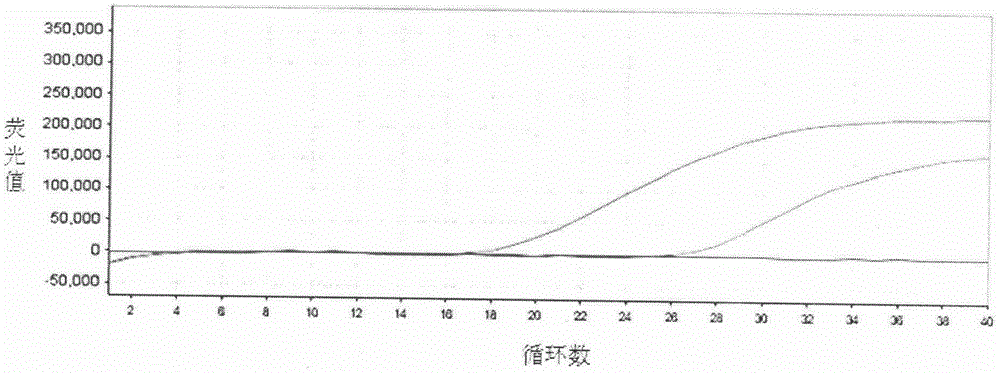
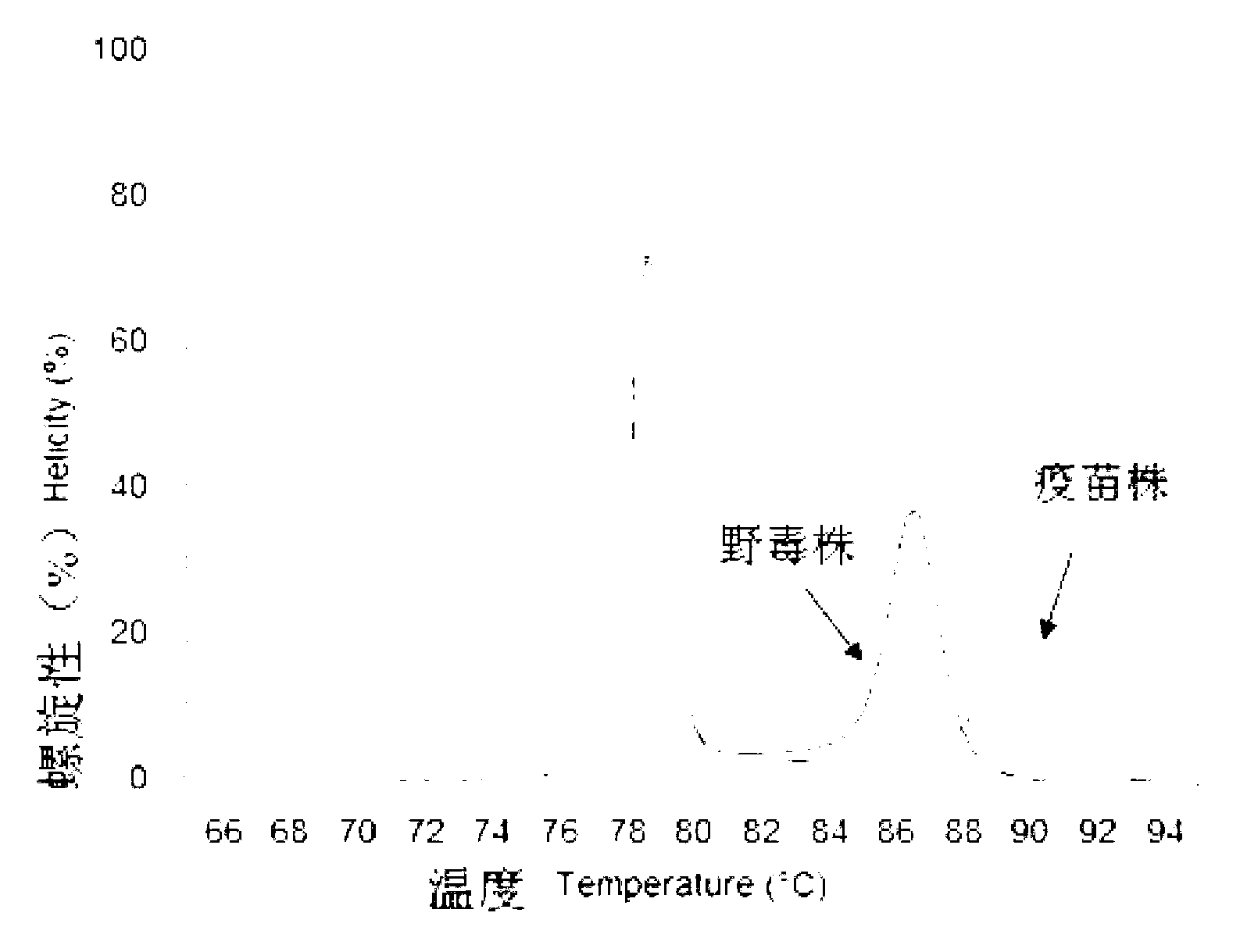
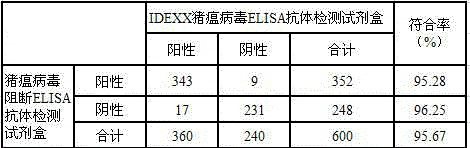
Patents
Literature
169 results about "Hog cholera virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multiple real time fluorescence quantifying PCR method for detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and classical swine fever virus
InactiveCN101260442ALow costImprove application stabilityMicrobiological testing/measurementClassical swine fever virus CSFVRegression analysis
The invention discloses a multiplex real-time fluorescent quantitation PCR method capable of detecting porcine circovirus, porcine parvovirus, porcine pseudorabies virus and hog cholera virus at the same time. The method of the invention detects that the four virus are all in good linear relations at the same time, all ten times serial dilution points of constructed normal plasmid are all in one straight line, CT value and copy number are in good linear relation, and regression analysis shows that the related coefficient of the CT value and the copy number is R<2> more than 0.99.The multiplex real-time fluorescent quantitation PCR method has the advantages of excellent specificity, sensibility and stability, which can rapidly, sensitively and differentially detect the four virus with serious harm to the economy and can be used for early diagnosis of virus infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
Method for preparing live vaccines of hog cholera and product thereof
InactiveCN101879311ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsVaccine ProductionFreeze-drying
The invention discloses a method for preparing live vaccines of hog cholera and a product thereof. The preparation method comprises the following steps of: (1) culturing porcine passage cell lines; (2) inoculating the porcine passage cell lines with live vaccine production seed viruses of the hog cholera to obtain attenuated vaccine strains of the hog cholera; (3) performing virus multiplication on the attenuated vaccine strains of the hog cholera; (4) measuring the virus titer of multiplication virus suspension by adopting an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the virus suspension which is detected to be qualified for vaccine matching and freeze-drying. The preparation method has the advantages of producing the live vaccines of the hog cholera by using the cell lines so as to achieve small quality differences among batches and the characteristics of simple and stable process, easy operation, high yield, low cost, the feasibility and extendibility of industrial production and the like, and measuring the virus titer of the multiplication virus suspension by adopting the immunofluorescence method so as to achieve sensitive, fast, specific and accurate detection, high repeatability and reliable results. The live vaccines of the hog cholera prepared by the method can completely protect pigs from the attacks of violent hog cholera viruses.
