Methods and nucleic acids for analyses of cellular proliferative disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
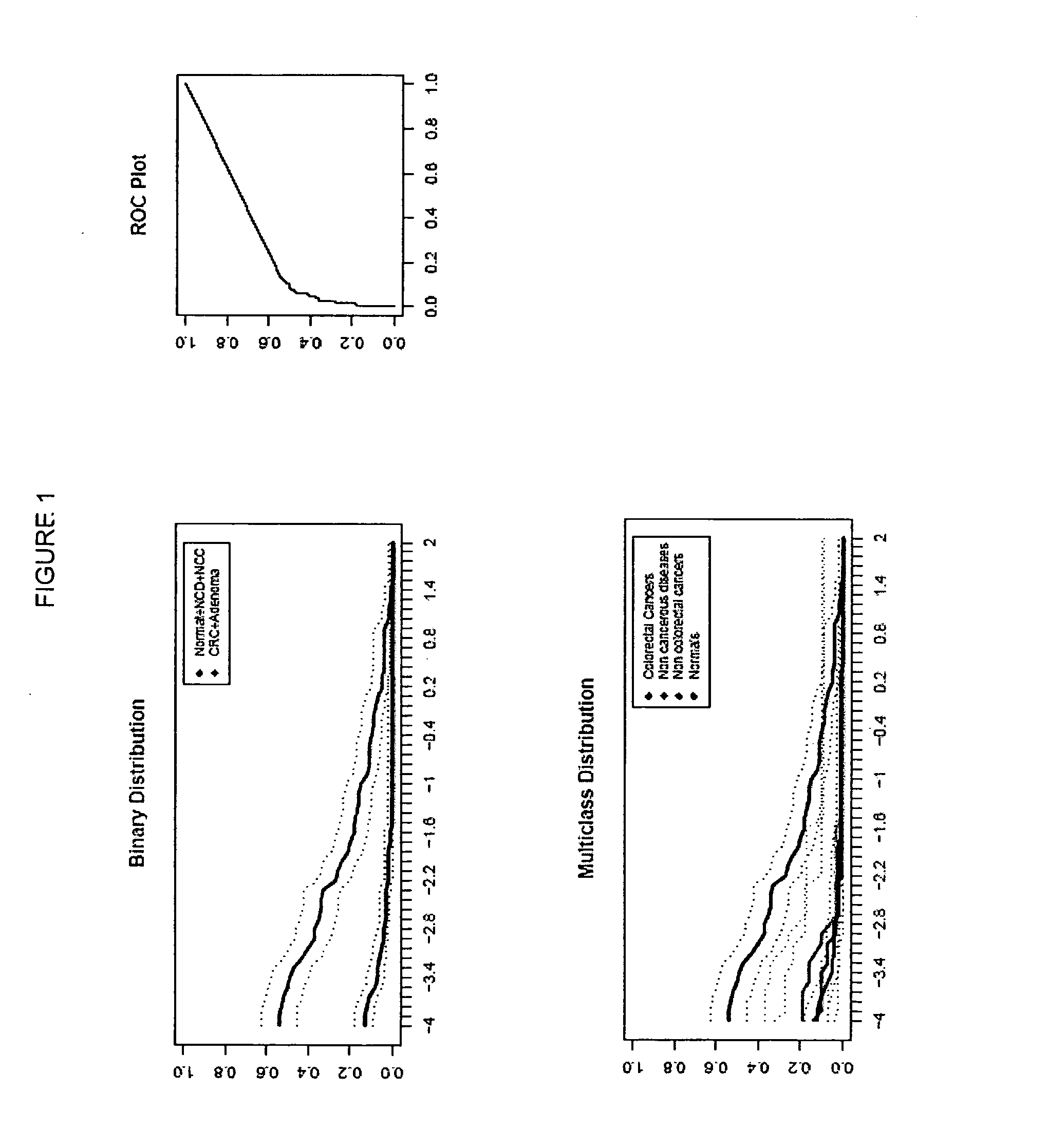
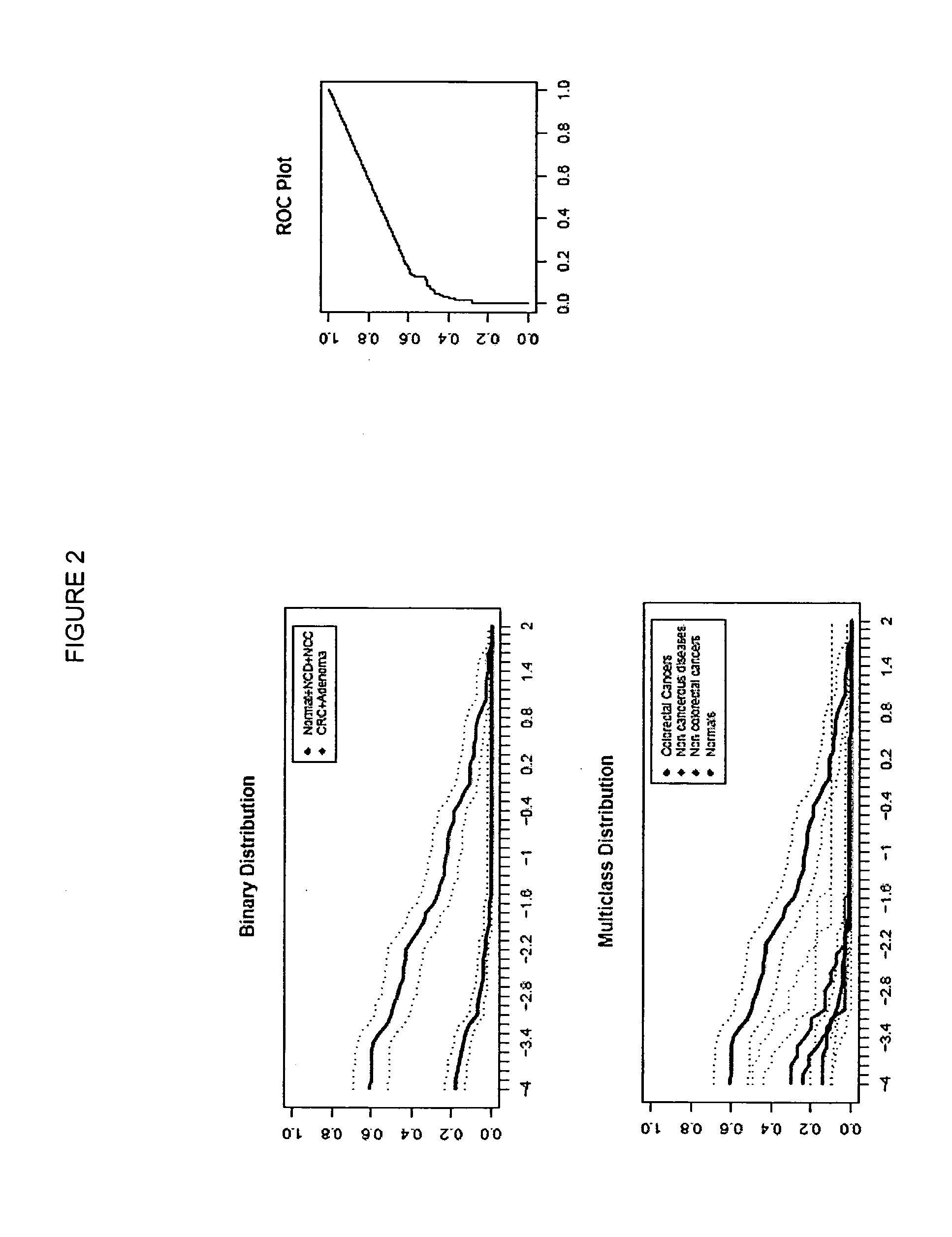
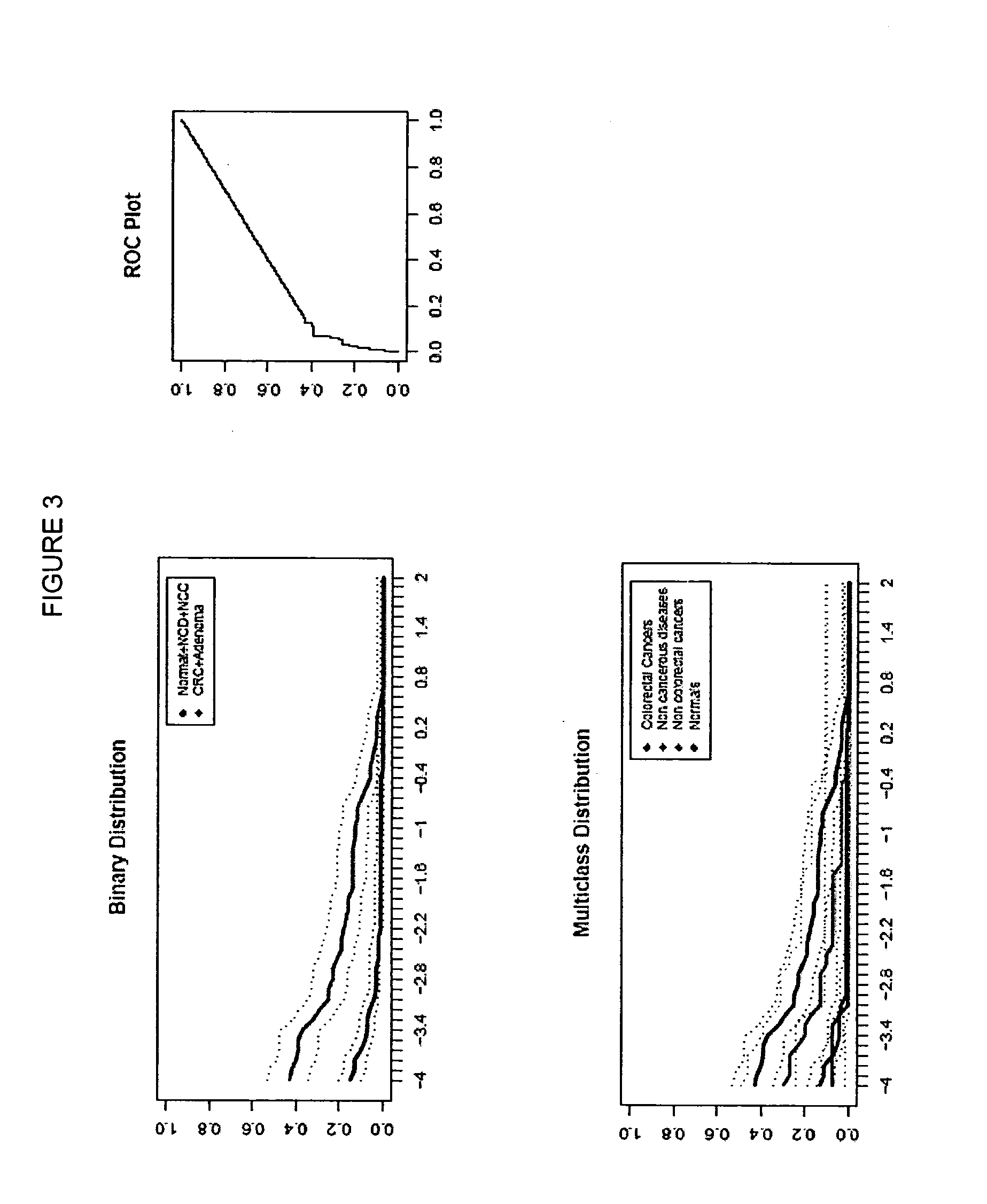
[0262]In the following investigation, the performance of selected genes according to Table 2 were analyzed as colorectal carcinoma detection markers by means of the HM (Heavymethy) assay. Target regions of each gene were bisulfite converted and amplified by means of non-MSP primers, in the presence of a blocker oligonucleotides designed to suppress amplificates that had not been methylated prior to bisulfite treatment. Amplificates were then detected by means of Lightcycler (dual) probes.
[0263]Plasma samples from the following patient classes were analyzed:
