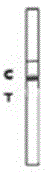Patents
Literature
67 results about "Pestivirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
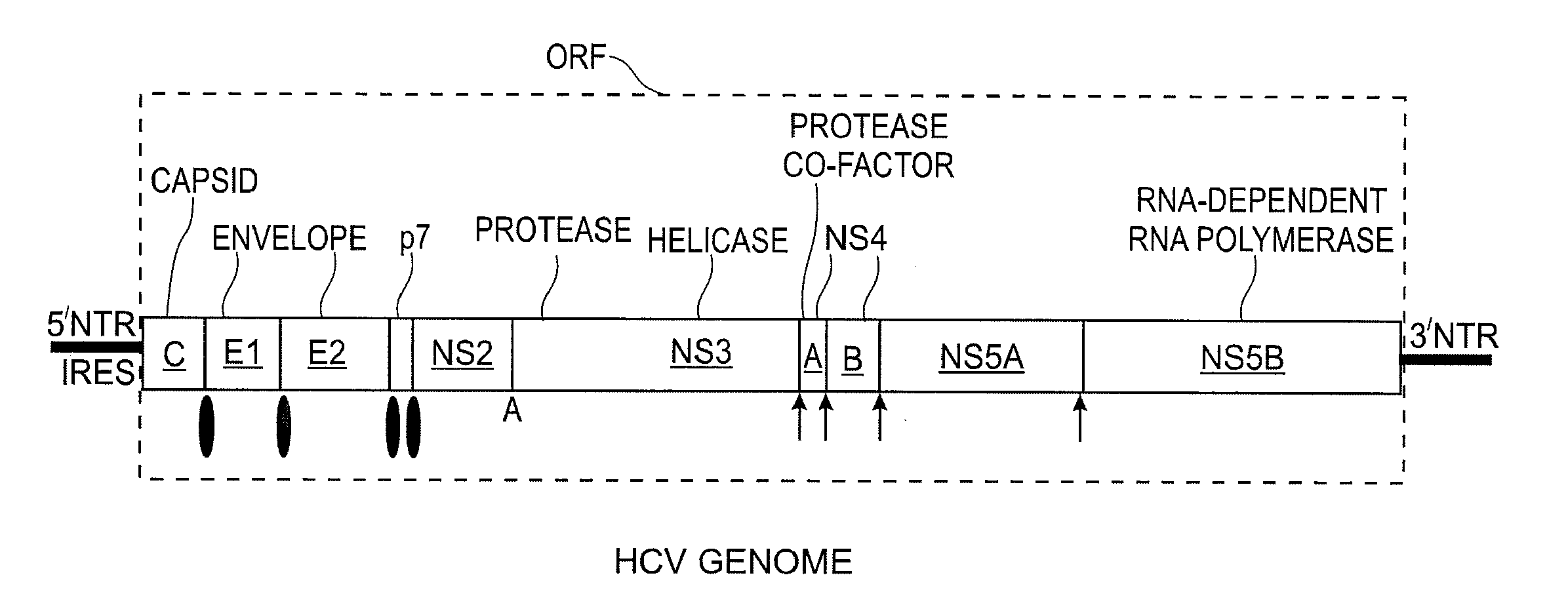
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae (which includes cattle, sheep, and goats) and the family Suidae (which includes various species of swine). Currently, 11 species are placed in this genus, including the type species Pestivirus A. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.
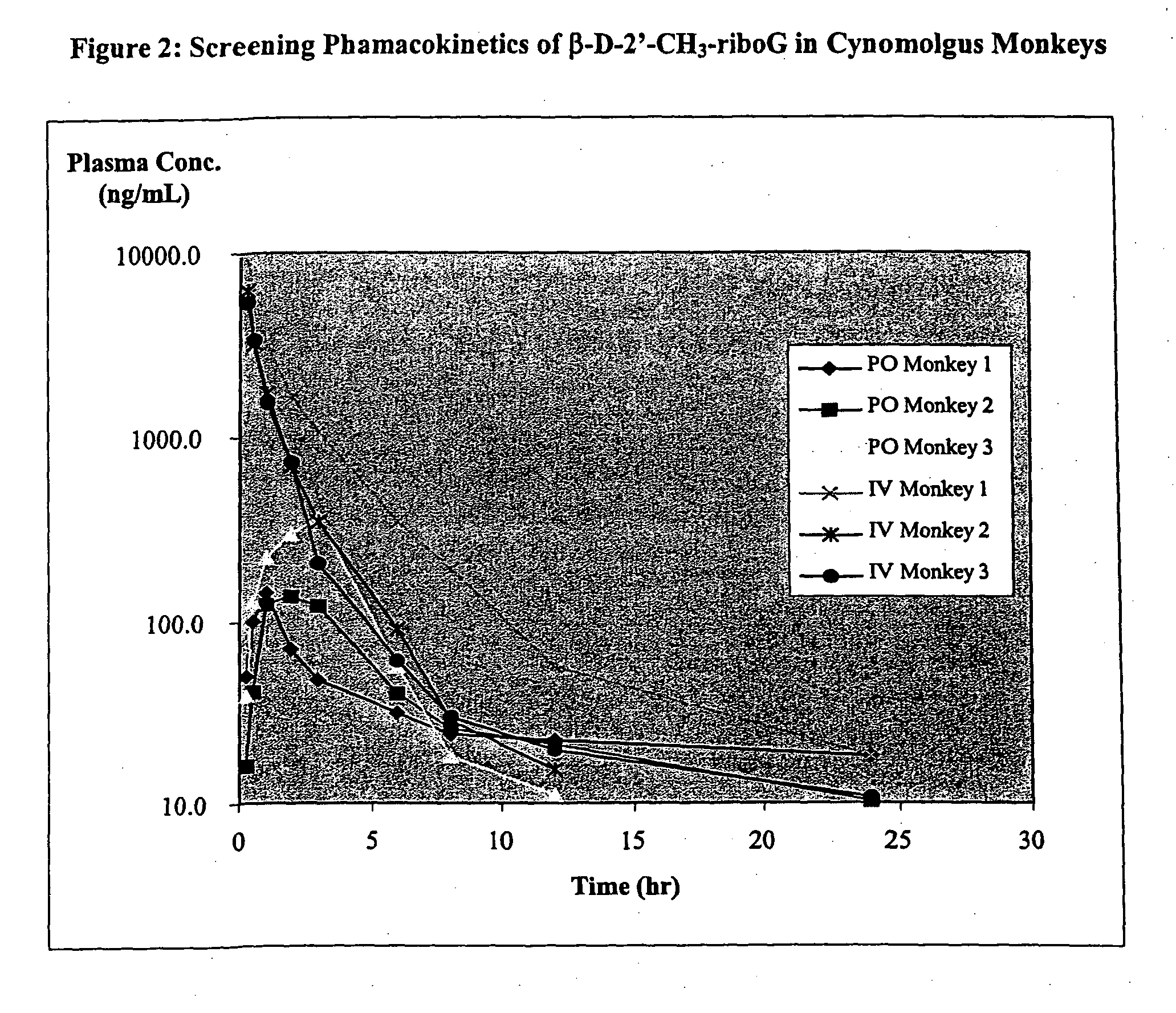
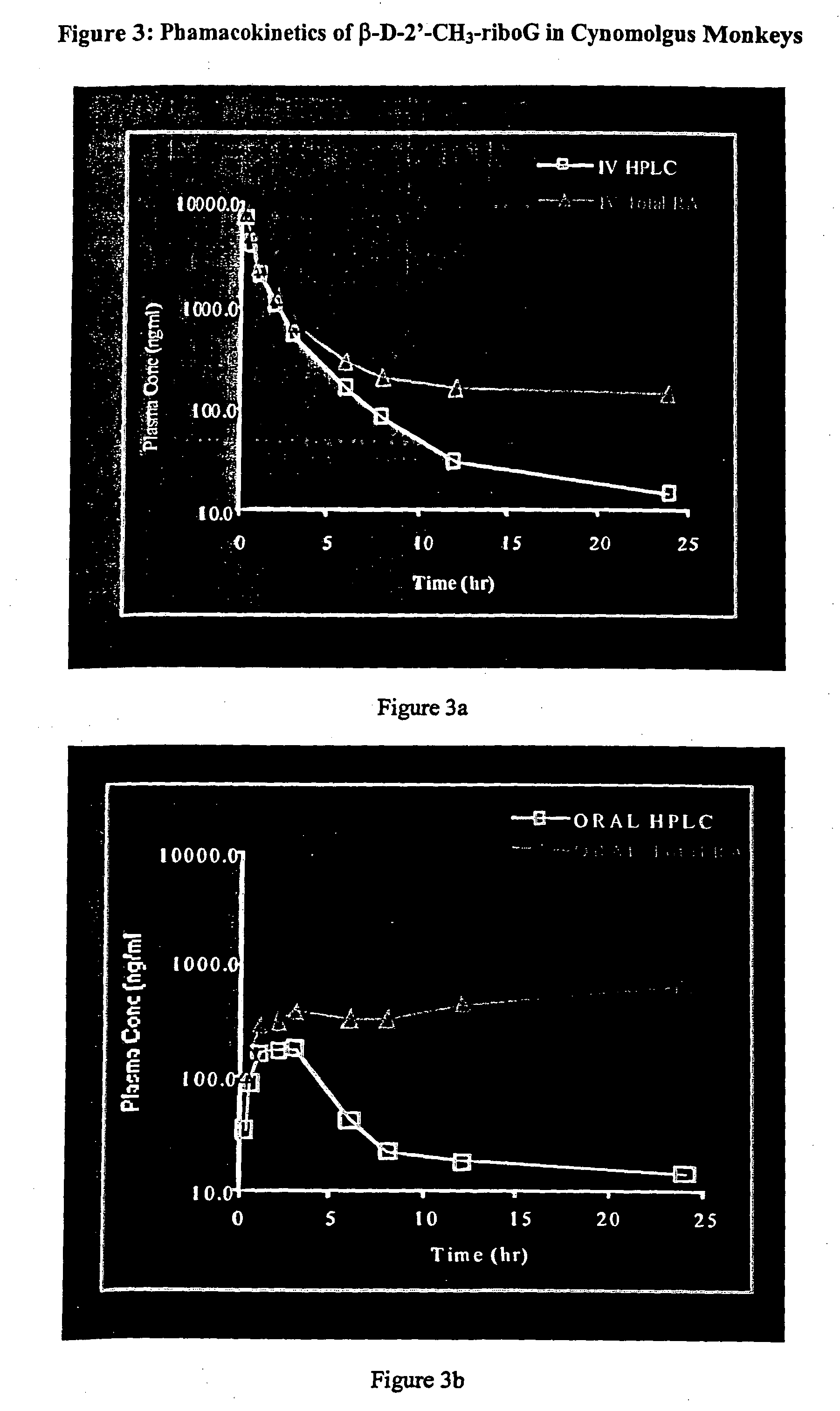
5-Aza-7-deazapurine derivatives for treating Flaviviridae
This invention is directed to a method for treating a host, especially a human, infected with hepatitis C, flavivirus and / or pestivirus, comprising administering to that host an effective amount of an anti-flavivirus or anti-pestivirus, biologically active compound has a 5-aza-7-deazapurine moiety. The 5-aza-7-deazapurine moiety may be substituted or unsubstituted, and may comprise a nucleoside analogue, or a salt or prodrug thereof. The compound of the present invention may be administered alone or in combination with another anti-hepatitis C, anti-flavivirus and / or anti-pestivirus agent.
