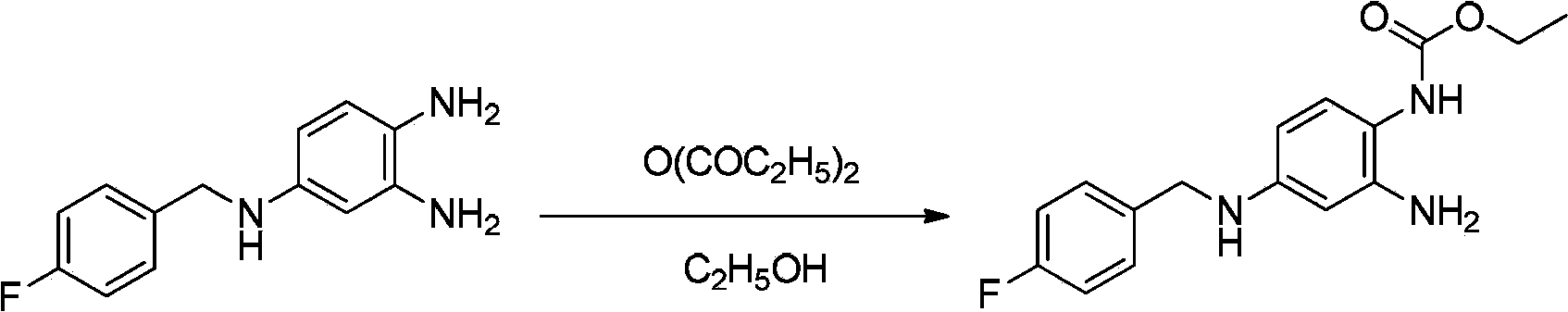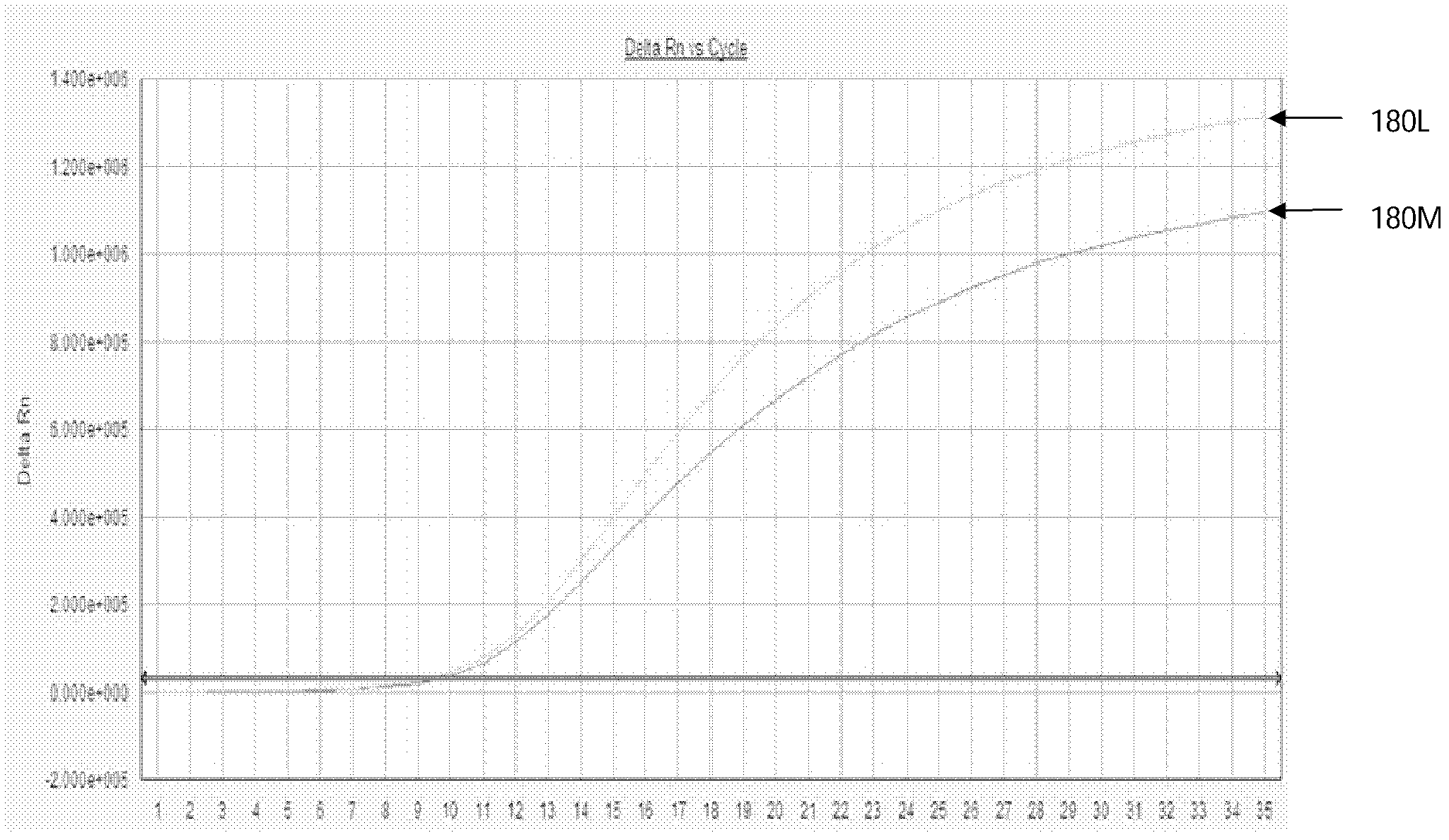Patents
Literature
49 results about "Diethyl pyrocarbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diethyl pyrocarbonate (DEPC), also called diethyl dicarbonate (IUPAC name), is used in the laboratory to inactivate RNase enzymes in water and on laboratory utensils. It does so by the covalent modification of histidine (most strongly), lysine, cysteine, and tyrosine residues.
Preservative composition of blood DNA
The invention provides a preservative composition of blood DNA. The preservative composition of blood DNA comprises, by weight, 20 to 50 parts of a preservative, 5 to 15 parts of an anticoagulant, 0.5 to 5 parts of a nuclease inhibitor, 1 to 5 parts of a metabolic inhibitor and 40 to 70 parts of water, wherein the nuclease inhibitor comprises one or more of diatomite, bentonite, ethyl alcohol, formamide, vanadyl-ribonucleoside complex, dithioerythritol, diethyl pyrocarbonate, proteinase-K, heparin, beta-mercaptoethanol, hydroxylamine-O-copper ions, ammonium sulfate, dithiothreitol, cysteine, and 4-formylbenzoic acid and oxovanadium Schiff base complex.
Owner:上海迅伯生物科技有限公司 +1
Quantitative detection kit, detection method, primer and probe for hepatitis B virus nucleic acid
ActiveCN102146487AAvoid pollutionAvoid harmMicrobiological testing/measurementFluorescence/phosphorescencePositive controlA-DNA
The invention discloses a quantitative detection kit, detection method, primer and probe for hepatitis B virus nucleic acid, belonging to the technical field of biology. The invention relates to a gene detection technology of a virus causing various types of acute, chronic and severe hepatitis of human beings, which is suitable for qualitative and quantitative detection of the hepatitis B virus. The kit comprises PCR (Polymerase Chain Reaction) reaction liquid, wherein the reaction liquid comprises DEPC (Diethyl Pyrocarbonate) treating water, Taq enzyme, dNTPs (Deoxynucleotide Triphosphates),10*PCR Buffer, a solution containing Mg<2+> ions, a hepatitis B virus positive primer: 5'-TTGTCCTGGYTATCGYTGGAT-3', a hepatitis B virus negative primer: 5'-TGAGGCATAGCAGCAGGATGA-3', a hepatitis B virus probe: 5'-CTGCGGCGTTTTAT-3'; and the kit also comprises a DNA (Deoxyribonucleic Acid) extracting solution, a negative control material, a working standard product, a positive control material and acritical positive control material. The hepatitis B virus nucleic acid is detected quantificationally by adopting a real-time fluorescent quantitative PCR technology. The invention has the characteristics of specificity, sensitivity, quickness and easiness and convenience for operation.
Owner:WUHAN BIOTECH GENE ENG
Method for separating RNA (Ribonucleic Acid) from human serum/blood plasma sample and PCR (Polymerase Chain Reaction) verification method thereof
InactiveCN101914523AEasy accessEconomic gainMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceReverse transcriptase
The invention discloses a method for separating RNA (Ribonucleic Acid) from a human serum / blood plasma sample, which sequentially comprises the steps of: 1, pretreating; 2, adding a Trizol reagent into the pretreated sample in an RNase-free centrifuge tube, uniformly mixing, standing at room temperature; 3, extracting chloroform; 4, adding isopropanol into the centrifuge pipe obtained from the step 3, uniformly mixing, standing at room temperature, centrifuging to obtain white sediment, washing and centrifuging the white sediment, and discarding supernatant; and 5, airing the sediment obtained from the step 4, adding diethyl pyrocarbonate for treating water to ensure that the sediment is dissolved to obtain a purified serum / blood serum RNA sample. The invention also discloses a method forcarrying out RT (Reverse Transcriptase)-fluorescence quantitative PCR (Polymerase Chain Reaction) verification by using the RNA obtained by the steps. The RNA with high quality and content can be effectively obtained by adopting the method of the invention.
