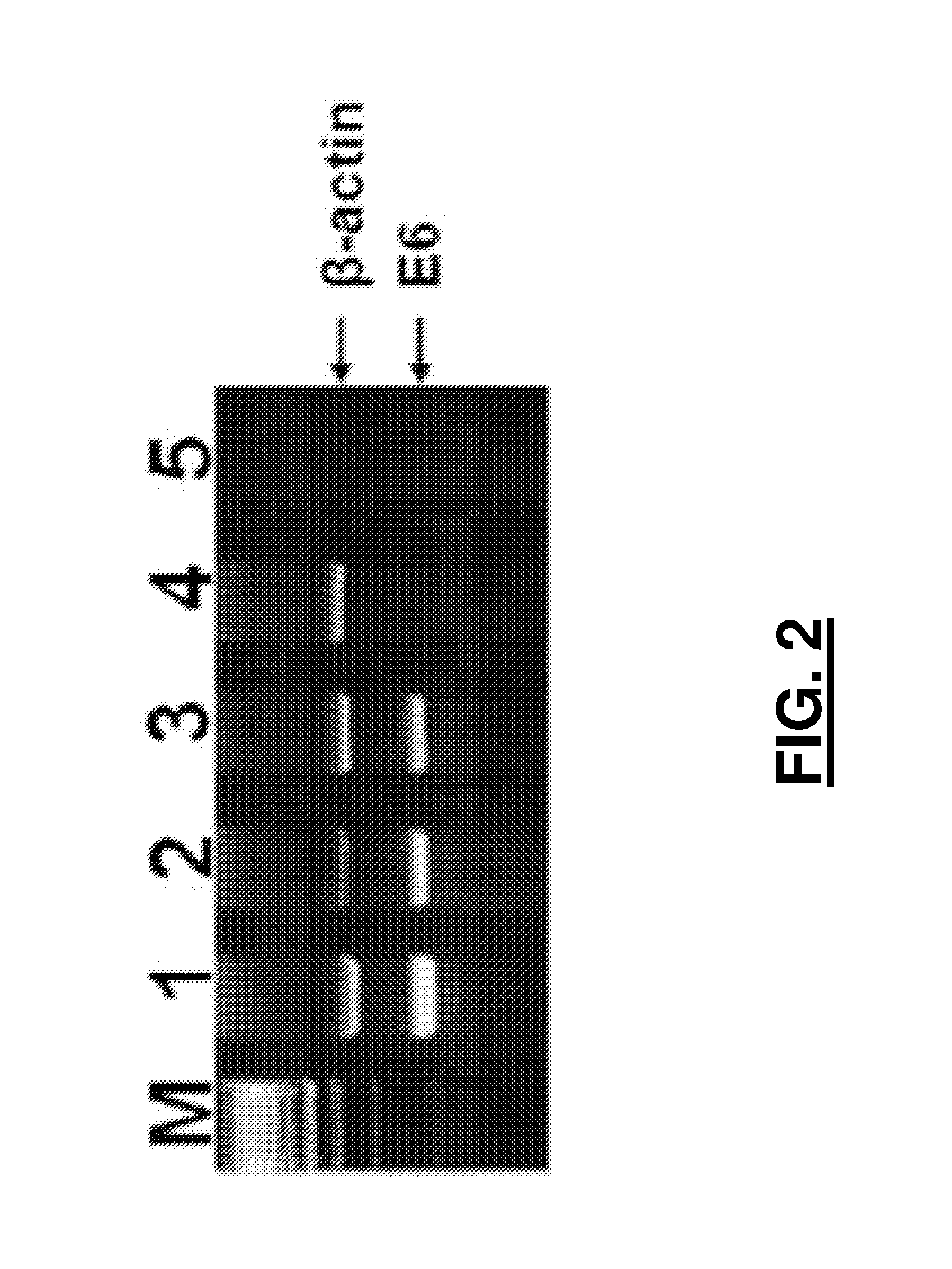
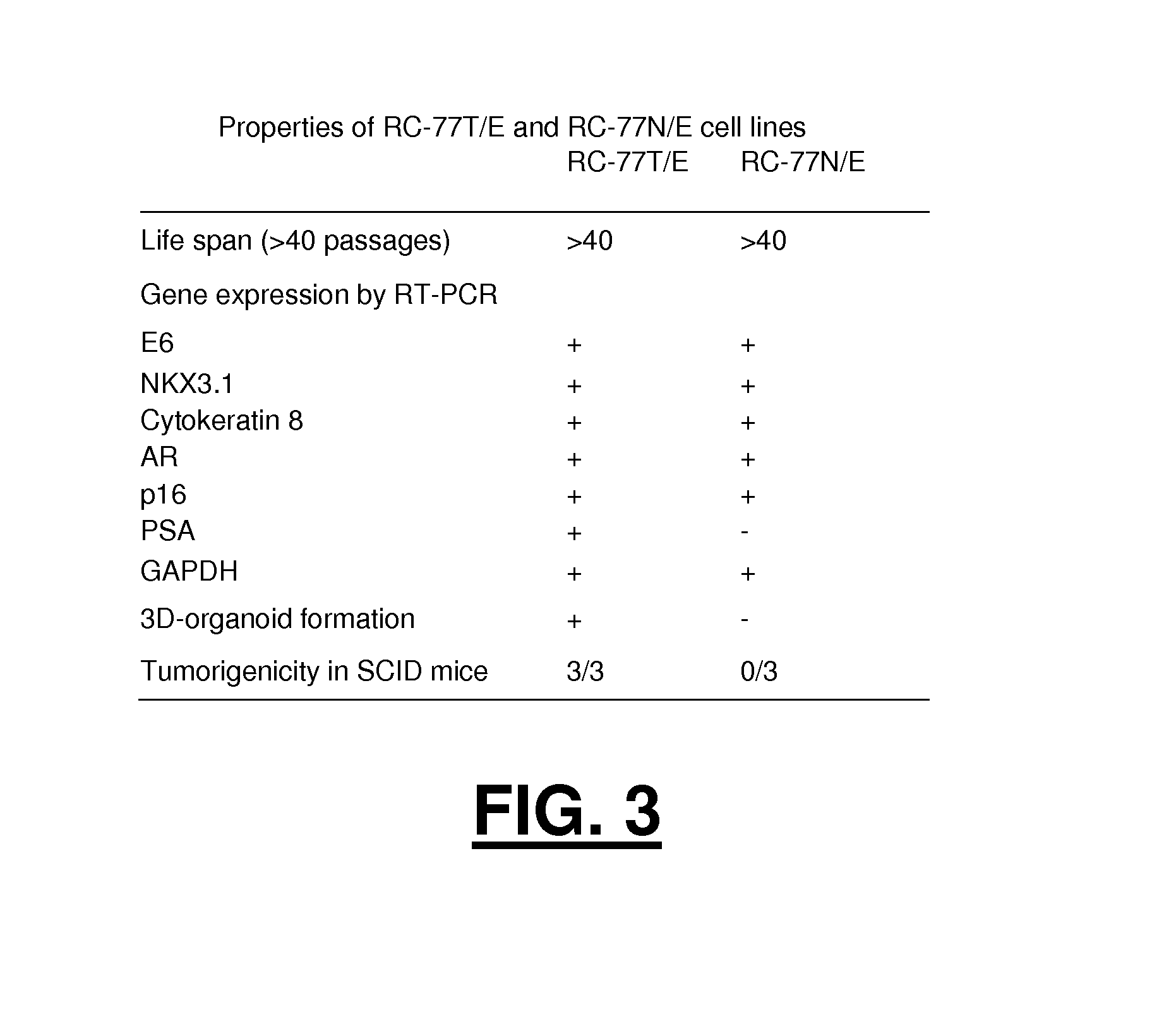
Patents
Literature
39 results about "Prostate cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The prostate is made up of three kinds of cells: glandular (epithelial) cells, smooth muscle cells, and stromal cells. The glandular cells produce part of the prostatic fluid.
Non-invasive method to detect prostate cancer
InactiveUS6383759B1Microbiological testing/measurementImmunoglobulinsAbnormal tissue growthNon invasive
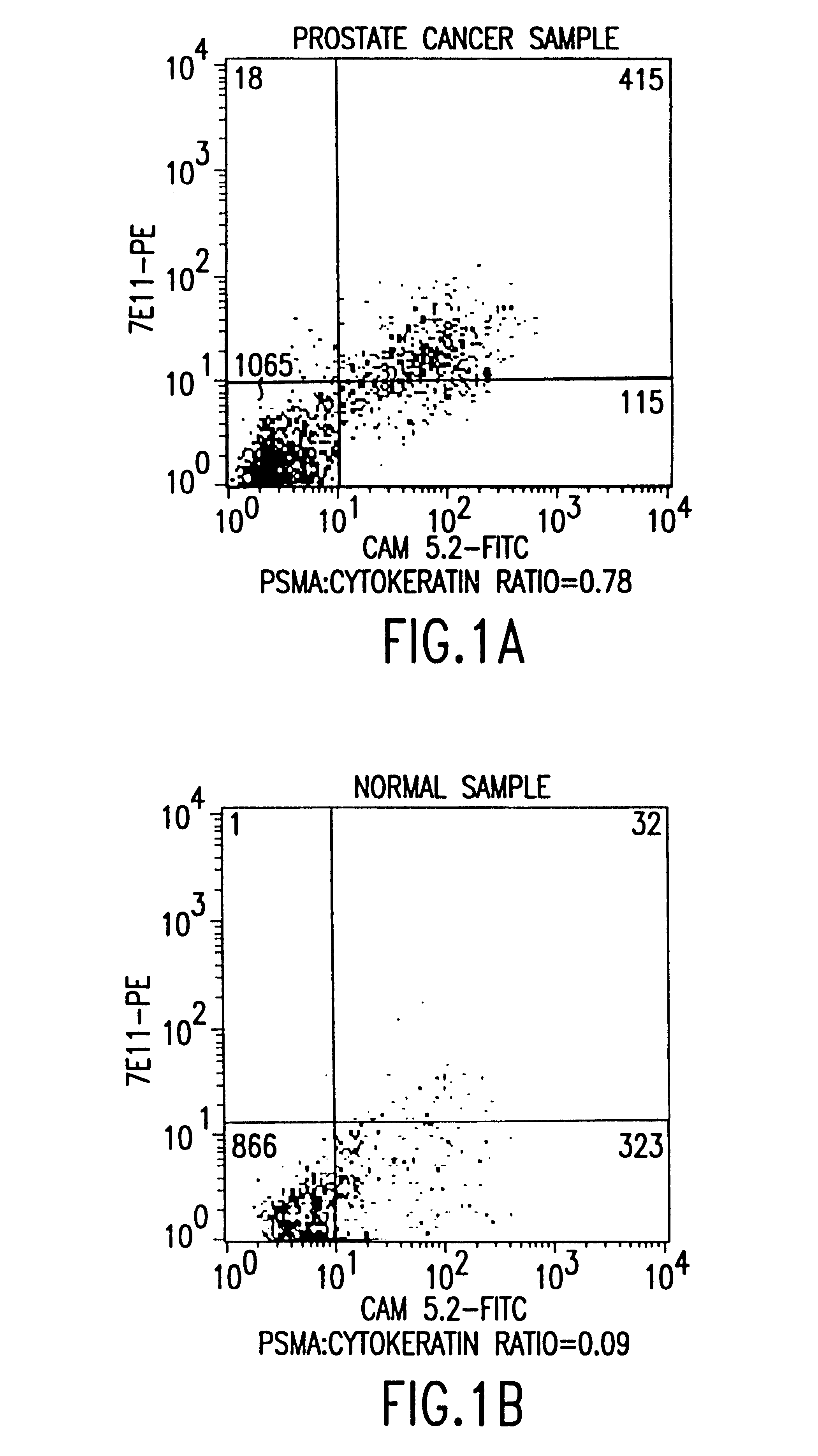
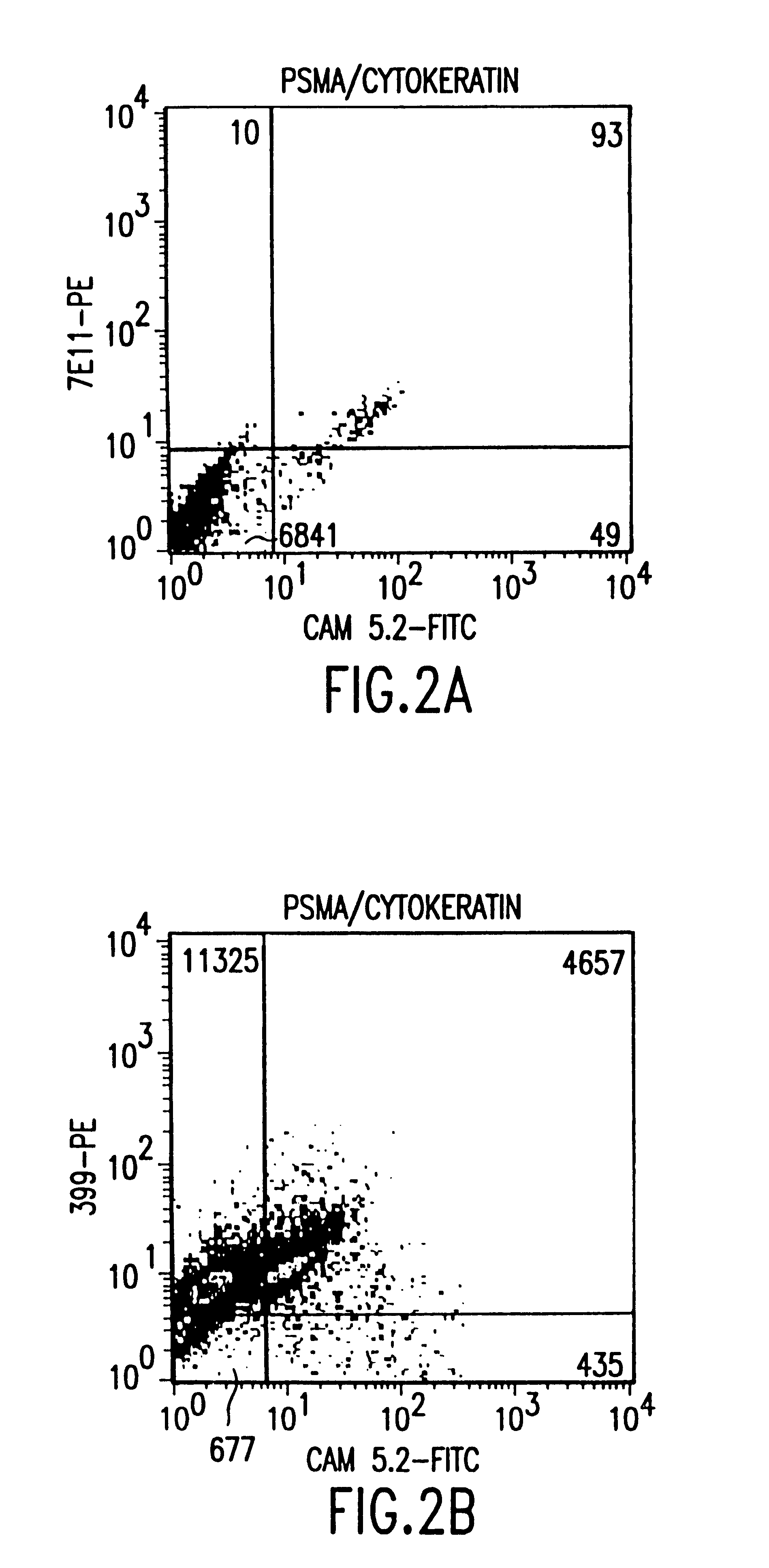
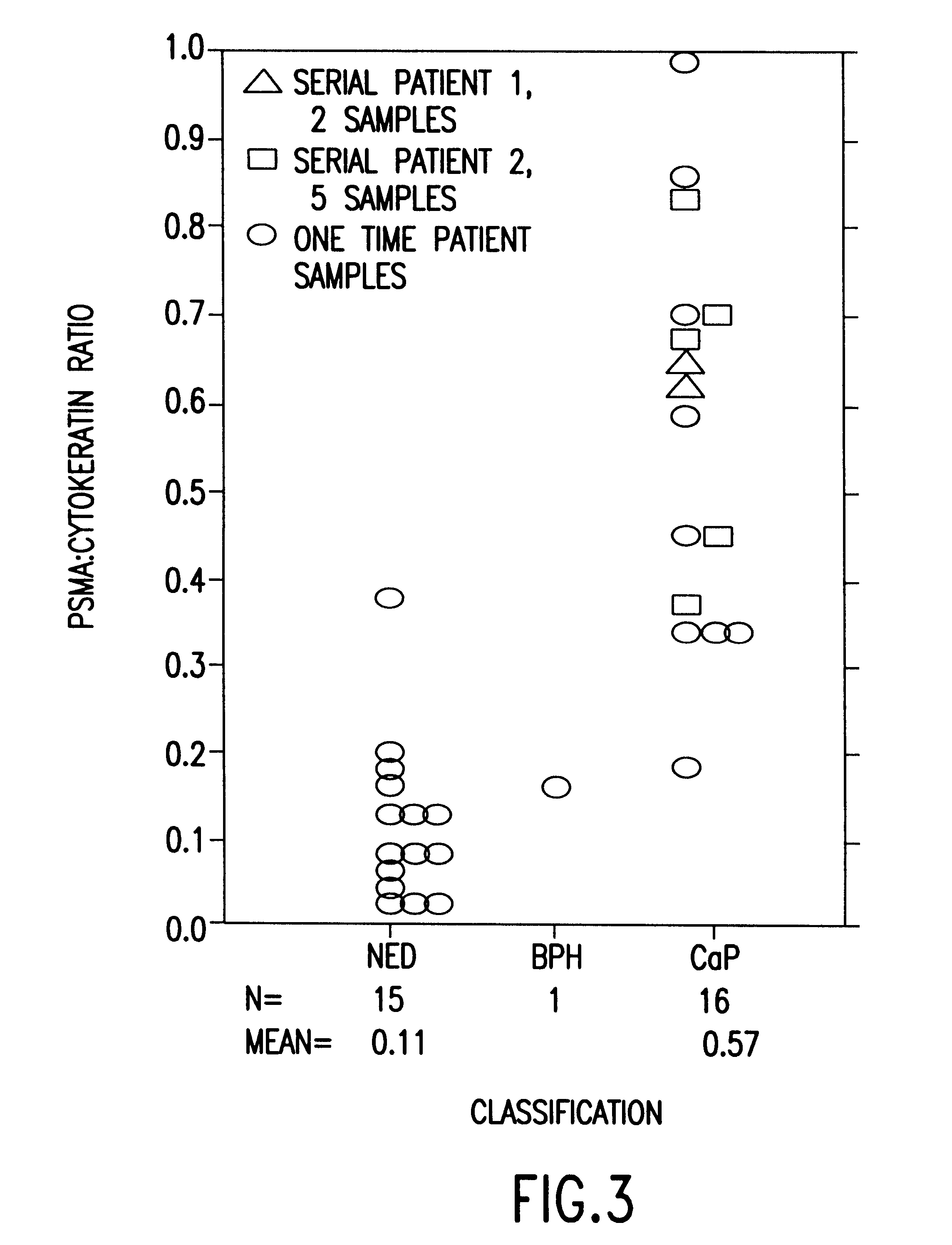
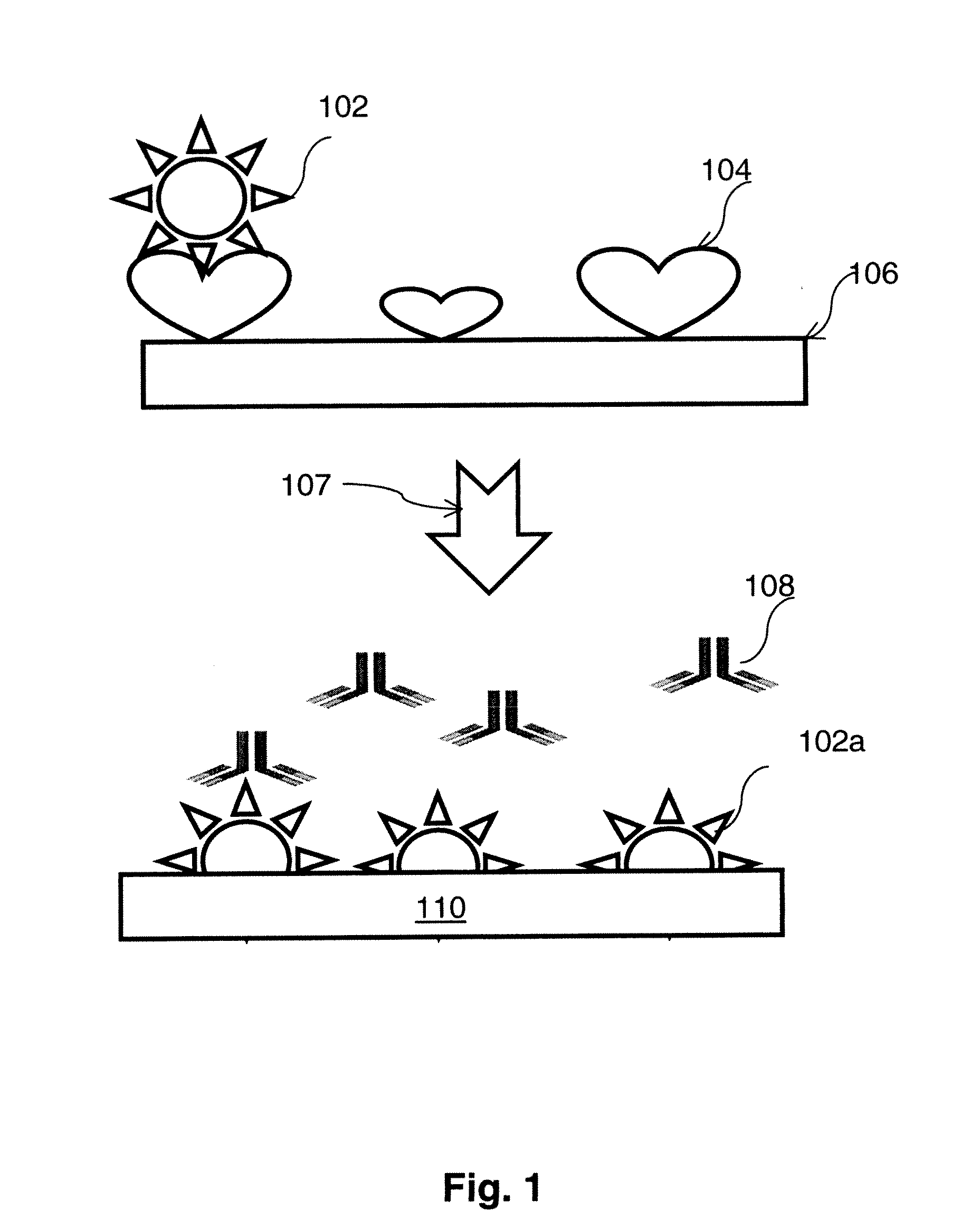
The present invention is directed to methods of detecting prostate cancer in a sample of a body fluid with prostate cell marker-specific and epithelial cell marker-specific antibodies as well as to kits comprising such antibodies for use in the detection of prostate cancer. The