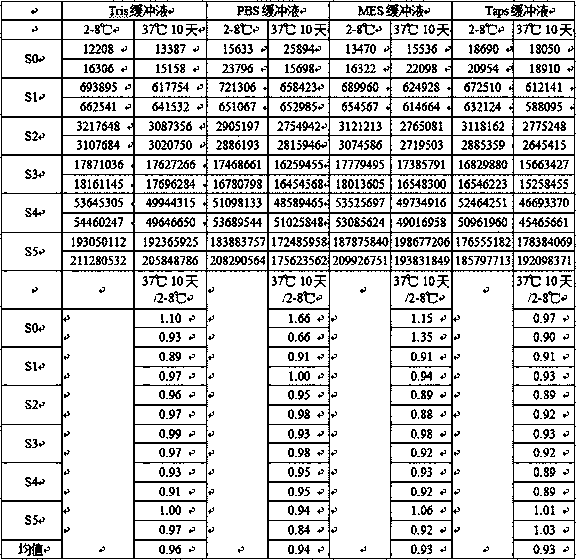
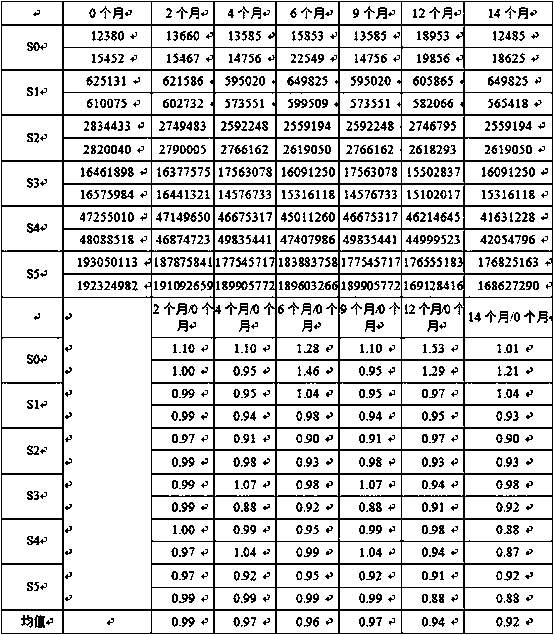
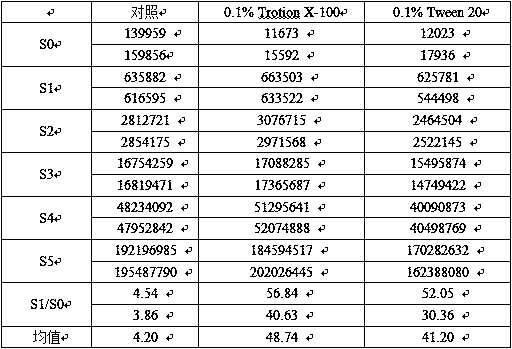
Patents
Literature
81 results about "Aprotinin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
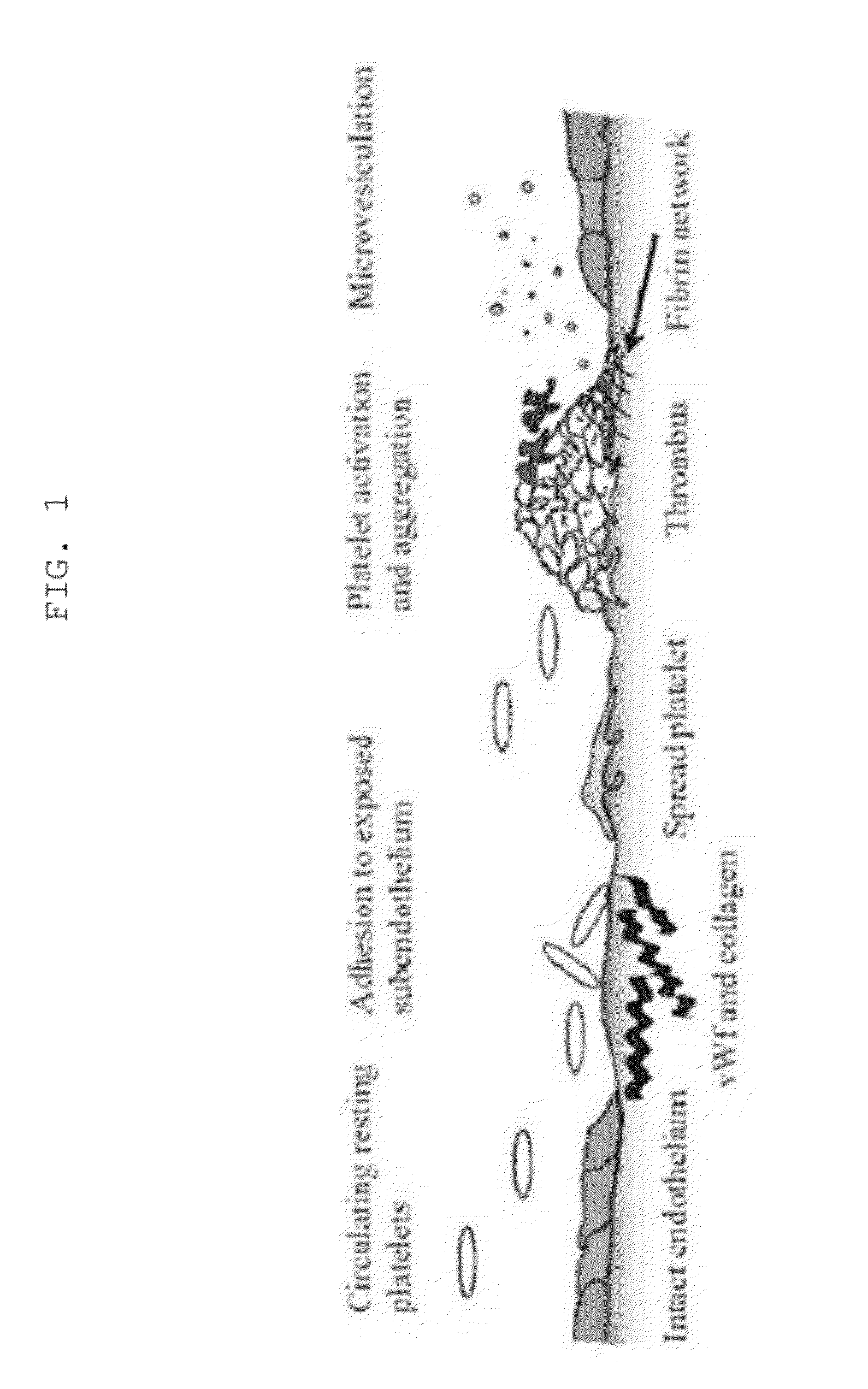
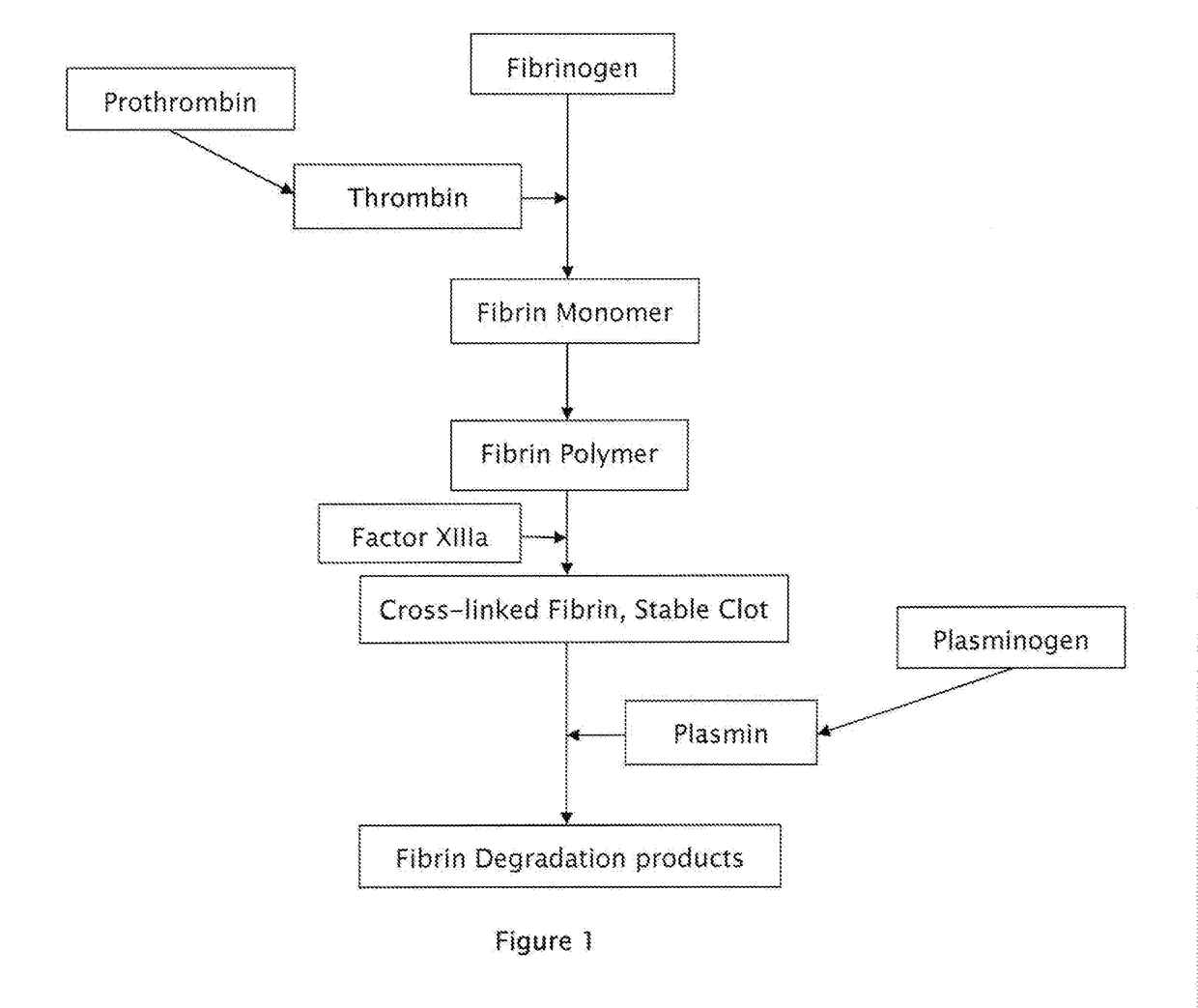
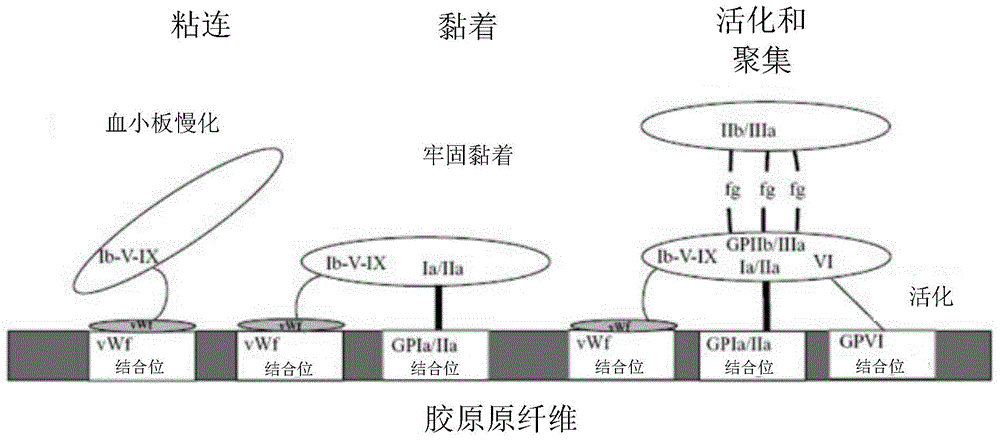
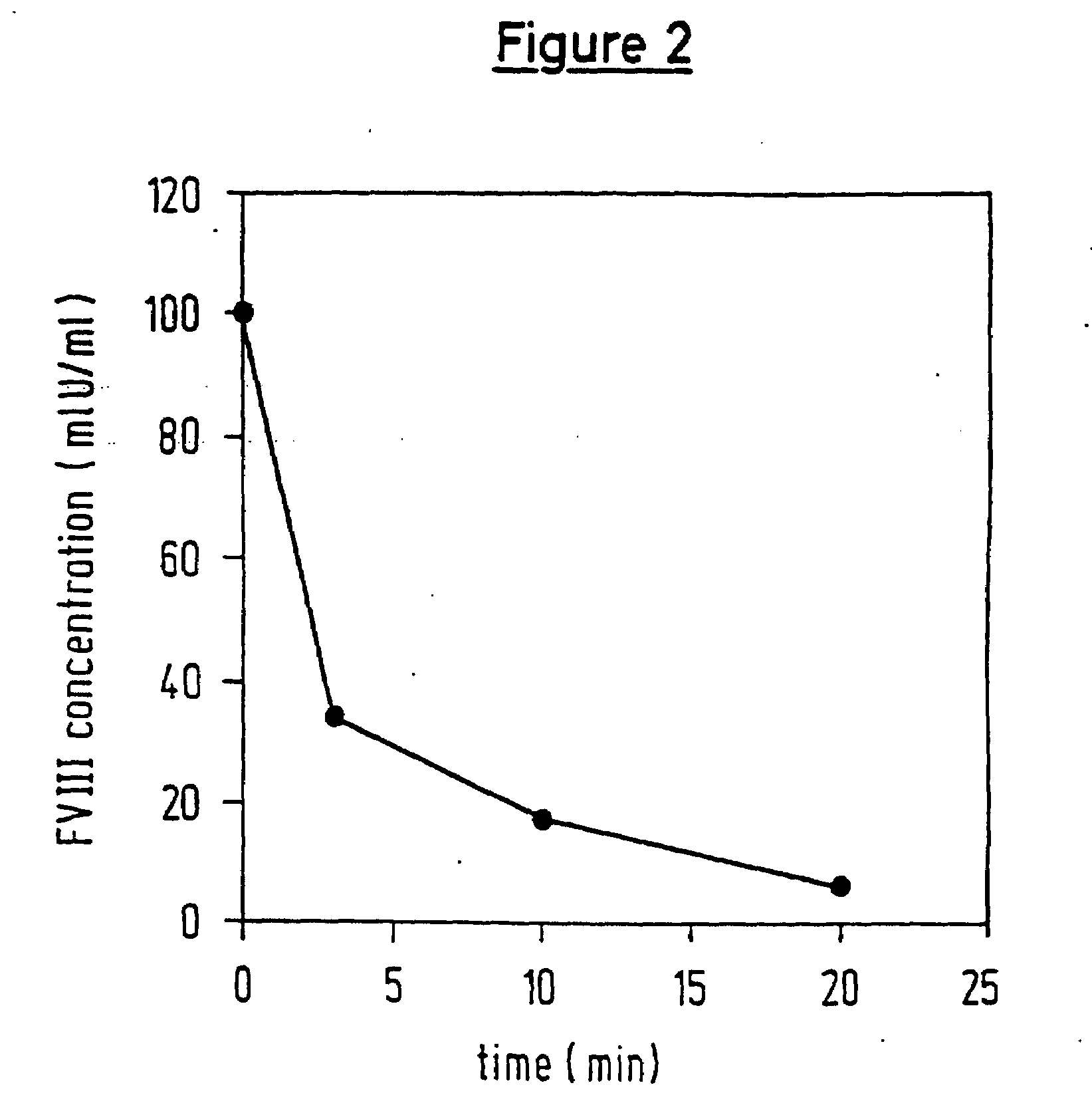
The drug aprotinin (Trasylol, previously Bayer and now Nordic Group pharmaceuticals), is a small protein bovine pancreatic trypsin inhibitor (BPTI), or basic trypsin inhibitor of bovine pancreas, which is an antifibrinolytic molecule that inhibits trypsin and related proteolytic enzymes. Under the trade name Trasylol, aprotinin was used as a medication administered by injection to reduce bleeding during complex surgery, such as heart and liver surgery. Its main effect is the slowing down of fibrinolysis, the process that leads to the breakdown of blood clots. The aim in its use was to decrease the need for blood transfusions during surgery, as well as end-organ damage due to hypotension (low blood pressure) as a result of marked blood loss. The drug was temporarily withdrawn worldwide in 2007 after studies suggested that its use increased the risk of complications or death; this was confirmed by follow-up studies. Trasylol sales were suspended in May 2008, except for very restricted research use. In February 2012 the European Medicines Agency (EMA) scientific committee reverted its previous standpoint regarding aprotinin, and has recommended that the suspension be lifted. Nordic became distributor of aprotinin in 2012.
