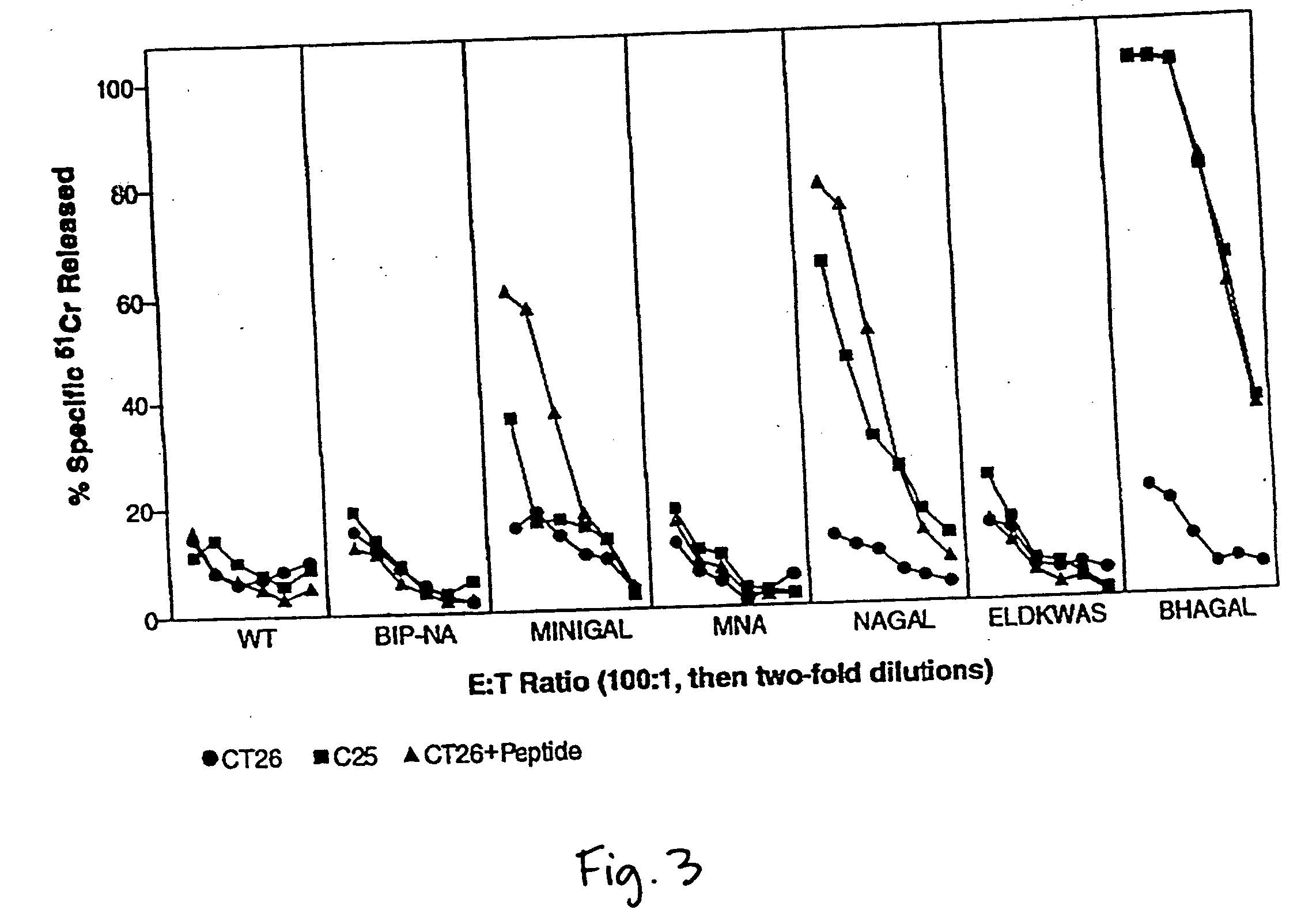
[0028] The engineering of recombinant influenza viruses expressing TAAs, and their use as immunogenic compositions or vaccines to induce tumor regression in mammals, including humans, is described. One drawback to the use of viruses such as vaccinia for constructing recombinant or chimeric viruses for use in vaccines is the lack of variation in its major epitopes. This lack of variability in the viral strains places strict limitations on the repeated use of chimeric vaccinia virus, in that a first vaccination will generate host resistance to the strain so that the same virus cannot infect the host in a second inoculation. Inoculation of a resistant individual with chimeric vaccinia virus will, therefore, not induce immune stimulation. The considerable advantage of using influenza virus, a negative-strand RNA virus, for vaccination, is that it demonstrates a wide variability of its major epitopes. Thousands of variants of influenza virus have been identified, each strain evolving by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| immunogenic composition | aaaaa | aaaaa |
| host resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com