present invention is also directed to methods of detecting prostate cancer in a sample of a body fluid with prostate cell marker-specific and tumor associated marker-specific antibodies as well as to kits comprising such antibodies for use in the detection of prostate cancer.
Owner:GERALD P MURPHY CANCER FOUND
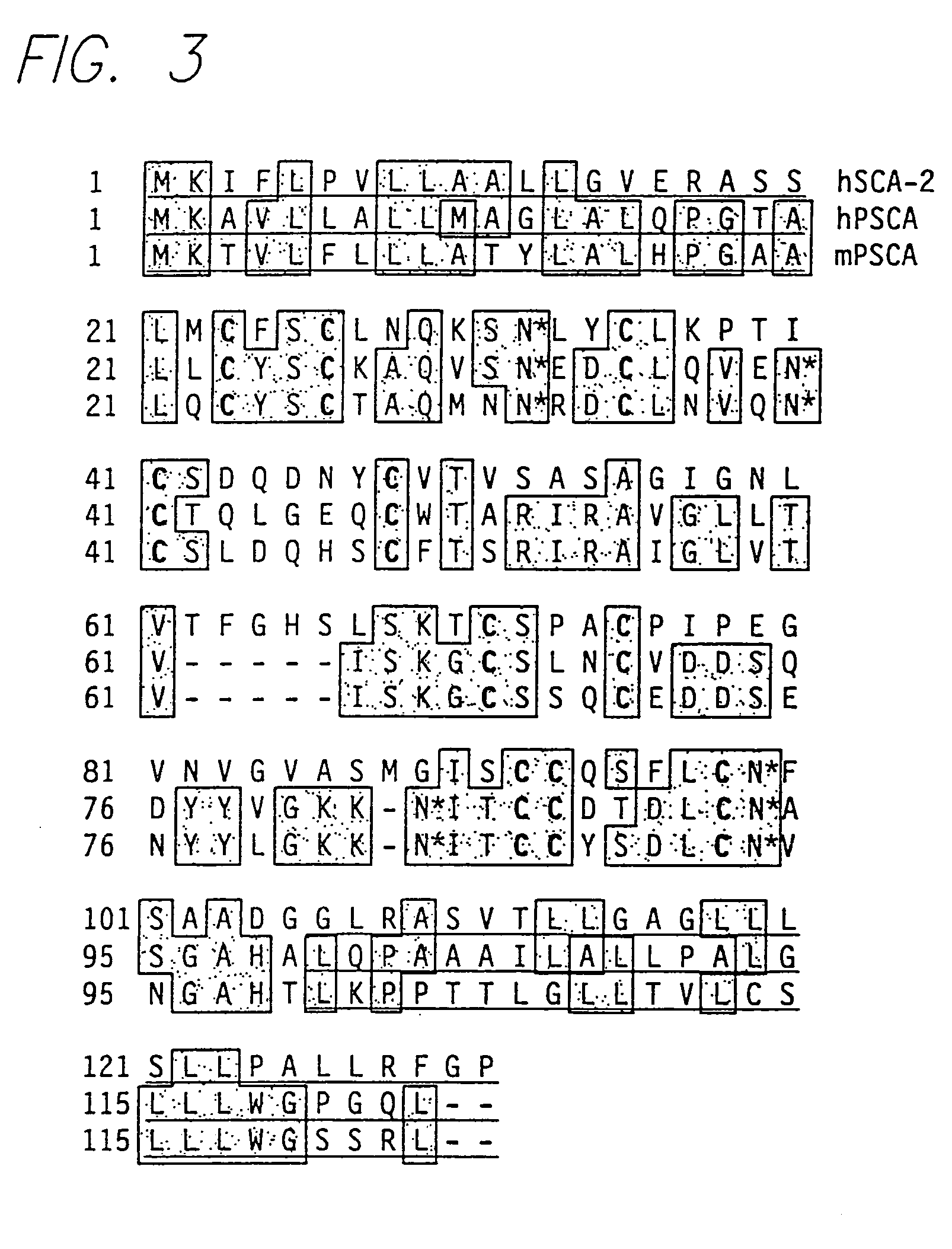
PSCA antibodies and hybridomas producing them
InactiveUS20050026229A1Promote immune-mediated destructionHigh expressionOrganic active ingredientsTumor rejection antigen precursorsAntigenProstatic epithelium
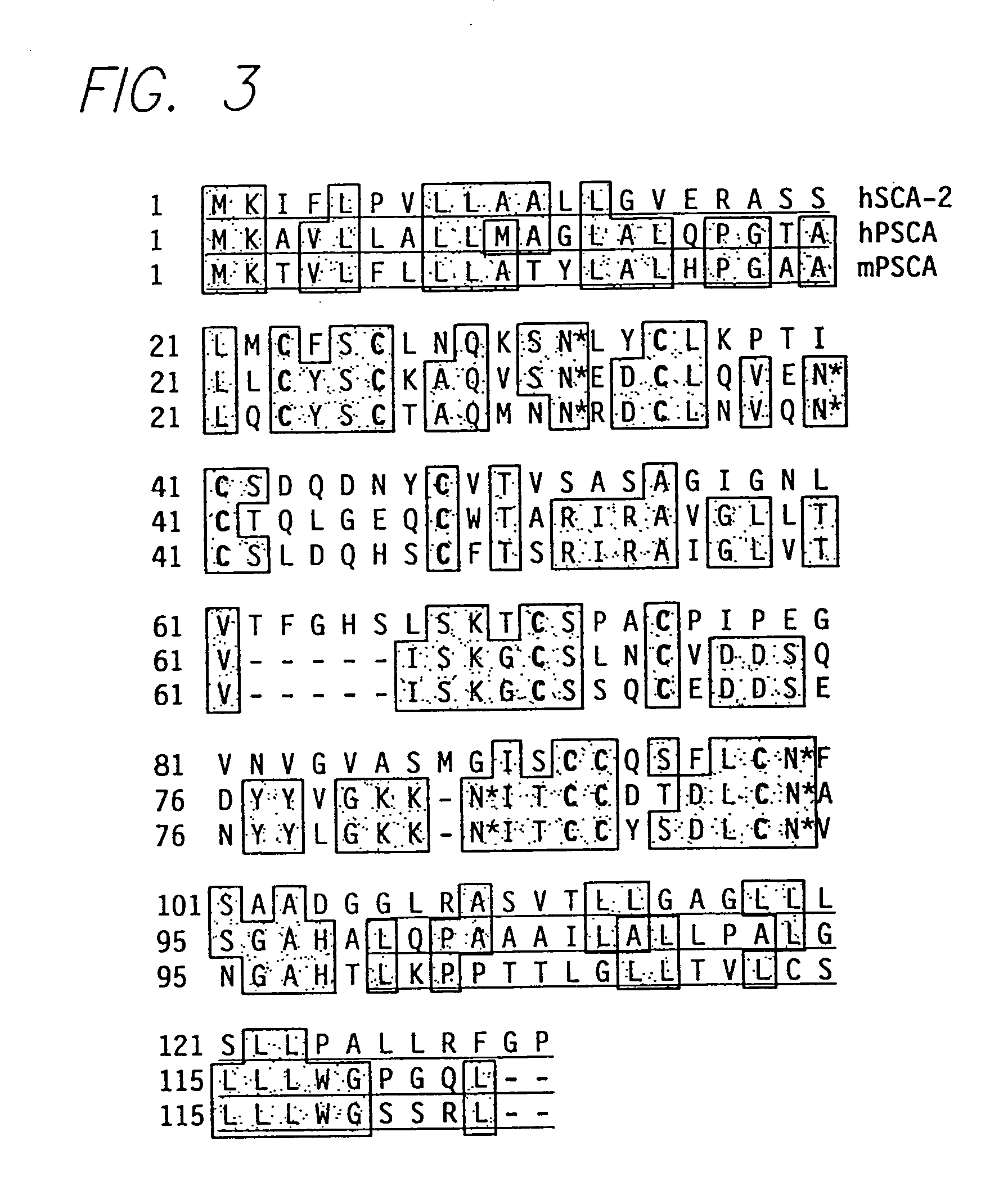
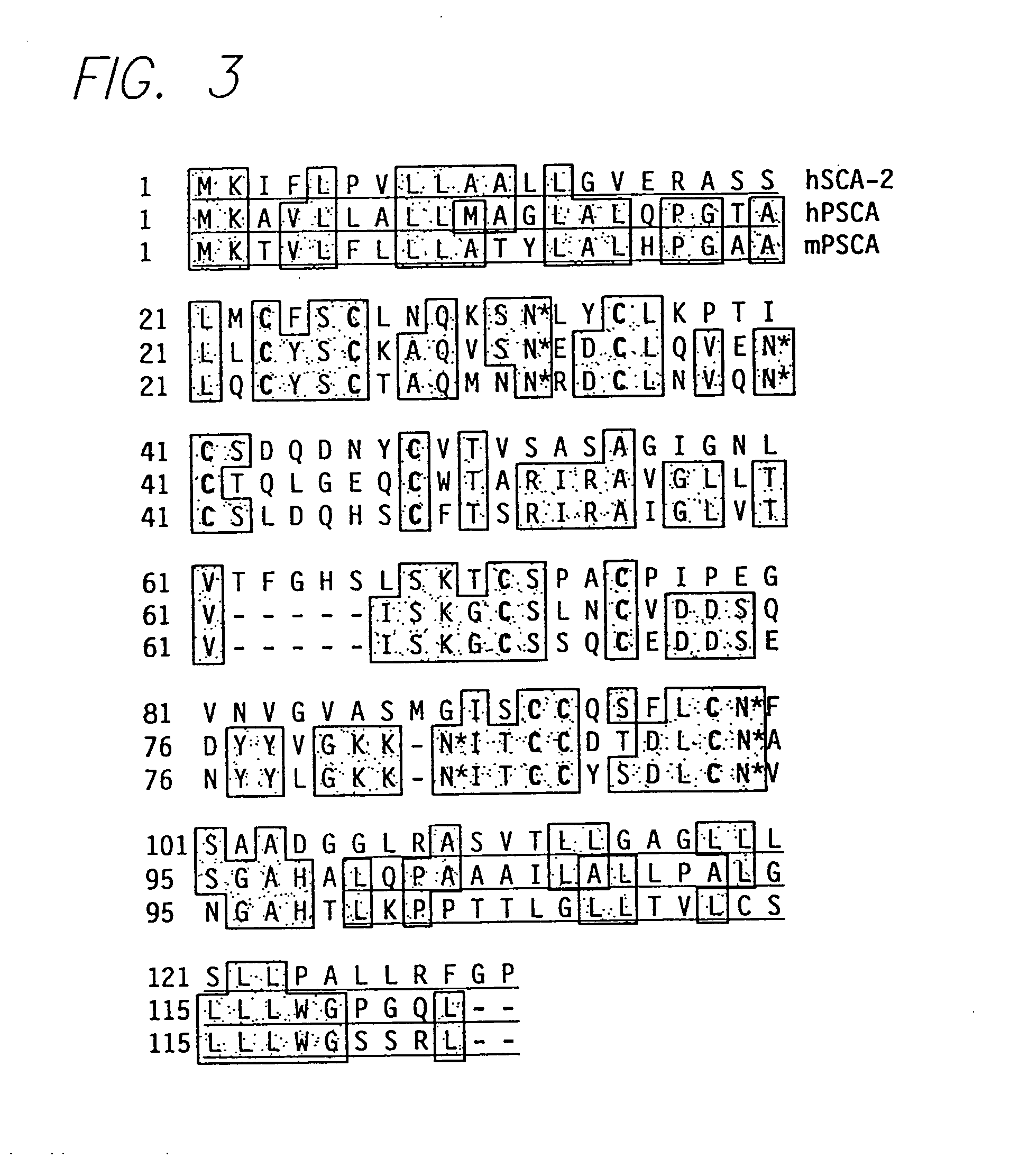
The invention provides a novel prostate cell-surface antigen, designated Prostate Stem Cell Antigen (PSCA), which is widely over-expressed across all stages of prostate cancer, including high grade prostatic intraepithelial neoplasia (PIN), androgen-dependent and androgen-independent prostate tumors.