Owner:ZHEJIANG SCI-TECH UNIV
Blood virus RAN protective agent and blood sampling tube
ActiveCN109679946AAvoid degradationAvoid pollutionBioreactor/fermenter combinationsBiological substance pretreatmentsSodium thiocyanatePollution
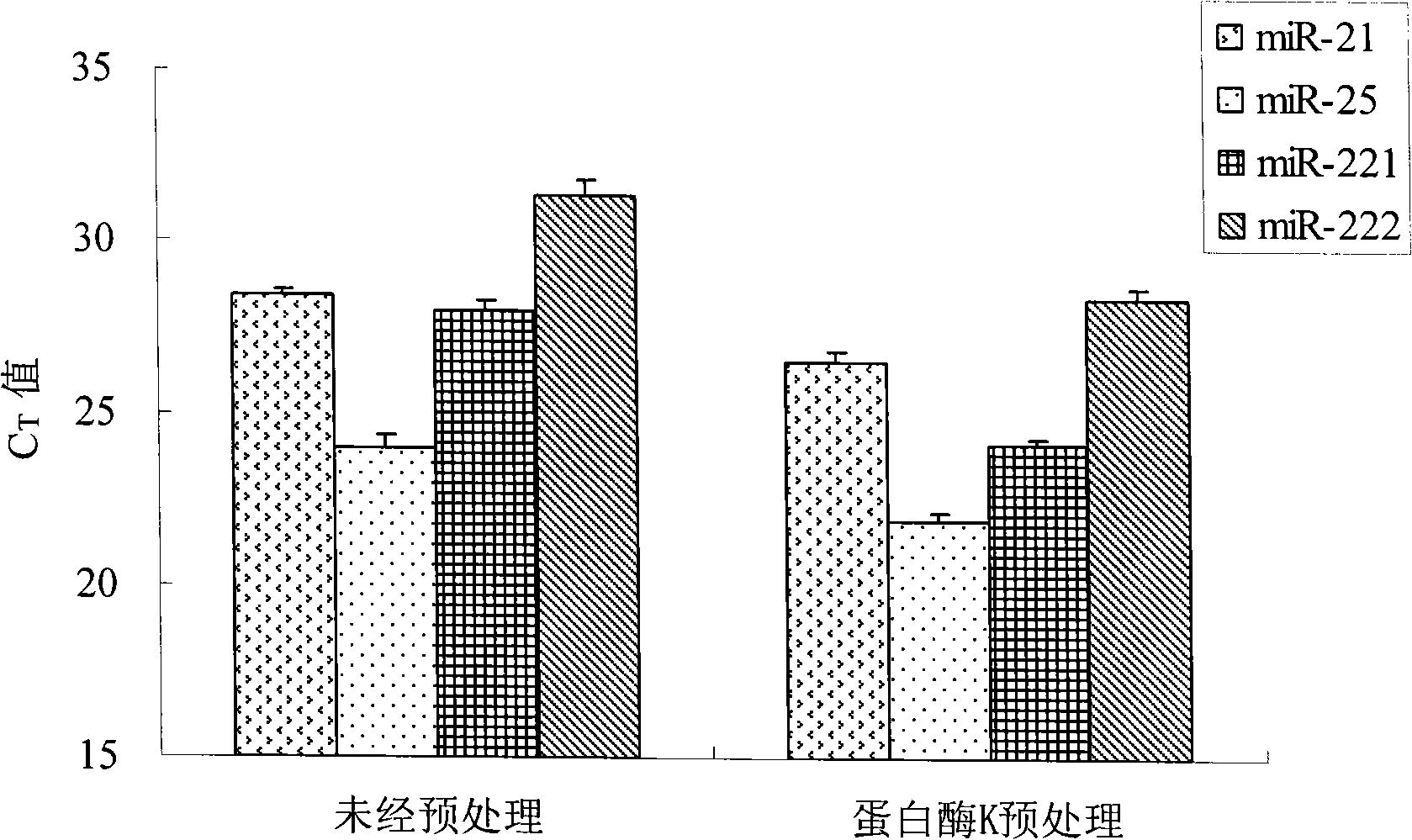
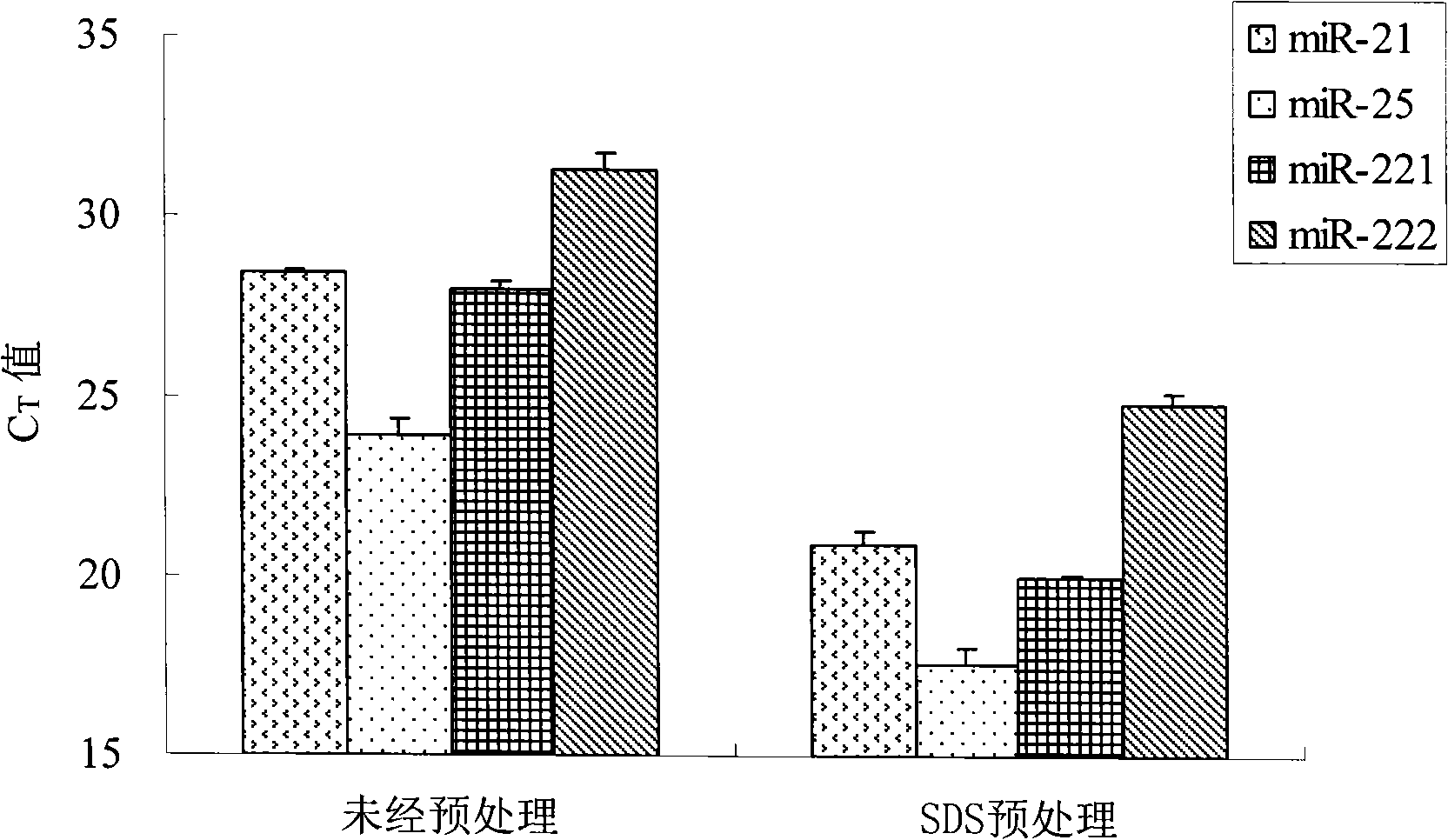
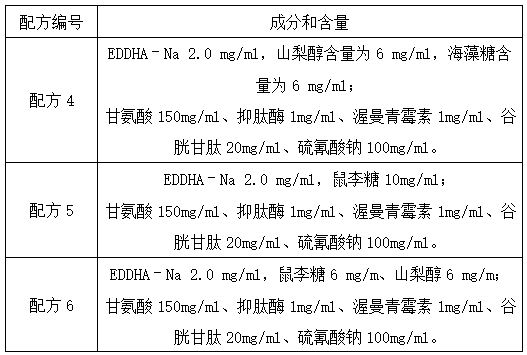
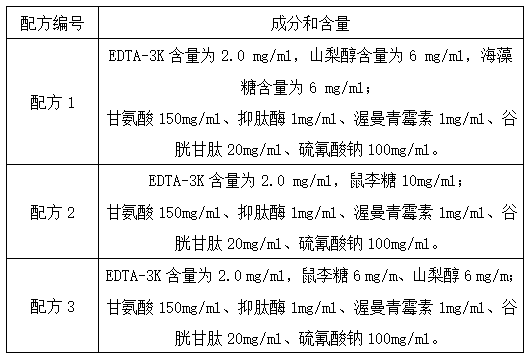
The invention provides a blood virus RAN protective agent and a blood sampling tube. The blood virus RAN protective agent is prepared by dissolving the following components in DEPC (diethyl pyrocarbonate) solution 100-500mg / ml glycine, 1-5mg / ml aprotinin, 1-5mg / ml wortmannin, 10-40mg / ml glutathione, 100-500mg / ml sodium thiocyanate, 1-3mg / ml anticoagulant and 5-20mg / ml membrane protective agent. The blood virus RAN protective agent provided by the invention is capable of supplying long-term protection to virus RNA in blood under room temperature, preventing degradation and pollution of virus RNA and effectively guaranteeing accuracy and validity of blood sample.
Owner:NINGBO AJCORE BIOSCIENCES INC
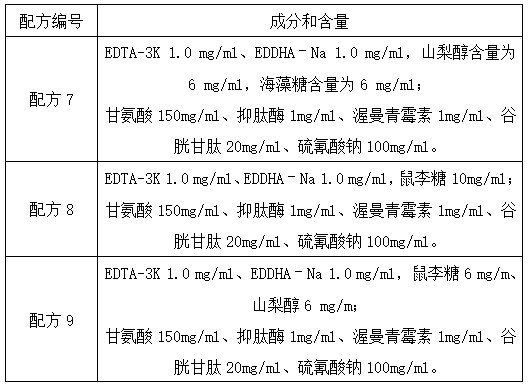
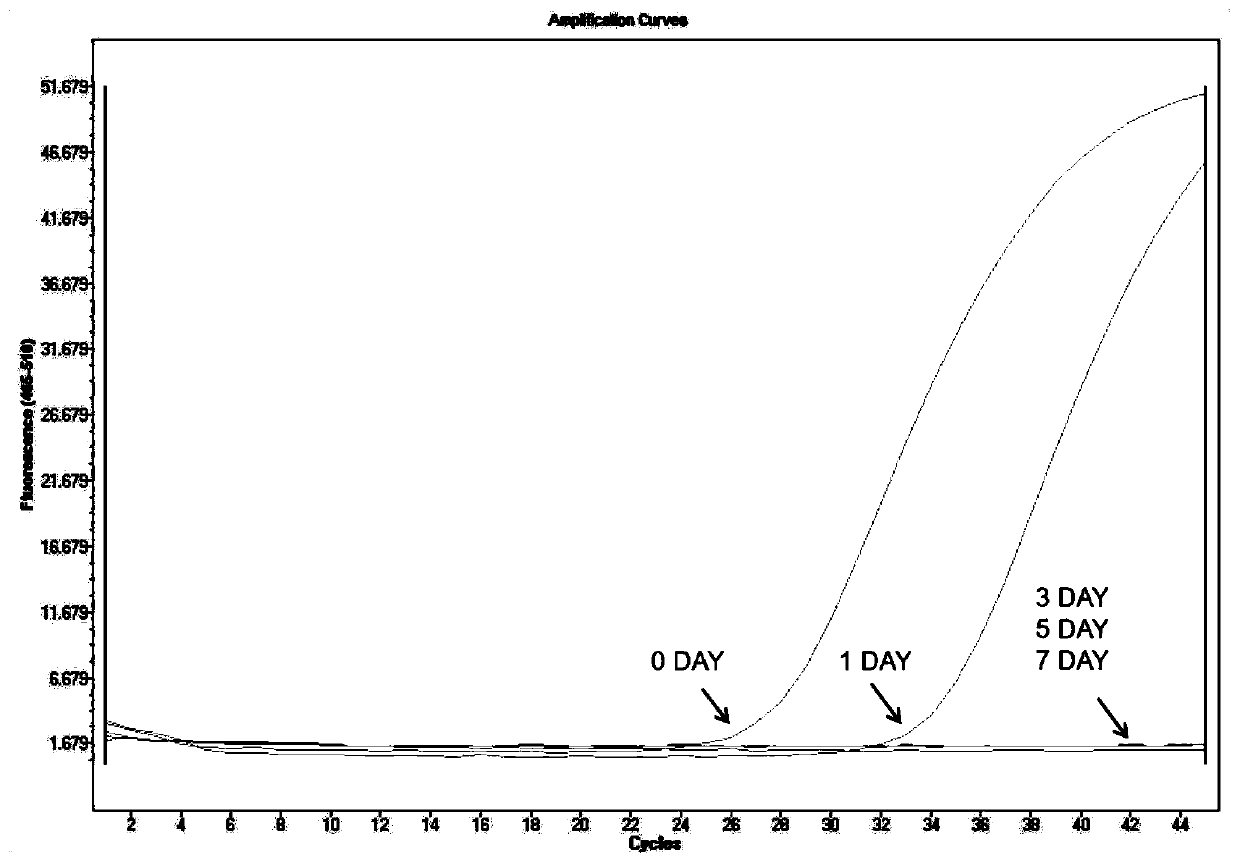
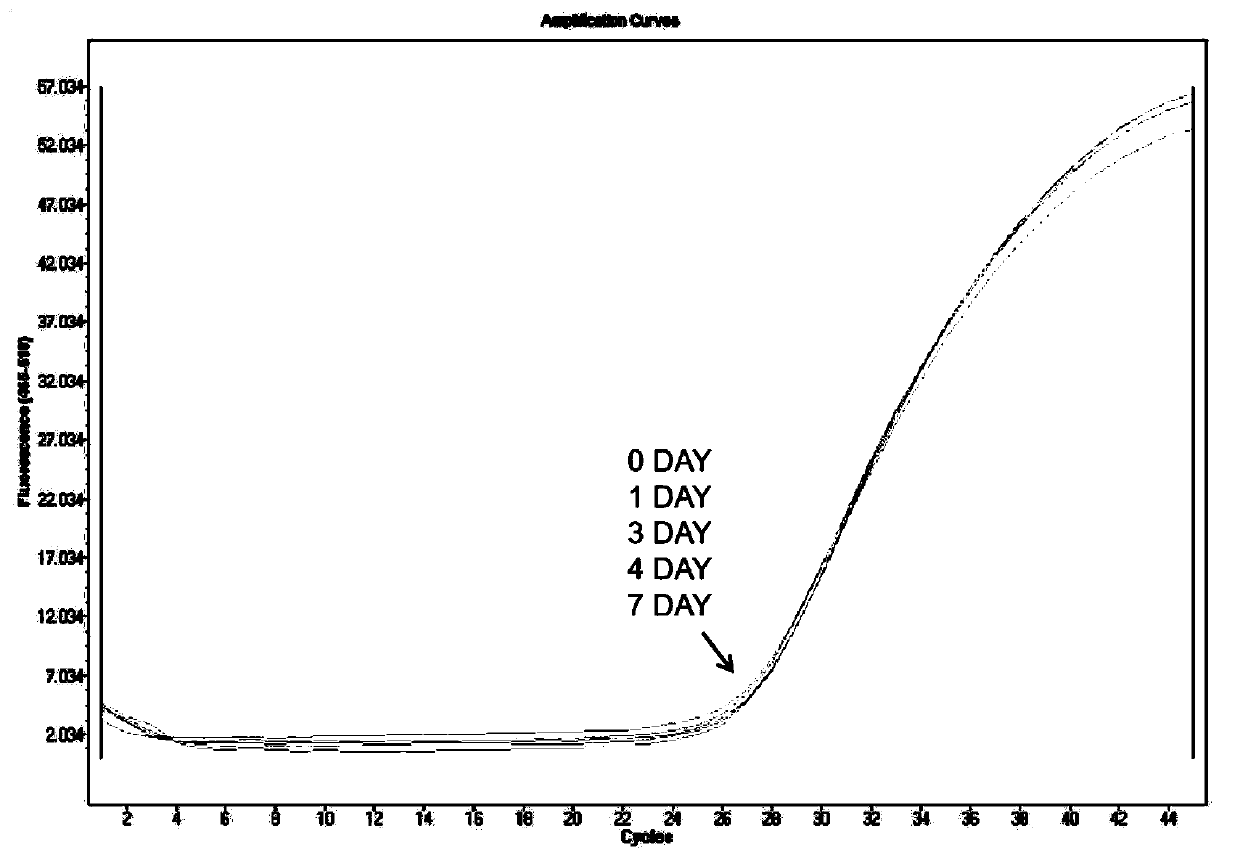
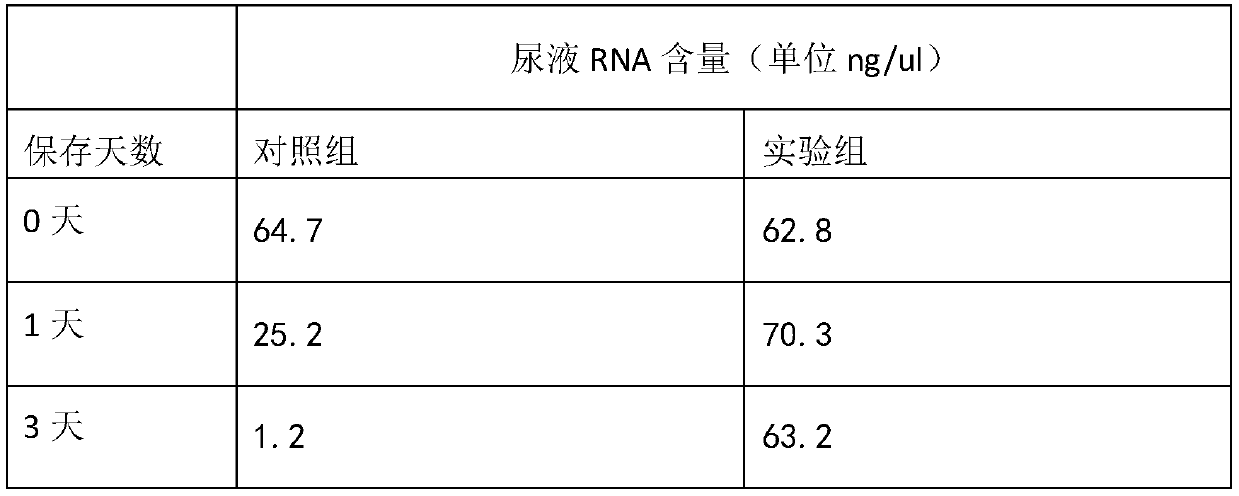
Urine sample RNA stabilizing solution and preparation method thereof
PendingCN110760567AAchieve preservationRealize transportationMicrobiological testing/measurementUrine sampleUltrapure water
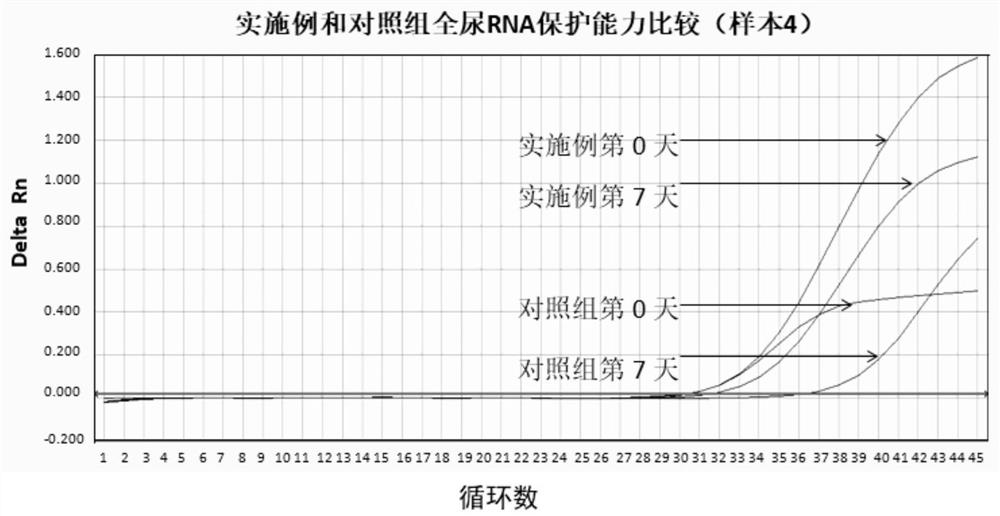
The invention discloses a urine sample RNA stabilizing solution and a preparation method thereof. The urine sample RNA stabilizing solution is prepared from 0.01 to 2M of dithiothreitol, 2M to 6M of guanidinium isothiocyanate, 1 to 200 mM of ethylenediamine tetraacetic acid, 0.05 to 0.5 mM of diethyl pyrocarbonate and ultrapure water. The preparation method comprises the following steps: adding acertain amount of the chemical reagent into ultrapure water, adjusting the pH value to 4-4.2 with hydrochloric acid, fixing the volume, and carrying out sealed storage. The urine sample RNA stabilizing solution provided by the invention can effectively maintain the stability of RNA in a urine sample, can realize preservation and transportation of large-volume urine, and is suitable for subsequentRNA extraction and fluorescent RT-PCR.
Owner:HANGZHOU YORK BIOTECH CO LTD
Kit for diagnosing reovirus genes of grass carps and application thereof
ActiveCN101906482AHigh sensitivityAvoid spreading the epidemicMicrobiological testing/measurementMicroorganism based processesForward primerRNA extraction
The invention relates to a kit for diagnosing the reovirus genes of grass carps, comprising an RNA (Ribonucleic Acid) extraction reagent for viruses in visceral tissues, an RAN extraction reagent for cell infecting viruses, an RT (Reverse Transcriptase) reaction reagent and a PCR (Polymerase Chain Reaction) reaction reagent, wherein the RNA extraction reagent for the viruses in the visceral tissues comprises the components of DEPC (Diethyl Pyrocarbonate) water, protease K (1 mg / mL, pH is 8.0), SDS (Sodium Dodecyl Sulfate) (1%), phenol / chloroform / isoamylol (25 / 24 / 1), chloroform / isoamylol (24 / 1), isopropanol and 70% ethanol; the RAN extraction reagent for the cell infecting viruses comprises the components of Trizol, chloroform, isopropanol, 70% ethanol and the DEPC water; the RT reaction reagent comprises the components of 5*AMV Buffer, dNTP, the DEPC water, a reverse primer R1, an RNA enzyme inhibitor RNAsin (R1) and a reverse transcriptase AMV; and the PCR reaction reagent comprises the components of 10*PCR buffer (containing Mg<2+>) and DNTP Mixture (which are respectively 2.5 mmol / L), a specific oligonucleotide primer F1, the primer R1, the DEPC water and Taq E (5U / muL), the forward primer F1:5'-ATCCCGTATATCTATGGCTT-3', and the reverse primer R1:5'TTGGAGACGAACATAGACGC-3. The kit is used for diagnosing the reovirus genes of the grass carps.
Owner:ZHEJIANG INST OF FRESH WATER FISHERIES
Reverse transcription-polymerase chain reaction (RT-PCR) detection kit for infectious haematopoietic necrosis viruses (IHNV) and preparation method of kit
ActiveCN103233005AEasy to operateMicrobiological testing/measurementMicroorganism based processesOperating instructionPosition control