Owner:武华
African hog cholera virus fluorescent quantitative PCR detecting reagent and preparation and use thereof
InactiveCN101463396AFast detection methodSensitive highMicrobiological testing/measurementLower limitFluorescence
The invention discloses a fluorescence quantitative PCR detection reagent for African swine fever virus, and a preparation method and the application thereof. A set of specific primers and Taqman probes are designed and synthesized to be used for detecting ASFV P54 in relevant porcine products. A standard curve drawn in the invention provides a standard for the quantitative detection of ASFV P54. The invention establishes a fast and simple real-time fluorescence quantitative PCR detection system with strong specificity and high flexibility. The detection time is only several hours, and the detection lower limit can be 15 copies. The invention can be applied to the diagnosis and quarantine technology towards the imported relevant porcine products at port, and the invention provides reliable and effective technical condition for the import quarantine work of the country without ASF.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Reagent, detection method and application for detection of African hog cholera virus
InactiveCN109735657AShort detection timeRapid prevention and controlMicrobiological testing/measurementMicroorganism based processesAgricultural scienceRecombinase Polymerase Amplification
The invention discloses a reagent, detection method and application for the detection of African hog cholera virus. The reagent comprises a specific primer pair and a probe, upstream and downstream primers of the primer pair are respectively sequences shown in Seq ID No. 1 and 2, and the probe is a sequence shown in Seq ID No. 3 or its reverse complement sequence; in the probe sequence shown in the SEQ ID No.3, a 28th base modifies fluorescent quenching group-dT, a 31st base is replaced with a base analog, a 33rd base modifies a fluorescent group-dT, and a 3' end modifies C3 Spacer. The reagent is sensitive, specific and efficient for detection of the African hog cholera virus by recombinase polymerase amplification; compared with conventional conventional or real-time fluorescent PCR, thereagent and the detection method have short detection time and simple operation, are especially suitable for on-site testing, and are of great significance for the rapid prevention and control of theAfrican hog cholera virus and guarantee of production safety.