Owner:INDENIX PHARM LLC +3
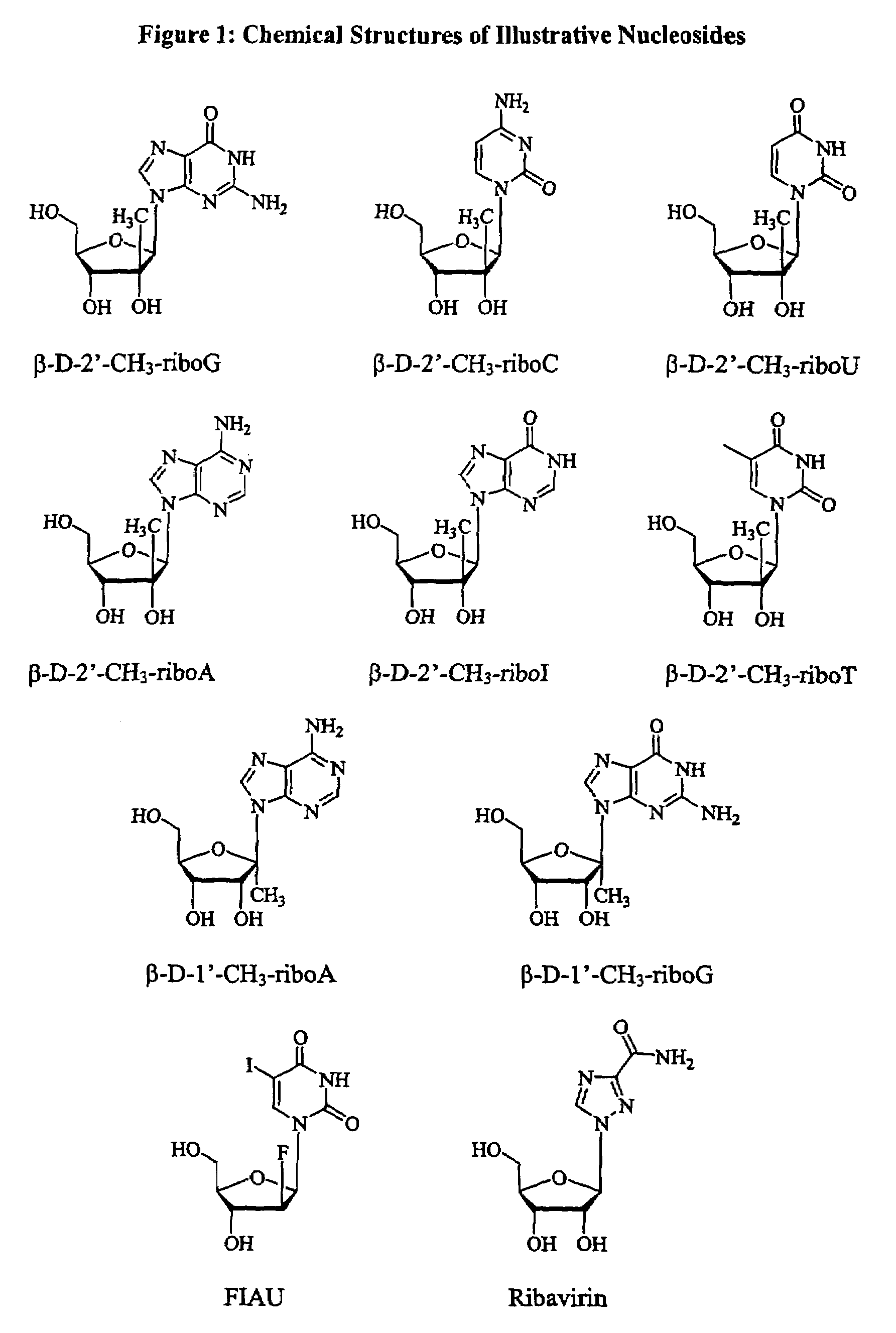
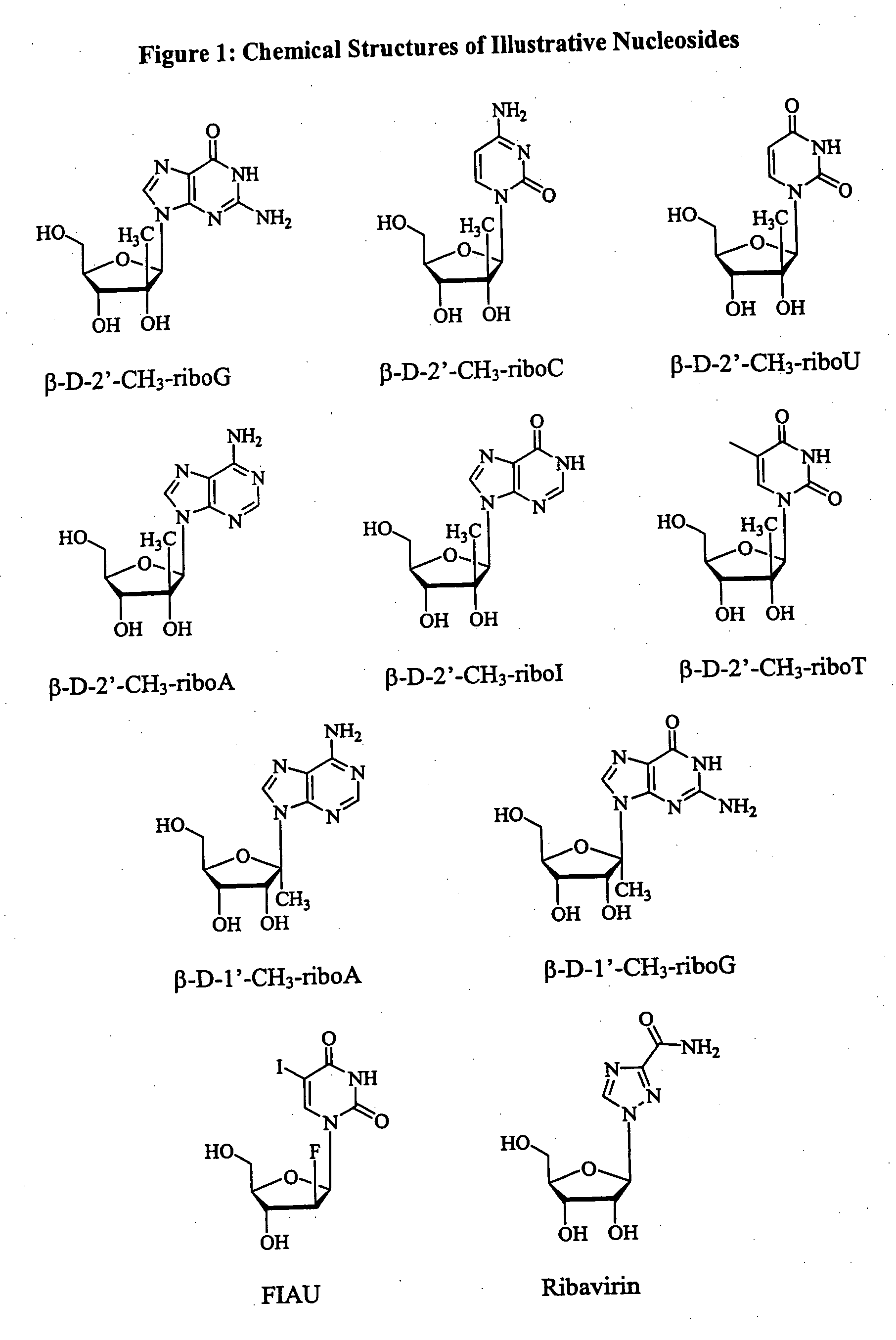
Methods and compositions for treating flaviviruses and pestiviruses
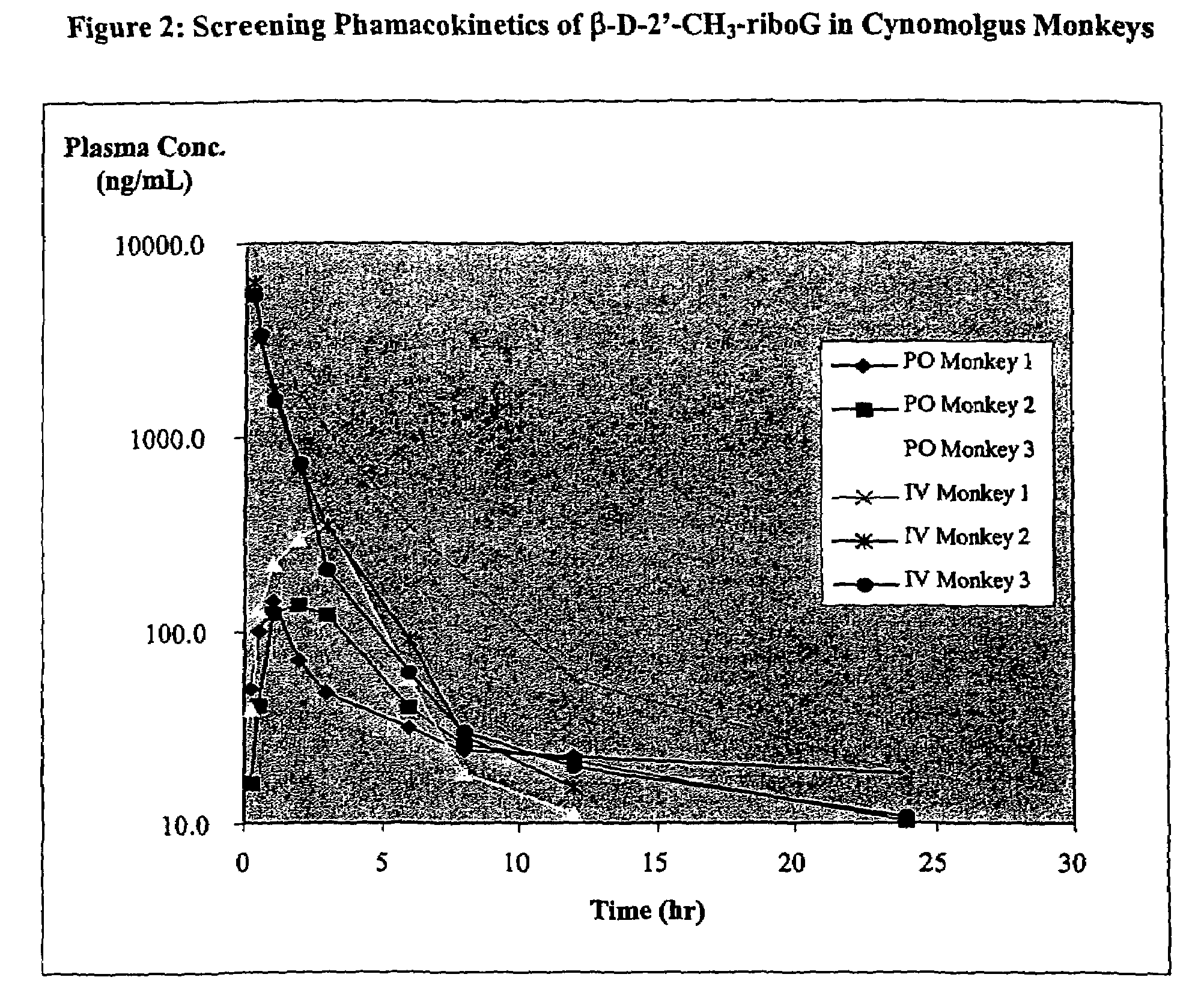
A method and composition for treating a host infected with flavivirus or pestivirus comprising administering an effective flavivirus or pestivirus treatment amount of a described 1′, 2′ or 3′-modified nucleoside or a pharmaceutically acceptable salt or prodrug thereof, is provided.
Owner:INDENIX PHARM LLC
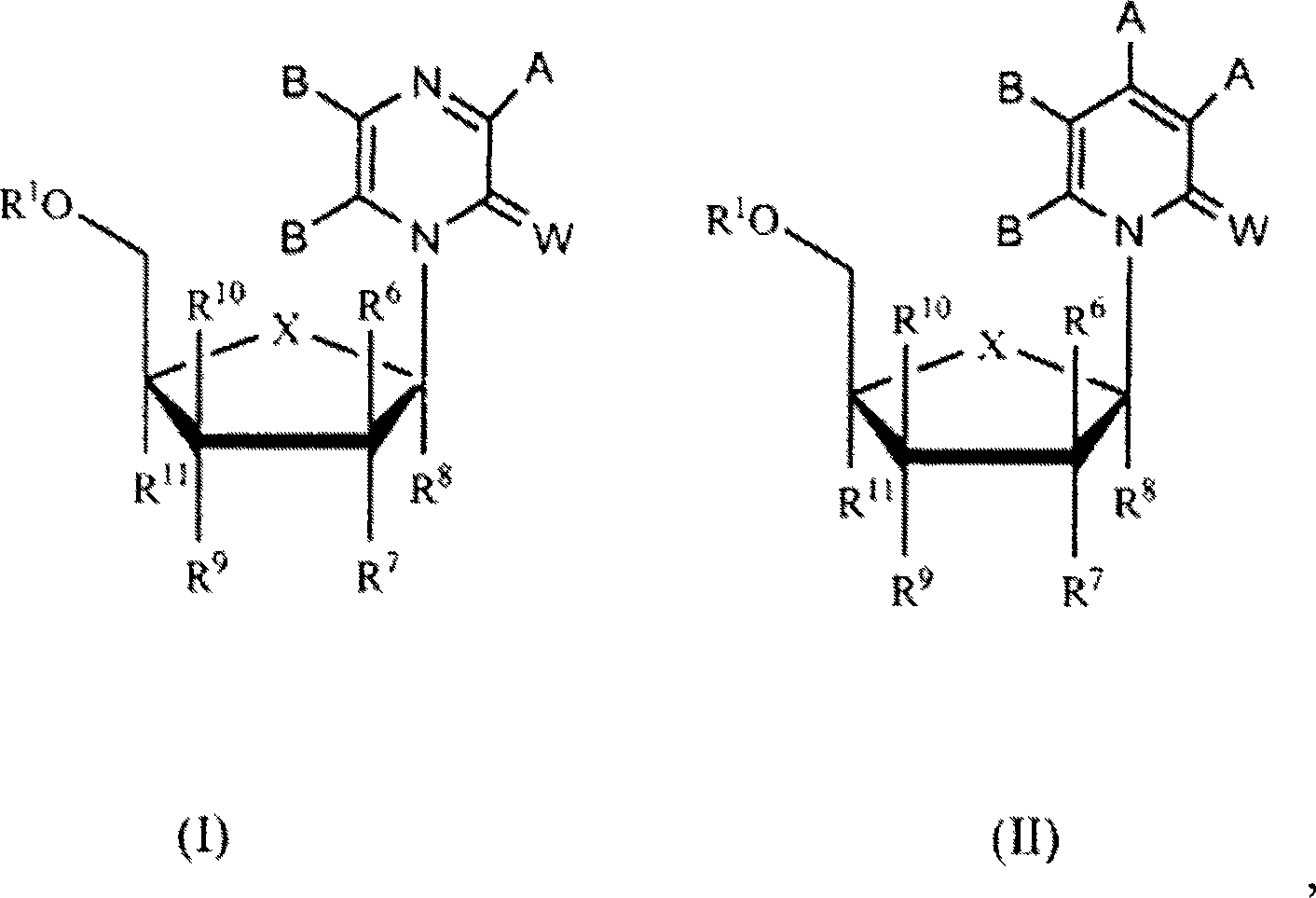
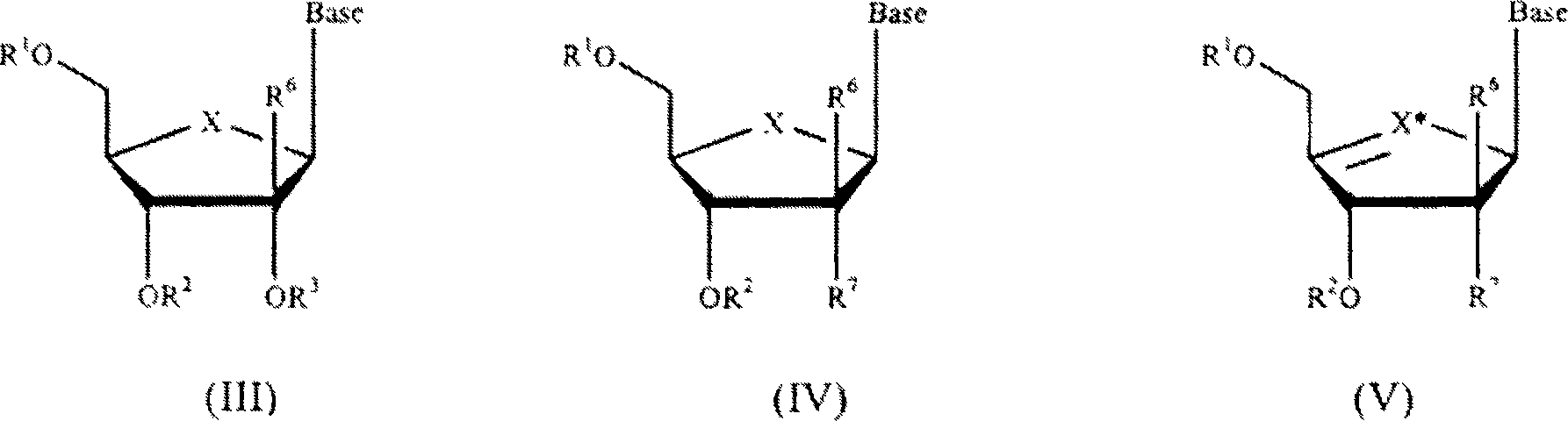
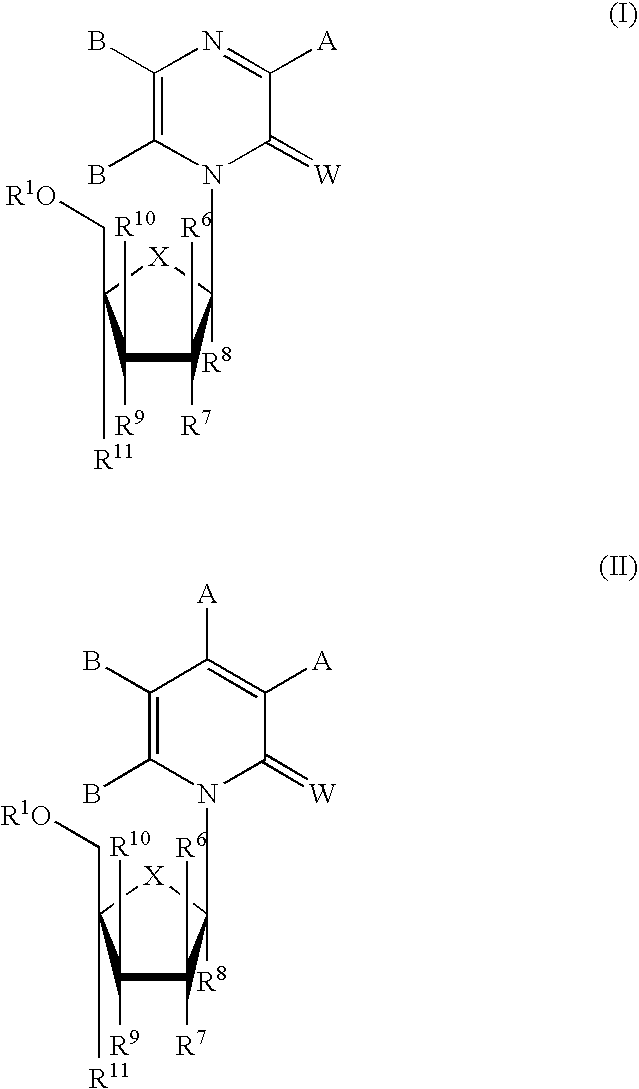
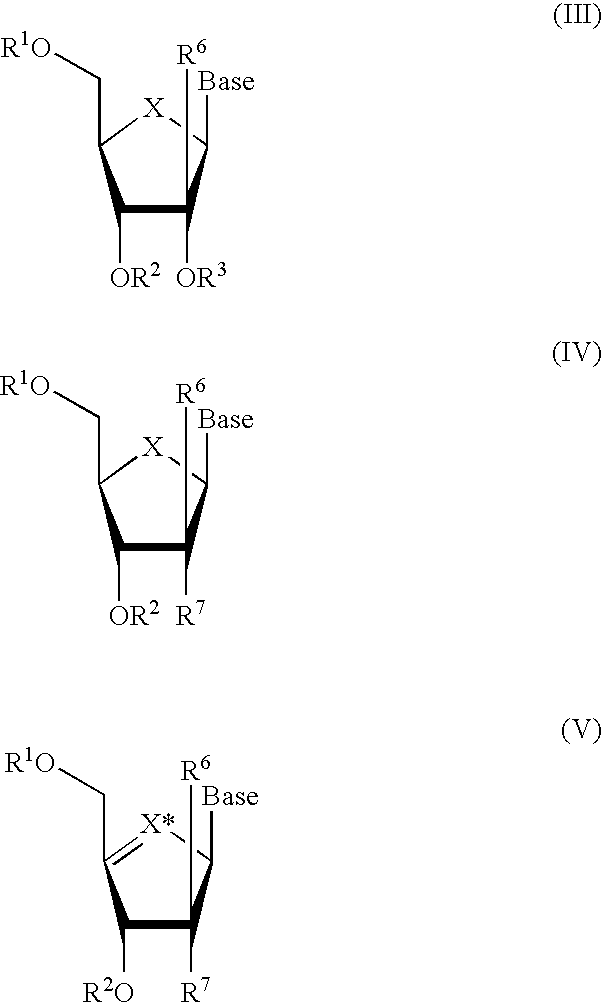
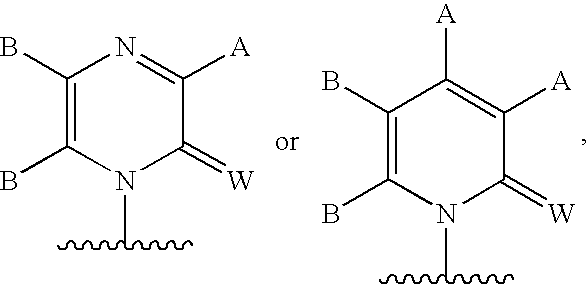
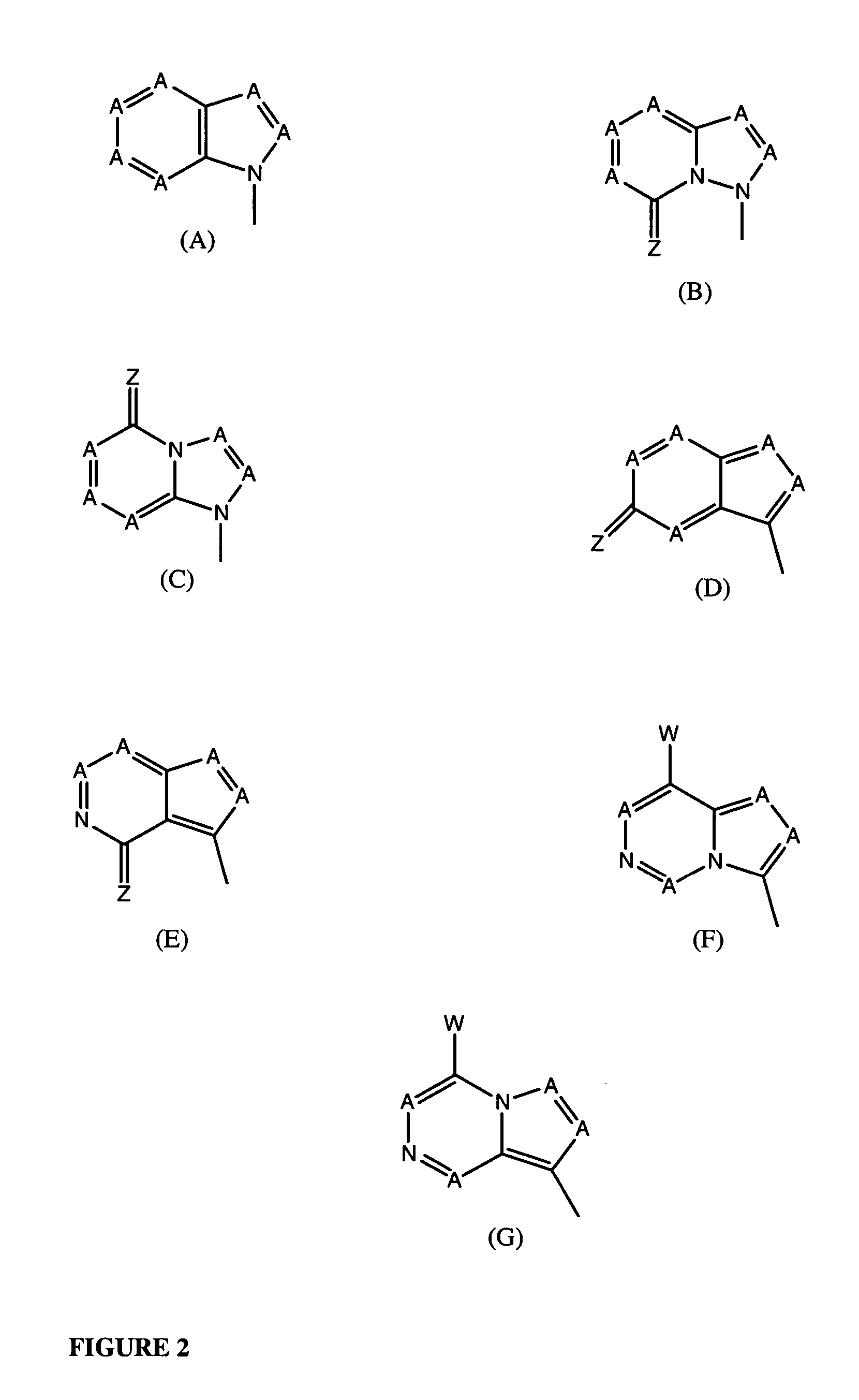
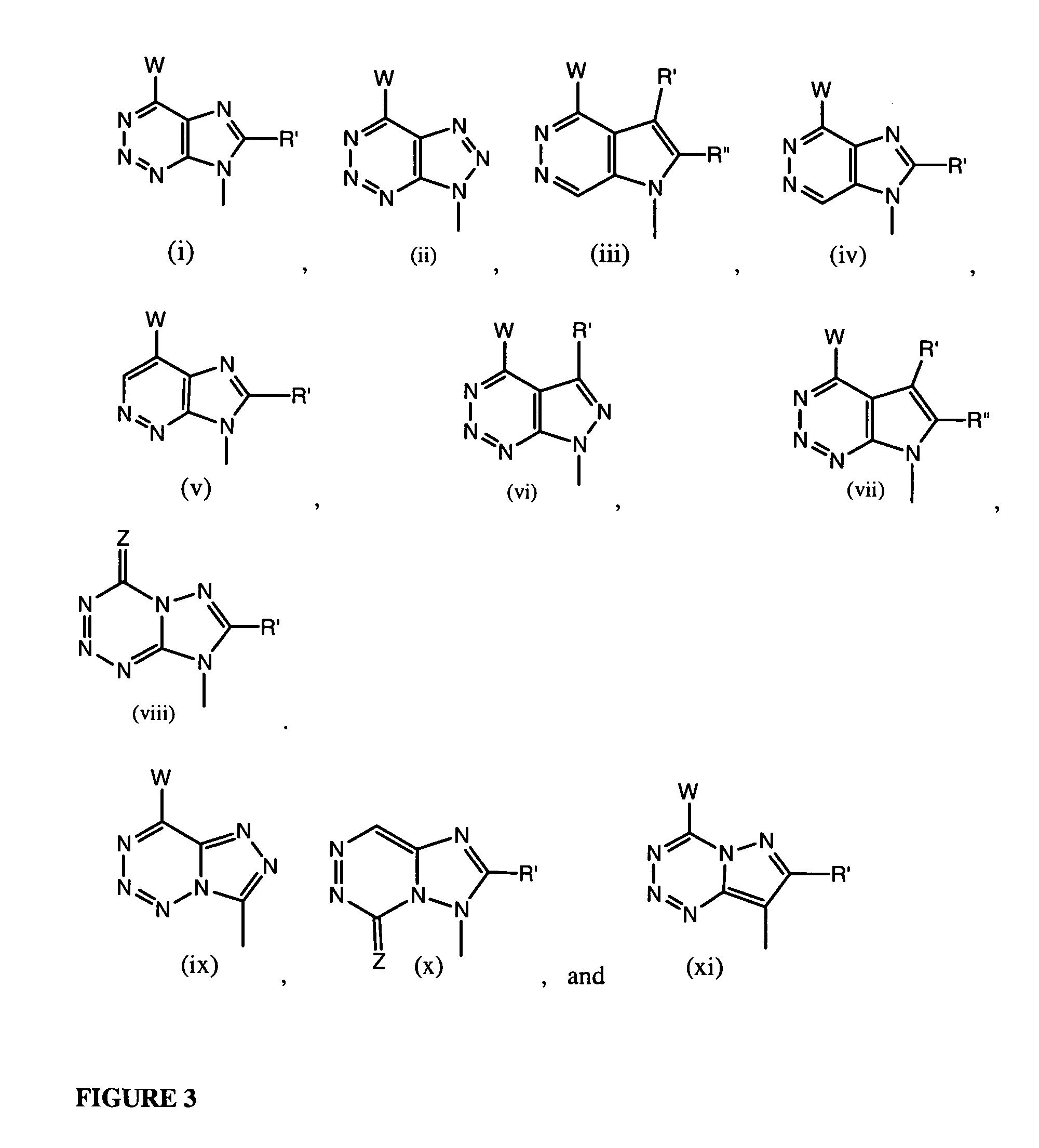
Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections
InactiveUS20040082574A1Potent and selective activityPotent activityBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
Purine nucleoside analogues for treating Flaviviridae including hepatitis C
This invention is directed to a method for treating a host, especially a human, infected with hepatitis C, flavivirus and / or pestivirus, comprising administering to that host an effective amount of an anti-HCV biologically active pentofuranonucleoside where the pentofuranonucleoside base is an optionally substituted 2-azapurine. The optionally substituted pentofuranonucleoside, or a salt or prodrug thereof, may be administered alone or in combination with one or more optionally substituted pentofuranonucleosides or other anti-viral agents.
Owner:THE CENT NAT DEL LA RECH SCIQUE +2
Antiviral agents for treatment of Flaviviridae infections
InactiveUS20040266723A1Alleviating and preventing and delaying onsetEffective conditioningBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a composition for and a method of treating Flaviviridae (Hepacivirus, Flavivirus, Pestivirus) infections, including BVDV and HCV, in a host, including animals, and especially humans, using a small molecule or its pharmaceutically acceptable salt or prodrug.
Owner:PHARMASSET
Purine nucleoside analogues for treating flaviviridae including hepatitis C
This invention is directed to a method for treating a host, especially a human, infected with hepatitis C, flavivirus and / or pestivirus, comprising administering to that host an effective amount of an anti-HCV biologically active pentofuranonucleoside where the pentofuranonucleoside base is an optionally substituted 2-azapurine. The optionally substituted pentofuranonucleoside, or a salt or prodrug thereof, may be administered alone or in combination with one or more optionally substituted pentofuranonucleosides or other anti-viral agents.