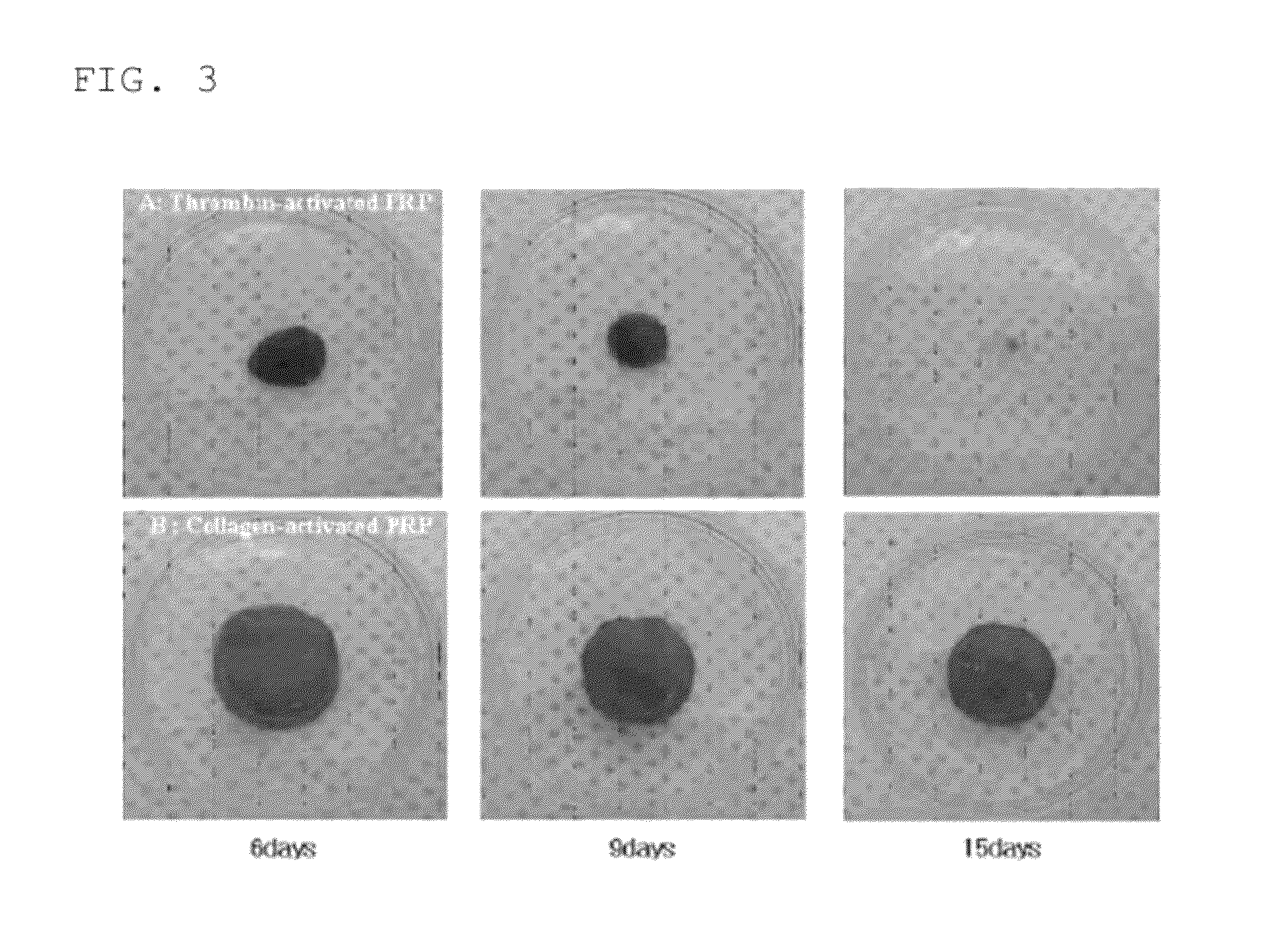
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm<3>, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsilon-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
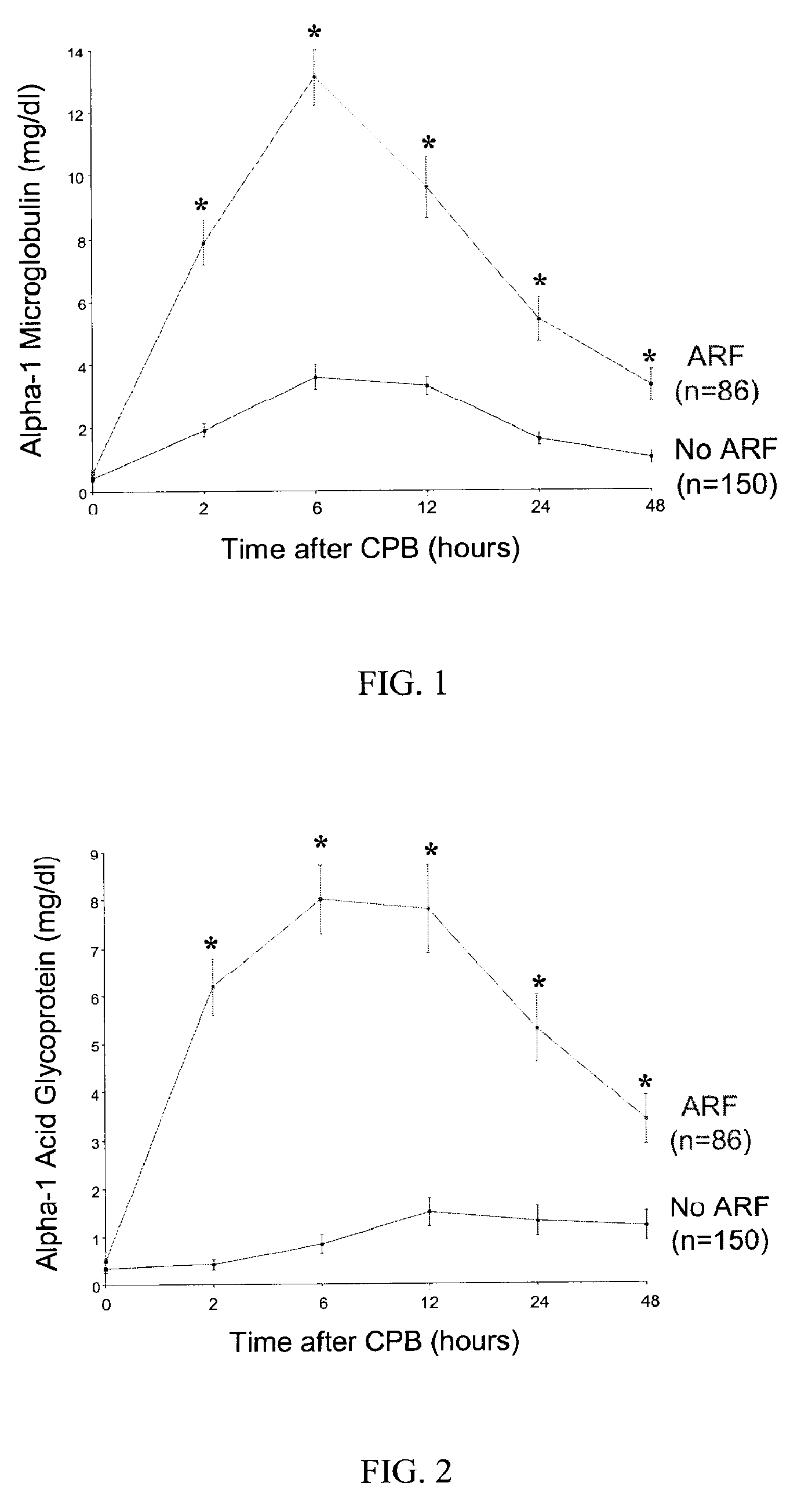
Method and Kit for the Early Detection of Impaired Renal Status
InactiveUS20070248989A1Pharmaceutical containersMedical packagingPoint of careExtracorporeal circulation
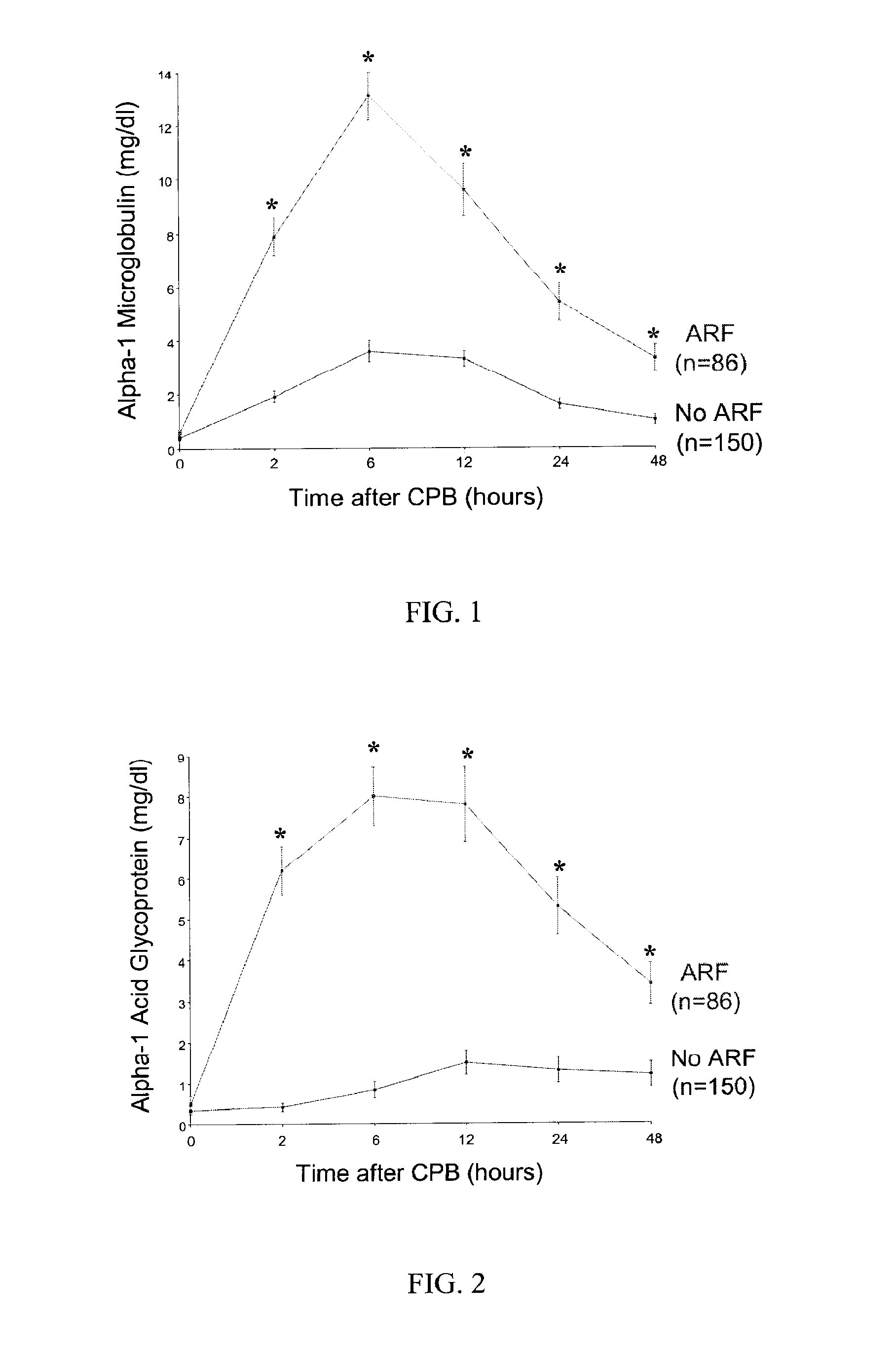
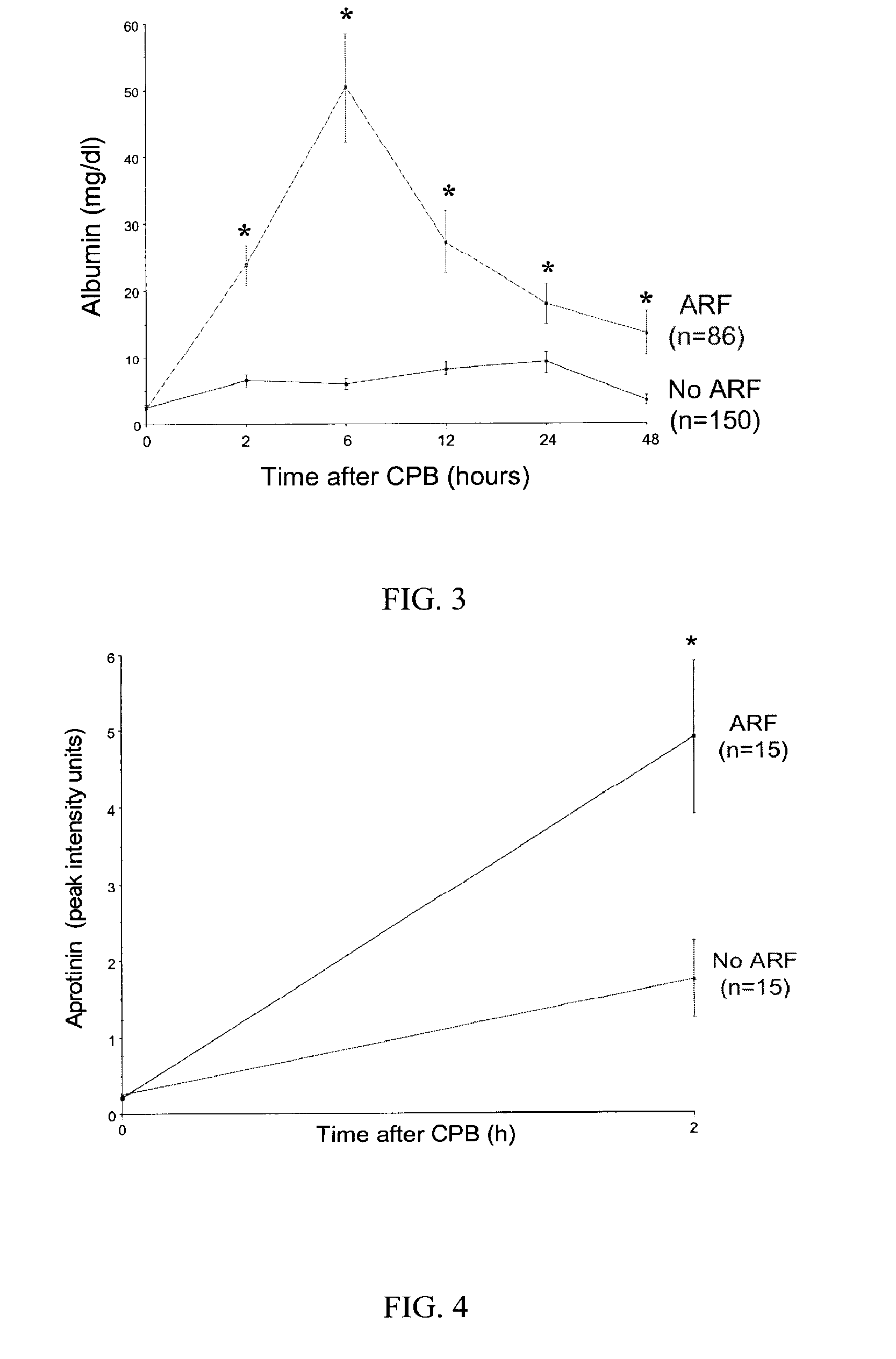
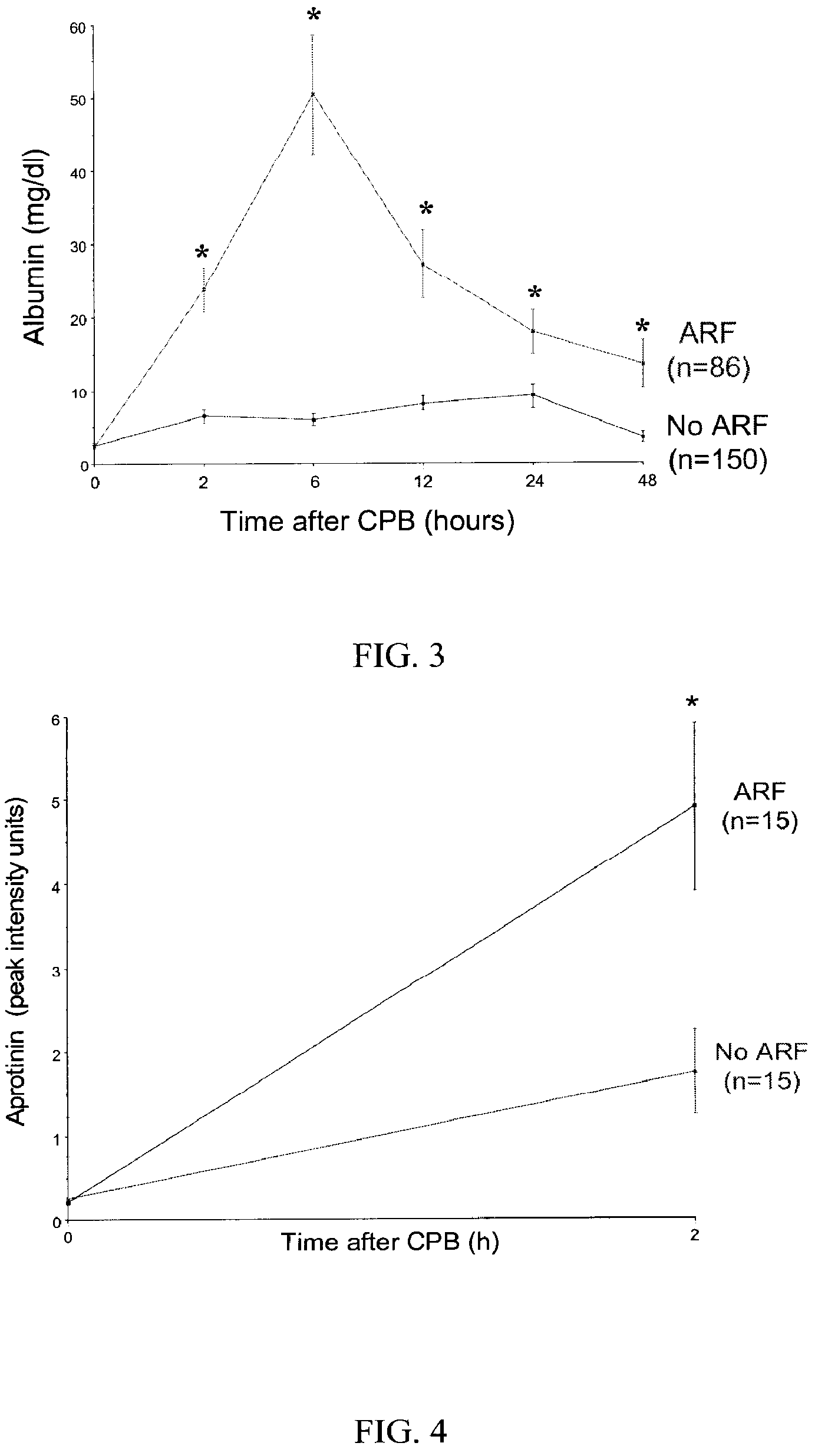
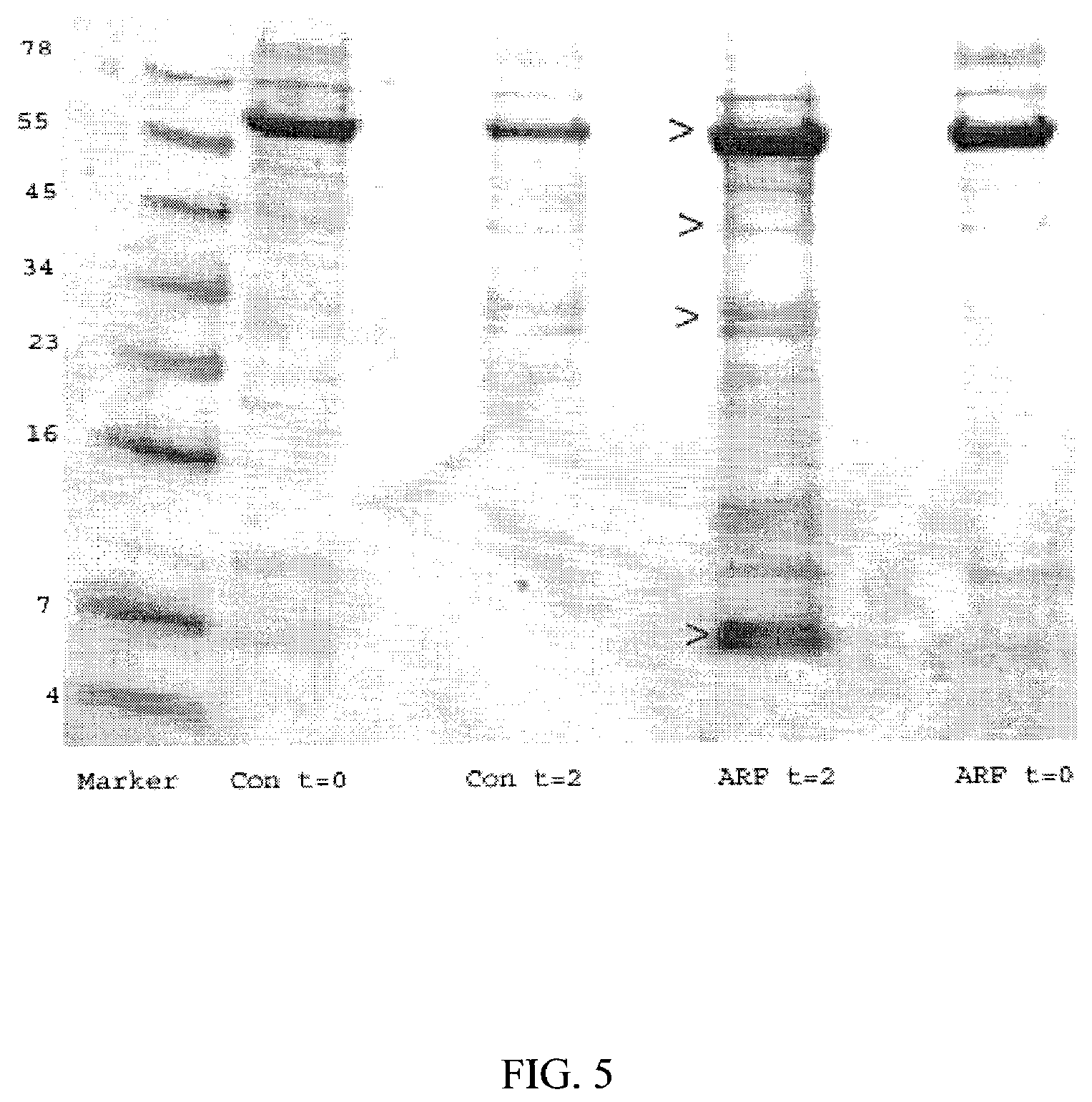
A method and kit for identifying the presence of an early biomarker of impaired renal status following a renal event in a mammalian subject. The method typically comprises (a) providing a body fluid sample obtained from a mammalian subject following a renal event; and (b) detecting in the provided sample the presence of a protein selected from the group consisting of aprotinin, alpha-1-microglobulin (A1M), alpha-1-acid-glycoprotein (A1AG), microalbumin, and combinations thereof, the presence thereof serving as an early biomarker of a change in renal status. The method can include a kit for point-of-care detection of the early biomarker of impaired renal status. Identification of the presence or absence of the early biomarker typically directs a caregiver's therapeutic decision regarding managing treatment of the subject for impaired renal status The invention also includes a method of assessing the administration of aprotinin during cardiopulmonary bypass surgery and provides for methods where the level of aprotinin in the subject's urine directs a caregiver's therapeutic decision regarding the intra-operative administration of aprotinin.
