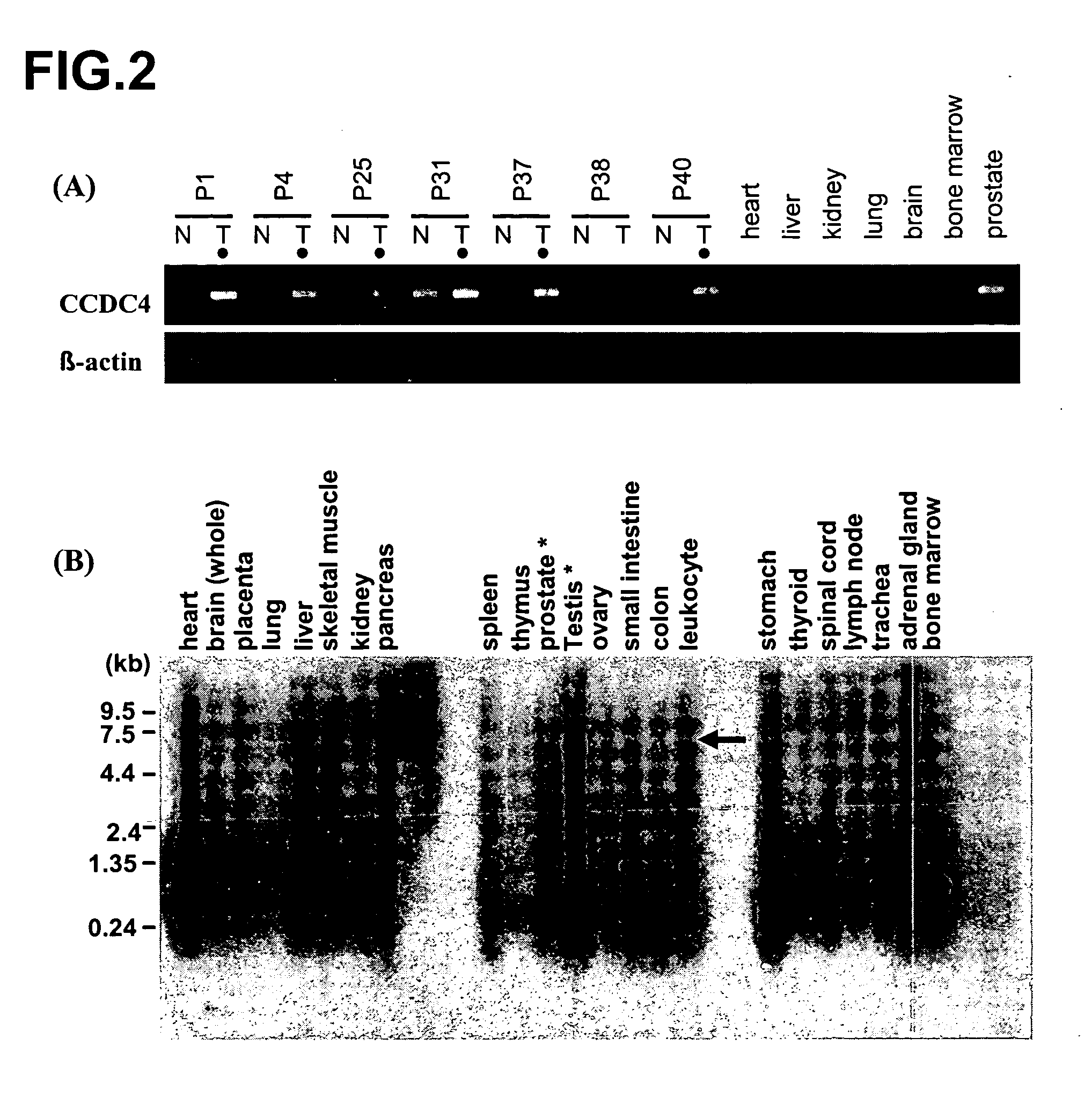
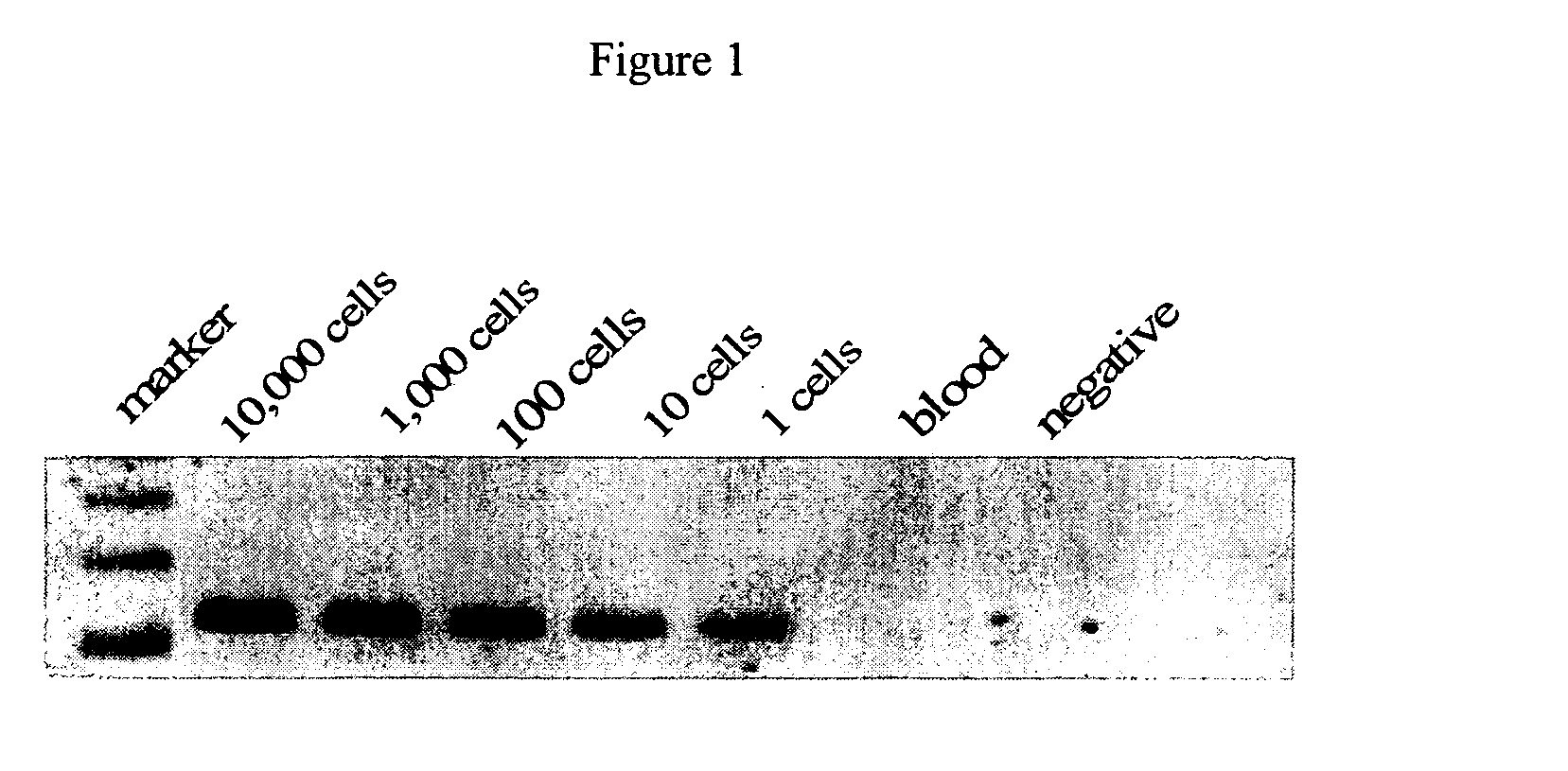
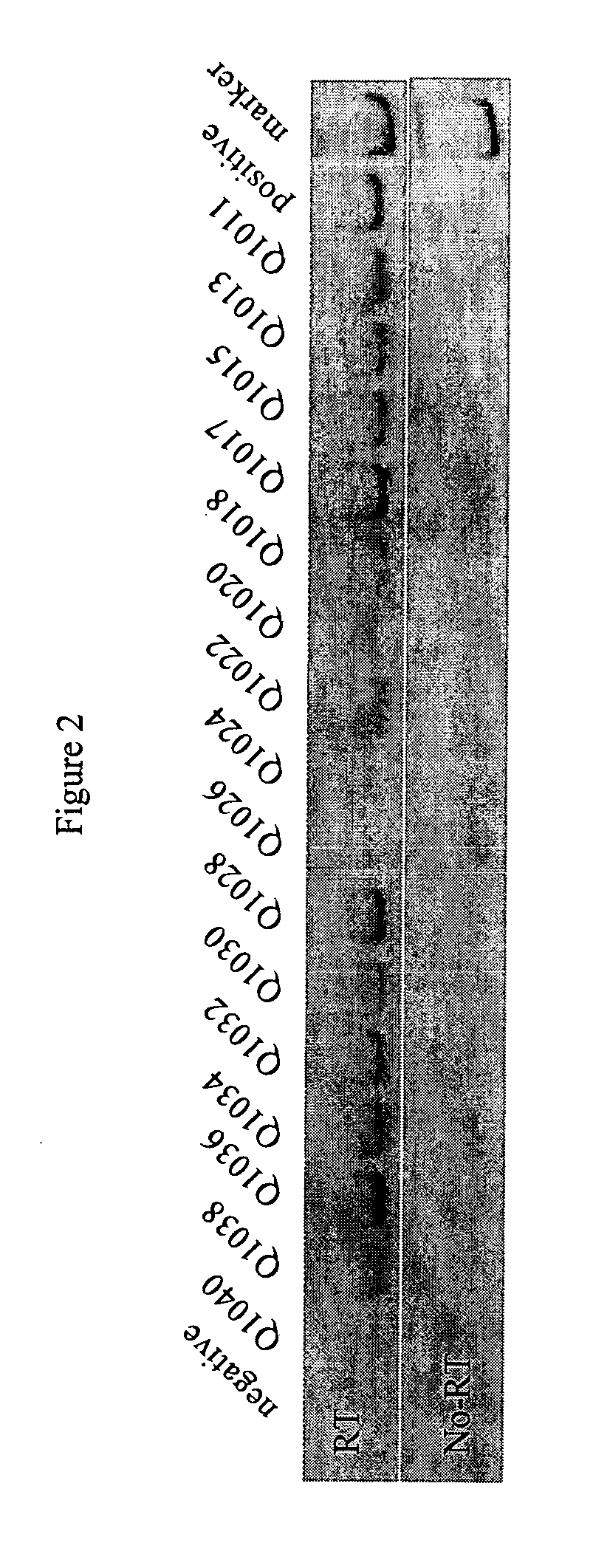
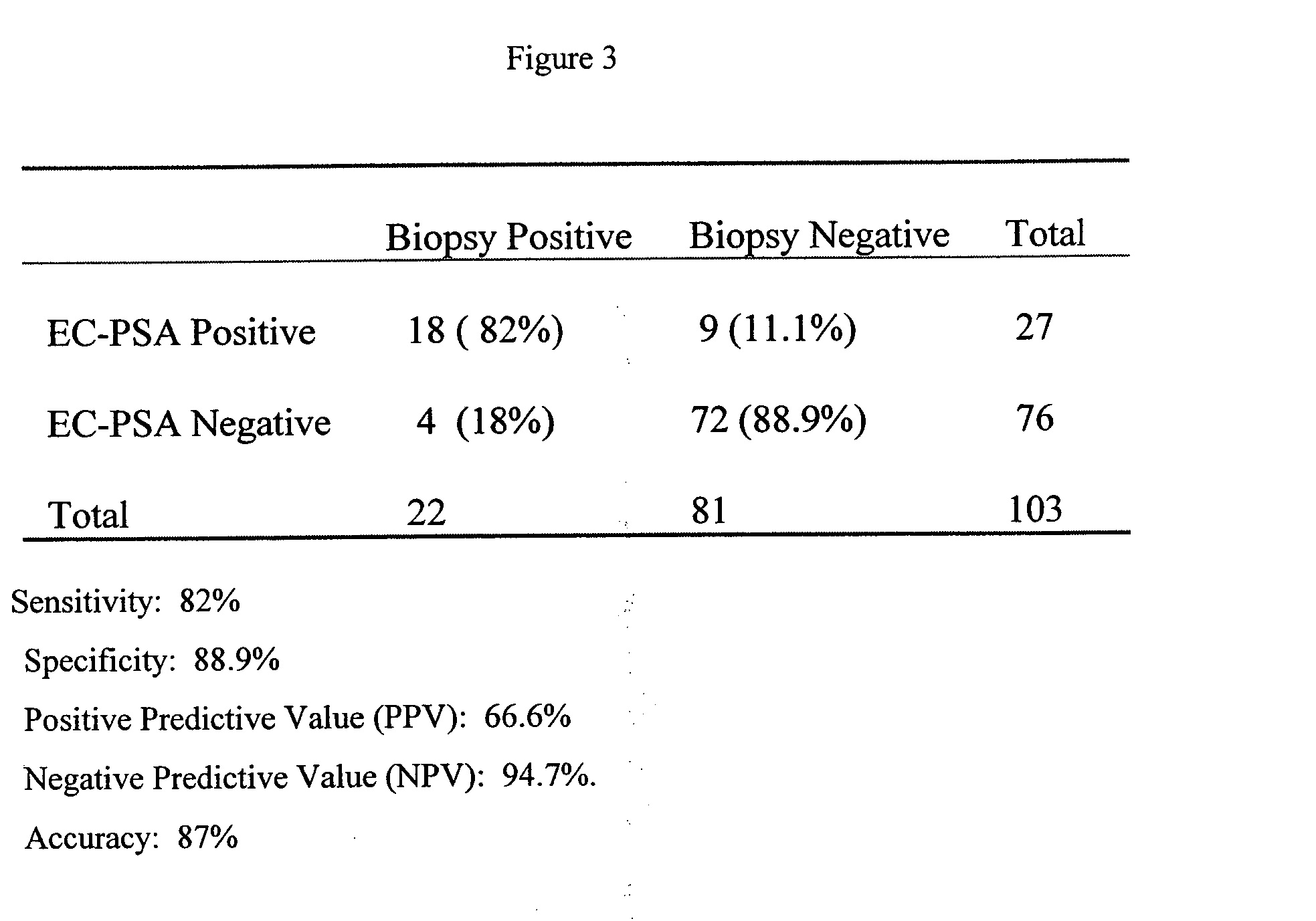
Patents
Literature
62 results about "Prostatic epithelium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
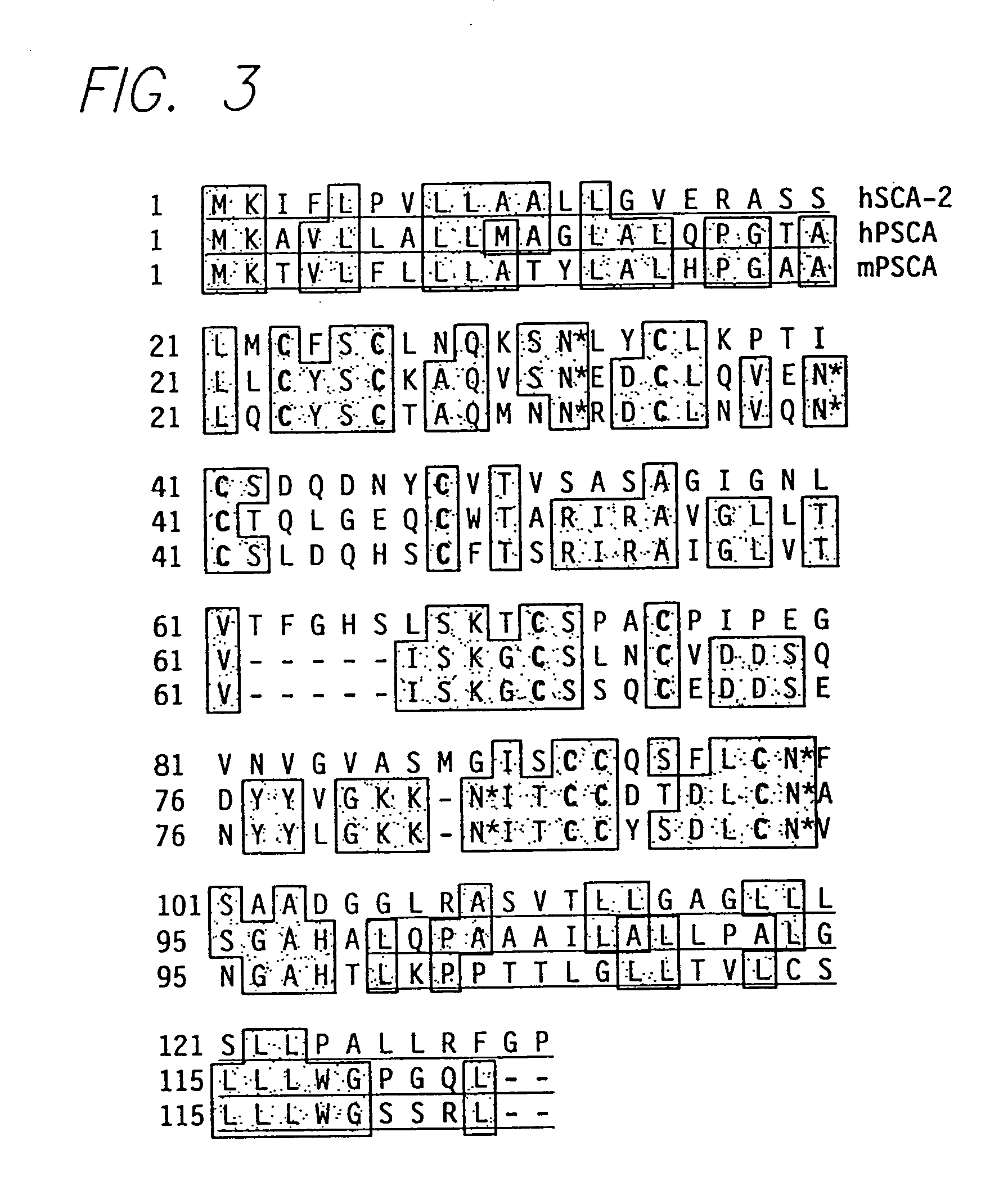
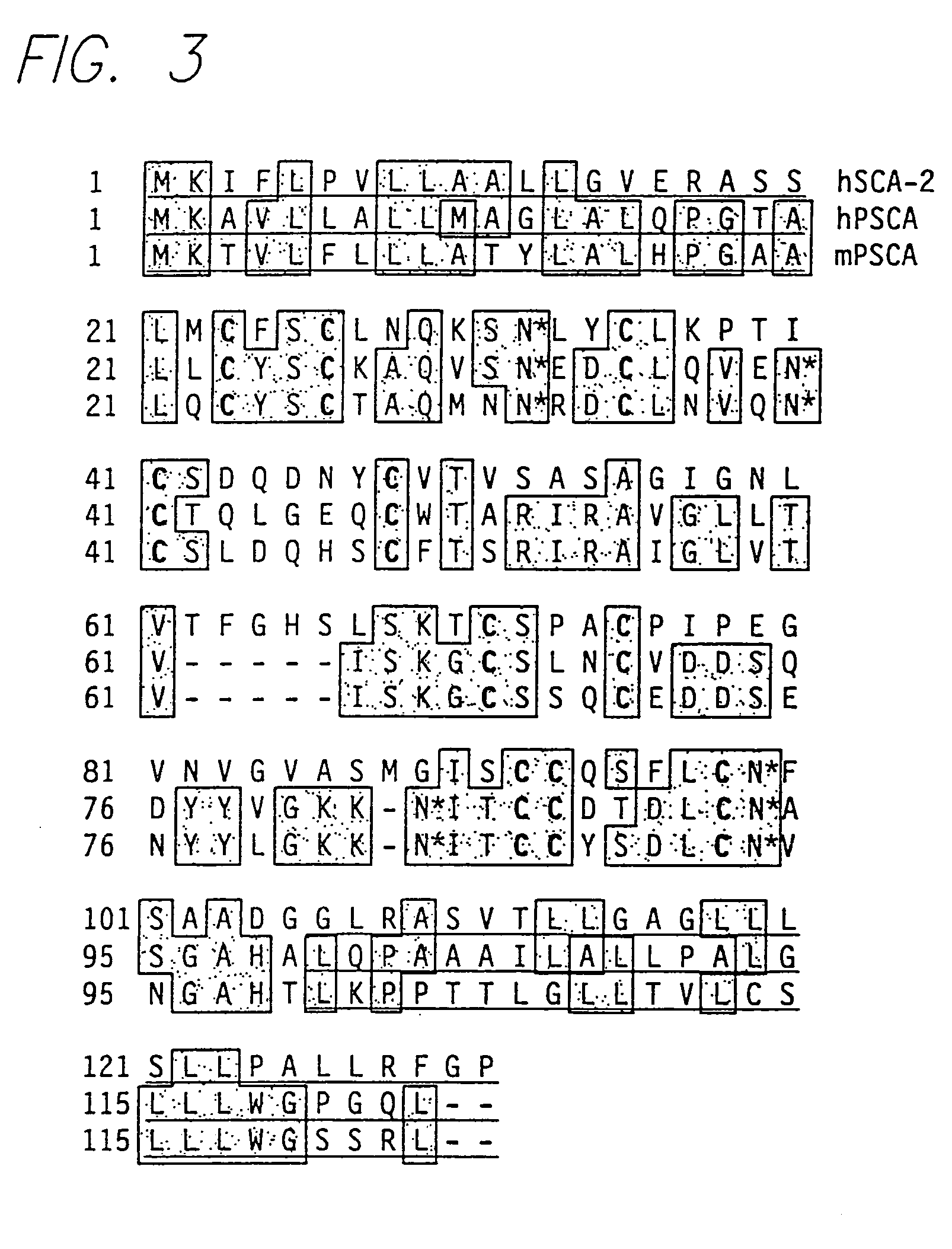
PSCA antibodies and hybridomas producing them
InactiveUS20050026229A1Promote immune-mediated destructionHigh expressionOrganic active ingredientsTumor rejection antigen precursorsAntigenProstatic epithelium
The invention provides a novel prostate cell-surface antigen, designated Prostate Stem Cell Antigen (PSCA), which is widely over-expressed across all stages of prostate cancer, including high grade prostatic intraepithelial neoplasia (PIN), androgen-dependent and androgen-independent prostate tumors.
