Reagent kit, method, primers and probe for quantitative detection of nucleic acid of swine fever virus
A swine fever virus and nucleic acid quantification technology, applied in biochemical equipment and methods, microbial determination/inspection, material excitation analysis, etc., can solve the problems of high detection cost and hardware requirements, high false positive rate, poor repeatability, etc. Achieve highly specific, simplified experimental procedures, and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The composition of embodiment 1 kit
[0075] The kit contains: PCR reaction solution, including DEPC-treated water, HotStart Taq DNA polymerase with 5’→3’ exolytic activity, M-MLV reverse transcriptase, dNTP Mix, 10×one-step PCRBuffer, MgCl 2 Solution, CSFV Forward Primer, CSFV Reverse Primer, CSFV LNA Probe; RNA extraction solution is divided into Solution 1, Solution 2, Solution 3, Solution 4 and Solution 5. Solution 1 is Trizol reagent, solution 2 is chloroform, solution 3 is isopropanol, solution 4 is 75% ethanol, solution 5 is DEPC-treated water; negative quality control product is a plasmid DNA fragment that does not contain classical swine fever virus E2 gene; The positive quality control product is a high-concentration swine fever virus genomic DNA fragment; the critical positive quality control product is a low-concentration swine fever virus gene DNA fragment; the working standard is a 127-base-pair nucleus containing the swine fever virus E2 gene The PSG-TS ...
Embodiment 2
[0109] Method for detecting classical swine fever virus nucleic acid on ABI 7300 fluorescent quantitative PCR instrument with kit of the present invention
