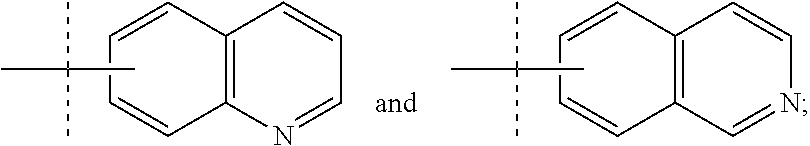Patents
Literature
53 results about "Family Flaviviridae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Flaviviridae is a family of viruses. Humans and other mammals serve as natural hosts. They are primarily spread through arthropod vectors (mainly ticks and mosquitoes).
5-Aza-7-deazapurine derivatives for treating Flaviviridae
This invention is directed to a method for treating a host, especially a human, infected with hepatitis C, flavivirus and / or pestivirus, comprising administering to that host an effective amount of an anti-flavivirus or anti-pestivirus, biologically active compound has a 5-aza-7-deazapurine moiety. The 5-aza-7-deazapurine moiety may be substituted or unsubstituted, and may comprise a nucleoside analogue, or a salt or prodrug thereof. The compound of the present invention may be administered alone or in combination with another anti-hepatitis C, anti-flavivirus and / or anti-pestivirus agent.
Owner:INDENIX PHARM LLC +3
2′-branched nucleosides and Flaviviridae mutation
ActiveUS7824851B2High sensitivityReduce Flaviviridae infectionBiocideSsRNA viruses positive-senseAmino acidMutant strain