Owner:RGT UNIV OF CALIFORNIA
Apparatus and method for treatment of benign prostatic hyperplasia
InactiveUS20060217703A1Short timeReduce relaxationElectrotherapyElectrical/wave energy microorganism treatmentOncologyNeoplastic cell
An apparatus and a method for treatment of benign prostatic hyperplasia are disclosed. The apparatus includes an applicator piece carrying a set of electrodes shaped and positioned to create a substantial electric field in the volume of hyperplasia and a pulse generator adapted for delivery of electrical pulses above the upper electroporation limit for the neoplastic cells. The amplitude, duration and number of the electrical pulses are generally selected to cause necrosis of a significant fraction of the volume of benign prostatic hyperplasia. The apparatus may include a high frequency system for heating the prostatic tissue and a cooling system for cooling the urethra. The combined action of heating and cooling may increase the temperature of the prostate cells to 45 degrees C. to 55 degrees C., while keeping the urinary tract at a temperature 15 degrees C. to 20 degrees C. This temperature distribution can increase the selectivity of the treatment by increasing susceptibility of the neoplastic cells to the electroporation treatment and decreasing it for the normal urethral tissues.
Owner:ANGIODYNAMICS INC
Engineered Anti-Prostate Stem Cell Antigen (PSCA) Antibodies for Cancer Targeting
ActiveUS20090311181A1In-vivo radioactive preparationsAntibody mimetics/scaffoldsAntigenBladder cancer
The invention provides novel humanized antibody fragments that specifically bind prostate cell-surface antigen (PSCA), a protein which is overexpressed in variety of cancers, including prostate, bladder, and pancreatic cancer. Methods are provided for the use of the compositions of the invention for the treatment of cancer, diagnosis of cancer, to provide a prognosis of cancer progression, and for cancer imaging.
Owner:RGT UNIV OF CALIFORNIA
Application of compounds in isorhodanic ester classes for treating diseases of prostate and skin cancer