Owner:RGT UNIV OF CALIFORNIA
Treatment and diagnosis of prostate cancer
InactiveUS7666425B1Effective diagnosisEffective treatmentPeptide/protein ingredientsMicrobiological testing/measurementAntigenProstatic epithelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
Treatment and diagnosis of cancer
InactiveUS7163680B2Effective diagnosisEffective treatmentBiological material analysisNanomedicineAntigenVascular endothelium
Owner:CORNELL RES FOUNDATION INC
Treatment and diagnosis of prostate cancer
InactiveUS7112412B1Effective diagnosisEffective treatmentPeptide/protein ingredientsMicrobiological testing/measurementAntigenProstatic epithelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
Genes and polypeptides relating to prostate cancers
InactiveUS20050259483A1Reduce the overall heightTumor rejection antigen precursorsTumor specific antigensProstatic epitheliumGene
Objective methods for detecting and diagnosing prostate cancer (PRC) or prostatic intraepithelial neoplasia (PIN) are described herein. In one embodiment, the diagnostic method involves the determining an expression level of PRC-associated gene that discriminate between PRC or PIN and nomal cell. The present invention further provides methods of screening for therapeutic agents useful in the treatment of either or both of PRC and PIN, methods of treating either or both of PRC and PIN and method of vaccinating a subject against either or both of PRC and PIN.
Owner:ONCOTHERAPY SCI INC
Enhanced diagnostic potential of prostate-specific antigen expressing cells
The present invention is directed to sensitive and specific methods and kits for the detection of prostate cancer in a patient. The invention uses RT-PCR to detect the expression or change in expression of PSA in epithelial cells enriched from whole blood. The methods and kits on the invention represent a a significant improvement over previous methods of CaP diagnosis. According to one embodiment, prostate epithelial cells are isolated from blood and the RNA subjected to RT-PCR. The resulting cDNA is subjected to traditional PCR amplification with primers able to distinguish between the genomic copy of the gene and the cDNA copy resulting from CaP gene expression. This method provides an assay which is more sensitive, specific and reproducible as compared to conventional methods. Results disclosed herein demonstrate a correlation between the presence of PSA-expressing prostate epithelial cells with cancer recurrence which provides a diagnostic tool for the early detection of prostate disease.
Owner:GAO CHUN L +2
PSCA: prostate stem cell antigen and uses thereof
InactiveUS20050152909A1Organic active ingredientsTumor rejection antigen precursorsAntigenProstatic epithelium
The invention provides a novel prostate cell-surface antigen, designated Prostate Stem Cell Antigen (PSCA), which is widely over-expressed across all stages of prostate cancer, including high grade prostatic intraepithelial neoplasia (PIN), androgen-dependent and androgen-independent prostate tumors.
Owner:RGT UNIV OF CALIFORNIA
Treatment of benign prostatic hyperplasia using energolytic agents
InactiveUS20060172953A1PSA level is decreasedDegradation is usually manifestedBiocideCarbohydrate active ingredientsProstatic epitheliumProstate hyperplasia
The invention provides a method for treatment or prophylaxis of benign prostatic hyperplasia by administration of an agent that interferes with energy metabolism, particularly the production of ATP and NADH / NADPH, in prostate epithelial cells.
Owner:THRESHOLD PHARM INC
Treatment and diagnosis of cancer
InactiveUS20060275212A1Effective diagnosisEffective treatmentIn-vivo radioactive preparationsBiological material analysisAntigenVascular endothelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of cancerous tissues, particularly cancerous tissues proximate to or containing vascular endothelial cells, which express an extracellular domain of prostate specific membrane antigen. The labeled biological agents can also be used to detect normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate or other cancers. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
Treatment and diagnosis of prostate cancer
InactiveUS20040105865A1Quick upgradeImprove abilitiesIn-vivo radioactive preparationsPeptide/protein ingredientsProstatic epitheliumAntigen
The present invention is directed to the use of antibodies or binding portions thereof or probes which recognize an antigen of normal, benign, hyperplastic, and cancerous prostate epithelial cells or portions thereof. These antibodies or binding portions thereof or probes can be labeled and used for detection of such cells. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed is a hybridoma cell line which produces a monoclonal antibody recognizing antigens of normal, benign, hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
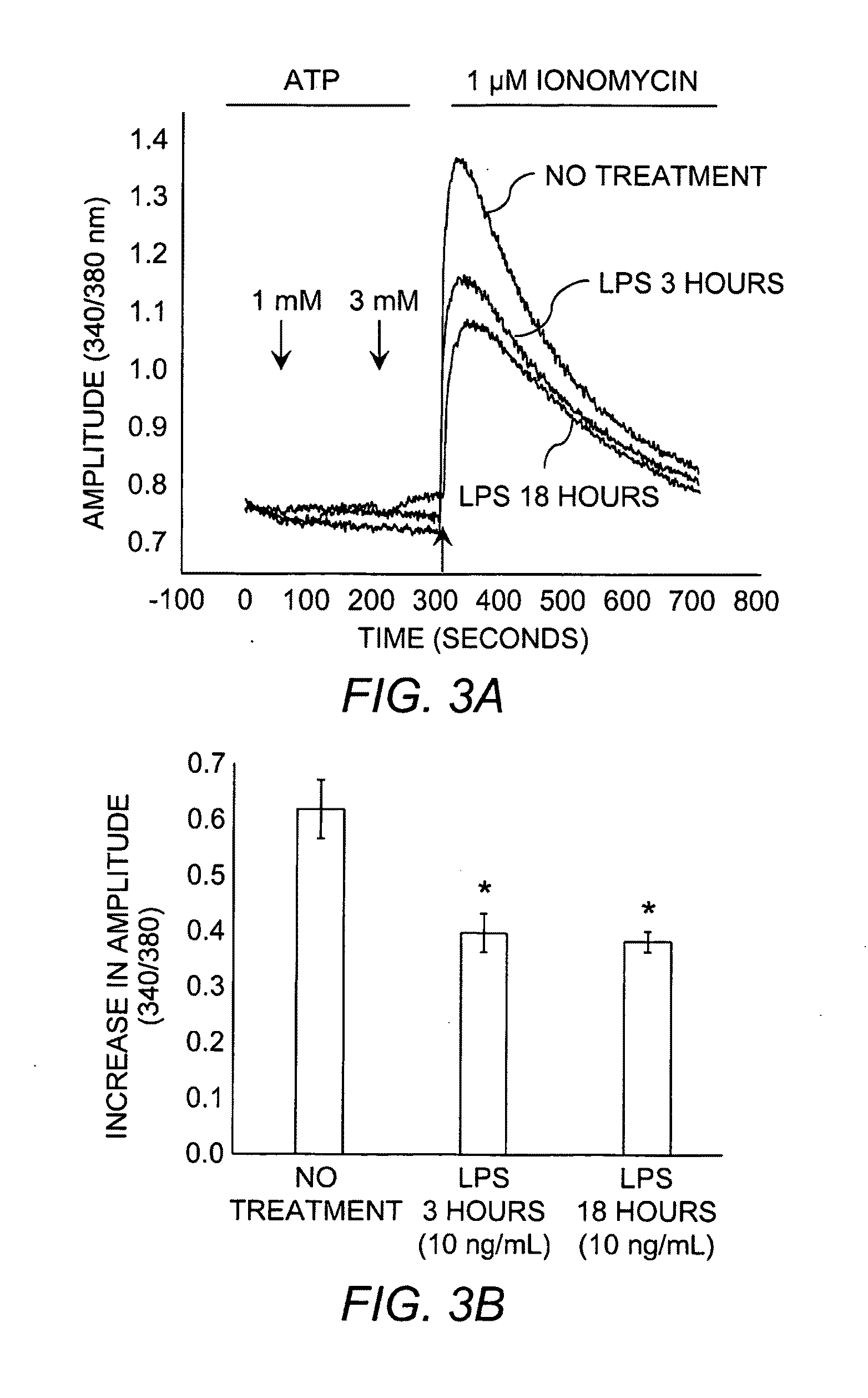
Compositions and Methods for Modulating Store-Operated Calcium Entry
Compositions and methods for modulating activity of store-operated calcium entry in cells or tissues are provided. The compositions comprise the P311 protein, fused to a cell-penetrating peptide and formulated for delivery to tissues and cells. This protein has been shown to increase the levels of store-operated calcium entry (SOCE) in gingival cells, skeletal muscle cells, and prostate epithelial cells. Also provided are methods for preventing and treating diseases that involve administration of the P311 fusion protein, as well as methods for increasing levels of SOCE in cells.
Owner:RUTGERS THE STATE UNIV
Diagnostic and prognostic methods for prostate cancers
InactiveUS20050112706A1Useful for ClassificationBiological testingTesting metalsOrganismHuman prostate
The invention includes prognostic and diagnostic methods and compositions for characterizing disease states, biopsy samples, biological samples, cells, and tissues. In various embodiments of the invention, a bioassay may be used to analyze the activity of the androgen receptor (AR) in human prostate epithelial (HPE) cells derived from patient samples. The activity of the AR may be used to characterize the sample in regard to AR activity. Characterization of a sample may be useful in classify the sample and / or in determining a treatment regiment. In other embodiments, a sample may be compared to other known samples to identify other distinguishing characteristics, such as disease markers, and / or determine a treatment regiment based on the comparison of samples.
Owner:VANDERBILT UNIV
Compositions and methods for diagnosing, treating, and preventing prostate conditions
ActiveUS20100029560A1Organic active ingredientsPeptide/protein ingredientsDiseaseProstatic epithelium
Disclosed are compositions and methods for diagnosing, preventing, and treating prostate cancer and prostate intraepithelial neoplasia (PIN).