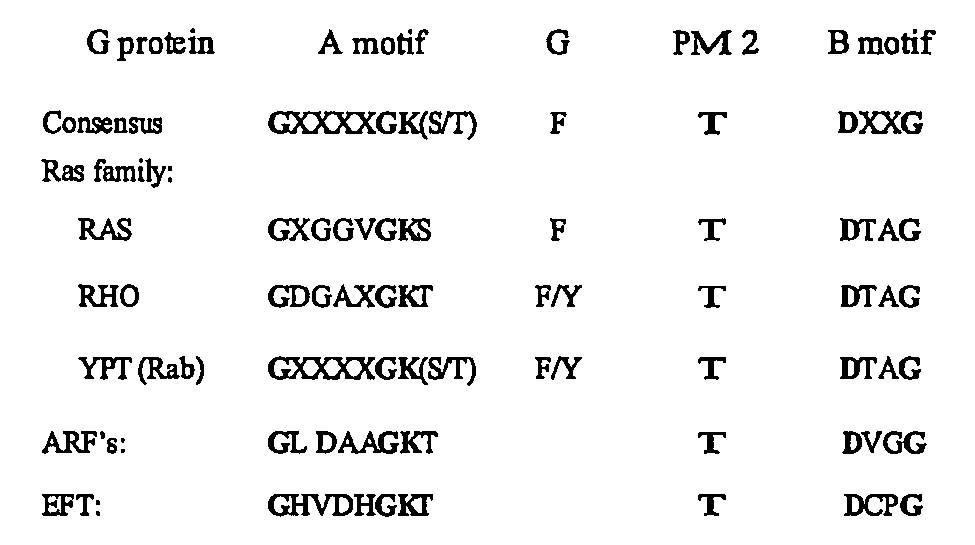
The present invention discloses a method for the treatment of Flaviviridae infection that includes the administration of a 2′-branched nucleoside, or a pharmaceutically acceptable prodrug and / or salt thereof, to a human in need of therapy in combination or alternation with a drug that directly or indirectly induces a mutation in the viral genome at a location other than a mutation of a nucleotide that results in a change from seine to a different amino acid in the highly conserved consensus sequence, XRXSGXXXT (Sequence ID No. 63), of domain B of the RNA polymerase region, or is associated with such a mutation. The invention also includes a method to detect a mutant strain of Flaviviridae and a method for its treatment.
Owner:INDENIX PHARM LLC
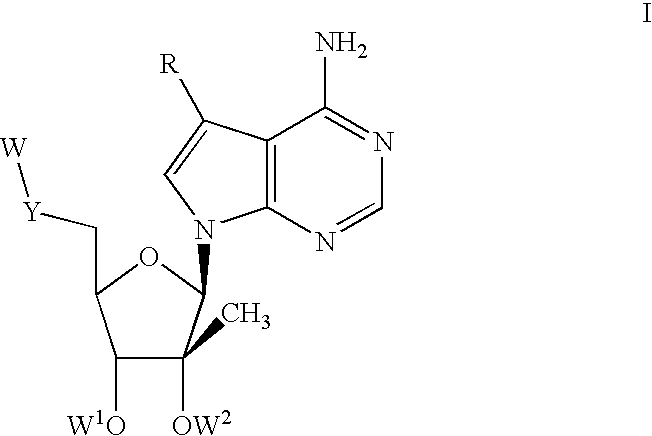
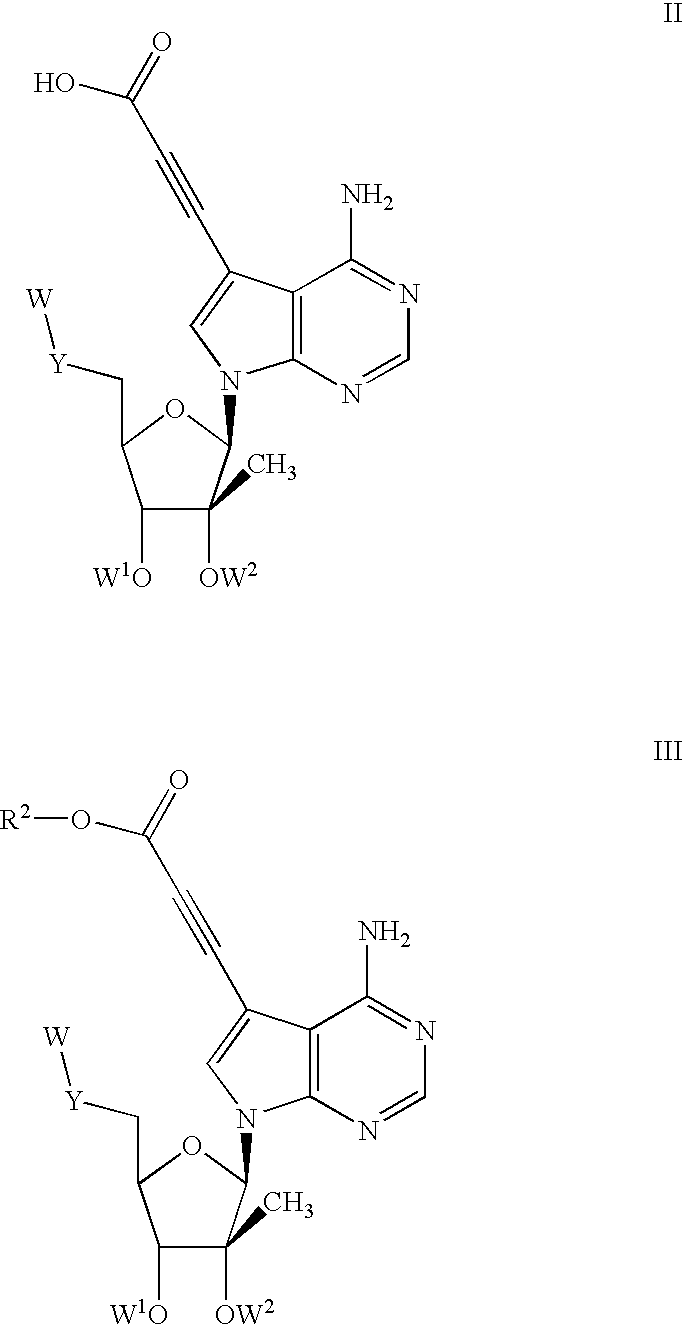
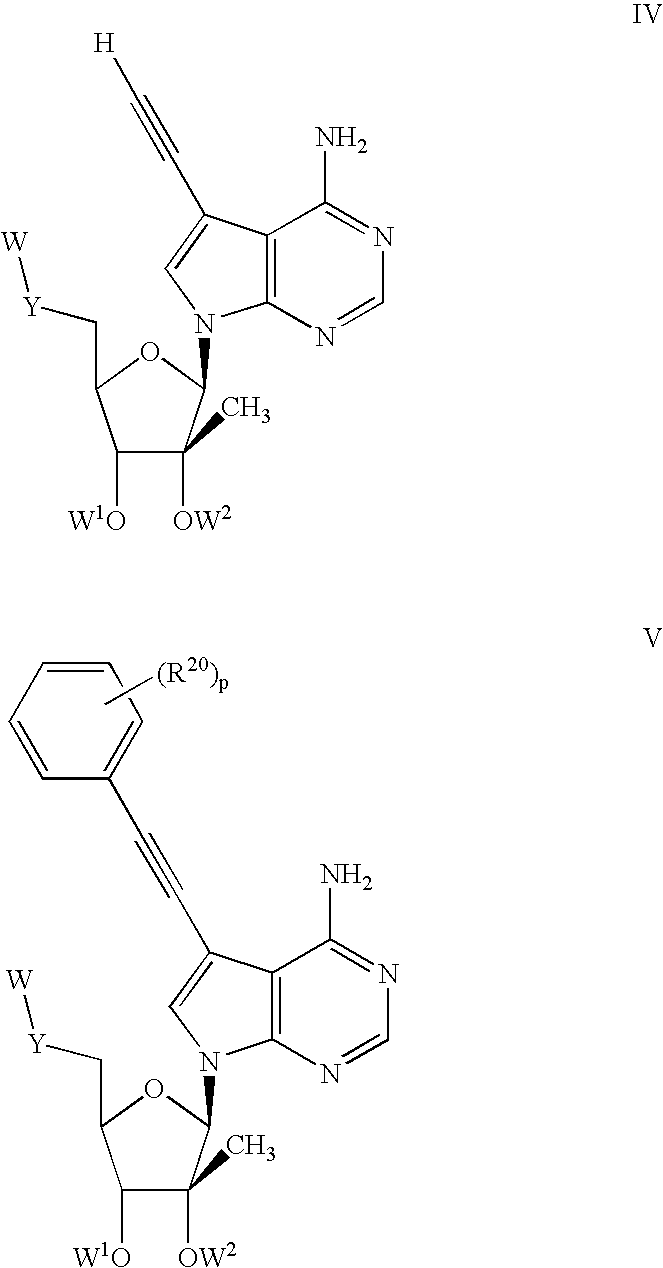
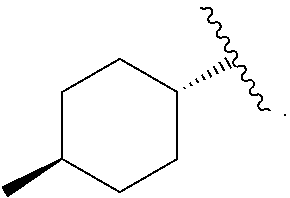
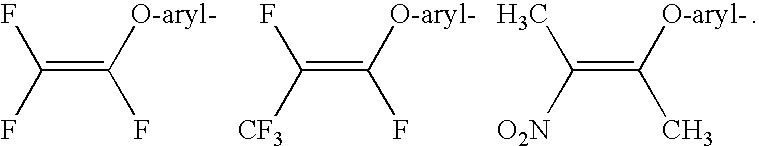
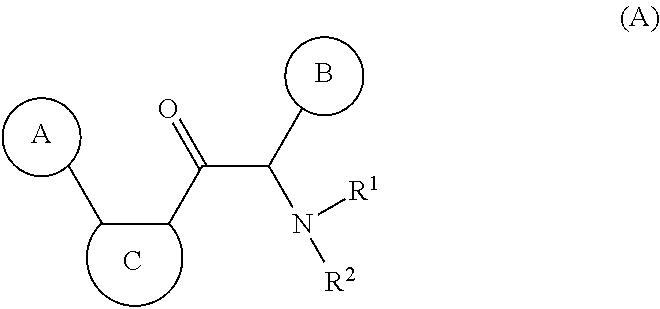
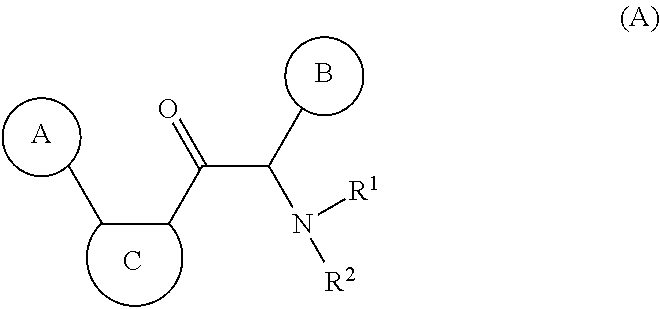
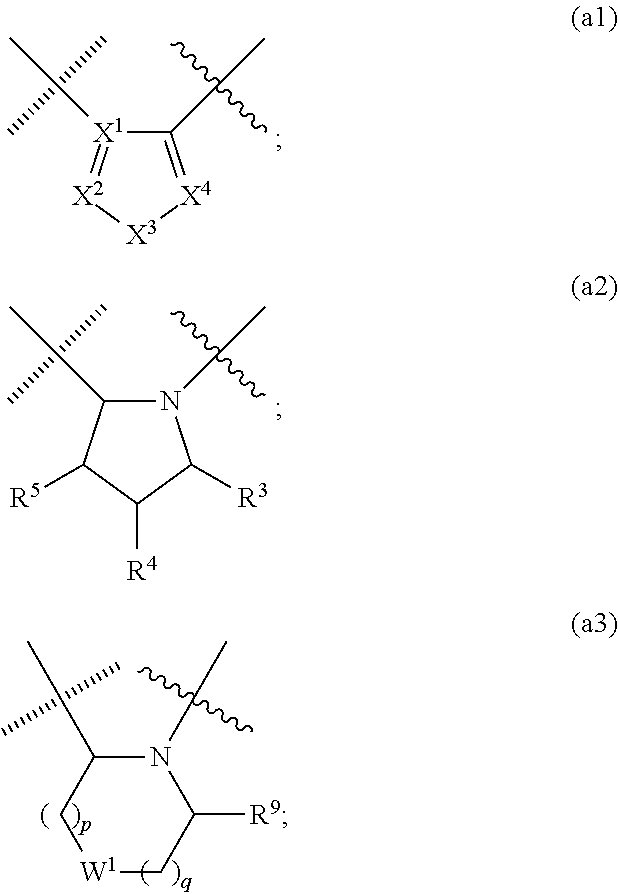
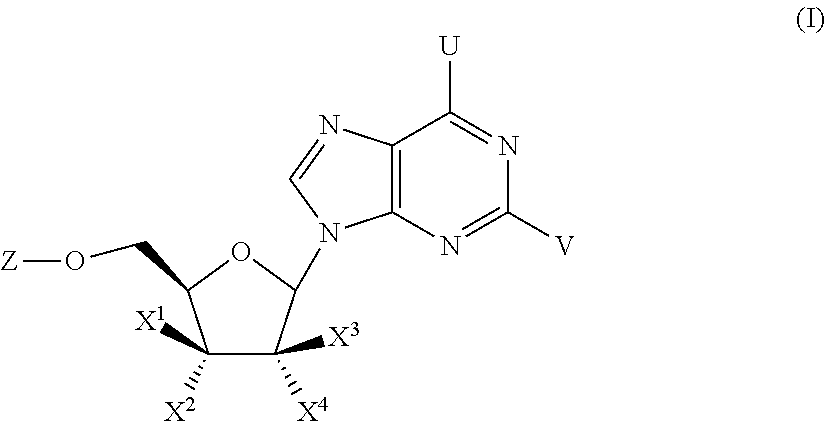
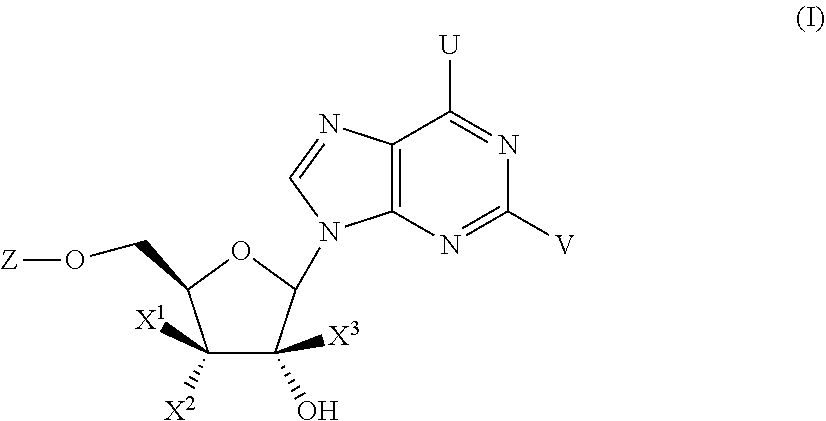