The invention provides a reverse transcription-polymerase chain reaction (RT-PCR) detection kit for infectious haematopoietic necrosis viruses (IHNV) and a preparation method of the kit, and relates to an RT-PCR detection kit. The RT-PCR detection kit for the IHNV is provided with a kit body, an operating instruction and detection reagents, wherein the operating instruction and the detection reagents are arranged in the kit body; and the detection reagents comprise lysate, adsorption liquid, a washing solution, diethyl pyrocarbonate (DEPC) treating water, reverse transcription reaction liquid, IHNV-PCR reaction liquid, position control and negative control. The preparation method comprises the following steps of: preparing the kit body; preparing the detection reagents; and placing the operating instruction, the lysate, the adsorption liquid, the washing solution, the DEPC treating water, the reverse transcription reaction liquid, the IHNV-PCR reaction liquid, the position control and the negative control into the kit body to obtain the RT-PCR detection kit for the IHNV. The reagents are complete and the operation is simple.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Method for extracting total RNA
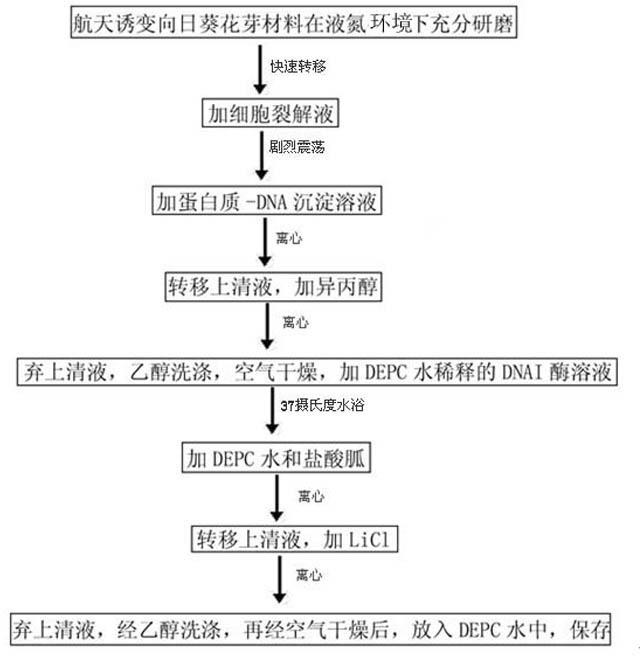
The invention relates to an optimized method for extracting total RNA of a plant material, which comprises the steps of: 1, grinding a fresh plant material into fine powder under a liquid nitrogen condition, and transferring to a centrifuge tube containing a cell lysing solution; 2, adding a protein-DNA settling solution in the centrifuge tube in the step 1; 3, transferring supernate to a new centrifuge tube, and adding isopropanol; 4, discarding the supernate, washing sediments with alcohol, drying in air, and adding a DNA enzyme I solution diluted with DEPC (diethyl pyrocarbonate) water; 5, adding the DEPC water and a guanidine hydrochloride solution; 6, transferring the supernate to the new centrifuge tube, and adding LiCl solution; and 7, centrifuging, washing with alcohol, drying in air, and dissolving in the DEPC water for storing at low temperature. The invention has the advantages of simple operation steps, convenience of operation and no need of extracting the total RNA with conventional organic solvents (phenol and chloroform), thereby preventing polluting the environment without hurting a human body.
Owner:杨军
One step method ribonucleic acid extraction agent
The present invention relates to an one-step method ribonucleic acid extraction reagent EZRNA. Its formula includes (by 1 L total amount) guanidinium isothiocyanate 3-5 M, sodium citrate 18-25 M, sodium laurate (by wt%) 0.3-0.6%, 2-mercaptoethanol 0.6-1.2 M and balanced acidic phenol (by volume%) 40-55%. Besides, said invention also provides the concrete steps of the invented one-step method ribonucleic acid extraction process by using said reagent.
Owner:JIERUI BIOENG SHANGHAI
Kit capable of quickly detecting classical swine fever virus/porcine reproductive and respiratory syndrome virus/pseudorabies virus/porcine parvovirus
ActiveCN105734172AEasy to operateShort timeMicrobiological testing/measurementMicroorganism based processesFluorescenceEnzyme system
The invention relates to a kit capable of quickly detecting classical swine fever virus / porcine reproductive and respiratory syndrome virus / pseudorabies virus / porcine parvovirus, in particular to a multiplex real-time fluorescence polymerase chain reaction technology capable of detecting CSFV (classical swine fever virus), PRRSV (porcine reproductive and respiratory syndrome virus), PRV (pseudorabies virus) and PPV (porcine parvovirus) simultaneously.The kit mainly comprises a RT-PCR (reverse transcription-polymerase chain reaction) mixture, primer probe mixed liquor, an RT-PCR enzyme system and DEPC (diethyl pyrocarbonate) H2O as well as packaging boxes for packaging reagent bottles or tubes separately and in a centralized manner.Through multiple fluorescence channels for separate detection, the kit applying a one-step real-time fluorescence PCR mode is capable of detecting and identifying the classical swine fever virus, the porcine reproductive and respiratory syndrome virus, the pseudorabies virus and the porcine parvovirus quickly and accurately and can be widely applied to multiple fields such as early clinical diagnosis of porcine reproductive disturbance diseases, port inspection and quarantine, plague prevention and scientific research.