Owner:MUSC FOUND FOR RES DEV
Method of identifying and treating invasive carcinomas
InactiveUS6864093B1Reduce intrusionSugar derivativesMicrobiological testing/measurementProstate cancer cellMda mb 231
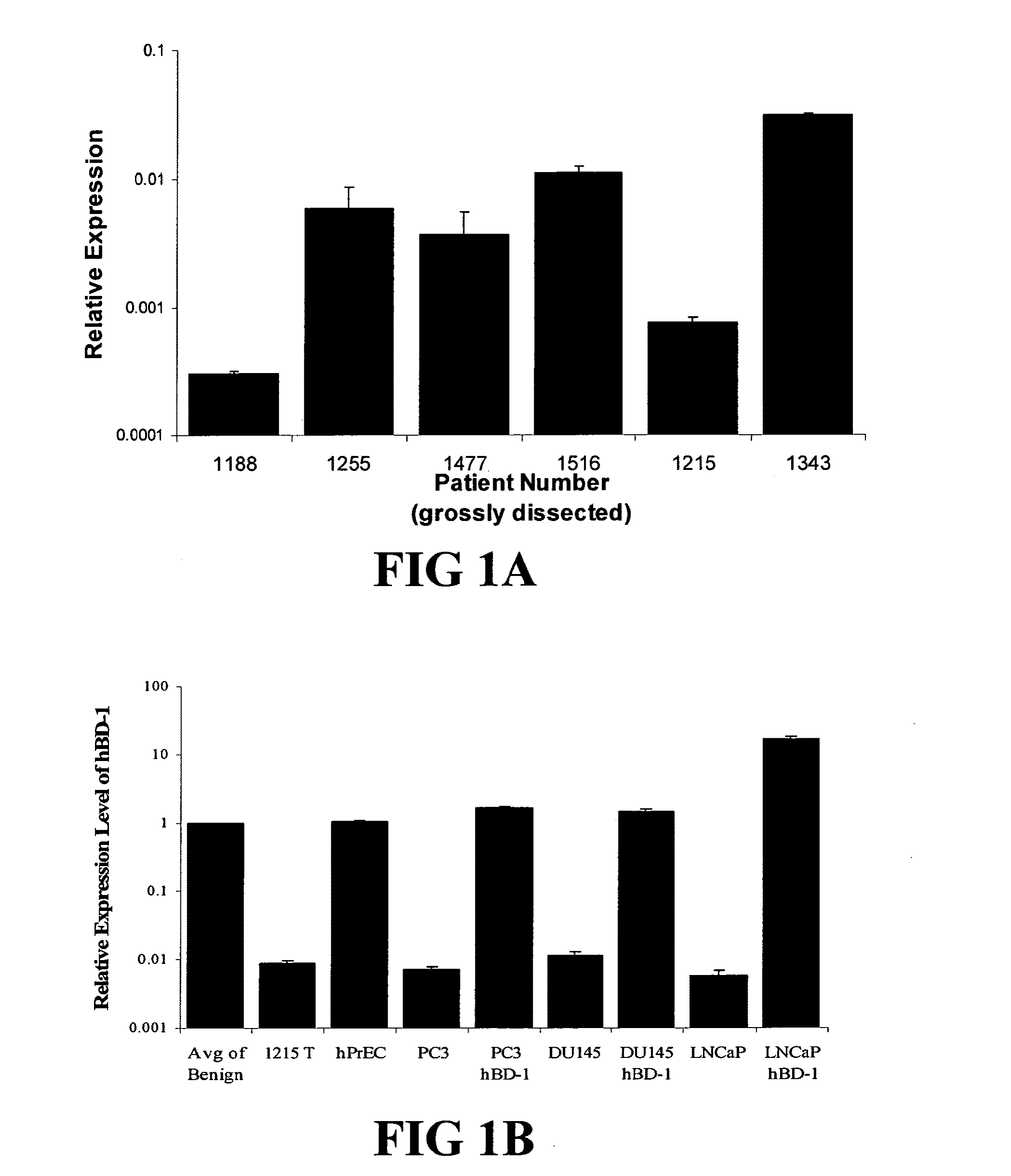
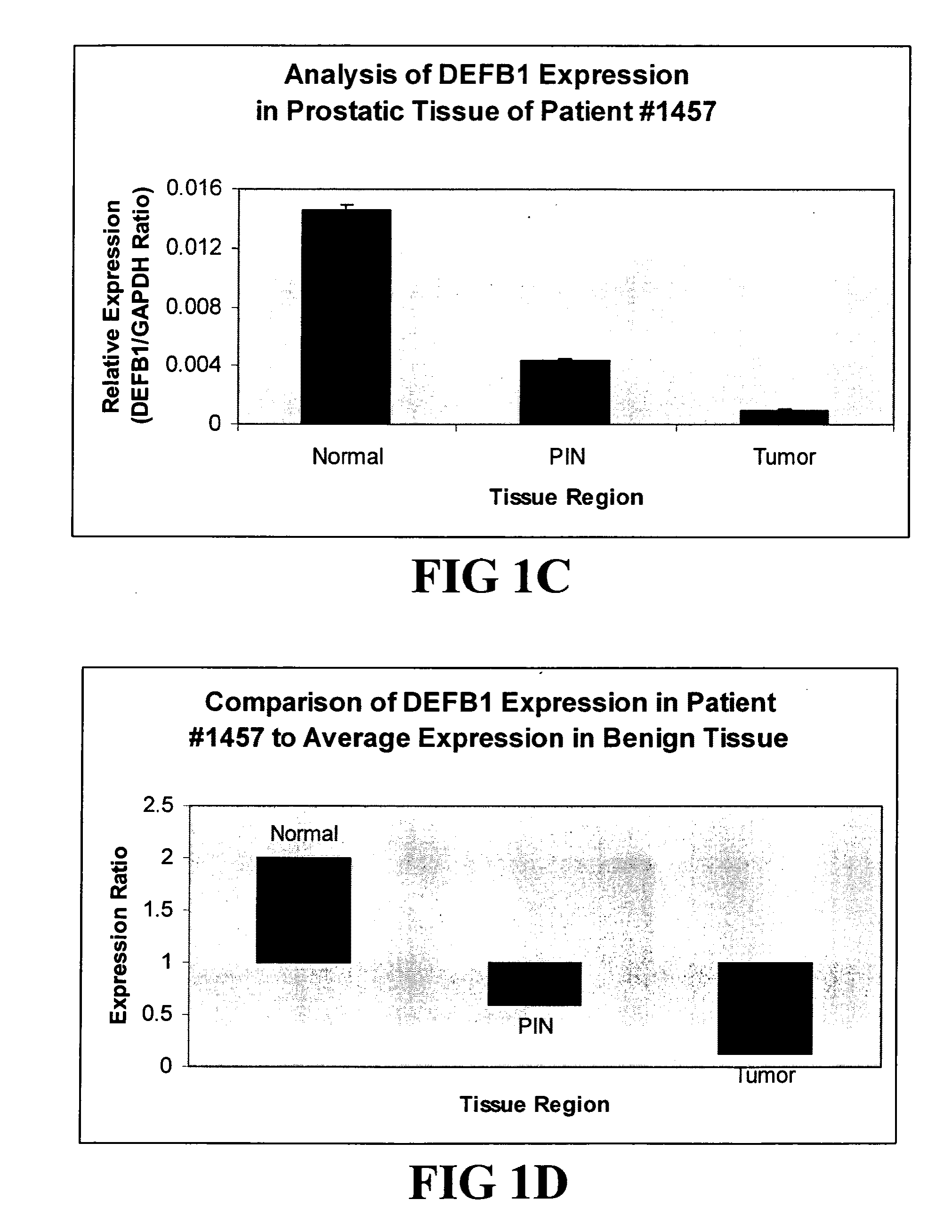
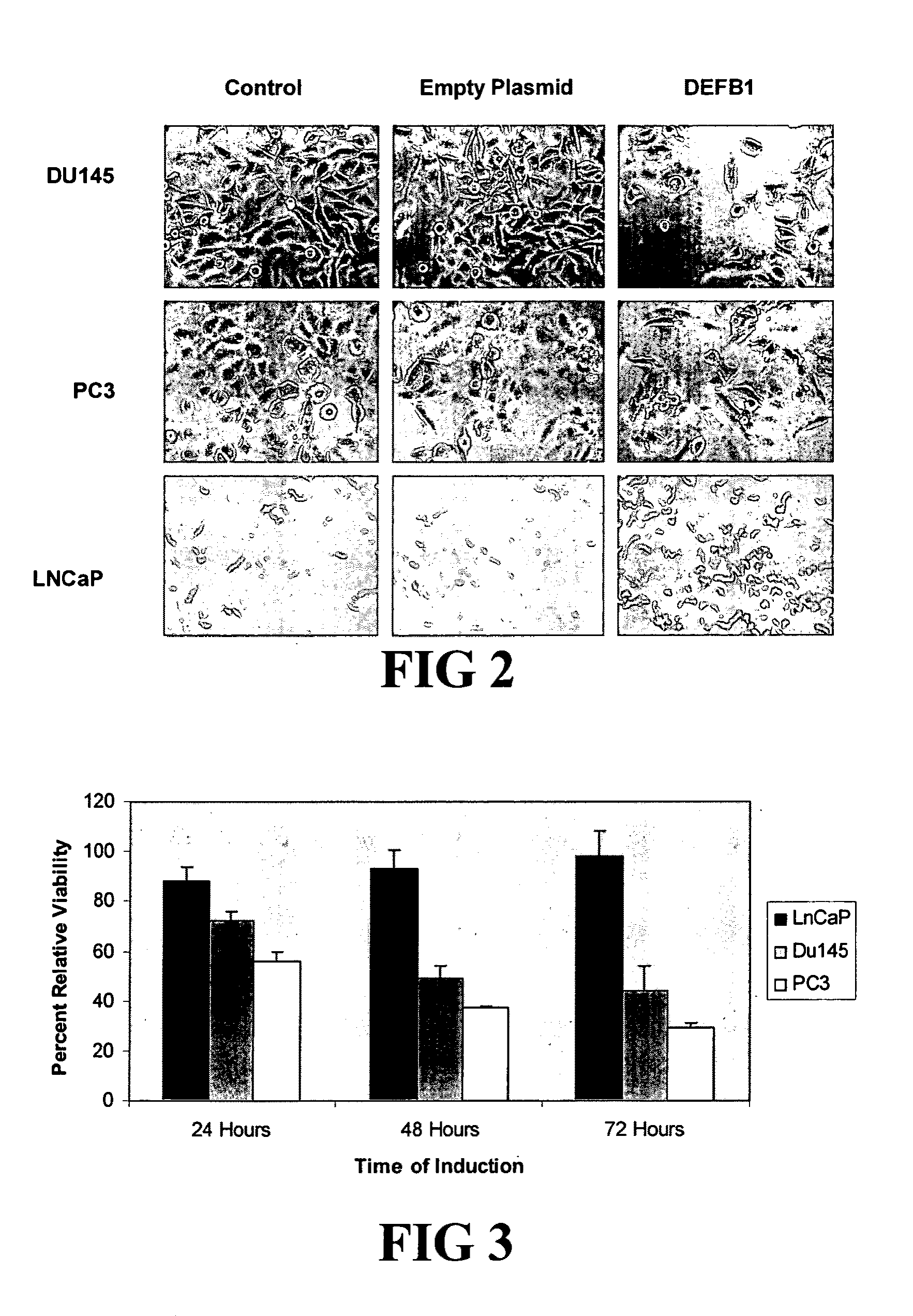
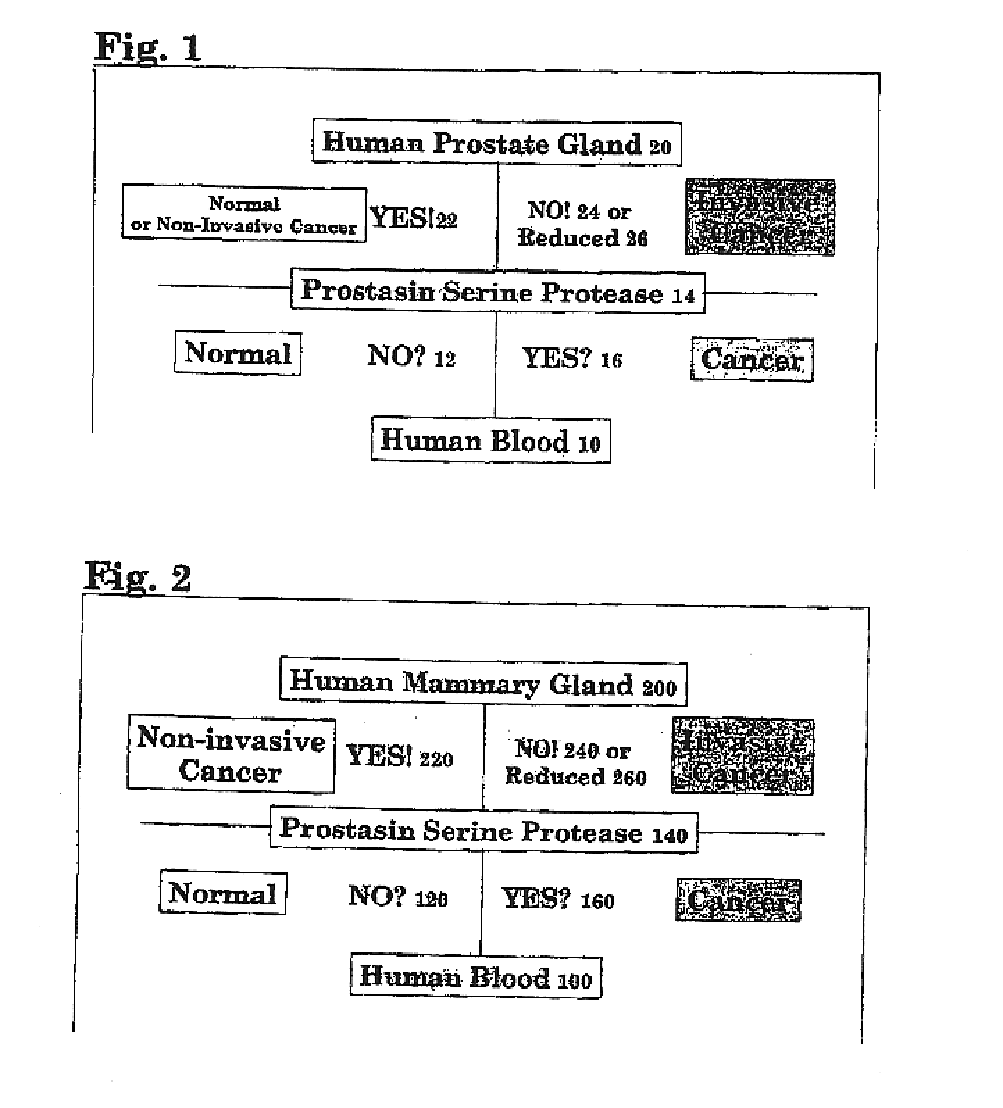
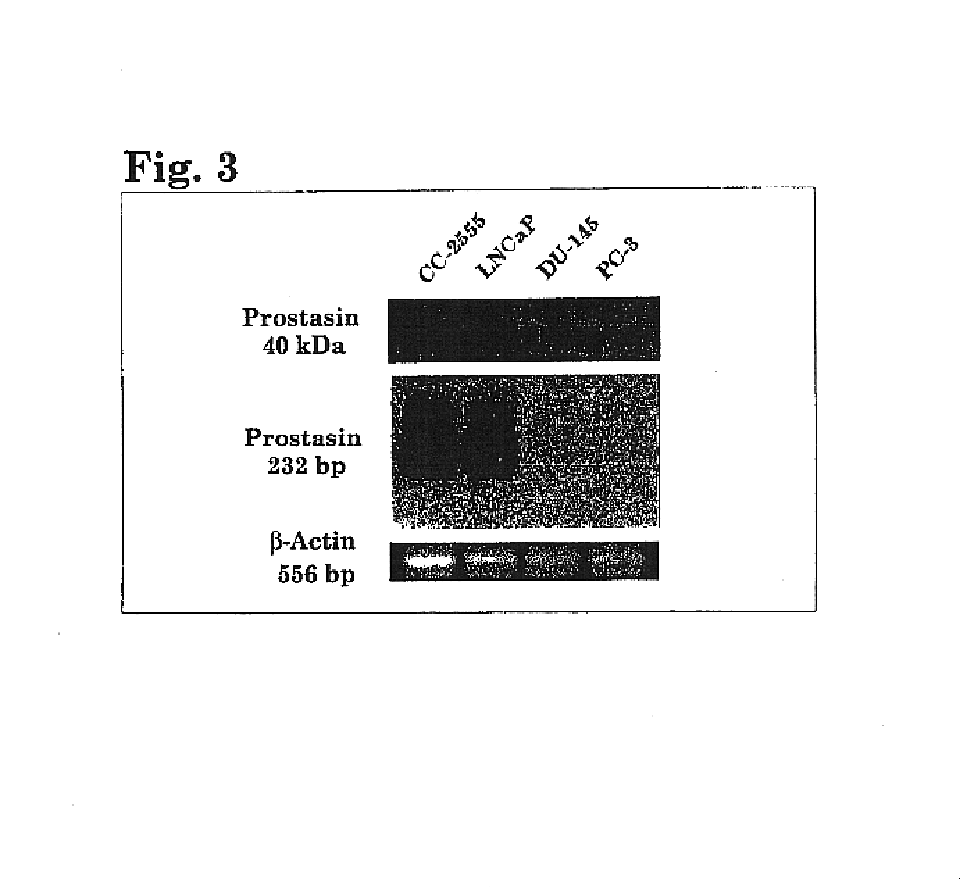
Prostasin protein has been found to be a useful marker for determination of the invasiveness of and as a means to treat human carcinomas. Using RT-PCR and western blot analyses, prostasin protein and mRNA expression were found in normal human prostate epithelial cells and the human prostate cancer cell line LNCaP, but not in the highly invasive human prostate cancer cell lines DU-145 and PC-3. Imunohistochemistry studies of human prostate cancer specimens revealed a down-regulation of prostasin in high-grade tumors. Using RT-PCR and western blot analyses, prostasin protein and mRNA expression were found in a non-invasive human breast cancer cell line, MCF-7, while invasive human breast cancer cell lines MDA-MB-231 and MDA-MB-435s were found not to express either the prostasin protein or the mRNA. A non-invasive human breast cancer cell line, MDA-MB-453, was shown to express prostasin mRNA but not prostasin protein. Transfection of DU-145 and PC-3 cells with a full-length human prostasin cDNA restored prostasin expression and reduced the in vitro invasiveness by 68% and 42%, respectively. Transfection of MDA-MB-231 and MDA-MB-435s cells with a full-length human prostasin cDNA restored prostasin expression and reduced the in vitro invasiveness by 50% for either cell line. The prostasin gene promoter region was found to be hypermethylated at specific sites in invasive cancer cells.