ActiveCN101091705AIncreased ability to remove harmful substancesImprove in vitro dissolutionUrinary disorderEster active ingredientsDiseaseProstate cancer cell
The present invention relates to a method capable of using natural and artificial synthetic isosulfocyanate compound or its derivative to prevent and cure prostatic diseases and skin carcinoma. The internal tests show that various isosulfocyanate compounds or their derivatives can induce prostatic cell II phase drug metabolic detoxication enzyme-glutathione transferase so as to can inhibit the hyperplasia of prostate and inflammation, and can prevent and cure prostatic carcinoma and skin carcinoma.
Owner:JC (WUXI) CO INC
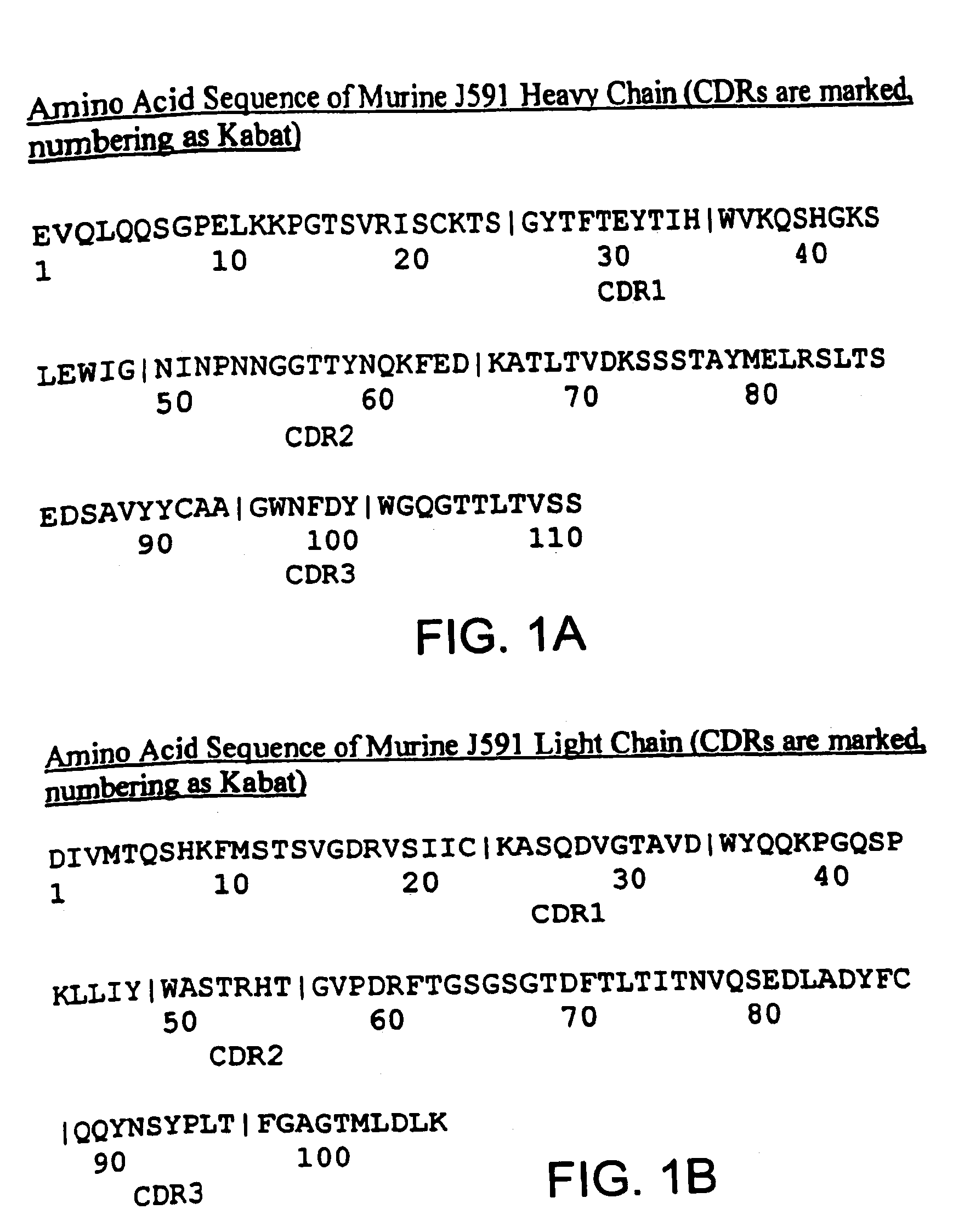
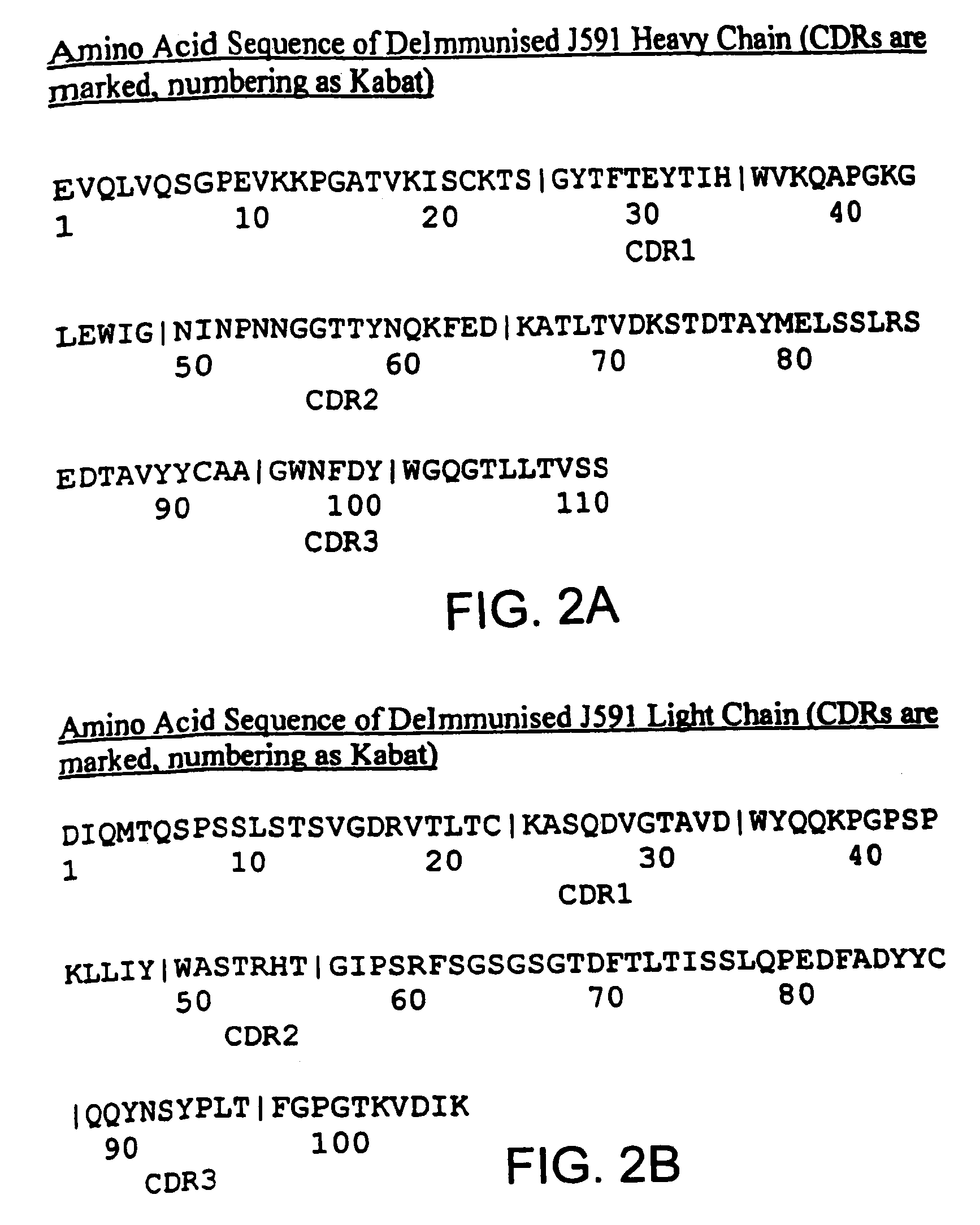
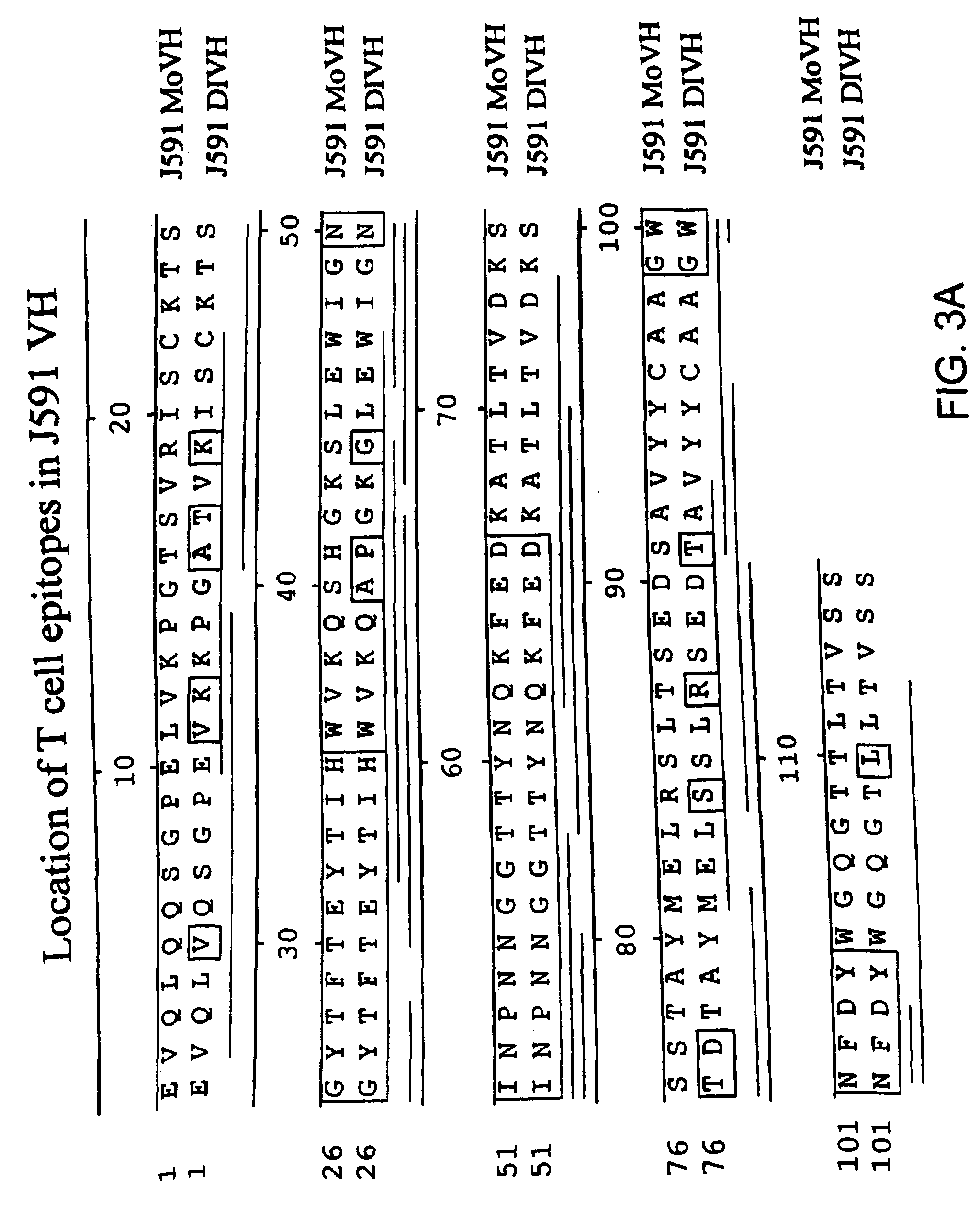
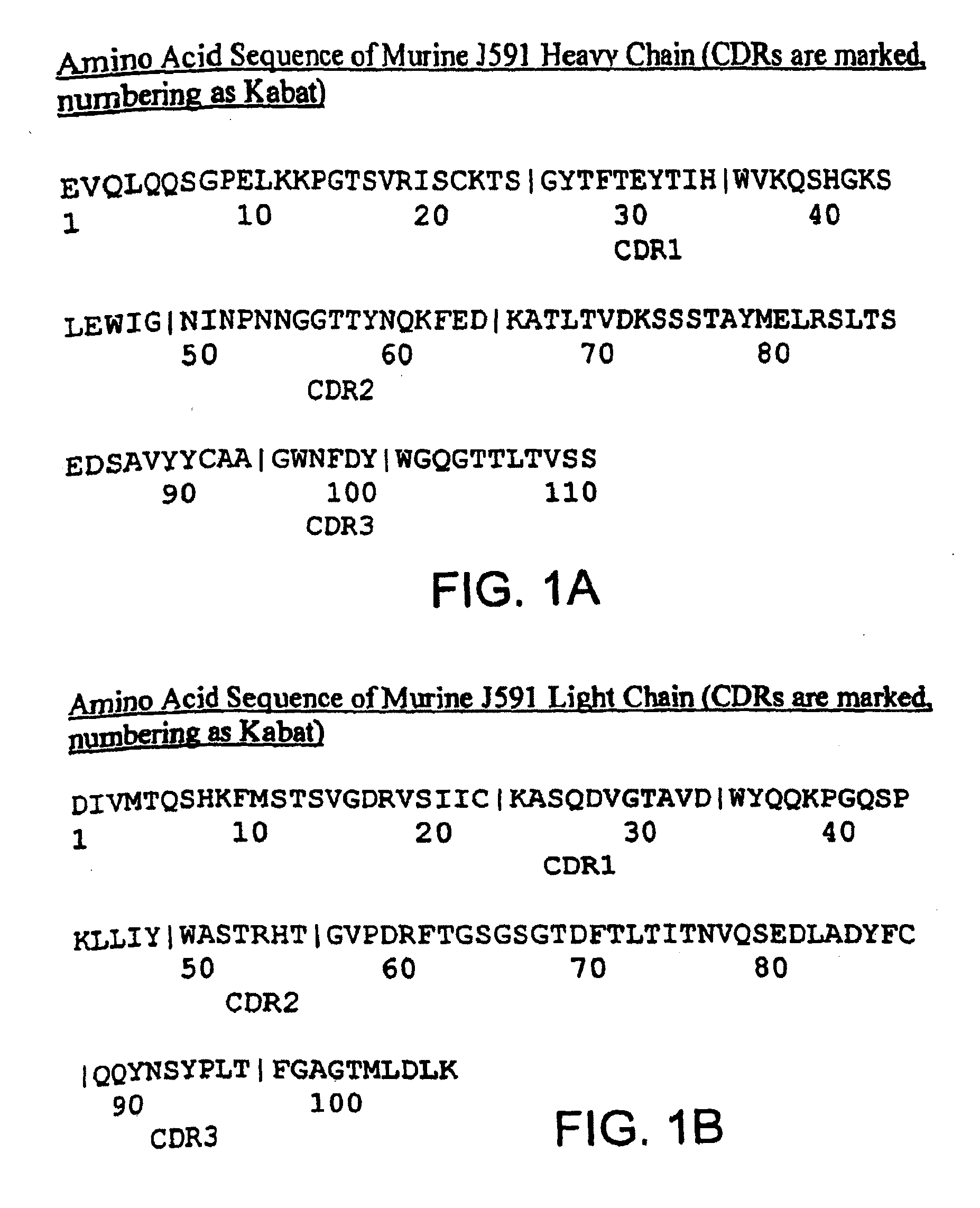
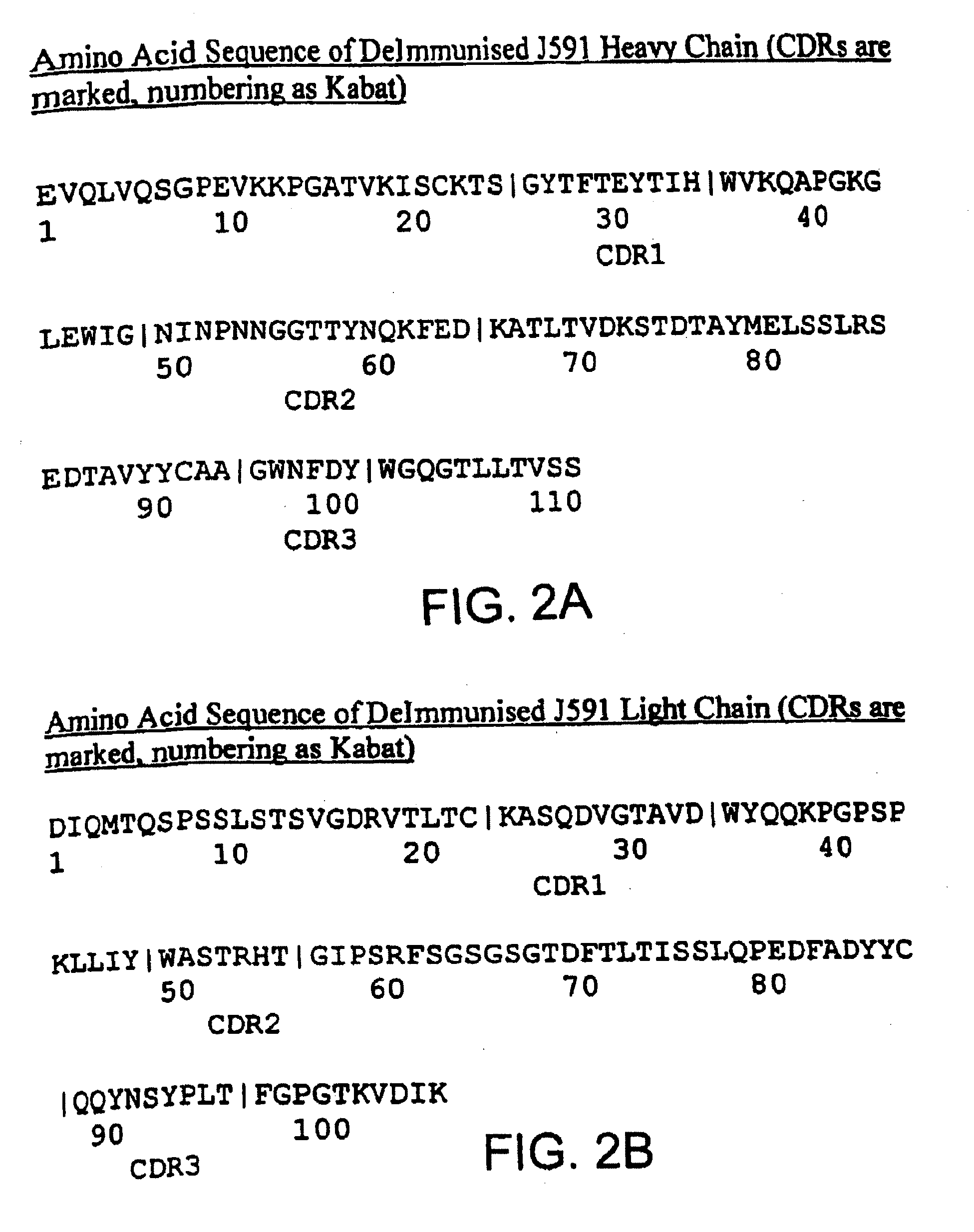
Methods for treating prostate cancer using modified antibodies to prostate-specific membrane antigen
InactiveUS7666414B2Relieve painReduce needIn-vivo radioactive preparationsSugar derivativesAntigen Binding FragmentAnti-PSMA Antibody
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
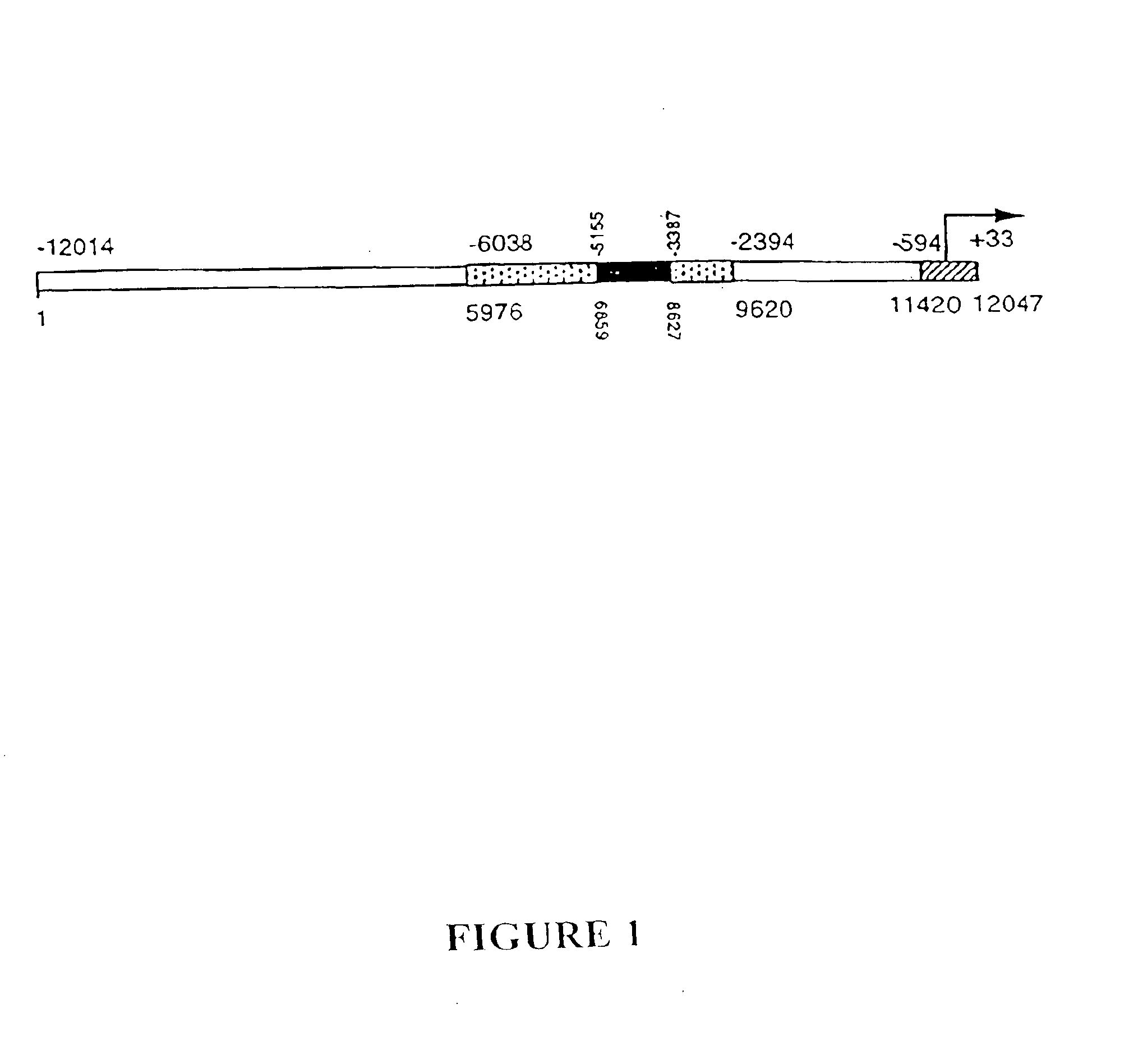
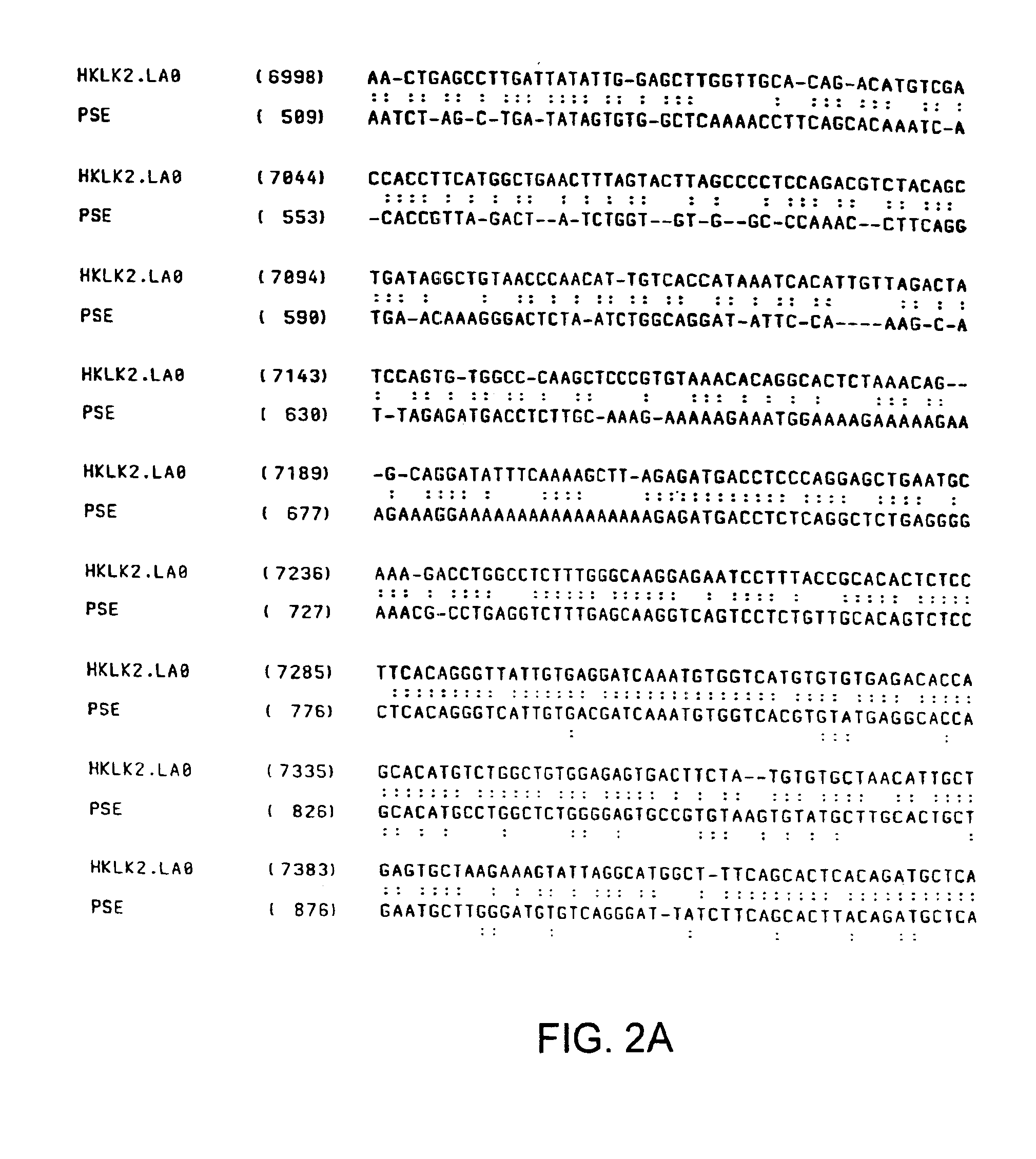
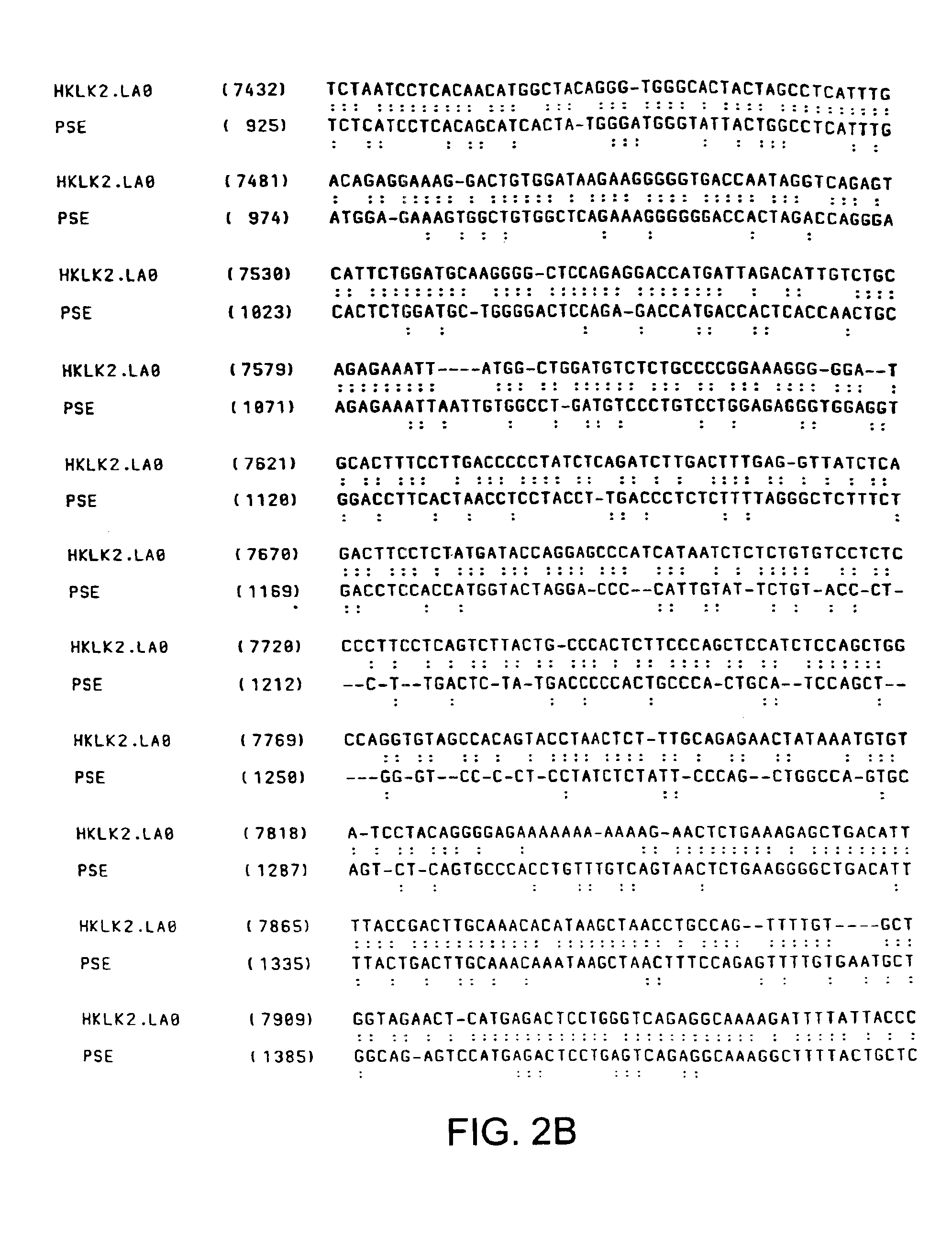
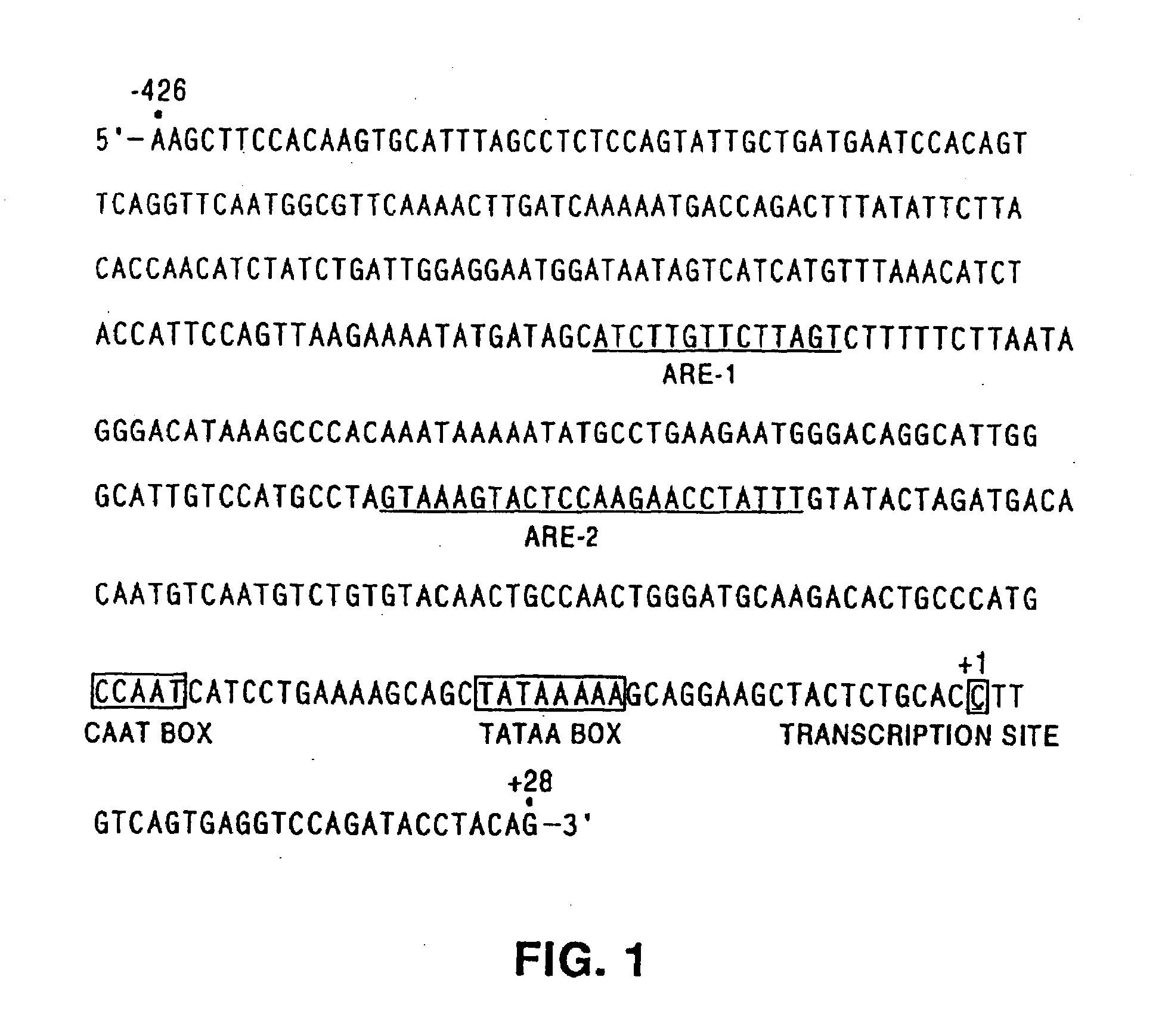
Human glandular kallikrein enhancer, vectors comprising the enhancer and methods of use thereof
Enhancers which preferentially increase the transcription of cis-linked coding sequences in prostate cells are provided. Methods of using DNA constructs comprising the enhancers to control transcription of heterologous polynucleotides are also provided. Delivery vehicles comprising the enhancers and methods of using the vehicles are also provided. Adenovirus vectors in which one or more genes are under transcriptional control of the enhancers of the invention are also provided. Further provided are methods of using the adenovirus vectors of the invention to confer selective cytotoxicity in mammalian cells.