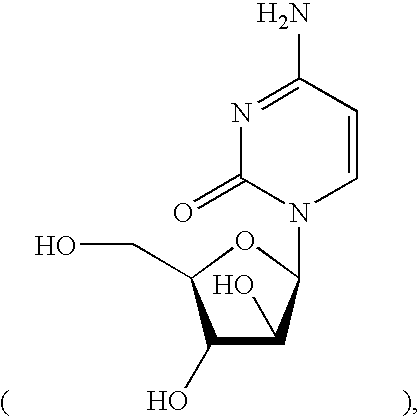
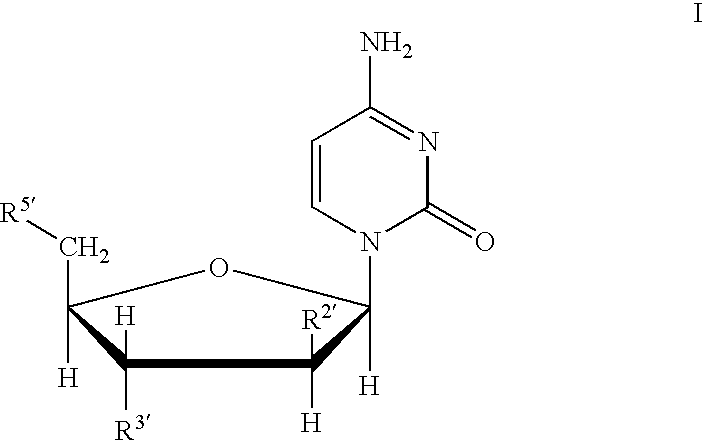
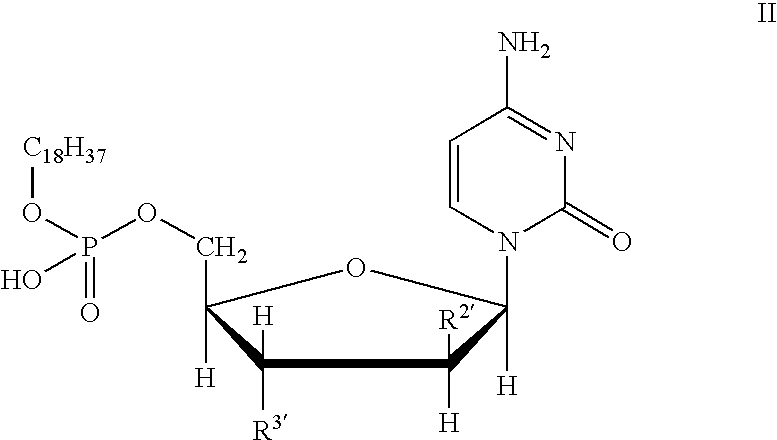
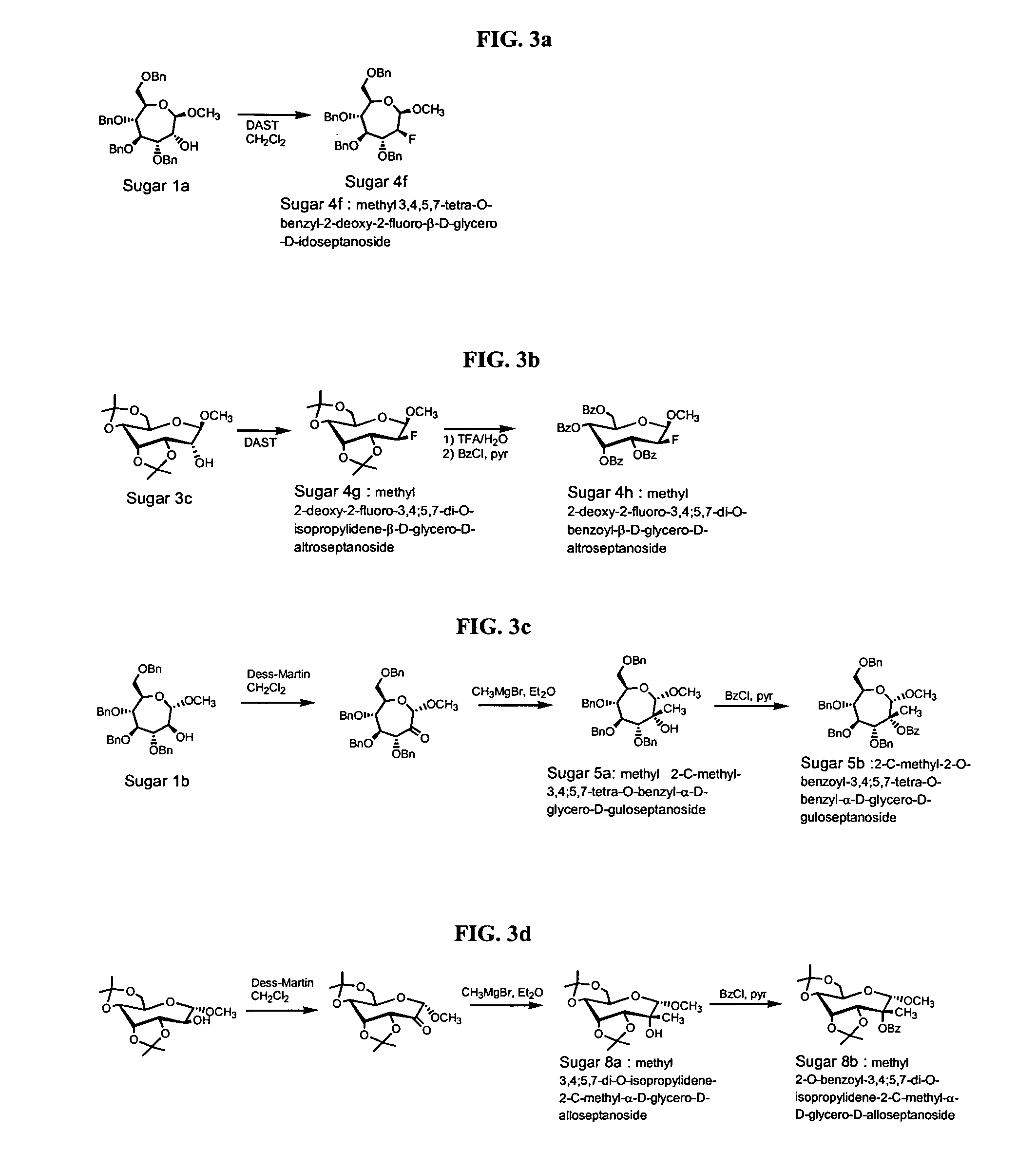
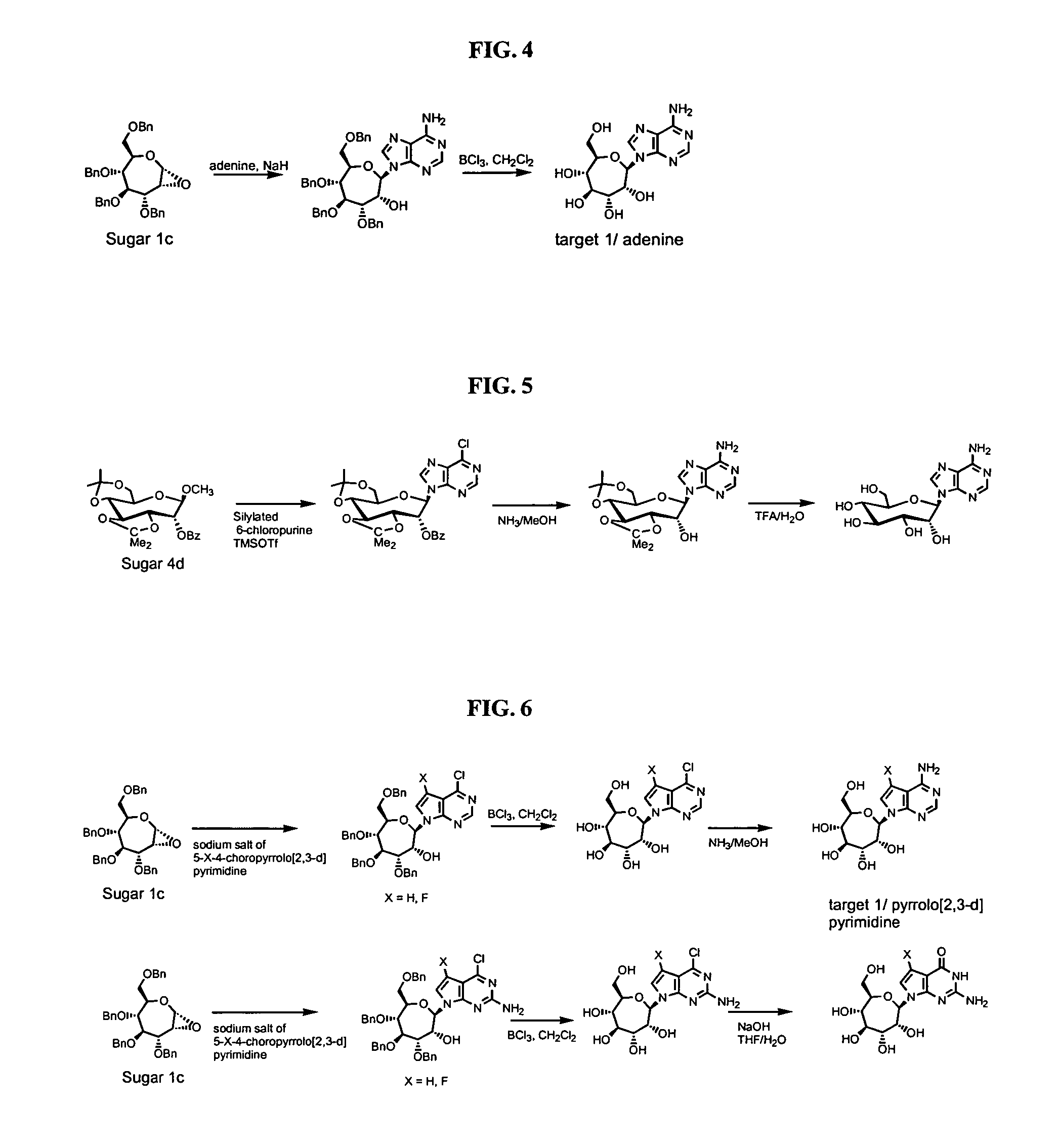
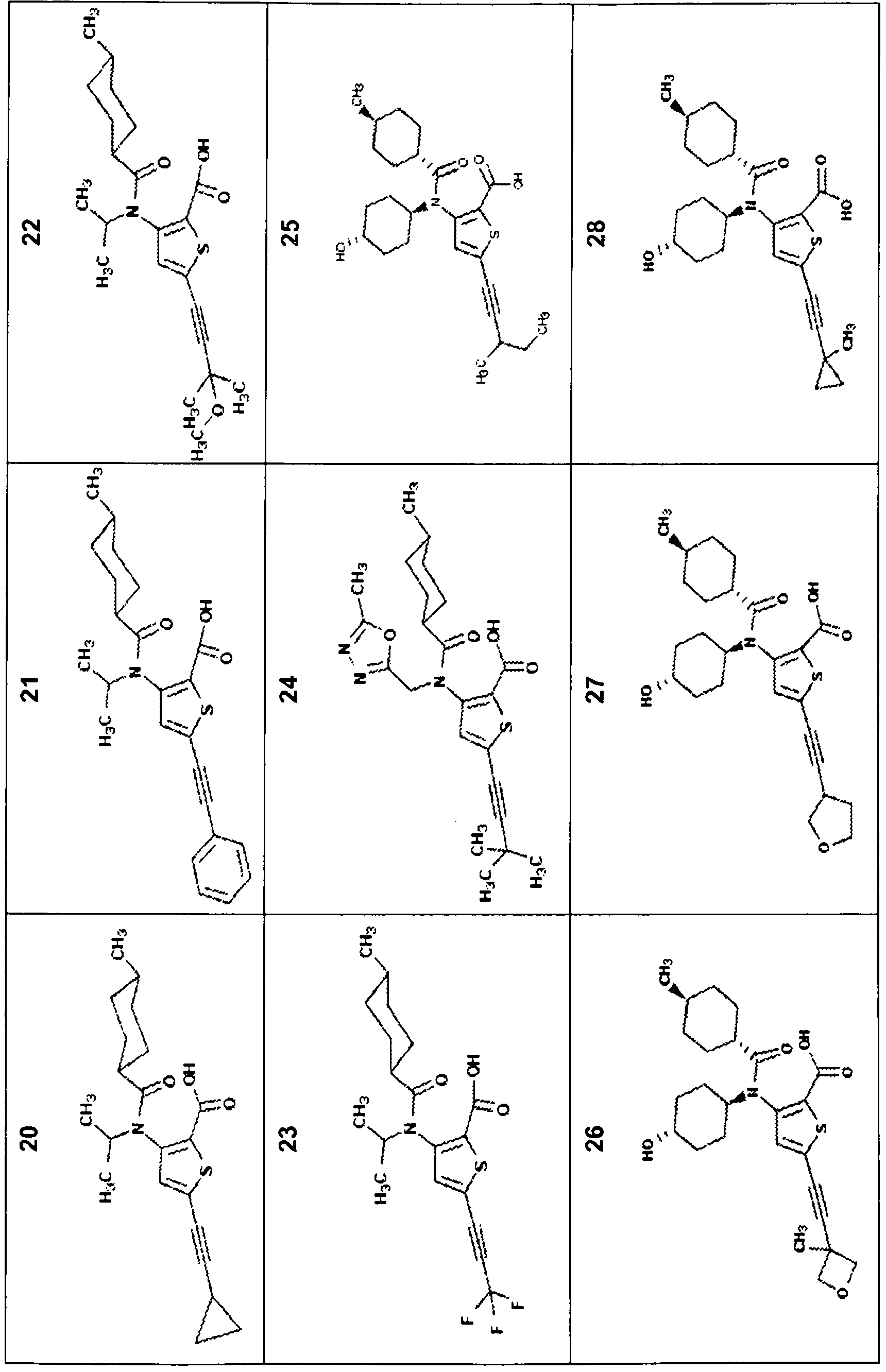
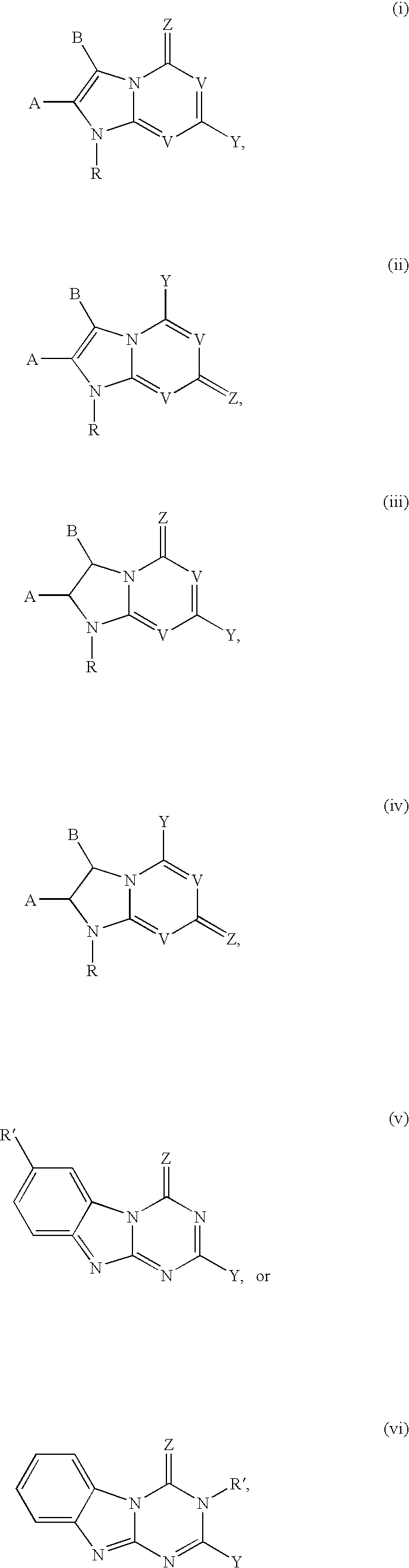
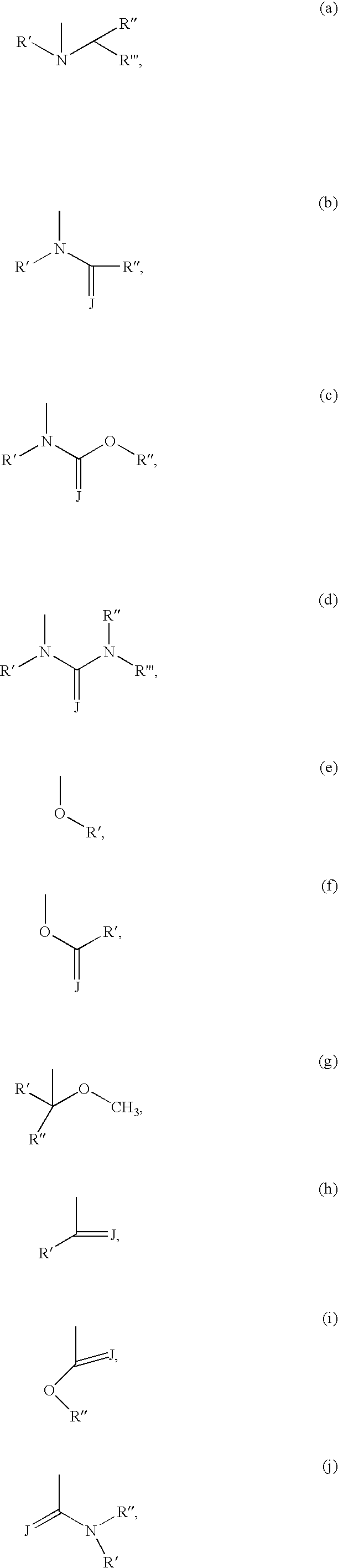
Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections
InactiveUS20040082574A1Potent and selective activityPotent activityBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
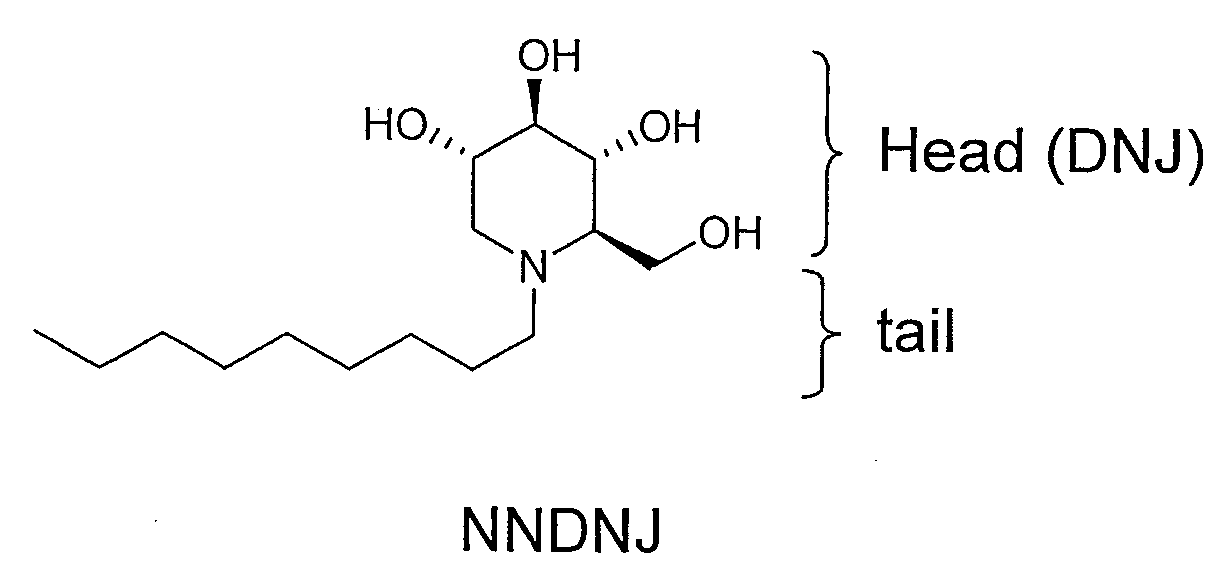
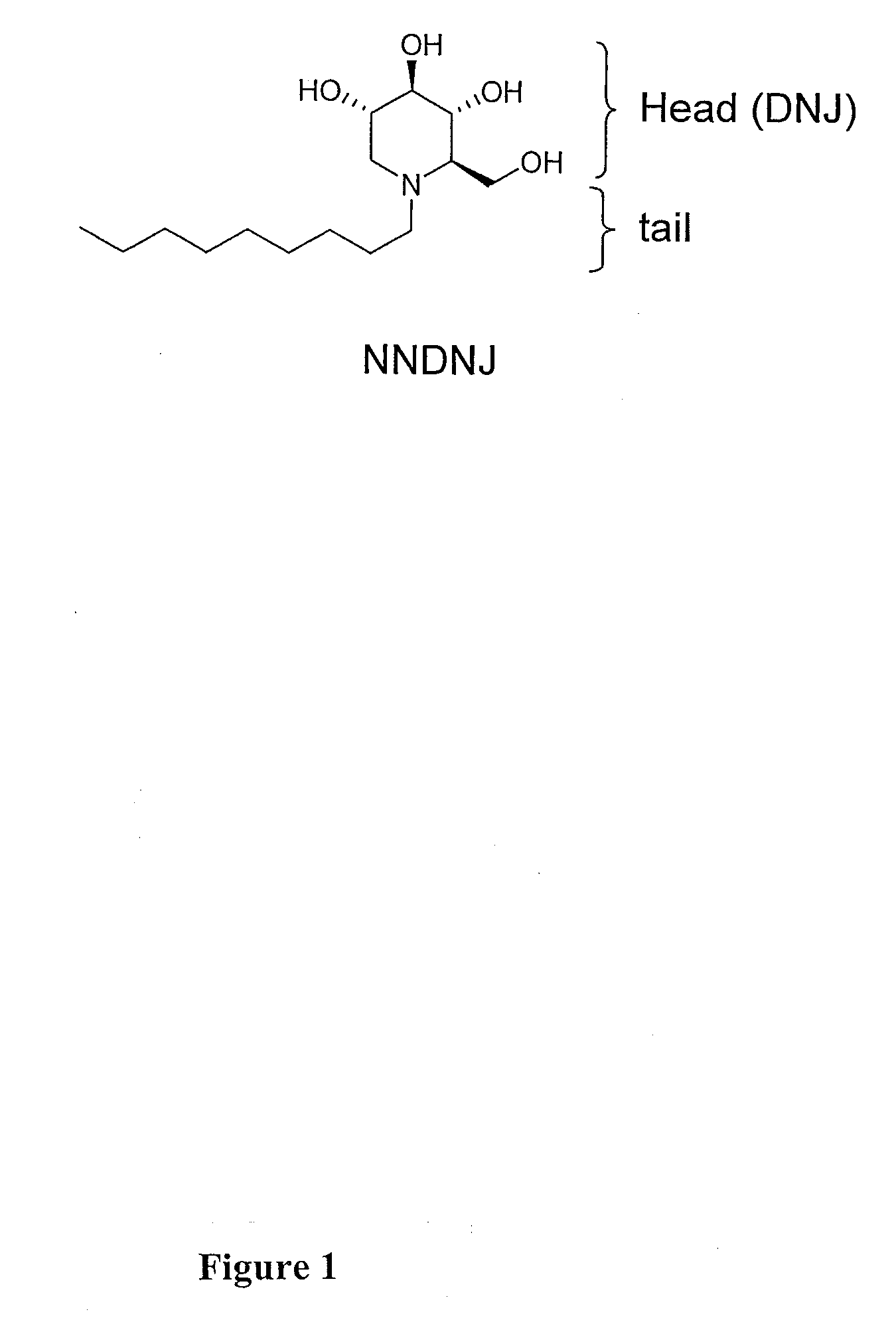
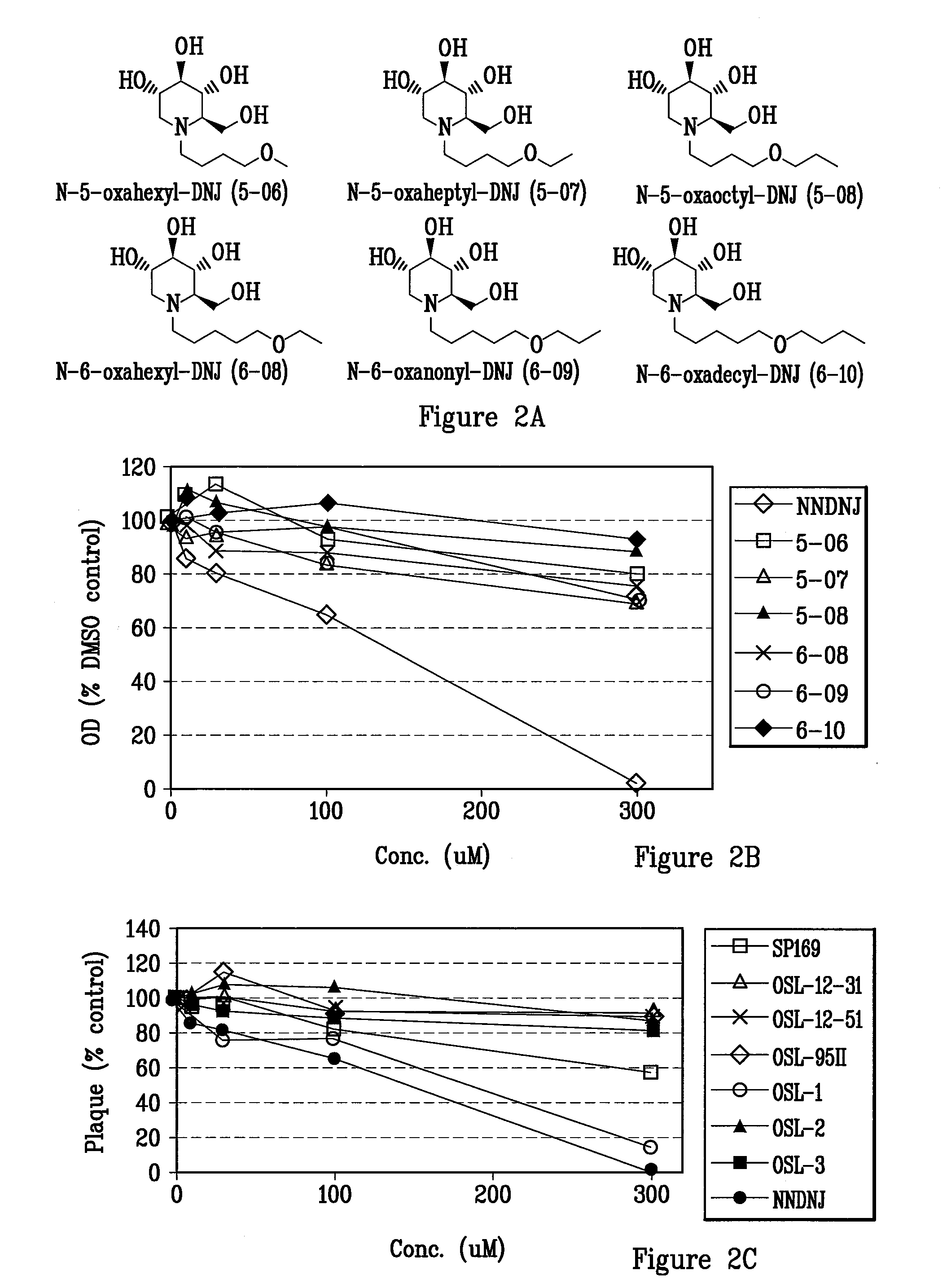
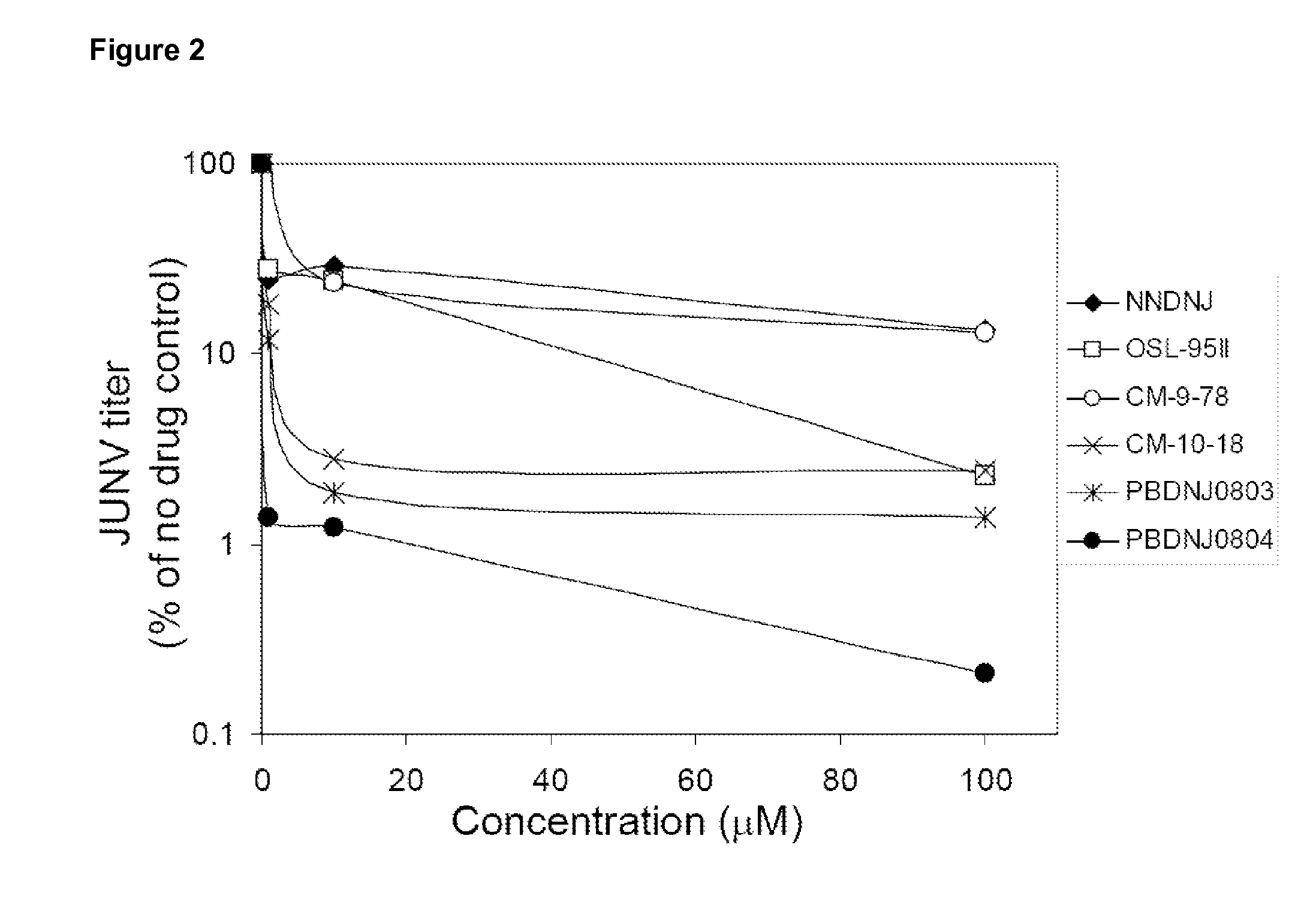
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
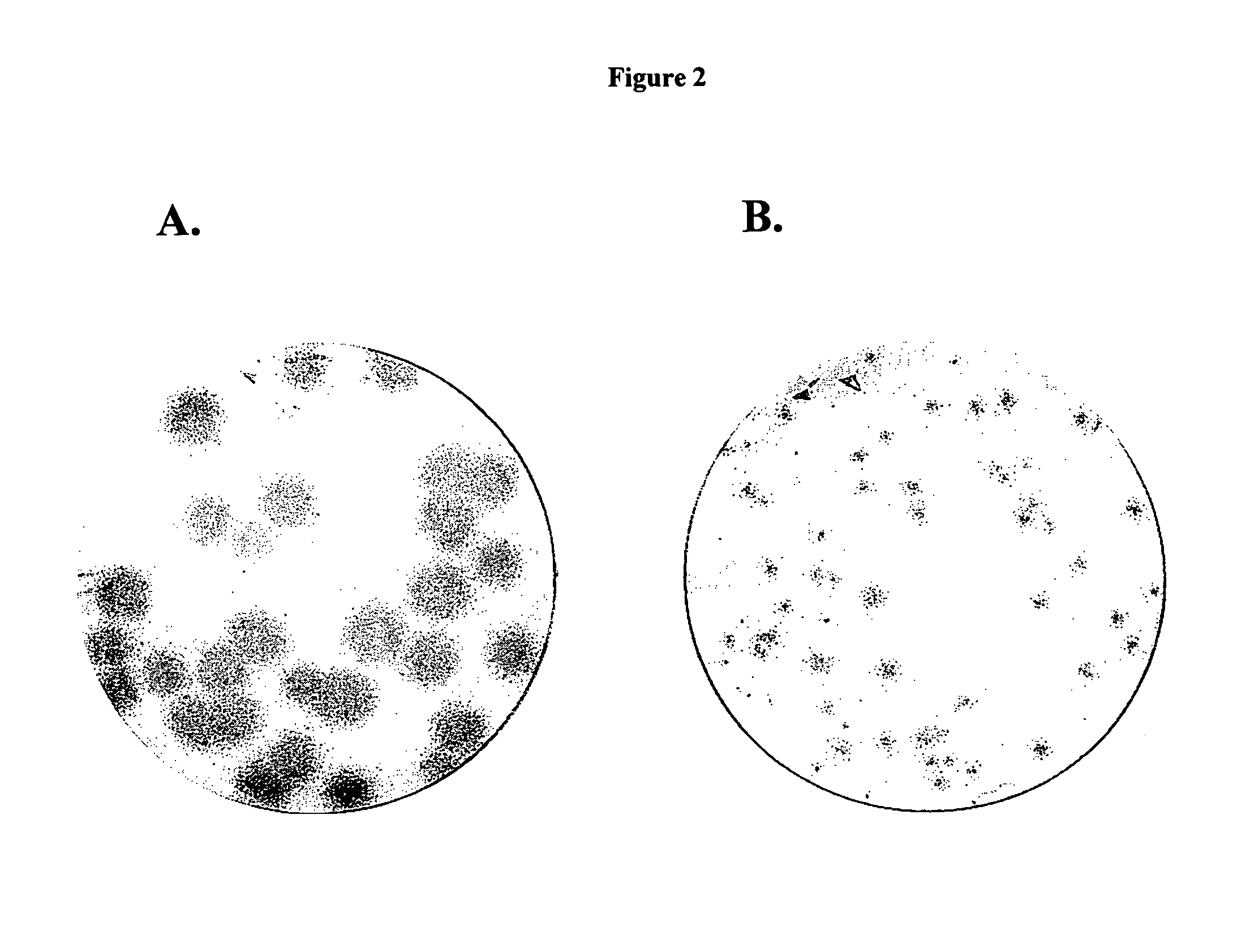
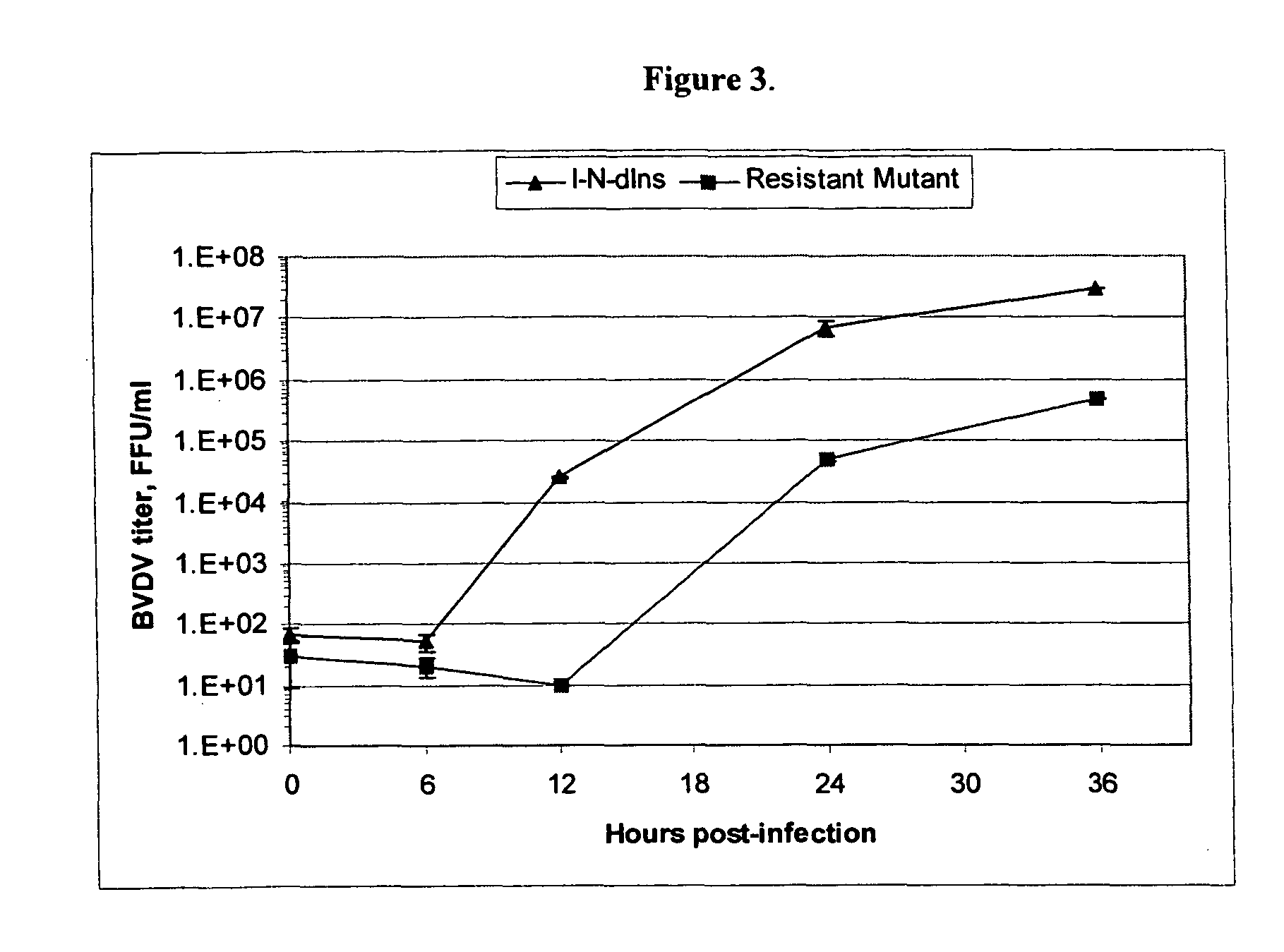
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
Nucleoside derivatives for treating hepatitis C virus infection
Disclosed are 6-hydroxyamino- or a 6-alkoxyamino-7-deazapurine-ribofuranose derivatives, salts, pharmaceutical compositions, and methods of use thereof for treating viral infections caused by a flaviviridae family virus, such as hepatitis C virus.