Owner:INDENIX PHARM LLC +2
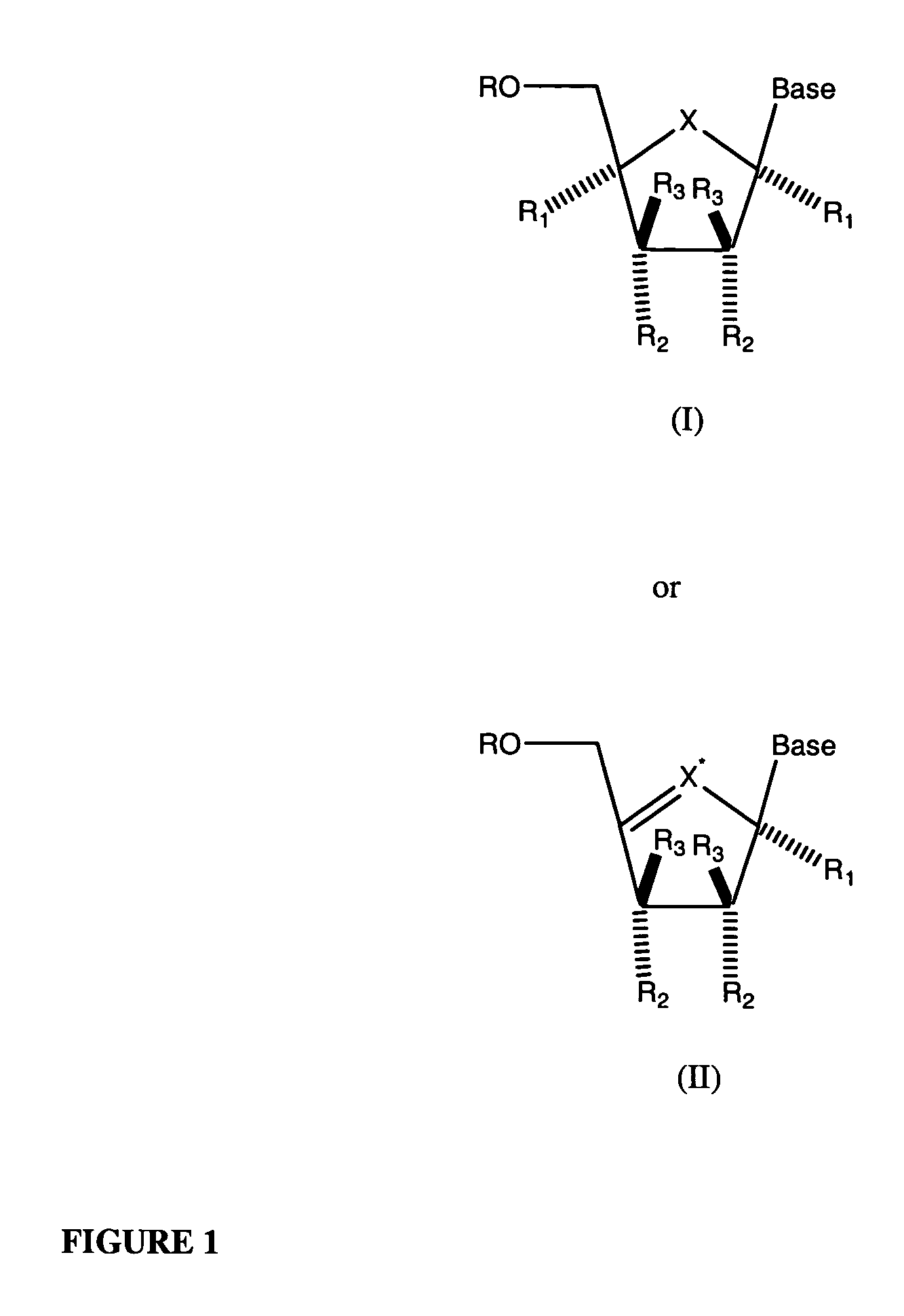
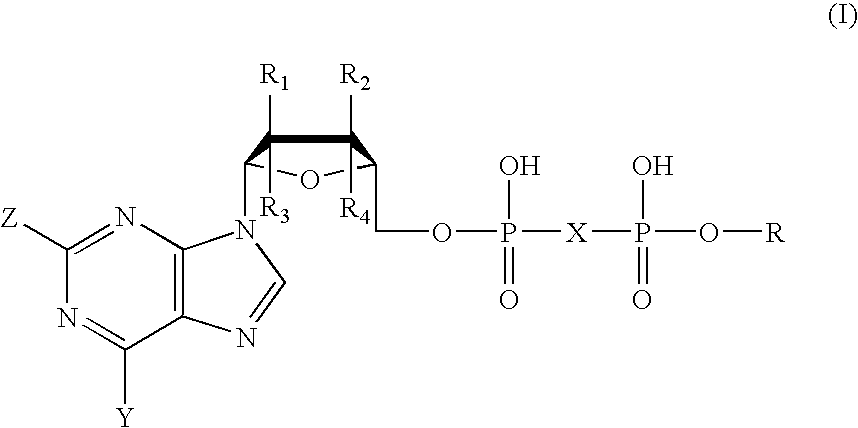
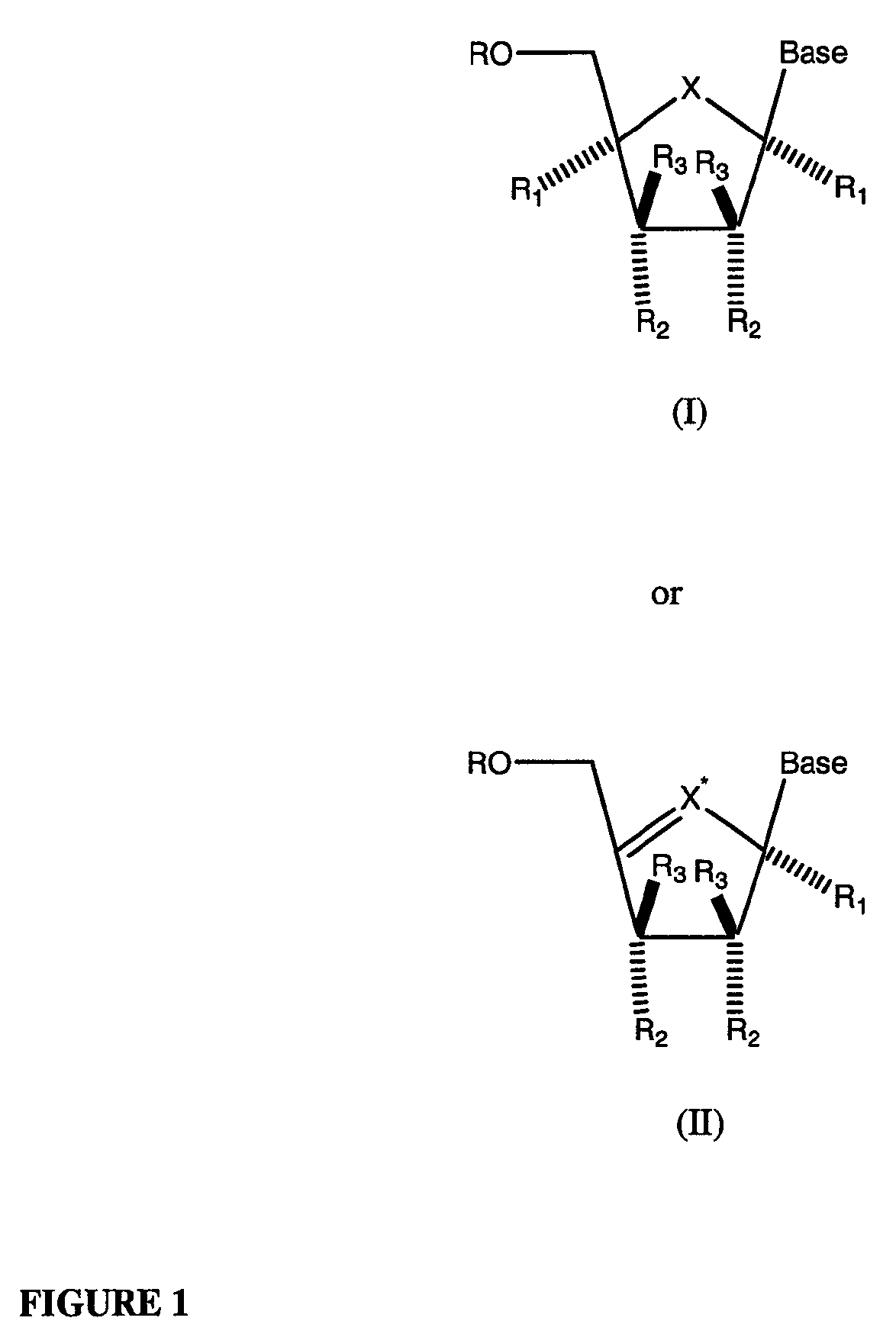
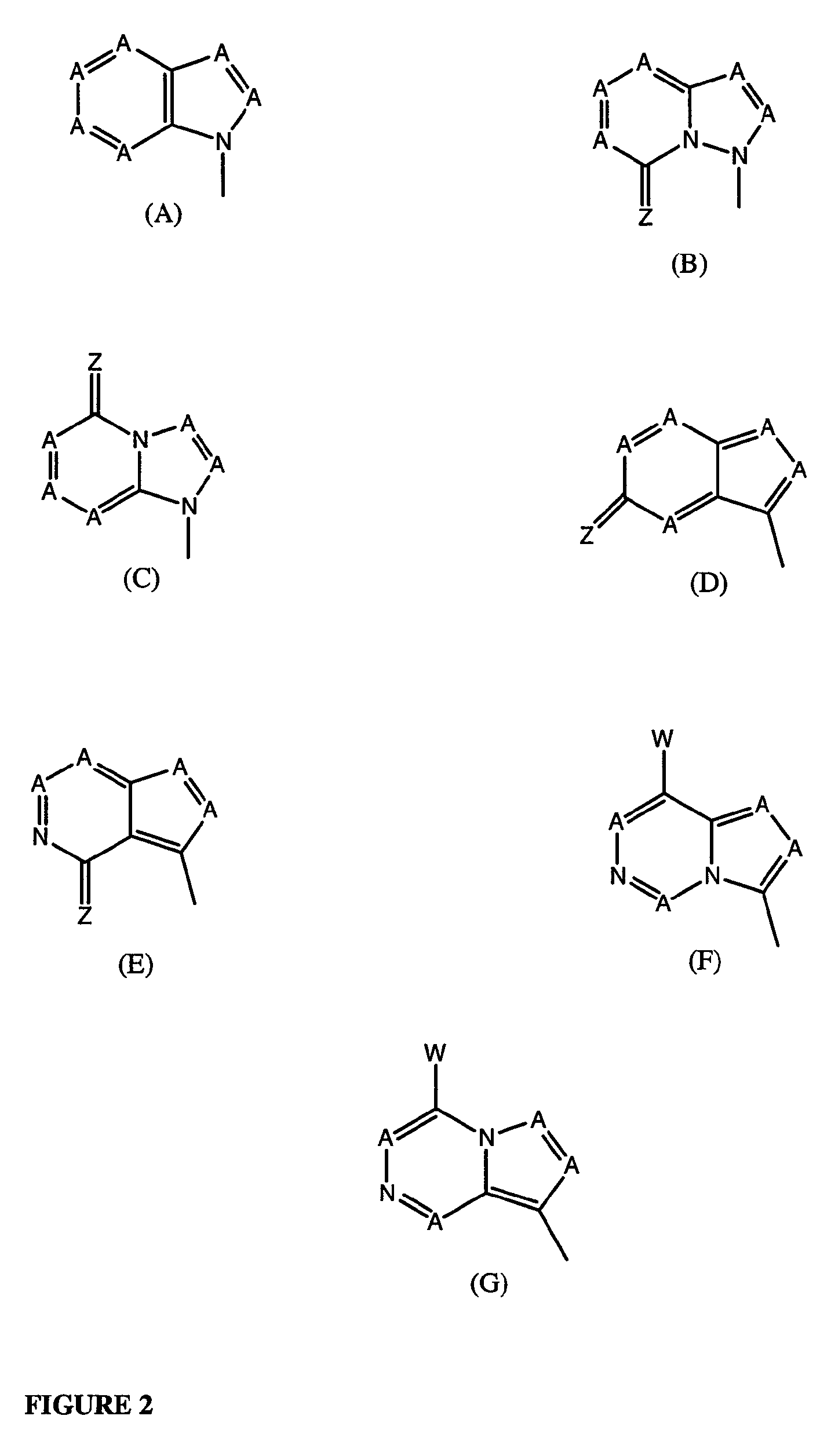
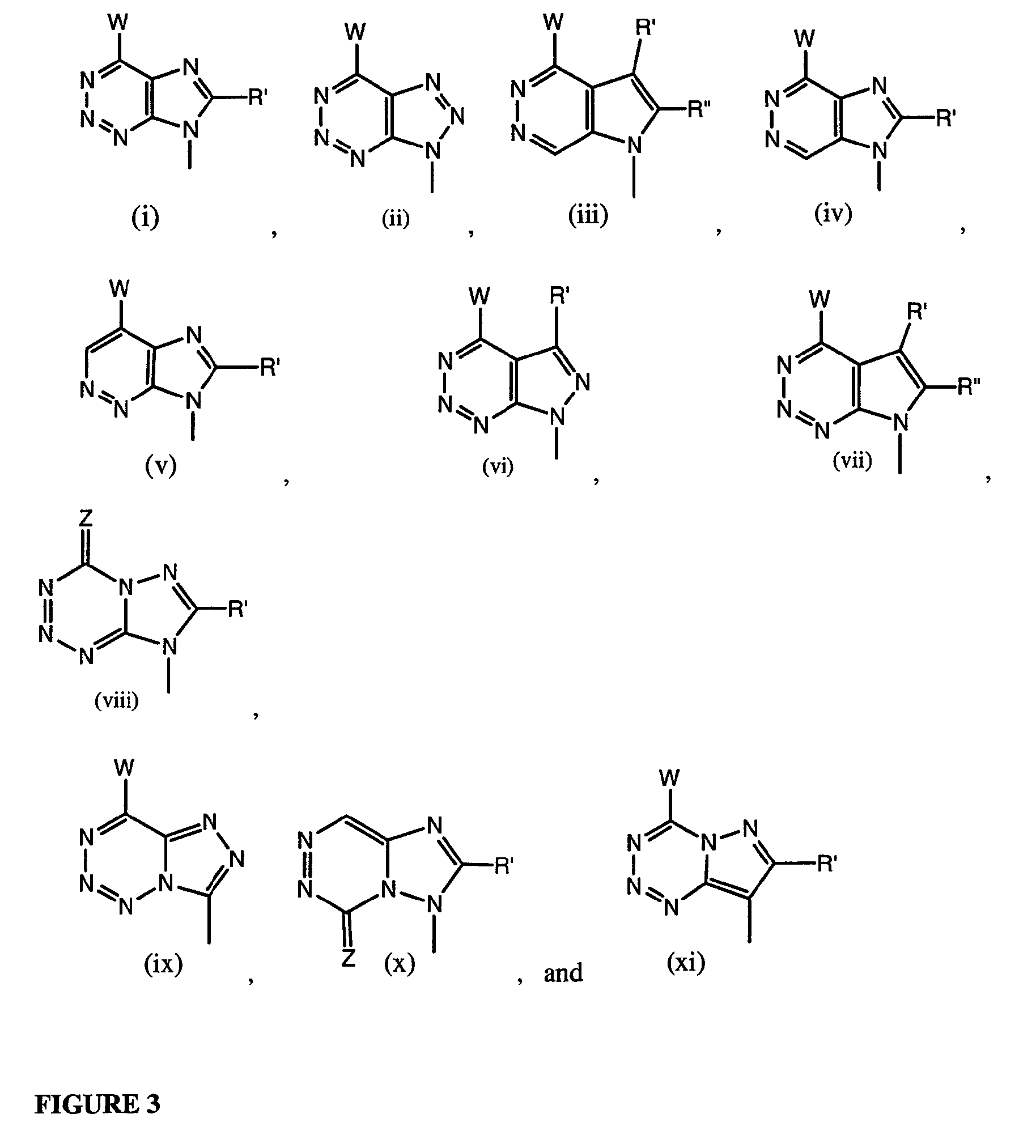
Compounds with the bicyclo[4.2.1]nonane system for the treatment of Flaviviridae infections
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
Nucleosides With Non-Natural Bases as Anti-Viral Agents
A method and composition for treating a host infected with flavivirus, pestivirus or hepacivirus comprising administering an effective flavivirus, pestivirus or hepacivirus treatment amount of a described base-modified nucleoside or a pharmaceutically acceptable salt or prodrug thereof, is provided.
Owner:INDENIX PHARM LLC +2
Methods and compositions for treating flaviviruses and pestiviruses
A method and composition for treating a host infected with flavivirus or pestivirus comprising administering an effective flavivirus or pestivirus treatment amount of a described 1′, 2′ or 3′-modified nucleoside or a pharmaceutically acceptable salt or prodrug thereof, is provided.