[0110] (1) Collect samples: collect serum, nasopharyngeal swab, throat swab or disease tissue;
[0111] Serum samples: Collect 5ml of venous blood into 10ml screw-top plastic centrifuge tubes with gaskets (without anticoagulant), centrifuge at 1,000g for 10min, draw serum under aseptic conditions, and divide into several 1ml gaskets with gaskets Put them in screw-top plastic serum tubes (100 μl / tube), and transport them to the laboratory within 48 hours of refrigeration (4-8°C).
[0112] Throat swab and nasopharyngeal swab specimens: first wet the cotton swab with normal saline (do not use specimen transport solution containing penicillin to prevent allergies), nasopharyngeal swab is collected by inserting the cotton swab parallel to the palate into the nostril, and staying After a few seconds, absorb the secretions and w...
Embodiment 3
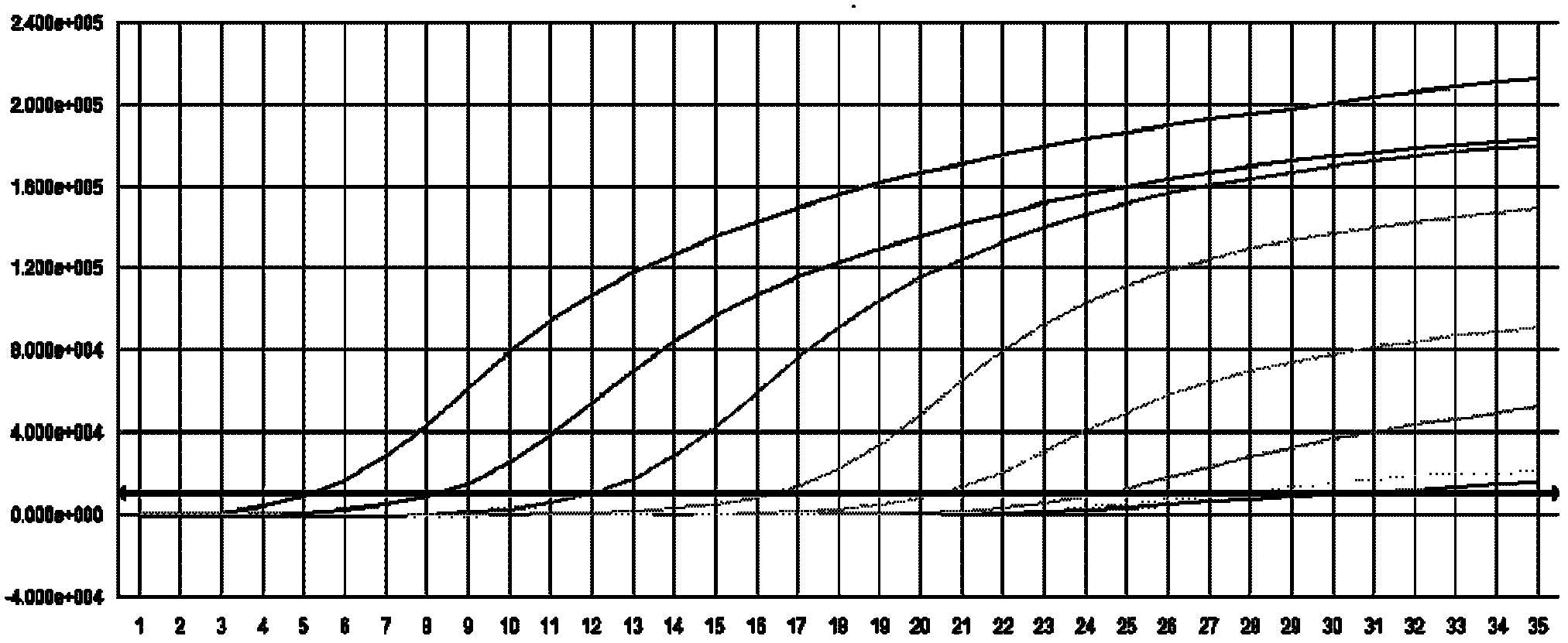
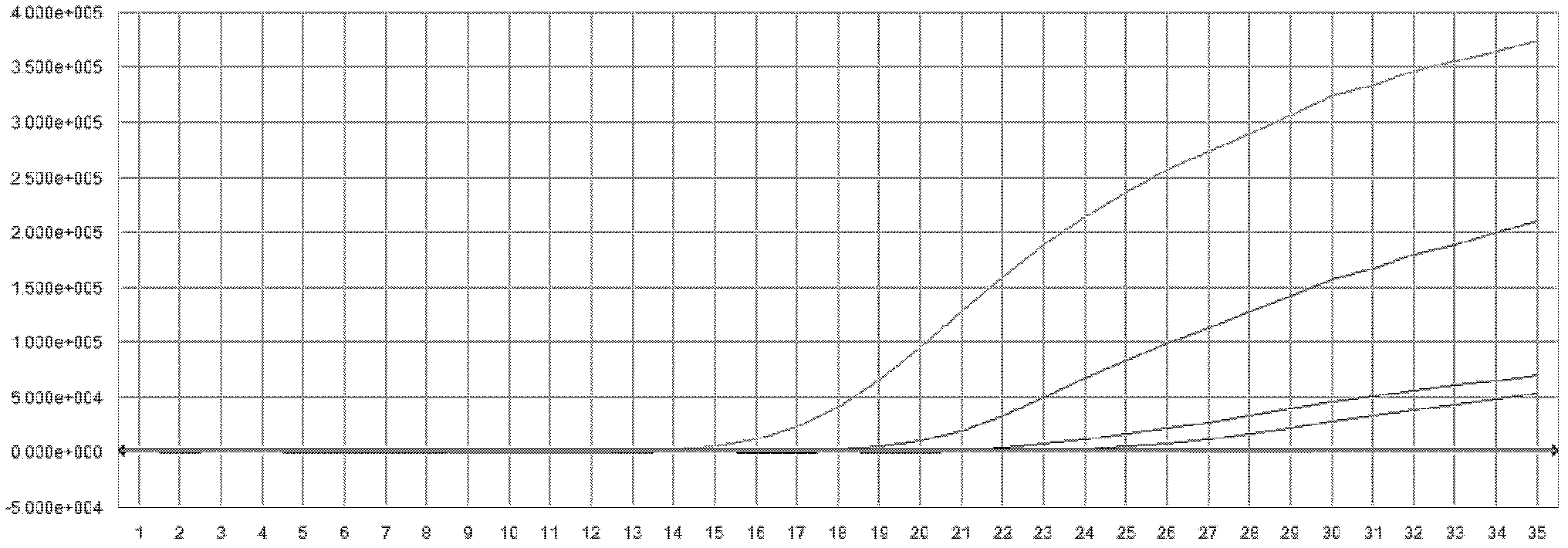
[0131] The kit of the present invention is used to detect the classical swine fever virus nucleic acid of the clinically confirmed sample according to the method of Example 2. Sample source ×× hospital patient's confirmed sample, the test result of embodiment 3 of the present invention is as follows figure 2 As shown, the test results are shown in the table below:
[0132] serial number
[0133] Clinically confirmed samples 1, 2, 3, 4, and 6 are positive samples, and samples 5 and 7 are negative samples. The test results are consistent, and the accuracy rate is 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com