Owner:CELLS GENESYS INC
PSCA: prostate stem cell antigen and uses thereof
InactiveUS20050152909A1Organic active ingredientsTumor rejection antigen precursorsAntigenProstatic epithelium
The invention provides a novel prostate cell-surface antigen, designated Prostate Stem Cell Antigen (PSCA), which is widely over-expressed across all stages of prostate cancer, including high grade prostatic intraepithelial neoplasia (PIN), androgen-dependent and androgen-independent prostate tumors.
Owner:RGT UNIV OF CALIFORNIA
Human Zip1, Zinc and Citrate for Prostate Cancer Screening
InactiveUS20110046204A1Genetic material ingredientsFermentationCITRATE ESTERProstate cancer screening
The present invention provides methods of detecting prostate cancer employing biomarkers, including hZIP1, zinc and citrate. Also provided are antibodies to detect hZIP1 protein or peptides and an expression vector comprising a genetic sequence effective to increase uptake of zinc into a prostate cell upon expression thereof. Furthermore, methods of treating prostate cancer and of increasing uptake of zinc into a prostate cell are provided.
Owner:UNIV OF MARYLAND
Prostate Cancer Glycan Markers and Autoantibody Signatures
ActiveUS20090258792A1High degreeSaccharide librariesLibrary screeningProstate cancer cellGriffonia simplicifolia
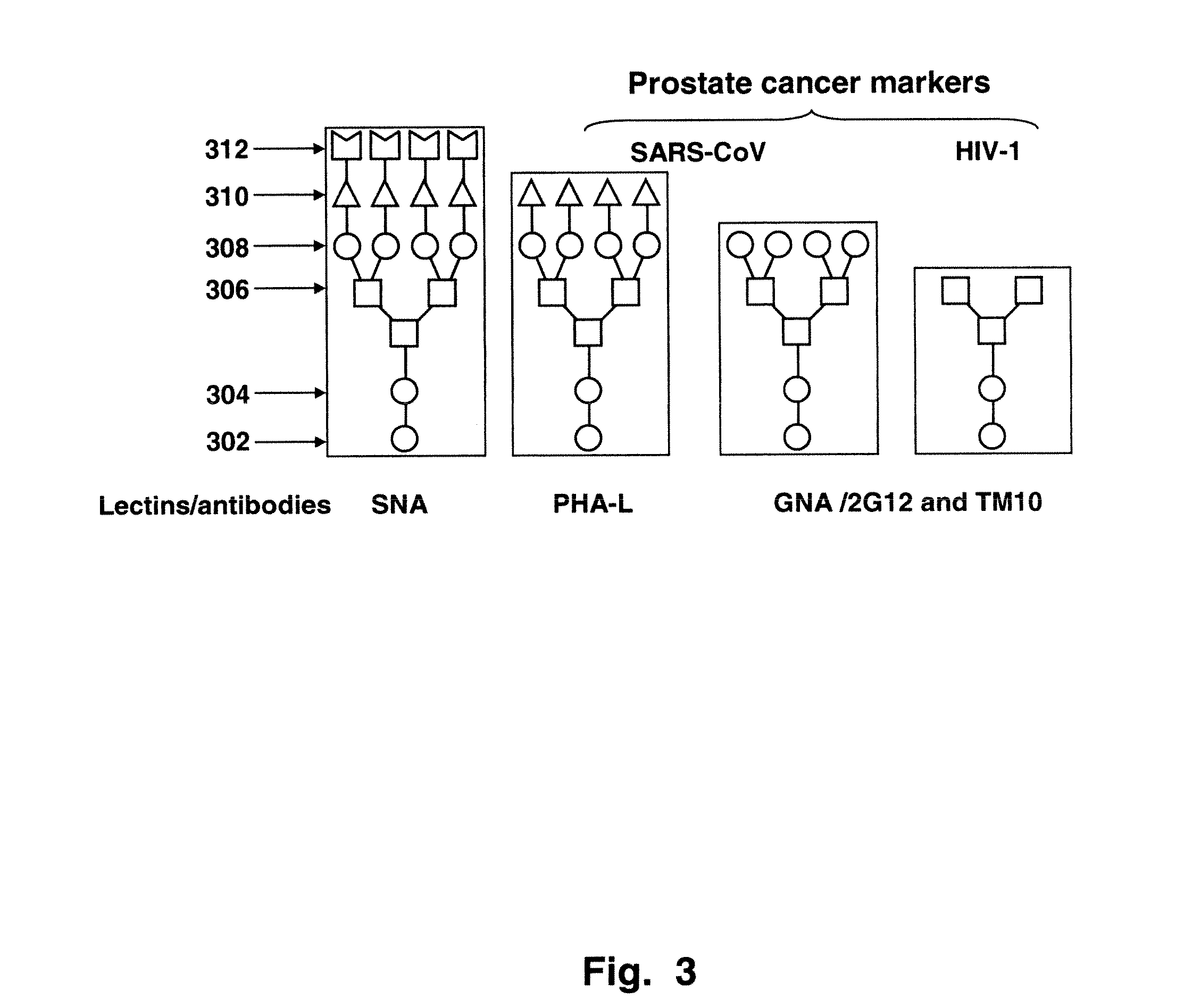
Disclosed are methods for probing the immunogenic sugar moieties of prostate cancer cells. The methods detect a number of glyco-epitopes that are highly and differentially expressed among prostate cancers of various Gleason grades. The glyco-epitopes exist on the surfaces of prostate cells. The methods also comprise the detection of autoantibodies in prostate cancer subjects. The antibodies bound to a glyco-motif of N-glycans that is normally “cryptic.” This target is highly expressed in prostate cancers. Lectins and antibodies that detect these glyco-epitopes that expressed in prostate cancer tissues include Euonymus europaeus lectin (EEL); Psophocarpus Tetragonolobus Lectin-I (PTL-I); Griffonia Simplicifolia Lectin-I-A4 (GSL-I-A4); Griffonia Simplicifolia Lectin-I-B4 (GSL-I-B4); Sambucus nigra I agglutinin (SNA-I; Phaseolus vulgaris-L (PHA-L; Galanthus nivalis agglutinin (GNA); Narcissus pseudonarcissus agglutinin (NPA); Artocarpus integrifolia agglutinin (Jacalin); and mAb TM10 (IgM).
Owner:SRI INTERNATIONAL
Genes overexpressed in prostate disorders as diagnostic and therapeutic targets
InactiveUS20060134688A1Reduced expression levelUseful in treatmentGenetic material ingredientsMicrobiological testing/measurementDiseaseCancer research
Disclosed are methods for diagnosing, monitoring the progression of, and treating a prostate disorder based upon genes that are differentially expressed in prostate disorders. Also disclosed are methods for identifying agents useful in the treatment of a prostate disorder, methods for monitoring the efficacy of a treatment for a prostate disorder, methods for inhibiting the proliferation of a prostate cell, and prostate-specific vectors including the promoter of these genes.
Owner:IRM
Single cell-derived organoids
The present invention relates to organoids derived from a single cell, such as a prostate cancer cell, and methods and compositions relating to the production and use thereof, including cell culture medium for producing organoids and methods of personalized treatment for prostate cancer. The invention further provides a humanized mouse comprising a prostate organoid derived from a patient's prostate cell.
Owner:RUTGERS THE STATE UNIV
Methods of treating prostate cancer with Anti-prostate specific membrane antigen antibodies
InactiveUS20090280120A1Relieve painReduce needImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntigen Binding FragmentOncology
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
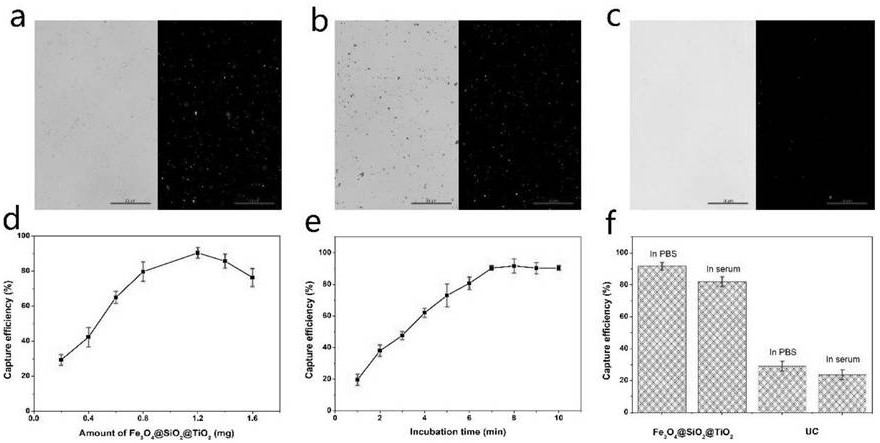
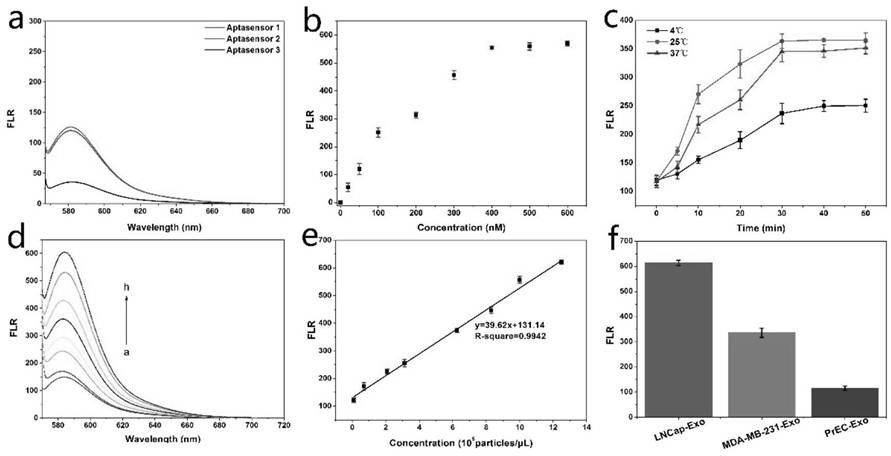
Method for detecting prostate cancer exosome based on Fe3O4@ SiO2@ TiO2 nanoparticle enrichment and PSMA sensor
PendingCN112129939AFirmly connectedEasy to separateFluorescence/phosphorescenceProstate cancer cellOncology
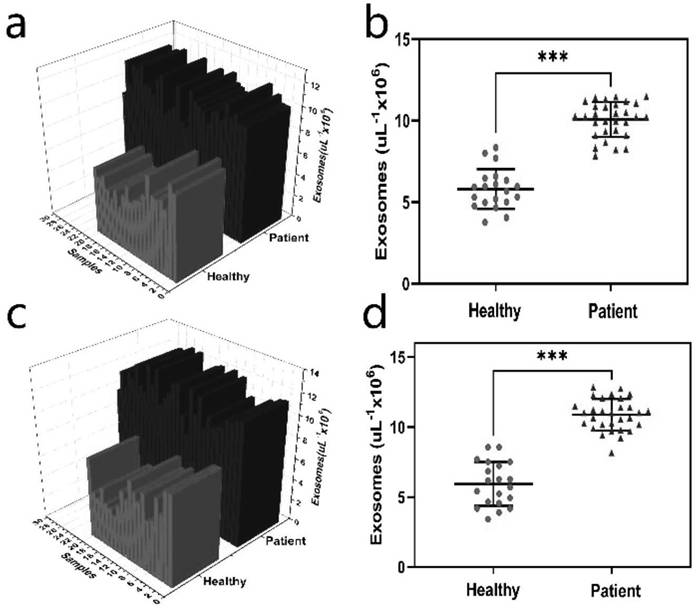
The invention discloses a method for detecting prostate cancer exosome based on Fe3O4@ SiO2@ TiO2 nanoparticle enrichment and a PSMA sensor. The method comprises the following steps of: enriching exosome by adopting Fe3O4@ SiO2@ TiO2 nanoparticles, and then constructing a hairpin-shaped PSMA aptamer sensor to quantify the exosome by measuring the change of fluorescence intensity. By utilizing thedetection method, the exosome of prostate cancer cells can be successfully distinguished from the exosome of normal prostate cells, and the detection limit is 9 * 10 <3> exosomes / microliter. The method is used for clinical prostate cancer patient serum samples and normal human serum samples, can quickly and accurately detect prostate cancer patients, and is verified by a traditional ELISA method. The exosome enrichment method is simple and quick, the enrichment efficiency is high, exosome enrichment can be completed within 8 minutes, and the enrichment efficiency is 91.5%. The sensitivity ishigh, the specificity is high, the detection limit is 9 * 10 <3> exosomes / microliter, and clinical prostate cancer patients and healthy people can be remarkably distinguished.
Owner:NINGBO UNIV
Regulatory constructs comprising intron 3 of prostate specific membrane antigen gene
InactiveUS7074400B1Strong prostate specificityHigh level expressionOrganic active ingredientsBiocideInteinVascular endothelium
The invention provides regulatory constructs comprising intron 3 of the prostate specific membrane antigen gene (PSMA). An isolated nucleic acid molecule encoding the partial sequence of intron 3 of PSMA, a vector and a recombinant expression cassette are disclosed. The invention also provides a method of directing expression of a coding sequence in a prostate cell, a bladder cell, a breast cell and a vascular endothelial cell using the said constructs. This invention further provides a method of treatment of cancer using the said constructs.