Owner:CENT FLORIDA UNIV OF
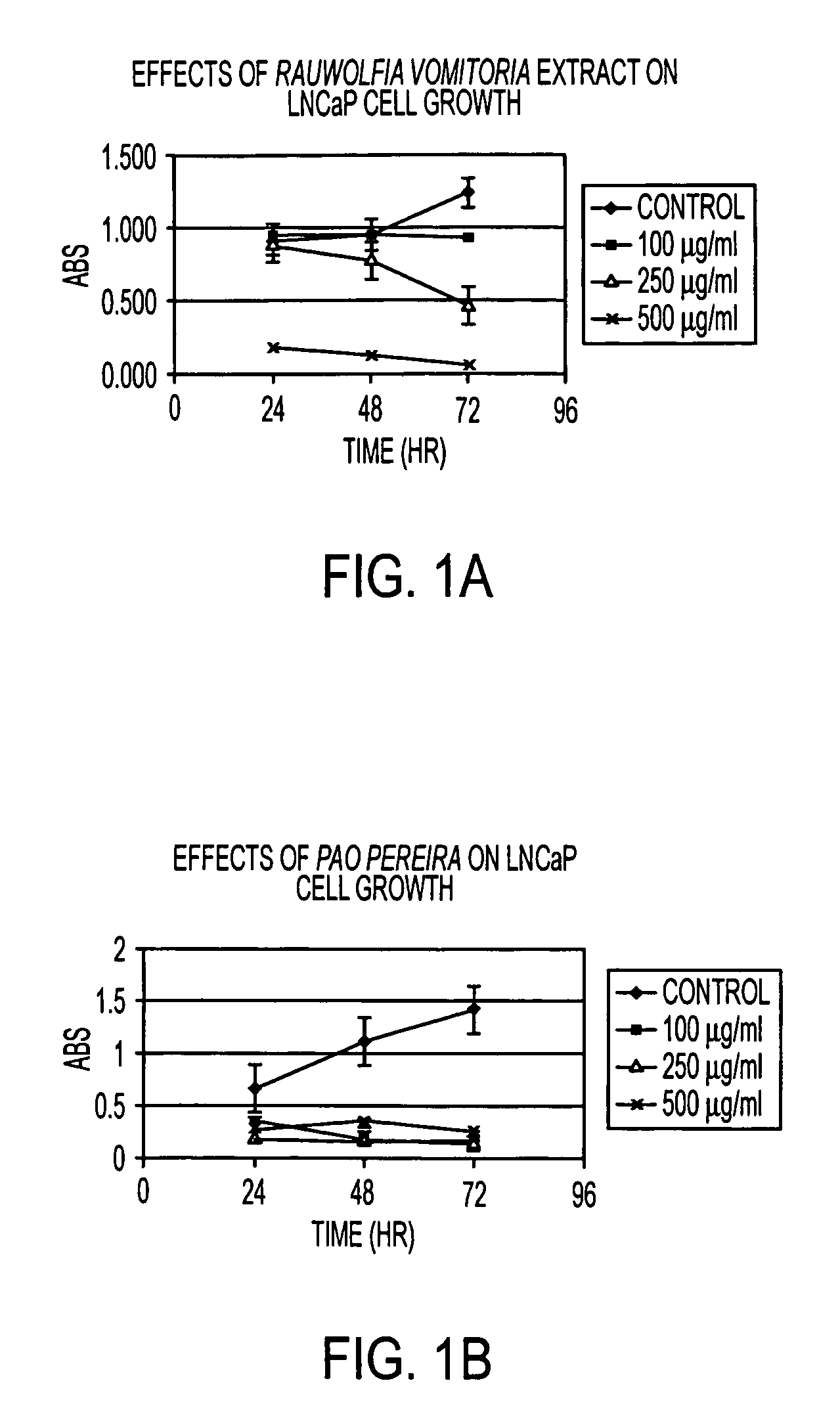
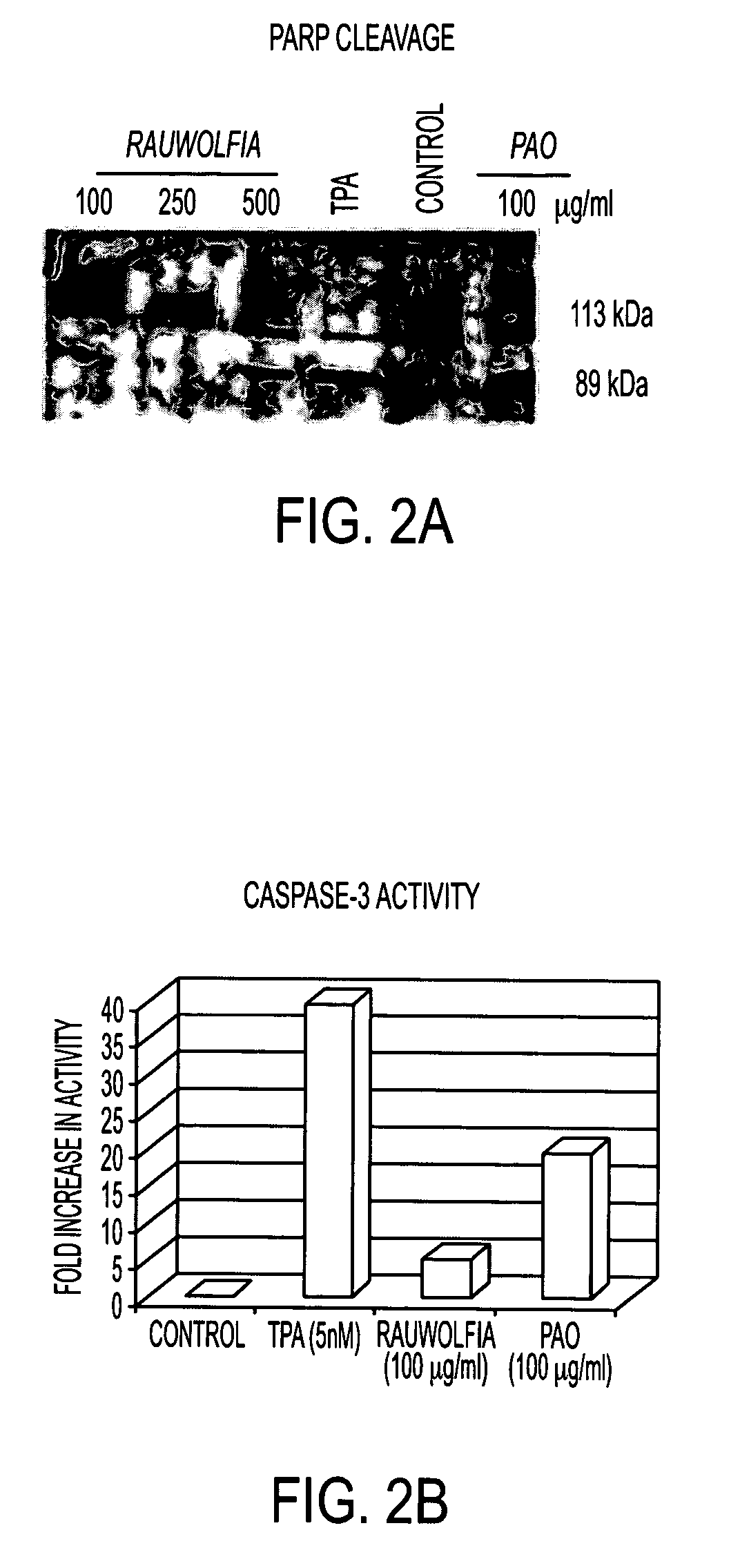
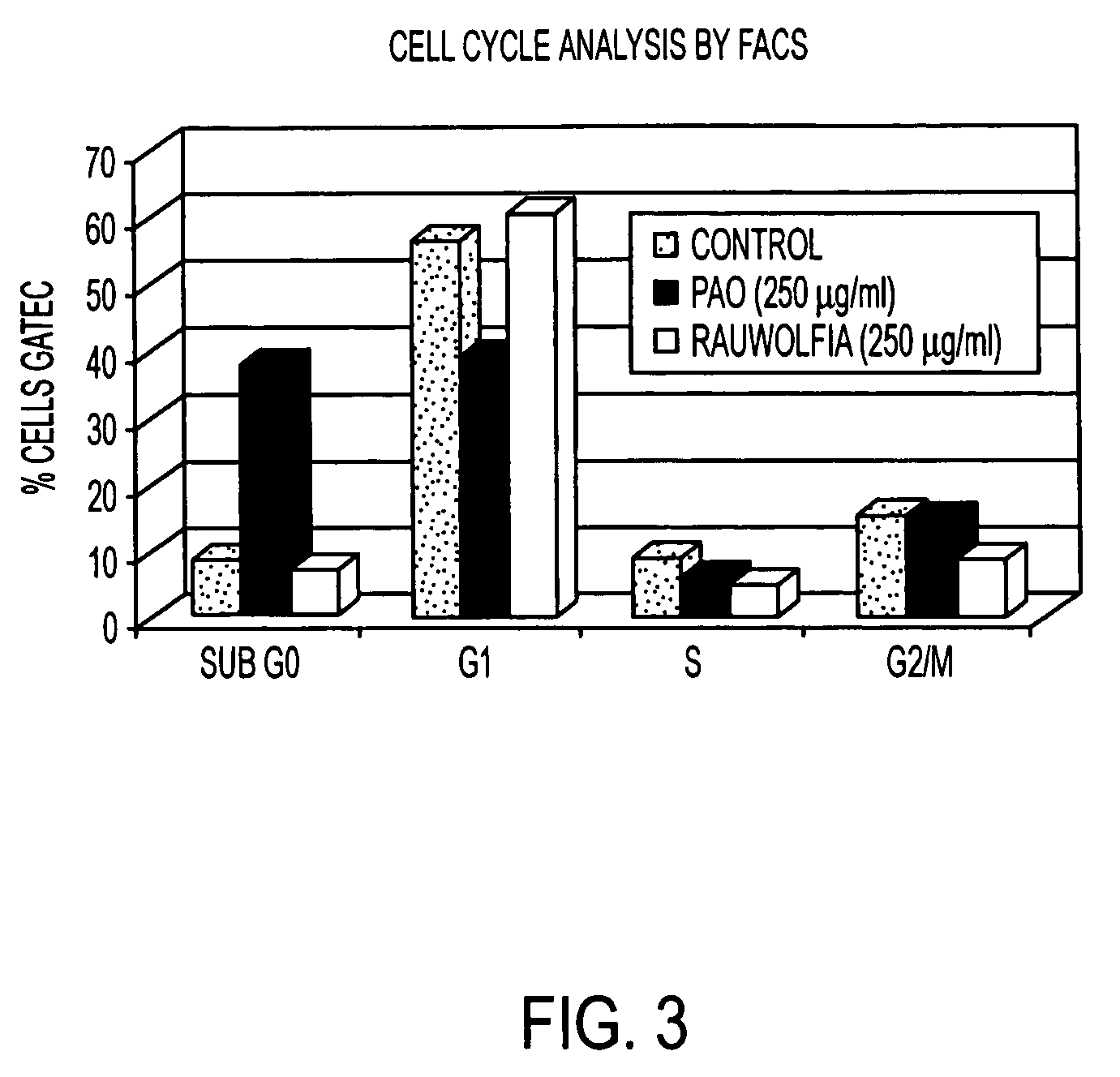
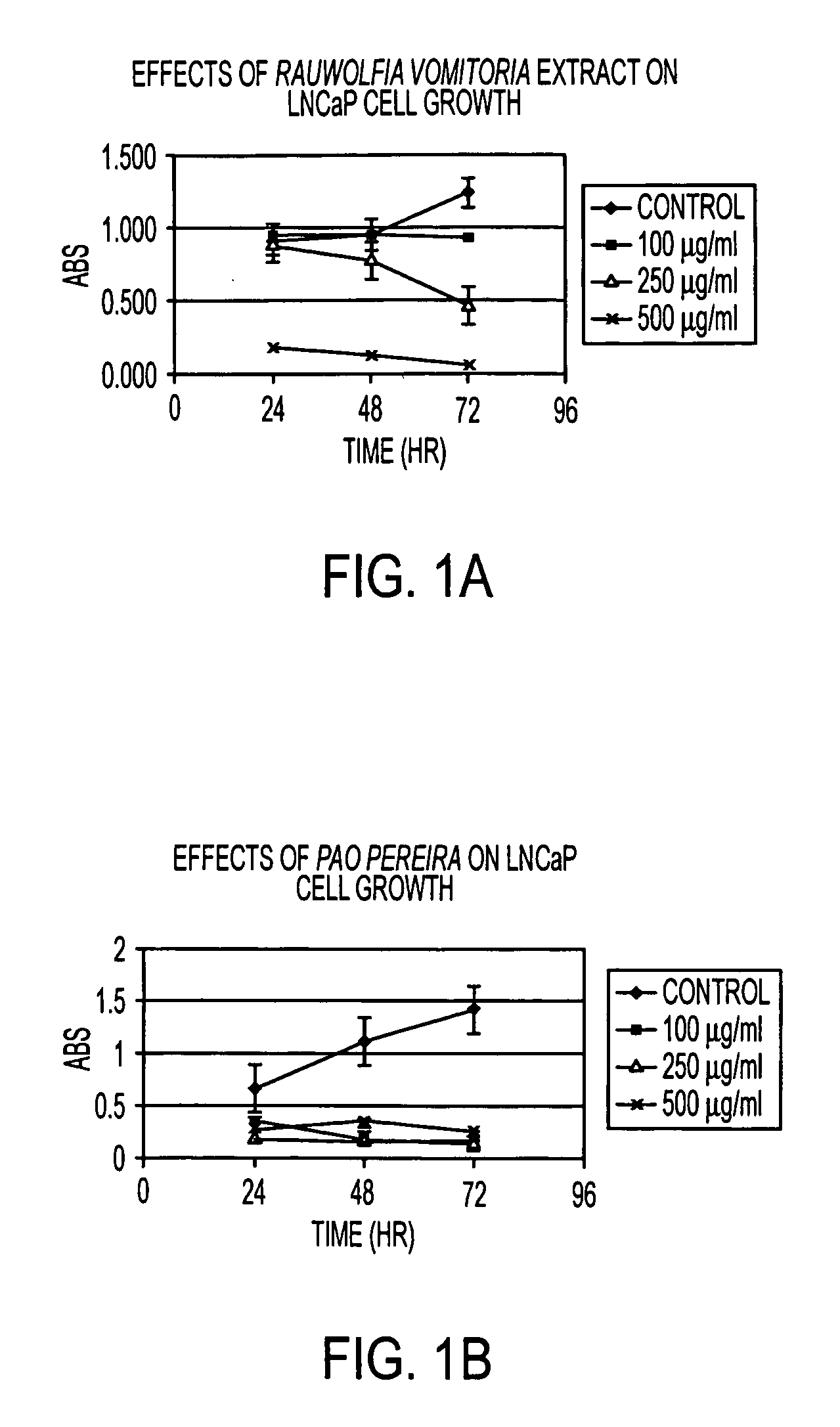
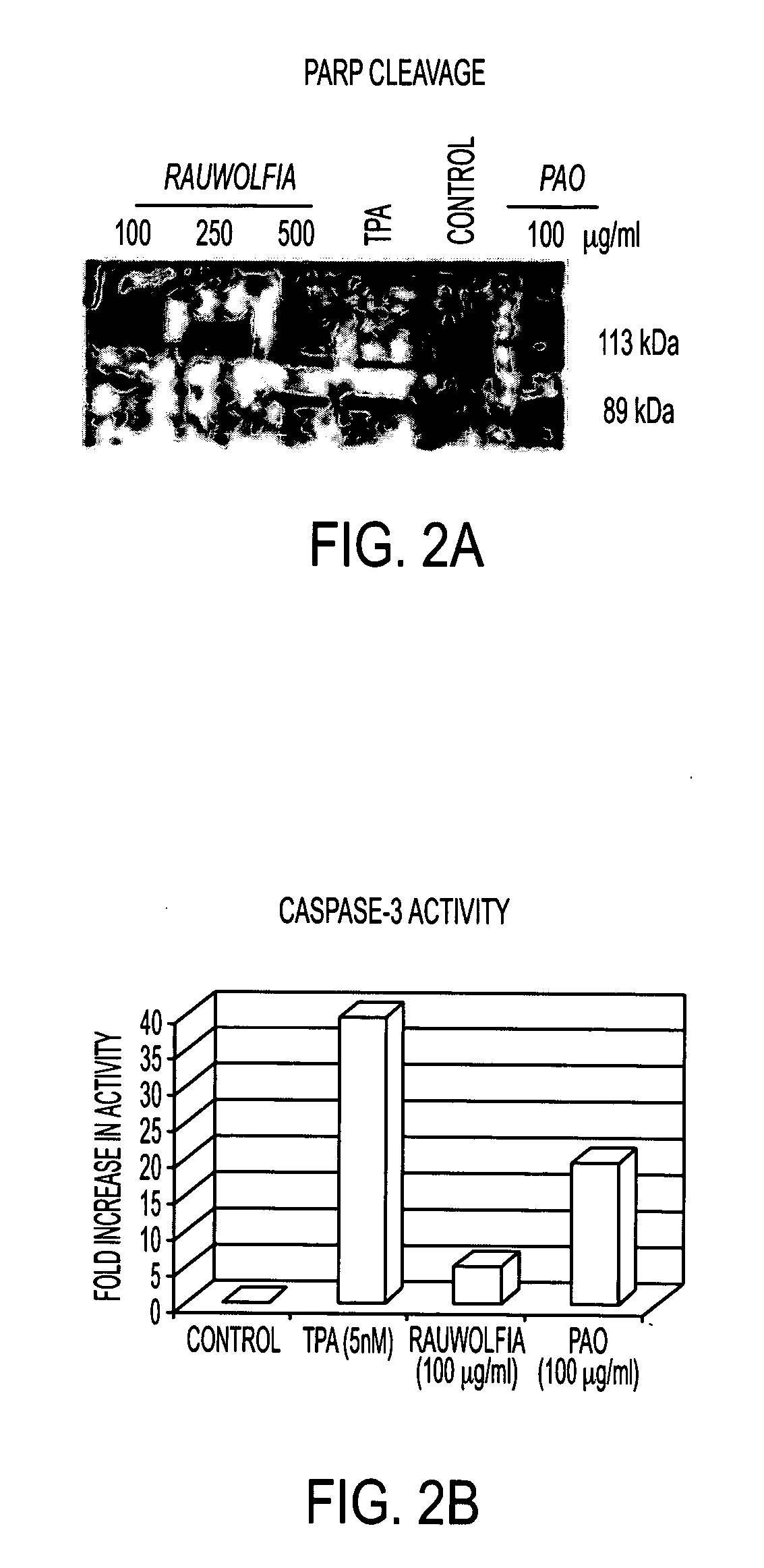
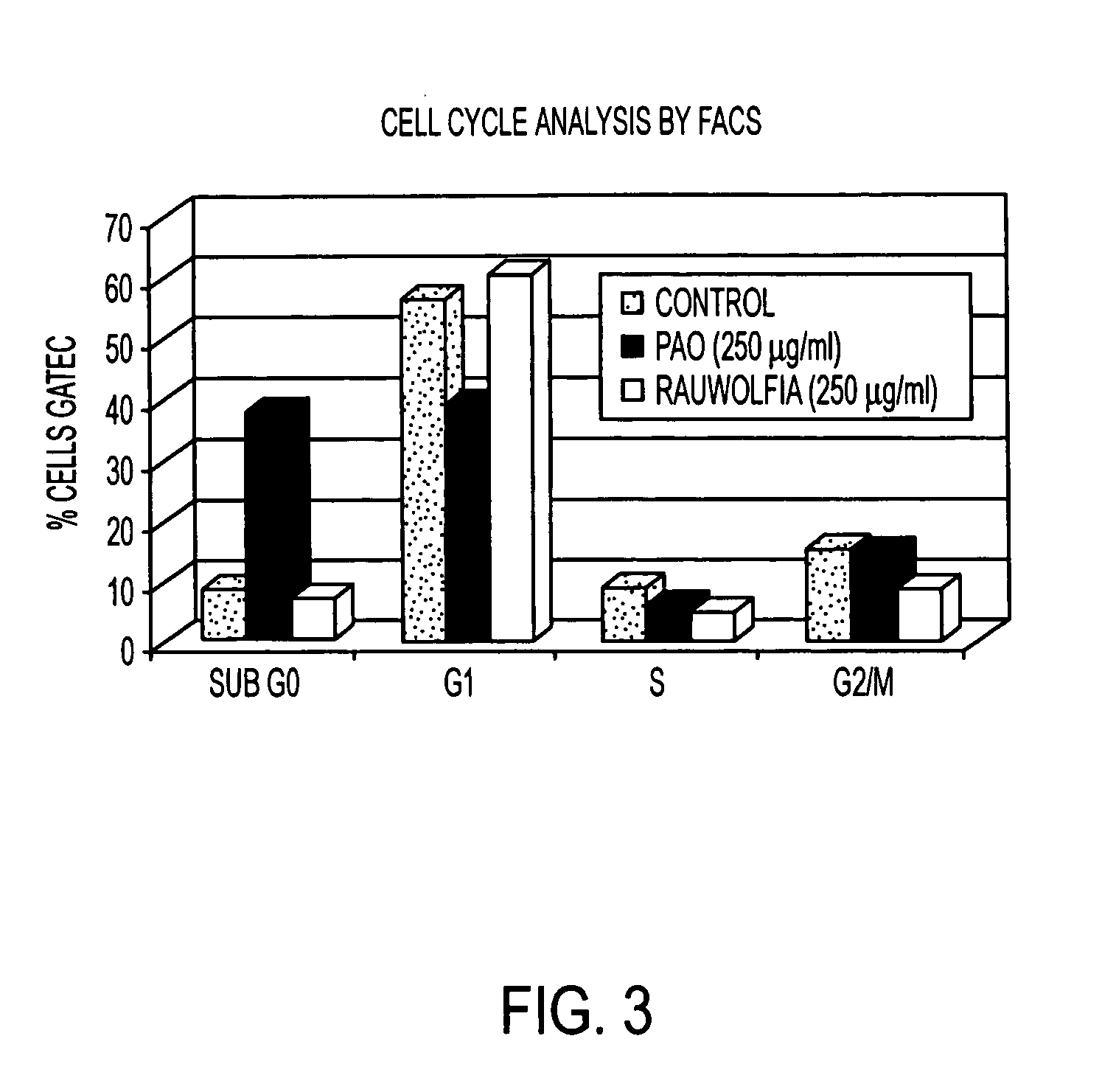
Flavopereirine and alstonine combinations in the treatment and prevention of prostate cancer
InactiveUS7341749B2Alleviating and avoiding onsetInhibit progressBiocideUnknown materialsDiseaseDouble-time
A method and composition for preventing prostate cancer and / or reducing PSA levels and / or alleviating the symptoms of BPH (Benign Prostatic Hyperplasia) or PIN (prostatic intraepithelial neoplasia) by administration of an effective amount of a mixture of flavopereirine and alstonine. A method and composition for treating low-grade prostate cancer and preventing the onset of metastatic disease and / or reducing the doubling time of PSA levels in men with positive biopsies showing relatively low Gleason scores and morphologies characteristic of non-invasive, slow-progressing prostate cancer. The flavopereirine and alstonine can be in the form of natural extracts derived from Pao Pereira and Rauwolfia Vomitoria, respectively. Alternatively, these two active compounds can be administered in purified form. The composition can be in included in a kit along with instructions for use in a treatment regimen.