Owner:SHENZHEN AUDAQUE DATA TECH
Preparation method and application of electrochemical immunosensor based on HS-beta-CD-Ag-GOD conjugate
ActiveCN104459124ACatalytic reductionDoubly magnifiedBiological material analysisMaterial analysis by electric/magnetic meansImmune profilingBovine serum albumin
The invention belongs to the technical fields of immunoassay and biosensing and discloses a preparation method and application of an electrochemical immunosensor based on an HS-beta-CD-Ag-GOD conjugate. The electrochemical immunosensor is used for rapidly detecting a hog cholera virus antigen CSFV. The manufacturing scheme comprises the following steps: modifying a working electrode a bare platinum carbon electrode by using MWCNTs-CD-Fc-Ab1, and sequentially adding bovine serum albumin, hog cholera virus antigen CSFV and an Ab2-HS-beta-CD-Ag-GOD conjugate. The HS-beta-CD-Ag-GOD conjugate can convert glucose into gluconic acid, two protons and two electrons are transferred to oxygen molecules, hydrogen peroxide is generated, and an HS-beta-CD-Ag nanometer material serves as a mimic enzyme, the reduction of the hydrogen peroxide is catalyzed, the dual amplification of an electrochemical signal is realized, the high sensitivity is realized, and the detection limit can be low to 0.33pg / mL.
Owner:UNIV OF JINAN
RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN105567871AShorten test timeLow reaction temperatureMicrobiological testing/measurementMicroorganism based processesBiologyDifferential diagnosis
The invention discloses an RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting a high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof. The kit comprises a pair of primers and a probe, the sequences of the primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe is shown as SEQ ID NO.3. It is proved through experiments that the kit can detect adverse effects of the high-pathogenicity porcine reproductive and respiratory syndrome virus (HP-PRRSV), a hog cholera virus, a C-type porcine reproductive and respiratory syndrome virus, a porcine circovirus type II, a porcine pseudorabies virus and a foot and mouth disease virus in a specificity mode. It is proved through experiments that the kit can detect out templates of at least 70 copies at the temperature of 40 DEG C on the condition of 20 min amplification, and the conformity between the kit and RT-qPCR is high. This shows that the kit can detect HP-PRRSV fast, efficiently and sensitively and provides an effective technological means for differential diagnosis of HP-PRRSV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Fluorescent quantitative PCR rapid diagnosis reagent box for specific detection of classical swine fever virus wild virus infection
InactiveCN101328506ASolve the problem of false positives that cannot be ruled out for immunization vaccinesStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceNucleic acid detectionSpecific detection
The invention relates to a fluorescence quantitative PCR rapid diagnosis kit for specifically detecting the Hog cholera virus and wild virus infection and an application method thereof, belonging to the virus nucleic acid detection field. The fluorescence quantitative PCR rapid diagnosis kit is applied to the rapid quantitative detection of the CSFV wild virus clinically and in scientific research and can remove the false positive caused by the CSFV HCLV vaccine immunization. Compared with the prior art, the fluorescence quantitative PCR rapid diagnosis kit has the advantages that: the Hog cholera virus and wild virus infection and the vaccine immunization can be verified and diagnosed, the problem of the incapability of removing the false positive caused by the vaccine immunization is solved; the specificity is good, the high specificity hybridization double control of the primer high specificity amplification and the fluorescence probe is realized, the accuracy is high, the false positive is low; the sensitivity is high; the detection speed is high, only one hour is needed and two to three hours are needed when the extraction process of the nucleic acid and the preparation process of the cDNA are added; and the post treatment is not necessary, the processes such as hybridization, electrophoresis and photo are not necessary and no pollution is caused.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Method for constructing hog-cholera virus infectious cDNA carrier having molecule mark
ActiveCN101864445AFermentationVector-based foreign material introductionTotal rnaTechnological system
The invention relates to a virus antibody research and provides a method for constructing a hog-cholera virus infectious cDNA carrier having a molecule mark. The method comprises the following steps of: (1) diluting a hog-cholera live vaccine to 1 portion per milliliter as the experimental material, after extracting the head RNA, amplifying 6 corresponding cDNA fragments through RT-PCR section according to designed 6 pairs of primers, (2) leading in the corresponding molecule marks from F1 and F5 of cDNA fragments, (3) reforming plasmids required by overall length cDNA cloning, and (4) constructing the overall length cDNA carrier. A hog-cholera virus lapinized vaccine C stem infectious cDNA carrier pA-FL22 having a molecule mark can be acquired through the method of the invention. The permissive cells of RNA electro transferred hog-cholera virus acquired by the carrier can save the infectious progeny virus which can stably inherit the molecule mark led in the construction. By using the saving technique system of the infectious cDNA carrier and the progeny virus, the copy stem and the pathopoiesia reason of the virus can be deeply researched and the foundation for developing new marked vaccines can be settled.