Owner:DAAN GENE CO LTD
Reagent kit, method, primers and probe for quantitative detection of nucleic acid of swine fever virus
ActiveCN102676690AAvoid pollutionAvoid harmMicrobiological testing/measurementFluorescence/phosphorescenceForward primerRNA extraction
The invention discloses a reagent kit, a method, primers and a probe for quantitative detection of nucleic acid of a swine fever virus and belongs to the technical field of biology. The reagent kit comprises PCR (polymerase chain reaction) liquid including DEPC (diethyl pyrocarbonate) treating water, Taq enzyme, M-MLV reverse transcriptase, RNase inhibitor, dNTP Mix, 10X one-step RT-PCR (reverse transcription-polymerase chain reaction) Buffer, MgC12 solution, the forward primer of the swine fever virus, the reverse primer of the swine fever virus and the LNA (locked nucleic acid) probe of the swine fever virus, wherein the forward primer of the swine fever virus refers to: 5'-AACGGYAGTGCTTTCTAYC-3', the reverse primer of the swine fever virus refers to: 5'-GTGGRAAAGGCTTCTCTC-3', and the LNA probe of the swine fever virus refers to: 5'-accActTctGtyCtac-3'. The reagent kit further comprises RNA (ribonucleic acid) extracting solution, negative quality control serum, working standard serum, positive quality control serum and critical positive quality control serum. The real-time fluorescent quantitative PCR technology is used for quantitatively detecting the nucleic acid of the swine fever virus, and accordingly the reagent kit has the advantages of specificity, sensitivity, rapidness and simplicity and convenience to operate.
Owner:湖北万德瑞生命科学技术有限公司
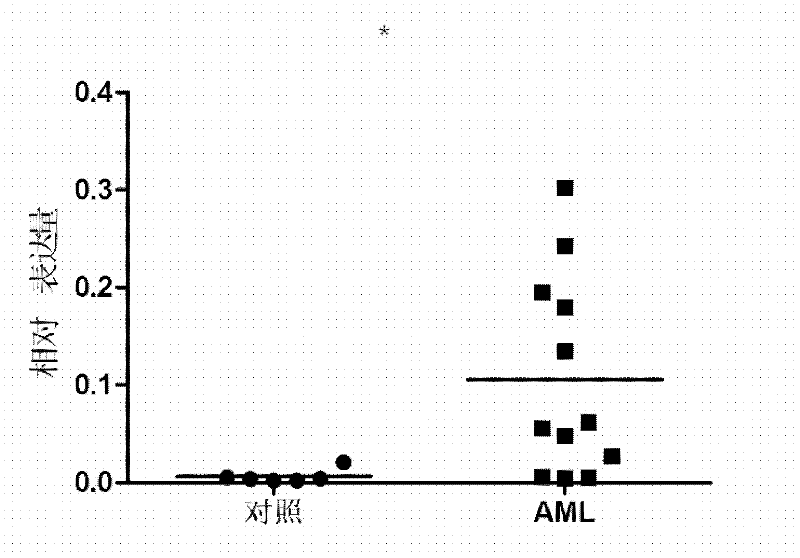
Kit for diagnosing acute myelocytic leukemia
InactiveCN102643899AConvenient, Accurate and Sensitive DiagnosisReduce false positive rateMicrobiological testing/measurementFluorescenceReverse transcriptase
The invention discloses a kit for diagnosing acute myelocytic leukemia. The kit comprises a reverse transcription system and a PCR (Polymerase Chain Reaction) amplification system, and is characterized in that: the reverse transcription system comprises repetition T oligonucleotide Ologod T, reverse transcription reaction solution, an M-MLV reverse transcriptase, an RNA (Ribonucleic Acid) enzyme inhibitor and dNTPs (Deoxynucleotide Triphosphate), wherein the PCR amplification system comprises an SYBRGreen PCR system and a primer pair; the reverse transcription reaction solution comprises diethyl pyrocarbonate treated water and M-MLV reverse transcriptase buffer solution; the SYBRGreen PCR system comprises PCR buffer solution, template cDNA (complementary Deoxyribonucleic Acid), dNTPs and an SYBRGreen fluorescent dye; and the primer pair is one capable of amplifying human Lc3 synthetase beta3Gn-T5 and human beta-actin. According to the invention, a method for detecting expression of the Lc3 synthetase beta3Gn-T5 by using the SYBRGreen technology is constructed, and the kit capable of conveniently, accurately and sensitively diagnosing the acute myelocytic leukemia is provided.
Owner:SUZHOU UNIV
Rapid extraction kit for animal tissue genome RNA and extraction method and application
The invention relates to a rapid extraction kit for animal tissue genome RNA and an extraction method and application. The rapid extraction kit comprises lysate, flushing liquid 1, flushing liquid 2, absolute ethyl alcohol and a diethyl pyrocarbonate solution; when in extracting, solid animal tissue is milled, and liquid animal tissue is directly extracted; after a sample is cracked, the genome RNA can be absorbed to an RNA absorbing column, and then the diethyl pyrocarbonate solution is used for flushing to obtain the complete genome RNA. The kit is applicable to extraction and purifying of the genome RNA of the animal tissue; the whole extraction costs 5 minutes; the extraction efficiency is high, and the detection sensitivity is high; reliable experience basis is provided to early diagnose various RAN bacteria or viral pathogen infection. The kit is applicable to all research and development institutions, clinical pathogen detection, molecular genetics research, clinical gene diagnosis, animal disease diagnosis and disease control centers, and has a wide market prospect and high economic benefit.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
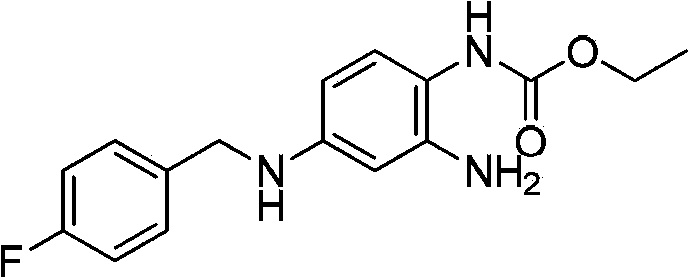
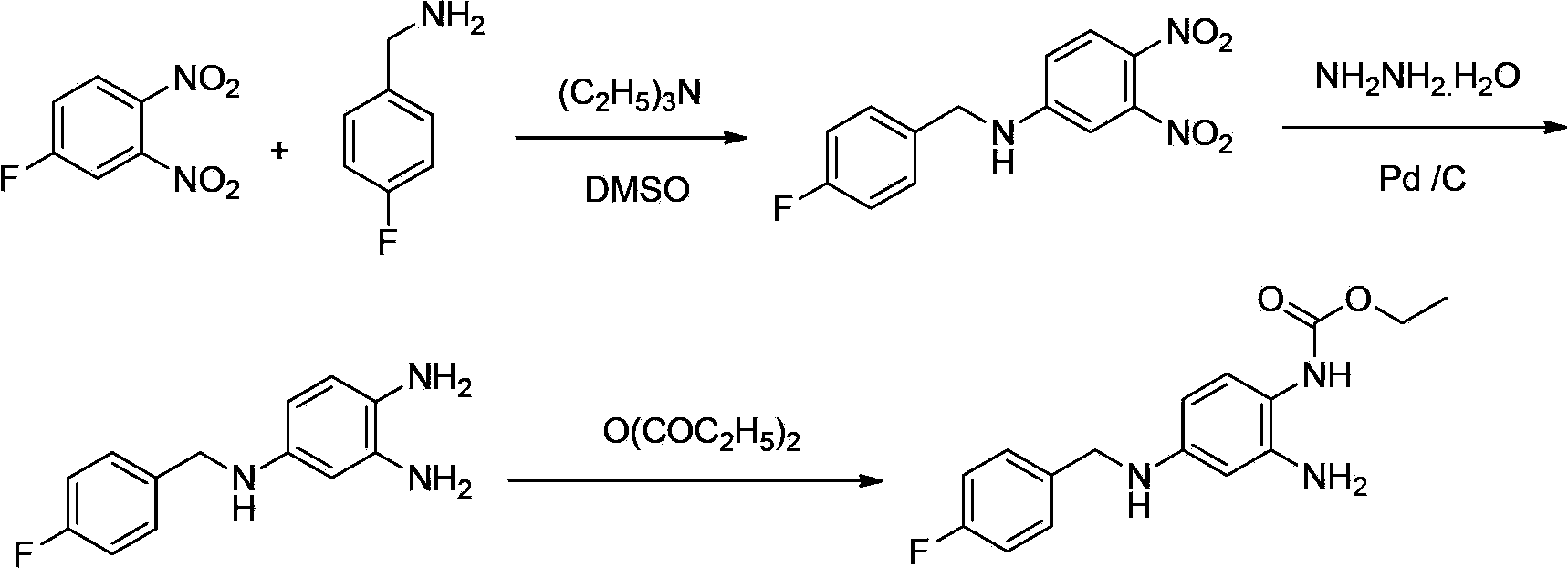
Method of synthesizing retigabine
InactiveCN103570587AEasy to operateLow costCarbamic acid derivatives preparationOrganic compound preparationNitrobenzeneSolvent
The invention discloses a method of synthesizing retigabine. The method comprises the following steps: carrying out catalytic hydrogenation on a raw material 2-amino-4-(4-florobenzyl amino)-1-nitrobenzene in a reaction solvent by taking Pd / C as a catalyst; filtering a reaction liquid; and adding diethyl pyrocarbonate into the filtrate to carry out condensation reaction to obtain retigabine. The method disclosed by the invention connects Pd / C catalytic hydrogenation and acylation in series, so that the method is simple in line operation, reduces the cost and improves the safety. The yield of the method disclosed by the invention for synthesizing retigabine reaches 48% which is remarkably improved compared with that reported at present, so that the method has a good industrial application prospect.