Owner:INDENIX PHARM LLC
Vaccine comprising an attenuated pestivirus
Attenuated pestiviruses, in particular attenuated BVDV, wherein at least one mutation is in the coding sequence for glycoprotein Erns and at least another mutation in the coding sequence for Npro which preferably leads to combined inactivation of the RNase activity residing in glycoprotein Erns in addition to the inactivation of the (hypothesized) immunomodulating activity residing in Npro. Methods for attenuating pestiviruses such as BVDV, nucleic acids encoding the pestiviruses, in particular BVDV, compositions and vaccines comprising the attenuated pestiviruses, in particular BVDV, of the invention.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
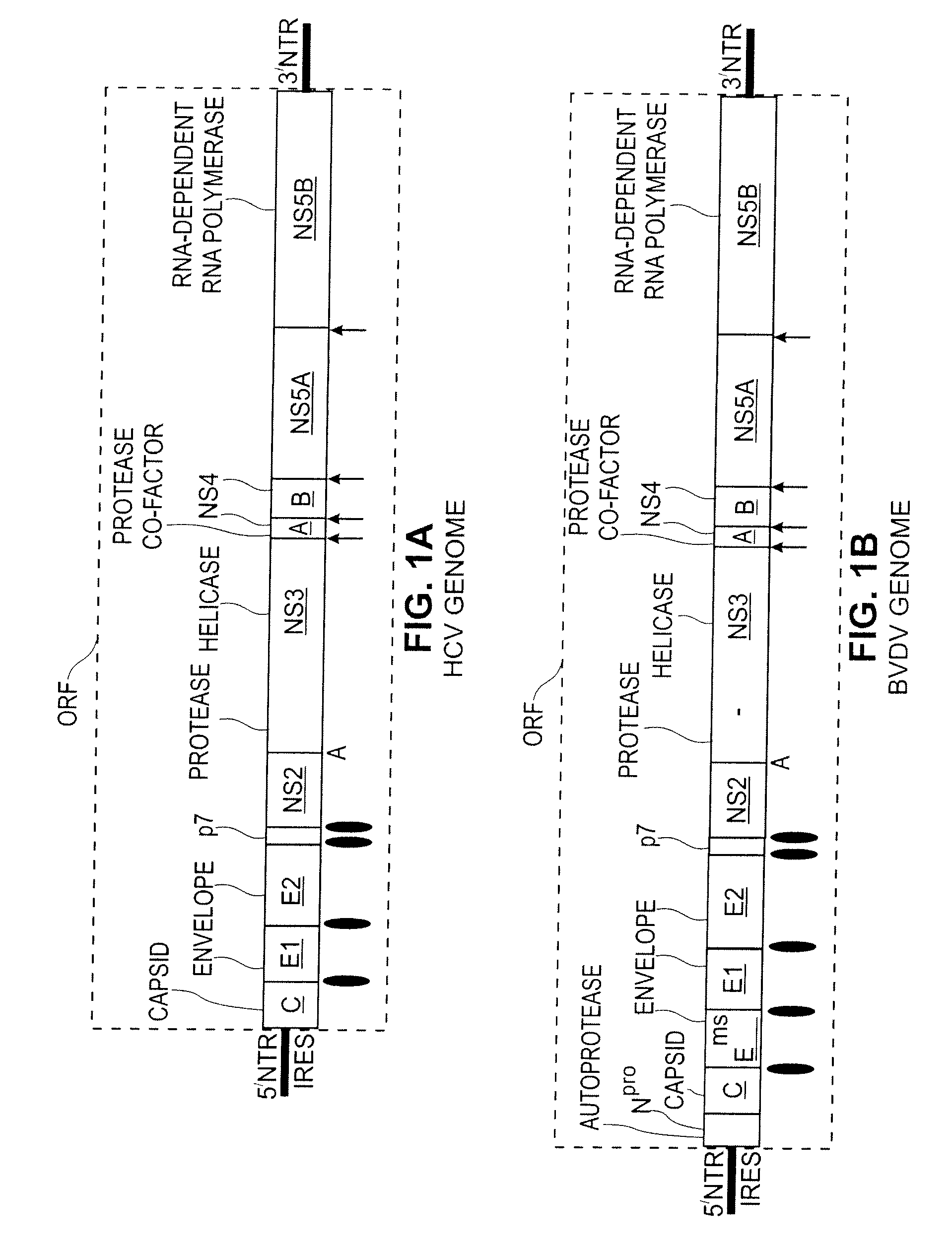
Replication Stable and RNase Resistant Chimeras of Pestivirus with Insertion in 3' Nontranslated Region (3'NTR)
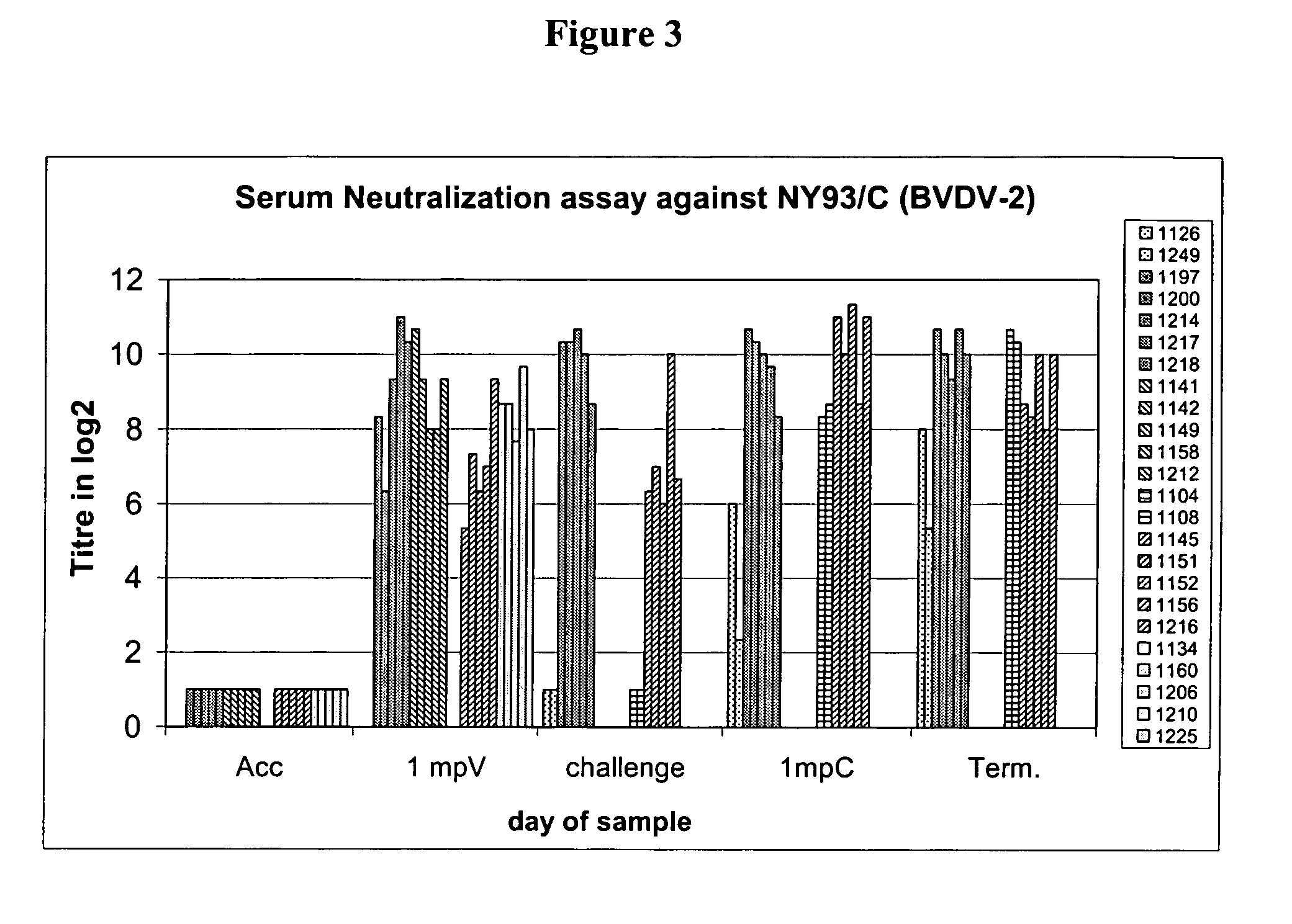
The invention relates to the field of nucleic acid amplification, particularly to quality control materials for use in viral RNA assays. It specifically relates to the construction of a recombinant Pestivirus by the identification of a region in the 3′NTR of the viral RNA genome where additional sequence elements can be stably inserted. Chimeric Pestivirus with sequence insertions in the 3′ nontranslated region (3′NTR) of the viral RNA genome were stable in replication and capable of forming infectious, RNase resistant virus particles. This chimeric Pestivirus with a 3′NTR insertion can be utilized as a quality control material in analytical assays for RNA targets, including external, internal controls, quantitative standards in PCR and NAT nucleic acid assays.
Owner:LIFE TECH CORP
E2 subunit vaccine comprising recombinant pestivirus E2 protein
InactiveUS6919085B2Increased and improved yieldProduce improveSsRNA viruses positive-senseViral antigen ingredientsCell culture mediaProtein C
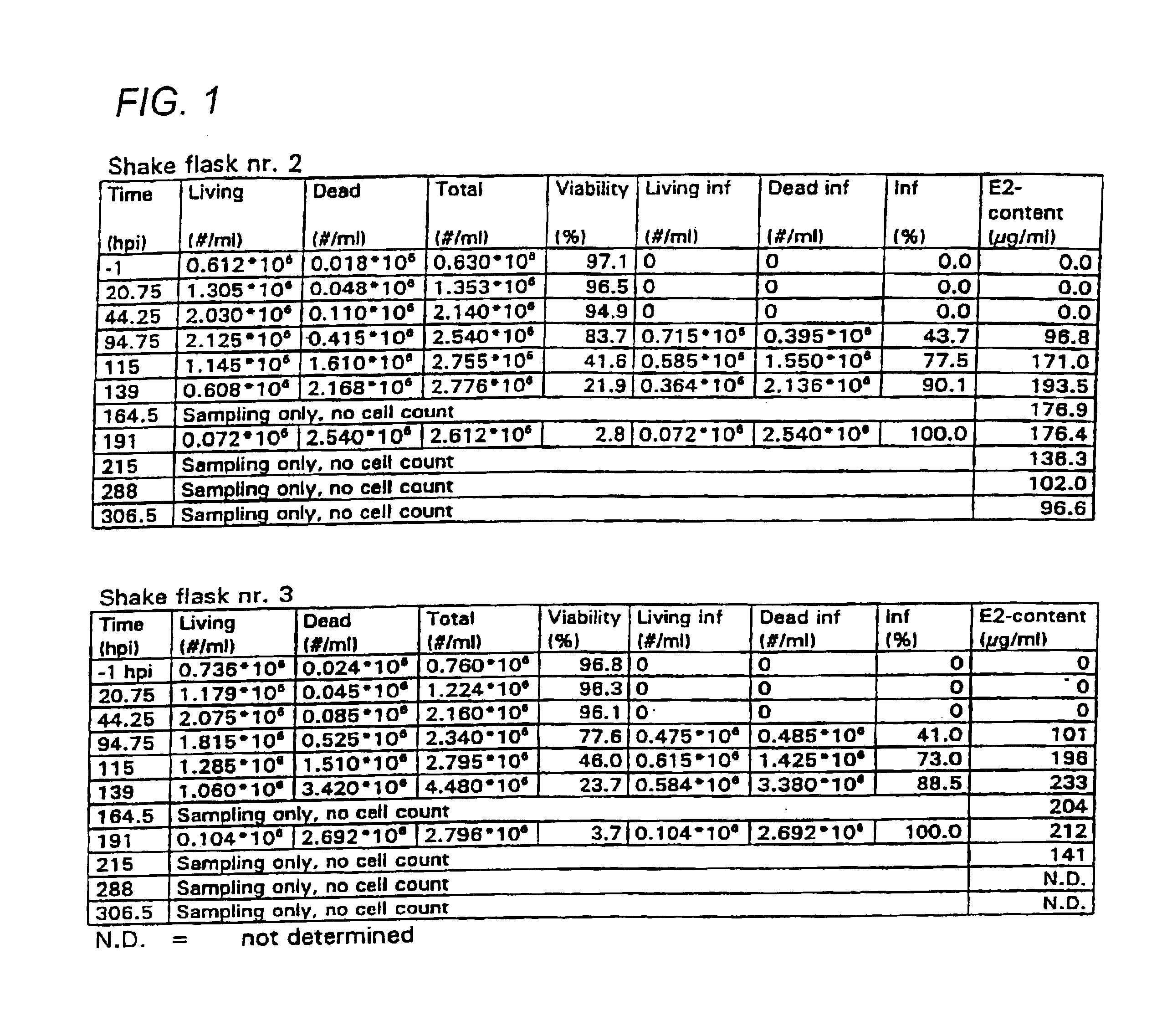
The invention relates to a method of increasing protein expression in baculo vector virus expression systems. The invention provides a method to produce a recombinant protein in insect cell culture which comprises selecting a recombinant baculovirus expressing said protein, growing insect cells in growth medium in a culture vessel and infecting the cells with an inoculum of at least one baculovirus at a cell density of 1×105 to 5×106 cells / ml with an m.o.i of <0.01. The invention also provides a method to produce recombinant pestivirus E2 or Em9 protein or fragments thereof in insect cell culture characterized by a final concentration of the protein fragments in the growth medium at harvest of at least 100 μg / ml. The invention also provides a method of producing recombinant FSH, α-units and / or β-units, and complexes and fragments thereof, at a concentration in the growth medium at harvest of at least 15 μ / ml.