Owner:SMITHKLINE BECKMAN CORP
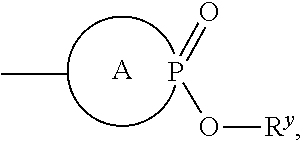
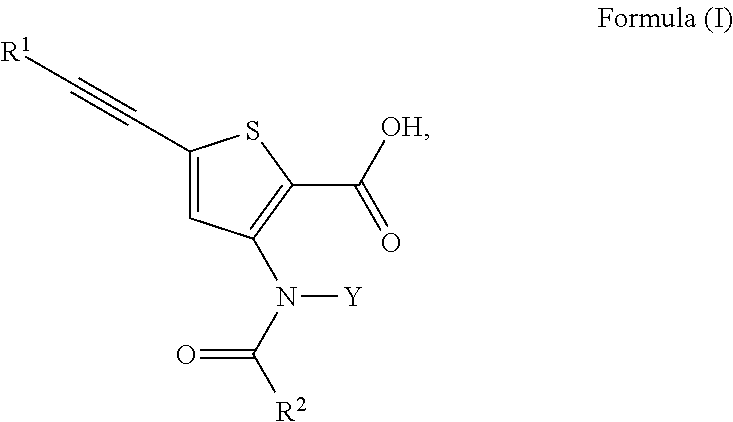
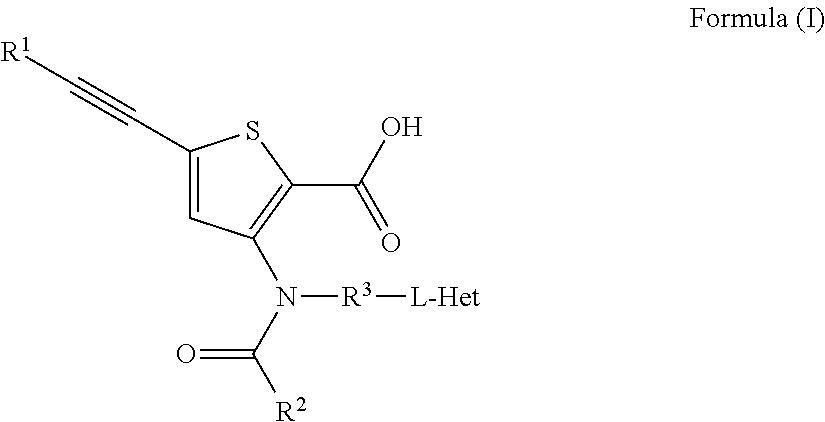
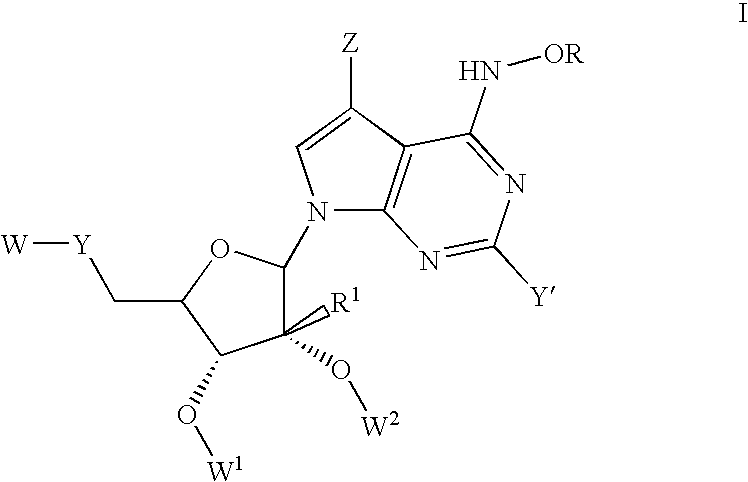
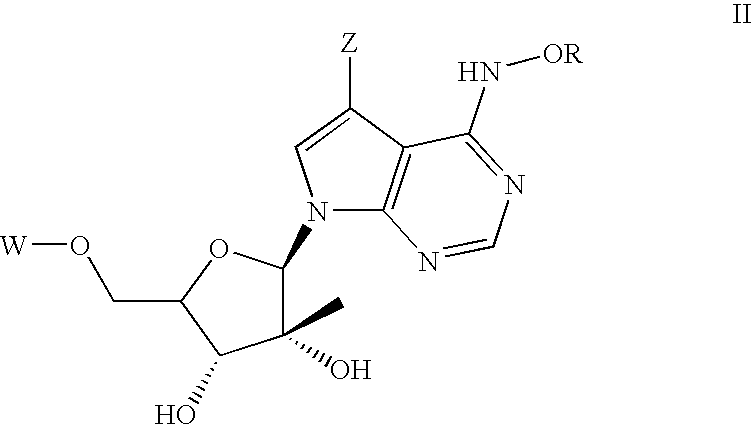
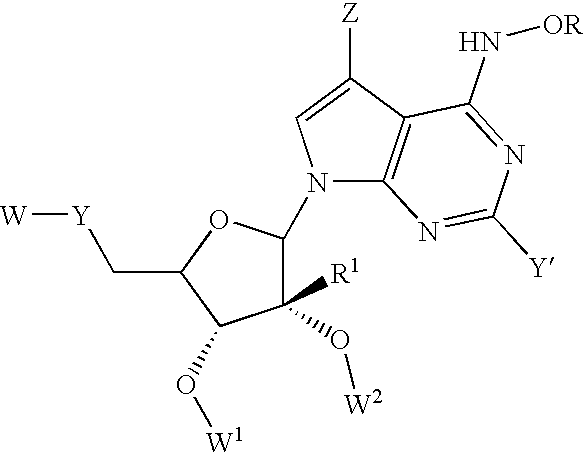
Anti-Viral Compounds
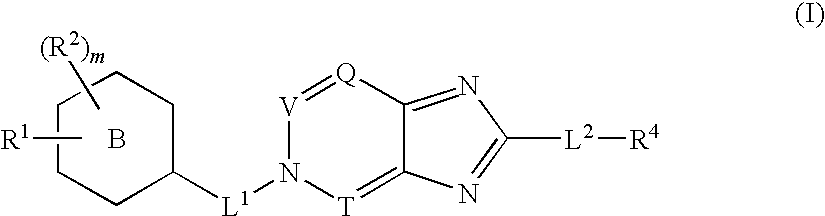
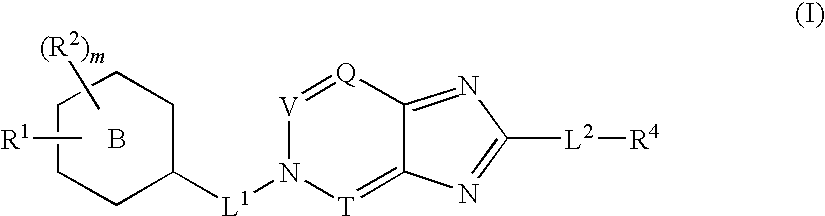
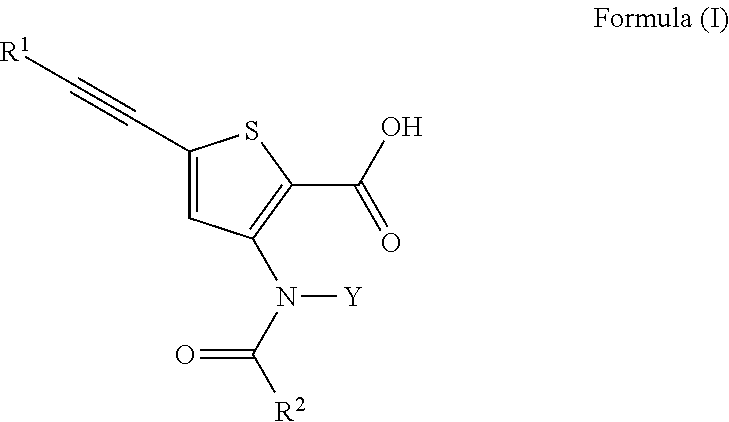
Disclosed are compounds, stereoisomers, tautomers, pharmaceutically acceptable salts, or prodrugs thereof of having Formula (I), their preparation, use, and compositions thereof for treating an infection mediated at least in part by a virus in the Flaviviridae family of viruses, wherein A, L1, V, W, T, Z, R, Y1, and p are as defined herein.
Owner:SMITHKLINE BECKMAN CORP
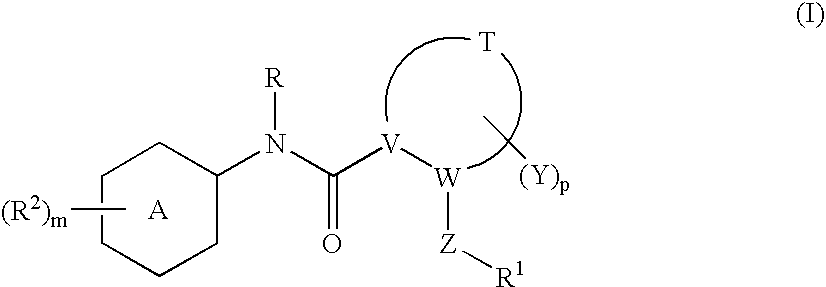
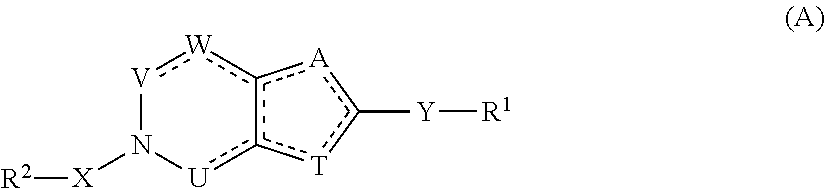
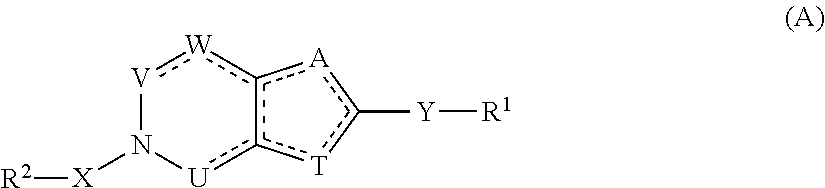
Compounds with the bicyclo[4.2.1]nonane system for the treatment of Flaviviridae infections
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
Nucleoside compounds for treating viral infections
Disclosed are compounds, compositions and methods for treating viral infections caused by a flaviviridae family virus, such as hepatitis C virus.