Owner:BEIJING BEILU PHARM CO LTD
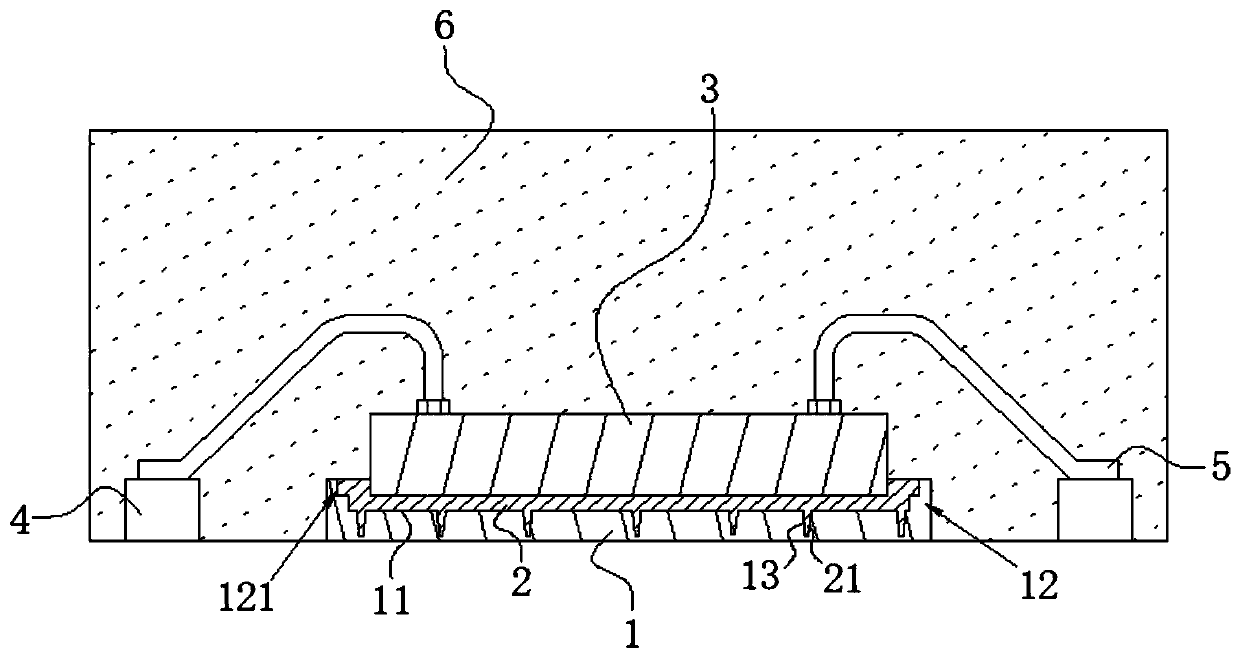
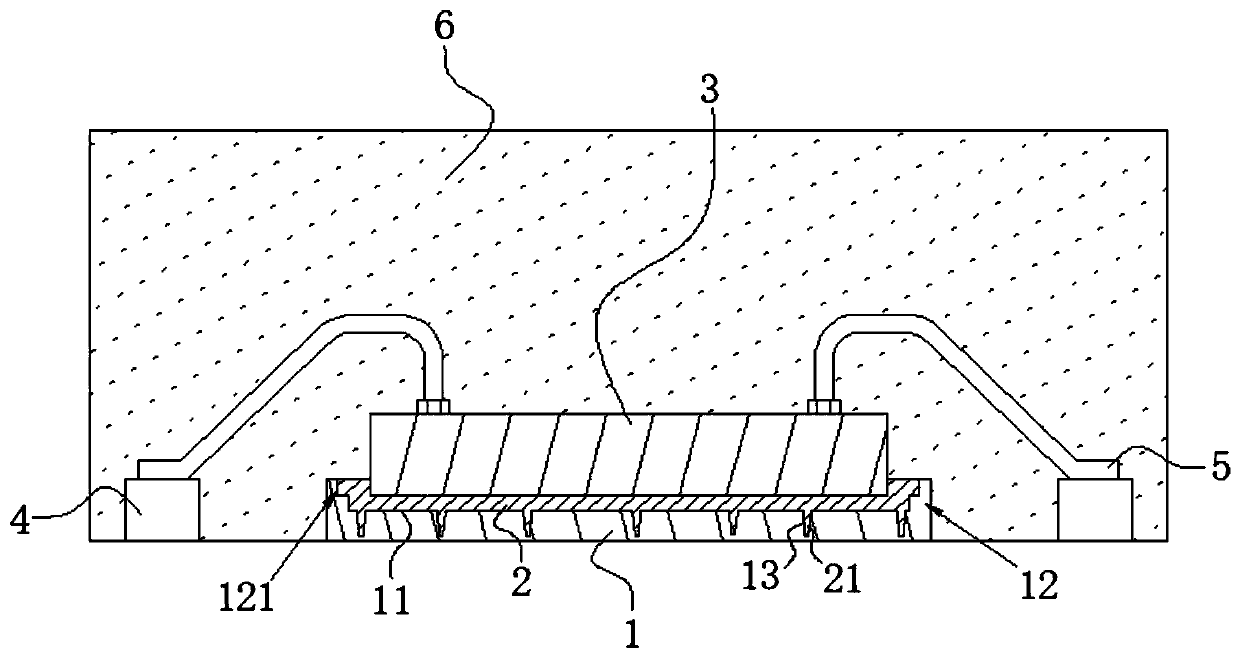
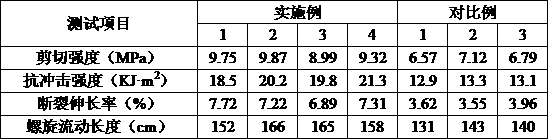
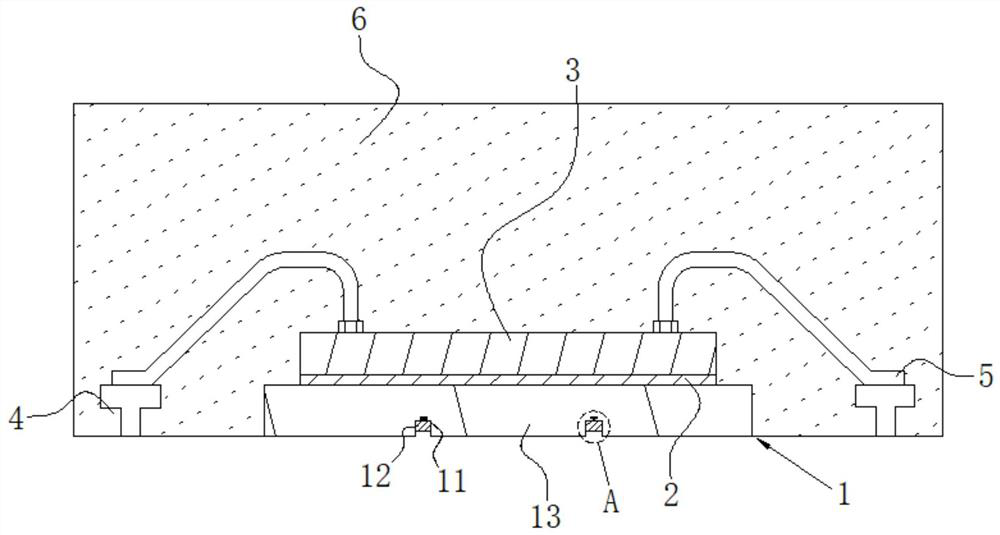
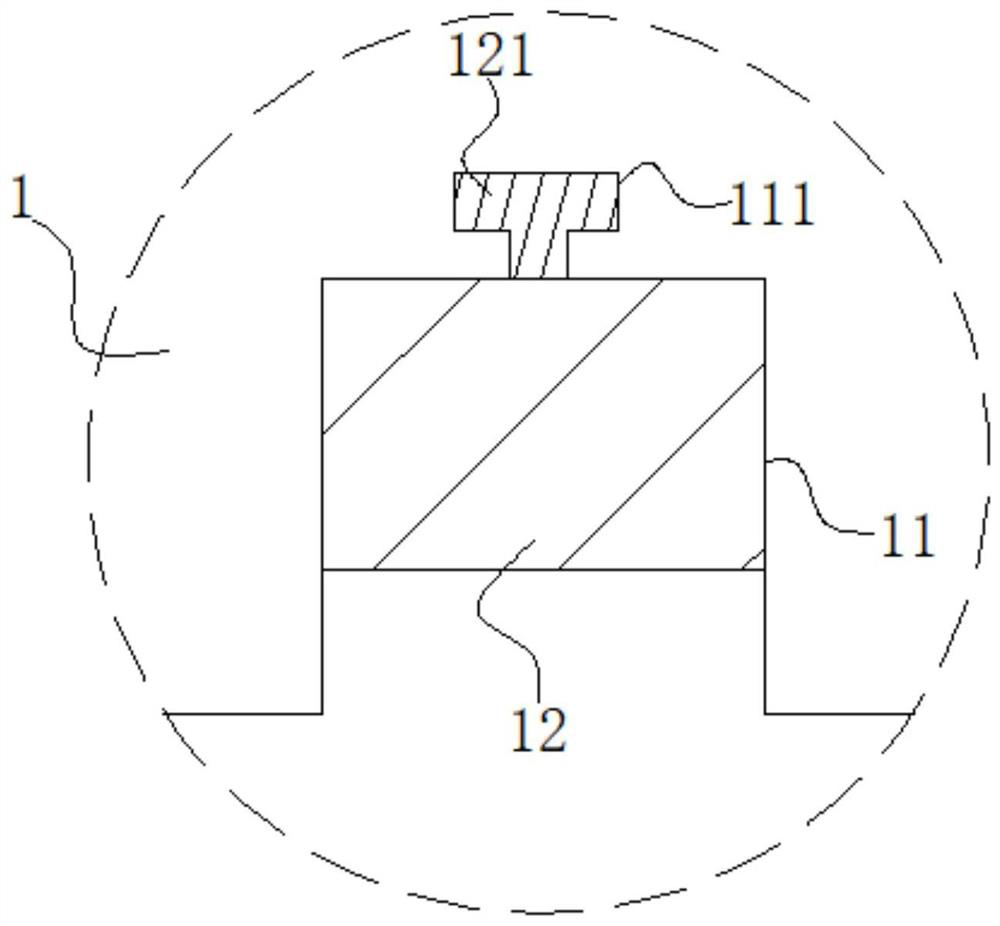
High-stability DFN packaging device
ActiveCN109904131AImprove cooling effectIncrease contact areaSemiconductor/solid-state device detailsSolid-state devicesNitrile rubberPolyethylene glycol
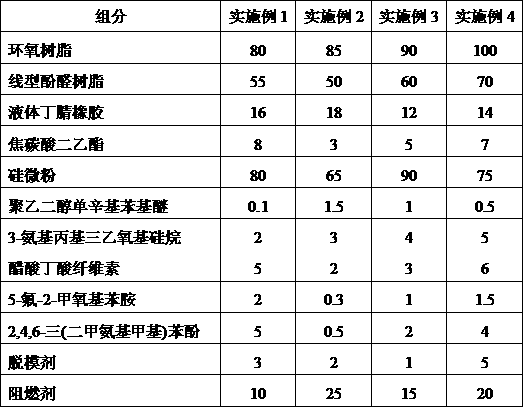
The invention discloses a high-stability DFN packaging device. An epoxy insulator of the high-stability DFN packaging device comprises the following raw materials in parts by weight: 80-100 parts of epoxy resin, 50-70 parts of linear phenolic resin, 12-18 parts of liquid nitrile rubber, 3-8 parts of diethyl pyrocarbonate, 65-90 parts of silica powder, 0.1-1.5 parts of octylphenylpolyethylene glycol, 2-5 parts of 3-aminopropyltriethoxysilane, 2-6 parts of cellulose acetate butyrate, 0.3-2 parts of 5-fluoro-2-methoxyaniline, 0.5-5 parts of 2,4,6-tri(dimethylamiomethyl)phenol, 1-5 parts of a release agent and 10-25 parts of a flame retardant. The high-stability DFN packaging device is excellent in heat dissipation effect and mechanical property and is stable and reliable in packaging structure, thereby having a wide application prospect.