Owner:NIH
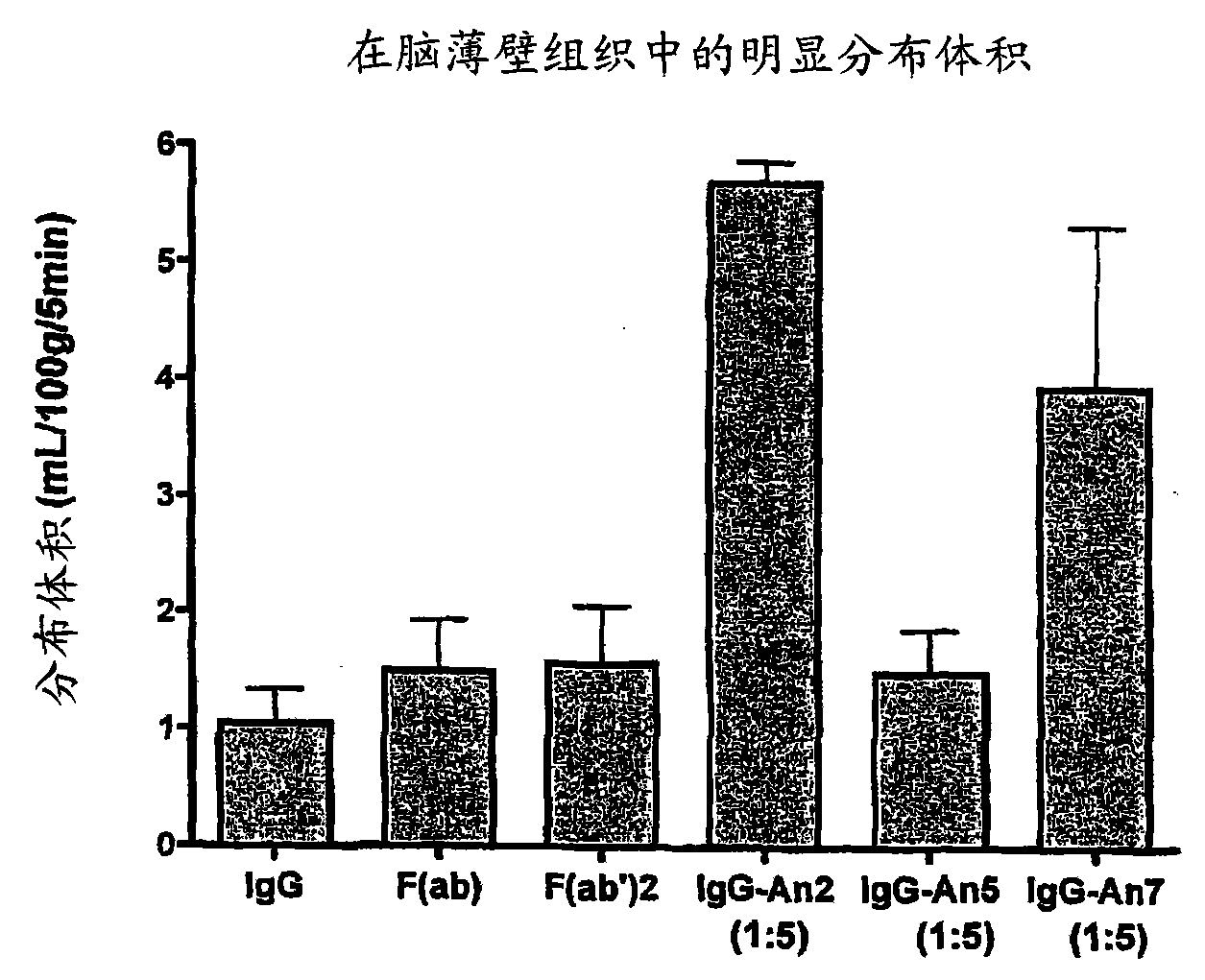
Delivery of antibodies to the central nervous system
InactiveUS20090016959A1Improve biological activityNon-invasiveCompounds screening/testingNervous disorderDiseaseAprotinin
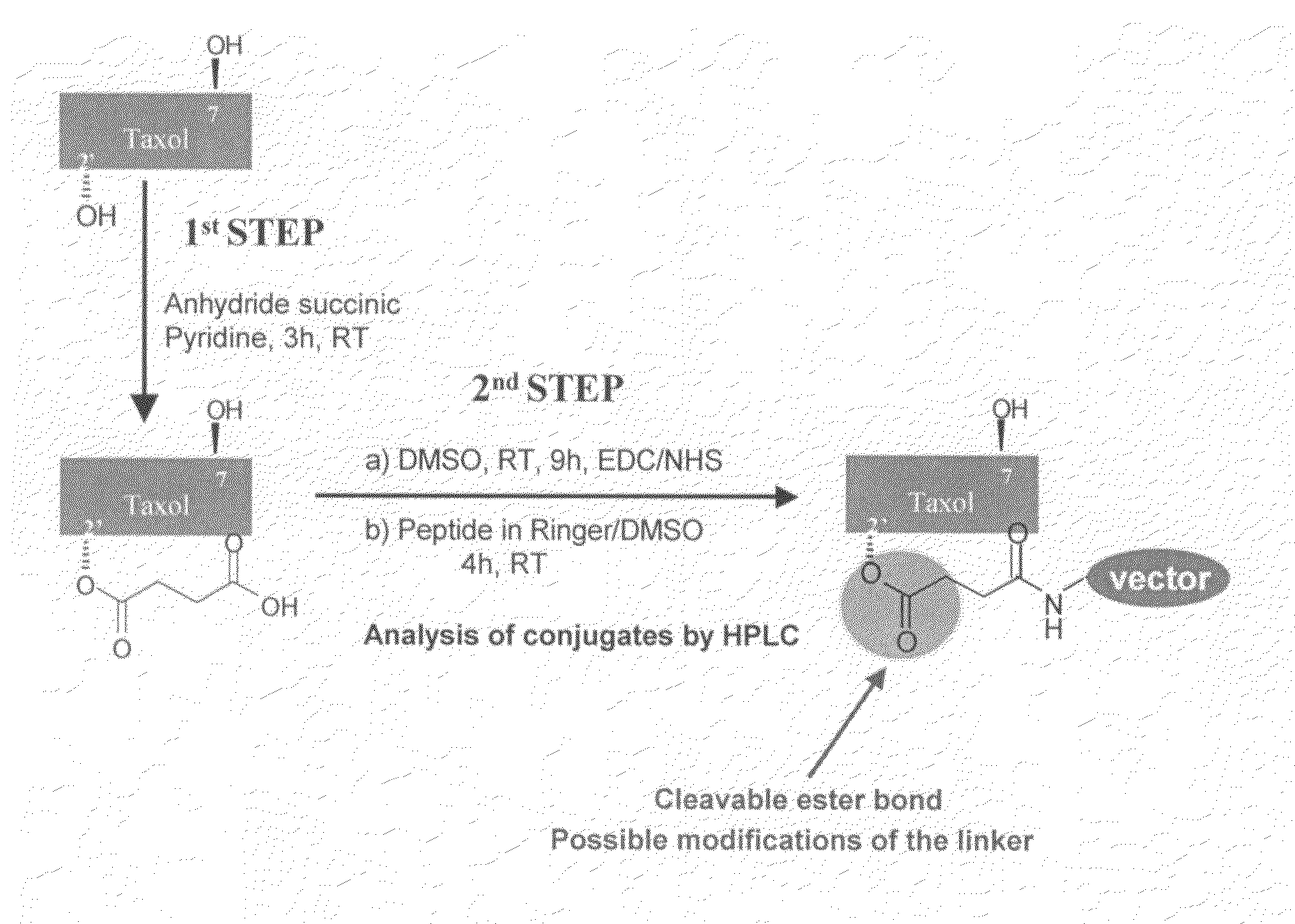
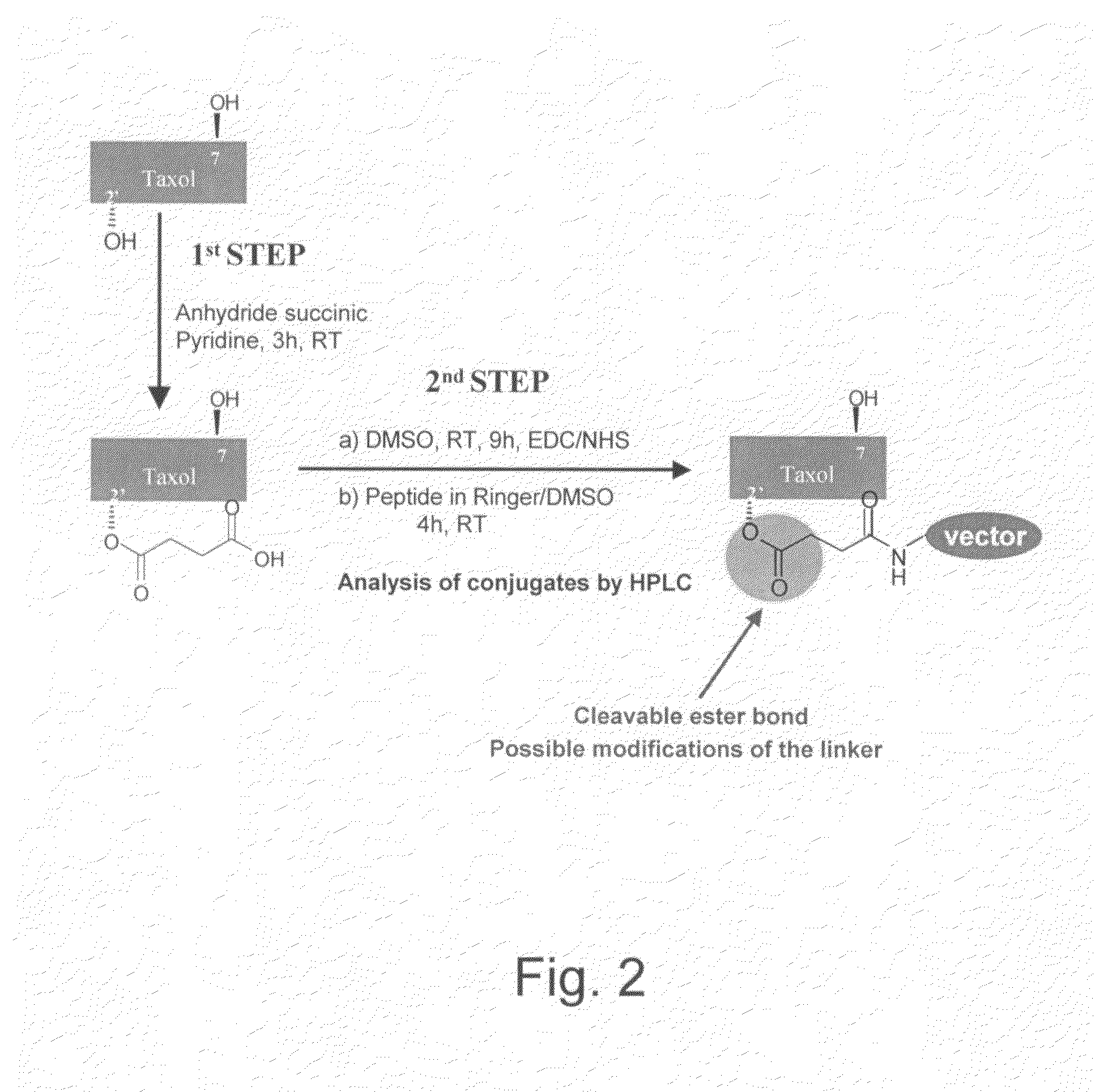
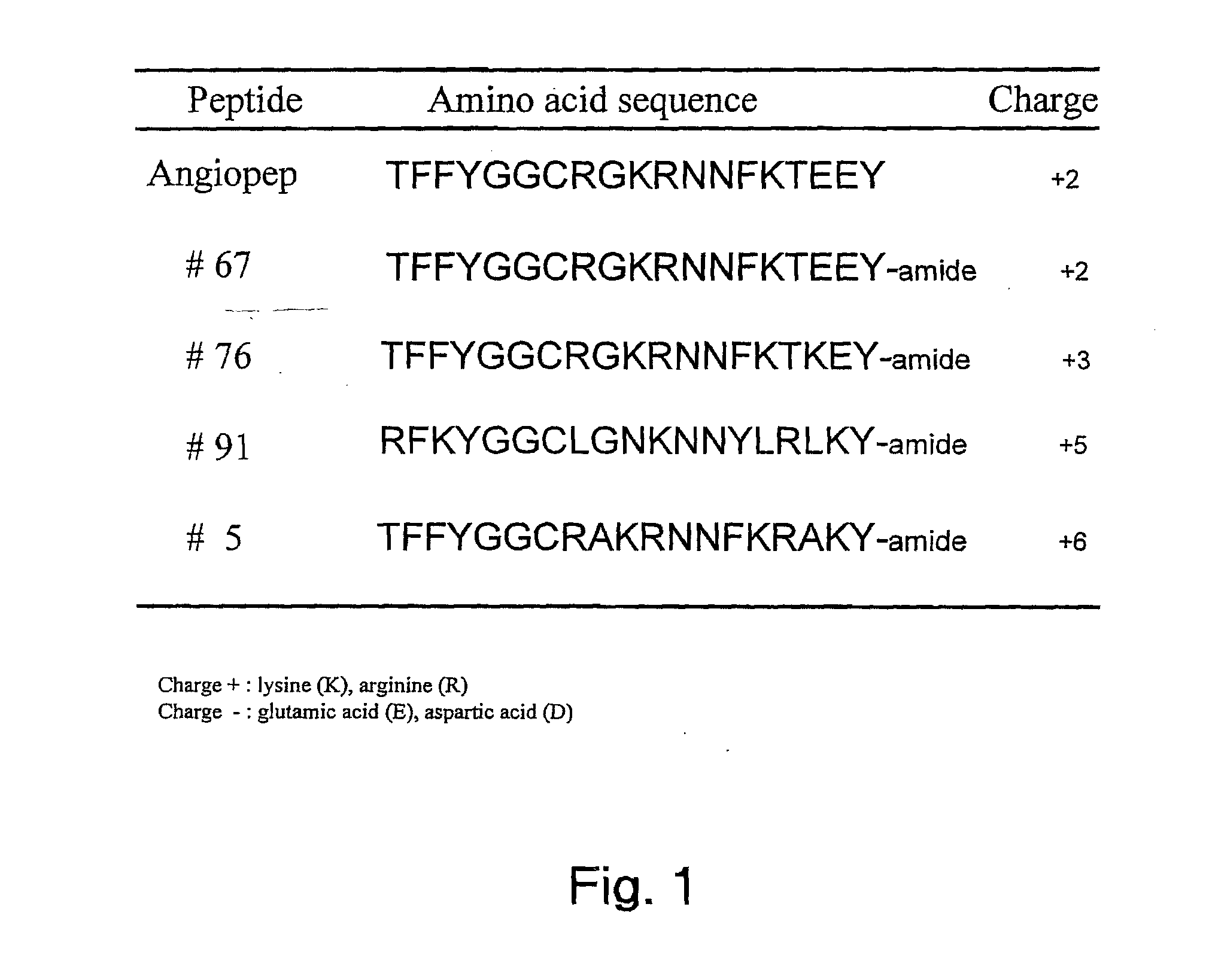
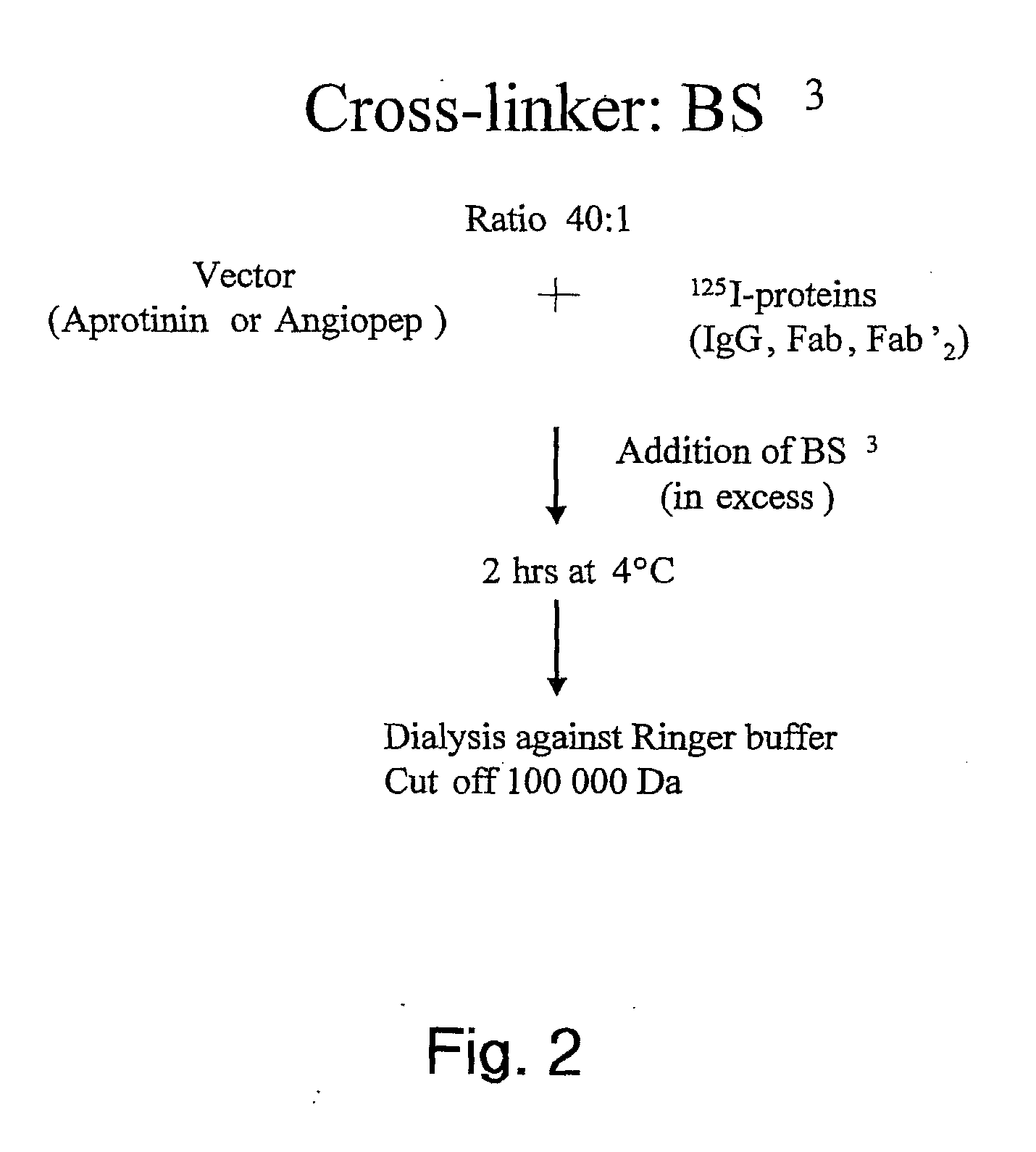
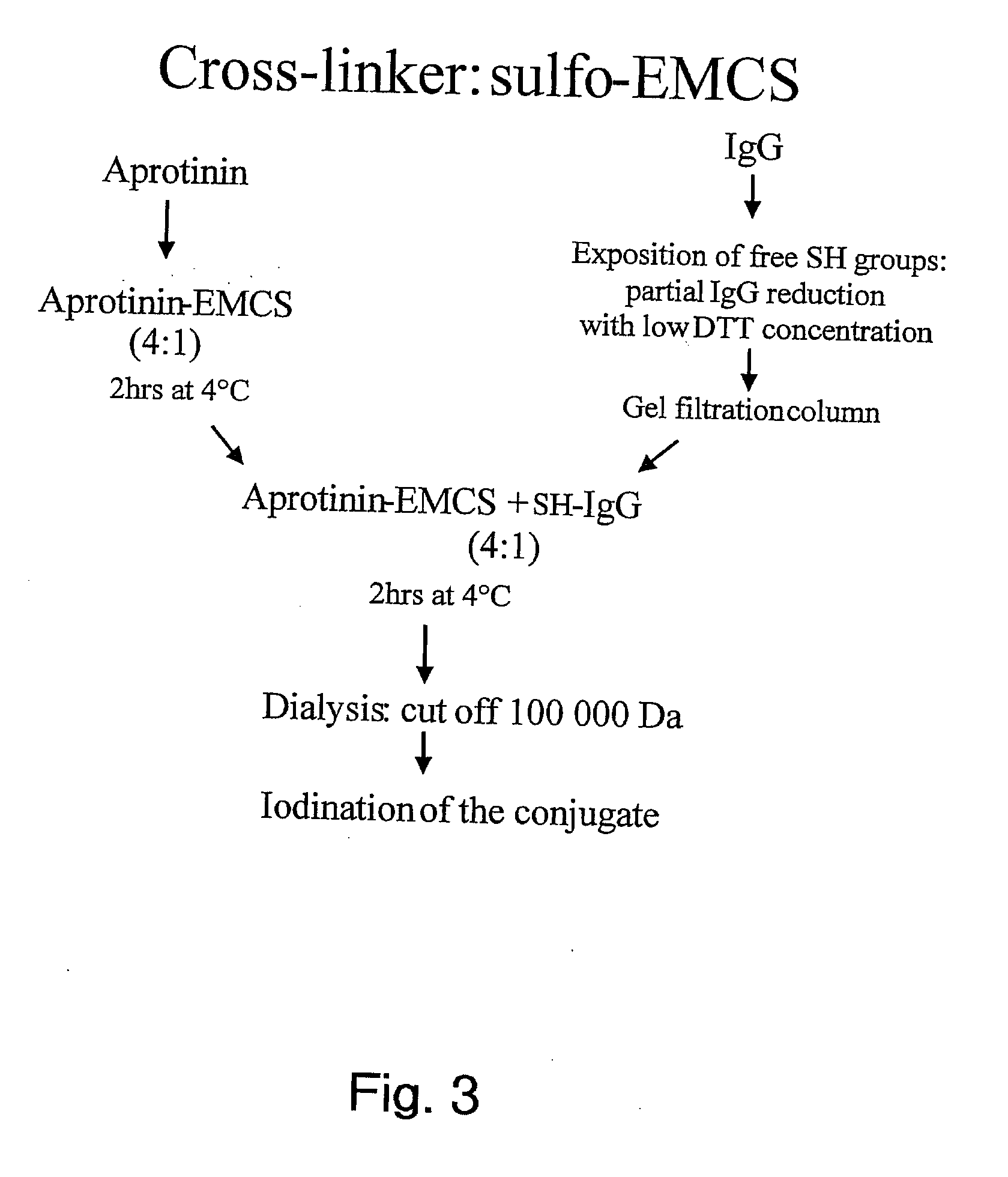
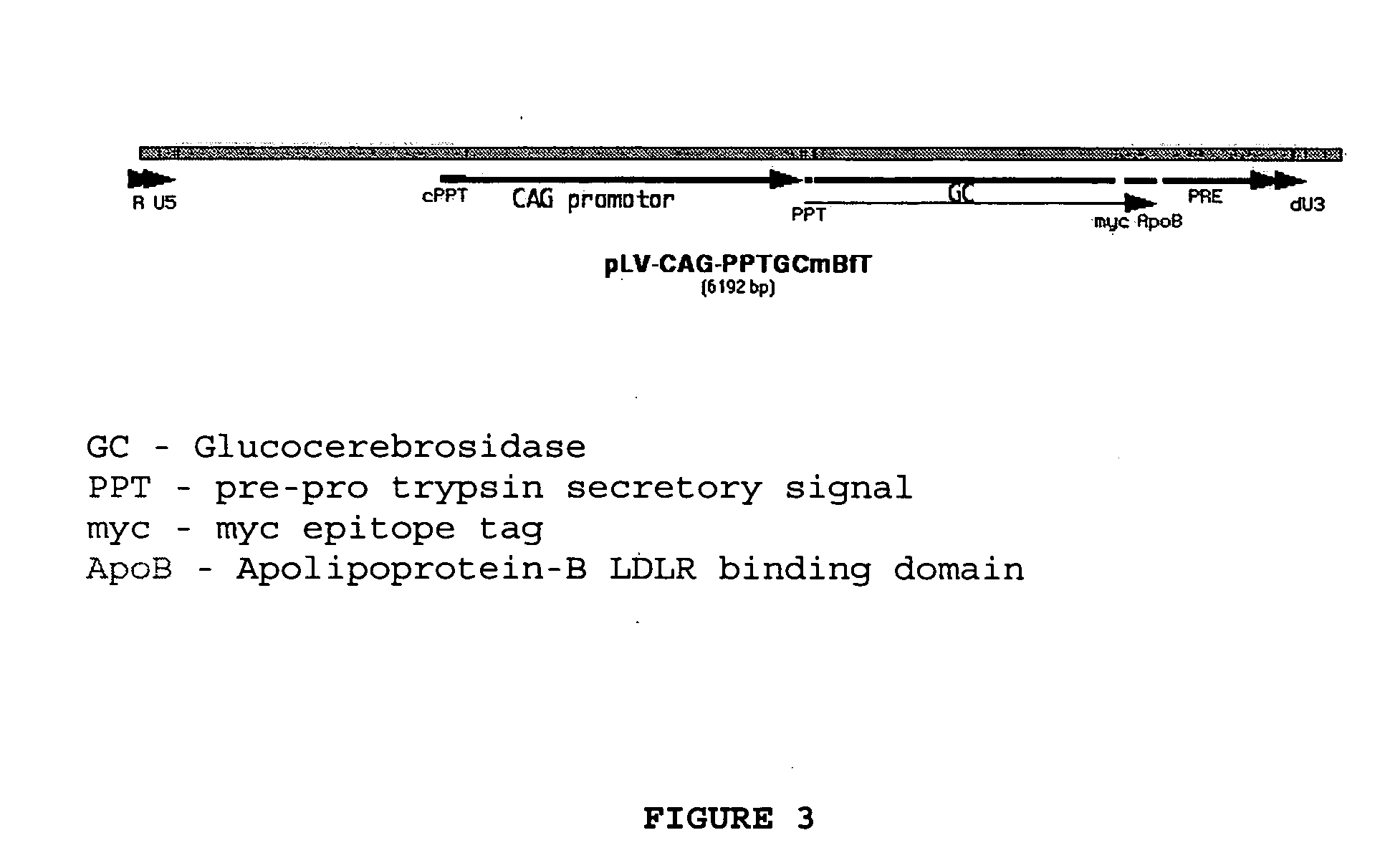
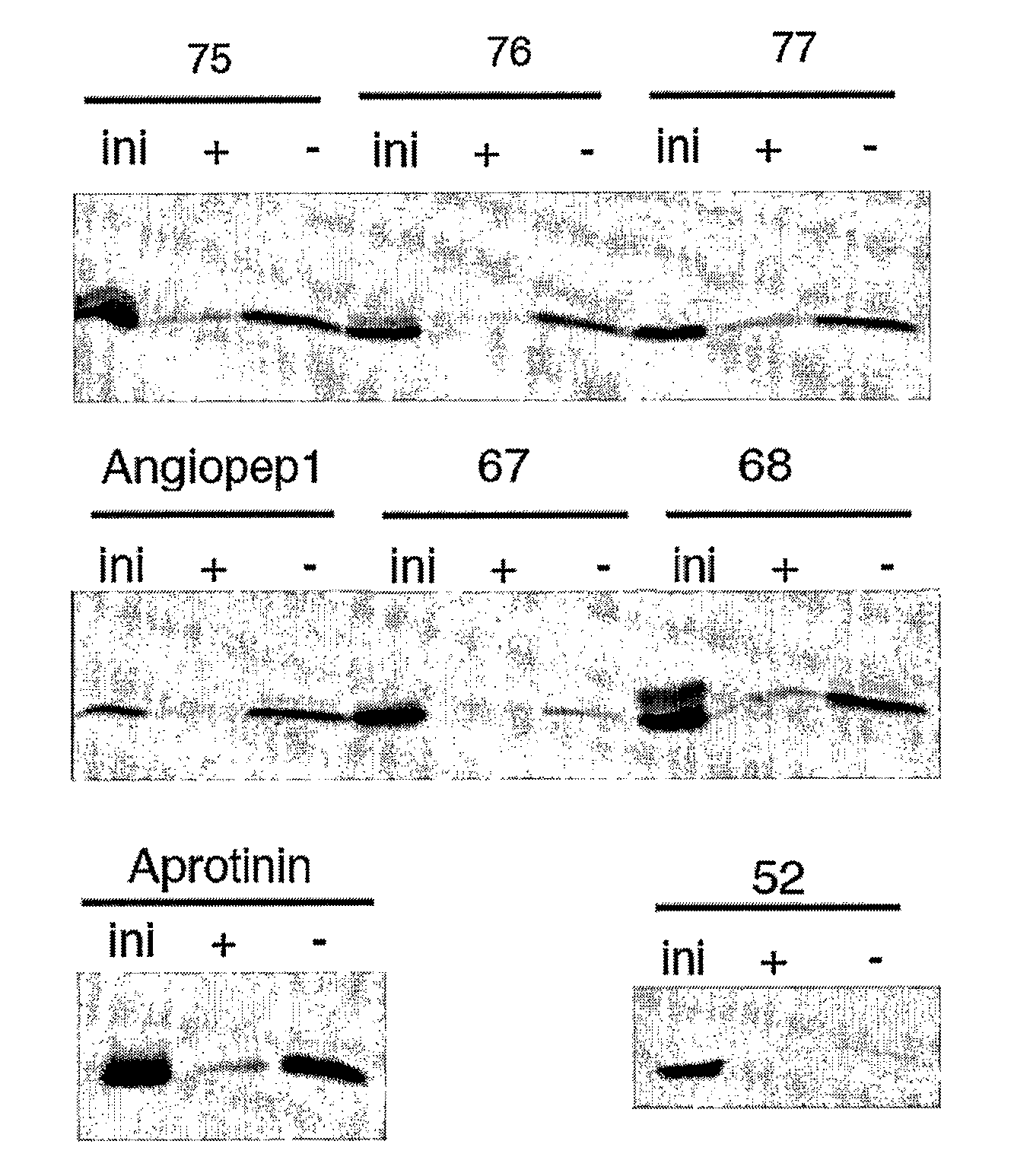
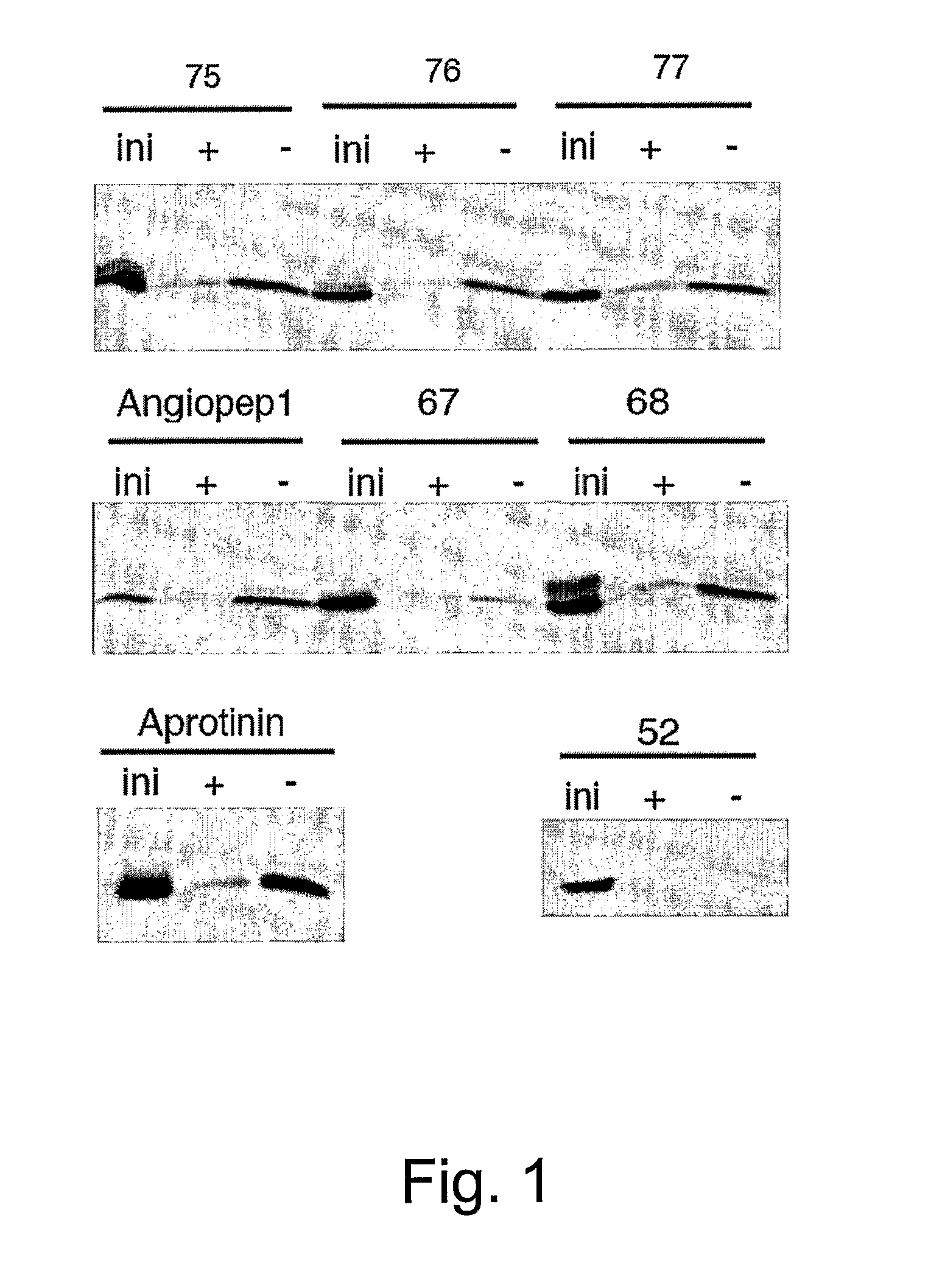
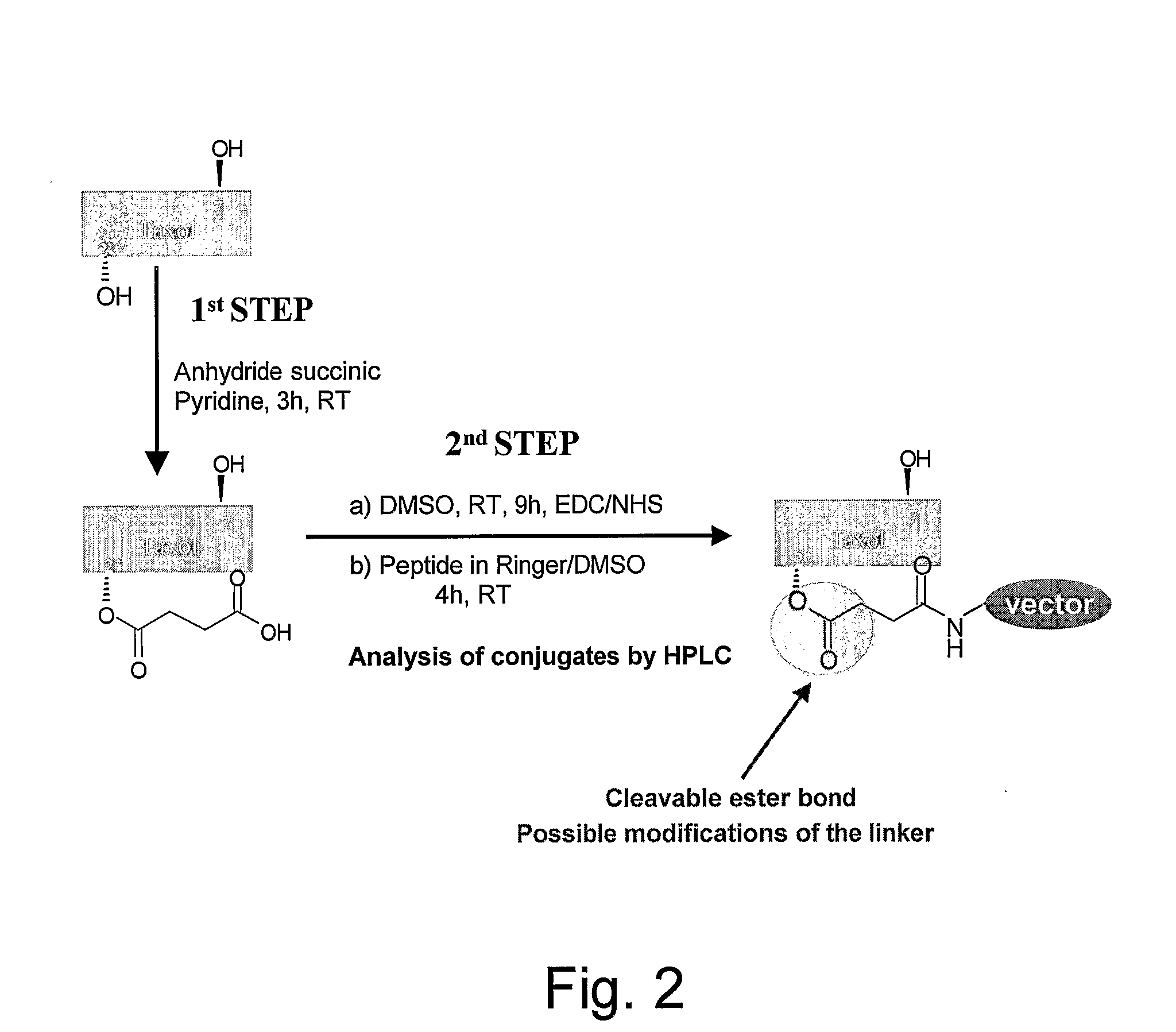
The invention relates to improvements in the field of drug delivery. More particularly, the invention relates to polypeptide derived from aprotinin and from aprotinin analogs as well as conjugates and pharmaceutical compositions comprising these polypeptides. The present invention also relates to the use of these polypeptide for transporting an antibody or antibody fragment across the blood-brain barrier of an individual and in the treatment and diagnosis of neurological diseases.
Owner:ANGLACHEM INC
Aprotinin-like polypeptides for delivering agents conjugated thereto to tissues
ActiveUS20100297120A1Altered tissue distributionPromote accumulationPeptide/protein ingredientsPeptide preparation methodsDiseaseAprotinin
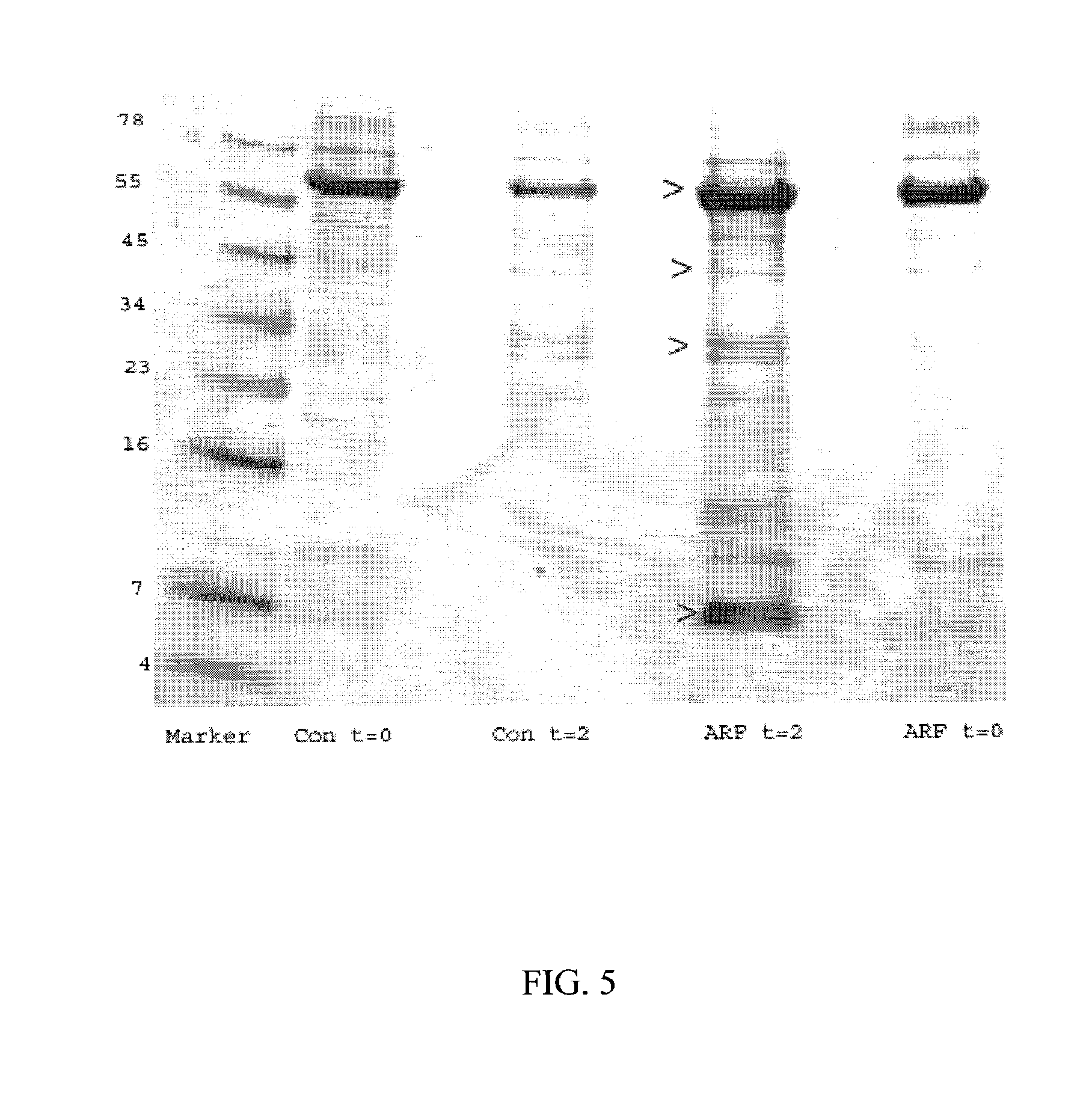
Based on our identification of a polypeptide (Angiopep-7) that is efficiently transported to cells such as liver, lung, kidney, spleen, and muscle, the invention provides polypeptides, conjugates including the polypeptides, and methods for treating diseases associated with these cell types. Unlike other aprotinin related polypeptides identified herein (including Angiopep-3, Angiopep-4a Angiopep-4b Angiopep-5, and Angiopep-6) which efficiently cross the blood-brain barrier (BBB), Angiopep-7 is not efficiently transported across the BBB.
Owner:ANGLACHEM INC
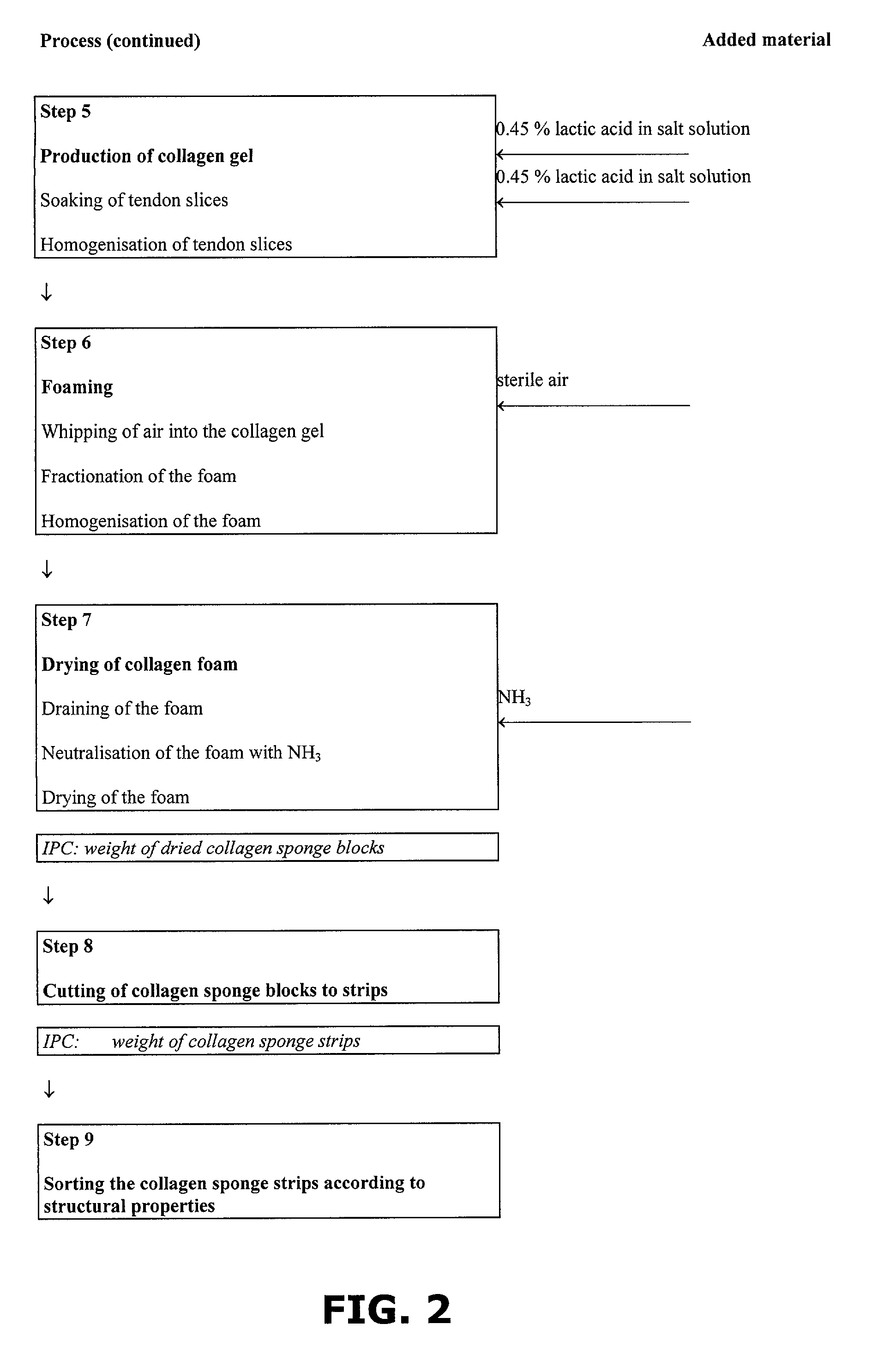
Method of preparing a collagen sponge, a device for extracting a part of a collagen foam, and an elongated collagen sponge
InactiveUS7098315B2Improve featuresHigh densitySurgical adhesivesPeptide/protein ingredientsAprotininFibrin glue