Owner:MOLECULAR INT RES +1
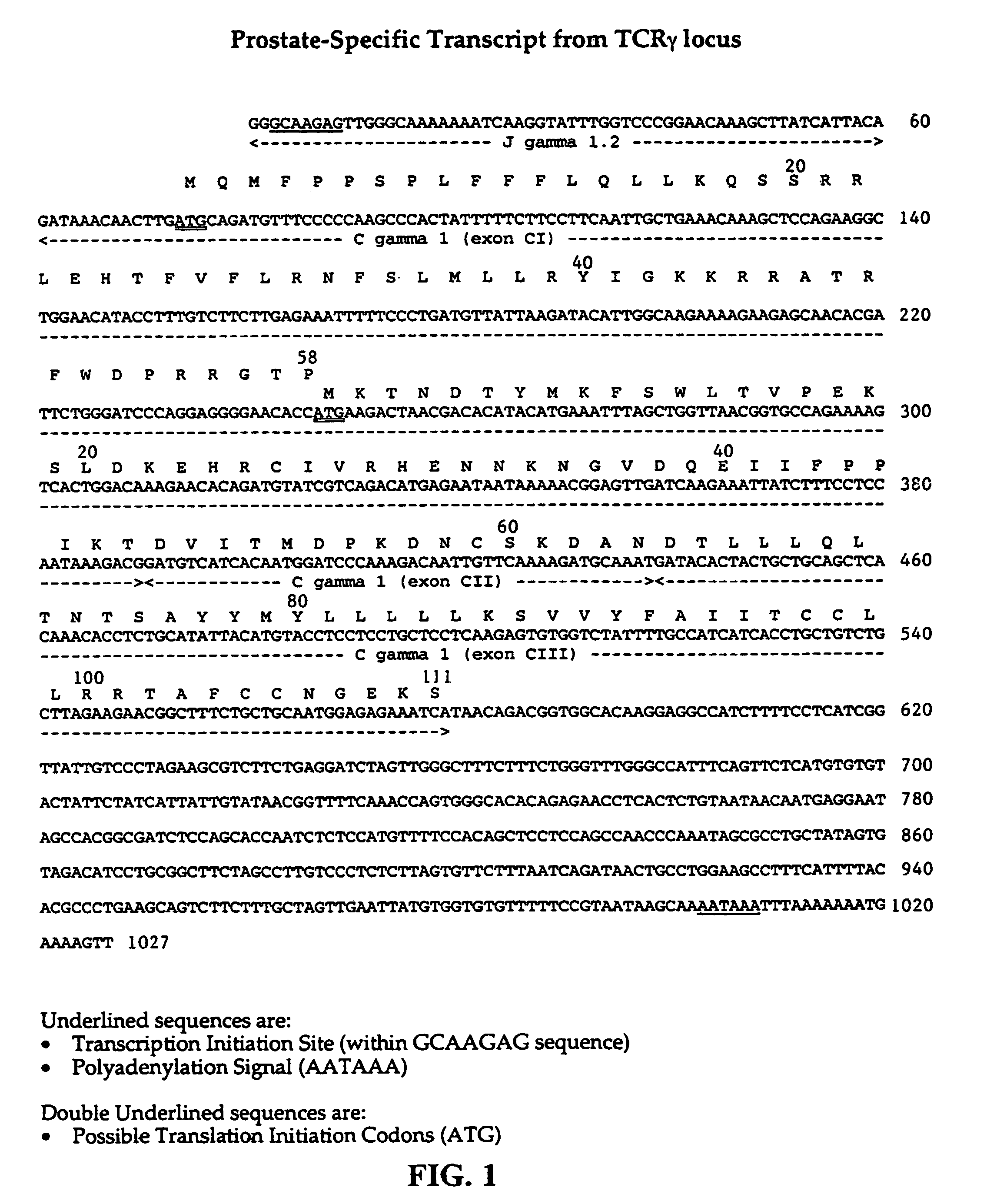
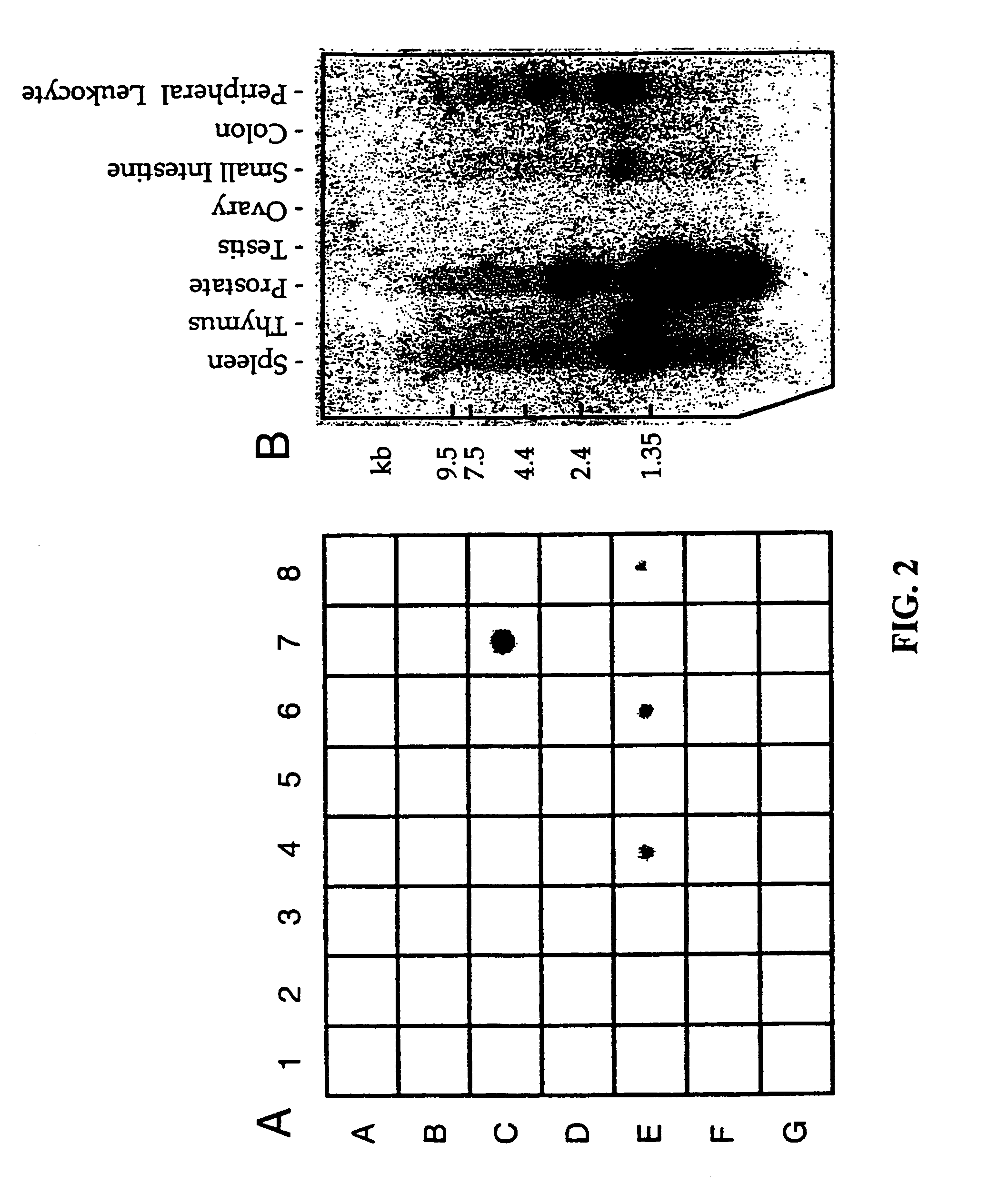
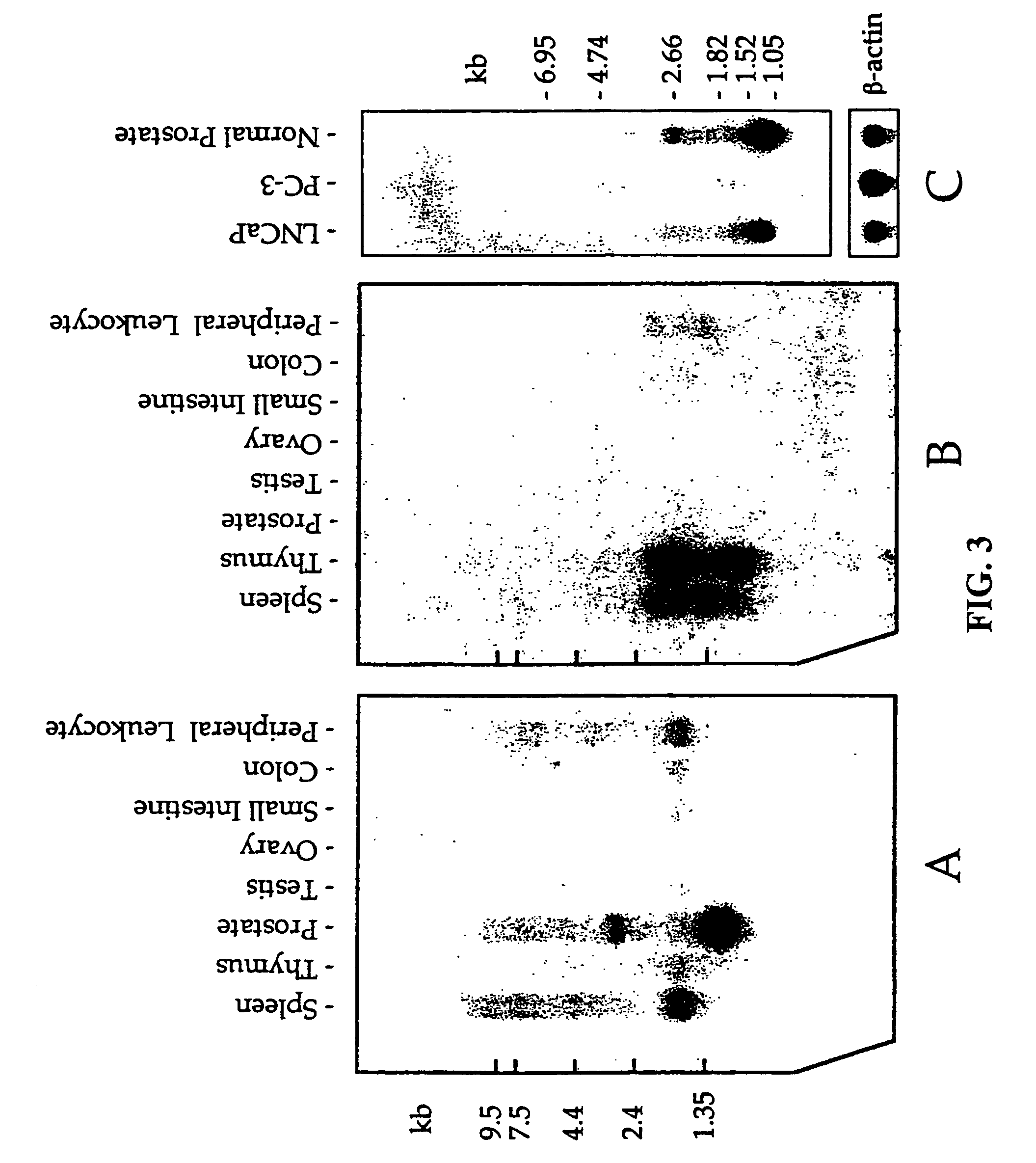
T-cell receptorγ alternate reading frame protein, (TARP) and uses thereof
InactiveUS7052703B1Organic active ingredientsPeptide/protein ingredientsProstate cancer cellProstatic epithelium
This invention provides nucleic acids containing sequences from a TCRγ transcript from prostate epithelial cells and many breast cancer cells and a T-cell receptor gamma Alternate Reading frame Protein (“TARP”) expressed from the translation of those sequences. Vaccines made from TARP are useful in raising immune responses to cells in which the protein is expressed, including prostate cancer cells and cells of many breast cancers. The invention also provides methods for diagnosing the presence of prostate cancer and TARP-expressing breast cancers, as well as methods of administering TARP and nucleic acids encoding TARP to subjects.
Owner:UNITED STATES OF AMERICA
Protein biomarkers that distinguish prostate cancer from non-malignant cells
InactiveUS20060088830A1Minimize potentialMicrobiological testing/measurementBiological testingProstatic epitheliumNon malignant
This invention provides organic biomolecule markers (e.g., proteins) useful for differentiating prostate cancer, prostate intraepithelial neoplasia or benign prostate hyperplasia, from a negative diagnosis (i.e. normal and benign prostate epithelial cells).