[0264]Colorectal carcinoma (131 total)
Stage 0=1
Stage I=13
Stage II=32
Stage III=27
Stage IV=8
Unclassified=50
[0265]Healthy colorectal (colonoscopy verified)=169
[0266]Non-cancerous diseases (NCD)=29
[0267]Cancers of non-colorectal origin (NCC)=31
[0268]In total 360 samples were analyzed.
DNA Extraction and Bisulfite Treatment
[0269]The DNA was isolated from the all samples by means of the Magna Pure method (Roche) according to the manufact...
example 2
Performance of Marker in Prostate Cancer Diagnosis
[0290]In the following investigation, the performance of selected markers in detecting prostate carcinoma was determined by means of the HM (HeavyMethyl) assay. Target regions of each gene were bisulfite converted and amplified by means of non-MSP primers, in the presence of a blocker oligonucleotide designed to suppress amplificates that had not been methylated prior to bisulfite treatment. Amplificates were then detected by means of Lightcycler (dual) probes and the level of methylation was determined by reference to control assays.
Samples
[0291]For this experiment, we collected matched plasma and urine from a total of 191 men, including 91 males with biopsy-confirmed prostate cancer, 51 males with no cancer detected by biopsy (subsequently diagnosed with BPH), and 50 young healthy males. In all analyses, the positive class is comprised of the Prostate cancer samples.
[0292]In designing the Present clinical study, the primary difficu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com