A method of preparing a collagen sponge comprises mixing air into a collagen gel, so as to obtain a collagen foam which is dried. From the dried product thereby obtained, collagen sponge is obtained by isolating parts of sponge with a chamber diameter of more than 0.75 mm and less than 4 mm, or parts with an average chamber diagonal dimension of 3 mm. The collagen sponge may be used as a material for sealing wounds, possibly with a coating comprising a fibrin glue, such as a combination of fibrinogen, thrombin and aprotinin. A device for extracting a part of a collagen foam and for degenerating another part of the collagen foam to a collagen gel is disclosed. An elongated collagen sponge having a through-going hole or bore and a flexible wall may be used for re-establishing walls in a mammalian gastrointestinal funnel or trachea system.
Owner:TOPAZ INVESTMENT AS
Carrier with solid fibrinogen and solid thrombin
InactiveUS7052713B2Safety and efficacyShorten hemostasis timePowder deliverySurgical adhesivesNatural sourceFiber
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibers. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Method and kit for the early detection of impaired renal status
InactiveUS7662578B2Pharmaceutical containersMedical packagingPoint of careExtracorporeal circulation
Owner:NIH
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin. The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Compositions and methods for targeting a polypeptide to the central nervous system
InactiveUS20050100986A1Nervous disorderPeptide/protein ingredientsPseudomonas aeruginosa exotoxin AInsulin-like growth factor
The invention provides a chimeric CNS targeting polypeptide having a BBB-receptor binding domain and a payload polypeptide domain. The chimeric CNS targeting polypeptide can have a BBB-receptor binding domain consisting of a receptor binding domain from ApoB, ApoE, aprotinin, lipoprotein lipase, PAI-1, pseudomonas exotoxin A, transferrin, α2-macroglobulin, insulin-like growth factor, insulin, or a functional fragment thereof. Nucleic acids encoding a chimeric CNS targeting polypeptide are also provided. Further provided is a method of delivering a polypeptide to the CNS of an individual. The method consists of administering to the individual an effective amount of a chimeric CNS targeting polypeptide, said chimeric CNS targeting polypeptide comprising a BBB-receptor binding domain and a payload polypeptide domain. The method also can deliver a polypeptide to the lysosomes of CNS cells.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Aprotinin Polypeptides for Transporting a Compound Across the Blood-Brain Barrier
