Compounds and methods for the treatment or prevention of flaviviridae viral infections
A compound and composition technology, applied in the field of ER and D, can solve problems such as hindering therapy compliance, failing to provide sustained viral response, and reducing doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] Embodiment 1: the synthesis of compound of the present invention
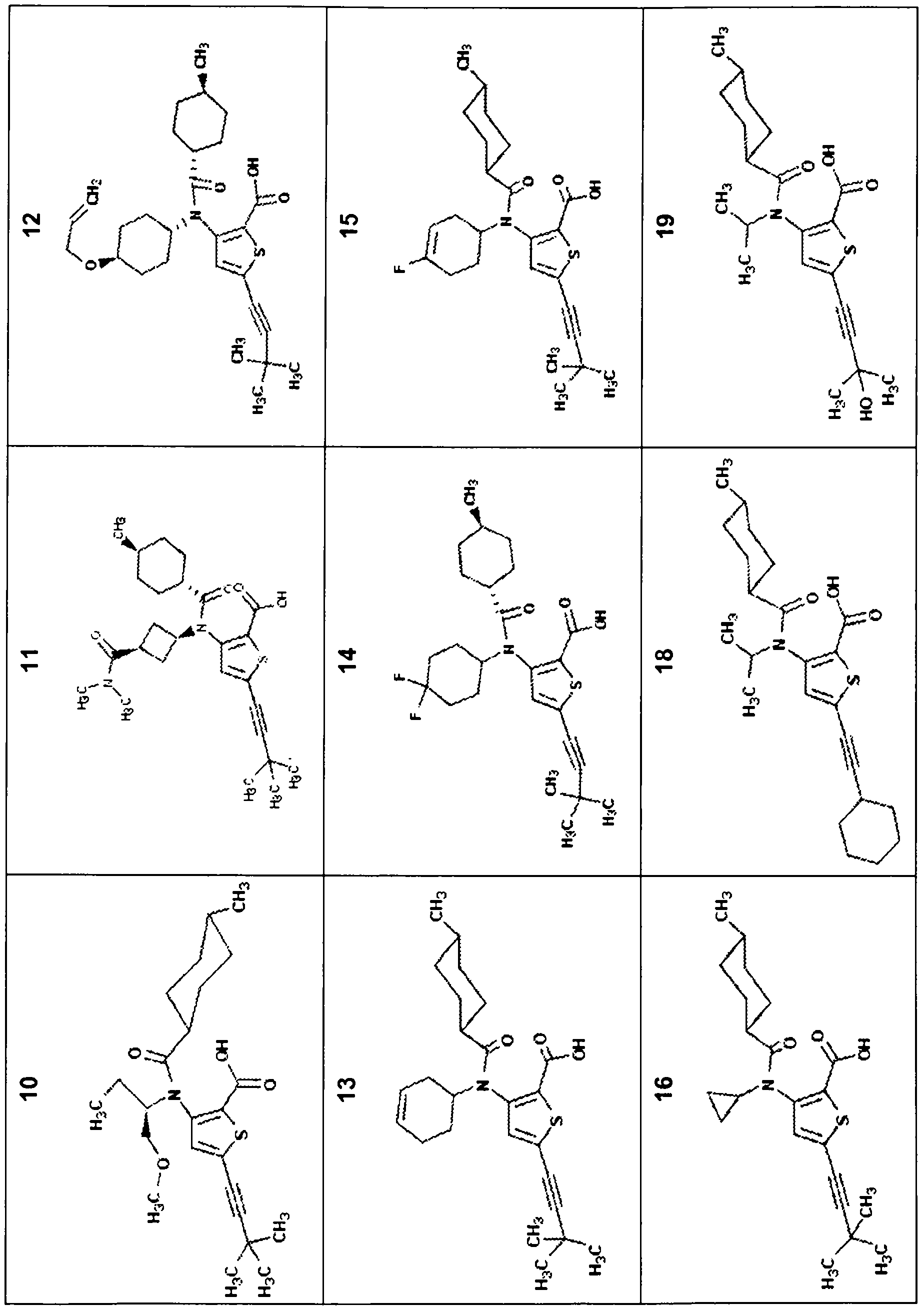
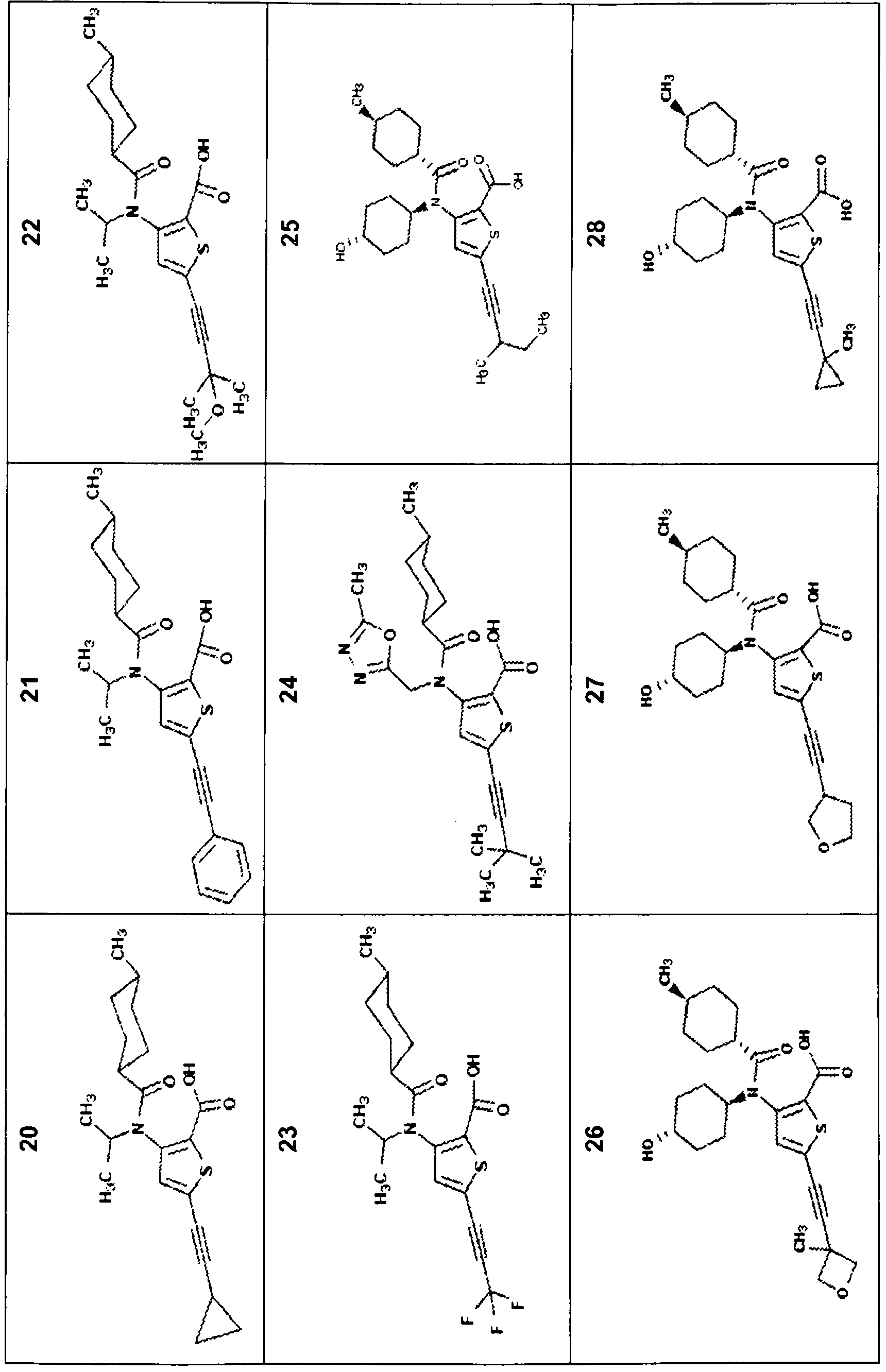
[0209] Compounds of the invention described herein may be prepared by any suitable method known in the art, eg US6,881,741, US2005 / 0009804, US2006 / 0276533, WO2002 / 100851 and WO08 / 58393. The preparation of some typical compounds is described in detail below. The synthesis of some typical compounds of the invention is described below. In general, the compounds of the invention may be prepared by synthetic methods as those optionally with any desired suitable modifications.
[0210] A. General Analytical Methods
[0211] As used herein, the term RT(min) refers to the LCMS retention time in minutes associated with a compound. Unless otherwise indicated, the method used to obtain the reported retention times is as follows:
[0212] Column: YMC-Pack Pro C18, 50mm×4.6mm id
[0213] Gradient: 10-95% methanol / H 2 O. Flow rate: 1.5ml / min. UV-vis detection.
[0214] B. General Analytical and Synthetic Meth...
