Treatment and diagnosis of prostate cancer
a prostate cancer and prostate cancer technology, applied in the field of prostate cancer treatment and diagnosis, can solve the problems of inability to accurately evaluate the involvement of nodal cells, inability to detect prostate cancer, and inability to accurately detect prostate cancer, etc., to achieve the effect of preventing or delaying the development or progression of prostate cancer, small in size, and easy destruction by cytotoxic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Human Tissues
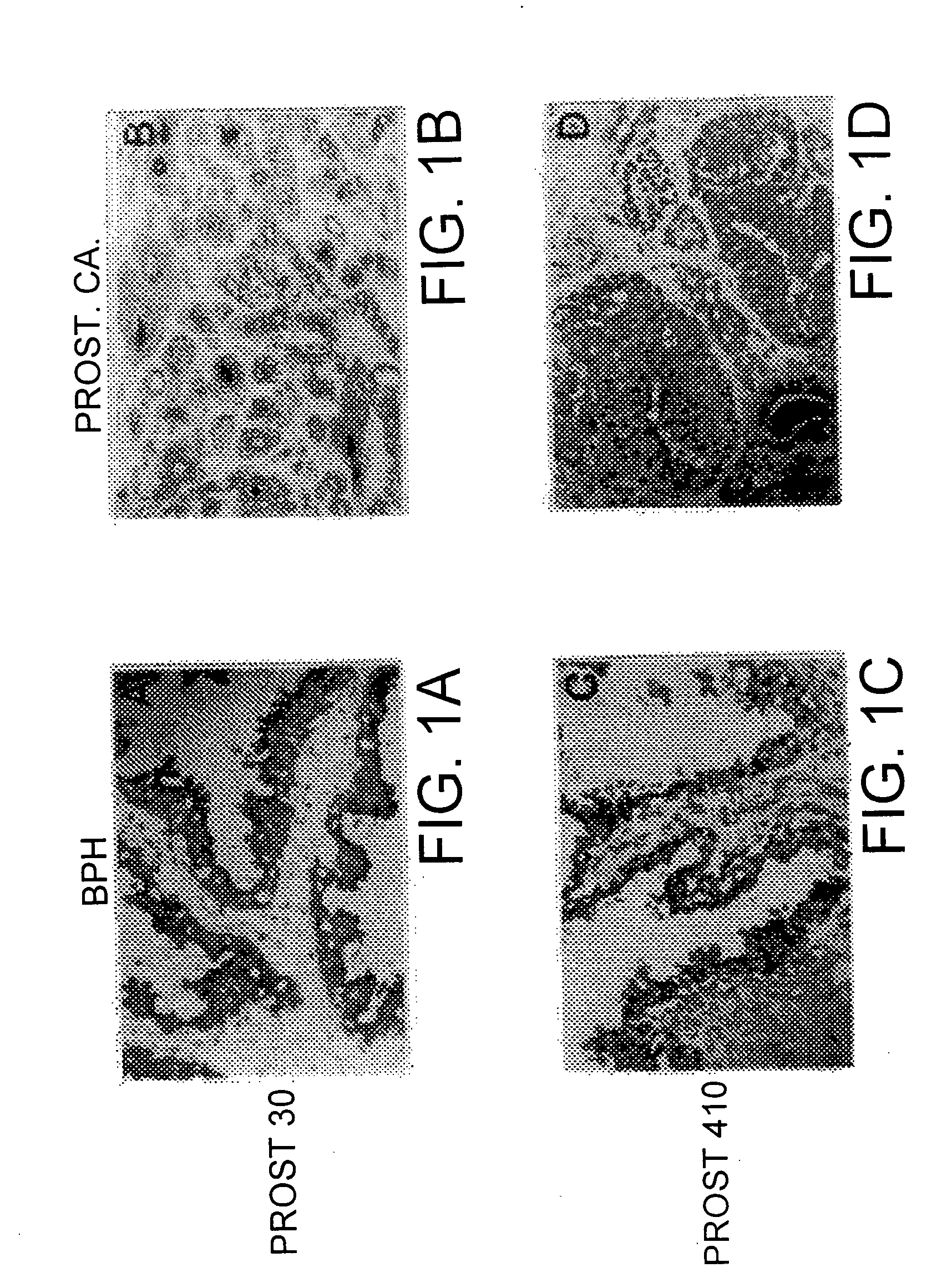
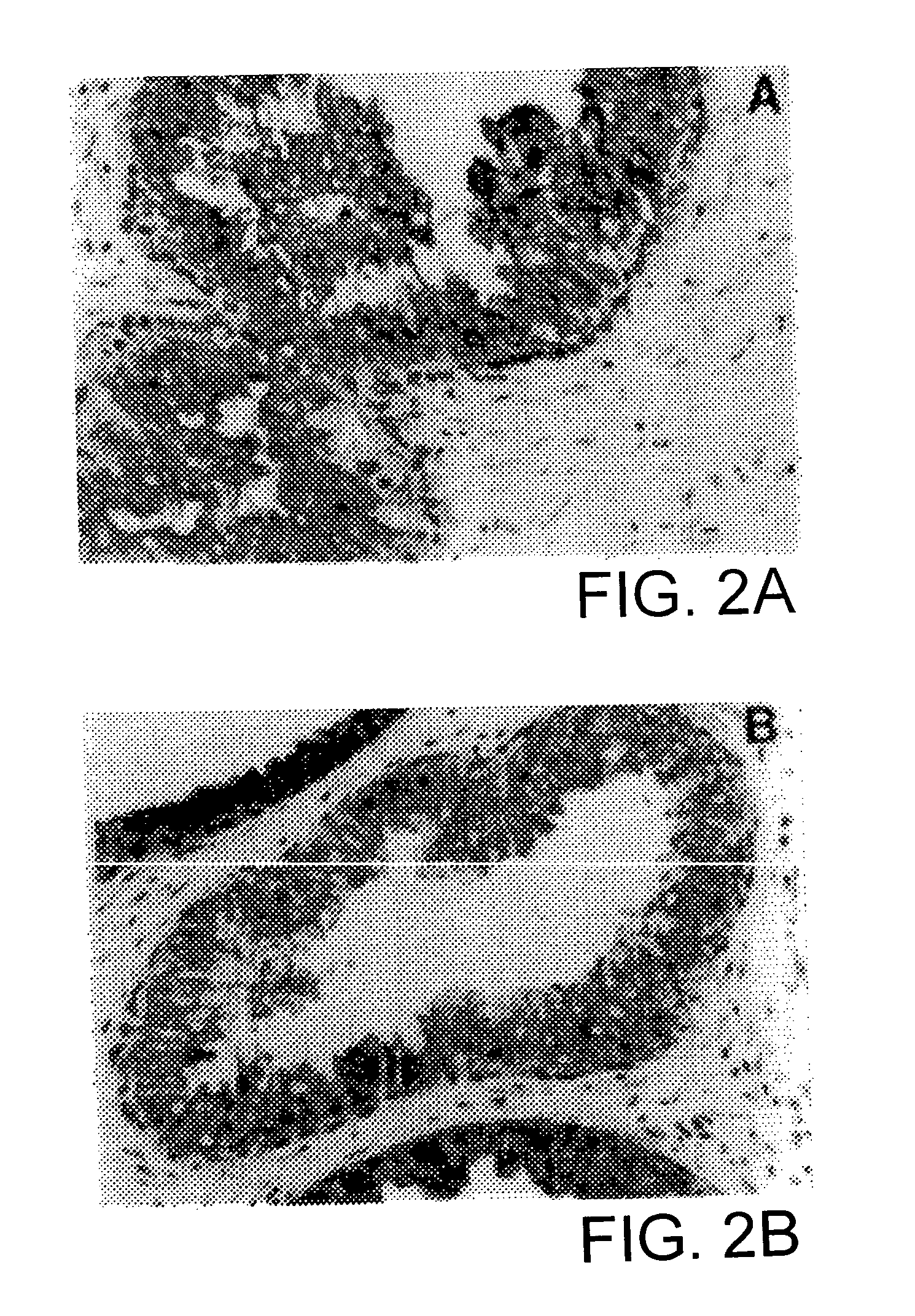
[0067] Fresh specimens of benign and malignant tissues were provided by the Tumor Procurement Service of the Department of Pathology at the Memorial Sloan-Kettering Cancer Center.
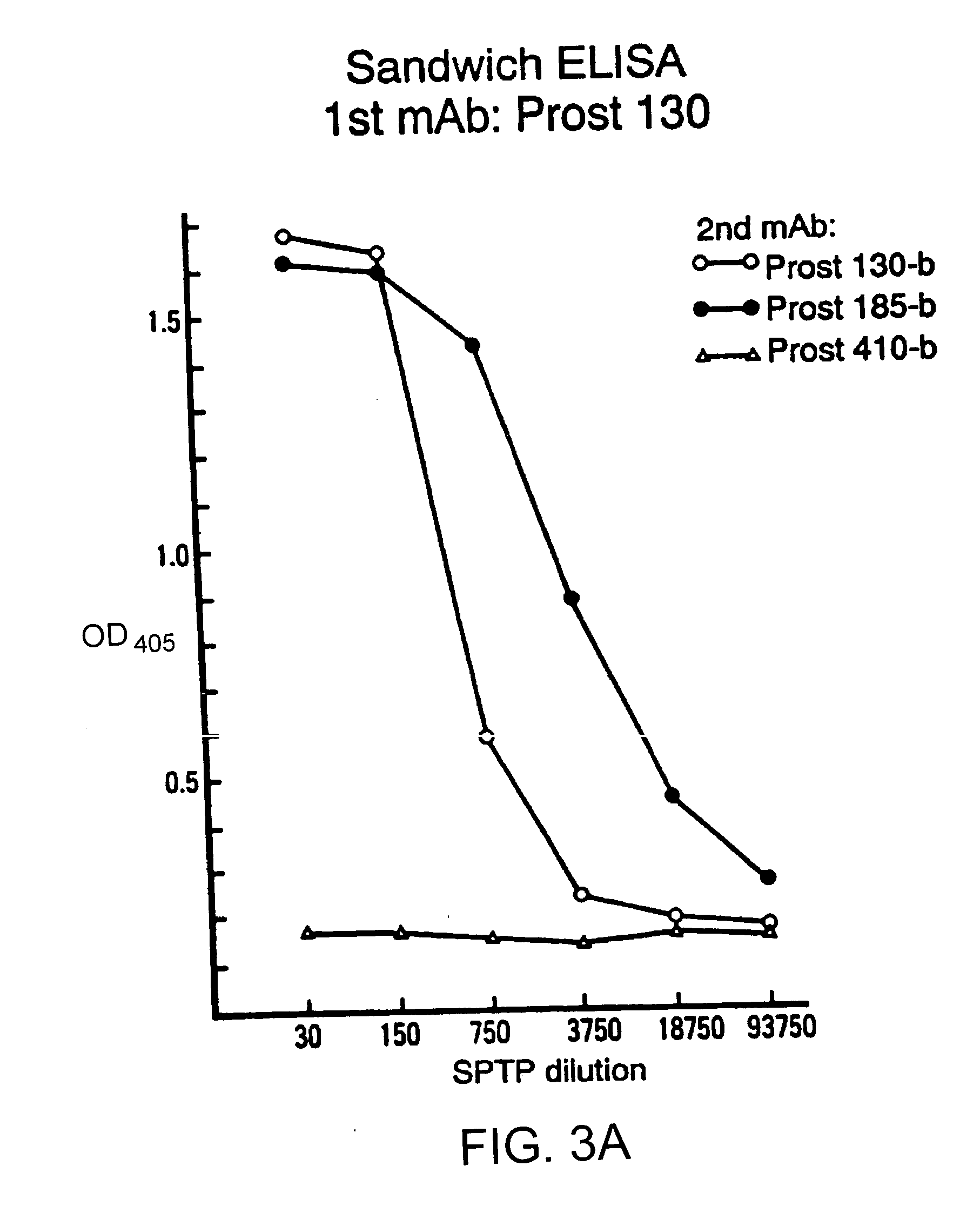
[0068] A soluble tissue preparation ("SPTP") was prepared by initial mechanical mincing of fresh benign and malignant prostates. The tissue was homogenized for 1 min in a blade homogenizer in phosphate buffered saline ("PBS"), pH 7.2, containing 0.2 mM phenylmethylsulfonyl