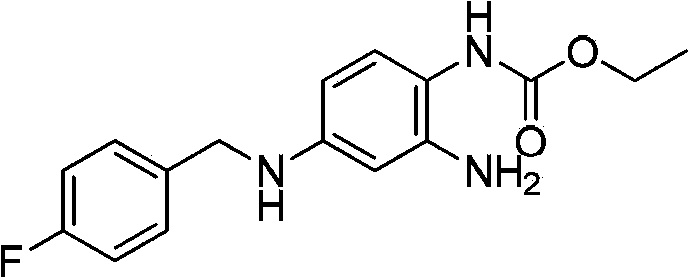
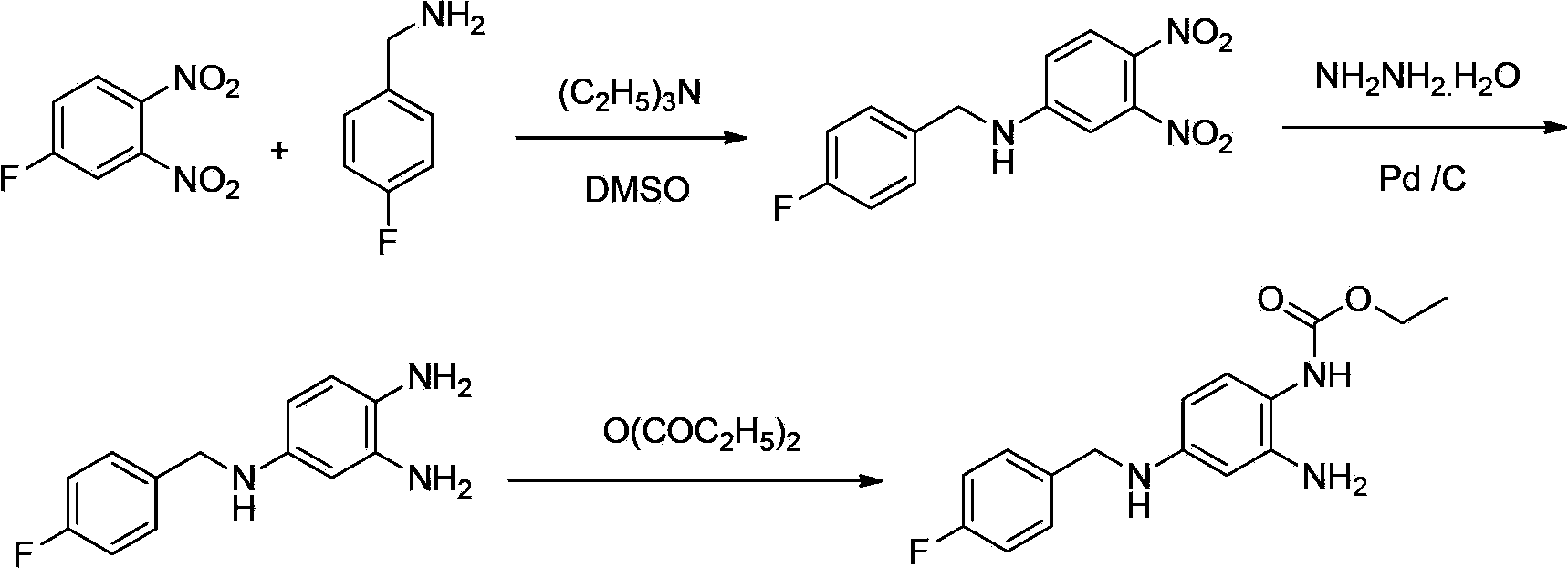
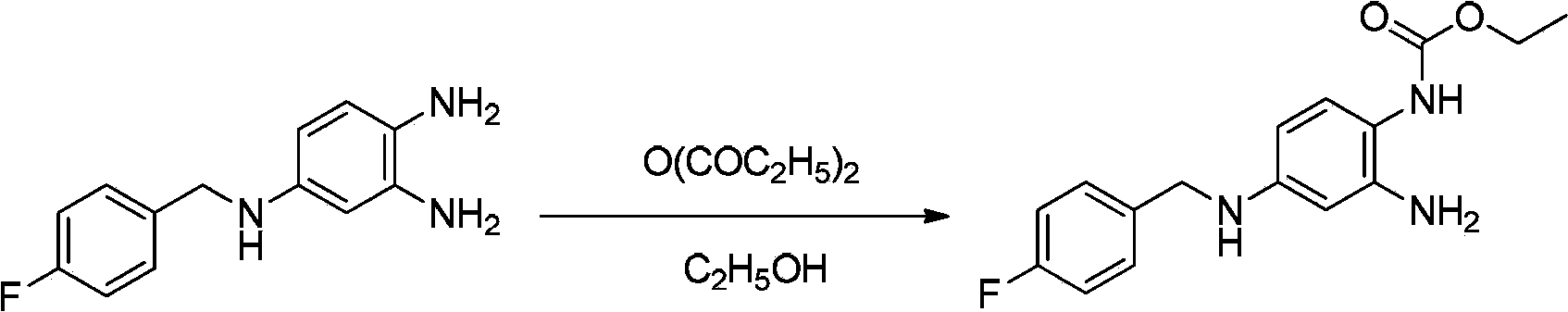Method of synthesizing retigabine
A retigabine and condensation reaction technology, which is applied in the field of synthesizing retigabine, can solve the problems of dangerous use and high toxicity, and achieve the effects of cost reduction, yield improvement, and simple route operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of 2-amino-4-(4-fluorobenzylamino)-1-nitrobenzene
[0033] Add 20g of 2-nitro-1,4-phenylenediamine into a 500mL reaction flask, add 280mL of toluene, stir, then add 17.8g of 4-fluorobenzaldehyde, heat to reflux for 6-10h to separate water. The complete reaction of 2-nitro-1,4-phenylenediamine was detected by TLC as the end point of the reaction. After cooling down to room temperature, continue to stir for 3 hours, filter and dry to obtain 4.62Kg of 2-amino-5-[(4-fluorophenylmethylene)amino]-1-nitrobenzene with a yield of 91.2%.
[0034] Take 20g of 2-amino-5-[(4-fluorophenylmethylene)amino]-1-nitrobenzene and add it to a 500mL reaction flask, add 160mL of tetrahydrofuran, 40mL of ethanol, stir, and add sodium borohydride 5.9 g, react at 50°C for 5-8h after the addition. TLC detected that the reaction of 2-amino-5-[(4-fluorophenylmethylene)amino]-1-nitrobenzene was complete as the end point of the reaction. Evaporate the solvent, add 100mL ethyl...
Embodiment 2
[0035] Example 2: Synthesis of retigabine N-(2-amino-4-(4-fluorobenzylamino)phenyl) ethyl carbamate Take 4g of 10% Pd / C (water content 50%) to 500mL reaction Add 400 mL of ethanol and 20 g of 2-amino-4-(4-fluorobenzylamino)1-nitrobenzene into the bottle, and stir. Under normal pressure, hydrogen gas was