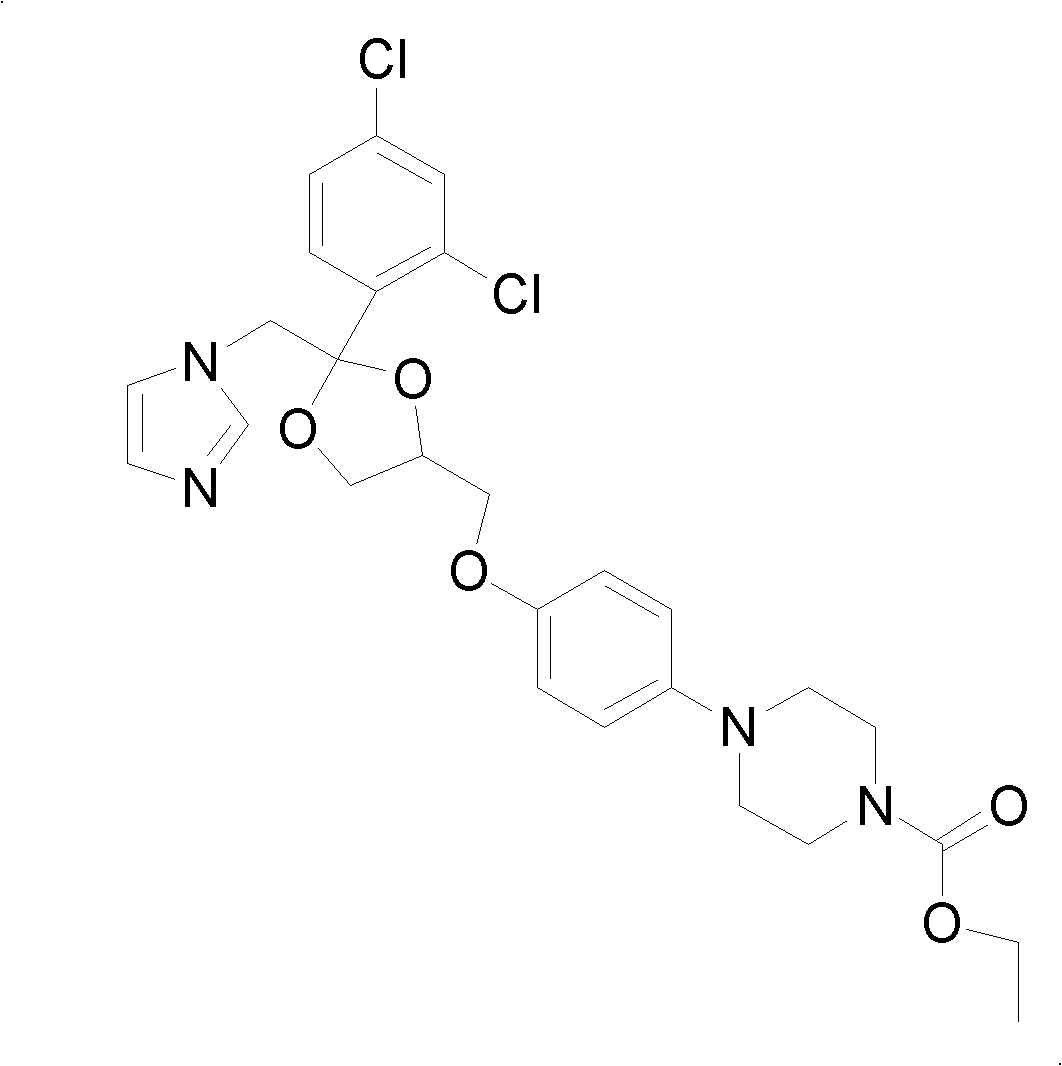
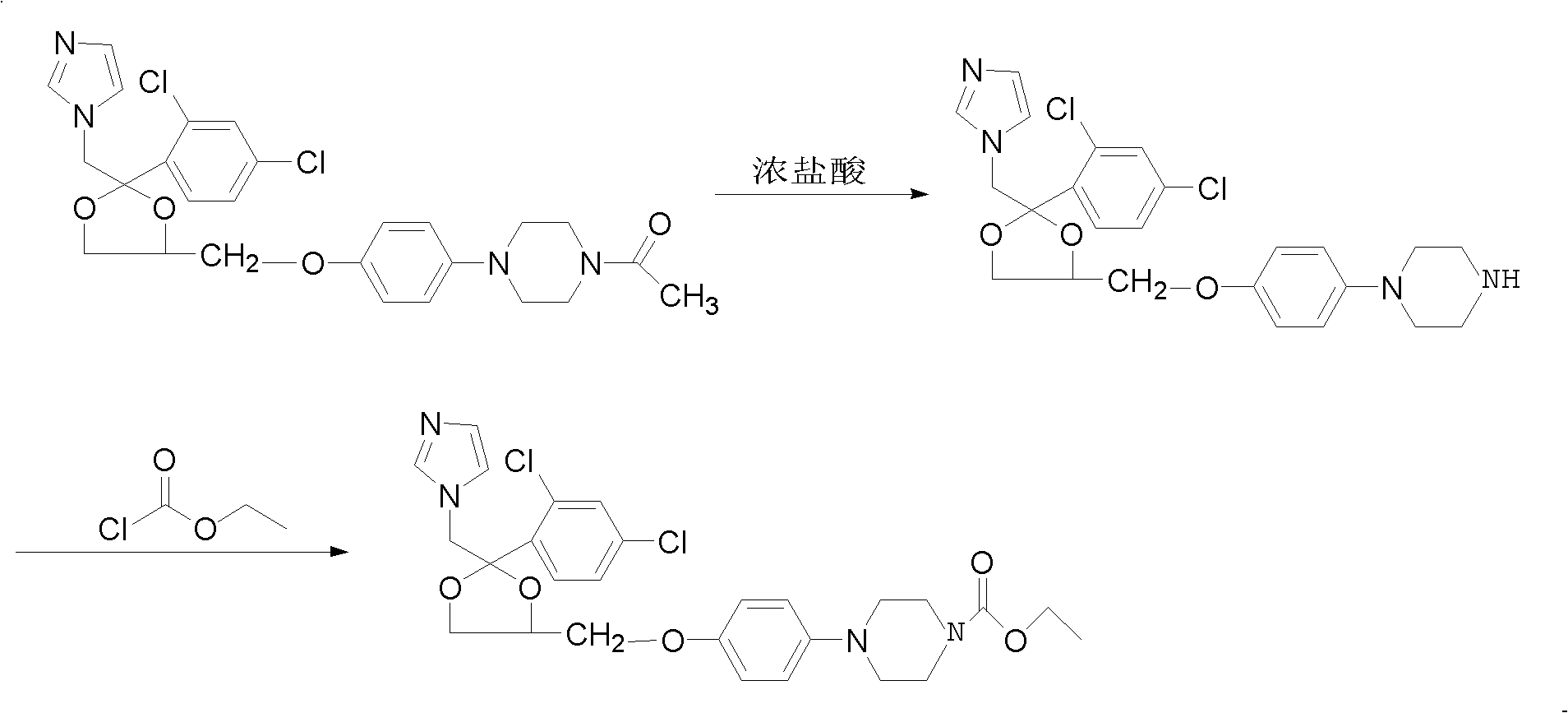
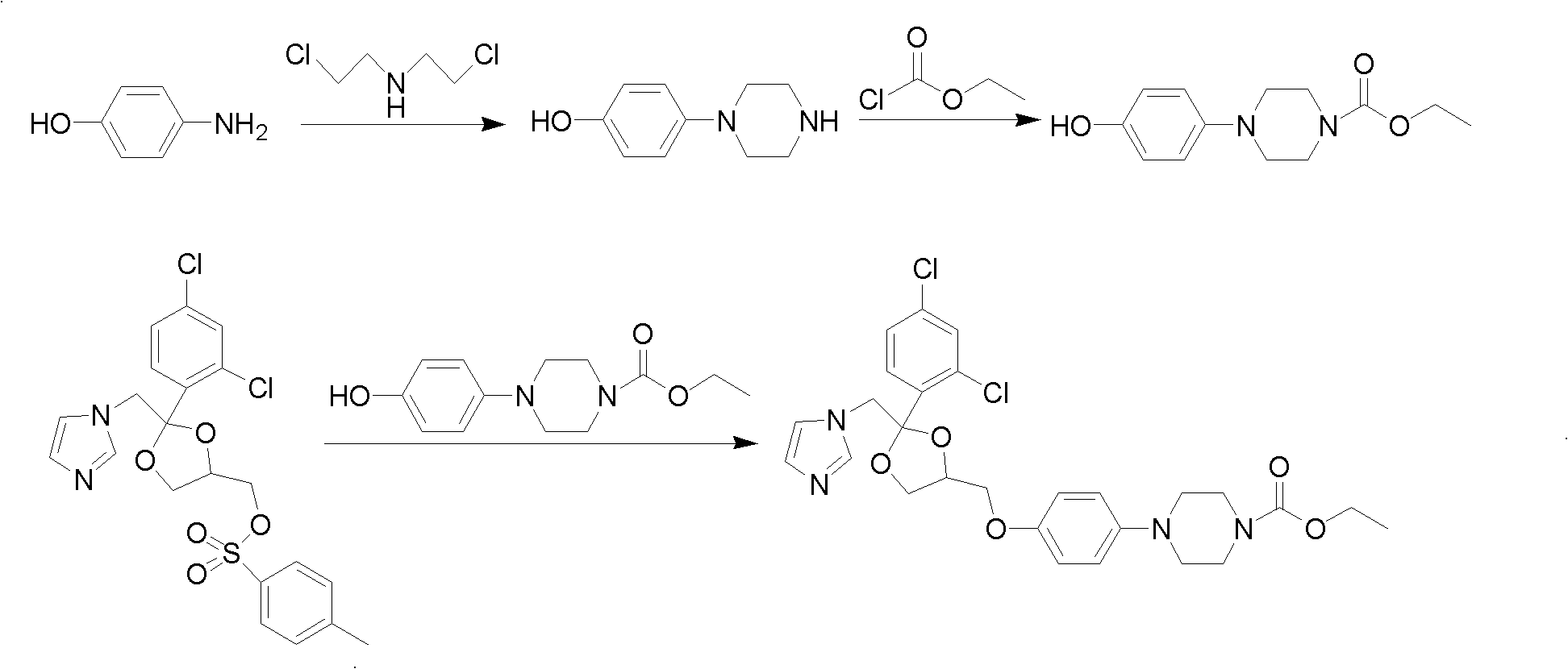
Method for preparing imidazole antifungal medicine derivate
An antifungal drug and imidazole technology, which is applied in the field of preparation of imidazole antifungal drug derivatives, can solve the problems of high toxicity of raw materials and auxiliary materials, complicated reaction process, unfavorable large production, etc., and achieves improved market competitiveness, simple operation, The effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 10g of ketoconazole to a 500ml three-neck flask, add 100ml of 1mol / L NaOH solution, stir to mix evenly, add 50ml of absolute ethanol, and