Epithelial cell growth inhibitors
a growth inhibitor and epithelial cell technology, applied in the direction of antibody medical ingredients, peptide/protein ingredients, peptide sources, etc., can solve the problems of difficult detection of disease, significant mortality, tumor formation, etc., and achieve the effect of inhibiting epithelial cell growth and preventing tumor formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Multiple Tissue Expression of ECGI
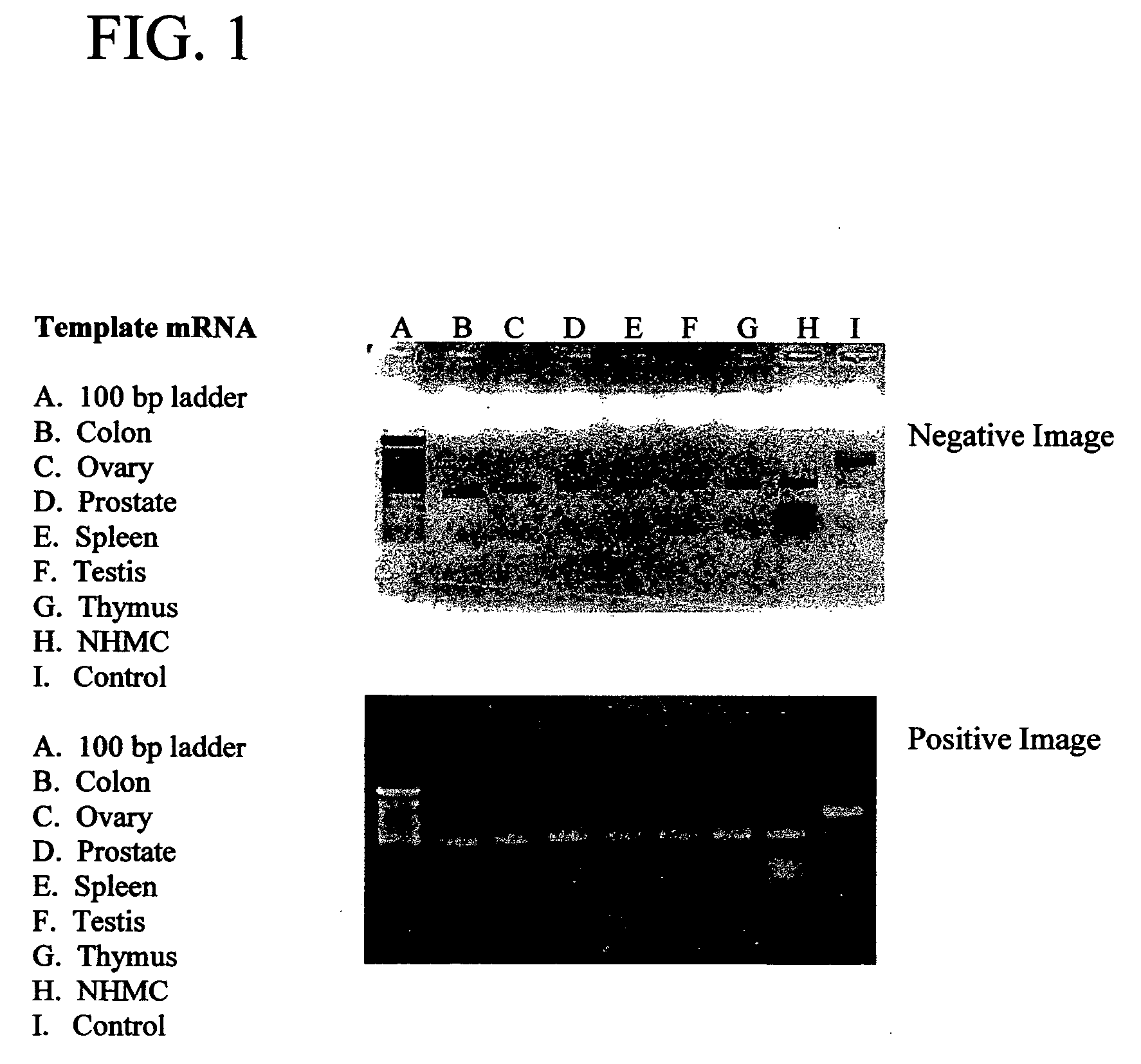
[0043] Northern blot analysis was performed on a multiple tissue expression array (Clonetech, Inc. #7775-1) to demonstrate the expression of ECGI in a variety of epithelial cell tissues. A digoxin-labeled EcoR1 fragment of Mammastatin, containing approximately 1800 base pairs of the 3′ region of pMammC, SEQ ID NO: 3 (approximately nucleotide 359-end) was used as a probe. The DIG-labeled Mammastatin cDNA was hybridized to the array in 10 ml easy HYB solution (Roche) for 16 hours at 65° C., with 65° C. washes, anti-DIG antibody hybridization and CSPD development performed according to the manufacture's instructions. The blot was then exposed to Kodak X-OMAT film for 30 minutes at room temperature.