Owner:SMITHKLINE BECKMAN CORP
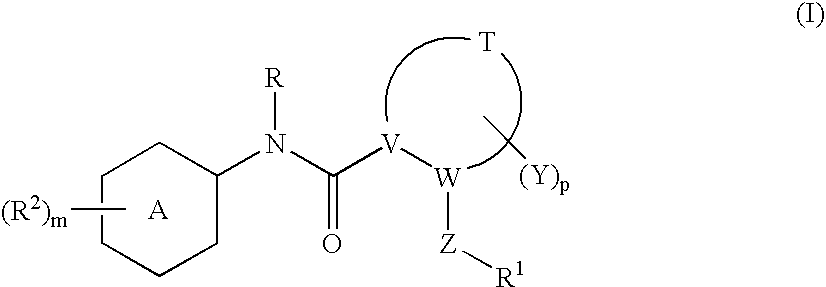
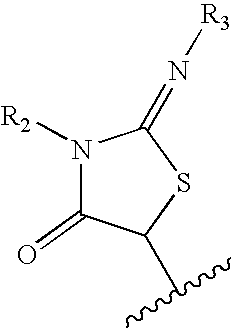
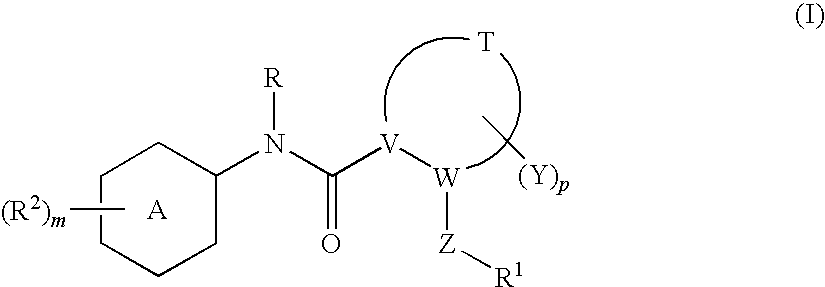
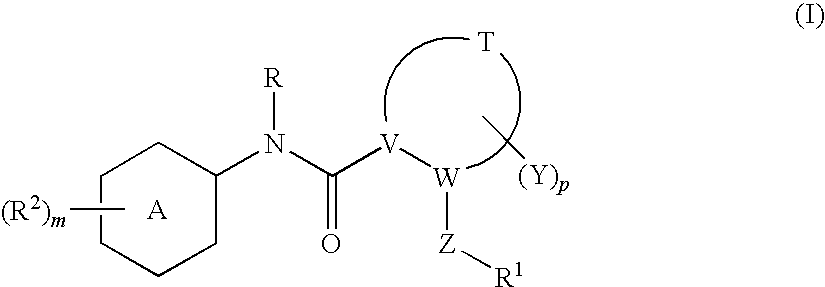
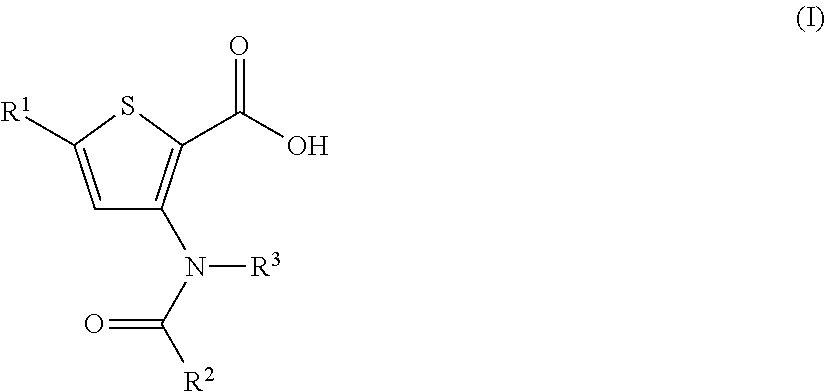
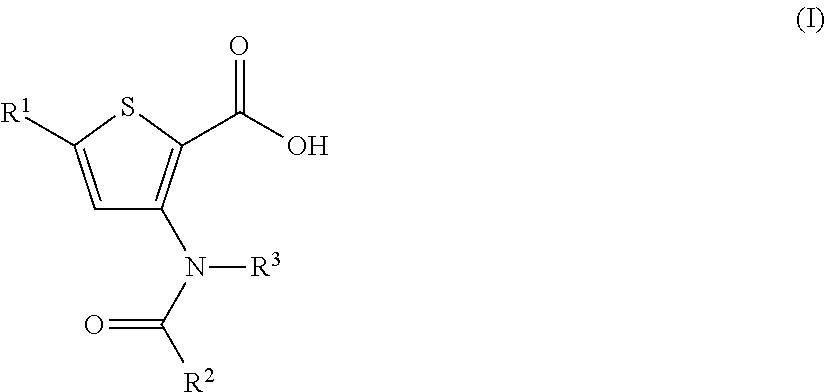
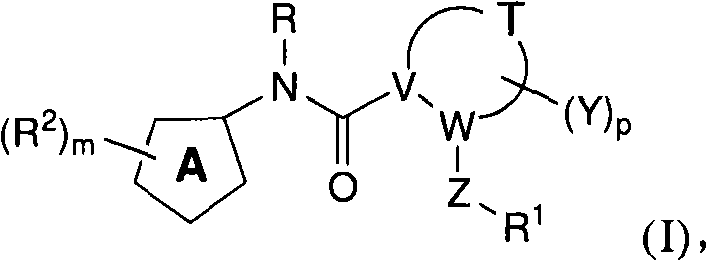
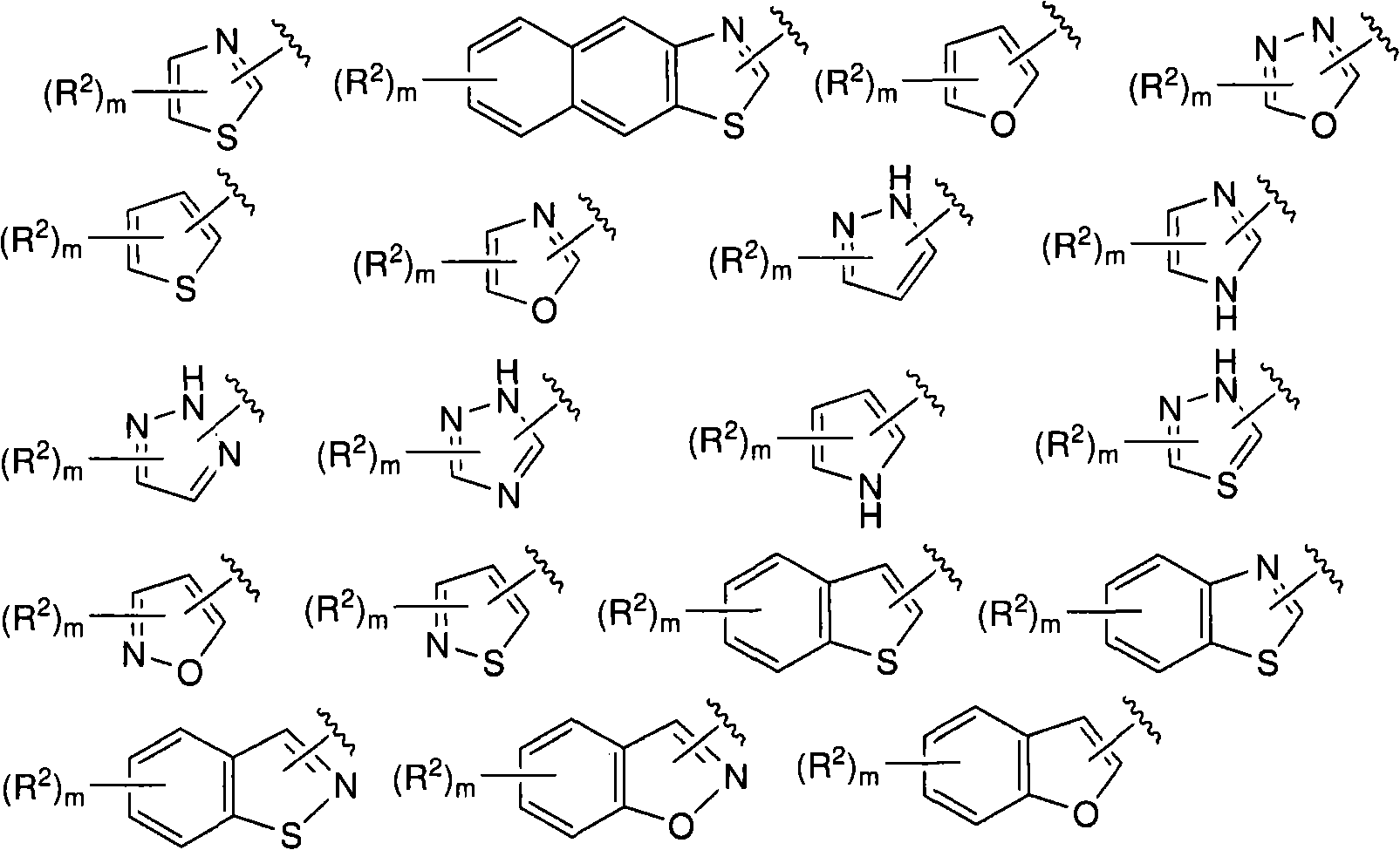
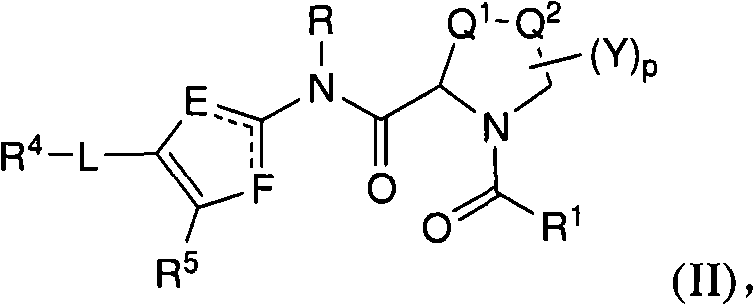
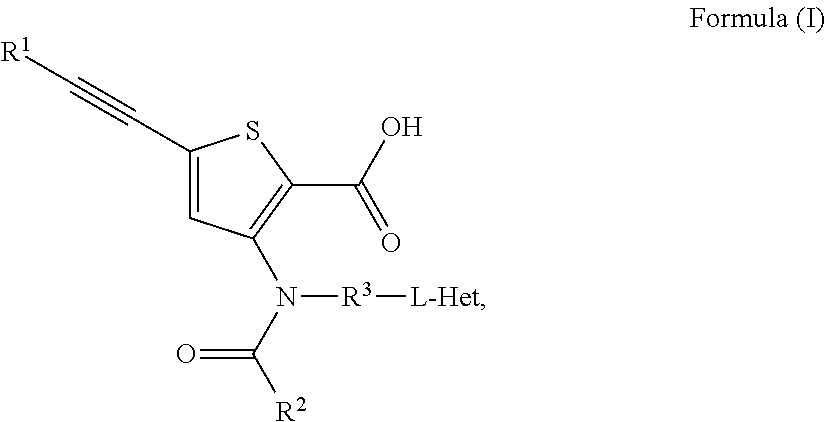
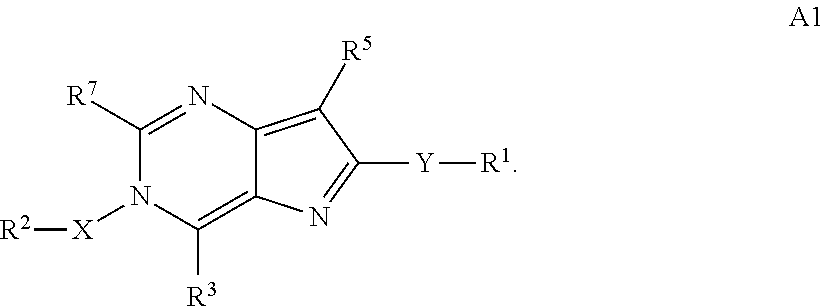
N-(6-membered aromatic ring)-amido Anti-viral compounds
Disclosed are compounds having Formula (I) and the compositions and methods relating to these compounds, for treating or preventing a viral infection mediated at least in part by a virus in the Flaviviridae family of viruses, wherein A, R2, m, R, V, W, T, Z, R1, Y, and p are disclosed herein.
Owner:SMITHKLINE BECKMAN CORP
Inhibitors of Flaviviridae viruses
Provided are compounds of Formula I:and pharmaceutically acceptable salts and esters thereof. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections.
Owner:GILEAD SCI INC
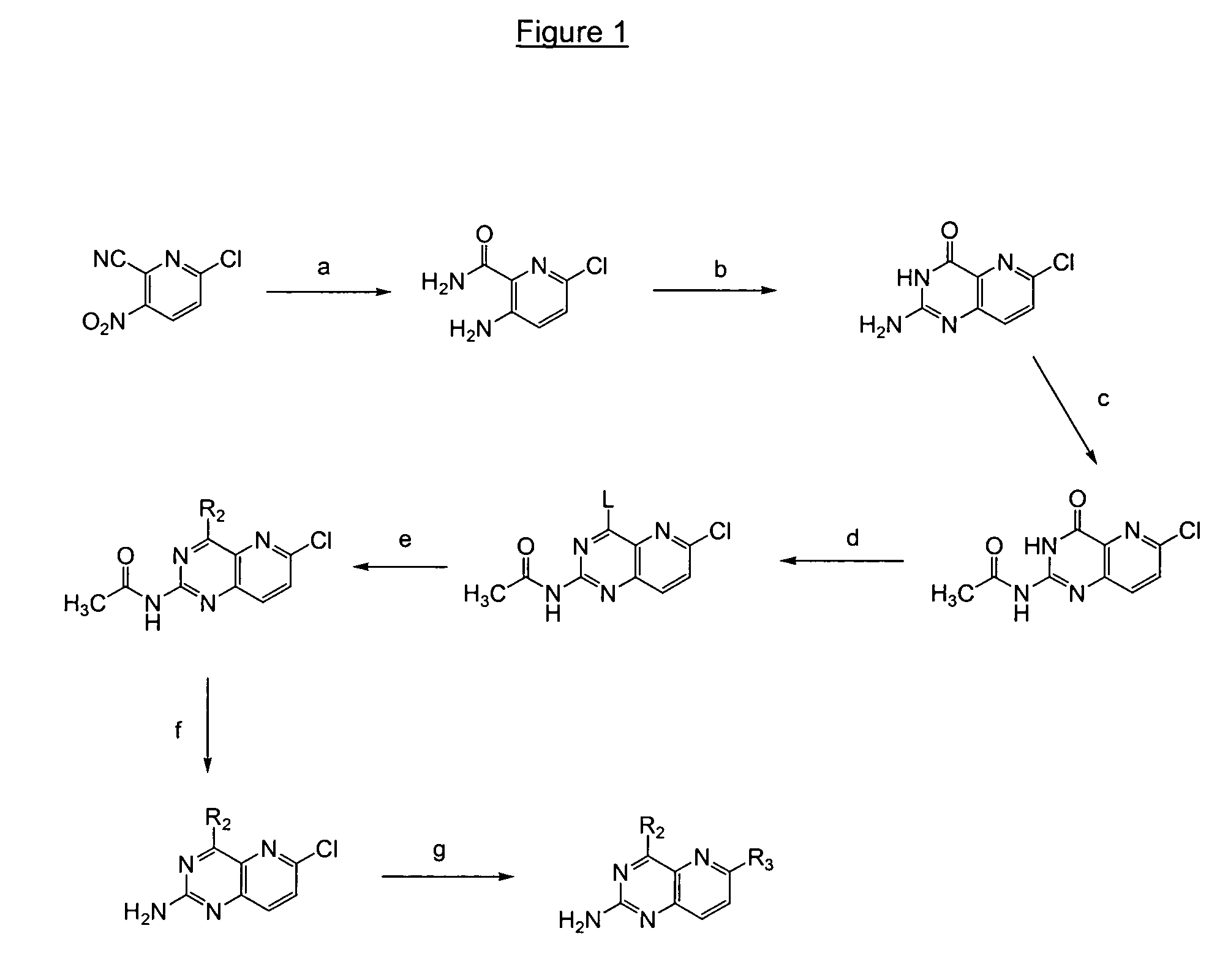
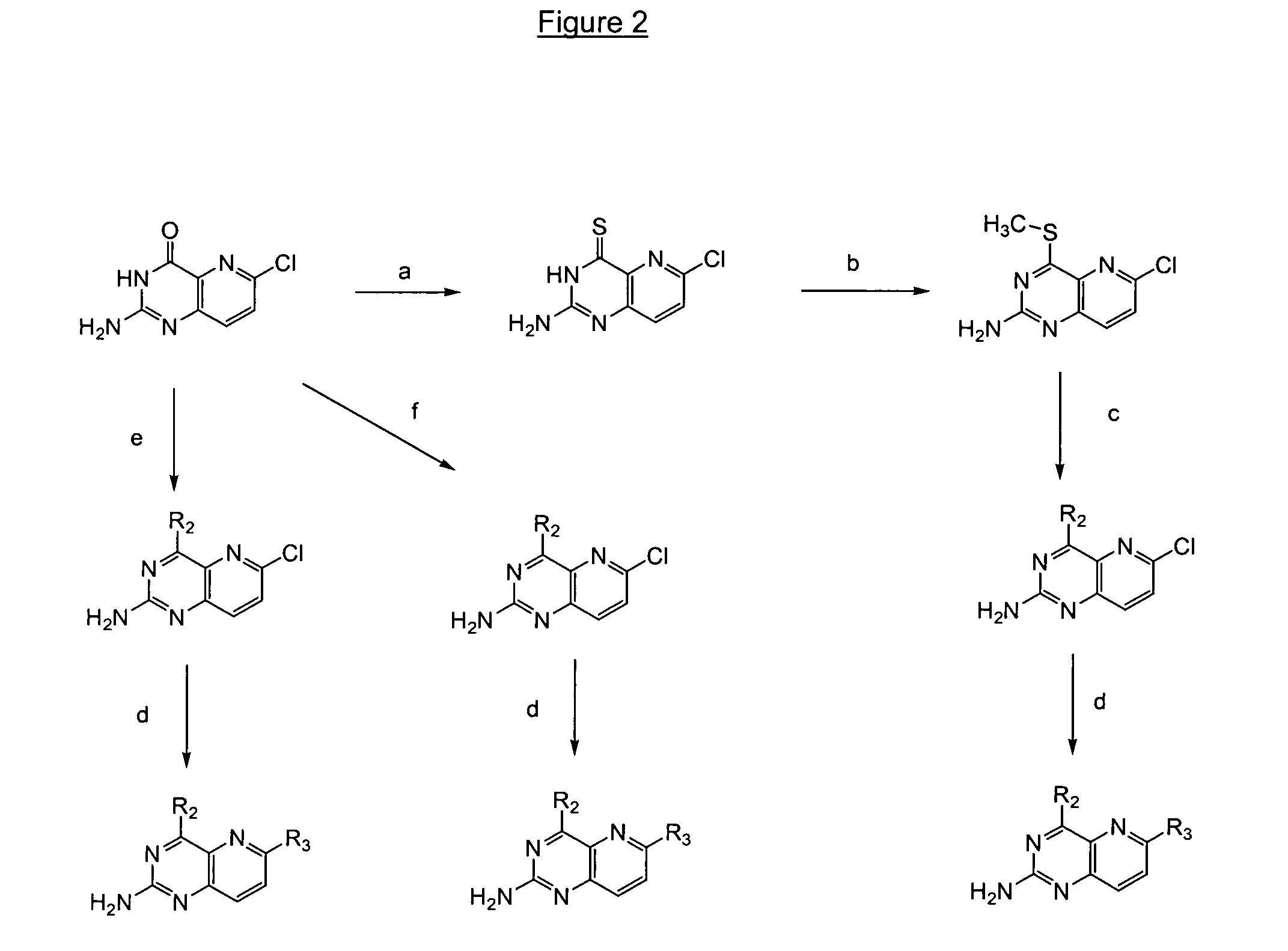
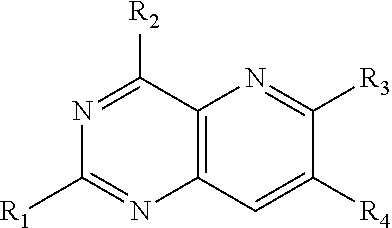
Pyrido(3,2-d)pyrimidines useful for treating viral infections
2-amino-pyrido(3,2-d)pyrimidine derivatives with a specific substitution pattern on positions 4 and 6 of the core structure are useful in the treatment or prevention of an infection due to a virus from the Flaviviridae family, especially HCV, when administered to a patient in a therapeutically effective amount.