InactiveUS20080299039A1Non-invasiveImprove biological activityOrganic active ingredientsNervous disorderDiseaseAprotinin
The invention relates to improvements in the field of drug delivery. More particularly, the invention relates to polypeptides derived from aprotinin and from aprotinin analogs as well as conjugates and pharmaceutical compositions comprising these polypeptides or conjugates. The present invention also relates to the use of these polypeptide for transporting a compound or drug across the blood-brain barrier of a mammal and in the treatment and diagnosis of neurological diseases.
Owner:ANGLACHEM INC
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin. The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsi-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Compositions and methods for targeting a polypeptide to the central nervous system
InactiveUS20060198833A1Nervous disorderSugar derivativesPseudomonas aeruginosa exotoxin AInsulin-like growth factor
The invention provides a chimeric CNS targeting polypeptide having a BBB-receptor binding domain and a payload polypeptide domain. The chimeric CNS targeting polypeptide can have a BBB-receptor binding domain consisting of a receptor binding domain from ApoB, ApoE, aprotinin, lipoprotein lipase, PAI-1, pseudomonas exotoxin A, transferrin, α2-macroglobulin, insulin-like growth factor, insulin, or a functional fragment thereof. Nucleic acids encoding a chimeric CNS targeting polypeptide are also provided. Further provided is a method of delivering a polypeptide to the CNS of an individual. The method consists of administering to the individual an effective amount of a chimeric CNS targeting polypeptide, said chimeric CNS targeting polypeptide comprising a BBB-receptor binding domain and a payload polypeptide domain. The method also can deliver a polypeptide to the lysosomes of CNS cells.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Pharmaceutical compositions comprising combinations of factor VII polypeptides and aprotinin polypeptides
InactiveUS20060025336A1Improved and reliable and widely applicableGood coagulationPeptide/protein ingredientsDepsipeptidesAprotininFactor VII
Compositions comprising factor VII or a factor VII-related polypeptide and aprotinin or an aprotinin-related polypeptide and uses thereof are provided.
