Fluorescent quantitative PCR rapid diagnosis reagent box for specific detection of classical swine fever virus wild virus infection
A technology for swine fever virus and fluorescence quantification, which is applied in the direction of fluorescence/phosphorescence, biochemical equipment and methods, and microbial measurement/inspection. Vaccine immune differential diagnosis technology and other issues, to achieve the effect of fast detection, good specificity and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Composition and preparation of a fluorescent quantitative PCR rapid diagnostic kit for the specific detection of wild virus infection of classical swine fever virus
[0033] 1. Reagent composition:
[0034] Trizol RNA lysate is a product of Invitrogen; Taq enzyme (5U / μl), dNTPs (10mM), MgCl 2 (25mM), M-MLV enzyme (50U / μl) were purchased from Promega Company; PCR primer pair FCSFV and RCSFV, quantitative standard primers were synthesized by Shanghai Sangong Bioengineering Company; Taqman-MGB probe was purchased from Shanghai Jikang Bioengineering Co., Ltd. Synthesized by the company; Shimen standard strain of classical swine fever virus is preserved by our laboratory.
[0035] 2. Reagent preparation:
[0036] A) Reverse transcription reaction solution: 1×RT Buffer, dNTPs 0.5mM, M-MLV enzyme 50U, primer RCSFV 0.4μM; the sequence of primer RCSFV is 5′-TGCCCACAGTAGGACTAGCAAAC-3′23nt;
[0037] B) Fluorescence quantitative reaction solution: 1×PCR Buffer, MgCl 2 4mM, dNTP...
Embodiment 2
[0043] Application method of the fluorescence quantitative PCR rapid diagnostic kit for specific detection of classical swine fever virus wild virus infection
[0044] 1. Sample processing
[0045] A) The sample to be tested is a tissue sample: take 50-100 mg of the sample to be tested in a clean, sterilized and dried homogenizer, add PBS solution at a volume ratio of 1:5, fully homogenate, centrifuge at 4000 rpm for 10 minutes, and take 100 μl Transfer the supernatant (for each reaction) into a sterile 1.5ml centrifuge tube, numbered for later use;
[0046] B) The sample to be tested is a liquid sample (such as: whole blood, serum, nasal swab, etc.), directly take 100 μl and transfer it into a sterile 1.5ml centrifuge tube, numbered for future use.
[0047] 2. Extraction of RNA from the tested sample
[0048] Take n sterilized 1.5ml centrifuge tubes, where n is the sum of the number of tested samples and the positive control, critical positive quality control and negative q...