Owner:ZHEJIANG UNIV
Method for producing antigen protein in use for hog cholera vaccine
A process for preparing the antigen protein used for hog cholera vaccine includes extracting hog cholera virus RNA, reverse transcription to obtain the immune gene E2 of hog cholera virus, using E2 as template for PCR while inserting them to expression carrier of Bichia yeast, introducing the recombinant expression carrier to Bichia yeast, screening the recombinant Bichia yeast, discriminating its expression product testing its immunoactivity, determining the chosen recombinant yeast, testing and analyzing its culture condition, choosing the optimal culture condition, culturing the recombinant yeast and preparing the antigen protein of said vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Heat-resistant freeze-drying protecting agent for vaccine and preparing method
InactiveCN102641504AHigh degree of aging resistanceSave solutionPowder deliveryAntiviralsFreeze-dryingDistilled water
The invention relates to a heat-resistant freeze-drying protecting agent for a vaccine and a preparing method and particularly relates to a heat-resistant freeze-drying protecting agent for a hog cholera vaccine, which is formed by mixing and proportioning the following substances according to weight percent: 1 to 2 percent of sodium carboxymethylcellulose, 4 to 6 percent of xylo-oligosaccharide,1 to 2 percent of glutamine, 0.2 to 0.8 percent of sodium selenite and the balance distilled water. The preparing method of the heat-resistant freeze-drying protecting agent for the hog cholera vaccine comprises the following steps of: (1) mixing the sodium carboxymethylcellulose and the distilled water as A liquid; (2) mixing the xylo-oligosaccharide and the distilled water as B liquid; (3) mixing the glutamine, the sodium selenite and the distilled water as C liquid; and (4) mixing the A liquid, the B liquid and the C liquid according to 1:1:1 (V / V) as the heat-resistant freeze-drying protecting agent. According to the heat-resistant freeze-drying protecting agent for the hog cholera live vaccine, which is designed by the invention, the activity of viruses can be kept in the freeze-drying process and the preserving process, and the reduction of the activity of a modified-live vaccine for hog cholera viruses is relieved.
Owner:青岛爱博生物科技有限公司
Test paper for identifying and detecting virulent strain and low virulent strain of hog cholera virus
The invention relates to livestock epidemic disease infection and immune identification and detection instruments, and in particular relates to a piece of test paper for identifying and detecting a virulent strain and a low virulent strain of hog cholera viruses. The test paper consists of a support plate, a sample pad, a gold mark pad, a detection membrane and a water absorbing pad, wherein a virulent infection detection line T1 '|', a low virulent infection detection line T2 '|' and a quality control line C '|' are arranged on the detection membrane. When the test paper is used, three red strips '|||' mean virulent virus infection of hog cholera viruses, two red strips '||' mean vaccine immunity of hog cholera viruses, and one red strip '|' means hog cholera viruse negative. The test paper is high in specificity, high in sensitivity, wide in reaction spectrum and simple, convenient and rapid to operate, can be used for detecting conventional low virulent vaccine strains and multiple epidemic virulent strains, can be widely used for identifying and detecting hog cholera virus infection and immune, and can be easily popularized and applied in production practice.
Owner:HENAN ACAD OF AGRI SCI
Monoclone antibody and application thereof
ActiveCN105837686AImprove accuracyMake up for single preventionImmunoglobulins against virusesAntiviralsHeavy chainSwine Fever Virus
The invention provides a monoclone antibody and application thereof. The monoclone antibody comprises a heavy chain as shown in SEQ ID No. 2 and a light chain as shown in SEQ ID No. 4. An enzyme-linked immunosorbent assay (ELISA) antibody detection kit (a blocking method) prepared with the monoclone antibody is good in sensitivity and high in specificity, and pigs infected with hog cholera virus can be detected more accurately. An ELISA antibody detection kit (a competition method) prepared with the monoclone antibody is short in detection time and can be used for quickly preliminarily screening pigs infected with the hog cholera virus and support further purification against the hog cholera virus. The monoclone antibody further has neutralizing activity and can further be used for preparing medicines for preventing and / or treating hog cholera relevant diseases.
Owner:LUOYANG PULIKE WANTAI BIOTECH
BAS-ELISA kit for detecting hog cholera virus Erns and E2 antigen
ActiveCN103760365AHigh sensitivityImprove stabilityBiological material analysisBiological testingBiotin-streptavidin complexElisa kit
The invention discloses a BAS-ELISA kit for detecting hog cholera virus Erns and E2 antigen, and belongs to the field of biological technologies and diagnosis and research of animal-borne diseases. The kit comprises an ELISA plate coated with a CSFV-Erns monoclonal antibody and a resistant-CSFV-E2 monoclonal antibody, a sample diluent, CSFV positive control and CSFV negative control, a scrubbing solution, a biotinylation rabbit-resistant CSFV-Erns polyclonal antibody and a biotinylation rabbit-resistant CSFV-E2 polyclonal antibody mixed solution, ELISA streptavidin, an enzyme developing substrate and a stop solution, wherein the two monoclonal antibodies and corresponding polyclonal antibodies have different epitopes. The kit adopts a biotin-avidin detecting system, and implements the combined detection for the hog cholera virus Erns and E2 protein, so that the sensitivity, specificity and repeatability in the detection of the hog cholera virus are greatly improved; the kit can be applied to the diagnosis and research of the hog cholera.