Owner:NOVO NORDISK AS
Liquid-state calibration product for determining ProGRP content
InactiveCN108107224AReduce uncontrollable factorsAccurate clinical test resultsBiological material analysisBiological testingAntigenEthylene diamine
The invention discloses a liquid-state calibration product for determining the ProGRP content. The liquid-state calibration product for determining the ProGRP content is prepared from the following components: 10 to 200mmol / L of buffer solution, 0.1 to 0.5 percent (w / v) of preservative, 1 to 5 percent (w / v) of protein stabilizer, 5 to 10 percent (w / v) of sugar stabilizer, 1 to 5 percent (w / v) of EDTA (Ethylene Diamine Tetraacetic Acid), 0.1 to 0.5 percent (w / v) of surfactant, 0 to 5000pg / mL of ProGRP antigen, and 1 to 10mg / L of aprotinin. The liquid-state calibration product for determining the ProGRP content provided by the invention has the advantages that no freeze drying is needed, no redissolving is needed when in use, uncontrollable factors probably occurred in a redissolving processare reduced, and a clinical detection result is more accurate. The sugar stabilizer, a metalloproteinase inhibitor (EDTA), the aprotinin and other components are added, so that the stability of the ProGRP protein is effectively improved, and the prepared ProGRP liquid-state calibration product is better in stability and can be stably stored for 12 months by being refrigerated at 2 to 8 DEG C.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Tissue sealant in which collagen and fibrin are mixed, and method for preparing same
InactiveUS20150320904A1High strengthReduce degradationPeptide/protein ingredientsInfusion syringesTissue sealantWeakness
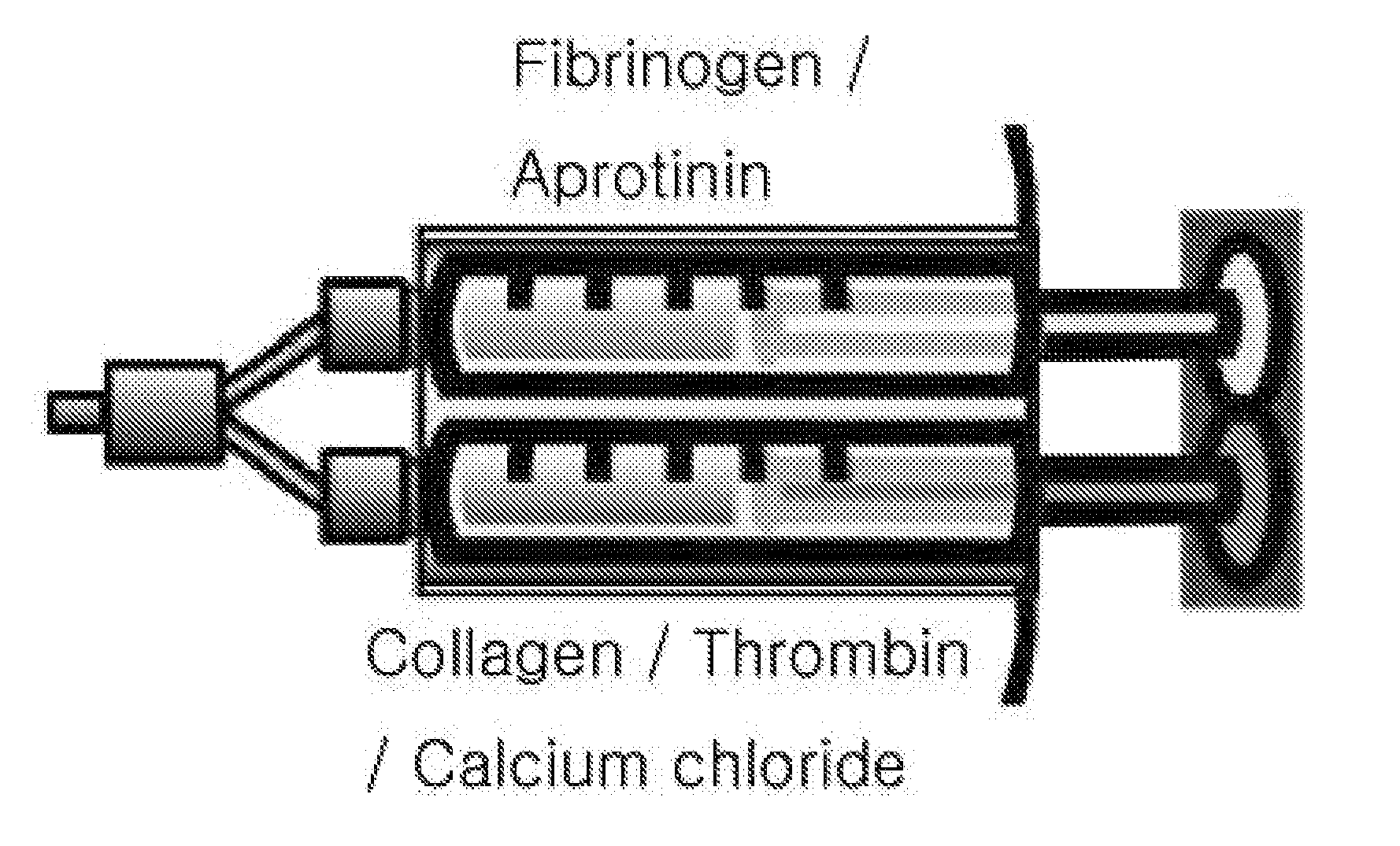
The present invention relates to a tissue sealant in which collagen and fibrin are mixed and to a method for preparing same. To this end, the method of the present invention comprises the steps of: mixing a first material using fibrinogen and aprotinin; mixing a second material using thrombin, calcium chloride, and collagen; and preparing a third material by mixing the first material and the second material. The tissue sealant prepared by the method may supplement weaknesses, i.e. strength and degradability, of the fibrin sealant which is currently available in the commercial marketplace. Further, the tissue sealant of the present invention is cytophilic and activates blood platelets contained in blood so as to induce tissue regeneration. Thus, quality and reliability of products can be significantly improved so as to satisfy various needs of consumers who are users, thereby presenting good image.
Owner:SEWON CELLONTECH CO LTD
Composition for inducing tissue regeneration by activating platelet-rich plasma (PRP)
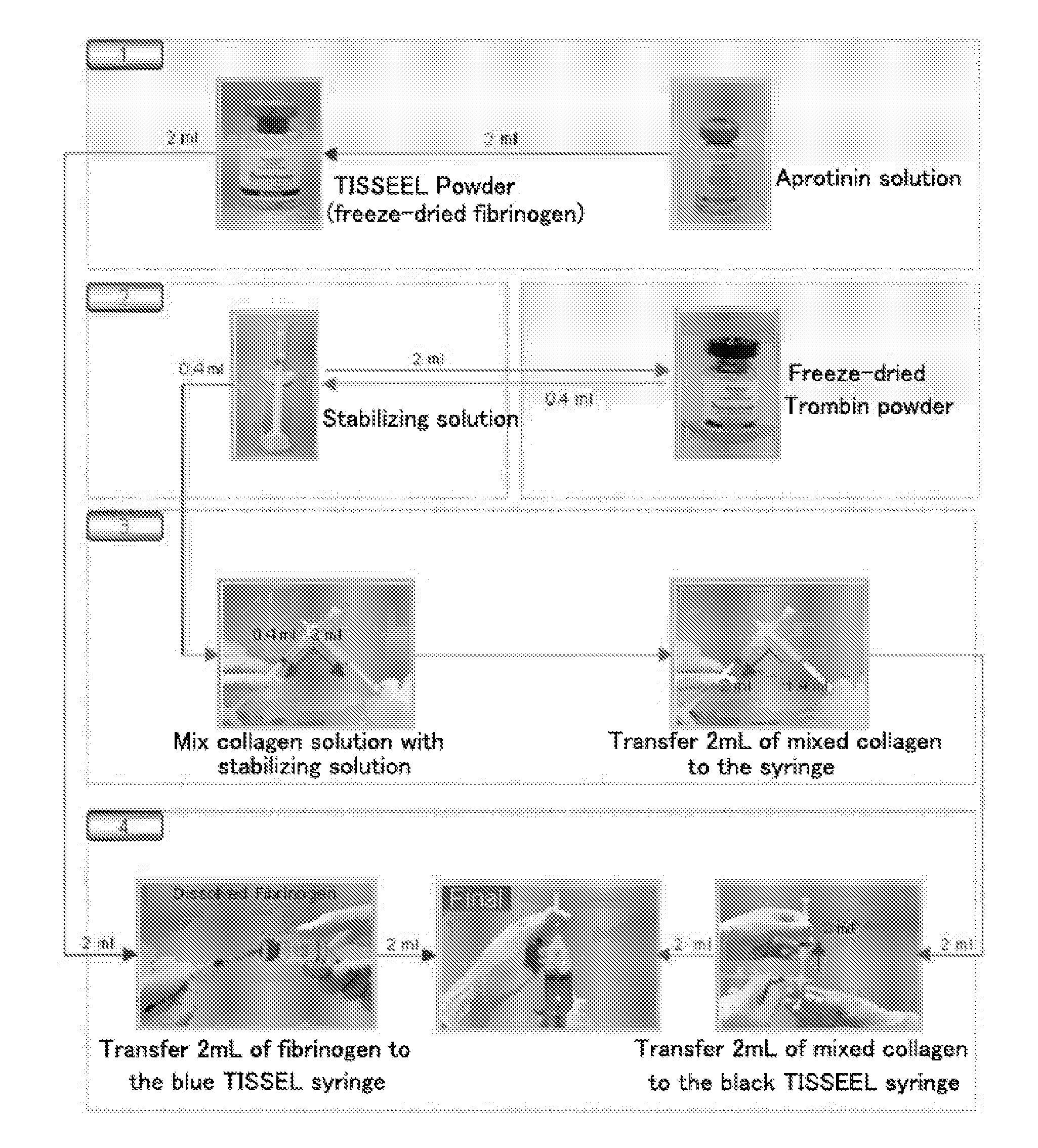
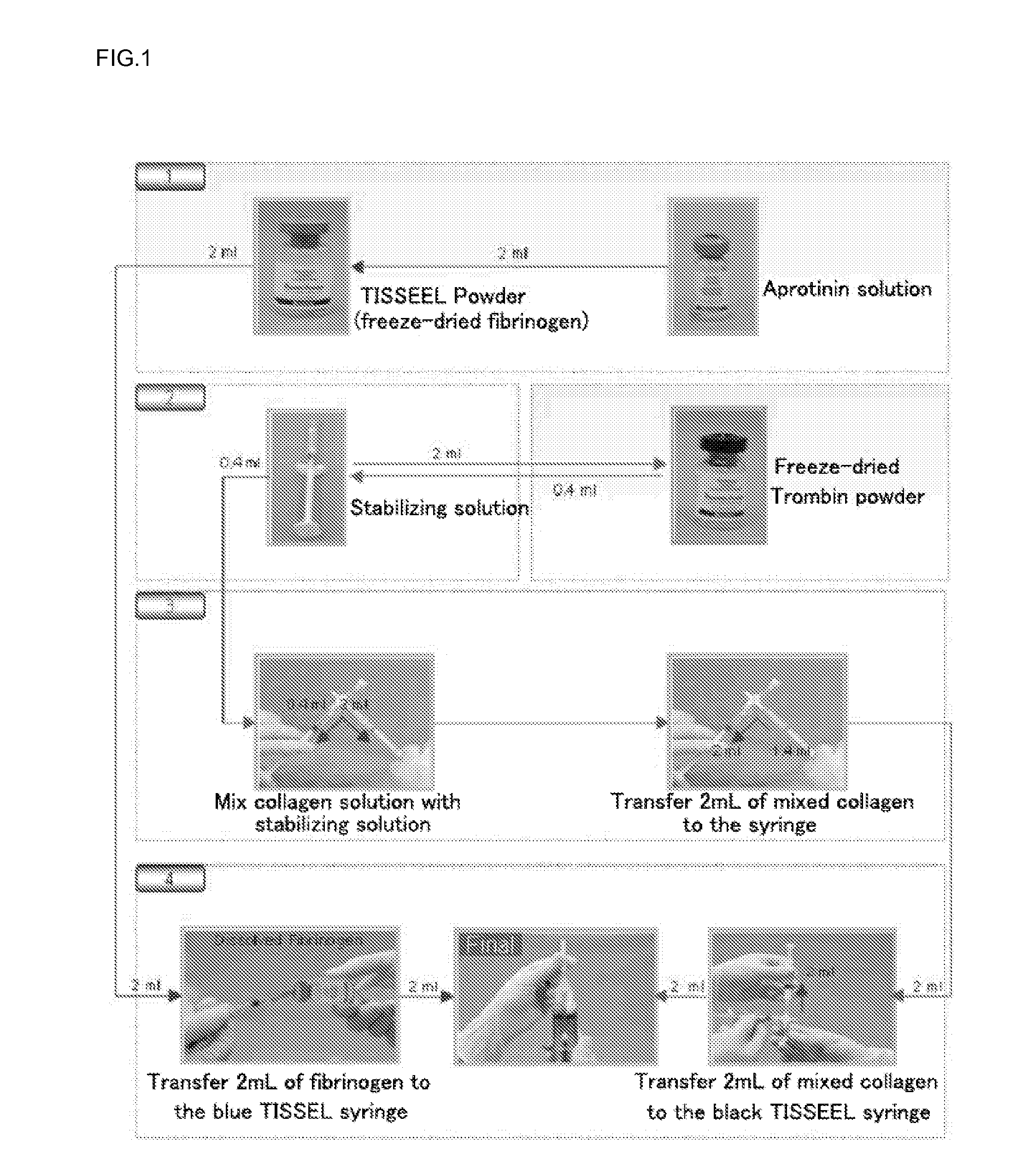
ActiveUS9011929B2Improve the problemImprove reactionPeptide/protein ingredientsMammal material medical ingredientsFiberFreeze-drying
The present invention relates to a composition for cartilaginous tissue repair and to a production method therefor. The present invention comprises the steps of: (a) dissolving freeze-dried fibrinogen in an aprotinin solution; (b) dissolving freeze-dried thrombin in a stabilizing solution; (c) mixing an enriched collagen solution with thrombin and the stabilizing solution; and installing the fibrinogen solution (a) to one side of a dual kit and the solution (c) containing the collagen to the other side, and then mixing and injecting into damaged cartilaginous tissue. In the present invention, which is constituted as described above, biomaterials such as collagen and fibrin are mixed so as to allow damaged cartilaginous tissue to be repaired to a state allowing transplantation onto the tissue, and efficient regeneration is induced, thereby making it possible to reduce surgery-related stress on people and animals while inducing relatively rapid and efficient cartilage repair and regeneration.