under the protection of nitrogen, heat the material to 50°C and react for 10 hours. After cooling the reaction material to room temperature, ice water was added to precipitate a solid, which was filtered and dried to obtain 2.7 g of a white solid. The content of the deacetylated product detected by HPLC was greater than 98%, and the yield was 29.2%.
Embodiment 2
[0033] Add 10g of ketoconazole to a 500ml three-neck flask, add 100ml of 1mol / L NaOH solution, stir to mix evenly, add 50ml of absolute ethanol, and under the protection of nitrogen, heat the material to 85°C and reflux for 4 hours. After the reaction material was cooled to room temperature, cold water was added to precipitate a solid. After filtration and drying, 8.7 g of a white solid was obtained. The content of deacetylated product was greater than 98% as detected by HPLC, and the yield was 94%.
Embodiment 3
[0035] Add 10g of ketoconazole to a 500ml three-neck flask, add 100ml of 1mol / L sodium carbonate solution, stir to mix evenly, add 5ml of triethylamine, heat to 90°C under the protection of nitrogen, and reflux for 4 hours. After the reaction material was cooled to room temperature, cold water was added to precipitate a solid, and 8.9 g of a white solid was obtained by filtration and drying. The content of the deacetylated product was greater than 98% as detected by HPLC, and the yield was 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com