Embodiment 1B
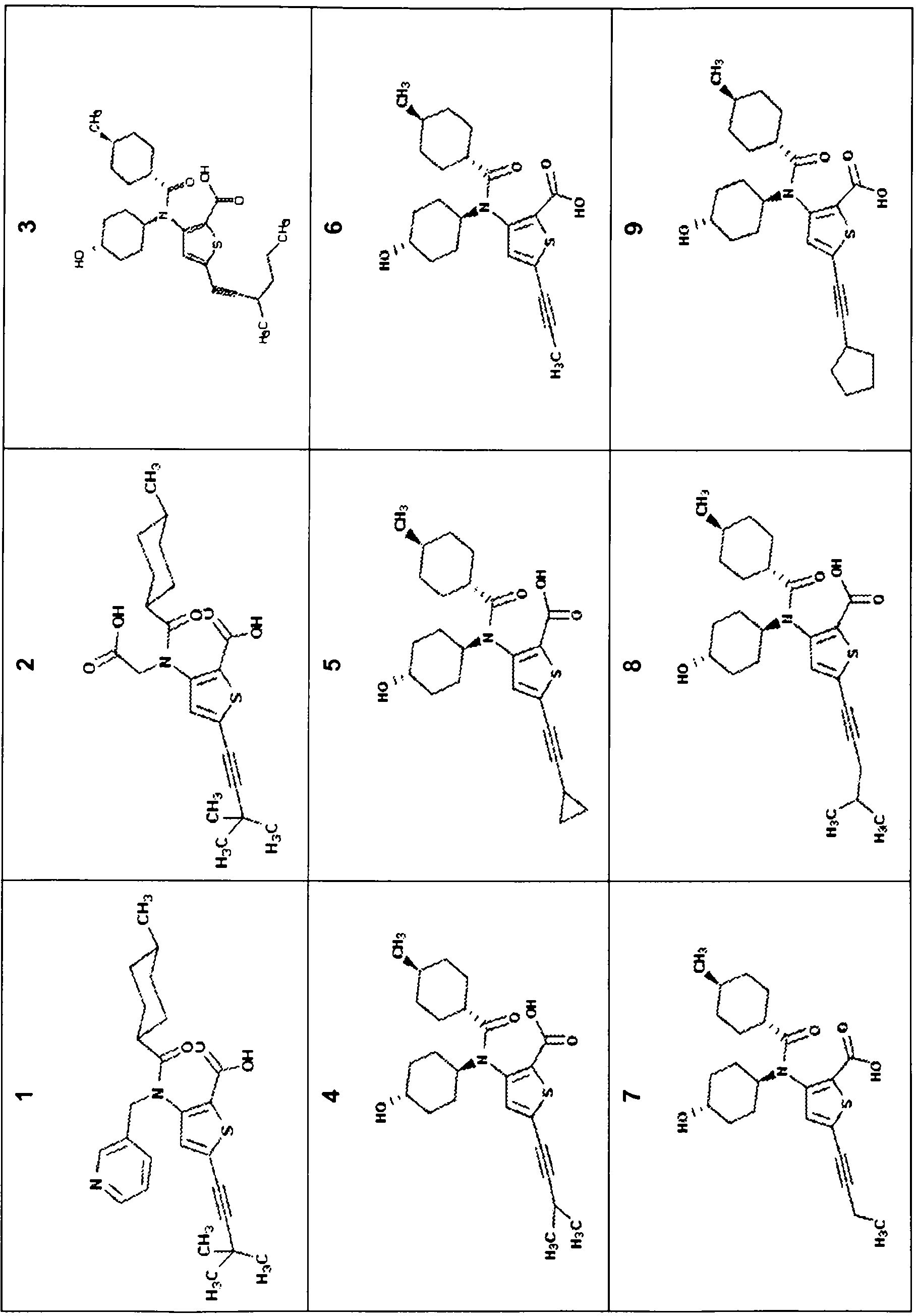
[0395] Preparation of compound 3
[0396] Compound 3 was prepared by the general method in the general scheme below.
[0397] MS: m / z (obs.): 460.6 [M+H] + ;Rt=6.05min
[0398] 1 H NMR (300MHz,MeOD)δ6.99(s,1H),4.39(dd,J=15.9,7.6Hz,1H),2.75(dd,J=13.4,6.7Hz,1H),2.05-1.84(m, 4H),1.56(ddd,J=18.4,12.9,10.4Hz,10H),1.32(ddd,J=14.5,11.3,4.8Hz,8H),1.13-0.85(m,5H),0.76(t,J= 21.8Hz, 5H).
[0399] general plan
[0400]
[0401] step 1
[0402] To 5-iodo-3-[(1,4-dioxaspiro[4.5]dec-8-yl)-(4-trans-methylcyclohexanecarbonyl)amino]thiophene-2-carboxylic acid methyl ester ( 1 mmol) in DMF (10-20 mL) was added Et 3 N(1mmol),CuI(0.1-0.25mol%),tris(dibenzylideneacetone)dipalladium(0)(Pd 2 (dba) 3 ) (0.01-0.05mol%) and 2-substituted but-1-yne (1mmol). The mixture was heated at 60 °C overnight, then diluted with ethyl acetate, washed with water and brine, dried (Na 2 SO 4 ), and then concentrate. The product was purified by silica gel chromatography (10-90% EtOAc in hexanes) to affo...
Embodiment 2
[0977] Embodiment 2: HCV replicon test
[0978] A. Principle
[0979] The following method describes the HCV replicon assay using the Huh7 hepatoma cell line (genotype 1b) (hereinafter referred to as cell line ET) containing a highly cell culture-adapted replicon. ET cells contain a highly cell culture-adapted replicon I 389 luc-ubi-neo / NS3-3' / 5.1 construct, which carries an intact copy of the firefly luciferase gene in addition to the neomycin gene (Krieger, N; Lohmann, V; Bartenschlager, R. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 2001, 75, 4614-4624). A replicon cell line W11.8 containing the HCV1a genotype was also used. These two cell lines (genotype 1b and 1a) were able to measure RNA replication and translation by measuring luciferase activity (for genotype 1b) or by measuring NS5A levels (for genotype 1a) using an ELISA assay. It has been demonstrated that luciferase activity closely follows replicon RNA levels ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com