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
Diagnostic methods and markers
InactiveUS20110287010A1Organic active ingredientsPeptide/protein ingredientsProstatic epitheliumIntraepithelial Neoplasms
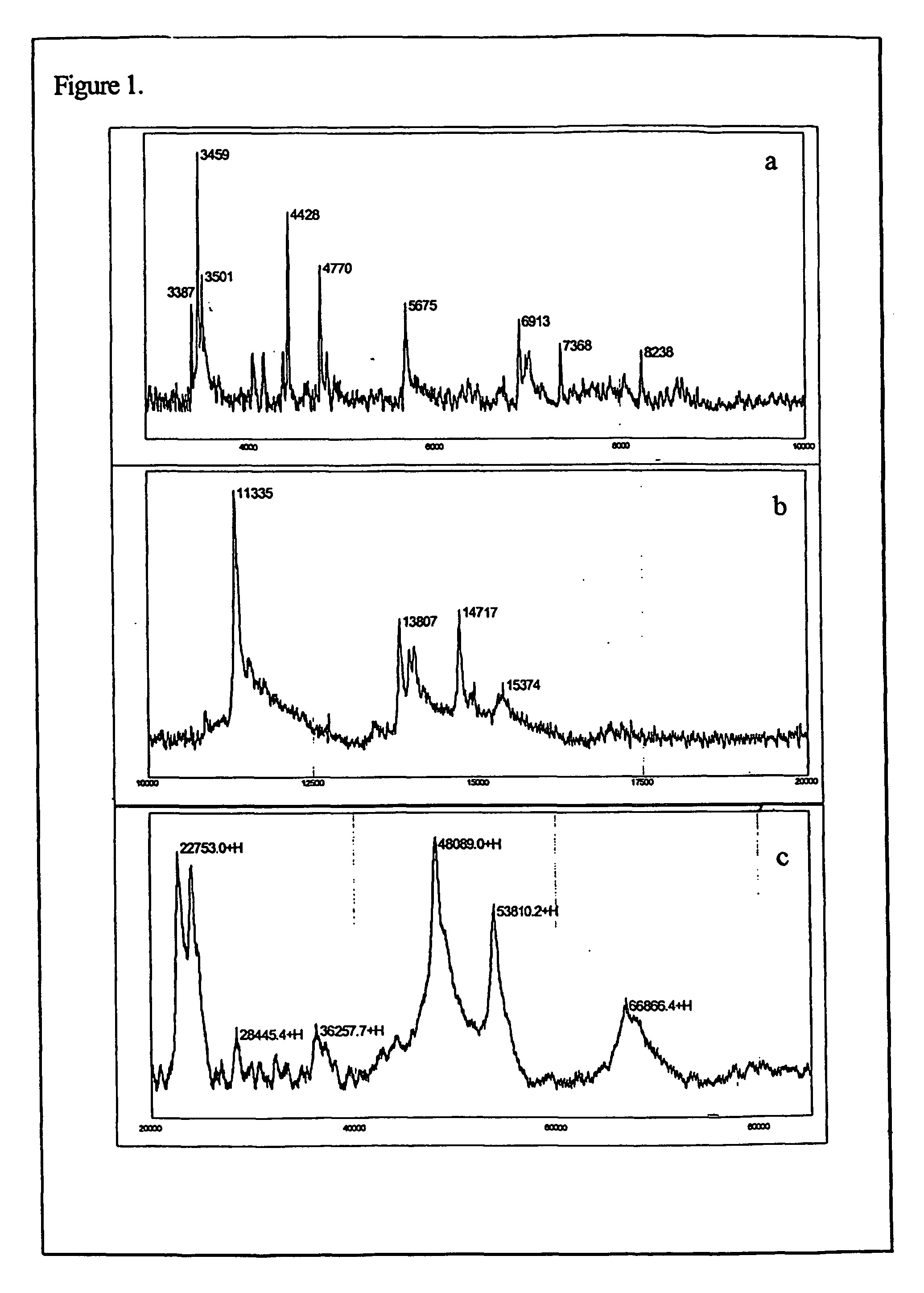
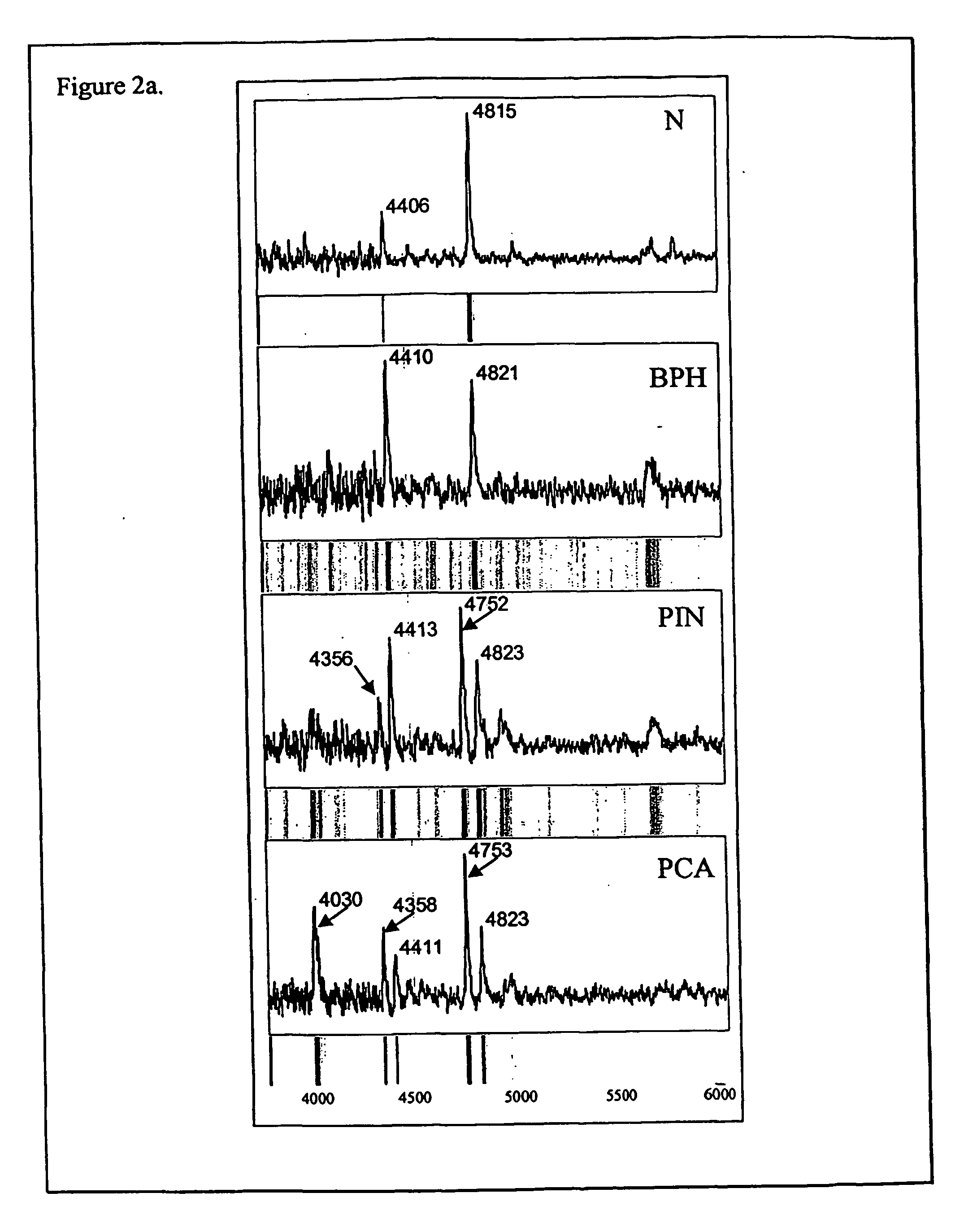
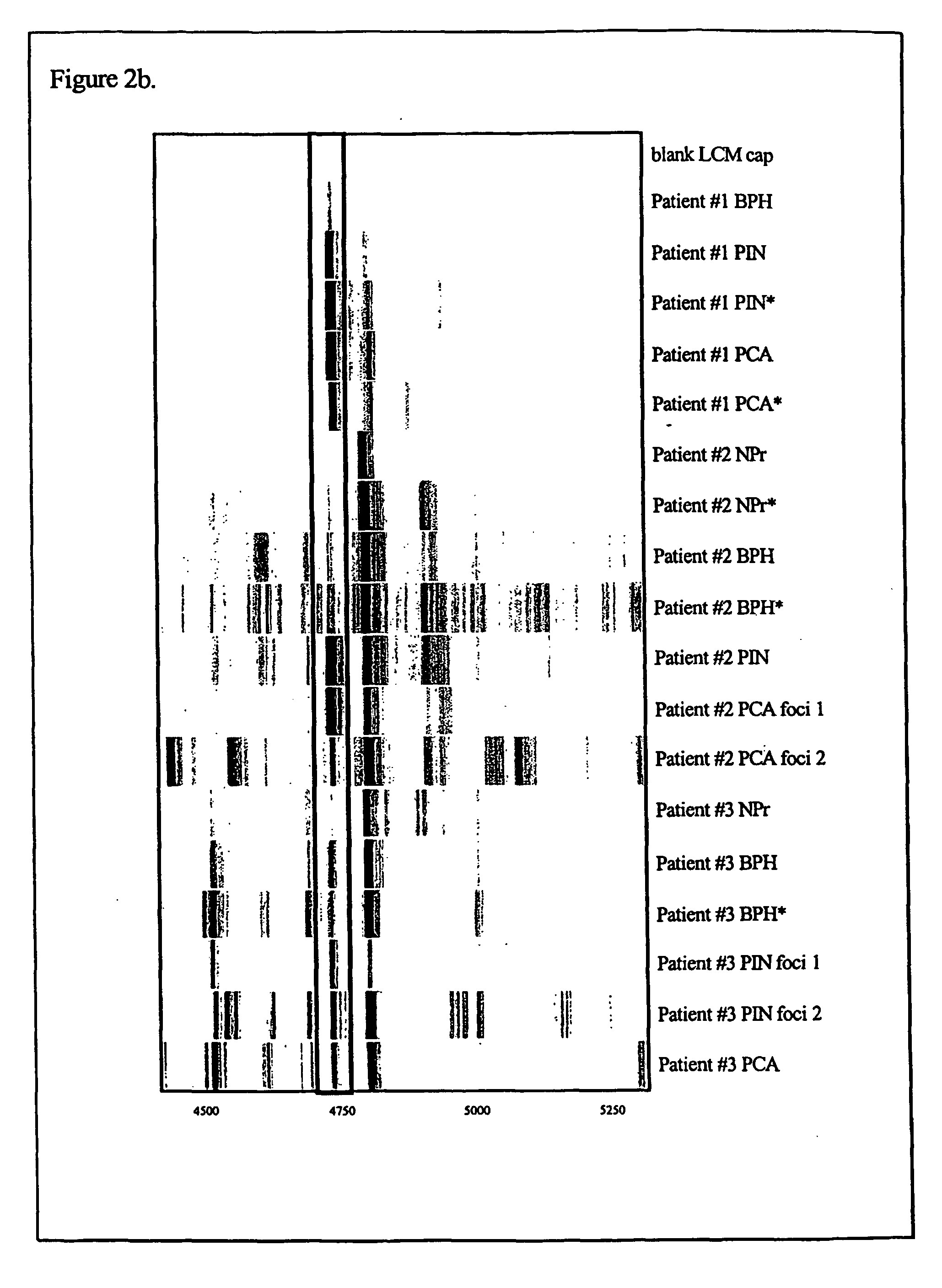
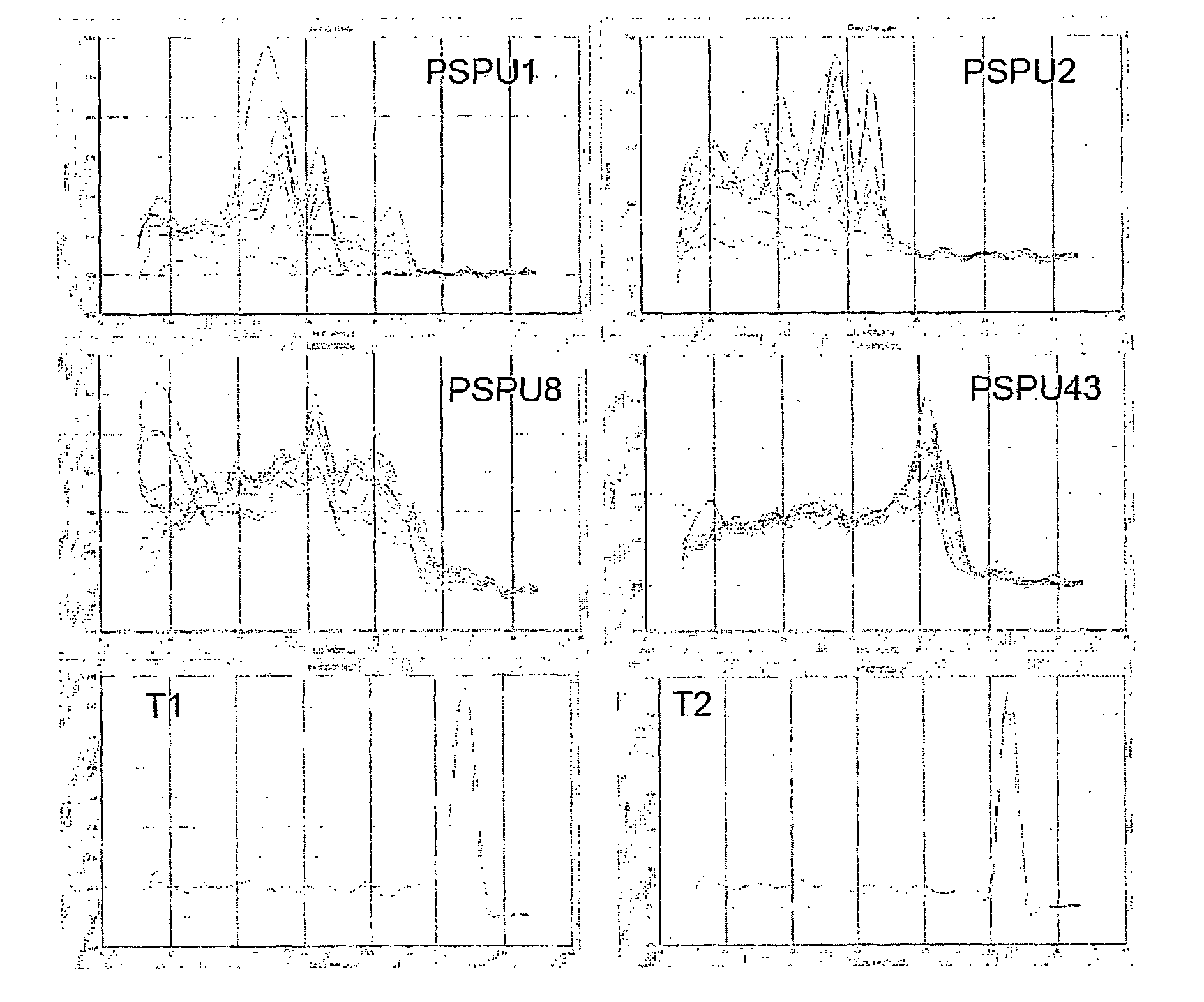
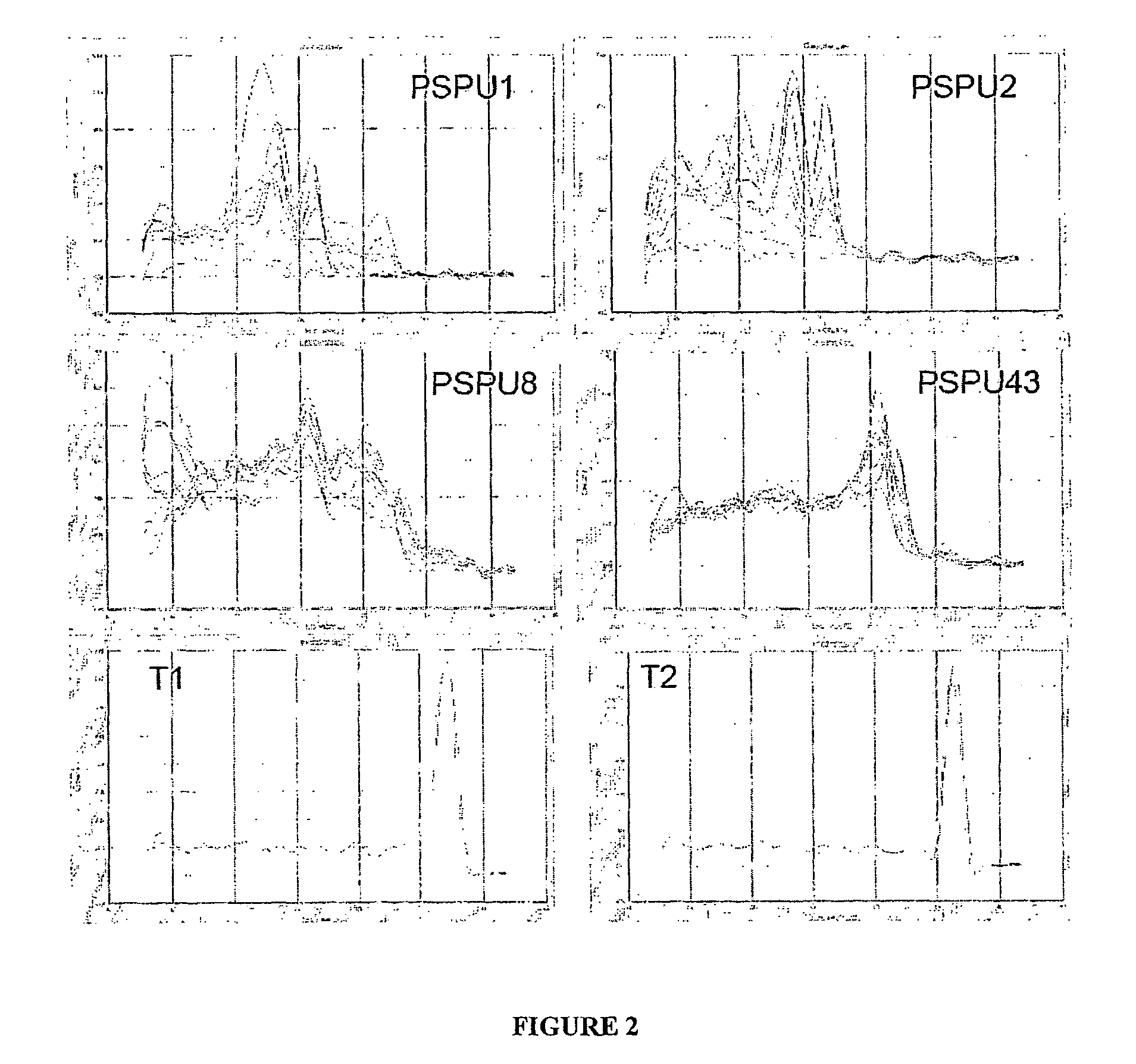
The present invention relates to methods of detecting, monitoring and treating prostate cancer (PRC) OR prostatic intraepithelial neoplasia (PIN) or a predisposition to same. Provided for use in the methods is a novel cancer marker, PSPU43, as well as bioassays and kits.