Owner:COMMONWEALTH SCI & IND RES ORG
Marker and kit for auxiliary diagnosis of prostatic cancer
ActiveCN113462780AAchieve early diagnosisPromote proliferationOrganic active ingredientsMicrobiological testing/measurementOncologyCancer research
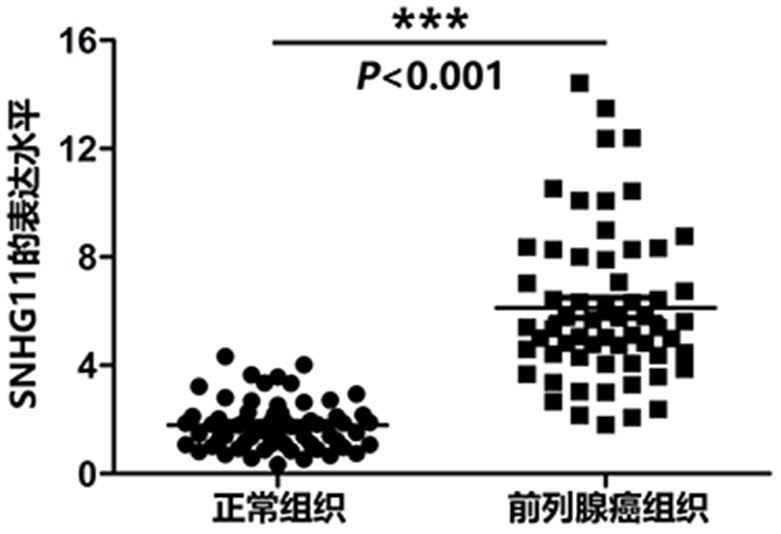
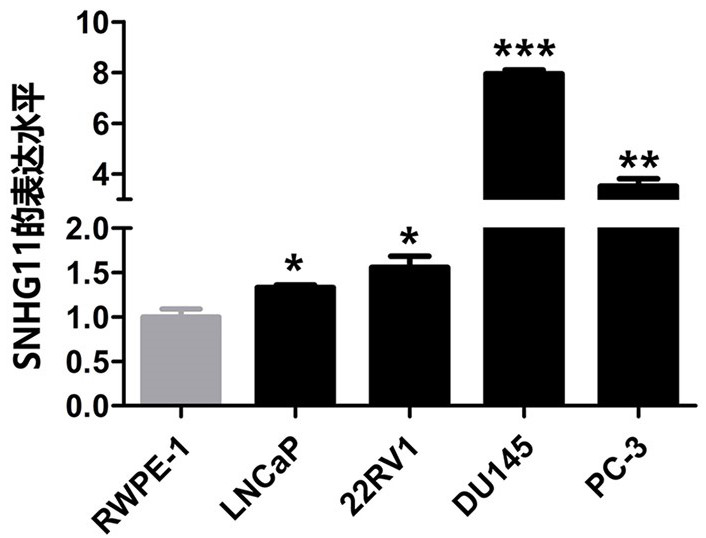
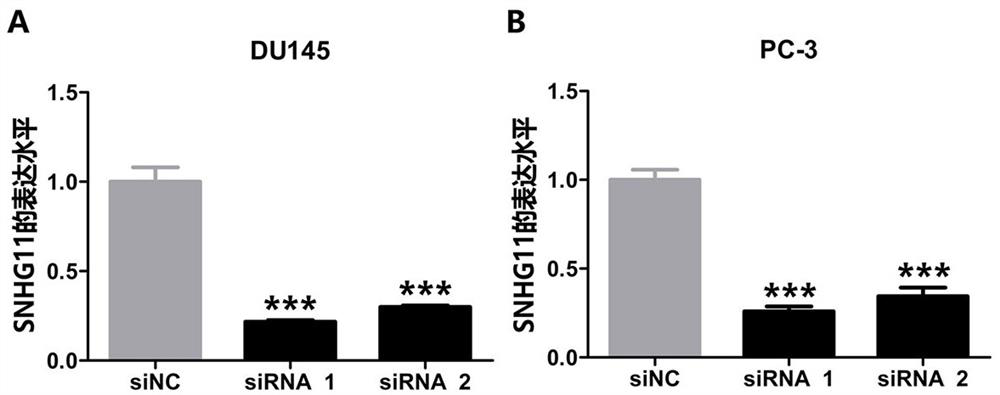
The invention belongs to the technical field of medical biology, and discloses a molecular marker-LncRNA SNHG11 for auxiliary diagnosis of prostatic cancer. The LncRNA SNHG11 is highly expressed in cancer tissues of prostatic cancer, and is lowly expressed in paracancerous normal tissues; moreover, the expression level of the LncRNA SNHG11 in a prostatic cancer cell line is obviously higher than that of a normal prostate cell line; proliferation, migration and invasion of prostatic cancer cells can be obviously inhibited by knocking down the expression level of the LncRNA SNHG11 gene. Therefore, the LncRNA SNHG11 can be used as a molecular marker for auxiliary diagnosis of the prostatic cancer; by detecting the expression level of the LncRNA SNHG11 in a sample, auxiliary diagnosis can be carried out on the prostatic cancer, and a reference basis is provided for clinical doctors to diagnose the prostatic cancer.
Owner:PEOPLES HOSPITAL OF HENAN PROV
Use of human prostate cell lines in cancer treatment
InactiveUS20050249756A1Promote growthSufficient characteristicBacterial antigen ingredientsPeptide/protein ingredientsGamma irradiationHuman prostate
The invention here relates to a product comprised of a cell line or lines intended for use as an allogeneic immunotherapy agent for the treatment of cancer in mammals and humans. All of the studies of cell-based cancer vaccines to date have one feature in common, namely the intention to use cells that contain at least some TSAs and / or TAAs that are shared with the antigens present in patients' tumour. In each case, tumour cells are utilised as the starting point on the premise that only rumour cells will contain TSAs or TAAs of relevance, and the tissue origins of the cells are matched to the tumour site in patients. A primary aspect of the invention is the use of immortalised normal, non-malignant cells as the basis of an allogeneic cell cancer vaccine. Normal cells do not possess TSAs or relevant concentrations of TAAs and hence it is surprising that normal cells are effective as anti-cancer vaccines. For prostate cancer, for example, a vaccine may be based on one or a combination of different immortalised normal cell lines derived from the prostate. The cell lines are lethally irradiated utilising gamma irradiation at 50-300 Gy to ensure that they are replication incompetent prior to use in the mammal or human.
Owner:ONYVAX
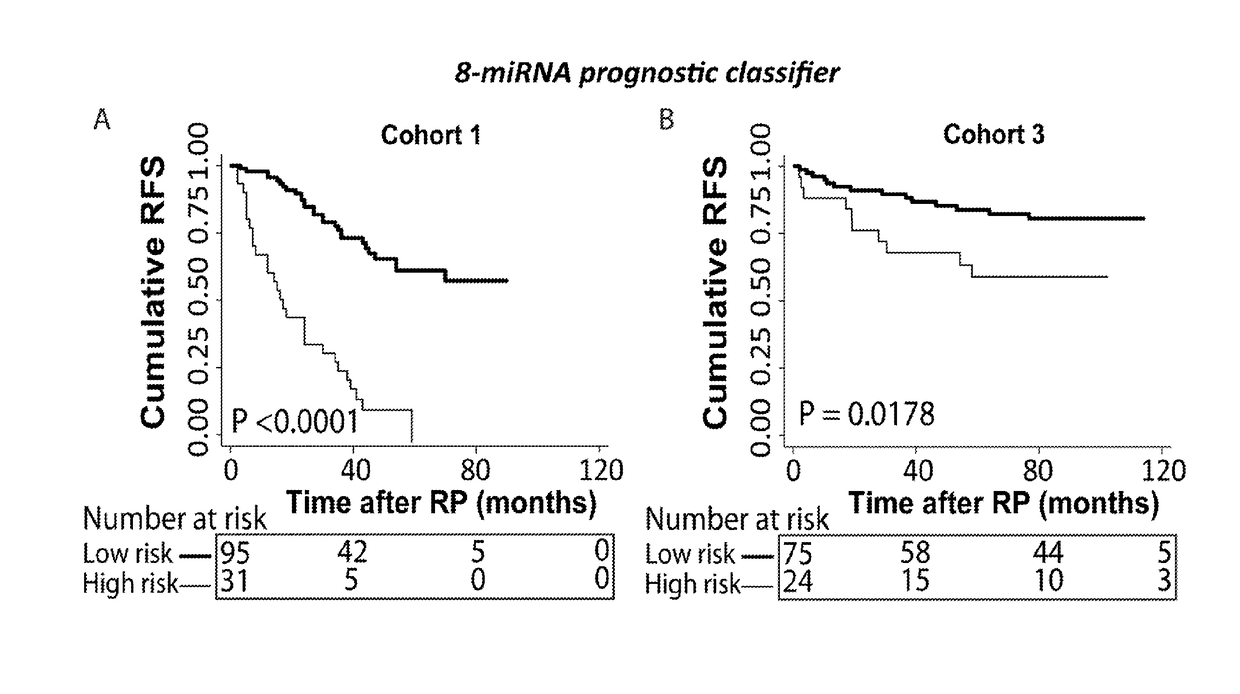
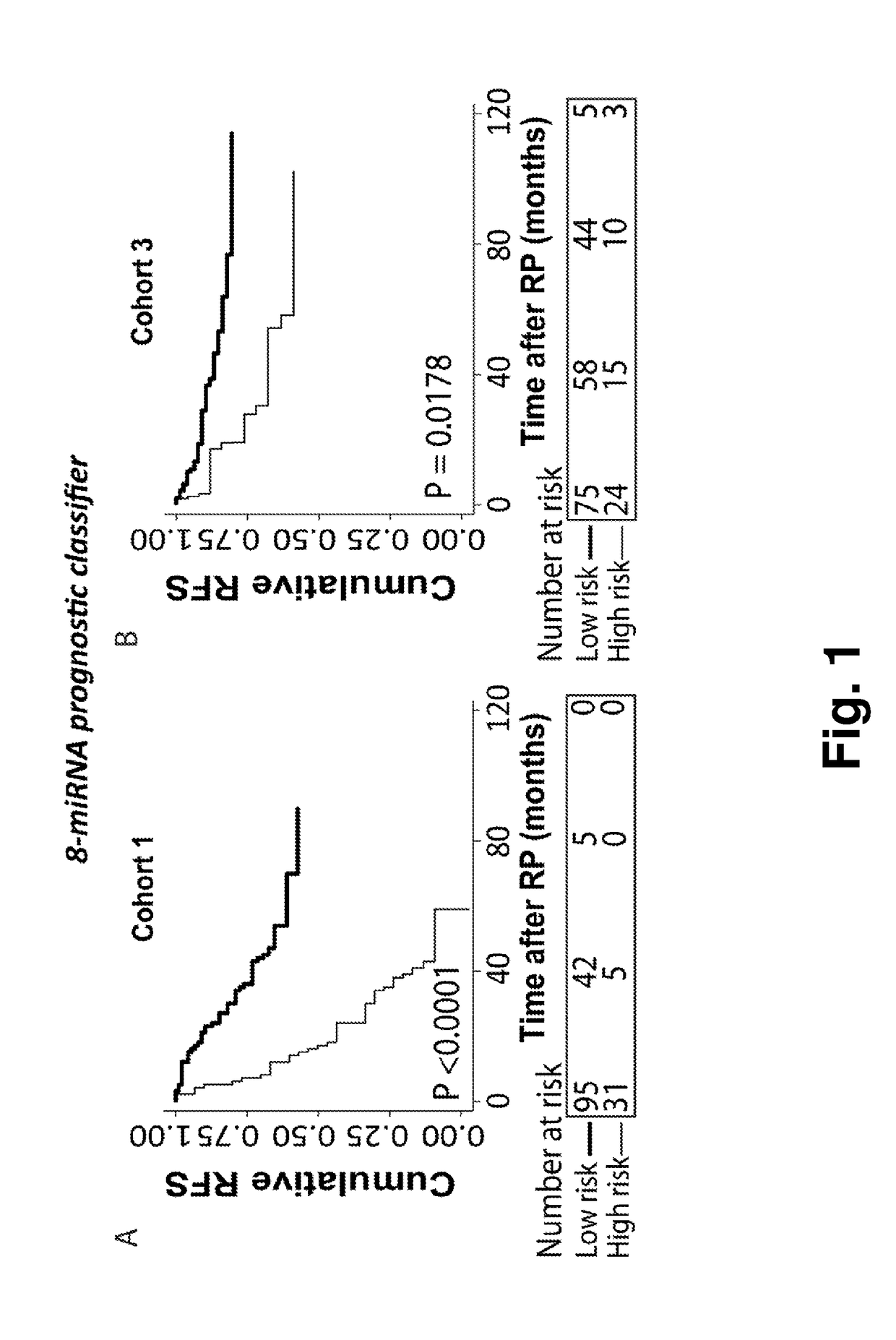
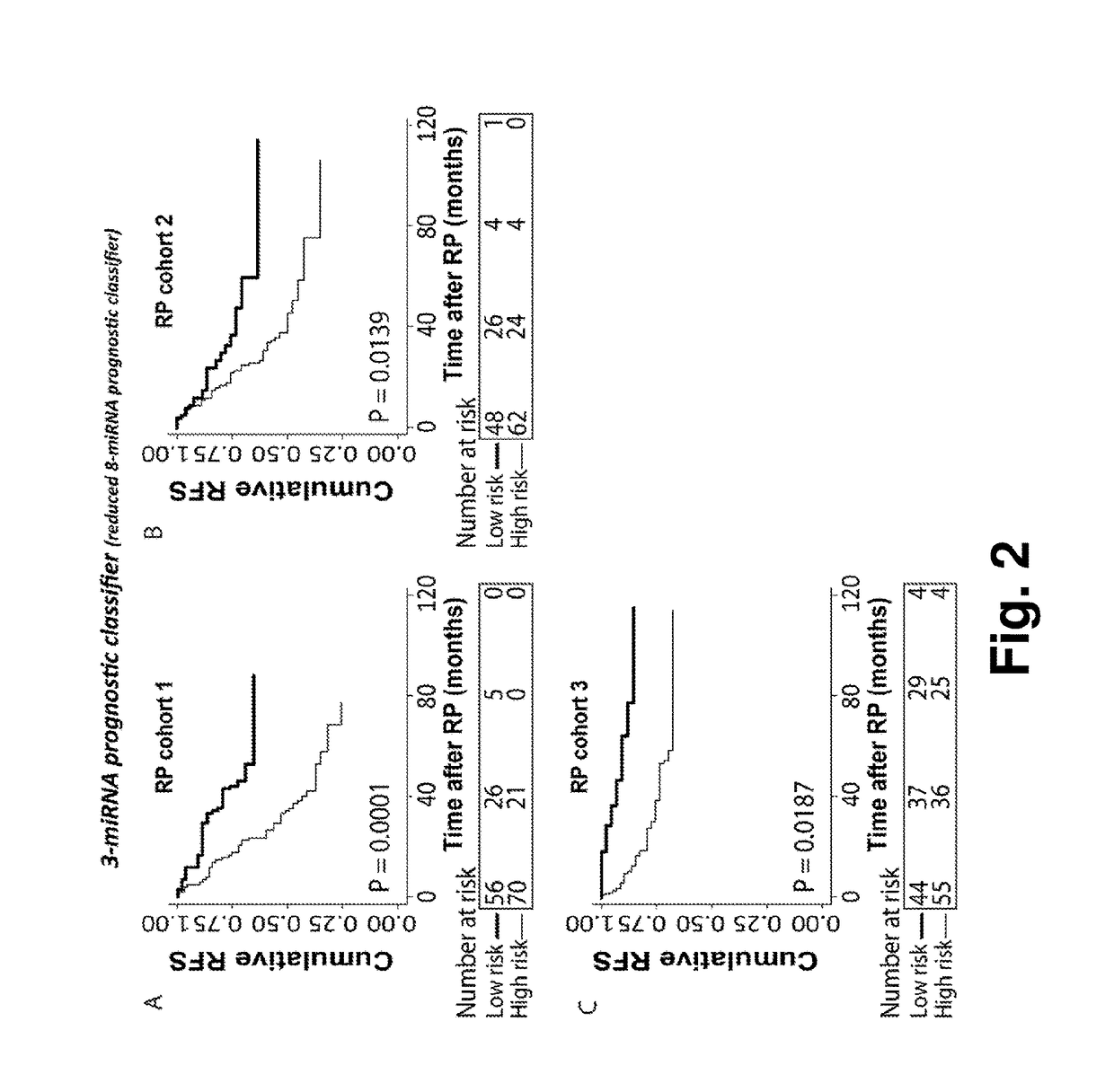
A microrna-based method for assessing the prognosis of a prostate cancer patient
The present application concerns a new in vitro method for assessing the prognosis of a prostate cancer patient, comprising measuring the expression level of at least two miRs selected from group of miRs consisting of: miR-106a-5p, miR-10b-5p, miR-133a-3p, miR-152-3p, miR-185-5p, miR-193a-5p, miR-221-3p, miR-23a-3p, miR-30d-3p, miR-326, miR-374b-5p, miR-615-3p and miR-625-3p in a RNA sample from prostate cells obtained from said patient, wherein a changed expression level of said at least 2 miRs, as compared to a reference expression profile, is indicative of the prognosis of said prostate cancer patient.
Owner:REGION MIDTJYLLAND +2
Methods and nucleic acids for analyses of cellular proliferative disorders
InactiveUS20110171637A1High sensitivityStrong specificitySugar derivativesComponent separationDiseaseGenome
The invention provides methods, nucleic acids and kits for detecting, or for detecting and distinguishing between or among prostate cell proliferative disorders or for detecting, or for detecting and distinguishing between or among colorectal cell proliferative disorders. The invention discloses genomic sequences the methylation patterns of which have utility for the improved detection of and differentiation between said class of disorders, thereby enabling the improved diagnosis and treatment of patients.
Owner:EPIGENOMICS AG
PSCA antibodies and hybridomas producing them
InactiveUS20080318254A9Promote immune-mediated destructionHigh expressionOrganic active ingredientsTumor rejection antigen precursorsAntigenProstatic epithelium
The invention provides a novel prostate cell-surface antigen, designated Prostate Stem Cell Antigen (PSCA), which is widely over-expressed across all stages of prostate cancer, including high grade prostatic intraepithelial neoplasia (PIN), androgen-dependent and androgen-independent prostate tumors.
Owner:RGT UNIV OF CALIFORNIA
Adenovirus vectors specific for cells expressing androgen receptor and methods of use thereof
Replication-competent adenovirus vectors specific for cells which allow a probasin transcriptional response element (PB-TRE) to function, such as cells which express the androgen receptor (AR), and methods of use of such viruses are provided. These viruses comprise an adenoviral gene under control of a transcriptional regulatory portion of a PB-TRE, which is in turn dependent upon AR expression. The gene can be, for example, a gene required for viral replication or the adenovirus death protein gene (ADP). The viruses can also comprise at least one additional adenoviral gene under control of at least one additional prostate-specific transcriptional response element, such as that controlling prostate-specific antigen expression (PSA-TRE). Thus, virus replication can be restricted to target cells exhibiting prostate-specific gene expression, particularly prostate carcinoma cells. An adenovirus of the present invention can further comprise a heterologous gene such as a reporter under transcriptional control of a PB-TRE. The adenovirus vectors can be used to detect and monitor samples for the presence of prostate cells as well as to selectively kill malignant cells producing prostate-specific gene products.