Owner:西安航思半导体有限公司
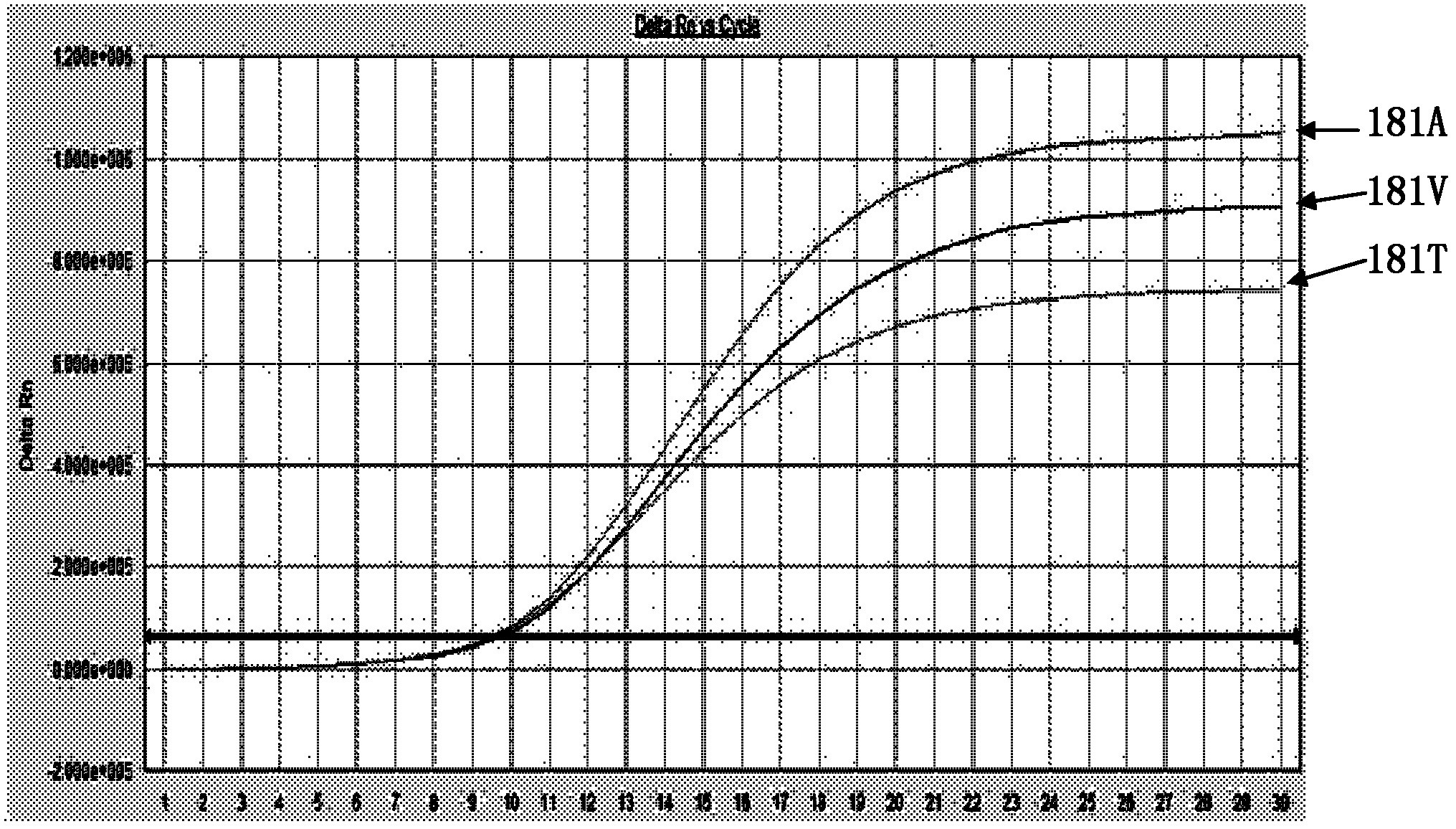
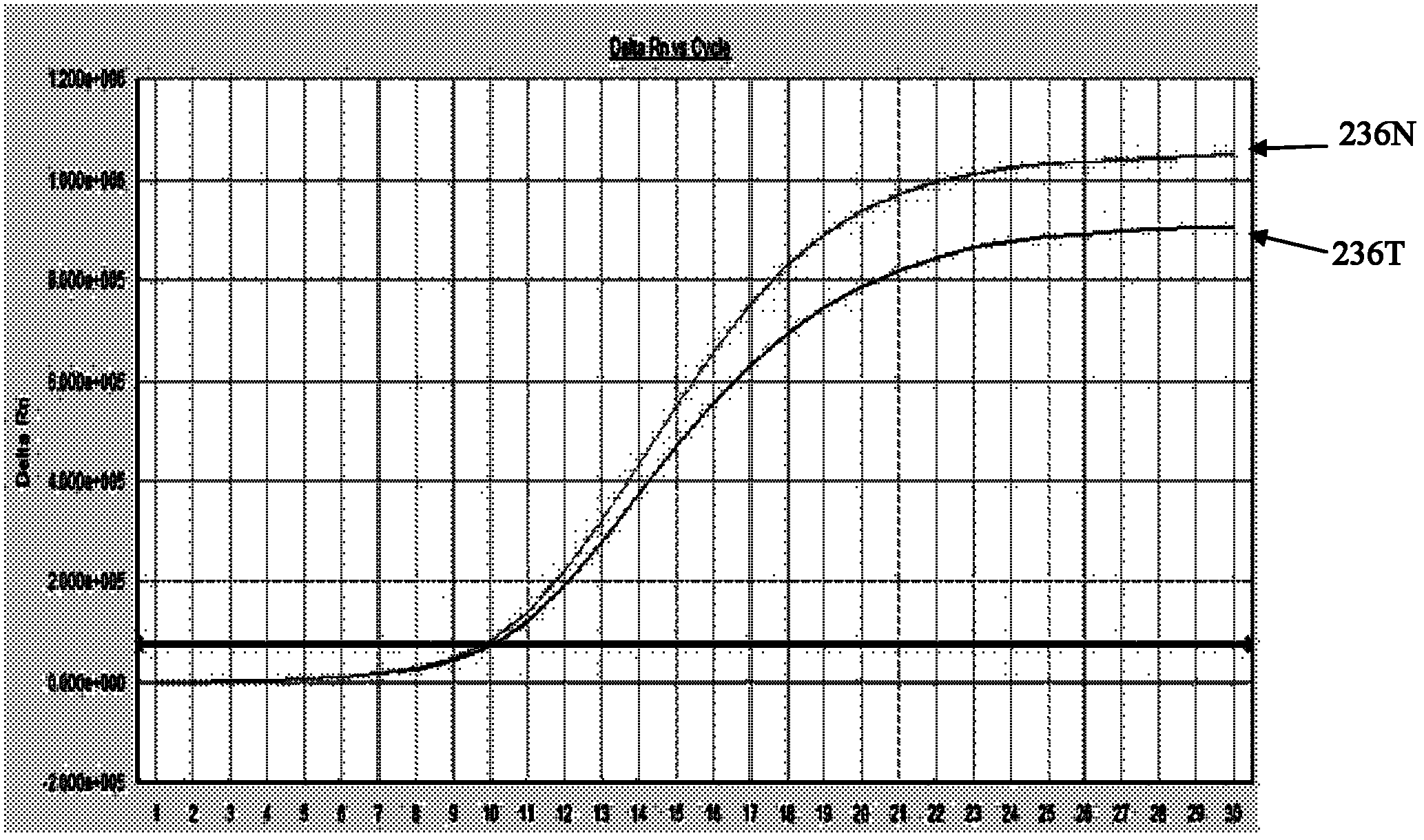
Hepatitis B virus Adefovir dipivoxil drug-resistance nucleic acid quantitative detection reagent kit, detection method, primers and probes thereof
ActiveCN102304589AAvoid pollutionAvoid harmMicrobiological testing/measurementFluorescence/phosphorescenceForward primerPositive control
The invention discloses a hepatitis B virus Adefovir dipivoxil drug-resistant nucleic acid quantitative detection reagent kit, a detection method, primers and probes thereof. The reagent kit contains fluorescence quantitative PCR (Polymerase Chain Reaction) reaction liquid, including DEPC (Diethyl Pyrocarbonate) treated water, DNA (Deoxyribose Nucleic Acid) polymerases with the 5'->3' exonucleolytic activity, dNTPs (Deoxyribonucleotide Triphosphates), 10*fluorescence quantitative PCR Buffer, solution containing Mg2+ions, rt181 site forward primers, rt181 site reverse primers, rt181A site probes, rt181V site probes, rt181T site probes as well as rt236 site forward primers, rt236 site reverse primers, rt236N site probes and rt236 site probes peculiar to a rtN236T detection reagent kit; and DNA extracting solution, negative controls, working standards, positive controls and critical positive controls are also contained in the reagent kit. According to the detection method, the fluorescence quantitative PCR (Polymerase Chain Reaction) technology is adopted for quantitatively detecting hepatitis B virus Adefovir dipivoxil drug-resistant strains; according to the fluorescence quantitative PCR (Polymerase Chain Reaction) method, the step of PCR (Polymerase Chain Reaction) extension is avoided; and the probes can mark a variety of fluorescent marks; and meanwhile, the reagent kit has the advantages of specificity, sensitivity, quickness and convenience in operation.
Owner:WUHAN BIOTECH GENE ENG
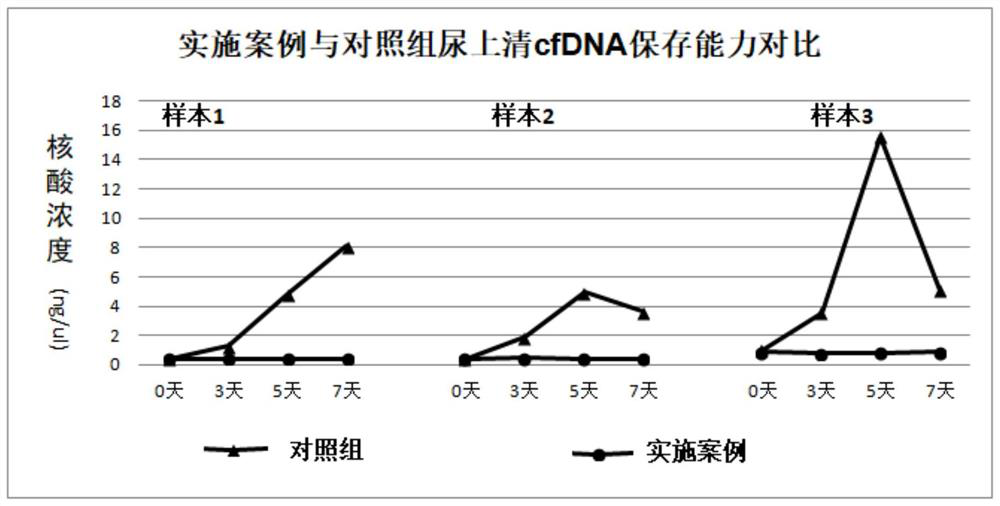
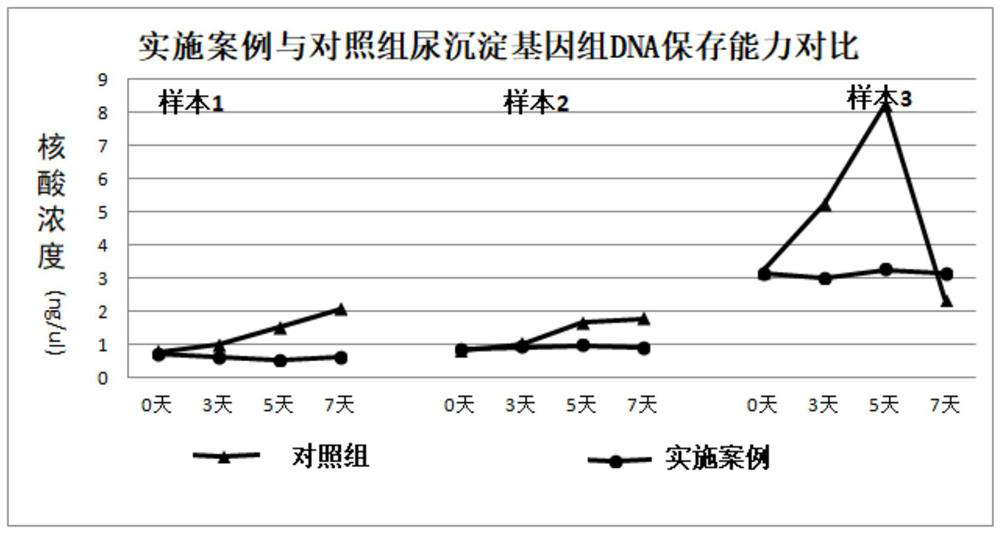
Urine preservation solution, preservation method and urine preservation tube
PendingCN114875022AAvoid degradationAvoid interferenceAnalysis material containersDead animal preservationActive agentA-DNA
The invention discloses a urine preservation solution which is an aqueous solution containing the following substances in percentage by mass: 0.02%-0.1% of an RNA (Ribonucleic Acid) enzyme inhibitor, 2%-10% of a DNA (Deoxyribose Nucleic Acid) enzyme inhibitor, 0.2%-0.8% of a nonionic surfactant, 1%-5% of a cell fixing agent, 1%-5% of a preservative and 1%-5% of an aldehyde group quencher, the RNA enzyme inhibitor at least comprises diethyl pyrocarbonate, and the DNA enzyme inhibitor at least comprises ethylenediamine tetraacetic acid. The invention further discloses a urine preservation method and a urine preservation tube. After urine is in vitro and is preserved by adopting the urine preservation solution provided by the invention, relatively accurate free DNA and RNA information can still be provided, the degradation of nucleic acid substances is inhibited, interference signals including but not limited to genome DNA and microorganism interference are inhibited, and an overall detection solution result still has relatively high sensitivity and relatively high accuracy. The operation is simple and the shelf life is long.
Owner:SANSURE BIOTECH INC
Method for preparing DFN package device with high thermal conductivity
ActiveCN109950158AIncrease contact areaImprove cooling effectPlastic/resin/waxes insulatorsSemiconductor/solid-state device detailsEpoxyPolymer science
The invention discloses a method for preparing a DFN package device with high thermal conductivity, comprising the following steps of: S1, uniformly mixing silicon micro-powders and a flame retardantwith 3-aminopropyltriethoxysilane for surface treatment; S2, adding epoxy resin, linear phenolic resin, liquid nitrile rubber, diethyl pyrocarbonate, polyethylene glycol monooctyl phenyl ether, cellulose acetate butyrate, 5-fluoro-2-meth-oxyaniline, 2,4,6-tris(dimethylaminomethyl)phenol and a release agent, and uniformly mixing the same; and S3, mixing the mixture at 90-110 degrees centigrade for3 to 5 minutes, cooling, pulverizing and sieving a product. The DFN package device with high thermal conductivity prepared by the method has a low incidence rate of internal pores, and avoids electrical property failure due to the deterioration of the thermal conductivity caused by the pores.
Owner:西安航思半导体有限公司
Preparation method of high temperature resistant qfn packaging structure
ActiveCN109904125BGuaranteed stabilityImprove mechanical propertiesSemiconductor/solid-state device detailsSolid-state devicesMeth-Engineering
Owner:西安航思半导体有限公司
Reagent kit, method, primers and probe for quantitative detection of nucleic acid of swine fever virus
ActiveCN102676690BAvoid pollutionAvoid harmMicrobiological testing/measurementFluorescence/phosphorescenceForward primerRNA extraction
The invention discloses a reagent kit, a method, primers and a probe for quantitative detection of nucleic acid of a swine fever virus and belongs to the technical field of biology. The reagent kit comprises PCR (polymerase chain reaction) liquid including DEPC (diethyl pyrocarbonate) treating water, Taq enzyme, M-MLV reverse transcriptase, RNase inhibitor, dNTP Mix, 10X one-step RT-PCR (reverse transcription-polymerase chain reaction) Buffer, MgC12 solution, the forward primer of the swine fever virus, the reverse primer of the swine fever virus and the LNA (locked nucleic acid) probe of the swine fever virus, wherein the forward primer of the swine fever virus refers to: 5'-AACGGYAGTGCTTTCTAYC-3', the reverse primer of the swine fever virus refers to: 5'-GTGGRAAAGGCTTCTCTC-3', and the LNA probe of the swine fever virus refers to: 5'-accActTctGtyCtac-3'. The reagent kit further comprises RNA (ribonucleic acid) extracting solution, negative quality control serum, working standard serum, positive quality control serum and critical positive quality control serum. The real-time fluorescent quantitative PCR technology is used for quantitatively detecting the nucleic acid of the swine fever virus, and accordingly the reagent kit has the advantages of specificity, sensitivity, rapidness and simplicity and convenience to operate.