fluoride (Sigma Chemical, St. Louis, Mo.) and 20 u / ml aprotinin (Calbiochem, San Diego, Calif.). The homogenate was centrifuged at 100,000 g for 60 min at 4.degree. C., and the supernatant was decanted and saved.
example 2
[0069] Cultured cell lines of human cancers were from the laboratory of Human Tumor Immunology, Memorial Sloan-Kettering Cancer Center. The prostate cancer cell lines PC-3 (Mickey, D. D., et al., "Characterization Of A Human Prostate Adenocarcinoma Cell Line (DU145) As A Monolayer Culture And As A Solid Tumor In Athymic Mice," Prog. Clin. Biol. Res., 37:67-84 (1980), which is hereby incorporated by reference), DU-145 (Mickey, D. D., et al., "Characterization Of A Human Prostate Adenocarcinoma Cell Line (DU145) As A Monolayer Culture And As A Solid Tumor In Athymic Mice," Prog. Clin. Biol. Res., 37:67-84 (1980), which is hereby incorporated by reference), and LNCaP (Horoszewicz, J. S., et al., "LNCaP Model Of Human Prostatic Carcinoma," Cancer Res., 43:1809-1818 (1983), which is hereby incorporated by reference) were obtained from the American Type Culture Collection (Rockville, Md.). Hybridomas were initially cloned in RPMI-1640 medium supplemented with 10% FCS, 0.1 mM...
example 3
Preparation of Mouse Monoclonal Antibodies
[0070] A BALB / c mouse was immunized subcutaneously with mechanically minced tissues from fresh benign hyperplastic and malignant prostate tissues three times at 1 week intervals. One week later, a final intraperitoneal immunization was administered. Three days later spleen cells were fused with SP-2 mouse myeloma cells utilizing standard techniques. Ueda, R., et al., "Cell Surface Antigens Of Human Renal Cancer Defined By Mouse Monoclonal Antibodies: Identification Of Tissue-Specific Kidney Giycoproteins," Proc. Natl. Acad. Sci. USA, 78:5122-5126 (1981); which is hereby incorporated by reference. Supernatants of the resulting clones were screened by immunohistochemistry. Clones which were reactive with benign prostate tissues, but not with normal lymph node, were selected and subcloned 3 times by limiting dilution. The immunoglobulin class of cultured supernatant from each clone was determined by immunodiffusion using specified rabbit antise...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com