Owner:WUHAN CHOPPER BIOLOGY
Hog cholera virus truncated E2 protein and application of same
ActiveCN108107217APreserve antigenicityReduce manufacturing costBiological testingBovine Viral Diarrhea VirusesBiology
The invention discloses a hog cholera virus truncated E2 protein which is designed on the basis of protein spatial structure, and an application of the same. In the invention, according to the crystalstructure of bovine viral diarrhea virus E2 protein, the spatial structure of the hog cholera virus E2 protein is simulated, and then the hog cholera virus E2 protein is subjected to truncated expression, wherein the amino acid sequence of the truncated protein E2B / C / D / A is represented as the SEQ ID No.1. The truncated protein can maintain the complete antigenicity of the E2 protein, and has no cross reaction with a bovine viral diarrhea virus antibody. The invention further constructs a CHO cell line which stably expresses the truncated protein E2B / C / D / A and is assigned the accession numberof CGMCC No.14722. The invention also discloses an indirect ELISA kit which is used for detection of a hog cholera virus antibody, wherein the enveloped antigen is the hog cholera virus truncated protein E2B / C / D / A. The kit is used for specifically detecting the hog cholera virus antibody with high specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for identifying wild strain and vaccine strain of hog cholera virus
ActiveCN103320535AStrong specificityPromote amplificationMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVTGE VACCINE
The invention discloses a method for identifying a wild strain and a vaccine strain of a hog cholera virus. According to the method, a primer specially used for identifying the wild strain and the vaccine strain of the hog cholera virus is designed in a conservative region shared by the wild strain and the vaccine strain. The primer is high in specificity and can well multiply the wild strain and the vaccine low virulent strain; according to a reverse transcription-polymerase chain reaction technology and a high resolution melting (HRM) technology, the wild strain and the vaccine strain can be obviously identified. The method is a simple, quick and practical novel technology for identifying the wild strain and the vaccine strain of the hog cholera virus.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Recombinant porcine adenovirus vector
InactiveUS7323177B1Good suitImprove protectionSsRNA viruses positive-senseViral antigen ingredientsDiseaseAntigen
Owner:VECTOGEN +2
Traditional Chinese medicine composition for improving immunity and resisting viruses for livestock and poultry, as well as preparation method and application thereof
InactiveCN106309464ASimple recipeEasy to administerAnthropod material medical ingredientsHydroxy compound active ingredientsDiseaseChlorogenic acid
The invention relates to a traditional Chinese medicine composition for improving immunity and resisting viruses for livestock and poultry, as well as a preparation method and application thereof. The composition is prepared from the following components in parts by weight: 0.5-3 parts of baicalin, 0.2-1.6 parts of oxymatrine, 0.1-1 part of chlorogenic acid, 0.5-2 parts of epigoitrin, 0.1-2 parts of berberine, 0.5-1.5 parts of gallic acid and 1.5-5 parts of astragalus polysaccharide. The traditional Chinese medicine composition is used for directly or indirectly killing viruses by the active components of natural herbs, can get rid of antibiotics in the feeding process, and has excellent effects for treating and preventing chicken new castle disease viruses, hog cholera viruses and the like.
Owner:CHANGSHA BROAD OCEAN BIO SCI & TECHN CO LTD
Hog cholera virus inhibition ELISA antibody detection kit and application thereof
ActiveCN104483490AAchieve mass productionIncreased sensitivitySsRNA viruses positive-senseVirus peptidesPositive controlHorse radish peroxidase
The invention discloses a hog cholera virus inhibition ELISA antibody detection kit and application thereof. The kit comprises hog cholera virus E2 protein which is expressed by virtue of a baculovirus expression system and a cell suspension culture process; the hog cholera virus E2 protein is purified to serve as a coated plate antigen and an immunogen to prepare a monoclonal antibody of the hog cholera virus E2 protein, and horse radish peroxidase (a hybridoma cell strain 4A7 CCTCC NO.C2014229) is marked. The kit further comprises an antigen coated plate, a positive control, a negative control, sample diluent, a scrubbing solution, a developing solution A, a developing solution B and a stop solution. According to the application of the hog cholera virus inhibition ELISA antibody detection kit in the detection of the hog cholera virus antibody, the kit is used for detecting other positive serums except hog cholera viruses, the cross reaction is avoided, the specificity is strong, the sensitivity is high, the detection time is short, and the repeatability is good; compared with an import kit, the detection coincidence rate reaches above 95%.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Prokaryotic expression protein of VP73 gene from African swine fever virus and preparation method thereof
InactiveCN102101889ANo risk of poisoningHigh detection sensitivityMicroorganism based processesPeptide preparation methodsAfrican swine fever virus AntibodySwine Fever Virus
The invention relates to a method for preparing a genetic engineering product, in particular to a prokaryotic expression protein of VP73 gene from African swine fever virus (ASFV) and a preparation method thereof. The preparation method comprises: artificially synthesizing the whole-length sequence of VP73 gene according to the sequence of the VP73 gene from ASFV in GenBank, constructing a recombinant expression vector pET32a-VP73, sequencing, verifying, transforming prokaryotic expression recipient bacteria E.coli BL21(DE3), and inducing expression by isopropyl-1-thio-beta-d-galactopyranoside (IPTG), wherein the molecular weight of the recombinant fusion protein is about 65KD. Protein purified by nickel column affinity chromatography can undergo a specific immune imprinting reaction with ASFV positive serum and avoid cross reaction with viruses such as swine fever virus, hog cholera virus, porcine circovirus, porcine reproductive and respiratory syndrome virus, swine influenza virus and pseudorabiesvirus. Experiments show the expressed protein has high detection sensitivity, and high specificity. When the antigen is used for detection, risk of spreading poison is avoided. And the antigen can be used as a detection antigen for use in an enzyme-linked immuno sorbent assay (ELISA) method for identifying an ASFV antibody.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Real-time fluorescent quantitative PCR primer, kit and detection method for detecting porcine circovirus 3