Owner:GILEAD SCI INC
N-(6-membered aromatic ring)-amido anti-viral compounds
Owner:SMITHKLINE BECKMAN CORP
Methods and compositions for identifying anti-hcv agents
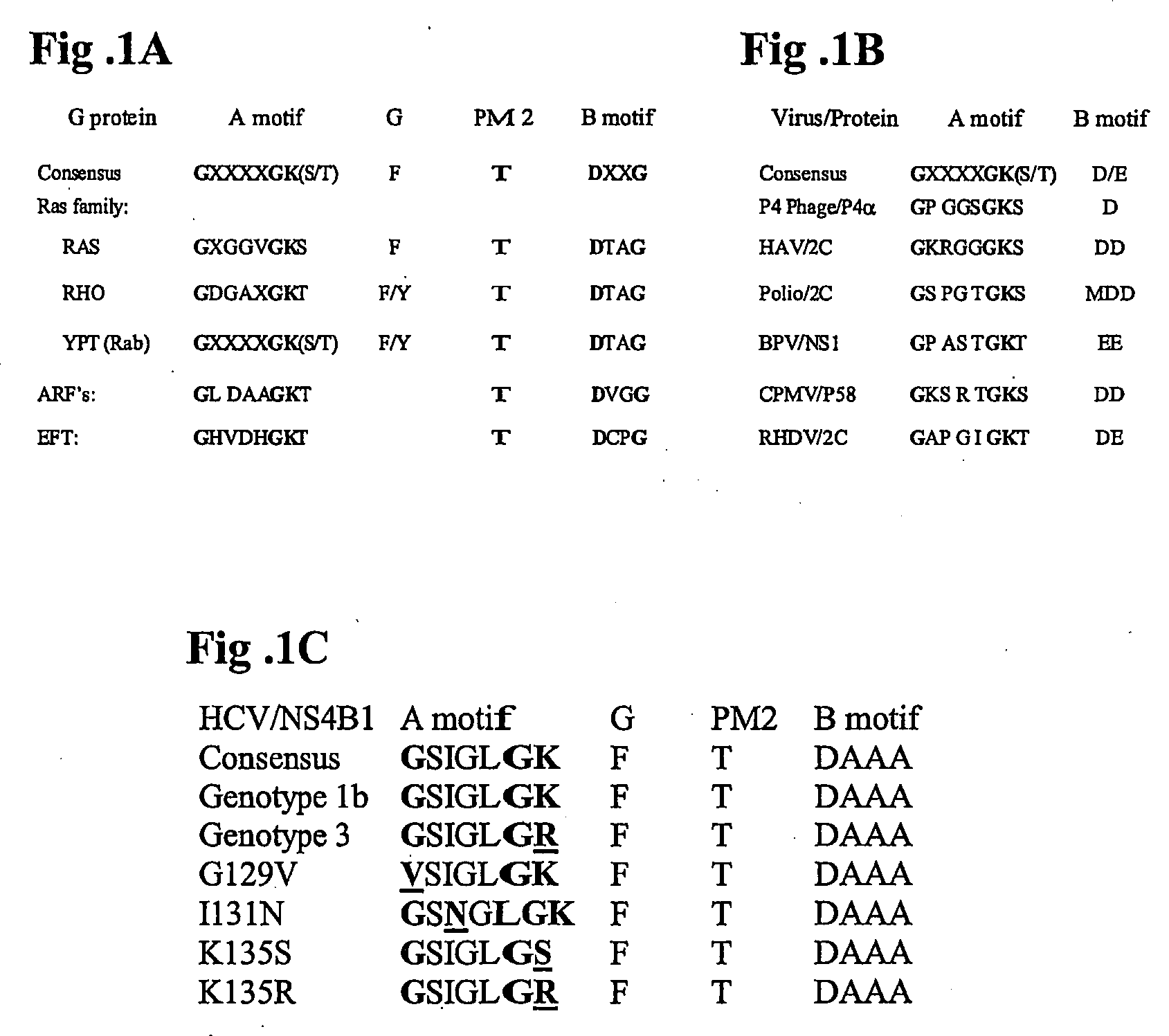
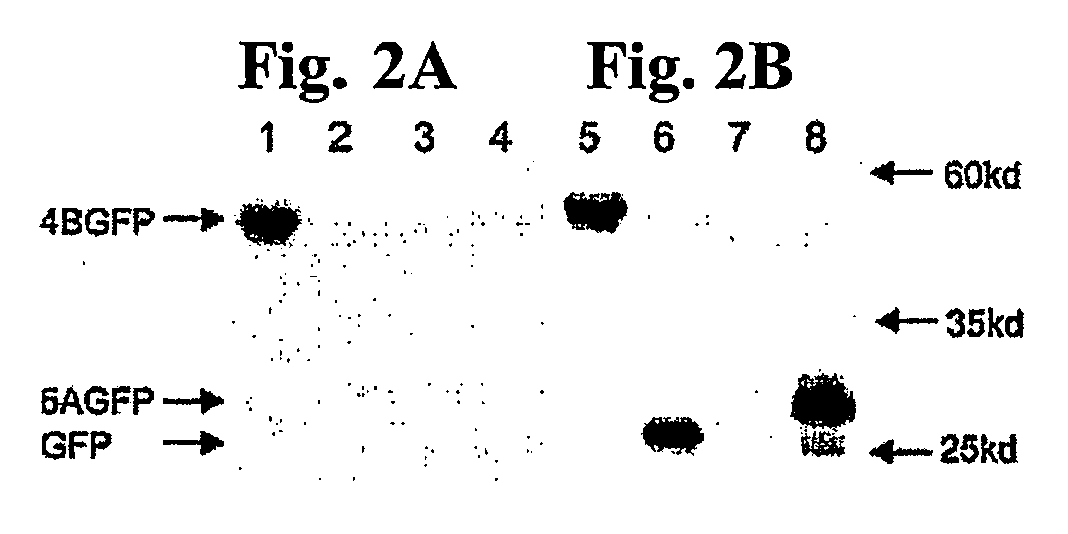
The invention provides methods and compositions for identifying agents for treating infection by viruses that encode a nucleotide-binding NS4B protein, or functional equivalent thereof, e.g., hepatitis C virus (HCV) or other members of the family Flaviviridae. In general, the methods involve contacting an NS4B nucleotide binding motif (NBM)-containing polypeptide with a candidate agent, and determining the effect of the candidate agent on nucleotide binding activity, a nucleotide hydrolyzing activity, or a nucleotide-dependent RNA binding activity of the polypeptide. A candidate agent that inhibits NS4B polypeptide binding to a nucleotide is an anti-viral agent, e.g., an anti-HCV agent. The invention also features a polynucleotide encoding a NS4B polypeptide having a modified NBM (e.g., which is impaired in NTP binding). The subject methods and compositions find use in a variety of therapeutic and screening applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Anti-viral compounds, compositions, and methods of use
Disclosed are compounds and compositions of Formula (I), pharmaceutically acceptable salts and solvates thereof, and their preparation and uses for treating viral infections mediated at least in part by a virus in the Flaviviridae family of viruses.
Owner:SMITHKLINE BECKMAN CORP
Inhibitors of Flaviviridae viruses
Provided are compounds of Formula I: and pharmaceutically acceptable salts and esters thereof. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections.
Owner:GILEAD SCI INC
Novel viral replication inhibitors
ActiveUS20160297810A1Promote efficient proliferationOrganic chemistryAntiviralsRNA Virus InfectionsMedicine
The present invention relates to a series of novel compounds, methods to prevent or treat viral infections in animals by using the novel compounds and to said novel compounds for use as a medicine, more preferably for use as a medicine to treat or prevent viral infections, particularly infections with RNA viruses, more particularly infections with viruses belonging to the family of the Flaviviridae, and yet more particularly infections with the Dengue virus. The present invention furthermore relates to pharmaceutical compositions or combination preparations of the novel compounds, to the compositions or preparations for use as a medicine, more preferably for the prevention or treatment of viral infections. The invention also relates to processes for preparation of the compounds.
Owner:KATHOLIEKE UNIV LEUVEN
Inhibitors of flaviviridae viruses
Provided are compounds of Formula I:and pharmaceutically acceptable salts and esters thereof. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections.
Owner:GILEAD SCI INC
Iminosugar compounds with antiflavirus activity
An anti-viral compounds effective against viruses belonging to the Flaviviridae family, wherein the anti-viral compounds are 1,5-dideoxy-1,5-imino-D-glucitol derivative compounds having the general formula (I)wherein R2, R3, R4 and R5 are the same or different and are selected from the group consisting of hydrogen, acyl, benzyl, alkyl, aryl, sulfonyl, phosphonyl, silyl, R6 is at least one of alkyl or branched alkyl, heteroalkyl or aryl, R6′ is a bridging group selected from at least one of bicycle[2.2.1]heptyl, bicycle[3.2.1]octyl, oxa analogs, admonyl and cubyl, n′=2-10, n″=1-10, enantiomers and stereoisomers of said compounds and physiologically acceptable salts or solvates of said compounds, enantiomer or stereoisomer.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
PYRIDO(3,2-d)PYRIMIDINES USEFUL FOR TREATING VIRAL INFECTIONS
ActiveUS20090324543A1Significant HCV replication inhibiting activityDesirable propertyBiocideOrganic chemistryFamily FlaviviridaePyrimidine
2-amino-pyrido(3,2-d)pyrimidine derivatives with a specific substitution pattern on positions 4 and 6 of the core structure are useful in the treatment or prevention of an infection due to a virus from the Flaviviridae family, especially HCV, when administered to a patient in a therapeutically effective amount.
Owner:GILEAD SCI INC
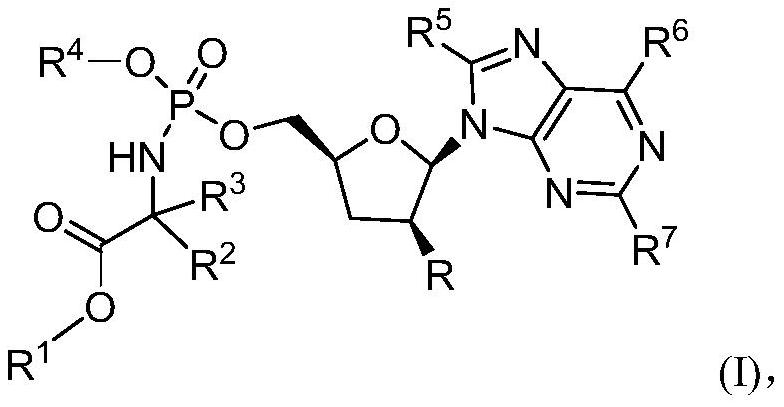
Substituted Purine Nucleosides, Phosphoramidate and Phosphordiamidate Derivatives for Treatment if Viral Infections
This invention is directed to compounds of Formula (I) having the structure that are useful in the treatment of viral infections in mammals, particularly in humans, mediated, at least in part, by a virus in the Flaviviridae family of viruses.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
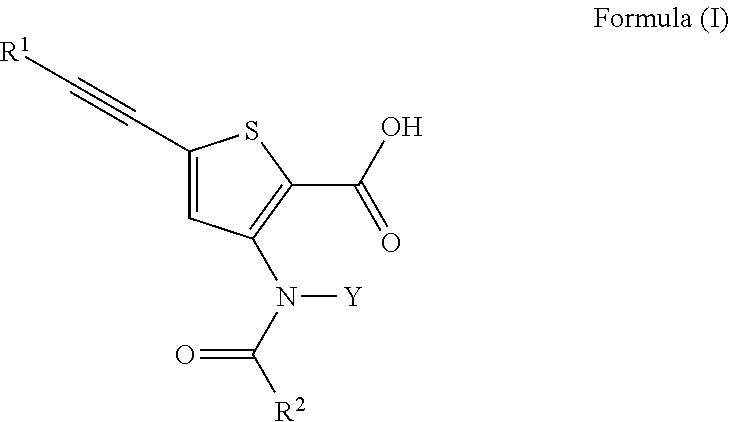
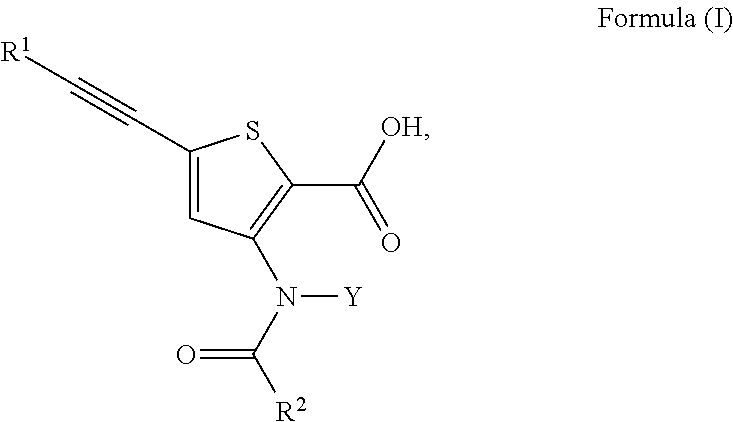
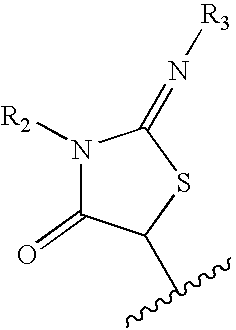
N-(5-membered aromatic ring)-amido anti-viral compounds
Disclosed are compounds having Formula (I) and the compositions and methods thereof for treating or preventing a viral infection mediated at least in part by a virus in the Flaviviridae family of viruses, wherein A, R<2>, m, R, V, W, T, Z, R<1>, Y and p are disclosed herein.
Owner:SMITHKLINE BECKMAN CORP
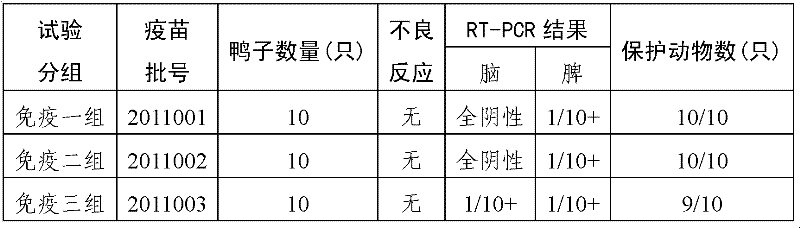
Duck BYD virus inactivated vaccine and preparation method thereof
InactiveCN102533669AImproving immunogenicityGood immune effectViral antigen ingredientsMicroorganism based processesNtaya virusesFamily Flaviviridae
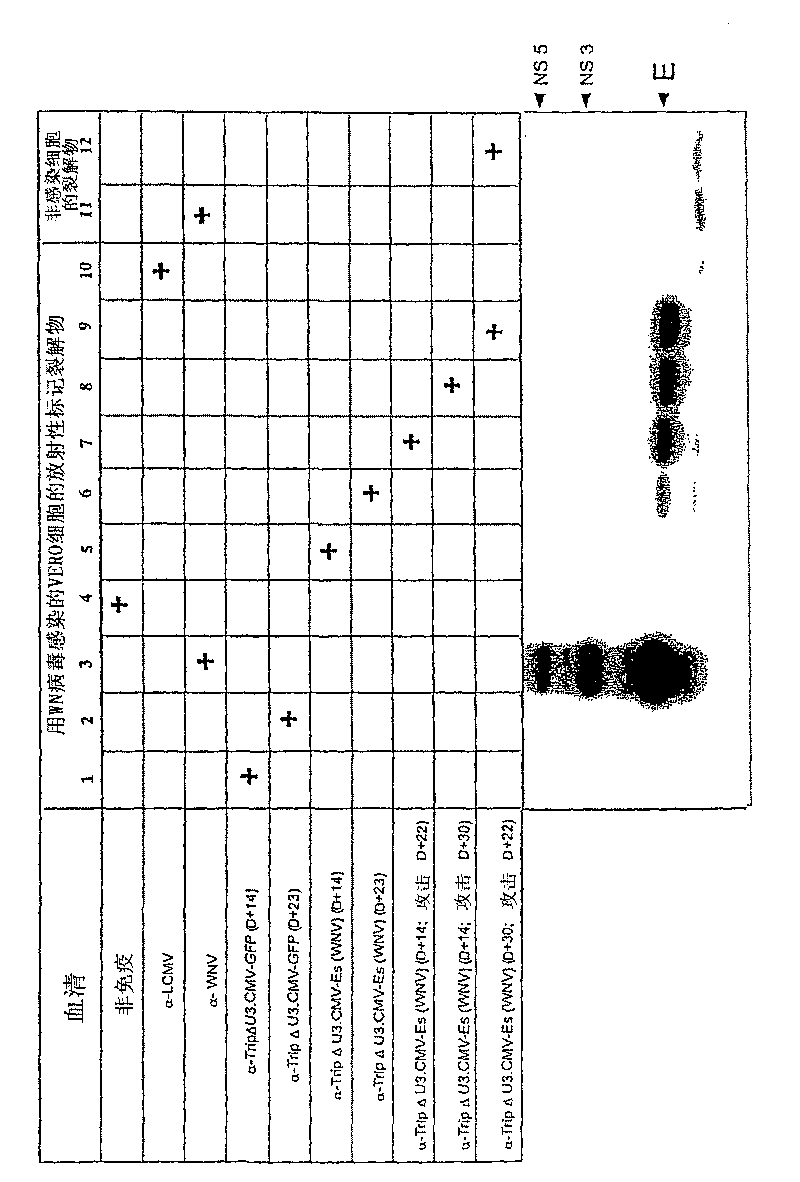
The invention provides a vaccine for preventing duck egg reduction syndrome caused by BYD virus and a preparation method thereof. The invention provides a duck BYD virus (Family Flaviviridae, Genus Flavivirus, Ntaya virus group, Duck BYD virus) JXSP strain, and the preservation number is CGMCC No.5266. The virulent strain provided by the invention is duck BYD virus virulent strain with excellent immunogenicity. The virulent strain is vaccinated onto a sensitive cell to obtain cell sap, which is emulsified after being inactivated so as to obtain the safe, effective and controllable duck BYD virus inactivated vaccine, so that the prevention and the control of the duck egg reduction syndrome can be favored.
Owner:CHINA AGRI UNIV
Inhibitors of flaviviridae viruses
Provided are compounds of Formula I:and pharmaceutically acceptable salts and esters thereof. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections.
Owner:GILEAD SCI INC
Treatments for Flaviviridae virus infection
The present invention provides methods for treating infections, in a host, by viruses belonging to the Flaviviridae family, such as HCV, comprising administering an Ara-C homologue to the host.
Owner:MERCK SHARP & DOHME LLC
Seven-membered ring nucleosides
InactiveUS20070185063A1Prevent and retard progressionInhibition of replicationBiocideSugar derivativesRNAViral replication
The present invention provides nucleoside analogue compounds that treat a host infected with a Flaviviridae virus infection, or other viruses that exhibit RNA-dependent RNA viral replication, compositions comprising these compounds and methods of using the compounds for the treatment and / or prophylaxis of viral infection, especially hepatitis C, in an infected host.