Owner:STICHTING INST VOOR DIERHOUDERIJ & DIERGEZONDHEID +1
Vaccine comprising an attenuated pestivirus
Attenuated pestiviruses, in particular attenuated BVDV, wherein at least one mutation is in the coding sequence for glycoprotein Erns and at least another mutation in the coding sequence for Npro which preferably leads to combined inactivation of the RNase activity residing in glycoprotein Erns in addition to the inactivation of the (hypothesized) immunomodulating activity residing in Npro. Methods for attenuating pestiviruses such as BVDV, nucleic acids encoding the pestiviruses, in particular BVDV, compositions and vaccines comprising the attenuated pestiviruses, in particular BVDV, of the invention.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
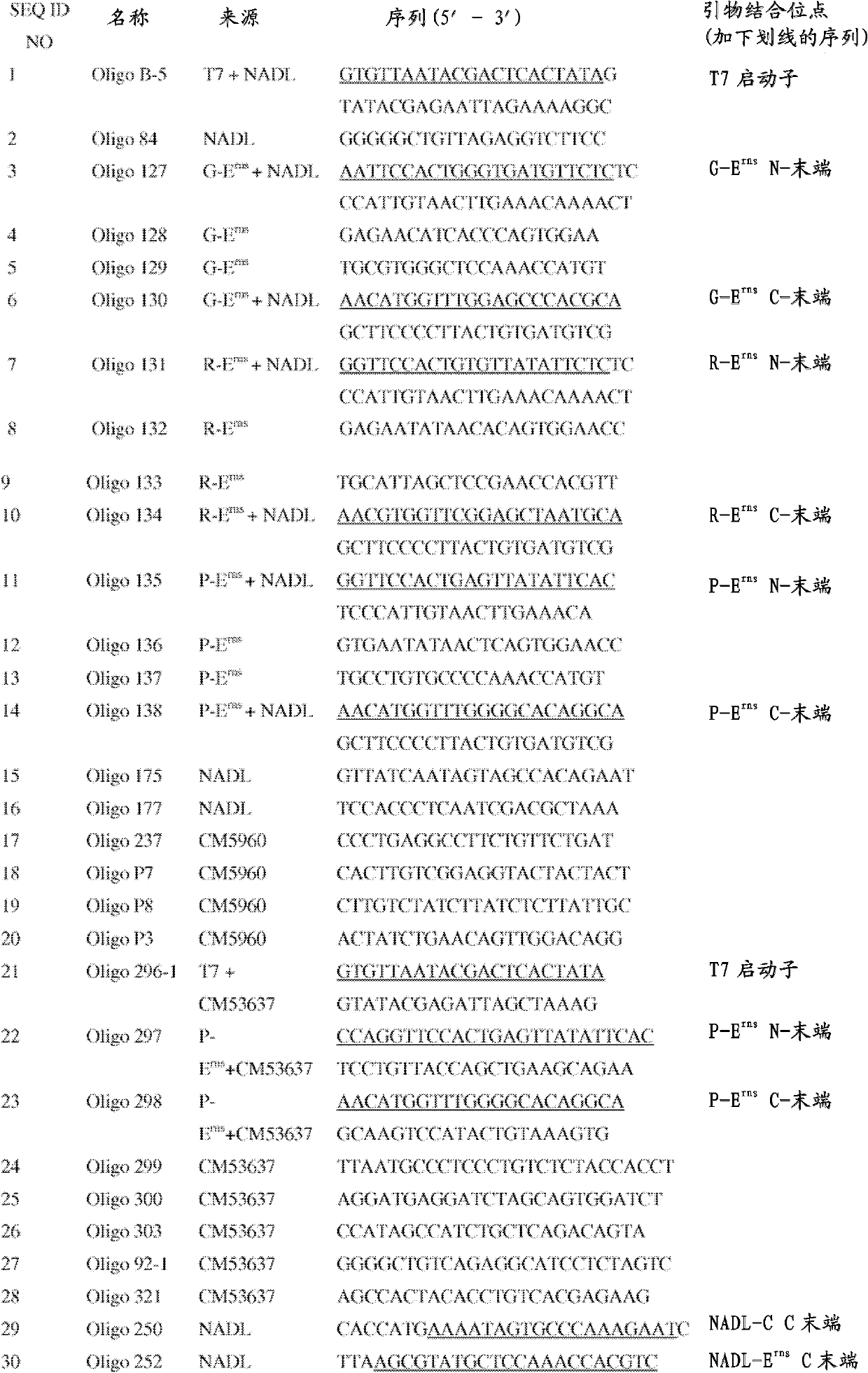
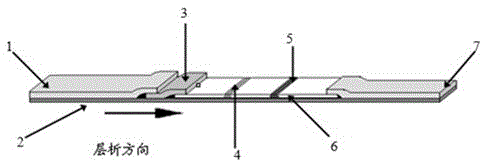
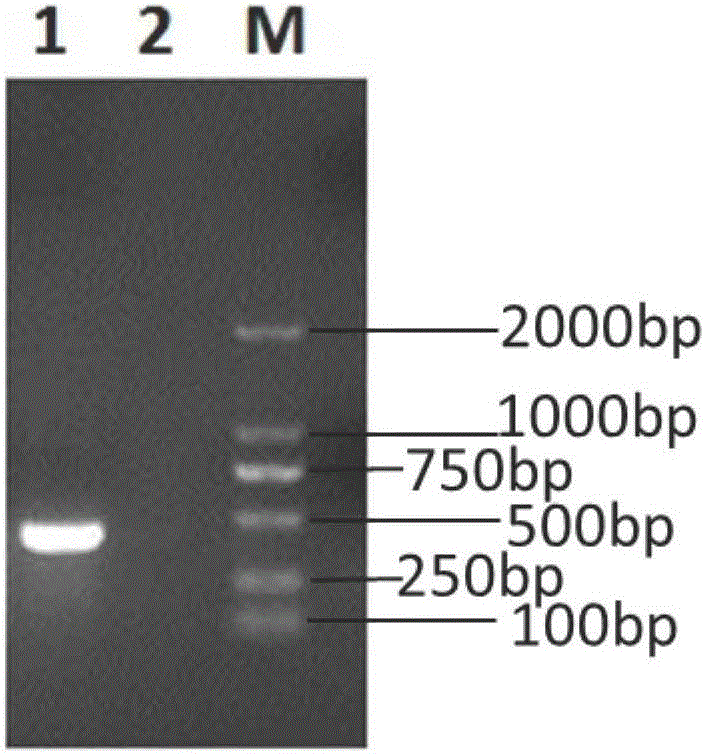
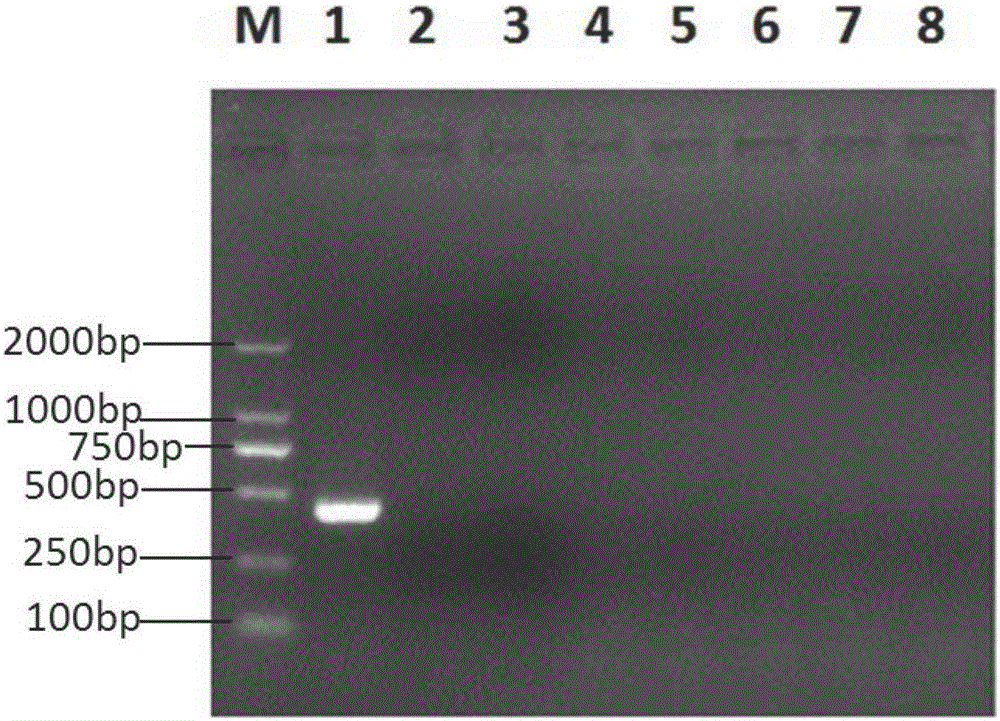
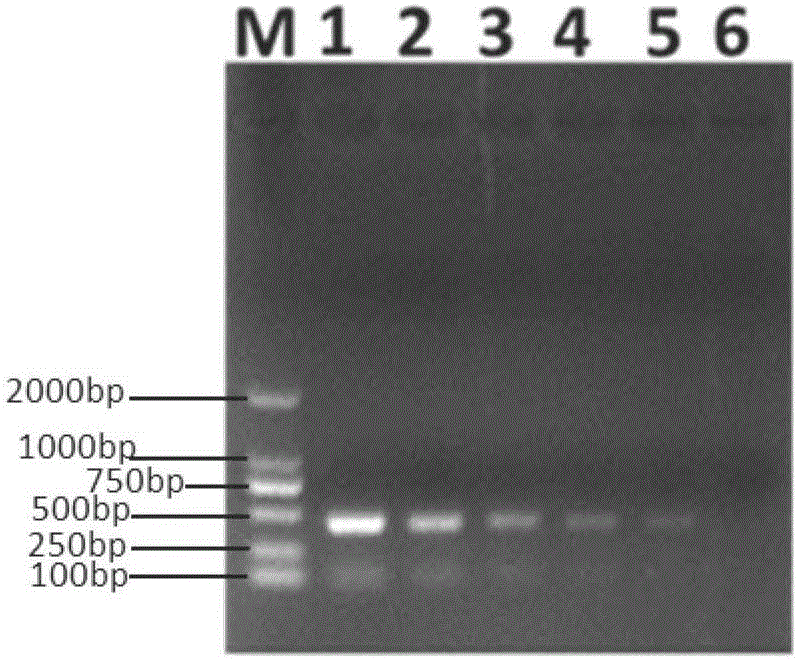
Pig atypical pestivirus RT-PCR detection specific primer, kit and detection method
ActiveCN106801109AImprove featuresHigh sensitivityMicrobiological testing/measurementMicroorganism based processesPestivirusNucleotide sequencing
The invention belongs to the technical fields of animal virology and molecular biology, and particularly relates to a pig atypical pestivirus RT-PCR detection specific primer, a kit and a detection method. The nucleotide sequences of the specific primer are as shown in SEQ ID NO:1 and SEQ ID NO:2; the kit comprises the primer, a Prime Script 1 step enzyme mixture, a 2*1 step buffer solution and nuclease-free water. The invention also discloses a method for detecting pig atypical pestivirus by using the specific primer or the kit. The primer provided by the invention has high specificity and sensitivity and can be used for detecting and identifying the pig atypical pestivirus from various clinical samples, so that prevention and control measures can be taken quickly and timely, and economic loss is reduced.
Owner:WENS FOOD GRP CO LTD
Flavivirus fusion inhibitors
InactiveUS20050271677A1Reduce HIV- loadImprove developmentSsRNA viruses positive-sensePeptide/protein ingredientsPestivirusVirosome
The present invention relates to peptides and methods of inhibiting fusion between the virion envelope of Flaviviruses and membranes of the target cell, the process that delivers the viral genome into the cell cytoplasm. The invention provides for methods which employ peptides or peptide derivatives to inhibit Flavivirus:cell fusion. The present invention is based in part on the discovery that E1 envelope glycoprotein of hepaciviruses and E2 envelope glycoprotein of pestivirus have previously undescribed structures, truncated class II fusion proteins. The present invention provides peptides and methods of treatment and prophylaxis of diseases induced by Flaviviruses.
Owner:TULANE EDUCATIONAL FUND +1
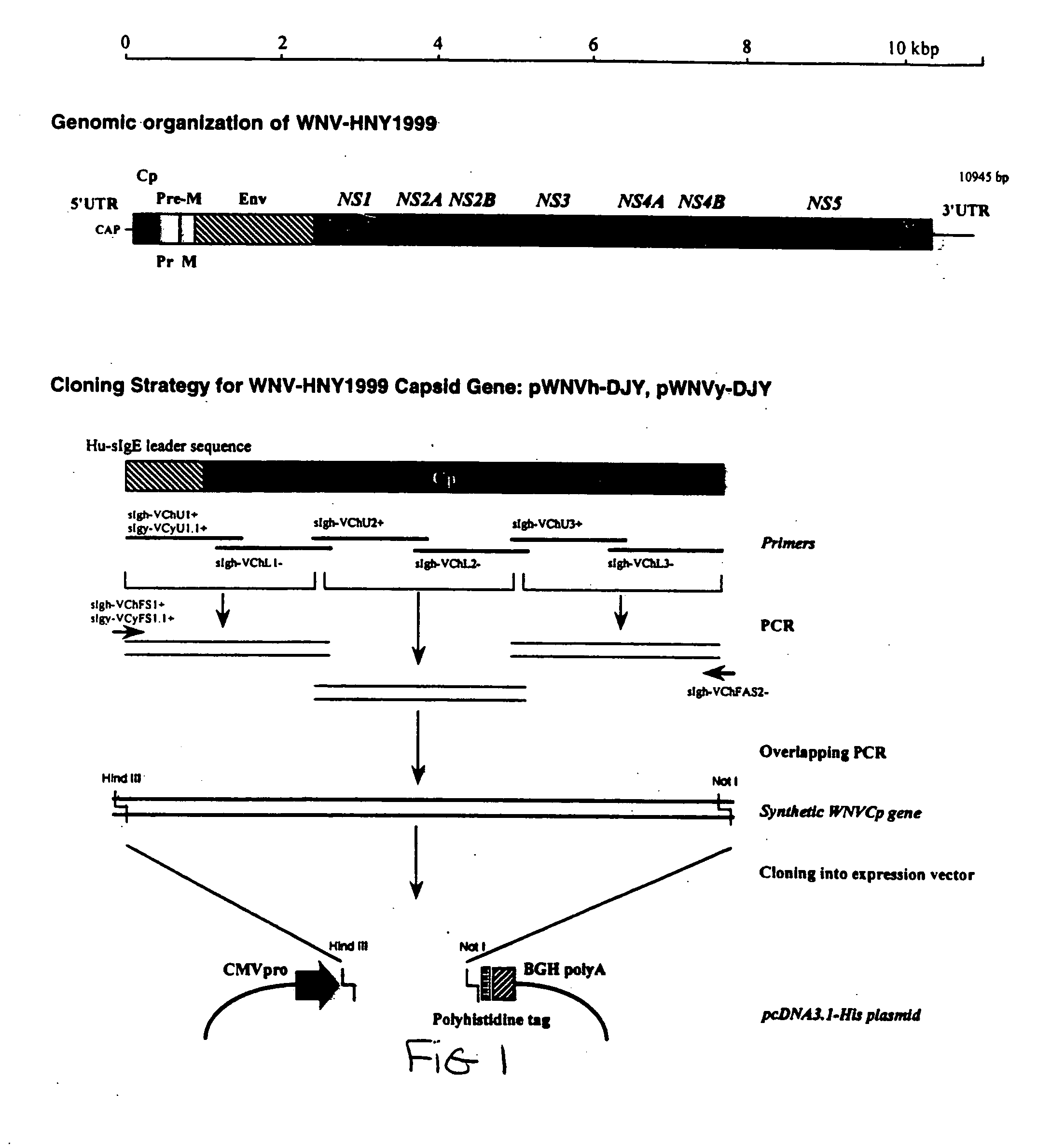
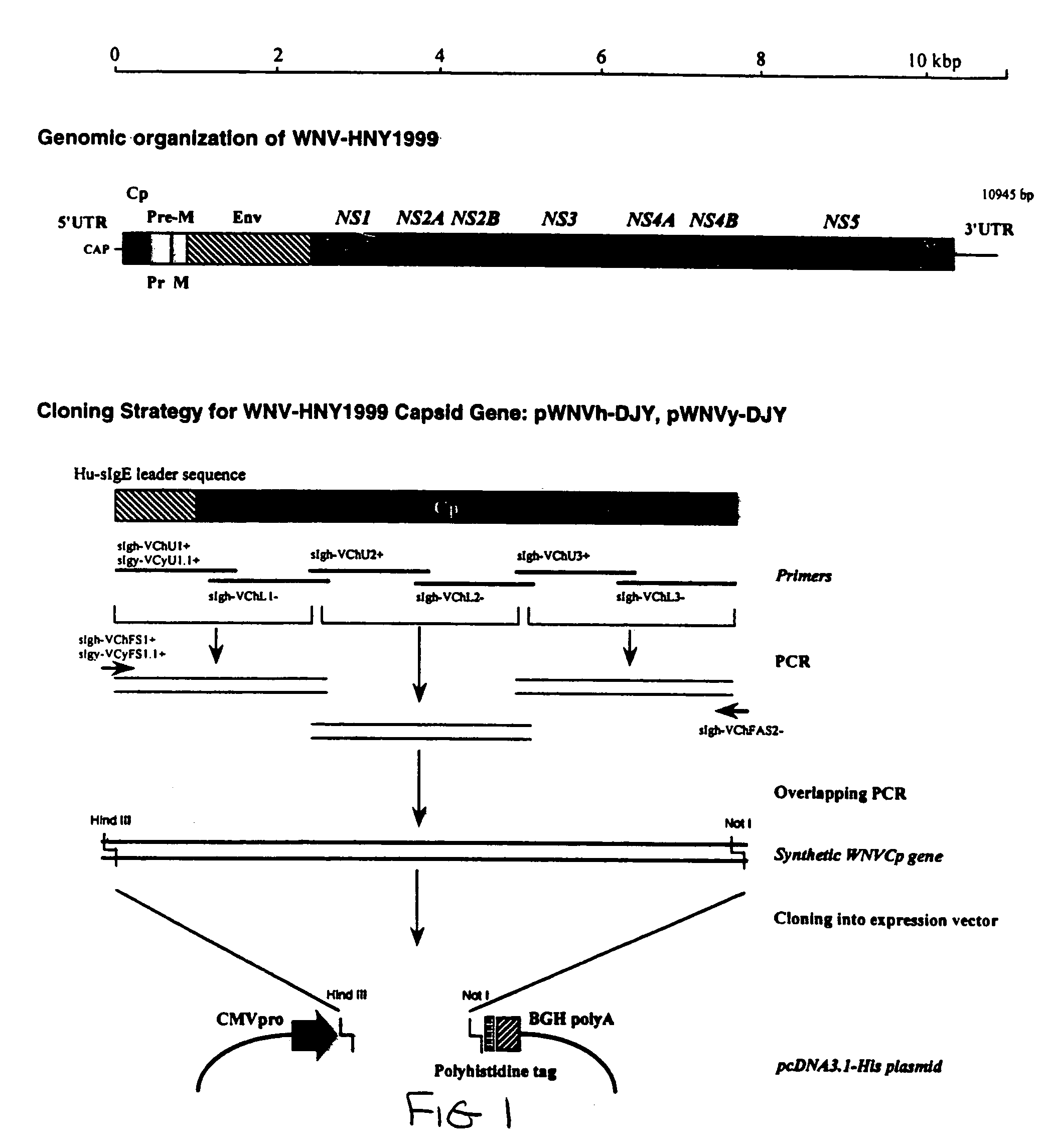
Method of inducing cell death using West Nile virus capsid protein
This invention provides methods of inducing cell death with Flavivirus or Pestivirus capsid protein, such as West Nile virus (WNV) capsid protein, and functional fragments thereof. The invention also provides methods of treating patients suffering from diseases characterized by hyperproliferating cells by administering pharmaceutical compositions comprising WNV or other virus including Flavivirus or Pestivirus capsid or other protein or a nucleic acid molecule encoding the same. Methods of identifying compounds which have anti-viral and / or anti-WNV and / or anti-Flavivirus and / or anti-Pestivirus capsid or other protein activity are disclosed. The invention also provides vaccine compositions comprising capsid or other proteins, or fragments thereof, or nucleic acids encoding same, from WNV or other virus including Flavivirus or Pestivirus and a pharmaceutically acceptable carrier. The invention also provides diagnostic methods and kits for identifying individuals exposed to WNV or other viruses including Flavivirus or Pestivirus.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
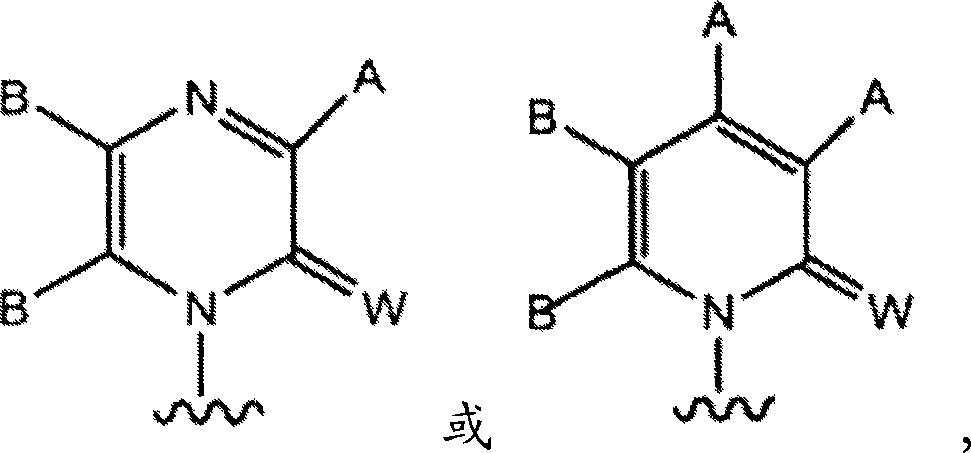
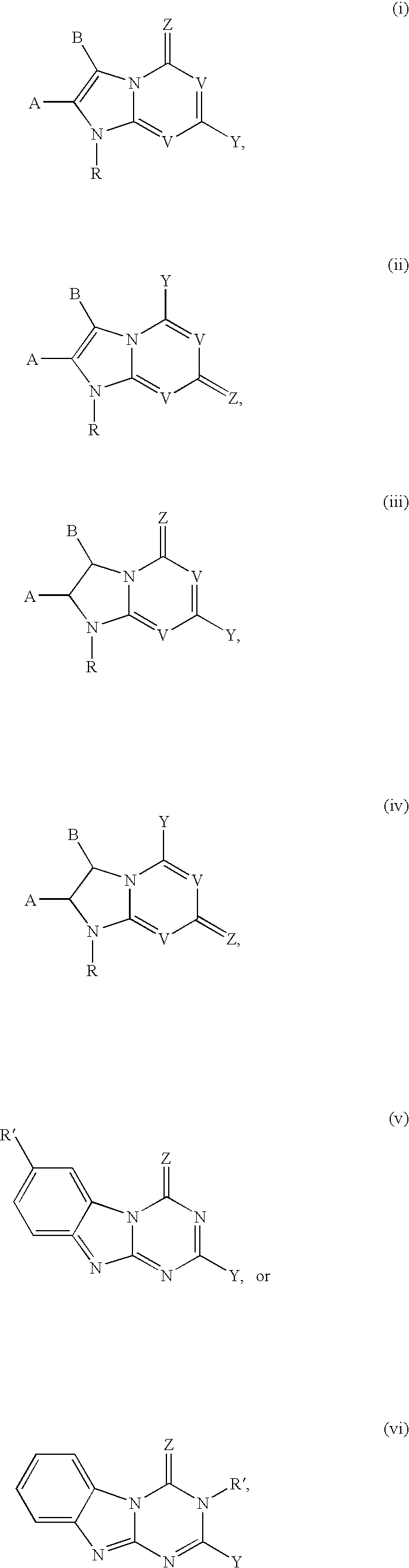
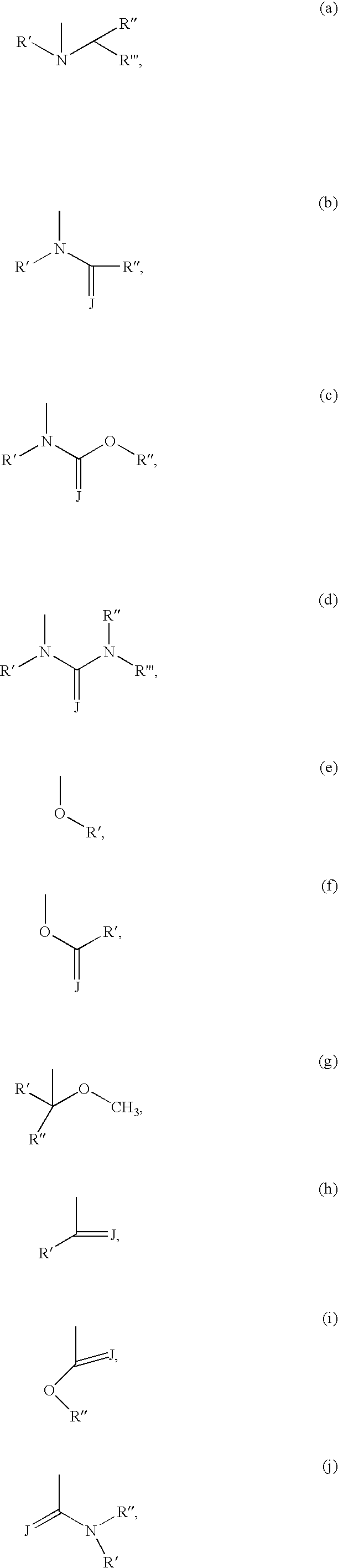
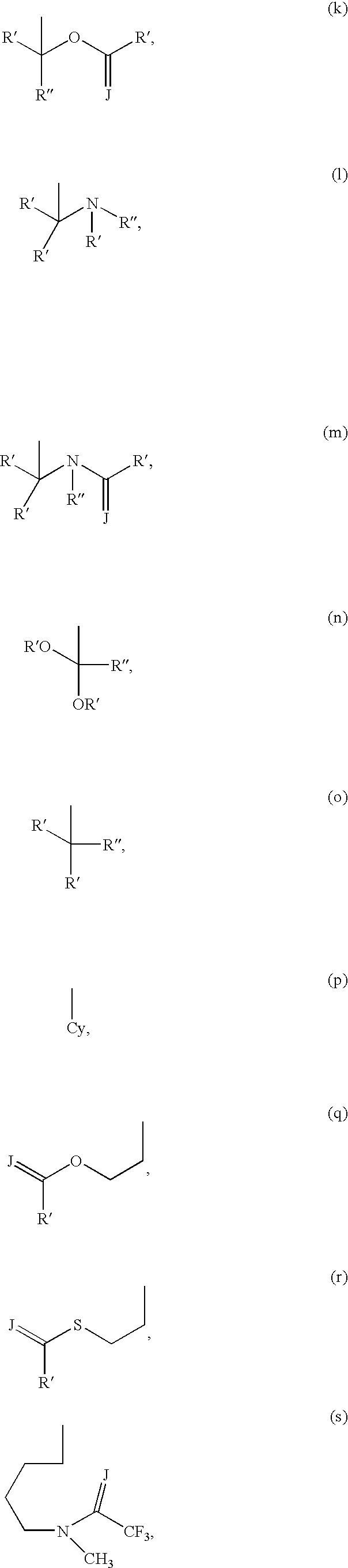
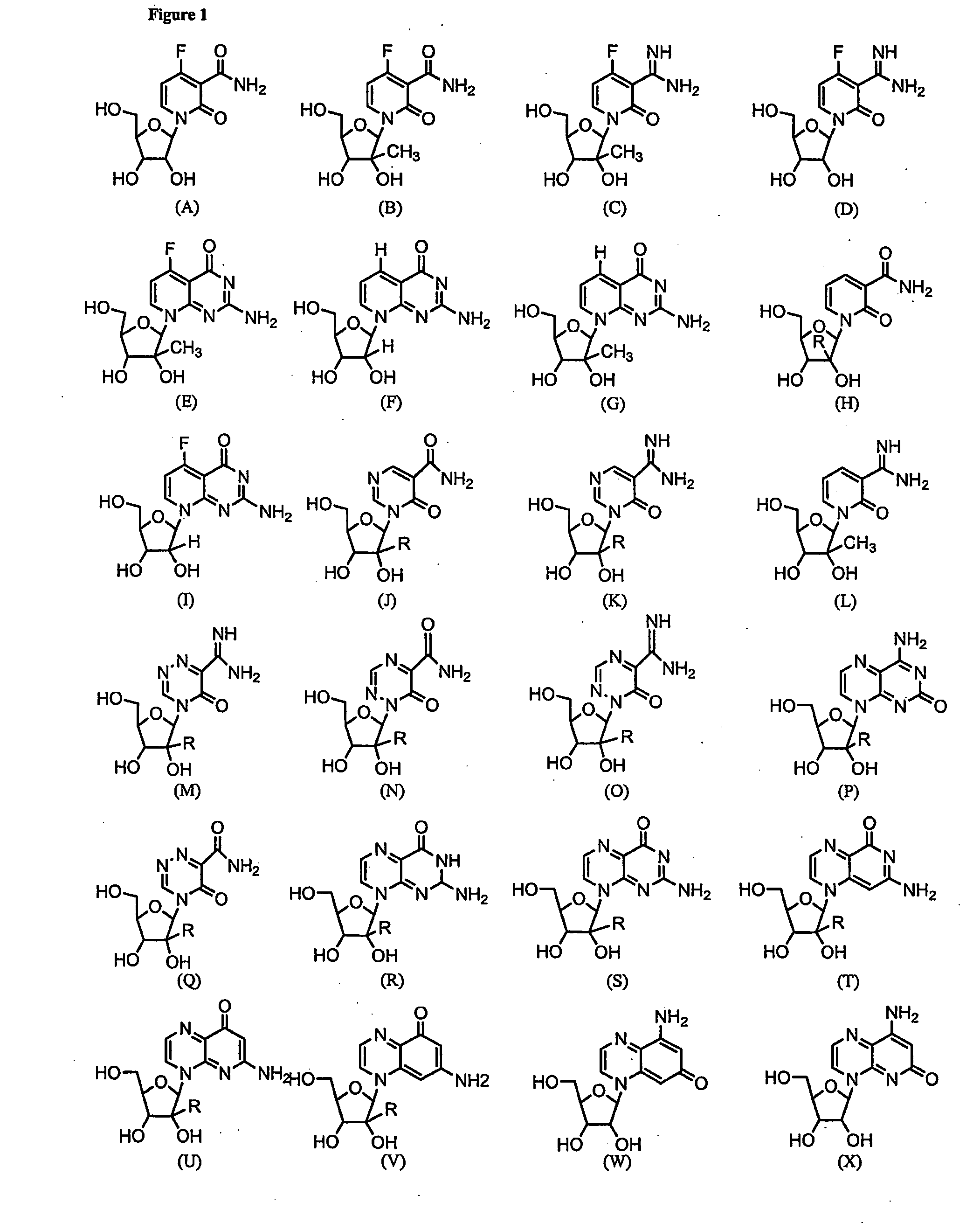
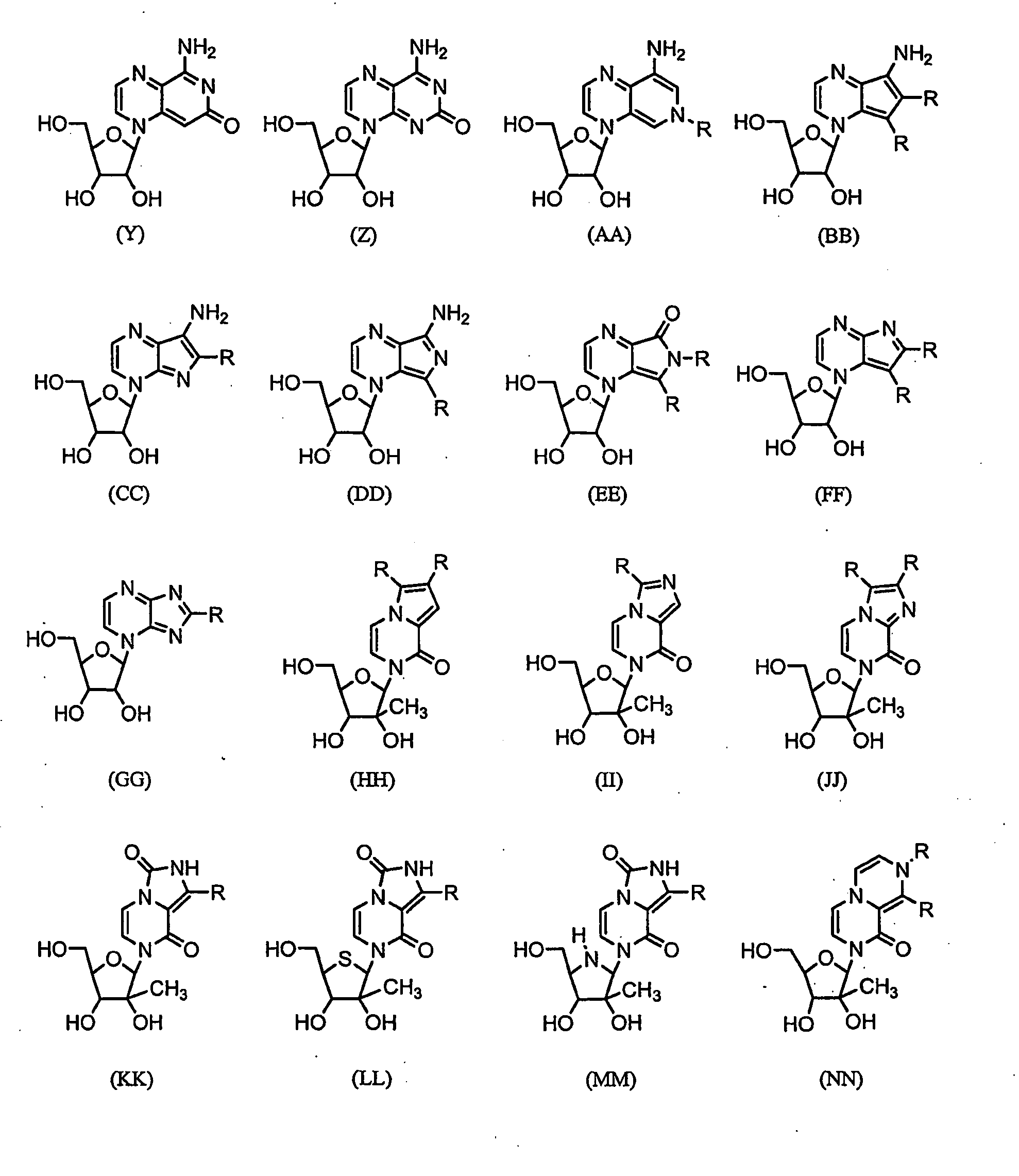
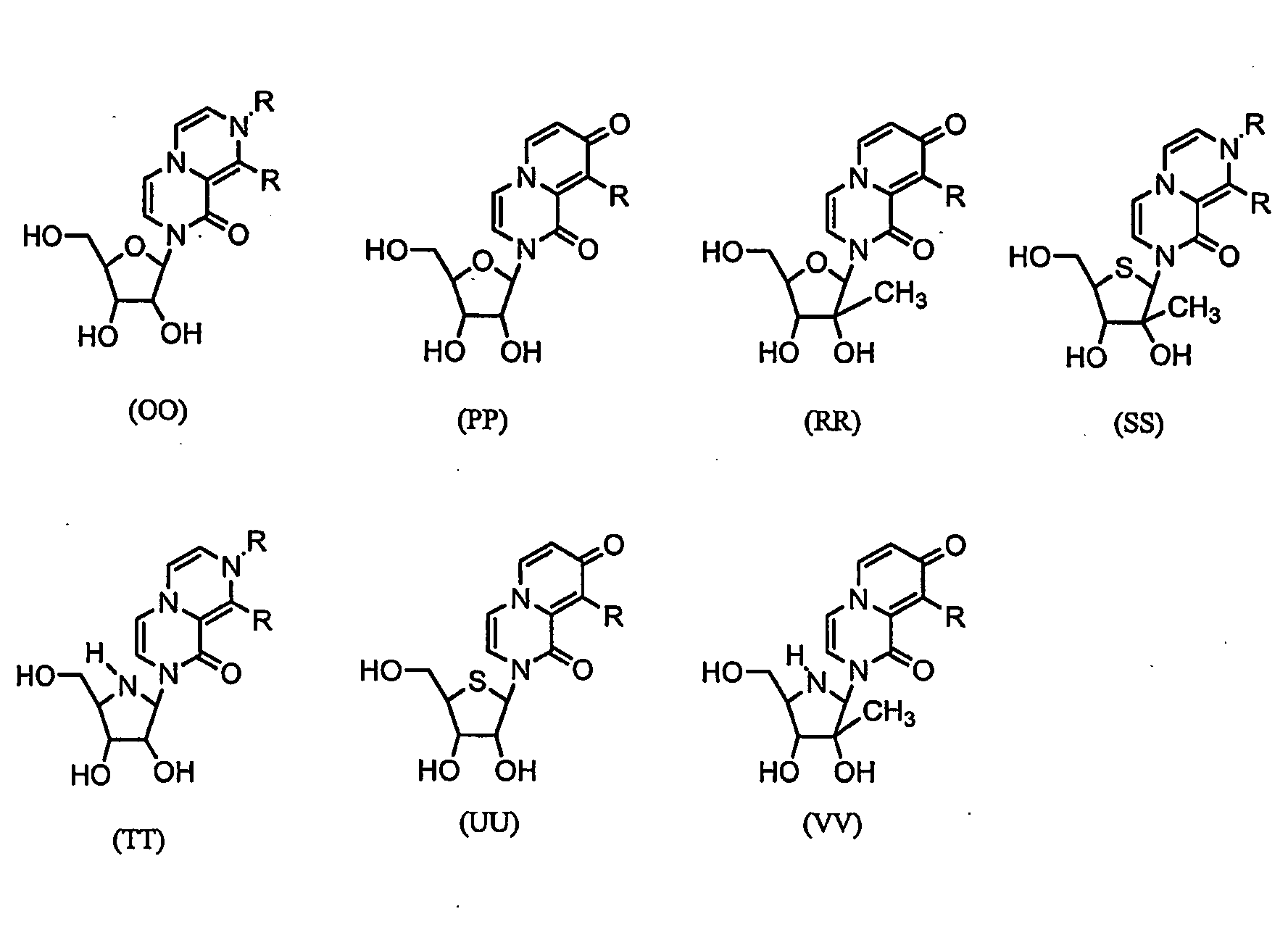
Imidazo [1, 2-a] pyrrolo [3, 2-c] pyridine compounds useful as pestivirus inhibitors
Owner:UNIV CLERMONT AUVERGNE +2
Bovine viral diarrhea virus with a modified erns protein