introduced and heated to 40-60°C for about 8 hours. TLC detection of 2-amino-4-(4-fluorobenzylamino) 1-nitrobenzene completely reacted as the end point of the reaction. The reaction solution was filtered after cooling down to room temperature. The filtrate was added to a 500 mL three-necked flask, and 12.4 g of diethyl pyrocarbonate was added dropwise at a temperature controlled at 12°C. The end point of the raw material reaction was detected by TLC, and stirring was continued for 1 h. Cool down to below 10°C and stir for 3 hours, filter, wash the filter cake 3 times with ice ethanol, 500 mL each time, and dry to obtain a crude product. The crude product was recrystallized f...
Embodiment 3
[0036] Example 3: Synthesis of retigabine N-(2-amino-4-(4-fluorobenzylamino)phenyl) ethyl carbamate Take 4g of 10% Pd / C (50% water content) into 500mL reaction Add 400 mL of isopropanol and 20 g of 2-amino-4-(4-fluorobenzylamino)1-nitrobenzene into the bottle, and stir. Under normal pressure, hydrogen gas was introduced and heated to 40-60°C for about 8 hours. TLC detection of 2-amino-4-(4-fluorobenzylamino) 1-nitrobenzene completely reacted as the end point of the reaction. The reaction solution was filtered after cooling down to room temperature. The filtrate was added to a 500 mL three-necked flask, and 12.4 g of diethyl pyrocarbonate was added dropwise at a temperature of 15°C. The end point of the raw material reaction was detected by TLC, and stirring was continued for 1 h. Cool down to below 10°C and stir for 3 hours, filter, wash the filter cake 3 times with ice ethanol, 500 mL each time, and dry to obtain a crude product. The crude product was recrystallized from t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com