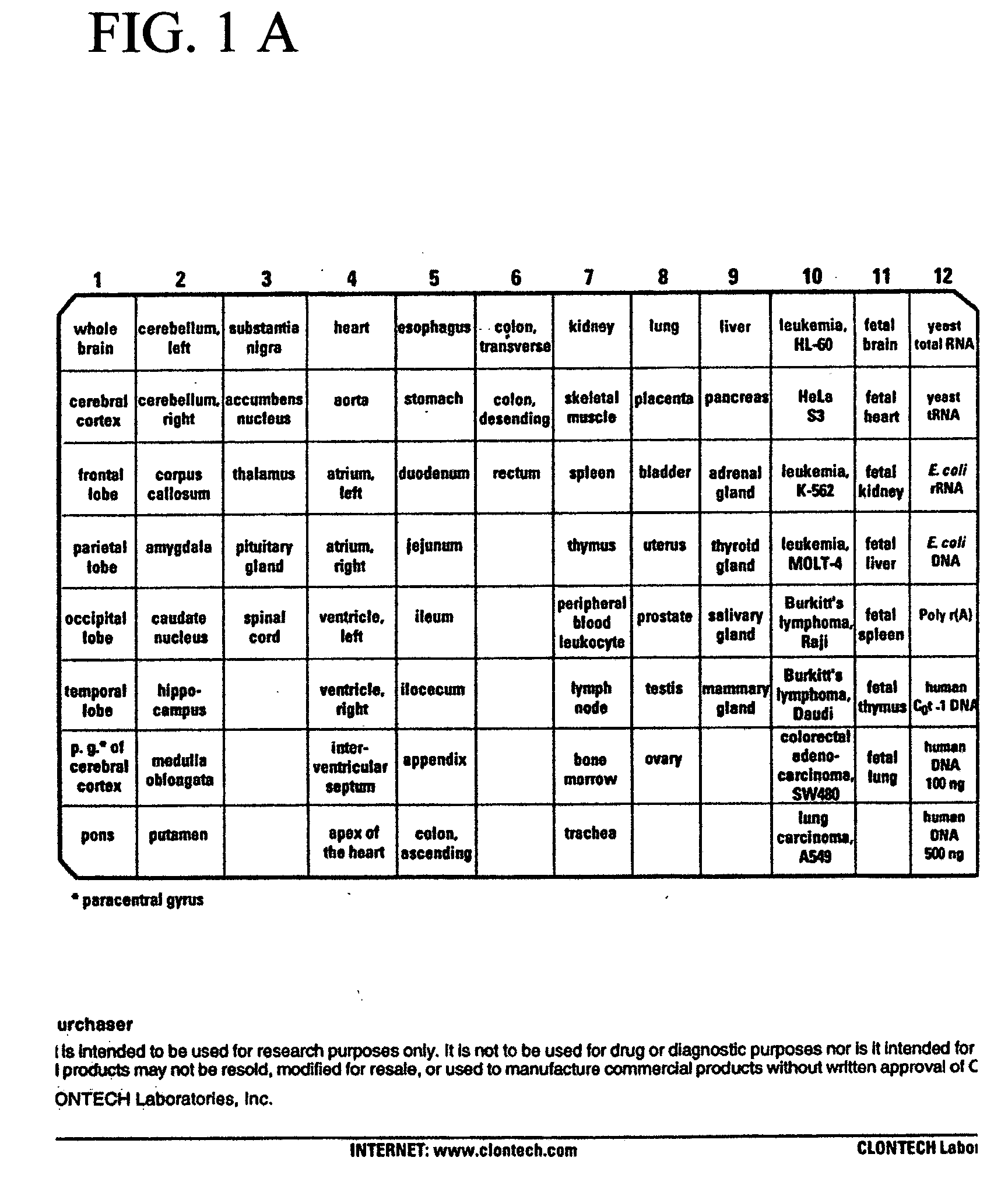
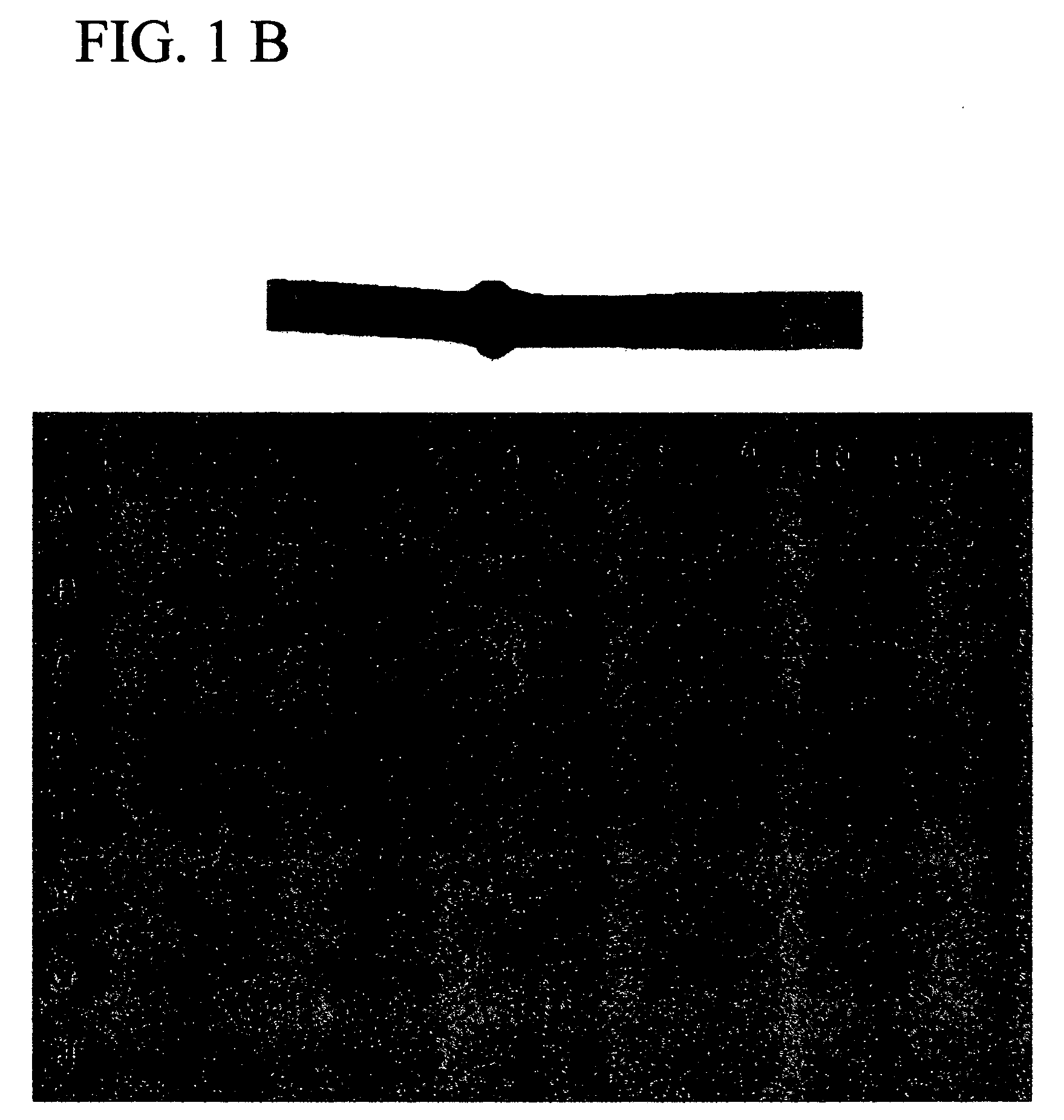
[0044] The tissue plan of the multiple tissue expression array is shown in FIG. 1A. Hybridization of the Mammastatin cDNA to the mRNA of the array is shown in FIG. 1B, and demonstrates the variety of epithelial cell tissues expressing a Mammastatin-like ECG...
example 2
Normal Versus Cancerous Prostate Cells
[0045] Normal prostate cells obtained from surgical samples and cancerous prostate cells, LnCap, obtained from the American Type Culture Collection (ATCC) were incubated and analyzed for the production of a prostate ECGI. The cells were cultured in DMEM / P12 media with 40 μM calcium, supplemented with 5% Chelex-treated horse serum, 10 ng / mL EGF, 10 μg / mL insulin, 100 ng / mL Cholera toxin and 1 μg / mL hydrocortisone for four days. Conditioned media samples were then collected and analyzed.
[0046] Normal human mammary cells obtained from patient samples were incubated in the same medium and Mammastatin secreted into the culture medium was used as a control. Serum obtained from breast cancer patients was also analyzed and used as a control.
[0047] Sample fluids were collected and loaded by suction onto a nitrocellulose membrane on a dot blot apparatus. The membranes were then probed with the anti-Mammastatin antibody 7G6, and antibody binding was det...
example 3
Differential Expression of ECGI in Prostate, Colon, and Ovary
[0049] Normal prostate cells (Clonetech, Inc.), LnCap prostate cancer cells (A.T.C.C.), MCF7 breast cancer cells (A.T.C.C.) and normal human mammary cells (obtained from hospital tissue) were incubated as described above for Example 2. After at least 48 hours incubation, cells were lysed in sample loading buffer and analyzed for the presence of ECGI by Western blot, using the anti-Mammastatin antibody, 7G6 as a probe. Normal human mammary cell protein (NHMC) lysate (1 mg / ml) was used as a Mammastatin control (A). The data are shown in FIG. 3.
[0050] Normal prostate cell lysate (D) contained a protein that was recognized by anti-Mammastatin antibody, while prostate cancer cells (LnCap) (B) and breast cancer cells (MCF7) (C) did not. The protein recognized in the prostate cell lysate (D) was of a similar size to that of Mammastatin (A).
Colon and Prostate
[0051] Normal prostate cells (Clonetech, Inc.), LnCap pros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com