Owner:CELLS GENESYS INC
Lytic peptides having Anti-proliferative activity against prostate cancer cells
Lytic peptides, including fusion peptides of lytic peptides conjugated with luteinizing hormone-releasing hormone or modified versions thereof to target luteinizing hormone-releasing hormone receptors, are disclosed. The lytic peptides show anti-proliferative activity against human prostate cancer cell lines, but are nontoxic to normal primary human prostate epithelial cells or to bone marrow stromal cells in co-culture. The lytic peptides have specificity for and anti-proliferative activity against prostate cancer tumor cells, and low toxicity for normal prostate cells, making the peptides useful in therapies for prostate cancer.
Owner:TUSKEGEE UNIVERSITY
Immortalized human prostate cell lines
InactiveUS20140286868A1Reflection characteristicLow toxicityCompounds screening/testingCompound screeningAbnormal tissue growthHuman cell
Immortalized human cell lines derived from prostate cells are disclosed. Immortalized cells derived from a human epithelial prostate tissue cancer tumor are provided, as well as immortalized cells derived from healthy human epithelial prostate tissue from the same patient. Methods for utilizing such immortalized cell lines for researching, screening, and evaluating antimalignancy therapies and drug candidates are also disclosed.
Owner:TUSKEGEE UNIVERSITY
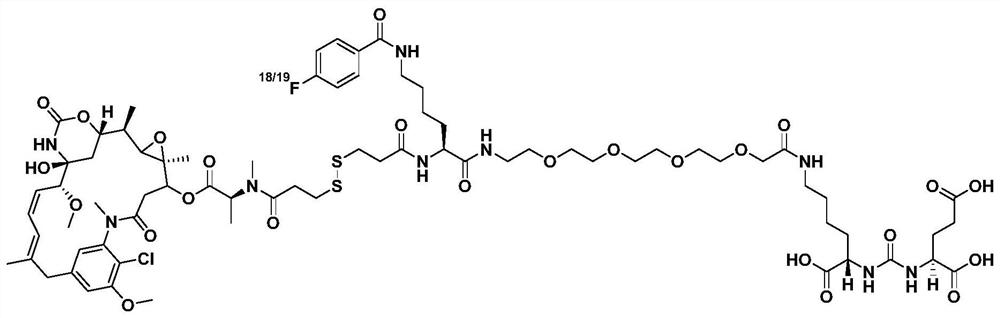
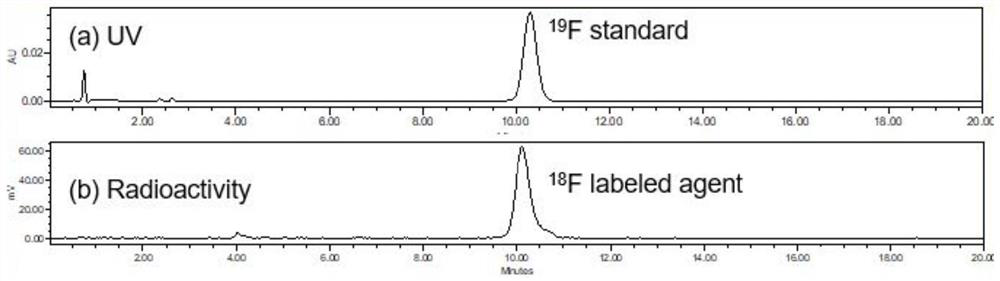
<18/19>F-labeled PSMA targeted diagnosis and treatment integrated small-molecular drug conjugate, preparation method and application of <18/19>F-labeled PSMA targeted diagnosis and treatment integrated small-molecular drug conjugate
ActiveCN112675311APrecision killSmall toxicityOrganic active ingredientsRadioactive preparation carriersProstate cancer cellTumor target
The invention relates to a tumor targeted diagnosis and treatment integrated drug compound, in particular to an <18 / 19>F-labeled PSMA targeted diagnosis and treatment integrated small molecular drug conjugate, a preparation method and an application of the <18 / 19>F-labeled PSMA targeted diagnosis and treatment integrated small molecular drug conjugate. By utilizing a PSMA targeted small molecular ligand, a DM1 drug with a high toxicity killing effect is delivered to PSMA positive prostate cancer cells in a targeted manner, the effect of effectively killing castration-resistant prostate CRPC cells with relative drug resistance is expected to be achieved, besides, the toxic and side effects of the DM1 on the whole body are effectively controlled and reduced, and the tolerance of a patient is improved; and besides, on the basis, a tumor diagnosis and treatment integrated mode is introduced, molecular imaging diagnosis is used for assisting in visualizing the in-vivo transfer process of drugs, predicting the curative effect of the patient, knowing the in-vivo distribution of the drugs, achieving real-time monitoring and individualized administration of the tumor treatment effect and achieving diagnosis and treatment integration.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Prostate extracellular matrix gel for cultivating prostate cells
InactiveCN105316280AGood biocompatibilityProlonged proliferative phaseArtificial cell constructsVertebrate cellsBiocompatibility TestingGrowth cell
The invention relates to prostate extracellular substrate gel for cultivating prostate cells. The prostate extracellular matrix gel has the advantages that the prostate extracellular matrix gel is white and semi-transparent and is excellent in biocompatibility; the human prostate epithelial cells can be cultivated by the aid of the prostate extracellular matrix gel and are in tufted three-dimensional spatial growth modes which are consistent with in-vivo cell growth physiological status of mammals, and accordingly in-vivo natural growth environments for the cells in the mammals can be effectively simulated; proliferative stages are long (11 days) and are prolonged by 6 days as compared with proliferative stages of cells cultivated in the prior art.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
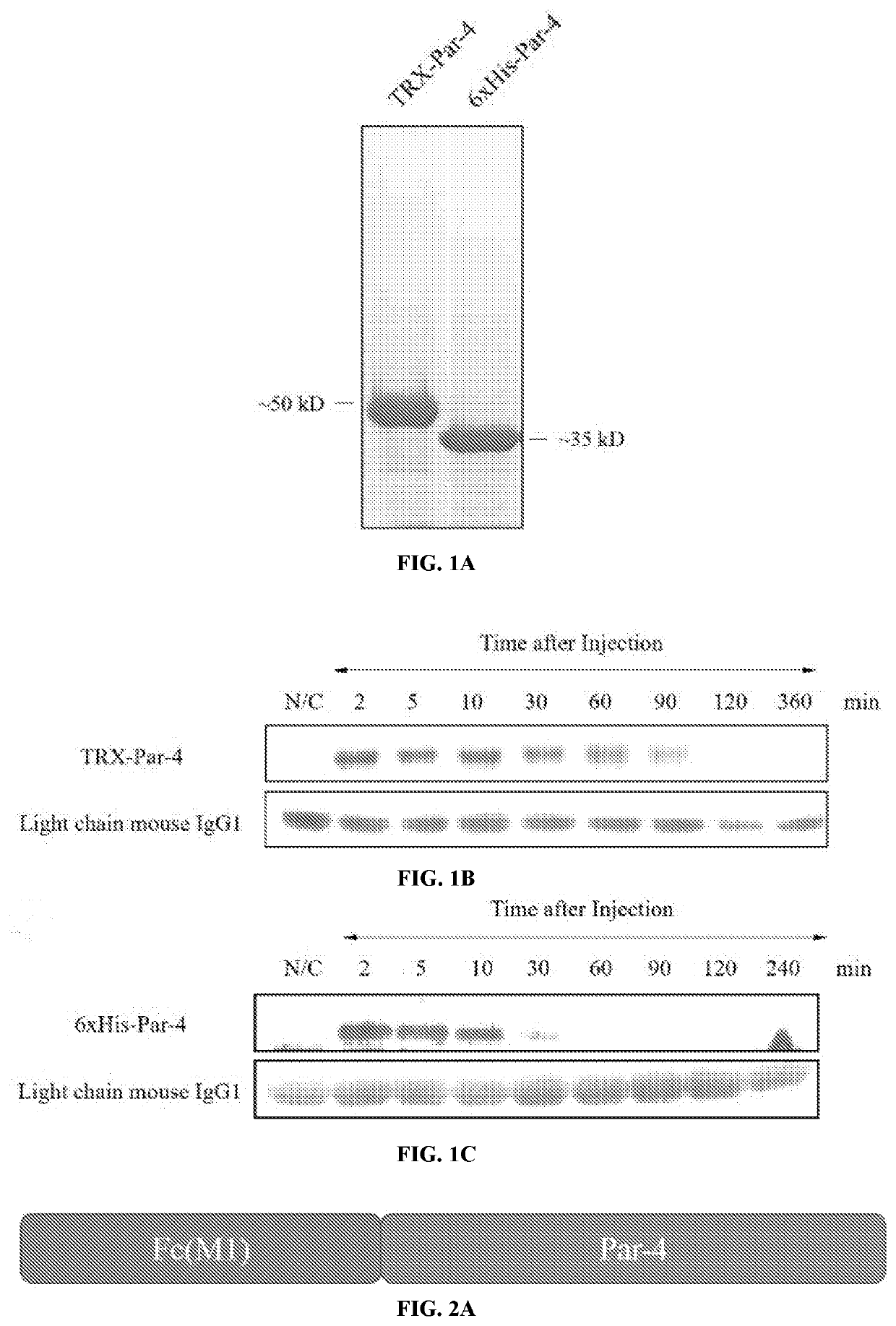
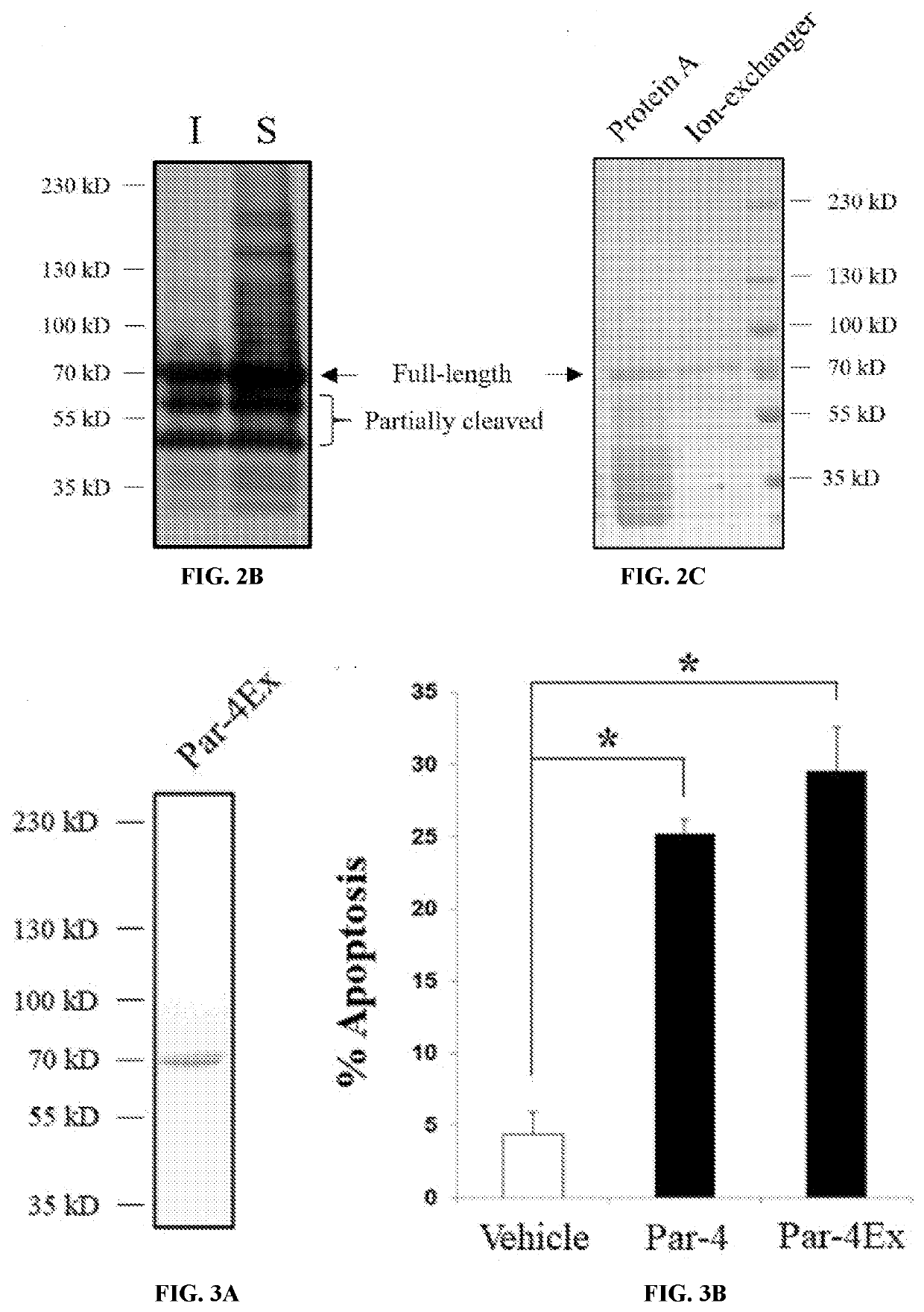
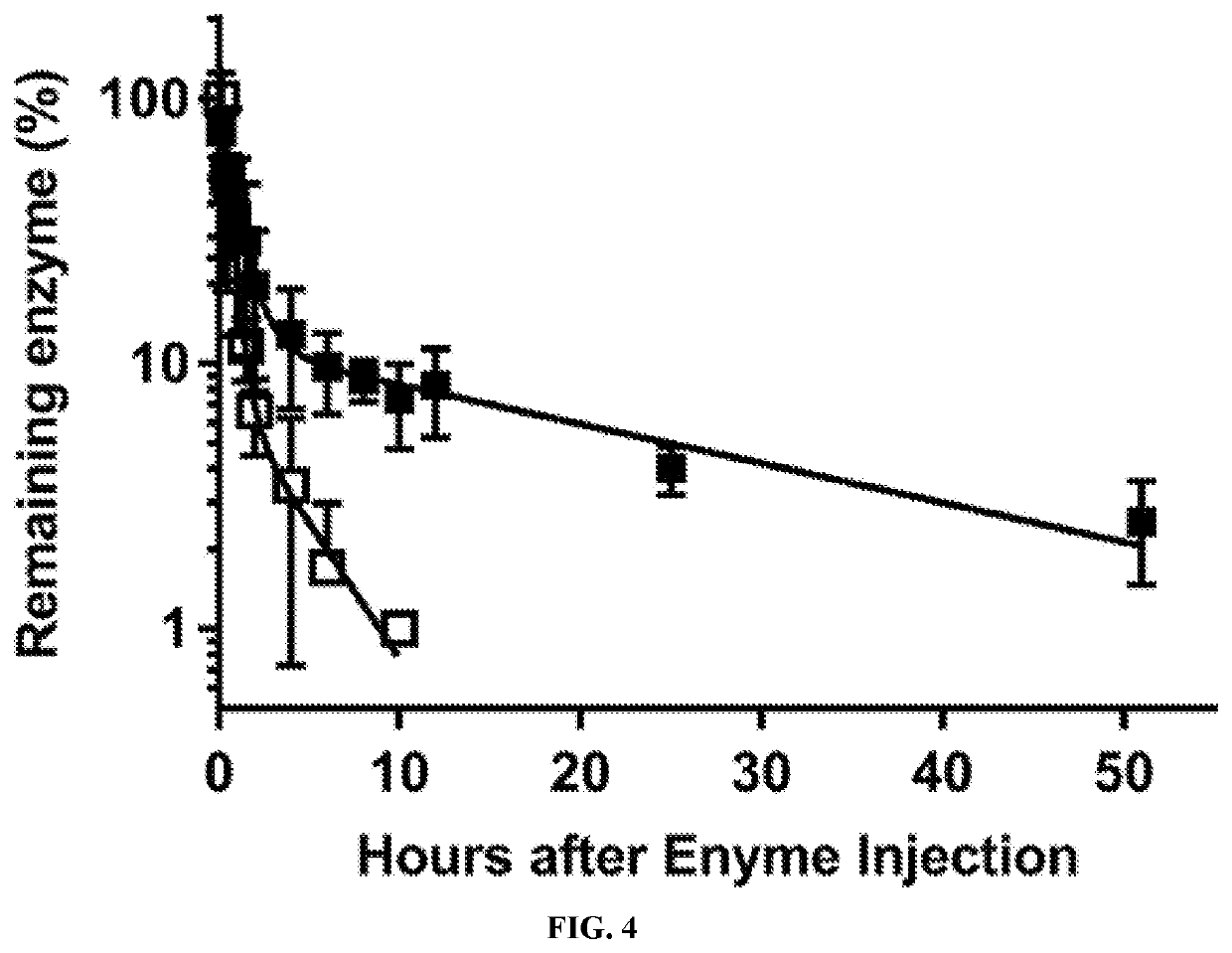
A Novel Prostate Apoptosis Response-4 (Par-4) Protein Entity with an Extended Duration of Action for Therapeutic Treatment of Cancer
PendingUS20220153805A1Improving biological half lifeProvide stabilityCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsCancer cellApoptosis
Disclosed herein are polypeptide molecules having Prostate apoptosis response-4 (Par-4) pro-apoptotic activity in cancer cells and enhanced biological half-life, and related methods. Specifically, the disclosure provides a composition comprising Par-4 polypeptide fusion protein, and a method of inducing apoptosis in a cancer cell using the composition, wherein the cancer cell is metastatic.