Owner:CELLONTECH
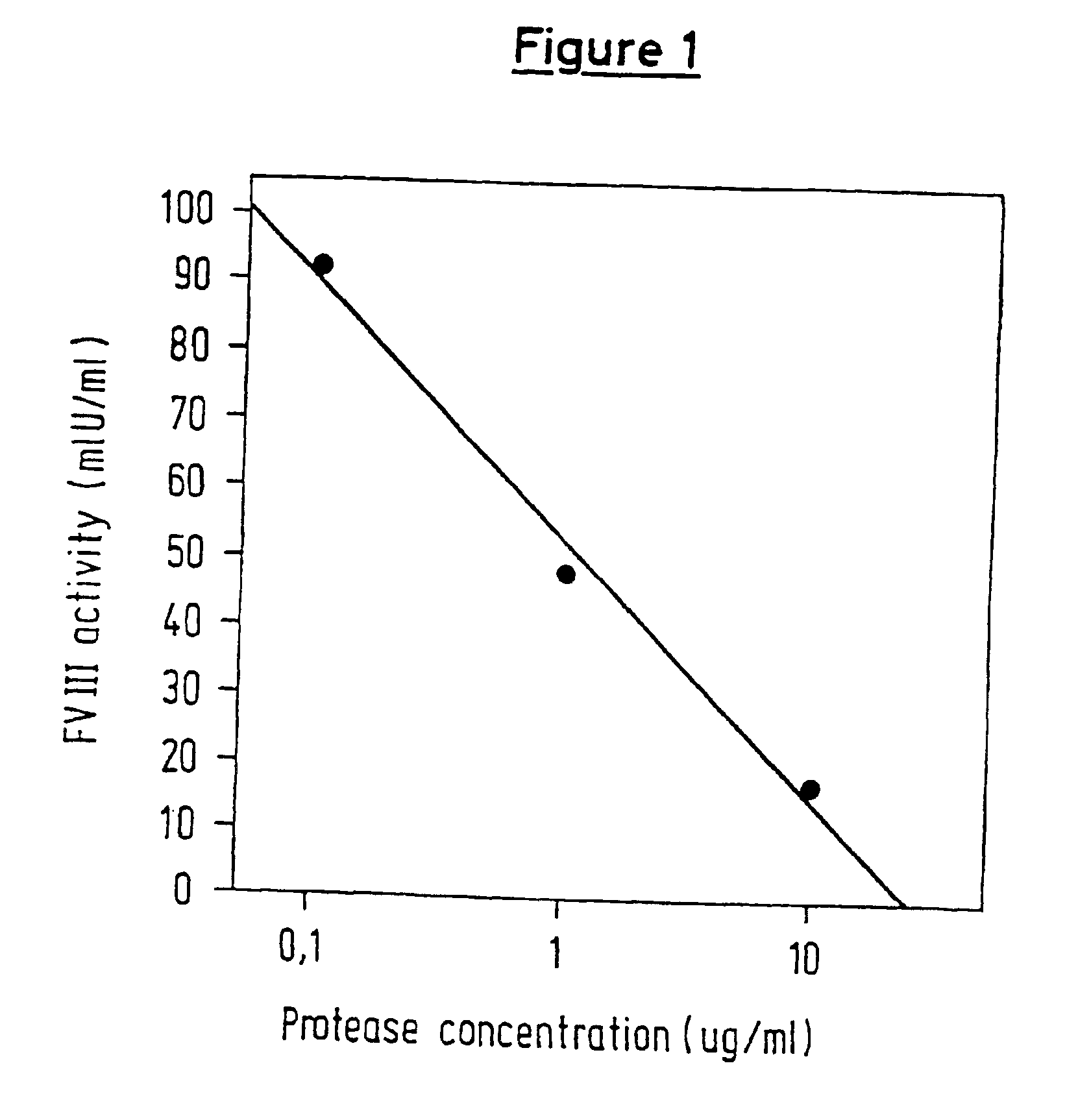
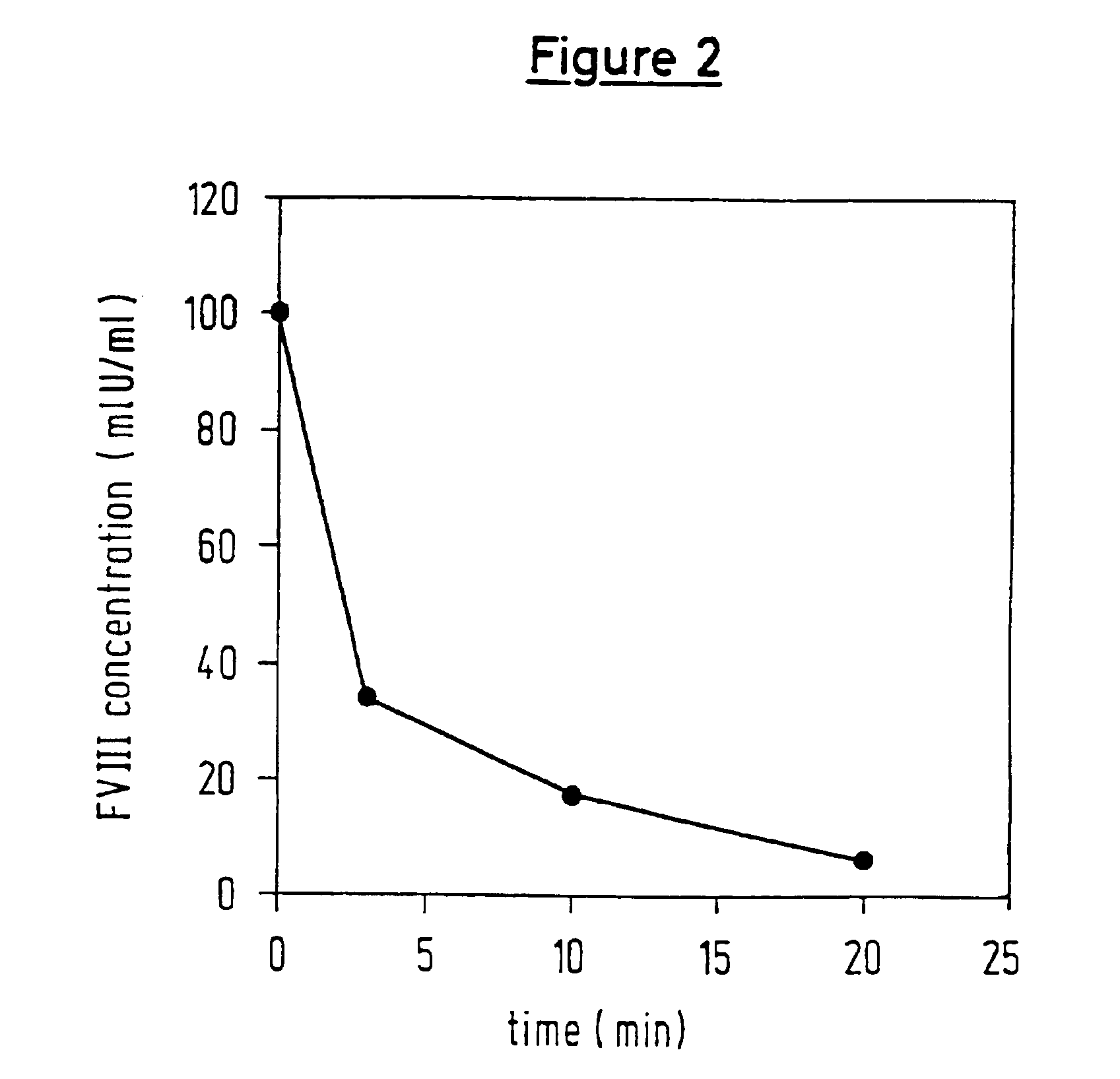
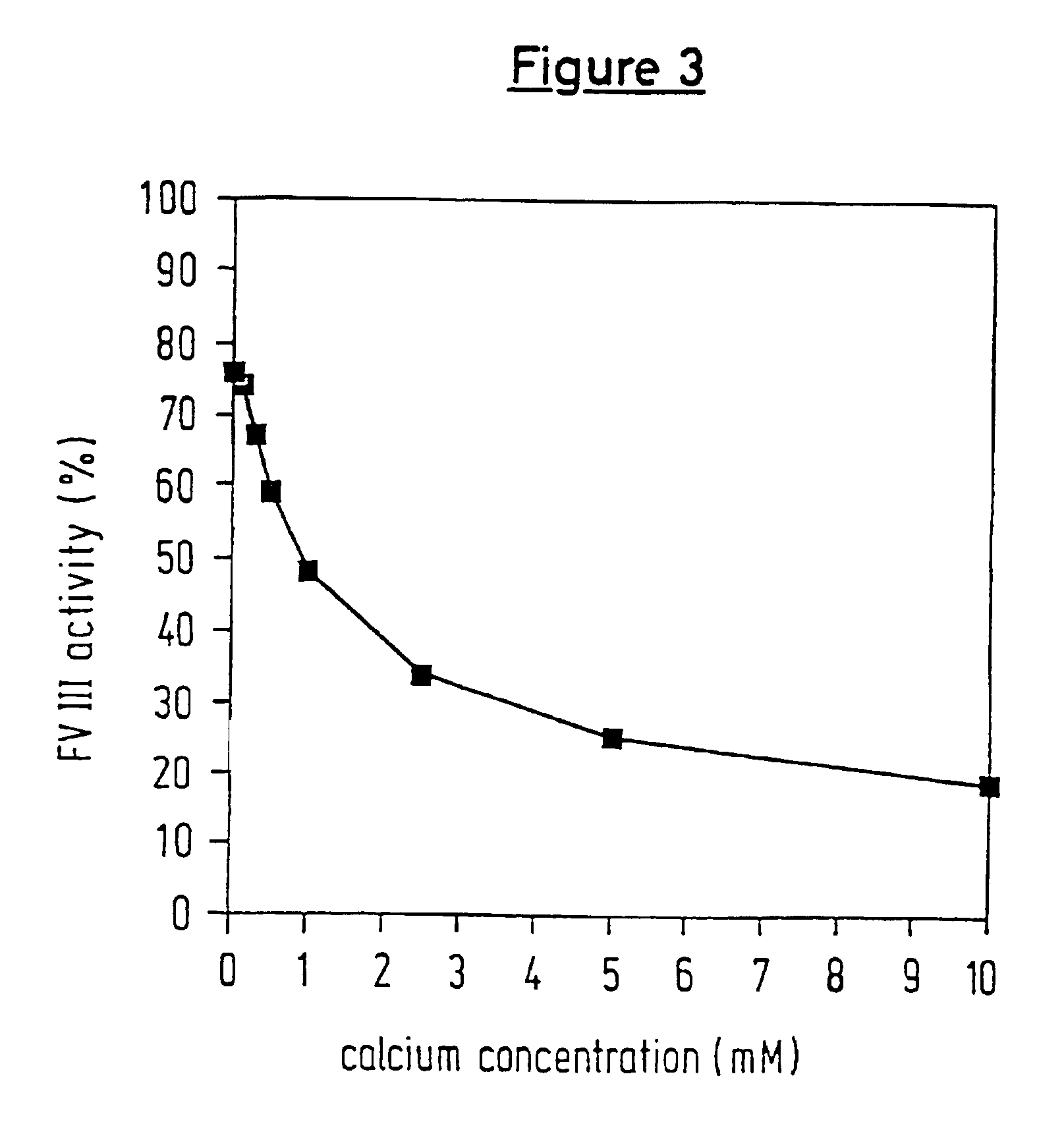
Protease for activating clotting factor VII
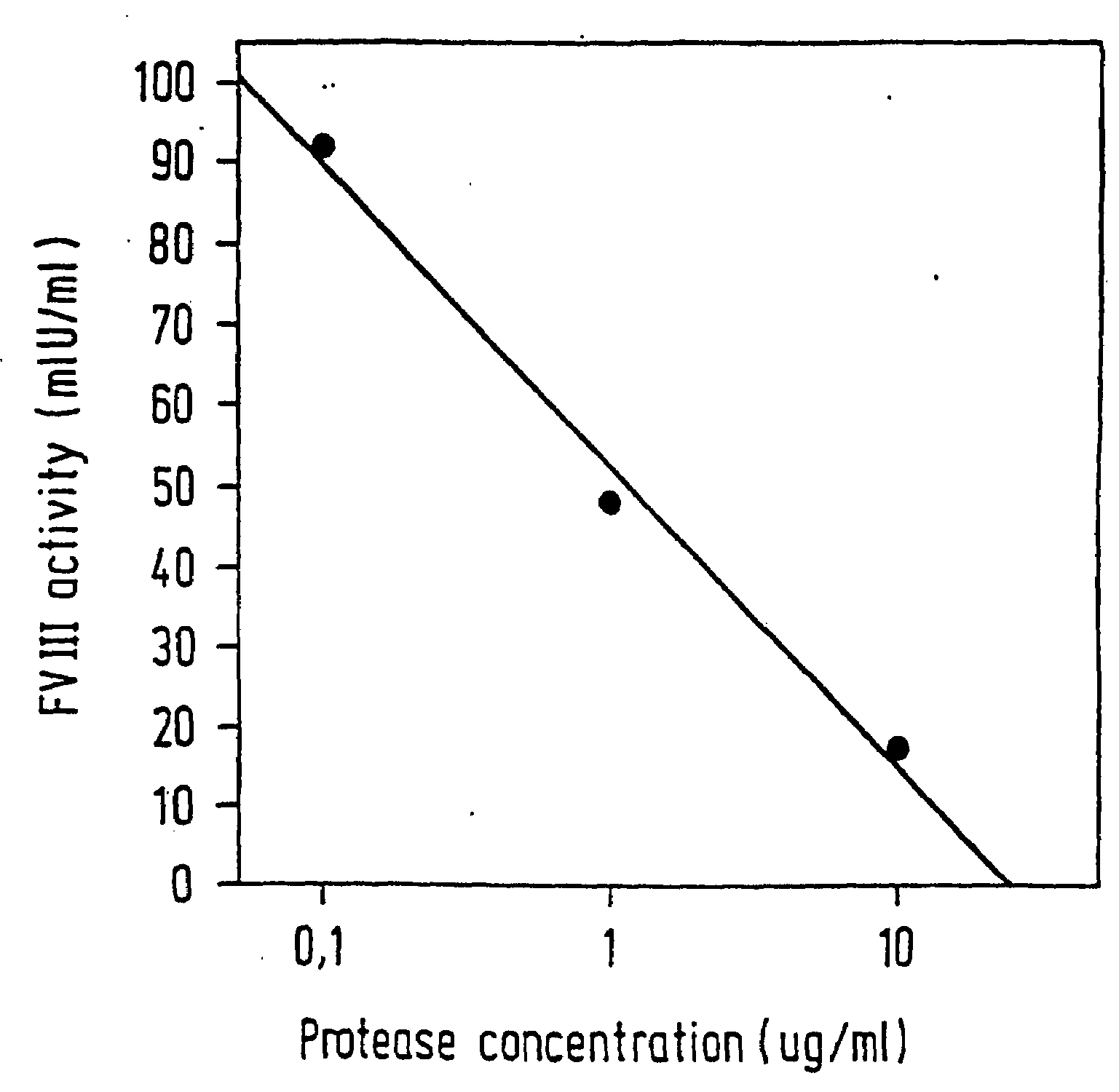
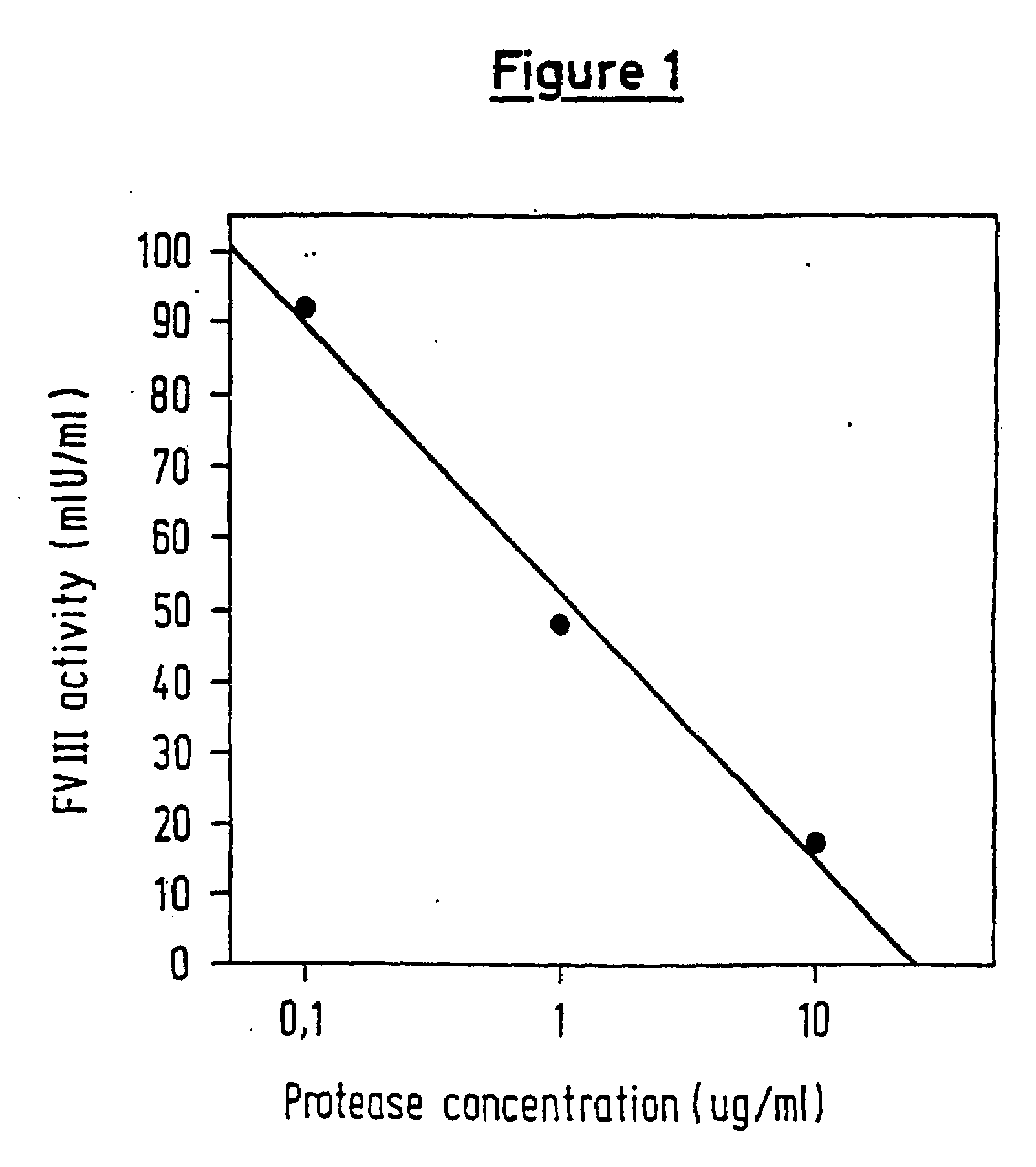
InactiveUS6911334B2High amidolytic activityHigh activityPeptide/protein ingredientsHydrolasesZymogenStaining
A protease for activating the blood clotting factor VII is described, wherein the protease is inhibited by the presence of aprotinin and is increased in its activity by calcium ions and / or heparin or heparin-related substances, and wherein in SDS-PAGE, on subsequent staining in the non-reduced state, the protease has one or more brands in the molecular weight range from 50 to 75 kDa, and in the reduced state, the protease has a band at 40 to 55 kDa and one or more bands in the molecular weight range from 10 to 35 kDa. The proenzyme of this protease is also characterized. Further, a process for obtaining this protease and its use in hemorrhage prophylaxis or hemostasis is described. Moreover, a test system for the qualitative and quantitative detection of a protease which activates the blood clotting factor VII is described.
Owner:CSL BEHRING GMBH
A suspension comprising fibrinogen, thrombin and alcohol and a method of coating a carrier with the same
A suspension of fibrinogen, thrombin, alcohol and optionally aprotinin is obtained by mixing fibrinogen in alcohol with thrombin in alcohol. The suspension contains fibrinogen and thrombin particles with a Folk Ward mean diameter of 25-100 mum. The thrombin may be human, bovine or recombinant. The fibrinogen may be human or recombinant. A method for coating a carrier, such as a collagen sponge, with the suspension, and a method for drying the coating is disclosed. The coated collagen carrier may be used as a ready-to-use absorbable composition for tissue gluing, tissue sealing and hemostasis wherein the carrier is coated with solidly fixed components of fibrin glue, i.e. fibrinogen and thrombin.
Owner:TAKEDA NYCOMED
Aprotinin-like polypeptides for delivering agents conjugated thereto to tissues
Based on our identification of a polypeptide (Angiopep-7) that is efficiently transported to cells such as liver, lung, kidney, spleen, and muscle, the invention provides polypeptides, conjugates including the polypeptides, and methods for treating diseases associated with these cell types. Unlike other aprotinin related polypeptides identified herein (including Angiopep-3, Angiopep-4a Angiopep-4b Angiopep-5, and Angiopep-6) which efficiently cross the blood-brain barrier (BBB), Angiopep-7 is not efficiently transported across the BBB.