Owner:INDENIX PHARM LLC +1
Heterocyclic flaviviridae virus inhibitors
InactiveUS20130171102A1Improve oral bioavailabilityPromote hydrolysisBiocideOrganic chemistryFamily FlaviviridaeStereochemistry
Owner:K U LEUVEN RES & DEV
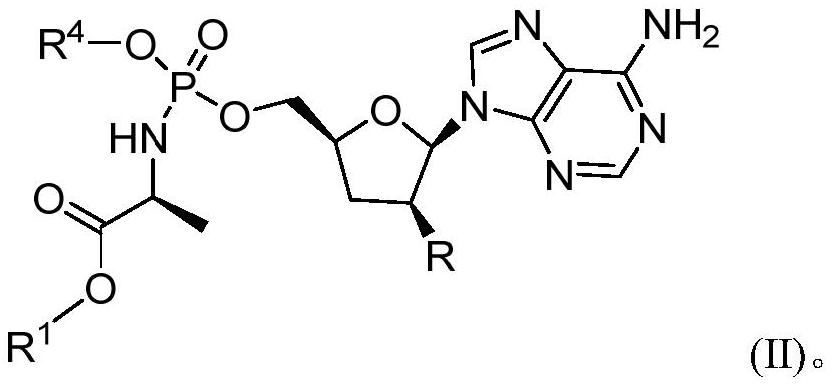
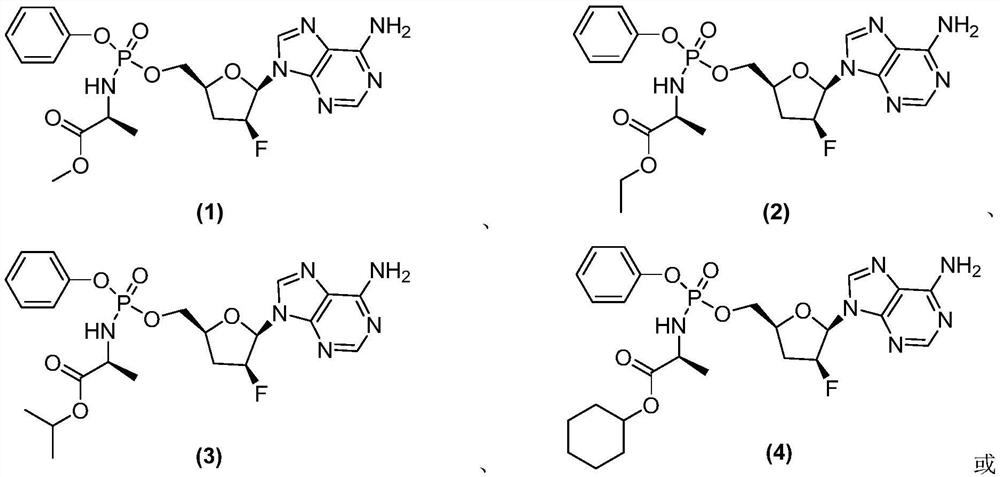
Phosphoramidate derivative of nucleoside compound and application thereof
ActiveCN112062800AInhibition of DiffusionExcellent anti-2019-nCoV activityOrganic active ingredientsSugar derivativesEnterovirusFamily Flaviviridae
The invention belongs to the technical field of medicines, and relates to a phosphoramidate derivative of a nucleoside compound and application thereof, and a pharmaceutical composition containing thecompound. The phosphoramidate derivative can be used as an antiviral reagent, especially an anti-SARS-CoV-2 reagent. The invention also relates to a method for preparing the compound and the pharmaceutical composition, and application of the compound and the pharmaceutical composition in preventing or treating viral infection, including but not limited to flaviviridae viral infection, filamentousvirology viral infection, enterovirus viral infection, orthomyxoviridae viral infection, paramyxoviridae viral infection and coronavirus viral infection, especially SARS-CoV-2 infection.
Owner:SUNSHINE LAKE PHARM CO LTD
Immortalized avian cell lines and use thereof
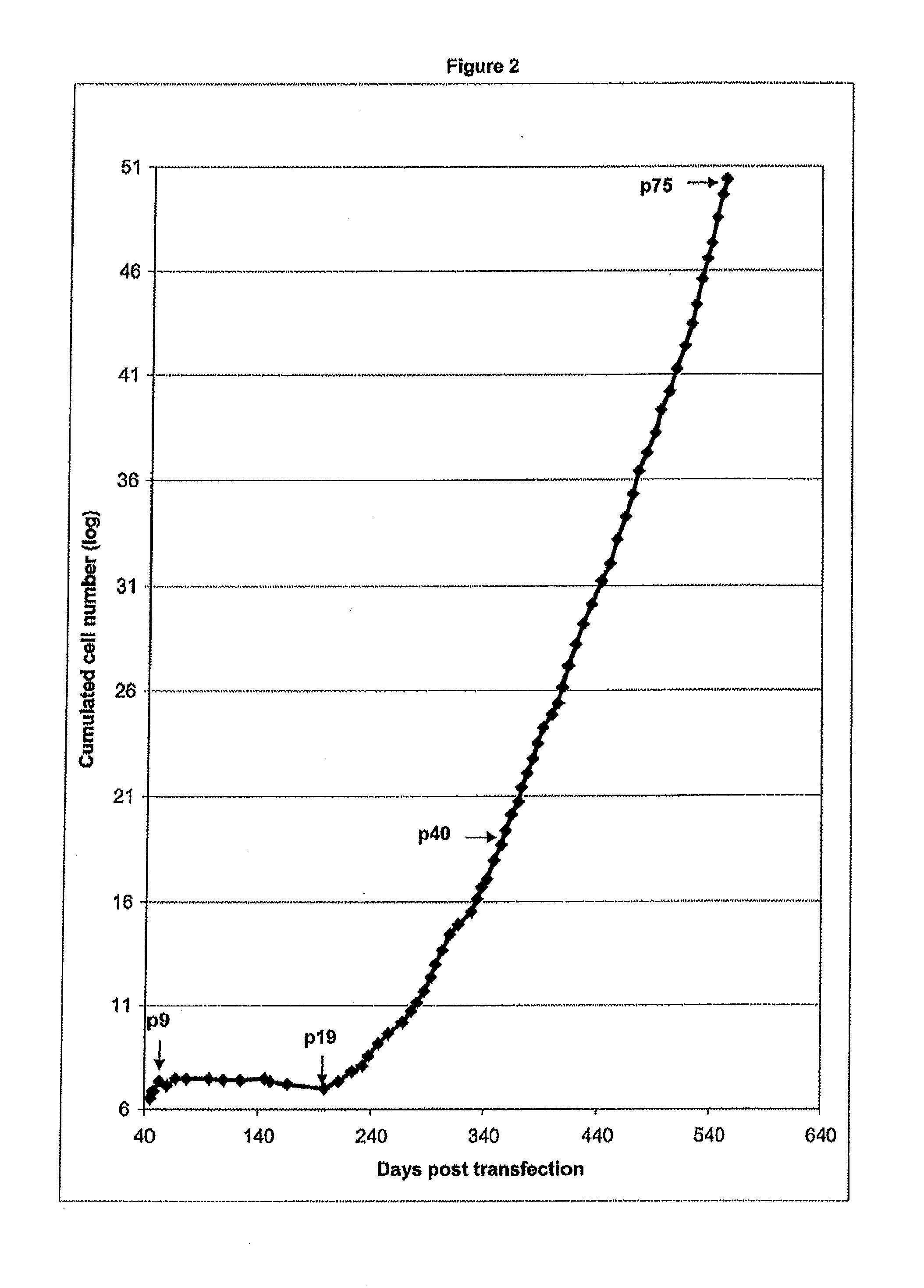
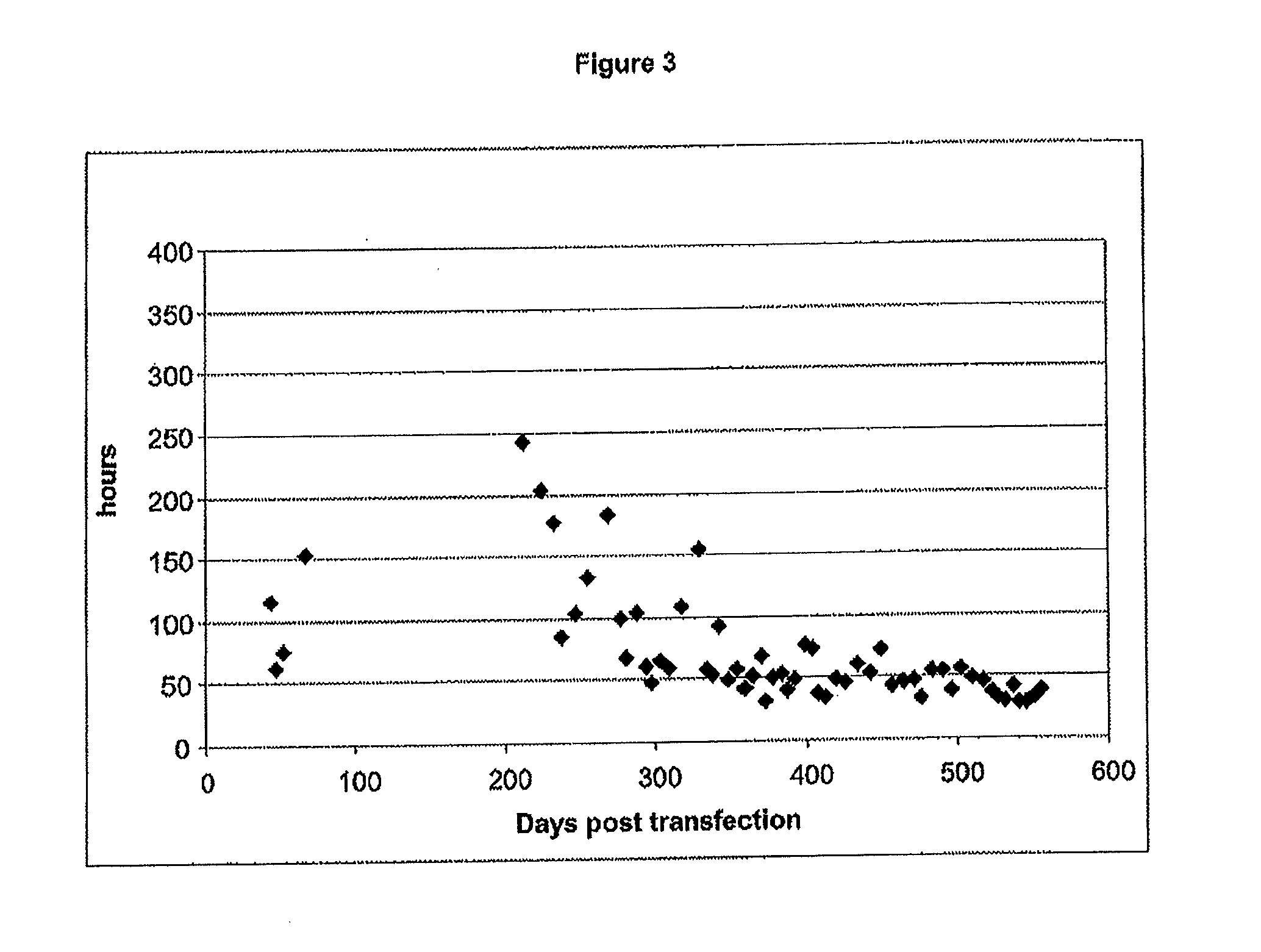
InactiveUS20110212488A1Easy maintenanceImprove stabilityAntibacterial agentsNervous disorderFlaviviridaeVaccinia
The present invention relates to specific immortalized avian cell lines expressing telomerase reverse transcriptase (TERT), and exhibiting distinct biologics production patterns. More particularly, the present invention relates to immortalized avian cell line capable of either amplifying Flaviviridae but not capable of amplifying Vaccinia virus strain Copenhagen (W—COP) nor Modified Vaccinia virus Ankara (MVA), or capable of amplifying both Flaviviridae and Poxyiridae. The invention further relates to the use of said immortalized avian cell lines and related methods for producing biologics, including viruses and proteins.
Owner:TRANSGENE SA