The present invention relates to chimeric pestiviruses having utility as immunogenic compositions and vaccines wherein said chimeric pestivirus comprises a bovine viral diarrhea virus which does not express its homologous Ems protein, further wherein said chimeric pestivirus expresses a heterologous Ems protein derived from another pestivirus, or a natural, synthetic or genetic variant of said heterologous Erns protein. Also described herein are methods and kits for treating or preventing the spread of bovine viral diarrhea virus infection, as well as methods and kits for differentiating between vaccinated and wild-type infected animals.
Owner:ZOETIS SERVICE LLC
Pestivirus mutants and vaccines containing the same
The present invention is directed to attenuated pestivirus mutants, which have a reduced ability to replicate as exhibited by a small plaque size. The mutations are in the 5′ nontranslated region of the viral genome. These mutant viruses are useful as live vaccines in the control of bovine viral diarrhea, border disease and classical swine fever.
Owner:INTERVET INT BV
Production of recombinant proteins by autoproteolytic cleavage of a fusion protein
The invention relates to a process for the recombinant production of a heterologous polypeptide of interest, comprising, (i) cultivation of a bacterial host cell which is transformed with an expression vector which comprises a nucleic acid molecule which codes for a fusion polypeptide, the fusion polypeptide comprising a derivative of an autoprotease N of Pestivirus, wherein at least one cysteine residue of the naturally occuring autoprotease N of Pestivirus is replaced by another amino acid residue, and a second polypeptide which is connected to the first polypeptide at the C-terminus of the first polypeptide in a manner such, that the second polypeptide is capable of being cleaved from the fusion polypeptide by the autoproteolytic activity of the first polypeptide, said second polypeptide being a heterologous polypeptide, wherein cultivation occurs under conditions which cause expression of the fusion polypeptide and formation of corresponding cytoplasmic inclusion bodies, (ii) isolation of the inclusion bodies from the host cell, (iii) solubilization of the isolated inclusion bodies, (iv) induction of autoproteolytic cleavage of the heterologous polypeptide of interest from the fusion polypeptide, and (v) isolation of the cleaved heterologous polypeptide of interest.
Owner:SANDOZ AG +1
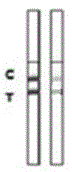
Rabbit pestivirus antibody rapid detection card and preparation method thereof
The invention relates to a rabbit pestivirus antibody rapid detection card and a preparation method thereof. The preparation method comprises the following concrete steps: (1) preparing an anti-rabbit-pestivirus VP60 monoclonal antibody, concretely performing immunization program, cell fusion, hybridomas screening and cloning, and antibody preparation and purification; and (2) preparing the rabbit pestivirus antibody rapid detection card, concretely decocting a colloidal gold solution, marking the anti-rabbit-pestivirus VP60 monoclonal antibody, processing a colloidal-gold pad, processing a sample pad, and determining C and T lines. The rabbit pestivirus antibody rapid detection card is capable of rapidly, sensitively and accurately detecting rabbit pestivirus antibody, and overcomes the problems that domestic rabbit pestivirus antibody detection mainly employs erythrocyte hemagglutination and ELISA detection.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Non-spreading pestivirus
InactiveUS20050220813A1Prevent negative consequenceSafely vaccinatedSsRNA viruses positive-senseViral antigen ingredientsPestivirusWild type
The invention relates to vaccines used in the eradication or control of pestivirus infections, particularly those used in pigs or ruminants. The invention provides nucleic acid, pestivirus-like particles and a pestivirus vaccine, comprising the nucleic acid or particles, which is capable of eliciting a proper immune response without having the ability to spread throughout the vaccinated animal, thereby avoiding the negative consequences of viral spread. Preferably, the immune response allows for serological discrimination between vaccinated animals and wild-type pestivirus infected animals.
Owner:INTERVET INT BV
Vaccine diagnostics
ActiveUS9291624B2Microbiological testing/measurementImmunoassaysBovine Viral Diarrhea VirusesPestivirus
The present invention relates to improved diagnostic methods and kits for differentiating between (a) animals administered a chimeric pestivirus, and (b) animals infected with a wild-type bovine viral diarrhea virus (BVDV) or immunized with a conventional BVDV vaccine.
Owner:ZOETIS SERVICE LLC
Compositions and methods of using capsid protein from Flaviviruses and Pestiviruses