InactiveCN107338330AIncreased sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationCircovirusPorcine reproductive and respiratory syndrome virus
The invention belongs to the technical field of molecular biology, and particularly relates to a real-time fluorescent quantitative PCR primer, kit and detection method for detecting the porcine circovirus 3. The nucleotide sequence of the real-time fluorescent quantitative PCR primer for detecting the porcine circovirus 3 is shown in SEQ ID NO.1-2. The invention further provides the kit containing the primer mentioned above and the detection method for detecting the porcine circovirus 3. The primer, the kit and the detection method are high in specificity, high in sensitivity and stable in repeatability, wave crests of the melting curve of amplified products are single, no primer dimer is caused, and the primer and the kit have no cross reaction with genomes of the porcine circovirus 2, the hog cholera virus, the porcine pseudorabies virus and the porcine reproductive and respiratory syndrome virus; sensitivity is 102 copies per microliter and is 100 times higher than that of the conventional PCR; and the variation coefficients of inter-group and intra-group repeated tests are all smaller than 3.0%. The foundation is laid for research on molecular epidemiology, prevention and control of the pestilence.
Owner:SOUTH CHINA AGRI UNIV
ST cell lines for stably expressing T7 RNA polyase, constructing process and applications thereof
InactiveCN101285054AEnzymesVector-based foreign material introductionMicroorganism preservationFluorescence
The invention discloses a cell line ST / T7 capable of stably expressing T7RNA polymerase, the microorganism preservation number of which is: CGMCC No.24444. In the invention, a T7RNA polymerase gene is inserted into a eukaryotic expression vector pIRES2-EGFP to get the pIRES2-EGFP-T7 to transfect ST cells to obtain the cell line ST / T7 capable of stably expressing T7RNA polymerase. Through RT-PCR detection, expression plasmid containing a red fluorescent protein gene controlled by T7 promotors can be used to transfect the cell line so as to observe the expression of red fluorescent protein in a transcription product amplified form a ST / T7 cell to the T7RNA polymerase. The cell line ST / T7 can provide reverse T7RNA polymerase for use in reverse genetic manipulation of RNA-virus such as hog cholera virus, etc., and can be used as transcription and expression systems in vitro for research on genic structures and functions.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Ribonucleic acid interference (RNAi) for inhibiting porcine reproduction and respiratory syndrome virus replication and preparation method of RNAi
ActiveCN102660545AInhibit biological functionAnimal cellsInactivation/attenuationSlow virus infectionGreen monkey kidney
The invention discloses ribonucleic acid interference (RNAi) for inhibiting porcine reproductiion and respiratory syndrome virus (PRRSV) replication and a preparation method of RNAi, The RNAi comprises a small interfering RNA (siRNA) sequence. The preparation method comprises the steps of constructing a short hairpin RNA (shRNA) slow virus expression vector, preparing replication-defective slow virus, infecting slow virus Marc-145 cells (green monkey kidney cells) and the like. The invention also discloses a method for verifying the effect of inhibiting PRRSV from replication. The RNAi sequence has the obvious effect of inhibiting the PRRSV replication on sensitive cells. According to the invention, the exploration of RNA interference on in vitro and vivo replication of hog cholera virus is carried out, a slow-virtue-mediated stably-integrated RNA interfering technology for special conserved gene segments of a targeted hog cholera virus genome is constructed, and transgenic animals with the siRNA of targeted hog PRRSV are hopeful to construct. The necessary experimental data is accumulated for gene function research of RNAi applied to PRRSV and prevention and treatment of hog cholera, and early-stage preparation is provided for disease resistance breeding of animals.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
RT-LAMP nucleic acid test-strip kit for determining hog cholera virus and application
ActiveCN103602761ALow costReduce use costMicrobiological testing/measurementMicroorganism based processesHybridization probeBetaine
The invention discloses a RT-LAMP nucleic acid test-strip kit for determining hog cholera virus and application. The kit comprises a primer group with nucleotide sequences shown as SEQ ID NO. 1-6 and nucleic acid detection test strips. The application method of the kit comprises as follows: preparing a RT-LAMP reaction system which comprises AMV retrovirus, a 1* reaction buffer, strand displacement DNA polymerase, a dNTP mixture, betaine, MgSO4, a FIP primer, a BIP primer, a hybridization probe, a LoopB primer, a F3 primer, a B3 primer and RNA of a sample to be measured; and carrying out a reaction at a constant temperature, after testing the obtained products by using the nucleic acid detecting test strip, judging and reading directly: the result is positive when two red bands appear, and one band is in the detection zone while the other band is in the control zone. The kit has the advantages of simple operation, low cost, easy observation of the reaction result, good specificity and easy popularization and application in large scope.