Owner:ANGLACHEM INC
Composition for inducing tissue regeneration by activating platelet-rich plasma (PRP), and method for manufacturing same
ActiveUS20120201897A1Improve the problemImprove reactionPeptide/protein ingredientsMammal material medical ingredientsFiberPlatelets blood
The present invention relates to a composition for cartilaginous tissue repair and to a production method therefor. The present invention comprises the steps of: (a) dissolving freeze-dried fibrinogen in an aprotinin solution; (b) dissolving freeze-dried thrombin in a stabilizing solution; (c) mixing an enriched collagen solution with thrombin and the stabilizing solution; and installing the fibrinogen solution (a) to one side of a dual kit and the solution (c) containing the collagen to the other side, and then mixing and injecting into damaged cartilaginous tissue. In the present invention, which is constituted as described above, biomaterials such as collagen and fibrin are mixed so as to allow damaged cartilaginous tissue to be repaired to a state allowing transplantation onto the tissue, and efficient regeneration is induced, thereby making it possible to reduce surgery-related stress on people and animals while inducing relatively rapid and efficient cartilage repair and regeneration.
Owner:CELLONTECH
Composition and method for repairing nerve damage and enhancing functional recovery of nerve
InactiveUS20060083734A1Efficient repairPromote functional recoveryOrganic active ingredientsBiocideFibrin glueAprotinin
The present invention relates to a fibrin glue composition for repairing a nerve damage, and / or enhancing the functional recovery of a damaged nerve which comprises an effective amount of nerve growth factor and / or nerve repair enhancer, fibrinogen, aprotinin and divalent calcium ions. The invention also relates to a method for repairing nerve damages, and / or enhancing the functional recovery of a damaged nerve which comprises topically applying to a damaged nerve the fibrin glue composition of the invention.
Owner:CHENG HENRICH
Thymopentin oral microsphere preparation and preparation method thereof
ActiveCN101721677AImprove oral bioavailabilityDoes not reduce clinical efficacyPeptide/protein ingredientsDigestive systemClinical efficacyMicrosphere
The invention discloses a thymopentin oral microsphere preparation and a preparation method thereof. The oral microsphere preparation comprises the following raw materials: thymopentin, gelatin, a lactide-glycolide copolymer, dichloromethane, polyvinylalcohol-124, an aprotinin or trypsin inhibitor, and sodium glycyl-cholate or deoxysodium cholate or sodium caprate. The thymopentin oral microsphere preparation has high bioavailability, can replace injection administration without reducing clinical curative effects and overcomes a lot of pains and inconvenience of patients caused by frequent injections.
Owner:北京博恩特药业有限公司
Composition of novel powder formulations of tranexamic acid
InactiveUS20180116986A1Facilitate administrationFacilitate methodPowder deliverySpray deliveryHydrophilic polymersEngineering
Powder composition of Tranexamic acid have been provided for the treatment of wound and bleeding. The powder composition may also contain aprotinin and epsilon-aminocaproic acid as active antifibrinolytic agent. The composition may also contain antibiotic(s), anti-inflammatory agent(s), local anesthetic(s) and hydrophilic polymer(s). The powder composition in this patent application is applied to mucosal or non-mucosal surfaces, but it is not for an oral administration.
Owner:JOSHI HEMANT N +2
Composition for cartilaginous tissue repair and a production method therefor
InactiveUS20120207736A1Reduce the burden onRapid and effective regenerationPeptide/protein ingredientsPharmaceutical delivery mechanismFiberFreeze-drying
The present invention relates to a composition for cartilaginous tissue repair and to a production method therefor. The present invention comprises the steps of: (a) dissolving freeze-dried fibrinogen in an aprotinin solution; (b) dissolving freeze-dried thrombin in a stabilizing solution; (c) mixing an enriched collagen solution with thrombin and the stabilizing solution; and installing the fibrinogen solution (a) to one side of a dual kit and the solution (c) containing the collagen to the other side, and then mixing and injecting into damaged cartilaginous tissue. In the present invention, which is constituted as described above, biomaterials such as collagen and fibrin are mixed so as to allow damaged cartilaginous tissue to be repaired to a state allowing transplantation onto the tissue, and efficient regeneration is induced, thereby making it possible to reduce surgery-related stress on people and animals while inducing relatively rapid and efficient cartilage repair and regeneration.
Owner:SEWON CELLONTECH CO LTD
Blood virus RAN protective agent and blood sampling tube
ActiveCN109679946AAvoid degradationAvoid pollutionBioreactor/fermenter combinationsBiological substance pretreatmentsSodium thiocyanatePollution
The invention provides a blood virus RAN protective agent and a blood sampling tube. The blood virus RAN protective agent is prepared by dissolving the following components in DEPC (diethyl pyrocarbonate) solution 100-500mg / ml glycine, 1-5mg / ml aprotinin, 1-5mg / ml wortmannin, 10-40mg / ml glutathione, 100-500mg / ml sodium thiocyanate, 1-3mg / ml anticoagulant and 5-20mg / ml membrane protective agent. The blood virus RAN protective agent provided by the invention is capable of supplying long-term protection to virus RNA in blood under room temperature, preventing degradation and pollution of virus RNA and effectively guaranteeing accuracy and validity of blood sample.
Owner:NINGBO AJCORE BIOSCIENCES INC
Tissue sealant in which collagen and fibrin are mixed, and method for preparing same
InactiveCN104902937AHigh strengthReduce degradationPeptide/protein ingredientsInfusion syringesFiberTissue sealant
Owner:SEWON CELLONTECH CO LTD
Methods and Compositions for Repairing Common Peroneal Nerve Lesions
InactiveUS20080109035A1Restore function of damagedEfficient repairBiocideNervous disorderFibrin glueAprotinin
Methods and compositions are provided for repairing common peroneal nerve (CPN) lesions and enhancing functional recovery of a damaged CPN. The methods of the present invention include applying a fibrin glue mixture to the area of a surgically repaired CPN. The fibrin glue mixture contains growth factor, fibrinogen, aprotinin and divalent calcium ions.
Owner:CHENG HENRICH
Process for producing high purity fibrinogen and thrombin for fibrin sealant
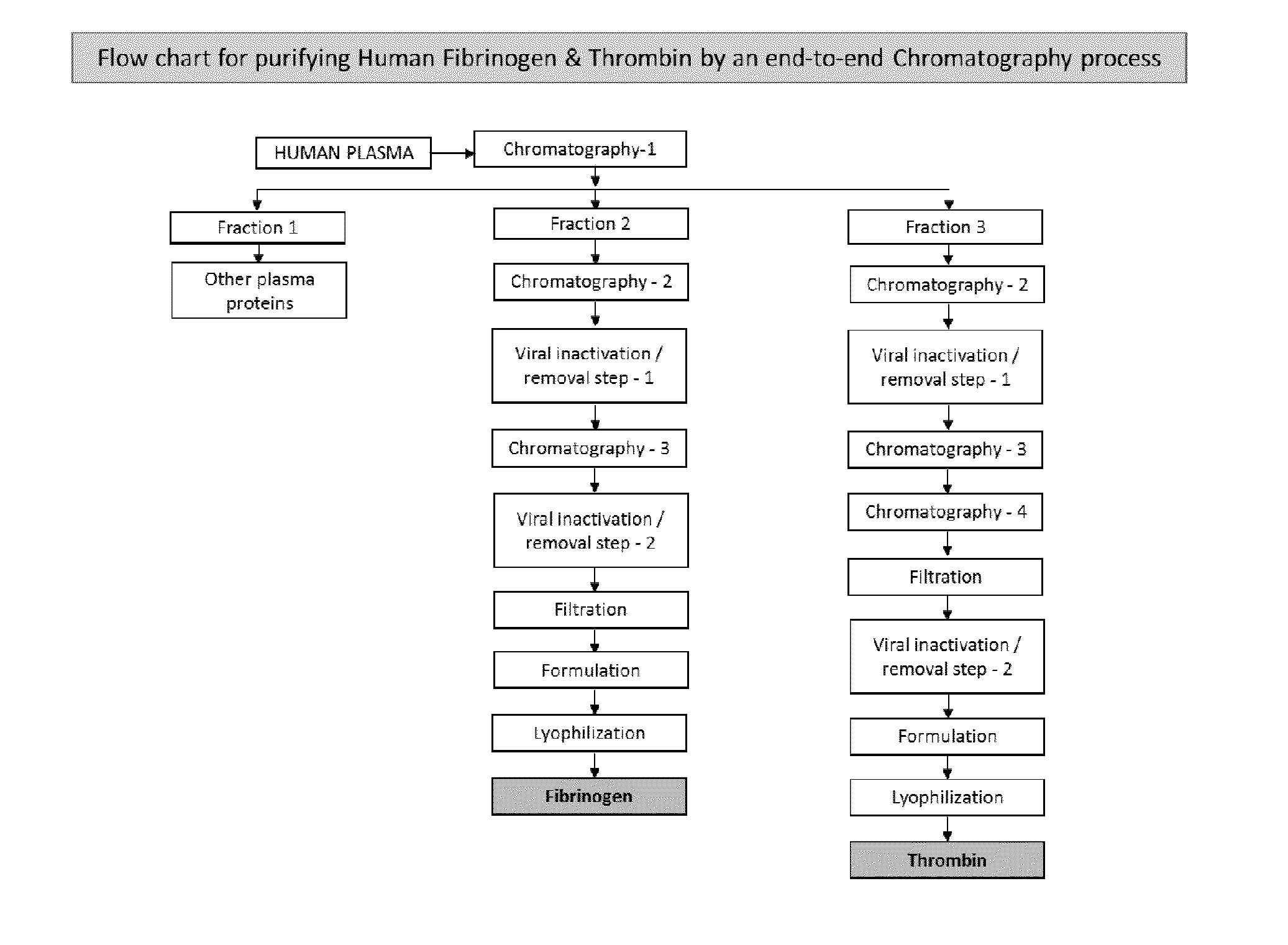
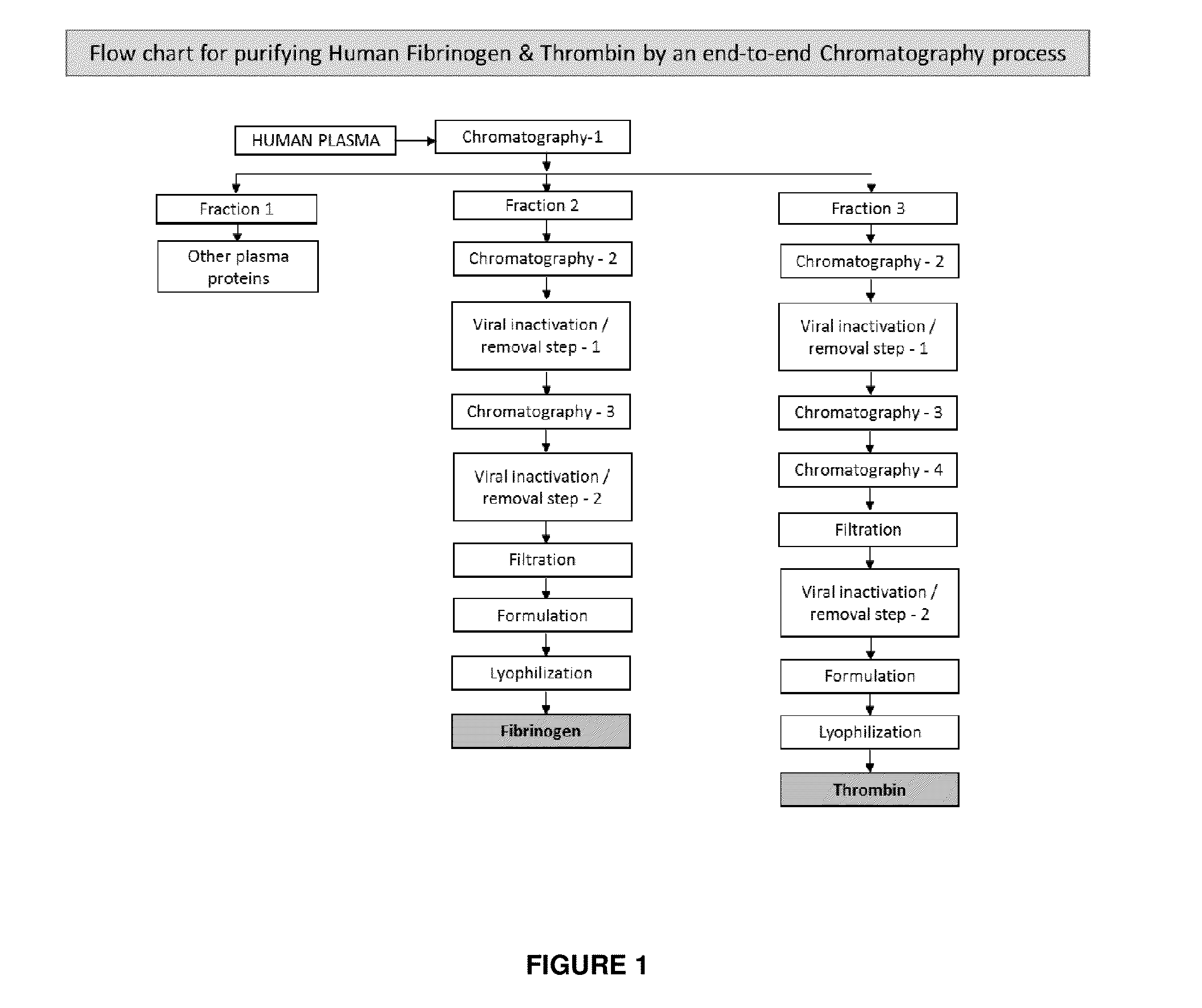
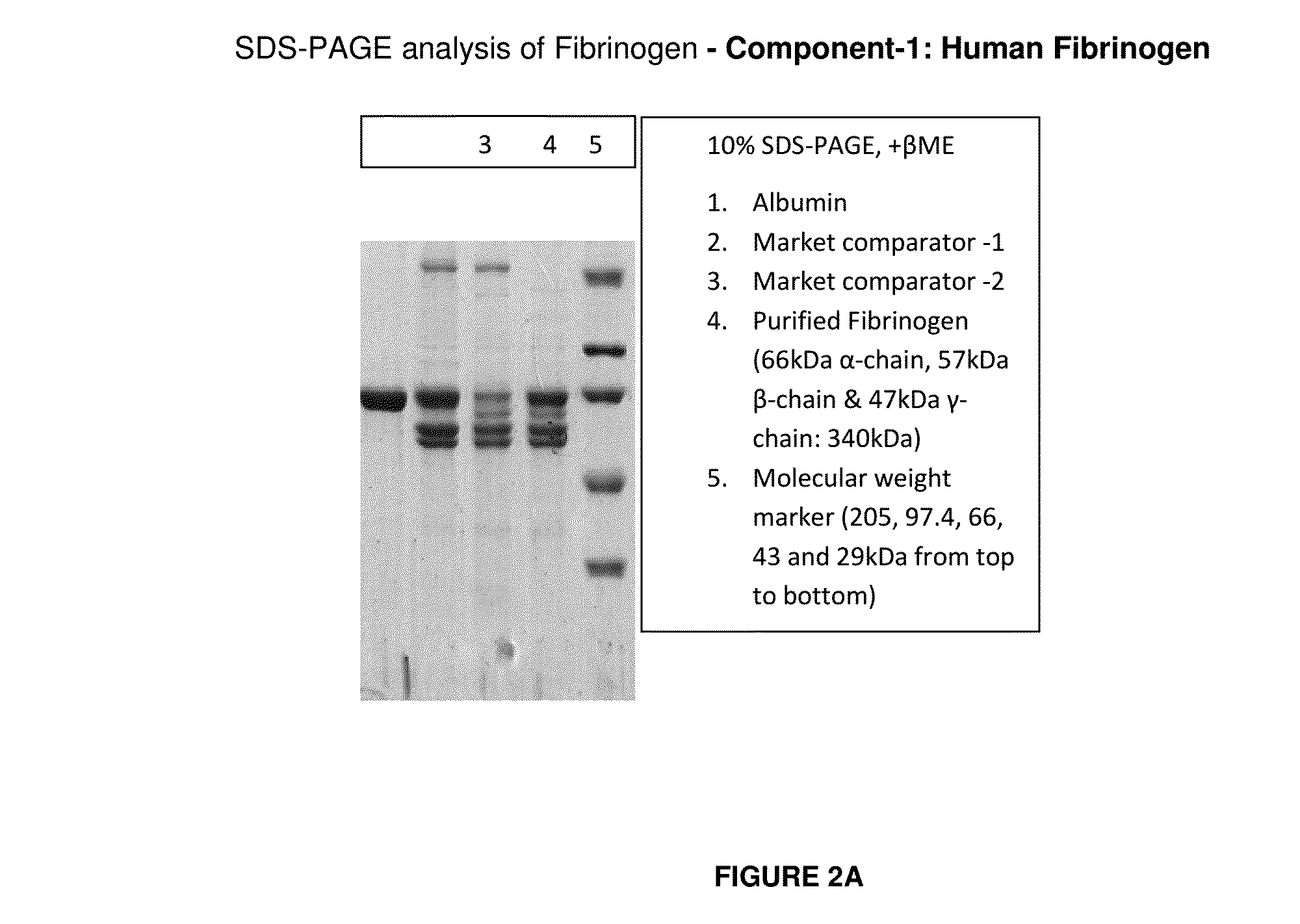
ActiveUS20160137719A1Low and undetectable level of contaminantReduce the amount requiredSurgical adhesivesFibrinogenAprotininThrombin activity
The present invention relates to a fibrin sealant kit comprising purified fibrinogen and thrombin. The invention particularly relates to an improved chromatographic process for the purification of thrombin and fibrinogen components devoid of any significant plasminogen and other impurities. The absence of plasminogen facilitates the exclusion of a proteolytic inhibitor (aprotinin) from among the kit components.
Owner:ICHOR BIOLOGICS PTE LTD
Protease for activating clotting factor VII
Owner:CSL BEHRING GMBH
New nasal spray formulation of calcitonin aqueous solution
The invention relates to a new nasal spray formulation of a calcitonin aqueous solution, belonging to the technical field of formulations. By the screening of various auxiliary materials, the invention obtains a composition different from that reported in any literature documents from a plurality of prescriptions and applications thereof in preparing medicaments for treating osteoporosis, Paget disease, malignant hypercalcemia and the like. The main composition ingredients of the new formulation comprise 0.05-1mg / ml of calcitonin, 10-50mmol / L of phosphate buffer, 0.5-50mmol / L of disodium ethylene diamine tetraacetate, 0.06-2ug / ml of trasylol, 0.5-2 percent of sodium chloride or 0.5-10 percent of mannite, 0.01-5 percent of brij and 0.01-0.2 percent of benzalkonium bromide, thus preparing the formulation with unit volume being 0.1-10ml.
Owner:BEIJING SL PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com