This invention provides methods of inducing cell death with Flavivirus or Pestivirus capsid protein, such as West Nile virus (WNV) capsid protein, and functional fragments thereof. The invention also provides methods of treating patients suffering from diseases characterized by hyperproliferating cells by administering pharmaceutical compositions comprising WNV or other virus including Flavivirus or Pestivirus capsid or other protein or a nucleic acid molecule encoding the same. Methods of identifying compounds which have anti-viral and / or anti-WNV and / or anti-Flavivirus and / or anti-Pestivirus capsid or other protein activity are disclosed. The invention also provides vaccine compositions comprising capsid or other proteins, or fragments thereof, or nucleic acids encoding same, from WNV or other virus including Flavivirus or Pestivirus and a pharmaceutically acceptable carrier. The invention also provides diagnostic methods and kits for identifying individuals exposed to WNV or other viruses including Flavivirus or Pestivirus.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Organic Compounds
ActiveUS20100062490A1Improve solubilityLow piHydrolasesPeptide preparation methodsHeterologousInclusion bodies
The invention relates to a process for the recombinant production of a heterologous polypeptide of interest, comprising, (i) cultivation of a bacterial host cell which is transformed with an expression vector which comprises a nucleic acid molecule which codes for a fusion polypeptide, the fusion polypeptide comprising a derivative of an autoprotease Npro of Pestivirus, wherein at least one cysteine residue of the naturally occuring autoprotease Npro of Pestivirus is replaced by another amino acid residue, and a second polypeptide which is connected to the first polypeptide at the C-terminus of the first polypeptide in a manner such, that the second polypeptide is capable of being cleaved from the fusion polypeptide by the autoproteolytic activity of the first polypeptide, said second polypeptide being a heterologous polypeptide, wherein cultivation occurs under conditions which cause expression of the fusion polypeptide and formation of corresponding cytoplasmic inclusion bodies, (ii) isolation of the inclusion bodies from the host cell, (iii) solubilization of the isolated inclusion bodies, (iv) induction of autoproteolytic cleavage of the heterologous polypeptide of interest from the fusion polypeptide, and (v) isolation of the cleaved heterologous polypeptide of interest.
Owner:BOEHRINGER INGELHEIM RCV GMBH & CO KG +1
Chimeric pestivirus with insertion in 3' nontranslated region (3' NTR) with stable replication and rnase resistance
Owner:LIFE TECH CORP
Vaccine composition, and preparation method and application thereof
ActiveCN104248761ASolve the problem of immune failureLow costAntibacterial agentsBacterial antigen ingredientsDiseasePestivirus
The invention relates to the field of biological products, and specifically relates to a Pestivirus suis and streptococcus suis vaccine composition and a preparation method thereof. The composition includes Pestivirus suis and streptococcus suis antigens. The invention also relates to application of the vaccine composition to preparation of a composition for the prevention and / or treatment of diseases associated with Pestivirus suis and streptococcus suis, and infections caused by Pestivirus suis and streptococcus suis.
Owner:PU LIKE BIO ENG
Methods and compositions for treating flaviviruses, pestiviruses and hepacivirus
A method and composition for treating a host infected with flavivirus, pestivirus or hepacivirus comprising administering an effective flavivirus, pestivirus or hepacivirus treatment amount of a described base-modified nucleoside or a pharmaceutically acceptable salt or prodrug thereof, is provided.
Owner:IDENIX (CAYMAN) LTD +1
Pestivirus vaccines for congenital tremors
ActiveUS20170058266A1Reduce severityReduction in daily weight gainSsRNA viruses positive-senseNervous disorderAntigenDisease
The present invention relates to a vaccine for protecting a piglet against diseases associated with a novel pestivirus. The vaccine commonly includes a pestivirus antigen and, optionally an adjuvant. Methods for protecting pigs against diseases associated with pestivirus, including but not limited to congenital tremors and methods of producing the pestivirus vaccine are also provided.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH +1
Methods and Compositions for Treating Flaviviruses, Pestiviruses and Hepacivirus
A method and composition for treating a host infected with flavivirus, pestivirus or hepacivirus comprising administering an effective flavivirus, pestivirus or hepacivirus treatment amount of a described base-modified nucleoside or a pharmaceutically acceptable salt or prodrug thereof, is provided.
Owner:THE CENT NAT DEL LA RECH SCIQUE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00002.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00003.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00000.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-C00001.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00001.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00002.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00003.png)