Owner:湖北万德瑞生命科学技术有限公司
Cell storage liquid and preparation method of sputum cell reference product
InactiveCN108094407AImprove integrityEasy to preparePreparing sample for investigationDead animal preservationNucleic acid detectionReference product
The invention provides a cell storage liquid having simple composition and aiming at maintaining nucleic acid stability in the cells, and a method for preparing a cell and sputum reference product byusing the cell storage liquid. The cell storage liquid is prepared from 75 percent ethanol, 0.9 percent of sodium chloride, 4 percent RNAsecure and the balance of diethyl pyrocarbonate treatment water. The method has the advantages that the cost is low; the preparation method is simple; no toxic and side effects exist. By using the method, the normal cell form can be maintained under the normal temperature condition (20 DEG C); meanwhile, the nucleic acid completeness in the cells can be effectively protected. The invention aims at providing a reference method for the preparation of a reference product of a nucleic acid detection type product of sputum or other tissue samples.
Owner:ZHEJIANG JFK BIOLOGICAL TECH
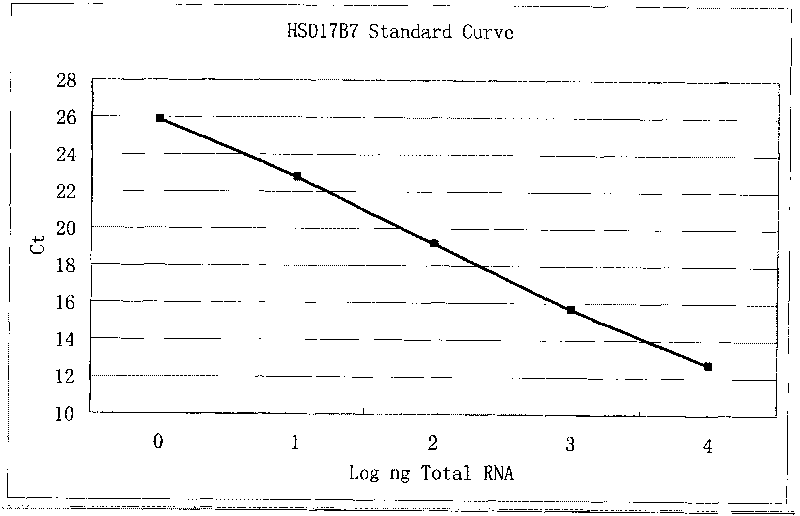
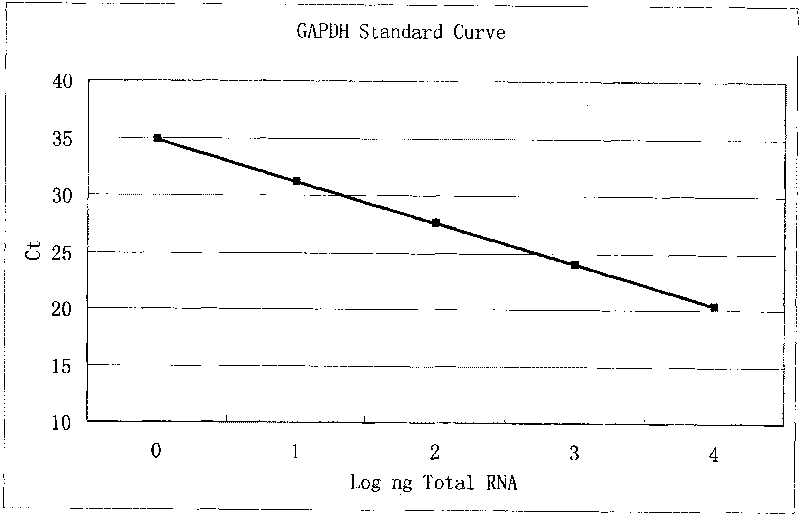
Kit for detecting expression level of 17 beta-hydroxysteroid dehydrogenase 7 in human prostate cancer
InactiveCN101760525AReduce false positive rateHigh detection sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceBiotechnologyFluorescence
The invention belongs to the field of biotechnology and relates to a real-time fluorescent quantitative PCR kit for detecting the mRNA expression level of 17 beta-hydroxysteroid dehydrogenase 7 (17 beta-HSD7) in human prostate cancer samples. The kit contains 2 * reverse transcription reaction solution, 20 * reverse transcriptase, water treated by diethyl pyrocarbonate, 2 * polymerase chain reaction solution, 20 * primers and fluorescent probes for detection and a standard product. The invention establishes a method for utilizing the Taqman technology for detecting the expression of the 17 beta-HSD7, and the detection practices prove that the kit and the detection method are feasible. As the method adopts the PCR amplification technology, the method can greatly improve the detection sensitivity of the 17 beta-HSD7 and obtain enough information in a very small number of samples; due to the application of the fluorescent probes, the specificity is also greatly improved and the false positive rate of the conventional PCR amplification is reduced.
Owner:FUDAN UNIV
Method for separating RNA (Ribonucleic Acid) from human serum/blood plasma sample and PCR (Polymerase Chain Reaction) verification method thereof
InactiveCN101914523BEfficiently obtainedImprove extraction efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseFluorescence
The invention discloses a method for separating RNA (Ribonucleic Acid) from a human serum / blood plasma sample, which sequentially comprises the steps of: 1, pretreating; 2, adding a Trizol reagent into the pretreated sample in an RNase-free centrifuge tube, uniformly mixing, standing at room temperature; 3, extracting chloroform; 4, adding isopropanol into the centrifuge pipe obtained from the step 3, uniformly mixing, standing at room temperature, centrifuging to obtain white sediment, washing and centrifuging the white sediment, and discarding supernatant; and 5, airing the sediment obtained from the step 4, adding diethyl pyrocarbonate for treating water to ensure that the sediment is dissolved to obtain a purified serum / blood serum RNA sample. The invention also discloses a method forcarrying out RT (Reverse Transcriptase)-fluorescence quantitative PCR (Polymerase Chain Reaction) verification by using the RNA obtained by the steps. The RNA with high quality and content can be effectively obtained by adopting the method of the invention.
Owner:ZHEJIANG SCI-TECH UNIV
High stability dfn packaged device
ActiveCN109904131BImprove cooling effectIncrease contact areaSemiconductor/solid-state device detailsSolid-state devicesEpoxyCellulose
Owner:西安航思半导体有限公司
Hepatitis B virus lamivudine resistant RNA quantitative detection primers and probes
ActiveCN102251059BAvoid pollutionAvoid harmMicrobiological testing/measurementMicroorganism based processesForward primerPositive control
The invention discloses a hepatitis B virus lamivudine resistant RNA quantitative detection kit, a detection method, primers and probes. The kit comprises fluorescent quantitative polymerase chain reaction (PCR) solution which contains diethyl pyrocarbonate (DEPC) treatment water, DNA polymerase with 5'-3' exonucleolytic activity, dNTPs, 10X fluorescent quantitative PCR Buffer, solution containing Mg<2+>, rt204 locus forward primer, a rt204 locus backward primer, a rt204M locus probe, a rt204I locus probe, a rt204V locus probe, a rt180 locus forward primer, a rt180 locus backward primer, a rt180L locus probe and a rt180M locus probe. The kit also comprises DNA extraction solution, a negative control, a working standard, a positive control and a critical positive control. In the invention, a real-time fluorescent quantitative PCR technique is adopted to quantitatively detect a lamivudine resistant hepatitis B virus strain, the fluorescent quantitative PCR method is changed into a '2-step method', and the step of PCR extension is avoided; and the probes can mark various fluorescent marks and have the advantages of specificity, sensitiveness, quickness and simple and convenient operation.
Owner:WUHAN BIOTECH GENE ENG
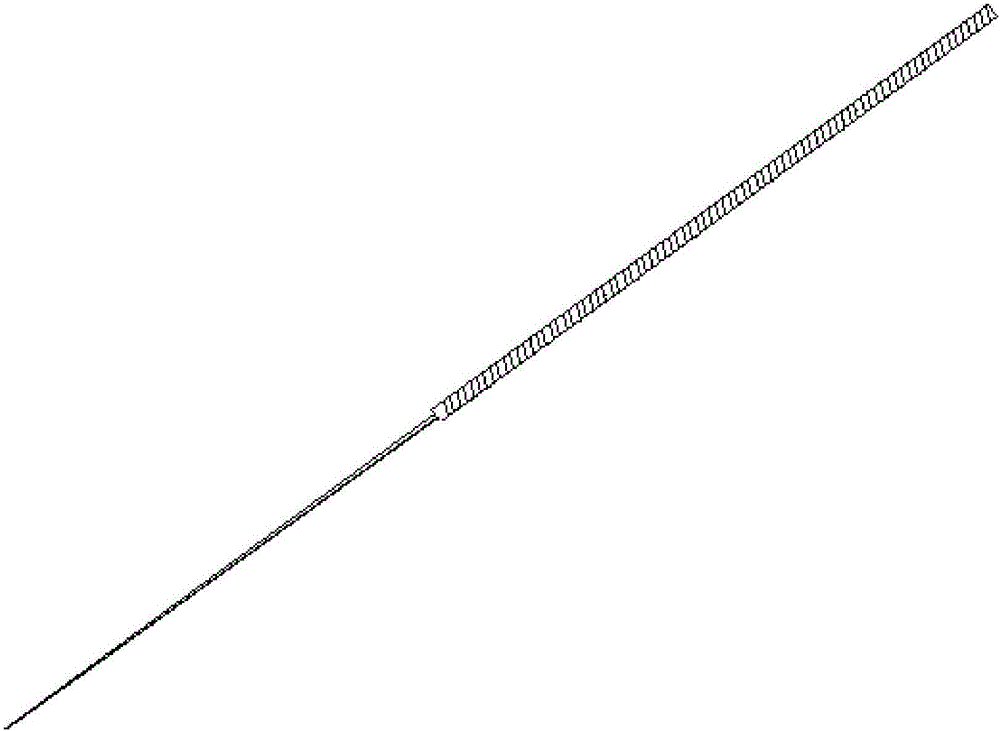
Plant mRNA (messenger ribonucleic acid) extraction method
PendingCN105802959ASimple processAccurate location extractionMicrobiological testing/measurementDNA preparationTotal rnaHigh pressure
The invention discloses a plant mRNA (messenger ribonucleic acid) extraction method, belongs to the technical field of molecular biology, and particularly relates to the technical field of extracting mRNA from plant materials through acupuncture needles.The plant mRNA extraction method is provided to solve the problems of operation complexity, low efficiency and damage to plant materials in conventional mRNA extraction methods.The plant mRNA extraction method includes 1, treating an acupuncture needle; 2, putting the dried acupuncture needle into a mixed solution of trimethoxysilane, xylene and diisopropylethylamine for incubation; 3, embedding the acupuncture needle to enable poly-thymine to be adsorbed to the surface of the acupuncture needle; 4, autoclaving the embedded acupuncture needle subjected to ultrapure water elution; 5, punching the naturally dried acupuncture needle into a test position of a target gene of a tender plant material, keeping the acupuncture needle at the test position for 1-3 minutes, pulling out the acupuncture needle, and putting the acupuncture needle into a PCR (polymerase chain reaction) tube of diethyl pyrocarbonate water to complete plant mRNA extraction.The plant mRNA extraction method has the advantages that total RNA extraction is not needed, the mRNA can be extracted from the plant materials in 1-3 minutes, and simplicity, convenience and rapidness of the operation process are achieved.
Owner:NORTHEAST FORESTRY UNIVERSITY
Kit used for RNA extraction and extraction method thereof
The invention provides a kit used for RNA extraction and an extraction method thereof. The kit used for RNA extraction comprises diethyl pyrocarbonate, sterile double distilled water, guanidinium isothiocyanate, sodium dodecyl sulfate, polyvinylpyrrolidone, beta-mercaptoethanol, NaAC, phenol water, chloroform-isoamylol, ethyl alcohol, glycerinum and sorbitol. By means of the technical scheme, thekit has the advantages that the design is reasonable, tissue RNA of the human bodies, mice and tobaccos can be well extracted, the extraction steps are simple, the cost is low, and the kit is easy towide apply. The required RNA sample size is low, the RNA extraction rate is high, the integrity of RNA obtained through extraction is very high, and the kit is completely suitable for downstream research work, such as sequencing, RT-PCR and N-count.