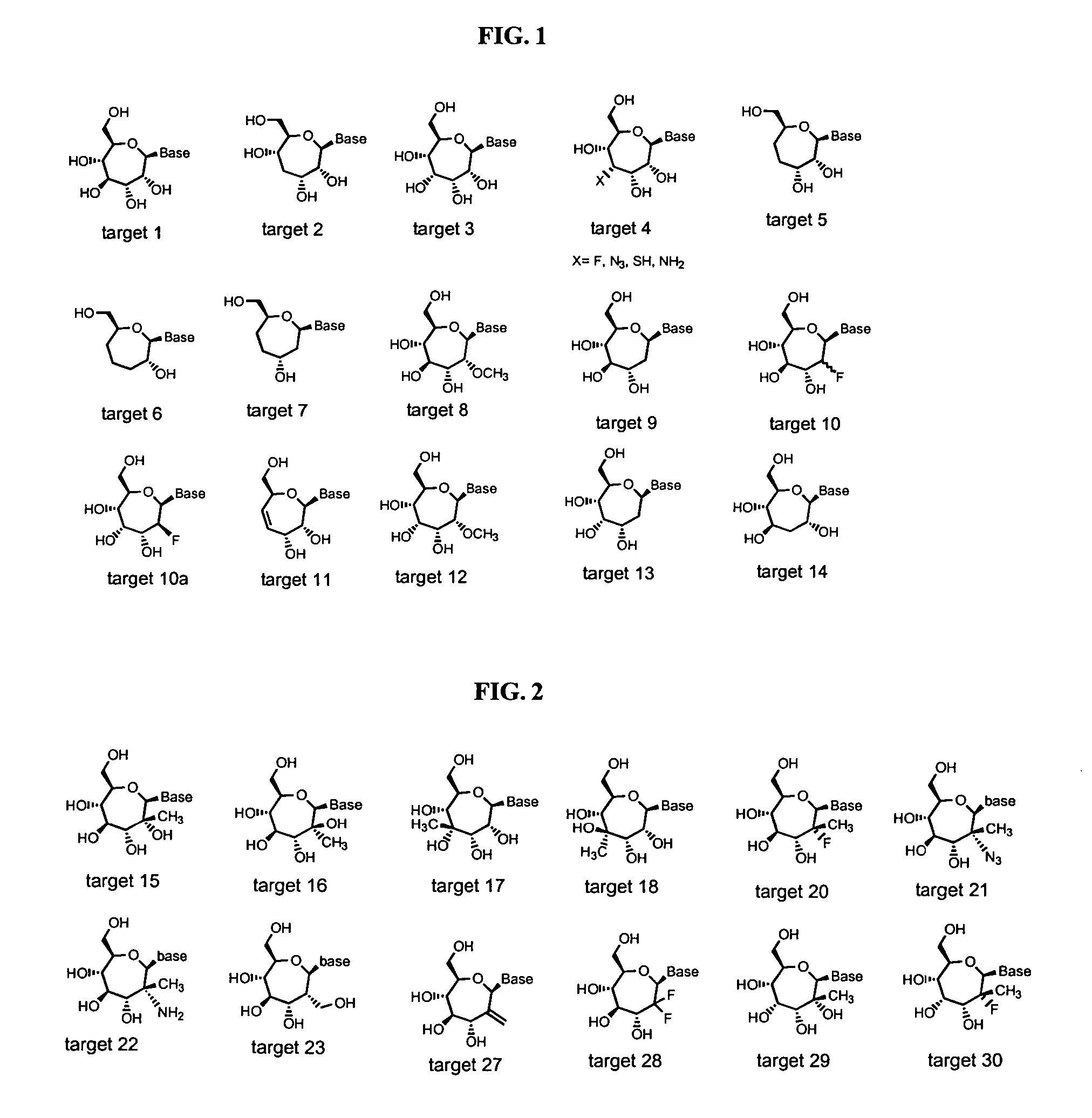
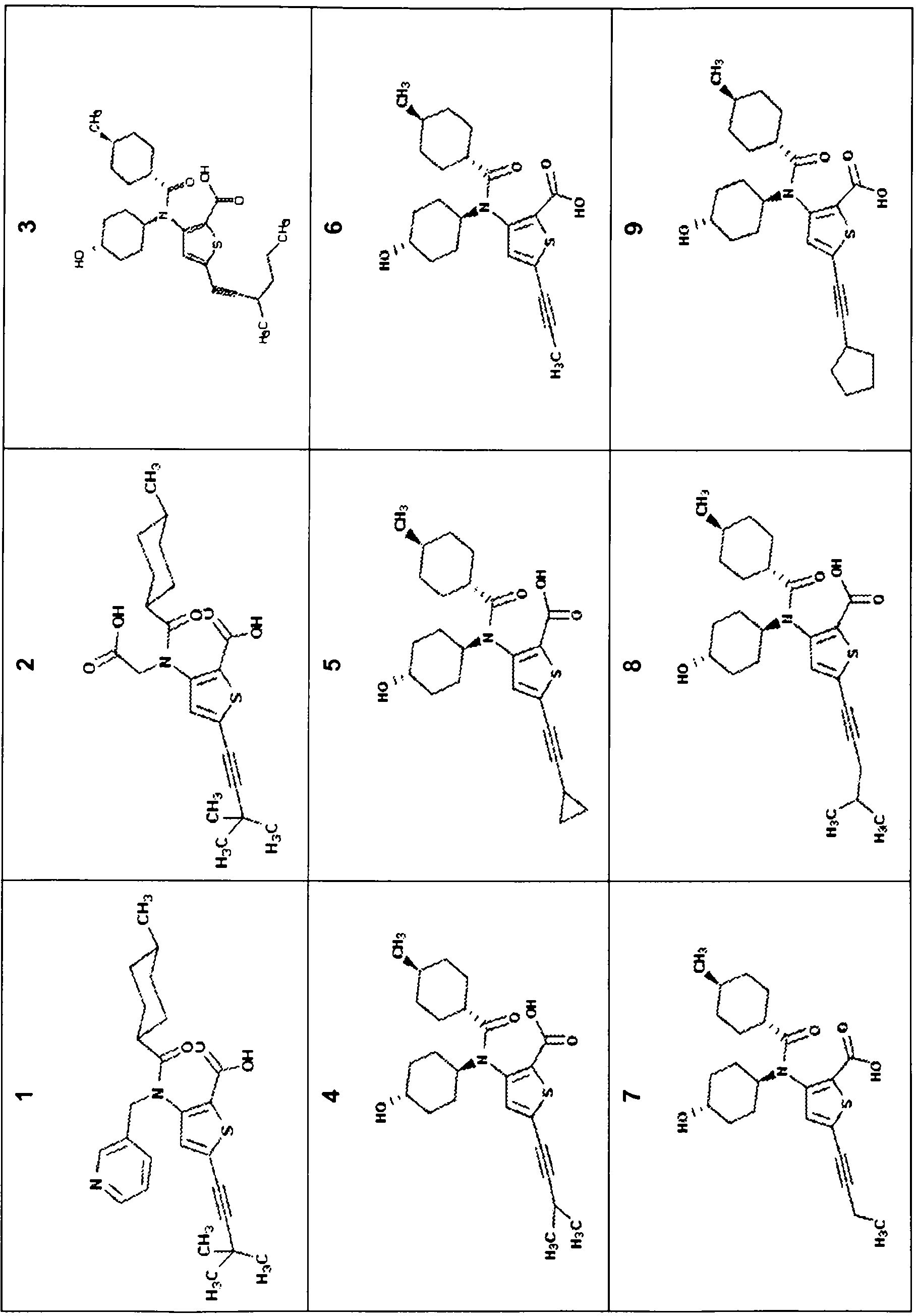
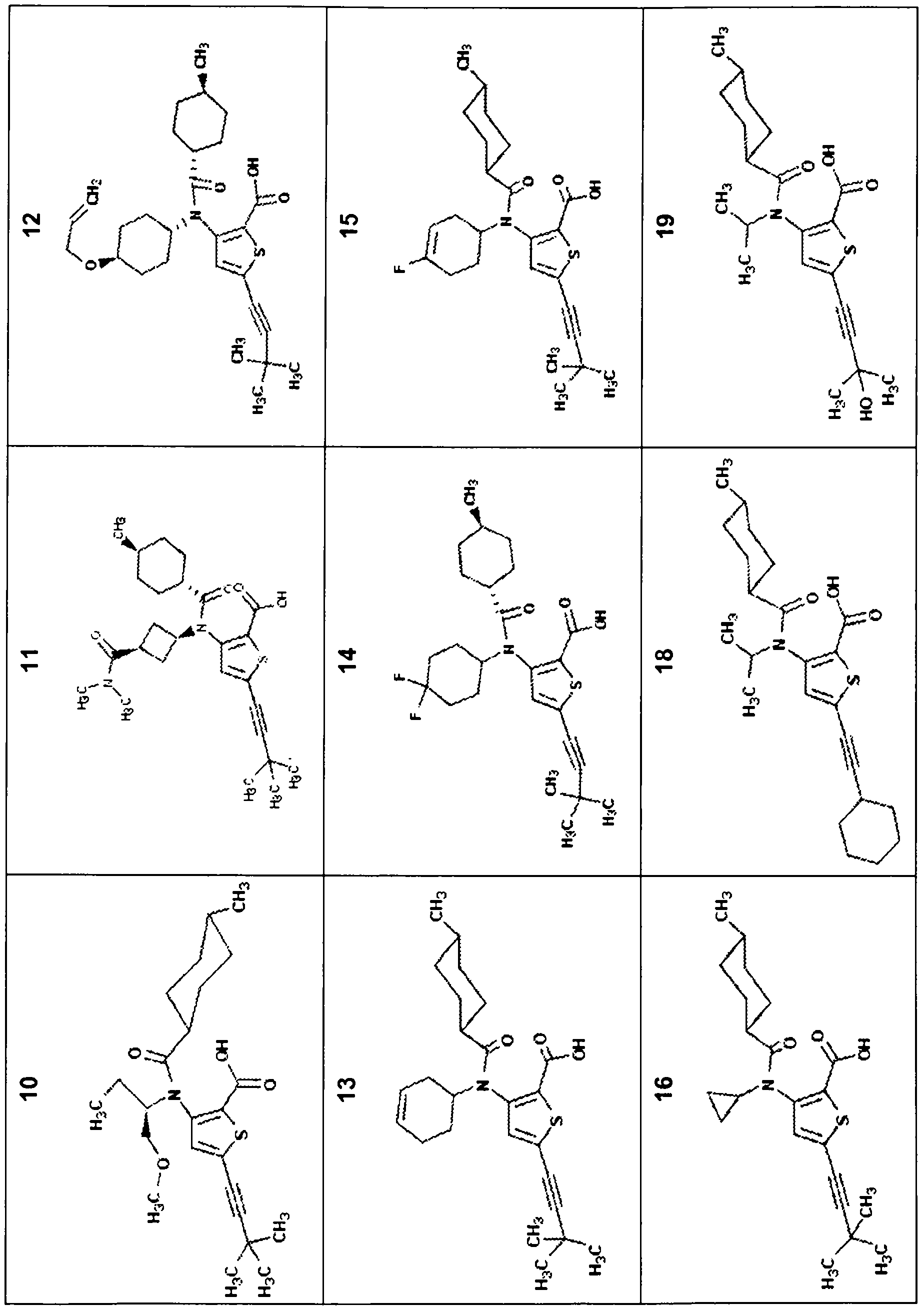
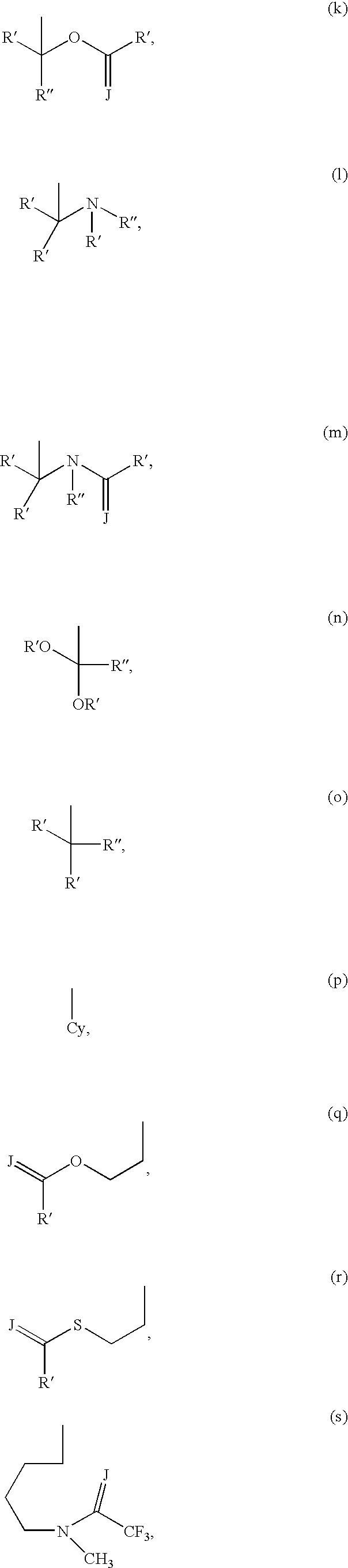
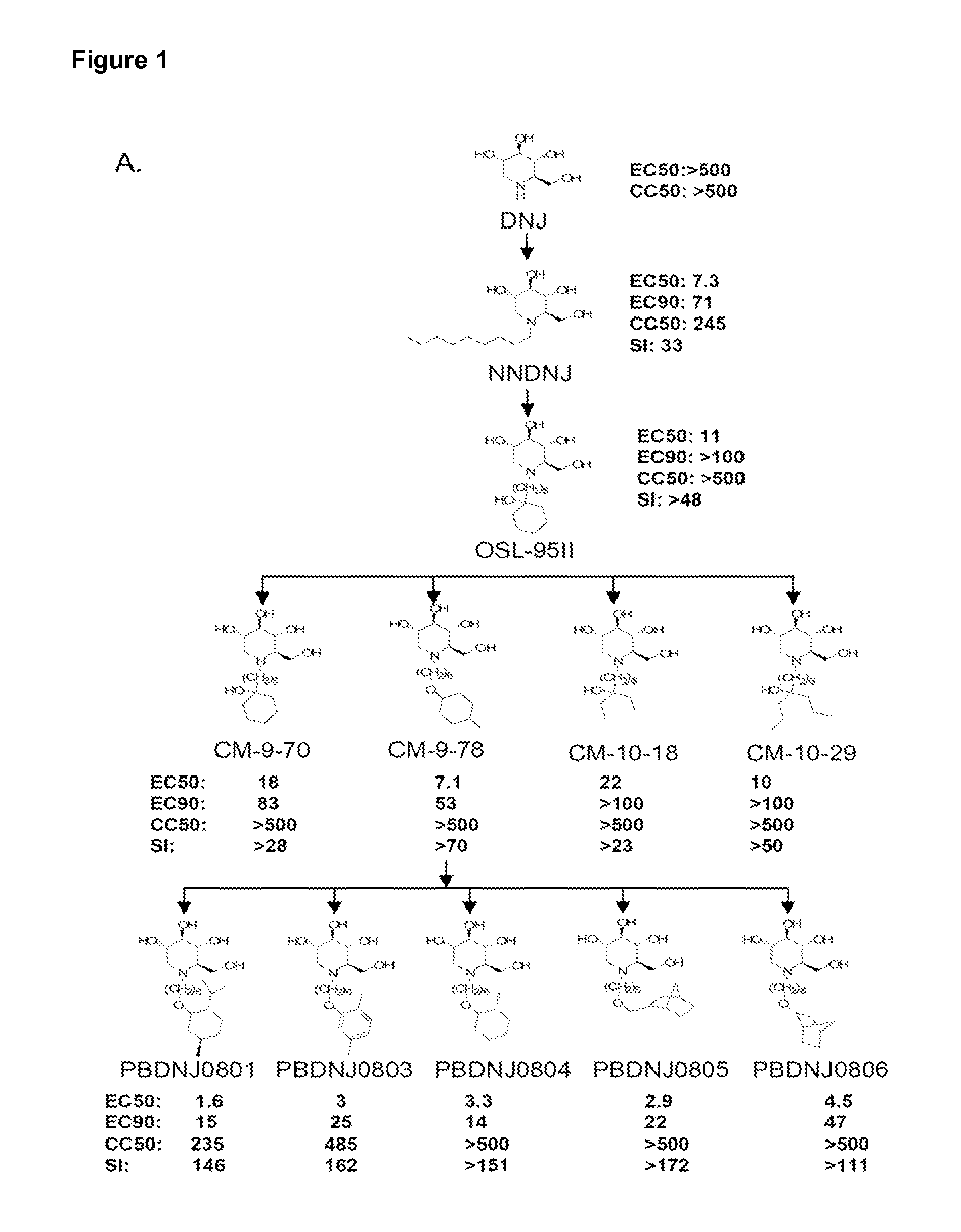
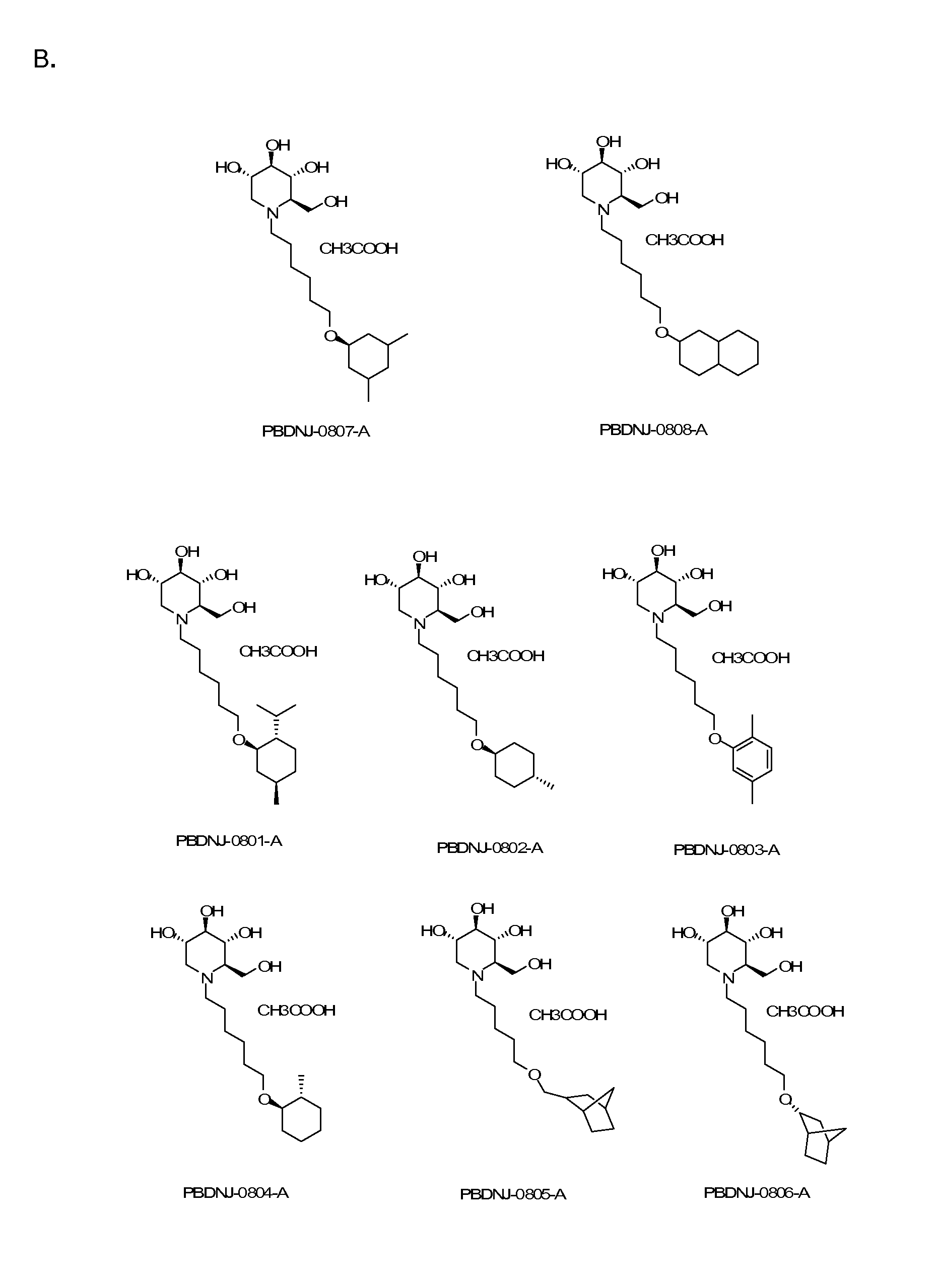
Compounds and methods for the treatment or prevention of flaviviridae viral infections
A compound is selected from the structural formulae depicted in FIG. 1 or a pharmaceutically acceptable salt thereof. A pharmaceutical composition comprises a compound selected from the structural formulae depicted in FIG. 1 or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier of excipient. A method of treating a HCV infection in a subject comprises administering to the subject a therapeutically effective amount of selected from the structural formulae depicted in FIG. 1 or a pharmaceutically acceptable salt thereof. A method of inhibiting or reducing the activity of HCV polymerase in a subject or in a biological in vitro sample comprises administering to the subject or to the sample a therapeutically effective amount of selected from the structural formulae depicted in FIG. 1 or a pharmaceutically acceptable salt thereof.
Owner:VERTEX PHARMA INC
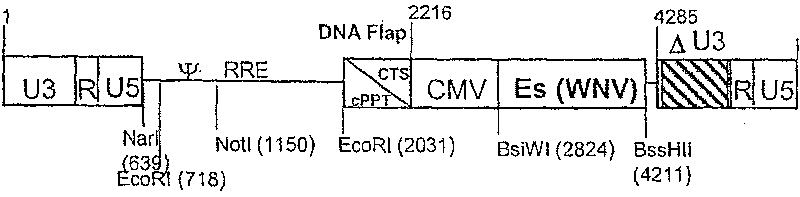
Recombinant lentiviral vector for expression of a flaviviridae protein and applications thereof as a vaccine
ActiveCN1981043BImproving immunogenicityFree from repeated administrationSsRNA viruses positive-senseGenetic material ingredientsNucleotideFamily Flaviviridae
Use of a recombinant lentiviral vector comprising a polynucleotide fragment encoding at least one protein of a virus of the family Flaviviridae or an immunogenic peptide of at least 8 amino acids of said protein, for preparing a pharmaceutical composition intended for the prevention and / or the treatment of a Flaviviridae infection in a sensitive species.
Owner:INST PASTEUR +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00002.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00003.png)
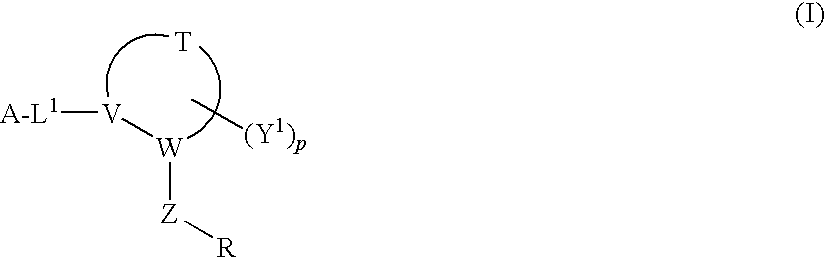
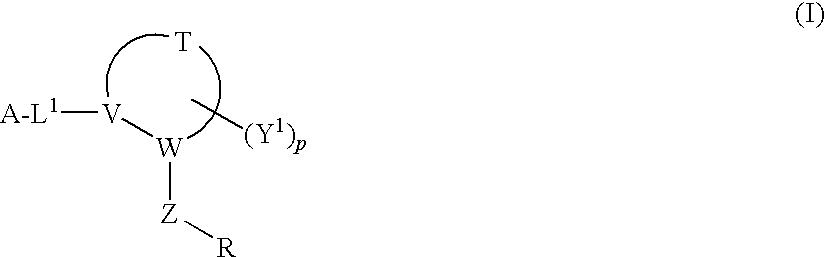
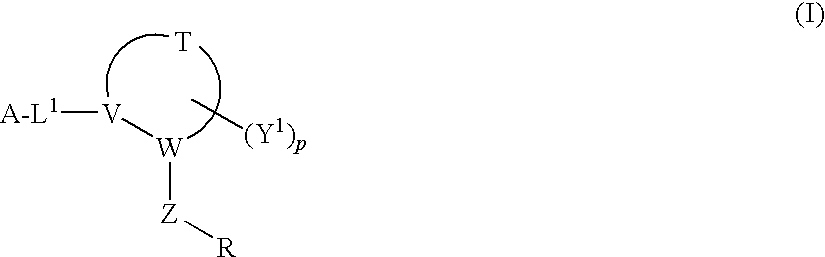
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00000.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-C00001.png)