Owner:广州易安生物技术有限公司
Preparation method of engineered vaccine based on CSF-FMD duplex gene
InactiveCN103933581APrevent and Control InfectionReduce economic lossGenetic material ingredientsAntiviralsGenetic engineeringAnimal body
The invention relates to a preparation method of an engineered vaccine based on a CSF-FMD duplex gene, and discloses research and application of a duplex nucleic acid vaccine based on hog cholera virus-O type hog foot-and-mouth disease virus. Main materials of the duplex nucleic acid vaccine based on hog cholera virus-O type hog foot-and-mouth disease virus are as follows: pcDNA3.1-CSFV-E2-FMDV-O-VP1 eukaryotic plasmids. The preparation method disclosed by the invention researches nucleic acid vaccines for CSFVE2 gene and the FMDV-OVP1, can stimulate an animal body to generate corresponding high-level antibody and show very strong specificity. For the hog infection and propagation caused by CSFV and FMDV-O, the E2 gene and the VP1 gene achieve prevention and control effect on two viruses, so that safe and effective effects can be achieved.
Owner:GUIZHOU UNIV
Primers and probe for detecting hog cholera virus based on a digital PCR technology, a kit and a method thereof
InactiveCN108950066AAccurate detectionThe result is accurateMicrobiological testing/measurementMicroorganism based processesRNA extractionPositive control
The invention discloses a pair of primers and a probe for detecting hog cholera virus based on a digital PCR technology, a kit and a method thereof. The primers and the probe include an upstream primer FP, a downstream primer BP and a probe. The detection kit includes a primer set, 2*RT-ddPCR Supermix, RNase-free distilled water, a viral total RNA extraction reagent, a negative control and a positive control. The detection method comprises the following steps: extracting a sample RNA to be tested, quantitatively detecting the sample RNA to be detected by the microdrop digital PCR technique, judging whether the sample to be tested contains hog cholera virus by the copy number obtained by the amplification analysis, and determining the content thereof. The invention has the advantages of accuracy, sensitivity and wide applicability, has no dependency on a standard curve, can realize absolute quantification, can achieve early detection, early treatment and early prevention of diagnosis ofswine fever, and can effectively control the outbreak of the epidemic.
Owner:JINAN UNIVERSITY
Enzyme-linked immuno sorbent assay (ELISA) kit created on the basis of hog cholera virus recombinant protein nopaline synthase (NS2)
The invention relates to an enzyme-linked immuno sorbent assay (ELISA) kit created on the basis of hog cholera virus recombinant protein nopaline synthase (NS2). The ELISA kit comprises an elisa plate wrapped by the hog cholera virus recombinant protein NS2, a ten-time concentration detergent, a serum diluent, a standard serum, an anti-swine Intravenous gamma globulin (IgG) enzyme-labeled antibody, an nzyme-labeled antibody diluent, a sealing agent, a color developing agent A, a color developing agent B and a stop solution. The ELISA kit can detect hog cholera virus antibodies. The method created by the ELISA kit has specificity, sensitivity and operability. In a sensitivity test, the coincidence rate between the method of the ELISA kit and an immunofluorescence technical method is 82%, and the method of the ELISA kit is more sensitive than the immunofluorescence technical method. The detection rate of other etiological agents through the method of the ELISA kit is zero. Compared with the hemagglutination inhibition (HI) method, the method of the ELISA kit has the advantages of being fast and low in cost, and having easily judging results, and can carry out detection work after hog cholera lapinized virus vaccine immunity.