Owner:UNIV OF KENTUCKY RES FOUND
Compositions and methods relating to prostate specific genes and proteins
InactiveUS20060019322A1Peptide/protein ingredientsGenetic material ingredientsDiseaseAntiendomysial antibodies
The present invention relates to newly identified nucleic acids and polypeptides present in normal and neoplastic prostate cells, including fragments, variants and derivatives of the nucleic acids and polypeptides. The present invention also relates to antibodies to the polypeptides of the invention, as well as agonists and antagonists of the polypeptides of the invention. The invention also relates to compositions comprising the nucleic acids, polypeptides, antibodies, variants, derivatives, agonists and antagonists of the invention and methods for the use of these compositions. These uses include identifying, diagnosing, monitoring, staging, imaging and treating prostate cancer and non-cancerous disease states in prostate, identifying prostate tissue, monitoring and identifying and / or designing agonists and antagonists of polypeptides of the invention. The uses also include gene therapy, production of transgenic animals and cells, and production of engineered prostate tissue for treatment and research.
Owner:ALI SHUJATH +3
Methods and nucleic acids for the analysis of gene expression associated with the development of prostate cell proliferative disorders
InactiveUS20170067119A1High sensitivityStrong specificityMicrobiological testing/measurementFhit geneGene expression
The invention provides methods, nucleic acids and kits for detecting prostate cell proliferative disorders. The invention discloses genomic sequences the methylation patterns of which have utility for the improved detection of said disorder, thereby enabling the improved diagnosis and treatment of patients.
Owner:EPIGENOMICS AG
Early prostate cancer antigens (EPCA), polynucleotide sequences encoding them, and their use
InactiveUS7598042B2Early detectionLimiting the number of biopsies in an individualOrganic active ingredientsTumor rejection antigen precursorsAntigenDisease
A novel prostate cancer marker is described that is found in cancerous and normal prostate cells of individuals that have prostate cancer but is not found in the prostate of individuals without prostate cancer. The marker also is present in normal tissue adjacent to tumor tissue in individuals having prostate cancer. The marker, however, is absent in the prostate of individuals without prostate cancer. Methods employing the novel prostate cancer marker of the invention to predict the occurrence of the prostate disease, monitor the progression of prostate cancer and effect the treatment of prostate cancer, also are described.
Owner:UNIVERSITY OF PITTSBURGH +1
Novel serpentine transmembrane antigens expressed in human cancers and uses thereof
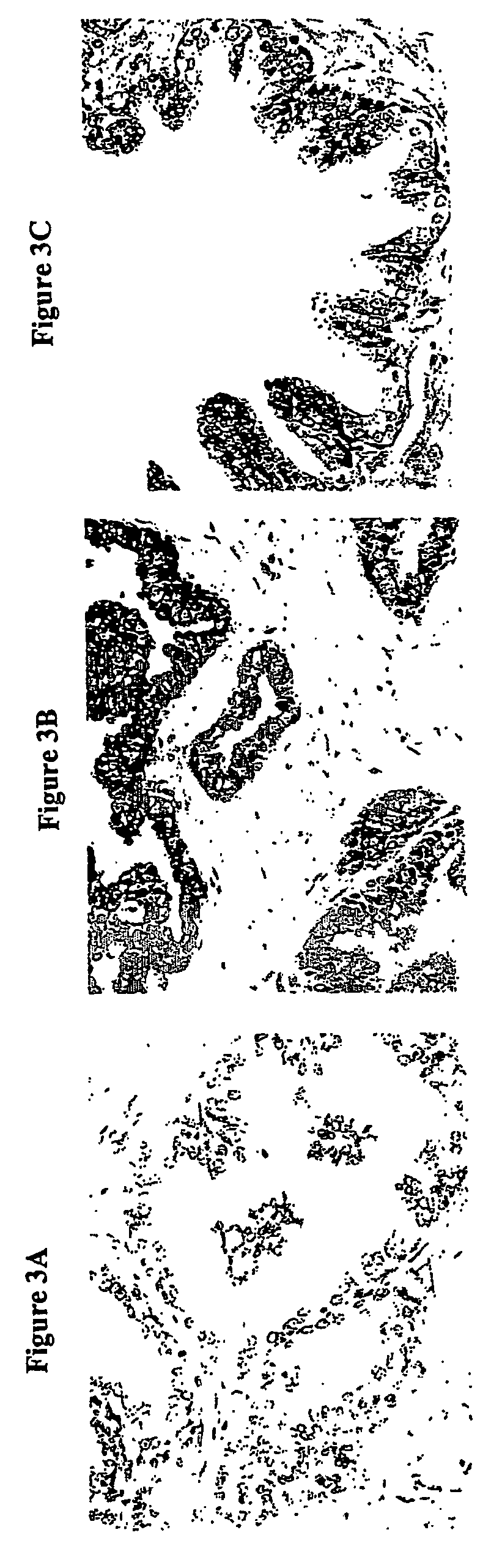
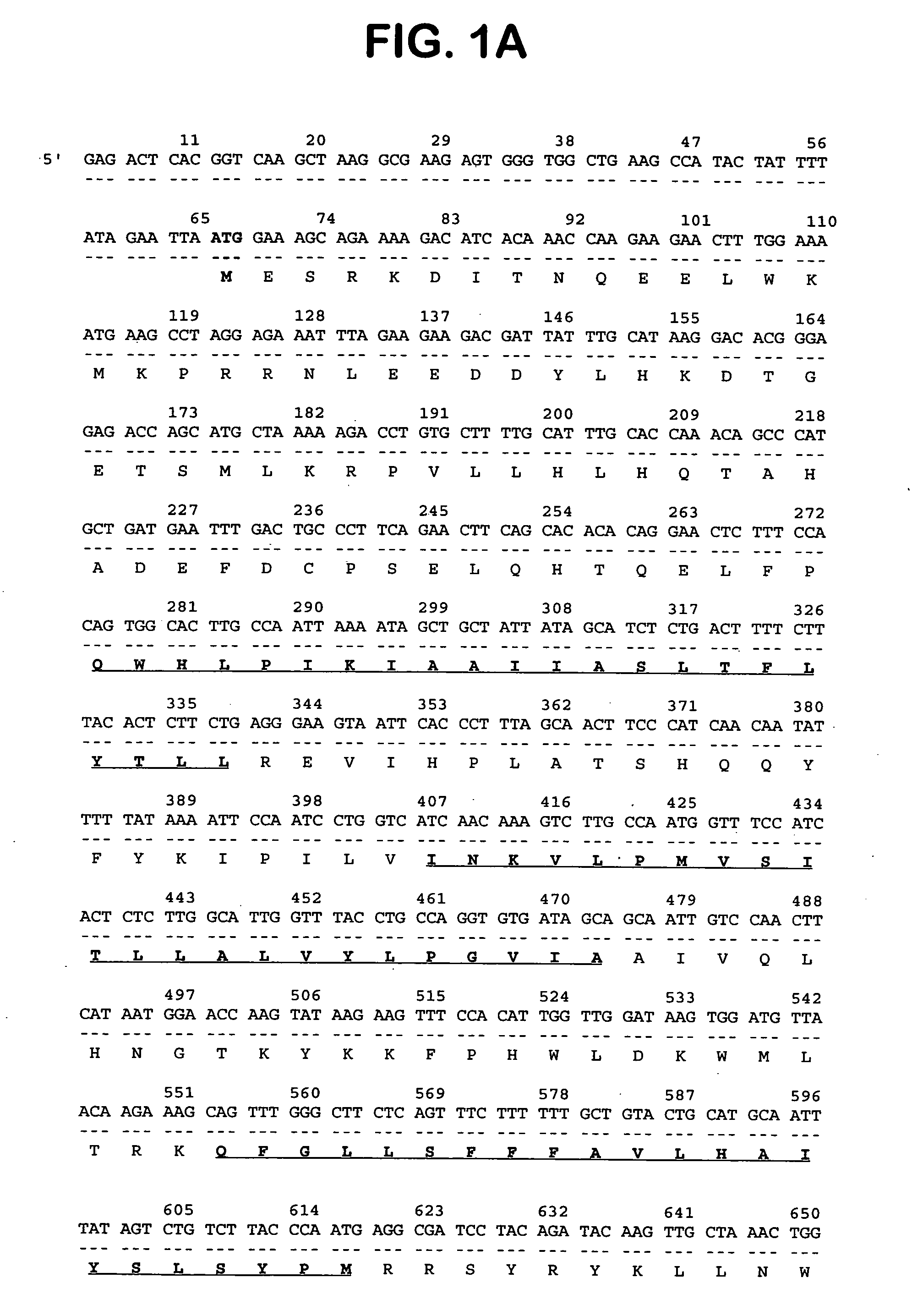
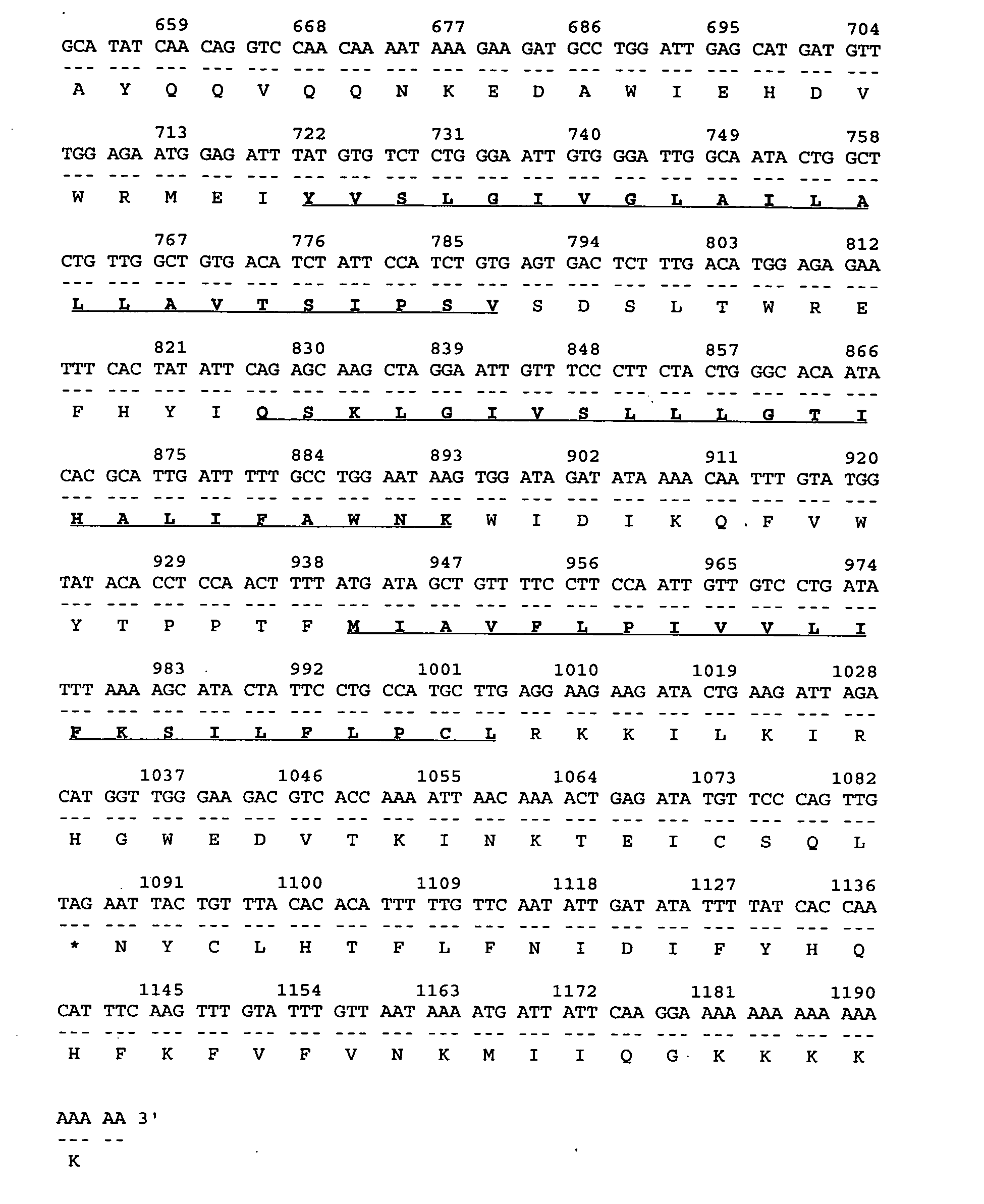
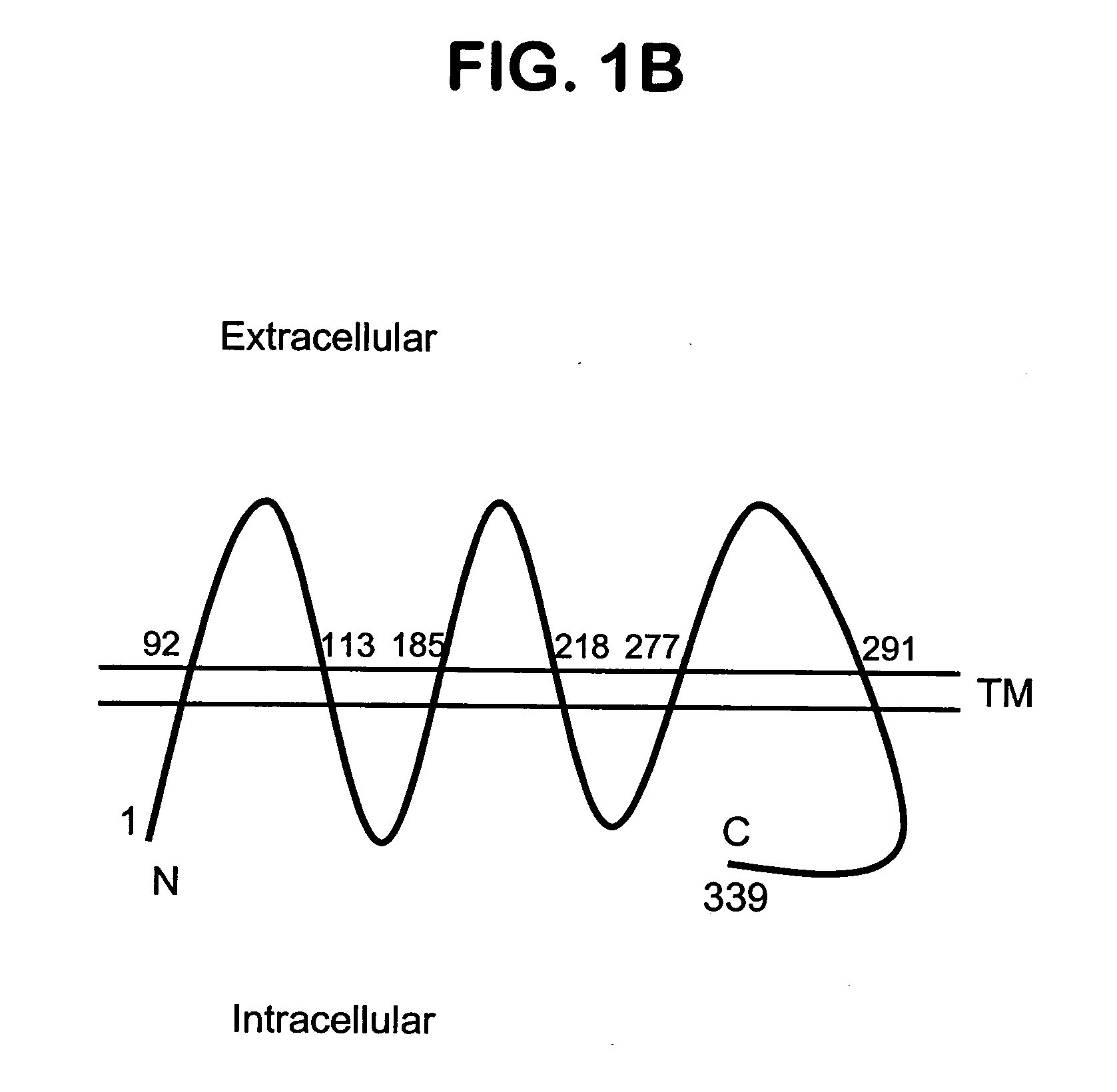
Described is a novel family of cell surface serpentine transmembrane antigens. Two of the proteins in this family are exclusively or predominantly expressed in the prostate, as well as in prostate cancer, and thus members of this family have been termed “STRAP” (Serpentine TRansmembrane Antigens of the Prostate). Four particular human STRAPs are described and characterized herein. The human STRAPs exhibit a high degree of structural conservation among them but show no significant structural homology to any known human proteins. The prototype member of the STRAP family, STRAP-1, appears to be a type IIIa membrane protein expressed predominantly in prostate cells in normal human tissues. Structurally, STRAP-1 is a 339 amino acid protein characterized by a molecular topology of six transmembrane domains and intracellular N- and C-termini, suggesting that it folds in a “serpentine” manner into three extracellular and two intracellular loops. STRAP-1 protein expression is maintained at high levels across various stages of prostate cancer. Moreover, STRAP-1 is highly over-expressed in certain other human cancers.
Owner:AGENSYS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com