Owner:OTAGO INNOVATION
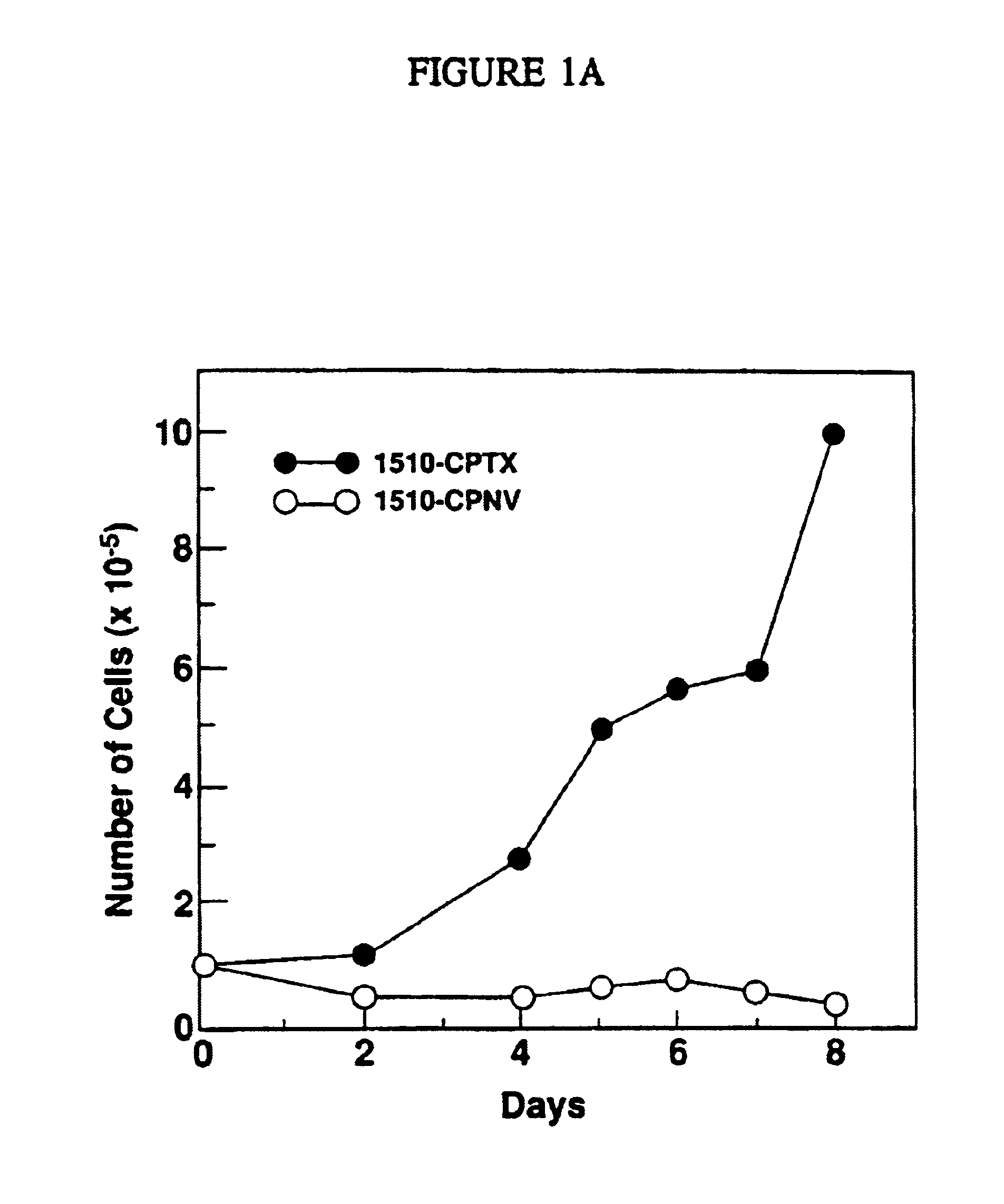
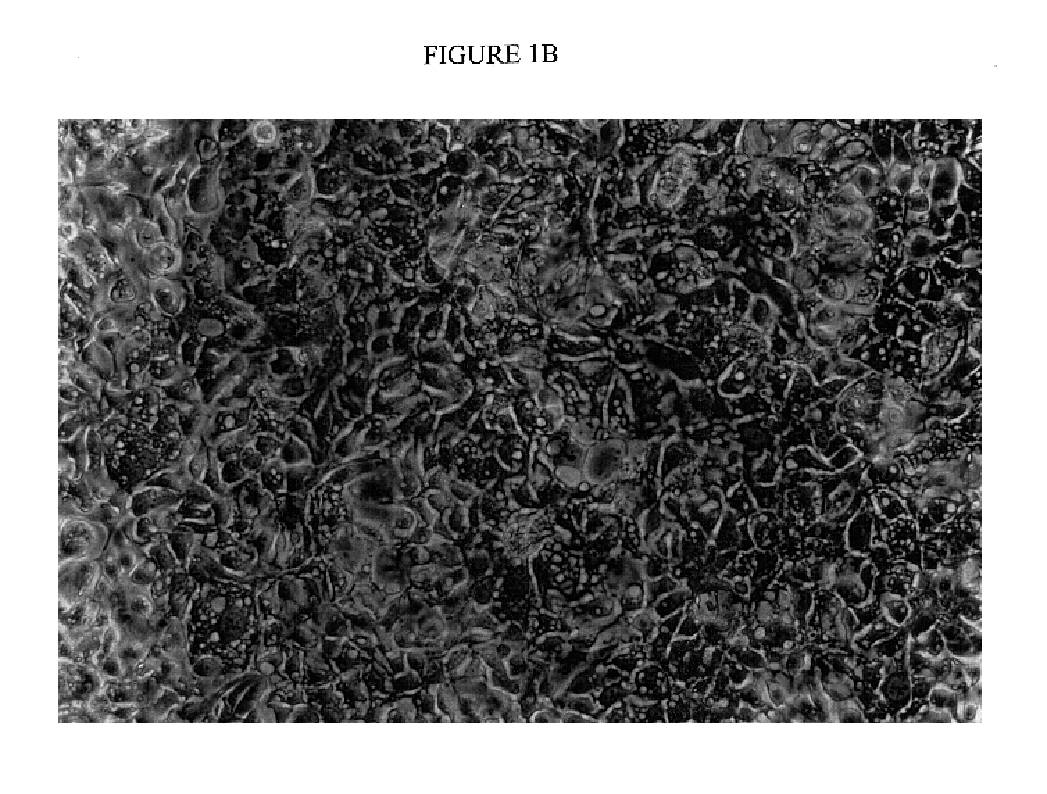
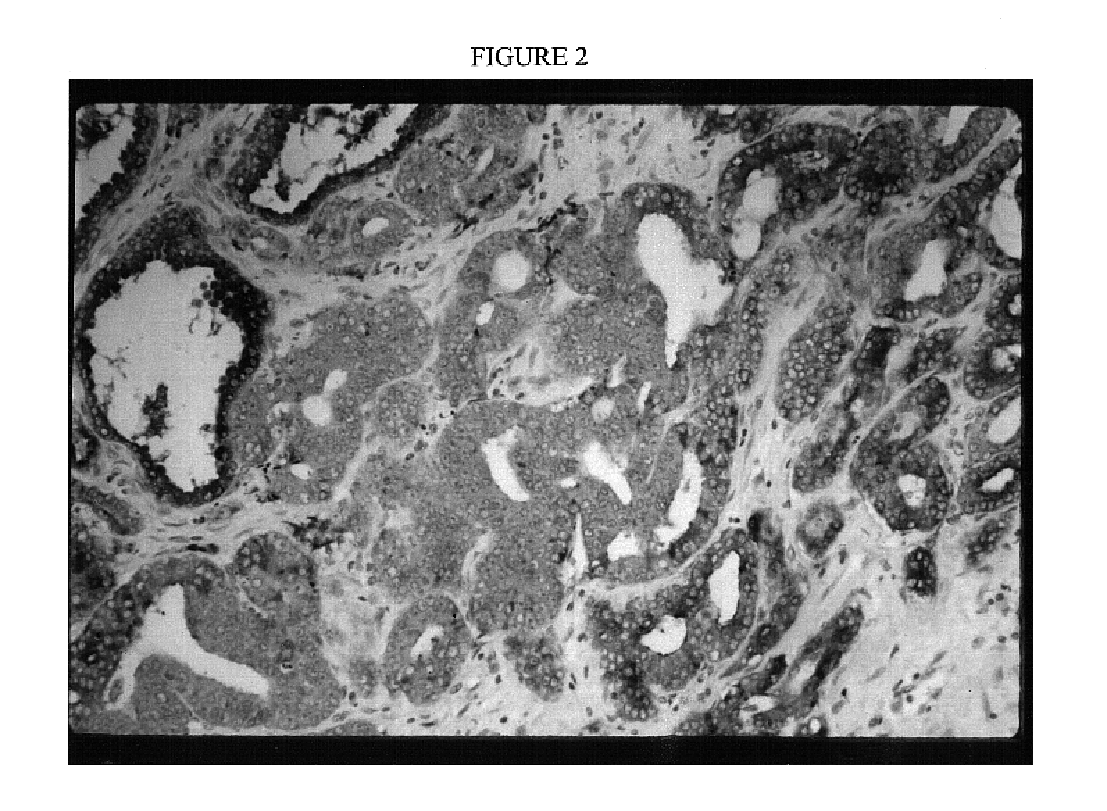
Immortal human prostate epithelial cell lines and clones and their applications in the research and therapy of prostate cancer
InactiveUS6982168B1Efficient productionPeptide/protein ingredientsImmunoglobulins against animals/humansProstatic epitheliumHuman prostate
The present invention relates to immortalized, malignant, human, adult prostate epithelial cell lines or cell lines derived therefrom useful in the diagnosis and treatment of prostate cancer. More particularly, the present invention relates to cloned, immortalized, malignant, human, adult prostate epithelial cell lines and uses of these cell lines for the diagnosis and treatment of cancer. Furthermore, the present invention provides for the characterization of said cell lines through the analysis of specific chromosomal deletions.
Owner:HEALTH & HUMAN SERVICES DEPT OF GOVERNMENT OF THE US SEC THE
Treatment and diagnosis of cancer
InactiveCN1244921APeptide/protein ingredientsMicrobiological testing/measurementAntigenAntiendomysial antibodies
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of cancerous tissues, particularly cancerous tissues proximate to or containing vascular endothelial cells, which express an extracellular domain of prostate specific membrane antigen. The labeled biological agents can also be used to detect normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate or other cancers. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
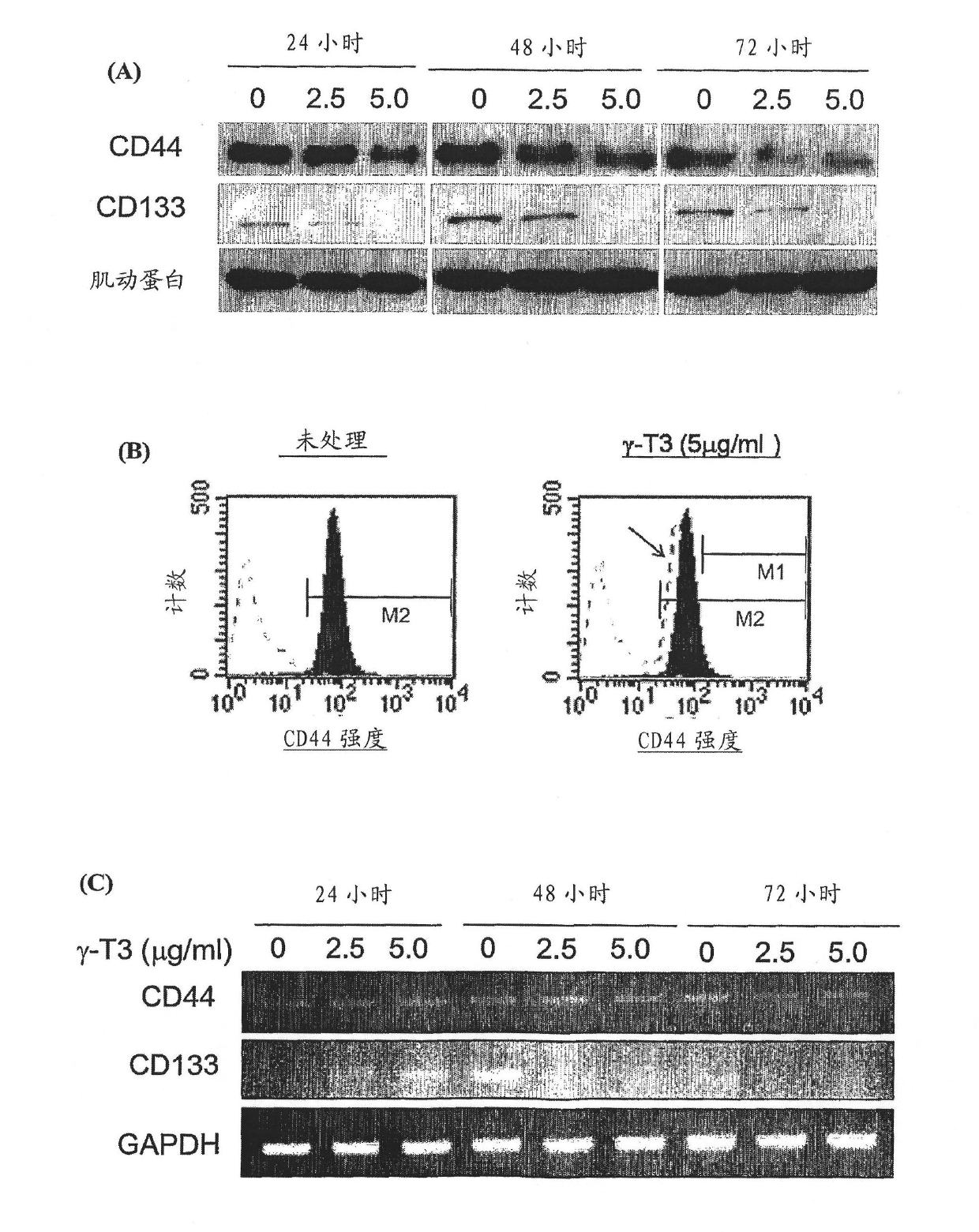
Use of tocotrienol composition for the prevention of cancer
The present invention is directed to a method of preventing cancer or preventing the recurrence of cancer after undergoing a cancer treatment by administering a composition comprising at least one of Gamma-tocotrienol or Gamma-tocotrienol, wherein the cancer is selected from the group consisting of melanoma, prostate cancer, prostate intraepithelial neoplasia, colon cancer, liver cancer, bladder cancer, breast cancer and lung cancer. The present invention is further directed to a composition comprising at least one of Gamma-tocotrienol or Delta-tocotrienol and Docetaxel and / or Dacarbazine, and to a method of inhibiting or arresting or reversing of cancer by administering a composition comprising at least one of Gamma-tocotrienol or Gamma-tocotrienol together with Docetaxel and / or Dacarbazine. The present invention is also directed to methods of manufacturing those compositions.
Owner:DAVOS LIFE SCI PTE
Methods of detecting prostate cancer
Proteins specific for prostate epithelial cells, normal or neoplastic, are identified and used for diagnosis, development of antibodies, and for evaluating drugs that react with the neoplastic specific proteins. Affinity based probes are used that react specifically with the active site to provide a measure of the enzyme activity of the cells. Prostate epithelial neoplastic cells can be used in screening candidate drugs for their effect in changing the proteome profile as to the serine-threonine hydrolase enzymes, using the affinity based probes for determining the profile.
Owner:BURNHAM INST FOR MEDICAL RES
Differentially expressed genes in prostate cancer
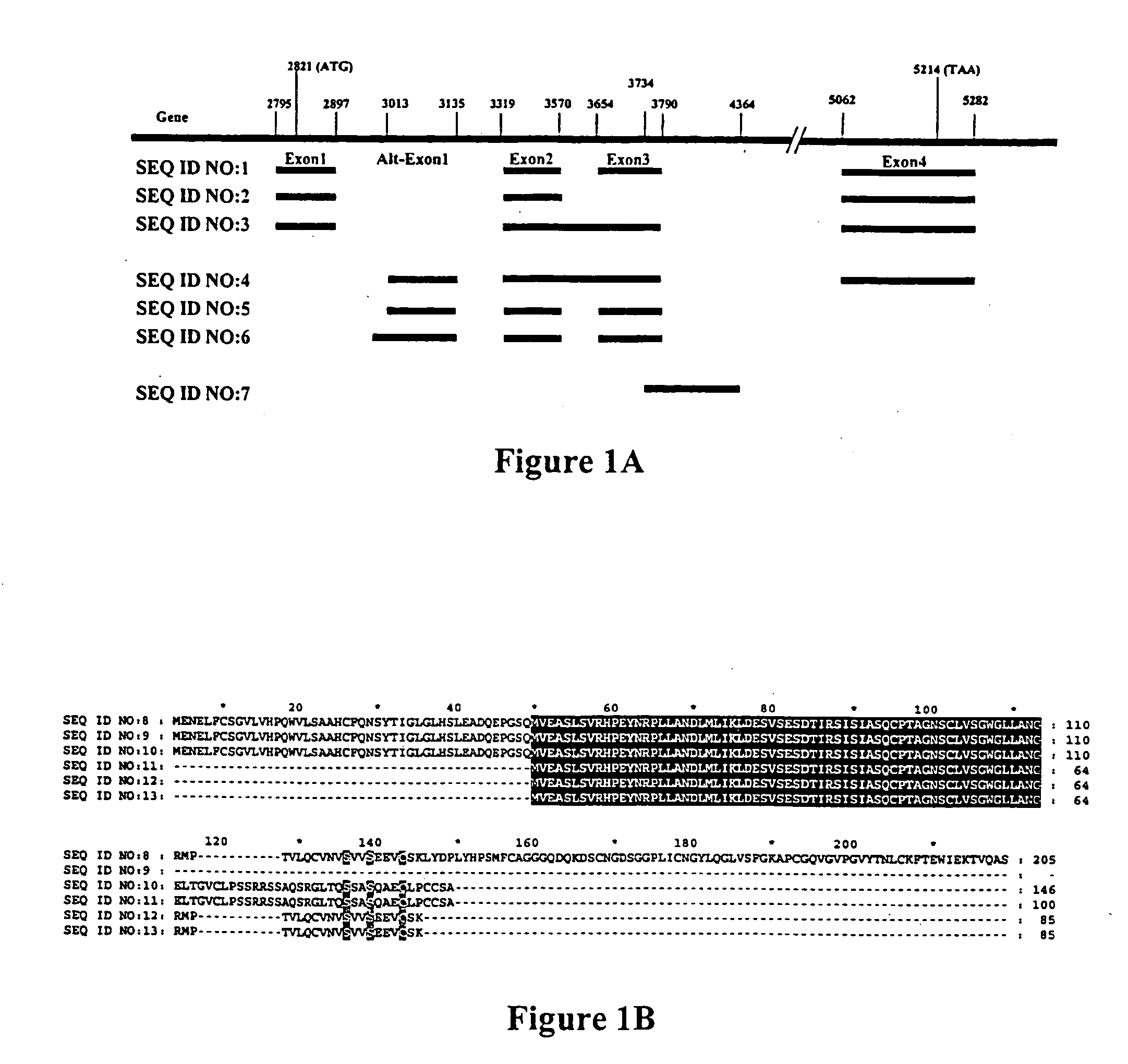
SEQ ID NO: 1, SEQ ID NO: 2, and SEQ ID NO: 3 encode an intracellular protein that is expressed in prostate epithelial cells in a hormone dependent manner. Encoded proteins SEQ ID NO: 4, SEQ ID NO: 5, and SEQ ID NO: 6 have predominantly perinuclear, nuclear and predominantly nuclear location localization within a cell, respectively. In contemplated methods of detecting a neoplastic cell in a system, a predetermined amount of at least one of SEQ ID NO: 4, SEQ ID NO:5, and SEQ ID NO: 6, or at least one of SEQ ID NO: 7, SEQ ID NO: 8, and SEQ ID NO: 9 is correlated with the presence of a neoplastic cell and detected within the system employing specific binding of a labeled probe. In a method of identifying differentially expressed genes, a tissue specific array of cDNA prepared by suppression subtractive hybridization is arranged on a solid phase. Two nucleic acid preparations are individually hybridized with the array, wherein the first and second nucleic acid preparations are prepared from treated and untreated target tissue. A comparison of the hybridization patterns reveals differentially expressed genes.