Embodiment 3
[0064] Application of Fluorescence Quantitative PCR Rapid Diagnosis Kit for Specific Detection of Wild Virus Infection of Classical Classical Classical Swine Fever Virus
[0065] 1. Sensitivity test
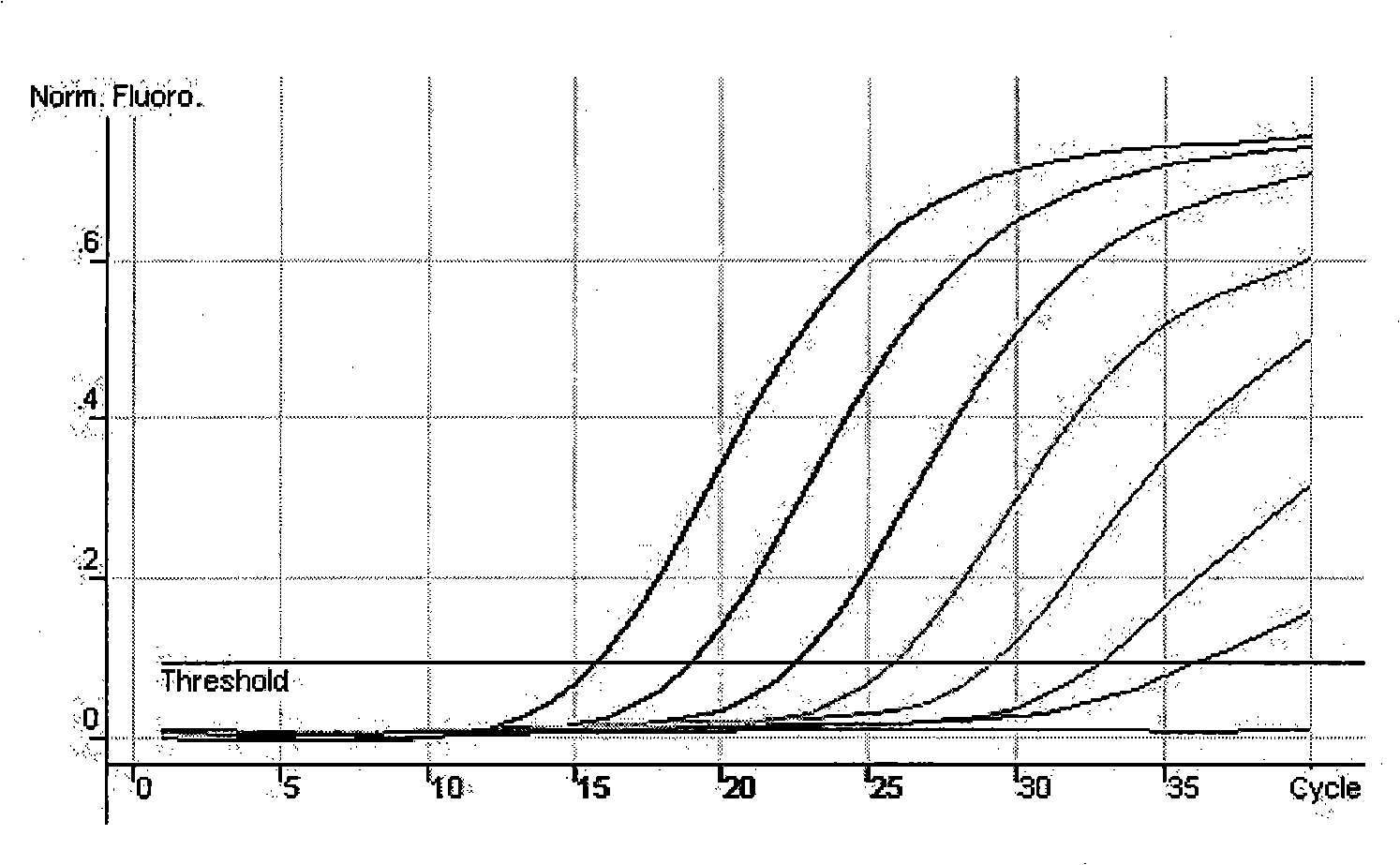
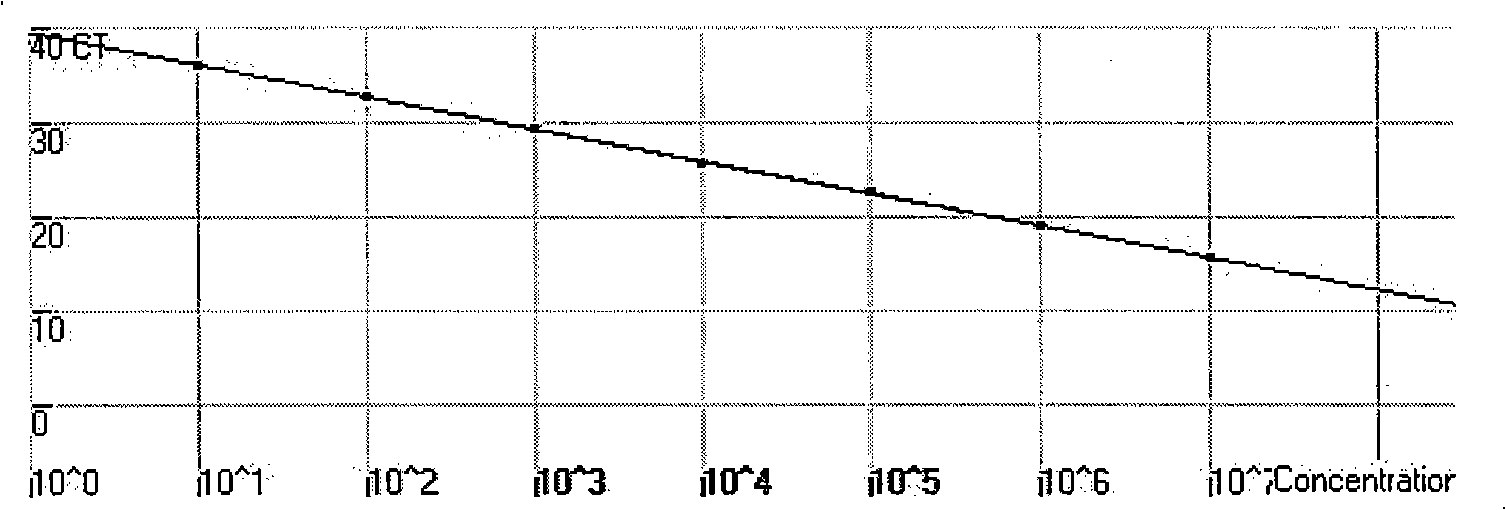
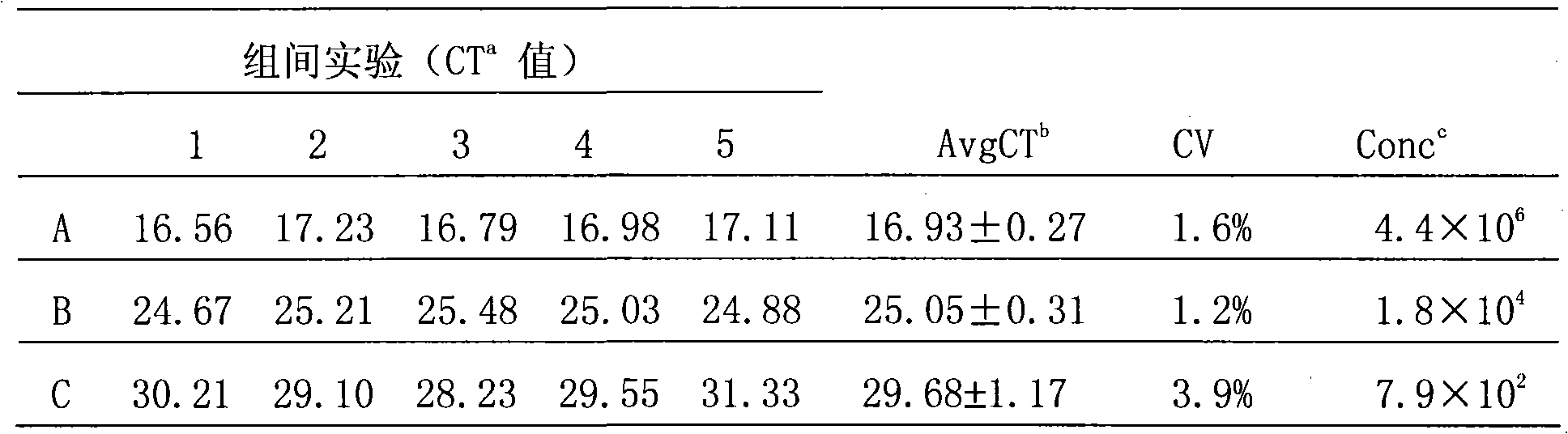
[0066] 10-fold serially diluted standard (1.0×10 1 ~1.0×10 7 copy / μl) as a template, detected on a fluorescent quantitative PCR instrument, to obtain a real-time PCR amplification curve and a standard curve, see the attached figure 1 And attached figure 2 . attached figure 1 It shows that when the standard plasmid concentration ≥ 10 copies / μl, the kinetic curve is on the rise, therefore, the detection sensitivity of the method is 10 copies / μl (the detection sensitivity is the cDNA template concentration of the tested sample, if converted to the detected The sample concentration should be 2.5 x 10 3 copy / m
[0067] 1). attached figure 2 It shows that the linear range of quantitative detection of this method is 1.0×10 1 ~1.0×10 7 copies / μl, correlation coefficient R=0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com