Owner:ZHENGZHOU HOUYI PHARMA
Cell strain of monoclonal antibodies of E2 protein resisting hog cholera virus and application thereof
ActiveCN107058239AMicroorganism based processesImmunoglobulins against virusesElisa kitSerological assay
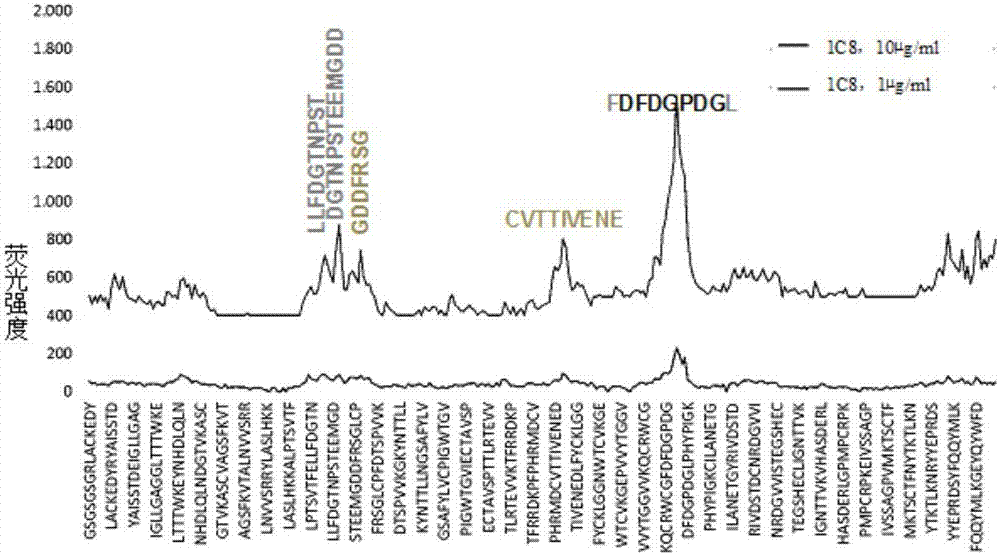
The invention relates to a cell strain of monoclonal antibodies of E2 protein resisting hog cholera virus and an application thereof. A hybridoma cell strain stably secreting the protein E2 resisting the hog cholera virus is obtained by screening, the monoclonal antibodies CSFV-1C8 secreted by the cell strain can generate specific reaction with the E2 protein of CSFV, and does not react with E2 protein of bovine viral diarrhoea / mucosal disease virus; and the monoclonal antibodies can recognize the E2 protein of the hog cholera virus specifically, and the epitope of recognition is FDFDGPDGL. The monoclonal antibodies and the epitope of the E2 protein of the hog cholera virus recognized by the monoclonal antibodies can be prepared into a reagent for detecting the CSFV, and thus laying foundation for establishing a serological detection method of antibodies of the hog cholera virus. A competitive ELISA kit for detecting the antibodies for the hog cholera and established by the invention has the advantages of specificity, sensitivity, simplicity and convenience in operation and the like and is suitable for large-scale screening of the antibodies for the hog cholera.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Method for preparing hogcholera vaccine
InactiveCN101797380ASolve efficiency problemsSolve pollutionMicroorganism based processesAntiviralsCulture fluidFreeze-drying
The invention discloses a method by adopting cell micro-carrier suspension culture system to prepare hogcholera (CSF) vaccine, which comprises the following steps that: (1) cells for preparing the vaccine is inoculated into a carrier tank containing culture liquid and micro-carriers, and the cells are uniformly mixed with the micro-carriers, so the cells are attached onto the micro-carriers; (2) when the quantity of the cells is increased to 5 to 40 times of the initial inoculation concentration, the hog cholera virus (HCLV) is inoculated onto the cells so as to breed the virus according to the virus multiple of infection (M.O.I) is of the ratio of 0.01 to 1; and (3) the prepared virus liquid is mixed, appropriate freezing drying protective agent is added, the virus liquid is quantitatively packed after being uniformly mixed with the freezing drying protective agent to be frozen and dried so as to obtain the hogcholera vaccine (CSF). The method which is adopted to produce the hogcholera vccine has the advantages that the concentration of the cultured cells is high, the cells can be continuously cultured, the virus yield is high, the immunity effect of the vaccine is high, the safety is good, and the like, and has complete immunity protection effect against the attack of the hog cholera virus.
Owner:PU LIKE BIO ENG
Indirect method of enzyme-linked immunosorbent assay for diagnosing antibody of hog cholera
The invention discloses the method for detecting antibody by using indirect enzyme-linked immunosorbent assay (ELISA) with recombinant protein being as antigen. The method includes following steps: in E2 gene of structural protein of hog cholera virus, a segment of gene of encoding antigen is connected to carrier of pGEM-T Easy plasmid so as to obtain recombinant plasmid; connecting purified recombinant plasmid to expression vector pPROEX-HTb, and shifting it in bacillus coli; culturing bacillus coli, expression carrier of the said segment of gene is expressed in bacillus coli; after picking up and denaturing the expressed protein by inclusion body, carrying out purifying, renaturing operations. Antibody in sample of hog blood serum is detected by using indirect ELISA under renatured fusion protein being as antigen. Advantages of the method are simple operation, easy of culturing and short production cycle.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com