Owner:北京华颉基因医疗技术有限公司
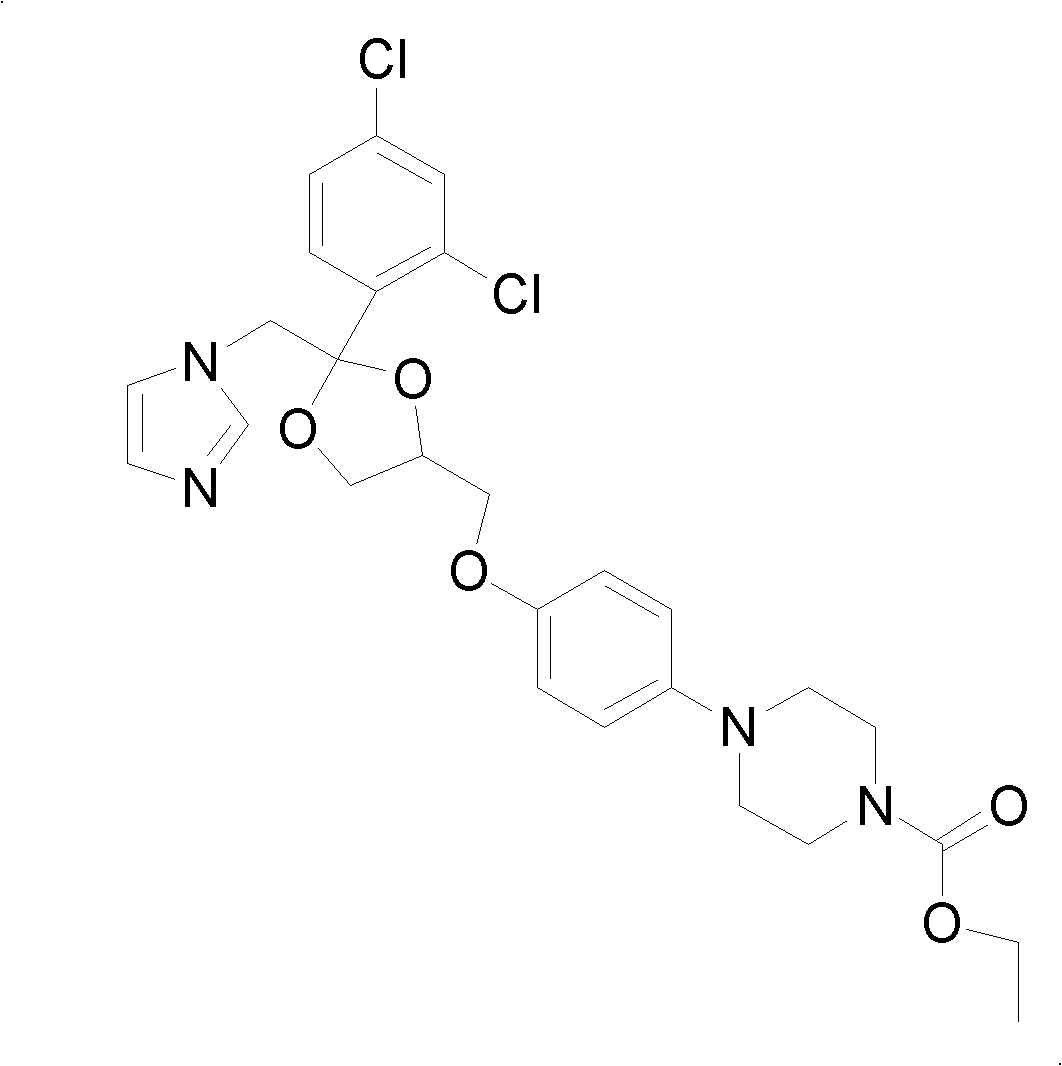
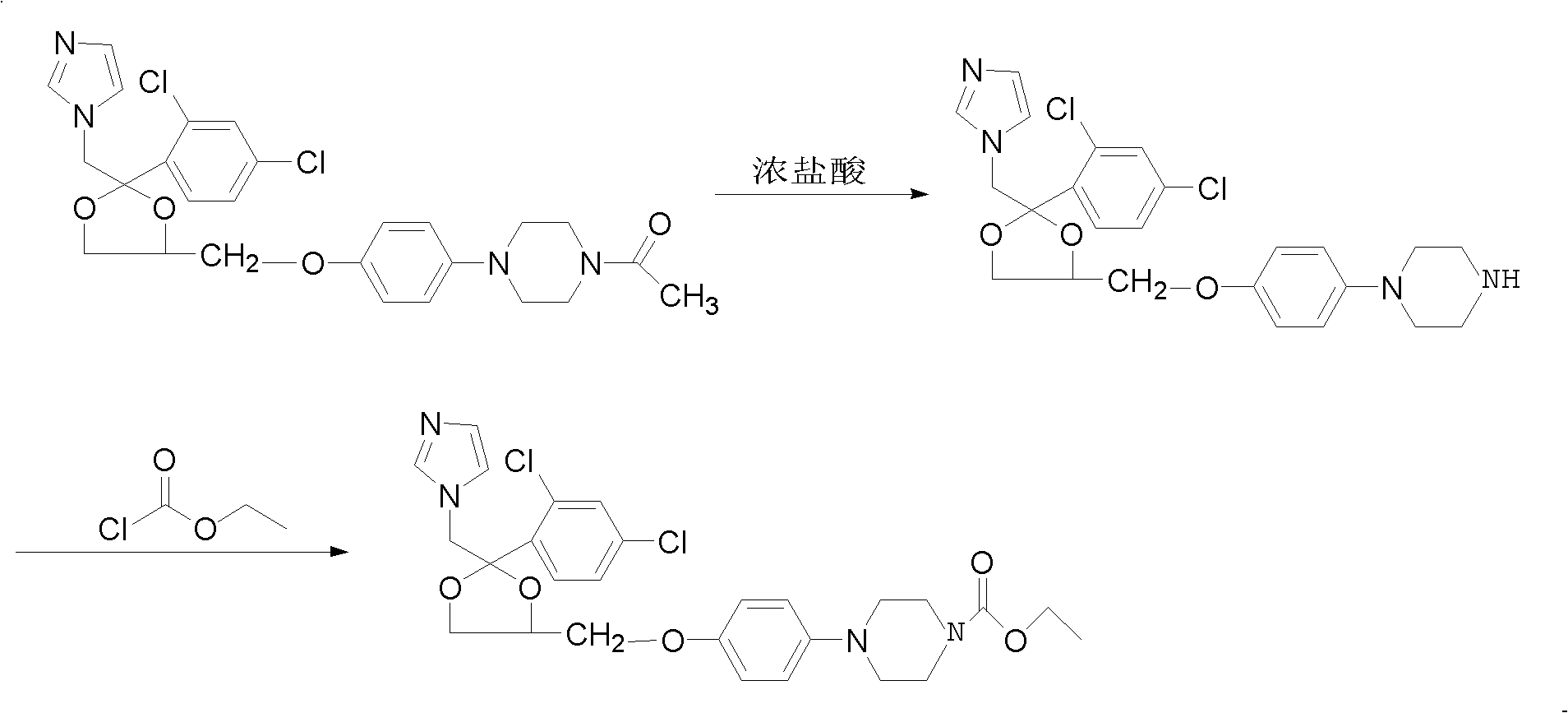
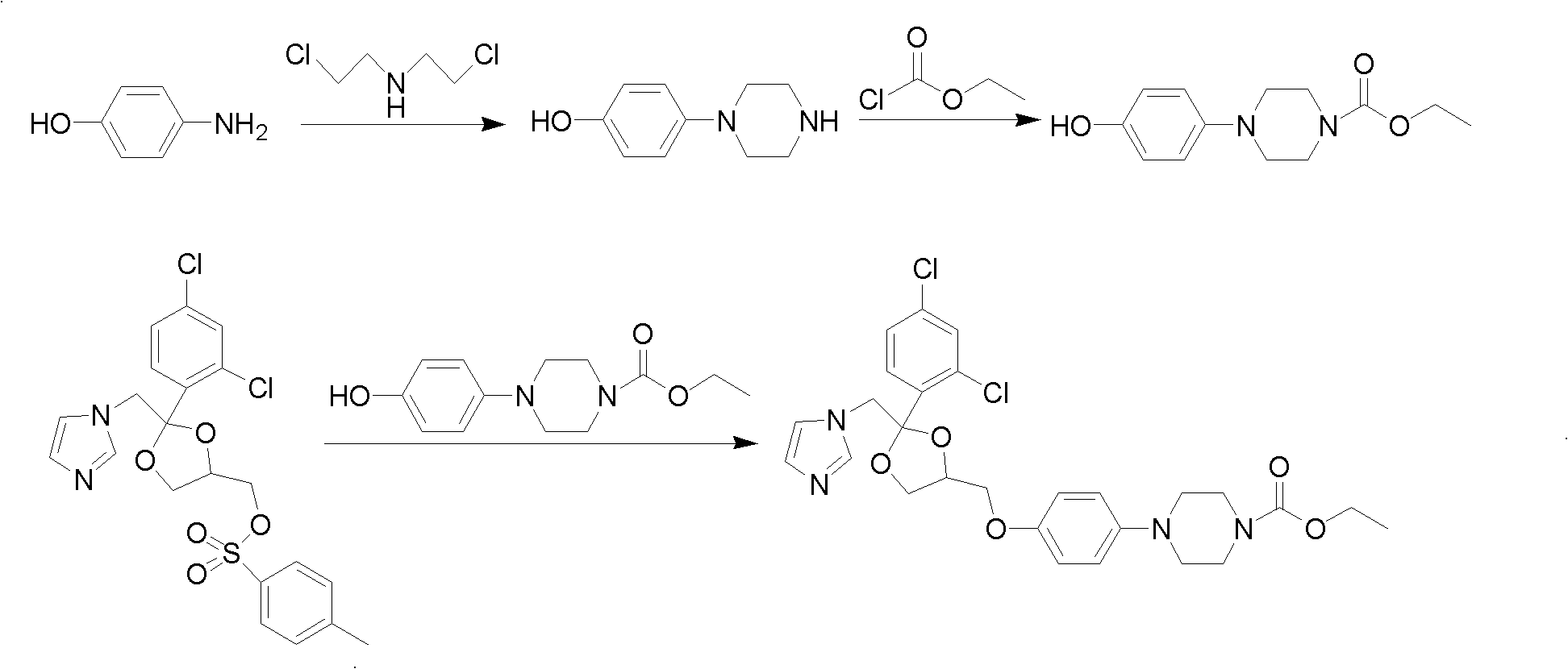
Method for preparing imidazole antifungal medicine derivate
InactiveCN101993436BHigh yieldImprove protectionAntimycoticsOrganic chemistryOrganic solventImidazole antifungal
The invention discloses a method for preparing an imidazole antifungal medicine derivate, which is characterized by comprising the following steps of: (a) heating ketoconazole in an organic solvent in the existence of alkaline substances, and removing acetyl to obtain a deacetylated product; and (b) dissolving the deacetylated product in the organic solvent, slowly dropping diethyl pyrocarbonate in the existence of a condensing agent, and reacting to obtain the imidazole antifungal medicine derivate. The invention greatly improves the yield of the reaction product, greatly reduces the cost required by production as all reagents can be repeatedly used and enhances the market competitive capacity of the product. The invention also has the advantages of simple operation, less reaction steps,high purity, less by-products, good reaction stability, and the like, is suitable for industrialized production, also has greater application prospect and less pollution as concentrated hydrochloric acid is not used in the reaction process and is beneficial to environmental protection.
Owner:杭州欧拓普生物技术有限公司
Reverse transcription-polymerase chain reaction (RT-PCR) detection kit for infectious haematopoietic necrosis viruses (IHNV) and preparation method of kit
ActiveCN103233005BEasy to operateMicrobiological testing/measurementMicroorganism based processesOperating instructionPosition control
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Detection kit for acute hemorrhagic conjunctivitis and detection method thereof
ActiveCN101864496BStrong specificitySimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescencePositive controlEnzyme system
The invention relates to a detection kit for an enterovirus 70-type (EV70) and Coxackie A24-type virus variants (CA24v) which are relevant to acute hemorrhagic conjunctivitis and a detection method thereof. The kit comprises a RT-PCR (reverse transcription-polymerase chain reaction) reaction buffer solution, enterovirus 70-type (EV70) and Coxackie A24-type virus variant (CA24v) specific forward and reverse primer and fluorescent probe mixed liquor necessary for PCR amplification, a RT-PCR reaction enzyme system, DEPC H2O (diethyl pyrocarbonate) and EV70 and CA24v positive controls as well as negative controls. The invention has stable and reliable result and simple and rapid operation and can rapidly detect the acute hemorrhagic conjunctivitis caused by the enterovirus 70-type and Coxackie A24-type virus variants, and thereby, the early anti-epidemic work can be completed.
Owner:DAAN GENE CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com