Owner:SAATCIOGLU FAHRI
Fkbp52 targeting agent pharmaceutical compositions
InactiveUS20160030369A1BiocidePeptide/protein ingredientsCancer preventionLiquid chromatography mass spectroscopy
Liposomes comprising an FKBP52 targeting agent (FTA) are disclosed. Pharmaceutical compositions comprising an FTA, a solvent, and a surfactant are disclosed. Pharmaceutical compositions comprising a cyclodextrin and / or a derivative thereof and an FTA are also disclosed. Method of detecting one or more compounds in a sample by liquid chromatography / tandem mass spectrometry (LC / MS / MS), methods of treating or preventing cancer, benign prostatic hyperplasia (BPH), prostatic intraepithelial neoplasia (PIN), prostatitis, enlarged prostate, or insulin-independent diabetes, and methods of inhibiting spermatogenesis or fertilized oocyte implantation in a mammal are also provided.
Owner:UNITED STATES OF AMERICA +2
Immunogenic compositions comprising a xenogenic prostate protein p501s
InactiveUS20060140965A1Enhancing induction of immunityBreaking the tolerance against the autologous antigenGenetic material ingredientsCancer antigen ingredientsHuman prostateImmunogenicity
The present invention relates to pharmaceutical / immunogenic compositions and methods for inducing an immune response against tumour-related antigens. More specifically, the invention relates to non-human prostate-specific antigens, more precisely to the non-human prostate-specific P501S, which can be used as xenogeneic antigen in prostate cancer vaccine therapy and as diagnostic agents for prostate tumours in humans, to immunogenic compositions containing them, to methods of manufacture of such compositions and to their use in medicine. Methods for formulating vaccines for immunotherapeutically treating P501S-expressing prostate tumors, prostatic hyperplasia, and prostate intraepithelilial neoplasia (PIN) are also provided.
Owner:GLAXO GROUP LTD +1
Treatment of benign prostatic hyperplasia using energolytic agents
InactiveCN1738629AMicrobiological testing/measurementCarbohydrate active ingredientsProstatic epitheliumProstate hyperplasia
The invention provides a method for treatment or prophylaxis of benign prostatic hyperplasia by administration of an agent that interferes with energy metabolism, particularly the production of ATP and NADH / NADPH, in prostate epithelial cells.
Owner:THRESHOLD PHARM INC
Serine/threonine hydrolase proteins and screening assays
Proteins specific for prostate epithelial cells, normal or neoplastic, are identified and used for diagnosis, development of antibodies, and for evaluating drugs that react with the neoplastic specific proteins. Affinity based probes are used that react specifically with the active site to provide a measure of the enzyme activity of the cells. Prostate epithelial neoplastic cells can be used in screening candidate drugs for their effect in changing the proteome profile as to the serine-threonine hydrolase enzymes, using the affinity based probes for determining the profile.
Owner:BURNHAM INST
PSCA antibodies and hybridomas producing them
InactiveUS20080318254A9Promote immune-mediated destructionHigh expressionOrganic active ingredientsTumor rejection antigen precursorsAntigenProstatic epithelium
The invention provides a novel prostate cell-surface antigen, designated Prostate Stem Cell Antigen (PSCA), which is widely over-expressed across all stages of prostate cancer, including high grade prostatic intraepithelial neoplasia (PIN), androgen-dependent and androgen-independent prostate tumors.
Owner:RGT UNIV OF CALIFORNIA
Flavopereirine and alstonine combinations in the treatment and prevention of prostate cancer
InactiveUS20050266107A1Contributes to effectiveness of preventiveAlleviating and avoiding onsetBiocideUnknown materialsDiseaseDouble-time
A method and composition for preventing prostate cancer and / or reducing PSA levels and / or alleviating the symptoms of BPH (Benign Prostatic Hyperplasia) or PIN (prostatic intraepithelial neoplasia) by administration of an effective amount of a mixture of flavopereirine and alstonine. A method and composition for treating low-grade prostate cancer and preventing the onset of metastatic disease and / or reducing the doubling time of PSA levels in men with positive biopsies showing relatively low Gleason scores and morphologies characteristic of non-invasive, slow-progressing prostate cancer. The flavopereirine and alstonine can be in the form of natural extracts derived from Pao Pereira and Rauwolfia Vomitoria, respectively. Alternatively, these two active compounds can be administered in purified form. The composition can be in included in a kit along with instructions for use in a treatment regimen.
Owner:MOLECULAR INT RES +1
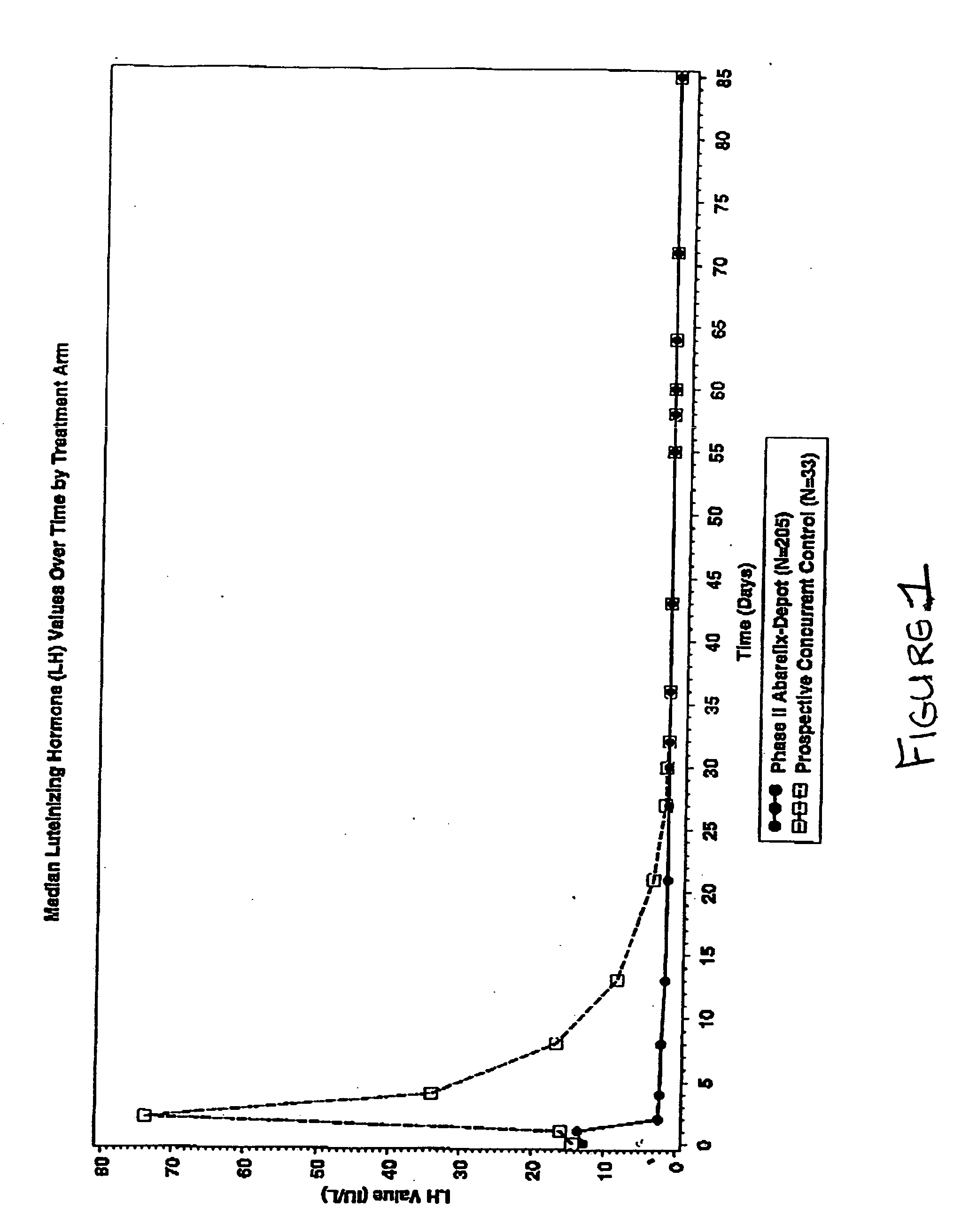
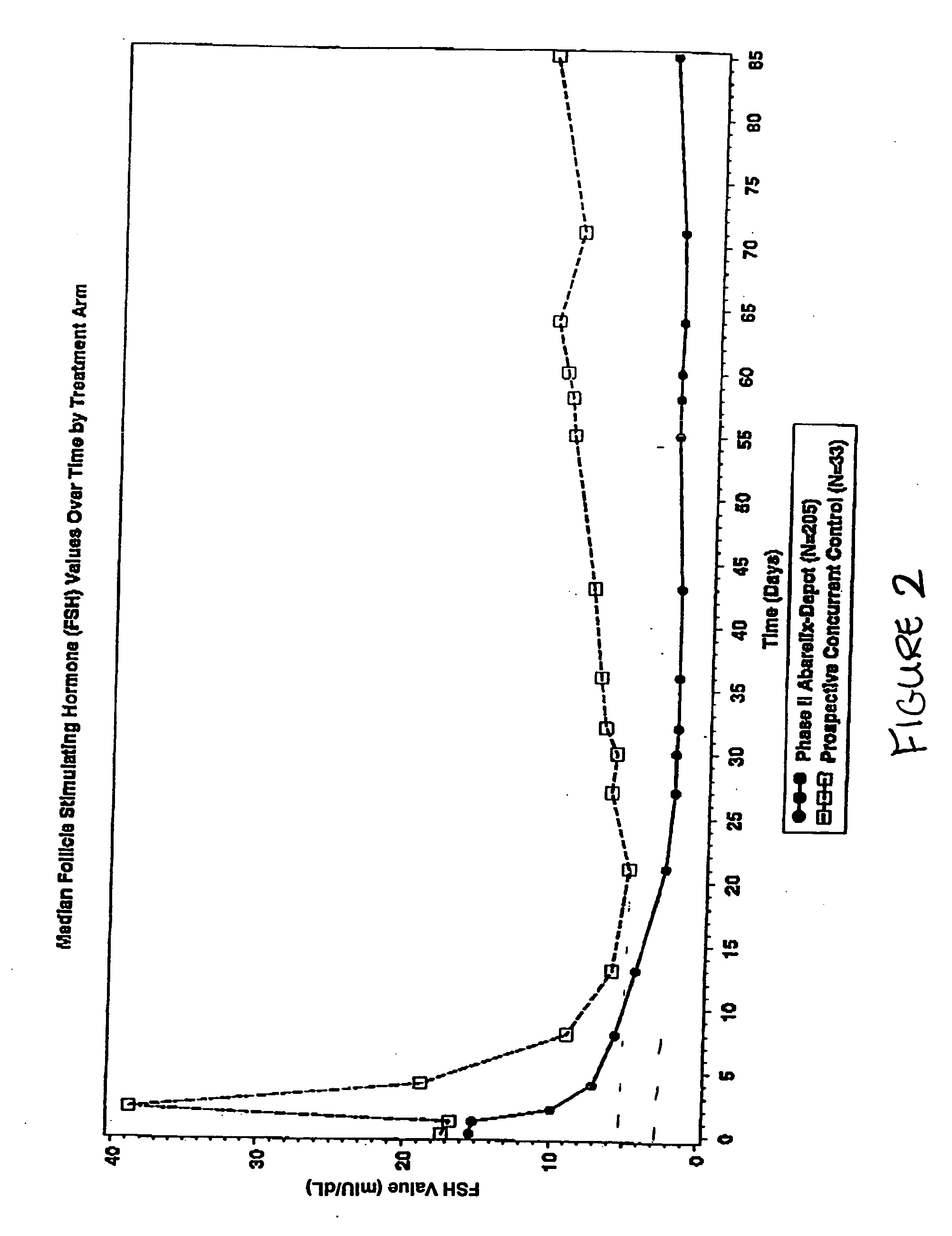
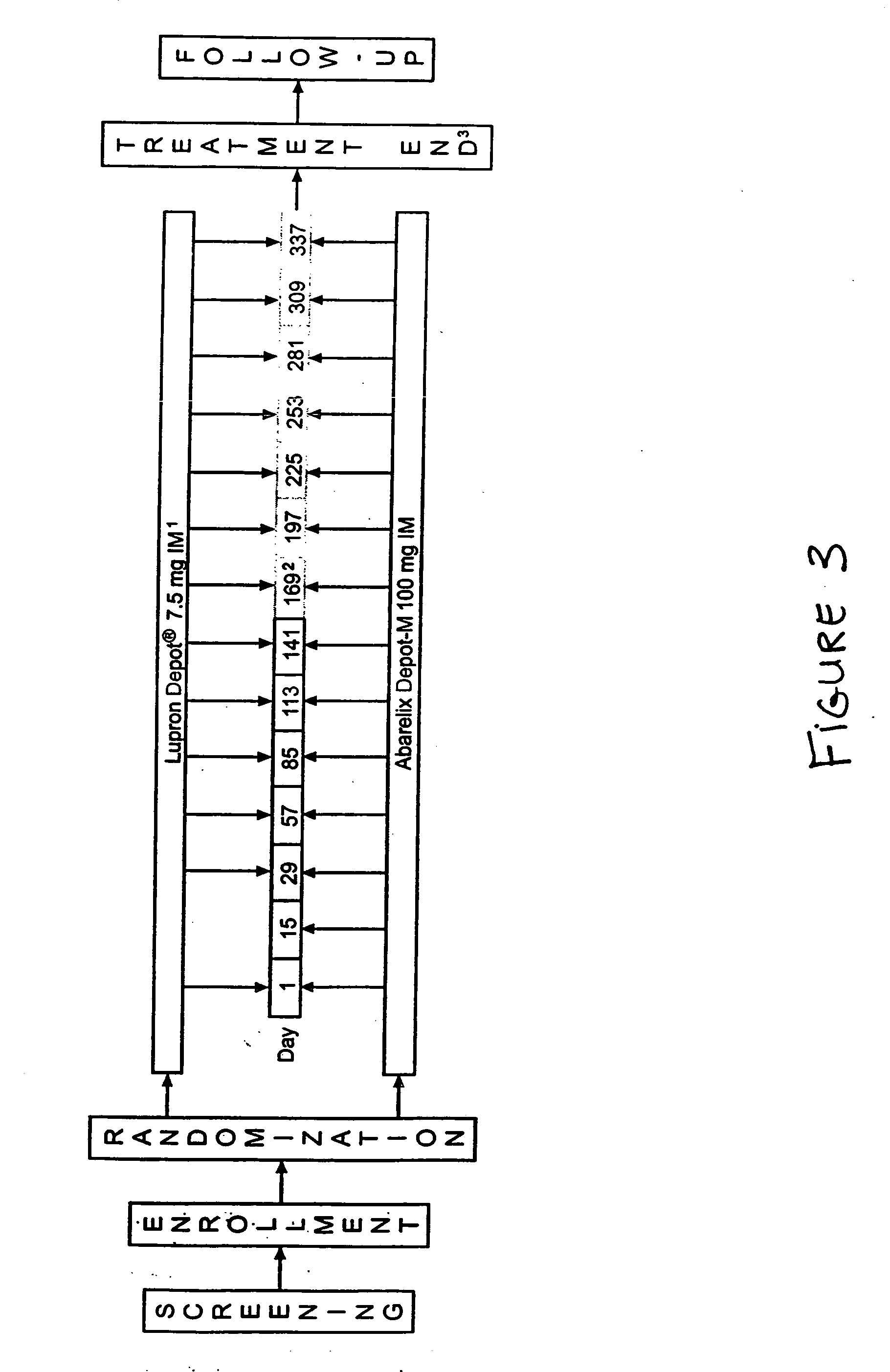
Methods for treating FSH related conditions with GnRH antagonists
InactiveUS20070015714A1Lower Level RequirementsRestore libidoOrganic active ingredientsDepsipeptidesGnRH AntagonistIn vivo
Owner:PRAECIS PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com